Back to Journals » International Journal of Nanomedicine » Volume 19
Engineering Strategies of Plant-Derived Exosome-Like Nanovesicles: Current Knowledge and Future Perspectives
Authors Li Y , Wang Y, Zhao H, Pan Q , Chen G
Received 17 September 2024
Accepted for publication 23 November 2024
Published 30 November 2024 Volume 2024:19 Pages 12793—12815
DOI https://doi.org/10.2147/IJN.S496664
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. RDK Misra
Yuhan Li,1,* Yulong Wang,1,* Hongrui Zhao,2 Qi Pan,3 Guihao Chen3
1Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Intensive Care Medicine Department, Yuhuangding Hospital, Yantai, People’s Republic of China; 3Department of Cardiology, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qi Pan; Guihao Chen, Email [email protected]; [email protected]
Abstract: Plant-derived exosome-like nanovesicles (PELNs) from edible plants, isolated by ultracentrifugation, size exclusion chromatography or other methods, were proved to contain a variety of biologically active and therapeutically specific components. Recently, investigations in the field of PELN-based biomedicine have been conducted, which positioned those nanovesicles as promising tools for prevention and treatment of several diseases, with their natural origin potentially offering superior biocompatibility and bioavailability. However, the inadequate targeting and limited therapeutic effects constrain the utility and clinical translation of PELNs. Thus, strategies aiming at bridging the gap by engineering natural PELNs have been of great interest. Those approaches include membrane hybridization, physical and chemical surface functionalization and encapsulation of therapeutic payloads. Herein, we provide a comprehensive overview of the biogenesis and composition, isolation and purification methods and characterization of PELNs, as well as their therapeutic functions. Current knowledge on the construction strategies and biomedical application of engineered PELNs were reviewed. Additionally, future directions and perspectives in this field were discussed in order to further enrich and expand the prospects for the application of engineered PELNs.
Keywords: plant-derived exosome-like nanovesicles, nanomedicine, engineered extracellular vesicles, drug delivery systems, targeted therapy
Introduction
Extracellular vesicles derived from animal cells, especially mammal stem cells, are being extensively investigated in nanomedicine as an alternative for combating diseases like myocardial infarction and inflammation due to their superior biocompatibility and potential therapeutic benefits, and several clinical studies have been conducted.1–5 However, low yield and high cost pose challenges for the clinical translation of animal-derived exosomes.6 Recent researches on milk-derived extracellular vesicles, which are characterized by the advantages of low cost, high yield, biocompatibility, tissue tropism, and good uptake, have encountered significant obstacles, including considerable inconsistencies in milk collection and processing batches, harsh storage conditions, as-yet-unexplored purification methods, and allergies to specific populations.7 In contrast, plant-derived exosome-like nanovesicles (PELNs) from edible plants have made a useful exploration in the field of exosome biomedicine.8,9 These lipid bilayer nanovesicles with a diameter of 50–1000 nm are highly analogous to animal-derived exosomes, exhibiting negative charges that range from −25 to −15 mV.10–12 Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) analyses have demonstrated that plant-derived exosomes exhibit a cup-shaped morphology and a homogeneous structure.13 Despite initial potential concerns due to cross-kingdom applications, PELNs were proved to contain a variety of biologically active and therapeutically specific components, including proteins, lipids and nucleic acids, and exerted protective effects in several models, with a higher yield, a lower cost, relatively small differences in source batches, and no significant toxicity observed in a series of pilot studies.14–18 These properties indicate the potential for applications in antimicrobial, anti-inflammatory, anti-tumor, intestinal homeostasis regulation, and pro-regenerative medicine.19 Nevertheless, the immunogenicity resulting from the species gap, coupled with the inadequate targeting, renders plant-derived exosomes susceptible to a short half-life and an insignificant therapeutic impact, thereby constraining their utility in nanomedicine advancement.5,20,21
Therefore, strategies aiming at bridging the gap by engineering natural PELNs have been of great interest. For instance, the use of membrane hybridization, physical and chemical modifications allow the capacity for targeted delivery and improved tissue uptake, thereby enhancing efficacy; encapsulating bioactive molecules, such as protein, expression vectors, siRNA, and DNA, promotes the therapeutic effects of PELNs. Dietary components have been demonstrated to regulate the cellular metabolism and function, and a bulk of gene expression in diseases, providing a rationale for the use of natural and engineered nanovesicles derived from edible plants as a similarly beneficial green synthetic strategy for utilizing food raw materials to promote health.22 Herein, current knowledge on the biomedical application of natural PELNs and approaches to engineer PELNs to enhance their therapeutic efficacy were reviewed.23–29 Additionally, future directions and prospective in this field were discussed in order to further enrich and expand the prospects for the application of engineered PELNs.
Overview of PELNs
Biogenesis and Compositions of PELNs
PELNs have been derived from edible plants and traditional herbs, including strawberries, amaranth, ginseng.30–32 Generally, it is accepted that there are at least three biogenesis pathways, namely: 1) the multivesicular bodies (MVB) pathway; 2) the exocyst–positive organelle (EXPO) pathway; and 3) the vacuolar pathway.6 The most frequently observed pathway is the MVB pathway, in which inward budding of the endosomal membrane forms the MVB and fusion of the MVB with the plasma membrane (PM) leads to the release of exosome-like nanovesicles.33 In contrast, the remaining two pathways represent unconventional forms of secretion.
PELNs are characterized by a diversity of internal and surface components. Despite sharing similar isolation and characterization methods, as well as comparable therapeutic effects, animal-derived exosomes and PELNs exhibit structural and compositional differences, conferring PELNs with distinctive advantages. Some of the membrane surface molecules have been instrumental in the identification and characterization of PELNs, despite the lack of clarity the specific membrane surface markers.34 In a study conducted by Zhang et al, it was demonstrated that the proteins present in ginger-derived exosome-like nanoparticles (GELNs) using HPLC/MS are predominantly cytoplasmic in nature, comprising actin and proteolysis enzymes. Additionally, the analysis revealed the presence of several membrane channel and transporter proteins, including aquaporin and chloride channels.35 In light of the findings of such studies, engineering techniques that induce the coupling or expression of CD47, CD55, CD59 and CD200 on the membrane surface or enrichment of MHC-I can evade recognition and clearance by the immune system, thereby reducing the immunogenicity of PELNs. Study conducted by Sushrut Kamerkar revealed that the CD47 protein, in collaboration with the Signal Regulatory Protein α (SIRPα), renders PELNs-like membrane structures less susceptible to phagocytosis by the monocyte-macrophage system. This consequently resulted in a notable reduction in the clearance rate.36
In addition to proteins, lipids such as phosphatidic acid (PA), phosphatidylethanolamine (PE), phosphatidylcholine (PC), digalactosyl monoacylglycerol (DGMG), digalactosyl diacylglycerol (DGDG) and monogalactosyl diacylglycerol (MGDG), and nucleic acid, including DNA, mRNA, miRNA and non-coding RNA, form the basis of PELNs engineering.37 Given the pivotal functions of miRNAs in regulating inflammation, the intestinal barrier, tumors and infantile immunological processes, it is perhaps unsurprising that they have been the subject of the most extensive research.38 For instance, a study on grapefruit-derived nanovectors (GNVs) demonstrated that miR-18a impeded colon cancer liver metastasis by stimulating the generation of M1 macrophages via the Interferon (IFN)-γ/ Interferon Regulatory Factor (Irf) 2 pathway, which provides a potential therapeutic approach for colon cancer treatment.39 It is notable that the more extensive nucleic acid components of PELNs, as well as biologically active substances, have the potential for engineering applications. The diverse range of DNA and RNA (miRNAs, microRNAs, long-chain non-coding RNAs, cyclic RNAs, and small RNAs) endow PELNs with the capacity to communicate at the cellular level and regulate the state of target cells.40 The distinctive bioactive constituents, including vitamin C, polyphenols, flavonoids, and carotenoids, endow PELNs with their efficacious therapeutic properties.34,41
Isolation and Purification of PELNs
In the pretreatment stage, isolation methods can be classified into two main categories: nanovesicles that occur naturally in plants and those isolated from plant roots, leaves, and fruits through destructive means.42 After obtaining plant juice, ultracentrifugation and sucrose density gradient centrifugation are the main techniques employed in engineered PELNs.43,44 The initial step involved grinding the plant material into a juice and filtering it. Subsequently, centrifugation was performed at varying centrifugal forces (1000–10,000×g) for 20–60 minutes to eliminate the presence of large sediments, cellular debris, and dead cells. The resulting supernatant was then subjected to ultracentrifugation (100,000–150,000×g) for 1–2 hours. To achieve the purification of PELNs, the precipitates were then resuspended and gently spread on sucrose density gradient layers, after which they were subjected to ultracentrifugation (100,000–150,000×g) for 1–2 h.45,46 Under the influence of centrifugal force, the components of varying densities and sizes form distinct bands in each region of the density gradient.43 The addition of a thin layer of high-density isotonic material as a buffer layer at the bottom of the centrifuge tubes allows the avoidance of impairment to the structural integrity of the PELNs that may otherwise result from the application of high centrifugal forces, thus preventing the formation of clumps.47
Polyethylene glycol (PEG) precipitation method, Ultrafiltration centrifugation (UC), and Size-exclusion chromatography (SEC), on account of their distinctive characteristics, can also be subjected to further development and incorporated into the engineered nanovesicle preparation process.10 Among these, precipitation with PEG exploits the property that PEG can competitively bind water molecules to separate PELNs from solution.48 UC using microporous ultrafiltration membranes with specific pore sizes is used to separate nanoparticles of different sizes and dimensions; while SEC utilizes particle hydrodynamics and chromatography.49,50 To enhance the productivity of engineered PELNs, Anagha Priya Suresh et al developed a low pH-based method to increase the yield of plant-derived nanoparticles from fresh ginger rhizomes. A polyethylene glycol (PEG6000)-based precipitation method for the isolation of ginger PELNs has recently been developed. It was demonstrated that the production of nanovesicles could be enhanced at pH levels below 7.0, specifically at pH 4 and 5.51
Despite the absence of standardized procedures for the isolation of PELNs, numerous studies have conducted comprehensive and valuable investigations into the merits and drawbacks of diverse methodologies (Figure 1). The current consensus in the field includes the following key perspectives: Ultracentrifugation, while straightforward and capable of high throughput, is costly, time-consuming, and potentially harmful to PELNs. Precipitation offers a simple and cost-effective approach but struggles with impurity separation, such as proteins and plant debris. Size exclusion chromatography (SEC) effectively maintains the integrity and consistency of PELNs, though it requires specialized equipment and may lead to the co-isolation of polysaccharide. Density gradient centrifugation (DGC) provides high purity and is cost-effective; however, its extended processing time and low yield make it unsuitable for scalable production. Tangential Flow Filtration (TFF) offers high purity, throughput, and structural integrity for PELNs at a scalable level, but is relatively expensive. Immunoaffinity capture enables precise separation of large, diluted samples with high purity, though it is time-consuming and costly.52,53 The combination of separation methods has the potential to provide a solution to the aforementioned issues. For instance, recent studies have developed a gradient filtration method combined with high-speed centrifugation for the separation and purification of the PELNs. This engineered approach expands the means of producing PELNs on a large scale, as well as the source, while maintaining the therapeutic properties of PELNs, thereby enriching the prospective applications of PELNs.54
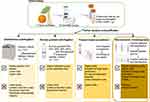 |
Figure 1 Widely-used isolation and purification approaches of PELNs and their advantages and limitations. |
Characterization of PELNs
It is essential to employ a range of characterization techniques to identify the various subgroups of PELNs, as this constitutes a fundamental step in the development of PELNs-based therapeutics or drug nanocarriers. Morphological analysis of PELNs can be conducted using TEM, which is employed for ultrastructural examination of the subcellular state. Additionally, atomic force microscopy (AFM) can be utilized to elucidate the structural and dimensional characteristics of individual PELN.23 By employing electron microscopy, Francesca Perut et al demonstrated that PELNs extracted from Fragaria exhibited a striking morphological homogeneity, with a diameter range of 30 to 191 nm. Moreover, they observed a distinct cup-shaped or rounded morphology, reminiscent of exosome-like nanovesicles derived from mammalian cells.32 As the gold standard for the characterization of PELNs, concentration can be measured using Nanoparticle Tracking Analysis (NTA). Resistive Pulse Sensing (RPS) allows the size and concentration of PDENs in suspension to be determined without affecting the quantification of PELNs.55 Flow cytometry represents a viable methodology for the detection of biomarkers associated with PELNs, which is capable of high-speed multi-channel analysis with low sample concentration.56 Furthermore, the zeta analyzer can be employed to observe the repulsive nature against aggregation or dispersity and the membrane potential.10–12 Techniques including small‐angle X‐ray scattering, NTA, dynamic light scattering (DLS), and tunable resistive pulse sensing have also been extensively employed for the characterization of the morphology of single nanovesicles.57–59
The identification of a uniform protein marker is a challenging endeavor, primarily due to the low protein content of PELNs in comparison to nanovesicles of animal origin, coupled with the significant inter-sample variability observed across different sources. Pinedo et al conducted a comprehensive analysis of surface proteins in PELNs, identifying Heat Shock Protein 70 (HSP70), Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH), and S-adenosine-homocysteine lyase as the most prevalent protein families in PELNs. It is possible that these proteins may serve as surface markers for PELNs.10 He et al demonstrated that Tetraspanin (TET) 8 co-localized with the Arabidopsis MVB marker Rab5-like GTPase, Ara6, inside the plant cells, suggesting that TET8-positive EVs are derived from MVBs and could be considered a marker of PELNs.60,61 Exo70 and TET3 were also used for PELN characterization; however, their universality is still worth exploring.62 In conclusion, further research is required on the specific protein marker of engineered PELNs in order to facilitate a deeper understanding and modification of these systems.34
Therapeutic Potential of PELNs
PELNs have garnered significant attention due to interspecies communication and are expected to serve as potent therapeutic agents for disease treatment.63 Compared to single-component agents, PELNs have shown enhanced pharmacological efficacy attributed to their multi-component system.64 PELNs are characterized by their natural lipid bilayer composition, which confers superior biocompatibility and bioavailability compared to synthetic delivery systems, enabling them to effectively evade immune phagocytosis in vivo and exhibit exceptional resistance to degradation. Besides, the ability of PELNs to effectively overcoming biological barriers such as the blood-brain barrier without triggering inflammation or necrosis has been revealed.65–67 Recent studies have elucidated the pleiotropic functions of PELNs, encompassing anti-tumor, anti-inflammation, intestinal homeostasis regulation, anti-infection and regenerative promotion functions (Figure 2).19,68–70
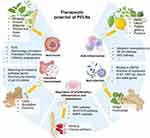 |
Figure 2 The sources, therapeutic potentials and underlying mechanisms of unmodified PELNs. |
Anti-Tumor
PELNs have been shown to play a pivotal role in suppressing tumor growth. Mechanistic studies of PELNs derived from different plant sources suggest that PELNs not only exert direct influence on the physiological activities of tumor cells, including proliferation, apoptosis, metabolic processes, drug-resistance, but also indirectly remodel the tumor microenvironment, without significant influence on normal cells.71,72 These multifaceted actions contribute to the mitigation of tumor progression and the reduction of its invasive characteristics.73,74
Chen et al discovered that tea flower-derived ELNs (TELNs) are rich in bioactive substances, which trigger oxidative stress in breast cancer cells, resulting in mitochondrial damage, cell cycle inhibition, and the subsequent cell apoptosis. Interestingly, in vivo studies have demonstrated that TELNs, intravenously or orally administrated, can effectively suppress the proliferation and metastasis of breast cancer.71 Similarly, the level of reactive oxygen species (ROS) in breast cancer cells increased 2.5 times compared with untreated cells after 8-hour co-incubation with TELNs.75 ELNs derived from Raphanus sativus L. var. caudatus Alef microgreens exhibit anti-proliferative effects in HCT116 colon cancer cells by inducing DNA damage, which alters the cellular biochemical compositions in the regions of nucleic acids and carbohydrates.76 In a study by Yan et al, Brucea javanica-derived ELNs could deliver 10 functional miRNAs to 4T1 cells, effectively inhibiting breast tumor growth by targeting the phosphatidylinositol 3-kinase (PI3K) / Akt / mammalian target of rapamycin (mTOR) signaling pathway and promoting ROS/caspase-mediated apoptosis. Concurrently, they could also modulate the physiological functions of endothelial cells, thereby suppressing Vascular endothelial growth factor (VEGF)-mediated angiogenesis.77 Immunomodulation in the tumor microenvironment (TME) are critical issues for improving cancer treatment,78 and PELNs play a role in normalization of immune systems. Especially, the distribution and bioavailability of PELNs could be crucial for certain types of tumors. Some PELNs such as Ginseng-derived ELNs could exhibit efficient penetration through closely spaced epithelial cells and excellent targeting ability towards blood-brain barrier and glioma, promote M1 macrophage polarization within the TME, and facilitate the secretion of CCL5 and CXCL9.8,79 This reprogramming facilitates the recruitment of CD8+ T cells into the tumor bed. The enhanced presence of these cytotoxic T cells synergizes with the PD-1 monoclonal antibody therapy, reinforcing the overall immunotherapeutic impact against cancer.
Anti-Inflammation
Inflammation is a protective mechanism initiated by external stimulation. However, an overreaction might cause acute or chronic diseases and tissue impairment. PELNs isolated from several sources are reported to modulate the expression of inflammation-related genes and ameliorate the inflammatory response.10,37 For example, garlic ELNs are abundant in miR-396e, which exerts a significant influence on the metabolic reprogramming of macrophages by targeting 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3 (PFKFB3) and prevents obesity via macrophage-adipocyte cross-talk.80 Further investigations suggest a similar role of garlic ELNs in enhancing lipid metabolism in hepatocytes through the macrophage-hepatocyte cross-talk.81
ELNs isolated from Panax notoginseng alleviate cerebral ischemia/reperfusion injury (I/R) by inducing M2 polarization of microglia, which is at least partly mediated by lipid components.82 Portulaca oleracea-derived ELNs suppress pro-inflammatory cytokines, enhance IL-10, and ameliorate acute colitis in mice, with gut microbiota modulation potential for ulcerative colitis therapy.31 Mechanistically, PELNs treatment alters the microbial metabolism and causes the reprogramming of conventional CD4+ T cells into double-positive CD4+CD8+ T cells. Ginger and lemon ELNs mediate the activation of nuclear factor erythroid 2-related factor 2 (Nrf2), which contributes to cytoprotective effects against peroxidation damage.83
Intestinal Homeostasis
The gut microbiota significantly influences physiological functions, with its dynamic balance closely tied to colonic health. Recent studies indicate that PELNs carrying miRNAs can be assimilated by intestinal bacteria, thereby altering the microbial metabolism and regulating the host’s physiological processes.84,85 The abundance and diversity of gut microbiota in Dextran Sulfate Sodium (DSS)-induced colitis mouse model were significantly improved treated by turmeric-derived ELNs (TELNs).69 Moreover, TELNs mitigate colitis-related symptoms by restoring the intestinal epithelial barrier, facilitating M2 macrophage polarization, and reshaping the immune microenvironment. Target gene analysis indicates that tartary buckwheat-derived ELNs can influence key genes in the physiological processes of Escherichia coli and Lactobacillus rhamnosus, which stimulate the proliferation and enrich the diversity of gut microbiota.86 Consumption of garlic-derived ELNs (GENs) notably reduced the expression levels of toll-like receptor 4 (TLR4), myeloid differentiation primary response gene 88 (MyD88) and NF-κB, and decreased the secretion of pro-inflammatory cytokines in DSS-induced colitis. Another study has reported that Peu-miR2916-p3 enriched in GENs specifically promoted the growth of Bacteroides thetaiotaomicron, a beneficial intestinal bacterium known to ameliorate colitis symptoms, indicating synergistic effects exerted by the multiple components of PELNs.87
Regenerative Potential
The regenerative potential of PELNs has been widely investigated, encompassing wound healing, cell differentiation and tissue repair.88,89 Ginseng ELNs can promote cell proliferation, migration, and angiogenesis via the extracellular signal-regulated kinase (ERK) and Akt/mTOR signaling pathway; and GELNs accelerate mouse skin wound healing and reduce inflammation in vivo.90 Rhizoma Drynariae-derived nanovesicles are observed to facilitate human bone marrow mesenchymal stem cells (hBMSCs) proliferation and the expression of Estrogen Receptor α (ERα). Additionally, they can stimulate the differentiation of hBMSCs to osteogenic lineage, as evidenced by increased expression of bone morphogenetic protein 2 (BMP2) and runt-related transcription factor 2 (RUNX2)91 Zhou et al found that Gouqi (Chinese wolfberry)-derived nanovesicles (GqDNVs) improve the cross-sectional area of quadriceps muscle and grip strength in dexamethasone-induced muscle atrophy model, mainly through the activation of AMP-activated protein kinase (AMPK). Furthermore, the energy-targeted metabolome analysis indicates that GqDNVs enhance the metabolism of oxidative phosphorylation, demonstrating their therapeutic capabilities for muscle regeneration.92
Anti-Infection
PELNs are known to confer resistance against various infections in plants, while the specific contributions of PELNs to antibiotic systems within the biomedical domain have not been extensively elucidated. miRNAs might play a crucial role in such cross-kingdom regulation. GELNs can be selectively internalized within the periodontal pathogen Porphyromonas gingivalis through a phosphatidic acid (PA)-mediated process, which involves specific interactions with the hemin-binding protein 35 (HBP35) present on the bacterial cell surface. Moreover, GELN-derived PA and aly-miR159a-3p significantly decreased the expression of type IX secretion system (T9SS), thereby affecting the virulence of the bacterium.93 This is another example of synergy where cargo molecules interacted with multiple pathogenic factors in the recipient bacteria simultaneously. miR858a and miR858b enriched in Houttuynia cordata-derived exosome-like nanoparticles specifically target the neuraminidase (NP) gene within the H1N1 influenza virus. Similarly, miR166a-3p has been identified to target the open reading frame 1ab (ORF1ab) in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), providing potential defense against viral replication and pathogenesis.40 Nsp12, an activator of nuclear factor kappa-B (NF-κB) pathway and producer of a range of inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β, is inhibited by ginger ELNs containing aly-miR396a-5p, suggesting a therapeutic strategy for mitigating the impact of SARS-CoV-2 on lung cells.94 Bacterial interaction may serve as another mechanism of their anti-infection effects. Lei et al reported that the treatment of lemon-derived exosome-like nanoparticles provide protection against Clostridioides difficile (C. diff) infection in a probiotic-dependent manner, which is mediated by the elevation of aryl hydrocarbon receptor (AhR) ligands indole-3-lactic acid (I3LA) and indole-3-carboxaldehyde (I3Ald) and subsequently induce the expression of IL-22. The observed increase in lactic acid production is implicated in the reduction of C. diff fecal shedding, ascribed to the inhibition of C. diff proliferation and the suppression of indole biosynthesis.95
Strategies for PELN Engineering
Similar to exosomes or exosome-like nanovesicles derived from other sources, PELNs exhibit the capacity to facilitate intercellular communication by selectively delivering bioactive substances to target cells, thus making them a promising therapeutic modality. Nevertheless, their inherent defects, such as limited natural effects and the lack of targeting, have sparked interest in innovative engineering strategies, including surface modification, cargo encapsulation, and membrane fusion or coating, which have been developed to significantly enhance their therapeutic potential (Figure 3).96 Reports on engineered nanovesicles prepared from animal cells, edible plants and other sources indicate that the choice of strategies is closely related to the intended objectives and anticipated benefits. Surface modification and membrane fusion are generally aimed at enhancing stability and targeting efficiency, while drug loading is typically intended to improve protective efficacy. Furthermore, although still in its infancy in PELN, the synergistic application of multiple engineering techniques is a promising research approach in terms of a series of studies on animal extracellular vesicles or artificial nanoparticles showing particularly encouraging results.97–99
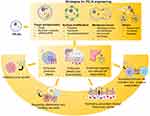 |
Figure 3 Strategies for PELN engineering and corresponding benefits. |
Membrane Surface Modification
Biological modification is the most widely used strategy for nanovesicle membrane modification (Table 1), which is pivotal for increasing the circulation stability, improving biocompatibility, achieving active delivery to designated sites to reduce off-target effects, and exerting additional biological effects in certain special circumstances.64,100,101 The modification strategies for animal cell-derived extracellular vesicles involve both parental cell engineering and direct manipulation. Parental cell-based modification is founded on the genetic alteration to exhibit the desired protein on the membrane which subsequently transfer to the surface of produced MEVs. Alvarez-Erviti et al firstly harnessed Lamp2b, an exosomal membrane protein, to fuse with the neuro-specific peptide rabies virus glycoprotein (RVG) for targeted delivery of siRNA.102 However, the unique process of PELNs isolation and undetermined membrane markers often renders parental cell-based genetic methods difficult to realize, thereby many researchers have focused on direct modification through physical or chemical approaches, which means combining specific substances (ligands, homing peptide, polymers or other small molecules) with the surface membrane of PELNs through special binding methods. Covalent bonding is considered the subject of investigation for engineering EVs surface modification.65 For instance, the cyclic RGD peptide (cRGD), a family of peptides which endow natural exosomes with high affinity with αvβ3 integrin receptors overexpressing on the surface of tumor cells or tumor vascular endothelial cells, has been applied in engineering PELNs. Lemon and orange ELNs are endowed with tumor-targeting ability after functionalization with heparin-cRGD, with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) as catalysts.103,104 Aptamers are short oligonucleotides or peptides with specific structures, which could target tumor cells with high affinity and specificity.105 Conjugation with HA1, an aptamer specific for Human epidermal growth factor receptor 2 (HER2)-overexpressing breast cancer cells endows GNVs with HER2+ breast cancer cell-targeting ability.
 |
Table 1 Membrane Surface Modification of PELNs and Enhanced Therapeutic Effects |
Additionally, decorations with several polymers and small molecules have also been reported. For instance, Moon et al extracted ELNs from grapefruit, inserted 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) into the membrane and conjugated aptamer R11-3 onto the surface using click chemistry, which facilitated penetration through blood-brain barrier and brain cellular uptake.110 Zhuang et al developed GNVs hybridized with polyethyleneimines (pGNVs) and combined with folic acid (FA). These modified GNVs were capable of delivering miR-17 efficiently intranasally to the mouse brain tumor that overexpresses folic acid receptor (FR).114,117 Similarly, Li et al applied arrow-shaped RNA to display FA on ginger ELNs membrane and successfully achieved targeted delivery of siRNA survivin to KB cancer cells. Furthermore, in vivo distribution of ELNs can be monitored through fluorescent dye labeling on the membrane surface.64,118 Targeted delivery of siRNA to duodenal epithelium via folate-modified ginger nanovectors presents a feasible strategy for alleviating iron burden.119 Besides, polyethylene glycol (PEG) decoration could significantly prolong systemic half-life and reduce non-specific immune clearance of Asparagus cochinchinensis ELNs.17 Qiao et al constructed a biomimetic nanoparticle combining electrodynamic Pd-Pt nanosheets and ELNs derived from ginger. With high biocompatibility and stability in vivo, the platform presented efficient targeting and accumulation at infection sites, coupled with sustained generation of ROS to achieve synergistic electrodynamic and photothermal anti-bacterial therapy.108 Chen et al explored extracellular vesicle-engineered structural droplet drugs (ESDDs) functionalization strategies by programming the self-assembly of lemon-derived ELNs on the doxorubicin (DOX)@squalene–PBS surface, optimizing the delivery of DOX across the blood-brain barrier for improved glioblastoma chemotherapy and demonstrating prolonged circulation time.106 Additionally, such strategy is proved to yield synergistic benefits by modifying with bioactive molecules. For instance, Ruan et al reported that surface modification of stromal cell-derived factor 1 (SDF-1) enhanced the migratory effects of EVs on neural stem cells (NSCs) without compromising their ability to promote NSCs differentiation.101 However, covalent functionalization of PELN with ligands or bioactive therapeutics have not been explored yet; to date, there have been few attempts to modify the surface of PELN using non-covalent modification method based on charge and hydrophobicity, positioning them as a potential research directions. Conclusively, surface modifications of PELNs enable enhanced targeting capability and improved pharmacokinetics profiles.
Cargo Encapsulation
In addition to transporting endogenous agents to target cells, the lipid layer and internal aqueous phase of PELNs endow them with the capability to encapsulate exogenous hydrophilic or hydrophobic molecules, such as chemotherapeutic drugs, mRNAs, miRNAs, siRNAs, proteins, while shielding them from degradation (Table 2).120–123 This attribute renders PELNs an exemplary option for drug delivery systems (DDS). In comparison with artificially synthesized nanoparticles for DDS, like liposomes or micelles, PELNs have proven to be a better choice in certain conditions in terms of higher stability, lower immunogenicity, higher efficacy and stronger cellular uptake.64,73,124,125
 |
Table 2 Cargo Encapsulation of PELNs and Enhanced Therapeutic Effects |
To accomplish optimal delivery, several factors need to be addressed: enhancing encapsulation efficiency, ensuring the structural integrity of the nanoparticle, and maintaining the drug molecule’s bioactivity. Multiple techniques can be employed to encapsulate drugs within PELNs, encompassing co-incubation, electroporation, sonication, click chemistry, freeze–thaw, osmotic shock, etc.124 For instance, due to the highly hydrophobic property, the bioavailability of curcumin, a lipophilic polyphenol serving as an anticancer, antibiotic and anti-inflammatory agent, is low which still prevents its clinical application.122 Ramila Mammadova et al constructed curcumin-loaded tomato-derived ELNs by extrusion, direct loading by co-incubation and sonication, discovering that sonication enhanced the inherent anti-inflammatory properties.122 Shoko Itakura et al adopted a novel method for loading siRNAs into grapefruit-derived nanoparticles by optimizing pressure conditions in the microfluidic device, which presented in vitro gene knockdown effects.127 Luiza Garaeva et al combined passive and active loading strategies, and demonstrated that ELNs derived from grapefruit serve as potent vehicles for delivery of bovine serum albumin (BSA) and heat shock protein 70 (HSP70) into both human peripheral blood mononuclear cells and colon cancer cells to exert cytoprotective activity.121 Numerous strategies are explored to achieve superior loading efficiency. Zhuang et al explored an innovative strategy for the intranasal delivery of therapeutic miR-17 to brain tumor cells, utilizing GNVs as carriers. Notably, GNV-coated polyethylenimine (PEI)/RNA complex not only increased the miR-17 loading efficiency from 5.91±0.6% to 86.2±5.7%, but also mitigate the cytotoxic effects typically associated with PEI.114 Similar paradigm has been reported where enhanced biomedical effects were observed in drug-loaded PELNs comparing with free drug.109,125,133,137 Recently, green tea-derived PELNs were reported to deliver an antagomir targeting heart-apoptosis-associated piwi-interacting RNA (HAAPIR) to the lesion of aortic dissection.133 The nanosystem effectively regulates vascular remodeling, mitigating AD occurrence and progression through the myocyte enhancer factor 2D (Mef2D) and matrix metallopeptidase 9 (MMP9) pathways. It’s interesting to note that PELN and the antagomir could be synergistic since PELN itself showed limited but significant effects. Bitter melon-derived extracellular vesicles (BMEVs) demonstrated significant therapeutic benefits when combined with 5-Fluorouracil (5-FU) for the treatment of oral squamous cell carcinoma (OSCC), outperforming the single-agent administration. This combination therapy was associated with a downregulation of the NOD-like receptor family pyrin domain containing 3 (NLRP3) expression, potentially attenuating the drug resistance of OSCC to 5-FU.138 As natural transporting platforms with low toxicity and high absorption rates, PELNs have an intriguing potential for delivering vaccine mRNA, which accelerates their application from bench to bedside. In addition, antigen-incorporated PELN vaccine ensures enduring immunity without the risks associated with vaccine-induced virulence reversion or pre-existing immunity.10,116 ELNs derived from orange juice have been identified as effective carriers for the delivery of SARS-CoV-2 mRNA vaccines via oral and intranasal routes. This approach has been shown to effectively immunize mice, eliciting a targeted humoral and cellular immune response.134
Nevertheless, the challenge of achieving efficient cargo loading without altering membrane integrity and contents remains significant. Matricellular contents restrict the loading of exogenous drugs, and the delivery of hydrophilic compounds is constrained by lipid bilayer membranes. This ultimately limits the potential of exosomes as carriers.139 As a result, technologies such as electroporation have thrived,140 but might lead to the aggregation and fusion of EVs, which can result in changes to their surface potential. On the other hand, ultrasonic treatment offers a simple and rapid alternative, markedly improving the loading efficiency of active ingredients into the vesicles.65 Besides, A standardized approach to assess the loading efficiency of PELNs is yet to be established, due to the impact of the loading technique, the duration of the loading process, the ratio of drug to carrier, and the origin of the carrier itself.
Membrane Hybridization and Coating
Although membrane surface modification endows traditional PELNs with unique characteristics not originally present, their applications are still constrained by the singular functionality of the ligands and intricate preparation methods, such as weak accumulation capacity.106 However, harnessing the inspiration of bionic design principles, the direct construction of biomimetic nanocarriers using cellular components from biological autologous sources holds the potential to endow PELNs with lower immunogenicity, extended circulation times, and enhanced targeting capabilities (Table 3).141,142 Hybrid membrane nanovesicles (HMNVs), synthesized by homologous or heterologous membrane origins, allow for the efficient and large-scale fabrication, and can be tailored to possess multiple functionalities within a single vesicle type. Furthermore, HMNVs derived from the fusion of two distinct cell types exhibit a combination of biological functions.143 Zhang et al developed an in situ cancer vaccine by fusing bacteria-derived outer membrane vesicles with thylakoid nanovesicles of spinach. Such bacteria-plant hybrid vesicles (BPNs) increase homing to tumor tissues, prompt immune cell activation and tumor-associated antigen presentation, eliciting potent CD8+ T lymphocyte responses. Additionally, BPNs mitigate the immunosuppressive tumor microenvironment and enhance the overall immune response.144 Employing this concept, functional hybrid cancer vesicles are designed by fusing ginseng-derived ELNs extracellular vesicles-like particles (G-EVLPs) with membranes of autologous tumors. G-EVLPs facilitate dendritic cell phagocytosis and tumor-specific cytotoxic T lymphocyte activation, potentially preventing tumor recurrence and metastasis.145
 |
Table 3 Membrane Hybridizing of PELNs and Enhanced Therapeutic Effects |
As mentioned above, HMNVs can exhibit the respective biological characteristics of different origins, which provides inspiration for precision of targeting. When transitioning to a state of “camouflage”, the cell membrane-coated nanoparticles (CMNPs) acquire the characteristics of the donor cells and the capability to evade immune detection.141 For instance, The recruitment ability into inflamed sites was imparted to grapefruit nanovectors after coating with enriched membranes of activated leukocytes, and inflammatory related receptor C-X-C Motif Chemokine Receptor 2 (CXCR2) and Leukocyte Function-associated Antigen 1 (LFA-1) played a key role.147 Neutrophil-camouflaged Panax ginseng root-derived ELNs target lung inflammation, and loaded miRNA-182-5p mitigates the symptom of sepsis.146 More research is needed to investigate the pleiotropy of PELNs interacting with heterogeneous membranes and the application of this bionic nanoparticle in more disease models.
Other Modification Strategies
Confronted with the restrictions of single-site modifications for PELNs, researchers have devised a solution through dual or multifaceted modification strategies. These encompass a synergistic application of surface ligand modifications, cargo loading, and membrane fusion techniques to develop multifunctional vesicles. For example, ginger-derived nanovectors modified with FA facilitated the precise delivery of DOX to Colon-26 tumor cells, resulting in stronger suppression of tumor growth.109 Huang et al constructed fused nanovesicles (FV@CX5461) from grapefruit and gingiva‐derived mesenchymal stem cells, encapsulating them with immunosuppressant CX5461. The C-C-Motif Receptor 6 (CCR6) activity increased homing to inflammation tissue and mediated the effective immune microenvironment reconfiguration of FV@CX5461.148 Meanwhile, hydroxyapatite crystal binding peptide (ESTP) modified grapefruit ELNs can be used for vascular calcification-targeted delivery of sodium thiosulfate.126 Zeng et al introduced an optimal method for drug carrying that leverages π-π stacking interactions to augment the combined therapeutic impact of DOX and Indocyanine green (ICG). Integrin-targeted peptide covalent conjugation Aloe-Derived Nanovesicles further enhanced their targeting specificity towards breast cancer cells.149
Other engineering approaches have also been reported in several studies. Wang et al first fabricated nanovectors from lipids extracted from grapefruit (GNVs), utilizing a high-pressure homogenization technique for reassembly. They further verified GNVs’ capability to encapsulate and transport chemotherapeutic agents or siRNAs, and explored various modifications to enhance the targeting specificity for improved therapeutic delivery.114,128 In the context of facilitating wound healing, utilizing a composite hydrogel system for EVs loading presents a superior approach for the protection and controlled release of EVs. This method circumvents issues like rapid degradation and depletion, ensuring a sustained therapeutic effect.150 ELNs derived from Olea europaea leaves integrated with a hyaluronic acid and tannic acid hydrogel have demonstrated efficacy in mitigating ultraviolet-induced skin damage and promoting skin regeneration.151 However, more investigations are required for the benefits and clinical transition potentials of those novel methods.
Perspective and Current Challenges
PELNs have shown great promise for the advancement of drug delivery, messaging, and tissue repair applications due to their environmentally friendly nature, excellent biocompatibility, superior gastrointestinal tolerance, and absence of human pathogens. Nevertheless, the current understanding of the biogenesis, signature markers, and substance transport of PELNs remains inadequate. Targeted delivery, yield, high heterogeneity, and lack of standardized GMP have also become pivotal issues that require immediate attention in the context of the current clinical translation and large-scale utilization of PELNs.14,136,152 Four specific pressing issues are listed here as examples: comparison among sources, storage and administration, endosomal escape and the effects of protein corona.
Comparison Among Sources
Engineered PELNs present distinctive features in comparison to exosomes derived from animal cells and milk-derived extracellular vesicles. While there is general consensus in the scientific community on the fundamental methodology for obtaining nanovesicles, significant variations remain in processing extracted materials, largely due to source differences. For PELNs, a primary challenge lies in breaking down and clearing cell walls. In terms of nanovesicle characterization, animal cell-derived exosomes and milk-derived extracellular vesicles display well-defined protein markers, including transmembrane/lipid-binding proteins (eg, CD63, CD9) and cytoplasmic proteins (eg, TSG101), whereas PELNs lack robust evidence for such markers. Regarding purification, exosomes from animal cells often contain impurities like cellular debris and vesicles of organelle or nuclear membrane origin, and milk-derived extracellular vesicles are characterized by high protein and lipid particle concentrations. Batch variability of nanovesicles due to instability between cell passaging cultures and animal-derived individuals has limited the engineering development of both of these nanovesicles. PELNs, however, may contain small cellular debris unique to their plant origin, posing specific challenges for purification. Furthermore, the biological activity of nanovesicles typically relates to their surface molecules and contents. Unlike the animal-derived bioactive compounds in cell-derived exosomes and milk-derived vesicles, PELNs offer plant-derived nucleic acids and small molecule compounds with a broad therapeutic potential.7,10,153,154 A comparative analysis of the characteristics of PELNs will facilitate the identification of future research avenues and the urgent need for further investigation. Due to previous reports that different PELNs exert complex biological effects, careful selection of sources is crucial.
Storage and Administration
Methods to prevent degradation, damage and inhomogeneous composition of engineered PELNs during storage and preparation represent an additional avenue of research that is worthy of continued investigation.155 Currently, the most prevalent method of administration of natural and modified PELNs currently under investigation is oral, and has been demonstrated to be efficacious.6 In addition to this, intravenous, intraperitoneal, nebulized inhalation and microneedles have also been explored as potential delivery modes.156 While PELNs delivery modalities such as intravenous injection demonstrate some targeted delivery capability, they also face significant challenges, including degradation and immune clearance of the drug in the body’s circulation. These challenges result in limited therapeutic efficacy.18,157 Nebulized inhalation allows for direct delivery to the alveoli, thereby optimizing drug concentration for pulmonary application.158 In contrast, microneedling represents an emerging and efficient delivery strategy for PELNs, combining minimally invasive, localized drug delivery with the pro-angiogenic, pro-tissue regeneration and anti-inflammatory properties of PELNs, offering a promising avenue for clinical application.156
Endosomal Escape
Endolysosomal trapping of PELNs and endosomal escape strategies contribute to the enhancement of PELNs activity upon entry into the cell, thus allowing for the more effective exertion of the inherent biological effects.159 As a lipid complex, PELNs have the potential to escape through a mechanism called flip-flop. Recent research has begun to employ methods used by viruses and bacteria for endosomal escape. To date, a number of endosomal escape agents have been purified or synthesized from various sources, including bacteria-derived agents like listeriolysin O (LLO), virus-derived agents such as influenza-derived fusion peptide diINF-7, plant-derived agents like ricin, human/animal-derived agents such as epidermal growth factor receptor (EGFR), and other synthetic chemical agents.160 By employing a specific class of ionizable lipids, the researchers were able to fabricate lipid nanoparticles for the purpose of facilitating cytoplasmic nucleic acid transfer, such as 1.2-di-O-octadecenyl-3-trimethylammonium propane (DOTMA), DLin-KC2-DMA, and DLin-MC3-DMA. In an acidic environment (endosomes), this class of lipid complexes exhibits a positive charge, which results in membrane fusion and endosome rupture. This process allows the lipid complexes to escape into the cytosol. This type of technology also has the potential to modify PELNs, thereby enhancing efficacy and ensuring a certain level of delivery.161,162
Effects of Protein Corona
A further study has demonstrated that the protein corona formed when PELNs are cultivated or administered under specific conditions has a deleterious impact on nanomedicine in numerous instances. This is evidenced by the disabling of PELNs targeting, the activation of immune responses and the rapid clearance of the nanoparticles. Following injection, PELNs interact with components of the circulation, resulting in the formation of a protein corona. This process alters the properties of the nanoparticles, influencing their subsequent fate. Despite the incomplete understanding of the formation mechanism and the imperfect characterization technique, the significant advantages of this engineered modification include enhanced targeting of PELNs, prolonged circulation time and reduced aggregation embolism.163 Jun-Yong Wu et al developed and utilized angiopep-2 (Ang) modified multifunctional exosome mimics (Ang-EM) for the purpose of controlling protein corona. The findings revealed that Ang-EM exhibited diminished protein corona formation due to its capacity to absorb fewer serum proteins. Furthermore, Ang-EM demonstrated an enhanced ability to target glioblastoma and a notable suppression of glioblastoma growth in mice through its mediated brain delivery of docetaxel (DTX).164 Besides, safety of PELNs is a prerequisite for extensive research and clinical translation. The ability of all types of PELNs to reach target cells without any toxicity and exert possible biological effects remains to be further explored. Meanwhile, clinical translation is limited by some unreported side effects. The potential cytotoxicity of some PELNs and the underlying mechanisms remain unclear.165 Additionally, establishing of transgenic plants may prove an attractive option as endotoxin-free bioreactors for engineered nanomedicine, with the capacity to express human-compatible recombinant proteins and other biologically active components with therapeutic potential, thus enhancing the effects of PELNs.166 More investigations are required on those topics.
It is incontestable that natural and engineered PELNs offer considerable advantages. A more comprehensive approach to research and optimization, coupled with a systematic consideration of the current state of research and challenges, could facilitate the further expansion of the prospects for the application of PELNs as drugs and drug delivery systems. The full realization of the potential of engineered PELNs in clinical therapy will necessitate interdisciplinary collaboration and sustained efforts.
Conclusion
In conclusion, PELNs represent a promising frontier in nanomedicine, offering a viable alternative to animal cell-derived extracellular vesicles due to their superior biocompatibility, low immunogenicity, and capacity for large-scale production. Their unique properties have positioned PELNs as a promising tool in the arsenal against various diseases. In addition, Strategies for PELN engineering have been instrumental in enhancing the therapeutic efficacy by improving their targeting specificity, bioavailability, and cellular uptake. The innovative engineering techniques have also paved the way for the encapsulation of a wide range of therapeutic molecules, including chemotherapeutic drugs, nucleic acids, and proteins, further expanding the utility of PELNs as drug delivery systems. To facilitate the translation of PELNs from bench to bedside, issues such as heterogeneity and the lack of standardized manufacturing practices need to be addressed. Additionally, the optimization of PELN isolation and purification methods is crucial for ensuring the purity and stability of these nanovesicles. With challenges abound, ongoing research and interdisciplinary collaboration hold the key to unlocking the full potential of PELNs, paving the way for their integration into clinical practice and the advancement of personalized medicine.
Funding
This work is supported by National Natural Science Foundation of China (82400565 and 82100313).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhao W, Zhang H, Liu R, Cui R. Advances in immunomodulatory mechanisms of mesenchymal stem cells-derived exosome on immune cells in scar formation. Int J Nanomed. 2023;18:3643–3662. doi:10.2147/ijn.S412717
2. Zhao Z, Zhang L, Ocansey DKW, Wang B, Mao F. The role of mesenchymal stem cell-derived exosome in epigenetic modifications in inflammatory diseases. Front Immunol. 2023;14:1166536. doi:10.3389/fimmu.2023.1166536
3. Kwon HH, Yang SH, Lee J, et al. Combination treatment with human adipose tissue stem cell-derived exosomes and fractional CO2 laser for acne scars: a 12-week Prospective, Double-blind, Randomized, Split-face Study. Acta Derm Venereol. 2020;100(18):adv00310. doi:10.2340/00015555-3666
4. Johnson J, Law SQK, Shojaee M, et al. First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing. J Extracell Vesicles. 2023;12(7):e12332. doi:10.1002/jev2.12332
5. Mondal J, Pillarisetti S, Junnuthula V, et al. Hybrid exosomes, exosome-like nanovesicles and engineered exosomes for therapeutic applications. J Control Release. 2023;353:1127–1149. doi:10.1016/j.jconrel.2022.12.027
6. Wang X, Xin C, Zhou Y, Sun T. Plant-derived vesicle-like nanoparticles: the next-generation drug delivery nanoplatforms. Pharmaceutics. 2024;16(5):588. doi:10.3390/pharmaceutics16050588
7. Salehi M, Negahdari B, Mehryab F, Shekari F. Milk-derived extracellular vesicles: biomedical applications, current challenges, and future perspectives. J Agric Food Chem. 2024;72(15):8304–8331. doi:10.1021/acs.jafc.3c07899
8. Han X, Wei Q, Lv Y, et al. Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol Ther. 2022;30(1):327–340. doi:10.1016/j.ymthe.2021.08.028
9. Kumar A, Sundaram K, Teng Y, et al. Ginger nanoparticles mediated induction of Foxa2 prevents high-fat diet-induced insulin resistance. Theranostics. 2022;12(3):1388–1403. doi:10.7150/thno.62514
10. Bai C, Liu J, Zhang X, et al. Research status and challenges of plant-derived exosome-like nanoparticles. Biomed Pharmacother. 2024;174:116543. doi:10.1016/j.biopha.2024.116543
11. Suharta S, Barlian A, Hidajah AC, et al. Plant-derived exosome-like nanoparticles: a concise review on its extraction methods, content, bioactivities, and potential as functional food ingredient. J Food Sci. 2021;86(7):2838–2850. doi:10.1111/1750-3841.15787
12. Papadopoulos KS, Piperi C, Korkolopoulou P. Clinical Applications of Adipose-Derived Stem Cell (ADSC) exosomes in tissue regeneration. Int J Mol Sci. 2024;25(11):5916. doi:10.3390/ijms25115916
13. Mu N, Li J, Zeng L, et al. Plant-derived exosome-like nanovesicles: current progress and prospects. Int J Nanomed. 2023;18:4987–5009. doi:10.2147/ijn.S420748
14. You JY, Kang SJ, Rhee WJ. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact Mater. 2021;6(12):4321–4332. doi:10.1016/j.bioactmat.2021.04.023
15. Wei Y, Cai X, Wu Q, et al. Extraction, isolation, and component analysis of turmeric-derived exosome-like nanoparticles. Bioengineering. 2023;10(10):1199. doi:10.3390/bioengineering10101199
16. Di Gioia S, Hossain MN, Conese M. Biological properties and therapeutic effects of plant-derived nanovesicles. Open Med. 2020;15(1):1096–1122. doi:10.1515/med-2020-0160
17. Zhang L, He F, Gao L, et al. Engineering exosome-like nanovesicles derived from Asparagus cochinchinensis can inhibit the proliferation of Hepatocellular Carcinoma cells with better safety profile. Int J Nanomed. 2021;16:1575–1586. doi:10.2147/ijn.S293067
18. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
19. Zhu Z, Liao L, Gao M, Liu Q. Garlic-derived exosome-like nanovesicles alleviate dextran sulphate sodium-induced mouse colitis via the TLR4/MyD88/NF-κB pathway and gut microbiota modulation. Food Funct. 2023;14(16):7520–7534. doi:10.1039/d3fo01094e
20. Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–494. doi:10.1016/j.actbio.2019.05.054
21. Xia Y, Zhang J, Liu G, Wolfram J. Immunogenicity of extracellular vesicles. Adv Mater. 2024;36(33):e2403199. doi:10.1002/adma.202403199
22. Huang S, He C, Li J, Gao YZ, Wang Z, Wei Y. Emerging paradigms in exploring the interactions among diet, probiotics, and cancer immunotherapeutic response. Innovation. 2023;4(4):100456. doi:10.1016/j.xinn.2023.100456
23. García-Manrique P, Matos M, Gutiérrez G, Pazos C, Blanco-López MC. Therapeutic biomaterials based on extracellular vesicles: classification of bio-engineering and mimetic preparation routes. J Extracell Vesicles. 2018;7(1):1422676. doi:10.1080/20013078.2017.1422676
24. Dad HA, Gu TW, Zhu AQ, Huang LQ, Peng LH. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29(1):13–31. doi:10.1016/j.ymthe.2020.11.030
25. Lu X, Han Q, Chen J, et al. Celery (Apium graveolens L.) exosome-like nanovesicles as a new-generation chemotherapy drug delivery platform against tumor proliferation. J Agric Food Chem. 2023;71(22):8413–8424. doi:10.1021/acs.jafc.2c07760
26. Iriawati I, Vitasasti S, Rahmadian FNA, Barlian A. Isolation and characterization of plant-derived exosome-like nanoparticles from Carica papaya L. fruit and their potential as anti-inflammatory agent. PLoS One. 2024;19(7):e0304335. doi:10.1371/journal.pone.0304335
27. Cheng Q, Dai Z, Shi X, et al. Expanding the toolbox of exosome-based modulators of cell functions. Biomaterials. 2021;277:121129. doi:10.1016/j.biomaterials.2021.121129
28. Koh E, Lee EJ, Nam GH, et al. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–129. doi:10.1016/j.biomaterials.2017.01.004
29. Rosso G, Cauda V. Biomimicking extracellular vesicles with fully artificial ones: a rational design of EV-BIOMIMETICS toward effective theranostic tools in nanomedicine. ACS Biomater Sci Eng. 2023;9(11):5924–5932. doi:10.1021/acsbiomaterials.2c01025
30. Kim J, Zhang S, Zhu Y, Wang R, Wang J. Amelioration of colitis progression by ginseng-derived exosome-like nanoparticles through suppression of inflammatory cytokines. J Ginseng Res. 2023;47(5):627–637. doi:10.1016/j.jgr.2023.01.004
31. Zhu MZ, Xu HM, Liang YJ, et al. Edible exosome-like nanoparticles from portulaca oleracea L mitigate DSS-induced colitis via facilitating double-positive CD4(+)CD8(+)T cells expansion. J Nanobiotechnol. 2023;21(1):309. doi:10.1186/s12951-023-02065-0
32. Perut F, Roncuzzi L, Avnet S, et al. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules. 2021;11(1):87. doi:10.3390/biom11010087
33. Liu G, Kang G, Wang S, Huang Y, Cai Q. Extracellular vesicles: emerging players in plant defense against pathogens. Front Plant Sci. 2021;12:757925. doi:10.3389/fpls.2021.757925
34. Kameli N, Dragojlovic-Kerkache A, Savelkoul P, Stassen FR. Plant-derived extracellular vesicles: current findings, challenges, and future applications. Membranes. 2021;11(6):411. doi:10.3390/membranes11060411
35. Zhu H, He W. Ginger: a representative material of herb-derived exosome-like nanoparticles. Front Nutr. 2023;10:1223349. doi:10.3389/fnut.2023.1223349
36. Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi:10.1038/nature22341
37. Yi Q, Xu Z, Thakur A, et al. Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol Res. 2023;190:106733. doi:10.1016/j.phrs.2023.106733
38. Subudhi PD, Bihari C, Sarin SK, Baweja S. Emerging role of Edible Exosomes-Like Nanoparticles (ELNs) as hepatoprotective agents. Nanotheranostics. 2022;6(4):365–375. doi:10.7150/ntno.70999
39. Teng Y, Mu J, Hu X, et al. Grapefruit-derived nanovectors deliver miR-18a for treatment of liver metastasis of colon cancer by induction of M1 macrophages. Oncotarget. 2016;7(18):25683–25697. doi:10.18632/oncotarget.8361
40. Zhu H, Chang M, Wang Q, Chen J, Liu D, He W. Identifying the potential of miRNAs in houttuynia cordata-derived exosome-like nanoparticles against respiratory RNA viruses. Int J Nanomed. 2023;18:5983–6000. doi:10.2147/ijn.S425173
41. Stanly C, Alfieri M, Ambrosone A, Leone A, Fiume I, Pocsfalvi G. Grapefruit-derived micro and nanovesicles show distinct metabolome profiles and anticancer activities in the A375 human melanoma cell line. Cells. 2020;9(12):2722. doi:10.3390/cells9122722
42. Chen X, He L, Zhang C, et al. Exploring new avenues of health protection: plant-derived nanovesicles reshape microbial communities. J Nanobiotechnol. 2024;22(1):269. doi:10.1186/s12951-024-02500-w
43. Gao Q, Chen N, Li B, et al. Natural lipid nanoparticles extracted from Morus nigra L. leaves for targeted treatment of hepatocellular carcinoma via the oral route. J Nanobiotechnol. 2024;22(1):4. doi:10.1186/s12951-023-02286-3
44. Kilasoniya A, Garaeva L, Shtam T, et al. Potential of plant exosome vesicles from grapefruit (Citrus × paradisi) and tomato (Solanum lycopersicum) juices as functional ingredients and targeted drug delivery vehicles. Antioxidants. 2023;12(4). doi:10.3390/antiox12040943
45. Sung J, Yang C, Viennois E, Zhang M, Merlin D. Isolation, purification, and characterization of ginger-derived nanoparticles (GDNPs) from ginger, rhizome of Zingiber officinale. Bio-Protocol. 2019;9(19):e3390. doi:10.21769/BioProtoc.3390
46. Zhan W, Deng M, Huang X, et al. Pueraria lobata-derived exosome-like nanovesicles alleviate osteoporosis by enhacning autophagy. J Control Release. 2023;364:644–653. doi:10.1016/j.jconrel.2023.11.020
47. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804. doi:10.7150/thno.18133
48. Kalarikkal SP, Prasad D, Kasiappan R, Chaudhari SR, Sundaram GM. A cost-effective polyethylene glycol-based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci Rep. 2020;10(1):4456. doi:10.1038/s41598-020-61358-8
49. Kreimer S, Ivanov AR. Rapid isolation of extracellular vesicles from blood plasma with size-exclusion chromatography followed by mass spectrometry-based proteomic profiling. Methods Mol Biol. 2017;1660:295–302. doi:10.1007/978-1-4939-7253-1_24
50. Ludwig N, Whiteside TL, Reichert TE. Challenges in exosome isolation and analysis in health and disease. Int J Mol Sci. 2019;20(19):4684. doi:10.3390/ijms20194684
51. Suresh AP, Kalarikkal SP, Pullareddy B, Sundaram GM. Low pH-based method to increase the yield of plant-derived nanoparticles from fresh ginger rhizomes. ACS Omega. 2021;6(27):17635–17641. doi:10.1021/acsomega.1c02162
52. Omrani M, Beyrampour-Basmenj H, Jahanban-Esfahlan R, et al. Global trend in exosome isolation and application: an update concept in management of diseases. Mol Cell Biochem. 2024;479(3):679–691. doi:10.1007/s11010-023-04756-6
53. Mao X, Li T, Qi W, et al. Advances in the study of plant-derived extracellular vesicles in the skeletal muscle system. Pharmacol Res. 2024;204:107202. doi:10.1016/j.phrs.2024.107202
54. Wu J, Ma X, Lu Y, et al. Edible pueraria lobata-derived exosomes promote M2 macrophage polarization. Molecules. 2022;27(23):8184. doi:10.3390/molecules27238184
55. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomed. 2020;15:6917–6934. doi:10.2147/ijn.S264498
56. Shin H, Oh S, Hong S, et al. Early-stage lung cancer diagnosis by deep learning-based spectroscopic analysis of circulating exosomes. ACS Nano. 2020;14(5):5435–5444. doi:10.1021/acsnano.9b09119
57. Patel GK, Khan MA, Zubair H, et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci Rep. 2019;9(1):5335. doi:10.1038/s41598-019-41800-2
58. Yang F, Liao X, Tian Y, Li G. Exosome separation using microfluidic systems: size-based, immunoaffinity-based and dynamic methodologies. Biotechnol J. 2017;12(4). doi:10.1002/biot.201600699
59. Lai JJ, Chau ZL, Chen SY, et al. Exosome processing and characterization approaches for research and technology development. Adv Sci. 2022;9(15):e2103222. doi:10.1002/advs.202103222
60. Cai Q, Qiao L, Wang M, et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2018;360(6393):1126–1129. doi:10.1126/science.aar4142
61. He B, Cai Q, Qiao L, et al. RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat Plants. 2021;7(3):342–352. doi:10.1038/s41477-021-00863-8
62. Trentini M, Zanolla I, Tiengo E, et al. Link between organic nanovescicles from vegetable kingdom and human cell physiology: intracellular calcium signalling. J Nanobiotechnol. 2024;22(1):68. doi:10.1186/s12951-024-02340-8
63. Kim J, Li S, Zhang S, Wang J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J Pharm Sci. 2022;17(1):53–69. doi:10.1016/j.ajps.2021.05.006
64. Yang LY, Li CQ, Zhang YL, Ma MW, Cheng W, Zhang GJ. Emerging drug delivery vectors: engineering of plant-derived nanovesicles and their applications in biomedicine. Int J Nanomed. 2024;19:2591–2610. doi:10.2147/ijn.S454794
65. Wu C, Li J, Huang K, et al. Advances in preparation and engineering of plant-derived extracellular vesicles for nutrition intervention. Food Chem. 2024;457:140199. doi:10.1016/j.foodchem.2024.140199
66. Xiao J, Feng S, Wang X, et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ. 2018;6:e5186. doi:10.7717/peerj.5186
67. Nail HM, Chiu CC, Leung CH, Ahmed MMM, Wang HD. Exosomal miRNA-mediated intercellular communications and immunomodulatory effects in tumor microenvironments. J Biomed Sci. 2023;30(1):69. doi:10.1186/s12929-023-00964-w
68. Shi C, Huang K, Soto J, et al. Piperlongumine inhibits proliferation and oncogenic MYCN expression in chemoresistant metastatic retinoblastoma cells directly and through extracellular vesicles. Biomed Pharmacother. 2023;161:114554. doi:10.1016/j.biopha.2023.114554
69. Gao C, Zhou Y, Chen Z, et al. Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis. Theranostics. 2022;12(12):5596–5614. doi:10.7150/thno.73650
70. Şahin F, Koçak P, Güneş MY, Özkan İ, Yıldırım E, Kala EY. In vitro wound healing activity of wheat-derived nanovesicles. Appl Biochem Biotechnol. 2019;188(2):381–394. doi:10.1007/s12010-018-2913-1
71. Chen Q, Li Q, Liang Y, et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm Sin B. 2022;12(2):907–923. doi:10.1016/j.apsb.2021.08.016
72. Kim K, Yoo HJ, Jung JH, et al. Cytotoxic effects of plant sap-derived extracellular vesicles on various tumor cell types. J Funct Biomater. 2020;11(2):22. doi:10.3390/jfb11020022
73. Chen X, Ji S, Yan Y, et al. Engineered plant-derived nanovesicles facilitate tumor therapy: natural bioactivity plus drug controlled release platform. Int J Nanomed. 2023;18:4779–4804. doi:10.2147/ijn.S413831
74. Zhao Y, Tan H, Zhang J, et al. Plant-derived vesicles: a new era for anti-cancer drug delivery and cancer treatment. Int J Nanomed. 2023;18:6847–6868. doi:10.2147/ijn.S432279
75. Chen Q, Zu M, Gong H, et al. Tea leaf-derived exosome-like nanotherapeutics retard breast tumor growth by pro-apoptosis and microbiota modulation. J Nanobiotechnol. 2023;21(1):6. doi:10.1186/s12951-022-01755-5
76. Kaimuangpak K, Rosalina R, Thumanu K, Weerapreeyakul N. Macromolecules with predominant β-pleated sheet proteins in extracellular vesicles released from Raphanus sativus L. var. caudatus Alef microgreens induce DNA damage-mediated apoptosis in HCT116 colon cancer cells. Int J Biol Macromol. 2024;269(Pt 1):132001. doi:10.1016/j.ijbiomac.2024.132001
77. Yan G, Xiao Q, Zhao J, et al. Brucea javanica derived exosome-like nanovesicles deliver miRNAs for cancer therapy. J Control Release. 2024;367:425–440. doi:10.1016/j.jconrel.2024.01.060
78. Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267–1284. doi:10.1101/gad.314617.118
79. Kim J, Zhu Y, Chen S, et al. Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood-brain-barrier penetration and tumor microenvironment modulation. J Nanobiotechnol. 2023;21(1):253. doi:10.1186/s12951-023-02006-x
80. Bian Y, Li W, Jiang X, et al. Garlic-derived exosomes carrying miR-396e shapes macrophage metabolic reprograming to mitigate the inflammatory response in obese adipose tissue. J Nutr Biochem. 2023;113:109249. doi:10.1016/j.jnutbio.2022.109249
81. Liu J, Li W, Bian Y, et al. Garlic-derived exosomes regulate PFKFB3 expression to relieve liver dysfunction in high-fat diet-fed mice via macrophage-hepatocyte crosstalk. Phytomedicine. 2023;112:154679. doi:10.1016/j.phymed.2023.154679
82. Li S, Zhang R, Wang A, et al. Panax notoginseng: derived exosome-like nanoparticles attenuate ischemia reperfusion injury via altering microglia polarization. J Nanobiotechnol. 2023;21(1):416. doi:10.1186/s12951-023-02161-1
83. Urzì O, Cafora M, Ganji NR, et al. Lemon-derived nanovesicles achieve antioxidant and anti-inflammatory effects activating the AhR/Nrf2 signaling pathway. iScience. 2023;26(7):107041. doi:10.1016/j.isci.2023.107041
84. Del Pozo-Acebo L, López de Las Hazas MC, Margollés A, Dávalos A, García-Ruiz A. Eating microRNAs: pharmacological opportunities for cross-kingdom regulation and implications in host gene and gut microbiota modulation. Br J Pharmacol. 2021;178(11):2218–2245. doi:10.1111/bph.15421
85. Niu G, Jian T, Gai Y, Chen J. Microbiota and plant-derived vesicles that serve as therapeutic agents and delivery carriers to regulate metabolic syndrome. Adv Drug Deliv Rev. 2023;196:114774. doi:10.1016/j.addr.2023.114774
86. Liu Y, Tan ML, Zhu WJ, et al. In vitro effects of tartary buckwheat-derived nanovesicles on gut microbiota. J Agric Food Chem. 2022;70(8):2616–2629. doi:10.1021/acs.jafc.1c07658
87. Wang X, Liu Y, Dong X, et al. peu-MIR2916-p3-enriched garlic exosomes ameliorate murine colitis by reshaping gut microbiota, especially by boosting the anti-colitic Bacteroides thetaiotaomicron. Pharmacol Res. 2024;200:107071. doi:10.1016/j.phrs.2024.107071
88. Feng H, Yue Y, Zhang Y, et al. Plant-derived exosome-like nanoparticles: emerging nanosystems for enhanced tissue engineering. Int J Nanomed. 2024;19:1189–1204. doi:10.2147/ijn.S448905
89. Narauskaitė D, Vydmantaitė G, Rusteikaitė J, et al. Extracellular vesicles in skin wound healing. Pharmaceuticals. 2021;14(8). doi:10.3390/ph14080811
90. Yang S, Lu S, Ren L, et al. Ginseng-derived nanoparticles induce skin cell proliferation and promote wound healing. J Ginseng Res. 2023;47(1):133–143. doi:10.1016/j.jgr.2022.07.005
91. Zhao Q, Feng J, Liu F, et al. Rhizoma drynariae-derived nanovesicles reverse osteoporosis by potentiating osteogenic differentiation of human bone marrow mesenchymal stem cells via targeting ERα signaling. Acta Pharm Sin B. 2024;14(5):2210–2227. doi:10.1016/j.apsb.2024.02.005
92. Zhou X, Xu S, Zhang Z, et al. Gouqi-derived nanovesicles (GqDNVs) inhibited dexamethasone-induced muscle atrophy associating with AMPK/SIRT1/PGC1α signaling pathway. J Nanobiotechnol. 2024;22(1):276. doi:10.1186/s12951-024-02563-9
93. Sundaram K, Miller DP, Kumar A, et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of porphyromonas gingivalis. iScience. 2019;21:308–327. doi:10.1016/j.isci.2019.10.032
94. Teng Y, Xu F, Zhang X, et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol Ther. 2021;29(8):2424–2440. doi:10.1016/j.ymthe.2021.05.005
95. Lei C, Mu J, Teng Y, et al. Lemon exosome-like nanoparticles-manipulated probiotics protect mice from C. d iff infection. iScience. 2020;23(10):101571. doi:10.1016/j.isci.2020.101571
96. Shao M, Jin X, Chen S, Yang N, Feng G. Plant-derived extracellular vesicles -a novel clinical anti-inflammatory drug carrier worthy of investigation. Biomed Pharmacother. 2023;169:115904. doi:10.1016/j.biopha.2023.115904
97. Tao SC, Li XR, Wei WJ, et al. Polymeric coating on β-TCP scaffolds provides immobilization of small extracellular vesicles with surface-functionalization and ZEB1-Loading for bone defect repair in diabetes mellitus. Biomaterials. 2022;283:121465. doi:10.1016/j.biomaterials.2022.121465
98. Lai J, Pan Q, Chen G, et al. Triple hybrid cellular nanovesicles promote cardiac repair after ischemic reperfusion. ACS Nano. 2024;18(5):4443–4455. doi:10.1021/acsnano.3c10784
99. Pan Q, Xu J, Wen CJ, Xiong YY, Gong ZT, Yang YJ. Nanoparticles: promising tools for the treatment and prevention of myocardial infarction. Int J Nanomed. 2021;16:6719–6747. doi:10.2147/ijn.S328723
100. Zhang M, Hu S, Liu L, et al. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct Target Ther. 2023;8(1):124. doi:10.1038/s41392-023-01382-y
101. Ruan H, Li Y, Zheng D, et al. Engineered extracellular vesicles for ischemic stroke treatment. Innovation. 2023;4(2):100394. doi:10.1016/j.xinn.2023.100394
102. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi:10.1038/nbt.1807
103. Long F, Pan Y, Li J, et al. Orange-derived extracellular vesicles nanodrugs for efficient treatment of ovarian cancer assisted by transcytosis effect. Acta Pharm Sin B. 2023;13(12):5121–5134. doi:10.1016/j.apsb.2023.04.006
104. Xiao Q, Zhao W, Wu C, et al. Lemon-derived extracellular vesicles nanodrugs enable to efficiently overcome cancer multidrug resistance by endocytosis-triggered energy dissipation and energy production reduction. Adv Sci. 2022;9(20):e2105274. doi:10.1002/advs.202105274
105. Ma C, Yang Z, Wang J, et al. Exosomes miRNA-499a-5p targeted CD38 to alleviate anthraquinone induced cardiotoxicity: experimental research. Int J Surg. 2024;110(4):1992–2006. doi:10.1097/js9.0000000000001118
106. Chen J, Pan J, Liu S, et al. Fruit-derived extracellular-vesicle-engineered structural droplet drugs for enhanced glioblastoma chemotherapy. Adv Mater. 2023;35(45):e2304187. doi:10.1002/adma.202304187
107. Li Z, Wang H, Yin H, Bennett C, Zhang HG, Guo P. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci Rep. 2018;8(1):14644. doi:10.1038/s41598-018-32953-7
108. Qiao Z, Zhang K, Liu J, et al. Biomimetic electrodynamic nanoparticles comprising ginger-derived extracellular vesicles for synergistic anti-infective therapy. Nat Commun. 2022;13(1):7164. doi:10.1038/s41467-022-34883-5
109. Zhang M, Xiao B, Wang H, et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther. 2016;24(10):1783–1796. doi:10.1038/mt.2016.159
110. Moon K, Hur J, Kim KP, Lee K, Kang JY. Surface-functionalizable plant-derived extracellular vesicles for targeted drug delivery carrier using grapefruit. Adv Mater Interfaces. 2023;10(22):2300220. doi:10.1002/admi.202300220
111. Niu W, Xiao Q, Wang X, et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21(3):1484–1492. doi:10.1021/acs.nanolett.0c04753
112. Tang Z, Jun Y, Lv Y, et al. Aptamer-conjugated and doxorubicin-loaded grapefruit-derived nanovectors for targeted therapy against HER2(+) breast cancer. J Drug Target. 2020;28(2):186–194. doi:10.1080/1061186x.2019.1624970
113. Yan W, Tao M, Jiang B, et al. Overcoming drug resistance in colon cancer by aptamer-mediated targeted co-delivery of drug and siRNA using grapefruit-derived nanovectors. Cell Physiol Biochem. 2018;50(1):79–91. doi:10.1159/000493960
114. Zhuang X, Teng Y, Samykutty A, et al. Grapefruit-derived nanovectors delivering therapeutic miR17 through an intranasal route inhibit brain tumor progression. Mol Ther. 2016;24(1):96–105. doi:10.1038/mt.2015.188
115. Xu Y, Yan G, Zhao J, et al. Plant-derived exosomes as cell homogeneous nanoplatforms for brain biomacromolecules delivery ameliorate mitochondrial dysfunction against Parkinson’s disease. Nano Today. 2024;58:102438. doi:10.1016/j.nantod.2024.102438
116. Orefice NS, Di Raimo R, Mizzoni D, Logozzi M, Fais S. Purposing plant-derived exosomes-like nanovesicles for drug delivery: patents and literature review. Expert Opin Ther Pat. 2023;33(2):89–100. doi:10.1080/13543776.2023.2195093
117. Narmani A, Rezvani M, Farhood B, et al. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev Res. 2019;80(4):404–424. doi:10.1002/ddr.21545
118. Hong R, Luo L, Wang L, et al. Lepidium meyenii Walp (Maca)-derived extracellular vesicles ameliorate depression by promoting 5-HT synthesis via the modulation of gut-brain axis. Imeta. 2023;2(3):e116. doi:10.1002/imt2.116
119. Wang X, Zhang M, Flores SRL, et al. Oral gavage of ginger nanoparticle-derived lipid vectors carrying Dmt1 siRNA blunts iron loading in murine hereditary hemochromatosis. Mol Ther. 2019;27(3):493–506. doi:10.1016/j.ymthe.2019.01.003
120. Del Pozo-Acebo L, López de Las Hazas MC, Tomé-Carneiro J, et al. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol Res. 2022;185:106472. doi:10.1016/j.phrs.2022.106472
121. Garaeva L, Kamyshinsky R, Kil Y, et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci Rep. 2021;11(1):6489. doi:10.1038/s41598-021-85833-y
122. Mammadova R, Maggio S, Fiume I, et al. Protein biocargo and anti-inflammatory effect of tomato fruit-derived nanovesicles separated by density gradient ultracentrifugation and loaded with curcumin. Pharmaceutics. 2023;15(2):333. doi:10.3390/pharmaceutics15020333
123. Zhang M, Wang X, Han MK, Collins JF, Merlin D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine. 2017;12(16):1927–1943. doi:10.2217/nnm-2017-0196
124. Cao M, Diao N, Cai X, et al. Plant exosome nanovesicles (PENs): green delivery platforms. Mater Horiz. 2023;10(10):3879–3894. doi:10.1039/d3mh01030a
125. Johnsen KB, Gudbergsson JM, Duroux M, Moos T, Andresen TL, Simonsen JB. On the use of liposome controls in studies investigating the clinical potential of extracellular vesicle-based drug delivery systems - A commentary. J Control Release. 2018;269:10–14. doi:10.1016/j.jconrel.2017.11.002
126. Feng W, Teng Y, Zhong Q, et al. Biomimetic grapefruit-derived extracellular vesicles for safe and targeted delivery of sodium thiosulfate against vascular calcification. ACS Nano. 2023;17(24):24773–24789. doi:10.1021/acsnano.3c05261
127. Itakura S, Shohji A, Amagai S, et al. Gene knockdown in HaCaT cells by small interfering RNAs entrapped in grapefruit-derived extracellular vesicles using a microfluidic device. Sci Rep. 2023;13(1):3102. doi:10.1038/s41598-023-30180-3
128. Wang Q, Zhuang X, Mu J, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4:1867. doi:10.1038/ncomms2886
129. Corvigno S, Liu Y, Bayraktar E, et al. Enhanced plant-derived vesicles for nucleotide delivery for cancer therapy. NPJ Precis Oncol. 2024;8(1):86. doi:10.1038/s41698-024-00556-3
130. Fang Z, Song M, Lai K, Cui M, Yin M, Liu K. Kiwi-derived extracellular vesicles for oral delivery of sorafenib. Eur J Pharm Sci. 2023;191:106604. doi:10.1016/j.ejps.2023.106604
131. Farheen J, Iqbal MZ, Lu Y, Tang Z, Kong X. Vitis vinifera Kyoho-derived exosome-like nanoparticles-based drug delivery and therapeutic modalities for breast cancer therapy. J Drug Delivery Sci Technol. 2024;92:105332. doi:10.1016/j.jddst.2023.105332
132. Li S, Ye Z, Zhao L, Yao Y, Zhou Z. Evaluation of antioxidant activity and drug delivery potential of cell-derived extracellular vesicles from citrus reticulata blanco cv. “dahongpao”. Antioxidants. 2023;12(9):1706. doi:10.3390/antiox12091706
133. Liu Y, Qi H, Zong J, et al. Oral piwi-interacting RNA delivery mediated by green tea-derived exosome-like nanovesicles for the treatment of aortic dissection. Adv Healthc Mater. 2024:e2401466. doi:10.1002/adhm.202401466
134. Pomatto MAC, Gai C, Negro F, et al. Plant-derived extracellular vesicles as a delivery platform for RNA-based vaccine: feasibility study of an oral and intranasal SARS-CoV-2 vaccine. Pharmaceutics. 2023;15(3):974. doi:10.3390/pharmaceutics15030974
135. Sharma S, Mahanty M, Rahaman SG, et al. Avocado-derived extracellular vesicles loaded with ginkgetin and berberine prevent inflammation and macrophage foam cell formation. J Cell Mol Med. 2024;28(7):e18177. doi:10.1111/jcmm.18177
136. Umezu T, Takanashi M, Murakami Y, et al. Acerola exosome-like nanovesicles to systemically deliver nucleic acid medicine via oral administration. Mol Ther Methods Clin Dev. 2021;21:199–208. doi:10.1016/j.omtm.2021.03.006
137. Zhang Y, Chopp M, Zhang ZG, et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69–81. doi:10.1016/j.neuint.2016.08.003
138. Yang M, Luo Q, Chen X, Chen F. Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J Nanobiotechnol. 2021;19(1):259. doi:10.1186/s12951-021-00995-1
139. Bashyal S, Thapa C, Lee S. Recent progresses in exosome-based systems for targeted drug delivery to the brain. J Control Release. 2022;348:723–744. doi:10.1016/j.jconrel.2022.06.011
140. Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585–598. doi:10.1080/10717544.2020.1748758
141. Kang W, Xu Z, Lu H, et al. Advances in biomimetic nanomaterial delivery systems: harnessing nature’s inspiration for targeted drug delivery. J Mater Chem B. 2024;12(29):7001–7019. doi:10.1039/d4tb00565a
142. Li A, Zhao Y, Li Y, Jiang L, Gu Y, Liu J. Cell-derived biomimetic nanocarriers for targeted cancer therapy: cell membranes and extracellular vesicles. Drug Deliv. 2021;28(1):1237–1255. doi:10.1080/10717544.2021.1938757
143. Sun M, Yang J, Fan Y, et al. Beyond extracellular vesicles: hybrid membrane nanovesicles as emerging advanced tools for biomedical applications. Adv Sci. 2023;10(32):e2303617. doi:10.1002/advs.202303617
144. Zhuang WR, Wang Y, Lei Y, et al. Phytochemical engineered bacterial outer membrane vesicles for photodynamic effects promoted immunotherapy. Nano Lett. 2022;22(11):4491–4500. doi:10.1021/acs.nanolett.2c01280
145. Wang H, Mu J, Chen Y, et al. Hybrid ginseng-derived extracellular vesicles-like particles with autologous tumor cell membrane for personalized vaccination to inhibit tumor recurrence and metastasis. Adv Sci. 2024;11(17):e2308235. doi:10.1002/advs.202308235
146. Ma C, Liu K, Wang F, et al. Neutrophil membrane-engineered Panax ginseng root-derived exosomes loaded miRNA 182-5p targets NOX4/Drp-1/NLRP3 signal pathway to alleviate acute lung injury in sepsis: experimental studies. Int J Surg. 2024;110(1):72–86. doi:10.1097/js9.0000000000000789
147. Wang Q, Ren Y, Mu J, et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015;75(12):2520–2529. doi:10.1158/0008-5472.Can-14-3095
148. Huang R, Jia B, Su D, et al. Plant exosomes fused with engineered mesenchymal stem cell-derived nanovesicles for synergistic therapy of autoimmune skin disorders. J Extracell Vesicles. 2023;12(10):e12361. doi:10.1002/jev2.12361
149. Zeng L, Shi W, Wang H, et al. Codelivery of π-π stacked dual anticancer drugs based on aloe-derived nanovesicles for breast cancer therapy. ACS Appl Mater Interfaces. 2022;14(24):27686–27702. doi:10.1021/acsami.2c06546
150. Zheng Y, Pan C, Xu P, Liu K. Hydrogel-mediated extracellular vesicles for enhanced wound healing: the latest progress, and their prospects for 3D bioprinting. J Nanobiotechnol. 2024;22(1):57. doi:10.1186/s12951-024-02315-9
151. Wang Z, Yuan J, Xu Y, et al. Olea europaea leaf exosome-like nanovesicles encapsulated in a hyaluronic acid / tannic acid hydrogel dressing with dual “defense-repair” effects for treating skin photoaging. Mater Today Bio. 2024;26:101103. doi:10.1016/j.mtbio.2024.101103
152. Adamo G, Fierli D, Romancino DP, et al. Nanoalgosomes: introducing extracellular vesicles produced by microalgae. J Extracell Vesicles. 2021;10(6):e12081. doi:10.1002/jev2.12081
153. Weng Z, Zhang B, Wu C, et al. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol. 2021;14(1):136. doi:10.1186/s13045-021-01141-y
154. Pincela Lins PM, Pirlet E, Szymonik M, Bronckaers A, Nelissen I. Manufacture of extracellular vesicles derived from mesenchymal stromal cells. Trends Biotechnol. 2023;41(7):965–981. doi:10.1016/j.tibtech.2023.01.003
155. Ana ID, Barlian A, Hidajah AC, Wijaya CH, Notobroto HB, Kencana wungu TD. Challenges and strategy in treatment with exosomes for cell-free-based tissue engineering in dentistry. Future Sci OA. 2021;7(10):Fso751. doi:10.2144/fsoa-2021-0050
156. Wang X, Cheng W, Su J. Research progress of extracellular vesicles-loaded microneedle technology. Pharmaceutics. 2024;16(3). doi:10.3390/pharmaceutics16030326
157. Hu L, Wang J, Zhou X, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016;6(1):32993. doi:10.1038/srep32993
158. Dinh PC, Paudel D, Brochu H, et al. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat Commun. 2020;11(1):1064. doi:10.1038/s41467-020-14344-7
159. Ahmad A, Khan JM, Paray BA, Rashid K, Parvez A. Endolysosomal trapping of therapeutics and endosomal escape strategies. Drug Discov Today. 2024;29(8):104070. doi:10.1016/j.drudis.2024.104070
160. Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151(3):220–228. doi:10.1016/j.jconrel.2010.11.004
161. Schlich M, Palomba R, Costabile G, et al. Cytosolic delivery of nucleic acids: the case of ionizable lipid nanoparticles. Bioeng Transl Med. 2021;6(2):e10213. doi:10.1002/btm2.10213
162. Cullis PR, Hope MJ. Lipid nanoparticle systems for enabling gene therapies. Mol Ther. 2017;25(7):1467–1475. doi:10.1016/j.ymthe.2017.03.013
163. Chen Z, Chen X, Huang J, Wang J, Wang Z. Harnessing protein corona for biomimetic nanomedicine design. Biomimetics. 2022;7(3):126. doi:10.3390/biomimetics7030126
164. Wu JY, Li YJ, Wang J, et al. Multifunctional exosome-mimetics for targeted anti-glioblastoma therapy by manipulating protein Corona. J Nanobiotechnol. 2021;19(1):405. doi:10.1186/s12951-021-01153-3
165. Cong M, Tan S, Li S, et al. Technology insight: plant-derived vesicles-How far from the clinical biotherapeutics and therapeutic drug carriers? Adv Drug Deliv Rev. 2022;182:114108. doi:10.1016/j.addr.2021.114108
166. Mirzaee M, Osmani Z, Frébortová J, Frébort I. Recent advances in molecular farming using monocot plants. Biotechnol Adv. 2022;58:107913. doi:10.1016/j.biotechadv.2022.107913
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

