Back to Journals » International Journal of Nanomedicine » Volume 19
Emerging Nanotherapeutic Approaches for Diabetic Wound Healing
Authors Shi S , Hu L, Hu D, Ou X, Huang Y
Received 28 April 2024
Accepted for publication 8 July 2024
Published 27 August 2024 Volume 2024:19 Pages 8815—8830
DOI https://doi.org/10.2147/IJN.S476006
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Shaoyan Shi, Leiming Hu, Dong Hu, Xuehai Ou, Yansheng Huang
Department of Hand Surgery, Honghui Hospital, Xi’an Jiaotong University, Xi’an, 710000, People’s Republic of China
Correspondence: Yansheng Huang, Department of Hand Surgery, Honghui Hospital, Xi’an Jiaotong University, Xi’an, 710000, People’s Republic of China, Email [email protected]
Abstract: Diabetic wounds pose a significant challenge in modern healthcare due to their chronic and complex nature, often resulting in delayed healing, infections, and, in severe cases, amputations. In recent years, nanotherapeutic approaches have emerged as promising strategies to address the unique pathophysiological characteristics of diabetic wounds. This review paper provides a comprehensive overview of the latest advancements in nanotherapeutics for diabetic wound treatment. We discuss various nanomaterials and delivery systems employed in these emerging therapies. Furthermore, we explore the integration of biomaterials to enhance the efficacy of nanotherapeutic interventions. By examining the current state-of-the-art research, challenges, and prospects, this review aims to offer valuable insights for researchers, clinicians, and healthcare professionals working in the field of diabetic wound care.
Keywords: diabetic wounds, nanoparticle, biomaterials, DELIVERY system
Introduction
Diabetic wounds represent a significant and growing healthcare challenge worldwide. The prevalence of diabetes mellitus has been steadily rising over the past few decades, and it is estimated that by 2045, more than 700 million individuals will be affected by this metabolic disorder.1,2 A distressing consequence of uncontrolled diabetes is the development of diabetic wounds, which, due to their chronic and complex nature, can lead to severe complications such as infections, amputations, and substantial reductions in the quality of life for those afflicted.3,4 The diabetic wound healing microenvironment plays a crucial role in the pathogenesis and persistence of these wounds, which is characterized by persistent inflammation, impaired angiogenesis, excessive oxidative stress, and a reduced ability to combat infections.5,6 Additionally, the presence of advanced glycation end products (AGEs) and altered extracellular matrix (ECM) components further complicate the healing process. Therefore, despite substantial efforts to develop effective treatments, diabetic wound treatment remains a formidable task for healthcare providers and researchers alike.
In recent years, nanotechnology has ushered in a new era of biomedical research and therapeutic development, and its application in diabetic wound treatment holds great promise.6,7 Nanotherapeutic approaches leverage the unique properties of nanomaterials to overcome some of the limitations of conventional wound healing strategies. These nanomaterials, often with dimensions on the nanometer scale (1–100 nanometers), can exhibit enhanced biocompatibility, controlled drug release, targeted delivery, and multifunctionality.8,9 Such attributes make nanotherapeutics particularly well-suited for addressing the intricate challenges associated with diabetic wounds.
This review paper aims to provide a comprehensive overview of the latest advancements in nanotherapeutics for diabetic wound treatment. By exploring various nanomaterials, delivery systems, and the integration of biomaterials, this review seeks to shed light on the potential of nanotechnology to revolutionize the field of diabetic wound healing (Figure 1).
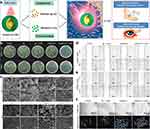 |
Figure 1 The promotion effect of near-infrared (NIR)-activated AuAgCu2O nanoshells on wound healing. (a) Illustration of NIR-activated AuAgCu2O nanoshells for targeting antibiotic-resistant bacteria and enhancing wound healing. (b) Images showing microbial colony formation after specific treatment. (c) Scanning Electron Microscope (SEM) images showing the effects of various treatments on ESBL E. coli and MRSA. (d and e) Scratch assay images of human keratinocyte cells (HaCaT) and human corneal epithelial cells (HCEpC) treated with specific treatment. (f) Images and their digital conversions depicting the formation of vascular-like structures by human umbilical vein endothelial cells grown in Matrigel, treated with specific methods. Reproduced with permission from Qiao Y, He J, Chen W, et al. Light-activatable synergistic therapy of drug-resistant bacteria-infected cutaneous chronic wounds and nonhealing keratitis by cupriferous hollow nanoshells. ACS Nano. 2020;14(3):3299–3315. Copyright 2020 American Chemical Society.10 |
The Underlying Mechanism of Diabetic Wound Healing
Diabetic wound healing is a complex and intricate process influenced by various factors related to the underlying pathology of diabetes mellitus. Chronic hyperglycemia, a hallmark of diabetes, exerts profound effects on multiple cellular and molecular pathways, disrupting the delicate balance required for efficient wound healing.11,12 Understanding the nuances of these mechanisms is imperative for the development of targeted therapeutic interventions.
Impact of Chronic Hyperglycemia on Cellular Functions
Chronic hyperglycemia, a defining characteristic of diabetes, engenders a cascade of deleterious effects on various cell types crucial for wound healing. High glucose levels have been implicated in impairing the functionality of endothelial cells, leading to compromised vasodilation and impaired angiogenesis. Furthermore, hyperglycemia disrupts the normal functioning of fibroblasts, impeding collagen synthesis and deposition essential for wound closure. The dysregulation of inflammatory cells, such as macrophages, further exacerbates the inflammatory phase of wound healing, hindering the timely progression to subsequent stages.
Impaired Angiogenesis in Diabetic Wounds
Angiogenesis, the formation of new blood vessels, is a critical aspect of wound healing that is notably compromised in individuals with diabetes. Chronic hyperglycemia contributes to endothelial dysfunction and abnormal signaling pathways, culminating in reduced capillary density around the wound site. This impaired angiogenesis results in inadequate oxygen and nutrient supply to the wound, perpetuating a hostile microenvironment that hampers the healing process. Nanotherapeutic strategies must address these specific challenges to promote angiogenesis and restore proper vascularization in diabetic wounds.
Compromised Immune Responses in Diabetic Wound Healing
The immune system’s response to injury is intricately regulated during wound healing, but in diabetes, this process is dysregulated. Chronic hyperglycemia contributes to an exaggerated and prolonged inflammatory response, characterized by abnormal cytokine profiles and dysfunctional immune cell recruitment. This chronic inflammation not only delays the transition to the proliferative phase but also exacerbates tissue damage, fostering a microenvironment conducive to infection. Nanotherapeutic interventions should target immune dysregulation to modulate the inflammatory milieu and promote a balanced immune response conducive to effective wound healing.
Nanomaterials for Diabetic Wound Healing
Nanotechnology has opened up new horizons in the quest for innovative therapies to tackle the intricate challenges associated with diabetic wounds. In this section, we explore some of the remarkable nanomaterials that have gained prominence as potential candidates for diabetic wound healing, with a focus on metallic nanoparticles.
Metallic Nanoparticles
Silver Nanoparticles
Silver nanoparticles (AgNPs) have garnered significant attention in the field of wound care, and their antimicrobial properties have been widely explored. In the context of diabetic wounds, which are particularly susceptible to infections, AgNPs offer a multifaceted approach to healing.13 One of the primary advantages of AgNPs is their potent antimicrobial activity. They can effectively combat a broad spectrum of microorganisms, including bacteria, fungi, and even antibiotic-resistant strains. In diabetic wounds, where infections can lead to severe complications, the antimicrobial prowess of AgNPs can help mitigate the risk.14 In a previous study, researchers reported that silver nanoparticles exhibited potent antibacterial effects against various human pathogens in a dose-dependent manner, including S. aureus, B. subtilis, E. coli and S. typhi. Furthermore, the AgNPs showed the ability to suppress the expression of resistance genes, thereby potentially enhancing the effectiveness of drugs.15 AgNPs have also been found to possess anti-inflammatory properties. Chronic inflammation is a hallmark of diabetic wounds, contributing to delayed healing. AgNPs can modulate the inflammatory response, potentially accelerating the resolution of inflammation and promoting the subsequent stages of wound healing. For instance, Chen et al reported that the medium-sized silver particles incorporated into carbomer gel exhibited stronger anti-inflammatory effects, reducing bacterial colonization, suppressing proinflammatory cytokine expression, promoting anti-inflammatory M2 macrophage generation, and directly blocking inflammatory responses, suggesting their effectiveness and safety for wound therapy.16 In addition, AgNPs have been shown to stimulate angiogenesis, aiding in the revascularization of the wound site and facilitating the delivery of oxygen and nutrients.17 Overall, the multifaceted properties of AgNPs make them promising candidates for diabetic wound care, offering antimicrobial, anti-inflammatory, and pro-angiogenic effects that collectively support the healing process and mitigate the risk of complications associated with these challenging wounds.
Gold Nanoparticles
Gold nanoparticles (AuNPs) represent another class of metallic nanomaterials with promising applications in diabetic wound healing. AuNPs exhibit anti-inflammatory and antioxidant properties, which can be particularly beneficial in the context of diabetic wounds. By reducing oxidative stress and dampening excessive inflammation, these nanoparticles can create a more favorable environment for wound healing.18 AuNPs can serve as drug delivery vehicles, enabling the controlled release of therapeutic agents at the wound site. This targeted drug delivery can enhance the efficacy of wound healing treatments while minimizing systemic side effects. For instance, Li et al aimed to enhance diabetic wound healing by stabilizing keratinocyte growth factor (KGF), traditionally limited by instability, through conjugation with AuNPs, resulting in increased stability and improved binding affinity, leading to accelerated wound recovery in diabetic rats.19 In addition to their role as drug carriers, AuNPs can be used in photothermal therapy. When exposed to near-infrared light, these nanoparticles generate heat, which can be harnessed to selectively target and destroy microbial pathogens in the wound without harming healthy tissue. For instance, Qiao et al designed a composite structured cupriferous hollow nanoshell (AuAgCu2O NS) with controllable photothermal therapeutic effects and released silver and copper ions (Figure 1a), which exhibits synergistic antimicrobial activity against drug-resistant bacteria (Figure 1b and c), promotes wound healing through angiogenesis and fibroblast migration, and facilitates re-epithelialization in diabetic mice with infection-complicated chronic wounds and keratitis (Figure 1d and e).10 Overall, the versatile applications of AuNPs in diabetic wound healing, ranging from drug delivery to photothermal therapy, offer promising avenues for improving treatment outcomes and addressing the complexities of diabetic wound management.
Zinc Oxide Nanoparticles
Zinc oxide nanoparticles (ZnONPs) have emerged as versatile nanomaterials with a range of applications, including wound healing in diabetic patients. ZnONPs possess notable antibacterial properties, making them effective against various bacterial strains commonly implicated in diabetic wound infections.20 Their ability to inhibit bacterial growth and biofilm formation can help reduce the risk of wound-related complications. Similar to other metallic nanoparticles, ZnONPs exhibit anti-inflammatory effects. By modulating the inflammatory response, these nanoparticles can contribute to a more favorable wound healing environment, potentially accelerating the closure of diabetic ulcers.21 ZnONPs have been shown to enhance epithelialization, the process of forming a new epidermal layer over the wound, which is a critical step in wound closure, and the promotion of epithelialization can expedite the healing process in diabetic wounds.22 Furthermore, ZinONPs has been applicated in Clinical. In a previous clinical trial, Loera-Valencia et al conducted a randomized controlled clinical trial on 26 Type 2 diabetes patients with foot ulcers, finding that calcium alginate dressings with ZnO nanoparticles (CAZnODs) led to significantly faster wound closure (75% vs 71% in the control group) and shorter healing time (48 vs 72 days), suggesting their potential to promote tissue regeneration and prevent complications like secondary infections in diabetic foot ulcer treatment.23
Polymeric Nanoparticles
Polymeric nanoparticles represent another category of nanomaterials that have garnered considerable attention in the realm of diabetic wound healing. These nanoparticles, with their diverse properties and versatility, offer unique advantages for addressing the specific challenges posed by diabetic wounds.24 In this section, we delve into two noteworthy polymeric nanoparticles: PLGA nanoparticles and chitosan nanoparticles.
PLGA Nanoparticles
Poly(lactic-co-glycolic acid) nanoparticles, commonly referred to as PLGA nanoparticles, are biodegradable and biocompatible polymeric carriers that have shown promise in the realm of drug delivery and wound healing.25 PLGA nanoparticles excellent in controlled drug release, allowing for sustained and localized delivery of therapeutic agents to the wound site.26 This controlled release can enhance the effectiveness of various wound-healing compounds, such as cells, growth factors, antimicrobial agents, and anti-inflammatory drugs. In a previous study, Zhang et al developed a novel therapeutic approach using PLGA nanoparticles loaded with the anti-inflammatory and angiogenic cytokine IL-8, incorporated onto acellular dermal matrix (ADM), to enhance the proliferation, differentiation, and survival of mesenchymal stem cells (MSCs) in diabetic chronic wounds, resulting in improved wound healing and tissue regeneration, suggesting the potential of this PLGA@IL-8/ADM scaffold as an effective delivery system for exogenous cells in diabetic wound therapy.27 PLGA nanoparticles gradually degrade into non-toxic byproducts, eliminating the need for removal or extraction after drug release. This biodegradability aligns with the goal of minimizing patient discomfort and intervention. The small size of PLGA nanoparticles enhances the bioavailability of encapsulated drugs. This is particularly advantageous in diabetic wound treatment, where achieving optimal drug concentrations at the wound site is crucial for therapeutic success. In a previous study, researchers introduced a novel approach using PLGA-PEI/NO nanoparticles to deliver nitric oxide (NO) to MRSA biofilm-infected wounds, a common complication in diabetic patients, where sustained NO delivery is crucial due to its short lifespan and limited diffusion. The nanoparticles exhibit prolonged NO release and strong binding to the biofilm matrix, resulting in enhanced antibacterial effects. In diabetic mice, these nanoparticles accelerate wound healing by dispersing the biofilm and reducing bacterial load, offering a promising strategy for treating biofilm-infected chronic wounds.28 Furthermore, PLGA nanoparticles could be designed to combine with targeted cells in diabetic wound. For instance, Wei et al introduced an innovative drug delivery system, F@GP, utilizing galactose-modified PLGA nanoparticles loaded with Fe3O4, which selectively targets senescent cells in diabetic wounds, inducing ferroptosis and promoting cell proliferation, thus accelerating wound healing.29 Overall, PLGA nanoparticles offer a promising platform for the development of innovative therapeutic approaches for diabetic wound healing, leveraging their unique properties for controlled drug delivery, targeted therapy, and enhanced tissue regeneration.
Chitosan Nanoparticles
Chitosan nanoparticles, derived from chitin, a natural polymer found in crustacean shells, have gained attention for their biocompatibility, biodegradability, and wound-healing properties.30 Chitosan nanoparticles possess inherent antimicrobial properties, making them effective against a wide range of pathogens. This property is invaluable in diabetic wound care, where infections are a constant threat. In a previous study, Yu et al designed supramolecular photothermal nanoparticles (MCC/CS NPs) (Figure 2a), which combined mono-carboxyl corrole (MCC) with chitosan, and showed potent antimicrobial effects against E. coli and MRSA under the near-infrared laser (Figure 2b and c), and effectively combat drug-resistant bacteria, expedite wound healing and angiogenesis in diabetic wound models (Figure 2d and e).31 Chitosan nanoparticles have been shown to promote the proliferation of fibroblasts and keratinocytes, two essential cell types involved in wound healing. This acceleration of cell growth can facilitate the closure of diabetic ulcers. For instance, Algandaby et al focused on chitosan nanoparticle-loaded Teucrium polium (TP-NP) for diabetic rat wound healing, revealing accelerated wound closure, epithelium regeneration, granulation tissue formation, and collagen synthesis, along with reduced oxidative stress and inflammation, and upregulated angiogenic factors.32 Similarly, chitosan nanoparticles were employed for delivering a plasmid encoding human CA5-HIF-1α, enhancing angiogenesis and wound closure in diabetic mice.33 Chitosan nanoparticles also exhibit hemostatic properties, aiding in the rapid cessation of bleeding from the wound site. This can be particularly beneficial in cases of deep diabetic foot ulcers with compromised vascular supply. For instance, Younas et al introduced a novel pullulan-based and nanoparticle-loaded smart microneedle patch, incorporating moxifloxacin-loaded nanoparticles along with lidocaine and thrombin, which demonstrates rapid hemostasis/analgesia, sustained bactericidal action, and accelerated wound healing.34 Overall, chitosan nanoparticles hold significant potential in improving outcomes for diabetic patients with chronic wounds through a multifaceted approaches.
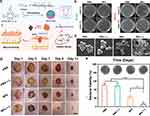 |
Figure 2 Chitosan-based nanoparticles (MCC/CS NPs) enhance diabetic wound healing through anti-bacterial potential. (a) A diagram showing how MCC/CS NPs are made and used to fight bacteria. (b) Images of agar dishes containing E. coli and MRSA after being treated with MCC/CS NPs with or without exposure to a 660 nm laser. (c) SEM images showing the surface morphology of MRSA after specific treatments. (d) Photos of a healing wound at different stages of treatment, captured on days 1, 3, 7, 9, and 13. (e) Photos and corresponding data showing the growth of bacterial colonies from wound samples under specific treatment. Reproduced from Yu Y, Tian R, Zhao Y, et al. Self-assembled corrole/chitosan photothermal nanoparticles for accelerating infected diabetic wound healing. Adv Healthc Mater. 2023;12(16):e2201651. Copyright, 2022 Wiley‐VCH GmbH.31 |
Lipid-Based Nanoparticles
Lipid-based nanoparticles have emerged as versatile carriers in the realm of nanotherapeutic approaches for diabetic wound healing. Their unique structure and properties make them well-suited for targeted drug delivery and addressing specific challenges associated with diabetic wounds.35 In this section, we examine two prominent lipid-based nanoparticles: liposomes and solid lipid nanoparticles (SLNs).
Liposomes
Liposomes are spherical vesicles composed of lipid bilayers that encase an aqueous core. These lipid-based nanoparticles have several attributes that make them appealing for diabetic wound treatment. Liposomes are biocompatible and biodegradable, minimizing the risk of adverse reactions or toxicity when applied to wounds.36 This compatibility with biological systems enhances their suitability for wound healing applications. Liposomes can efficiently encapsulate a wide range of therapeutic agents, including antibiotics, growth factors, and anti-inflammatory drugs. This encapsulation enables controlled and targeted drug delivery to the wound site, optimizing treatment outcomes.37 The lipid bilayers of liposomes mimic cell membranes, facilitating their interaction with cells at the wound site. This property enhances the penetration of drugs or bioactive compounds into tissues, including those with impaired circulation often seen in diabetic wounds. In a previous study, red blood cell membrane (RBCM)-mimicking liposomes containing curcumin (RC-Lips) were developed to address the challenge of excessive inflammation in diabetic wound healing by efficiently adsorbing α-hemolysin (Hlα), reducing its damage to keratinocytes, facilitating liposome uptake into macrophages, regulating M2 macrophage polarization, and promoting wound healing in diabetic mice through downregulation of interleukin-1β (IL-1β) and upregulation of interleukin-10 (IL-10).38 Liposomes provide a protective shield for encapsulated drugs, shielding them from degradation and enzymatic activity in the wound environment. This protection helps maintain the efficacy of therapeutic agents during transit to the wound site. For instance, purpurolide C (PC), derived from Penicillium purpurogenum, promotes diabetic wound healing by inhibiting inflammatory macrophage activation, but its instability and low solubility hinder its use. Hence, the novel delivery system PC@MLIP MN, integrating macrophage liposomes and GelMA-based microneedle patches, enhances PC delivery to tissues, addressing solubility and permeability issues and offering a promising strategy for diabetic wound management (Figure 3).39 In another study, Liposome-encapsulated PDTPTBT, under NIR irradiation, efficiently eradicates multiple bacteria, including MRSA and E. coli, causing membrane damage and cytoplasm leakage, thereby showing promise in treating multidrug-resistant bacterial infections, as demonstrated in animal models, notably suppressing mortality and accelerating wound healing in a diabetic skin infection model.40 Recently, liposomes have emerged as effective carriers for delivering therapeutic genes to target cells in diabetic wounds.41 This innovative approach holds great promise for precisely modulating the expression of key genes involved in wound repair processes, such as those related to inflammation, angiogenesis, and tissue regeneration. With the development of advanced technologies, liposomes will offering tailored solutions to address specific challenges encountered in diabetic wound healing processes.
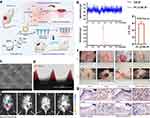 |
Figure 3 Purpurolide C-loaded microneedles enhanced diabetic wound repair. (a) Schematic shows purpurolide C (PC) combined with macrophage-liposomes (MLIP) within Gelatin methacryloyl (GelMA)-based microneedles (MNs) to enhance diabetic wound healing. (b) UPLC-QqQ-MS/MS results showed that the loading efficiency of PC in the PC@MLIP system is 5.91 ± 0.57% w/w. (c) SEM) image of the PC@MLIP microneedles. (d) Confocal microscopy reveals the placement of PC@MLIP (marked with Cy7se, shown in red) at the microneedle tips. (e) In vivo fluorescence imaging demonstrates sustained release of PC from the microneedles for up to five days post-application. (f) The PC@MLIP microneedles substantially enhance the healing rate of diabetic wounds, outperforming direct PC injections. (g) Immunohistochemical staining of local iNOS+ cells three days post-injury showcases the precise regulatory effects of the PC@MLIP system on wound healing processes. Adapted from Acta Pharm Sin B, volume 13(12), Liu Y, Xia G, Chen Y, et al. Purpurolide C-based microneedle promotes macrophage-mediated diabetic wound healing via inhibiting TLR4-MD2 dimerization and MYD88 phosphorylation. 5060–5073, Copyright 2023, with permission from Elsevier.39 |
Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLNs) represent another lipid-based nanoparticle system that has shown promise in diabetic wound healing. SLNs are composed of lipids in a solid state at room temperature and have unique advantages.42 Firstly, SLNs offer sustained drug release profiles, ensuring a prolonged presence of therapeutic agents at the wound site. This controlled release pattern can be particularly beneficial for chronic diabetic wounds requiring long-term treatment. SLNs exhibit a high drug-loading capacity, allowing for the encapsulation of a significant amount of therapeutic agents. In a previous study, Arantes et al developed a chitosan film embedded with all-trans retinoic acid-loaded solid lipid nanoparticles (SLN-ATRA), which successfully loaded high-efficiency drugs and promoted controlled drug release, showing potential as an effective treatment for diabetic wounds.43 SLNs can enhance the permeation of drugs through the skin and into the wound bed, which property is vital in cases where diabetic wounds require topical treatment to reach deeper layers of tissue. For instance, encapsulating sesamol in solid lipid nanoparticles not only increases its stability and retention in the skin but also doubles its effectiveness against skin pathogens and greatly improves its ability to heal wounds by managing bacterial activity and oxidative stress.44
Organic Nanoparticles
Organic nanomaterials have garnered considerable attention in the field of diabetic wound healing due to their ability to mimic natural biological processes and structures.45 In this section, we delve into four prominent organic nanomaterials: extracellular vesicles (EVs), nanofibers, Metal-Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs).
Extracellular Vesicles
Extracellular vesicles are small, membrane-bound particles secreted by various cell types, including stem cells and immune cells, which play a pivotal role in intercellular communication and tissue repair.46 In the context of diabetic wounds, the secreted EVs from cells may be different with those normal wounds. Guda et al developed a novel method to isolate and characterize keratinocyte-originated EVs (hExoκ) from human chronic wound fluid, revealing surface KRT14 as a reliable marker and demonstrating their reduced abundance and altered composition in diabetic wounds, suggesting their potential role in diabetic wound chronicity and providing valuable insights into EVs malfunction in diabetic complications.47 However, EVs from normal cells or organs hold significant promise as natural carriers of bioactive molecules, which contain a cargo of growth factors, cytokines, and nucleic acids, stimulating regenerative processes such as angiogenesis, collagen synthesis, and cell migration, all of which are crucial for diabetic wound healing.48 For instance, Teng et al investigated the potential of EVs derived from human umbilical cord MSCs in enhancing diabetic wound healing, revealing their ability to promote proliferation of endothelial and fibroblast cells, reduce wound area and inflammation, increase collagen deposition, and stimulate anti-inflammatory macrophages and angiogenesis, thus elucidating their mechanism in improving wound repair.49 EVs can also exert immunomodulatory effects by regulating the inflammatory response at the wound site. It is well known that excessive neutrophil extracellular traps (NETs) on diabetic wound healing impair the repair process, and Chu et al reported that utilizing hypoxic preconditioned MSCs-derived small EVs could mitigate NET formation (Figure 4a and b), thereby promoting wound repair through miR-17-5p-mediated regulation of the TLR4/ROS/MAPK pathway (Figure 4c–e).50 EVs offer a cell-free alternative to traditional cell-based therapies, overcoming many of the challenges associated with cell transplantation. Their small size allows for easier administration and targeted delivery to the wound area.51 EVs can be isolated from various cell sources, including mesenchymal stem cells (MSCs) and immune cells, and tailored to the specific needs of individual patients. Recently, the engineered EVs have been enhanced to expand their functionality, such as through modification to efficiently incorporate miR146a and adhere to a silk fibroin patch, resulting in a notable improvement in diabetic wound healing.52 Additionally, loading EVs into biomaterials enables sustained release, thereby augmenting their stability and effectiveness. For instance, incorporating macrophage-derived EVs in a novel hydrogel system provides an effective therapy for diabetic wounds by combining anti-swelling and photothermal effects, scavenging reactive oxygen species, inhibiting inflammation, and promoting angiogenesis.53 Overall, EVs present a promising avenue for diabetic wound healing.
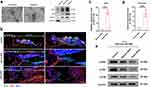 |
Figure 4 EVs derived from MSCs promote diabetic wound repair through miR- miR-17-5p-mediated signaling pathway. (a) TEM of Normo-sEVs and Hypo-sEVs. Scale bars, 200 nm. (b) The marks of sEVs were detected by Western blot. (c and d) miR-17-5p level was detected by qRT-PCR in sEVs and sEVs-treated neutrophil. (e) Western blot was used to evaluate the protein level of p-ERK, p-JNK and p-p38. Adapted from Bioact Mater, volume 27, Chu Z, Huang Q, Ma K, et al. Novel neutrophil extracellular trap-related mechanisms in diabetic wounds inspire a promising treatment strategy with hypoxia-challenged small extracellular vesicles. 257–270, copyright 2023, with permission from Elsevier.50 |
Nanofibers
Nanofibers, with their ultra-thin diameters measured in nanometers, have become key tools in healing diabetic wounds. These tiny fibers bring several benefits to wound care. First, their large surface area relative to their volume makes them excellent at absorbing drugs and other healing molecules, allowing for the efficient delivery of treatments directly to the wound site.54 In a previous study, chitosan-based nanofiber meshes with a nanocapsule-in-nanofiber structure have been developed to effectively load both hydrophobic and hydrophilic drugs, enhancing the treatment of difficult wounds by offering co-loading capacity, biocompatibility, and controlled release features.55 Additionally, nanofibers can replicate the structure and function of the extracellular matrix, creating a supportive environment that enhances cell attachment, movement, and tissue growth.56 Nanofiber-based dressings can also be designed to slowly release medications or growth factors over time, which helps in maintaining long-lasting treatment effects. Moreover, these fibers function as protective barriers over wounds, helping to ward off infections while keeping the wound moist, which is vital for healing.57 They can even be crafted into three-dimensional structures to aid the repair of more severe wounds. For instance, Huang et al developed a three-dimensional chitosan/polyvinyl alcohol-tannic acid nanofiber sponge (3D-TA), which offers a fluffy, highly porous structure with superior water handling, hemostatic properties, and enhanced antibacterial and antioxidant capabilities without antibiotics, showing promise for future clinical wound dressing applications.58 Finally, many nanofibers are biodegradable, decomposing into harmless substances as the wound heals, which reduces the need for frequent dressing changes and other medical interventions.59
Metal-Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs) in Diabetic Wound Healing
Metal-organic frameworks (MOFs) and Covalent organic frameworks (COFs) are two types of innovative nanomaterials that have gained attention for their potential in various applications, including diabetic wound healing.60 These materials are part of a broader category of crystalline porous materials and are noted for their unique structural characteristics and compatibility with biological tissues, making them suitable for medical applications.
Recent advancements in biomedical research have led to the development of innovative treatment methods utilizing metal-organic frameworks (MOFs) that show promising potential in addressing the unique challenges of diabetic wound healing. One such advancement is the antioxidative system known as MOF/Gel, designed for chronic wound healing in diabetic rats.61 This system integrates a MOF nanozyme with antioxidant enzyme-like activity within a hydrogel. The MOF/Gel continuously scavenges reactive oxygen species, modulating the oxidative stress microenvironment in chronic diabetic wounds, and facilitates a transition from the inflammation phase to the proliferation phase. This treatment has demonstrated efficacy comparable to that of human epidermal growth factor Gel, a widely used clinical drug for wound treatments, highlighting its potential as a safe and convenient option for clinical demands. Another notable approach involves engineered therapeutic hydrogels, which harness the antioxidative and oxygen-generating capabilities of MOFs to enhance diabetic wound healing.62 These hydrogels are constructed from natural polymers and a MOF-derived catalase-mimic nanozyme that captures elevated ROS and synergistically produces oxygen. This mechanism protects critical skin cells from ROS and hypoxia-mediated death and promotes a shift in macrophage activity from a pro-inflammatory to an anti-inflammatory state, accelerating the healing process.(Figure 5a) Furthermore, the application of multifunctional nanoplatforms such as Au NCs@PCN for bacterial-infected diabetic wounds has shown excellent results.63 These platforms leverage gold nanoclusters and zirconium-based MOFs to generate ROS and photothermal effects under NIR laser irradiation, effectively killing bacteria and significantly reducing wound coverage in diabetic rats (Figure 5b). The introduction of porous MOF liquids, such as ZIF-91 porous liquid (ZIF-91-PL), marks a significant advancement in drug delivery systems for diabetic wound care.64 These liquids offer high curcumin loading capacity and sustained release, enhancing healing when crosslinked with modified gelatin to form hydrogels (Figure 5c and d). Lastly, novel porous MOF microneedle (MN) patches capable of photothermal-responsive delivery of nitric oxide (NO) are under development.65 These patches promote deeper NO delivery to the wound site, accelerating vascularization and tissue regeneration (Figure 5e). In summary, the integration of MOFs into diabetic wound care offers a multi-faceted approach that addresses inflammation, bacterial infection, and oxidative stress, showing substantial promise for improving the outcomes of chronic diabetic wounds. These innovative treatments exemplify a significant stride towards overcoming the limitations of current therapies and provide a foundation for future advancements in diabetic wound management.
On the other hand, COFs consist of a rigid organic framework formed through covalent bonds. These frameworks are characterized by their predictable structures, robustness, and the versatility to modify their features according to specific needs. In diabetic wound healing, COFs have shown potential in enhancing controlled drug release and supporting tissue repair processes. Their precise structure allows for the engineering of their physical and chemical properties, impacting their ability to carry and release drugs.66 This precise control is crucial in developing delivery systems that are optimized for the specific requirements of diabetic wounds, focusing on the appropriate timing and dosage of therapeutic agents.67 In a previous study, an innovative nanoagent was created using 2D reductive COF, which was coated with immuno-engineered exosomes to effectively address diabetic wounds by mitigating oxidative stress, reducing inflammation, enhancing angiogenesis, and eliminating bacterial infections, demonstrating significant improvements over conventional treatments.68(Figure 6a) In another study, a novel therapeutic gel combining COFs with microalgae was developed to tackle the complex challenges of diabetic chronic wound healing, by synergizing oxygen release, angiogenesis promotion, and inflammatory response reduction, showing promising in vivo results for intensive wound care69 (Figure 6b and c).
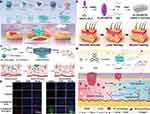 |
Figure 5 MOF-based therapies for diabetic wound management. (a) Depicts the creation of nanozyme-enhanced self-protective hydrogels that function as reactive oxygen species (ROS)-driven oxygen generators, aimed at improving diabetic wound healing. Reproduced with permission from Li Z, Zhao Y, Huang H, et al. A nanozyme-immobilized hydrogel with endogenous ROS-scavenging and oxygen generation abilities for significantly promoting oxidative diabetic wound healing. Adv Healthc Mater. 2022;11(22):e2201524. Copyright 2022 Wiley‐VCH GmbH.62 (b) Details the preparation of Au NCs@PCN for treating multidrug-resistant bacterial infections in wounds under NIR radiation, enhancing bacterial membrane disruption and promoting tissue repair. Reproduced with permission from Zhao X, Chang L, Hu Y, et al. Preparation of photocatalytic and antibacterial MOF nanozyme used for infected diabetic wound healing. ACS Appl Mater Interfaces. 2022;14(16):18194–18208. Copyright 2022 American Chemical Society.63 (c) Illustrates the use of a ZIF-91-PL composite hydrogel coating designed for diabetic wound healing. Weng P, Liu K, Yuan M, et al. Development of a ZIF-91-porous-liquid-based composite hydrogel dressing system for diabetic wound healing. Small. 2023;19(25):e2301012. Copyright 2023 Wiley‐VCH GmbH.64 (d) Shows immunofluorescence staining of wound sites treated with ZIF-91-PL gel on day 14, highlighting endothelial cells (CD31) and smooth muscle cells (α-SMA). Reproduced with permission from Weng P, Liu K, Yuan M, et al. Development of a ZIF-91-porous-liquid-based composite hydrogel dressing system for diabetic wound healing. Small. 2023;19(25):e2301012. Copyright 2023 Wiley‐VCH GmbH.64 (e) Outlines the synthesis and healing effects of Ni-HHTP on chronic diabetic wounds by mimicking natural enzymes, modulating macrophages towards an anti-inflammatory state, and activating the TGF-β signaling pathway to accelerate wound healing. Reproduced with permission from Liu J, Chen Z, Liu H, et al. Nickel-based metal-organic frameworks promote diabetic wound healing via scavenging reactive oxygen species and enhancing angiogenesis. Small. 2024;20(10):e2305076. Copyright 2023 Wiley‐VCH GmbH.65 |
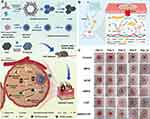 |
Figure 6 Nanoagent-Based Theranostic and Healing Processes in Diabetic Wounds. (a) Illustration of PCOF and E-Exo integration into the PCOF@E-Exo nanoagent for infected diabetic wounds. Reproduced with permission from Sun B, Wu F, Wang X, et al. An optimally designed engineering exosome-reductive COF integrated nanoagent for synergistically enhanced diabetic fester wound healing. Small. 2022;18(26):e2200895. Copyright 2022 Wiley‐VCH GmbH.68 (b) Diagram of bMPG/COF gel promoting wound healing with the treatment protocol. (c) Healing progression photographs at days 0, 3, 6, 9, and 12 with bMPG/COF gel application. Reproduced with permission from Jin N, Wu J, Ye S, et al. Injectable dynamic ROS-responsive COF-modified microalgae gels for in vivo bFGF delivery to treat diabetic wounds. ACS Appl Mater Interfaces. 2024;16(15):18608–18626. Copyright 2024 American Chemical Society.69 |
Challenges and Future Directions
As the field of nanotherapeutics and biomaterials for diabetic wound treatment continues to develop, it faces several challenges that need to be addressed to push the boundaries of what’s possible and improve patient outcomes.
Safety Concerns and Toxicity
The safety and biocompatibility of nanotherapeutic agents and biomaterials are crucial. It is important to fully assess their potential toxicity, immune responses they might elicit, and any long-term health effects.70 Different materials should be compared extensively, including biocompatibility assessment and toxicity analysis. Developing standardized testing methods will also aid in the comparison of different materials and help in their approval by regulatory bodies.71 A comprehensive approach to safety assessment should be adopted, ensuring that all possible interactions and effects are thoroughly evaluated. This includes studying the materials in various biological environments and over extended periods to understand their long-term impact.
Regulatory Hurdles
Obtaining regulatory approval for new nanotherapeutic treatments involves navigating complex and stringent safety and efficacy requirements. This process can be lengthy and requires significant resources. Effective collaboration among scientists, regulatory bodies, and industry stakeholders is essential for smoothing these pathways.72 Simplifying regulatory frameworks, establishing clear guidelines for nanotherapeutic products, and maintaining open lines of communication are key strategies to accelerate clinical application.
Clinical Translation
One of the primary challenges in the field is moving from laboratory research to clinical practice. This includes overcoming issues such as scalability and cost-effectiveness of production.73 Researchers should focus on translational research that is scalable, cost-efficient, and applicable in real-world settings. Collaborations including clinicians, engineers, and regulatory professionals, are crucial to successfully moving projects from the lab bench to clinical trials. Additionally, launch more clinical translation cases can provide valuable insights and facilitate further exploration in this area. Emphasis should be placed on sharing successful examples of nanotherapeutics in clinical settings, highlighting the pathways taken to achieve regulatory approval and real-world application. By increasing the visibility of these cases, the field can better understand the necessary steps and potential obstacles in the translation process.
Multifunctional Additive Materials
Future research should focus on the development of multifunctional additive materials that integrate antibacterial, anti-inflammatory, antioxidant, and angiogenesis properties. Such biomaterials can provide a comprehensive approach to wound healing, addressing multiple aspects of the healing process simultaneously. By incorporating these multifunctional properties, the effectiveness of nanotherapeutic treatments for diabetic wounds can be significantly enhanced.
Conclusion
The use of nanotherapeutics and biomaterials presents a promising path forward for enhancing diabetic wound care.74,75 These technologies have the potential to transform treatment approaches, thereby improving patient outcomes and mitigating the social and economic impacts of diabetes-related complications (Table 1). Continued research and collaboration are essential for advancing this field and redefining the future of diabetic wound treatment. Furthermore, numerous studies have demonstrated that stem cell-derived exosomes exhibit superior biocompatibility, immunomodulatory capabilities, and regenerative properties compared to metal-based nanoparticles. Therefore, prioritizing the development and clinical application of stem cell-derived exosomes could lead to more effective and targeted therapies for diabetic wounds.
 |
Table 1 Summary of Nanoparticle-Based Therapeutic Approaches for Diabetic Wound Healing |
Acknowledgments
We deeply appreciate the support from all participants.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic foot ulcers: a review. JAMA. 2023;330(1):62–75. doi:10.1001/jama.2023.10578
2. Matoori S, Veves A, Mooney DJ. Advanced bandages for diabetic wound healing. Sci Transl Med. 2021;13:585. doi:10.1126/scitranslmed.abe4839
3. Dixon D, Edmonds M. Managing diabetic foot ulcers: pharmacotherapy for wound healing. Drugs. 2021;81(1):29–56. doi:10.1007/s40265-020-01415-8
4. Wang G, Lin Z, Li Y, et al. Colonizing microbiota is associated with clinical outcomes in diabetic wound healing. Adv Drug Deliv Rev. 2023;194:114727. doi:10.1016/j.addr.2023.114727
5. Shao Z, Yin T, Jiang J, He Y, Xiang T, Zhou S. Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing. Bioact Mater. 2023;20:561–573. doi:10.1016/j.bioactmat.2022.06.018
6. Huang F, Lu X, Yang Y, et al. Microenvironment-based diabetic foot ulcer nanomedicine. Adv Sci. 2023;10(2):e2203308. doi:10.1002/advs.202203308
7. Banerjee K, Madhyastha R, Nakajima Y, Maruyama M, Madhyastha H. Nanoceutical adjuvants as wound healing material: precepts and prospects. Int J Mol Sci. 2021;22(9):4748. doi:10.3390/ijms22094748
8. Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14(1):85. doi:10.1186/s13045-021-01096-0
9. Liu T, Lu Y, Zhan R, Qian W, Luo G. Nanomaterials and nanomaterials-based drug delivery to promote cutaneous wound healing. Adv Drug Deliv Rev. 2023;193:114670. doi:10.1016/j.addr.2022.114670
10. Qiao Y, He J, Chen W, et al. Light-activatable synergistic therapy of drug-resistant bacteria-infected cutaneous chronic wounds and nonhealing keratitis by cupriferous hollow nanoshells. ACS Nano. 2020;14(3):3299–3315. doi:10.1021/acsnano.9b08930
11. Li Y, Li J, Zhao C, Yang L, Qi X. Hyperglycemia-reduced NAD(+) biosynthesis impairs corneal epithelial wound healing in diabetic mice. Metabolism. 2021;114:154402. doi:10.1016/j.metabol.2020.154402
12. Moura J, Madureira P, Leal EC, Fonseca AC, Carvalho E. Immune aging in diabetes and its implications in wound healing. Clin Immunol. 2019;200:43–54. doi:10.1016/j.clim.2019.02.002
13. Xu L, Wang YY, Huang J, Chen CY, Wang ZX, Xie H. Silver nanoparticles: synthesis, medical applications and biosafety. Theranostics. 2020;10(20):8996–9031. doi:10.7150/thno.45413
14. Fathil MAM, Katas H. Antibacterial, anti-biofilm and pro-migratory effects of double layered hydrogels packaged with lactoferrin-DsiRNA-silver nanoparticles for chronic wound therapy. Pharmaceutics. 2023;15(3):991. doi:10.3390/pharmaceutics15030991
15. El-Aassar MR, Ibrahim OM, Fouda MMG, et al. Wound dressing of chitosan-based-crosslinked gelatin/ polyvinyl pyrrolidone embedded silver nanoparticles, for targeting multidrug resistance microbes. Carbohydr Polym. 2021;255:117484. doi:10.1016/j.carbpol.2020.117484
16. Chen CY, Yin H, Chen X, et al. Angstrom-scale silver particle-embedded carbomer gel promotes wound healing by inhibiting bacterial colonization and inflammation. Sci Adv. 2020;6(43). doi:10.1126/sciadv.aba0942
17. Ullah S, Hussain Z, Ullah I, et al. Mussel bioinspired, silver-coated and insulin-loaded mesoporous polydopamine nanoparticles reinforced hyaluronate-based fibrous hydrogel for potential diabetic wound healing. Int J Biol Macromol. 2023;247:125738. doi:10.1016/j.ijbiomac.2023.125738
18. Wang S, Yan C, Zhang X, et al. Antimicrobial peptide modification enhances the gene delivery and bactericidal efficiency of gold nanoparticles for accelerating diabetic wound healing. Biomater Sci. 2018;6(10):2757–2772. doi:10.1039/C8BM00807H
19. Li S, Tang Q, Xu H, et al. Improved stability of KGF by conjugation with gold nanoparticles for diabetic wound therapy. Nanomedicine. 2019;14(22):2909–2923. doi:10.2217/nnm-2018-0487
20. Elsawy H, Sedky A, Abou Taleb MF, El-Newehy MH. Antidiabetic wound dressing materials based on cellulosic fabrics loaded with zinc oxide nanoparticles synthesized by solid-state method. Polymers. 2022;14(11):2168. doi:10.3390/polym14112168
21. Tavakoli S, Mokhtari H, Kharaziha M, Kermanpur A, Talebi A, Moshtaghian J. A multifunctional nanocomposite spray dressing of Kappa-carrageenan-polydopamine modified ZnO/L-glutamic acid for diabetic wounds. Mater Sci Eng C Mater Biol Appl. 2020;111:110837. doi:10.1016/j.msec.2020.110837
22. Chen Y, Xiang Y, Zhu T, Chen S. A dZnONPs enhanced hybrid injectable photocrosslinked hydrogel for infected wounds treatment. Gels. 2022;8(8):463. doi:10.3390/gels8080463
23. Loera-Valencia R, Neira RE, Urbina BP, Camacho A, Galindo RB. Evaluation of the therapeutic efficacy of dressings with ZnO nanoparticles in the treatment of diabetic foot ulcers. Biomed Pharmacother. 2022;155:113708. doi:10.1016/j.biopha.2022.113708
24. Narisepalli S, Salunkhe SA, Chitkara D, Mittal A. Asiaticoside polymeric nanoparticles for effective diabetic wound healing through increased collagen biosynthesis: in-vitro and in-vivo evaluation. Int J Pharm. 2023;631:122508. doi:10.1016/j.ijpharm.2022.122508
25. Paul PS, Cho JY, Wu Q, et al. Unconjugated PLGA nanoparticles attenuate temperature-dependent beta-amyloid aggregation and protect neurons against toxicity: implications for Alzheimer’s disease pathology. J Nanobiotechnol. 2022;20(1):67. doi:10.1186/s12951-022-01269-0
26. Almukainzi M, Elekhnawy E, Saleh A. Gentiopicroside PLGA nanospheres: fabrication, in vitro characterization, antimicrobial action, and in vivo effect for enhancing wound healing in diabetic rats. Int J Nanomed. 2022;17:1203–1225. doi:10.2147/IJN.S358606
27. Zhang Y, Jiang W, Kong L, Fu J, Zhang Q, Liu H. PLGA@IL-8 nanoparticles-loaded acellular dermal matrix as a delivery system for exogenous MSCs in diabetic wound healing. Int J Biol Macromol. 2023;224:688–698. doi:10.1016/j.ijbiomac.2022.10.157
28. Hasan N, Cao J, Naeem M, et al. PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mater Sci Eng C Mater Biol Appl. 2019;103:109741. doi:10.1016/j.msec.2019.109741
29. Wei X, Zheng Z, Liu M, et al. Enzyme-responsive nanospheres target senescent cells for diabetic wound healing by employing chemodynamic therapy. Acta Biomater. 2023;172:407–422. doi:10.1016/j.actbio.2023.10.015
30. Taheriazam A, Entezari M, Firouz ZM, et al. Eco-friendly chitosan-based nanostructures in diabetes mellitus therapy: promising bioplatforms with versatile therapeutic perspectives. Environ Res. 2023;228:115912. doi:10.1016/j.envres.2023.115912
31. Yu Y, Tian R, Zhao Y, et al. Self-assembled corrole/chitosan photothermal nanoparticles for accelerating infected diabetic wound healing. Adv Healthc Mater. 2023;12(16):e2201651. doi:10.1002/adhm.202201651
32. Algandaby MM, Esmat A, Nasrullah MZ, et al. LC-MS based metabolic profiling and wound healing activity of a chitosan nanoparticle-loaded formula of Teucrium polium in diabetic rats. Biomed Pharmacother. 2023;168:115626. doi:10.1016/j.biopha.2023.115626
33. Born LJ, Bengali S, Hsu ATW, et al. Chitosan particles complexed with CA5-HIF-1alpha plasmids increase angiogenesis and improve wound healing. Int J Mol Sci. 2023;24(18):14095. doi:10.3390/ijms241814095
34. Younas A, Dong Z, Asad M, Asad M, Li M, Zhang N. A chitosan/fucoidan nanoparticle-loaded pullulan microneedle patch for differential drug release to promote wound healing. Carbohydr Polym. 2023;306:120593. doi:10.1016/j.carbpol.2023.120593
35. Suh JW, Lee KM, Ko EA, et al. Promoting angiogenesis and diabetic wound healing through delivery of protein transduction domain-BMP2 formulated nanoparticles with hydrogel. J Tissue Eng. 2023;14:20417314231190641. doi:10.1177/20417314231190641
36. Kandregula B, Narisepalli S, Chitkara D, Mittal A. Exploration of lipid-based nanocarriers as drug delivery systems in diabetic foot ulcer. Mol Pharm. 2022;19(7):1977–1998. doi:10.1021/acs.molpharmaceut.1c00970
37. Ding Q, Ding C, Liu X, et al. Preparation of nanocomposite membranes loaded with taxifolin liposome and its mechanism of wound healing in diabetic mice. Int J Biol Macromol. 2023;241:124537. doi:10.1016/j.ijbiomac.2023.124537
38. Tang Q, Dong M, Xu Z, et al. Red blood cell-mimicking liposomes loading curcumin promote diabetic wound healing. J Control Release. 2023;361:871–884. doi:10.1016/j.jconrel.2023.07.049
39. Liu Y, Xia G, Chen Y, et al. Purpurolide C-based microneedle promotes macrophage-mediated diabetic wound healing via inhibiting TLR4-MD2 dimerization and MYD88 phosphorylation. Acta Pharm Sin B. 2023;13(12):5060–5073. doi:10.1016/j.apsb.2023.05.032
40. Huang Y, Li D, Chen X, et al. A NIR-II emissive polymer AIEgen for imaging-guided photothermal elimination of bacterial infection. Biomaterials. 2022;286:121579. doi:10.1016/j.biomaterials.2022.121579
41. Wang Y, Ji JY, Guo K, et al. Gene liposome nanocomplex-loaded dermal substitute promotes diabetic chronic wound healing and angiogenesis in rat. Biomed Pharmacother. 2023;163:114794. doi:10.1016/j.biopha.2023.114794
42. Ganesan P, Ramalingam P, Karthivashan G, Ko YT, Choi DK. Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int J Nanomed. 2018;13:1569–1583. doi:10.2147/IJN.S155593
43. Arantes VT, Faraco AAG, Ferreira FB, et al. Retinoic acid-loaded solid lipid nanoparticles surrounded by chitosan film support diabetic wound healing in in vivo study. Colloids Surf B Biointerfaces. 2020;188:110749. doi:10.1016/j.colsurfb.2019.110749
44. Deol PK, Kaur IP, Dhiman R, et al. Investigating wound healing potential of sesamol loaded solid lipid nanoparticles: ex-vivo, in vitro and in-vivo proof of concept. Int J Pharm. 2024;654:123974. doi:10.1016/j.ijpharm.2024.123974
45. Guo Y, Li Y, Fan R, et al. Silver@Prussian blue core-satellite nanostructures as multimetal ions switch for potent zero-background SERS bioimaging-guided chronic wound healing. Nano Lett. 2023;23(18):8761–8769. doi:10.1021/acs.nanolett.3c02857
46. Dixson AC, Dawson TR, Di Vizio D, Weaver AM. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat Rev Mol Cell Biol. 2023;24(7):454–476. doi:10.1038/s41580-023-00576-0
47. Guda PR, Sharma A, ElMasry MS, et al. Nanoscopic and functional characterization of keratinocyte-originating exosomes in the wound fluid of non-diabetic and diabetic chronic wound patients. Nano Today. 2023;2:52.
48. Al-Masawa ME, Alshawsh MA, Ng CY, et al. Efficacy and safety of small extracellular vesicle interventions in wound healing and skin regeneration: a systematic review and meta-analysis of animal studies. Theranostics. 2022;12(15):6455–6508. doi:10.7150/thno.73436
49. Teng L, Maqsood M, Zhu M, et al. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate diabetic wound healing via promoting M2 macrophage polarization, angiogenesis, and collagen deposition. Int J Mol Sci. 2022;23(18). doi:10.3390/ijms231810421
50. Chu Z, Huang Q, Ma K, et al. Novel neutrophil extracellular trap-related mechanisms in diabetic wounds inspire a promising treatment strategy with hypoxia-challenged small extracellular vesicles. Bioact Mater. 2023;27:257–270. doi:10.1016/j.bioactmat.2023.04.007
51. Liao Y, Zhang Z, Ouyang L, Mi B, Liu G. Engineered extracellular vesicles in wound healing: design, paradigms, and clinical application. Small. 2024;20(7):e2307058. doi:10.1002/smll.202307058
52. Li Q, Hu W, Huang Q, et al. MiR146a-loaded engineered exosomes released from silk fibroin patch promote diabetic wound healing by targeting IRAK1. Signal Transduct Target Ther. 2023;8(1):62. doi:10.1038/s41392-022-01263-w
53. Li W, Wu S, Ren L, Feng B, Chen Z. Development of an antiswelling hydrogel system incorporating M2-exosomes and photothermal effect for diabetic wound healing. ACS Nano. 2023;17(21):22106–22120. doi:10.1021/acsnano.3c09220
54. Jiang Z, Zheng Z, Yu S, et al. Nanofiber scaffolds as drug delivery systems promoting wound healing. Pharmaceutics. 2023;15(7):1829. doi:10.3390/pharmaceutics15071829
55. Lin ZI, Tsai TH, Yu KC, Nien YH, Liu RP. Creation of chitosan-based nanocapsule-in-nanofiber structures for hydrophobic/hydrophilic drug co-delivery and their dressing applications in diabetic wounds. Macromol Biosci. 2023;23(10):e2300145. doi:10.1002/mabi.202300145
56. Zhao Y, Tian C, Liu Y, et al. All-in-one bioactive properties of photothermal nanofibers for accelerating diabetic wound healing. Biomaterials. 2023;295:122029. doi:10.1016/j.biomaterials.2023.122029
57. Mirbagheri MS, Akhavan-Mahdavi S, Hasan A, Kharazmi MS, Jafari SM. Chitosan-based electrospun nanofibers for diabetic foot ulcer management; recent advances. Carbohydr Polym. 2023;313:120512. doi:10.1016/j.carbpol.2022.120512
58. Huang Z, Wang D, Sonderskov SM, et al. Tannic acid-functionalized 3D porous nanofiber sponge for antibiotic-free wound healing with enhanced hemostasis, antibacterial, and antioxidant properties. J Nanobiotechnol. 2023;21(1):190. doi:10.1186/s12951-023-01922-2
59. John JV, Sharma NS, Tang G, et al. Nanofiber aerogels with precision macrochannels and LL-37-mimic peptides synergistically promote diabetic wound healing. Adv Funct Mater. 2023;33(1). doi:10.1002/adfm.202206936
60. Xiong Y, Feng Q, Lu L, et al. Metal-organic frameworks and their composites for chronic wound healing: from bench to bedside. Adv Mater. 2024;36(2):e2302587. doi:10.1002/adma.202302587
61. Chao D, Dong Q, Yu Z, et al. Specific nanodrug for diabetic chronic wounds based on antioxidase-mimicking MOF-818 nanozymes. J Am Chem Soc. 2022;144(51):23438–23447. doi:10.1021/jacs.2c09663
62. Li Z, Zhao Y, Huang H, et al. A nanozyme-immobilized hydrogel with endogenous ROS-scavenging and oxygen generation abilities for significantly promoting oxidative diabetic wound healing. Adv Healthc Mater. 2022;11(22):e2201524. doi:10.1002/adhm.202201524
63. Zhao X, Chang L, Hu Y, et al. Preparation of photocatalytic and antibacterial MOF nanozyme used for infected diabetic wound healing. ACS Appl Mater Interfaces. 2022;14(16):18194–18208. doi:10.1021/acsami.2c03001
64. Weng P, Liu K, Yuan M, et al. Development of a ZIF-91-porous-liquid-based composite hydrogel dressing system for diabetic wound healing. Small. 2023;19(25):e2301012. doi:10.1002/smll.202301012
65. Liu J, Chen Z, Liu H, et al. Nickel-based metal-organic frameworks promote diabetic wound healing via scavenging reactive oxygen species and enhancing angiogenesis. Small. 2024;20(10):e2305076. doi:10.1002/smll.202305076
66. Li Y, Wang L, Liu H, et al. Ionic covalent-organic framework nanozyme as effective cascade catalyst against bacterial wound infection. Small. 2021;17(32):e2100756. doi:10.1002/smll.202100756
67. Zhu X, Feng T, Chen Y, et al. Reactive oxygen-correlated photothermal imaging of smart COF nanoreactors for monitoring chemodynamic sterilization and promoting wound healing. Small;2024. e2310247. doi:10.1002/smll.202310247
68. Sun B, Wu F, Wang X, et al. An optimally designed engineering exosome-reductive COF integrated nanoagent for synergistically enhanced diabetic fester wound healing. Small. 2022;18(26):e2200895. doi:10.1002/smll.202200895
69. Jin N, Wu J, Ye S, et al. Injectable dynamic ROS-responsive COF-modified microalgae gels for in vivo bFGF delivery to treat diabetic wounds. ACS Appl Mater Interfaces. 2024;16(15):18608–18626. doi:10.1021/acsami.4c01509
70. Xu H, Li S, Ma X, et al. Cerium oxide nanoparticles in diabetic foot ulcer management: advances, limitations, and future directions. Colloids Surf B Biointerfaces. 2023;231:113535. doi:10.1016/j.colsurfb.2023.113535
71. Liu M, Wei X, Zheng Z, et al. Recent advances in nano-drug delivery systems for the treatment of diabetic wound healing. Int J Nanomed. 2023;18:1537–1560. doi:10.2147/IJN.S395438
72. Sharifi S, Hajipour MJ, Gould L, Mahmoudi M. Nanomedicine in healing chronic wounds: opportunities and challenges. Mol Pharm. 2021;18(2):550–575. doi:10.1021/acs.molpharmaceut.0c00346
73. Hou L, Zhang X, Du H. Advances in mesenchymal stromal cells and nanomaterials for diabetic wound healing. Diabetes Metab Res Rev. 2023;39(4):e3638. doi:10.1002/dmrr.3638
74. Mandakhbayar N, Ji Y, El-Fiqi A, et al. Double hits with bioactive nanozyme based on cobalt-doped nanoglass for acute and diabetic wound therapies through anti-inflammatory and pro-angiogenic functions. Bioact Mater. 2024;31:298–311. doi:10.1016/j.bioactmat.2023.08.014
75. Pal D, Das P, Chaudhuri S, et al. Biomaterials-based strategies to enhance angiogenesis in diabetic wound healing. ACS Biomater Sci Eng. 2024;10(5):2725–2741. doi:10.1021/acsbiomaterials.4c00216
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

