Back to Journals » International Journal of Nanomedicine » Volume 20
Emerging Targeted Delivery Strategies of Nanosystems for Ischemic Stroke Treatment
Authors Ren JX, Ma HY, Yin WJ, Li YK, Lei SY, Liu JC, Yang Y, Guo ZN
Received 25 January 2025
Accepted for publication 11 May 2025
Published 24 June 2025 Volume 2025:20 Pages 8143—8171
DOI https://doi.org/10.2147/IJN.S519328
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Xing Zhang
Jia-Xin Ren,1,* Hong-Yin Ma,1,* Wen-Jing Yin,1 Yi-Kai Li,2 Shuang-Yin Lei,1 Jia-Cheng Liu,1 Yi Yang,1 Zhen-Ni Guo1,3
1Stroke Center, Department of Neurology, the First Hospital of Jilin University, Chang Chun, People’s Republic of China; 2The First Norman Bethune Clinical Medical College, Jilin University, Chang Chun, People’s Republic of China; 3Neuroscience Research Center, Department of Neurology, the First Hospital of Jilin University, Chang Chun, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yi Yang, Department of Neurology, the First Hospital of Jilin University, Xinmin Street 1#, Changchun, 130021, People’s Republic of China, Tel +86-13756661217, Fax +86-431-88782378, Email [email protected] Zhen-Ni Guo, Department of Neurology, the First Hospital of Jilin University, Xinmin Street 1#, Changchun, 130021, People’s Republic of China, Tel +86-18186872986, Fax +86-431-88782378, Email [email protected]
Abstract: Ischemic stroke is a leading cause of death and severe disability worldwide. Current treatments mainly focus on reperfusion and neuroprotection. However, due to limitations like a narrow treatment window, single therapeutic targets, and side effects, drug therapy effectiveness is often unsatisfactory. Additionally, the blood-brain barrier (BBB) and the pathophysiological changes following ischemia pose challenges in stroke treatment. Recent developments in nanomaterials have enabled the design of multifunctional drug delivery nanosystems to advance stroke therapeutic approaches. These novel treatments significantly overcome the shortcomings of current therapies and improve efficacy. This review comprehensively summarizes innovative strategies for drug delivery nanosystems, which include crossing the BBB to target the ischemic region and controllable release in a responsive manner. Smart nanosystems, due to the modification of specific ligands and/or cell membranes, can cross the BBB to target the ischemic region for precise treatment, achieving controlled release and specific accumulation of drugs through intelligent molecular switching. Combining different strategies to build drug delivery nanosystems allows for positioning and targeted accumulation of drugs in the ischemic region, thereby extending the therapeutic time window and synergistically improving the neuroprotective effects. Such combinations provide a powerful strategy for developing novel ischemic stroke treatments and establishing targeting delivery nanosystems. Furthermore, the review summarizes the challenges encountered by multifunctional drug delivery nanosystems including clinical translation, drug loading capacity, and safety concerns, and potential solutions.
Keywords: ischemic stroke, nanosystems, targeting therapy, blood-brain barrier, controllable release
Graphical Abstract:

Introduction
Ischemic stroke (IS) is the leading cause of death and severe disability worldwide.1 The mainstay of IS treatment is early revascularization and neuroprotection. Although many promising drugs have shown significant therapeutic effects in animal models, the benefit of clinical functional outcomes remains unsatisfactory. Thus, most of these drugs have not yet been successfully applied in clinics.2–4 The main therapeutic limitations include the inability to cross the blood-brain barrier (BBB), low lesion targeting capability and bioavailability, and evident systemic side effects.5 The BBB is a natural biological barrier that restricts drug transport between blood circulation and the central nervous system. Although BBB destruction occurs, to some extent, in the ischemic area after IS, cumulative doses of the drug in the infarct core and penumbra remain insufficient to reach a therapeutic effect. Moreover, traditional therapeutic strategies of neuroprotection drugs are mostly focused on one or two aspects of the complex pathophysiological mechanisms during IS progression, including calcium dysregulation, oxidative stress, neuro-inflammation, cellular apoptosis, and BBB impairment, which fail to exert beneficial efficacy comprehensively. Consequently, overcoming the BBB, targeting the ischemic lesion, and exploring multifunctional drugs with diverse and synergistic efficacy are priorities for the new-generation IS treatment.
Nanomedicine, which combines nanotechnology and medicine, is an emerging field in the advancement of drug delivery systems. Drug delivery nanosystems has become an effective solution with considerable prospects for application due to recent advancements in nanotechnology.6 Constructed nanosystems can cross the BBB to target the ischemic region for precise treatment through strategies such as ligand modification and the use of biomimetic cell membranes. Moreover, the nanosystem functions as a platform for drug delivery and release, achieving the specific accumulation and controlled release of drugs in the ischemic penumbra through intelligent molecular switching. Intelligent molecular switching are stimulus-responsive components integrated into nanomaterials, including pH-sensitive polymers, enzyme-sensitive peptides, thioketal linkers, and thrombolytic peptides, among others. These elements regulate drug release specifically within certain pathological microenvironments, such as those characterized by acidity, elevated ROS, or high levels of thrombolytic enzymes, thereby facilitating controlled drug delivery. The targeted strategies of drug delivery nanosystems can improve bioavailability, prolong circulation time and reduce toxic side effects on normal tissues.7 Synergistic drug treatment can be achieved by building multifunctional nanosystems to induce multi-mechanism therapeutic effects and alleviate the complex cascade of pathological responses after IS.
Therefore, this review summarizes the strategies underlying the targeted drug delivery of nanosystems in IS, including overcoming the BBB, targeting ischemic lesions, and responsively releasing drugs. These insights aim to usher in new cutting-edge ideas for drug delivery nanosystems and facilitate the advancement and application of nanosystems in IS.
Pathophysiology of Ischemic Stroke
IS progression involves necrosis of the ischemic core, which is defined as the area where cerebral vessels occlude, and deterioration of the penumbra in the later phase. The blood from collateral vessels cannot compensate for the occlusion in time, resulting in oxygen and glucose depletion at the ischemic site, which leads to irreversible downstream neuronal death.8 The penumbra represents a peri-infarcted area with limited affected neurons, thus potentially reversible injury following early reperfusion.9 However, cerebral ischemia-reperfusion injury (CIRI) is a pathological response to further neuronal damage and increased dysfunction after reconstruction of the cerebral blood supply. The cellular and molecular mechanisms underlying the development of IS include excitotoxicity, calcium dysregulation, oxidative stress, neuro-inflammation, apoptosis, and BBB impairment, as detailed below.10
Excitotoxicity and Calcium Dysregulation
Glutamate functions as a potent and rapid neurotransmitter. However, prolonged exposure to high levels of glutamate can lead to neurotoxic effects in the ischemic area through the excessive response of glutamate receptors, which facilitates the entry of Na+, Ca2+, and water into the cells.11 The accumulation of water and ions leads to cellular swelling and death. The calcium influx also triggers catabolic processes through Ca2+-dependent proteases, lipases, and nucleases, serving as an extra trigger for neuron death.12,13 The release of stored Ca2+ from the mitochondria further accelerates a cascade of catastrophic events that leads to acute cell death.14
Oxidative Stress
The brain is more susceptible to oxidative stress due to its massive oxygen -consumption and high lipid content.15 In the penumbra, following reperfusion and restoration of oxygen supply, there is a significant increase in the levels of reactive oxygen species (ROS), including superoxide radical anions, hydroxyl radicals, singlet oxygen, nitric oxide, and peroxynitrite, which disrupt the balance between oxidants and antioxidants. The interaction between the cellular components of neurons and oxidants results in protein oxidation and nitridation, lipid peroxidation, and DNA damage, exacerbating the damage to ischemic brain tissue.4,16 Furthermore, hydroxyl radicals compromise mitochondrial membrane integrity and impair the electron transport chain during oxidative phosphorylation. Excessive ROS generation triggers mitochondrial damage and leads to cellular apoptosis.17
Neuro-Inflammation
In the acute phase of IS, the endothelium of cerebral blood vessels promptly upregulates adhesion molecules, such as selectins, facilitating the adherence of leukocytes and platelets after reperfusion. BBB impairment, meningeal infiltration, and choroid plexus entry are examples of the diverse pathways through which immune cells, especially neutrophils and monocytes, access the brain during ischemic events.18–20 Microglia function as major immune cells in the maintenance of CNS stability.21 After the onset of cerebral ischemia, damaged brain cells release danger-associated molecular patterns that can activate microglia, transform microglia into distinct phenotypes, and engage in their organized division of labor.22 The classical activation (M1 type) of microglia is characterized by the secretion of pro-inflammatory mediators, which lead to the release of more chemokines and cytokines, promoting the influx of leukocytes, and exacerbating brain damage. The alternative activation (M2 type) of microglia primarily functions to repair damage to the brain tissue.23,24 The balance between the two types of activated microglia is vital for regulating the neuroinflammatory process in IS.25,26
Apoptosis
Various cell death pathways are associated with the pathophysiological processes of IS, such as intrinsic and extrinsic apoptosis, necroptosis, autophagy, ferroptosis, parthanatos, phagoptosis, and pyroptosis. Apoptosis, the predominant form of programmed cell death, can be activated through intrinsic or extrinsic pathways. Following the occurrence of IS, the intrinsic pathway of apoptosis is initiated, involving mitochondrial dysfunction, the release of cytochrome C, and apoptosis-inducing factor, or both, leading to caspase-dependent or independent cell death. Rapid translocation of apoptosis-inducing factors released by mitochondria to the nucleus mediates large-scale deoxyribo nucleic acid (DNA) damage and cell death in a caspase-independent manner. Furthermore, the nuclear pathway of neuronal cell death is activated in response to DNA damage by either phosphorylation and activation of p53 or the translocation of nuclear phosphoproteins from mitochondria.27
BBB Impairment
The BBB is a highly selective and regulated structure that limits the exchange of substances between blood circulation and the CNS. It is composed of the cerebral microvascular endothelium, surrounded by astrocytes, pericytes, neuronal terminals, the basement membrane, and the extracellular matrix. Collectively, these elements constitute a neurovascular unit that is essential for the health and stability of the CNS.28,29 The causes of BBB deficits after IS are mainly the progressive impairment of the transcellular barrier mechanism followed by paracellular barrier mechanisms. Studies have shown that BBB function is impaired as early as 6 hours after IS. Although the tight junction was found to exhibit severe structural deficits two days later, the number of endothelial caveolae and the rate of transcytosis increased as early as 6 hours after IS.18 The enzymatic breakdown of the basement membrane and tight junction and the loss of endothelial cells, pericytes, and astrocytes can be observed during a 24–48-hour period after IS onset. Therefore, the biphasic increase in BBB permeability exists after IS, which expands the therapeutic window for neuroprotective treatment and enhances drug delivery. After the breakdown of the BBB, leukocytes infiltrate and adhere to the walls of endothelial cells.30,31 Excessive ROS accumulation and impairment of endogenous antioxidant mechanisms lead to the peroxidation of DNA, proteins, and lipids, resulting in tissue damage and breakdown of the BBB.32,33 Matrix metalloproteinase (MMP) levels are significantly elevated following the activation of microglia and astrocytes. The loss of adenosine triphosphate and oxygen and other induced cytokines, such as hypoxia-inducible factor-1α, triggers the activation of the MMP family, particularly MMP9 and MMP2. In conjunction with other chemokines, cytokines, and vascular endothelial growth factor released by activated microglia and astrocytes, the tight junction between the cells and basal lamina undergoes degradation, leading to compromised BBB integrity. This degradation increases BBB permeability, facilitates the infiltration of blood-derived immune cells, and leads to vasogenic edema, hemorrhagic transformation, and an elevated mortality rate (Figure 1).31,34
Current Clinical Treatments for IS and Limitations
Reperfusion Treatments for IS
Reperfusion therapy is the primary treatment for IS, which mainly includes endovascular intervention and thrombolysis. Endovascular therapy has emerged as a highly effective intervention for IS caused by large vessel occlusion, including mechanical thrombectomy, thrombus aspiration, angioplasty and stenting placement.35 The meta-analysis of large-scale clinical trials on endovascular treatment of IS demonstrated that recanalization was achieved in 71% of patients following thrombus extraction. However, only 46% of patients experienced a favorable prognosis, and futile recanalization was observed in 50% of the cases. The causes of futile recanalization include tissue damage, cerebral edema, no-reflow phenomenon, reperfusion injury, procedural features, and variations in postprocedural management.36 In addition, the application of endovascular therapy remains constrained by the requirement for advanced stroke center infrastructure, specialized neurointerventional teams, and substantial healthcare expenditures. These limitations highlight the urgent need for adjuvant neuroprotective strategies to improve therapeutic benefits.
So far, the only drug approved by the US Food and Drug Administration is recombinant tissue-type plasminogen activator (rt-PA), a fibrin-specific thrombolytic agent. The clot-dissolving mechanism of rt-PA involves activating endogenous plasminogen into plasmin, which rapidly dissolves the fibrinogen surrounding the thrombus.37 However, due to the narrow therapeutic window (<4.5 h), only a small proportion of patients are eligible for rt-PA treatment, and hemorrhagic transformation and CIRI limit their efficacy. The extremely short half-life of rt-PA is approximately 4–6 minutes, mainly due to the presence of fast-acting endogenous inhibitors, such as fibrinogen activator inhibitor-1, in the circulation.38 Therefore, continuous intravenous infusion is necessary to achieve effective thrombolysis, which poses a risk of bleeding complications. Despite strict adherence to these indications, there is a 2–7% risk of fatal intracranial hemorrhage.39 Additionally, there is a risk that the dissolved embolic components from thrombolysis may spread to distal small arteries, potentially leading to fluctuating symptoms and worsening functional prognosis.40
Drug Treatment
Neuroprotection is achieved by inhibiting the pathogenic cascade to from preserving, recovering, or regenerating the nervous system.41 Various neuroprotectants are currently being investigated in preclinical research. Neuroprotective agents that target different pathophysiological processes can be divided into four categories: glutamate antagonists, calcium-channel blockers, free radical scavengers, and immunosuppressants.42 Although these promising candidates have shown significant therapeutic effects in animal models, they have yet to be successfully translated into clinical use.4,43 Efforts to develop neuroprotective agents such as edaravone and butylphthalide have also faced challenges related to the limited bioavailability and potential side effects of these drugs in clinical trials.44 The limited permeability of drugs to the brain may decrease the efficacy of neuroprotectants due to the protective function of the BBB. Moreover, the need for precise site-specific delivery of the drugs within a narrow treatment window, their side effects, and their short half-life after administration all present challenges for the clinical use of neuroprotective agents.4,5 To circumvent the BBB limitations of conventional drug delivery, intranasal administration has emerged as a promising strategy for bypassing the BBB.45,46 This approach utilizes olfactory and trigeminal neural pathways to facilitate rapid (within 15 minutes) transport of substances from the nasal cavity to the brain, achieving cerebral drug concentrations that are 3 to 5 times higher than those attained via intravenous administration. This method enhances drug absorption efficiency, minimizes extensive systemic exposure, and reduces hepatic and renal toxicity. At present, the intranasal route for the treatment of IS facilitates the delivery of small molecules, proteins, gene vectors, stem cells, and drug-loaded vesicles.47,48 Nevertheless, challenges such as limited drug retention time due to rapid nasal clearance, which impacts drug absorption, uneven drug distribution resulting from individual variability, and drug degradation by local enzymes in the nasal cavity, present significant obstacles. Consequently, the current therapeutic approach to intranasal drug delivery remains primarily in the basic research phase, with clinical translation facing substantial bottlenecks.49 Therefore, overcoming the limitations of current stroke therapeutics in crossing the BBB and targeting the ischemic region are key challenges that need to be addressed in the pharmacological treatment of stroke. How can therapeutic drugs be intelligently released only to the ischemic region to improve targeting and biological responsiveness? The drug delivery nanosystems designed using various strategies offer a promising avenue, and the current strategies for the use of nanosystems are described in detail below.
Targeted Delivery Strategies of Nanosystems
The function of drug delivery nanosystems for IS is site-specific targeting and releasing, including crossing the BBB to target the focal lesion and releasing drugs in a target-responsive manner. Detailed information on drug-delivery nanosystems is listed in Table 1.
 |
Table 1 Targeted Delivery Strategies of Nanosystems |
First, the ability to cross the BBB and reach the focal zone primarily relies on the presence of ligands on the nanocarriers that specifically bind to receptors mainly on the brain microvascular endothelial cells (BMECs) highly expressed at the focal site, including transferrin receptor (TfR), low-density lipoprotein receptor (LDLR), integrin receptors, and so forth. In addition to these receptors that are highly expressed on BMECs, the nanosystems can also target ischemic neurons, microglia, and mitochondria. Another strategy is to deliver the drug to the ischemic site by using biomimetic cell membranes or cellular components as nanocarriers, including neutrophil membranes, erythrocytes, macrophages, microglia, cancer cells, neural stem cells, and extracellular vehicles (EVs). Natural cell membranes and cellular components can also be modified with specific peptides to increase their targeting properties. In addition, external ultrasound or magnetic fields can be used to assist the nanocarriers in crossing the BBB and targeting the ischemic region.
Second, target-responsive drug release is a promising method for overcoming dose limitations, reducing systemic toxicity, and enhancing drug concentration at the lesion site. This strategy involves designing intelligent and controllable “switches” that respond to specific microenvironmental changes in the ischemic region. Specific chemical groups or other protein structures are attached to the nanocarriers to respond to the changes in the ischemic region, including changes in pH, ROS, thrombin aggregation, hypoxia, and inflammatory changes. The strategy of using nanosystems for thrombolytic therapy when the stroke has thromboembolic causes is to target the thrombus. Similarly, receptors expressed on thrombus-forming erythrocytes, platelets, and fibronectin are targeted via specific ligands, and thrombi are targeted via cell membranes and cellular components. Recently, multi-targeted, cascade-delivered nanosystems can both target thrombi and reach the ischemic site to achieve multiple functions, including thrombolysis and neuroprotection.
Strategies for Crossing BBB and Targeting Ischemic Region
Crossing the BBB and targeting the ischemic region are the primary hurdles for drug delivery nanosystems. Although some degree of BBB disruption occurs in the ischemic region after IS, the accumulated drug in the infarct core and penumbra remains insufficient to have therapeutic effects. Researchers have simulated the natural biological crossing of the BBB and selected various receptor–ligand pairs, cell membrane coatings, extracellular or cell membrane-derived vesicles, exosomes, peptides, and proteins to cross the BBB for drug delivery successfully. Regulating the permeability of the BBB also constitutes an effective strategy to enhance transport by crossing BBB. Furthermore, the current nanosystems not only cross the BBB, but also precisely target the infarcted brain region to exert multifunctional neuroprotective effects, such as anti-apoptosis, anti-inflammation, and anti-oxidative stress.
Receptor-Ligand Mediated Strategies
Transporter receptors on the surface of the BBB are responsible for the intracranial transport of biomolecules. Receptor-mediated transcytosis (RMT) is one of the most widely used and effective strategies to help biomolecular drugs cross the BBB. The receptors contain TfR, LDLR, integrin receptors and other ligands decorated on the surface of nanoparticles, which not only bind to BMECs to achieve BBB crossing, and even to target lesion site to fulfill specific brain cells targeting ability, taking the example of ischemic neurons and activated microglia. This so-called “molecular Trojan horse technology” provide potential strategies for achieving trans-BBB and targeted drug delivery.80
TfR-Medicated Targeting
Transferrin is responsible for transporting iron across the BBB by binding to TfR expressed on BMECs. Previous studies have shown that TfR is significantly overexpressed in the vascular endothelium 1 day and 2 days after in middle cerebral artery occlusion (MCAO) rat model to mimic middle cerebral artery occlusion.81 Raza et al developed transferrin-coupled pH-responsive nanomicelles to encapsulate NOD-like receptor thermal protein domain associated protein 3(NLRP3) inflammasome inhibitor. The nanomicelles were synthesized by using poly (2-methyl-2-oxazoline) (a hydrophilic and pH-responsive polymer), poly (l-lactic-co-glycolic acid) (PLGA) and holo-transferrin. These nanomicelles specifically bind to TfR and cross the BBB. The nanomicelles were found to significantly increase the overall survival of MCAO rats and act as a therapeutic agent for CIRI injury by inhibiting the activation of NLRP3 inflammasome.82 In addition, a specific ligand peptide of TfR, namely HAIYPRH (T7) mediates the transport of the nanocarriers across the BBB. Gu et al and Fan et al found that nanomaterials with T7 loading were translocated between BMECs in contrast to non-targeted liposomes.74,83 Furthermore, Li et al used a mild biomimetic mineralization technique to build a transferrin-based manganese dioxide nanozyme (MnO2@Tf, MT) loaded with neuroprotective agent edaravone (Eda-MnO2@Tf, EMT). The decoration of transferrin enabled EMT to be targeted to the ischemic BMECs. Then, the EMT not only crossed the BBB and aggregated at the ischemic lesion with good biocompatibility and biosafety. MnO2 nanozyme and edaravone endowed the EMT with superoxide dismutase and catalase-like antioxidant activity to scavenge overwhelmed ROS, ameliorate ROS-mediated damage and reduce the levels of inflammatory factors. Additionally, EMT released Mn2+ ions in the weak acid environment of the ischemic lesion area, which can be used for dynamic tracking of the treatment process through magnetic resonance imaging.84
LDLR-Medicated Targeting
LDLR is expressed on BMECs and other peripheral tissues and is responsible for transporting lipoproteins such as cholesterol, tocopherol, and apolipoproteins.85 LDLR-related protein receptors 1 and 2, which are structurally similar to LDLR, are also expressed in BMECs. Various ligands, including L-carnosine, angiopep-2 (ANG), lactoferrin, and melanotransferrin, can bind to these receptors and mediate nanocarriers across the BBB via RMT.52 Therefore, Shi et al ingeniously developed an edaravone-loaded ceria nanoparticle for crossing the BBB and combating IS. The engineered nanoparticles performed a core-shell structure with monodisperse ultrasmall ceria as the core and organic ANG/polyethylene glycol (PEG) loaded by edaravone as the shell. Firstly, PEG was able to significantly improve biocompatibility and monodispersity and prolong the half-life in the blood. Second, ANG was the targeted ligand on cerium oxide nanoparticles that binds specifically to the LDLR of the BBB via receptor-mediated transcytosis and makes nanoparticle high accumulation in intracerebral lesions. Meanwhile, the core of the ceria nanoparticles and the absorbed edaravone synergistically eliminate ROS, which mitigates oxidative stress and reduces infarct size.52 In addition, Liu et al also created a nanoprobe decorated by ANG peptides, which penetrated BBB and were activated by a highly specific biomarker of neuroinflammation through up-conversion with near-infrared emission. The ANG peptides can bind to the LDLR overexpressed on the BBB and then deliver the nanoprobe into the brain tissue to allow non-invasive neuroinflammation assessment.86
Integrin Receptor-Medicated Targeting
Integrin αvβ3, an important member of the integrin family, is significantly upregulated on stressed vascular endothelial cells during cerebral ischemia.87,88 One of the identified ligands for integrin is the RGD integrin-binding peptide.89 Arg-Gly-Asp (RGDyC) is selective for αvβ3 integrin peptides and also binds to integrin receptors on the surface of leukocytes (neutrophils and monocytes).90 Therefore, drug delivery systems enriched with the αvβ3-specific ligand are one of the potential ways to target lesions.
Xin et al constructed c (RGDyK)-modified reactive nitrogen species stimuli-responsive liposomes loaded with NF-κB inhibitor, caffeic acid phenethyl ester (R-lipo-CAPE) to remodel the neurovascular unit and reduce the progression of CIRI. The lipid-containing o-phenylenediamine structure was first synthesized and self-assembled with phospholipids, cholesterol, c (RGDyK)-modified phospholipids, and CAPE in proportion to create a targeted R-Lipo-CAPE in response to reactive nitrogen species stimulation. The c (RGDyK) peptide promoted the targeted accumulation of R-Lipo-CAPE to cross the BBB and reach the ischemic lesion. Subsequently, the o-phenylenediamine lipid hydrolyzed in response to RNS activation accumulated in the lesion focus, causing alterations in the lipid bilayer’s curvature and regional instability, which ultimately led to the intelligent release of CAPE. The results suggested that R-lipo-CAPE remodels neurovascular unit and exhibits good protection against IS by inhibiting NF-κB activation in neurons, glial cells and endothelial cells, and exerting anti-nitrosative stress, anti-neuronal apoptosis, modulation of microglia polarization and repair of vascular endothelial cells.63 Similarly, Deng et al encapsulated the plasmid DNA containing the mutant form of the hypoxia-inducible factor 1-α (HIF-1α-AA) in a hyperbranched cationic amylopectin derivative (DMAPA-Amyp) nanocarrier, which was decorated by RGD peptides to increase aggregation in BMECs at the infarct region and penumbra, which significantly contributed to the recovery of neurological function.57
Other Ligands-Medicated Targeting
Stroke-Homing Peptides
The stroke-homing peptides (SHp) are peptides obtained by in vivo screening of phage libraries with a high affinity for glutamate receptors on the cell surface during neuronal apoptosis.51,91 Modifying targeted peptide on the surface of nanoparticles is a feasible and promising strategy. Self-assembled nanoparticles with peptides have abundant metal-binding sites, making them effective biomineralization scaffolds. These nanoparticles are more advantageous in terms of biocompatibility and targeting than inorganic nanomaterials. Therefore, Fan et al designed a novel peptide-templated manganese dioxide nanozyme (PNzyme/MnO2), which combined the ability of nanozymes to scavenge ROS with the thrombolytic activity of functional peptides (Figure 2a).74 There were two functional peptide chains included to construct peptide templates for the thrombin response. The functional peptide chain 1 contained the following structural motifs: a fibrin binding sequence, a thrombolytic sequence (CREKA), a sequence recognized and cleaved by thrombin, a transferrin receptor binding sequence, and a hydrophobic metal ion binding peptide. The functional peptide chain 2 consisted of a sequence that targeted ischemic neuronal tissues and a hydrophobic sequence that bound metal ions. A mixture of the two peptide chains self-assembled into nanoparticles and was biomineralized with MnO2 initiated by KMnO4 to form PNzyme/MnO2. Based on self-assembling polypeptides with different functional motifs, the PNzyme/MnO2 performed step-wise targeting thrombus, BBB and ischemic lesion. First, the PNzyme/MnO2 was particularly localized at the thrombus site via the fibrin-binding motif CREKA, which is then cleaved by thrombin to release the thrombolytic peptide, GRPAK to exert thrombolytic effects. Then, the rest of PNzyme/MnO2 bound to the TfR on endothelial cells via the T7 sequence HAIYPRH. Finally, the stroke homing sequence CLEVSRKNC targeted to the apoptotic neurons in the IS tissue to exert antioxidant effect. Moreover, the PNzyme/MnO2 prolonged the blood-circulation time and exhibited effective thrombolytic action, and reduced the ischemic damages and neuroinflammation in brain tissues. Similarly, Wang et al and Zhang et al used SHp-modified nanoparticles that could selectively target the site of cerebral ischemia and co-localize with neurons undergoing apoptosis in ischemic brain tissue.72,73 In addition, the targeted delivery of nanoparticles to the stroke site can be achieved by attaching other stroke-targeting peptides (eg, T6 or T7 peptides) to the surface of liposomes, cyclodextrins, or nanozymes.83
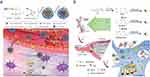 |
Figure 2 The different ligands-medicated targeting for crossing BBB and targeting ischemic region. (a) Schematic illustration of the biomineralization synthesis of a novel peptide-templated manganese dioxide nanozyme (PNzyme/MnO2) and its application as a smart multifunctional therapeutic for the treatment of ischemic stroke. Reprinted from Wang Z, Zhao Y, Hou Y, et al. A thrombin-activated peptide-templated nanozyme for remedying ischemic stroke via thrombolytic and neuroprotective actions. Adv Mater. 2024;36(10):e2210144. © 2023 Wiley-VCH GmbH.74 (b) Triple targeting SS31-HA-QT and its fluorescent indicator IR-780-SS31-HA-QT exerts neuroprotective effect against cerebral ischemia. Reprinted from Cen J, Zhang R, Zhao T, et al. A water-soluble quercetin conjugate with triple targeting exerts neuron-protective effect on cerebral ischemia by mitophagy activation. Adv Healthcare Mater. 2022;11(22):e2200817. © 2022 Wiley-VCH GmbH.64 |
Mitochondria-Targeting Peptide Bendavia
The mitochondria-targeting peptide Bendavia (SS-31) (a member of the Szeto-Schiller peptide family) is a short peptide that freely crosses cell membranes and selectively binds to cardiolipin, independent of the mitochondrial transmembrane potential, which is capable of increasing the mitochondrial accumulation 1000 to 5000-fold.64,92 Wang et al instructed the SS-31 peptide-modified multifunctional nanoparticles with ROS-responsiveness and mitochondrial-targeted (SPNPs). The SPNPs encapsulated in a thermo-sensitive gel were delivered to ischemic penumbra via nose-to-brain pathway. Apparently, the SPNPs were shown using MitoTracker Red staining to achieve mitochondrial targeting with the help of SS-31 rapid accumulation in the ischemic penumbra. The thioketal cross linked skeleton of SPNPs were fractured in response to the ROS in the oxidative microenvironment, which exerted ROS-scavenging activity and achieved controlled release of loaded Puerarin. Triggered by abundant ROS at mitochondria, ROS-responsive skeleton in SPNPs and SS-31 could eliminate ROS to reduce oxidative stress and cascade inflammatory response. The encapsulated Puerarin release can repair mitochondrial function by maintaining adenosine triphosphate metabolism and membrane function, thereby inhibiting cytochrome C-mediated apoptosis.46 Additionally, Guo et al constructed a novel delivery system through decorating SS-31 peptide to a water-soluble conjugate hyaluronic acid -Quercetin, which was capable of penetrating the BBB and indiscriminately targeting mitochondria. The delivery system could be targeting to the mitochondria of damaged neurons and exerted a neuroprotective effect by activating mitochondrial autophagy (Figure 2b).64,77
Chlorotoxin
Chlorotoxin (CTX) is a 36-amino acid peptide closely related to the cerebral ischemic microenvironment, for which the upregulated MMP-2 has a high specificity and affinity.93 CTX had been shown to transiently enhance the BBB permeability and drug delivery to the brain.94,95 As we all know, conventional targeted drug delivery is achieved using ligands with a high affinity for the ischemic microenvironment, which allows a small fraction of the nanoparticles to enter the ischemic region of the brain. Departing from the aforementioned targeting mechanisms, Zhou et al innovatively used an autocatalytic targeting approach to release the BBB modulator-lexiscan, which in turn transiently enhances BBB permeability, allowing more nanoparticles to enter the ischemic region.50 Through this secondary autocatalytic mechanism, the delivery process created a positive feedback loop that promoted drug accumulation in the ischemic region. Based on this concept, Zhou et al synthesized the novel nanoparticles using PLGA anchored by CTX and encapsulated as the BBB modulator. The nanoparticles were not only efficiently and precisely accumulated in the ischemic brain, but also the targeting efficiency autocatalytically became superior with following administration. The results showed that the aggregation of nanoparticles on the infarct side was 12 times higher than it on the healthy side.
Docosahex Aenoic Acids
Approximately 50% of the polyunsaturated fatty acids (PUFA) on cell membranes in the CNS are docosahex aenoic acids (DHA).96 DHA can pass through the BBB owing to its ability to absorb plasma-unesterified PUFA.97,98 Wang et al encapsulated Cu4.6O nanoparticles in the diselenide bond-conjugated zein and DHA, which was coated by plate membrane and rt-PA was further conjugated on the plate membrane. The platelet membrane represents a highly promising endogenous carrier for the targeted delivery to thrombotic sites. The structural domains of platelet surface membrane proteins, such as CD62p and CD41, facilitate the evasion of immune clearance and enhance the targeting efficacy towards blood clots. Through the synthesis methods, the nanoparticle was finally constructed, which possessed multiple functions of ROS responsiveness, targeted thrombolysis and neuroprotection. The nanoparticles evaded phagocytosis by macrophages via plate membrane, crossed the BBB based on the DHA receptor-mediated endocytosis of BMECs, and Cu4.6O and DHA would be released in ROS-rich ischemic microenvironment. Therefore, the nanoparticles exerted strong ROS scavenging and neuroprotection abilities and had good therapeutic effects on embolic MCAO rats.75 Besides, liposomes can selectively aggregate in the ischemic hemisphere and maintain long-term liposome colocalization in the neurovascular unit, thereby enhancing drug accumulation after stroke.30 Consequently, Wang’s group proposed a strategy to form covalent complexes and encapsulate Ginkgolide B liposomes with PUFA. The amount of Ginkgolide B liposomes-DHA targeted to the ischemic hemisphere was 2.2 times that of the free solution, which increased the accumulation of Ginkgolide B in the ischemic area.99
Complement Component 5a
Inflammation-related molecules highly expressed in the ischemic region can be used in targeting strategies. Complement component 5a (C5a), an anaphylatoxin consisting of 74 amino acids, is highly expressed in the ischemic penumbra, and can thus be a potential target for drug delivery. C5a binds to its receptor on microglia and releases a variety of pro-inflammatory cytokines that further promote inflammatory responses.100,101 Given their capability of precise chemical modification and great potential to target ischemia, anti-C5a aptamers specifically target C5a and block C5a binding to the C5a receptor to inhibit inflammatory responses. Therefore, based on the biocompatibility and abundance of binding sites of poly (acrylic acid), Peng et al combined the anti-C5a aptamer onto the poly (acrylic acid)-encapsulated and fluorescein isothiocyanate-labeled SiO2 nanospheres, which could be regarded as scaffolds of anti-C5a aptamer and other drugs.65 The “navigation” - amino-modified anti-C5a aptamers were covalently cross linked with poly (acrylic acid) of nanospheres by pH-sensitive amide bonds. The fluorescein isothiocyanate label could track the real-time delivery institution in vivo. Effectively, the nanospheres targeted the ischemic penumbra, alleviated oxidative stress and polarized microglial phenotype to suppress inflammatory response in ischemic stroke.
C-X-C Motif Chemokine Receptor 4
C-X-C motif chemokine receptor 4 (CXCR4) is a chemokine receptor that is highly expressed in ischemic tissues. AMD3100, a fully characterized CXCR4 antagonist, has previously been used as a ligand for targeted drug delivery in CXCR4-overexpressing diseases.102,103 Thus, Sheth et al attached AMD3100 to the surface of antioxidant betulinic amine, which was preferentially abundant in the ischemic brain and could enhance medication release in acidic environments. This approach could be used as an effective vehicle for targeted delivery of therapeutic drugs to the ischemic brain in addition to treating ischemic stroke.55 Furthermore, based on the fact that nanoparticles can expand or shrink their size by reacting to enzymes enriched in the stroke microenvironment, Zhou et al assessed the delivery efficiency of nanoparticles in different formulations to MCAO mice. Zhou’s group constructed nanoparticles using block copolymers consisting of PEG, poly (ε-caprolactone), and enzyme-cleavable peptides. Then the AMD3100 was conjugated to the surface of nanoparticles above, which performed the maximum delivery efficiency. After thrombin trigger, the average size of the nanoparticles gradually decreased from 218.2 nm to 79.3 nm over 24 h and accumulated 30 times more in the ischemic region than control nanoparticles without ligands.53
Cell-Membrane Mediated Strategies
The emergence of cell membrane-based biomimetic carriers provides new avenues for BBB recognition and transport as well as for biomultifunctionality and high biocompatibility. In recent years, cell membrane-camouflaged nanocarriers derived from neutrophils, macrophages, microglia, erythrocytes, neural stem cells, and cancer cells have shown great potential as IS therapeutic drug delivery systems.78,104,105 At the onset of ischemia, leukocytes in the peripheral circulation are activated and recruited to the ischemic brain region, where they communicate with endothelial cells via receptor-ligand interactions and transport, cross the BBB, and release payload drugs into the inflamed reconstituted endothelium. Encapsulating cell membranes from leukocytes have been shown to confer nanoparticles the ability to precisely target inflammatory lesions. Besides largely improving targeting capability, as the native component in the systematic circulation, cell membrane coating by as drug carriers also improves bio-capacity and reduce immunogenicity, and thus extend the circulation time of the system in vivo and eliminate potential peripheral side effects.106
Neutrophils
As the fastest immune cells to respond to inflammation, neutrophils are the first leukocytes to be activated and recruited from the periphery to the ischemic brain region. Neutrophils can detect the concentrations of inflammatory chemokines through their cytokine receptors (including CXCR2, CXCR4, integrin CD11b, lymphocyte function-associated antigen 1 (LFA1), very late antigen 4 (VLA-4) and migrate to the site of injury.107,108 Intercellular adhesion molecule (ICAM)-1 expression is upregulated in inflamed BMECs after IS.109 Neutrophils also interact with endothelial cells through the expression of integrin β2, macrophage-1 antigen, and LFA1 on neutrophil membranes.110,111 Moreover, RMT is enhanced after IS, and neutrophils actively move towards endothelial junctions by rolling, adhering, and crawling, eventually migrating to the BBB.112,113 N-acetyl Pro-Gly-Pro (PGP) is an endogenous tripeptide derived from extracellular matrix degradation, which can act as a ligand with high affinity to CXCR on neutrophils.114 Based on the properties of neutrophils, the main ideas of researchers to design nanomaterials include nesting neutrophil membranes to the surface of synthetic substrates or drug delivery function by means of interaction with peripheral blood neutrophils.
On the one hand, nanoparticles can be encapsulated directly using natural neutrophil membranes. Therefore, Wang et al constructed a biomimetic nanosystem (Leo@NM-Lipo) consisting of nanoliposomal leonurine nested with neutrophil membrane. In vivo and in vitro experiments indicated that the cell membrane-to-nanocore weight ratio significantly altered the targeting and therapeutic efficacy of biomimetic nanosystems in addition to changing their physicochemical characteristics. Finally, Leo@NM-Lipo constructed with a low cell membrane protein-to-lipid ratio of 1:10 greatly increased the delivery efficiency of leonurine across the BBB and performed the multiple roles including anti-oxidant damage, anti-inflammation and anti-apoptosis, which remodeled the inflammatory microenvironment, reduced infarct size and improved neurological recovery.60 Additionally, the drug delivery system encapsulated by the neutrophil membranes exerted a 5.16-fold targeting effect on the inflammatory microenvironment.73
On the other hand, neutrophils can act as a “hitchhiker” in drug delivery.67,115 Wang et al developed cross-linked dendrigraft poly-L-lysine nanoparticles decorated with PGP. The nanoparticles crossed the BBB and targeted to ischemic brain by PGP binding to neutrophils and neutrophil-mediated inflammatory migration, which demonstrated notable binding effectiveness to neutrophils and effective transport to recipient cells.116 Zhou et al used a strategy based on bacterial-derived outer membrane vesicles (OMV) hitchhiking on neutrophils to enhance the intracranial delivery of pioglitazone for the treatment of IS. OMVs contain lipopolysaccharides, which are specifically recognized by neutrophils through the toll-like receptor-specific recognition, which promotes endocytosis of vesicles by neutrophils. OMV was extracted by differential centrifugation and pioglitazone was loaded into OMV by electroporation. Ultimately, OMV@PGZ was able to ride on neutrophils to penetrate the BBB into the infarcted brain. When neutrophils reach the ischemic region, excess ROS promoted neutrophil chromatin depolymerisation and formation of neutrophil extracellular traps. OMV@PGZ exhibited the neuroprotective effect through preventing lipid peroxidation, mitochondrial damage, ferroptosis, and the activation of nucleotide oligomerization-like receptor protein 3 inflammasomes while minimizing reperfusion injury (Figure 3a).66
 |
Figure 3 The cell-membrane mediated strategies for crossing BBB and targeting ischemic region. (a) Schematic of ischemic stroke mediated by OMV@PGZ nanoparticles. OMV was extracted by differential centrifugation, and the PGZ was loaded into the OMV by electroporation. After the tail vein enters the blood circulation, OMV@PGZ is recognized and captured by the TLR receptor of neutrophils and brought to the stroke site. Then two pharmacological pathways occur, one is to inhibit the assembly of NLRP3 inflammasomes, and the other is to inhibit ferroptosis. Reprinted from Pan J, Wang Z, Huang X, et al. Bacteria-derived outer-membrane vesicles hitchhike neutrophils to enhance ischemic stroke therapy. Adv Mater. 2023;35(38):e2301779. © 2023 Wiley-VCH GmbH.66 (b) Schematic of engineered nanoerythrocyte vesicles formation. Reprinted from Yin N, Zhao Y, Liu C, et al. Engineered nanoerythrocytes alleviate central nervous system inflammation by regulating the polarization of inflammatory microglia. Adv Mater. 2022;34(27):e2201322. © 2022 Wiley-VCH GmbH.117 (c) Schematic illustration of the preparation of the nanojacket-cloaked drug-loaded liposome (PP@PCL) (c-A) and therapeutic mechanisms for precise ischemic stroke management. Reprinted from Tang L, Yin Y, Liu H, et al. Blood-brain barrier-penetrating and lesion-targeting nanoplatforms inspired by the pathophysiological features for synergistic ischemic stroke therapy. Adv Mater. 2024;36(21):e2312897. © 2024 Wiley-VCH GmbH.118 Abbreviations: NLRP3, NOD-like receptor thermal protein domain associated protein 3; OMV, outer membrane vesicles; PGZ, pioglitazone; TLR, toll-like receptor; HA, hyaluronic acid; MCAO/R, middle cerebral artery occlusion/reperfusion. |
Furthermore, neutrophils are associated with proinflammation, and reducing neutrophil infiltration into ischemic areas is a potential approach to treating stroke. Inspired by this, Zhou et al designed rod-shaped piceatannol-encapsulated PLGA nanoparticles, which were prepared by solvent evaporation method to fabricate Pic@Spheres, followed by stretching the spheres to rod-shaped nanoparticles by film stretching method. Then, the nanoparticles with an aspect ratio of 5 were found to be more likely to be endocytosed by neutrophils. Therefore, the nanoparticles were filtered out to load with piceatannol, which blocked neutrophil adhesion to endothelial cells and inhibited neutrophil infiltration into the BBB. In addition, even if a small fraction of nanoparticles-carrying neutrophils entered the BBB, inflammatory cytokines were reduced in the ischemic brain. The nanoparticles released piceatannol in the ischemic region, inhibited spleen tyrosine kinase signalling from microglia, and alleviated neuroinflammation.119
Macrophages and Microglia
During CIRI, macrophages are recruited to and infiltrate the ischemic brain within hours.120 ICAM-1 and P-selectin are overexpressed on the luminal side of stressed vascular endothelial cells,121 and these molecules can interact with the CD44 and CD11b ligands on leukocytes recruited from the peripheral blood to promote infiltration into the lesion, whereas the recruitment is not affected by the degree of opening of the BBB.122,123 Therefore, Jiang et al developed a macrophage‐disguised honeycomb manganese dioxide (MnO2) nanosphere loaded with fingolimod (Ma@(MnO2+FTY)).62 The macrophage enabled Ma@(MnO2+FTY) to have inflammation-directed chemotaxis and promoted the recruitment of CD11b and CD44 to the lesion via a volume-dependent caveolin- mediated endocytic pathway into the cells, thus crossing the BBB and accurately delivering drugs to the ischemic penumbra. The manganese dioxide nanospheres could consume excess hydrogen peroxide, convert it to oxygen demand, and break it down in acidic lysosomes to release fingolimod, which reduced oxidative stress, promoted phenotypic transition of microglia, and ultimately reversed the pro-inflammatory microenvironment and enhanced the survival of damaged neurons.79
Microglia are versatile macrophages found in the brain. Ischemia-activated anti- inflammatory M2 microglia are preferentially recruited to infarcted areas because lymphocyte LFA-1 on the surface of M2 microglia has a strong affinity for ICAM-1.59,124 Previous studies using an in vitro BBB model have shown that the encapsulation of M2-microglia membranes significantly enhanced the BBB penetration of the nanovehicles, leading to an outstanding ability to target cerebral infarction regions.71 Therefore, Sha et al designed a drug-free biomimetic nanovehicle, which was assembled from polyfluorocarbon and Pluronic P123 camouflaged with M2 microglia membranes. The perfluoro undecane-derived amphiphilic polyfluorocarbon was of exceeding ischemia-oxygenation capacity and the Pluronic P123 was uniquely characterized in downregulating MMP-9 and stabilized nanoparticles with excellent biocompatibility. The biomimetic nanovehicle targeted cerebral infarction regions by M2 microglia membranes, exerted neuroprotection effects and ameliorated neurological deficits by increasing oxygen to ischemic regions and inhibiting MMP-9 secretion.71 Additionally, Lin et al developed a magnetic field driven, mitochondria-targeted ceria nanosystem (MMTCe) anchored by BV2 cell membranes, which used an external magnetic field to achieve effective delivery of nanosystems in the brain to target damaged mitochondria and repair the ischemic microenvironment.125
Red Blood Cells
Red blood cells (RBC)-based bionic systems have proliferated for drug delivery.126–128 Critically, molecular signaling on the surface of the erythrocyte membrane, through hydrophilic glycans and negatively charged sialic acid residues, gives the nanoparticles advanced natural properties, such as excellent biocompatibility, better immune evasion, and longer blood circulation time. Typically, erythrocyte membranes are not targeted, but could theoretically increase the chance of passing the BBB by extending the half-life. Current erythrocyte membranes on IS therapy usually contain other targeting modifications at the same time to increase targeting. Xin et al constructed targeted “core–shell” nanoparticles (designated as SHp-RBC-NP), which is consisted of RBC membrane shell with stroke homing peptide (SHp) inserted, and a dextran polymer core modified with ROS-responsive boronic ester. The RBC membrane shell of SHp-RBC-NP reduced reticuloendothelial system clearance and prolong in vivo circulation time through the “don’t eat me” signaling molecule CD47 on the erythrocyte membranes used erythrocyte membrane.51 Through the pharmacokinetic and pharmacodynamic studies in vivo, the SHp-RBC-NP group had a longer circulation time (more than 48 hours) than the control group. At 24 and 48 h, there was still about 17% and 12% of the SHp-RBC-NP remaining in the blood circulation, while the plain nanoparticles were found to be swiftly removed (could not be detected after 24 h). The successful coating of RBC membranes onto the nanoparticles was validated by the stealth capability of the RBC membrane, which is crucial for the smart bioengineered nanoparticles’ main in vivo mimicking properties.
Modifying nanoparticles by combining erythrocyte membranes with peptides can perform multiple functions of crossing the BBB and precisely targeting ischemic regions. Shi’s group fabricated engineered nanoerythrocytes by physical extrusion, which were doubly modified by MG1 peptide and RVG29 peptide for cascade targeting.117 RVG29 peptide (YTIWMPENPRPGTPCDIFTNSRGKRASNGC) was conjugated to distearoyl phosphoethanolamine-selenium-selenium-poly (ethylene glycol) (3400)-maleimide (DSPE-Se-Se-PEG3400-Mal) with a ROS-responsive long arm through maleimide chemistry to enhance BBB targeting, which bond to cerebrovascular endothelial cells and improved delivery efficiency effectively. And the MG1 peptide (CHHSSSARC) was also coupled to DSPE-PEG2000-Mal (with a short arm) for precise M1 microglia recognition. After crossing the BBB, shedding of the outer RVG excited by ROS exposed MG1, which enabled precise recognition of microglia. Consequently, the inflammatory microglia absorbed the engineered nanoerythrocytes, which resulted in the release of exogenous heme. Hemoglobin initiated M2 polarization of microglia by inducing heme oxygenase-1, which in turn promotes the expression of endogenous anti-inflammatory cytokines and the production of two potent antioxidants, carbon monoxide and bilirubin, which significantly alleviated the inflammatory environment and had an excellent therapeutic role in the MCAO model. Promisingly, the engineered nanoerythrocytes provided a powerful tool for the precise delivery by encapsulating therapeutic drugs (Figure 3b).
Cancer Cells
Clinically, some malignant tumors (such as breast cancer cells) have been found to readily cross the BBB to form brain metastases.129,130 After metastasis, syndecan-1 on the membrane of 4T1 tumor cells binds to platelet endothelial cell adhesion molecule-1 on BMECs, platelets, and leukocytes (monocytes and neutrophils) to achieve adhesion. Similarly, it was found that vascular cell adhesion molecule 1 was overexpressed on the cell membranes of 4T1 cancer cells, which showed high affinity for VLA-4 on leukocytes (eg, lymphocytes, monocytes, and eosinophils).131,132 Therefore, Sha’s group developed the biomimetic nanovehicles by camouflaging a succinobucol-loaded pH-sensitive polymeric nanovehicle with a 4T1 cell membrane. After encapsulation of 4T1 cell membrane, the nanovehicles could be preferentially delivered to the ischemic hemisphere, which is 4.79 times higher than that of the normal hemisphere in the MCAO rat model. The 4T1 cell membrane not only made the nanovehicles penetrate the BBB to target inflammation sites in the ischemic parenchyma, but also had a prolonged circulation time through the signaling of CD47, which was highly expressed on the cell surface. Additionally, the nanovehicles were preferably delivered in a biomimetic manner to the stroke-affected sites, and were internalized by a wide variety of brain cells, and responded to the intracellular acidic environment by releasing succinobucol, which had antioxidant and anti-inflammatory effects, thereby attenuating CIRI.61 Furthermore, Wang et al originally developed a bio-derived nanojacket by fusing 4T1 tumor cell membrane with platelet membrane, which further clothes on the surface of paeonol and polymetformin-loaded liposome to obtain biomimetic nanoplatforms (PP@PCL) for ischemic stroke treatment.118 The PP@PCL consisted of hydrophobic therapeutic agent PAE (encapsulated in the phospholipid bilayer of liposome) and hydrophilic and cationic polymetformin, which was fabricated by coextrusion according to well-established protocols. The innovative nanoplatforms exhibited the capability of penetrating the BBB, characteristic of CM, as well as the ischemic lesion-targeting ability inherent to PM, making it an exceptional delivery mechanism for overcoming the BBB to achieve precise drug transport. Significantly, the PP@PCL not only safeguarded damaged neuronal cells from additional harm by scavenging excessive ROS, mitigating mitochondrial dysfunction, and inhibiting neuroinflammation, but also restructured the ischemic microenvironment by polarizing microglial cell phenotypes, promoting angiogenesis, and reducing the secretion of pro-inflammatory cytokines, which remarkably alleviated ischemia-reperfusion injury and performed the neuroprotective influence (Figure 3c).
Neural Stem Cells
Neural stem cells (NSCs) can spontaneously migrate to the vicinity of IS damage and communicate with damaged cells for precise targeting.56,133 Thus, NSCs are regarded as carriers in CNS diseases. Endogenous NSCs actively home to damaged brain regions after IS, and then proliferate and differentiate into damaged cell phenotypes to self-repair and rebuild neural circuits. However, spontaneous recovery based on endogenous NSCs is insufficient for full functional recovery.134,135 Previous studies have found that implanted NSCs secrete trophic factors, such as human insulin-like growth factor 1, vascular endothelial growth factor, epidermal growth factor and basic fibroblast growth factor to reduce the infarcted area.136 Gao et al engineered gene transfection of NSCs overexpressing brain-derived neurotrophic factor (BDNF), and after intravenous injection in MCAO mice, the transfected NSCs (BDNF-NSCs) crossed the BBB and homed to the ischemic region just as efficiently as the original NSCs and produced BDNF more efficiently, leading to a significant increase in BDNF levels, which increased survival rate of the mice to a 60% and enabled faster and better neurological reconstruction.56
Extracellular Vesicles
EVs are a heterogeneous collection of membrane-bound carriers with complex cargoes including proteins, lipids, and nucleic acids.137 As natural intercellular shuttles, EVs have low immunogenicity and biodegradability, ability to encapsulate endogenous bioactive molecules, and capacity to cross the BBB. Previous studies have demonstrated that EVs can break through the intact BBB in vivo through transcytosis.138 Stem cell-derived EVs have emerged as a new therapeutic agent for immunosuppression.139 Gao et al used neural progenitor cells-derived EVs harboring the Arg-Gly-Asp (RGD) peptide (ACDCRGDCFC),57 which is a ligand capable of binding to integrin αvβ3 (integrin αvβ3 is significantly expressed on endothelial cells in ischemic tissue, but not on blood vessels in normal tissue).140 After intravenous injection, neural progenitor cell-derived EVs were found to be capable of targeting ischemic brain lesions and exhibiting intrinsic anti-inflammatory activity.141 Stem cells are activated and recruited to the vicinity of the lesion after IS. Recruited stem cells can interact with damaged BMECs and cross the inflamed BBB through VLA-4, and thus enter the lesion site through the combination of VCAM-1/VLA-4.142 Gao et al conceived stem cell-derived bio-responsive vesicles equipped with the functional ligand VLA-4 and packed with metformin, which could reverse the inflammatory response in BMECs, rapidly restore BBB integrity, and downregulate VCAM-1 expression.68 With the alleviation of inflammation after therapeutic drug release, repair of BBB integrity, and downregulation of VCAM-1 expression, stem cell-derived vesicle migration and action are terminated.143 Altered targeting capacity due to changes in the inflammatory environment confers stem cell bioreactivity, allowing for a smarter and more controllable drug delivery platform.
Strategies for Increased Permeability of the BBB
Selective, controlled and reversible transient increase in BBB permeability is a potential delivery strategy of crossing BBB. Ultrasound and microbubble-mediated opening of the BBB represents a non-invasive and effective technique for targeted drug delivery to specific brain regions. The acoustic bubbles and targeted microbeads were proved to induce reversible Ca2+ signaling and enhance paracellular transport in endothelial monolayer.144 Some clinical trials are currently being conducted to demonstrate that ultrasound and microbubble-mediated techniques can facilitate localized, safe, temporary, and reversible opening of the blood-brain barrier in the human hippocampus (NCT03671889, NCT02253212). Davies et al explored Acoustic Cluster Therapy (ACT), which induced controlled volumetric oscillations of ACT bubbles at a lower frequency (500 kHz), thereby exerting biomechanical forces on capillary walls to enhance the regulation of BBB permeability.145 Laser-induced stimulation of nanoparticles also modulates the BBB permeability.146,147 Qin et al designed picosecond laser excitation of tight junction-targeted gold nanoparticles. Their study demonstrated that the interaction between gold nanoparticles and laser pulses can transiently open the BBB, with the permeability alterations being adjustable through laser intensity and entirely reversible. The findings indicated that targeting endothelial glycoproteins achieved a targeting efficiency over ten times greater than that of targeting tight junctions. Importantly, this strategy for modulating the BBB did not induce hemodynamic changes or cause significant disruption to the structure of the neurovascular unit.148
Strategies for Targeted Controlled Release at Ischemic Region
To improve therapeutic efficacy and minimize complications, drug delivery systems have been designed to not only accumulated in the targeted area, but also trigger the release of the payload at the ischemic lesion site.149 Modified moieties on nanomaterials can respond to the complex microenvironment at the ischemic site, for example to the pH conditions, ROS levels, presence of inflammatory factors.72 Alternatively, hypoxia and thrombin responsiveness subtly employ ischemic site characteristics to implement a spatiotemporally targeted controlled release.
ROS-Responsive Release
High ROS levels in ischemic regions can serve as intelligent and sensitive triggers for controlling drug release and can be applied to the development of site-specific drug delivery systems. Mitochondrial dysfunction in cerebral ischemia leads to oxidative stress and increased production of ROS, a class of oxygen-derived chemicals including hydrogen peroxide (H2O2), superoxide anions (O2∙-), superoxide and hydroxyl radicals.150,151 For increased ROS levels at the IS site, ROS-sensitive groups (aryl-boronate esters/acids, sulphides, thioethers, thioketal linkage, and proline) nanozymes can be engineered to construct ROS-responsive nanomaterials to deliver therapeutic drugs in a spatially and temporally controlled manner.152,153
Among these sites, thioketal linkers sensitive to H2O2 and O2∙- are ideal candidates for the construction of ROS-responsive nanoparticles.154 It has been shown that sulfide groups can be converted to more polar hydrophilic groups (sulfoxide and sulfone) upon exposure to ROS, and often this increase in polarity/solubilization is used as a ROS-responsive release mechanism for encapsulated payloads for targeted drug release.155 Drug-loaded nanopharmaceutical delivery systems prepared by Wang et al ‘s with the thioketal linker crosslink skeleton had been shown to break under the action of microenvironmental ROS, exerting ROS-eliminating activity and achieving the controlled release of loaded Puerarin, leading to oxidative stress-reducing therapeutic effects.46 The nanoparticles were cross linked using the H2O2-sensitive cross linker, and the cumulative release of Puerarin from the system was approximately 48% in the presence of H2O2. In contrast, only approximately 18% of the Puerarin was released in the absence of H2O2. This suggested that the Puerarin release behavior was dependent on H2O2 and that the accelerated release profile occurred in a ROS environment. In addition, Jiang et al69 and Wang et al75 doped diselenide bonds into nanomaterials, which converted the diselenide bonds within the materials to hydrophilic selenic acid under ROS stimulation to achieve synchronous ROS depletion and responsive drug release at the ischemic hemidiaphragm. The intelligent multifunctional delivery system developed by Xin et al, on the other hand, exploited the ability of thioketal functional group to be rapidly cleaved into non-toxic mercaptan and acetone products when exposed to ROS to release drugs in response to pathological ROS environment.70
Furthermore, borate, a smart ROS-responsive biomaterial, can be doped into nanoparticles via self-assembly.156,157 Xin et al designed a ROS microenvironment using overexpressed ROS in IS lesions as a smart drug release switch, with phenylboronic ester modified dextran polymer vesicles carrying the neuroprotectant NR2B9C as the core, covered with a red blood cell membrane coat modified with the cerebral ischemia-homing peptide SHp. Ultraviolet-visible spectra showed that 95% of the nanoparticles were hydrolyzed within 30 min in a medium containing 1 mm H2O2, suggesting that functional ROS triggered the sensitivity of nanoparticles made of phenylboronic ester modified dextran, promoting the rapid release of neuroprotective drugs in response to ROS to exert neuroprotective effects.51 By using ROS-responsive charge-reversal poly[(2-acryloyl) ethyl (p-boronic acid benzyl) diethylammonium bromide] (B-PDEA), Gao et al could effectively condense the BDNF gene into polymeric B-PDEA nanoparticles, which could effectively protect DNA and be taken up by the cells. After internalization, B-PDEA oxidizes intracellular ROS into negatively charged polyacrylic acid, which rapidly releases the BDNF plasmid for efficient transcription and secretion of high levels of BDNF.56 The ROS- responsiveness of B-PDEA not only led to the rapid release of plasmids with high levels of ROS from NSCs in the IS region, but also depleted some ROS, thereby alleviating ROS stress.158
Using cyclodextrin-sourced materials, Zhang et al72 and Wang et al73 constructed a ROS-responsive nanocarrier, which was triggered by pathological signals with a significantly enhanced function owing to a significant reduction in particle size, morphological alterations, and chemical switching of cellular surface uptake by brain endothelial cells. This ROS-responsive and transformable nanoplatform exhibited significantly higher brain drug accumulation in a mouse model of IS than non-responsive nanocarriers.
pH-Responsive Release
Ischemic injury lowers pH by shifting the metabolism of the ischemic brain from aerobic to anaerobic glycolysis, resulting in the accumulation of lactate and protons. Through magnetic resonance spectroscopic imaging, the ischemic region of the brain was found to be acidic with a pH of 6.0 to 6.8. Acidosis persisted for more than 2 days, while the pH of the contralateral normal tissue remained neutral.55 Considering the inherent pH gradient, brain- targeted nanoparticles with pH responsiveness may hold great promise for drug release in specific intracellular cavities. Typically, pH-responsive NPs are designed to deform (by swelling, dissolving, or collapsing) under the pH conditions of intracellular lysosomes, or have unstable linkers capable of cleavage under acidic pH conditions for efficient intracellular drug release.
Peng et al designed the “nano-courier”, which was based on the biocompatibility of poly acrylic acid and abundant binding sites. The ligand molecules were covalently cross linked to the receptor through pH-sensitive amide bonds, resulting in the controlled release of acetylcholine and melatonin triggered by the low pH in the ischemic region.65 Similarly, Sha et al combined an amphiphilic pH-sensitive polymer of methoxypoly (ethylene glycol)-block-poly (2-diisopropyl methacrylate) onto a nano-loaded platform that released almost no drug at pH 7.4 but almost all at pH 4.7, further confirming that the pH-responsive approach was capable of the rapid release of loaded drugs.61
To design pH responsiveness, researchers had also considered the pH environment in which the neuroprotective drugs function. Sheth et al found that betulinic acid (BA) was one of the most potent antioxidants for the treatment of stroke, but that BA was released slowly at physiological pH. Encapsulated drug release is less than 30% in the first 24h, with complete release taking more than 6 days.159 Therefore, given the narrow therapeutic time window of acute IS, BA was chemically modified to betulinic amine through the chemically conversion of the carboxyl group at the edge of the BA ring to an amino end group. This resulted in preferential release in the acidic ischemic brain tissues, with a release of 75% in 24 hours.55
Thrombin-Responsive Release
Thrombin is a trypsin-like serine proteinase that plays a crucial role in the process of thrombosis, in which a large amount of thromboplastin is activated and induces the accumulation of thrombin at the thrombus site.160 Thus, the thrombin-triggered release of rt-PA into the thrombus has great potential with a rapid response rate owing to its high sensitivity. Up-regulated thrombin triggers rt-PA release around the thrombus, converting fibrinogen to fibrinolytic enzymes, and inducing gradual loosening of the thrombus, which dissolves under the high shear forces of blood flow.161 Fan et al devised a novel peptide-templated manganese dioxide nanozyme (PNzyme/MnO2), where thrombolysis is activated by thrombin. The thrombolytic peptide Gly-Arg-Pro- Ala-Lys (GRPAK) is released from the peptide after cleavage by thrombin at the thrombogenic site to initiate thrombolysis.74
Zhou et al suggested that, in addition to the surface affixation of targeted ligands, the delivery of nanoparticles in stroke therapy can be improved by designing nanoparticles that respond to the thrombin in hypoxic microenvironment and subsequently change their size. Variable-size nanoparticles were synthesized using block copolymers with peptides that can be cleaved by enzymes that are enriched in an ischemic microenvironment. This study confirmed that MMP-9 is an alternative protease for protease-triggered drug delivery in the treatment of stroke, and that thrombin and MMP-9 expression in the ischemic brain was significantly elevated 3–8 hours after ischemic injury. A block copolymer was used to synthesize swellable nanoparticles containing either thrombin-cleavable NH2-norleucine-TPRSFL-C-SH or NH2-LGRMGLPGK-C-SH (MMP-9 cleavable peptide), both of which responded within 5 min in the presence of protease. Comparing the drug delivery efficiency of the two, it was found that the nanomaterials containing thrombin- cleavable peptides were 2.2 times more efficient than those containing protease-cleavable peptides.53 Similarly, Jiang et al conjugated polymers via the thrombin cleavable peptide NH2- norleucine TPRSFL-C-SH.162 When used for nanoparticle construction, the resulting nanoparticles responded to thrombin, a protease that is highly enriched in the ischemic microenvironment, by shrinking in size to promote antioxidant release. This study further confirmed that designing nanoparticles to respond to the ischemic microenvironment by shrinking their size significantly enhances brain penetration and improves the efficiency of ligand-mediated targeting, although stimulus responsiveness may not provide higher efficiency than ligand-mediated approaches.54
Hypoxia-Responsive Release
Engineered nanomedicine delivery systems based on the tissue microenvironmental response of lesions to achieve site-specific targeted delivery have received significant attention and represent a promising new strategy for targeted delivery. Therefore, the construction of an efficient targeted delivery system that incorporates the tissue microenvironmental characteristics of IS will provide a new way to enhance the efficiency of neuroprotective drug delivery and improve patient prognosis.
When assessing the microenvironment of infarcted tissue, it was found that a hypoxic environment coexists with the upregulation of cytochrome P450 reductase and inducible nitric oxide synthase enzymes, confirming the feasibility of the hypoxic response in IS.76 The upregulated co-existence of reductases confirmed the feasibility of a hypoxic response in IS. The hypoxic features of IS provided an important breakthrough for further research on therapeutic strategies for IS. Gao et al prepared azocalixarene (CA), -grafted self-assembled peptide hydrogels, which were expected to serve as an intelligent hypoxia-responsive drug library for the on-demand release of anti-inflammatory drugs catalyzed by compliant reductases under hypoxic conditions, while maintaining their stability in the normal physiological state. CA had been utilized as a hypoxia-responsive controlled- release drug carrier. The complexation between CA and the drug was stable under normal conditions and hypoxia-responsive release was achieved by mimicking the reductase. Hypoxia-responsive hydrogels were prepared by phosphate buffer saline-induced β- fold formation following a click reaction between CA containing methacrylate groups and thiol-containing Q11 peptides.
Clinical Application and Challenges of the Targeted Delivery Strategies of Nanosystems
Fewer clinical trials of nanosystems for IS have been conducted in phase ǀ/ǁ clinical trials, mainly focusing on exosomes from mesenchymal stem cells (NCT03384433/NCT06138210/NCT05326724), liposomes (NCT00507806) and metal nanoparticles (NCT06495671). In the small clinical sample size study (NCT02963376), intravenous dodecafluoropentane emulsion was safe with clinically improvements in NIHSS scores and mRS of IS patients (n=24, no statistically differences).163 As an adjunctive therapy of reperfusion treatment, nanoparticle-mediated magnetically enhanced diffusion restored blood flow of blocked vessels by overcoming stagnant blood flow (NCT06495671). The magnetic nanoparticles may reduce the clot lysis time for reperfusion by 40% when compared to free rtPA at the same drug dosage.164 Besides, nanosystems can be used as novel biomarkers to assess prognosis and guide the treatment of IS patients making clinical treatment more personalized and targeted (NCT05370105/ NCT06319742).
However, the clinical trials and translation of nanodrug delivery systems encounter significant challenges. Primarily, the complexity of safety assessments for nanosystems poses a major obstacle, as issues such as long-term toxicity and potential bioaccumulation remain inadequately understood. Additionally, challenges such as low drug loading capacity contribute to the risks associated with clinical applications, thereby confining the research and development of nanomedicines to preclinical studies. Furthermore, technical bottlenecks in the large-scale production of drug delivery nanosystems hinder the achievement of efficient, stable, and cost-effective large-scale preparation, thereby restricting their widespread application.165
To address these challenges, researchers are advancing the development of intelligent responsive nanosystems designed to achieve precise drug release in response to microenvironmental changes within the body, thereby enhancing the performance potential of drug delivery nanosystems. Concurrently, during the construction of multifunctional nanosystems, researchers also focused on how to increase the drug loading capacity by innovative strategies and techniques.166 Three principal methodological frameworks, alongside several innovative technologies, have been developed to achieve high drug-loading in nanoparticles: post-loading, co-loading, and pre-loading strategies. (1) The post-loading strategy typically utilizes carrier materials characterized by a high specific surface area or porous structure (eg, SiO2, carbon, metal-organic frameworks), which are subsequently combined with a drug solution. This allows for the drug to be loaded onto the nanoparticles through various mechanisms, including adsorption, electrostatic interactions, encapsulation, and hydrophobic forces. (2) The co-loading method involves coupling the drug to a polymer or macromolecule, where the drug is covalently attached to the carrier via cleavable bonds (eg, disulfide bonds, ester bonds), thereby enhancing the efficiency of drug loading.167 (3) The pre-loading strategy entails the initial formation of drug nanoparticles, followed by the encapsulation of these nanoparticles with an additional material layer, resulting in nanoparticles with a drug core and a protective shell structure. To achieve synchronous and efficient delivery of both drugs, Chen et al encapsulated a prodrug into a nanocarrier, which enabled the drug loading capacity to reach as high as 99%, nearly achieving quantitative drug loading.168,169
Furthermore, the researchers have advanced novel technologies, such as modular design, microfluidics, 3D printing, and artificial intelligence-driven methodologies, which facilitate the precise construction and large-scale production of delivery nanosystems. By separating the functional modules (performing functions such as targeted, responsive release, imaging, etc). from the drug-loaded modules, the drug loading capacity can be increased by avoiding the functional modules from crowding the drug space through plug-to-direct assembly.170 Wan et al designed an innovative “dual chimeric nanoantibody-lipid-anchoring protein” scheme, which not only increased the preparation efficiency by more than three times, but also reduced the payload loss during preparation.171 Hammond etc. performed the engineering nanolayered particles for modular drug delivery, which allows for high drug loadings in nano-scale thin film coatings, thus enabling the delivery of quantities of drug. 3D printing opens a possibility for compartmentalization of modular drug delivery, which can spatially separate drug and functional excipient modules.172 Zhao et fabricated the microfluidic devices using polyethylene tubings by 3D and the nanoparticles were varied to synthesize by the flow ratio between the polymer solution and water. The microfluidic-assisted synthesis made the drug loading up to 50% and achieved continuous and homogeneous production of nano systems.173 AI techniques can simulate different stages of biological processes and determine the proportions and optimal design parameters of the various components used to build delivery nanosystems. First, by simulating the behavior of nanosystems in complex biological environments (eg blood brain barrier), the penetration and distribution of nanosystems are enhanced to overcome biological barriers. Second, by predicting morphology, potentiation and targeted ligands, and optimizing the density and orientation of targeted ligands on the surface of the nanosystems, the drug loading rate and cellular uptake and transport of nanodrugs are increased. Based on the above-mentioned functions, AI-assisted drug delivery makes complex drug systems easy to build and enables faster, more efficient and more precise nanosystems manufacturing.174 Besides, Santos et al proposed the DELIVER framework containing design, experimental, manufacturing, preclinical, clinical, regulatory and business considerations, which guide researchers to critically examine nanomedicine design and development at an early stage to mitigate risk and research time, as well as to accelerate clinical translation and maximize the impact of nanosystems.175
Future and Prospects
Over the years, researchers have pursed improvements in the targeting and controllability of drugs in stroke therapy. Based on the unique characteristics of nanoparticles including increased drug solubility, reduced adverse drug reactions and enhanced permeability and retention effect, smart drug delivery nanosystems can be constructed by diverse strategies to achieve targeting goal in IS. In this review, we provided a comprehensive summary of the targeting strategies of drug delivery nanosystems in stroke therapy to support further advances in the development of IS treatment. On the one hand, nanosystems can overcome the BBB and precisely target ischemic sites, mainly through receptor-ligand mediated strategies and cell-membrane mediated strategies, which improves drug accumulation at ischemic sites. In an innovative way, the researchers went beyond using ligands with high affinity for the ischemic site to achieve targeted delivery, and built on this by allowing the nanoparticles to locally release BBB modulators, which in turn transiently enhanced BBB permeability, allowing more nanoparticles to enter the ischemic region. Through this secondary autocatalytic mechanism, the delivery process generates a positive feedback loop that promotes further drug accumulation in the targeted region. On the other hand, specific changes in the microenvironment after ischemia have been used to equip nanosystems with targeted-release switches to improve delivery efficiency. Furthermore, nanosystems that combine thrombolysis and neuroprotectants, drug delivery systems that can target thrombolysis and deliver neuroprotectants to the ischemic brain in a sequential order, further compensate for the limitations of current treatments. Probably, combining approaches is needed to achieve a synergistic increase in the functionality of the nanosystems, and overlay the therapeutic efficacies of revascularization and neuroprotection. In the future, further preclinical and clinical studies are urgently warranted to evaluate the efficacy and safety of nanosystems for facilitating the transformation of scientific innovation to the clinical application.
Abbreviations
BBB, blood-brain barrier; BDNF, brain-derived neurotrophic factor; BMECs, brain microvascular endothelial cells; CNS, central nervous system; CIRI, cerebral ischemia-reperfusion injury; CTX, Chlorotoxin; CXCR, C-X-C motif chemokine receptor; DNA, deoxyribo nucleic acid; EVs, extracellular vehicles; H2O2, hydrogen peroxide; ICAM, intercellular adhesion molecule; IS, ischemic stroke; LDLR, low-density lipoprotein receptor; LFA1, lymphocyte function-associated antigen 1; MMP, matrix metalloproteinase; NLRP3, NOD-like receptor thermal protein domain associated protein 3; PLGA, poly (l-lactic-co-glycolic acid); PUFA, polyunsaturated fatty acids; RMT, receptor-mediated transcytosis; ROS, reactive oxygen species; TfR, transferrin receptor; VLA-4, very late antigen 4.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82301464, China), Science and Technology Department of Jilin Province (YDZJ202401656ZYTS, China) and Doctor of excellence program, the First Hospital of Jilin University (JDYY-DEP-2022009, China) to Hong-Yin Ma, Science and Technology Department of Jilin Province (YDZJ202302CXJD061 and 20220303002SF, China) and Jilin Provincial Key Laboratory (YDZJ202302CXJD017, China) to Yi Yang, and the Talent Reserve Program of the First Hospital of Jilin University (JDYYCB-2023002, China) to Zhen-Ni Guo.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97(20 Suppl 2):S6–s16. doi:10.1212/WNL.0000000000012781
2. Wang BN, Wu CB, Chen ZM, et al. DL-3-n-butylphthalide ameliorates diabetes-associated cognitive decline by enhancing PI3K/Akt signaling and suppressing oxidative stress. Acta Pharmacol Sin. 2021;42(3):347–360. doi:10.1038/s41401-020-00583-3
3. Xu J, Wang A, Meng X, et al. Edaravone dexborneol versus edaravone alone for the treatment of acute ischemic stroke: a Phase III, randomized, double-blind, comparative trial. Stroke. 2021;52(3):772–780. doi:10.1161/STROKEAHA.120.031197
4. Chamorro Á, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15(8):869–881. doi:10.1016/S1474-4422(16)00114-9
5. Kaviarasi S, Yuba E, Harada A, Krishnan UM. Emerging paradigms in nanotechnology for imaging and treatment of cerebral ischemia. J Controlled Release. 2019;300:22–45. doi:10.1016/j.jconrel.2019.02.031
6. Correa-Paz C, da Silva-Candal A, Polo E, et al. New approaches in nanomedicine for ischemic stroke. Pharmaceutics. 2021;13(5):757. doi:10.3390/pharmaceutics13050757
7. Jiang C, Zhou Y, Chen R, et al. Nanomaterial-based drug delivery systems for ischemic stroke. Pharmaceutics. 2023;15(12):2669. doi:10.3390/pharmaceutics15122669
8. Zauner A, Daugherty WP, Bullock MR, Warner DS.Brain oxygenation and energy metabolism: part I-biological function and pathophysiology. Neurosurgery. 2002;51(2):289–301.
9. Tuo QZ, Zhang ST, Lei P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med Res Rev. 2022;42(1):259–305. doi:10.1002/med.21817
10. Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi:10.1016/j.neuron.2010.07.002
11. Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annual Rev Neurosci. 1990;13:171–182. doi:10.1146/annurev.ne.13.030190.001131
12. Ankarcrona M, Dypbukt JM, Bonfoco E, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15(4):961–973. doi:10.1016/0896-6273(95)90186-8
13. Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4(5):399–415. doi:10.1038/nrn1106
14. Choe CU, Lewerenz J, Fischer G, et al. Nitroxyl exacerbates ischemic cerebral injury and oxidative neurotoxicity. J Neurochem. 2009;110(6):1766–1773. doi:10.1111/j.1471-4159.2009.06266.x
15. Adibhatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2010;12(1):125–169. doi:10.1089/ars.2009.2668
16. Moro MA, Almeida A, Bolaños JP, Lizasoain I. Mitochondrial respiratory chain and free radical generation in stroke. Free Radic Biol Med. 2005;39(10):1291–1304. doi:10.1016/j.freeradbiomed.2005.07.010
17. Datta A, Sarmah D, Mounica L, et al. Cell death pathways in ischemic stroke and targeted pharmacotherapy. Transl Stroke Res. 2020;11(6):1185–1202. doi:10.1007/s12975-020-00806-z
18. Knowland D, Arac A, Sekiguchi KJ, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82(3):603–617. doi:10.1016/j.neuron.2014.03.003
19. Otxoa-de-Amezaga A, Gallizioli M, Pedragosa J, et al. Location of neutrophils in different compartments of the damaged mouse brain after severe ischemia/reperfusion. Stroke. 2019;50(6):1548–1557. doi:10.1161/STROKEAHA.118.023837
20. Kivisäkk P, Mahad DJ, Callahan MK, et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci USA. 2003;100(14):8389–8394. doi:10.1073/pnas.1433000100
21. Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14(9):1142–1149. doi:10.1038/nn.2887
22. Qin C, Zhou LQ, Ma XT, et al. Dual functions of microglia in ischemic stroke. Neurosci Bull. 2019;35(5):921–933. doi:10.1007/s12264-019-00388-3
23. Yenari MA, Kauppinen TM, Swanson RA. Microglial activation in stroke: therapeutic targets. Neurotherapeutics. 2010;7(4):378–391. doi:10.1016/j.nurt.2010.07.005
24. Zeng J, Bao T, Yang K, et al. The mechanism of microglia-mediated immune inflammation in ischemic stroke and the role of natural botanical components in regulating microglia: a review. Front Immunol. 2022;13:1047550. doi:10.3389/fimmu.2022.1047550
25. Qin C, Fan WH, Liu Q, et al. Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke. 2017;48(12):3336–3346. doi:10.1161/STROKEAHA.117.018505
26. Chu X, Cao L, Yu Z, et al. Hydrogen-rich saline promotes microglia M2 polarization and complement-mediated synapse loss to restore behavioral deficits following hypoxia-ischemic in neonatal mice via AMPK activation. J Neuroinflamm. 2019;16(1):104. doi:10.1186/s12974-019-1488-2
27. Uzdensky AB. Apoptosis regulation in the penumbra after ischemic stroke: expression of pro- and antiapoptotic proteins. Apoptosis. 2019;24(9–10):687–702. doi:10.1007/s10495-019-01556-6
28. Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–185. doi:10.1124/pr.57.2.4
29. Candelario-Jalil E, Dijkhuizen RM, Neuroinflammation MT. Stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke. 2022;53(5):1473–1486. doi:10.1161/STROKEAHA.122.036946
30. Al-Ahmady ZS, Jasim D, Ahmad SS, et al. Selective liposomal transport through blood brain barrier disruption in ischemic stroke reveals two distinct therapeutic opportunities. ACS Nano. 2019;13(11):12470–12486. doi:10.1021/acsnano.9b01808
31. Yang C, Hawkins KE, Doré S, Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol. 2019;316(2):C135–c153. doi:10.1152/ajpcell.00136.2018
32. Wang L, Zhang X, Xiong X, et al. Nrf2 regulates oxidative stress and its role in cerebral ischemic stroke. Antioxidants. 2022;11(12):2377.
33. Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39(1):51–70.
34. Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42(11):3323–3328. doi:10.1161/STROKEAHA.110.608257
35. Xiong Y, Li S, Wang C, et al. Chinese stroke association guidelines on reperfusion therapy for acute ischaemic stroke 2024. Stroke Vasc Neurol;2025. svn–2024–003977. doi:10.1136/svn-2024-003977
36. Nie X, Leng X, Miao Z, Fisher M, Liu L. Clinically ineffective reperfusion after endovascular therapy in acute ischemic stroke. Stroke. 2023;54(3):873–881. doi:10.1161/STROKEAHA.122.038466
37. Murray V, Norrving B, Sandercock PA, Terént A, Wardlaw JM, Wester P. The molecular basis of thrombolysis and its clinical application in stroke. J Internal Med. 2010;267(2):191–208. doi:10.1111/j.1365-2796.2009.02205.x
38. Colucci M, Paramo JA, Collen D. Inhibition of one-chain and two-chain forms of human tissue-type plasminogen activator by the fast-acting inhibitor of plasminogen activator in vitro and in vivo. J Lab Clin Med. 1986;108(1):53–59.
39. Yaghi S, Willey JZ, Cucchiara B, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2017;48(12):e343–e361. doi:10.1161/STR.0000000000000152
40. Heo JH, Lee KY, Kim SH, Kim DI. Immediate reocclusion following a successful thrombolysis in acute stroke: a pilot study. Neurology. 2003;60(10):1684–1687. doi:10.1212/01.WNL.0000063323.23493.98
41. Haupt M, Gerner ST, Bähr M, Doeppner TR. Neuroprotective strategies for ischemic stroke-future perspectives. Int J Mol Sci. 2023;24(5):4334. doi:10.3390/ijms24054334
42. Li C, Sun T, Jiang C. Recent advances in nanomedicines for the treatment of ischemic stroke. Acta pharmaceutica Sinica B. 2021;11(7):1767–1788. doi:10.1016/j.apsb.2020.11.019
43. Haupt M, Gerner ST, Bähr M, Doeppner TR. Quest for quality in translational stroke research-A new dawn for neuroprotection? Int J Mol Sci. 2022;23(10):5381. doi:10.3390/ijms23105381
44. Marto JP, Strambo D, Livio F, Michel P. Drugs associated with ischemic stroke: a review for clinicians. Stroke. 2021;52(10):e646–e659. doi:10.1161/STROKEAHA.120.033272
45. Zhou X, Deng X, Liu M, et al. Intranasal delivery of BDNF-loaded small extracellular vesicles for cerebral ischemia therapy. J Contr Rel. 2023;357:1–19. doi:10.1016/j.jconrel.2023.03.033
46. Zhang Y, Zhang H, Zhao F, et al. Mitochondrial-targeted and ROS-responsive nanocarrier via nose-to-brain pathway for ischemic stroke treatment. Acta pharmaceutica Sinica B. 2023;13(12):5107–5120. doi:10.1016/j.apsb.2023.06.011
47. Bseiso EA, Sheta NM, Abdel-Haleem KM. Recent progress in nanoparticulate-based intranasal delivery for treating of different central nervous system diseases. Pharmaceut Dev Technol. 2024;29(9):913–929. doi:10.1080/10837450.2024.2409807
48. Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Delivery Rev. 2012;64(7):614–628. doi:10.1016/j.addr.2011.11.002
49. Safarov R, Fedotova O, Uvarova A, Gordienko M, Menshutina N. Review of intranasal active pharmaceutical ingredient delivery systems. Pharmaceuticals. 2024;17(9):1180. doi:10.3390/ph17091180
50. Han L, Cai Q, Tian D, et al. Targeted drug delivery to ischemic stroke via chlorotoxin-anchored, lexiscan-loaded nanoparticles. Nanomedicine. 2016;12(7):1833–1842. doi:10.1016/j.nano.2016.03.005
51. Lv W, Xu J, Wang X, Li X, Xu Q, Xin H. Bioengineered boronic ester modified dextran polymer nanoparticles as reactive oxygen species responsive nanocarrier for ischemic stroke treatment. ACS nano. 2018;12(6):5417–5426. doi:10.1021/acsnano.8b00477
52. Bao Q, Hu P, Xu Y, et al. Simultaneous blood-brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS nano. 2018;12(7):6794–6805. doi:10.1021/acsnano.8b01994
53. Guo X, Deng G, Liu J, et al. Thrombin-responsive, brain-targeting nanoparticles for improved stroke therapy. ACS nano. 2018;12(8):8723–8732. doi:10.1021/acsnano.8b04787
54. Wu H, Peng B, Mohammed FS, et al. Brain targeting, antioxidant polymeric nanoparticles for stroke drug delivery and therapy. Small. 2022;18(22):e2107126. doi:10.1002/smll.202107126
55. Zhang S, Peng B, Chen Z, et al. Brain-targeting, acid-responsive antioxidant nanoparticles for stroke treatment and drug delivery. Bioact Mater. 2022;16:57–65. doi:10.1016/j.bioactmat.2022.02.033
56. Jiang XC, Xiang JJ, Wu HH, et al. Neural stem cells transfected with reactive oxygen species-responsive polyplexes for effective treatment of ischemic stroke. Adv Mater. 2019;31(10):e1807591. doi:10.1002/adma.201807591
57. Deng L, Zhang F, Wu Y, et al. RGD-modified nanocarrier-mediated targeted delivery of HIF-1α-AA plasmid DNA to cerebrovascular endothelial cells for ischemic stroke treatment. ACS Biomater Sci Eng. 2019;5(11):6254–6264. doi:10.1021/acsbiomaterials.9b01362
58. Dong X, Gao J, Zhang CY, Hayworth C, Frank M, Wang Z. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano. 2019;13(2):1272–1283. doi:10.1021/acsnano.8b06572
59. Feng L, Dou C, Xia Y, et al. Neutrophil-like cell-membrane-coated nanozyme therapy for ischemic brain damage and long-term neurological functional recovery. ACS Nano. 2021;15(2):2263–2280. doi:10.1021/acsnano.0c07973
60. Tang Z, Meng S, Song Z, et al. Neutrophil membrane fusogenic nanoliposomal leonurine for targeted ischemic stroke therapy via remodeling cerebral niche and restoring blood-brain barrier integrity. Mater Today Bio. 2023;20:100674. doi:10.1016/j.mtbio.2023.100674
61. He W, Mei Q, Li J, et al. Preferential targeting cerebral ischemic lesions with cancer cell-inspired nanovehicle for ischemic stroke treatment. Nano Lett. 2021;21(7):3033–3043. doi:10.1021/acs.nanolett.1c00231
62. Li C, Zhao Z, Luo Y, et al. Macrophage-disguised manganese dioxide nanoparticles for neuroprotection by reducing oxidative stress and modulating inflammatory microenvironment in acute ischemic stroke. Adv Sci. 2021;8(20):e2101526. doi:10.1002/advs.202101526
63. Lu H, Li S, Dai D, et al. Enhanced treatment of cerebral ischemia-Reperfusion injury by intelligent nanocarriers through the regulation of neurovascular units. Acta Biomater. 2022;147:314–326. doi:10.1016/j.actbio.2022.05.021
64. Cen J, Zhang R, Zhao T, et al. A water-soluble quercetin conjugate with triple targeting exerts neuron-protective effect on cerebral ischemia by mitophagy activation. Adv Healthcare Mater. 2022;11(22):e2200817. doi:10.1002/adhm.202200817
65. Xiao X, Gao Y, Liu S, et al. A “nano-courier” for precise delivery of acetylcholine and melatonin by C5a-targeted aptamers effectively attenuates reperfusion injury of ischemic stroke. Adv Funct Mat. 2023;33(23):2213633.
66. Pan J, Wang Z, Huang X, et al. Bacteria-derived outer-membrane vesicles hitchhike neutrophils to enhance ischemic stroke therapy. Adv Mater. 2023;35(38):e2301779.
67. Mu Q, Yao K, Syeda MZ, et al. Ligustrazine nanoparticle hitchhiking on neutrophils for enhanced therapy of cerebral ischemia-reperfusion injury. Adv Sci. 2023;10(19):e2301348. doi:10.1002/advs.202301348
68. Wu H, Jiang X, Li Y, et al. Hybrid stem cell-derived bioresponsive vesicles for effective inflamed blood-brain barrier targeting delivery. Nano Today. 2023;49:101800. doi:10.1016/j.nantod.2023.101800
69. Li C, Wu Y, Chen Q, et al. Pleiotropic microenvironment remodeling micelles for cerebral ischemia-reperfusion injury therapy by inhibiting neuronal ferroptosis and glial overactivation. ACS Nano. 2023;17(18):18164–18177. doi:10.1021/acsnano.3c05038
70. Sun S, Lv W, Li S, et al. Smart liposomal nanocarrier enhanced the treatment of ischemic stroke through neutrophil extracellular traps and cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes (cGAS-STING) pathway inhibition of ischemic penumbra. ACS nano. 2023;17(18):17845–17857. doi:10.1021/acsnano.3c03390
71. He W, Zhang X-Y, Gong X, et al. Drug-free biomimetic oxygen supply nanovehicle promotes ischemia-reperfusion therapy in stroke. Adv Funct Mat. 2023;33(21):2212919.
72. Yang Q, Pu W, Hu K, et al. Reactive oxygen species-responsive transformable and triple-targeting butylphthalide nanotherapy for precision treatment of ischemic stroke by normalizing the pathological microenvironment. ACS Nano. 2023;17(5):4813–4833. doi:10.1021/acsnano.2c11363
73. Dong Z, Tang L, Zhang Y, et al. A homing peptide modified neutrophil membrane biomimetic nanoparticles in response to ROS/inflammatory microenvironment for precise targeting treatment of ischemic stroke. Adv Funct Mat. 2024;34(4):2309167.
74. Wang Z, Zhao Y, Hou Y, et al. A thrombin-activated peptide-templated nanozyme for remedying ischemic stroke via thrombolytic and neuroprotective actions. Adv Mater. 2024;36(10):e2210144. doi:10.1002/adma.202210144
75. Kong J, Zou R, Chu R, et al. An ultrasmall Cu/Cu(2)O nanoparticle-based diselenide-bridged nanoplatform mediating reactive oxygen species scavenging and neuronal membrane enhancement for targeted therapy of ischemic stroke. ACS Nano. 2024;18(5):4140–4158. doi:10.1021/acsnano.3c08734
76. Zheng W, Yao S-Y, Hu H, et al. Hypoxia-responsive calixarene-grafted self-assembled peptide hydrogel for inflammation suppression in ischemic stroke. Nano Today. 2024;54:102064. doi:10.1016/j.nantod.2023.102064
77. Liao J, Li Y, Fan L, et al. Bioactive ceria nanoenzymes target mitochondria in reperfusion injury to treat ischemic stroke. ACS Nano. 2024;18:5510–29.
78. Zhao Y, Li Q, Niu J, et al. Neutrophil membrane-camouflaged polyprodrug nanomedicine for inflammation suppression in ischemic stroke therapy. Adv Mater. 2024;36(21):e2311803. doi:10.1002/adma.202311803
79. Ma J, Zhan M, Sun H, et al. Phosphorus dendrimers co-deliver fibronectin and edaravone for combined ischemic stroke treatment via cooperative modulation of microglia/neurons and vascular regeneration. Adv Healthcare Mater. 2024;13(29):e2401462. doi:10.1002/adhm.202401462
80. Bajracharya R, Caruso AC, Vella LJ, Nisbet RM. Current and emerging strategies for enhancing antibody delivery to the brain. Pharmaceutics. 2021;13(12):2014. doi:10.3390/pharmaceutics13122014
81. Omori N, Maruyama K, Jin G, et al. Targeting of post-ischemic cerebral endothelium in rat by liposomes bearing polyethylene glycol-coupled transferrin. Neurol Res. 2003;25(3):275–279. doi:10.1179/016164103101201508
82. Prakash R, Vyawahare A, Sakla R, et al. NLRP3 inflammasome-targeting nanomicelles for preventing ischemia-reperfusion-induced inflammatory injury. ACS Nano. 2023;17(9):8680–8693. doi:10.1021/acsnano.3c01760
83. Zhao Y, Jiang Y, Lv W, et al. Dual targeted nanocarrier for brain ischemic stroke treatment. J Controlled Release. 2016;233:64–71. doi:10.1016/j.jconrel.2016.04.038
84. Zhao Q, Du W, Zhou L, et al. Transferrin-enabled blood-brain barrier crossing manganese-based nanozyme for rebalancing the reactive oxygen species level in ischemic stroke. Pharmaceutics. 2022;14(6):1122. doi:10.3390/pharmaceutics14061122
85. Feczkó T, Piiper A, Ansar S, et al. Stimulating brain recovery after stroke using theranostic albumin nanocarriers loaded with nerve growth factor in combination therapy. J Controlled Release. 2019;293:63–72. doi:10.1016/j.jconrel.2018.11.017
86. Zhang M, Zuo M, Wang C, et al. Monitoring neuroinflammation with an HOCl-activatable and blood-brain barrier permeable upconversion nanoprobe. Anal Chem. 2020;92(7):5569–5576. doi:10.1021/acs.analchem.0c00526
87. Lu Y, Han S, Zheng H, et al. A novel RGDyC/PEG co-modified PAMAM dendrimer-loaded arsenic trioxide of glioma targeting delivery system. Int J Nanomed. 2018;13:5937–5952. doi:10.2147/IJN.S175418
88. Huang H, Huang Q, Wang F, Milner R, Li L. Cerebral ischemia-induced angiogenesis is dependent on tumor necrosis factor receptor 1-mediated upregulation of α5β1 and αVβ3 integrins. J Neuroinflamm. 2016;13(1):227. doi:10.1186/s12974-016-0697-1
89. Zhang X, Yao S, Liu C, Jiang Y. Tumor tropic delivery of doxorubicin-polymer conjugates using mesenchymal stem cells for glioma therapy. Biomaterials. 2015;39:269–281. doi:10.1016/j.biomaterials.2014.11.003
90. Qin J, Chen D, Hu H, Cui Q, Qiao M, Chen B. Surface modification of RGD-liposomes for selective drug delivery to monocytes/neutrophils in brain. Chem Pharm Bull. 2007;55(8):1192–1197. doi:10.1248/cpb.55.1192
91. Hong HY, Choi JS, Kim YJ, et al. Detection of apoptosis in a rat model of focal cerebral ischemia using a homing peptide selected from in vivo phage display. J Controlled Release. 2008;131(3):167–172. doi:10.1016/j.jconrel.2008.07.020
92. Yamada Y, Satrialdi, Hibino M, Sasaki D, Abe J, Harashima H. Power of mitochondrial drug delivery systems to produce innovative nanomedicines. Adv Drug Delivery Rev. 2020;154-155:187–209. doi:10.1016/j.addr.2020.09.010
93. Chang DI, Hosomi N, Lucero J, et al. Activation systems for latent matrix metalloproteinase-2 are upregulated immediately after focal cerebral ischemia. J Cerebral Blood Flow Metab. 2003;23(12):1408–1419. doi:10.1097/01.WCB.0000091765.61714.30
94. Carman AJ, Mills JH, Krenz A, Kim DG, Bynoe MS. Adenosine receptor signaling modulates permeability of the blood-brain barrier. J Neurosci. 2011;31(37):13272–13280. doi:10.1523/JNEUROSCI.3337-11.2011
95. Gao X, Qian J, Zheng S, et al. Overcoming the blood-brain barrier for delivering drugs into the brain by using adenosine receptor nanoagonist. ACS Nano. 2014;8(4):3678–3689. doi:10.1021/nn5003375
96. Mayurasakorn K, Williams JJ, Ten VS, Deckelbaum RJ. Docosahexaenoic acid: brain accretion and roles in neuroprotection after brain hypoxia and ischemia. Curr Opin Clin Nutr Metab Care. 2011;14(2):158–167. doi:10.1097/MCO.0b013e328342cba5
97. Adkins Y, Soulika AM, Mackey B, Kelley DS. Docosahexaenoic acid (22:6n-3) ameliorated the onset and severity of experimental autoimmune encephalomyelitis in mice. Lipids. 2019;54(1):13–23. doi:10.1002/lipd.12130
98. Oppedisano F, Maiuolo J, Gliozzi M, et al. The potential for natural antioxidant supplementation in the early stages of neurodegenerative disorders. Int J Mol Sci. 2020;21(7):2618. doi:10.3390/ijms21072618
99. Li Y, Zhang M, Li S, et al. Selective ischemic-hemisphere targeting Ginkgolide B liposomes with improved solubility and therapeutic efficacy for cerebral ischemia-reperfusion injury. Asian J Pharm Sci. 2023;18(2):100783. doi:10.1016/j.ajps.2023.100783
100. Zhang SR, Phan TG, Sobey CG. Targeting the immune system for ischemic stroke. Trends Pharmacol Sci. 2021;42(2):96–105. doi:10.1016/j.tips.2020.11.010
101. Ma Y, Liu Y, Zhang Z, Yang GY. Significance of complement system in ischemic stroke: a comprehensive review. Aging Dis. 2019;10(2):429–462. doi:10.14336/AD.2019.0119
102. Wang Y, Xie Y, Oupický D. Potential of CXCR4/CXCL12 chemokine axis in cancer drug delivery. Current Pharmacol Rep. 2016;2(1):1–10. doi:10.1007/s40495-015-0044-8
103. Cashen AF, Nervi B, DiPersio J. AMD3100: CXCR4 antagonist and rapid stem cell-mobilizing agent. Future Oncol. 2007;3(1):19–27. doi:10.2217/14796694.3.1.19
104. Hao C, Sha M, Ye Y, Wang C. Cell membrane-derived nanovehicles for targeted therapy of ischemic stroke: from construction to application. Pharmaceutics. 2023;16(1):6. doi:10.3390/pharmaceutics16010006
105. Liao J, Gong L, Xu Q, et al. Revolutionizing neurocare: biomimetic nanodelivery via cell membranes. Adv Mater. 2024;36(26):e2402445. doi:10.1002/adma.202402445
106. Liao J, Fan L, Li Y, et al. Recent advances in biomimetic nanodelivery systems: new brain-targeting strategies. J Controlled Release. 2023;358:439–464. doi:10.1016/j.jconrel.2023.05.009
107. Adrover JM, Del Fresno C, Crainiciuc G, et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity. 2019;50(2):390–402.e310. doi:10.1016/j.immuni.2019.01.002
108. Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The neutrophil. Immunity. 2021;54(7):1377–1391. doi:10.1016/j.immuni.2021.06.006
109. Enzmann GU, Pavlidou S, Vaas M, Klohs J, Engelhardt B. ICAM-1(null) C57BL/6 mice are not protected from experimental ischemic stroke. Transl Stroke Res. 2018;9(6):608–621. doi:10.1007/s12975-018-0612-4
110. Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cerebral Blood Flow Metab. 2015;35(6):888–901. doi:10.1038/jcbfm.2015.45
111. Parodi A, Quattrocchi N, van de Ven AL, et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8(1):61–68. doi:10.1038/nnano.2012.212
112. Georgieva JV, Hoekstra D, Zuhorn IS. Smuggling drugs into the brain: an overview of ligands targeting transcytosis for drug delivery across the blood-brain barrier. Pharmaceutics. 2014;6(4):557–583. doi:10.3390/pharmaceutics6040557
113. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi:10.1038/nri3399
114. Weathington NM, van Houwelingen AH, Noerager BD, et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nature Med. 2006;12(3):317–323. doi:10.1038/nm1361
115. Locke LW, Chordia MD, Zhang Y, et al. A novel neutrophil-specific PET imaging agent: cFLFLFK-PEG-64Cu. J Nuclear Med. 2009;50(5):790–797. doi:10.2967/jnumed.108.056127
116. Zhang C, Ling CL, Pang L, et al. Direct macromolecular drug delivery to cerebral ischemia area using neutrophil-mediated nanoparticles. Theranostics. 2017;7(13):3260–3275.
117. Yin N, Zhao Y, Liu C, et al. Engineered nanoerythrocytes alleviate central nervous system inflammation by regulating the polarization of inflammatory microglia. Adv Mater. 2022;34(27):e2201322. doi:10.1002/adma.202201322
118. Tang L, Yin Y, Liu H, et al. Blood-brain barrier-penetrating and lesion-targeting nanoplatforms inspired by the pathophysiological features for synergistic ischemic stroke therapy. Adv Mater. 2024;36(21):e2312897. doi:10.1002/adma.202312897
119. Song Z, Fang J, Wang Z, Xiao R, Guo X, Zhou S. Rod-shaped polymeric nanoparticles intervene neutrophils for efficient ischemic stroke therapy. Adv Funct Mat. 2023;33(19):2212326.
120. Gelderblom M, Leypoldt F, Steinbach K, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–1857. doi:10.1161/STROKEAHA.108.534503
121. Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuromol Med. 2010;12(2):193–204.
122. Arumugam TV, Salter JW, Chidlow JH, Ballantyne CM, Kevil CG, Granger DN. Contributions of LFA-1 and Mac-1 to brain injury and microvascular dysfunction induced by transient middle cerebral artery occlusion. Am J Physiol Heart Circulatory Physiol. 2004;287(6):H2555–2560. doi:10.1152/ajpheart.00588.2004
123. Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66(3):232–245. doi:10.1016/j.surneu.2005.12.028
124. Enam SF, Kader SR, Bodkin N, et al. Evaluation of M2-like macrophage enrichment after diffuse traumatic brain injury through transient interleukin-4 expression from engineered mesenchymal stromal cells. J Neuroinfl. 2020;17(1):197. doi:10.1186/s12974-020-01860-y
125. Liao J, He W, Zhang S, et al. Magnetic field driven ceria nanosystems for mitochondria targeted therapy of ischemic stroke. Adv Funct Mater;2025. 2423291. doi:10.1002/adfm.202423291
126. Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci USA. 2011;108(27):10980–10985. doi:10.1073/pnas.1106634108
127. Hu CM, Fang RH, Zhang L. Erythrocyte-inspired delivery systems. Adv Healthcare Mater. 2012;1(5):537–547. doi:10.1002/adhm.201200138
128. Danielyan K, Ganguly K, Ding BS, et al. Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation. 2008;118(14):1442–1449. doi:10.1161/CIRCULATIONAHA.107.750257
129. Wang C, Wu B, Wu Y, Song X, Zhang S, Liu Z. Camouflaging nanoparticles with brain metastatic tumor cell membranes: a new strategy to traverse blood–brain barrier for imaging and therapy of brain tumors. Adv Funct Mat. 2020;30(14):1909369.
130. Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–5678. doi:10.1158/1078-0432.CCR-10-1564
131. Vainio SK, Dickens AM, Tuisku J, et al. Cessation of anti-VLA-4 therapy in a focal rat model of multiple sclerosis causes an increase in neuroinflammation. EJNMMI Res. 2019;9(1):38. doi:10.1186/s13550-019-0508-7
132. Calabresi PA, Pelfrey CM, Tranquill LR, Maloni H, McFarland HF. VLA-4 expression on peripheral blood lymphocytes is downregulated after treatment of multiple sclerosis with interferon beta. Neurology. 1997;49(4):1111–1116. doi:10.1212/WNL.49.4.1111
133. Zhang T, Li F, Xu Q, et al. Ferrimagnetic nanochains-based mesenchymal stem cell engineering for highly efficient post-stroke recovery. Adv Funct Mat. 2019;29(24):1900603.
134. Faiz M, Sachewsky N, Gascón S, Bang KW, Morshead CM, Nagy A. Adult neural stem cells from the subventricular zone give rise to reactive astrocytes in the cortex after stroke. Cell Stem Cell. 2015;17(5):624–634. doi:10.1016/j.stem.2015.08.002
135. Ziaee SM, Tabeshmehr P, Haider KH, et al. Optimization of time for neural stem cells transplantation for brain stroke in rats. Stem Cell Investig. 2017;4:29. doi:10.21037/sci.2017.03.10
136. Wakabayashi K, Nagai A, Sheikh AM, et al. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. 2010;88(5):1017–1025. doi:10.1002/jnr.22279
137. Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–188. doi:10.1016/j.tcb.2016.11.003
138. Morad G, Carman CV, Hagedorn EJ, et al. Tumor-derived extracellular vesicles breach the intact blood-brain barrier via transcytosis. ACS Nano. 2019;13(12):13853–13865. doi:10.1021/acsnano.9b04397
139. Bian B, Zhao C, He X, et al. Exosomes derived from neural progenitor cells preserve photoreceptors during retinal degeneration by inactivating microglia. J Extracell Vesicles. 2020;9(1):1748931. doi:10.1080/20013078.2020.1748931
140. Arosio D, Casagrande C, Manzoni L. Integrin-mediated drug delivery in cancer and cardiovascular diseases with peptide-functionalized nanoparticles. Curr Med Chem. 2012;19(19):3128–3151. doi:10.2174/092986712800784748
141. Tian T, Cao L, He C, et al. Targeted delivery of neural progenitor cell-derived extracellular vesicles for anti-inflammation after cerebral ischemia. Theranostics. 2021;11(13):6507–6521. doi:10.7150/thno.56367
142. Rosenblum S, Wang N, Smith TN, et al. Timing of intra-arterial neural stem cell transplantation after hypoxia-ischemia influences cell engraftment, survival, and differentiation. Stroke. 2012;43(6):1624–1631. doi:10.1161/STROKEAHA.111.637884
143. Nitzsche F, Müller C, Lukomska B, Jolkkonen J, Deten A, Boltze J. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cells. 2017;35(6):1446–1460. doi:10.1002/stem.2614
144. Lin J, Qiao C, Jiang H, et al. Reversible Ca(2+) signaling and enhanced paracellular transport in endothelial monolayer induced by acoustic bubbles and targeted microbeads. Ultrason Sonochem. 2025;112:107181. doi:10.1016/j.ultsonch.2024.107181
145. Olsman M, Mühlenpfordt M, Olsen EB, et al. Acoustic cluster therapy (ACT®) enhances accumulation of polymeric micelles in the murine brain. J Controlled Release. 2021;337:285–295. doi:10.1016/j.jconrel.2021.07.019
146. Perolina E, Meissner S, Raos B, Harland B, Thakur S, Svirskis D. Translating ultrasound-mediated drug delivery technologies for CNS applications. Adv Drug Delivery Rev. 2024;208:115274. doi:10.1016/j.addr.2024.115274
147. Du ZW, Li YS, Jiang XC, Gao JQ. Nanoparticles designed based on the blood-brain barrier for the treatment of cerebral ischemia-reperfusion injury. Small. 2025;e2410404. doi:10.1002/smll.202410404
148. Li X, Cai Q, Wilson BA, et al. Mechanobiological modulation of blood-brain barrier permeability by laser stimulation of endothelial-targeted nanoparticles. Nanoscale. 2023;15(7):3387–3397. doi:10.1039/D2NR05062E
149. Gunawan ST, Kempe K, Bonnard T, et al. Multifunctional thrombin-activatable polymer capsules for specific targeting to activated platelets. Adv Mater. 2015;27(35):5153–5157. doi:10.1002/adma.201502243
150. Tao W, He Z. ROS-responsive drug delivery systems for biomedical applications. Asian J Pharm Sci. 2018;13(2):101–112. doi:10.1016/j.ajps.2017.11.002
151. Liang J, Liu B. ROS-responsive drug delivery systems. Bioeng Transl Med. 2016;1(3):239–251. doi:10.1002/btm2.10014
152. Huang G, Zang J, He L, et al. Bioactive nanoenzyme reverses oxidative damage and endoplasmic reticulum stress in neurons under ischemic stroke. ACS Nano. 2022;16(1):431–452. doi:10.1021/acsnano.1c07205
153. Tapeinos C, Physical PA. Chemical, and biological structures based on ros-sensitive moieties that are able to respond to oxidative microenvironments. Adv Mater. 2016;28(27):5553–5585. doi:10.1002/adma.201505376
154. Rinaldi A, Caraffi R, Grazioli MV, et al. Applications of the ROS-responsive thioketal linker for the production of smart nanomedicines. Polymers. 2022;14(4):687. doi:10.3390/polym14040687
155. Rajkovic O, Gourmel C, d’Arcy R, et al. Reactive oxygen species-responsive nanoparticles for the treatment of ischemic stroke. Adv Therapeut. 2019;2(7):1900038.
156. Broaders KE, Grandhe S, Fréchet JM. A biocompatible oxidation-triggered carrier polymer with potential in therapeutics. J Am Chem Soc. 2011;133(4):756–758. doi:10.1021/ja110468v
157. Lippert AR, Van de Bittner GC, Chang CJ. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc Chem Res. 2011;44(9):793–804. doi:10.1021/ar200126t
158. Liu X, Xiang J, Zhu D, et al. Gene delivery: fusogenic reactive oxygen species triggered charge-reversal vector for effective gene delivery. AdvMater. 2016;28(9):1714.
159. Deng G, Ma C, Zhao H, et al. Anti-edema and antioxidant combination therapy for ischemic stroke via glyburide-loaded betulinic acid nanoparticles. Theranostics. 2019;9(23):6991–7002. doi:10.7150/thno.35791
160. Furie B, Furie BC. Mechanisms of thrombus formation. New Engl J Med. 2008;359(9):938–949. doi:10.1056/NEJMra0801082
161. Nesbitt WS, Westein E, Tovar-Lopez FJ, et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15(6):665–673. doi:10.1038/nm.1955
162. Whitney M, Savariar EN, Friedman B, et al. Ratiometric activatable cell-penetrating peptides provide rapid in vivo readout of thrombin activation. Angew Chem. 2013;52(1):325–330. doi:10.1002/anie.201205721
163. Culp WC, Onteddu SS, Brown A, et al. Dodecafluoropentane emulsion in acute ischemic stroke: a phase ib/ii randomized and controlled dose-escalation trial. J Vasc Intervent Radiol. 2019;30(8):1244–1250.e1241. doi:10.1016/j.jvir.2019.04.020
164. Chen HA, Ma YH, Hsu TY, Chen JP. Preparation of peptide and recombinant tissue plasminogen activator conjugated poly(Lactic-Co-Glycolic Acid) (PLGA) magnetic nanoparticles for dual targeted thrombolytic therapy. Int J Mol Sci. 2020;21(8). doi:10.3390/ijms21082690
165. Greish K, Mathur A, Bakhiet M, Taurin S. Nanomedicine: is it lost in translation? Therap Delivery. 2018;9(4):269–285. doi:10.4155/tde-2017-0118
166. Peng B, Mohammed FS, Tang X, Liu J, Sheth KN, Zhou J. Nanotechnology approaches to drug delivery for the treatment of ischemic stroke. Bioact Mater. 2025;43:145–161. doi:10.1016/j.bioactmat.2024.09.016
167. Liu Y, Yang G, Jin S, Xu L, Zhao CX. Development of high-drug-loading nanoparticles. ChemPlusChem. 2020;85(9):2143–2157. doi:10.1002/cplu.202000496
168. Zhou H, Yang Z, Jin G, et al. Prodrug-designed nanocarrier co-delivering chemotherapeutic and vascular disrupting agents with exceptionally high drug loading capacity. J Contr Rel. 2025;382:113628. doi:10.1016/j.jconrel.2025.113628
169. Rahman MM, Wang J, Wang G, et al. Chimeric nanobody-decorated liposomes by self-assembly. Nat Nanotechnol. 2024;19(6):818–824. doi:10.1038/s41565-024-01620-6
170. Zaleski MH, Omo-Lamai S, Nong J, et al. Nanocarriers’ repartitioning of drugs between blood subcompartments as a mechanism of improving pharmacokinetics, safety, and efficacy. J Controlled Release. 2024;374:425–440. doi:10.1016/j.jconrel.2024.07.070
171. Correa S, Dreaden EC, Gu L, Hammond PT. Engineering nanolayered particles for modular drug delivery. J Controlled Release. 2016;240:364–386. doi:10.1016/j.jconrel.2016.01.040
172. Eleftheriadis GK, Genina N, Boetker J, Rantanen J. Modular design principle based on compartmental drug delivery systems. Adv Drug Delivery Rev. 2021;178:113921. doi:10.1016/j.addr.2021.113921
173. Baby T, Liu Y, Yang G, Chen D, Zhao CX. Microfluidic synthesis of curcumin loaded polymer nanoparticles with tunable drug loading and pH-triggered release. J Colloid Interface Sci. 2021;594:474–484. doi:10.1016/j.jcis.2021.03.035
174. Mottafegh A, Joo J-U, Na G-S, Sharma V, Kim D-P. AI-assisted autonomous manufacturing of tailored drug-loaded nanoparticles by multi-step continuous-flow platform. Chem Eng J. 2024;500:157454. doi:10.1016/j.cej.2024.157454
175. Joyce P, Allen CJ, Alonso MJ, et al. A translational framework to DELIVER nanomedicines to the clinic. Nat Nanotechnol. 2024;19(11):1597–1611. doi:10.1038/s41565-024-01754-7
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


