Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Elevated MPV: A Key Indicator of Acute Anterior Circulation Stroke Prognosis in Mechanical Thrombectomy
Authors Tian SY , Yu MJ, Mei K , Xu B, Xiao LC, Wen HB, Shang FR
Received 21 February 2025
Accepted for publication 12 May 2025
Published 19 May 2025 Volume 2025:21 Pages 715—725
DOI https://doi.org/10.2147/TCRM.S522253
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Garry Walsh
Shi-Yuan Tian,1 Min-Jie Yu,2 Kefu Mei,1 Bing Xu,1 Lian-Chen Xiao,1 Hong-Bin Wen,1 Fu-Rong Shang1
1Department of Neurology, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, People’s Republic of China; 2School of Medicine, Wuhan University of Science and Technology, Wuhan, Hubei, People’s Republic of China
Correspondence: Fu-Rong Shang; Hong-Bin Wen, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, People’s Republic of China, Email [email protected]; [email protected]
Objective: The aim of this study is to investigate the relationship between admission platelet indices and 90-day clinical outcomes in patients with acute ischemic stroke (AIS) undergoing mechanical thrombectomy (MT).
Methods: A retrospective analysis was conducted on 247 AIS patients with anterior circulation large vessel occlusion (LVO) treated with MT between July 2021 and April 2024. Platelet indices (PIs) were measured at admission. Participants were stratified into two groups based on 90-day modified Rankin Scale (mRS) outcomes. Multivariate regression analysis and receiver operating characteristic (ROC) curves were employed to evaluate relationships between admission platelet indices, clinical parameters, and functional outcomes.
Results: Among 247 enrolled patients, those with unfavorable outcomes (mRS 3– 6) exhibited significantly higher platelet distribution width (PDW) and Mean Platelet Volume (MPV) levels compared to the favorable outcome group (mRS 0– 2). Elevated MPV remained an independent predictor of unfavorable outcomes after multivariate adjustment (OR=2.747, 95% CI: 1.791– 4.216, P< 0.001). ROC analysis identified a MPV threshold > 10.75 fL for predicting unfavorable prognosis, demonstrating 55.4% sensitivity and 81.2% specificity.
Conclusion: PDW is associated with unfavorable 90-day outcomes in patients with acute anterior circulation LVO following MT, while elevated MPV may serve as a prognostic indicator for unfavorable functional outcomes in patients with acute anterior circulation LVO following MT.
Keywords: mean platelet volume, mechanical thrombectomy, large vessel occlusion, stroke prognosis, platelet indices
Introduction
Large Vessel Occlusion (LVO) is a leading cause of disability and mortality in ischemic stroke and represents a significant global disease burden. Early recanalization of occluded vessels to salvage the ischemic penumbra is the primary therapeutic strategy for acute ischemic stroke (AIS). Mechanical thrombectomy (MT) has emerged as an effective treatment for AIS caused by large artery occlusion.1 However, despite MT intervention, 29–67.4% of patients still experience poor functional outcomes.2 Consequently, early and accurate prognostic prediction is critical to improving neurological recovery in this population.
AIS triggers a cascade of inflammatory responses, and numerous inflammatory biomarkers have been implicated in disease progression.3 Previously reported inflammatory markers associated with unfavorable outcomes in AIS include the neutrophil-to-lymphocyte ratio (NLR), high-sensitivity C-reactive protein (hs-CRP), procalcitonin (PCT), and interleukin-6 (IL-6).4 Platelet indices (PIs), encompassing parameters such as platelet count (PC), platelet distribution width (PDW), mean platelet volume (MPV), and plateletcrit (PCT), are essential markers for evaluating platelet morphology and proliferative kinetics.5 While PIs have been investigated as inflammatory biomarkers in various diseases, such as inflammation and infection, thrombosis, autoimmune diseases, hematological diseases, malignant tumors, cardiovascular diseases,6–11 their role in AIS patients with LVO remains underexplored. Peng F et al2 reported that elevated MPV levels independently predict poor outcomes in anterior circulation LVO stroke patients treated with MT. Similarly, Dębiec A et al12 suggested MPV as a potential biomarker for predicting prognosis following reperfusion therapy in LVO stroke. In contrast, Sabença F et al13 found no association between elevated MPV and unfavorable 3-month outcomes in anterior circulation LVO patients undergoing MT. Li et al14 identified PDW as an independent predictor of poor 3-month outcomes after MT in anterior circulation AIS patients. However, these markers have predominantly been studied in isolation, and existing findings remain inconsistent. To date, the study by Chen et al4 is the only investigation linking platelet indices to 3-month outcomes in anterior circulation LVO patients after endovascular thrombectomy (EVT), yet their results indicated that MPV, PDW, and MPV/PC correlated with stroke severity but failed to serve as independent predictors of poor outcomes.
Given these discrepancies, this retrospective study aims to explore the relationship between platelet indices and both stroke severity as well as 3-month clinical outcomes in anterior circulation LVO patients following MT.
Material and Methods
Strategy and Population
This retrospective cohort study enrolled 561 consecutive patients with acute anterior circulation large vessel occlusion (LVO) who underwent mechanical thrombectomy (MT) at the Department of Neurology, Xiangyang Central Hospital, between July 2021 and April 2024. The study protocol received ethical approval from the Institutional Review Board of Xiangyang Central Hospital (Approval No. 2024–129), with waived informed consent due to its retrospective design. The inclusion criteria were as follows: (1) Age ≥18 years; (2) Diagnosis of AIS within 6 hours of symptom onset; (3) Radiologically confirmed anterior circulation LVO via computed tomography angiography (CTA) or digital subtraction angiography (DSA); (4) Baseline stroke severity of National Institutes of Health Stroke Scale (NIHSS) score ≥6 and Alberta Stroke Program Early CT Score (ASPECTS) ≥6; (5) Baseline modified Rankin Scale (mRS) scores ≤ 2 before the onset of the disease; (6) Compliance with intravenous thrombolysis eligibility per the 2018 Chinese Guidelines for Acute Ischemic Stroke Management;15 (7) Postoperative modified Thrombolysis in Cerebral Infarction (mTICI) score ≥2b. The exclusion criteria were as follows: (1) Incomplete clinical or laboratory records; (2) Loss to 90-day follow-up; (3) Comorbidities including inflammation and infection, active malignancies, hematological disorders, or autoimmune diseases.
Data Collection
Comprehensive baseline characteristics were systematically documented for all participants, encompassing three principal domains: (1) demographic parameters including gender, age, smoking status, and alcohol consumption; (2) clinical profiles incorporating medical history of hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, and prior stroke; (3) therapeutic interventions with specific recording of thrombolysis administration status and number of endovascular thrombectomy attempts. Standardized stroke evaluation protocols were implemented using the NIHSS for neurological deficit quantification, ASPECTS for neuroimaging analysis, and the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification system for etiological categorization. Postprocedural monitoring specifically focused on two critical complications: malignant cerebral edema16 and parenchymal hemorrhage type 2 (PH2).17 Short-term functional outcomes were assessed at 90-day follow-up using the mRS.
Blood Sample Analysis
Venous blood samples were collected into standardized tubes containing anticoagulant (EDTA) at the time of admission and stored at room temperature. Platelet indices were measured within 1 hour of sample collection using an automated hematology analyzer (Mindray BC-7500 plus, China).
Therapeutic Strategy
Patients meeting the eligibility criteria for intravenous thrombolysis were administered alteplase intravenously at a dosage of 0.9 mg/kg prior to MT. The procedures were performed under general anesthesia. The initial MT approach employed the SWIM technique, which combines a stent retriever with intermediate catheter aspiration. Reperfusion was assessed using post-thrombectomy angiography; if residual stenosis exceeded 70%, further interventions such as balloon angioplasty or stenting were considered. Device selection and technical decisions were made at the operator’s discretion. Postoperative care followed standardized neurocritical management protocols.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation or median (interquartile range) and compared using Student’s t-test or Mann–Whitney U-test, depending on whether the data followed a normal distribution. Categorical variables were presented as numbers (percentages) and analyzed using the chi-square test or Fisher’s exact test. Variables with statistical significance (P < 0.1) in univariate analysis were subsequently entered into multivariate logistic regression analysis to identify independent risk factors for adverse outcomes. The goodness of fit for the predictive model was assessed using the Hosmer-Lemeshow test. Correlation analysis was performed using Spearman’s rank correlation method. A two-tailed P-value < 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS version 19.0 statistical software (IBM Corp., Armonk, NY, USA). A receiver operating characteristic (ROC) curve was used to determine the sensitivity and specificity for the MPV at the cut-off level to predict clinical outcome.
Results
Population Selection
In this retrospective cohort study, we initially identified 561 consecutive patients with anterior circulation ischemic stroke confirmed by CTA or DSA. Following rigorous application of predefined inclusion/exclusion criteria, 314 patients were excluded through sequential screening: 26 for baseline mRS scores >2 before the onset of the disease, 58 demonstrating ASPECTS<6, 102 who did not undergo MT, 18 with final mTICI <2b, 77 lacking preoperative mean platelet volume measurements, and 33 lost to follow-up. The remaining 247 patients constituted the final analytic cohort, comprising 117 individuals achieving favorable clinical outcomes (mRS 0–2) and 130 with unfavorable outcomes (mRS 3–6) at 90-day follow-up (Figure 1).
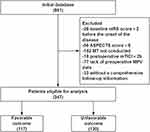 |
Figure 1 Flowchart of Study Population Selection. |
Baseline Characteristics
Comparative analysis of the 247-patient cohort revealed significant intergroup disparities in baseline and clinical parameters (Table 1). Patients with unfavorable outcomes (n=130) demonstrated older age (70 vs 67, P = 0.010), lower male predominance (60.0% vs 76.9%, P = 0.004), and higher prevalence of hypertension (76.2% vs 64.1%, P = 0.038) and atrial fibrillation (43.8% vs 28.2%, P = 0.011) compared to the favorable outcome group (n=117). The unfavorable cohort exhibited higher NIHSS scores (16 vs 14, P = 0.001) and underwent a more frequent number of MT attempts (2 vs 1, P = 0.003). Etiological stratification revealed distinct distribution patterns with statistical significance (P = 0.011): large-artery atherosclerosis predominated in the favorable outcome group (59.0% vs 40.0%), whereas cardioembolic etiology was more frequent in the unfavorable outcome group (46.2% vs 32.5%). Post-procedural complications differed substantially between groups, with the unfavorable cohort demonstrating markedly higher incidence of malignant cerebral edema (37.7% vs 3.4%, P < 0.001) and parenchymal hemorrhage type 2 (43.8% vs 9.4%, P < 0.001). Platelet indices analysis revealed significant intergroup differences: the unfavorable outcome group showed elevated MPV (10.9 fL vs 9.9 fL, P < 0.001) and marginally higher PDW (16.3fL vs 16.1fL, P = 0.026) compared to the favorable outcome group.
 |
Table 1 Baseline Characteristics of Patients Stratified by Outcome |
Logistic Regression Analysis of Factors Associated with Unfavorable Outcomes
Multivariable logistic regression analysis incorporating variables screened by univariate analysis (P < 0.1 threshold) identified four independent predictors of unfavorable outcomes (Table 2). Prior to multivariate regression analysis, we conducted collinearity diagnostics among the study variables. Variance inflation factor (VIF) analysis demonstrated acceptable multicollinearity thresholds for all included covariates: MPV (VIF = 1.12), malignant cerebral edema (VIF = 1.43), ASPECTS (VIF = 1.14), PH2 (VIF = 1.33), TOAST classification (VIF = 1.41), age (VIF = 1.34), sex (VIF = 1.11), hypertension (VIF = 1.18), atrial fibrillation (VIF = 1.50), NIHSS score (VIF = 1.08), intravenous thrombolysis (VIF = 1.12), number of thrombectomy attempts (VIF = 1.15), and PDW (VIF = 1.04). All VIF values remained well below the critical threshold of 5, indicating no substantial multicollinearity concerns. The final model demonstrated: TOAST classification (OR = 1.825, 95% CI 1.019–3.270; P = 0.043), malignant cerebral edema (OR = 7.428, 95% CI 2.214–24.928; P = 0.001), PH2 (OR = 3.648, 95% CI 1.462–9.100; P = 0.006), and elevated MPV (OR = 2.747, 95% CI 1.791–4.216; P < 0.001). The Hosmer-Lemeshow test further supported the model’s adequate calibration (χ² = 5.287, degrees of freedom df = 8, p = 0.727), indicating that the model fits well, with significant explanatory power and proper calibration.
 |
Table 2 Logistic Regression Analysis of Factors Associated with Unfavorable Outcomes |
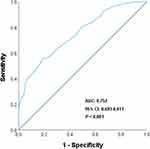
ROC Curve Analysis for MPV Prognostic Utility
ROC curve analysis was employed to assess the predictive performance of MPV for unfavorable outcomes in anterior circulation LVO-related AIS. The analysis yielded an AUC of 0.752 (95% CI 0.693–0.811; P < 0.001), indicating moderate discriminative capacity. At the empirically derived optimal cutoff of 10.75 fL (Youden index), MPV demonstrated a specificity of 81.2% and sensitivity of 55.4% for predicting poor outcomes (Figure 2).
Stratified Analysis by MPV Threshold
Using this cutoff, patients were dichotomized into low MPV (≤10.75 fL) and high MPV (>10.75 fL) cohorts. Univariate analysis revealed that the proportion of patients with a mRS score of 0–2 at 90-day follow-up was significantly higher in the MPV ≤10.75 group versus the MPV >10.75 group (62.1% vs 23.4%; P < 0.001). Differences in the distribution of the mRS scores between the two groups were shown in Figure 3.
 |
Figure 3 Differences in the distribution of the mRS at 90-day follow-up between MPV>10.75fL and MPV≤10.75fL. |
Discussion
The objective of this study was to investigate the association between platelet indices and short-term outcomes in patients with acute anterior circulation LVO undergoing MT. Our findings demonstrate that elevated PDW correlates with poor 90-day outcomes in acute anterior circulation patients after MT, while increased MPV serves as an independent predictor of poor 90-day functional outcomes following MT in this population.
Platelets participate in thrombus formation through adhesion, release reactions, and aggregation—processes that may be triggered by multiple factors such as inflammation and subendothelial vascular layer exposure. Since the 1960s, researchers have reported potential associations between platelet activation and AIS.18 Larger platelets, as biomarkers of platelet activation, secrete more prothrombotic mediators and express higher levels of adhesion molecules, resulting in enhanced aggregatory activity.19 Platelet indices (PIs), including MPV, PDW, PCT, and PC, serve as biomarkers of platelet size. Previous studies demonstrate that elevated MPV is associated with worse functional outcomes in AIS patients.20,21 Additionally, research suggests potential associations between cerebral infarction and either reduced PC or elevated PDW.22,23 However, limited evidence exists on the impact of PIs in acute LVO ischemic stroke. Although studies by Peng F et al2 and Dębiec A et al12 identified elevated MPV as an independent predictor of poor outcomes in acute anterior circulation LVO patients, these analyses focused solely on MPV without considering potential confounding effects of PC, PDW, and PCT. In contrast, Chen et al4 investigated multiple PIs but found no independent prognostic value for MPV, PDW, or MPV/PC in predicting post-EVT outcomes, instead, elevated NLR emerged as an independent predictor. We hypothesize that NLR may confound the predictive value of platelet indices. The study conducted by Sabença et al13 concluded that MPV was not a reliable predictor of clinical prognosis in patients with anterior circulation large vessel occlusion. To explore potential reasons for this finding, we compared the studies by Peng F et al2 and Dębiec A et al12 with the following observations: Firstly, Sabença et al13 did not specify the duration from symptom onset to mechanical thrombectomy, whereas both Peng F et al2 and Dębiec A et al12 included patients treated within 6 hours of symptom onset. This distinction is significant because the interval between stroke onset and blood sample collection, as well as the storage conditions of the samples, can influence MPV measurements. It is well-documented that MPV values tend to increase over time in anticoagulant tubes, a phenomenon that is particularly pronounced when storage exceeds 2 hours, although the increase is typically less than 0.5 fL within this timeframe.24,25 Consequently, minimizing the storage duration of blood samples enhances the accuracy of MPV measurement. Secondly, the study conducted by Sabença et al13 predominantly involved participants who experienced strokes due to cardioembolism. In contrast, the studies by Peng F et al2 and Dębiec A et al12 primarily included individuals with strokes attributed to large artery atherosclerosis. This discrepancy in stroke etiology may contribute to the divergent conclusions observed across these studies. Previous research has indicated a correlation between elevated mean platelet volume (MPV) and the incidence of large artery atherosclerotic strokes.26,27 Furthermore, additional factors, such as variations in racial demographics and the use of different automated cell counters for MPV measurement, may also account for inconsistencies in research findings, potentially leading to contradictory outcomes among the studies.
Based on the inconsistencies in the aforementioned conclusions and the differences in included factors, we have designed this research protocol to investigate the relationship between platelet indices and the 90-day prognosis in patients undergoing mechanical thrombectomy for anterior circulation large vessel occlusion. Our study demonstrates that elevated MPV (>10.75 fL) independently predicts poor 90-day outcomes in anterior circulation AIS patients after MT, with 55.4% sensitivity and 81.2% specificity. The AUC of ROC is 0.752. In comparison to the studies conducted by Peng F et al and Dębiec A et al, our research is the first to demonstrate a larger AUC (Peng F et al, 0.65; Dębiec A et al, 0.66), suggesting enhanced diagnostic efficacy. Furthermore, MPV threshold for diagnosing adverse prognosis is higher in our study (Peng F et al, 10.4; Dębiec A et al, 10.2), thereby enabling a broader range of patients to benefit. Lastly, our study exhibits greater specificity (Peng F et al, 66.7%; Dębiec A et al, 73%), although our sensitivity is marginally lower (Peng F et al, 62.1%; Dębiec A et al, 66%).
Platelets play a crucial role in inflammatory responses by releasing cytokines and chemokines, which serve to recruit white blood cells and facilitate adhesion at sites of injury and along the endothelium. During inflammatory processes, platelets can interact with white blood cells, forming platelet-leukocyte aggregates.28 In individuals experiencing inflammation, elevated levels of pro-inflammatory cytokines, particularly interleukin-6 (IL-6), are associated with increased platelet release. This phenomenon is linked to IL-6’s ability to stimulate thrombopoietin production and its direct impact on megakaryocytes. IL-6 induces an increase in megakaryocyte nuclear ploidy and cytoplasmic volume, thereby enhancing platelet production. Furthermore, under inflammatory conditions, interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) can also activate platelet precursor cells, leading to an elevated platelet count.29–31 The course of an inflammatory condition is also associated with increased percentage of large platelets, probably due to intracellular synthesis of procoagulatory and proinflammatory factors, degranulation of granules, and initiation of the platelet pool stored in the spleen.32
MPV serves as a reflection of platelet size and the rate of platelet production in the bone marrow, thereby functioning as an indicator of platelet activation and the severity of inflammation. Patients exhibiting elevated MPV levels generally demonstrate enhanced thrombogenic capacity, which is attributed to larger platelets that contain more dense granules and thromboxane B2, express higher levels of adhesion molecules, and secrete increased amounts of 5-hydroxytryptamine and β-thromboglobulin.19 MPV plays a critical role in the interplay between thrombosis and inflammation. Research indicates that large platelets exhibit greater enzymatic and metabolic activity compared to smaller platelets, and they secrete and express a higher quantity of inflammatory mediators, including adhesion proteins, platelet-derived growth factor, transforming growth factor-β, platelet factor 4, and interleukin-1, among others. These inflammatory mediators facilitate platelet adhesion and aggregation, thereby promoting atherosclerosis and elevating the risk of thrombosis following the rupture of atherosclerotic plaques.33–36 Research has demonstrated that during the acute phase of ischemic stroke, patients exhibit elevated mean platelet volume (MPV) values, which persist for several hours to days post-ischemia. This phenomenon indicates a persistent ongoing platelet activation and a release of more reactive large platelets within circulation.37,38 While Li et al14 identified elevated PDW as an independent predictor of poor 3-month outcomes post-MT, our study links PDW to stroke severity but not to independent outcome prediction. Neither our study nor Chen et al’s4 found prognostic associations for PC, suggesting that platelet quality rather than quantity predominantly influences thrombogenesis. Similarly, PCT (%)—calculated as (PC × MPV)/1000039—showed no prognostic correlation, reflecting its composite nature and dependence on constituent parameters.
This study has several limitations. First, dynamic monitoring and assessment of platelet indices are not conducted over the extended disease duration. Second, Due to the inclusion and exclusion criteria of this study being designed to increase research sensitivity, eligible stroke patients represent a small but homogeneous cohort of patients, which may limit the general applicability of the findings to all types of stroke patients. Further prospective studies and external validation are required. Third, the data regarding inflammatory factors, antiplatelet drugs, anticoagulants and pre-hospital medication in this study are incomplete, which indirectly affects the correlation and reliability of the research results. Fourth, in this study, the sensitivity of MPV in predicting adverse prognosis is relatively low, which may lead to false-negative results. It is necessary to combine other predictive indicators to improve the accuracy and reliability of the diagnosis.
Conclusion
Our study demonstrates that elevated PDW is associated with unfavorable 90-day outcomes in patients with acute anterior circulation LVO following MT, while increased MPV independently predicts unfavorable 90-day functional outcomes in anterior circulation AIS patients receiving MT.
Abbreviations
LVO, large vessel occlusion; MT, mechanical thrombectomy; NIHSS, the National Institute of Health Stroke Scale; PIs, Platelet indices; PLT, platelet; MPV, mean platelet volume; PDW, platelet distribution width; PCT, Plateletcrit; ROC, receiver operating characteristic; TOAST, Trial of Org 10172 in Acute Stroke Treatment; AIS, Acute ischemic stroke; EVT, endovascular thrombectomy; DSA, digital subtraction angiography; mTICI, modified thrombolysis in cerebral infarction; ASPECTS, Alberta Stroke Program Early Computed; SD, standard deviation; IQR, interquartile range; mRS, modified rankin scale.
Ethical Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Xiangyang Central Hospital (approval number: 2024-129). The Ethics Committee granted a waiver for informed consent due to the retrospective nature of the study. We will treat all patient data with the utmost respect for privacy, ensuring that it is managed in a manner that preserves the dignity and confidentiality of the individuals involved.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Lin Y, Schulze V, Brockmeyer M, et al. Endovascular thrombectomy as a means to improve survival in acute ischemic stroke: a meta-analysis. JAMA Neurol. 2019;76(7):850–854. doi:10.1001/jamaneurol.2019.0525
2. Peng F, Zheng W, Li F, et al. Elevated mean platelet volume is associated with poor outcome after mechanical thrombectomy. J Neurointerv Surg. 2018;10(1):25–28. doi:10.1136/neurintsurg-2016-012849
3. Yang Y, Xie D, Zhang Y. Increased platelet-to-Lymphocyte ratio is an independent predictor of hemorrhagic transformation and in-hospital mortality among acute ischemic stroke with large-artery atherosclerosis patients. Int J Gen Med. 2021;14:7545–7555. doi:10.2147/IJGM.S329398
4. Chen Z, He Y, Su Y, et al. Association of inflammatory and platelet volume markers with clinical outcome in patients with anterior circulation ischaemic stroke after endovascular thrombectomy. Neurol Res. 2021;43(6):503–510. doi:10.1080/01616412.2020.1870359
5. Budak YU, Polat M, Huysal K. The use of platelet indices, plateletcrit, mean platelet volume and platelet distribution width in emergency non-traumatic abdominal surgery: a systematic review. Biochem Med. 2016;26:178–193. doi:10.11613/BM.2016.020
6. Morrell CN, Aggrey AA, Chapman LM, et al. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123(18):2759–2767. doi:10.1182/blood-2013-11-462432
7. Gasparyan AY, Ayvazyan L, Mikhailidis DP, et al. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17(1):47–58. doi:10.2174/138161211795049804
8. Schmoeller D, Picarelli MM, Paz Munhoz T, et al. Mean Platelet Volume and Immature Platelet Fraction in Autoimmune Disorders. Front Med. 2017;4:146. doi:10.3389/fmed.2017.00146
9. Li Y, Wang S, Xiao H, et al. Evaluation and validation of the prognostic value of platelet indices in patients with leukemia. Clin Exp Med. 2023;23(6):1835–1844. doi:10.1007/s10238-022-00985-z
10. Vizioli L, Muscari S, Muscari A. The relationship of mean platelet volume with the risk and prognosis of cardiovascular diseases. Int J Clin Pract. 2009;63(10):1509–1515. doi:10.1111/j.1742-1241.2009.02070.x
11. Leader A, Pereg D, Lishner M. Are platelet volume indices of clinical use? A multidisciplinary review. Ann Med. 2012;44(8):805–816. doi:10.3109/07853890.2011.653391
12. Dębiec A, Pogoda-Wesołowska A, Piasecki P, et al. Mean Platelet Volume as a Potential Marker of Large Vessel Occlusion and Predictor of Outcome in Acute Ischemic Stroke Patients Treated with Reperfusion Therapy. Life. 2021;11(6):469. doi:10.3390/life11060469
13. Sabença F, Carvalho A, Rocha M, et al. Mean platelet volume and mechanical thrombectomy. J Stroke Cerebrovasc Dis. 2020;29(8):104971. doi:10.1016/j.jstrokecerebrovasdis.2020.104971
14. Li Y, Li T, Zhao L, et al. Platelet Distribution Width: a Significant Predictor of Poor Outcome After Mechanical Thrombectomy. J Stroke Cerebrovasc Dis. 2022;31(3):106273. doi:10.1016/j.jstrokecerebrovasdis.2021.106273
15. Peng B, Chinese Society of Neurology, Chinese Stroke Society. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin J Neurol. 2018;51:666–682.
16. Jüttler E, Unterberg A, Woitzik J, et al. DESTINY II Investigators. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 2014;370(12):1091–1100. doi:10.1056/NEJMoa1311367
17. Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352(9136):1245–1251. doi:10.1016/S0140-6736(98)08020-9
18. Hollenhorst RW. Vascular status of patients who have cholesterol emboli in the retina. Am J Ophthalmol. 1966;61(5):1159–1165. doi:10.1016/0002-9394(66)90238-8
19. Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996;7(2):157–161. doi:10.1097/00001721-199603000-00011
20. Staszewski J, Pogoda A, Data K, et al. The mean platelet volume on admission predicts unfavorable stroke outcomes in patients treated with IV thrombolysis. Clin Interv Aging. 2019;14:493–503. doi:10.2147/CIA.S195451
21. Xie D, Xiang W, Weng Y, et al. Platelet volume indices for the prognosis of acute ischemic stroke patients with intravenous thrombolysis. Int J Neurosci. 2019;129(4):344–349. doi:10.1080/00207454.2018.1536054
22. Yuan J, Cai J, Zhao P, et al. Association between low-density lipoprotein cholesterol and platelet distribution width in acute ischemic stroke. Front Neurol. 2021;12:631227. doi:10.3389/fneur.2021.631227
23. Sadeghi F, Kovács S, Zsóri KS, et al. Platelet count and mean volume in acute stroke: a systematic review and meta-analysis. Platelets. 2020;31(6):731–739. doi:10.1080/09537104.2019.1680826
24. Boos CJ, Balakrishnan B, Lip GY. The effects of coronary artery disease severity on time-dependent changes in platelet activation indices in stored whole blood. J ThrombosisThrombolysis. 2008;25(2):135–140. doi:10.1007/s11239-007-0034-8
25. Greisenegger S, Endler G, Hsieh K, et al. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events? Stroke. 2004;35(7):1688–1691. doi:10.1161/01.STR.0000130512.81212.a2
26. Quan W, Chen Z, Yang X, et al. Mean platelet volume/platelet count ratio as a predictor of 90-day outcome in large artery atherosclerosis stroke patients. Int J Neurosci. 2017;127(11):1019–1027. doi:10.1080/00207454.2017.1296438
27. Arévalo-Lorido JC, Carretero-Gómez J, Villar-Vaca P. Mean platelet volume predicting carotid atherosclerosis in atherothrombotic ischemic stroke. Ir J Med Sci. 2012;181(2):179–183. doi:10.1007/s11845-011-0755-8
28. Barnard MR, Krueger LA, Frelinger III AL, Furman MI, Michelson AD. Whole blood analysis of leukocyte-platelet aggregates. Curr Protoc Cytom. 2003;24(1). doi:10.1002/0471142956.cy0615s24
29. Senchenkova EY, Komoto S, Russell J, et al. Interleukin-6 mediates the platelet abnormalities and thrombogenesis associated with experimental colitis. Am J Pathol. 2013;183(1):173–181. doi:10.1016/j.ajpath.2013.03.014
30. Korniluk A, Koper-Lenkiewicz OM, Kamińska J, et al. Mean Platelet Volume (MPV): new Perspectives for an Old Marker in the Course and Prognosis of Inflammatory Conditions. Mediators Inflamm. 2019;2019:9213074. doi:10.1155/2019/9213074
31. Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115(12):3339–3347. doi:10.1172/JCI26674
32. Schwertz H, Köster S, Kahr WH, et al. Anucleate platelets generate progeny. Blood. 2010;115(18):3801–3809. doi:10.1182/blood-2009-08-239558
33. Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Investig. 2005;115(12):3378–3384. doi:10.1172/JCI27196
34. Nording HM, Seizer P, Langer HF. Platelets in inflammation and atherogenesis. Front Immunol. 2015;6:98. doi:10.3389/fimmu.2015.00098
35. Kamath S, Blann A, Lip G. Platelet activation: assessment and quantification. Eur Heart J. 2001;22(17):1561–1571. doi:10.1053/euhj.2000.2515
36. Chu SG, Becker RC, Berger PB, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8(1):148–156. doi:10.1111/j.1538-7836.2009.03584.x
37. Ntaios G, Gurer O, Faouzi M, et al. Mean platelet volume in the early phase of acute ischemic stroke is not associated with severity or functional outcome. Cerebrovasc Dis. 2010;29(5):484–489. doi:10.1159/000297964
38. Ciancarelli I, De Amicis D, Di Massimo C, et al. Mean Platelet Volume During Ischemic Stroke is a Potential Pro-inflammatory Biomarker in the Acute Phase and During Neurorehabilitation Not Directly Linked to Clinical Outcome. Curr Neurovasc Res. 2016;13(3):177–183. doi:10.2174/1567202613666160517122109
39. Akpinar I, Sayin MR, Gursoy YC, et al. Plateletcrit. A platelet marker associated with saphenous vein graft disease. Herz. 2014;39(1):142–148. doi:10.1007/s00059-013-3798-y
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.