Back to Journals » International Journal of Nanomedicine » Volume 19
Enhanced Antimicrobial Properties of Polymeric Denture Materials Modified with Zein-Coated Inorganic Nanoparticles
Authors Naguib GH , Abd El-Aziz GS , Mira A, Kayal RA , Al-Turki L, Mously H, Alnowaiser A, Mazhar J, Hamed MT
Received 30 April 2024
Accepted for publication 31 August 2024
Published 9 September 2024 Volume 2024:19 Pages 9255—9271
DOI https://doi.org/10.2147/IJN.S476261
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Eng San Thian
Ghada H Naguib,1,2 Gamal S Abd El-Aziz,3 Abdulghani Mira,1 Rayyan A Kayal,4 Lulwa Al-Turki,5 Hisham Mously,5 Abeer Alnowaiser,6 Jumana Mazhar,7 Mohamed T Hamed5,8
1Department of Restorative Dentistry, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia; 2Department of Oral Biology, Cairo University School of Dentistry, Cairo, Egypt; 3Department of Clinical Anatomy, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia; 4Department of Periodontology, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia; 5Department of Oral and Maxillofacial Prosthodontics, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia; 6Department of Pediatric Dentistry, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia; 7King Abdulaziz University, Jeddah, Saudi Arabia; 8Department of Fixed Prosthodontics, Cairo University School of Dentistry, Cairo, Egypt
Correspondence: Ghada H Naguib, Department of Restorative Dentistry, Faculty of Dentistry, King Abdulaziz University, P.O Box 80209, Jeddah, 21589, Saudi Arabia, Tel +966558190589, Email [email protected]
Background: Polymeric denture materials can be susceptible to colonization by oral microorganisms. Zein-coated magnesium oxide nanoparticles (zMgO NPs) demonstrate antimicrobial activity. The aim of this study was to investigate the antimicrobial effect and adherence of different oral microorganisms on hybrid polymeric denture materials incorporated with zMgO NPs.
Methods: Five types of polymeric denture materials were used. A total of 480 disc-shaped specimens were divided by material type (n=96/grp), then subdivided by zMgO NPs concentration: control with no nanoparticles and other groups with zMgO NPs concentrations of 0.3%, 0.5% and 1% by weight. Characterization of the polymeric denture materials incorporating zMgO NPs was done, and the antimicrobial activity of all groups was tested against four types of microorganisms: 1) Streptococcus mutans, 2) Staphylococcus aureus, 3) Enterococcus faecalis and 4) Candida albicans. The samples underwent an adherence test and an agar diffusion test. Experiments were done in triplicates.
Results: The characterization of the hybrid samples revealed variation in the molecular composition, as well as a uniform distribution of the zMgO NPs in the polymeric denture materials. All hybrid polymeric denture materials groups induced a statistically significant antimicrobial activity, while the control groups showed the least antimicrobial activity. The agar diffusion test revealed no release of the zMgO NPs from the hybrid samples, indicating the NPs did not seep out of the matrix.
Conclusion: The zMgO NPs were effective in reducing the adherence of the tested microorganisms and enhancing the antimicrobial activity of the polymeric denture materials. This antimicrobial effect with the polymeric dentures could aid in resisting microbial issues such as denture stomatitis.
Keywords: antimicrobial, nanoparticles, denture, magnesium oxide, biomaterial
Graphical Abstract:

Introduction
Contemporary science is in need of modern materials with practical and effective attributes, and this demand has brought forth the “hybrid ideology”, which is to establish a conglomeration of materials with enhanced and renovated characteristics.1 Hybrid nanomaterials have essential properties as a result of the inorganic and organic commixture, and can be modified to embody desirable traits.2–4 Researchers worldwide are engrossed by hybrid nanomaterials with magnetic nanoparticles and conjugated polymers.5
Recently, the interest of using the nanoparticles (NPs) in healthcare has heightened as result of the NPs’ distinctive chemical and physical characteristics, particularly their reactivity, stability, and capacity to bind and damage bacterial membranes.6–8 Several studies on NPs have reported significant antibacterial activity in their interaction with the peptidoglycan cell wall and bacterial membrane. Furthermore, NPs can interrupt the synthesis of bacterial proteins and prevent DNA duplication.9–11 In dentistry, several innovations through NPs have emerged in the treatment and prevention of dental infections.12–15 Salvo and Sandoval reported on the multiple uses of copper NPs in angiogenesis, Parnia et al used titanium NPs to improve implant osseointegration, and Irfan et al utilized zinc oxide NPs-based surgical sutures to expedite wound healing.16–19
Despite the significant advancements in nanomaterials, several research gaps remain, particularly the lack of standardization in evaluation methods and the biological impacts of metal oxide nanoparticles on the body. It is imperative that the nanoparticles be safe and beneficial. Magnesium oxide nanoparticles (MgO NPs) are metal-based NPs that are essential, biocompatible, and biodegradable in the body, and they are capable of inhibiting gram-positive, gram-negative, and endospore-forming bacteria.20 MgO NPs have been reported to exhibit antibacterial activity against Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa. 21 Nanosized MgO had been recently introduced in dentistry to improve the dental materials’ antimicrobial properties. However, dental application of nanosized MgO is affected by the NPs’ agglomeration.22–24 In order to preserve the antimicrobial property of these NPs, a coating or surfactant is needed to prevent their clustering.
Zein is a natural polymer that comprises 44–79% of the corn endosperm protein.25 Being biodegradable, biocompatible, and versatile, zein has been exploited in various fields. Lately, pharmaceutical companies have used it as a coating for release-control and targeted delivery of drugs.26–28 The inherent characteristics of zein sustain the particles’ state of dispersion by reducing their hydrophobicity.29 Coating MgO NPs with the natural polymer zein was effective in the dispersion of the NPs and in the prevention of its agglomeration. Furthermore, the inclusion of zein with the NPs and the dental materials tested did not compromise the properties of either the NPs or the dental material. There was improvement of the dental material’s properties through the enhancement of the antimicrobial activity provided by the zein-coated magnesium oxide nanoparticles (zMgO NPs).22–24
Polymeric denture materials are commonly employed for dental applications.30 They have good physical and mechanical properties but can be susceptible to colonization by the oral microorganisms, which depicts a serious impediment. The epidemiological studies have reported that the prevalence of denture stomatitis among denture wearers ranged from 15% to over 70%.31,32 Studies reported that many complex factors are associated with denture stomatitis such as the age and health of the denture wearer, the lifespan, the composition, and the cleaning regimen of the denture material together with the nature of the colonizing oral microorganisms.33 A higher prevalence of denture stomatitis is seen in older people due to long-term denture use, ill-fitting dentures, lack of oral hygiene, several medications, and impaired immunity. Nonetheless, children and young adults wearing acrylic partial dentures can also be affected by denture stomatitis.34–36 The primary microorganisms that colonize denture bases are Gram-positive Streptococcus spp., Streptococcus oralis, Streptococcus mutans, Streptococcus mitis, Streptococcus gordonii, Streptococcus sanguinis, and Streptococcus parasanguinis, and other species including Veillonella spp., Neisseria spp., Rothia spp., Abiotrophia spp., Gamella spp. and Granulicatella spp. 37 The most prevalent fungi are Candida albicans. 33
Antimicrobial hybrid polymers can be used to decrease plaque formation; however, being soluble can accelerate the release of the antimicrobial agent and increases plaque accumulation, thereby retroactively negating the benefit. The antimicrobial agent needs to be immobilized in the polymer to halt the attachment of microorganisms onto the surface of the material.38,39 Therefore, this study was designed to investigate the antimicrobial effect and adherence of the oral microorganisms Streptococcus mutans (S. mutans), Staphylococcus aureus (S. aureus), Enterococcus faecalis (E. faecalis), and Candida albicans (C. albicans) on the denture polymers incorporated with zMgO NPs. The null hypothesis was that there is no difference in adherence or antimicrobial effect of hybrid denture polymers after incorporating zMgO NPs.
Materials and Methods
Ethical Approval
This study was carried out after obtaining an approval from the Research Ethics Committee in King Abdulaziz University, Faculty of Dentistry (#47-12-19).
Materials
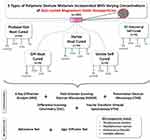
An overview of the study design is demonstrated in Figure 1. Five different types of polymeric denture materials were used in this study: Heat-cured Pro base Hot (HCPb) (Ivoclar Vivadent, New York, USA), Vertex heat-cured (HCV) (Vertex Dental, the Netherlands), DPI heat-cured (HCDPI) (DPI, Dental Products of India, India), Vertex self-cured (SCV) (Vertex Dental, the Netherlands), and self-cured GC Nature-Cryl Pour (SCGC) (GC, Illinois, USA). Natural corn polymer zein was procured from Sigma–Aldrich (St. Louis, MO, USA). The agar plates and thioglycolate were purchased from Saudi Prepared Laboratory Media Company (SPLM, Riyadh, Saudi Arabia) while discs were bought from Becton, Dickinson, and Company (BD, New Jersey, 07417, USA). Distinctive microorganism strains were purchased from the American Type Culture Collection (ATCC, VA, USA). All other chemicals, solutions and kits were purchased from Sigma–Aldrich (St. Louis, MO, USA).
Preparation of MgO NPs
The hydrothermal approach was adapted to prepare the MgO NPs. Magnesium acetate was stirred in distilled water (6.44 g/75 mL) for 30 minutes at 25°C. After incorporation of the mixture of water and urea (25 mL/1.2 g), the blend was autoclaved for 15 minutes. Upon reaching room temperature, the mixture was then centrifuged, filtered, and dried for 24 hours at 60°C and calcinated for 1 hour at 600°C.40
Coating of MgO NPs
The zein was used to coat the synthesized MgO NPs as done in accordance with a previous study.22 The zein polymer (0.02 g) was dissolved in a combination of 0.1 NaOH and ethanol. Then, drops of the zein solution were added to a 15mL combination of 0.9% (w/v) polyvinyl alcohol and MgO (0.02 g) at 10°C and 20 kHz frequency while utilizing 750 W of ultrasonic shear. At room temperature, the solution was stirred at 500 rpm to evaporate the ethanol. Afterwards, centrifuging at 3000 rpm was done for 45 minutes to purify the MgO NPs and get rid of the superfluity of the polyvinyl alcohol. Following removal of the supernatant, a 5 mL buffer was used to dissolve the pellet. Finally, the combination was lyophilized after the addition of 2% (w/v) of trehalose (VirTis Bench Top Lyophilizer, SP Industries, Stone Ridge, NY, USA).
Preparation of Hybrid Polymeric Denture Discs
480 samples of hybrid polymeric denture materials were prepared. For the self-cured polymeric material, a mold (10x2 mm) was directly used. The heat-cured samples’ mold was made from blue inlay wax with the same dimensions (10x2 mm), invested, and then processed according to the manufacturer’s instructions.
The polymeric denture materials were incorporated with zMgO NPs of 0.0%, 0.3%, 0.5%, and 1.0% (n=96) through precise calibration by weight down to 0.0001 g (Mettler Toledo™, Fischer Scientific, USA).41 The NPs were vortexed with the powder of the polymeric denture materials at 2000 rpm. The heat-cured and self-cured denture base materials were processed according to the manufacturer’s instructions to ensure that complete setting was achieved.42
Characterization of the Denture Base Materials with zMgO NPs
X-Ray Diffraction (XRD)
The crystalline structure of the five types of polymeric denture materials before and after incorporation of zMgO NPs was identified using an X-ray diffractometer (XRD; Rigaku, Ultima IV, Japan) with the intensity as a function of Bragg’s angle. The range of 2θ (30–80◦) was used to scan the XRD spectra.
Field Emission Scanning Electron Microscopy (FESEM)
Images of the specimens before and after incorporation of the zMgO NPs were investigated for their surface morphology and the distribution of the NPs at 1000X with the lower secondary electron detector at 5 kV.43
Transmission Electron Microscopy (TEM)
Transmission electron microscopy was done to assess the surface morphology of the zMgO NPs using a JEOL 2011 High-Resolution Electron Microscope (Jeol Ltd., Tokyo, Japan) at an acceleration voltage of 100 kV. The zMgO NPs with ethanol were placed onto carbon-coated copper grids, after which they were dried under ambient conditions.
Differential Scanning Calorimetry (DSC)
Differential Scanning Calorimetry was employed as a thermo-analytical technique to characterize the behavior of the zMgO NPs at different temperatures.44 DSC-60 Plus Differential Scanning Calorimeter (Shimadzu, Kyoto, Japan) was set with an airflow rate of 50 mL per minute. Empty sealed aluminum pans were used as the control, while the test material was 1–2 mg of dry MgO nanowires sealed in the other pans. The samples were assessed in a thermal range of 25–800°C at a rate of 10°C every minute. This was repeated with the zein polymer as the test material, and then the zMgO NPs.
Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR spectra of the five types of polymeric denture materials before and after incorporation of zMgO NPs were investigated. FTIR Type 8000 Series Fourier transformation (Shimadzu Co., Japan) was used in the absorbance mode at a wave number of 400–4000 cm−1.45
Preparation of Microbial Cultures
The tested oral microorganisms were S. mutans: 10449 (ATCC 25175), S. aureus: Seattle 1945 (ATCC 25923), E. faecalis: Portland (ATCC 29212), and C. albicans: 3147 (ATCC 10231). They were obtained from the American Type Culture Collection (ATCC, VA, USA) to assess the hybrid polymeric denture materials’ antimicrobial activity.
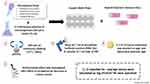
Adherence Test
A schematic of the adherence test can be seen in Figure 2. An aseptic 24-wells plate was used to perform the adherence test. 0.5 McFarland solution of microorganisms (40 µL) in broth (15 mL) (SPLM, Riyadh, Saudi Arabia) was prepared. Discs of hybrid polymeric denture materials with different concentrations of zMgO NPs 0.0%, 0.3%, 0.5% and 1% were inoculated with the separately prepared bacterial and fungal suspensions and incubated with 240 rpm of continuous shaking (Shaker-Adamo, Piracicaba, Brazil) at 37°C for 24 hours.
To detach non-adherent cells, the specimens were rinsed with 1 mL of phosphate buffered solution three times and transmitted to a 1 mL tube of phosphate buffered solution. The suspension was then vortexed for 60 seconds to disperse the adhered cells. After dilution of the suspension 10, 100, and 1000 times, 0.1 mL of every suspension was planted on agar and Sabouraud dextrose agar (Difco Labs., Detroit, MI, USA). The antimicrobial effect of the hybrid polymeric denture specimens was investigated after 48 hours of incubation at 37°C by decrease in colony counts. The experiment was done three times. Results of the average values were calculated as log CFU/cm2 for each specimen.
Agar Diffusion Assay
Freshly prepared microorganisms were used to inoculate sterile agar plates. Hybrid polymeric denture materials discs of various groups of 0.0%, 0.3%, 0.5% and 1% of zMgO NPs were positioned in the inoculated plates of the incubator (Thermo Fischer Scientific, Waltham, MA, USA) at 37°C for 24 hours. Experiments were done in triplicates and inhibition zones were gauged.46
Statistical Analysis
All data (mean value ± SD) were investigated using two-way analysis of variance (ANOVA) trailed by the least significant test at p<0.05 using the Statistical Package for Social Sciences (SPSS Version 23, IBM Inc. Armonk, NY, USA).
Results
Characterization of Hybrid Polymeric Denture Materials
X-Ray Diffraction Analysis (XRD)
The zMgO NPs demonstrated respective peaks at 36.98°, 42.95°, 62.40°, 74.80° and 78.75° 2θ as a result of (111), (200), (220), (311), and (222) planes, respectively, affirming the cubic shape of MgO (Figure 3). However, XRD spectra of the hybrid polymeric materials with 1% zMgO NPs showed no definite diffraction peaks. This could be due to the amorphous structure of the polymer of the hybrid polymeric denture materials and due to the minute amount of the incorporated zMgO NPs.
 |
Figure 3 Diagrammatic representation of XRD spectra of: (A) zMgO NPs; (B) denture base materials with zMgO NPs. |
Field Emission Scanning Electron Microscopy (FESEM)
The morphological characteristics of the specimens HCPb, HCV, HCDPI, SCV, and SCGC incorporating 1% zMgO NPs can be seen in Figure 4. Micrograph analysis showed a uniform dispersion of the inorganic NPs in the polymer matrix of the five types of hybrid polymeric denture materials.
Transmission Electron Microscopy (TEM)
The assessment of the procured TEM images illustrated that the zMgO nanowires were about 30–60 nm wide and 2–3 μm long. This was corroborated by the XRD and SEM results (Figure 5).
 |
Figure 5 TEM micrograph analysis of the zein-coated MgO nanowires. |
Differential Scanning Calorimetry (DSC)
The DSC spectra of the nanowires showed a single peak (endothermic) at 254°C for the MgO nanowires (Figure 6A). The peak appeared distinct, illustrating that the nanowires were pure. The pure zein polymer was represented by a single peak (endothermic) at 381.5 °C(Figure 6B). In Figure 6C, the zMgO NPs scan demonstrated two distinct peaks at 180.6°C and 280.8°C—lower values than the pure MgO or zein polymer alone. Furthermore, the melting point of the MgO nanowires went from 254°C to 180.6°C. The melting point of the zein polymer also lowered from 381°C to 280.8°C.
Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR absorption bands for the specimens after incorporation of 1% zMgO NPs showed a shift and change in the bands’ intensity with the appearance of new peaks in comparison with the control polymeric denture materials without the zMgO NPs.
FTIR of the Heat-Cured Polymeric Denture Materials
As seen in Figure 7, in the heat-cured hybrid polymeric denture materials incorporating 1% zMgO NPs, there was a shortening and a shift in the C=C and C-N bands at 800 cm−1 and 950 cm−1, respectively, in all groups involving zMgO NPs, thereby matching the pattern of the polymethylmethacrylate. Additionally, there was a shift in O-H band at 1500 cm−1 for zMgO (HCV), at 1490cm−1 for zMgO (HCP), and at 1550cm−1 for zMgO (HCDPI), likely due to the adsorbed water molecules on the surface of the nanowires. A shortening in the C=O band at 1700cm−1 for zMgO (HCV), at 1730 cm−1 for zMgO (HCP), and at 1690cm−1 for zMgO (HCDPI) was seen, expressing the spectra of zMgO nanowires Also, a stretching of the C=O, C≡C, and O-H bands at 2350 cm−1, 3000 cm−1, and 3100 cm−1, respectively, for zMgO (HCV) was seen due to the acrylate carboxyl group. The disappearance of C-H bands in zMgO (HCPC) and the appearance of O-H bands at 3750 cm−1, 3850 cm−1, 3900 cm−1, and 3950 cm−1 in zMgO (HCDPI) was also noted, corresponding to the hydrogen bond of the hydroxyl groups of the zein and MgO NPs.
 |
Figure 7 FTIR Spectra of heat-cured denture base materials with and without zMgO NPs. Abbreviations: HCV, Vertex heat-cured; HCDPI, DPI heat-cured; HCPb, Heat-cured Pro-base Hot. |
FTIR of the Self-Cured Polymeric Denture Materials
In the self-cured polymeric denture materials incorporating 1% zMgO NPs, both materials zMgO (SCCV) and zMgO (SCGC) displayed a shortening and shift of the C-O band at 950 cm−1 and a shortening and shift of the O-H band at 1400 cm−1. The presence of the -OH functional group was likely due to the adsorbed water molecules. A shortening in the C=O band at 1720 cm−1, 2800 cm−1, 2900 cm−1, and 3000 cm−1 was also seen. Moreover, a shortening of the N-H and O-H bands of amine and alcohol appeared at 3550 cm−1 and 3700 cm−1, corresponding to the hydrogen bond of the hydroxyl groups of zein and MgO (Figure 8).
 |
Figure 8 FTIR Spectra of the self-cured denture base materials with and without MgO NPs. Abbreviations: SCGC, self-cured GC Nature-Cryl Pour; SCV, Vertex self-cured. |
Adherence Test
The results of the antimicrobial activity of the zMgO NPs in the adherence test can be seen in Figure 9. After 24 hours of incubation, all the concentrations showed a statistically significant effect against S. mutans when compared to the control groups (p<0.01). The effect of zMgO NPs against S. mutans was close to that of the C. albicans. For HCPb, there was no bacterial growth (0 CFU) with all added concentrations of zMgO NPs. For HCV and HCDPI, the bacteria count for added concentrations of 0.3%, 0.5%, and 1% zMgO NPs was 1, 1, and 1 CFU, respectively. With the self-cured samples SCV and SCGC, the bacteria count for zMgO NPs concentrations of 0.3%, 0.5%, and 1% zMgO NPs was 10, 10, and 10 CFU, respectively.
In comparison to the control groups, the effect of zMgO NPs on the S. aureus was highly significant (p<0.001). For HCPb, all groups with zMgO NPs showed growth of 1 CFU. The HCV bacteria count with zMgO NPs concentrations of 0.3%, 0.5%, and 1% zMgO NPs was 10, 10, and 1 CFU, respectively. Meanwhile, the HCDPI bacteria count for 0.3%, 0.5%, and 1% zMgO NPs was 10, 1, and 1 CFU, respectively. For the self-cured SCV, the bacterial count for 0.3%, 0.5%, and 1% zMgO NPs was 10, 1, and 1 CFU, respectively. The SCGC bacteria count for 0.3%, 0.5%, and 1% zMgO NPs was 10, 10, and 1 CFU, respectively.
The effect of zMgO NPs against E. faecalis presented the lowest effect in comparison to S. mutans, S. aureus, and C. albicans. All the concentrations showed a significant effect against E. faecalis in association of the control group (p<0.01). For HCPb, the bacteria count for 0.3%, 0.5%, and 1% zMgO NPs was 10, 10, and 1 CFU, respectively. For HCV, the bacteria count for 0.3%, 0.5%, and 1% zMgO NPs was 100, 10, and 10 CFU, respectively. For the HCDPI, the bacteria count for 0.3%, 0.5%, and 1% zMgO NPs was 10, 10, and 1 CFU, respectively. SCV with 0.3%, 0.5%, and 1% zMgO NPs both showed a bacteria count of 10, 1, and 1 CFU, respectively. Lastly, the SCGC with 0.3%, 0.5%, and 1% zMgO NPs demonstrated a bacteria count of 10, 10 and 1 CFU, respectively.
The effect of the zMgO on C. albicans was highly significant with all concentrations of HCPb, HCV, HCDPI, SCV, and SCGC in comparison to the control groups as there was no growth of bacteria (p<0.001). There was a significant difference between the control group and the groups with added zMgO NPs in SCGC (p<0.01). Furthermore, there was bacterial growth of 100, 10, and 0 CFU on SCGC 0.3%, 0.5%, and 1% zMgO NPs, respectively.
Agar Diffusion Assay
The agar diffusion test was used to assess the release of the zMgO NPs. Results showed no release of inorganic NPs as seen by the absence of the inhibition zone with all of the microorganisms: S. mutans, S. aureus., E. faecalis, and C. albicans (p<0.01). (Figure 10).
Discussion
Hybrid polymers containing both inorganic and organic components are gaining traction as a result of their distinct and integral characteristics. Dentistry aims to devise materials with good physical and mechanical properties that are also highly biocompatible with the oral environment. Polymeric denture materials assert satisfying characteristics for use in the patient’s mouth and have a long history of use for the replacement of teeth.47
The FDA approved the usage of MgO NPs for safe applications in biomedicine.7,23,40 In our previous work, we found that the incorporation of the zein polymer as a coating agent prevented the agglomeration of MgO NPs and improved the NPs’ interaction with microorganisms.22,23 This study is part of a series of investigations involving the dental implications of zMgO NPs.22–24,48–58 Herein, the zMgO NPs were incorporated with different polymeric denture materials in order to investigate the antimicrobial effect and adherence of some of the most common oral pathogens: S. mutans, S. aureus., E. faecalis, and C. albicans. Previous studies had reported that an increase in NPs concentration increases their antimicrobial effect; our previous studies revealed that adding zMgO NPs up to 1% increases the antimicrobial activity.23,29,55 Similarly, several studies showed that the antimicrobial property of NPs was not linearly dependent on their concentration, but rather affected by their dispersion and distribution in the matrix.59 Therefore, we chose to assess zMgO NPs in various concentrations, those being 0% (control), 0.3%, 0.5%, and 1%.
In the present study, the formation of the hybrid polymers was confirmed by XRD, FESEM, TEM, DSC, and FTIR. The XRD of each material is a unique portrayal of its composition and structure. The crystalline nature of the NPs was displayed by its sharp, intense peaks. The hybrid polymeric denture materials did not exhibit high peaks due to the minute amount of the incorporated zMgO NPs. Upadhyay et al reported that the amorphous background together with the elastic and inelastic scattering of the material contributes to its XRD analysis while others stated that the load and size of NPs cause the XRD peaks to be broad.60 Moreover, the amorphous hybrid polymeric denture materials could have suppressed the crystallinity of the zMgO NPs as a result of their hydrophilic interaction with them.61,62 This pattern was also observed in similar studies as Pantazi et al in which PMMA with titanium oxide NPs displayed broad diffraction peaks, indicating the presence of significantly small crystallites in the material’s structure.63 Other metal oxide NPs also exhibit a crystalline nature, but demonstrated varying results within their respective material, probably due to the distinctive types of NPs and the properties of the base material.18,39,64
In the FESEM analysis, the zMgO NPs were uniformly distributed in the matrix of all the hybrid polymeric denture materials. The absence of aggregation of zMgO NPs ascertains the correlation of dispersion to the antimicrobial power of the NPs. The uniform dispersion is essential to the antimicrobial activity, as the nano-size allows for optimum performance.18,65 Moreover, the TEM graphs illustrated that the nanowires were about 30–60 nm wide and 2–3 μm long, similar to the zinc oxide and silver NPs in the investigation conducted by Irfan et al, in which the NPs’ size were 60 nm and 50 nm, respectively.66 Furthermore, it was reported that this size range is optimal from a nanomaterials’ standpoint.66,67
Characterization was also fulfilled through DSC, which allows for analysis of the heat energy uptake of the investigated specimens. The DSC thermogram of the zein-coated MgO nanowires expressed a shift of the endothermic peaks to lower temperatures. This could be a result of the physical interaction of the polymer with the NPs, although there was no formation of a new component, similar to the thermal behavior of zein in the synthesis of NPs in other studies.68
The FTIR spectroscopy allows for the investigation into the different functional groups in a material to compare their absorbance and transmission peaks to the database, providing illuminating data on the material’s behavior. It is a useful technique that appraises the binding function of NPs to the polymer and the polymerization of polymeric denture materials. In our study, the FTIR results of the hybrid polymeric denture materials recorded new peaks and changes of intensity in some peaks when compared with the control. Variation in the molecular components of the materials was reflected as a shift in the absorbance band intensity and its position in the vibration spectra. This may be attributed to the variation in composition of the materials, interactions, and distribution between the polymeric denture matrix and the zMgO NPs.69 Additionally, hybrid polymers are difunctional molecules that have a methyl methacrylate group at each end with double bonds that can undergo free radical polymerization on activation, producing a strong cross-linked network of organic molecules, enhancing the mechanical and biological properties of the reinforced polymer.70 The heat-cured denture base materials undergo high pressure and heat to promote the formation of long polymer chains and therefore lead to a reduced amount of residual monomer and a superior rate of monomer conversion. Heat and pressure may have affected the interaction of the zMgO NPs with the polymer matrix. The self-cured denture base materials usually have excess monomers that can interact with the zMgO NPs, which explains the increase in peaks and the variation in the peaks’ intensity in the self-cured materials.69 Furthermore, the NPs’ shape plays an important role in the materials’ reaction. Nanowires have shown promising catalytic reactions such as reduction, which will prove to be a valuable asset in the future of catalysis.59,71
There was a statistically significant decrease in adherence of bacteria and fungi on the surface of the hybrid polymeric denture materials. This could be attributed to the antimicrobial effect of zMgO NPs as well as the increase in contact surface due to the increase of surface area to volume ratio, leading to a rise in surface reactivity and decrease in adherence of microorganisms to the hybrid polymeric denture materials.12,72 This was in agreement with a recent study by Meran et al, who either coated or impregnated flexible denture material disks with 5% MgO NPs and found these hybrid disks could prevent the attachment of S. mutans.73 Similarly, Gad et al investigated the addition of zirconium dioxide NPs and silver NPs to acrylic resin powder and found a reduction in the adhesion of C. albicans.74 Likewise, Gligorijević et al modified cold and heat-cured denture base resins with silver NPs and evaluated the growth of C. albicans and S. aureus. The results presented microbicidal effect of the 10% silver NPs-PMMA hybrid against bacterial and fungal strains.75
A drawback of adding antimicrobial nanofillers to the resin matrix is their tendency to be released in a wet environment to exert their antimicrobial property, affecting their concentration in the material. Thus, modifications of hybrid polymers so they act as contact inhibitors against microorganisms attaching to the material’s surface were pursued by the immobilization of the antimicrobial components in the matrix.38
The agar diffusion test showed no or very slight release of zMgO NPs as seen by the absence of the inhibition zone in all of the microorganisms: S. mutans, S. aureus., E. faecalis, and C. albicans. This could be accredited to their copolymerization with monomers in the polymeric denture materials and being covalently linked to the polymeric network.69 Therefore, they did not seep out of the matrix but rather hindered the approximation of microorganisms to the polymer façade.76 As a result, the incorporation of zMgO NPs in polymeric denture materials generated hybrid polymers with immobilized antimicrobial NPs. Therefore, the null hypothesis was rejected. Moritz and Geszke-Moritz stated that the immobilization of NPs in various matrices should be planned to provide consistent release of the antibacterial agent, and that the optimal solution establishes antibacterial activity with as little release of the agent to the environment. The hybrid disk of this study meets that requirement, although further testing should be conducted to fully affirm its capabilities in all conditions.77
These polymeric denture materials are expected to operate in the oral environment, which is a consistently changing landscape in terms of temperature, acidity, and dynamic movement. However, it was a challenge emulating that in the study. Further assessment of the specimens in saliva should be done to analyze its washing effect. The influence of saliva’s enzymatic action could also provide valuable data on the antimicrobial effect of these hybrid specimens. Furthermore, as heat and pressure are already variables to be considered in the production of heat-cured polymeric denture materials, thermocycling was not done in the study. However, a future study to supplement the results found here should be considered, as the denture material should withstand thermal and mechanical influence.
Conclusion
The addition of zMgO NPs to polymeric denture materials imparted a potent antimicrobial and anti-adherent activity against the tested microorganisms S. mutans S. aureus, E. faecalis and C. albicans. Furthermore, the inorganic nanoparticles remained immobilized in the denture material. Adoption of these inorganic nanoparticles with polymeric denture materials is a viable consideration to disrupt oral diseases; however, further in-vitro and clinical investigations are required to validate the findings.
Abbreviations
NPs, nanoparticles; MgO NPs, magnesium oxide nanoparticles; zMgO NPs, zein-incorporated magnesium oxide nanoparticles; XRD, X-Ray Diffraction; FESEM, Field Emission Scanning Electron Microscopy; TEM, Transmission Electron Microscopy; DSC, Differential Scanning Calorimetry; FTIR, Fourier Transform Infrared Spectroscopy; HCPb, Heat-cured Pro base Hot; HCV, Vertex heat-cured; HCDPI, DPI heat-cured; SCV, Vertex self-cured; SCGC, self-cured GC Nature-Cryl Pour.
Data Sharing Statement
Data is available from the corresponding author upon reasonable request.
Consent for Publication
Not Applicable.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funds, grants, or other support was received.
Disclosure
The authors have no competing interests to declare that are relevant to the content of this article.
References
1. Gad MM, Al-Thobity AM, Rahoma A, Abualsaud R, Al-Harbi FA, Akhtar S. Reinforcement of PMMA denture base material with a mixture of ZrO2 nanoparticles and glass fibers. Int J Dent. 2019;2019(1):2489393. doi:10.1155/2019/2489393
2. El-Rashidy AA, Abdelraouf RM, Habib NA. Effect of two artificial aging protocols on color and gloss of single-shade versus multi-shade resin composites. BMC Oral Health. 2022;22(1):321. doi:10.1186/s12903-022-02351-7
3. Lu W, Li C, Wu J, et al. Preparation and characterization of a polyetherketoneketone/hydroxyapatite hybrid for dental applications. J Funct Biomater. 2022;13(4):220. doi:10.3390/jfb13040220
4. Palacios T, Tarancón S, Pastor JY. On the mechanical properties of hybrid dental materials for CAD/CAM restorations. Polymers. 2022;14(16):3252. doi:10.3390/polym14163252
5. Karpacheva GP. Hybrid Magnetic Nanocomposites Containing Polyconjugated Polymers. Springer; 2016:131–146.
6. Haleem A, Javaid M, Singh RP, Rab S, Suman R. Applications of nanotechnology in medical field: a brief review. Glob Health J. 2023;7(2):70–77. doi:10.1016/j.glohj.2023.02.008
7. Sindhwani S, Chan WCW. Nanotechnology for modern medicine: next step towards clinical translation. J Internal Med. 2021;290(3):486–498. doi:10.1111/joim.13254
8. Irfan M, Bagherpour S, Munir H, et al. GC–MS metabolomics profile of methanol extract of Acacia modesta gum and gum-assisted fabrication and characterization of gold nanoparticles through green synthesis approach. Int J Biol Macromol. 2023;252:126215. doi:10.1016/j.ijbiomac.2023.126215
9. Cao W, Zhang Y, Wang X, et al. Development of a novel resin-based dental material with dual biocidal modes and sustained release of Ag+ ions based on photocurable core-shell AgBr/cationic polymer nanocomposites. J Mater Sci Mater Med. 2017;28(7):103. doi:10.1007/s10856-017-5918-3
10. Urnukhsaikhan E, Bold B-E, Gunbileg A, Sukhbaatar N, Mishig-Ochir T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci Rep. 2021;11(1). doi:10.1038/s41598-021-00520-2
11. Sharmin S, Rahaman MM, Sarkar C, Atolani O, Islam MT, Adeyemi OS. Nanoparticles as antimicrobial and antiviral agents: a literature-based perspective study. Heliyon. 2021;7(3):e06456. doi:10.1016/j.heliyon.2021.e06456
12. Li Z, Sun J, Lan J, Qi Q. Effect of a denture base acrylic resin containing silver nanoparticles on Candida albicans adhesion and biofilm formation. Gerodontology. 2016;33(2):209–216. doi:10.1111/ger.12142
13. Alrahlah A, Fouad H, Hashem M, Niazy A, Albadah A. Titanium oxide (TiO2)/Polymethylmethacrylate (PMMA) denture base nanocomposites: mechanical, viscoelastic and antibacterial behavior. Materials. 2018;11(7):1096. doi:10.3390/ma11071096
14. Ferrando-Magraner E, Bellot-Arcís C, Paredes-Gallardo V, et al. Antibacterial properties of nanoparticles in dental restorative materials. a systematic review and meta-analysis. Medicina. 2020;56(2):55. doi:10.3390/medicina56020055
15. Kamonkhantikul K, Arksornnukit M, Takahashi H. Antifungal, Optical, and Mechanical Properties of Polymethylmethacrylate Material Incorporated with silanized Zinc Oxide Nanoparticles. Dove Press; 2017:2353.
16. Salvo J, Sandoval C. Role of copper nanoparticles in wound healing for chronic wounds: literature review. Burns Trauma. 2022;10.doi: 10.1093/burnst/tkab047.
17. Parnia F, Yazdani J, Javaherzadeh V, Maleki Dizaj S. Overview of nanoparticle coating of dental implants for enhanced osseointegration and antimicrobial purposes. J Pharm Pharm Sci. 2017;20:148–160. doi:10.18433/J3GP6G
18. Irfan M, Munir H, Ismail H. Characterization and fabrication of zinc oxide nanoparticles by gum Acacia modesta through green chemistry and impregnation on surgical sutures to boost up the wound healing process. Int J Biol Macromol. 2022;204:466–475. doi:10.1016/j.ijbiomac.2022.02.043
19. Ihsan S, Munir H, Meng Z, et al. Tragacanth gum-based copper oxide nanoparticles: comprehensive characterization, antibiofilm, antimicrobial and photocatalytic potentials. Int J Biol Macromol. 2024;268:131600. doi:10.1016/j.ijbiomac.2024.131600
20. Nguyen N-YT, Grelling N, Wetteland CL, Rosario R, Liu H. Antimicrobial activities and mechanisms of magnesium oxide nanoparticles (nMgO) against pathogenic bacteria, yeasts, and biofilms. Sci Rep. 2018;8(1):16260. doi:10.1038/s41598-018-34567-5
21. Wetteland CL, Nguyen NYT, Liu H. Concentration-dependent behaviors of bone marrow derived mesenchymal stem cells and infectious bacteria toward magnesium oxide nanoparticles. Acta Biomater. 2016;35:341–356. doi:10.1016/j.actbio.2016.02.032
22. Naguib GH, Hassan AH, Al-Hazmi F, et al. Zein based magnesium oxide nanowires: effect of anionic charge on size, release and stability. Dig J Nanomater Biostruct. 2017;12:741–749.
23. Naguib GH, Hosny KM, Hassan AH, et al. Zein based magnesium oxide nanoparticles: assessment of antimicrobial activity for dental implications. Pak J Pharm Sci. 2018;31(1 Suppl):245–250.
24. Naguib GH, Nassar HM, Hamed MT. Antimicrobial properties of dental cements modified with zein-coated magnesium oxide nanoparticles. Bioact Mater. 2021;8:49–56. doi:10.1016/j.bioactmat.2021.06.011
25. Berardi A, Bisharat L, AlKhatib HS, Cespi M. Zein as a pharmaceutical excipient in oral solid dosage forms: state of the art and future perspectives. AAPS Pharm Sci Tech. 2018;19(5):2009–2022. doi:10.1208/s12249-018-1035-y
26. Raza A, Hayat U, Bilal M, Iqbal HMN, Wang J-Y. Zein-based micro- and nano-constructs and biologically therapeutic cues with multi-functionalities for oral drug delivery systems. J Drug Delivery Sci Technol. 2020;58:101818. doi:10.1016/j.jddst.2020.101818
27. Pérez L, Sentís A, Hafidi Z, et al. Zein nanoparticles containing arginine-based surfactants: physicochemical characterization and effect on the biological properties. Int J Mol Sci. 2023;24(3):2568. doi:10.3390/ijms24032568
28. De Marco I. Zein microparticles and nanoparticles as drug delivery systems. Polymers. 2022;14(11):2172. doi:10.3390/polym14112172
29. Yan X, Li M, Xu X, Liu X, Liu F. Zein-based nano-delivery systems for encapsulation and protection of hydrophobic bioactives: a review. RevFront Nutr. 2022;9. doi:10.3389/fnut.2022.999373.
30. Alqutaibi AY, Baik A, Almuzaini SA, et al. Polymeric denture base materials: a review. Polymers. 2023;15(15):3258. doi:10.3390/polym15153258
31. Mousavi SA, Ghotaslou R, Kordi S, et al. Antibacterial and antifungal effects of chitosan nanoparticles on tissue conditioners of complete dentures. Int J Biol Macromol. 2018;118:881–885. doi:10.1016/j.ijbiomac.2018.06.151
32. Sartawi SY, Abu-Hammad SA, Salim N, Al-Omoush S. Denture stomatitis revisited: a summary of systematic reviews in the past decade and two case reports of papillary hyperplasia of unusual locations. Int J Dent. 2021;2021:1–8. doi:10.1155/2021/7338143
33. Redfern J, Tosheva L, Malic S, Butcher M, Ramage G, Verran J. The denture microbiome in health and disease: an exploration of a unique community. Lett Appl Microbiol. 2022;75(2):195–209. doi:10.1111/lam.13751
34. Sardari F, Khalili P, Hakimi H, Mahmoudaghaei S, Abedi P. The prevalence of denture stomatitis in cigarette and hookah smokers and opium addicts: findings from rafsanjan cohort study. BMC Oral Health. 2021;21(1):455. doi:10.1186/s12903-021-01807-6
35. McReynolds DE, Moorthy A, Moneley JOC, Jabra‐Rizk MA, Sultan AS. Denture stomatitis—An interdisciplinary clinical review. J Prosthodontics. 2023;32(7):560–570. doi:10.1111/jopr.13687
36. Abuhajar E, Ali K, Zulfiqar G, et al. Management of chronic atrophic candidiasis (denture stomatitis)—a narrative review. Int J Environ Res Public Health. 2023;20(4):3029. doi:10.3390/ijerph20043029
37. Yitzhaki S, Reshef L, Gophna U, Rosenberg M, Sterer N. Microbiome associated with denture malodour. J Breath Res. 2018;12(2):027103. doi:10.1088/1752-7163/aa95e0
38. Fadilah NIM, Isa ILM, Zaman WSWK, Tabata Y, Fauzi MB. The effect of nanoparticle-incorporated natural-based biomaterials towards cells on activated pathways: a systematic review. Polymers. 2022;14(3):476. doi:10.3390/polym14030476
39. Venditti I. Metal nanoparticles–polymers hybrid materials I. Polymers. 2022;14(15):3117. doi:10.3390/polym14153117
40. Al-Hazmi F, Alnowaiser F, Al-Ghamdi AA, et al. A new large – scale synthesis of magnesium oxide nanowires: structural and antibacterial properties. Superlattices Microstruct. 2012;52(2):200–209. doi:10.1016/j.spmi.2012.04.013
41. Shakir T, Abass S. The effect of Magnesium Oxide (MgO) nano- fillers on the antibacterial activity and some properties of heat cured acrylic resin. Int J Sci Res. 2018;7.
42. Bakr T, Hasan R. Evaluation of some properties of heat curing denture base materials cured by different curing techniques. Zanco J Med Sci. 2017;21(2):1796–1806. doi:10.15218/zjms.2017.036
43. Jawad I, Qasim A, Hasan R. Fourier Transform infrared(FT-IR) Spectroscopy of modified heat cured acrylic resin denture base material. Tsinghua Sci Technol. 2016;5:130–140.
44. Gill P, Moghadam TT, Ranjbar B. Differential scanning calorimetry techniques: applications in biology and nanoscience. J Biomol Tech. 2010;21(4):167–193.
45. Kowalczuk D, Pitucha M. Application of FTIR method for the assessment of immobilization of active substances in the matrix of biomedical materials. Materials. 2019;12(18):2972. doi:10.3390/ma12182972
46. Herman JL, Wang Y, Lilly EA, et al. Synthesis, antifungal activity, and biocompatibility of novel 1,4-Diazabicyclo[2.2.2]Octane (DABCO) Compounds and DABCO-containing denture base resins. Antimicrob. Agents Chemother. 2017;61(4):10.1128/aac.02575–16. doi:10.1128/aac.02575-16
47. Saveleva MS, Eftekhari K, Abalymov A, et al. Hierarchy of hybrid materials—the place of inorganics-in-organics in it, their composition and applications. Review Front Chem. 2019;7. doi:10.3389/fchem.2019.00179
48. Naguib GH, Bakhsh T, Mazhar J, et al. Noninvasive assessment of novel nanohybrid resin cement adaptation using cross-polarization optical coherence tomography. Dent Mater. 2024;40(4):643–652. doi:10.1016/j.dental.2024.02.004
49. Naguib G, Nasser M, Mirdad L, et al. Surface characteristics of composite resin enhanced by new antibacterial nanofillers. Int J Curr Adv Res. 2018;7:15965–15969. doi:10.24327/ijcar.2018.15969.2930
50. Naguib GH, Abd El-Aziz GS, Kayal RA, et al. Cytotoxic effects of dose dependent inorganic magnesium oxide nanoparticles on the reproductive organs of rats. Ann Med. 2023;55(2). doi:10.1080/07853890.2023.2258917
51. Naguib G, Mously H, Magdy W, et al. Color behavior of composite resin enhanced with different shapes of new antimicrobial polymer coated nanoparticles. BMC Oral Health. 2023;23(1):771. doi:10.1186/s12903-023-03495-w
52. Naguib GH, El-Aziz GS A, Mously HA, Bukhary SM, Hamed MT. Assessment of the dose-dependent biochemical and cytotoxicity of zein-coated MgO nanowires in male and female albino rats. Ann Med. 2021;53(1):1850–1862. doi:10.1080/07853890.2021.1991587
53. Naguib G, Mously H, Mazhar J, et al. Bond strength and surface roughness assessment of novel antimicrobial polymeric coated dental cement. Discov Nano. 2024;19(1):123. doi:10.1186/s11671-024-04074-w
54. Naguib GH, Abd El-Aziz GS, Almehmadi A, et al. Evaluation of the time-dependent osteogenic activity of glycerol incorporated magnesium oxide nanoparticles in induced calvarial defects. Heliyon. 2023;
55. Naguib GH, Abd El-Aziz GS, Mously HA, et al. In vitro investigation of the antimicrobial activity of mouth washes incorporating zein-coated magnesium oxide nanoparticles. Clin Cosmetic Invest Dentistry. 2021;13:395. doi:10.2147/CCIDE.S327912
56. Naguib GH, Abuelenain D, Mazhar J, Alnowaiser A, Aljawi R, Hamed MT. Maximizing dental composite performance: strength and hardness enhanced by innovative polymer-coated MgO nanoparticles. J Dent. 2024;149:105271. doi:10.1016/j.jdent.2024.105271
57. Naguib GH, Mazhar J, Alnowaiser A, et al. Mechanical behaviour of novel nanohybrid resin composite using two light cure systems. Int Dent J. 2024. doi:10.1016/j.identj.2024.07.004
58. Naguib GH, Bakhsh TA, Turkistani AA, Mously HA, Fattouh M, Hamed MT. Noninvasive adaptation appraisal of antimicrobial nano-filled composite. Int Dent J. 2023;73(4):533–541. doi:10.1016/j.identj.2022.11.004
59. Narayan N, Meiyazhagan A, Vajtai R. Metal Nanoparticles as Green Catalysts. Materials. 2019;12(21):3602. doi:10.3390/ma12213602
60. Upadhyay S, Parekh K, Pandey B. Influence of crystallite size on the magnetic properties of Fe3O4 nanoparticles. J Alloys Compd. 2016;678:478–485. doi:10.1016/j.jallcom.2016.03.279
61. Igawa K, Yoshinari N, Okumura M, Ohtsu H, Kawano M, Konno T. Crystalline-amorphous-crystalline transformation in a highly brilliant luminescent system with trigonal-planar gold(I) centers. Sci Rep. 2016;6(1):1–8. doi:10.1038/srep26002
62. Alshehri SM, Tiwari RV, Alsulays BB, et al. Investigation of the combined effect of MgO and PEG on the release profile of mefenamic acid prepared via hot-melt extrusion techniques. Pharm Dev Technol. 2017;22(6):740–753. doi:10.3109/10837450.2016.1138129
63. Pantazi A, Totu E, Dorobantu D, Cristache C, Enachescu M. Poly(methyl metacrylate) nanocomposites for two-piece CAD/CAM solution as an alternative to monolithic removable prosthesis. Materiale Plastice. 2018;55(4):634–639. doi:10.37358/MP.18.4.5091
64. Lazouzi G, Vuksanović MM, Tomić NZ, et al. Optimized Preparation of Alumina Based Fillers for Tuning Composite Properties. Elsevier Ltd; 2018:7442–7449.
65. Gronwald B, Kozłowska L, Kijak K et al. Nanoparticles in Dentistry—Current Literature Review.Multidisciplinary Digital Publishing Institute;2023:102
66. Irfan M, Munir H, Ismail H. Moringa oleifera gum based silver and zinc oxide nanoparticles: green synthesis, characterization and their antibacterial potential against MRSA. Biomater Res. 2021;25(1):17. doi:10.1186/s40824-021-00219-5
67. Kora AJ, Arunachalam J. Green fabrication of silver nanoparticles by gum tragacanth (astragalus gummifer): a dual functional reductant and stabilizer. J Nanomater. 2012;2012(1):869765. doi:10.1155/2012/869765
68. Podaralla S, Perumal O. Influence of formulation factors on the preparation of zein nanoparticles. AAPS Pharm Sci Tech. 2012;13(3):919–927. doi:10.1208/s12249-012-9816-1
69. Mohsin HA, Abdul-Hadi NF, Mustafa MJ. Evaluating some mechanical and physical properties of vertex thermosens denture base material in comparison with heat cure acrylic denture base material. International Journal of Science and Research. 2017;6(5):394–397. doi:10.21275/ART20172925
70. Elmadani AA, Radović I, Tomić NZ, et al. Hybrid denture acrylic composites with nanozirconia and electrospun polystyrene fibers. PLoS One. 2019;14(12):e0226528. doi:10.1371/journal.pone.0226528
71. Trindell JA, Duan Z, Henkelman G, Crooks RM. Well-defined nanoparticle electrocatalysts for the refinement of theory. Chem. Rev. 2020;120(2):814–850. doi:10.1021/acs.chemrev.9b00246
72. Rodríguez-Hernández A-P, Vega-Jiménez AL, Vázquez-Olmos AR, Ortega-Maldonado M, Ximenez-Fyvie L-A. Antibacterial properties in vitro of magnesium oxide nanoparticles for dental applications. Nanomater. 2023;13(3):502. doi:10.3390/nano13030502
73. Meran ZD, Hassan PA, Salaie RN. Comparing the antibacterial effect of coated and impregnated flexible dentures with magnesium oxide nanoparticles against streptococcus mutans. Coatings. 2023;13(8):1429. doi:10.3390/coatings13081429
74. Gad MM, Abualsaud R, Rahoma A, Al-Thobity AM, Akhtar S, Fouda SM. Double-layered acrylic resin denture base with nanoparticle additions: an in vitro study. J Prosthetic Dent. 2022;127(1):174–183. doi:10.1016/j.prosdent.2020.08.021
75. Gligorijević N, Mihajlov-Krstev T, Kostić M, et al. Antimicrobial properties of silver-modified denture base resins. Nanomater. 2022;12(14):2453. doi:10.3390/nano12142453
76. Aničić N, Kurtjak M, Jeverica S, Suvorov D, Vukomanović M. Antimicrobial polymeric composites with embedded nanotextured magnesium oxide. Polymers. 2021;13(13):2183. doi:10.3390/polym13132183
77. Moritz M, Geszke-Moritz M. The newest achievements in synthesis, immobilization and practical applications of antibacterial nanoparticles. Chem Eng J. 2013;228:596–613. doi:10.1016/j.cej.2013.05.046
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.