Back to Journals » International Journal of Nanomedicine » Volume 20
Enhanced Sonodynamic Therapy via Au Nanoclusters Deposited on TiO2 Nanosheets
Authors Huang L, Wei L, Li D, Zhang W , Liu L
Received 8 February 2025
Accepted for publication 30 April 2025
Published 14 May 2025 Volume 2025:20 Pages 6121—6131
DOI https://doi.org/10.2147/IJN.S516314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sachin Mali
Lei Huang,1,* Lineng Wei,2,* Dan Li,2 Weiqing Zhang,2 Lidong Liu1
1Department of Medical Imaging Center, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, 530021, People’s Republic of China; 2Department of Experimental Research, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, 530021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weiqing Zhang, Email [email protected] Lidong Liu, Email [email protected]
Purpose: This study aimed to enhance the efficacy of sonodynamic therapy (SDT) for breast cancer by engineering TiO2 nanosheets modified with Au nanoclusters (TiO2-Au), thereby improving reactive oxygen species (ROS) generation under ultrasound (US) irradiation.
Methods: TiO2-Au sonosensitizers were synthesized via a deposition–precipitation with urea (DPU) method and characterized by TEM, XRD, and XPS. ROS generation efficiency was quantified using DPBF, TMB, and NBT probes, along with electron spin resonance (ESR). In vitro therapeutic performance was assessed in 4T1 breast cancer cells via flow cytometry, Calcein-AM/PI staining, and cell counting kit-8 (CCK-8) assay. In vivo efficacy and biosafety were validated in 4T1 tumor-bearing BALB/c mice through tumor growth monitoring, histological analysis, blood biochemistry, and hemolysis assays.
Results: TiO2-Au10.5 exhibited enhanced electron–hole separation, reduced bandgap (from 3.2 to 2.8 eV), and significantly boosted ROS generation under US irradiation. In vitro, TiO2-Au10.5 combined with US induced a 4.25-fold increase in intracellular ROS and a 4.7-fold higher apoptosis rate compared to TiO2 + US. In vivo, TiO2-Au10.5 + US achieved a tumor growth inhibition index of 76.9% without significant toxicity, as evidenced by normal blood markers, no hemolysis, and no damage to major organs.
Conclusion: Au nanocluster modification effectively tunes the sonodynamic performance of TiO2 nanosheets by modulating electron–hole separation and ROS production. Notably, varying the Au content enabled precise regulation of SDT efficacy, with TiO2-Au10.5 achieving optimal therapeutic outcomes. These findings highlight TiO2-Au as a safe, potent, and composition-tunable sonosensitizer platform for precise and effective cancer therapy.
Keywords: TiO2 nanosheets, gold nanoclusters, Au content modulation, reactive oxygen species, sonodynamic therapy
Introduction
Cancer remains a prominent cause of worldwide fatality and the conventional treatment methods such as surgery, chemotherapy, and radiation often result in significant adverse effects, drug resistance, and non-specific targeting.1 Consequently, there is a growing interest in developing more selective, less invasive, and safer treatment modalities.2,3 Sonodynamic therapy (SDT) holds significant potential as a targeted cancer treatment strategy by utilizing low-intensity focused ultrasound (LIFU) and sonosensitizers to generate cytotoxic reactive oxygen species (ROS) within tumor tissues.4,5 Sonosensitizers are central to the mechanism and effectiveness of SDT. Upon activation by ultrasound (US), they undergo a sonochemical reaction that generates electron-hole pairs. These pairs subsequently engage in interactions with oxygen molecules present within the tumor microenvironment, resulting in the generation of singlet oxygen (1O2).6,7 The accumulation of ROS induces oxidative stress, leading to apoptosis and necrosis in cancer cells.4,8,9 There is a strong need to enhance the therapeutic effectiveness of SDT by developing more efficient sonosensitizers.
Titanium dioxide (TiO2) nanoparticles have garnered significant attention in SDT due to their unique physicochemical properties, biocompatibility, and ability to generate ROS upon ultrasound activation, making them promising candidate for cancer treatment.10–12 However, one of the main challenges is the relatively low ROS yield observed under standard clinical ultrasound frequencies.13–15 To overcome this limitation, it is crucial to improve the processes governing electron-hole pair formation and recombination during ultrasound activation.13,15,16 Noble metals like gold (Au), ruthenium (Ru) and platinum (Pt) have been successfully integrated with TiO2 to narrow its bandgap, improving electron-hole separation and reducing recombination.15–19 Furthermore, the incorporation of Au exploits the surface plasmon resonance (SPR) phenomenon, leading to an amplification of the localized electromagnetic field surrounding the TiO2 nanoparticles. This further enhances ultrasound responsiveness and ROS generation.20–22 The modification of noble metal on TiO2 significantly increases ROS production, thereby enhancing the cancer cell-killing efficiency of SDT.7 However, the high cost and potential biocompatibility concerns associated with noble metals limit their widespread clinical application. Thus, determining the optimal noble metal content to maximize ROS generation while preventing excessive particle aggregation remains a challenge.
In this work, we report for the first time novel Au nanoclusters anchored in varying amounts on TiO2 nanosheets (denoted as TiO2-Au) and systematically investigate their performance in SDT for breast cancer treatment (Scheme 1). As anticipated, the band gap of TiO2 decreases progressively with increasing Au nanocluster content. This phenomenon can be ascribed to the increased separation of electron-hole (e−/h+) pairs due to the band gap activation of TiO2. When the Au content reaches 10.5% (TiO2-Au10.5), the band gap is reduced to 2.8 eV, which is considerably smaller than the pristine TiO2 (3.2 eV). As a result, TiO2-Au10.5 significantly boosts SDT efficiency with a high ROS quantum yield. Comprehensive in vitro and in vivo tumor model experiments confirm that TiO2-Au10.5 greatly enhances the therapeutic effect of SDT against breast cancer compared to pristine TiO2. This research highlights a promising sonosensitizer for improving SDT efficiency.
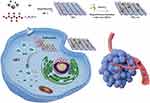 |
Scheme 1 Schematic illustration of TiO2-Au for tumor therapy under US exposure. |
Materials and Experimental
Materials
All chemicals and reagents were analytical grade and used without any further purification. Tetrabutyl titanate (Ti(OBu)4, Chloroauric acid (HAuCl4) were purchased from Sigma-Aldrich. 1,3-diphenylisobenzofuran (DPBF) was purchased from Macklin (Shanghai, China). 3,3’,5,5’-tetramethylbenzidine (TMB) was purchased from Aladdin (Shanghai, China). BBoxiProbe® O28 was purchased from Bestbio (Shanghai, China). Cell counting kit-8 (CCK-8) was provided by Zoman Biotechnology (Beijing, China). NBT, Calcein AM/PI cell double-staining kit, ROS assay kit and Hoest33342 were purchased from Beyotime Biotechnology (Shanghai, China). Annexin V-FITC/PI kit was purchased from Bestbio (Shanghai, China). Fetal bovine serum (FBS), penicillin–streptomycin solution and Dulbecco’s Modified Eagle’s medium (DMEM) were purchased from Gibco (Shanghai, China). Streptomycin, penicillin, and trypsin were obtained from Solarbio (Beijing, China). The aqueous solution used in the experiment was prepared from deionized water of Milli-Q water purification system.
Experimental
Preparation of TiO2-Au Sonosensitizers
TiO2 nanosheets were synthesized using a standard hydrothermal method.23 Subsequently, Au atoms were deposited onto the TiO2 substrate via the deposition-precipitation with urea (DPU) process to synthesize the TiO2-Au10.5 sonosensitizers.17 In brief, a 200 mL of urea solution (0.42 M) containing 0.2 g of TiO2 nanosheets was mixed with 3 mL of a solution containing HAuCl4 (50 mm) in water. The resulting solution underwent heating and stirring at a temperature of 80 °C for a duration of 14 hours while being shielded from light. Finally, the TiO2-Au10.5 sonosensitizers were thoroughly washed and freeze-dried for further use.
Determination of ROS Generation
The generation of 1O2 in the TiO2-Au10.5-mediated SDT process were detected by using 1,3-diphenylisobenzofuran (DPBF) as probes, respectively. Typically, a certain concentration of probes dissolved in dimethyl sulphoxide (DMSO) was mixed with 200 µg/mL as-synthesized sonosensitizers, which was exposed to US irradiation (1.0 MHz, 50% duty cycle, 1.5 W/cm²) at fixed time intervals in the dark. Then the concentrations of probes were recorded by UV-vis spectra. The generation of hydroxyl radical (•OH) in the TiO2-Au-mediated SDT process was detected by using 3,3’,5,5’-tetramethylbenzidine (TMB) as probes, and the TiO2-Au (200 µg/mL) was exposed to US irradiation (1.0 MHz, 50% duty cycle, 1.5 W/cm²) for 5 minutes reaction in the dark. Then the concentrations of probes were recorded by UV-vis spectra. The generation of O2− in the TiO2-Au-mediated SDT process was detected by using NBT as probes, and the TiO2-Au (200 µg/mL) was exposed to US irradiation (1.0 MHz, 50% duty cycle, 1.5 W/cm²) for 5 minutes reaction in the dark. Then the concentrations of probes were recorded by UV-vis spectra. The long-term stability of TiO2-Au10.5 sonosensitizers in physiological environments was assessed by monitoring their optical properties. Freshly prepared TiO2-Au10.5 sonosensitizers were compared with samples stored in PBS and DMEM for one week. Stability was evaluated by measuring the relative absorbance at 412 nm (At/A₀) over time using UV-vis spectroscopy.
Intracellular ROS Determination
The 4T1 cell line used in this study was obtained from the ATCC (RRID: CVCL_0125) repository. The total induction of ROS in the 4T1 cells caused by different treatment (Control, US, TiO2 + US, TiO2-Au1.3 + US, TiO2-Au6.4 + US, and TiO2-Au10.5 + US) was determined by flow cytometer with the sonosensitizer concentration set at 200 µg/mL 4T1 cells were seeded in 24-well plates with a density of 5×104 cells overnight for 12 h, depending on the group. Subsequently, cells were stained with DCFH-DA following the manufacturer’s instructions according to the manufacturer’s instructions. Finally, the cells were digested and flow cytometer was employed for determination. For the BBoxiProbe® O28 staining assay, cells were first stained with Hoest 33342 (5 μg/mL) for 15 min at 37°C to label the nuclei, followed by staining with O28. Fluorescence imaging was then acquired.
Calcein AM/PI Staining and Annexin V-FITC/PI Apoptosis Assay
4T1 cells were subjected to six treatment conditions: Control, US, TiO2 + US, TiO2-Au1.3 + US, TiO2-Au6.4 + US, and TiO2-Au10.5 + US, with the sonosensitizer concentration set at 200 µg/mL. For the Calcein-AM/PI staining assay, 1 µL of Calcein-AM and 2 µL of PI sequentially added to the treated cells. After staining for 30 minutes, fluorescence images were acquired.
Apoptosis was assessed by staining the cells with FITC-labeled Annexin V and PI, Briefly, cells were resuspended in Annexin V binding buffer, stained with 5 µL of Annexin V-FITC and 5 µL of PI, and incubated for 15 min. The samples were then analyzed by flow cytometry, and apoptotic rates were quantified.
Cell Cytotoxicity Evaluation
The Cell Counting Kit-8 (CCK-8) assay was utilized to evaluate cytotoxicity and cell viability. 4T1 cells were exposed to different concentrations of TiO2-Au10.5 (0, 50, 100, 200, and 300 µg/mL) for a duration of 24 hours. Following rinsing, the treated cells were supplemented with CCK-8 reagent and incubated at a temperature of 37°C for an additional period of 2 hours. The survival rate of the cells was subsequently determined by measuring the optical density (OD) at a wavelength of 450 nm using a microplate reader.
In vivo Antitumor Efficacy Evaluation
Female BALB/c mice weighing around 20 g were used as animal models. All animal experiments were conducted in accordance with the GB/T 35892–2018: Laboratory Animal-Guideline for Ethical Review of Animal Welfare, in addition to obtaining approval from the Ethical Committee of Guangxi Medical University Cancer Hospital (approval number: KY-2022-272). Each mouse was subcutaneously injected with 8×105 4T1 cells into their right flank. The tumor-bearing mice were then divided randomly into three groups, each consisting of four mice: Control, US, and TiO2-Au10.5+US. Each group received four intravenous injections of sonosensitizers at a dose of 10 mg/kg. Following the administration, ultrasound (US) irradiation will be applied at a power density of 1.5 W/cm² for a duration of five minutes. Throughout the treatment period, daily records were maintained for body weights and tumor volume. Tumor tissues and major organs were collected for histological analysis using Hematoxylin and Eosin (H&E) staining techniques. Additionally, hematological analysis was performed utilizing a fully automated biochemical analyzer. Blood samples from treated mice were collected over a span of 14 days prior to sacrifice; routine blood tests were promptly conducted while serum separation was achieved through centrifugation at 3000 rpm for a duration of 15 minutes at a temperature of 4 °C in preparation for biochemical analysis.
Hemolysis Assay
Red blood cells (RBC) were obtained from BALB/c mice and diluted with PBS to 2% suspension. TiO2-Au1.3, TiO2-Au6.4, and TiO2-Au10.5 were added to 2% red blood cell suspension to the desired concentrations (25, 50, 100, and 200 µg/mL). It was then incubated at 37 °C for 3 h. An identical red cell suspension incubated with PBS and ultrapure water was used as a negative and positive control under the same conditions. All samples were centrifuged at 3000 rpm for 10 min before accurately absorbing the same volume of the supernatant into a 96-well plate. The hemoglobin release at 540 nm was measured by Varioskan Flash microplate reader.
Statistical Analysis
The statistical significance of experimental data was assessed by employing one-way analysis of variance (ANOVA) with the assistance of GraphPad Prism 9 software. The criteria for determining significance were based on the p-value (P) satisfied the following criteria: *P < 0.05, **0.001 < P < 0.01, ***P < 0.001, while “ns” denoted no significant distinction.
Discussion and Results
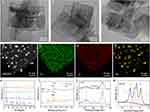
As shown in Scheme 1, TiO2 nanosheets were synthesized using a modified hydrothermal method. The morphology of the as-synthesized TiO2 was confirmed to be well-defined nanosheets through transmission electron microscopy (TEM) images TiO2 (Figure S1). Analysis of the lattice spacing revealed distinct lattice fringes measuring 0.19 nm and 0.35 nm, which correspond to the (200) and the (101) crystal planes of TiO2, respectively.24 These TiO2 nanosheets was employed as supports for Au nanoclusters through the deposition-precipitation with urea (DPU) process to yield TiO2-Au. By varying the amount of HAuCl4 added (8.5 and 50.9 mg), the Au content on the TiO2 nanosheets was easily controlled, ranging from 1.3% to 10.5% (Figure 1A–C). Energy dispersive spectroscopy (EDS) elemental mapping of TiO2-Au10.5 confirmed the presence of Ti, O, and Au, verifying the successful deposition of Au on TiO2 (Figure 1D–G). The average size of the Au nanoclusters was determined to be approximately 1.7 nm. X-ray diffraction (XRD) was employed to examine the crystal structure of TiO2 prior and following Au deposition (Figure 1H). The observed peaks were attributed to those of anatase-phase TiO2 (JCPDS no. 21–1272). The XRD patterns of TiO2-Au1.3 and TiO2-Au6.4 exhibited no discernible Au characteristic diffraction peak, probably due to the low loading and ultrafine size of Au nanocluster. When the Au content increased to 10.5%, a subtle Au peak was observed in the TiO2-Au10.5, confirming the presence of Au at higher loading levels. X-ray photoelectron spectroscopy (XPS) analysis confirmed the presence of Au in TiO2-Au10.5 (Figure 1I). Notably, a 0.1 eV blue shift in the Ti 2p binding energy following Au modification (Figures 1J and S2–3).25,26 This shift indicates a partial reduction of Ti4+, suggesting that the Au modification alters the electronic properties of TiO2. The O 1s XPS spectra displayed three characteristic peaks at 529.7 eV, 530.3 eV and 531.6 eV, attributed to O-Ti, vacancy O and O-H bonds, respectively (Figure S4).27,28 The abundance of Ti-OH groups endowed the TiO2-Au sonosensitizer with excellent hydrophilicity. In the high-resolution Au 4f spectrum, two peaks corresponding to Auδ+ species were observed alongside peaks attributed to Au0 states, suggesting the coexistence of Au–O sites and metallic Au clusters within the composite (Figure 1K).28,29
The generation capacity of 1O2 species was first evaluated using 1,3-diphenylisobenzofuran (DPBF) as a specific sensing probe.30 The UV-vis spectrophotometer was utilized to track the changes in DPBF absorbance following various treatments. Figures 2A–C illustrates a significant reduction in DPBF absorbance with increased Au loading and extended US irradiation. The intensity was reduced by 50% after treatment with TiO2-Au10.5 under US irradiation for 120 seconds. The rate of DPBF degradation for US-treated TiO2-Au groups, especially TiO2-Au10.5, was significantly faster than that of the US-treated TiO2 group (Figure 2D). While 1O2 generation efficiency improves with increasing Au content, the enhancement peaks at 10.5% and begins to decline beyond this point, likely due to excessive Au hindering electron-hole separation. These results indicate that TiO2-Au10.5 achieves an optimal balance between ROS generation and SDT efficiency, outperforming pristine TiO2 in the sonodynamic process. In addition, electron spin resonance (ESR) measurements were conducted to detect the 1O2 production using 2,2,6,6-tetramethylpiperidine (TEMP) as spin trap reagents. Under US irradiation, TiO2-Au groups exhibited stronger characteristic TEMP/1O2 adducts signals compared to pristine TiO2 (Figure 2E), which is consistent with the findings in the above colorimetric assays. Stability assessments demonstrated that TiO2-Au10.5 nanoparticles maintained excellent structural integrity in physiological environments (PBS and DMEM) over one week, as evidenced by consistent optical absorbance properties (Figure S5). This robust stability ensures the nanoparticles’ reliable functionality for subsequent applications in reactive oxygen species (ROS) generation studies.
Hydroxyl radical (•OH) production was evaluated using 3,3′,5,5′-tetramethylbenzidine (TMB) and the specific red fluorescent probe BBoxiProbe® O28. The increase of •OH levels was detected in TiO2-Au treated by US for 5 minutes (Figures S6-S8). At the same time, nitrotetrazolium blue chloride (NBT) was employed as a probe to investigate the generation of O2− by TiO2-Au. However, no significant increase in absorption values at 595 nm (the absorption peak of the generated compound when O2− was produced) was detected following US irradiation (Figure S9). These results indicated that TiO2-Au was unable to generate O2−. These results indicate that TiO2-Au heterostructures exhibited higher ROS quantum yield than TiO2. It can be ascribed to that Au nanoclusters as electron sinks modified the bandgap structures of TiO2, thereby enhancing the separation efficiency of e−/h+ pairs in SDT process.20,31 As shown in Figure 2F, the bandgap of TiO2 was decreased from 3.2 eV to 2.8 eV after Au nanoclusters deposition. Additionally, the presence of Au clusters significantly improved light absorption in the 450–800 nm range compared to pristine TiO2 (Figure S10), which corresponds well with the emission spectrum of sonoluminescence in aqueous media. Consequently, both improved e−/h+ separation and enhanced light absorption lead to a substantial increase ROS production in TiO2-Au heterostructure.
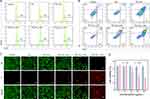
The intracellular ROS levels and therapeutic efficiency of TiO2-Au in vitro were investigated using breast cancer 4T1 cells. Flow cytometry analysis was conducted to assess the ROS levels using DCFH-DA as probes (Figure 3A). It was found that the fluorescence intensity of DCFH-DA probes increased as Au content in TiO2-Au increased, indicating that TiO2-Au can effectively produce 1O2 under US irradiation within the cellular environment. Notably, the fluorescence intensity in the TiO2-Au10.5 + US group was 4.25-fold higher than that in TiO2 + US group, demonstrating elevated ROS levels. Next, the apoptosis rate of treated 4T1 cells were assessed using flow cytometry (Figure 3B), revealing a correlation between increased ROS levels and higher apoptosis rates. The TiO2-Au10.5 + US group exhibited an apoptosis rate of 48.2%, which was 4.7-fold higher than the TiO2 + US group. The differentiation between live and dead cells were further established through the co-staining of Calcein acetoxymethyl ester and propidium iodide (Calcein-AM/PI). As shown in Figure 3C, the TiO2-Au10.5 + US group exhibited significantly stronger red fluorescence, indicating a higher proportion of dead cells, while the other groups showed intense green fluorescence, suggesting that most cells remained alive. Without US irradiation, no significant cytotoxic effects were observed, even at high concentrations of TiO2 and TiO2-Au10.5 (up to 300 μg/mL, Figures 3D and S11). However, upon US exposure, the cell mortality rate in the TiO2-Au10.5 nanoparticle group exceeded 60%, indicating a pronounced cytotoxic effect compared to the TiO2 group alone, suggesting a synergy between the gold doping (Au) and ultrasound exposure that enhances the therapeutic effect.
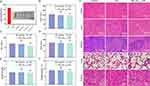
Building on the promising in vitro antitumor results, we further assessed the in vivo efficacy of TiO2-Au-mediated SDT in 4T1 tumor-bearing BALB/c mice. The mice were randomly assigned to three groups (n=4 per group): Control (PBS), US and TiO2-Au10.5+US. As illustrated in Figure 4A, treatments were administered over a 14-day period, with tumor size and weight recorded every two days (Figure 4B and C and S12). While the US and TiO2-Au10.5 groups exhibited minimal tumor inhibition compared to the control, the combined TiO2-Au10.5+US treatment markedly suppressed tumor growth, achieving tumor growth inhibition (TGI) indices of 76.9% based on tumor volume (Figure 4D–F). Histological analysis of tumor tissues via hematoxylin and eosin (H&E) staining revealed substantial tissue damage in the TiO2-Au10.5+US group (Figure 4G), whereas the Control, US, and TiO2-Au10.5 groups displayed normal cell morphology with intact membrane and nuclear structures. These findings underscore the significant tumor-suppressive effect of TiO2-Au10.5+US, attributable to enhanced SDT.
The biocompatibility of TiO2-Au10.5 sonosensitizers was thoroughly evaluated. As shown in Figures 5A and S13, even at a high concentration of 200 µg/mL, no hemolysis was observed, demonstrating excellent hemocompatibility. Furthermore, the results of biochemical analysis indicated that crucial indicators of liver and kidney function, including ALT (alanine aminotransferase), AST (aspartate aminotransferase), ALP (alkaline phosphatase), BUN (blood urea nitrogen), and CREA (creatinine) levels, remained within the normal range across all groups subjected to treatment (Figures 5B–F), indicating minimal risk of inflammation, hepatotoxicity, or nephrotoxicity. Furthermore, histological examination of major organs via H&E staining showed no significant tissue damage (Figure 5G), confirming the good biocompatibility of TiO2-Au10.5. Additionally, body weight increased steadily across all groups during the treatment period (Figure 4B), suggesting minimal toxicity or adverse effects from the TiO2-Au10.5 treatment.
Conclusions
In summary, we investigated the utilization of Au nanocluster-modified TiO₂ nanosheets (TiO₂-Au) to enhance sonodynamic therapy (SDT) for breast cancer. The addition of Au clusters to TiO2 led to a notable decrease in its bandgap, which consequently promoted the separation of electron-hole pairs and enhanced the generation of reactive oxygen species (ROS). In vitro experiments revealed that when activated by ultrasound (US), TiO₂-Au10.5 exhibited a significant augmentation in cell apoptosis compared to pristine TiO₂. Furthermore, in vivo studies provided compelling evidence showcasing substantial tumor suppression and high tumor growth inhibition (TGI) in mice treated with TiO₂-Au10.5 + US, Biocompatibility studies, including hemolysis assays, histological analysis, and biochemical assessments, showed no significant toxicity. These findings strongly suggest that TiO₂-Au10.5 holds great promise as an effective and biocompatible sonosensitizer for cancer therapy.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, [Weiqing Zhang], upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors appreciate financial supports from the Joint Project on Regional High-Incidence Diseases Research of Guangxi Natural Science Foundation (2024GXNSFAA010157). Advanced Innovation Teams and Xinghu Scholars Program of Guangxi Medical University, and start-up funding for high-level talents from Guangxi Medical University Cancer Hospital.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Bray F, Laversanne M, Sung HYA, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
2. Guo XL, Yang ND, Ji WH, et al. Mito‐bomb: targeting mitochondria for cancer therapy. Adv Mater. 2021;33(43):e2007778. doi:10.1002/adma.202007778
3. Liang S, Deng XR, Ma PA, Cheng ZY, Lin J. Recent advances in nanomaterial‐assisted combinational sonodynamic cancer therapy. Adv Mater. 2020;32(47):
4. McHale AP, Callan JF, Nomikou N, Fowley C, Callan B. Sonodynamic therapy: concept, mechanism and application to cancer treatment. Ther Ultrasound. 2016;880:429–450.
5. Wang PH, Chen J, Zhong RM, et al. Recent advances of ultrasound-responsive nanosystems in tumor immunotherapy. Eur J Pharm Biopharm. 2024;198:114246. doi:10.1016/j.ejpb.2024.114246
6. Bai S, Yang NL, Wang XW, et al. Ultrasmall iron-doped titanium oxide nanodots for enhanced sonodynamic and chemodynamic cancer therapy. ACS Nano. 2020;14(11):15119–15130. doi:10.1021/acsnano.0c05235
7. Chen P, Zhang P, Shah NH, Cui Y, Wang Y. A comprehensive review of inorganic sonosensitizers for sonodynamic therapy. Int J Mol Sci. 2023;24(15):12001. doi:10.3390/ijms241512001
8. Wei LN, Wang ZY, Lu XX, et al. Interfacial strong interaction-enabling cascade nanozymes for apoptosis-ferroptosis synergistic therapy. J Colloid Interface Sci. 2024;653:20–29. doi:10.1016/j.jcis.2023.09.036
9. Chen QQ, Zhang M, Huang H, et al. Single atom-doped nanosonosensitizers for mutually optimized sono/chemo-nanodynamic therapy of triple negative breast cancer. Adv Sci. 2023;10(6):e2206244. doi:10.1002/advs.202206244
10. Wen SW, Zhang WY, Yang JL, Zhou ZJ, Xiang Q, Dong HF. Ternary Bi2WO6/TiO2-Pt heterojunction sonosensitizers for boosting sonodynamic therapy. ACS Nano. 2024;18(34):23672–23683. doi:10.1021/acsnano.4c08236
11. Perota G, Zahraie N, Vais RD, Zare MH, Sattarahmady N. Au/TiO2 nanocomposite as a triple-sensitizer for 808 and 650 nm phototherapy and sonotherapy: synergistic therapy of melanoma cancer in vitro. J Drug Deliv Sci Technol. 2022;76:103787. doi:10.1016/j.jddst.2022.103787
12. Zlotver I, Sosnik A. Glucosylated hybrid TiO2/polymer nanomaterials for actively targeted sonodynamic therapy of cancer. Small. 2023;20(4):e2305475. doi:10.1002/smll.202305475
13. Wang XW, Zhong XY, Bai LX, et al. Ultrafine titanium monoxide (TiO1+x) nanorods for enhanced sonodynamic therapy. J Am Chem Soc. 2020;142(14):6527–6537. doi:10.1021/jacs.9b10228
14. Liu W, Shao RR, Guo LY, et al. Precise design of TiO2@CoOx heterostructure via atomic layer deposition for synergistic sono‐chemodynamic oncotherapy. Adv Sci. 2024;11(14):e2304046. doi:10.1002/advs.202304046
15. Li GL, Wu SC, Liu JG, Wang KY, Chen XY, Liu HX. Narrow bandgap schottky heterojunction sonosensitizer with high electron-hole separation boosted sonodynamic therapy in bladder cancer. Adv Mater. 2024;36(26):e2401252. doi:10.1002/adma.202401252
16. Qiao X, Xue L, Huang H, Dai X, Chen Y, Ding H. Engineering defected 2D Pd/H-TiO2 nanosonosensitizers for hypoxia alleviation and enhanced sono-chemodynamic cancer nanotherapy. J Nanobiotechnology. 2022;20(1):186. doi:10.1186/s12951-022-01398-6
17. Lu XX, Qiao K, Shaik F, et al. Evoking robust immunogenic cell death by synergistic sonodynamic therapy and glucose depletion using Au clusters/single atoms modified TiO2 nanosheets. Nano Res. 2023;16(7):9730–9742. doi:10.1007/s12274-023-5562-9
18. Zhao YM, Liu JH, He MT, et al. Platinum-itania schottky junction as nanosonosensitizer, glucose scavenger, and tumor microenvironment-modulator for promoted cancer treatment. ACS Nano. 2022;16(8):12118–12133. doi:10.1021/acsnano.2c02540
19. Liang S, Deng XR, Xu GY, et al. A novel Pt-TiO2 heterostructure with oxygen‐deficient layer as bilaterally enhanced sonosensitizer for synergistic chemo‐sonodynamic cancer therapy. Adv Funct Mater. 2020;30(13):1908598. doi:10.1002/adfm.201908598
20. Park SH, Kim S, Park JW, Kim S, Cha W, Lee J. In-situ and wavelength-dependent photocatalytic strain evolution of a single Au nanoparticle on a TiO2 film. Nat Commun. 2024;15(1):5416. doi:10.1038/s41467-024-49862-1
21. Wang D, Zhang J-T. Metal-based inorganic nanocrystals for biological sonodynamic therapy applications: recent progress and perspectives. Rare Met. 2023;43(2):413–430. doi:10.1007/s12598-023-02450-6
22. Elahi N, Kamali M, Baghersad MH. Recent biomedical applications of gold nanoparticles: a review. Talanta. 2018;184:537–556. doi:10.1016/j.talanta.2018.02.088
23. Yang YQ, Wang XW, Qian HS, Cheng L. Titanium-based sonosensitizers for sonodynamic cancer therapy, Appl. Mater Today. 2021;25:101215.
24. Qu J, Wang Y, Mu X, et al. Determination of crystallographic orientation and exposed facets of titanium oxide nanocrystals. Adv Mater. 2022;34(37):2203320. doi:10.1002/adma.202203320
25. Ning S, Dai X, Tang W, et al. Cancer cell membrane-coated C-TiO2 hollow nanoshells for combined sonodynamic and hypoxia-activated chemotherapy. Acta Biomater. 2022;152:562–574. doi:10.1016/j.actbio.2022.08.067
26. Bharti B, Kumar S, Lee H-N, Kumar R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment, Sci. Rep. 2016;6(1):32355.
27. Xu FY, Meng K, Cheng B, Wang SY, Xu JS, Yu JG. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat Commun. 2020;11(1):4613. doi:10.1038/s41467-020-18350-7
28. Zhang Y, Liu JX, Qian K, et al. Structure sensitivity of Au‐TiO2 strong metal-support interactions. Angew Chem Int Ed. 2021;60(21):12074–12081. doi:10.1002/anie.202101928
29. Amin MO, Al-Hetlani E. Development of efficient saldi substrate based on Au-TiO2 nanohybrids for environmental and forensic detection of dyes and NSAIDs. Talanta. 2021;233:122530. doi:10.1016/j.talanta.2021.122530
30. Entradas T, Waldron S, Volk M. The detection sensitivity of commonly used singlet oxygen probes in aqueous environments. J Photochem Photobiol B: Biol. 2020;204:111787. doi:10.1016/j.jphotobiol.2020.111787
31. Cao XS, Li MX, Liu QY, Zhao JJ, Lu XH, Wang JW. Inorganic sonosensitizers for sonodynamic therapy in cancer treatment. Small. 2023;19(42):e2303195. doi:10.1002/smll.202303195
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
M1 Macrophage-Derived Sonoresponsive Nanoparticles for Sonodynamic Anticancer Therapy
Chen S, Wang J, Liao H, Tang K, Xu Y, Wang L, Niu C
International Journal of Nanomedicine 2022, 17:4725-4741
Published Date: 10 October 2022