Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Electroacupuncture Protects Against Cerebral Ischemia-Reperfusion Injury: Reducing Inflammatory Response and Cell Pyroptosis by Inhibiting P2X7/NLRP3/GSDMD Pathway
Authors Chen S , Hao P , Liang Y , Cao Y , Han W, Sun S
Received 6 August 2024
Accepted for publication 18 November 2024
Published 27 November 2024 Volume 2024:20 Pages 2335—2346
DOI https://doi.org/10.2147/NDT.S485884
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yu-Ping Ning
Sifang Chen,1 Panfu Hao,1 Yueguang Liang,1 Yu Cao,2 Wei Han,3 Shanbin Sun1
1Department of Rehabilitation Medicine, The Second Affiliated Hospital of Anhui University of Traditional Chinese Medicine, Hefei, People’s Republic of China; 2Department of Acupuncture and Massage, Anhui University of Chinese Medicine, Hefei, People’s Republic of China; 3Department of Acupuncture and Moxibustion, The Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, People’s Republic of China
Correspondence: Shanbin Sun, Department of Rehabilitation Medicine, The Second Affiliated Hospital of Anhui University of Traditional Chinese Medicine, No. 300 Shouchun Road, Hefei, 230061, People’s Republic of China, Email [email protected]
Purpose: To investigate the mechanism underlying the effects of Tongdu Tiaoshen electroacupuncture in the treatment of cerebral ischemia-reperfusion (I/R) injury.
Methods: Sixty adult male Sprague-Dawley (SD) rats were randomly allocated to five groups (n=12): Sham, I/R, electroacupuncture (EA), BBG (P2X7R inhibitor), and MCC950 (NLRP3 inhibitor). The EA group received acupuncture at Shenting (GV24), Baihui (GV20), and Dazhui (GV14) points with a stimulation frequency of 2/5 Hz, intensity of 2 mA, and a duration of 40 min. Neurologic deficit scoring was then performed, and cerebral infarction volume was determined using triphenyl tetrazolium chloride (TTC) staining. Morphological changes in neurons in cortical areas were observed using an electron microscope, interleukin-18 (IL-18) and interleukin-1β (IL-1β) levels were assessed via the enzyme-linked immunosorbent assay (ELISA). Moreover, the expression of P2X7R/NLRP3/GSDMD pathway-related proteins and mRNAs was quantified by immunofluorescence staining, Western blot assay, and real-time fluorescence quantitative (RT-PCR) analysis.
Results: Electroacupuncture improved neurologic deficit scores, cerebral infarct size, and cell pyroptosis, and reduced IL-1β and IL-18 levels. Furthermore, electroacupuncture and inhibitor treatment significantly downregulated the expression of P2X7R, ASC, Caspase-1, NLRP3, and GSDMD involved in the P2X7/NLRP3/GSDMD signaling pathway.
Conclusion: Tongdu Tiaoshen electroacupuncture may exert cerebral protection by inhibiting neuroinflammation through the P2X7/NLRP3/GSDMD pathway and reducing cellular pyroptosis.
Keywords: Tongdu Tiaoshen, electroacupuncture, cerebral ischemia-reperfusion injury, inflammatory, pyroptosis
Introduction
The incidence of stroke in younger populations has been increasing in recent years.1 A stroke occurs every five seconds globally, according to World Health Organization,2 and ischemic stroke (IS) accounts for over 70% of all strokes. Although thrombolysis is the mainstay treatment for ischemic stroke, a significant number of patients will not receive it in time.3,4 Optimal therapeutic benefits of thrombolysis are achieved in the first 3–4.5 hours after stroke onset, and in some cases, thrombolysis may cause cerebral ischemia-reperfusion(I/R) injury if administered outside the optimal window.5 I/R can aggravate brain dysfunction, and its pathophysiological mechanism involves a series of complex cascade reactions of neuro-injury and nerve cell death throughout the injury process.6–8
One preclinical study found that electroacupuncture can effectively treat IS,9 presumably by altering various signaling pathways.10–12 Another previous study found that electroacupuncture preconditioning against I/R injury is associated with cellular pyroptosis.13 However, the underlying molecular mechanisms remain unclear.
Pyroptosis is a form of programmed cell death with inflammatory characteristics and is thought to be closely related to stroke.14,15 Cellular pyroptosis is closely related to nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammatory vesicles, with gasdermin D (GSDMD) serving as an essential substrate protein downstream.16,17 Studies have suggested that the purinergic P2X receptor 7(P2X7R) is a membrane ion channel upstream of NLRP3 inflammatory vesicles. Moreover, P2X7R/NLRP3 pathways modulate microglia-mediated inflammatory responses.18 However, whether acupuncture targets cellular pyroptosis via the P2X7R/NLRP3/GSDMD pathway during cerebral I/R injury is not well understood. For this reason, in this study we investigated the mechanism of action of Tongdu Tiaoshen electrocupuncture in cerebral I/R. Specifically, we explored whether it regulates inflammation and cellular pyroptosis via the P2X7R/NLRP3/GSDMD.
Material and Methods
Animals
Sixty adult male SD rats weighing 230±20 g were donated by Jinan Pengyue Laboratory Animal Breeding Co., Ltd. (Production license: SCXK (Lu) 20220006). The animals were kept at room temperature (21–25 degrees Celsius) with a relative humidity of 60–65% and had unrestricted access to purified water and rodent chow. The rats were randomly divided into groups (n=12), as follows: Sham, I/R, EA, BBG (P2X7R inhibitor), and MCC950 (NLRP3 inhibitor). This study followed the protocols of the Anhui University of Traditional Chinese Medicine Animal Research Committee and was approved by the Anhui University of Traditional Chinese Medicine’s Experimental Animal Ethics Committee (AHUCM-rats-2023098).
Cerebral I/R Injury Model
A cerebral I/R injury model was established using the Longa wire occlusion method. All rats were fasted for 12 h prior to surgery and anesthetized with an intraperitoneal injection of 3% sodium pentobarbital (30 mg/kg-1) (Shanghai ShangPharma Xinya Pharmaceutical Co., Shanghai, China). The rats were then fixed on a rat plate in the supine position, and the neck was sterilized. A longitudinal incision (about 2 cm long) was made at 0.3 mm from the left anterior midline of the neck. The left cervical triangle was exposed, and the left common carotid, internal carotid arteries, and external carotid were gently separated. The internal carotid artery was occluded with a vascular clip, and a small incision was made 3 mm distal to the common carotid artery bifurcation. Prepared embolic wire was subsequently advanced into the internal carotid artery until resistance was met. The tip of the bolus wire was then gently withdrawn to the common carotid artery after 2 h of ischemia. However, for rats in the Sham group, only vascular isolation procedures were performed, there was no insertion of wire plugs. Rats were individually housed and subjected to Zea Longa neurobehavioral scoring upon awakening.19 A score of 1 to 3 indicated successful model establishment. Animals that did not achieve this score were excluded. The experimental design is shown in Figure 1A.
Groupings and Treatments
EA Group
Disposable sterile acupuncture needles (0.25 mm×25 mm) were sourced from the Suzhou Tianxie Acupuncture Instrument Co., Suzhou, China. The acupoints were selected as Shenting (GV24), Baihui (GV20), and Dazhui (GV14).13 Briefly, the rats were placed on a splint in the prone posture to immobilize them, then the needles were horizontally inserted at the Shenting (GV24) and Baihui (GV20) points but straight at Dazhui (GV14). The depth of penetration into the skin was 2 mm for all points. EA stimulation was applied to the acupoints for 40 min using an SDZ-II instrument (Suzhou, China) with a dispersive wave pattern, a frequency of 2/5 Hz, and an intensity of 2 mA. The EA was administered every 12 hours for a total of 7 treatments. The positioning of acupoints and insertion depth were based on the “Atlas of Commonly Used Animal Acupoints” (1991) published by Hua Xingbang20 as well as “Experimental Acupuncture and Moxibustion” (2010) published by Zhang Lufen.21 Acupoint location diagram is shown in Figure 1B.
BBG (P2X7R Inhibitor) Group
Inhibitor injection was performed 2 hours after model establishment. Here, 50 mg/kg BBG (GlpBio, Montclair, USA) was intraperitoneally injected into the rats after dilution with 0.9% saline.22
MCC950 (NLRP3 Inhibitor) Group
Inhibitor injection was performed 2 hours after model establishment, in which a sample of 50 mg/kg MCC950 (GlpBio, Montclair, USA) dissolved in 0.9% saline was intraperitoneally injected into rats.23 Rats in the Sham and Model groups were immobilized only, and did not receive EA or inhibitor interventions. Neurological deficits were scored after 72 hours, and brain tissue samples were collected immediately after the score was determined.
Neurological Deficit Scoring
The Longa score19 was used to assess the neurological deficits of the rats after 72 hours of intervention. The specific scoring criteria are detailed in Table 1.
 |
Table 1 Neurological Deficit Scoring |
Triphenylte Trazolium Chloride (TTC)
Frozen sections of brain tissue (three rats in each group) were prepared and sliced to 2 mm thickness. These sections were then fixed with 4% paraformaldehyde (Solarbio, Beijing, China) overnight after incubation in 2% TTC solution (Solarbio, Beijing, China) in the dark for 30 minutes. The cerebral infarcted areas were colored white, and the normal areas were colored red after the removal of each fixed brain piece and drying. The infarcted area was analyzed using Image J software (NIH, Bethesda, USA), and the percentage of the brain infarct volume was calculated as follows: brain infarct volume percentage=infarct zone volume/cerebral hemisphere volume×100%.
Transmission Electron Microscopy
Cortical tissue from the ischemic hemisphere was fixed using electron microscopy fixative, followed by dehydration at room temperature, osmotic embedding, and polymerization. Ultrathin sections (70 nm) were obtained using a Leica ultramicrotome (Wetzlar, Germany) and stained with 2% aqueous uranyl acetate for 20 min and lead citrate for 15 min. After washing and overnight drying, samples were visualized under transmission electron microscopy (HITACHI, Tokyo, Japan).
Enzyme-Linked Immunosorbent Assay (ELISA)
Buffer PBS (0.01M, pH=7.4) was added to cerebral cortical tissue samples, at a weight to volume ratio of 1:9, ground and homogenized, and centrifuged for 20 min after which the supernatant was collected. IL-18 and IL-1β levels were detected following the guidelines of the IL-18 (ER20267M, Vio Biotechnology Co., Shanghai, China) and the IL-1β (ER20275M, Vio Biotechnology Co., Shanghai, China) kits, respectively. Optical density (OD) was measured at 450 nm using a microplate reader (DR-200Bc, Wuxi, China).
Immunofluorescence Staining
Paraffin sections (5μm thick) were prepared from brain cortical tissue, followed sequentially by dewaxing to water, antigen repair, circle painting, blocking of endogenous peroxidase activity, and closure with 3% BSA. The sections were then incubated with primary antibodies P2X7R (1:500; AF4626, Affinity, Suzhou, China), NLRP3 (1:500; 381207, ZENBIO, Chengdu, China), and GSDMD (1:500; 20,770-1-AP, Proteintech, Wuhan, China) at 4°C overnight. These resulting slides were placed in PBS (PH7.4) on a shaker and washed 3 times, for 5 min each. The sections were also incubated with secondary antibody (1:400; PV-9000, ZSGB-BIO, Beijing, China) at room temperature for 50 min. Before being stained with DAPI for 5 min. Slices were then re-stained three times with PBS, sealed with glycerol, and visualized using a fluorescent microscope (NIKON, Tokyo, Japan).
Western Blot Assay
Tissue samples (100 mg) were added to the cell lysate (tissue: cell lysate=100 mg:1 mL) followed by cerebral cortical tissue homogenization using an electric homogenizer to extract the total protein. Proteins were quantified using a BCA protein kit (Beyotime, Shanghai, China), and a protein sample of 30 μg was subjected to electrophoresis and then transferred to PVDF membranes (Millipore, Boston, USA). The membranes were blocked with 5% BSA, and incubated with primary antibodies ASC (1:1000; 340097, ZENBIO, Chengdu, China), Caspase-1 (1:1000; 342947, ZENBIO, Chengdu, China), P2X7R (1:1000; AF4626, Affinity, Suzhou, China), NLRP3 (1:1000; 381207, ZENBIO, Chengdu, China), and GSDMD (1:1000; 20,770-1-AP, Proteintech, Wuhan, China) at 4°C overnight. Membranes were also incubated with secondary antibody (1:10,000; ZB-2301, ZSGB-BIO, Beijing, China) at room temperature for 2 h. Finally, each membrane was washed and then imaged using gel imaging analysis software, and the resulting band intensities were quantified using ImageJ software (NIH, Bethesda, USA).
Real-Time Fluorescence Quantitative (RT-PCR) Analysis
Total RNA was extracted from the brain cortex tissue using an RNA extraction kit (Biosharp, Beijing, China). cDNA was then synthesized using the Hifair II 1st Strand cDNA Synthesis Kit (YEASEN, Shanghai, China), and the PCR reaction system was configured using the cDNA template. PCR primers were added for PCR amplification using SYBR Green qPCR mix (Biosharp, Beijing, China) (total volume; 5Μl) using the following reaction procedures: 95 °C/2 min, 95 °C/15 s, 60 °C/30 s, 40 cycles. Different transcript quantities were normalized using β-actin as the reference gene, and the results were analyzed using real-time PCR system (LightCycler 480, Roche, Basel, Switzerland). The relative mRNA expressions of GSDMD, NLRP3, and P2X7R were measured using the 2−∆∆Ct method. Primer sequences used in this study are presented in Table 2.
 |
Table 2 Primers Utilized in This Study |
Data Analysis
SPSS 26.0 (IBM Corp. Armonk, NY, USA) was used for all data analysis, where all data points were expressed as mean ± standard deviation (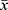 ±s). One-way ANOVA was used to make comparisons between the two groups, and the Bonferroni test was used to make multiple comparisons. Statistical graphs were plotted using GraphPad Prism 9.0 software (GraphPad Software, San Diego, USA).
±s). One-way ANOVA was used to make comparisons between the two groups, and the Bonferroni test was used to make multiple comparisons. Statistical graphs were plotted using GraphPad Prism 9.0 software (GraphPad Software, San Diego, USA).
Results
Electroacupuncture is Protective Against Ischemic Brain Injury
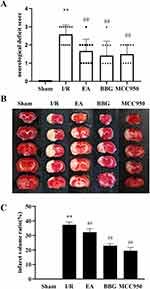
After the intervention in each group, neurologic deficit scores were evaluated, and all rats in the Sham group had a score of 0. Compared to the Sham group, neurological deficit scores were higher in the I/R group (P < 0.01). However, neurological deficit scoring was lower in the EA, BBG, and MCC950 groups than in the I/R group (P < 0.01) (Figure 2A). Histological examination revealed uniformly red-stained cerebral cortices with adequate blood supply in the Sham group. In contrast, brain tissue sections from the remaining groups displayed white infarcts of varying sizes (Figure 2B). Compared to the Sham group, the volume of cerebral infarction was higher in the I/R group (P < 0.01), but the volume of cerebral infarction was considerably lower in the EA, BBG, and MCC950 groups than in the I/R group (P < 0.01) (Figure 2C).
Electroacupuncture Improves Brain Cortical Cellular Pyroptosis and Reduces the Expression of Inflammatory Factors in Cerebral I/R
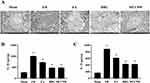
Morphological examination revealed intact cell structure and morphology in the sham group. Conversely, I/R-injured cells exhibited marked ultrastructural alterations, including compromised cell membrane integrity, extensive intracellular edema, and nuclear abnormalities characterized by localized nuclear envelope invaginations. Compared to the I/R group, cell morphology characteristics were better in the EA, BBG, and MCC950 groups, with only slight depression of the nuclear membrane (Figure 3A). ELISA results indicated that levels of IL-1β and IL-18 were significantly higher in the I/R group than in the Sham group as well (P < 0.01). Nonetheless, IL-1β and IL-18 levels were significantly higher in the EA, BBG, and MCC950 groups (P < 0.01) than in the I/R group (Figure 3B and C).
Electroacupuncture Decreases the Positive Expression of P2X7R, NLRP3, and GSDMD in Cerebral I/R
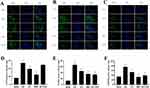
Compared to the Sham group, the I/R group exhibited significantly elevated expression levels of NLRP3, GSDMD, and P2X7R (P < 0.01). In contrast, both the EA and BBG groups showed significantly reduced expression of these proteins compared to the I/R group (P < 0.01). Moreover, MCC950 treatment markedly decreased NLRP3 and GSDMD expression relative to the I/R group (P < 0.01) (Figure 4A–F).
Electroacupuncture Reduces the Protein and mRNA Expression of the P2X7R/NLRP3/GSDMD Signaling Pathway in Cerebral I/R
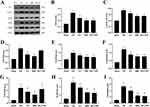
Western blotting showed that the protein expressions of P2X7R, ASC, Caspase-1, NLRP3, and GSDMD were significantly higher in the I/R group than in the Sham group (P<0.01). Compared to the I/R group, the protein expressions of ASC, Caspase-1, NLRP3, and GSDMD were lower in the EA, BBG, and MCC950 groups as well (P < 0.01). The EA and BBG groups also exhibited lower P2X7R protein expression levels compared to the I/R group (P < 0.01) (Figure 5A–F). Furthermore, real-time quantitative PCR showed that the mRNA expressions of P2X7R, NLRP3, and GSDMD were substantially higher in the I/R group than in the Sham group (P < 0.01). However, these mRNA expressions were significantly lower in the EA group than in the I/R group (P < 0.01). The mRNA expression levels of P2X7R, NLRP3, and GSDMD were significantly decreased in the BBG group compared to the I/R group (P < 0.01), and similarly, NLRP3 and GSDMD mRNA expression was lower in the MCC950 group compared to the I/R group (P < 0.01) (Figure 5G–I).
Discussion
Cerebral I/R injury is located in the brain, and in traditional Chinese medicine, brain problems can be treated by acupoints of the Du meridian. The method of Tongdu Tiaoshen electroacupuncture used in this study is a characteristic example of this therapy. Tongdu Tiaoshen can invigorate Yang-Qi, and dredge collaterals.24 According to the academic idea of acupuncture by Prof. Zhang Daozong of our hospital, and combining with the research foundation of the team in the early stage, the acupoints were selected as Shenting (GV24), Baihui (GV20), and Dazhui (GV14). Although studies have also shown that Tongdu Tiaoshen electroacupuncture can reduce cerebral injury, which may be related to the regulation of neuronal cell death,13,25 the specific mechanism involved remains unclear. Cai et al26 suggest that the protective effect of electroacupuncture on cerebral I/R injury may be due to the inhibition of the pyroptosis pathway of caspase-1 but with no known specific molecular pathway.
In the present study, both electroacupuncture and inhibitors resulted in lower volumes of cerebral infarction in cerebral I/R injury rats. Transmission electron microscopy also showed that disruption of cytosolic membrane integrity, cellular swelling, and significant expansion of organelles occurred in some cells after cerebral I/R. Furthermore, rats in the EA, BBG, and MCC950 groups showed better cellular pyroptosis, consistent with previous results.27 The EA group and the inhibitor group had similar results, both of which involved alleviation of neurological deficits and inhibition of pyroptosis, which may be related to the P2X7R/NLRP3 pathway.
Several forms of cell death are involved in the early phase of stroke,28 and recent research indicates that pyroptosis plays an essential role in stroke onset and neuroinflammation,14,29,30 suggesting that cellular focal death-related pathways may serve as potential key targets in stroke. Extracellular ATP, a damage-associated molecular pattern (DAMP), activates the immune cell P2X7 receptors, triggering NLRP3 inflammasome assembly and subsequent caspase-1 activation in inflammatory environments. Caspase-1 then releases pro-inflammatory cytokines and intracellular material, resulting in pyroptosis.18,31 Ye et al discovered that stroke considerably increases the levels of P2X7R, NLRP3, and ASC in ischemic brain tissue. However, NLRP3 inhibitor (MCC950) and P2X7R antagonist (BBG) can both markedly reduce the aforementioned expressions.27
Our experimental results indicate that the P2X7R/NLRP3 inflammatory vesicle pathway is crucial for neuronal death in cerebral I/R injury rats. The anti-inflammatory properties of BBG, a P2X7R antagonist, have been investigated using many animal models already,22 and MCC950 (CP456, 773) is currently the most extensively researched NLRP3 inhibitor.23 In the present study, P2X7R, ASC, Caspase-1, NLRP3, and GSDMD expression levels were significantly lower in the EA group than in the I/R group. Moreover, BBG and MCC950 resulted in significantly lower expression levels of the aforementioned markers in ischemic brain tissue, suggesting that electroacupuncture ameliorates neurological deficits in cerebral I/R injury by inhibiting the P2X7R/NLRP3 pathway and suppressing cellular pyroptosis.
In addition, the levels of IL-18 and IL-1β were much higher in the I/R group than in the EA group, suggesting the anti-inflammatory effect of electroacupuncture. Inflammatory response is present in the entire pathological process of cerebral I/R injury.32 Interleukins act as messengers between cells and tissues throughout the inflammatory process, maintaining the balance of the immune response.33 Acupuncture of local acupoints activates somatic afferent pathways, transmits information to neurons, and acts on immune cells to exert anti-inflammatory effects.34 Therefore, elucidating the anti-inflammatory action pathway of electroacupuncture may help to bring about precision acupuncture therapy.
P2X7R activation is one of the most important steps in the assembly of NLRP3 inflammatory vesicles.18 NLRP3 protein is connected to the ASC after its activation, where it interacts with the recruited pro-Caspase-1, thus forming NLRP3 inflammatory vesicles. Subsequently, pro-caspase-1 is hydrolyzed to generate active caspase-1, triggering GSDMD release and the synthesis of pro-inflammatory cytokines, including IL-1β and IL-18, thus intensifying inflammatory reactions.35–37 Pyroptosis actively participates in the inflammatory reaction in IS patients and neuronal death,38 studies have reported that pyroptosis surrounding the infarcted region is increased in the I/R phase. Furthermore, GSDMD ablation significantly lowers pyroptosis following I/R by preventing microglia from secreting mature IL-18 and IL-1β.39 Feng Shao et al40,41 found that GSDMD is an essential protein that drives pyroptosis, and Wang et al42 demonstrated that GSDMD knockdown confers neuroprotective effects by blocking microglia pyroptosis and neuroinflammation in I/R injury mice. Importantly, the results of our present study show that the expression of GSDMD in the EA and the inhibitor groups was significantly lower than that in the Model group, suggesting that EA may inhibit NLRP3 inflammasome and block the expression of GSDMD, thereby alleviating cell pyroptosis and exerting neuroprotection.
Although some protein inhibitors have shown good results in animal experimental results, they lack specificity or are accompanied by adverse effects and thus cannot be tested in large-scale clinical trials. For example, one Phase II clinical trial found that MCC950 increases serum liver enzyme levels for rheumatoid arthritis and was thus stopped.43 However, electroacupuncture has no negative effects and has been widely used in medical treatments. The limitations of this study are that a rescue experiment was not performed, no agonist intervention was given, and more rigor should be applied during experimental design in the future.
Conclusion
Tongdu Tiaoshen electroacupuncture may offer cerebral protection by suppressing neuroinflammation, possibly via the P2X7/NLRP3/GSDMD pathway and by attenuating cellular pyroptosis. The rapid and multifaceted nature of stroke, however, presents challenges for therapeutic interventions that target a single pathway. Nevertheless, neuroprotection remains a critical factor in stroke prognosis. Therefore, future research should aim to explore the interlocking effects of multiple signaling pathways and the joint intervention of multiple targets in the search for sufficient basic evidence in support of the use of electroacupuncture in stroke rehabilitation.
Data Sharing Statement
The datasets used in this study may be obtained from the corresponding author upon reasonable request.
Ethics Approval
This study followed the protocols of the Anhui University of Traditional Chinese Medicine Animal Research Committee and was approved by the Anhui University of Traditional Chinese Medicine’s Experimental Animal Ethics Committee (AHUCM-rats-2023098).
Funding
The Natural Science Foundation of China (No. 81973933), National Chinese Medicine Advantageous Speciality Construction Project (National TCM Medical Affairs Letter [2024] No. 90), Key Specialty Construction Project of Anhui Province (Anhui Medical Secretary of Health [2022] No. 105), and the Project of Anhui Provincial Department of Education (No. 2022AH050439) supported this work.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol. 2019;18(5):417–418. doi:10.1016/S1474-4422(19)30030-4
2. Feigin VL, Norrving B, Mensah GA. Global Burden of Stroke. Circ Res. 2017;120(3):439–448. doi:10.1161/CIRCRESAHA.116.308413
3. Yang N, Lee H, Wu C. Intravenous thrombolysis for acute ischemic stroke: from alteplase to tenecteplase. Brain Circ. 2023;9(2):61–63. doi:10.4103/bc.bc_70_22
4. Yan Y, Wang Z, Liu X, et al. Identification of brain endothelial cell-specific genes and pathways in ischemic stroke by integrated bioinformatical analysis. Brain Circ. 2023;9(4):228–239. doi:10.4103/bc.bc_40_23
5. Widimsky P, Snyder K, Sulzenko J, Hopkins LN, Stetkarova I. Acute ischaemic stroke: recent advances in reperfusion treatment. Eur Heart J. 2023;44(14):1205–1215. doi:10.1093/eurheartj/ehac684
6. Tang L, Liu S, Li S, Chen Y, Xie B, Zhou J. Induction Mechanism of Ferroptosis, Necroptosis, and Pyroptosis: a Novel Therapeutic Target in Nervous System Diseases. Int J Mol Sci. 2023;24(12):10127. doi:10.3390/ijms241210127
7. Uzuner N, Uzuner GT. Risk factors for multiple recurrent ischemic strokes. Brain Circ. 2023;9(1):21–24. doi:10.4103/bc.bc_73_22
8. Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17(11):1391–1401. doi:10.1038/nm.2507
9. Chen SF, Han W, Sun SB. Impacts of the combined treatment of Tongdu Tiaoshen moxibustion and rehabilitation training on the motor function recovery of the upper limbs in the patients with apoplectic hemiplegia. World J Acupunct Moxibustion. 2020;30(2):97–101. doi:10.1016/j.wjam.2020.05.013
10. Zhang CL, Li YF, Hui JR, Wang J. Progress of Experimental Researches on Mechanisms of Acupuncture Underlying Improvement of Ischemic Cerebral Vascular Disease by Regulating Different Intracellular Signaling Pathways. Zhen Ci Yan Jiu. 2018;43(8):531–536. doi:10.13702/j.1000-0607.180120
11. Tang B, Li Y, Xu X, Du G, Wang H. Electroacupuncture Ameliorates Neuronal Injury by NLRP3/ASC/Caspase-1 Mediated Pyroptosis in Cerebral Ischemia-Reperfusion. Mol Neurobiol. 2024;61(4):2357–2366. doi:10.1007/s12035-023-03712-1
12. Jiang T, Wu MY, Zhang ZQ, et al. Electroacupuncture attenuated cerebral ischemic injury and neuroinflammation through α7nAChR-mediated inhibition of NLRP3 inflammasome in stroke rats. Mol Med. 2019;25(1):22. doi:10.1186/s10020-019-0091-4
13. Tong TT, Wang Y, Li KW, et al. Effect of Tongdu Tiaoshen electroacupuncture pretreatment on PPARγ-mediated pyroptosis of cerebral cortex in rats with cerebral ischemia reperfusion injury. Zhong Guo Zhen Jiu. 2023;43(7):783–792. doi:10.13703/j.0255-2930.20221010-k0004
14. Long J, Sun Y, Liu S, et al. Targeting pyroptosis as a preventive and therapeutic approach for stroke. Cell Death Discov. 2023;9(1):155. doi:10.1038/s41420-023-01440-y
15. Zheng Y, Xu X, Chi F, Cong N. Pyroptosis: a Newly Discovered Therapeutic Target for Ischemia-Reperfusion Injury. Biomolecules. 2022;12(11):1625. doi:10.3390/biom12111625
16. Xu J, Núñez G. The NLRP3 inflammasome: activation and regulation. Trends Biochem Sci. 2023;48(4):331–344. doi:10.1016/j.tibs.2022.10.002
17. Ting HW, Wan H, Hu L, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25(12):1285–1298. doi:10.1038/cr.2015.139
18. Pelegrin P. P2X7 receptor and the NLRP3 inflammasome: partners in crime. Biochem Pharmacol. 2021;187:114385. doi:10.1016/j.bcp.2020.114385
19. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi:10.1161/01.str.20.1.84
20. Hua XB, Zhou HL. Development of acupoint atlas for rats. Laboratory Animals Animal Exp. 1991;1991(1):1–5.
21. Zhang LF. Experimental Acupuncture and Moxibustion. China: Beijing: Chemical Industry Press; 2010:219–220.
22. Saber S, Youssef ME, Sharaf H, et al. BBG enhances OLT1177-induced NLRP3 inflammasome inactivation by targeting P2X7R/NLRP3 and MyD88/NF-κB signaling in DSS-induced colitis in rats. Life Sci. 2021;270:119123. doi:10.1016/j.lfs.2021.119123
23. Coll RC, Schroder K, Pelegrín P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci. 2022;43(8):653–668. doi:10.1016/j.tips.2022.04.003
24. Haiyang W, Ying W, Wei H, Huihui L, Haisheng J, Xiuxiu L. Protective effect of Tongdu Tiaoshen acupuncture combined with Xiaoxuming decoction on dopaminergic neurons in Parkinson’s disease model. J Tradit Chin Med. 2023;43(3):484–493. doi:10.19852/j.cnki.jtcm.20230214.005
25. Wu XQ, Wang Y, Han W, et al. Effect of electroacupuncture pretreatment on ferroptosis in neurons of rats with cerebral ischemia-reperfusion injury. Zhen Ci Yan Jiu. 2023;48(8):754–763. doi:10.13702/j.1000-0607.20230148
26. Cai L, Yao ZY, Yang L, et al. Mechanism of Electroacupuncture Against Cerebral Ischemia-Reperfusion Injury: reducing Inflammatory Response and Cell Pyroptosis by Inhibiting NLRP3 and Caspase-1. Front Mol Neurosci. 2022;15:822088. doi:10.3389/fnmol.2022.822088
27. Ye X, Shen T, Hu J, et al. Purinergic 2X7 receptor/NLRP3 pathway triggers neuronal apoptosis after ischemic stroke in the mouse. Exp Neurol. 2017;292:46–55. doi:10.1016/j.expneurol.2017.03.002
28. Tuo QZ, Zhang ST, Lei P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med Res Rev. 2022;42(1):259–305. doi:10.1002/med.21817
29. Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6(1):128. doi:10.1038/s41392-021-00507-5
30. Vasudevan SO, Behl B, Rathinam VA. Pyroptosis-induced inflammation and tissue damage. Semin Immunol. 2023;69:101781. doi:10.1016/j.smim.2023.101781
31. Sun K, Zhang J, Yang Q, et al. Dexmedetomidine exerts a protective effect on ischemic brain injury by inhibiting the P2X7R/NLRP3/Caspase-1 signaling pathway. Brain Res Bull. 2021;174:11–21. doi:10.1016/j.brainresbull.2021.05.006
32. Ravichandran KA, Heneka MT. Inflammasomes in neurological disorders - mechanisms and therapeutic potential. Nat Rev Neurol. 2024;20(2):67–83. doi:10.1038/s41582-023-00915-x
33. Xu Y, Wang Y, Ji X. Immune and inflammatory mechanism of remote ischemic conditioning: a narrative review. Brain Circ. 2023;9(2):77–87. doi:10.4103/bc.bc_57_22
34. Fan ZZ, Dou BM, Li YW, et al. Exploration of the neuroimmune regulation mechanism of acupuncture anti-inflammatory. Chin J Trad Chin Med. 2024;39(3):1379–1383.
35. Bai R, Lang Y, Shao J, Deng Y, Refuhati R, Cui L. The Role of NLRP3 Inflammasome in Cerebrovascular Diseases Pathology and Possible Therapeutic Targets. ASN Neuro. 2021;13:1–38. doi:10.1177/17590914211018100
36. Wann SR, Lo HR, Chang YT, Liao JB, Wen ZH, Chi PL. P2X7 receptor blockade reduces pyroptotic inflammation and promotes phagocytosis in Vibrio vulnificus infection. J Cell Physiol. 2023;238(10):2316–2334. doi:10.1002/jcp.31114
37. Feng M, Wei S, Zhang S, Yang Y. Anti-Inflammation and Anti-Pyroptosis Activities of Mangiferin via Suppressing NF-κB/NLRP3/GSDMD Signaling Cascades. Int J Mol Sci. 2022;23(17):10124. doi:10.3390/ijms231710124
38. Liu X, Luo P, Zhang W, Zhang S, Yang S, Hong F. Roles of pyroptosis in atherosclerosis pathogenesis. Biomed Pharmacother. 2023;166:115369. doi:10.1016/j.biopha.2023.115369
39. Mao R, Zong N, Hu Y, Chen Y, Xu Y. Neuronal Death Mechanisms and Therapeutic Strategy in Ischemic Stroke. Neurosci Bull. 2022;38(10):1229–1247. doi:10.1007/s12264-022-00859-0
40. Wang K, Sun Q, Zhong X, et al. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell. 2020;180(5):941–955.e20. doi:10.1016/j.cell.2020.02.002
41. Ding J, Wang K, Liu W, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111–116. doi:10.1038/nature18590
42. Wang K, Sun Z, Ru J, et al. Ablation of GSDMD Improves Outcome of Ischemic Stroke Through Blocking Canonical and Non-canonical Inflammasomes Dependent Pyroptosis in Microglia. Front Neurol. 2020;11:577927. doi:10.3389/fneur.2020.577927
43. Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17(8):588–606. doi:10.1038/nrd.2018.97
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.