Back to Journals » Journal of Pain Research » Volume 18
Efficacy of Transcutaneous Electrical Acupoint Stimulation on Modulating Upper Extremity Sympathetic Skin Response in Alleviating Cancer Survivors With Chemotherapy-Induced Peripheral Neuropathy: A Propensity Score-Matched Cohort Study
Authors Xu Y, Wu J , Jiang Q, Lv Y, Zhou J, Wang Z, Zhao H, Du D
Received 15 October 2024
Accepted for publication 9 January 2025
Published 21 January 2025 Volume 2025:18 Pages 293—303
DOI https://doi.org/10.2147/JPR.S500717
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Yongming Xu,1,* Junzhen Wu,1,* Qingqing Jiang,2 Yingying Lv,1 Jin Zhou,1 Zhiyu Wang,3 Hui Zhao,3 Dongping Du1
1Department of Pain Management Center, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Neurology, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 3Department of Internal Oncology, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dongping Du, Department of Pain Management Center, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China, Tel +86-21-24058896, Email [email protected] Hui Zhao, Department of Neurology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, 600 Yishan Road, Shanghai, 200233, People’s Republic of China, Tel +86-21-24058328, Fax +86-21-240598328, Email [email protected]
Objective: Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect of chemotherapy and it is currently intractable We compared the efficacy of transcutaneous electrical acupoint stimulation (TEAS) against non-TEAS groups and investigated the variables that predict effective relief of upper extremity pain in cancer survivors with CIPN.
Methods: We retrospectively collected data of cancer survivors who developed CIPN between May 2017 to March 2022. All eligible CIPN patients were divided into TEAS group (received TEAS) and non-TEAS group (did not receive TEAS) in our department. A 1:1 ratio propensity score matching (PSM) was used to balance the baseline features. The change of numerical rating scale (NRS), Short-Form McGill Pain Questionnaire-2 (SF-MPQ-2), and sympathetic skin response (SSR) parameters are all assessed after treatment. The procedure was considered a clinically effective relief if the patients’ NRS scores were reduced by 50% or more, and overall patients with effective relief were all counted after treatment. Furthermore, a multivariable logistic regression model was utilized to evaluate the predictors of effective relief following CIPN treatment.
Results: : A total of 102 cancer survivors with CIPN were analyzed after PSM (51 in each group). The change of NRS, SF-MPQ-2, SSR latency and SSR amplitude in TEAS group were significantly higher than those in non-TEAS group at 3 weeks after therapy (all P< 0.01). In addition, the effective relief rate was significantly higher in TEAS group than in non-TEAS group (P=0.026). Multivariate logistic regression on the total study cohort showed that TEAS group (OR 2.783, P = 0.025) and the baseline SSR amplitude of the upper extremity < 1265 μV (OR 12.191, P = 0.000) were independent predictive factors for the clinical efficacy.
Conclusions: : TEAS significantly decreased the severity of CIPN. TEAS group and baseline SSR amplitude of the upper extremity < 1265 μV were the independent predictive factors for the clinical efficacy after treatment.
Keywords: transcutaneous electrical acupoint stimulation, chemotherapy-induced peripheral neuropathy, sympathetic skin response, cancer survivors, neuropathic pain
Introduction
Chemotherapy induced peripheral neuropathy (CIPN) is a persistent neuropathic pain that is a common cause of decreased chemotherapy dose and medication discontinuation, which can result in less effective cancer treatment and reduced quality of life.1 CIPN cannot be absolutely cured with currently available drugs, and it can impact the peripheral nervous system from the cell body to the axon and any of the peripheral nerve fiber types. Paclitaxel‐based and platinum‐based chemotherapy has been effectively utilized in clinical practice to treat breast cancer, lung cancer, and ovarian cancer2 and the major manifestation of CIPN is sensory symptoms such as numbness and paresthesia in the extremities.3 Meanwhile, the neurotoxicity of chemotherapy might affect nerves globally in the body thereby involving the autonomic nerves.
Nowadays, the prevalence of autonomic dysfunction and neuropathic pain for patients treated with chemotherapy remains undefined. Treatments for CIPN have been suggested, including oral analgesics, exercise rehabilitation therapy, acupuncture, transcutaneous electrical nerve stimulation (TENS).4–7 Most oral medicines fail to improve limb numbness, tingling, paresthesia, or burning sensation. Transcutaneous electrical acupoint stimulation (TEAS) is a therapeutic method combining stimulation of acupuncture with TENS, and it attaches surface electrode patch to deliver electricity, which stimulates the acupoint instead of traditional acupuncture.8 The application of TEAS has been increasing because of its painless experience, non-invasive insertion, and practicable process.9,10 The analgesic impact of TEAS on patients with CIPN is still barely understood, although acupuncture treatment has been proven to have certain therapeutic effects.11,12
Acupoints are essential spots on the body for acupuncture treatment because they facilitate the activation and accelerate the self-healing process under certain circumstances.13 However, the mechanism remains unclear. The primary pathogenic characteristic of hypersensitive acupoints is neurogenic inflammation, which is caused mainly by nociceptor activation and neuroimmune interactions.14 Previous research has demonstrated that the sympathetic nervous system plays an important role in the encoding of nociceptive information, and that reducing sympathetic activity significantly improves sensory hyperalgesia.15 In animals, electroacupuncture treatment can improve inflammation and hyperalgesia, potentially by suppressing the sprouting of sympathetic nerve fibers and neurogenic inflammation.16 Meanwhile, it is reported that sensory input from disease-sensitized acupoints contributes to homeostasis while simultaneously participating in the transmission of therapeutic signals during acupuncture and the role of sprouting sympathetic nerve plays in acupoint function.17 In clinical practice, we hope to evaluate the potential benefits of sympathetic response on the plasticity of acupoint function. Sympathetic skin response (SSR) has recently been proposed as a non-invasive technique to examining the function of the sympathetic system because it is considered an obtainable index of the function of sympathetic postganglionic fibers.18 SSR had been used to evaluate patients with somatic and autonomic neuropathy, while abnormal SSR results were found in intractable pain diseases, including CIPN.19–21
Consequently, we utilized propensity score matching (PSM) to evaluate the efficacy of TEAS against non-TEAS groups for CIPN and investigated the variables that predict effective relief of upper extremity pain in cancer survivors with CIPN.
Methods
Patients
From May 2017 to March 2022, 387 patients who received paclitaxel combined with platinum (cisplatin or carboplatin) chemotherapy treatment in the Shanghai Sixth People’s Hospital. Among these individuals, 174 patients who developed CIPN later were included in the study. Variables include age, sex, BMI, disease duration, type of cancer (breast cancer, lung cancer, ovarian cancer), severity of upper limbs CIPN before treatment and 3 weeks after treatment. Thereafter, the numerical rating scale (NRS), Short-Form McGill Pain Questionnaire-2 (SF-MPQ-2), SSR test were used to evaluate the severity of upper limbs CIPN. The study protocol was ethically authorized by the Shanghai Sixth People’s Hospital ethics committee (No. 2016–102). All human-related operations were carried out in compliance with the National Research Council’s ethical requirements. In accordance with the Declaration of Helsinki, the researchers explained the significance of the study to all participants. All recruited patients provide written informed consent to participate in the trial.
Inclusion Criteria
The following were the inclusion requirements: adult breast, lung, and ovarian cancer survivors who experience painful CIPN for at least 2 months and completed chemotherapy at least 3 months ago will be recruited; presenting grade>2 CIPN symptoms according to the National Cancer Institute Common Terminology Criteria; the patients with NRS score of 4 or more on a scale of 0–10 (with 0 indicating no pain and 10 indicating the worst imaginable pain); clear consciousness, no memory or attention impairment; symptoms and signs of limb sensory or motor neuropathy related to chemotherapy. The exclusion criteria were as follows: patients who have incomplete medical records; patients with peripheral neuropathy caused by other diseases; history of oral anticholinergic drugs; SSR failed to be exported; infection. Thus, 142 individuals were chosen, and fourteen of them lost contact during the post-therapy follow-up. Ultimately, a total of 128 individuals were selected for this comparative analysis.
CIPN patients were subsequently divided into two groups: those who received TEAS (TEAS group) and those who did not (non-TEAS group). All patients from both groups received duloxetine or pregabalin as the analgesic treatment. Due to patient condition or medical preference, TEAS was not routinely used within the time period of our records, and those were performed by 3 experienced doctors. Eventually, 56 patients received TEAS treatment, whereas 72 patients did not.
Treatment Protocols
TEAS Treatment
The TEAS spots were chosen based on traditional Chinese medical philosophy, specifically bilateral Hegu (LI4), Waiguan (TE5), Quchi (LI11) and second Baxie (Extra 27). These acupoints were identified using classical anatomical localization (Figure 1). In three weeks, the TEAS group had 15 TEAS treatments (twice stimulation each day for five consecutive days, followed by a two-day rest interval, with each stimulation lasting 30 minutes). After disinfecting the skin at the acupoints, patients received square-shaped electrode patches that were attached to the Hwato electronic acupuncture treatment instrument (Suzhou Electronic Needle Therapy Instrument SDZ-II). The device generated “disperse-dense” waves with alternating frequencies of 2 hz and 10 hz for 30 min. The stimulation intensity was adjusted to maintain a slight twitching of regional muscle according to the patients’ maximum tolerance.
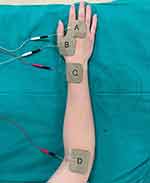 |
Figure 1 Acupoints selected for chemotherapy-induced peripheral neuropathy in this study: (A) Baxie (Extra 27), (B) Hegu (LI4), (C) Waiguan (TE5), (D) Quchi (LI11). |
SSR Test
Every single patient, whether TEAS or non-TEAS, had an SSR test prior to treatment. The SSR was investigated using the standard method.18 During the examination, all patients were placed supine, SSR recordings were recorded between 2 and 5 p.m., and the room temperature was 23–26°C. Blood pressure, heart rate, body temperature, and blood glucose levels were all evaluated and determined to be normal. The SSR test employs electrodiagnostic equipment (Focus, Dantec Keypoint) to detect an induced change in the skin’s electrical potential in order to measure autonomic function. Electrical stimuli are employed to examine SSR parameters in the bilateral upper limbs, variations in palm activity potential are recorded, and the dorsum of the hand serves as a reference. Alcohol is used for sterilizing electrodes before connecting them to the palm and dorsum of the hand for SSR recording. We were able to record the SSR of the upper limbs by applying a pulse width of 0.2 ms and a current intensity of 15 mA to the median nerve at the arm and wrist. SSR parameters have two potential values. The latency was calculated as the time spent between the start of the electrical stimulus and the first deviation from baseline, and the amplitude as the distance between the baseline and the greatest positive peak. To avoid habituation, record SSR with three repeated stimuli on each limb, with a 60 second pause between each stimulus. The average latency and amplitude values from a total of six SSRs were utilized. An SSR outlet failed when there is no response to stimulation at both ends. Finally, three weeks after treatment, the final SSR of the bilateral upper limbs would be examined again.
Data Recording
Baseline characteristics, including age, sex, body mass index (BMI), symptom duration, cancer type, baseline NRS score, baseline SF-MPQ-2 score, baseline SSR latency and amplitude of the upper limbs were collected from the patients’ medical records. The NRS was used to assess upper limb pain, which ranged from 0 (no pain) to 10 (severe pain). SF-MPQ-2 contains 22 items and uses a 0–10 NRS scale. It can comprehensively evaluate the pain properties of CIPN. Change in NRS, SF-MPQ-2, SSR latency and SSR amplitude were compared between the two treatment groups, reflecting the change in ratings of severity from baseline to final follow-up. Outpatient follow-up with the patients was performed after 3 weeks to assess the efficacy of treatment. The percentage of pain relief was determined using the following formula: (NRS base - NRS after therapy)/NRS base 100%. The procedure was considered effective relief if the patients showed more than a 50% relief in their NRS scores, and the effective relief rates were calculated for each group. Meanwhile, the patients were retested for SSR in the outpatient department after 3 weeks. All adverse effects (eg, dizziness, abnormal increased pain, numbness, paresthesia and motor weakness) were recorded during the procedure.
Statistical Analysis
Normally distributed continuous variables were presented as mean ± standard deviation (SD) and compared using the independent samples t-test. Variables with a skewed distribution were summarised using medians and interquartile ranges (IQR) and compared by a paired Wilcoxon test or signed rank-sum test. Categorical variables were presented as numbers and were compared by the Chi-square test or Fisher’s exact test.
To balance important patient characteristics between groups, we performed propensity score matching. The propensity score was calculated using a logistic regression analysis with the dependent variable being the type of interventional procedure and the independent covariates being the following baseline clinical and demographic variables: age, sex, BMI, duration, baseline NRS score, baseline SF-MPQ-2 score, baseline SSR latency and amplitude. A 1:1 nearest neighbor matching algorithm that pairs patients with the closest propensity scores was used. A caliper width of 0.2 units was used. The procedure of propensity scores yielded 2 matched cohorts of 51 patients. Unpaired cases were discarded from analysis. We compared standardized differences for all covariates between prematch and postmatch.
To analyze prognostic factors of the clinical response of treatment, univariate χ 2 analysis was first performed. Afterward, the factor with P < 0.2 in the results of the univariate analysis was used as an independent variable entered the multivariate binomial logistic regression model to adjust clinical predictive effect and rule out any confounder effects. The odds ratio (OR) and its 95% confidence interval (CI) were calculated. Multicollinearity was checked before we performed the multivariate logistic regression analysis. The Variance Inflation Factor (VIF) was calculated, and a VIF above 10 indicates that the model has multicollinearity.
All data were analyzed with SPSS software (SPSS version 25.0; IBM Corp., Chicago, IL, USA). P values <0.05 were considered statistically significant.
Results
From May 2017 to March 2022, 387 patients who underwent paclitaxel combined with platinum chemotherapy were evaluated for eligibility, with 128 participants (non-TEAS group, n=72; TEAS group, n=56) enrolling in the analysis (Figure 2).
 |
Figure 2 Flow chart. |
Demographic and Baseline Characteristics of Participants
The demographic characteristics for the total study cohort are presented (Table 1). Significant statistical differences were detected for NRS score and the latency of SSR between the groups before matching. After PSM, 51 patients remained in each group and a good matching balance was achieved (Figure 2).
 |
Table 1 Demographic Characteristics |
Comparison of Outcome Measurements and Treatment Effectiveness Between Two Groups
The outcome measurements and treatment effectiveness were consistent before and after propensity score matching. The NRS and SF-MPQ-2 score decreased much more in TEAS group than non-TEAS group (P<0.01); simultaneously, the latency and amplitude of SSR in TEAS group was significantly changed compared to non-TEAS group (P<0.01); the overall effective relief rate of TEAS group was higher than non-TEAS group (non-TEAS group, 49%; TEAS group, 73.5%; P=0.026) (Table 2).
 |
Table 2 Outcome Measurements and Treatment Effectiveness Between Two Groups |
Predictive Factors for Clinical Efficacy After Therapy in the Total Cohort
The univariable analysis revealed that age, symptom duration, the baseline SSR amplitude of the upper extremity and treatment group were significant explanatory variables (P<0.20) (Table 3). In the multivariable regression analysis, all VIF scores were <1.5. There was no multicollinearity in our model, and so all the four factors were included in the multivariable regression model. Finally, the two predictive factors were identified by multivariate logistic regression: the baseline SSR amplitude of the upper extremity <1265 µV (OR 12.191, 95% CI: 4.096–30.293, P = 0.000) and TEAS group (OR 2.783, 95% CI: 1.137–6.815, P = 0.025) (Table 4).
 |
Table 3 Risk Factors for Clinical Efficacy After Therapy in the Total Cohort (n=128) |
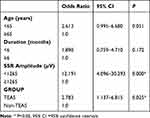 |
Table 4 Multivariate Logistic Regression Analysis for Clinical Efficacy After Therapy (n=128) |
Discussion
After our research we found that TEAS can greatly decrease the severity of CIPN, improving patients’ quality of life and promoting rehabilitation. With the frequency of malignant tumors rising with every passing year, anti-tumor medications are becoming more and more important in the long-term management of malignant tumors. These medications’ short- and long-term adverse effects are also causing a growing amount of worry.22 In recent years, oncology, neurology, and pain specialists have begun to devote more attention to neurotoxicity. The neurotoxicity of chemotherapy medications is second only to blood system toxicity as a reason of dose decrease or withdrawal.23 CIPN is a major, dose-limiting side effect of treatment with neurotoxic cancer treatment and it occurs in around 60% of patients three months, and in approximately 30% of patients six months or longer after chemotherapy.24 Furthermore, CIPN is frequently referred to as distal, symmetrical, and sensory neuropathy,25 which has a negative impact on patients’ quality of life26 and a lot of cancer survivors continue to suffer from neuropathic symptoms after discontinuing medication.27 Oral medicine is the most basic treatment for CIPN-related symptoms, with duloxetine being the most recommended, providing modest to moderate relief in CIPN discomfort.28 In our study, all CIPN patients were given either duloxetine or pregabalin to relieve their limb symptoms. Recently, acupuncture has gained popularity in integrative oncology clinics due to clinical evidence supporting its utility in minimizing symptoms of CIPN and improving prognosis of patients.11,12,29 However, the impacts of TEAS on CIPN and its underlying mechanism are not fully clear. Therefore, we attempted to uncover it.
TEAS is a form of noninvasive therapy that has advantages over needle-based electrostimulation and conventional acupuncture. It could potentially be useful in clinical applications, especially for pain management. A number of studies that investigated the efficacy of TEAS in pain management reported reductions in overall opioid dosages and pain relief including chronic postsurgical pain8 and cancer-related pain.30 To our knowledge, this is the first study aimed at the efficacy of TEAS treatment in CIPN utilizing subjective assessment (NRS and SF-MPQ-2) and objective SSR measurement. Several studies regard acupuncture as a treatment choice for mild to severe CIPN in practice12 which may result in notable improvement in CIPN symptoms.31 In our study, patients treated with TEAS experienced considerable pain relief basing on changes in NRS and SF-MPQ-2 before and after treatment (Table 2). Furthermore, the results we have obtained imply that TEAS therapy is associated with a better recuperation and a decreased occurrence of aberrant sensations in CIPN patients. CIPN can result in long-term impairment and its deficiencies frequently represent a large fiber polyneuropathy; nevertheless, small fiber involvement has the potential to result in neuropathic pain and autonomic dysfunction. Interestingly, multiple trials have proven that acupuncture can activate Aδ and C fibers, which may be injured by chemotherapy.32,33 Burning sensations in the limbs are thought to be caused by small fiber neuropathy, which affects somatic unmyelinated nerves. What may occur simultaneously are vasomotor or sudomotor abnormalities in CIPN, indicating autonomic fiber dysfunction.28 And that, several investigations have demonstrated that the pathophysiology of CIPN depends on the autonomic nervous system (ANS).34,35 Generally, questionnaires, clinical assessments, or neurophysiological testing have been used for the diagnosis of CIPN.23 However, due to the unavailability of an easy-to-understand assessment method, it is not well investigated at an early stage.36 Sudomotor function testing such as SSR has been proposed for early screening of peripheral neuropathy, because sweat glands are innervated by small autonomic C-fibers.37 Although autonomic dysfunction is infrequently seen and is rarely considered as a severe adverse effect of chemotherapy, it is highly likely to be a component of small unmyelinated nerve fiber neuropathy.38
SSR has been widely used as a quantitative objective testing for several types of autonomic nerve diseases20,39 and it is more useful than subjective scale testing as a marker of peripheral neuropathy.40 By detecting the sympathetic sudomotor fibers’ conduction function, SSR can compensate for the deficiencies of nerve conduction velocity.41 There have been very few studies exploring into the role of SSR changes in CIPN diagnosis and treatment. The study on chemotherapy-induced autonomic neuropathy assessed the effects of cisplatin or paclitaxel and revealed that SSR could not seem to be associated with the early detection of CIPN. However, its significance in identifying small fiber neuropathy abnormalities is valuable.19 When the ANS is affected by small fiber neuropathy, SSR latency will be prolonged, and the amplitude will decrease.39 Aside from extensive denervation of sweat glands or afferent sensory neurons, abnormal SSR may indicate autonomic efferent dysfunction18 and a high incidence of small fiber neuropathy.42 It should be mentioned that in our study, there were significant differences in the changes of SSR parameter on upper limbs treated with TEAS compared to non-TEAS, together with the effective relief rate (Table 2). Some investigations have indicated that acupoints perform more effectively and have a broader variety of effects.43 Stimulating the hypersensitive acupoint has shown to be a more successful therapeutic strategy for the treatment of neuropathic pain.44 This suggests that TEAS could have a positive impact on the symptoms of CIPN patients by stimulating acupoints.
To determine favorable prognostic factors for effective relief of CIPN patients after undergoing neurotoxic paclitaxel combined with cisplatin or carboplatin chemotherapy, we evaluated independent predictive factors such as age, sex, symptom duration, type of cancer, baseline SSR latency, baseline SSR amplitude and treatment group (Table 3). Finally, we identified two consistent favorable variables for CIPN, including the baseline SSR amplitude of the upper extremity <1265 µV and TEAS group (Table 4). The TEAS group is the most promising prognostic factor for effective CIPN treatment. The effects of TEAS on the pathophysiology of CIPN patients have not been researched, although they are likely to involve both central and peripheral mechanisms45 including entirely reversible the inhibition of nerve impulse generation and transmission, blocking the spinal reflex pathway, and minimizing sympathetic excitability.46 Based on these fundamentals, TEAS is anticipated to boost nerve regeneration and neurological restoration, consequently alleviating CIPN symptoms. In our research, the overall effective relief rate of the TEAS group was 73.5% (36/49), compared to 46.9% (23/49) of the non-TEAS group, indicating that TEAS has a significant advantage in treating CIPN.
There are several limitations in our study. First, because the study was retrospective and observational, possible bias and confounding were not entirely avoided. Second, we lacked more clinical assessment methods specific to CIPN (eg, Patient Neurotoxicity Questionnaire, nerve conduction velocity). Third, in the subsequent observation stage, the efficacy was judged by short-term pain alleviation with no long-term follow-up. Although we did not observe a long-term effect in our investigation, we conducted evaluation (NRS and SF-MPQ-2) at 3 weeks after CIPN treatment. Finally, because there is no recognized standard for normal SSR parameter values, we studied SSR parameter changes in our study, and the evaluation of SSR parameter values is dependent on neurologists’ explanations.
Conclusion
In summary, our results showed that, when compared to the non-TEAS group, the TEAS group can effectively relieve upper extremities pain in CIPN patients, with a significantly higher effective relief rate. The TEAS group and baseline upper extremity SSR amplitude <1265 µV were independent predictors of clinical efficacy after treatment. In terms of analgesic effectiveness and non-invasiveness, the TEAS intervention might be an appealing choice for cancer survivors with CIPN.
Data Sharing Statement
The datasets generated during/analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This research was funded by the National Natural Science Foundation of China (82271250) and the Science Technology Department of School of Medicine, Shanghai Jiaotong University (NO. TM202111).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Desforges AD, Hebert CM, Spence AL, et al. Treatment and diagnosis of chemotherapy-induced peripheral neuropathy: an update. Biomed Pharmacothe. 2022;147:112671. doi:10.1016/j.biopha.2022.112671
2. Cheng Y, Ji Y. Mitochondria-targeting nanomedicine self-assembled from GSH-responsive paclitaxel-ss-berberine conjugate for synergetic cancer treatment with enhanced cytotoxicity. J Control Release. 2020;318:38–49. doi:10.1016/j.jconrel.2019.12.011
3. Brewer JR, Morrison G, Dolan ME, Fleming GF. Chemotherapy-induced peripheral neuropathy: current status and progress. Gynecologic Oncol. 2016;140:176–183. doi:10.1016/j.ygyno.2015.11.011
4. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2014;32:1941–1967. doi:10.1200/JCO.2013.54.0914
5. McCrary JM, Goldstein D, Sandler CX, et al. Exercise-based rehabilitation for cancer survivors with chemotherapy-induced peripheral neuropathy. Supportive Care in Cancer. 2019;27:3849–3857. doi:10.1007/s00520-019-04680-w
6. Ben-Arye E, Hausner D, Samuels N, et al. Impact of acupuncture and integrative therapies on chemotherapy-induced peripheral neuropathy: a multicentered, randomized controlled trial. Cancer. 2022;128:3641–3652. doi:10.1002/cncr.34422
7. Gewandter JS, Chaudari J, Ibegbu C, et al. Wireless transcutaneous electrical nerve stimulation device for chemotherapy-induced peripheral neuropathy: an open-label feasibility study. Supportive Care in cancer. 2019;27:1765–1774. doi:10.1007/s00520-018-4424-6
8. Chen S, Ding Y, Zhang X, et al. Efficacy of transcutaneous electrical acupoint stimulation on chronic postsurgical pain after video-assisted thoracoscopic lobectomy: study protocol for a prospective randomized controlled trial. Pain Ther. 2024;13:269–280. doi:10.1007/s40122-024-00580-y
9. Zhou X, Cao SG, Tan XJ, et al. Effects of Transcutaneous Electrical Acupoint Stimulation (TEAS) on postoperative recovery in patients with gastric cancer: a randomized controlled trial. Cancer Manage Res. 2021;13:1449–1458. doi:10.2147/CMAR.S292325
10. Yan W, Kan Z, Yin J, Ma Y. Efficacy and Safety of Transcutaneous Electrical Acupoint Stimulation (TEAS) as an analgesic intervention for labor pain: a network meta-analysis of randomized controlled trials. Pain Ther. 2023;12:631–644. doi:10.1007/s40122-023-00496-z
11. D’Alessandro EG, Nebuloni Nagy DR, de Brito CMM, Almeida EPM, Battistella LR, Cecatto RB. Acupuncture for chemotherapy-induced peripheral neuropathy: a randomised controlled pilot study. BMJ Supportive Palliative Care 2022;12:64–72.
12. Lu W, Giobbie-Hurder A, Freedman RA, et al. Acupuncture for chemotherapy-induced peripheral neuropathy in breast cancer survivors: a randomized controlled pilot trial. oncologist. 2020;25:310–318. doi:10.1634/theoncologist.2019-0489
13. Ma Q. Somatotopic organization of autonomic reflexes by acupuncture. Current opinion in neurobiology. Curr OpinNeurobiol. 2022;76:102602. doi:10.1016/j.conb.2022.102602
14. Kim DH, Ryu Y, Hahm DH, Sohn BY, Shim I, Kwon OS. Acupuncture points can be identified as cutaneous neurogenic inflammatory spots. Sci Rep. 2017;7:15214. doi:10.1038/s41598-017-14359-z
15. Zhu X, Xie W, Zhang J, Strong JA, Zhang JM. Sympathectomy decreases pain behaviors and nerve regeneration by downregulating monocyte chemokine CCL2 in dorsal root ganglia in the rat tibial nerve crush model. Pain. 2022;163:e106–e120. doi:10.1097/j.pain.0000000000002321
16. Wang YL, Zhu HY, Lv X-Q. Electroacupuncture zusanli (ST36) relieves somatic pain in colitis rats by inhibiting dorsal root ganglion sympathetic-sensory coupling and neurogenic inflammation. Neural Plasticity. 2023;2023:9303419. doi:10.1155/2023/9303419
17. Cui X, Zhang Z, Xi H, Liu K, Zhu B, Gao X. Sympathetic-sensory coupling as a potential mechanism for acupoints sensitization. J Pain Res. 2023;16:2997–3004. doi:10.2147/JPR.S424841
18. Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003;13:256–270. doi:10.1007/s10286-003-0107-5
19. Argyriou AA, Koutras A, Polychronopoulos P, et al. The impact of paclitaxel or cisplatin-based chemotherapy on sympathetic skin response: a prospective study. Eur J Neurol. 2005;12:858–861. doi:10.1111/j.1468-1331.2005.01086.x
20. Xu Y, Wu J, Jiang Q, et al. Prediction of the efficacy of lumbar sympathetic block in patients with lower extremity complex regional pain syndrome type 1 based on the sympathetic skin response. Pain and Therapy. 2023;12:785–796. doi:10.1007/s40122-023-00499-w
21. Ng DQ, Tan CJ, Soh BC, et al. Impact of cryotherapy on sensory, motor, and autonomic neuropathy in breast cancer patients receiving paclitaxel: a randomized, controlled trial. Front Neurol. 2020;11:604688. doi:10.3389/fneur.2020.604688
22. Hou S, Huh B, Kim HK, Kim KH, Abdi S. Treatment of chemotherapy-induced peripheral neuropathy: systematic review and recommendations. Pain Physician. 2018;21:571–592.
23. Saad M, Tafani C, Psimaras D, Ricard D. Chemotherapy-induced peripheral neuropathy in the adult. Current Opin Oncology. 2014;26:634–641. doi:10.1097/CCO.0000000000000139
24. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155:2461–2470. doi:10.1016/j.pain.2014.09.020
25. Fukuda Y, Li Y, Segal RA. A mechanistic understanding of axon degeneration in chemotherapy-induced peripheral neuropathy. Front Neurosci. 2017;11:481. doi:10.3389/fnins.2017.00481
26. Timmins HC, Li T, Kiernan MC, et al. Taxane-induced peripheral neuropathy: differences in patient report and objective assessment. Supportive Care Cancer. 2020;28:4459–4466. doi:10.1007/s00520-020-05299-y
27. Bulls HW, Hoogland AI, Kennedy B, et al. A longitudinal examination of associations between age and chemotherapy-induced peripheral neuropathy in patients with gynecologic cancer. Gynecologic Oncol. 2019;152:310–315. doi:10.1016/j.ygyno.2018.12.002
28. D’Souza RS, Alvarez GAM, Dombovy-Johnson M, Eller J, Abd-Elsayed A. Evidence-based treatment of pain in chemotherapy-induced peripheral neuropathy. Curr Pain Headache Rep. 2023;27:99–116. doi:10.1007/s11916-023-01107-4
29. Huang MC, Chang SC, Liao WL, et al. Acupuncture may help to prevent chemotherapy-induced peripheral neuropathy: a randomized, sham-controlled, single-blind study. oncologist. 2023;28:e436–e447. doi:10.1093/oncolo/oyad065
30. Lyu Z, Tian S, Bao G, et al. Transcutaneous electrical acupoint stimulation for cancer-related pain management in patients receiving chronic opioid therapy: a randomized clinical trial. Supportive Care in Cancer. 2023;32:16. doi:10.1007/s00520-023-08240-1
31. Bao T, Patil S, Chen C, et al. Effect of acupuncture vs sham procedure on chemotherapy-induced peripheral neuropathy symptoms: a randomized clinical trial. JAMA Network Open 2020;3:e200681. doi:10.1001/jamanetworkopen.2020.0681
32. Zhi WI, Ingram E. Acupuncture for bortezomib-induced peripheral neuropathy: not just for pain. Integr. Cancer Ther 2018;17:1079–1086.
33. Gao X, Qin Q, Yu X, et al. Acupuncture at heterotopic acupoints facilitates distal colonic motility via activating M3 receptors and somatic afferent C-fibers in normal, constipated, or diarrhoeic rats. Neurogastroenterol Motil. 2015;27:1817–1830. doi:10.1111/nmo.12694
34. Kang L, Tian Y, Xu S, Chen H. Oxaliplatin-induced peripheral neuropathy: clinical features, mechanisms, prevention and treatment. J Neurol. 2021;268:3269–3282. doi:10.1007/s00415-020-09942-w
35. Staff NP, Cavaletti G. Platinum-induced peripheral neurotoxicity: from pathogenesis to treatment. J. Peripher. Nerv. Syst 2019;24(Suppl 2):S26–s39.
36. Fabry V, Gerdelat A, Acket B, et al. Which method for diagnosing small fiber neuropathy?. Front Neurol. 2020;11:342. doi:10.3389/fneur.2020.00342
37. Idiaquez J, Casar JC, Fadic R, Iturriaga R. Sympathetic and electrochemical skin responses in the assessment of sudomotor function: a comparative study. Neurophysiol Clin. 2023;53:102840. doi:10.1016/j.neucli.2022.102840
38. Noguchi Y, Kawashima Y, Maruyama M, et al. Risk factors for eye disorders caused by paclitaxel: a retrospective study. Biol Pharm Bull. 2018;41:1694–1700. doi:10.1248/bpb.b18-00444
39. On AY, Tanigor G. Relationships of autonomic dysfunction with disease severity and neuropathic pain features in fibromyalgia: is it really a sympathetically maintained neuropathic pain? Korean J Pain 2022;35:327–335.
40. Arunodaya GR, Taly AB. Sympathetic skin response: a decade later. J Neurol Sci. 1995;129:81–89. doi:10.1016/0022-510X(94)00265-P
41. Karsidag S, Morali S, Sargin M, Salman S, Karsidag K, Us O. The electrophysiological findings of subclinical neuropathy in patients with recently diagnosed type 1 diabetes mellitus. Diabetes Res Clin Pract. 2005;67:211–219. doi:10.1016/j.diabres.2004.07.017
42. von Bischhoffshausen S, Ivulic D, Alvarez P, et al. Recessive dystrophic epidermolysis bullosa results in painful small fibre neuropathy. Brain. 2017;140:1238–1251. doi:10.1093/brain/awx069
43. Cui X, Liu K, Gao X, Zhu B. Advancing the understanding of acupoint sensitization and plasticity through cutaneous C-Nociceptors. Front Neurosci. 2022;16:822436. doi:10.3389/fnins.2022.822436
44. Zucker NA, Tsodikov A, Mist SD, Cina S, Napadow V, Harris RE. Evoked pressure pain sensitivity is associated with differential analgesic response to verum and sham acupuncture in fibromyalgia. Pain Med. 2017;18:1582–1592. doi:10.1093/pm/pnx001
45. Westerhaus MJ, Loewy AD. Central representation of the sympathetic nervous system in the cerebral cortex. Brain Res. 2001;903:117–127. doi:10.1016/S0006-8993(01)02453-2
46. Lipov EG, Joshi JR, Sanders S, Slavin KV. A unifying theory linking the prolonged efficacy of the stellate ganglion block for the treatment of chronic regional pain syndrome (CRPS), hot flashes, and posttraumatic stress disorder (PTSD). Med Hypotheses. 2009;72:657–661. doi:10.1016/j.mehy.2009.01.009
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

