Back to Journals » International Journal of Nanomedicine » Volume 19
Efficacy of Oral Nanoparticle-Encapsulated Insulin in Reducing Oxidative Stress and Enhancing Tissue Integrity in a Diabetic Rat Model
Authors Kaddour N , Benyettou F, Moulai K, Mebarki A , Ghemrawi R, Amir ZC, Merzouk H, Trabolsi A, Mokhtari-Soulimane NA
Received 14 May 2024
Accepted for publication 5 October 2024
Published 28 October 2024 Volume 2024:19 Pages 10961—10981
DOI https://doi.org/10.2147/IJN.S468756
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Xing Zhang
Nawel Kaddour,1 Farah Benyettou,2 Kawtar Moulai,1 Abdelouahab Mebarki,1 Rose Ghemrawi,3,4 Zine-Charaf Amir,5 Hafida Merzouk,1 Ali Trabolsi,2 Nassima Amel Mokhtari-Soulimane1
1Laboratory of Physiology, Physiopathology, and Biochemistry of Nutrition, Department of Biology, Faculty of Sciences of Nature and Life, Earth Sciences and Universe (SNVSTU), University of Tlemcen, Tlemcen, 13000, Algeria; 2Chemistry Program, New York University Abu Dhabi (NYUAD), Abu Dhabi, 129188, United Arab Emirates; 3College of Pharmacy, Al Ain University, Abu Dhabi, P.O. Box 112612, United Arab Emirates; 4AAU Health and Biomedical Research Center, Al Ain University, Abu Dhabi, P.O. Box 112612, United Arab Emirates; 5Department of Anatomy and Pathological Cytology, University Hospital Center Mustapha Pacha, Algiers, Algeria
Correspondence: Nassima Amel Mokhtari-Soulimane, Faculty SNVSTU University of Tlemcen, BP 119, Rocade 2 Mansourah, Tlemcen, 13000, Algeria, Tel +213 557 229 623, Email [email protected]; [email protected] Ali Trabolsi, Chemistry Program, New York University Abu Dhabi (NYUAD), Abu Dhabi, 129188, United Arab Emirates, Tel +971 26284575, Email [email protected]
Introduction: Diabetes mellitus, a chronic metabolic disorder, leads to systemic organ damage characterized by oxidative stress and structural alterations, contributing to increased morbidity and mortality. Traditional subcutaneous insulin therapy, while managing hyperglycemia, often falls short in addressing the oxidative damage and preventing organ-specific complications. This study evaluates the therapeutic efficacy of a novel oral nanoparticle-mediated insulin (nCOF/Insulin) against these diabetes-induced changes, comparing it with traditional subcutaneous insulin in a streptozotocin (STZ)-induced diabetic rat model.
Methods: We induced diabetes in Wistar rats, dividing them into four groups: standard control, diabetic control, diabetic treated with subcutaneous insulin, and diabetic treated with oral nanoparticle-mediated insulin (nCOF/Insulin). Assessments included organ and body weights, histopathological examinations, and oxidative stress markers (MDA and PCOs) across various organs, including the brain, muscle, intestine, spleen, heart, liver, kidney, and adrenal glands. Additionally, we evaluated antioxidant parameters (GSH and catalase) and conducted immunohistochemical analysis of E-cadherin to assess intestinal integrity.
Results: Our findings reveal that STZ-induced diabetes significantly impacts organ health, with subcutaneous insulin providing limited mitigation and, in some cases, exacerbating oxidative stress. Conversely, oral nCOF/Insulin treatment effectively restored organ and body weights, reduced oxidative stress markers, and mitigated histological damage. This suggests that oral nCOF/Insulin not only offers superior glycemic control but also addresses the underlying oxidative stress.
Conclusion: nCOF/Insulin emerges as a promising treatment for diabetes, with the potential to improve patient quality of life by ameliorating oxidative stress and preventing organ-specific complications. This study underscores the need for further investigation into the long-term effects and mechanisms of action of oral nCOF/Insulin, aiming to revolutionize diabetes management and treatment strategies.
Keywords: oral nanoparticle-mediated insulin, diabetes mellitus, oxidative stress, organ toxicity, diabetic complications
Introduction
Diabetes mellitus is a group of metabolic disorders marked by persistent hyperglycemia.1 Over half a billion people are living with diabetes worldwide, which represents more than 10.5% of the world’s adult population.2 This widespread prevalence underscores its status as a major global health challenge.1
Diabetes mellitus, a complex metabolic disorder, is intricately linked to systemic oxidative stress, a key factor in its pathogenesis. It is now understood that the onset of diabetes originates from oxidative stress within pancreatic cells, which triggers inflammation and prompts the release of pro-inflammatory cytokines, ultimately leading to the death of pancreatic beta cells.3,4 This loss of beta cells results in hypoinsulinemia and hyperglycemia, which in turn creates a vicious cycle of oxidative stress throughout the organism.5,6 This systemic oxidative stress then triggers functional and structural alterations in various organs, leading to diabetes-related complications and consequently making diabetes one of the principal causes of mortality and morbidity worldwide.7–9
The contemporary understanding of diabetes has evolved to recognize it as a complex dysglycemic disorder, not just defined by chronic high blood sugar levels (hyperglycemia) but also involving two additional critical mechanisms: glucose variability, and hypoglycemic incidents. These mechanisms collectively contribute to triggering oxidative stress in diabetic patients.5 Oxidative stress is implicated in inducing various alterations across organs,10,11 amplifying the stress response, and perpetuating a cycle of tissue damage that compromises organ function.12–15 A self-sustaining pattern of oxidative stress pervades the entire body,6,16 ultimately resulting in diabetic complications.5,17
These mechanisms are particularly pertinent to type 1 diabetes (TD1), where therapeutic management primarily relies on subcutaneous insulin (SC Ins). Understanding these mechanisms not only sheds light on the complexities of diabetes pathology but also underscores the challenges inherent in current insulin therapy. While subcutaneous insulin effectively targets hyperglycemia, its limitations become apparent when considering the multifactorial nature of diabetes. The management of diabetes should not only be limited to addressing chronic hyperglycemia but should systematically consider glucose variability, and hypoglycemic incidents. However, the reliance on subcutaneous insulin injections fails to address the underlying dysglycemic mechanisms and oxidative stress induced by diabetes.3,12 Furthermore, the pharmacokinetic limitations of subcutaneous insulin necessitate frequent administrations to achieve glycemic control, inherently associated with risks of hypoglycemia and inadequate postprandial glucose management.18,19 Consequently, insulin therapy, as currently administered, does not adequately prevent diabetes-related complications and may even exacerbate them by further exacerbating oxidative stress.5,20 The challenge today is to develop therapies that address all the pathophysiological mechanisms of diabetes.
An ideal diabetes management system requires swift glucose level adjustments without causing insulin overdose or hypoglycemia. It must support lifelong therapy with easy, predictable use, minimal side effects, and stable performance over time. These features are crucial in developing a glucose-responsive insulin delivery system aimed at achieving a synthetic pancreas, addressing challenges like preventing burst release, ensuring ease of administration, and managing degradation and immune responses. This is why alternatives are currently being sought, with nanoparticle-based approache for oral insulin emerging as a promising option due to its ability to closely mimic physiological insulin.21–23 Nanoparticles can be engineered to encapsulate and deliver insulin in a controlled manner, offering several advantages over conventional insulin therapy (Figure 1). These advantages include improved pharmacokinetics, enhanced bioavailability, and targeted delivery to specific tissues or cells. To address these needs, covalent organic framework nanoparticles (nCOFs), have shown great promise. nCOFs, known for their robustness, tunability, and suitability for biological applications due to their stability in water and acidic environments, offer a novel approach to insulin delivery. In a previous study, we highlighted the use of a specific nCOF, synthesized from 2,6-diformylpyridine and 4,4′4″-(1,3,5-triazine-2,4,6-triyl)trianiline, which demonstrated high insulin loading capacity, biocompatibility, and effective glucose-triggered insulin release (nCOF/Insulin).19 The system allows insulin to be stored between nanosheet layers, enhancing its protection in the stomach’s harsh conditions and subsequent controlled delivery. Furthermore, this method directly absorbs insulin through the intestinal epithelium and delivers it to the liver, more accurately mimicking the natural central-to-peripheral insulin distribution. This approach ensures that the liver receives the highest concentration of insulin, acting as a buffer to prevent peripheral hyperinsulinemia. Moreover, the insulin release from the nCOF/Insulin system closely mimics physiological processes. Under natural conditions, insulin secretion is regulated in a pulsatile, non-linear manner in response to blood glucose levels, a pattern crucial for maintaining glucose homeostasis and aligning insulin levels with metabolic needs. Subcutaneous insulin (SC Ins) often results in a rapid spike in insulin levels, which does not replicate this natural pulsatile secretion. Our oral nCOF/Insulin formulation, however, demonstrates a controlled and gradual release of insulin, thereby reducing the risk of hypoglycemic episodes and offering a more natural and physiologically relevant delivery method. Additionally, the nCOF/Insulin system features a dose-dependent and self-regulated release mechanism. This on-off regulation mimics the pulsatile release of endogenous insulin, ensuring tight control of insulin levels and preventing both hypo- and hyperglycemia. These unique properties of the nCOF/Insulin system—central-to-peripheral distribution, non-linear and pulsatile release, and dose-dependent response—play a crucial role in its physiological efficacy. Collectively, these mechanisms enable the oral nCOF/Insulin formulation to more closely replicate natural insulin secretion, highlighting its advancement over traditional subcutaneous injections.19 Furthermore, our last study showed that the nCOF can prevent hemostasis disorder and endothelial injuries related to type 1 diabetes, while the traditional subcutaneous treatment exacerbates them. Specifically, we have shown that oral insulin delivered via nCOF addresses various mechanisms in the systemic circulation, such as dyslipidemia, inflammation, oxidative stress, and cortisol levels.24
This intriguing observation underscores the intricate interplay between insulin delivery methods and resulting physiological responses. Building upon these findings, the present study aims to delve deeper into this phenomenon by comparing the effects of subcutaneous and oral nCOF/Insulin on oxidative stress profiles and tissue integrity in a diabetic rat model. Through detailed histopathological analysis, we aim to elucidate the specific impacts of oral nCOF/Insulin, particularly its potential to mitigate diabetes-induced tissue alterations. Additionally, this study examines the non-toxicological profile of oral nCOF/Insulin compared to SC insulin, reinforcing its promise as a viable and effective alternative for diabetes management.
By offering a comprehensive evaluation of oral nCOF/Insulin’s efficacy and safety, this research provides critical insights into the potential of innovative nanoparticle insulin delivery strategies. While the study demonstrates promising short-term effects, the findings suggest that such strategies may also contribute to addressing the complex challenges of diabetes treatment and potentially mitigating diabetic complications in the long term, thereby advancing the field toward more effective and patient-friendly therapeutic options.
Methods
Insulin Loaded nCOF Nanoparticles (nCOF/Insulin)
Synthesis and Characterization of Insulin-Loaded nCOF Nanoparticles (nCOF/Insulin)
nCOFs were synthesized through a co-condensation of 2.6-diformylpyridine (DFP, 21 mg, 0.15 mmol) and 4,4′,4″-(1,3,5-triazine-2,4,6-triyl)trianiline (TTA, 12 mg, 0.03 mmol) in anhydrous 1,4-dioxane (3 mL) with 0.5 mL of acetic acid (13 M) to achieve a final concentration of 4.0 M. This mixture was reacted at room temperature for 10 minutes and subsequently dialyzed in water to remove unreacted materials, yielding a stable colloidal suspension of nCOFs. The resultant nanoparticles exhibited an average diameter of 123.7 ± 18.1 nm and a spherical morphology confirmed via Transmission Electron Microscopy (TEM). The surface charge was determined by zeta potential measurements, indicating a value of −16 mV, which suggests good colloidal stability.19
Drug Loading and Encapsulation Efficiency
For insulin loading, nCOFs (5 mg) were dispersed in 2 mL of HEPES buffer, to which a HEPES-buffered aqueous solution of insulin (10 mg/mL, 1 mL) was added, maintaining an nCOF ratio of 1:2. This mixture was stirred overnight at pH 7.4 and room temperature. The unbound insulin was removed by multiple centrifugation washes with deionized water. The insulin loading capacity reached 64.6 ± 1.7 wt%, with encapsulation efficiency confirmed through various advanced characterization techniques such as fluorescence and Fourier Transform Infrared (FTIR) Spectroscopies as well as X-ray Photoelectron Spectroscopy (XPS), which indicated specific interactions between insulin and the nCOF framework. Additional studies utilizing Powder X-ray Diffraction (PXRD) showed distinct peaks confirming the crystalline structure, while Brunauer-Emmett-Teller (BET) analysis demonstrated a significant surface area, ensuring the structural integrity and porosity essential for insulin encapsulation.19
Stability and Release Profile
Stability assessments under simulated gastrointestinal and bloodstream conditions confirmed that the nanoparticles maintained their integrity and protected the insulin payload. Insulin release studies demonstrated a controlled release profile, particularly under hyperglycemic conditions, suggesting a glucose-responsive release mechanism facilitated by the nanoscale dimensions and porous structure of the nCOFs, which enable rapid glucose-mediated insulin displacement.19
Biocompatibility and Efficacy Studies
In vitro and in vivo studies confirmed the biocompatibility and efficacy of the nCOF/insulin system for oral insulin delivery. Viability assays on several cell lines (Hep-G2, HCT-116, HCT-8, RKO, HeLa, A2780, MDAMB-231, MCF-7, HEK-293 and U251-MG) showed no cytotoxic effects, affirming the system’s suitability for oral use. Further TEM analysis demonstrated that nCOF/insulin did not alter cellular ultrastructure, suggesting safe endocytosis and internal processing. Hemolytic assays confirmed the formulation’s non-immunotoxic nature, underlining its safety for bloodstream entry. Ex vivo experiments showed that the nanoparticles effectively crossed the intestinal barrier, enhancing insulin permeability and demonstrating a promising method for diabetes treatment. Oral administration in diabetic rats led to significant reductions in blood glucose levels up to 10 hours without causing organ damage or affecting kidney and liver functions, highlighting the potential of nCOF/insulin to maintain glucose homeostasis and serve as a substitute for subcutaneous injections.19
In vivo Experiments
Animals
This study utilized twenty Wistar rats, comprising ten males and ten females, aged 12 weeks and weighing approximately 200 ± 20 g, which were obtained from the Pasteur Institute. The rats were housed in plastic cages with wood-chip bedding, under a constant temperature of 25 °C and a 12:12-hour light/dark cycle. The animals were provided with a standard pellet diet (Teklad Global 18% protein rodent diet, Harlan Laboratories, 58% carbohydrate, 24% protein, 18% fat) and had unrestricted access to water throughout the study. All procedures were conducted in strict accordance with national standards for the care and use of laboratory animals, and the study protocol was approved by the Animal Research Ethics Committee of the University Abou Bekr Belkaid of Tlemcen in 2020 (accreditation number: D01N01UN130120150006).
T1D Induction
T1D was induced via a single intraperitoneal injection of streptozotocin (STZ, dissolved in 10 mM citrate buffer at pH 4.5) at a dose of 45 mg.kg–1 of body weight.25,26 The rats were then returned to their cages and provided with food and water until the onset of diabetes. Blood glucose levels were monitored using a blood glucose monitoring system (AccuChek Performa, Hoffman-La Roche) by sampling from the rat’s tail vein. Rats with fasting glycemia ≥ 250 mg.dL–1 (13.7 mmol.L–1) were considered diabetic and selected for the study; the experimental protocol is summarized in Figure 2.
Study Design
Following the induction of diabetes, the rats were categorized into four distinct groups, each consisting of five animals. The groups were organized as follows: standard control (C), diabetic control (DC), diabetic rats treated with subcutaneous insulin (SC Ins, at a dosage of 5 IU kg–1), and diabetic rats treated with oral administration of nanoparticle-formulated insulin (Oral nCOF/Ins, at a dosage of 50 IU kg–1). After their allocation into groups, the rats underwent an overnight fasting period (12 hours) with free access to water. Subsequently, they received a single dose of insulin when applicable in the early morning. Throughout the remainder of the day, the rats had access to food and water. They were then fasted overnight once again. The sacrifice was conducted in the early morning following this overnight fasting period, during which the rats had free access to water.
In our study design, the dosages for subcutaneous (5 IU/kg) and oral (50 IU/kg) insulin delivery were selected based on the distinct bioavailability profiles of these administration routes. Subcutaneous delivery provides nearly 100% bioavailability, allowing lower doses to be effective. In contrast, oral administration involves challenges such as significant enzymatic degradation and first-pass metabolism, leading to markedly lower bioavailability. To compensate for this and ensure sufficient systemic insulin levels, a higher dose is used for oral administration. This dosage strategy is critical for comparing the therapeutic efficacy of the two delivery methods under equivalent systemic exposure conditions.
Sample Collection
Following an overnight fast, the animals were weighted and then euthanized under deep anesthesia with isoflurane, at a concentration of approximately 2.5% v/v.27,28 Then, various organs were harvested for analysis. The organs collected included the intestine, spleen, heart, liver, kidney, brain, and soleus muscle, all of which were immediately rinsed with ice-cold 0.1 M phosphate-buffered saline (PBS; pH 7.4) to remove any residual blood and debris. The weights of the spleen, heart, liver, and kidneys were accurately measured. For biochemical analyses, a portion of each tissue was homogenized in a solution of ice-cold 10 mM phosphate-buffered saline. This process was performed using an ultra-Turrax homogenizer (Bioblock Scientific, Illkirch, France) for three cycles of 10 seconds each, ensuring thorough disruption of the tissue. The homogenates were then centrifuged at 3000 rpm and 4 °C for 5 minutes to separate the supernatant fractions, which were collected for subsequent redox marker determinations.
For histological examination, samples were carefully sectioned using a sharp razor blade and fixed in a 10% formalin solution to preserve tissue architecture. These samples were then subjected to a dehydration process through a series of alcohol baths of increasing concentration, preparing them for embedding in paraffin blocks. This meticulous preparation allows for detailed microscopic examination of tissue structure and pathology.29,30
Biomarkers of Oxidative Stress and Antioxidant Defense
Malondialdehyde (MDA), a biomarker of lipid peroxidation, was quantified in tissue samples by measuring thiobarbituric acid reactive substances (TBARS). The procedure involved treating the samples overnight with Sodium Dodecyl Sulfate (SDS), followed by quantification of TBARS through their reaction with thiobarbituric acid (TBA) under acidic conditions. This reaction produces a pink chromophore, the intensity of which was measured spectrophotometrically at a wavelength of 532 nm.31 The MDA concentration was reported as micromoles of MDA per mg protein and expressed as percentage of the Control Protein Carbonyls (PCOs), indicators of protein oxidation, were assessed by determining the carbonyl group content via reaction with dinitrophenylhydrazine (DNPH), according to a well-established protocol.32 Protein precipitation was achieved using 20% trichloroacetic acid, followed by redissolution in DNPH. The absorbance of the resulting solution was measured spectrophotometrically at 370 nm, with PCO concentration expressed as nanomoles of PCOs per milligram of protein and expressed as percentage of the Control.
Catalase activity, an important enzymatic antioxidant defense, was assessed by measuring the rate of hydrogen peroxide decomposition using a spectrophotometric kinetic method.33 The decrease in absorbance due to hydrogen peroxide breakdown was monitored at 240 nm. Catalase activity was expressed as micromoles of hydrogen peroxide degraded per minute per milligram of protein and expressed as percentage of the Control.
Glutathione (GSH) levels, reflecting the tissue’s antioxidant capacity, were determined using a method that involves the reduction of 5.5′-dithiobis(2-nitrobenzoic acid) (DTNB) by reduced glutathione, leading to the formation of a yellow compound.32 The intensity of this color, directly proportional to the GSH concentration, was measured at 405 nm. GSH concentration was quantified as micromoles of GSH per milligram of protein and expressed as a percentage of the Control.
Histopathological Study
For the histopathological analysis, sections with a thickness of 5 μm were meticulously prepared from the paraffin-embedded blocks utilizing a microtome. Following preparation, these sections were stained with hematoxylin and eosin (H&E) to enhance the visualization of cellular and tissue structures.29,30 The stained sections were then examined and documented using an optical microscope (AX80, Olympus, Tokyo, Japan). During the microscopic examination, observations were documented based on criteria established through a comparison of the histological features of the samples to those of the control group. Each notable observation, such as deviations from normal tissue architecture or the presence of specific pathological features, was recorded with a photograph. To ensure a comprehensive assessment of tissue alterations, we systematically examined sections from each organ of every rat used in the study. This was followed by comparative imaging across other samples to ensure consistency and representativeness of the findings.
Immunohistochemical Staining
Immunohistochemical staining was employed to investigate the impact of nCOF/Insulin on the expression of E-cadherin, a critical cell adhesion protein essential for the cohesion and integrity of epithelial cell layers, in the intestines of diabetic rats. The procedure began with the collection of intestinal tissue samples from both the treatment group, receiving nCOF/Insulin, and the control group. These samples were then fixed in buffered paraformaldehyde, dehydrated through a graded series of ethanol, cleared in xylene, and finally embedded in paraffin. Tissue sections of 4–5 μm thickness were prepared using a microtome, deparaffinized, and rehydrated. Antigen retrieval was performed by heating the sections in a citrate buffer, followed by blocking of non-specific binding sites. The sections were incubated with Dako’s E-Cadherin antibody (Clone: NCH-38, Cat. No. M3612), according to the manufacturer’s recommendations. E-cadherin expression was visualized as violet staining.
Statistical Analysis
The data were analyzed using SPSS (IBM, SPSS Statistics, version 23, USA) and expressed as means ± standard error of the mean (SEM). Data were analyzed using one-way ANOVA with post hoc Tukey’s tests for multiple comparisons. A value of p < 0.05 was considered statistically significant.
Results
Rat Weight and Organ Weight
Diabetes significantly reduced the body weight of rats (Figure 3a) and increased the relative weights of their organs (Figure 3b), indicating metabolic distress. Subcutaneous insulin fails to restore body weights, and while it does normalize relative organ weights of the rat, heart, and kidney, it does not achieve the same effect in the liver. Notably, oral administration of nCOF/Insulin effectively counteracted these effects, reinstating body and relative organ weight to near-normal levels.
Oxidative Stress
Our findings suggest that diabetes disrupts the balance between oxidants and antioxidants. Diabetes induces oxidative stress, as evidenced by disturbances in MDA levels in all organs of untreated diabetic rats (Figure 3c, D.C.), along with an increase in protein carbonyl concentrations in the spleen, heart, and muscle (Figure 3d). Furthermore, antioxidant concentrations in certain organs are impacted, particularly in the intestine for GSH (glutathione), and in the heart and muscle for GSH and catalase, respectively (Figure 3e and f).
Injections of insulin in diabetic rats, administered subcutaneously (SC Ins), appeared to mitigate the increases in MDA induced by diabetes in some organs (liver, kidney, and brain, Figure 3c), as well as protein carbonyls in the spleen and heart, although not in the muscles (Figure 3d). However, subcutaneous insulin treatment also increased protein carbonyl concentrations in the intestine and muscle.
Administration of Oral nCOF/Insulin resulted in a reduction of oxidative stress levels induced by diabetes. MDA levels (Figure 3c) and protein carbonyl concentrations (Figure 3d) in diabetic rats treated with Oral nCOF/Insulin closely resembled those of non-diabetic rats (C.), indicating a restoration of oxidative balance. Moreover, the increased concentrations of GSH in the muscle (Figure 3e) and catalase in the intestine and heart (Figure 3f) underscore the antioxidant properties of Oral nCOF/Insulin on diabetic organs.
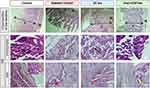
Histological Sections of Rat Intestine
Under microscopic examination, the intestinal lining of control rats displayed a highly organized structure, characterized by well-defined epithelial villi and a pronounced muscular layer (Figure 4, Control). At high magnification of the villi, lymphoid elements were observed in the lamina propria, a connective tissue layer at the core of the villi. Additionally, glands responsible for the secretion of digestive enzymes and mucus were located within the epithelial layer.
Diabetes markedly induced epithelial degeneration within the intestine, as evidenced by increased crypt depth and villus height in untreated diabetic rats (Figure 4, Diabetic Control), compared to control and insulin-treated groups. Detailed examination at higher magnification revealed a notable decrease in lymphoid elements in the Diabetic Control group, contrasting sharply with the Control and insulin-treated rats. Lymphoid elements are crucial for antibody synthesis, playing a vital role in regulating microbial growth and maintaining intestinal health.
In diabetic rats treated with oral nanoparticle-mediated insulin (Figure 4, Oral nCOF/Ins), the intestinal villi showcased endocrine cells and glands mirroring the structural integrity observed in non-diabetic controls (yellow arrows). This contrasts with the untreated diabetic group and those receiving subcutaneous insulin (Figure 4, Diabetic Control and SC Ins), where significant architectural disruption and cellular necrosis were observed (red asterisks), leading to obscured intestinal glands (red arrows).
Histological Sections of Rat Spleen
Microscopic examination of the spleen in control rats revealed a well-defined architecture, characterized by a clear distinction between the red pulp and the white pulp, the latter marked by white circles for identification (Figure 5, Control). The white pulp is further organized into a germinal zone (GZ) encircled by a marginal zone (MZ), reflecting the typical histological features of a healthy spleen.
Diabetes significantly alters spleen morphology, most notably reducing the white pulp to red pulp ratio, which is often associated with alterations in immune response (Figure 5, Diabetic Control). The germinal zone (GZ) within the white pulp of untreated diabetic rats appeared diminished and blurred, lacking the distinct marginal zone (MZ) observed in healthy spleens. This structural degradation was similarly noted in rats receiving subcutaneous insulin, with an unclear demarcation between the two pulp zones (Figure 5, SC Ins).
High-magnification observations revealed densely packed Billroth cords in the red pulp of control specimens, indicative of robust cellular activity (Figure 5, Control). In contrast, diabetic rats exhibited a significant reduction in red pulp cellularity, with noticeable thickening of the Billroth cords which appeared contracted (Figure 5, Diabetic Control, red arrow), suggesting compromised spleen function. This condition was exacerbated in rats treated with subcutaneous insulin (Figure 5, SC Ins, red arrow).
In the white pulp, observation of the central artery (CA) revealed lymphoid tissue surrounding it in control, a typical structure (Figure 5, Control, yellow arrows). However, in untreated diabetic rats, significant dilatation marked by black asterisks and an absence of lymphocytic cells, indicative of immunodepletion, was observed (Figure 5, Diabetic Control). Subcutaneous insulin treatment restored lymphocytes in diabetic rats, indicating a positive effect on lymphocyte presence (Figure 5, SC Ins, yellow arrow).
Remarkably, diabetic rats treated with oral nCOF/Insulin showed a restoration of the spleen’s structural integrity, with the white pulp/red pulp ratio and the clarity of the GZ and MZ closely resembling those of control rats and a red pulp with good cellularity and a white pulp rich in lymphocytes (yellow arrow) (Figure 5, Oral nCOF/Ins).
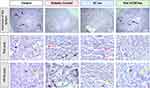
Histological Sections of Rat Heart
Microscopic examination of heart cross-sections from control rats delineated the heart’s layered architecture with remarkable clarity (Figure 6, Control), with the innermost layer, the endocardium (Ed), lines the heart chambers, playing a crucial role in maintaining a smooth, frictionless environment for blood flow. The myocardium (My), the thick, muscular middle layer, is instrumental in the heart’s contraction, facilitating blood circulation throughout the body. The outermost layer, the epicardium (Ep), serves as a protective sheath for the heart.
In contrast, untreated diabetic rats exhibited significant pathological changes, including thickening of the subendocardial area and notable myocardial dilation (Figure 6 Diabetic Control, black asterisk). These changes suggest a compromised cardiac function, likely attributable to the metabolic disturbances associated with diabetes.
Remarkably, both subcutaneous and oral nanoparticle-mediated insulin treatments appeared to mitigate these diabetic-induced cardiac alterations. The treated rats’ heart tissues closely resembled the healthy architecture observed in control rats, suggesting that the insulin treatment effectively repairs heart alterations induced by diabetes (Figure 6, SC Ins and Oral nCOF/Ins).
Histological Sections of Rat Liver
Microscopic examination revealed that the liver tissue of control rats and those treated with oral nCOF/Insulin exhibited a normal histological architecture. This was characterized by a well-defined centro-lobular vein (cv) surrounded by hepatocytes, which were evenly spaced by sinuses of regular thickness, indicative of healthy liver function (Figure 7, Control and Oral nCOF/Ins, black arrow).
In contrast, Diabetic Control and diabetic rats treated with subcutaneous insulin (SC Ins) showed severe pathological changes: untreated diabetic rats exhibited atrophied hepatocytes, leading to dilated sinuses (Figure 7, Diabetic Control, red arrow), which are symptoms of cellular degeneration due to insulin deficiency and compromised metabolic activity. Rats treated with subcutaneous insulin also showed signs of cellular distress, including cellular swelling, indicative of impending necrosis, and resulting in a reduction in sinus spacing (Figure 7, SC Ins, red arrow). Additionally, high-magnification of liver sections from untreated diabetic rats and those receiving subcutaneous insulin therapy displayed significant pathological alterations. Notably, these sections were marked by extensive necrotic areas (red asterisks), identifiable by necrotic cells with pale, white nuclei (red arrows), signaling severe tissue damage (Figure 7, Diabetic Control and SC Ins).
Conversely, the liver tissue of diabetic rats treated with oral nCOF/Insulin exhibited significant restoration of its normal structure. Hepatocytes in these rats displayed dark, prominent nuclei. Additionally, there was a notable presence of binucleated cells (double black arrows) which can be linked to enhanced metabolic activity and cellular regeneration (Figure 7, Oral nCOF/Ins).
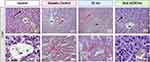
Histological Sections of Rat Kidney
Microscopic analysis of kidney sections from control rats displayed a pristine renal architecture, characterized by healthy glomeruli (G), intact renal tubules, and an intact brush border (black arrow), all indicative of optimal kidney function (Figure 8, Control). The nuclei within these structures were prominently visible underscoring the tissue’s health.
In contrast, kidney sections from untreated diabetic rats and those treated with subcutaneous insulin revealed significant histological alterations. Notably, these changes included glomerular degeneration (red asterisks), dilation of renal tubules (black asterisk), and a conspicuous loss of the brush border (red arrow) (Figures 8, Diabetic Control and SC Ins). These findings highlight the detrimental impact of diabetes on renal integrity and the insufficiency of subcutaneous insulin in mitigating these effects.
Remarkably, diabetic rats treated with oral nCOF/Insulin exhibited kidney sections that mirrored the healthy architecture observed in control rats, with intact glomeruli (G) and tubules featuring healthy tubular epithelial cells and a healthy brush border (Figure 8, Oral nCOF/Ins, black arrow).
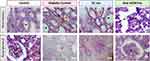
Histological Sections of Rat Adrenal Gland
The adrenal gland of control rats exhibits a well-organized structure, characterized by three distinct cortical zones: the Zona Glomerulosa (ZG), the Zona Fasciculata (ZF), and the Zona Reticularis (ZR), alongside a centrally located medulla (M) (Figure 9, Control). This organization is crucial for the gland’s endocrine function, with each zone responsible for producing specific hormones.
In untreated diabetic rats, significant morphological changes were observed in the adrenal glands, suggesting stress-induced alterations in adrenal function. In the first panel, the medulla (M) appeared unusually expanded (Figure 9, Diabetic Control), while alterations in the proportions of the different zones of the cortex were observed (highlighted by double red arrows). Additionally, at high magnification in the third panel, vacuolization within the Zona Fasciculata suggested hyperactivity and hypersecretion of hormones (Figure 9, Diabetic Control, red arrows). Furthermore, diabetic rats treated with subcutaneous insulin exhibited an enlarged adrenal cortex, particularly the ZF, which was approximately twice the size observed in control rats (Figure 9, SC Ins, double red arrows). At lower magnification, the ZF in SC Ins rats appeared indiscernible and resembled a large necrotic zone (red asterisk, first panel); however, higher magnification revealed vacuolated areas similar to those observed in untreated diabetic rats (red arrow, third panel).
Conversely, diabetic rats treated with oral nanoparticle-mediated insulin (nCOF/Insulin) showed adrenal glands with cortical zones (ZG, ZF, and ZR) and a medulla closely resembling the organized structure seen in control rats (Figure 9, Oral nCOF/Ins).
Immunohistochemical Analysis of E-Cadherin Expression
Observation of the intestine section stained with E-cadherin antibody in Figure 10 revealed that the intensity of E-cadherin staining in rats treated with oral nCOF/Insulin matched that of the control group, indicating no depletion of E-cadherin post-treatment. Similarly, untreated diabetic rats and those treated with subcutaneous insulin exhibited E-cadherin expression levels comparable to the control group.
Comprehensive histopathological examinations of muscle and brain tissues across the four groups did not reveal any significant changes.
Discussion
Our comprehensive study on the effects of diabetes and subsequent insulin treatments on various organs in rats provides significant insights into the pathological changes induced by diabetes and the therapeutic potential of oral nanoparticle-mediated insulin (nCOF/Insulin) in comparison to subcutaneous insulin.
Our findings indicate that streptozotocin-induced diabetes causes widespread organ toxicity, as evidenced by notable changes in organ weight, disruption of the oxidative-antioxidative equilibrium, and various histological modifications, primarily including necrosis, vasodilations, and karyolysis (loss of nuclei) in tissues of various organs. The observed reduction in body weight alongside increased organ weights in diabetic rats underscores the metabolic distress caused by diabetes. Additionally, the increase in relative organ weights provides insight into the toxicity within the organs. The restoration of body and organ weights to near-normal levels with oral nCOF/Insulin treatment highlights its efficacy in counteracting the metabolic imbalances and the underlying stress induced by diabetes, suggesting a promising therapeutic avenue for managing diabetes-induced cachexia and organ hypertrophy.
The imbalance between oxidants and antioxidants, leading to heightened oxidative stress, is a hallmark of diabetes pathology. Our findings of elevated malondialdehyde (MDA) levels and protein carbonyl concentrations across various organs in diabetic rats underscore the systemic nature of oxidative damage. Notably, oral nCOF/Insulin administration effectively reduced these oxidative stress markers, aligning them with those observed in non-diabetic rats. This suggests that oral nCOF/Insulin not only mitigates hyperglycemia but also addresses the underlying oxidative stress through effective insulinization, which implies metabolic improvement. This offers a comprehensive therapeutic strategy for diabetes management.
Brain
The long-term impact of diabetes on brain health is well-documented,34 with the immediate effects of increased oxidative stress being clearly observable in our findings. During diabetes, the brain undergoes impaired energy metabolism. This impairment can result from a combination of factors, including reduced glucose uptake and increased oxidative stress.35,36 The brain, with its lipid-rich composition, is especially susceptible to lipid peroxidation due to metabolic imbalances caused by a lack of insulin.37,38 Our study reveals that insulin therapies, administered both orally and subcutaneously, are effective in mitigating lipid peroxidation. This is evidenced by the reduced malondialdehyde (MDA) levels in the brains of diabetic rats. However, subcutaneous insulin therapy has limitations, particularly in inducing hypoglycemia. In contrast, our findings demonstrate that nCOF administration effectively reduces MDA levels in the brain, akin to subcutaneous insulin, but without the associated risk of hypoglycemia,36 as evidenced by our previous study.19 This highlights the potential of nCOF as a safer alternative treatment option for diabetes-related alterations affecting the brain.
Muscle
Streptozotocin is known to trigger oxidative stress in muscle tissue, resulting in functional decline.39,40 Moreover, the lack of insulin compromises the cellular uptake of glucose and amino acids, leading to increased proteolysis, muscle degradation, and the consequent weight loss observed in diabetes.41,42 In our study, we observed significant weight loss and increased oxidative stress in the skeletal muscle of diabetic rats and those treated with subcutaneous insulin, with a notably higher level of protein carbonylation in diabetic rats treated with subcutaneous insulin. This aligns with previous research, which has shown that subcutaneous injections of insulin lead to peripheral hyperinsulinemia, linked to elevated oxidative stress in muscle tissues.43,44 This phenomenon elucidates why insulin administered subcutaneously does not reestablish the equilibrium between pro-oxidants and antioxidants, but rather exacerbates oxidative stress in the muscles through the hyper-insulinemia it induces. Interestingly, the oral administration of nCOF/Insulin not only restored body weight but also reestablished the balance between pro-oxidant and antioxidant, highlighting the therapeutic potential of this delivery method.
Intestine
Our investigation into the intestinal alterations in diabetic rats reveals a significant deepening of crypts, highlighting the adverse effects of diabetes on intestinal health. These observations align with previous findings that attribute such histological changes to oxidative stress and apoptotic mechanisms induced by diabetes in the intestine.45,46 Our analysis indicates that these histological alterations are primarily due to an imbalance in the oxidant/antioxidant balance, notably characterized by a reduction in antioxidant levels in individuals with diabetes. Interestingly, insulin treatments, whether administered subcutaneously or orally, appear to mitigate these histological changes. However, with subcutaneous insulin administration, we observed significant necrotic areas within the intestinal tissue, accompanied by elevated levels of carbonylated proteins, a marker associated with chemical alterations in the intestine due to diabetes.14,47 The effectiveness of oral nCOF/Insulin treatment in restoring intestinal architecture underscores its potential in preventing diabetes-induced gastrointestinal complications.
The immunohistochemical analysis of E-cadherin was specifically used to assess intestinal barrier integrity, which is crucial for our study given that our newly formulated nanoparticles are absorbed in the intestine. E-cadherins are essential transmembrane adhesion molecules crucial for the cohesion of the intestinal epithelium.48 Our E-cadherin labeling in the intestine shows that despite the effective absorption of the nanoparticles, the integrity of the intestinal barrier is preserved, as indicated by the comparable levels of E-cadherin across all groups, including the control.
These findings are corroborated by those from our previous in vitro analysis where transmission electron microscopy (TEM) images revealed that the nCOF/insulin nanoparticles were located inside goblet cells of the intestinal tissue and were excreted into the gut lumen through cell secretion.19 This confirms that our nanoparticle formulation can traverse the intestinal barrier while maintaining its integrity and carrying the insulin cargo without causing significant pathological changes to intestinal tissues.
Spleen
Diabetes is associated with degenerative changes in splenic tissues induced by oxidative stress.11,49 In our study, streptozotocin-induced diabetes resulted in a marked reduction of the white pulp and the marginal zone within the spleen—key anatomical regions integral to the organ’s immune function. This reduction in white pulp has been characterized as stemming from an increase in apoptosis triggered by oxidative stress accumulation due to prolonged hyperglycemia.50 Such alterations in splenic structure contribute significantly to the immunodeficiency observed in diabetic conditions, underscoring the spleen’s critical role in the array of diabetic complications. Furthermore, our findings indicate that while subcutaneous insulin treatment may mitigate some aspects of this degeneration, it appears to offer only limited protection against the comprehensive degradation of splenic tissues. This observation suggests that subcutaneous insulin administration alone may not adequately prevent the onset of immunodeficiency disorders associated with diabetes, highlighting the need for more effective therapeutic strategies to preserve immune function in diabetic patients. The restoration of splenic architecture with oral nCOF/Insulin treatment suggests its role in preserving immune competence in diabetic conditions, potentially mitigating the risk of immunodeficiency and related complications.
Heart
Chronic hyperglycemia associated with diabetes is known to exert pathological effects on the structure and function of the myocardium,5,51 and for inducing subendocardial stress-related alterations.52 Microscopic examination of heart sections from diabetic rats treated with either subcutaneous insulin or oral nCOF/Insulin showed normal histological architecture, indicating that both treatments effectively restored cardiac integrity. This observation is consistent with existing research, which suggests that the pathological alterations in the heart induced by diabetes can potentially be reversed through insulin therapy.53,54
Liver
The pathophysiology of diabetes involves an impairment of antioxidants and an increase in oxidative stress at the hepatic cell level, leading to intracellular damage.55,56 These biochemical imbalances result in observable alterations under the microscope: hepatocyte ballooning, periportal necrosis, and dilatation.57 Our findings are in line with existing literature, and our histological sections reveal alterations in both untreated diabetic rats and those administered subcutaneous insulin. Although subcutaneous insulin administration was observed to decrease malondialdehyde (MDA) and protein carbonyls (PCOs) levels in the livers of diabetic rats, it failed to ameliorate tissue alterations. This aligns with previous findings suggesting that subcutaneous insulin is ineffective in mitigating liver oxidative stress damage.12 This collective evidence underscores the limitations of subcutaneous insulin in addressing diverse aspects of hepatic pathology associated with diabetes. This ineffectiveness can be attributed to insufficient insulinization, leading to inadequate restoration of glucose uptake and impairment in liver glucose metabolism.58 This underscores the critical role of the administration route in insulin assimilation by organs and its metabolism.
Kidney
The impact of diabetes on renal function and structure is extensively documented, aligning with our findings that corroborate previous research indicating significant histological changes within renal tubules, nephrons, and vasculature.42,59 These alterations are mediated by the impairment of oxidative species in the kidney.42,60 Notably, Ghavimishamekh et al explored the efficacy of both subcutaneous and oral insulin in mitigating these renal alterations in diabetic rats, reporting no observable improvements.42 Consistent with these findings, our study also revealed that subcutaneous insulin administration failed to ameliorate the diabetes-induced histological changes in the kidneys. However, our investigation into the effects of oral nCOF/Insulin presents a contrasting outcome, indicating a potential therapeutic advantage of this novel insulin delivery method in preserving renal integrity in the context of diabetes.
Adrenals
The adrenal glands, pivotal in the endocrine system,61 experience significant structural and compositional changes in the context of diabetes.62,63 Research indicates that diabetes, particularly when induced by streptozotocin (STZ), leads to the hypersecretion of adrenocorticotropic hormone (ACTH) from the pituitary gland. This process stimulates the growth of the zona fasciculata within the adrenal cortex while concurrently causing atrophy in the zona glomerulosa.64 Furthermore, it has been observed that subcutaneous insulin treatment impacts the adrenal glands by promoting the secretion of androgens, thereby contributing to structural alterations and notably increasing cortex thickness.39,65 These results demonstrate that insulin can influence various metabolic processes, including the secretion of other hormones. Interestingly, diabetic rats treated with oral nCOF/Insulin exhibit adrenal glands that resemble those of non-diabetic controls. This suggests that the different assimilation and metabolism of oral insulin, compared to subcutaneous insulin, may modulate hormonal responses in diabetic rats due to its gradual absorption.
Our study primarily focuses on the implications of insulin delivery routes, highlighting how oral nanoparticle-mediated insulin (nCOF/Insulin) can offer distinct advantages over subcutaneous administration. While the rapid repair observed in our study is notable, it should be interpreted in the context of the relatively recent onset of hyperglycemia in our model. It is worth noting that the tissue alterations observed in this study were assessed one week after streptozotocin administration, a period when the hyperglycemic effects of diabetes are becoming fully established. Our findings suggest that the observed damage may still be somewhat reversible. This potential for repair within a short timeframe may reflect the early stages of metabolic imbalance rather than long-term complications.
A key advantage of the nCOF/Insulin formulation is its controlled release of insulin over 10 hours, compared to the approximately one hour of coverage provided by subcutaneous insulin.19 This sustained release aligns with blood glucose concentrations throughout the 24-hour period when the animals had access to food. By ensuring continuous insulin delivery, the nCOF/Insulin formulation facilitates glucose uptake and potentially reduces cellular stress, contributing to the restoration of tissue architecture.
Moreover, restoring glycemic homeostasis is crucial for mitigating diabetes-induced damage. Our previous studies have demonstrated that the nCOF/Insulin formulation not only alleviates hyperglycemia but also addresses associated conditions such as hyperinsulinemia, hypertriglyceridemia, and inflammation.19,24 Additionally, the formulation may influence adrenal hormone levels. All these factors are known to impact tissue damage. By managing these conditions, the nCOF/Insulin formulation supports a more favorable tissue environment, likely contributing to the impressive results observed in our study.
This study serves as an exploratory investigation into the therapeutic potential of oral nCOF/Insulin, providing a comprehensive analysis of its short-term effects on multiple organs and offering novel insights into diabetes treatment. As a pioneering effort in nanoparticle-based therapies, it highlights the effectiveness of oral nCOF/Insulin in ameliorating metabolic disturbances and oxidative stress, demonstrating its potential to significantly impact diabetes management. However, the study’s short duration is a limitation that underscores the need for further research to fully understand the long-term efficacy and underlying mechanisms of this delivery method. While the rapid improvements observed are promising, future studies are essential to establish a more comprehensive view of its therapeutic benefits and validate its potential in clinical settings.
Conclusion
In conclusion, our study provides compelling evidence of the effectiveness of oral nanoparticle-mediated insulin delivery (nCOF/Insulin) in mitigating organ damage induced by diabetes in rat models. We have elucidated the pathophysiological changes that diabetes imposes on various organs, including alterations in organ and body weights, disruption of the oxidative-antioxidative balance, and histopathological changes. Our findings suggest that the innovative nCOF/Insulin treatment may help to reverse these detrimental effects and facilitate a return to normative physiological functions. Notably, our results indicate that oral nCOF/Insulin may support brain health by reducing lipid peroxidation, mitigate muscle wasting by reducing oxidative stress, and restore intestinal integrity. Furthermore, it appears effective in improving splenic immune function, myocardial structure, hepatic architecture, renal integrity, and adrenal gland composition.
While our research highlights the limitations of existing diabetes treatments and positions oral nanoparticle-mediated insulin delivery as a promising therapeutic strategy, we recognize that these findings are preliminary. The short treatment period used in this study necessitates further investigation to fully understand the long-term efficacy and safety of this approach. Additional research is essential to unravel the intricate mechanisms of action of nCOF/Insulin and to validate its therapeutic potential in humans. Future studies should aim to expand on our findings, exploring the long-term efficacy and safety of oral nCOF/Insulin, as well as its integration into existing diabetes treatment regimens. The journey towards a more effective diabetes treatment is far from over, but our study marks a significant step forward.
Abbreviations
nCOF/Insulin, Oral Insulin Loaded with Nanoparticles comprising covalent organic frameworks; TD1, Type 1 diabetes; STZ, Streptozotocin; C., Standard Control; D.C., Diabetic Control; SC Ins, Diabetic rats treated with subcutaneous insulin; Oral nCOF/Ins, Diabetic rats treated with oral administration of nanoparticle-formulated insulin; PBS, Phosphate Buffer Saline; MDA, Malondialdehyde; TBARS, Thiobarbituric Acid Reactive Substances; TBA, Thiobarbituric Acid; PCOs, Protein Carbonyls; DNPH, Dinitrophenylhydrazine; GSH, Glutathione; DTNB, 5,5′-Dithiobis(2-Nitrobenzoic Acid); HE, Hematoxylin and Eosin; GZ, Germinal Zone; MZ, Marginal Zone; CA, Central artery; Ed, Endocardium; My, Myocardium; Ep, Epicardium; CV, Centro-Lobular Vein; G, Glomerular Structures; ZG, Zona Glomerulosa; ZF, Zona Fasciculata; ZR, Zona Reticularis; M, Medulla.
Data Sharing Statement
All data are available in the main text.
Acknowledgments
We thank New York University Abu Dhabi (NYUAD, UAE) for its generous support of this research. This research was partially carried out using the Core Technology Platforms resources at New York University Abu Dhabi. We would like to acknowledge ASPIRE (AARE20-116) for their generous support. All authors read and approved the final manuscript.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. World health organization. Class Diabetes Mellitus. 2019.
2. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabet Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
3. Wronka M, Krzeminska J, Młynarska E, Rysz J, Franczyk B. The Influence of Lifestyle and Treatment on Oxidative Stress and Inflammation in Diabetes. Int J Mol Sci. 2022;23:19. doi:10.3390/ijms232415743
4. Delmastro MM, Piganelli JD. Oxidative Stress and Redox Modulation Potential in Type 1 Diabetes. Clin Dev Immunol. 2011;2011:15. doi:10.1155/2011/593863
5. Papachristoforou E, Lambadiari V, Maratou E, Makrilakis K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J Diabetes Res. 2020;2020:17. doi:10.1155/2020/7489795
6. Rotariu D, Elena E, Mirela D, et al. Oxidative stress – complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed Pharmacother. 2022;152:113238. doi:10.1016/j.biopha.2022.113238
7. Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta. 2014;1840(9):2709–2729. doi:10.1016/j.bbagen.2014.05.017
8. Yang H, Jin X, Wai C, Lam K, Yan S. Oxidative stress and diabetes mellitus. Clin Chem Lab Med. 2011;49(11):1773–1782. doi:10.1515/CCLM.2011.250
9. Xuan Y, Gào X, Anusruti A, et al. Association of Serum Markers of Oxidative Stress With Incident Major Cardiovascular Events, Cancer Incidence, and All-Cause Mortality in Type 2 Diabetes Patients: pooled Results From Two Cohort Studies. Diabetes Care. 2019;42(August):1436–1445. doi:10.2337/dc19-0292
10. Takahashi P, Xavier DJ, Lima JEBF, et al. Transcript Expression Profiles and MicroRNA Regulation Indicate an Upregulation of Processes Linked to Oxidative Stress, DNA Repair, Cell Death, and Inflammation in Type 1 Diabetes Mellitus Patients. J Diabetes Res. 2022;2022:15. doi:10.1155/2022/3511329
11. Sayed LH, Badr G, Omar HM, El-rahim AMA, Mahmoud MH. Camel whey protein improves oxidative stress and histopathological alterations in lymphoid organs through Bcl-XL/Bax expression in a streptozotocin-induced type 1 diabetic mouse model. Biomed Pharmacother. 2017;88:542–552. doi:10.1016/j.biopha.2017.01.076
12. Dal S, Jeandidier N, Seyfritz E, et al. Oxidative stress status and liver tissue defenses in diabetic rats during intensive subcutaneous insulin therapy. Exp Biol Med. 2015;2015(2):1–9. doi:10.1177/1535370215603837
13. Gutierres VO, Pires R, Carlos A, et al. Curcumin improves the effect of a reduced insulin dose on glycemic control and oxidative stress in streptozotocin ‐ diabetic rats. Phyther Res. 2019;2018:1–13. doi:10.1002/ptr.6291
14. Soulimane-Mokhtari NA, Guermouche B, Saker M, et al. Serum lipoprotein composition, lecithin cholesterol acyltransferase and tissue lipase activities in pregnant diabetic rats and their offspring receiving enriched n-3 PUFA diet. Gen Physiol Biophys. 2008;27(1):3–11.
15. Liao X, Shen M, Li T, et al. Combined Molybdenum Gelatine Methacrylate Injectable Nano-Hydrogel Effective Against Diabetic Bone Regeneration. Int J Nanomed. 2023;18(October):5925–5942. doi:10.2147/IJN.S428429
16. Mokhtari-soulimane N, Merzouk H, Merzouk SA. Elevation of oxidative stress markers in Type 1 diabetic children. J Diabetes Endocrinol. 2015;6(2):8. doi:10.5897/JDE2014.0083
17. Zhang Z, Miao L, Qian L, Wang N, Qi M, Wang R. Molecular Mechanisms of Glucose Fluctuations on Diabetic Complications. Front Endocrinol. 2019;10(September):1–11. doi:10.3389/fendo.2019.00640
18. De Vasconcelos Silva EL, de Oliveira JAC, de Moreira LMC, et al. Insulin-loaded nanoparticles based on acetylated cashew gum/chitosan complexes for oral administration and diabetes treatment. Int J Biol Macromol. 2023;2023:242. doi:10.1016/J.IJBIOMAC.2023.124737.
19. Benyettou F, Kaddour N, Prakasam T, et al. In vivo oral insulin delivery via covalent organic frameworks. Chem Sci. 2021;12:6037. doi:10.1039/d0sc05328g
20. Liu H, Cao SY, Hong T, Han J, Liu Z, Cao W. Insulin Is a Stronger Inducer of Insulin Resistance than Hyperglycemia in Mice with Type 1 Diabetes Mellitus (T1DM). J Biol Chem. 2009;284(40):27090–27100. doi:10.1074/jbc.M109.016675
21. Liu L, Zhou C, Xia X, Liu Y. Self-assembled lecithin / chitosan nanoparticles for oral insulin delivery: preparation and functional evaluation. Int J Nanomed. 2016;11:761–769. doi:10.2147/IJN.S96146
22. Song L, Zhi Z, Pickup JC. Nanolayer encapsulation of insulin- chitosan complexes improves efficiency of oral insulin delivery. Int J Nanomed. 2014;9(1):2127–2136. doi:10.2147/IJN.S59075
23. Damgé C, Maincent P, Ubrich N. Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats. J Control Release. 2007;117(2):163–170. doi:10.1016/j.jconrel.2006.10.023
24. Kaddour N, Benyettou F, Moulai K, et al. Heliyon Effects of subcutaneous vs. oral nanoparticle-mediated insulin delivery on hemostasis disorders in type 1 diabetes: a rat model study. Heliyon. 2024;10(9):e30450. doi:10.1016/j.heliyon.2024.e30450
25. Raghunathan S, Tank P, Bhadada S, Patel B. Evaluation of Buspirone on Streptozotocin Induced Type 1 Diabetes and Its Associated Complications. Biomed Res Int. 2014;2014(1):9. doi:10.1155/2014/948427
26. Sonawane RD, Vishwakarma SL, Lakshmi S, Rajani M, Padh H, Goyal RK. Amelioration of STZ-induced type 1 diabetic nephropathy by aqueous extract of Enicostemma littorale Blume and swertiamarin in rats. Mol Cell Biochem. 2010;304:1–6. doi:10.1007/s11010-010-0393-x
27. Nakatsu N, Igarashi Y, Aoshi T, et al. Isoflurane is a suitable alternative to ether for anesthetizing rats prior to euthanasia for gene expression analysis. J Toxicol Sci. 2017;42(4):491–497. doi:10.2131/jts.42.491
28. Oh SS, Narver HL. Mouse and Rat Anesthesia and Analgesia. Curr Protoc. 2024;4(2):1–25. doi:10.1002/cpz1.995
29. Slaoui M, Fiette L. Histopathology Procedures: from Tissue Sampling to Histopathological Evaluation. Drug Saf Eval Mol Biol. 2011;691:69–82. doi:10.1007/978-1-60761-849-2
30. Sucedaram Y, Johns EJ, Husain R, et al. Exposure to High-Fat Style Diet Induced Renal and Liver Structural Changes, Lipid Accumulation and Inflammation in Intact and Ovariectomized Female Rats. J Inflamm Res. 2021;14:690–710. doi:10.2147/JIR.S299083
31. Esmaeilpour T, Lotfealian A, Anvari M, et al. Effect of methamphetamine on ultraweak photon emission and level of reactive oxygen species in male rat brain. Neurosci Lett. 2023;801:137136. doi:10.1016/j.neulet.2023.137136
32. Bekhti Sari F, Mokhtari-soulimane N, Meriem B, et al. Histological Injury to Rat Brain, Liver, and Kidneys by Gold Nanoparticles is Dose-Dependent. ACS Omega. 2022. doi:10.1021/acsomega.2c00727
33. Tabaru IN, Turkhan A. Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: its application for the removal of hydrogen peroxide from artificial wastewater. Green Process Synth. 2024;13:1–14. doi:10.1515/gps-2024-0006
34. Huang M, Gao L, Yang L, Lin F, Lei H. Abnormalities in the brain of streptozotocin-induced type 1 diabetic rats revealed by diffusion tensor imaging. Neuroimage. 2012;1(1):57–65. doi:10.1016/j.nicl.2012.09.004
35. Derakhshan F, Toth C. Insulin and the Brain. Curr Diabetes Rev. 2013;9(2):102–116. doi:10.2174/157339913805076454
36. Nedelcovych MT, Gadiano AJ, Wu Y, et al. Pharmacokinetics of Intranasal versus Subcutaneous Insulin in the Mouse. ACS Chem Neurosci. 2018;9(4):809–816. doi:10.1021/acschemneuro.7b00434.Pharmacokinetics
37. Prince PSM, Kumar MR, Selvakumari CJ. Effects of Gallic Acid on Brain Lipid Peroxide and Lipid Metabolism in Streptozotocin-Induced Diabetic Wistar Rats. J Biochem Mol Toxicol. 2011;25(2):101–107.
38. Ramudu K, Mallikarjuna K, Kesireddy N, Sathyavelu K. Neuroprotective effect of ginger on anti-oxidant enzymes in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2011;49(4):893–897. doi:10.1016/j.fct.2010.12.013
39. Carolo K, Bueno BG, Pereira LF, et al. Yacon (Smallanthus sonchifolius) Leaf Extract Attenuates Hyperglycemia and Skeletal Muscle Oxidative Stress and Inflammation in Diabetic Rats. Evid Based Complement Altern Med. 2017;2017:9.
40. Guan YUE, Cui Z, Sun BEI, Han L, Li C, Chen L. Celastrol attenuates oxidative stress in the skeletal muscle of diabetic rats by regulating the AMPK-PGC1α-SIRT3 signaling pathway. Int J Mol Med. 2016;37:1229–1238. doi:10.3892/ijmm.2016.2549
41. Xu M, Qin H, Zheng Y, et al. Construction of a double-responsive modified guar gum nanoparticles and its application in oral insulin administration. Colloids Surf B Biointerfaces. 2022;220(12). doi:10.1016/J.COLSURFB.2022.112858
42. Ghavimishamekh A, Ziamajidi N, Dehghan A. Study of Insulin-Loaded Chitosan Nanoparticle Effects on TGF- b 1 and Fibronectin Expression in Kidney Tissue of Type 1 Diabetic Rats. Indian J Clin Biochem. 2018;34:418–426. doi:10.1007/s12291-018-0771-9
43. Kaul K, Apostolopoulou M, Roden M. Insulin resistance in type 1 diabetes mellitus. Metabolism. 2015;64(12):1629–1639. doi:10.1016/j.metabol.2015.09.002
44. Bergman BC, Howard D, Schauer IE, et al. Features of Hepatic and Skeletal Muscle Insulin Resistance Unique to Type 1 Diabetes. J Clin Endocrinol Metab. 2012;97:1663–1672. doi:10.1210/jc.2011-3172
45. Shirpoor A, Ilkhanizadeh B, Saadatian R, et al. Effect of Vitamin E on diabetes-induced changes in small intestine and plasma antioxidant capacity in rat. J Physiol Biochem. 2006;62(3):171–177.
46. Sukhotnik I, Shamir R, Bashenko Y. Effect of Oral Insulin on Diabetes-Induced Intestinal Mucosal Growth in Rats. Dig Dis Sci. 2011;56:2566–2574. doi:10.1007/s10620-011-1654-6
47. Bhor VM, Raghuram N, Sivakami S. Oxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin-induced diabetic rats. Int J Biochem Cell Biol. 2004;36:89–97. doi:10.1016/S1357-2725(03)00142-0
48. Sun T, Gao G, Li R, et al. Bone marrow-derived mesenchymal stem cell transplantation ameliorates oxidative stress and restores intestinal mucosal permeability in chemically induced colitis in mice. Am J Transl Res. 2015;7:891–901.
49. Tunali S, Yanardag R. Effect of vanadyl sulfate on the status of lipid parameters and on stomach and spleen tissues of streptozotocin-induced diabetic rats. Pharmacol Res. 2006;53:271–277. doi:10.1016/j.phrs.2005.12.004
50. Ghosh S, Chowdhury S, Sarkar P, Sil PC. Ameliorative Role of Ferulic Acid against Diabetes Associated Oxidative Stress Induced Spleen Damage. Food Chem Toxicol. 2018. doi:10.1016/j.fct.2018.05.029
51. Hajdu M, Knutsen MO, Vértes V, et al. Quality of glycemic control has significant impact on myocardial mechanics in type 1 diabetes mellitus. Sci Rep. 2022;12:1–13. doi:10.1038/s41598-022-24619-2
52. Guido MC, Marques AF, Tavares ER, De MMDT, Salemi VMC, Maranhão RC. The Effects of Diabetes Induction on the Rat Heart: differences in Oxidative Stress, Inflammatory Cells, and Fibrosis between Subendocardial and Interstitial Myocardial Areas. Oxid Med Cell Longev. 2017;2017:11.
53. Fein FS, Strobeck JE, Malhotra A, Scheuer J. Reversibility of Diabetic Cardiomyopathy with Insulin in Rats. Circ Res. 1981;49(6):1251–1261. doi:10.1161/01.RES.49.6.1251
54. Han X, Abendschein DR, Kelley JG, Gross RW. Diabetes-induced changes in specific lipid molecular species in rat myocardium. Biochem J. 2000;352:79–89.
55. Abdelrazek HMA, Kilany OE, Muhammad MAA, Tag HM, Abdelazim AM. Black Seed Thymoquinone Improved Insulin Secretion, Hepatic Glycogen Storage, and Oxidative Stress in Streptozotocin-Induced Diabetic Male Wistar Rats. Oxid Med Cell Longev. 2018;2018:10.
56. Farid A, Moussa P, Youssef M, Haytham M, Shamy A, Safwat G. Saudi Journal of Biological Sciences Melatonin relieves diabetic complications and regenerates pancreatic beta cells by the reduction in NF-kB expression in streptozotocin induced diabetic rats. Saudi J Biol Sci. 2022;29(7):103313. doi:10.1016/j.sjbs.2022.103313
57. Ozdemir O, Akalin PP, Baspinar N, Hatipoglu F. Pathological changes in the acute phase of streptozotocin-induced diabetic rats. Bull Vet Inst Pulawy. 2009;53(4):783–790.
58. Edgerton DS, Scott M, Farmer B, et al. Targeting insulin to the liver corrects defects in glucose metabolism caused by peripheral insulin delivery. JCI Insight. 2019;26(5):1–15. doi:10.1172/jci.insight.126974
59. Al-rasheed NM, Al-rasheed NM, Bassiouni YA, Hasan IH. Biomedicine & Pharmacotherapy Simvastatin ameliorates diabetic nephropathy by attenuating oxidative stress and apoptosis in a rat model of streptozotocin-induced type 1 diabetes. Biomed Pharmacother. 2018;105:290–298. doi:10.1016/j.biopha.2018.05.130
60. Zhou J, Zhang J, Sun Y, et al. A nano-delivery system based on preventing degradation and promoting absorption to improve the oral bioavailability of insulin. Int J Biol Macromol. 2023;2023:244. doi:10.1016/J.IJBIOMAC.2023.125263.
61. Soliman MA, Noya DA. The Possible Ameliorating Effect of Barley Grain on the Histological Structure of the Adrenal Cortex in Streptozotocin (STZ) -induced Diabetes in Adult Male Albino Rats. Egypt J Histol. 2020;554–568. doi:10.21608/ejh.2019.16258.1157
62. El-feky H, Salah A, Hussein Y, Sabry M. Histological Study of the Effect of Induced Diabetes on the Zona Glomerulosa of the Adrenal Cortex of Adult Male Albino Rat. Egypt J Histol. 2010;33(1):17–22.
63. Oyouni AAA, Al-amer OM, Abo F, et al. Melatonin ameliorates the adrenal and pancreatic alterations in streptozotocin-induced diabetic rats: clinical, biochemical, and descriptive histopathological studies. Front Vet Sci. 2022;9:14. doi:10.3389/fvets.2022.1016312/full
64. Mahar Y, AALI S, Naqvi A. The effect of l-Arginine and Insulin on Histological Changes in Streptozotocin treated Rat Adrenal Gland. Pak J Med Heal Sci. 2012;6(4):843–847.
65. Unluhizarci K, Karaca Z, Kelestimur F. Role of insulin and insulin resistance in androgen excess disorders. World J Diabetes. 2021;12(5):616–629. doi:10.4239/wjd.v12.i5.616
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.