Back to Journals » Journal of Pain Research » Volume 17
Efficacy of Preemptive Topical Lidocaine 5% Plaster in the Prevention of Post-Craniotomy Pain, a Randomized Clinical Trial
Authors Han X, Yang Y, Ren T, Ji N, Luo F
Received 4 October 2024
Accepted for publication 9 December 2024
Published 13 December 2024 Volume 2024:17 Pages 4251—4261
DOI https://doi.org/10.2147/JPR.S499264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Karina Gritsenko
Xueye Han,1 Yixuan Yang,2 Tong Ren,3 Nan Ji,3 Fang Luo2
1Department of Anesthesiology and Pain Medicine, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, 100027, People’s Republic of China; 2Department of Pain Management, Beijing Tiantan Hospital, Capital Medical University, Beijing, 100070, People’s Republic of China; 3Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, 100070, People’s Republic of China
Correspondence: Fang Luo, Department of Pain Management, Beijing Tiantan Hospital, Capital Medical University, Beijing, 100070, People’s Republic of China, Tel +86 010 59976664, Fax +86 010 67050177, Email [email protected] Nan Ji, Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, 100070, People’s Republic of China, Tel +86 010 59976516, Email [email protected]
Objective: The current landscape is characterized by a dearth of effective, safe, simple, and noninvasive methods for preventing pain following craniotomy. This clinical trial seeks to evaluate the efficacy profile of preemptive application of a topical 5% lidocaine patch in alleviating post-craniotomy pain.
Methods: This was a multi-centric, prospective randomized placebo-controlled triple-blind clinical trial. Eligible patients were randomly assigned to either the masked intervention group, who received lidocaine 5% white hydrogel plasters (N = 90), or the placebo control group who received plain hydrogel plasters of the same pattern, size, appearance and material as L5Ps, but free of lidocaine (N = 90). Primary outcome was the pain intensity (Visual Analogue Scale at 24 h) after craniotomy. Secondary outcomes included: intra-operative analgesics consumption, pain intensity, cumulative rescue analgesics consumption, sleeping scores, adverse effects such as skin reactions, etc. The intention-to-treat analyses and the per-protocol analyses were used.
Results: There were no statistically significant differences in the VAS scores at 24 h after craniotomy (P = 0.539). However, subgroup analysis for female and male patients showed that statistically significant differences were found in VAS scores in male patients (0 [0, 3] in the masked intervention group at 24 h after craniotomy and 3 [1, 4.5] in the placebo control group, P = 0.017). There were no obvious side effects directly associated with preemptive L5P.
Conclusion: Preemptive lidocaine 5% plaster as a safe technique was not found to be effective in reducing post-craniotomy pain, but potential gender disparities in the outcomes of this method warrant further investigation.
Trial Registration: ClinicalTrials.Gov (NCT 04169854).
Keywords: craniotomy, lidocaine 5% plaster, postoperative pain, triple-blind, randomized controlled trial
Introduction
In recent years, pain following craniotomy is still recognized as a real problem and continues to receive increasing attention. Uncontrolled post-craniotomy pain may contribute to arterial hypertension, cerebral hyperemia, edema, and other detrimental symptoms.1–3 Moreover, acute postoperative pain can also lead to the development of chronic pain.4,5 Therefore, controlling and, if possible, preventing post-craniotomy pain is essential for neurosurgical patients. A prospective study by Mordhorst et al,6 showed that up to 55% of patients had moderate-to-severe postoperative pain in the first 24 h following craniotomy. Two other similar studies also showed high rates (60%–80%) of postoperative pain in this population.4,7 Furthermore, post-craniotomy pain continues to be poorly managed and undertreated.1 Therefore, pain management in craniotomy patients is challenging and often requires multimodal analgesia.
Preoperative gabapentin can alleviate acute postoperative pain and decrease the incidence of vomiting. But it did not influence postoperative opioid consumption, and it has a potential risk of delayed awakening.8 Preemptive analgesia is an antinociceptive treatment that administering analgesics before surgical stimuli to prevent pain. The analgesic effect of medications such as lidocaine primarily arises from the preemptive blockage of noxious stimuli originating from skin incisions. These analgesics are absorbed by the A delta and C fibers of the skin and by blocking sodium channels in the neuronal membrane, and they prevent the generation and transmission of action potentials from the periphery to the cortex.9–12 This afferent pain transmission results in a decrease in pain perception. An additional mechanism involves the reduction of the acute inflammatory response, as analgesics inhibit the activation of neutrophils and decrease the local release of cytokines.13–15 Preemptive local anesthetic (LA) infiltration seems to be an attractive method owing to its simplicity, safety, and low cost. However, the benefits of a single LA infiltration are still controversial, since the LA infiltration cannot cover the entire operative region, and it only provides a short period of postoperative pain relief.16–18 Currently, there is a lack of effective, safe, and simple approach for the prevention of post-craniotomy pain,19 in particular, a noninvasive approach.
Lidocaine 5% plaster (L5P) is a 10 cm*14 cm white hydrogel plaster, containing 700 mg lidocaine, as a non-invasive adjunct to pain management, that is not limited by systemic side effects of analgesics and can make the lidocaine evenly penetrate around the surgical incision.11 L5P has been used for several years to treat local postherpetic neuralgia. In a recent study, Kim et al,20 suggested that L5P application before percutaneous endoscopic lumbar discectomy produced considerable relief from superficial somatic pain and increased postoperative satisfaction. Fiorelli et al21 reported that pre-emptive skin analgesia with L5P can significantly relieve post-thoracotomy pain while reducing opioid demands and opioid-related side effects. Lau et al22 deduced that the use of lidocaine patch reduces postoperative pain intensity after gynecological surgery at rest, without any adverse effects. To date, there have been no published reports on the potential of preemptive scalp application of L5P to prevent postoperative pain following craniotomy. While L5P has demonstrated its effectiveness in alleviating postoperative pain for certain types of surgeries, the efficacy may be compromised by inadequate adhesion between the patch and the scalp, potentially leading to suboptimal pain management. Therefore, this study aims to evaluate the efficacy of preemptive topical lidocaine 5% plaster in reducing postoperative pain in patients undergoing craniotomy.
Methods
This is a multi-centered, prospective randomized placebo-controlled triple-blind clinical trial. Clinicians, patients and staff responsible for follow-up were blinded to treatment allocation. Our study complies with the Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee of Beijing Tiantan Hospital, Capital Medical University (No. KY2020-008-02). The study was registered in the ClinicalTrials.gov (NCT 04169854; Principal investigator: Fang Luo; Date of registration: November 11, 2019). The study protocol had been published in the Chinese Medical Journal.12 The trail was conducted at the Beijing Tiantan Hospital, Peking University Third Hospital and Peking University International Hospital between October 15, 2020, and December 15, 2020, and the final follow-up completed on December 18, 2020. This manuscript adheres to the applicable Consolidated Standards of Reporting Trials (CONSORT) guidelines.23
Patient Population
Patients scheduled for elective craniotomy for non-emergency procedures were consecutively screened for participation. The inclusion criteria were as follows:
• Age 18 years or older,
• American Society of Anesthesiologists status I or II,
• Agree to sign informed consent to participate in the trial.
Patients were excluded from the study if they met at least one of the following criteria:
• Allergy to lidocaine or the hydrogel plaster,
• Prior neurological deficits,
• Chronic headache,
• Craniofacial pain or neuralgia,
• Glasgow Coma Scale less than 15,
• Current or previous cardiovascular or cerebrovascular accident,
• Expected delayed recovery or extubation,
• Uncontrolled arrhythmia,
• History of craniotomy,
• Mental illness,
• Psychiatric drug use or alcohol abuse,
• Failure to understand the use of a 100 mm visual analog scale (VAS) scores,
• Expected surgical incision length is more than 30 cm.
Withdrawal criteria:
• Patient still unconscious 2 hours after surgery,
• Unanticipated delayed extubation,
• Early re-operation within the first 48 hours,
• Voluntary withdrawal.
Randomization and Blinding
A computer-generated randomization table was prepared by an investigator with no further involvement in the trial. One hundred and eighty participants undergoing craniotomy were randomly assigned to the masked intervention group and the placebo control group at a 1:1 ratio. A sealed opaque envelope was used for allocation concealment and kept in a secure locker. After randomization, irrespective of intervention group allocation, a member of the research team informed the participant about treatment and follow-up plan, and informed the therapy provider so a visit can be arranged. Significantly, clinicians, patients and staff responsible for follow-up were blinded to group allocation.
Intervention and Control
Surgeons were asked to mark the planned incision site once informed consent was obtained (at least 3 days before surgery). For each participant in the masked intervention group, the masked L5P was applied over the skin to completely cover the marked incision site (the plaster was cut to suit the shape of the incision in advance, if necessary) as well as the head-holder sites. The masked intervention group was equipped with masked lidocaine 5% white hydrogel plasters measuring 10 cm*14 cm containing 700 mg of lidocaine. The placebo control group was equipped with plain hydrogel plasters of the same pattern, size, appearance and material as L5Ps but free of lidocaine for maintenance of blinding. In both groups, the plasters were applied for 12 h at night (from 6:00 PM to 6 AM) and were removed for 12 h during the day (from 6:00 AM to 6:00 PM). This process was repeated for three days before surgery. All craniotomies in this trial were performed in the morning within 3 h after removing the plasters. No further treatment with patch after surgery.
Solid food was prohibited for 8 h and water for 4 h in all patients prior to surgery. After transfer to the operating room, electrocardiogram, heart rate, mean arterial pressure (MAP) and peripheral oxygen saturation (SpO2) were continuously monitored. A peripheral venous cannula was inserted, and an intravenous (IV) infusion of crystalloid solution was started. Each participant was premedicated with IV midazolam 0.03 mg/kg before induction of general anesthesia. About 1.5–2.0 mg/kg propofol and 10–15 μg sufentanil were used for standard induction of anesthesia. Neuromuscular blockade was provided by intravenous cisatracurium (0.2 mg/kg) for tracheal intubation. After tracheal intubation, patients were mechanically ventilated with volume-controlled ventilation (6–8 mL/kg) to achieve an end-tidal CO2 level of 28 to 35 mmHg. Anesthesia was maintained with 4–8 mg/kg/hour propofol and 0.1–0.3 µg/kg/min remifentanil intravenously; anesthesiologists could adjust the remifentanil and propofol infusion dose as needed. Antihypertensive drugs or vasoactive drugs would be administered as needed, and crystalloid and colloid solutions would be infused as necessary, by the anesthesiologist in charge, to maintain the MAP and heart rate within 30% of baseline values. Extra neuromuscular blockers and sufentanil were given as needed.
A loading dose of 0.1μg/kg sufentanil was administered to each participant for post-operative analgesia in the post-anesthesia care unit. We define uncontrolled post-operative pain as a VAS score greater than 4, which has the potential to adversely impact sleep quality, and uncontrolled post-operative pain was to be treated with an intravenous rescue bolus of 2 μg of sufentanil.
Outcome Measures
The primary outcome of this study was the pain intensity at 24 h after craniotomy (measured using a visual analog scale [VAS]). Pain intensity was evaluated by two independent, well-trained researchers using a 100 mm scale, where 0 mm in the left end represents “no pain” and 100 mm in the right end represents “the greatest pain imaginable.” Secondary outcomes of this study included the following:
• Cumulative intra-operative analgesics (sufentanil and remifentanil) consumption.
• Pain intensity measured using the 100 mm VAS at 1, 4, 6, 12, 48, and 72 h after craniotomy in both groups.
• The time interval from the end of craniotomy to the first intravenous rescue sufentanil administration.
• Cumulative rescue analgesics consumption within 24, 48, and 72 h after craniotomy.
• The number of participants who did not receive any analgesics within 72 hours after surgery.
• Sleeping scores per the Pittsburgh Sleep Quality Index (PSQI) self-rated questionnaire for the first 3 days after craniotomy.
• Skin reactions attributed to L5P application.
By combining our clinical experience, previous studies and the placebo effect, a median 100 mm VAS score of 35 at 24 h after craniotomy was estimated for patients in the placebo control group.18 According to a recent study, a minimal improvement of 10 mm can sufficiently signify a clinical difference on a 100 mm VAS. Hence, 80 participants for each group were needed to detect significance with a two-sided alpha of 0.05 with a power of 85%. Assuming an estimated 10% drop-out rate, a total of 180 participants were included in this trial.
Statistical Analysis
Statistical analyses were performed using SPSS software (version 25.0, IBM, Armonk, NY, USA). Intention-to-treat analyses (ITT analyses) and per-protocol analyses (PP analyses) were used for the Visual Analogue Scale (VAS) within 24 h after craniotomy. Other data analyses were performed in line with the intention-to-treat principle. The Kolmogorov–Smirnov test was used for normality testing. Continuous variables with normalized distributions (the total amount of sufentanil consumption, the total amount of remifentanil consumption, length of scalp, etc.) were recorded as the mean and standard deviation and analyzed using Student’s t test. Non-normally distributed data (pain intensity, cumulative rescue analgesics consumption, skin reactions, etc.) were recorded as median (interquartile range) and analyzed using the Mann–Whitney U-test. Analyses of VAS scores and cumulative rescue analgesics consumption within 24, 48, and 72 h after craniotomy were performed using linear mixed models, with treatment, time, and treatment by time included as fixed effects and within-person correlation modeled as a random effect. Categorical variables were described as N and percentage and compared using the Pearson Chi-square test or Fisher exact test. In addition, the time of first rescue analgesic demand after craniotomy was compared by Log rank test and reported as hazard ratios with 95% CI. The median time of first rescue analgesic demand was estimated by Kaplan–Meier curves. Planned subgroup analyses were performed for gender. A P-value of <0.05 was considered statistically significant.
Throughout the trial process, data safety was monitored by the Data Monitoring Committee (DMC). A clinical research associate monitored whether the clinical trial was conducted in accordance with the prescribed protocols and standard operating procedures. However, some protocol amendments were made, and research members were informed with written notice after approval from the Institutional Review Board (IRB). Any severe adverse events (AEs) were reported to the DMC by the project investigator within 24 h.
Result
Of the total of 198 patients screened (Figure 1), 18 patients (15 patients did not meet the inclusion criteria and 3 patients had a history of craniotomy) were excluded. A total of 180 eligible patients (90 patients in the masked intervention group and 90 patients in the placebo control group) were randomized in a 1:1 ratio. All participants received the allocated intervention in compliance with the study protocol and were included in the ITT analysis for primary outcome. Sixteen patients were lost to follow-up: 9 patients in the intervention group (6 patients were not awake within 2 hours after surgery and 3 patients withdrew voluntarily) and 7 patients in the control group (5 patients were not awake within 2 hours after surgery and 2 patients withdrew voluntarily). Eighty-one patients in the masked intervention group and 83 patients in the placebo control group were ultimately included in PP analysis.
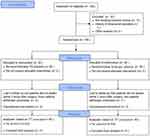 |
Figure 1 The CONSORT patient flow diagram showing numbers analyzed in each group. Abbreviation: CONSORT, Consolidated Standards of Reporting Trials. |
Baseline Characteristics
The patients’ demographic information was shown in Table 1. There were no significant differences in age, sex, Body Mass Index (BMI), ASA classification, location of scalp incision and length of scalp incision between the two groups. Despite the variation in the pathology type of tumors observed between the intervention group and the control group, no significant difference emerged in the fundamental nature of the tumors present in both groups. Consequently, the two groups were deemed to be well matched for the purposes of the study.
 |
Table 1 Baseline Characteristics |
Primary Outcome
There were no statistically significant differences in VAS scores at 24 h after craniotomy (3 [0, 5] in the masked intervention group and 3 [1, 4] in the placebo control group, P = 0.539, Table 2). The results of primary outcome in the PP sensitivity analysis were similar to those in the ITT analysis (Table 3). We conducted a subgroup analysis for female and male patients, and statistically significant differences were found in VAS scores at 24 h after craniotomy in male patients (0 [0, 3] in masked intervention group and 3 [1, 4.5] in the placebo control group, P = 0.017, Table 4).
 |
Table 2 Primary and Secondary Outcomes |
 |
Table 3 The Visual Analogue Scale (VAS) Within 24 h After Craniotomy of the per-Protocol Population |
 |
Table 4 Subgroup Analysis of VAS at 24h After Craniotomy |
Secondary Outcomes
There was no difference in the total amount of sufentanil consumption during surgery between the intervention (20.3 ± 0.9 µg) and the control group (20.7 ± 0.8 µg, Mean diff. = −0.4, P = 0.666, Table 2). Similarly, no significant difference was found in the total amount of remifentanil consumption during surgery between the intervention group (3494.4 ± 284.3 µg) and the control group (3507.8 ± 304.2 µg, Mean diff. = −13.4, P = 0.903, Table 2).
The difference in VAS scores at 1, 4, 6, 12, 48, and 72 h after craniotomy between two groups was not significant (Table 2). Ninety-one (50.6%) participants (49 participants in intervention group and 42 participants in the control group) had no analgesic consumption within 72 hours after surgery (P = 0.676). The first rescue analgesic demand did not differ distinctively by the type of intervention (hazard ratio [HR] 1.170, 95% CI 0.75 to 1.8; Log-rank P = 0.4878, Figure 2). There were no statistically significant differences observed in the cumulative rescue analgesic consumption within 24 hours after surgery (0 [0, 10] in the masked intervention group and 2 [0, 8] in the placebo control group, P = 0.729, Table 2). The PSQI was not significantly different between the two groups (6 [3, 11] in intervention group and 4.5 [3, 8] in the control group, P = 0.202, Table 2).
Scalp reactions were reported in 3 patients in each group, with no significant difference between the two groups (RR = 1.0 [0.2 to 4.8], P = 1.000). No patients withdrew from study due to adverse reactions. No other AEs related to study interventions, including skin reactions to the plaster, neurological side effects or complications from any lidocaine use, were recorded during the study.
Discussion
In this study, we sought to examine the post-craniotomy analgesic effect of preemptive lidocaine 5% plaster that has been shown to provide analgesic benefit in orthopedic and thoracic surgeries. Unfortunately, although this is a safe and non-invasive additional intervention, we did not find a reduced degree of pain with preemptive lidocaine 5% plaster in craniotomy patients. Thus, the clinical value of preemptive topical lidocaine 5% plaster for the prevention of post-craniotomy pain is limited.
In contrast to several previous studies, which indicated that preventive scalp infiltration with anesthetic significantly reduced pain intensity and the cumulative consumption of opioids post-surgery,1,24,25 we did not found that preemptive lidocaine 5% plaster was effective in reducing post-craniotomy pain. Lidocaine 5% plaster has limited penetration, and the hair stubble on the scalp impedes the complete adherence of L5P to the skin, leading to inadequate lidocaine concentration in the scalp. Consequently, a low drug concentration fails to provide sufficient preemptive analgesia.
Contrary to expectations and previous reports of analgesic benefits in percutaneous endoscopic lumbar discectomy and thoracotomy,20,21 there was no statistically significant difference between the VAS scores, cumulative rescue analgesics consumption and the time of first rescue analgesic administration between the two groups at any time point after craniotomy. Lidocaine was absorbed by the painful fibers in the skin, and by blocking the sodium channels in the neuronal membrane, and it inhibited the generation and propagation of action potentials from the periphery (the site of incision) to the cortex. This disruption of afferent pain transmission led to a decrease in pain perception.9–12 Therefore, we assume that the reason for this is because the skin on the waist and the chest is generally very smooth, so the L5P fits the skin more tightly, resulting in an even and easy absorption of medication through the skin. While in craniotomy, even with preoperative skin preparation, the scalp is textured with hair stubble which limits the ability of L5P to completely attach to the skin, resulting in reduced distribution and absorption of medication, leading to insufficient concentration of lidocaine in the scalp. Therefore, the intensity and duration of pain relief may fall short of expectations. The mechanism of lidocaine plaster is to alleviate surface pain by blocking local nerve conduction. In future studies, the pain of somatic surgery with flat and smooth surgical sites should be evaluated, to study the postoperative analgesic effect of preemptive L5P.
Interestingly, subgroup analysis showed that the VAS score at 24h was not different between the female patients in the two groups, but there was significant difference between the intervention group and the control group in male patients. Up to now, there has been no research that has reported gender-based differences in the mechanism of action of lidocaine 5% plaster. Lidocaine is an amide-based local anesthetic, exhibiting a greater lipid solubility than water solubility. We deduce that this might be because male patients experience more oil secretion on their scalp, making the medication more easily absorbed through the skin. As a result, the effectiveness of lidocaine is magnified. Consequently, additional research is essential to assess the effectiveness of preemptive lidocaine 5% plaster with respect to gender-specific differences.
Similar to previous studies,20–22 preemptive L5P did not reduce total intraoperative opioid consumption. In this study, preemptive L5P also did not improve PSQI. The relationship between pain and sleep quality is reciprocal; poor sleep quality can lead to increased sensitivity to pain, and high levels of pain are important predictors of poor sleep quality.26,27 Preemptive L5P in this study did not reduce postoperative pain intensity, nor did it logically improve postoperative sleep quality.
Only a small number of patients in both groups developed scalp reactions, and the incidence of adverse reactions in the intervention group was not higher than that in the control group. No patients withdrew from the study due to adverse reactions, and no other investigation-related adverse events were recorded during the study, which confirms that 5% lidocaine plaster is safe for preemptive topical use, consistent with the results reported in previous studies.20–22
Although this study boasts certain advantages, notably its randomized design, it is also subject to several limitations. First, only one plaster was used in all patients without considering the variations in patients’ scalp thickness that could affect the diffusion of lidocaine through the tissues. Ideally, the rate of lidocaine diffusion in patients with greater skin thickness was low, and more than one patch could be used. Second, the shape of the incision may have varied based on the surgical purposes. As mentioned above, the plaster may be placed obliquely or cut into pieces to suit the shape of the incision. This will inevitably lead to unequal distribution of lidocaine along either side of the incision and cause underlying confounding effects. Third, the pain was measured with VAS scores, which can be influenced by many variables in contrast to more sophisticated means of quantifying pain, such as the McGill pain questionnaire. Fourth, only one patch was applied to all patients for 12 hours, without considering that individual differences in the skin of patients might have affected the diffusion of lidocaine through tissues. Fifth, our study did not evaluate the postoperative bedside sedation scores, which may have influenced the reliability of the self-reported pain intensity by patients. Sixth, given the absence of significant differences, questions arise regarding statistical power, particularly for subgroup analyses; therefore, the results of the study should be interpreted with caution. Seventh, variations in scalp thickness can potentially influence the absorption rate of lidocaine, and since all patients utilized plaster of identical dimensions, this may lead to an uneven distribution of the medication. Eighth, the follow-up duration of our study was short, indicating a need for further research to evaluate the long-term pain outcomes associated with L5P. Finally, further exploration into whether the combination of this patch with other analgesic methods can achieve a superior analgesic effect is warranted.
In conclusion, the preemptive application of a 5% lidocaine plaster did not result in additional pain score reduction or a decrease in cumulative opioid consumption post-craniotomy. Nonetheless, subgroup analysis revealed a significant difference in outcomes between the intervention and control groups among male patients. Further research is warranted to assess gender-specific efficacy variations of preemptive 5% lidocaine plaster. Additionally, it would be beneficial to investigate whether integrating this plaster with other analgesic techniques could enhance pain relief.
Abbreviations
LA, local anesthetic; L5P, lidocaine 5% plaster; ASA, American Society of Anaesthesiologists; ITT, intention-to-treat; PP, per-protocol; VAS, Visual Analogue Scale; BMI, body mass index; PSQI, Pittsburgh Sleep Quality Index; DMC, Data Monitoring Committee; IRB, Institutional Review Board; AEs, adverse events.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author (Fang Luo, email: [email protected]). The data are not publicly available due to privacy or ethical restrictions.
Author Contributions
XYH, and YYX contributed equally to this work. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The Capital’s Funds for Health Improvement and Research (2020-2-2046). The Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. XMLX201707). Capital’s Funds for Health Improvement and Research (2022-1-4061). The National Key Research and Development Program of China (2022YFC3602200).
Disclosure
There is no conflict of interest among the authors in this study.
References
1. Bilotta F, Guerra C, Rosa G. Update on anesthesia for craniotomy. Curr Opin Anaesthesiol. 2013;26(5):517–522. doi:10.1097/01.aco.0000432513.92822.c2
2. An LX, Chen X, Ren XJ, Wu HF. Electro-acupuncture decreases postoperative pain and improves recovery in patients undergoing a supratentorial craniotomy. Am. J Chin Med. 2014;42(5):1099–1109. doi:10.1142/S0192415X14500682
3. Galvin IM, Levy R, Day AG, Gilron I. Pharmacological interventions for the prevention of acute postoperative pain in adults following brain surgery. Cochrane Database Syst Rev. 2019;2019(11):CD011931. doi:10.1002/14651858.CD011931.pub2
4. De Benedittis G, Lorenzetti A, Migliore M, Spagnoli D, Tiberio F, Villani RM. Postoperative pain in neurosurgery: a pilot study in brain surgery. Neurosurgery. 1996;38(3):466–470. doi:10.1097/00006123-199603000-00008
5. Mordhorst C, Latz B, Kerz T, et al. Prospective assessment of postoperative pain after craniotomy. Journal of Neurosurgical Anesthesiology. 2010;22(3):202–206. doi:10.1097/ANA.0b013e3181df0600
6. Hansen MS, Brennum J, Moltke FB, Dahl JB. Pain treatment after craniotomy: where is the (procedure-specific) evidence? A qualitative systematic review. Eur. J Anaesthesiol. 2011;28(12):821–829. doi:10.1097/EJA.0b013e32834a0255
7. Flexman AM, Ng JL, Gelb AW. Acute and chronic pain following craniotomy. Curr Opin Anaesthesiol. 2010;23(5):551–557. doi:10.1097/ACO.0b013e32833e15b9
8. Zeng M, Dong J, Lin N, et al. Preoperative Gabapentin Administration Improves Acute Postoperative Analgesia in Patients Undergoing Craniotomy: a Randomized Controlled Trial. Journal of Neurosurgical Anesthesiology. 2019;31(4):392–398. doi:10.1097/ANA.0000000000000533
9. Derry S, Wiffen PJ, Moore RA, Quinlan J. Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;2014(7):CD010958. doi:10.1002/14651858.CD010958.pub2
10. Goddard JM, Reaney RL. Lidocaine 5%-medicated plaster (Versatis) for localised neuropathic pain: results of a multicentre evaluation of use in children and adolescents. Br J Pain. 2018;12(3):189–193. doi:10.1177/2049463718756431
11. Habib AS, Polascik TJ, Weizer AZ, et al. Lidocaine patch for postoperative analgesia after radical retropubic prostatectomy. Anesth Analg. 2009;108(6):1950–1953. doi:10.1213/ane.0b013e3181a21185
12. Saber AA, Elgamal MH, Rao AJ, Itawi EA, Martinez RL. Early experience with lidocaine patch for postoperative pain control after laparoscopic ventral hernia repair. Int J Surg. 2009;7(1):36–38. doi:10.1016/j.ijsu.2008.09.003
13. Yon JH, Choi GJ, Kang H, Park JM, Yang HS. Intraoperative systemic lidocaine for pre-emptive analgesics in subtotal gastrectomy: a prospective, randomized, double-blind, placebo-controlled study. Can J Surg. 2014;57(3):175–182. doi:10.1503/cjs.009613
14. Xie W, Strong JA, Li H, Zhang JM. Sympathetic sprouting near sensory neurons after nerve injury occurs preferentially on spontaneously active cells and is reduced by early nerve block. J Neurophysiol. 2007;97(1):492–502. doi:10.1152/jn.00899.2006
15. Fiorelli A, Izzo AC, Frongillo EM, et al. Efficacy of wound analgesia for controlling post-thoracotomy pain: a randomized double-blind study. Eur J Cardiothorac Surg. 2016;49(1):339–347. doi:10.1093/ejcts/ezv097
16. Kjærgaard M, Møiniche S, Olsen KS. Wound infiltration with local anesthetics for post-operative pain relief in lumbar spine surgery: a systematic review. Acta Anaesthesiol Scand. 2012;56(3):282–290. doi:10.1111/j.1399-6576.2011.02629.x
17. Paladini G, Di Carlo S, Musella G, et al. Continuous Wound Infiltration of Local Anesthetics in Postoperative Pain Management: safety. J Pain Res. 2020;13:285–294. doi:10.2147/JPR.S211234
18. Li K, Li H, Luo D, et al. Efficacy of local infiltration analgesia with ropivacaine for postoperative pain management in cervical laminoplasty: a retrospective study. Sci Rep. 2020;10(1):4217. doi:10.1038/s41598-020-61229-2
19. Meng L, Chen Z, Yang J, Luo F. Pre-emptive topical lidocaine 5% plaster for prevention of post-craniotomy pain: a protocol for a multicentred, randomized, triple-blind, placebo-controlled clinical trial. Chin Med J. 2020;133(19):2375–2377. doi:10.1097/CM9.0000000000001066
20. Kim KH. Use of lidocaine patch for percutaneous endoscopic lumbar discectomy. Korean J Pain. 2011;24(2):74–80. doi:10.3344/kjp.2011.24.2.74
21. Fiorelli A, Pace C, Cascone R, et al. Preventive skin analgesia with lidocaine patch for management of post-thoracotomy pain: results of a randomized, double blind, placebo controlled study. Thorac Cancer. 2019;10(4):631–641. doi:10.1111/1759-7714.12975
22. Lau LL, Li CY, Lee A, Chan SK. The use of 5% lidocaine medicated plaster for acute postoperative pain after gynecological surgery: a pilot randomized controlled feasibility trial. Medicine. 2018;97(39):e12582. doi:10.1097/MD.0000000000012582
23. Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials- PubMed - NCBI. BMC Med. 2010;8(18). doi:10.1186/1741-7015-8-18
24. Yang X, Ma J, Li K, et al. A comparison of effects of scalp nerve block and local anesthetic infiltration on inflammatory response, hemodynamic response, and postoperative pain in patients undergoing craniotomy for cerebral aneurysms: a randomized controlled trial. BMC Anesthesiology. 2019;19(1). doi:10.1186/s12871-019-0760-4.
25. Song J, Li L, Yu P, Gao T, Liu K. Preemptive scalp infiltration with 0.5% ropivacaine and 1% lidocaine reduces postoperative pain after craniotomy. Acta Neurochir. 2015;157(6):993–998. doi:10.1007/s00701-015-2394-8
26. Chouchou F, Khoury S, Chauny JM, Denis R, Lavigne GJ. Postoperative sleep disruptions: a potential catalyst of acute pain? Sleep Med Rev. 2014;18(3):273–282. doi:10.1016/j.smrv.2013.07.002
27. Kehlet H. Postoperative pain, analgesia, and recovery—bedfellows that cannot be ignored. Pain. 2018;159(Suppl 1):S11–S16. doi:10.1097/j.pain.0000000000001243
28. Paladini G, Di Carlo S, Musella G, et al. Continuous Wound Infiltration of Local Anesthetics in Postoperative Pain Management: safety, Efficacy and Current Perspectives [Corrigendum]. J Pain Res. 2020;13:659. doi:10.2147/JPR.S252624
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Combination Treatment with Thread-Embedding Acupuncture and Electroacupuncture for Knee Osteoarthritis Patients with Postoperative Pain: A Randomized Controlled Feasibility Study
Lee YJ, Han CH, Jeon JH, Kim E, Park KH, Kim AR, Kim YI
Journal of Pain Research 2025, 18:89-103
Published Date: 8 January 2025


