Back to Journals » Journal of Pain Research » Volume 18
Efficacy of Percutaneous Spinal Endoscopic YESS Technique in Adjacent Segmental Disease Without Severe Instability After Lumbar Fusion Surgery: A Case Series
Authors Zou H, Huang L, Zhao L, Li P, Xiao Q, Rong X
Received 16 September 2024
Accepted for publication 23 February 2025
Published 30 March 2025 Volume 2025:18 Pages 1711—1720
DOI https://doi.org/10.2147/JPR.S488031
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Krishnan Chakravarthy
Haitao Zou,1 Lingan Huang,2 Litao Zhao,1 Pengcui Li,3 Qiongrun Xiao,1 Xueqin Rong1
1Department of Pain Medicine, Sanya Central Hospital (The Third People’s Hospital of Hainan Province), Sanya City, Hainan Province, People’s Republic of China; 2Department of Sports Medicine Center, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, People’s Republic of China; 3Shanxi Key Laboratory of Bone and Soft Tissue Injury Repair, Taiyuan, Shanxi Province, People’s Republic of China
Correspondence: Lingan Huang, Department of Orthopedic and Sports Medicine Center, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, 168 Litang Road, Changping District, Beijing, 102218, People’s Republic of China, Email [email protected]
Background: The adjacent segment disease (ASD) after lumbar fusion is inherently stressed abnormally, and the destruction of bony structures in surgery can further exacerbate that abnormality. The Yeung Endoscopic Spine Surgery (YESS) technique does not destroy facet joints, and therefore it may be a good indication for ASD. However, the efficacy of the YESS technique in ASD patients have not been reported.
Patients and Methods: From January 2018 to May 2023, 13 patients with adjacent spondylolisthesis secondary to lumbar fusion were treated with endoscopic YESS technique surgery. Patients’ visual analog score (VAS) and Oswestry disability index (ODI) score were analyzed preoperatively, 3 days postoperatively, and at the final follow-up. Gait analysis parameters and intervertebral height index of patients preoperatively and at 1 year postoperatively were used to objectively quantify patient pain, function, and radiographic changes.
Results: A total of 13 patients, 2 females and 11 males, had single-segment adjacent spondylolisthesis. The patients’ VAS and ODI scores at 3 days postoperatively decreased (P< 0.05) compared to preoperatively and further decreased (P< 0.05) at the final follow-up. There were no infections, wound complications or reoperations. The results of gait analysis showed no statistically significant difference in single-stance time and the gait cycle before and after the patients’ surgery (P> 0.05), but the patients’ velocity, step length, step time, step frequency and stride length were significantly improved at 1-year postoperatively (P< 0.05). Intervertebral height loss did not occur in all patients.
Conclusion: In the short term, spinal endoscopic YESS technique markedly improves clinical symptoms and gait parameters in patients with ASD without severe instability after lumbar fusion, while avoiding facet joint destruction and intervertebral height loss. Future studies with larger sample sizes and longer follow-up times are needed to clarify the long-term efficacy.
Keywords: adjacent segment degeneration, endoscopic decompression, YESS technique, gait analysis
Introduction
Lumbar spine degenerative diseases are a group of disorders involving degeneration of intervertebral discs and degeneration and hyperplasia of adjacent small joints and posterior structures.1 The adjacent segment degeneration global lifetime and point prevalences of lumbar spine degenerative diseases are 38.9% and 11.9%, respectively.2 These diseases have symptoms such as low back pain and neurogenic intermittent claudication that can cause disability in severe cases, substantially affecting the quality of life of middle-aged and older patients and increasing the economic burden on their families and society. Interbody fusion surgery is a classic surgical method for treating lumbar spine degenerative diseases, which can facilitate adequate decompression of neural tissues, restores spinal lordosis or corrects deformities and stable fusion of the intervertebral space.3–8 However, lumbar fusion-induced biomechanical changes may increase the mobility of the adjacent segment and the pressure on the adjacent disc, accelerating its degeneration and causing the development of adjacent segment disease (ASD).9–11 ASD is a potential long-term complication of lumbar fusion, involving degenerative diseases of the adjacent segment, fractures, infections, and scoliosis or kyphosis and substantially affecting the long-term outcomes in patients after lumbar fusion.12 The incidence of ASD requiring surgical intervention after lumbar fusion ranges from 5.2% to 49%.13 For patients with ASD in whom strict conservative treatment is ineffective after > 3 months and symptoms severely affect work and life or cause cauda equina syndrome, revision surgery can be considered. The revision surgery approach—extended open surgery or minimally invasive endoscopic surgery—depends on factors related to the initial surgery, patient factors, and possible complications.14–16
Percutaneous spinal endoscopy is an excellent option for older patients who do not want to undergo another open procedure, desire less trauma or suffer from comorbidities.17–19 Percutaneous endoscopic surgery is widely used in patients with lumbar spine degenerative disease20–23 and adjacent spondylolisthesis because it is associated with less trauma, fast recovery, and few complications.24 The ASD are inherently stressed abnormally, and the destruction of bony structures during endoscopic removal of the disc can further exacerbate that abnormality, with the possibility of reherniation in the long-term postoperative period. When 1/4 of the superior articular process is removed, lumbar stress abnormalities can occur. Yeung Endoscopic Spine Surgery (YESS) technique only removes soft tissues such as the joint capsule without resecting the superior articular process, and performs disc excision from the Kambin triangle, moving from the inside out. Therefore, it does not exacerbate the stress of ASD after lumbar fusion surgery, which may be a good indication for it. ASD after lumbar fusion may be its natural indication. However, research on the efficacy of YESS technique for ASD after lumbar fusion is not reported, and changes in gait parameters pre- and post-treatment are unknown. This study aimed to objectively assess the clinical outcomes and gait parameters of YESS technique surgery through a case series follow-up.
Materials and Methods
Patients
In this consecutive observational cohort study, patients with adjacent spondylolisthesis after lumbar fusion from the Hainan Pain Medicine Center were enrolled between 2017 and 2023. The following information was collected: previous fusion segment, time of appearance of provertebral disease after fusion, segment of onset of neighboring spondylosis, visual analog scores (VAS) (preoperative endoscopy, 3 days postoperatively, and final follow-up), and Oswestry disability index (ODI) scores (preoperatively, 3 days postoperatively, and final follow-up). This study was approved by the Ethics Committee of Sanya Central Hospital, and all patients who underwent percutaneous spinal endoscopy signed informed consent forms. This study complied with the Declaration of Helsinki.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows:
- VAS >5 for low back and lower extremity pain and preoperative ODI score >60;
- Advanced imaging findings corresponding to clinical signs and symptoms with compressive changes in the adjacent segments after previous lumbar fusion, including lumbar disc herniation, lumbar spinal stenosis, lumbar spondylolisthesis (I degree), and nerve root compression with lumbar mild instability.
- No significant loss of foraminal height at the neighboring segments.
- Complete postoperative follow-up information available.
The exclusion criteria were as follows:
- Severe osteophyte formation at the posterior margin of the vertebral body;
- Severe instability of lumbar vertebral segments;
- Patients with other spinal diseases, such as spinal fracture, scoliosis, tumor, infection, or ankylosing spondylitis;
- History of previous surgery of neighboring vertebral segments.
Endoscopic Surgical Technique
Position
Patients are either in the prone or lateral position. The lateral position is preferable for patients with advanced age, poor physical condition, or poor cardiopulmonary function who cannot tolerate the prone position. Intravenous intensive anesthesia is safer in the lateral recumbent position.
Anesthesia
Local anesthesia, alone or combined with intensive intravenous anesthesia, is preferred, as it is safer for older patients and those with poor organ function and offers real-time communication between patients and surgeons to enhance surgical safety.
Imaging and Navigation
Internal fixation can interfere with intraoperative (radiography or computed tomography) navigation for target puncture and placement. Proper fluoroscopy positioning and equipment adjustment are essential to eliminate “bilateral signs” of the vertebral body and articular processes.
Working Cannula Placement
Through the posterolateral approach, place sequential cannulas into the safe triangle.The lateral hypotenuse is composed of the exiting nerve roots that travel forward, downward, and laterally through the intervertebral foramen, the medial edge is the lateral margin of the dural sac, and the inferior edge is the plane of the superior endplate of the lower vertebra.
Direct visualization
After placing the endoscope, the hypertrophic ligamentum flavum is partially removed, and the lateral saphenous fossa is decompressed using an endoscopic power abrasive drill or gun forceps.
Decompression
All decompression operations are performed under direct visualization of the traversing nerve root, including comprehensive exploration and release of adhesions and compressive pathology from the exiting root. Internal decompression of the intervertebral disc should be performed first to create an internal workspace within the disc, and then the nucleus pulposus that has herniated into or outside the spinal canal should be addressed (in-out).
Criteria for Completing Decompression
The original symptoms of the patients should be significantly relieved, particularly when the surgery is performed under local anesthesia and sedation. The dura and nerve root morphology should be normal, and the nerve root should be unobstructed, with the nerve being able to move freely vertically within a 4-mm radius. The nerve root and dura should pulsate and be rhythmically consistent with the heart rate. The epineural vessels of the nerve root must demonstrate significant filling with no evident bleeding points.
Postoperative Rehabilitation
Respiratory Adjustment and Fascial Relaxation Therapy
Respiratory training and axial turning activities in bed are the primary activities performed by the patients within 1 week postoperatively. Pelvic stabilization exercises and muscle-strengthening training can restore tension in the thoracic and abdominal cavities and improve pelvic stability. A set of 5–10 repetitions of these exercises, lasting 5–10 s each, is performed every 2–3 h.
Acupuncture
Abdominal moxibustion and acupuncture may activate the abdominal muscle groups and relieve muscle tension in the lower back. The abdominal rectus and transverse muscles of the abdomen are used when the patients tuck in the abdomen for slow training and undergo moxibustion of the rectus abdominis region, when available.
Exercise Training
Active hip flexion and extension movements are recommended to enhance the rectus abdominis and iliopsoas muscle strength 2–4 weeks postoperatively. Other useful training exercises include lower-limb muscle strength, pelvic stability, and paravertebral muscle strength training 1 month postoperatively. The recommended training exercises include pelvic stability, abdominal muscle plyometric, gluteus maximus, lower-back muscle, quadriceps, and anterior tibial muscle resistance training (Figure 1).
Perioperative Pain Management
Preoperative analgesic measures primarily involve administering drugs that do not affect platelet function, such as acetaminophen, celecoxib, flurbiprofen ester, parecoxib, pregabalin, and sedatives or anxiolytic drugs. Preoperative pain education achieves doctor–patient consensus, enabling patients to actively cooperate with the treatment regimen and eliminating stress and anxiety.
Intraoperative multimodal analgesia is used for pain relief through local infiltration anesthesia, intravenous analgesics, and sedative drugs. Local anesthetics are typically mixtures of short- (lidocaine) and long- (ropivacaine) acting agents used for local infiltration. Analgesic and sedative drugs such as parecoxib, flurbiprofen ester, fentanyl, and dextromethorphan are administered intravenously to achieve a better analgesic effect. Local injections of long-acting anesthetic drugs (eg, ropivacaine) before incision suturing at the end of surgery can effectively reduce postoperative wound pain. The analgesic drugs are reduced or discontinued postoperatively because most patients experience rapid pain relief. “Rebound pain”, similar to preoperative lower-limb pain, may arise 2–4 weeks after endoscopic surgery. For pain with VAS >6, small doses of oral non-steroidal and other pain medications are recommended. In addition, trigger points and fascial release tactics are useful.
Gait Analysis and Radiographic Parameter
The gait pressure distribution flat test system (footscan 2 m HE; RSscan International, Beringen, Belgium) was placed on a flat, hard surface covered with a very thin, non-elastic cloth. Two-meter-long hard pads were attached to the front and back of the foot scans to serve as a cushion for participants to get on and off the force plate. Once the apparatus was calibrated, patients walked barefoot on the test board and each participant acclimatized to walking 5–10 times prior to the test to eliminate tension and to ensure that they crossed the test area with a natural and realistic gait.25 Three complete gait assessments were performed for each examiner and the average of the three recordings was used for gait index data.26 Preoperative and 1-year postoperative gait spatiotemporal parameters were collected including velocity, step length, step time, single-stance time, gait cycle, step frequency and stride length. Preoperative and final follow-up intervertebral height index of the operated segments were compared.
Statistical Processing
Data were analyzed using GraphPad Prism version 8.0.1 (GraphPad Software, Boston, MA, USA). VAS and ODI scores are described using means and standard deviations, and comparisons between periods were analyzed using an analysis of variance with a repeated-measures design. Comparison of spatiotemporal parameters of gait between pre- and post-surgery was performed using paired t-tests. P<0.05 indicated statistical significance.
Results
Descriptive Statistics
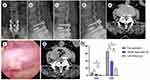
We enrolled 13 patients, including 2 women and 11 men, who were aged 59–82 years (mean age, 67.5 years). The follow-up period was 12–56 months, (mean, 21.6 months). ASD developed 1–15 years after open fusion (mean time, 5 years). Patient characteristics are summarized in Table 1. Seven patients had a history of single-segment open fusion surgery; four, double-segment fusion; and two, triple-segment fusion. ASD occurred in the cephalad and caudal vertebral bodies in nine and four patients, respectively, with single-segment onset. Symptoms improved in all patients immediately after percutaneous endoscopy. The VAS decreased from 7.1±0.568 preoperatively to 3.4±0.6992 postoperatively (P<0.05) and 1±0.816 at the final follow-up (P<0.05). The ODI score decreased from 65.8±3.521 preoperatively to 38.6±3.373 postoperatively (P<0.05) and 17.3±4.321 at the final follow-up (P<0.05) (Figure 2h). These results confirmed the efficacy of percutaneous spinal endoscopy for adjacent spondylolisthesis after fusion surgery.
 |
Table 1 Patient Characteristics |
Clinical Case
One of the thirteen patients was a 70-year-old woman with lumbar disc herniation with spinal stenosis who underwent lumbar 4 - sacrum 1 (L4–S1) open fusion surgery (Figure 2). Eight years postoperatively, the patient developed L3–4 ASD, which manifested as low back pain with radiating pain in both lower extremities that lasted beyond 3 months, for which conservative treatment was ineffective, causing the development of intermittent claudication (Figure 2a–e). The preoperative VAS and ODI score were 7 and 62, respectively. Intraoperative percutaneous endoscopy revealed severe adhesions from the previous surgical exposure and progressive lesions in all three regions of the intervertebral foramina. Endoscopy performed under direct visualization and decompression revealed hypertrophy of the L3–4 facet joint complex and severe nerve root compression. There was tethering and scarring of the outgoing and traversing nerve roots to the dura mater and ligamentum flavum. The herniated disc tissue were removed below the nerve root using endoscopic decompression (Figure 2f). The decompression procedure was successful, with significant improvement in clinical function and pain relief. Postoperative CT showed complete decompression on the right side, widening of the spinal canal and relief of nerve root compression (Figure 2g). The patient experienced significant postoperative symptomatic relief, with the VAS reduced to 4 and subsequently to 1 at the final follow-up. The ODI score was reduced to 17 at the final follow-up, indicating a favorable outcome.
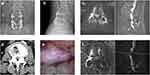
Another 59-year-old male patient underwent lumbar fusion (L3-5) 10 years ago (Figure 3a and b). The patient developed ASD of L2-3 with low back pain and radiating pain in the left lower extremity, Magnetic resonance (MR) showed lumbar disc herniation on the left side of L2-3 (Figure 3c), and no calcification on computed tomography (CT) (Figure 3d). The pre-operative VAS and ODI scores were 8 and 71, respectively. The herniated disc tissue was removed below the nerve root using the YESS technique (Figure 3e). Decompressive surgery was successful, with significant improvement in clinical function and pain relief. Postoperative MR showed left intervertebral disc removal and relieved nerve root compression (Figure 3f). The symptoms resolved significantly after surgery, with VAS scores reduced to 3 and further reduced to 1 at final follow-up. The ODI score decreased to 15 at the final follow-up, indicating a good outcome.
Gait Parameters and Radiography
There was a significant difference in gait spatiotemporal parameters between pre-operative and 1-year postoperative periods (Table 2). The results showed no statistically significant difference in single-stance time (366.0±63.16 VS 390.8±41.97, P>0.05) and the gait cycle (1312±172.1 VS 1282±61.19, P>0.05) before and after the patients’ surgery, which indicated that the patients’ walking patterns did not change. The patients’ velocity (0.51±0.19 VS 0.81±0.09, P<0.05), step length (34.36±9.08 VS 45.41±5.82, P<0.05), step time (713.9±99.80 VS 617.9±54.44, P<0.05), step frequency (85.44±11.20 VS 97.80±8.87, P<0.05) and stride length (61.22±20.98 VS 89.29±10.27, P<0.05) were significantly improved at 1-year postoperatively. This suggests that postoperative patients had faster walking speeds and shorter walking times as a result of reduced pain and improved function. In addition, there was no statistically significant difference in the intervertebral height index at the final follow-up compared to the preoperative period.
 |
Table 2 Comparison of Gait Parameters Between Preoperative and Postoperative Periods |
Discussion
Open revision surgery is the primary treatment modality for patients with ASD requiring surgery. This includes extended decompression, adjunctive internal fixation, and further lengthening of the fused segment.27,28 However, most patients hesitate to undergo repeat open surgery because of the associated pain and trauma and resulting scar formation and fibrosis of the muscle, which may affect the efficacy of secondary surgery. In comparison, percutaneous spinal endoscopy is less invasive, with faster recovery and fewer complications in degenerative spinal disease. It has been argued that the YESS technology is considered a transitional spinal endoscopic surgery because it performs discectomy from inside to outside through the safe triangle, and therefore cannot deal with extruded or sequestered discs. Previous studies have confirmed that the YESS technique is effective in treating stable isthmic spondylolisthesis,29 and our study further found that the YESS technique of spinal endoscopy for treating stable ASD after lumbar fusion is effective, with low recurrences and complications. In the meanwhile, spinal endoscopic YESS technique markedly relieved pain symptoms and improved gait parameters in patients, without intervertebral height loss. Therefore, the YESS technique, as a classic technique of spinal endoscopy, still has unique indications and application prospects.
Percutaneous endoscopic techniques are used in treating lumbar degenerative diseases because they offer less trauma, fast recovery, and few complications.30 In this study, all patients who underwent endoscopic surgery had a significant reduction in the VAS and ODI score at 3 days postoperatively, and both scores improved further during the follow-up period. Our clinical results show that percutaneous endoscopy can effectively reduce preoperative low back pain and improve lumbar spine function for neighboring spondylosis after lumbar fusion. These advantages are preferable for patients medically unfit to undergo general anesthesia or those who decline open surgery. Among the 13 patients in this study, 1 had a history of cerebral infarction combined with lupus nephritis and severe hypertension, and 1 had concurrent cerebral artery supply deficiency and severe osteoporosis. Both patients were in poor physical condition to tolerate anesthesia and the trauma of open extended surgery; however, minimally invasive percutaneous endoscopy enabled successful treatment of their adjacent segment spondylosis.
The YESS technique can achieve minimal trauma, adequate neurological decompression, and minimal overall structural damage to the spine while maximizing the preservation of lumbar segmental motion. There was no significant loss of intervertebral height in the operated segment at the final follow-up due to maximal maintenance of bony structures. Yuan et al explored the application of spinal endoscopic surgery in ASD and confirmed its good outcomes.31 However, it did not use the YESS technique and employed subjective scoring methods such as VAS scores. We introduced gait analysis to objectively assess the efficacy of the YESS technique by comparing preoperative and postoperative gait parameters. Gait analysis can automate and naturalize the examination process by completing the test in the most natural state of the patient. Unlike traditional subjective visual observation and physical measurements, gait analysis allowed precise quantification and objective evaluation of parameters.32,33 The results of gait analysis at 1 year postoperatively objectively showed that patients had greater stride and faster walking speed after spinal endoscopy, ie, pain relief and functional improvement. This may be closely related to the effective decompression, less invasive advantages and postoperative rehabilitation. Previous studies have shown that postoperative rehabilitation after spinal endoscopy can increase the effectiveness of surgery, promote postoperative recovery, and improve the patient’s gait parameters.34
Moreover, percutaneous spinal endoscopy is associated with low recurrence and complication rates. For example, our study, with a maximum follow-up duration of 56 months, showed no relevant postoperative complications in an 82-year-old patient. All 13 patients had no recurrence and low complication rates in the postoperative segments, consistent with the findings reported in the literature.35 Although excessive decompression may affect lumbar spine stability in endoscopic procedures, it was not found in this YESS technique study. And in a 10-year follow-up study of YESS technique, although some patients eventually underwent fusion surgery, 100% were satisfied with their decision to delay fusion as their first endoscopic surgery option.29
Percutaneous endoscopic surgery involves a steep learning curve, a clear understanding of anatomy, specialized skills, and strict indications to ensure efficacy.36 Overall, the YESS technique for ASD seems to have a smoother learning curve and lower risk due to its indirect stress reduction. A limitation of our study was its small sample size, owing to the limited application of YESS technique in ASD after lumbar fusion. Studies with larger sample sizes and longer follow-up periods are required to confirm the reliability of the results. Due to the characteristics of the YESS technique, we strictly controlled the indications to ensure good treatment outcomes, which was why the number of cases included was small. Based on the current results, we are confident that in future studies we will include more cases to increase the stability of the outcomes and obtain long-term follow-up results. One other limitation was that there was no comparison of the efficacy with other endoscopic techniques. Therefore, we used gait analysis of the patients between pre- and post-surgery to assess the efficacy of the procedure, to provide quantitative and objective data support. While avoiding the potential bias caused by the subjectivity of the VAS score and ODI score, it strongly confirms the effectiveness of the YESS technique in the treatment of such diseases.
Conclusions
In the short term, the YESS technique markedly improves clinical symptoms and gait parameters in patients with ASD without severe instability after lumbar fusion, without intervertebral height loss. Furthermore, the indirect decompression of this technique ensured low complications during the follow-up period and did not increase the recurrence rate, making it a promising alternative to conventional open surgery for ASD patient. However, further large-sample and long-term studies are required to fully evaluate the long-term efficacy and durability of the YESS techniques for treating this condition, as well as to characterize gait changes.
Data Sharing Statement
The data used to support the findings of this study are included within the article.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Acknowledgment
We acknowledge Kai-Uwe Lewandrowski, M.D. and Anthony T. Yeung, M.D. for minor language editing and reference list review.
Funding
This research was supported by the National Natural Science Foundation of China (No. 82172503), Hainan Provincial Medical and Health Research Program (No. 21A200349), and Hainan Province Clinical Medical Center.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhuang Y, Zhou F, Zhang Y, Jin Z. Curative effect of posterior lumbar interbody fusion in the treatment of single-segment lumbar degenerative disease and changes in adjacent segment quantitative score. Exp Ther Med. 2018;16(1):161–166. doi:10.3892/etm.2018.6159
2. Wong T, Patel A, Golub D, et al. Prevalence of long-term low back pain after symptomatic lumbar disc herniation. World Neurosurg. 2023;170:163–173.e1. doi:10.1016/j.wneu.2022.11.029
3. Schlesinger SM, Gelber BR, Gerber MB, Lorio MP, Block JE. Comparison of transforaminal lumbar interbody fusion in the ambulatory surgery center and traditional hospital settings, part 1: multi-center assessment of surgical safety. J Pers Med. 2023;13(2). doi:10.3390/jpm13020311
4. Yang Z, Chang J, Sun L, Chen C-M, Feng H. Comparing Oblique Lumbar Interbody Fusion with Lateral Screw Fixation and Transforaminal Full-Endoscopic Lumbar Discectomy (OLIF-TELD) and Posterior Lumbar Interbody Fusion (PLIF) for the treatment of adjacent segment disease. Biomed Res Int. 2020;2020:4610128. doi:10.1155/2020/4610128
5. Tassemeier T, Haversath M, Jäger M. Transforaminal lumbar interbody fusion with expandable cages: radiological and clinical results of banana-shaped and straight implants. J Craniovertebr Junction Spine. 2018;9(3):196–201. doi:10.4103/jcvjs.JCVJS_56_18
6. Kurra S, Lavelle WF, Silverstein MP, Savage JW, Orr RD. Long-term outcomes of transforaminal lumbar interbody fusion in patients with spinal stenosis and degenerative scoliosis. Spine J. 2018;18(6):1014–1021. doi:10.1016/j.spinee.2017.10.063
7. Abbasi H, Abbasi A. Oblique Lateral Lumbar Interbody Fusion (OLLIF): technical notes and early results of a single surgeon comparative study. Cureus. 2015;7(10):e351. doi:10.7759/cureus.351
8. Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1(1). doi:10.3978/j.issn.2414-469X.2015.10.05
9. Ashinsky B, Smith HE, Mauck RL, Gullbrand SE. Intervertebral disc degeneration and regeneration: a motion segment perspective. Eur Cell Mater. 2021;41:370–380. doi:10.22203/eCM.v041a24
10. Sakti YM, Mafaza A, Lanodiyu ZA, Sakadewa GP, Magetsari R. Management of distal adjacent segment disease due to central subsidence of PLIF using local anesthetic transforaminal foraminotomy and lumbar discectomy. Int J Surg Case Rep. 2020;77:269–275. doi:10.1016/j.ijscr.2020.10.089
11. Harada GK, Tao Y, Louie PK, et al. Cervical spine MRI phenotypes and prediction of pain, disability and adjacent segment degeneration/disease after ACDF. J Orthop Res. 2021;39(3):657–670. doi:10.1002/jor.24658
12. Virk SS, Niedermeier S, Yu E, Khan SN. Adjacent segment disease. Orthopedics. 2014;37(8):547–555. doi:10.3928/01477447-20140728-08
13. Lau D, Song Y, Guan Z, La Marca F, Park P. Radiological outcomes of static vs expandable titanium cages after corpectomy: a retrospective cohort analysis of subsidence. Neurosurgery. 2013;72(4):529–539. doi:10.1227/NEU.0b013e318282a558
14. Gu Y-T, Cui Z, Shao H-W, Ye Y, Gu A-Q. Percutaneous transforaminal endoscopic surgery (PTES) for symptomatic lumbar disc herniation: a surgical technique, outcome, and complications in 209 consecutive cases. J Orthop Surg Res. 2017;12(1):25. doi:10.1186/s13018-017-0524-0
15. Madhavan K, Chieng LO, McGrath L, Hofstetter CP, Wang MY. Early experience with endoscopic foraminotomy in patients with moderate degenerative deformity. Neurosurg Focus. 2016;40(2):E6. doi:10.3171/2015.11.FOCUS15511
16. Xie P, Feng F, Chen Z, et al. Percutaneous transforaminal full endoscopic decompression for the treatment of lumbar spinal stenosis. BMC Musculoskelet Disord. 2020;21(1):546. doi:10.1186/s12891-020-03566-x
17. Shen Y, Peng D, Dai Z, Zhong W. Reoperation rate and risk factors of elective spinal surgery for degenerative spondylolisthesis: still more challenges lie ahead. Spine J. 2016;16(2):270–271. doi:10.1016/j.spinee.2015.11.001
18. Epstein NE. Non-neurological major complications of extreme lateral and related lumbar interbody fusion techniques. Surg Neurol Int. 2016;7(Suppl 25):S656–S9. doi:10.4103/2152-7806.191071
19. Domínguez I, Luque R, Noriega M, Rey J, Alia J, Marco-Martínez F. Extreme lateral lumbar interbody fusion. Surgical technique, outcomes and complications after a minimum of one year follow-up. Rev Esp Cir Ortop Traumatol. 2017;61(1). doi:10.1016/j.recot.2016.09.001
20. Yeung AT. The evolution of percutaneous spinal endoscopy and discectomy: state of the art. Mt Sinai J Med. 2000;67(4):327–332.
21. Ba Z, Pan F, Liu Z, et al. Percutaneous endoscopical transforaminal approach versus PLF to treat the single-level adjacent segment disease after PLF/PLIF: 1-2 years follow-up. Int J Surg. 2017;42:22–26. doi:10.1016/j.ijsu.2017.04.021
22. Wu Q, Yuan S, Fan N, et al. Clinical outcomes of percutaneous endoscopic lumbar discectomy for the treatment of grade I and grade II degenerative lumbar spondylolisthesis: a retrospective study with a minimum five-year follow-up. Pain Physician. 2021;24(8):E1291–E8.
23. Song SK, Son S, Choi SW, Kim HK. Comparison of the outcomes of percutaneous endoscopic interlaminar lumbar discectomy and open lumbar microdiscectomy at the L5-S1 level. Pain Physician. 2021;24(4):E467–E75.
24. Kapetanakis S, Gkantsinikoudis N, Gkasdaris G, Charitoudis G. Treatment of adjacent segment disease with percutaneous transforaminal endoscopic discectomy: early experience and results. J Orthop Surg. 2020;28(3):2309499020960560. doi:10.1177/2309499020960560
25. Low DC, Dixon SJ. Footscan pressure insoles: accuracy and reliability of force and pressure measurements in running. Gait Posture. 2010;32(4):664–666. doi:10.1016/j.gaitpost.2010.08.002
26. Zhao R, Dong Z, Wei X, et al. Inflammatory factors are crucial for the pathogenesis of post-traumatic osteoarthritis confirmed by a novel porcine model: “Idealized” anterior cruciate ligament reconstruction” and gait analysis. Int Immunopharmacol. 2021;99:107905. doi:10.1016/j.intimp.2021.107905
27. Lee JC, Kim Y, Soh J-W, Shin B-J. Risk factors of adjacent segment disease requiring surgery after lumbar spinal fusion: comparison of posterior lumbar interbody fusion and posterolateral fusion. Spine. 2014;39(5):E339–E45. doi:10.1097/BRS.0000000000000164
28. Lu K, Liliang P-C, Wang H-K, et al. Reduction in adjacent-segment degeneration after multilevel posterior lumbar interbody fusion with proximal DIAM implantation. J Neurosurg Spine. 2015;23(2):190–196. doi:10.3171/2014.12.SPINE14666
29. Yeung A, Kotheeranurak V. Transforaminal endoscopic decompression of the lumbar spine for stable isthmic spondylolisthesis as the least invasive surgical treatment using the YESS surgery technique. Int J Spine Surg. 2018;12(3):408–414. doi:10.14444/5048
30. Lewandrowski K-U. Endoscopic transforaminal and lateral recess decompression after previous spinal surgery. Int J Spine Surg. 2018;12(2):98–111. doi:10.14444/5016
31. Yuan S, Lu X, Zang L, Mei Y, Fan N, Du P. Percutaneous transforaminal endoscopic discectomy for adjacent segment disease versus lumbar disc herniation in elderly patients. J Pain Res. 2024;17:2257–2265. doi:10.2147/JPR.S457225
32. Baker R, Esquenazi A, Benedetti MG, Desloovere K. Gait analysis: clinical facts. Eur J Phys Rehabil Med. 2016;52(4):560–574.
33. Klöpfer-Krämer I, Brand A, Wackerle H, Müßig J, Kröger I, Augat P. Gait analysis - Available platforms for outcome assessment. Injury. 2020;51(Suppl 2):S90–S6. doi:10.1016/j.injury.2019.11.011
34. Lyu Z, Bai J, Chen S, Liu J, Yu W. Efficacy of lumbar kinetic chain training for staged rehabilitation after percutaneous endoscopic lumbar discectomy. BMC Musculoskelet Disord. 2021;22(1):793. doi:10.1186/s12891-021-04674-y
35. Lewandrowski K-U. Incidence, management, and cost of complications after transforaminal endoscopic decompression surgery for lumbar foraminal and lateral recess stenosis: a value proposition for outpatient ambulatory surgery. Int J Spine Surg. 2019;13(1):53–67. doi:10.14444/6008
36. Wang N, Xie Y, Liu X, et al. Safety and clinical efficacy of endoscopic procedures for the treatment of adjacent segmental disease after lumbar fusion: a systematic review and meta-analysis. PLoS One. 2023;18(2):e0280135. doi:10.1371/journal.pone.0280135
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.