Back to Journals » International Journal of Nanomedicine » Volume 19
Emerging Trends in the Application of Extracellular Vesicles as Novel Oral Delivery Vehicles for Therapeutics in Inflammatory Diseases
Authors Zeng M, Liu M, Tao X, Yin X, Shen C, Wang X
Received 25 April 2024
Accepted for publication 8 August 2024
Published 21 August 2024 Volume 2024:19 Pages 8573—8601
DOI https://doi.org/10.2147/IJN.S475532
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Eng San Thian
Mingtang Zeng,1,* Maozhu Liu,2,* Xuelin Tao,1,* Xi Yin,1 Chao Shen,1 Xueyan Wang1
1Department of Pharmacy, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China; 2Center of Infectious Diseases, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xueyan Wang, Department of Pharmacy, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China, Email [email protected]
Abstract: Inflammation involves complex immune responses where cytokines such as TNF-α, IL-1, and IL-6 promote vasodilation and increased vascular permeability to facilitate immune cell migration to inflammation sites. Persistent inflammation is linked to diseases like cancer, arthritis, and neurodegenerative disorders. Although oral anti-inflammatory drugs are favored for their non-invasiveness and cost-effectiveness, their efficacy is often compromised due to gastrointestinal degradation and limited bioavailability. Recent advancements highlight the potential of extracellular vesicles (EVs) as nanocarriers that enhance drug delivery by encapsulating therapeutic agents, ensuring targeted release and reduced toxicity. These EVs, derived from dietary sources and cell cultures, exhibit excellent biocompatibility and stability, presenting a novel approach in anti-inflammatory therapies. This review discusses the classification and advantages of orally administered EVs (O-EVs), their mechanism of action, and their emerging role in treating inflammatory conditions, positioning them as promising vectors in the development of innovative anti-inflammatory drug delivery systems.
Keywords: extracellular vesicles, anti-inflammatory, drug delivery systems, food, oral administration
Introduction
Inflammation is a complex biological response involving multiple cells, molecules, and pathways. The activation of immune cells is at the heart of the inflammatory response during inflammation. Immune cells, including macrophages, neutrophils, lymphocytes, etc., upon external stimuli, release a series of inflammatory mediators and cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), by recognizing and binding to pathogens, cellular damage markers, etc. The release of inflammatory mediators leads to vasodilation and increased vascular permeability, resulting in increased blood flow to the site of inflammation, allowing immune cells and other inflammatory mediators to more easily enter the tissues. In fact, vasodilation and increased vascular permeability are important features of the inflammatory response. Dysregulation of the severity or duration of inflammation can lead to abnormal tissue remodeling, irreversible damage, and widespread pathology, contributing to the progression of various complex diseases such as cancer, arthritis, cardiovascular diseases, and neurological disorders.1,2 The intestinal route (including oral medication) is the primary means of treating inflammatory diseases, as it is minimally invasive (painless), relatively low-cost, and self-administrable.3 Clinically, oral medications used for anti-inflammatory purposes mainly include non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs), immunosuppressants, and biologics, which exhibit significant adverse effects.4–6 Moreover, factors such as drug degradation in the gastrointestinal tract and low permeability of intestinal epithelial cells lead to low bioavailability of orally administered drugs, resulting in limited efficacy in the treatment of inflammation. The diversification of drug properties, uncertainty of excipients, the presence of physiological barriers in the human body, and other factors pose challenges to the development of oral formulations.7,8
As an alternative to oral anti-inflammatory drugs, nanocarriers can safely transport active therapeutic molecules in oral administration, improving pharmacokinetics, biodistribution, and stability, while reducing toxicity. The application of nanotechnology and intelligent release systems controls drug release and delivery to specific sites to enhance the therapeutic effects of anti-inflammatory drugs and reduce side effects.9 Among the most studied nanocarriers, extracellular vesicles have unique natural properties due to their biological origins, making them suitable for biomedical applications.10 Extracellular vesicles are defined as phospholipid vesicles actively secreted by various cells, primarily released into the extracellular space through exocytosis to transmit information between cells and organisms. They contain substances identical to the parent cells and can carry harmless chemical substances into the biological environment to perform various functions.11 Moreover, they exhibit high stability under physiological conditions and possess advantageous properties related to the controlled and specific release to tune down endogenous or loaded therapeutic molecules by modifying their internal and surface components.12 Extracellular vesicles have demonstrated significant advantages in targeting, safety and pharmacokinetics over synthetic nanocarriers, leading them to be considered as next-generation drug delivery platforms.13
Over the past decades, extensive research has been conducted on the impact of active ingredients in food on human health and disease. Extracellular vesicles derived from edible plants and animals have attracted intense attention and have been confirmed to exhibit appropriate pharmacological activity as cross-species therapeutic agents. Additionally, orally administered extracellular vesicles derived from bacterial and cell culture media serve as natural nanocarriers due to their unique morphology and biological composition. These orally administered extracellular vesicles (O-EVs) have been proven effective in inflammation models and have been utilized in the development of novel drugs and targeted drug delivery research.14 Compared to conventional drug vectors, O-EVs offer advantages such as low immunogenicity,15 non-toxicity,16 environmental friendliness,17 good biocompatibility,18 and the ability to cross the blood-brain barrier without crossing the placental barrier,19 making them promising drug vectors for the treatment of inflammatory diseases.20 This paper summarizes the classification and potential advantages of O-EVs for anti-inflammatory purposes. Based on O-EVs, a special focus is placed on the latest trends in the management of various inflammatory diseases (including pulmonary inflammation, hepatic inflammation, intestinal inflammation, periodontitis, arthritis, etc). The drug delivery system of O-EVs for anti-inflammatory therapy and the mechanism of action of O-EVs in anti-inflammatory therapy are also discussed, with the aim of providing theoretical references for the development of O-EV-based oral formulations for anti-inflammatory therapy.
Classification of O-EVs
Animal Milk-Derived O-EVs
Nearly all cells possess the ability to release extracellular vesicles during both normal physiological functions and pathological states.21 As a result, EVs are found in the bodily fluids of a wide array of eukaryotic organisms, including animal blood, urine, saliva, amniotic fluid, and milk.22–24 It is worth noting that milk, a commonly consumed natural beverage known for its nutritional value, contains substantial amounts of EVs that are distinguished by the persistent integrity of their bilayer when examined in vitro. Given their attributes of low toxicity, stability, accessibility, and cost-effectiveness, milk EVs hold promise as highly effective carriers for drug delivery that can be feasibly produced on a large scale. The molecular weight of milk EVs surpasses that of most proteins inherent in milk, facilitating their isolation from other biomolecules through appropriately sized ultrafiltration membranes.25 This allows for the direct encapsulation of hydrophobic small molecule drugs such as paclitaxel and curcumin into milk EVs. However, the presence of abundant free proteins and fats in milk hinders the extraction, separation, and purification of milk EVs. Therefore, it is imperative to eliminate impurities such as fats, cellular debris, and a substantial portion of free proteins during the purification process of milk EVs. The techniques employed for the purification of milk EVs closely mirror traditional EV purification methods and include differential centrifugation, filtration, density gradient centrifugation, immune-affinity capture, size exclusion chromatography, microfluidic approaches, and others.26,27
In human milk-derived extracellular vesicles, the microRNA miR-148a has been identified as a key regulator in altering epigenetic mechanisms by targeting DNA methyltransferase 1 (DNMT1), thus playing a critical role in preventing necrotizing enterocolitis through modulation of p53 and Sirtuin 1.28 Studies have demonstrated that administration of cow and human milk-derived EVs alone can alleviate symptoms in dextran sulfate sodium (DSS)-induced colitis in mice, leading to early weight gain, downregulation of pro-inflammatory cytokine gene expression such as TNF-α and IL-6, and an increase in colon TGF-β1 protein levels.29 Notably, some EVs, including those sourced from commercial cow milk, carry TGF-β1, allowing for targeted transport of this cytokine to affected tissues.30–32 The therapeutic effects of EVs may also be attributed to encapsulated miRNAs, including miRNA-375 and miRNA-320.29 Oral administration of commercial cow milk-derived EVs to the small intestine in mouse models of ulcerative colitis has demonstrated anti-inflammatory properties, as evidenced by improvements in the macroscopic appearance of the ileum and colon segments and the consistency of fecal samples.33 Conversely, depletion of EVs from milk led to exacerbated symptoms of inflammatory bowel disease (IBD) in mice, associated with decreased levels of miRNA-200a-3p, which is abundant in milk-derived EVs, and increased serum concentrations of CXCL9.34 However, a significant consideration in utilizing miRNAs delivered via EVs is the potential for off-target effects. Off-target effects refer to unintended interactions of miRNAs with non-targeted mRNA sequences, leading to altered gene expression profiles that may not align with therapeutic goals. These effects can arise due to sequence complementarity between the miRNA and unintended mRNA targets, which may be unrelated to the disease state or therapeutic pathway of interest.35 Additionally, the presence of abundant miRNAs in EVs can saturate the RNA interference machinery within recipient cells, potentially leading to unpredictable biological outcomes.35 Strategies to mitigate off-target effects include optimizing the selection of miRNA sequences with minimal homology to non-targeted mRNAs, enhancing EV delivery specificity to target cells or tissues, and refining bioinformatics tools to predict and validate miRNA-mRNA interactions.36 Furthermore, comprehensive characterization of EV cargo composition, including miRNA profiling and functional assays, is crucial to understanding and minimizing off-target effects while maximizing therapeutic efficacy.37
In addition, milk EVs have shown promise as a therapeutic intervention for certain autoimmune conditions, as demonstrated in mouse models of arthritis. Arntz et al reported a dose-dependent reduction in collagen-induced arthritis following oral administration of bovine milk-derived EVs in drinking water one week prior to collagen immunization. Similarly, gavage delivery of EVs loaded with interleukin-1 receptor antagonist (IL-1Ra) resulted in dose-dependent improvements in ankle joint swelling, bone marrow cell depletion, and cartilage erosion.38 In addition to human milk, cow milk, and bovine milk-derived EVs, investigations have extended to exploring the anti-inflammatory potential of EVs derived from pig milk, camel milk, and goat milk (Table 1).
 |
Table 1 The Anti-Inflammatory Potential of EVs Derived from a Variety of Milk |
Plant-Derived O-EVs
Plant-derived O-EVs have emerged as a novel area of exploration in the field of therapeutics, displaying similarities to their animal-derived counterparts in terms of size, surface charge, morphology, and content. The International Society for Extracellular Vesicles (ISEV) recommends that plant-derived O-EVs be isolated using standardized methods such as differential centrifugation, density gradient centrifugation, and ultrafiltration (Figure 1). These methods aim to ensure the purity and consistency of PDEVs for downstream analyses. However, limitations include potential contamination from other plant-derived particles and variability in vesicle yield depending on the plant species and tissue source.46,47 Furthermore, certain EV extraction kits now offer rapid extraction of total EVs from plant juices, potentially serving as alternatives to traditional isolation methods in the future.48 A comparative analysis of animal and plant-derived O-EVs, detailing their origin, extraction process, composition, and advantages as drug carriers, is presented in Table 2. Plant-derived O-EVs exhibit favorable characteristics for serving as oral drug delivery vehicles (ODDVs), including stability in the gastrointestinal tract (GIT), high biocompatibility with human cells, biodegradability, low cytotoxicity, and immunogenicity.49,50 These plant-derived O-EVs have the capacity to encapsulate various therapeutic agents such as chemotherapeutic drugs, siRNA, DNA expression vectors, and proteins, facilitating targeted delivery to different tissues.51 Given that the GIT is the primary site of interaction with ingested fruits and plant EVs, numerous studies have concentrated on exploring the therapeutic potential of EVs in addressing GIT-related conditions. Methotrexate (MTX) is a lipophilic drug commonly administered orally to treat various intestinal diseases, such as cancer or Crohn’s disease, despite its significant toxic side effects.52 In a study by Songwen et al, MTX was encapsulated into grapefruit-derived nanovesicles (GDN) to enhance its pharmacokinetics, and the MTX-GDNs were orally administered to mice. The results showed that MTX-GDNs were able to significantly reduce MTX toxicity and improve its efficacy compared to free MTX in mice with dextran sulfate sodium-induced colitis.53 This research underscores the potential of utilizing plant-derived O-EVs as oral drug delivery systems (ODDS) for delivering small molecule biologics to mitigate inflammatory responses in humans. Furthermore, certain plant-derived O-EVs have been found to possess tolerance and immunogenicity properties that are crucial for regulating intestinal immune homeostasis and preventing intestinal inflammation. For instance, oral administration of broccoli EVs has been shown to suppress mouse colitis by activating dendritic cell AMP-activated protein kinase in three mouse colitis models.54 Similarly, O-EVs derived from grapes, grapefruits, and ginger may contain functional molecules with similar immunomodulatory effects that promote intestinal immune tolerance.55,56
 |
Table 2 Comparative Characteristics of O-EVs Derived from Animal, Plant, and Bacterial Sources |
Research has shown that animal-derived O-EVs are ingested through regular dietary intake in humans, transferring miRNA and nutrients and demonstrating exceptional pharmacological properties. However, a notable limitation lies in the difficulty of generating sufficient animal-derived O-EVs in vitro and isolating them from biological fluids. The production of EVs from cell cultures may present risks due to the presence of potentially harmful molecules, including substances with carcinogenic properties.57 In contrast, plant-derived O-EVs are readily available in natural sources, allowing for the economical production of large quantities of EVs.58 Plants such as grapes, tomatoes, and grapefruits are rich sources of EVs, with concentrations of 1.76 mg/g, 0.44 mg/g, and 2.21 mg/g, respectively, suggesting the feasibility of utilizing certain plant-based foods for mass EV production.59 Additionally, plant-derived O-EVs exhibit high biocompatibility, low immunogenicity, minimal toxicity, and reduced allergenicity in the human body.60 While animal and plant-derived O-EVs have been extensively studied, research on microbial-derived O-EVs lags behind, highlighting the need for further exploration to evaluate their potential utility.
Bacteria-Derived O-EVs
Gram-negative and Gram-positive bacteria exhibit the capacity to generate a diverse array of EVs with distinct compositions and contents through unique biological mechanisms.61 These EVs serve as a critical potential mechanism underlying the detrimental or beneficial effects of numerous pathogenic, symbiotic, and probiotic bacteria. The isolation and purification of bacterial-derived EVs can be achieved utilizing various methods, including low-speed centrifugation, sterile filtration, ultrafiltration, and ultracentrifugation.62 In certain instances, specialized purification techniques such as protein precipitation, affinity chromatography, or size-exclusion chromatography may be necessary to eliminate flagellar proteins, lipopolysaccharides (LPS), and protein aggregates.63–65 An essential role of bacterial-derived EVs is the transportation of biomolecules to specific remote locations of their originating bacteria.66 Building upon this principle, researchers have hypothesized that bacterial-derived EVs could serve as a novel oral delivery system. Apart from sharing similar advantages with EVs sourced from other origins, bacterial-derived EVs also encompass a variety of pathogen-associated molecular patterns (PAMPs) that can be readily identified and internalized by host phagocytic cells, predominantly neutrophils. This characteristic has the potential to facilitate targeted delivery and co-opting of neutrophils, thereby augmenting the delivery of drugs to sites of inflammation.67
Extracellular vesicles derived from pathogenic bacteria play a crucial role in enhancing bacterial pathogenicity. These EVs act as vehicles for the transfer of virulence factors, such as enzymes, DNA, and small RNAs, to host cells, resulting in cellular damage and eliciting inflammatory responses. Furthermore, EVs originating from pathogenic bacteria exhibit immunomodulatory and immunostimulatory properties, suggesting their potential therapeutic utility in managing inflammatory conditions through immunosuppression.68 Notably, these EVs possess the ability to selectively target specific cell types, offering a promising platform for targeted drug delivery.69,70 However, the clinical application of oral delivery systems based on EVs from pathogenic bacteria presents challenges, as these EVs may accumulate at unintended sites such as the kidneys and liver, potentially causing off-target toxicity. To address this issue, further research is warranted to detoxify pathogenic bacteria-derived EVs, thereby minimizing inflammatory responses and associated risks.
Extracellular vesicles derived from symbiotic and probiotic bacteria play a pivotal role in maintaining microbial and gastrointestinal tract (GIT) homeostasis through their interactions with host epithelial cells and immune responses, thereby conferring beneficial effects on the host.71,72 In light of the safety concerns surrounding EVs derived from pathogenic bacteria, it is desirable to prioritize the use of EVs derived from less harmful symbiotic and probiotic bacteria for drug delivery applications. Notably, Escherichia coli Nissle 1917, a well-known probiotic strain, has demonstrated efficacy in ameliorating ulcerative colitis. Oral administration of probiotic EVs has shown significant improvements in histological and clinical markers of inflammation in experimental colitis models,73,74 underscoring the potential of bacterial-derived EVs as a novel approach for addressing intestinal inflammatory processes with potentially reduced risks of adverse effects compared to conventional probiotic interventions. Furthermore, the immunomodulatory properties of various Lactobacillus EVs make them promising candidates for designing drug delivery systems targeting inflammatory diseases. A study highlighted the therapeutic potential of EVs from Lactobacillus casei, a probiotic strain with anti-inflammatory attributes, in mitigating intestinal inflammation through modulation of the endoplasmic reticulum stress pathway.75 Moreover, a novel therapeutic strategy mimicking the anti-inflammatory effects of probiotics was achieved by conjugating EVs from probiotic strains such as Lactobacillus casei and Lactobacillus plantarum onto microparticles. This innovative system demonstrated anti-inflammatory and barrier-protective properties in a GIT model, showcasing the potential of probiotic EVs as effective agents in combating inflammatory conditions.76
Extracellular vesicles derived from specific gut microbiota bacteria exhibit immunomodulatory effects, potentially contributing to the enhancement of intestinal barrier integrity in pathological conditions. In a colitis mouse model, EVs derived from fragile Bacteroides have demonstrated the ability to induce immunomodulatory effects and prevent intestinal inflammation.72 Akkermansia muciniphila, a prevalent species in the human gut microbiota, functions as a “self-beneficial probiotic” and is inversely related to metabolic inflammatory diseases such as obesity and type 2 diabetes.77 Intriguingly, oral administration of Akkermansia muciniphila and its EVs via gavage has been shown to mitigate weight gain in mice fed a high-fat diet, leading to reductions in fat tissue weight and total cholesterol levels. The substantial downregulation of TLR-4, TNF-α, and IL-6 gene expression in perirenal fat tissue and/or colon corroborated these findings. Additionally, oral administration of Akkermansia muciniphila and its EVs stimulated IL-10 gene expression in the colon of healthy mice.78 The anti-inflammatory and immunomodulatory properties of these probiotics or engineered probiotic EVs play a pivotal role in the clinical translation of therapeutic EVs. With advancements in various bacterial manipulation technologies, probiotic EVs represent a promising avenue for the development of orally delivered drug delivery vehicles, owing to their safety profile and beneficial pharmacological effects.
Cell-Derived O-EVs
Extracellular vesicles can be isolated from a variety of mammalian cell types, such as dendritic cells, epithelial cells, tumor cells, immune cells, and mesenchymal stem cells.79 Typically, EVs are obtained from cell culture supernatants and purified using conventional isolation methods. These cell-derived EVs act as carriers that amalgamate the advantages of cell-based drug delivery and nanotechnology to facilitate efficient drug transport and long-distance delivery, with the potential to address inflammatory conditions. Sun et al fabricated curcumin-loaded EVs sourced from diverse cell lineages, including human gland cancer cells, mouse breast tumor cells, and mouse lymphoma cells.80 Oral administration of curcumin EVs enhanced curcumin’s solubility, gastrointestinal stability, and bioavailability, demonstrating superior anti-inflammatory efficacy compared to conventional curcumin formulations. The specificity of the delivery system is dictated by EVs, which guide curcumin to relevant inflammatory cells without inducing toxicity, thereby augmenting its therapeutic potential. Generally, cell-derived EVs, particularly host-derived EVs, exhibit advantages as prospective oral delivery systems over non-host carriers due to their mitigated immune responses and associated adverse effects. Nevertheless, the limited yield and high cost of EV production pose challenges for large-scale manufacturing, impeding their clinical translation.
EV Separation Methods
Despite the availability of numerous methods for EV isolation in the literature and some ongoing controversies, one conclusion is unequivocal: there is no single best method for use. Each method has its limitations, and selecting the most appropriate one for specific purposes (such as isolating therapeutic EVs from specific cell types grown under certain conditions) requires matching these limitations with the requirements of the available sample type and application. Some of the most important parameters to consider among different isolation methods include throughput, cost, ease of use, time and equipment requirements, gentleness, vesicle loss during separation, and the final preparation’s purity. Common methods target general physicochemical characteristics of EVs (such as size, density, and charge) or focus on specific properties (such as the expression of surface markers). Therefore, depending on their respective isolation principles, methods may be non-specific (enriching a broad range of EVs and possible non-EV materials) or highly specific (isolating only particular vesicle populations). A brief overview of common EV isolation methods and their advantages and disadvantages is provided in Table 3. Regardless of the chosen method, strict standardization and frequent quality control are essential to ensure reproducible, pure, stable, and effective products.
 |
Table 3 Summary of EV Separation Methods |
Potential Advantages of O-EVs in Treating Inflammatory Diseases
Biocompatibility
O-EVs possess remarkable biocompatibility, as evidenced by their negligible influence on the physiological and biochemical indices of experimental animals upon oral administration.53,56 Ginger-derived nanoparticles (GDNPs) at a dosage of 100 µg/mL did not impair cellular viability when co-cultured with colon-26 and RAW 264.7 cells over a 24-hour period. In addition, the barrier integrity of the Caco2-BBE monolayer is not perturbed by GDNPs2. Subsequent to a 7-day regimen of administration (0.3 mg/mouse), the levels of pro-inflammatory cytokines were found to be unaltered, and no morphological or pathological deviations were discernible.56 In a separate inquiry, Ginger-derived lipid vehicles (GDLVs) harboring siRNA-CD98 were associated with diminished toxicity in comparison to their liposomal counterparts. The GDLVs maintained hemostasis in parameters such as leukocyte and erythrocyte counts, hemoglobin concentration, and hepatic and renal function indices, akin to the PBS control group.81 Similarly, Grapefruit-derived EVs affirmed their superior biocompatibility, with no fluctuations in serum IFN-γ, nor in hepatic transaminases including AST/ALT subsequent to oral administration (10 mg/kg).53 Ginger-Derived Nanovesicles (GDNVs) have also manifested exceptional biocompatibility, with no significant fluctuations in the levels of pro-inflammatory cytokines within murine blood (inclusive of IL-6, IL-1β, and TNF-α) following intravenous administration, and without any overt damage to organs.82 Furthermore, safety assessments of bovine milk-derived EVs have indicated that variables such as body mass, plasma cytokine profiles, and tissue integrity remained consistent, inferring their commendable tolerability and minimal immunogenicity.83 Plant-derived EVs (PDEVs) from diverse botanical sources, including lemon, ginseng, shiitake mushrooms, and asparagus, have also exhibited negligible toxicity post-administration.84 Although PDEVs have demonstrated favorable biocompatibility upon injection, it is hypothesized that this may result from the elimination of immunogenic components during preparation and purification. The direct injection of PDEVs into the body is not recommended due to the indeterminate nature of their contents. Conversely, for oral administration, PDEVs are deemed to be relatively innocuous.
Stability
O-EVs have demonstrated remarkable resilience and stability in gastrointestinal environments. For instance, grape-derived O-EVs exhibit resistance to degradation by salivary enzymes, gastric acid, and proteolytic enzymes, allowing them to successfully navigate the intestinal tract, traverse the mucus barrier, and be absorbed by murine intestinal stem cells.55 Similarly, O-EVs sourced from ginger have shown high stability in simulated gastrointestinal fluids, facilitating their absorption by intestinal epithelial cells and macrophages upon oral administration.56 Grapefruit-derived nanovesicles (GDN) have been found to maintain stability in acidic and simulated gastrointestinal conditions, showcasing exceptional resistance to enzymatic digestion.53 In a study utilizing GDN for methotrexate (MTX) encapsulation, it was demonstrated that GDN efficiently targeted resident macrophages, enhancing the anti-inflammatory efficacy of MTX while minimizing its adverse effects, which underscores the potential of EV-based oral drug delivery systems to improve drug bioavailability. Similarly, studies involving the delivery of siRNA by O-EVs, such as ginger-derived O-EVs (GDLV), have shown promising results in protecting and delivering siRNA payloads to target cells. GDLV effectively reduced the expression of specific genes, such as CD98, in colonic cells.81 Furthermore, encapsulation of nucleic acids within acerola EV-like nanovesicles (AELN) has been shown to significantly enhance nucleic acid stability, offering robust protection against degradation post-encapsulation.48
However, Munagala et al reported that the gastrointestinal stability of cow milk-derived O-EVs showed suboptimal results in nude mice after oral administration, which contrasts sharply with previous extensive research in this area.85 Studies have shown that liquid-ordered membranes exhibit greater resistance to detergent solubilization compared to liquid-disordered membranes.86 It appears that structurally ordered membranes are more resistant to digestion, although this factor alone does not fully elucidate the challenges faced by mammalian O-EVs in traversing the gastrointestinal environment, particularly considering that mammalian O-EVs also possess ordered membrane structures. Differential lipid composition may play a crucial role in the resilience of O-EVs to the gastrointestinal environment. Mammalian O-EVs predominantly consist of lipids such as cholesterol (CHOL), sphingomyelin (SM), phosphatidylcholine (PC), and phosphatidylethanolamine (PE),87 while plant-derived O-EVs are typically abundant in lipids like phosphatidic acid (PA), digalactosyldiacylglycerol (DGDG), monogalactosyldiacylglycerol (MGDG), PC, PE, and phosphatidylinositol (PI). Previous studies have suggested that lipid composition influences the formation and distribution of intracellular EVs.88,89 Therefore, the lipid composition may be linked to the ability of plant-derived O-EVs to resist gastrointestinal digestion. Moreover, the rapid clearance of exogenously administered exosomes from circulation within minutes to half an hour post systemic injection is predominantly mediated by macrophages.90 Understanding the reasons why EVs absorbed from the gastrointestinal tract exhibit longer circulating half-lives compared to systemically administered EVs is a critical issue that must be addressed to fully appreciate the potential clinical implications of O-EVs.
Drug Delivery System
Modification and Transformation
Nanocarriers offer numerous advantages over traditional pharmaceutical forms by providing protection against premature degradation, improved retention, and enhanced tissue penetration. As shown in Figure 2, extracellular vesicles have the ability to cross natural barriers such as the blood-brain barrier and remain in circulation for extended periods due to their lipid bilayer structure, which protects them from enzymatic degradation.91 Additionally, EVs derived from biocompatible cells are less likely to trigger immune responses, thus exhibiting immunological inertness.92 However, challenges such as cytotoxicity, ecotoxicity, suboptimal targeting efficiency, and uneven distribution hinder their clinical application. Consequently, artificial EVs have emerged as a potential alternative to natural ones, leveraging advanced techniques to functionalize engineered EVs, broaden their range of applications, and enhance their binding to specific target cells to improve therapeutic effectiveness.
Surface Modification
Conjugating peptides, proteins, targeted molecules, or compounds to the surface of vesicles can introduce additional functionalities while maintaining vesicle integrity. This can be achieved through coupled reactions or lipid assembly modification strategies, allowing for enhanced targeting specificity, improved bioavailability, fluorescence imaging capabilities, photothermal effects, and more. For instance, O-EVs modified with polyethylene glycol (PEG) can extend blood circulation time and enhance stability.93,94 Grapefruit-derived EVs can be covalently attached to heparin-based nanocarriers loaded with pH-sensitive doxorubicin, utilizing both the heparin carboxyl group and the membrane molecular amino group, resulting in high payload and targeted delivery capabilities.95 Saponin treatment has been shown to increase the loading of hydrophobic porphyrins in EVs without affecting drug delivery capacity.96 Permeabilization of EVs also improves the loading efficiency of catalase.97 However, saponin treatment may lead to a lower encapsulation rate of hydrophilic fluoropyran in EVs compared to co-incubation, possibly due to saponin displacing surface-bound pyran molecules.98
Physical Loading
The co-incubation method provides a straightforward approach for loading drugs into EVs. By co-incubating drugs and EVs at a specific temperature for a defined duration, drugs can be effectively incorporated into EVs through simple diffusion mechanisms. For example, efficient loading of miRNA mimics such as hsa-miR-340 and hsa-miR-146a into cherry-derived EVs can be achieved by co-incubating them on ice for 30 minutes.99 However, the co-incubation method often results in a lower encapsulation efficiency due to the lack of additional forces to aid drug diffusion. In addition, the nature of the drug plays a critical role in determining the encapsulation efficiency, with lipophilic drugs generally exhibiting higher encapsulation efficiency than hydrophilic drugs.96 Sonication is a widely employed method for drug loading that utilizes ultrasound energy and mechanical force to transiently modify the lipid bilayer structure of EVs, facilitating efficient drug penetration. Sonication has been demonstrated to provide improved drug loading efficiency compared to co-incubation.100,101 It is important to note that the biophysics of EVs decreases significantly after multiple cycles of sonication, gradually returning to normal within an hour post-sonication. While sonication treatment does not significantly affect the lipid and protein content of EVs, it may potentially cause structural distortions and compromise vesicle integrity.102 The application of extrusion technology involves the passage of vesicles and drugs through a filter with defined pore sizes, resulting in the transformation of larger vesicles into smaller ones as they traverse the filter. This process facilitates the encapsulation of small-molecule drugs within the vesicles, ensuring a homogeneous distribution of vesicle-drug complexes. Extrusion is a commonly utilized technique for drug encapsulation within cell membranes and is also employed for drug loading in O-EVs.103 Fuhrmann et al conducted a comprehensive assessment of various drug loading strategies and demonstrated that extrusion exhibits superior drug loading efficiency.96 Moreover, Mammadova et al successfully loaded curcumin into tomato-derived EVs using direct co-incubation, sonication, and extrusion methods to enhance its anti-inflammatory properties.104
Expanding upon these preparatory approaches, the coating of extracellular vesicles with metal-organic framework nanoparticles has emerged as a promising strategy to enhance targeted delivery, improve loading efficiency, facilitate encapsulation, and prevent premature release of cargo.105 Mao et al employed ultrasound to coat ginger-derived EVs onto custom-designed mesoporous silica nanoparticles for the efficient loading of high quantities of TNF-α antibody, enabling specific delivery to the colon through oral administration. The robust framework of mesoporous silica nanoparticles effectively impedes the aggregation of loaded antibodies, thereby averting instabilities arising from the clustering of large molecules during drug delivery and significantly augmenting the loading capacity.106 For the development of a more uniform and stable nano-delivery platform, researchers extracted total lipids from PDEVs using the Bligh and Dyer method, a liquid-liquid separation technique, followed by high-pressure homogenization to produce uniformly sized nanostructures for drug loading and subsequent modifications.51,107
Membrane Fusion
Lipid bilayers of extracellular vesicles have demonstrated a unique ability to spontaneously fuse with various membrane structures, particularly through freeze-thaw cycles. This phenomenon is particularly prominent in PDEVs, where repeated freeze-thaw cycles induce mild disruption in the lipid bilayer, creating pores that facilitate the diffusion of drug molecules into the vesicle. The fusion of mammalian EVs with liposomes not only enhances their colloidal stability but also increases their drug loading capacity and enables pH-sensitive sustained drug release.108 By harnessing membrane fusion techniques, researchers can manipulate the fusion and conversion of liposomes and natural O-EVs to enhance drug loading capabilities. A notable example is the incorporation of the antioxidant enzyme catalase into EVs isolated from macrophages using freeze-thaw cycles, resulting in a significantly higher drug loading rate of 14.7% compared to a direct co-incubation approach that yielded only 4.9% loading efficiency.97 Freeze-thaw cycles offer a distinct advantage over techniques such as ultrasound and squeezing by imposing minimal external forces, thereby preserving vesicle integrity and simplifying operating procedures. Additionally, the strategic coating of grapefruit-derived EVs with leukocyte membranes through extrusion enhances their targeting ability to inflamed tissues by enriching the vesicles with inflammation-related receptors.109 Nevertheless, prolonged exposure to repeated freeze-thaw cycles may lead to protein inactivation, EV aggregation, and an increase in particle size.110,111 Therefore, meticulous consideration and optimization of the freeze-thaw process is imperative to preserve the integrity and functionality of engineered vesicles.
In summary, the commonly used methods for engineering O-EVs are illustrated in Figure 3. These methods not only enhance the loading efficiency and targeting accuracy of the payload but also promote applications in drug delivery and biological therapies. Future research will continue to explore and optimize these strategies, as well as identify new approaches to further enhance their biological activity and therapeutic potential.
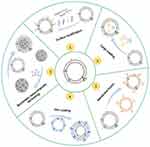 |
Figure 3 Modification and transformation of O-EVs. |
Loading Drugs
As previously mentioned, O-EVs encapsulate biologics with therapeutic properties such as proteins, lipids, nucleic acids, and natural bioactive compounds. Nonetheless, significant differences are observed in the cargo loading profiles of diverse EV populations, even when sharing the same EV classification but originating from different sources. External strategies can be utilized to enhance the loading of specific therapeutic agents, including pharmaceuticals, proteins, mRNA, miRNA, and siRNA.14
Chemical Medicine
Plant-derived extracellular vesicles possess the inherent bioactive properties of their botanical origins, making them a promising option for drug delivery applications due to their distinctive structural properties. The lipid bilayer arrangement of PDEVs allows for the incorporation of hydrophobic drugs, thereby enhancing drug availability, while also effectively encapsulating hydrophilic drugs to prevent potential adverse effects from drug leakage.60 For example, grapefruit-derived EVs have been utilized to encapsulate the anti-inflammatory drug MTX (GDN-MTX), maintaining the therapeutic effectiveness of MTX and facilitating improved uptake by intestinal macrophages. In a murine colitis model, GDN-MTX demonstrated significant efficacy in alleviating colitis symptoms, suppressing the release of pro-inflammatory cytokines by colonic macrophages, alleviating MTX-related side effects, and eliciting a potent anti-inflammatory response.53 Similarly, modifying lemon-derived EVs with functional heparin (HR) to create HRE, loaded with DOX, led to a slight increase in particle size (from 150 nm to 156 nm). The encapsulation of HRE notably enhanced the uptake efficiency of HRED, with its diverse internalization capacity effectively dissipating intracellular energy and inhibiting drug efflux in resistant cells.112 Moreover, grapefruit-derived nanovectors coated with inflammatory-related receptor enriched membranes of activated leukocytes (IGNVs) demonstrated improved targeting abilities at inflammatory sites in various inflammation models, prolonging drug circulation in the body and enhancing drug accumulation in specific tissues.109 Although PDEVs can traverse multiple physiological barriers, they are unable to cross the placental barrier. Upon administering fluorescently labeled grapefruit-derived EVs to pregnant mice, no fluorescent signal was observed in the placenta, emphasizing the safety profile of PDEVs and creating novel opportunities for drug administration in pregnant women.51
siRNA
The quest for suitable vectors remains a primary challenge in the field of gene therapy. Both viral and non-viral vectors have been employed for the delivery of genetic pharmaceuticals, yet they are commonly associated with significant immunogenicity and toxicity.113 Plant-derived extracellular vesicles have been identified as carriers of siRNA, capable of shielding siRNA from environmental degradation in vivo while facilitating targeted delivery and therapeutic effects. Zhang et al employed ginger-derived lipid vehicles (GDLVs) to load siRNA-CD98, which remained stable at 4 °C and demonstrated exceptional biocompatibility. GDLVs were able to effectively load siRNA-CD98 and exhibited transfection efficiencies in colon 26 and RAW 264.7 cells comparable to liposomes. Upon oral administration, GDLVs/siRNA-CD98 targeted the colon and effectively down-regulated CD98 expression,81 and a decrease in CD98 expression can reduce colitis and colitis-associated cancer. The traversal of the blood-brain barrier to deliver drugs to the brain represents a significant obstacle in the treatment of brain tumors.114 EVs derived from grapefruit have been shown to deliver functional siRNA to both GL26-luc and A549-luc cells, inhibiting the expression of the luciferase gene within these cells. Subsequently, grapefruit-derived EVs carrying JSI-124 were delivered intranasally to the brain, inhibiting GL-26 tumor growth and thereby prolonging the survival of mice.51 Similarly, grapefruit-derived nanovectors coated with folic acid (FA-GNVs) carrying PEI/miR17 were rapidly and selectively absorbed by GL-26 cells for intranasal administration, thus inhibiting the growth of mouse brain tumors by activating NK cells.115 Additionally, EVs derived from ginger, capable of producing and recombining lipids, offer a vehicle for targeted delivery of siRNA-loaded ginger nanovesicles against CD98. The doses required for this siRNA carrier are 10,000 times lower than that of naked siRNA, with toxicity below that of the commercial “DC-Chol/DOPE” liposomal formulation used for the treatment of intestinal inflammation.116 Herbal medicines traditionally used for lung diseases are often administered in decoctions produced by boiling herbs in water, with the active components in the extracts generally remaining stable ELNs (exosome-like nanoparticles) from traditional medicinal plants may also play a role in these decoctions. ELNs extracted from decoctions and those obtained in vitro both exhibit therapeutic effects in vivo, although the latter source of ELNs shows stronger therapeutic potential. ELNs loaded with siRNA, especially HJT-sRNA-m7 and PGY-sRNA-6, have demonstrated potent ability to combat fibrosis and inflammation, respectively. Comprising two main active components, sphingosine-HJT-sRNA-m7 and sphingosine-PGY-sRNA-6 benzoate, co-assembled with sphingosine and siRNAs, respectively improved stroke outcomes in mice when administered orally. These effects were evident in both bleomycin-induced pulmonary fibrosis and poly(I:C)-induced pulmonary inflammation.117
Anti-Inflammatory Mechanism
Regulation of the Immune Response
Inflammation is initiated by the local cellular response to infectious agents or injury, accompanied by the activation of innate immune cells. These cells produce cytokines and chemokines to amplify the local inflammatory process and initiate adaptive immunity.1 While inflammation is beneficial for protecting the body, excessive inflammation is considered a phenomenon caused by an imbalance in immune signaling. This imbalance adversely affects the treatment mechanisms and processes for many diseases, including autoimmune diseases and neurodegenerative disorders. Plant-derived extracellular vesicles, through modification and transformation, can target the inflammatory microenvironment, inhibiting deleterious immune responses within inflamed tissues and promoting the survival and regeneration of damaged parenchymal cells. PDEVs are capable of delivering their immunoregulatory cargo, such as RNA and proteins, to immune cells (M1 macrophages, dendritic cells, CD4+ Th1, and Th17 cells), and effecting phenotypic transformation into immunosuppressive cells (M2 macrophages, tolerogenic dendritic cells, and regulatory T-cells).
Macrophage Regulation
Macrophages, as versatile components of the immune system, exhibit distinct functional states based on the environmental stimuli they encounter. These states are characterized by specific patterns of cytokine production, surface marker expression, and metabolic profiles. Macrophages stimulated by interferon-gamma (IFN-γ), interleukin-1β (IL-1β), and lipopolysaccharides (LPS) are typically involved in host defense mechanisms and inflammatory responses. They secrete pro-inflammatory cytokines, produce reactive oxygen species (ROS), and enhance Th1 immune responses. On the other hand, macrophages exposed to interleukin-4 (IL-4) and interleukin-13 (IL-13) play roles in resolving inflammation and maintaining tissue homeostasis by producing anti-inflammatory cytokines such as IL-4, IL-10, IL-13, and transforming growth factor-beta (TGF-β). Recent studies have highlighted the potential of plant-derived extracellular vesicles in modulating macrophage functions. For instance, EVs derived from kudzu can be absorbed by isolated murine macrophages in vitro, leading to a shift towards an anti-inflammatory phenotype.118 EVs from onions have been shown to inhibit the expression of pro-inflammatory genes and block macrophage inflammation by preventing the phosphorylation of NF-κB.119 Additionally, oral administration of plant-derived EVs has shown promise in maintaining intestinal immune stability. Turmeric-derived EVs facilitate the transition of intestinal macrophages to an anti-inflammatory state, reducing levels of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6, IL-1α, and IFN-β), increasing the expression of IL-10, and upregulating tight junction proteins to protect intestinal barrier function and regulate intestinal immunity.120 Grapefruit-derived EVs, when taken up by intestinal macrophages, have been found to ameliorate dextran sulfate sodium (DSS)-induced colitis in mice by upregulating heme oxygenase-1 (HO-1) and suppressing the production of IL-1β and TNF-α in intestinal macrophages.53 Furthermore, the molecular mechanisms underlying these effects are beginning to be uncovered. Bian et al demonstrated that garlic-derived EVs deliver miRNA-396e to macrophages, which reduces the expression of fructose-2,6-bisphosphatase 3 (PFKFB3), a critical glycolytic enzyme. This process reprograms the metabolic activity of LPS-stimulated macrophages, reducing adipose inflammation and preventing obesity through macrophage-adipocyte interactions.121 Additionally, these garlic-derived EVs were found to modulate PFKFB3-mediated metabolic pathways through macrophage-hepatocyte interactions, thereby alleviating hepatic inflammation and enhancing hepatic lipid metabolism, providing a therapeutic approach for chronic non-alcoholic liver disease.122 In summary, plant-derived EVs represent a novel therapeutic strategy for modulating macrophage function and treating inflammatory diseases, though the detailed molecular mechanisms remain to be fully elucidated.
Regulation of DC Cells
Dendritic cells (DCs) are among the most proficient antigen-presenting cells within the immune system. They possess the capability to capture and process both exogenous and endogenous antigens, subsequently presenting these antigens to T-cells via surface molecules, such as major histocompatibility complex (MHC) class molecules. The antigen presentation process activates T-cells, triggering immune responses, including inflammatory reactions. EVs derived from broccoli not only inhibit the activation of intestinal DCs but also induce tolerogenic DCs by activating the AMP-activated protein kinase (AMPK) signaling pathway, thereby protecting mice from colitis.54 EVs sourced from Aster yomena callus can suppress the expression of surface molecules on DCs, enhance the internalization of extracellular antigens, and inhibit the antigen-presenting capability of DCs, leading to a reduction in the induction of excessive T-cells by mature DCs, thus alleviating asthma symptoms.123 According to in vitro studies, EVs derived from celery inhibit the phorbol 12-myristate 13-acetate (PMA)/ionomycin-mediated activation of CD4+ T lymphocytes in a dose-dependent manner.124
NLRP3 Inflammasome
The NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome serves as a critical signaling platform within the innate immune system, orchestrating inflammatory responses.125 Figure 4 provides a comprehensive summary of the activation mechanism of the NLRP3 inflammasome. Inhibition of the NLRP3 inflammasome presents a promising therapeutic approach for combating inflammatory diseases. Nanovesicles derived from shiitake mushrooms significantly attenuate NLRP3 inflammasome activation in bone marrow-derived macrophages (BMDMs) stimulated ex vivo with lipopolysaccharide (LPS) and palmitate, as well as ameliorate inflammation in a mouse model of acute liver injury induced by D-galactosamine and LPS.126 Nanovesicles from bitter melon containing selective microRNAs have shown promise in regulating NLRP3 mRNA expression, with 11 microRNAs identified as potential modulators.101 Chen et al have demonstrated that intraperitoneal administration of honey-derived extracellular vesicle-like nanoparticles (ELNs) mitigates inflammation in a mouse model of acute liver injury by inhibiting the NLRP3 inflammasome, thereby elucidating the in vivo anti-inflammatory function of these honey-derived entities. Notably, miR-4057, identified within the honey-derived ELNs, is an essential and critical component in the inhibition of the NLRP3 inflammasome.127 While this study provides evidence for the intrinsic anti-inflammatory activity of miRNAs in honey-derived ELNs, further research is warranted to explore whether oral administration of these ELNs also yields anti-inflammatory effects. Liu et al have discovered that garlic chive-derived ELNs (GC-ELNs) inhibit the NLRP3 inflammasome and its downstream pathways, including the activation of caspase-1, the release of inflammatory cytokines, and pyroptosis, in a dose-dependent manner.128 The efficacy of GC-ELNs was further demonstrated in mouse models of acute liver injury and diet-induced obesity via oral and intravenous administration, identifying 1.2-dioleoyl-sn-glycero-3-phosphocholine as the primary active ingredient of GC-ELNs. Nevertheless, the anti-inflammatory role of the contained miRNAs remains to be elucidated, necessitating further studies to determine their importance in immune modulation. Intriguingly, ginger-derived ELNs also exhibited potent inhibition of the NLRP3 inflammasome, where lipids, rather than miRNAs or proteins, have been shown to be the requisite bioactive molecules for inflammasome modulation.125 In most reported studies, miRNAs or proteins within ELNs were identified as active components. Hence, a comprehensive and systematic analysis of the anti-inflammatory properties of various plant-derived ELNs and milk EVs is necessary to delineate the full extent of anti-inflammatory activity and its correlation with ELN and EV components. Additionally, bionic nanocomposites crafted from ginger-derived EVs and metal-organic frameworks, capable of efficient oral delivery of anti-TNF-α antibodies, offer enhanced therapeutic efficacy in inflammatory bowel disease by circumventing systemic side effects associated with injectable antibodies and by inhibiting the NLRP3 inflammasome.106
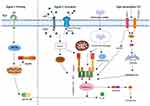 |
Figure 4 Activation mechanism of the NLRP3 inflammasome. |
TLR4/NF-κB Pathway
The TLR4/NF-κB signaling pathway has emerged as a pivotal axis in the modulation of anti-inflammatory immune responses, playing a critical role in the pathogenesis of inflammation as recent studies have elucidated. TLRs initiate signaling cascades through two primary routes: the MyD88-dependent and MyD88-independent pathways. The MyD88-dependent pathway is a well-characterized classical pathway that drives the production of NF-κB and pro-inflammatory cytokines, leading to downstream inflammatory responses. This sequence is triggered when TLR4 binds to its specific ligand, leading to the recruitment of MyD88 within the cell. The adaptor protein MAL bridges the interaction between MyD88 and the signaling protein IRAK, together with TNF receptor-associated factor 6 (TRAF6), which subsequently activates NF-κB inducing kinases. This results in the phosphorylation of IκB kinase and the release of NF-κB from the IκB/NF-κB complex. NF-κB then translocates into the nucleus, prompting the transcription and translation of inflammation-related genes that ultimately release a surge of inflammatory mediators. Extracellular vesicles from porcine milk have been demonstrated to confer protection against LPS-induced intestinal damage by diminishing cellular inflammation and apoptosis. This protective effect is partially attributed to the vesicle-encapsulated miRNAs (miR-4334, miR-219, miR-338), which exert an inhibitory effect on the TLR4/NF-κB and p53 pathways. These findings propose that incorporating miRNA-enriched EVs into infant formula could be an innovative preventative intervention for necrotizing enterocolitis.44 In addition, ginger-derived exosomes containing shogaol have been documented to avert alcohol-induced liver damage. These functional dietary EVs (FDEs) activate the Nrf2 pathway through the TLR/TRIF signaling, thereby upgrading detoxifying enzymes in the liver to mitigate the impact of reactive oxygen species.129 Similarly, extracellular vesicles from Lactobacillus rhamnosus GG (LGG-EVs) have been shown to mitigate colonic tissue damage and prevent colon shortening, as well as reduce intestinal inflammation. LGG-EVs significantly inhibit the activation of the TLR4-NF-κB-NLRP3 axis, leading to the suppression of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-2) post-treatment.130
NF-κB/TNF-α Pathway
TNF-α is a pivotal cytokine synthesized by a diversity of immune cells within the array of inflammatory markers, including macrophages and T-cells. TNF-α plays an integral role in the orchestration of inflammatory responses, immune regulation, and apoptosis. It possesses the capacity to activate the NF-κB signaling pathway, thereby inciting inflammatory responses and a plethora of other biological effects. Aquilano et al have posited that nanovesicles derived from dry nuts mitigate the TNF-α signaling pathway in adipocytes. This mitigation is evident in a diet-induced obesity model, wherein an improvement in glucose tolerance and a more favorable profile of inflammatory cytokines within visceral adipose tissue were observed.131 The driving force behind these changes has been identified as plant-derived microRNAs, miR-159a and miR-156c, derived from dried nuts, which exhibit a high degree of complementarity with the mammalian transcript TNFRSF1A.131 Concurrently, studies have shown that nanovesicles derived from blueberries neutralize the effects of TNF-α on human vascular endothelial cells.132 Exposure to Nsp12 in isolation does not instigate activation of the NF-κB pathway. However, the targeted delivery of ginger-derived miRNA to pulmonary epithelial cells and macrophages significantly inhibited Nsp12 expression, effectively forestalling the inflammatory consequences mediated by exosomal Nsp12 in lung tissues. Ginger EV-like nanoparticles (GELNs) extend their therapeutic capabilities by attenuating the production of TNF-α, IL-6, and IL-1β, concurrently suppressing the NF-κB pathway.133 In a murine model of lipopolysaccharide (LPS)-induced colitis, the oral administration of turmeric-derived nanoparticles with a specific population (TDNPs) displayed a preferential localization to inflamed colonic tissues, being predominantly internalized by colonic epithelial cells and macrophages. TDNPs offered relief from colitis symptoms by modulating pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) and upregulating the antioxidative gene HO-1, thereby inactivating the NF-κB pathway.134
AhR Pathway
The aryl hydrocarbon receptor (AhR) signaling pathway has been shown to enhance the production of interleukin-22 (IL-22) in the gut, thereby sustaining the levels of mucosal antimicrobial peptides and maintaining intestinal barrier function. Teng et al utilized AhR gene knockout mice, IL-22 gene knockout mice, and germ-free mice to irrefutably demonstrate that nanovesicles derived from ginger are absorbed by the probiotic Lactobacillus rhamnosus GG (LGG) and induce the production of indole-3-aldehyde (I3A), thus promoting the activation of the AhR pathway and the production of IL-22.89 In the context of gastrointestinal diseases, nanovesicles derived from Morus alba (mulberry) bark have been observed to exert protective effects against dextran sulfate sodium (DSS)-induced colitis by inducing various antimicrobial peptides and reducing pathogenic bacteria.135 These nanovesicles facilitate the activation of the AhR signaling pathway mediated by Heat Shock Protein Family A (HSP70) member 8 in intestinal epithelial cells, which leads to the induction of the COP9 signalosome subunit 8 (COPS8), culminating in the induction of antimicrobial peptides.135
Regulation of Oxidative Stress
Excessive inflammation can precipitate a state of persistent oxidative stress, which in turn can perpetuate the inflammatory condition. This establishes a feedback loop where inflammation and oxidative stress mutually amplify one another. The regulation of oxidative stress mediated by PDEVs can disrupt this pathological positive feedback loop, facilitating the restoration of homeostasis within the organism. Ginger-derived EVs deliver the secondary metabolite 6-gingerol to hepatocytes, where it activates Nrf2 nuclear translocation through a Toll-like receptor 4/Toll-interleukin-1 receptor (TIR)-domain-containing adapter-inducing interferon-β (TRIF)-dependent manner, mediating the expression of a suite of hepatic detoxification/antioxidant genes and suppressing the production of reactive oxygen species (ROS). This opens up novel therapeutic avenues for treating alcohol-induced liver damage.129 Blueberry-derived EVs can accelerate the nuclear translocation of Nrf2 to regulate the expression of detoxification/antioxidant genes and mitigate mitochondrial oxidative stress, thereby ameliorating non-alcoholic fatty liver disease.136 EVs from blueberries can also be taken up by primary endothelial cells in a dose-dependent manner and inhibit TNF-α-induced ROS production, thus protecting the vascular system against various stressors.132 Kim et al have explored carrot-derived EVs as a novel biomaterial with antioxidative properties and have demonstrated their ability to suppress the reduction of antioxidative molecules such as HO-1 and NAD(P)H:quinone oxidoreductase 1 (NQO-1) in cardiomyocytes and neuroblastoma cells via the Nrf-2 pathway, positioning them as potential candidates for the treatment of myocardial infarction and Parkinson’s disease.137 In addition, EVs derived from citrus lemon and strawberries have been shown to prevent oxidative stress in mesenchymal stem cells through their intrinsic bioactive compounds.138,139
Tissue Repair and Regeneration
Parenchymal cell injury is a hallmark of inflammation. Supporting the survival and regeneration of damaged parenchymal cells is beneficial for the resolution of chronic inflammation. The literature indicates that PDEVs hold extensive potential in tissue regeneration. Research by Ju et al has demonstrated that EVs derived from grapes can penetrate the intestinal mucosal barrier and be absorbed by intestinal stem cells in a mouse model of DSS-induced colitis. These grape-derived EVs significantly induce the proliferation of intestinal stem cells through the Wnt/β-catenin pathway, thereby accelerating the regeneration of intestinal epithelium and mediating the homeostatic reshaping of damaged intestinal tissue.55 Similarly, ginger-derived EVs, administered orally, are absorbed by intestinal epithelial cells, where they inhibit apoptosis and enhance cell survival and proliferation, contributing to the repair of damaged tissues.56 Experiments in vitro and in vivo by Xu et al have shown that EVs from ginseng can deliver miRNAs to bone marrow-derived mesenchymal stem cells, upregulating the PI3K signaling pathway to promote neural differentiation and regeneration.140
In another study, ginseng-derived EVs were found to significantly enhance the proliferation of human keratinocytes (HaCaT), fibroblasts, and human umbilical vein endothelial cells (HUVEC) through the extracellular signal-regulated kinase (ERK) and AKT/mammalian target of rapamycin (mTOR) pathways. These EVs increased the secretion of matrix metalloproteinase-1 (MMP-1), fibronectin-1, elastin-1, and type I collagen alpha 1 chain (COL1A1), additionally facilitating skin wound healing and reducing inflammation in a mouse skin wound model.141 Apple-derived EVs, by modulating TLR4-induced signaling, downregulated the NF-κB pathway in dermal fibroblasts. They regulated the expression of genes such as type III collagen alpha 1 chain (COL3A1), type I collagen alpha 2 chain (COL1A2), type VIII collagen alpha 1 chain (COL8A1), and type VI collagen alpha 1 chain (COL6A1), increasing collagen synthesis and downregulating metalloproteinases, thus possessing tremendous potential for skin regeneration and reducing extracellular matrix (ECM) degradation.142 Aloe vera-derived EVs significantly upregulated Nrf2, promoting the migration of HaCaT cells and fibroblasts, thereby enhancing the healing capacity of skin wounds. Moreover, they exhibited angiogenic activity, inducing angiogenesis in human umbilical vein endothelial cells, suggesting that PDEVs hold promise as natural biomaterials to promote skin regeneration.143,144 Angiogenesis is a complex biological process associated with the formation of new blood vessels and wound healing, involving a multitude of cells, mediators, and signaling pathways. EVs can influence the gene expression of pro-angiogenic pathways in relevant cells by delivering non-coding RNAs, with molecular pathways including signal transducer and activator of transcription 3 (STAT3), mitogen-activated protein kinase (MAPK), ERK1/2, Nrf2, and NF-κB.145 The current research on the mechanism of PDEV-induced angiogenesis is incomplete and warrants further exploration. Additionally, a series of studies have indicated that PDEVs isolated from a variety of plants, including cabbage, red cabbage, grapefruit, pomegranate, and wheat, can suppress apoptosis and promote epithelial cell repair by enhancing proliferation and migration.146–148
Regulation of Microbial Community Equilibrium
O-EVs have been found to exert immunomodulatory effects not only by acting on macrophages and other cell types but also by regulating the gut microbiota, thereby enhancing the integrity of the intestinal barrier. Teng et al have reported that EVs derived from plant sources are assimilated by the gut microbiome.89 This uptake, due to the vesicles’ internal constituents such as RNA, leads to alterations in the gut microbial spectrum and host physiology. For instance, ginger-derived EVs encapsulating specific microRNAs are preferentially absorbed by Lactobacillaceae, prompting targeted gene expression in Lactobacillus rhamnosus. This intricate mechanism of gene targeting elevates the production of indole-3-aldehyde (I3A), which subsequently induces the generation of interleukin-22 (IL-22). The aforementioned evidence suggests that the intake of dietary-derived EVs by the gut microbiota may improve intestinal barrier function and attenuate colitis in a mouse model through an IL-22-dependent mechanism.89 Plant-derived and milk-derived EVs containing miRNA are absorbed by bacteria and actively modulate the expression of specific genes that impact microbial growth.149,150 The presence of miRNA within EVs derived from bovine milk affects the gut microbiome profile, which can promote the growth of certain bacteria, including Firmicutes, Lachnospiraceae, and Tenericutes.150 Furthermore, EVs derived from Tartary buckwheat enhance the diversity of the gut microbiome and stimulate target functional genes that affect the physiological processes and growth of Lactobacillus rhamnosus and Escherichia coli.151
Intestinal epithelial cells play a pivotal role as a physical barrier, which is critical for the prevention and control of antigen and pathogenic toxin entry into systemic circulation, as well as the translocation of luminal nutrients, water, and electrolytes released by the gut microbiota and intestinal tissue.152 Several reports have documented the ameliorative effects of food-derived EVs and their cargo on intestinal barrier function. The miRNAs present in plant and milk-derived EVs can traverse the gastrointestinal tract and play a key role in enhancing the permeability and integrity of the intestinal barrier. They participate in various pathways, such as intestinal epithelial cell remodeling, gut microbiota modulation, and gut immune system improvement.153–155 Furthermore, orally administered fruit-derived EVs can promote the signaling processes of Wnt/β-catenin in intestinal barrier stem cells, thereby improving cell proliferation and enhancing the homeostasis and integrity of the intestinal wall.55 Zhang et al have documented that orally administered ginger-derived EVs induce cell proliferation and promote the expression of adherens junction proteins (namely E-cadherin and plakoglobin) through the internalization of EVs consumed by colonic epithelial cells in a mouse model of colitis.56 In a murine colitis model, broccoli-derived EVs regulate intestinal immune homeostasis by targeting dendritic cells (DCs), thus mitigating colitis.54 According to these extensive datasets, it has been hypothesized that food-derived EVs may provide novel insights into the modulation of intestinal barrier permeability and the gut immune system, as well as the regulation of the composition of the gut microbiome.
Application in Inflammatory Diseases
Pulmonary Inflammation (Including COVID-19)
EVs are endogenously produced by alveolar epithelial cells, macrophages, pulmonary microvascular endothelial cells, and neutrophils, and have been shown to modulate immunity in the context of pulmonary injury and inflammation.156 Recent advances suggest that PDEVs are capable of intercellular communication with animal cells across species, playing a crucial role in non-negligible immunomodulation. Immune compromise and pulmonary inflammation are hallmarks of coronavirus disease 2019 (COVID-19) infection.157 In a murine model exposed to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), researchers identified that exosomal Nsp12 and Nsp13 activate NF-κB in pulmonary macrophages, leading to the expression of inflammatory cytokines such as TNF-α, interleukin (IL)-6, and IL-1β. Ginger-derived EV-like nanoparticles (GELNs) were utilized for tracheal delivery, effectively transporting miRNA aly-miR396a-5p to the lungs. GELNs carrying miRNA inhibited the expression of viral S and Nsp12, which in turn suppressed the cytopathic effects (CPE) observed in SARS-CoV-2 infected Vero E6 cells. Furthermore, isolated exposure to Nsp12 did not activate NF-κB. The targeted delivery of ginger miRNA to pulmonary epithelial cells and macrophages could inhibit the expression of Nsp12, thereby preventing EV-mediated Nsp12-induced pulmonary inflammation. GELNs also attenuate the production of TNF-α, IL-6, and IL-1β, achieving therapeutic effect by inhibiting the NF-κB pathway.133 Additionally, oral administration of EVs derived from ginger to mice on a high-fat diet not only improved the host’s glucose tolerance and insulin response but also suppressed chronic inflammation.14,158,159 By delivering miRNAs, ginger significantly inhibited lipopolysaccharide (LPS)-induced intestinal inflammation and mitigated the progression of COVID-19 by targeting the SARS-CoV-2 viral genome.133,160,161 Notably, viral EVs induce a cascade of pro-inflammatory cytokines during disease progression, which can be counteracted by GELNs inducing endogenous EVs. This example illustrates the potential interactions between PDEVs and endogenous host EVs, although the exact mechanisms of these responses require further elucidation. In addition, novel utilization of the traditional Chinese herb Rehmanniae Radix has been explored. The administration of this herb mitigates LPS-induced pulmonary inflammation and restores dysbiosis of the gut microbiota through EVs-delivered miRNAs targeting the G protein-coupled receptor 161 (GPR161)-mediated pathway.162
Liver Inflammation
Hepatic inflammation is a hallmark pathology in non-alcoholic fatty liver disease (NAFLD), alcoholic liver disease (ALD), and chronic hepatitis B (HBV).163–165 Anti-inflammatory strategies are pivotal in the pharmacological intervention of these hepatic conditions. Ginger-derived extracellular vesicles (GDEVs) target hepatocytes, potentially due to the bioactive compound gingerol contained within GDEVs.166 In vitro studies have elucidated the anti-inflammatory effects of garlic-derived nanovesicles (GDVs), which is evidenced by the down-regulation of pro-inflammatory markers such as IFN-γ and IL-6 mRNA in HepG2 cells.167 The attenuation of hepatic inflammation may be attributed to the selective targeting of CD98 by GDVs, contributing to the reduced levels of pro-inflammatory cytokines (TNF-α, IFN-γ, and IL-6) observed both in vitro and in vivo. Additionally, the liver is prone to damage from pathogenic microbes and toxic agents, with the hyperactivation of the NLRP3 inflammasome being implicated in exacerbating inflammation and disease progression, leading to hepatic injury. Research has demonstrated that ginger rhizome-derived EV-like nanoparticles (G-ELNs) impede the activation of the NLRP3 inflammasome in primary macrophages by interfering with the downstream pathways, specifically through the autolysis of caspase-1 and the subsequent reduction in secretion of IL-1β and IL-18.125 Similarly, shiitake mushroom-derived EV-like nanoparticles (S-ELNs) significantly inhibit the formation of the NLRP3 inflammasome in primary macrophages, a mechanism distinct from that of shiitake polysaccharides, by preventing inflammasome assembly. This was demonstrated by immunofluorescence staining, which showed a significant reduction in ASC specks formation in S-ELN-treated cells, indicating that S-ELNs thwart the initial stages of the NLRP3 inflammasome pathway. Pre-treatment with S-ELNs has been shown to reduce the levels of pro-IL-1β and NLRP3 in mouse livers exposed to GalN/LPS-induced stress, suggesting an inhibitory effect on the secretion of IL-6 and the protein and mRNA expression levels of IL-1β. These findings suggest that S-ELNs may mitigate the severity of GalN/LPS induced acute liver injury in mice.126
Intestinal Inflammation
Current research predominantly focuses on the immunomodulatory properties of EVs derived from milk, including human, bovine, and porcine sources, as well as PDEVs, with a particular emphasis on their potential to protect against intestinal inflammation.168 These milk-derived EVs are orally bioavailable and have been demonstrated to navigate through the gastrointestinal tract to reach the colon, where they are taken up by intestinal epithelial cells, leading to the attenuation of inflammation and a possible reduction in the frequency of enterocolitis.28,169 Similarly, plant EVs derived from sources such as orange juice, grapefruit, broccoli, onions, leeks, and ginger have been shown to be effective in ameliorating colitis, modulating the gut microbiome, and maintaining intestinal homeostasis. Lactic acid bacteria appear to preferentially absorb GELNs through the lipid components of their cellular surface. These GELNs contain microRNAs targeting various genes identified in Lactobacillus rhamnosus GG (LGG). Specifically, GELN mdo-miR7267-3p targets the LGG monooxygenase gene ycnE, resulting in elevated levels of indole-3-aldehyde (I3A). Both GELN-RNA and I3A act as ligands on aryl hydrocarbon receptors, thereby inducing the production of the anti-inflammatory cytokine IL-22, which in turn mitigates colonic inflammation.89 This is a meta-analysis of the application of different species of O-EVs in anti-intestinal inflammation (Table 4).
 |
Table 4 The Application of Different Species of O-EVs in Anti-Intestinal Inflammation |
Periodontitis
Chronic conditions such as periodontitis, caused by a variety of oral and other pathogens, often result in progressive tissue deterioration that can lead to tooth loss and systemic illness. In such scenarios, routine surgical procedures and antibiotic therapy, while not entirely ineffective, have been shown to be inadequate. Nano-vesicles derived from ginger (sized at 204 nm) have been shown to protect the oral environment by inhibiting the pathogenicity of Porphyromonas gingivalis, a key bacterium associated with gum disease.174 In vivo studies have revealed that ginger-derived nano-vesicles significantly reduce alveolar bone loss and the release of inflammatory cytokines induced by P. gingivalis. The unsaturated PA (34:2) from ginger-derived nano-vesicles promotes the uptake of the vesicles by Lactobacillus gingivitis through interaction with bacterial surface heme-binding proteins. PA (34:2) and nano-vesicle miRNA selectively inhibit the expression of various virulence factors, restraining the attachment and invasion of P. gingivalis in oral epithelial cells. As a result, ginger-derived nano-vesicles hold promise for development as a therapeutic intervention for chronic periodontitis.
Arthritis
EVs from bovine milk contain miR-30-A, miR-223, miR-92-A, β-casein mRNA, and β-lactoglobulin mRNA, which are readily absorbed by RAW264.7 cells, splenocytes, and intestinal cells, and contribute to the deceleration of arthritis onset in a murine model of rheumatoid arthritis. Histological evidence of reduced cartilage pathology and bone marrow inflammation, as well as lowered serum levels of IL-6, monocyte chemoattractant protein-1 (MCP-1), and anti-collagen IgG2a, further substantiate these findings.38 This study represents the first demonstration that oral administration of milk-derived EVs can ameliorate arthritis, providing a rational basis for further exploration of their full therapeutic potential and their potential for prevention and mitigation in other inflammatory diseases. In this vein, oral administration of bovine milk-derived EVs in healthy mice resulted in a reduction of osteoclast activity (bone resorption) and an increase in the number of osteoblasts in trabecular bone regions.175,176 These findings warrant further investigation in models of bone-related diseases such as osteogenesis imperfecta and Paget’s disease.
Other Inflammatory Conditions
Oral administration of EVs also holds substantial promise in the treatment of skin inflammation.177 Methods for the extraction of highly purified and stable extracellular vesicles from ginseng have been reported, which have shown exceptional efficacy in stimulating neural differentiation. They hold substantial potential for neural regeneration and have been shown to have beneficial effects on healing and anti-inflammation in chronic skin wound models.140,141 Furthermore, Xu et al investigated the impact of orally administered oat-derived ELNs (O-ELNs) on alcohol-induced brain inflammation.178 The study discovered that PKH26-labeled O-ELNs crossed the blood-brain barrier (BBB) via free diffusion and active transport through endothelial cells in murine brains. The uptake of O-ELNs by microglial cells significantly diminished levels of pro-inflammatory cytokines (IL-6, IL-1β, TNFα), and suppressed the induction of the CD11bLy6G+ microglial lineage with CD45, thus reducing infiltrating cells and the presence of activated CD68Iba-1 microglia. This work suggests that O-ELNs effectively attenuate brain inflammation, yet the crucial regulatory role of the contained miRNAs remains to be elucidated.
Conclusions and Prospects
Recently, EVs have emerged as a novel cell-free anti-inflammatory strategy, offering numerous advantages including high biocompatibility and low cytotoxicity, environmental sustainability, reproducibility, cost-effectiveness, and inherent therapeutic activity. These nanocarriers capitalize on their intrinsic biological properties and can be further enhanced by the integration of site-specific regulatory modifications to facilitate efficient drug delivery. However, one of the primary challenges in anti-inflammatory applications of O-EVs is the lack of uniform and standardized isolation methods. Techniques such as ultracentrifugation, density gradient centrifugation, ultrafiltration, and size-exclusion chromatography have proven to be time-consuming and yield low productivity in the process of industrial transition. Inconsistencies in the results obtained through ultracentrifugation, as reported by researchers, may be influenced by variables such as centrifugal force, speed, and rotor type.179 Consequently, it is imperative to explore and establish an ideal method of separation that yields homogeneity, stability, and high production rates of O-EVs.
The understanding of the physiological properties of O-EVs, their drug delivery mechanisms, and targeted release is still relatively limited, and no definitive conclusions have been drawn regarding the exact mechanisms of specific binding of O-EVs to receptor cells. The transition from laboratory research to commercial production for clinical applications requires substantial efforts, such as the development of scalable and standardized production and preparation protocols, the exploration of long-term stable storage and transport modes, and the conduction of clinical trials to prove safety and efficacy. Clinical trials have been undertaken exploring the therapeutic effects of PDEVs, including the relief of oral mucositis during chemotherapy for head and neck cancer (NCT01668849) and the symptomatic improvement of inflammatory bowel disease using ginger-derived EVs alone or in combination with curcumin (NCT04879810). PDEVs also offer solutions for the transport of anti-cancer compounds by overcoming limitations in bioavailability, stability, and safety encountered with synthetic liposomes and EVs from non-food sources.
Investigating the biological properties and transport mechanisms of EVs represents one of the main challenges in their application as oral anti-inflammatory agents. The gastrointestinal tolerance of O-EVs, a noteworthy characteristic, has yet to be clearly described. Understanding the gastrointestinal tolerance mechanisms of O-EVs could significantly alter the predicaments faced by oral drug delivery. This tolerance may benefit from the ordered membrane structure or lipid composition of the O-EVs. To further explore the gastrointestinal tolerance of O-EVs, a comprehensive understanding of their physical properties may be the correct approach. The proteins, lipids, and miRNA within O-EVs appear to be involved in regulatory communication, with variations potentially arising from the EVs’ sources. However, the general regulatory mechanisms seem to be elusive, thus focusing on the regulation within specific species of O-EVs could be more productive. Additionally, differentiating between regulatory patterns and targeting mechanisms may aid in the delivery of various drugs, allowing researchers to select from various sources based on the applicability of O-EVs to diseases. The naturally active compounds carried by O-EVs represent an unpredictable variable that can synergize with drugs, potentially affecting therapeutic efficacy.60 Extensive research is necessary to elucidate the composition of naturally active compounds within O-EVs and the mechanisms by which they interact with drugs to achieve effective loading or synergistic therapeutic effects.
The oral administration application of O-EVs also faces challenges related to their insufficient targeting capabilities. Proteins are key factors in receptor recognition, and studies have shown that some plant-derived O-EVs contain few proteins and provide poor selectivity.180 Engineering has been extensively used in the study of mammalian O-EVs, giving them more malleable properties.181 In the future, ligands such as folic acid (FA) or peptides may be used to modify plant-derived O-EVs for enhanced cargo targeting capabilities. Membrane fusion techniques can be employed to amalgamate plant-derived O-EVs with artificially synthesized liposomes, mammalian EVs, and bacterial EVs to produce hybrid EVs that may offer a wider range of biological properties. These techniques could potentially improve the targeting and immune evasion capabilities of O-EVs. Given the breadth and variety of challenges faced by extracellular vesicles as oral delivery systems in inflammatory diseases, several different focal points for future research in this field are anticipated: (1) extensive exploration of new EV sources and their potential as oral delivery systems; (2) the potential and mechanisms of different EV sources in traversing the gastrointestinal barrier; (3) specificity of different EV sources to various cells and tissues; (4) more efficient drug loading and specificity or controlled drug release; (5) the cellular mechanisms of EV uptake and endosomal escape in target cells; (6) the development of surface modification methods for EVs tailored for targeted applications. Interestingly, the development of novel diagnostic tools using cell-derived EVs is well known.182 EV-like nanoparticles (ELNs) from dietary sources can be easily mass-produced, thus well-suited for such imaging technology development. This is an emerging field that must fully realize its potential. Recently, EVs from goat milk have been utilized to develop such optical imaging techniques. EVs labeled with commercial fluorophores have been developed into nanoprobes capable of localizing to sites of inflammation.183 In conclusion, despite the potential demonstrated by extracellular vesicles as oral delivery carriers in the treatment of inflammatory diseases, their application still faces numerous challenges. Future research should delve deeper into their biological mechanisms and optimize oral delivery systems to achieve broader clinical applications.
Data Sharing Statement
All data generated or analyzed during this study are included in this manuscript.
Funding
This research was supported by National Key Clinical Specialties Construction Program, the Sichuan Natural Science Foundation (2023NSFSC1646).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Bennett JM, Reeves G, Billman GE, Sturmberg JP. Inflammation-nature’s way to efficiently respond to all types of challenges: implications for understanding and managing ”the epidemic” of chronic diseases. Front Med. 2018;5:316. doi:10.3389/fmed.2018.00316
2. Gupta SC, Kunnumakkara AB, Aggarwal S, Aggarwal BB. Inflammation, a double-edge sword for cancer and other age-related diseases. Front Immunol. 2018;9:2160. doi:10.3389/fimmu.2018.02160
3. Hua S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract - influence of physiological, pathophysiological and pharmaceutical factors. Front Pharmacol. 2020;11:524. doi:10.3389/fphar.2020.00524
4. Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem. Pharmacol. 2020;180:114147. doi:10.1016/j.bcp.2020.114147
5. Sinniah A, Yazid S, Flower RJ. From NSAIDs to glucocorticoids and beyond. Cells. 2021;11:10. doi:10.3390/cells11010010
6. Dai C, Huang YH, Jiang M. Combination therapy in inflammatory bowel disease: current evidence and perspectives. Int Immunopharmacol. 2023;114:109545. doi:10.1016/j.intimp.2022.109545
7. Mathur P, Rawal S, Patel B, Patel MM. Oral delivery of anticancer agents using nanoparticulate drug delivery system. Curr Drug Metab. 2019;20:1132–1140. doi:10.2174/1389200220666191007154017
8. Liu L, Yao W, Rao Y, Lu X, Gao J. pH-Responsive carriers for oral drug delivery: challenges and opportunities of current platforms. Drug Deliv. 2017;24:569–581. doi:10.1080/10717544.2017.1279238
9. Din FU, Aman W, Ullah I, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int j Nanomed. 2017;12:7291–7309. doi:10.2147/IJN.S146315
10. Yang B, Chen Y, Shi J. Exosome biochemistry and advanced nanotechnology for next-generation theranostic platforms. Advanced Mat. 2019;31:e1802896. doi:10.1002/adma.201802896
11. Schuh C, Cuenca J, Alcayaga-Miranda F, Khoury M. Exosomes on the border of species and kingdom intercommunication. Transl Res. 2019;210:80–98. doi:10.1016/j.trsl.2019.03.008
12. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. doi:10.1126/science.aau6977
13. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748–759. doi:10.1038/s41565-021-00931-2
14. Shao M, Jin X, Chen S, Yang N, Feng G. Plant-derived extracellular vesicles -A novel clinical anti-inflammatory drug carrier worthy of investigation. Biomed Pharmacothe. 2023;169:115904. doi:10.1016/j.biopha.2023.115904
15. Feng J, Xiu Q, Huang Y, Troyer Z, Li B, Zheng L. Plant-derived vesicle-like nanoparticles as promising biotherapeutic tools: present and future. Advanced Mat. 2023;35:e2207826. doi:10.1002/adma.202207826
16. Di Giulio S, Carata E, Mariano S, Panzarini E. Plant extracellular vesicles: investigating their utilization as beneficial nutrients in diet. Appl Sci. 2023;13:6656. doi:10.3390/app13116656
17. Wu C, Li J, Huang K, et al. Advances in preparation and engineering of plant-derived extracellular vesicles for nutrition intervention. Food Chem. 2024;457:140199. doi:10.1016/j.foodchem.2024.140199
18. Somiya M, Yoshioka Y, Ochiya T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J Extracell Vesicles. 2018;7:1440132. doi:10.1080/20013078.2018.1440132
19. Elashiry M, Carroll A, Yuan J, et al. Oral microbially-induced small extracellular vesicles cross the blood–brain barrier. Int J Mol Sci. 2024;25:4509. doi:10.3390/ijms25084509
20. Song M, Cui M, Fang Z, Liu K. Advanced research on extracellular vesicles based oral drug delivery systems. J Cont Rel. 2022;351:560–572. doi:10.1016/j.jconrel.2022.09.043
21. Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208–1215. doi:10.1172/JCI81135
22. Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi:10.1016/j.imlet.2006.09.005
23. An Q, van Bel AJ, Hückelhoven R. Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal Behav. 2007;2:4–7. doi:10.4161/psb.2.1.3596
24. Xia X, Shi B, Wang L, et al. From mouse to mouse-ear cress: nanomaterials as vehicles in plant biotechnology. Exploration. 2021;1:9–20. doi:10.1002/EXP.20210002
25. Zhong J, Xia B, Shan S, et al. High-quality milk exosomes as oral drug delivery system. Biomaterials. 2021;277:121126. doi:10.1016/j.biomaterials.2021.121126
26. Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2013:2.
27. Buschmann D, Mussack V, Byrd JB. Separation, characterization, and standardization of extracellular vesicles for drug delivery applications. Adv Drug Delivery Rev. 2021;174:348–368. doi:10.1016/j.addr.2021.04.027
28. Guo MM, Zhang K, Zhang JH. Human breast milk-derived exosomal mir-148a-3p protects against necrotizing enterocolitis by regulating p53 and sirtuin 1. Inflammation. 2022;45:1254–1268. doi:10.1007/s10753-021-01618-5
29. Reif S, Elbaum-Shiff Y, Koroukhov N, Shilo I, Musseri M, Golan-Gerstl R. Cow and human milk-derived exosomes ameliorate colitis in dss murine model. Nutrients. 2020;12. doi:10.3390/nu13010012
30. Roefs MT, Sluijter JPG, Vader P. Extracellular Vesicle-Associated Proteins in Tissue Repair. Trends Cell Biol. 2020;30:990–1013. doi:10.1016/j.tcb.2020.09.009
31. Shelke GV, Yin Y, Jang SC, et al. Endosomal signalling via exosome surface TGFβ-1. J Extracell Vesicles. 2019;8:1650458. doi:10.1080/20013078.2019.1650458
32. Pieters BC, Arntz OJ, Bennink MB, et al. Commercial cow milk contains physically stable extracellular vesicles expressing immunoregulatory TGF-β. PLoS One. 2015;10:e0121123.
33. Stremmel W, Weiskirchen R, Melnik BC. Milk exosomes prevent intestinal inflammation in a genetic mouse model of ulcerative colitis: a pilot experiment. Inflamm Intestinal Disea. 2020;5:117–123. doi:10.1159/000507626
34. Wu D, Kittana H, Shu J, et al. Dietary depletion of milk exosomes and their microRNA cargos elicits a depletion of mir-200a-3p and elevated intestinal inflammation and chemokine (C-X-C Motif) Ligand 9 Expression in Mdr1a(-/-) Mice. Curr Dev Nutr. 2019;3:nzz122.
35. Fu P, Guo Y, Luo Y, et al. Visualization of microRNA therapy in cancers delivered by small extracellular vesicles. J Nanobiotechnol. 2023;21:457. doi:10.1186/s12951-023-02187-5
36. Munir J, Yoon JK, Ryu S. Therapeutic miRNA-enriched extracellular vesicles: current approaches and future prospects. Cells. 2020;9:2271. doi:10.3390/cells9102271
37. Smith NC, Wajnberg G, Chacko S, et al. Characterization of miRNAs in extracellular vesicles released from Atlantic salmon monocyte-like and macrophage-like cells. Front Immunol. 2020;11:587931. doi:10.3389/fimmu.2020.587931
38. Arntz OJ, Pieters BC, Oliveira MC, et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol Nutr Food Res. 2015;59:1701–1712. doi:10.1002/mnfr.201500222
39. Li Y, Zhang X, Zhang C, et al. Comparative study on the immunomodulatory function of extracellular vesicles from different dairy products. Food Funct. 2022;13:2504–2514. doi:10.1039/D1FO02394B
40. Tong L, Hao H, Zhang Z, et al. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics. 2021;11:8570–8586. doi:10.7150/thno.62046
41. Li B, Hock A, Wu RY, et al. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS One. 2019;14:e0211431.
42. Pisano C, Galley J, Elbahrawy M, et al. human breast milk-derived extracellular vesicles in the protection against experimental necrotizing enterocolitis. J Pediatric Surg. 2020;55:54–58. doi:10.1016/j.jpedsurg.2019.09.052
43. Xie MY, Chen T, Xi QY, et al. Porcine milk exosome miRNAs protect intestinal epithelial cells against deoxynivalenol-induced damage. Biochem. Pharmacol. 2020;175:113898. doi:10.1016/j.bcp.2020.113898
44. Xie MY, Hou LJ, Sun JJ, et al. Porcine milk exosome miRNAs attenuate LPS-induced apoptosis through Inhibiting TLR4/NF-κB and p53 pathways in intestinal epithelial cells. J Agri Food Chemi. 2019;67:9477–9491. doi:10.1021/acs.jafc.9b02925
45. Gao HN, Hu H, Wen PC, et al. Yak milk-derived exosomes alleviate lipopolysaccharide-induced intestinal inflammation by inhibiting PI3K/AKT/C3 pathway activation. Journal of Dairy Science. 2021;104:8411–8424. doi:10.3168/jds.2021-20175
46. Chen M, Lin S, Zhou C, Cui D, Haick H, Tang N. From conventional to microfluidic: progress in extracellular vesicle separation and individual characterization. Adv Healthcare Mater. 2023;12:e2202437.
47. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7:789–804. doi:10.7150/thno.18133
48. Umezu T, Takanashi M, Murakami Y, et al. Acerola exosome-like nanovesicles to systemically deliver nucleic acid medicine via oral administration. Mol Ther Meth Clin Develop. 2021;21:199–208. doi:10.1016/j.omtm.2021.03.006
49. Nemidkanam V, Chaichanawongsaroj N. Characterizing Kaempferia parviflora extracellular vesicles, a nanomedicine candidate. PLoS One. 2022;17:e0262884.
50. Di Gioia S, Hossain MN, Conese M. Biological properties and therapeutic effects of plant-derived nanovesicles. Open Med. 2020;15:1096–1122. doi:10.1515/med-2020-0160
51. Wang Q, Zhuang X, Mu J, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4:1867. doi:10.1038/ncomms2886
52. López-Molina D, Chazarra S, How CW, et al. Cinnamate of inulin as a vehicle for delivery of colonic drugs. Int J Pharm. 2015;479:96–102. doi:10.1016/j.ijpharm.2014.12.064
53. Wang B, Zhuang X, Deng ZB, et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mole Ther. 2014;22:522–534. doi:10.1038/mt.2013.190
54. Deng Z, Rong Y, Teng Y, et al. Broccoli-Derived nanoparticle inhibits mouse colitis by activating dendritic cell amp-activated protein kinase. Mole Ther. 2017;25:1641–1654. doi:10.1016/j.ymthe.2017.01.025
55. Ju S, Mu J, Dokland T, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mole Ther. 2013;21:1345–1357. doi:10.1038/mt.2013.64
56. Zhang M, Viennois E, Prasad M, et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi:10.1016/j.biomaterials.2016.06.018
57. Schillaci O, Fontana S, Monteleone F, et al. Exosomes from metastatic cancer cells transfer amoeboid phenotype to non-metastatic cells and increase endothelial permeability: their emerging role in tumor heterogeneity. Sci Rep. 2017;7:4711. doi:10.1038/s41598-017-05002-y
58. Pinedo M, de la Canal L, de Marcos Lousa C. A call for Rigor and standardization in plant extracellular vesicle research. J Extracell Vesicles. 2021;10:e12048.
59. Kim J, Li S, Zhang S, Wang J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J Pharm Sci. 2022;17:53–69. doi:10.1016/j.ajps.2021.05.006
60. Nemati M, Singh B, Mir RA, et al. Plant-derived extracellular vesicles: a novel nanomedicine approach with advantages and challenges. Cell Commun Signali. 2022;20:69. doi:10.1186/s12964-022-00889-1
61. Jahromi LP, Fuhrmann G. Bacterial extracellular vesicles: understanding biology promotes applications as nanopharmaceuticals. Adv Drug Delivery Rev. 2021;173:125–140. doi:10.1016/j.addr.2021.03.012
62. Huang W, Meng L, Chen Y, Dong Z, Peng Q. Bacterial outer membrane vesicles as potential biological nanomaterials for antibacterial therapy. Acta Biomater. 2022;140:102–115. doi:10.1016/j.actbio.2021.12.005
63. Alves NJ, Turner KB, DiVito KA, Daniele MA, Walper SA. Affinity purification of bacterial outer membrane vesicles (OMVs) utilizing a His-tag mutant. Res Microbiol. 2017;168:139–146. doi:10.1016/j.resmic.2016.10.001
64. Dauros Singorenko P, Chang V, Whitcombe A, et al. Isolation of membrane vesicles from prokaryotes: a technical and biological comparison reveals heterogeneity. J Extracell Vesicles. 2017;6:1324731. doi:10.1080/20013078.2017.1324731
65. Bonnington KE, Kuehn MJ. Protein selection and export via outer membrane vesicles. Biochimica Et Biophysica Acta. 2014;1843(8):1612–1619. doi:10.1016/j.bbamcr.2013.12.011
66. Li M, Zhou H, Yang C, et al. Bacterial outer membrane vesicles as a platform for biomedical applications: an update. J Cont Rel. 2020;323:253–268. doi:10.1016/j.jconrel.2020.04.031
67. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi:10.1038/nri3399
68. Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375–387. doi:10.1038/nri3837
69. Tashiro Y, Hasegawa Y, Shintani M, et al. Interaction of bacterial membrane vesicles with specific species and their potential for delivery to target cells. Front Microbiol. 2017;8:571. doi:10.3389/fmicb.2017.00571
70. Zhang LY, Yang X, Wang SB, Chen H, Pan HY, Hu ZM. Membrane derived vesicles as biomimetic carriers for targeted drug delivery system. Curr Top Med Chem. 2020;20:2472–2492. doi:10.2174/1568026620666200922113054
71. Alvarez CS, Badia J, Bosch M, Giménez R, Baldomà L. Outer membrane vesicles and soluble factors released by probiotic Escherichia coli nissle 1917 and Commensal ECOR63 enhance barrier function by regulating expression of tight junction proteins in intestinal epithelial cells. Front Microbiol. 2016;7:1981. doi:10.3389/fmicb.2016.01981
72. Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–520. doi:10.1016/j.chom.2012.08.004
73. Fábrega MJ, Aguilera L, Giménez R, et al. Activation of immune and defense responses in the intestinal mucosa by outer membrane vesicles of commensal and probiotic Escherichia coli strains. Front Microbiol. 2016;7:705. doi:10.3389/fmicb.2016.00705
74. Fábrega MJ, Rodríguez-Nogales A, Garrido-Mesa J, et al. Intestinal Anti-inflammatory Effects of Outer Membrane Vesicles from Escherichia coli Nissle 1917 in DSS-Experimental Colitis in Mice. Front Microbiol. 2017;8:1274. doi:10.3389/fmicb.2017.01274
75. Choi JH, Moon CM, Shin TS, et al. Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp Mol Med. 2020;52:423–437. doi:10.1038/s12276-019-0359-3
76. Kuhn T, Koch M, Fuhrmann G. Probiomimetics-novel lactobacillus-mimicking microparticles show anti-inflammatory and barrier-protecting effects in gastrointestinal models. Small. 2020;16:e2003158. doi:10.1002/smll.202003158
77. Cani PD, de Vos WM. Next-generation beneficial microbes: the case of akkermansia muciniphila. Front Microbiol. 2017;8:1765. doi:10.3389/fmicb.2017.01765
78. Yaghoubfar R, Behrouzi A, Ashrafian F, et al. Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci Rep. 2020;10:22119. doi:10.1038/s41598-020-79171-8
79. Cheng L, Hill AF. Therapeutically harnessing extracellular vesicles. Nat Rev Drug Discov. 2022;21:379–399. doi:10.1038/s41573-022-00410-w
80. Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mole Ther. 2010;18:1606–1614. doi:10.1038/mt.2010.105
81. Zhang M, Wang X, Han MK, Collins JF, Merlin D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine. 2017;12:1927–1943. doi:10.2217/nnm-2017-0196
82. Zhu H, He W. Ginger: a representative material of herb-derived exosome-like nanoparticles. Fronti Nutrit. 2023;10:1223349. doi:10.3389/fnut.2023.1223349
83. Mun D, Oh S, Kim Y. Perspectives on bovine milk-derived extracellular vesicles for therapeutic applications in gut health. Food Scien Animal Reso. 2022;42:197–209. doi:10.5851/kosfa.2022.e8
84. Fang Z, Liu K. Plant-derived extracellular vesicles as oral drug delivery carriers. J Cont Rel. 2022;350:389–400. doi:10.1016/j.jconrel.2022.08.046
85. Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371(1):48–61. doi:10.1016/j.canlet.2015.10.020
86. Osteikoetxea X, Sódar B, Németh A, et al. Differential detergent sensitivity of extracellular vesicle subpopulations. Org Biomol Chem. 2015;13:9775–9782. doi:10.1039/C5OB01451D
87. Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30–41. doi:10.1016/j.plipres.2017.03.001
88. Boura E, Ivanov V, Carlson LA, Mizuuchi K, Hurley JH. Endosomal sorting complex required for transport (ESCRT) complexes induce phase-separated microdomains in supported lipid bilayers. J Biol Chem. 2012;287:28144–28151. doi:10.1074/jbc.M112.378646
89. Teng Y, Ren Y, Sayed M, et al. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe. 2018;24:637–52.e8. doi:10.1016/j.chom.2018.10.001
90. Parada N, Romero-Trujillo A, Georges N, Alcayaga-Miranda F. Camouflage strategies for therapeutic exosomes evasion from phagocytosis. J Adv Res. 2021;31:61–74. doi:10.1016/j.jare.2021.01.001
91. Szatmári T, Hargitai R, Sáfrány G, Lumniczky K. Extracellular vesicles in modifying the effects of ionizing radiation. Int J Mol Sci. 2019;21:20. doi:10.3390/ijms21010020
92. Das CK, Jena BC, Banerjee I, et al. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol Pharmaceut. 2019;16:24–40.
93. Sasaki D, Kusamori K, Nishikawa M. Delivery of corn-derived nanoparticles with anticancer activity to tumor tissues by modification with polyethylene glycol for cancer therapy. Pharm Res. 2023;40:917–926. doi:10.1007/s11095-022-03431-7
94. Zhang L, He F, Gao L, et al. Engineering exosome-like nanovesicles derived from asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int j Nanomed. 2021;16:1575–1586. doi:10.2147/IJN.S293067
95. Niu W, Xiao Q, Wang X, et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21:1484–1492. doi:10.1021/acs.nanolett.0c04753
96. Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Cont Rel. 2015;205:35–44. doi:10.1016/j.jconrel.2014.11.029
97. Haney MJ, Klyachko NL, Zhao Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Cont Rel. 2015;207:18–30. doi:10.1016/j.jconrel.2015.03.033
98. Hettich BF, Bader JJ, Leroux JC. Encapsulation of hydrophilic compounds in small extracellular vesicles: loading capacity and impact on vesicle functions. Adv Healthcare Mater. 2022;11:e2100047.
99. Kalarikkal SP, Prasad D, Kasiappan R, Chaudhari SR, Sundaram GM. A cost-effective polyethylene glycol-based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci Rep. 2020;10:4456. doi:10.1038/s41598-020-61358-8
100. Haney MJ, Zhao Y, Jin YS, et al. Macrophage-derived extracellular vesicles as drug delivery systems for triple negative breast cancer (TNBC) therapy. J Neuroimmune Pharm. 2020;15:487–500. doi:10.1007/s11481-019-09884-9
101. Yang M, Luo Q, Chen X, Chen F. Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J Nanobiotechnol. 2021;19:259. doi:10.1186/s12951-021-00995-1
102. Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12:655–664. doi:10.1016/j.nano.2015.10.012
103. Zhu JY, Zheng DW, Zhang MK, et al. Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 2016;16:5895–5901. doi:10.1021/acs.nanolett.6b02786
104. Mammadova R, Maggio S, Fiume I, et al. Protein biocargo and anti-inflammatory effect of tomato fruit-derived nanovesicles separated by density gradient ultracentrifugation and loaded with curcumin. Pharmaceutics. 2023; 15. doi:10.3390/pharmaceutics16010015
105. Illes B, Hirschle P, Barnert S, Cauda V, Wuttke S, Engelke H. Exosome-coated metal–organic framework nanoparticles: an efficient drug delivery platform. Chem Mater. 2017;29:8042–8046. doi:10.1021/acs.chemmater.7b02358
106. Mao Y, Han M, Chen C, et al. A biomimetic nanocomposite made of a ginger-derived exosome and an inorganic framework for high-performance delivery of oral antibodies. Nanoscale. 2021;13:20157–20169. doi:10.1039/D1NR06015E
107. Sung J, Yang C, Collins JF, Merlin D. Preparation and Characterization of Ginger Lipid-derived Nanoparticles for Colon-targeted siRNA Delivery. Bio-Protocol. 2020;2020:10.
108. Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–494. doi:10.1016/j.actbio.2019.05.054
109. Wang Q, Ren Y, Mu J, et al. Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites. Cancer Res. 2015;75:2520–2529. doi:10.1158/0008-5472.CAN-14-3095
110. Fu S, Wang Y, Xia X, Zheng JC. Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact. 2020;20:100261. doi:10.1016/j.impact.2020.100261
111. Gelibter S, Marostica G, Mandelli A, et al. The impact of storage on extracellular vesicles: a systematic study. J Extracell Vesicles. 2022;11:e12162.
112. Xiao Q, Zhao W, Wu C, et al. Lemon-derived extracellular vesicles nanodrugs enable to efficiently overcome cancer multidrug resistance by endocytosis-triggered energy dissipation and energy production reduction. Adv Sci. 2022;9:e2105274. doi:10.1002/advs.202105274
113. Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541–555. doi:10.1038/nrg3763
114. Nair KGS, Ramaiyan V, Sukumaran SK. Enhancement of drug permeability across blood brain barrier using nanoparticles in meningitis. Inflammopharmacology. 2018;26:675–684. doi:10.1007/s10787-018-0468-y
115. Zhuang X, Teng Y, Samykutty A, et al. Grapefruit-derived nanovectors delivering therapeutic miR17 through an intranasal route inhibit brain tumor progression. Mole Ther. 2016;24:96–105. doi:10.1038/mt.2015.188
116. Rahimi Ghiasi M, Rahimi E, Amirkhani Z, Salehi R. Leucine-rich repeat-containing g-protein coupled receptor 5 gene overexpression of the rat small intestinal progenitor cells in response to orally administered grape exosome-like nanovesicles. Adv Biomed Res. 2018;7:125. doi:10.4103/abr.abr_114_18
117. Li X, Liang Z, Du J, et al. Herbal decoctosome is a novel form of medicine. Sci China Life Sci. 2019;62:333–348. doi:10.1007/s11427-018-9508-0
118. Wu J, Ma X, Lu Y, et al. Edible pueraria lobata-derived exosomes promote M2 macrophage polarization. Molecules. 2022;28:27. doi:10.3390/molecules28010027
119. Kang SJ, Kim SE, Seo M-J, Kim E, Rhee WJ. Suppression of inflammatory responses in macrophages by onion-derived extracellular vesicles. J Ind Eng Chem. 2022;115:287–297. doi:10.1016/j.jiec.2022.08.011
120. Gao C, Zhou Y, Chen Z, et al. Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis. Theranostics. 2022;12:5596–5614. doi:10.7150/thno.73650
121. Bian Y, Li W, Jiang X, et al. Garlic-derived exosomes carrying miR-396e shapes macrophage metabolic reprograming to mitigate the inflammatory response in obese adipose tissue. J Nutr Biochem. 2023;113:109249. doi:10.1016/j.jnutbio.2022.109249
122. Liu J, Li W, Bian Y, et al. Garlic-derived exosomes regulate PFKFB3 expression to relieve liver dysfunction in high-fat diet-fed mice via macrophage-hepatocyte crosstalk. Phytomedicine. 2023;112:154679. doi:10.1016/j.phymed.2023.154679
123. Kim WS, Ha JH, Jeong SH, et al. Immunological effects of aster yomena callus-derived extracellular vesicles as potential therapeutic agents against allergic asthma. Cells. 2022; 11. doi:10.3390/cells12010011
124. Taşlı PN. Usage of celery root exosome as an immune suppressant; Lipidomic characterization of Apium graveolens originated exosomes and its suppressive effect on PMA/ionomycin mediated CD4(+) T lymphocyte activation. J Food Biochem. 2022;46:e14393. doi:10.1111/jfbc.14393
125. Chen X, Zhou Y, Yu J. Exosome-like nanoparticles from ginger rhizomes inhibited NLRP3 Inflammasome Activation. Mol Pharmaceut. 2019;16:2690–2699. doi:10.1021/acs.molpharmaceut.9b00246
126. Liu B, Lu Y, Chen X, et al. Protective role of shiitake mushroom-derived exosome-like nanoparticles In D-galactosamine and lipopolysaccharide-induced acute liver injury in mice. Nutrients. 2020;13:12.
127. Chen X, Liu B, Li X, et al. Identification of anti-inflammatory vesicle-like nanoparticles in honey. J Extracell Vesicles. 2021;10:e12069. doi:10.1002/jev2.12069
128. Liu B, Li X, Yu H, et al. Therapeutic potential of garlic chive-derived vesicle-like nanoparticles in NLRP3 inflammasome-mediated inflammatory diseases. Theranostics. 2021;11:9311–9330. doi:10.7150/thno.60265
129. Zhuang X, Deng ZB, Mu J, et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713. doi:10.3402/jev.v4.28713
130. Tong L, Zhang X, Hao H, et al. Lactobacillus rhamnosus GG derived extracellular vesicles modulate gut microbiota and attenuate inflammatory in dss-induced colitis mice. Nutrients. 2021;14:13. doi:10.3390/nu14010013
131. Aquilano K, Ceci V, Gismondi A, et al. Adipocyte metabolism is improved by TNF receptor-targeting small RNAs identified from dried nuts. Commun Biol. 2019;2:317. doi:10.1038/s42003-019-0563-7
132. De Robertis M, Sarra A, V D, et al. Blueberry-derived exosome-like nanoparticles counter the response to TNF-α-induced change on gene expression in EA.hy926 Cells. Biomolecules. 2020;11:10. doi:10.3390/biom11010010
133. Teng Y, Xu F, Zhang X, et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mole Ther. 2021;29:2424–2440. doi:10.1016/j.ymthe.2021.05.005
134. Liu C, Yan X, Zhang Y, et al. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J Nanobiotechnol. 2022;20:206. doi:10.1186/s12951-022-01421-w
135. Sriwastva MK, Deng ZB, Wang B, et al. Exosome-like nanoparticles from Mulberry bark prevent DSS-induced colitis via the AhR/COPS8 pathway. EMBO Rep. 2022;23:e53365. doi:10.15252/embr.202153365
136. Zhao WJ, Bian YP, Wang QH, et al. Blueberry-derived exosomes-like nanoparticles ameliorate nonalcoholic fatty liver disease by attenuating mitochondrial oxidative stress. Acta Pharmacol Sin. 2022;43:645–658. doi:10.1038/s41401-021-00681-w
137. Kim DK, Rhee WJ, Ting SC. Antioxidative effects of carrot-derived nanovesicles in cardiomyoblast and neuroblastoma cells. Pharmaceutics. 2021;14:13. doi:10.3390/pharmaceutics14010013
138. Baldini N, Torreggiani E, Roncuzzi L, Perut F, Zini N, Avnet S. Exosome-like nanovesicles isolated from citrus limon l. exert antioxidative effect. Current Pharm Biotechnol. 2018;19:877–885. doi:10.2174/1389201019666181017115755
139. Perut F, Roncuzzi L, Avnet S, et al. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules. 2021;12:11. doi:10.3390/biom12010011
140. Xu XH, Yuan TJ, Dad HA, et al. Plant exosomes as novel nanoplatforms for MicroRNA transfer stimulate neural differentiation of stem cells in vitro and in vivo. Nano Lett. 2021;21:8151–8159. doi:10.1021/acs.nanolett.1c02530
141. Yang S, Lu S, Ren L, et al. Ginseng-derived nanoparticles induce skin cell proliferation and promote wound healing. J Ginseng Res. 2023;47:133–143. doi:10.1016/j.jgr.2022.07.005
142. Trentini M, Zanolla I, Zanotti F, et al. Apple derived exosomes improve collagen type i production and decrease MMPs during aging of the skin through downregulation of the nf-κb pathway as mode of action. Cells. 2022;12:11.
143. Kim M, Park JH, Gorantla S, Singhvi G. Isolation of aloe saponaria-derived extracellular vesicles and investigation of their potential for chronic wound healing. Pharmaceutics. 2022;15:14. doi:10.3390/pharmaceutics15010014
144. Kim MK, Choi YC, Cho SH, Choi JS, Cho YW. The antioxidant effect of small extracellular vesicles derived from aloe vera peels for wound healing. Tissue Eng and Regener Med. 2021;18:561–571. doi:10.1007/s13770-021-00367-8
145. Moeinabadi-Bidgoli K, Rezaee M, Hossein-Khannazer N, et al. Exosomes for angiogenesis induction in ischemic disorders. J Cell & Mol Med. 2023;27:763–787. doi:10.1111/jcmm.17689
146. Sánchez-López CM, Manzaneque-López MC, Pérez-Bermúdez P, Soler C, Marcilla A. Characterization and bioactivity of extracellular vesicles isolated from pomegranate. Food Funct. 2022;13:12870–12882. doi:10.1039/D2FO01806C
147. Savcı Y, Kırbaş OK, Bozkurt BT, et al. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021;12:5144–5156. doi:10.1039/D0FO02953J
148. Şahin F, Koçak P, Güneş MY, Özkan İ, Yıldırım E, Kala EY. In vitro wound healing activity of wheat-derived nanovesicles. Appl Biochem Biotechnol. 2019;188:381–394. doi:10.1007/s12010-018-2913-1
149. Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–215. doi:10.1038/nature16504
150. Díez-Sainz E, Lorente-Cebrián S, Aranaz P, Riezu-Boj JI, Martínez JA, Milagro FI. Potential mechanisms linking food-derived microRNAs. Gut microbiota and intestinal barrier functions in the context of nutrition and human health. Front Nutrition. 2021;8:586564.
151. Liu Y, Tan ML, Zhu WJ, et al. In vitro effects of tartary buckwheat-derived nanovesicles on gut microbiota. J Agri Food Chemi. 2022;70:2616–2629. doi:10.1021/acs.jafc.1c07658
152. König J, Wells J, Cani PD, et al. Human intestinal barrier function in health and disease. Clinic Translational Gastroen. 2016;7:e196. doi:10.1038/ctg.2016.54
153. Yun B, Kim Y, Park DJ, Oh S. Comparative analysis of dietary exosome-derived microRNAs from human, bovine and caprine colostrum and mature milk. J Animal Sci Technol. 2021;63:593–602. doi:10.5187/jast.2021.e39
154. Liao Y, Du X, Li J, Lönnerdal B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol Nutr Food Res. 2017;2017:61.
155. Suresh AP, Kalarikkal SP, Pullareddy B, Sundaram GM. Low pH-Based Method to Increase the Yield of Plant-Derived Nanoparticles from Fresh Ginger Rhizomes. ACS omega. 2021;6:17635–17641. doi:10.1021/acsomega.1c02162
156. Liu F, Peng W, Chen J, et al. Exosomes Derived From Alveolar Epithelial Cells Promote Alveolar Macrophage Activation Mediated by miR-92a-3p in Sepsis-Induced Acute Lung Injury. Front Cell Infect Microbiol. 2021;11:646546. doi:10.3389/fcimb.2021.646546
157. Mukund K, Nayak P, Ashokkumar C, et al. Immune response in severe and non-severe coronavirus disease 2019 (COVID-19) infection: a mechanistic landscape. Front Immunol. 2021;12:738073. doi:10.3389/fimmu.2021.738073
158. Kumar A, Sundaram K, Teng Y, et al. Ginger nanoparticles mediated induction of Foxa2 prevents high-fat diet-induced insulin resistance. Theranostics. 2022;12:1388–1403. doi:10.7150/thno.62514
159. Kumar A, Ren Y, Sundaram K, et al. miR-375 prevents high-fat diet-induced insulin resistance and obesity by targeting the aryl hydrocarbon receptor and bacterial tryptophanase (tnaA) gene. Theranostics. 2021;11:4061–4077. doi:10.7150/thno.52558
160. Yin L, Yan L, Yu Q, et al. Characterization of the MicroRNA profile of ginger exosome-like nanoparticles and their anti-inflammatory effects in intestinal caco-2 cells. J Agri Food Chemi. 2022;70:4725–4734. doi:10.1021/acs.jafc.1c07306
161. Kalarikkal SP, Sundaram GM. Edible plant-derived exosomal microRNAs: exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol Appl Pharmacol. 2021;414:115425. doi:10.1016/j.taap.2021.115425
162. Qiu FS, Wang JF, Guo MY, et al. Rgl-exomiR-7972, a novel plant exosomal microRNA derived from fresh Rehmanniae Radix, ameliorated lipopolysaccharide-induced acute lung injury and gut dysbiosis. Biomed Pharmacothe. 2023;165:115007. doi:10.1016/j.biopha.2023.115007
163. Wiering L, Tacke F. Treating inflammation to combat non-alcoholic fatty liver disease. J Endocrinol. 2023;2023:256.
164. Zhang F, Wang M, Zha Y, Zhou J, Han J, Zhang S. Daucosterol Alleviates Alcohol-Induced Hepatic Injury and Inflammation through P38/NF-κB/NLRP3 Inflammasome Pathway. Nutrients. 2023;16:15. doi:10.3390/nu16010015
165. Zhang L, Yang L, Gao Y, et al. Nomogram for evaluating obvious liver inflammation in treatment-naïve HBeAg positive chronic hepatitis B virus infection patients with normal ALT. Virulence. 2023;14:2158710. doi:10.1080/21505594.2022.2158710
166. Man F, Meng C, Liu Y, et al. The study of ginger-derived extracellular vesicles as a natural nanoscale drug carrier and their intestinal absorption in rats. AAPS Pharm Sci Tech. 2021;22:206. doi:10.1208/s12249-021-02087-7
167. Song H, Canup BSB, Ngo VL, Denning TL, Garg P, Laroui H. Internalization of garlic-derived nanovesicles on liver cells is triggered by interaction with CD98. ACS omega. 2020;5:23118–23128. doi:10.1021/acsomega.0c02893
168. Jiang Q, Wang L, Tian J, et al. Food-derived extracellular vesicles: natural nanocarriers for active phytoconstituents in new functional food. Crit Rev Food Sci Nutr. 2023; 1–21. doi:10.1080/10408398.2023.2242947
169. Melnik BC, Schmitz G. MicroRNAs: milk’s epigenetic regulators. Best Pract Res Clin Endocrinol Metab. 2017;31:427–442. doi:10.1016/j.beem.2017.10.003
170. Zu M, Xie D, Canup BSB, et al. ‘Green’ nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials. 2021;279:121178. doi:10.1016/j.biomaterials.2021.121178
171. Bruno SP, Paolini A, D’Oria V, et al. Extracellular vesicles derived from citrus sinensis modulate inflammatory genes and tight junctions in a human model of intestinal epithelium. Fronti Nutrit. 2021;8:778998. doi:10.3389/fnut.2021.778998
172. Zhu MZ, Xu HM, Liang YJ, et al. Edible exosome-like nanoparticles from portulaca oleracea L mitigate DSS-induced colitis via facilitating double-positive CD4(+)CD8(+)T cells expansion. J Nanobiotechnol. 2023;21:309. doi:10.1186/s12951-023-02065-0
173. Kang CS, Ban M, Choi EJ, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One. 2013;8:e76520. doi:10.1371/journal.pone.0076520
174. Sundaram K, Miller DP, Kumar A, et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of porphyromonas gingivalis. iScience. 2020;23:100869. doi:10.1016/j.isci.2020.100869
175. Oliveira MC, Arntz OJ, Blaney Davidson EN, et al. Milk extracellular vesicles accelerate osteoblastogenesis but impair bone matrix formation. J Nutr Biochem. 2016;30:74–84. doi:10.1016/j.jnutbio.2015.11.017
176. Oliveira MC, Di Ceglie I, Arntz OJ, et al. Milk-derived nanoparticle fraction promotes the formation of small osteoclasts but reduces bone resorption. J Cell Physiol. 2017;232:225–233. doi:10.1002/jcp.25414
177. Jang J, Jeong H, Jang E, et al. Isolation of high-purity and high-stability exosomes from ginseng. Front Plant Sci. 2022;13:1064412. doi:10.3389/fpls.2022.1064412
178. Xu F, Mu J, Teng Y, et al. Restoring oat nanoparticles mediated brain memory function of mice fed alcohol by sorting inflammatory dectin-1 complex into microglial exosomes. Small. 2022;18:e2105385. doi:10.1002/smll.202105385
179. Kameli N, Dragojlovic-Kerkache A, Savelkoul P, Stassen FR, Mujtaba IM. Plant-derived extracellular vesicles: current findings, challenges, and future applications. Membranes. 2021;12:11. doi:10.3390/membranes12010011
180. Pocsfalvi G, Turiák L, Ambrosone A, et al. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J Plant Physiol. 2018;229:111–121. doi:10.1016/j.jplph.2018.07.006
181. van der Koog L, Gandek TB, Nagelkerke A. Liposomes and extracellular vesicles as drug delivery systems: a comparison of composition, pharmacokinetics, and functionalization. Adv Healthcare Mater. 2022;11:e2100639. doi:10.1002/adhm.202100639
182. Huda MN, Nafiujjaman M, Deaguero IG, et al. Potential use of exosomes as diagnostic biomarkers and in targeted drug delivery: progress in clinical and preclinical applications. ACS Biomater Sci Eng. 2021;7:2106–2149. doi:10.1021/acsbiomaterials.1c00217
183. Santos-Coquillat A, González MI, Clemente-Moragón A, et al. Goat milk exosomes as natural nanoparticles for detecting inflammatory processes by optical imaging. Small. 2022;18:e2105421. doi:10.1002/smll.202105421
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



