Back to Journals » Cancer Management and Research » Volume 17
“Enhancing Liposarcoma Prognosis – A New Predictive Scoring System Integrating Histopathological Insights”
Authors Ciongariu AM, Țăpoi DA, Dumitru AV, Enache V, Marin A , Creangă Snr CA, Costache M
Received 7 November 2024
Accepted for publication 3 February 2025
Published 17 February 2025 Volume 2025:17 Pages 331—348
DOI https://doi.org/10.2147/CMAR.S504889
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Ana-Maria Ciongariu,1,2 Dana Antonia Țăpoi,1,2 Adrian-Vasile Dumitru,1,2 Valentin Enache,3 Andrei Marin,4 Cosmin A Creangă Snr,3 Mariana Costache1,2
1Department of Pathology, “Carol Davila” University of Medicine and Pharmacy, Bucharest, 020021, Romania; 2Department of Pathology, University Emergency Hospital Bucharest, Bucharest, 050098, Romania; 3Department of Pathology, Clinical Emergency Hospital BGucharest, Bucharest, 014461, Romania; 4Department of Plastic Surgery, “Carol Davila” University of Medicine and Pharmacy, Bucharest, 020021, Romania
Correspondence: Adrian-Vasile Dumitru, Email [email protected]; Dana Antonia Țăpoi, Email [email protected]
Introduction: Liposarcomas are malignant soft tissue tumours with heterogeneous features and variable prognosis. Each entity comprised in this group displays distinct morphology and harbours specific genetic alterations, which correlate with clinical behaviour and therapy response. The aim of this study is to analyse the clinical and histopathological features that can influence the prognosis of liposarcoma. We also present a newly designed scoring system that could be useful for predicting the risk of disease progression and death in patients with different liposarcoma subtypes.
Materials and Methods: We carried out a retrospective multicentric study on 77 liposarcomas diagnosed between 2009 and 2023 that were followed up to assess the presence of metastases and survival of the patients. We evaluated the age, gender, tumour location and dimensions, histological subtype, mitotic index, presence and percentage of necrosis, and their association with disease progression and survival.
Results: In this respect, progression-free survival was positively associated with lower mitotic index, somatic soft-tissue localization, well-differentiated and myxoid subtypes and absence of necrosis. Overall survival was negatively influenced by older age, higher mitotic index, dedifferentiated and pleomorphic subtypes and the presence of necrosis. Therefore, several clinical and histopathological features of liposarcomas, such as tumour location, mitotic index, and tumour necrosis can strongly predict the disease evolution.
Discussion: This study focuses on developing a new scoring system that considers histologic subtype, mitotic index, and tumour necrosis as indicators that could predict the risk of disease progression and overall survival in patients with liposarcoma. The system classifies liposarcomas of any histological subtype into low-risk and high-risk tumours. Diagnosing liposarcomas using this two-tiered system could be useful for providing personalized therapy, in order to avoid relapses, metastases and improve the disease’s prognosis.
Keywords: liposarcoma, necrosis, prognosis, survival
Introduction
Liposarcomas are rare malignant tumours of the adipose tissue that can be classified into multiple subtypes, reflecting their clinical course and treatment sensitivity, as well as their histopathological and biological peculiarities.1 This heterogeneous group comprises tumours with significantly variable prognoses, correlated with several clinical and histopathological features.1,2 Histopathological subtype and tumour location are well described as prognostic factors for liposarcoma.1,3 Well-differentiated liposarcoma typically associates favourable prognosis, although it harbours complex genetic abnormalities.4 Dedifferentiated liposarcoma has a more aggressive clinical course, as patients frequently develop metastases and have poor overall survival.5,6 Myxoid liposarcoma is a distinct entity implying specific molecular features and has a propensity for local recurrence and metastases involving soft tissue, bone, and lung.6,7 Pleomorphic liposarcoma is a rare subtype, also regarded as a high-risk malignancy, associating variable prognosis, depending on tumour location, local extension, and presence of metastases.8 Tumours located in the retroperitoneum, omentum or mediastinum have a worse prognosis, compared to lesions of the somatic soft tissue, as patients tend to develop recurrences and die, even in the absence of metastases.9–11 Tumours of these regions usually are dedifferentiated and well-differentiated liposarcomas, as well as pleomorphic and myxoid pleomorphic liposarcomas.9–12 Proliferative activity is important for the diagnosis and evaluation of sarcomas and can have prognostic significance in the matter of liposarcomas.13 The value of the mitotic index is recognized for assessing the risk of primary and recurrent tumours with any localization.13–15 Although mitotic activity tends to be higher in dedifferentiated and pleomorphic liposarcomas, it is significant for every liposarcoma subtype.14,15 Tumour necrosis is also regarded as an independent predictor of local recurrence and survival, especially in patients with liposarcomas of the retroperitoneum.16–19 Moreover, an assessment of tumour necrosis is performed to evaluate the treatment sensitivity of tumours at various locations.17,18
Materials And Methods
Study Participants
This retrospective study included 77 patients diagnosed with primary liposarcoma of the somatic soft tissue, viscera, abdominal cavity, mediastinum and breast in the Pathology department of the University Emergency Hospital of Bucharest, Romania, and in the Department of Pathology of the Clinical Emergency Hospital of Bucharest, Romania, between 2009 and 2022. The cases were collected in the context of a retrospective chart review. At first, 83 cases were identified but 6 of them were excluded due to lack of follow-up. The inclusion criteria for the patients were: a histopathological diagnosis of primary liposarcoma, confirmed using immunohistochemical analysis, an imagistic investigation used to assess the presence of metastases after the initial diagnosis, and a follow-up of at least two years to determine the progression-free survival (PFS) and overall survival (OS). Next-generation sequencing was also performed for cases with atypical histomorphology or non-specific immunophenotype, but investigations were carried out in a tertiary diagnostic centre, therefore results could not be displayed. PFS was calculated as the time between the date of primary diagnosis and metastatic spread, while OS was calculated as the time between the date of the initial diagnosis and death.
The study that we conducted was approved by the Ethics Committee of the University Emergency Hospital of Bucharest by decision nr. 79363/21.12.2023 and by the Ethics Committee of the Clinical Emergency Hospital Romania Bucharest by decision nr. 5210/ 03.07.2024 and was conducted in accordance with the principles of the Helsinki Declaration. Each patient signed an informed consent form.
Histopathological and Immunohistochemical Analysis
The tissue samples were processed using standard histopathological and immunohistochemical methods. Four pathologists (A.M.C., D.A.Ț., A.V.D, and V.E.) examined the sections and established the diagnosis. Differences in opinion were settled by consultation with other two pathologists (M.C. and C.C.). The immunohistochemical markers that we used for confirmation were: MDM2 (Murine Double Minute 2) - SMP14 mouse monoclonal 1:50-, Zeta; CDK 4 (Cyclin-dependent kinase-4) - ZR349, rabbit monoclonal 1:100, Zeta; S100 protein - 4C4.9, mouse monoclonal 1:150, Zeta and p53 – DO - 7 mouse monoclonal 1:100, Zeta. Results of immunohistochemical analysis are showed in Table 1.
Data Collection and Analysis
The following variables were collected for each patient: age, gender, tumour site, histological subtype of liposarcoma, tumour’s greatest dimension, mitotic index, presence and percentage of necrosis, status of the resection margins, presence/absence of recurrences and metastatic lesions. All cases were revised before performing statistical analysis and liposarcoma subtypes, grade and TNM staging were classified according to the 5th edition of The Who Classification of tumours of the soft tissue and bone and to guidelines of Fédération Nationales des Centres de Lutte Contre le Cancer. We thoroughly selected only patients without neoadjuvant therapy in order to investigate the native tumour heterogeneity and avoid evaluation of treatment induced cellular changes. Descriptive statistics were provided, including mean, standard deviation (SD), median and range for continuous variables and frequency with percentage. Continuous variables were analysed be performing Mann–Whitney or Student t tests. Fisher’s exact test was used for categorial variables, and relative risks with 95% confidence intervals (CI) were calculated. All the results were considered significant at p values <0.05. Statistical analysis was performed using GraphPad Prism 10.2.3. (GraphPad Software Inc, San Diego, CA, USA).
Results
The mean age at diagnosis was 57.26 years old (SD = 14.62, range: 27–91), 53.247% were women and 46.753% were men. During follow-up, 16 patients developed metastases and 20 died. The age of the patients with PFS was slightly younger (mean = 56.36, SD = 14.09, range: 27–91) than that of the patients with progressive disease (mean = 60.69, SD = 16.52, range: 33–85). However, this difference was not statistically significant (t-test; p = 0.295). On the contrary, the age at diagnosis of patients who survived during follow-up was significantly younger (mean = 54.12, SD = 13.49, range: 27–80) than of those who died during follow-up (mean = 66.20, SD = 14.29, range: 45–91) (t-test; p = 0.0011).
Among the patients who developed metastases during follow-up, 43.75% were women (n = 7) and 56.25% were men (n = 9) but this difference was not statistically significant (Fisher’s exact test, RR = 1.464; 95% CI = 0.6233–3.466; p = 0.4148). Among the patients who died during follow-up, 60% were women (n = 12) and 40% were men (n = 8) but no significant association was noted (Fisher’s exact test, RR = 0.7593; 95% CI = 0.3508–1.604; p = 0.6045).
Regarding the location of the tumours, the most commonly affected sites were the members (n = 46), followed by visceral soft tissue (n = 27) and trunk (n = 4) – Figure 1.
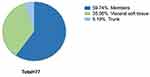 |
Figure 1 Distribution of primary tumour location. |
Out of the 27 cases of deep soft tissue liposarcomas, 40.74% of the patients developed metastases (n = 11), while 59.26% had no progressive disease (n = 16). Considering the 50 patients with liposarcomas in various locations, other than deep soft tissue (limbs, trunk), it was noted that only 10% of them developed metastases (n = 5) while 90% had no disease progression (n = 45) (Figure 2).
 |
Figure 2 Distribution of tumour location based on disease progression. |
By using Fisher’s exact test, we found out that the visceral soft tissue location of the tumours was statistically significant for disease progression (RR = 4.074, 95% CI = 1.643–10.29; p = 0.0027).
Among the patients with liposarcomas of the deep soft tissue, 40.74% died during the follow-up period (n = 11) and 59.26% survived (n = 16). Within the group of patients with other tumour locations, 18% died (n = 9) and 82% (n = 41) survived during follow-up (Figure 3).
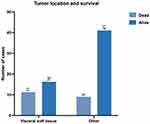 |
Figure 3 Distribution of tumour location based on overall survival. |
In this instance, visceral soft tissue location was not statistically significant for survival (Fisher’s exact test, RR = 2.263; 95% CI = 1.081–4.709; p = 0.0546).
Histopathological Characteristics of the Study Population
Tumour Dimension
The first feature that we analyzed was the tumours’ greatest dimension. The median dimension of the tumour in patients with PFS was 62 mm (range: 10–350), and the median tumour dimension in patients with metastases was 120 mm (range: 21–300) – Figure 4. Even though the median tumour dimension was greater in patients with progressive disease than in patients with PFS, this difference was not statistically significant (Mann–Whitney test, p = 0.1653).
 |
Figure 4 Median, minimum and maximum tumour dimension in patients in relation to PFS. |
The median tumour dimension in patients who survived during the follow-up period was 70 mm (range: 10–350), while the median tumour dimension in patients who died was 97.5 mm (range: 21–300) – Figure 5. Even though the median tumour dimension was greater in patients who died than in patients who survived during follow-up, this difference was not statistically relevant (Mann–Whitney test, p = 0.2553).
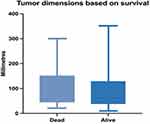 |
Figure 5 Median, minimum and maximum tumour dimension in relation to survival. |
Liposarcoma Subtype
Upon investigation of our group of patients, the most common histological subtype that we identified was myxoid liposarcoma (MLS) – 35 cases, followed by well-differentiated liposarcoma (WDLS) – 18 cases, while the least encountered were dedifferentiated liposarcoma (DDLS) and pleomorphic liposarcoma (PLS), each of them with 12 identified cases. All the liposarcoma subtypes identified within our studied group are presented in Figure 6.
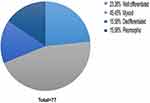 |
Figure 6 Distribution of liposarcoma subtype in the study group. |
The distribution of the histopathological subtype in relation to PFS and OS is presented in Table 2.
 |
Table 2 Histological Subtype in Relation to Disease Progression and Survival |
As DDLS and PLS were the most frequent diagnoses in both patients with progressive disease and deceased patients, we conducted Fisher’s exact tests comparing DDLS and PLS vs MLS and WDLS subtypes altogether. As a consequence, DDLS and PLS were significantly associated with both disease progression (RR = 6.625; 95% CI = 2.514–17.93; p < 0.0001) and overall survival (RR = 3.313; 95% CI = 1.578–6.964; p = 0,0021).
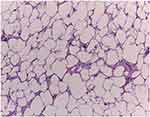
Histopathological aspects of low grade and high grade liposarcoma variants included in our study are presented comparatively in Figures 7–10.
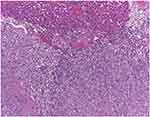 |
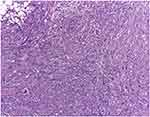
Figure 9 Dedifferentiated liposarcoma of the thigh with hepatic metastasis M, 78 B. Dedifferentiated liposarcoma secondary tumour determination infiltrating the hepatic parenchyma, H.E., 100x. |
Mitotic Index
The mean mitotic index within the group of patients with metastases was 8.375/10 hPF (median = 5, range: 2–23), while the group of patients without metastases showed a mean mitotic index of 3.934/10 hPF (median = 2, range: 1–25).
In this respect, a higher mitotic index is strongly associated with decreased PFS (Mann–Whitney test, p < 0.0001) – Figure 11.
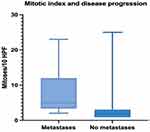 |
Figure 11 Median, minimum and maximum mitotic count in relation to PFS. |
The mean mitotic count in tumours of the patients who survived during follow-up was 3.562/10 hPF (median = 2, range: 1–23) and in those of patients who died was 8.65/10 hPF (median = 5, range 1–25).
Similar to OFS, a higher mitotic index is also significantly associated with decreased OS (Mann–Whitney test, p = 0.0011) – Figure 12.
 |
Figure 12 Median, minimum and maximum mitotic count in relation to survival. |
Mitotic activity in highly aggressive liposarcomas in our study group is emphasized in Figures 13–15.
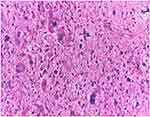 |
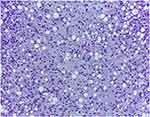
Figure 13 Pleomorphic liposarcoma of the retroperitoneum, M, 60: The tumour comprises markedly pleomorphic cells and exhibits numerous atypical mitotic figures, H.E., 200x. |
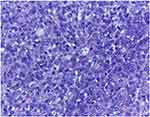 |
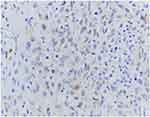
Figure 15 Dedifferentiated liposarcoma of the thigh, M, 32.: The non-lipogenic tumour component shows atypical mitotic activity, H.E., 200x. |
Tumour Necrosis
To go on, we evaluated the presence of tumour necrosis within liposarcomas in our study group. Even though most of the tumours included in this study displayed areas of necrosis, this feature was more frequently encountered in patients who developed metastases (Table 3).
 |
Table 3 Tumour Necrosis in Relation to PFS |
Results of Fisher's exact test showed that the presence of tumour necrosis is statistically significant for disease progression (RR = 8.571; 95% CI = 1.629–49.89; p = 0.007).
Additionally, tumour necrosis was also identified in most of the patients who died during follow-up (Table 4).
 |
Table 4 Tumour Necrosis in Relation to OS |
Results of Fisher’s exact test revealed that the presence of tumour necrosis is also statistically significant for decreased overall survival (RR = 3.7; 95% CI = 1.467–9.919; p = 0.0043).
Novel Algorithm for Predicting Disease Progression
The final part of our study focused on analyzing how statistically significant histopathological characteristics can predict the patients’ risk for disease progression and death. In this context, we designed a new algorithm entitled LEMON score (Liposarcoma Evaluation Mitosis Origin Necrosis) that evaluates the histologic subtype, mitotic index, and the presence of tumour necrosis and provides a two-tiered system, encompassing lesions of low-risk and high-risk (Table 5).
 |
Table 5 Risk Assessment in Liposarcoma by LEMON Score |
Based on this algorithm, tumours acquiring 3–5 points are classified as low risk, while tumours acquiring 6–8 points are classified as high risk.
According to this system, 43 patients included in the study group were diagnosed with low-risk tumours, and 34 patients were diagnosed with high-risk tumours. Among the patients with high-risk lesions, 41.18% (n = 14) developed metastases, while 58.82% (n = 20) had progression-free survival during follow-up. Regarding the group of patients with low-risk tumours, 4.65% (n = 2) developed metastases, while 95.35% (n = 41) had no disease progression (Figure 16).
 |
Figure 16 LEMON score in relation to disease progression. |
Examination of Fisher’s exact test results revealed that, based on the LEMON score, high-risk tumours are strongly associated with disease progression (RR = 8.853; 95% CI = 2.479–33.52; p = 0,0001).
Afterwards, the LEMON score was also used to assess OS. Among the patients with high-risk tumours, 41.18% (n = 14) died during the follow-up period, while 58.82% (n = 20) of them survived within the follow-up. On the contrary, patients diagnosed with tumours classified as low-risk died in a proportion of 13.95% (n = 6), while 86.05% (n = 37) of them survived during the follow-up (Figure 17).
 |
Figure 17 LEMON score in relation to survival. |
Results of Fisher’s exact test showed that, based on the LEMON score, high-risk tumours are also strongly associated with decreased overall survival (RR = 2.951; 95% CI = 1.321–6.82; p = 0.0091).
Limitations of the study consist of the following issues: the small number of cases, the lack of follow-up in some patient’s cases, and the reduced data upon the clinical history of the disease in the matter of each patient.
Discussion
Dedifferentiated and pleomorphic liposarcoma are associated with the most unfavorable prognosis, as patients develop metastases and have the worst survival rate.6,8,20–25 Well-differentiated and myxoid liposarcoma are usually associated with a lower metastatic potential and usually display locally aggressive behavior.5,7,26,27 Although some histological variants of liposarcoma have an adverse prognosis, there are some differences noted in the clinical course of patients with the same diagnosis.26,28 Dissimilar behaviour of high-grade liposarcomas is incompletely understood, and therefore additional prognostic factors have been investigated.1,29 In this respect, the age of the patients in this group was associated with decreased OS, but not with PFS. Nevertheless, it is unclear whether the poorer OS in older patients is strictly due to age itself or it could be explained by other comorbidities of such patients. It was acknowledged by some researchers in the scientific literature that age of the patients was not statistically significant for prognosis of patients with liposarcoma.30 However, some studies show that young age, histologic grade and location of the tumours are correlated with disease progression and therapy response of liposarcoma, therefore further surveys should be carried out.31,32 A potential association between tumour location and disease progression and survival has also been investigated, as lesions of the deep soft tissue and unusual, peculiar locations are associated with a poorer prognosis, compared to tumours of the extremities.5,32–34 In our study group, patients with liposarcoma of the deep soft tissue were significantly more prone to developing distant metastases. Although the impact of tumour location on disease progression and survival has modestly been studied, some authors noted a greater risk of metastases and death in retroperitoneal and intrathoracic tumours.35,36 For example, by conducting multivariate Cox analysis, Zhang et al demonstrated that histological subtype and tumour location are independent prognostic factors for disease progression and overall survival.37 Researchers identified a higher frequency of relapse and metastases in patients with liposarcoma of the retroperitoneum.37 Furthermore, according to some surveys, the location of the tumors is also significant for overall survival of patients with liposarcoma.33,34,38 Nevertheless, in our study group, the results of Fisher’s exact test showed that this factor is not statistically significant for overall survival.
Some studies report an unfavourable clinical outcome in patients with large/giant liposarcoma; therefore, the correlations between the size of tumours and prognosis have been investigated.39,40 Baudo et al performed univariate and multivariate statistical analysis on a group of patients with abdominal sarcomas and noted a poor prognosis of tumours larger than 17.1 cm.39 Moreover, studies regarding the effect of neoadjuvant therapy for liposarcoma suggest that the size of the lesions is important, as tumour downstaging implies improvement of survival.41 However, in our study group, tumour size was not statistically relevant for disease progression, even though the median tumour dimension was greater in patients with progressive disease and in those who died during follow-up, compared to patients with PFS and the ones who survived.
Liposarcoma subtype is an important histopathological feature and should always be reported, considering the heterogeneity of malignant adipocytic proliferations.1,6,42 From the results of Fisher’s exact test, we found out that histological subtype was a significant predictive factor for disease progression and survival. Dedifferentiated liposarcoma is associated with an important metastatic potential, as this was the most frequent diagnosis of patients with progressive disease. Within the scientific literature, most authors underline the aggressive clinical course of dedifferentiated liposarcoma.5,6,9,16 Peculiarities of its clinical evolution are related to histopathological and molecular features of dedifferentiated liposarcoma. In most of the cases, tumours encompass a non-lipogenic component with high-grade features and increased mitotic activity, frequently displaying atypical morphologies, such as rhabdoid or epithelioid cells.6,43,44 Genetic alterations and molecular features of this malignancy are related to its immunohistochemical characteristics, which are mandatory for an adequate diagnosis.22,44–46 Additionally, researchers suggest that further molecular and genomic analysis of dedifferentiated liposarcoma should be performed, to identify the prognostic significance of more genomic alterations.22 As an example, Jagosky et al conducted a study on the role of RECQL4, MN1 and NOTCH 1 in disease progression and survival, in addition to MDM2 and CDK4 amplification.22
Pleomorphic liposarcoma is also associated with adverse prognosis, and this histological subtype was one of the most frequently encountered in patients who died during follow-up along with dedifferentiated liposarcoma. Pleomorphic liposarcoma is a rare tumour with high mortality, sometimes displaying misleading histologic features, which should be taken into consideration as a differential diagnosis, especially for tumours of the abdominal cavity and mediastinum.47,48 Molecular study of this tumour is of limited use, as there is no specific genetic alteration with subsequent molecular and immunohistochemical peculiarities.11,49
A high mitotic index was associated with disease progression and poor survival during follow-up, according to Fisher’s exact test. The mean mitotic count was higher in tumours of patients with progressive disease and patients who died during follow-up, compared to those identified in patients with progression-free survival. According to Federation Nationales des Centres de Lutte Contre le Cancer grading system, the mitotic rate is an important parameter for all liposarcoma subtypes, considered useful for diagnosis and assessment of prognosis and therapeutic management.13,44,50 Mitotic cell-cycle progression is altered within malignant proliferations, as neoplastic cells bypass the normal controlling mechanisms of cell division.51 In dedifferentiated liposarcoma, malignant tumour cells control the Rb-dependent growth suppression and inactivate Rb through cyclin-dependent kinase 4 (CDK4) and CDK6 partnered with D-type cyclins.51,52 Immunohistochemical analysis of Ki 67 expression in liposarcoma is useful for evaluating the mitotic index and establishing the tumour grade.53 Lin et al suggest that assessment of mitotic index and identification of tumour necrosis can subclassify liposarcomas in two categories – low-grade and high-grade.53
Tumour necrosis is an important histologic factor associated with poor prognosis of liposarcoma.16,54,55 In our study group, tumour necrosis was statistically significant for disease progression and survival. By investigating the scientific literature, we found that necrosis is present in most high-grade liposarcomas and was significant for survival.54,55 Dantey et al underline the necessity of distinguishing high-grade liposarcoma from its low-grade counterpart and recommend the evaluation of necrosis for a correct grading.54 Authors acknowledge that Kaplan–Meier analysis revealed that most of the patients with high-grade tumours displaying necrotic foci died during follow-up.54
Considering the findings of our study and the information provided in the scientific literature and by the Fédération Nationales des Centres de Lutte Contre le Cancer guidelines, we elaborated the LEMON score, a newly developed system for assessment of risk in liposarcoma. To the best of our knowledge, this is the first system that considers and correlates histological subtypes with mitotic activity and tumour necrosis for the classification of malignant adipocytic tumours into low-grade and high-grade lesions. In our patients’ case, tumours classified as high-risk were strongly associated with metastases and poor survival during follow-up. Results of the Fisher’s exact test showed that the LEMON score is statistically significant for disease progression and overall survival. These results are particularly promising as the LEMON score can be rapidly evaluated during a standard histopathological examination of the tumours, without additional costs for the patient or the healthcare system. Furthermore, the algorithm we proposed is straightforward and does not require extensive experience in sarcoma pathology. Nevertheless, in spite of these obvious advantages, the LEMON score should be further investigated in order to be validated as an independent prognostic factor in larger series of patients.
The adequate treatment for patients with liposarcoma is established after a complete analysis of the histological subtype, local extension of the malignant tumour, and the presence of metastatic lesions. In our study group, most of the patients were treated using surgical excision of the tumour, followed by chemotherapy. In some cases, patients with locally advanced and multiply relapsed tumours of the extremities underwent amputation. At the moment, oncological treatment options for patients with liposarcoma are limited.4 The key to establishment of a suitable treatment consists of precisely rendering the diagnosis and assessing the risk, as liposarcoma is often associated with pitfalls.56,57 Immunohistochemical analysis can be a very useful ancillary method, as long as results of the staining are interpreted in correlation with the histological features of each tumour.56,57 In addition to diagnosis confirmation, revealing the immunophenotype of multiple liposarcoma subtypes indicates the corresponding molecular and genetic background of each entity and can also predict the disease prognosis.57 Although histopathological examination and immunostaining are valuable instruments for the management of liposarcoma, molecular analysis and genetic testing are sometimes required.56,57 For example, additional examination is need in cases of tumours with misleading histomorphology or peculiar location.58 Such malignant lesions can mimic other mesenchimal proliferations with dissimilar differentiation and even benign tumours.56,58,59 Atypical lipomatous tumour can resemble spindle cell lipomas and confirmation of MDM2 and CDK4 amplification is required for diagnosis.60 Analysis of Rb 1 status in patients with atypical lipomatous tumour/well differentiated liposarcoma can also be performed for distinguishing this entity from its high-grade counterpart, dedifferentiated liposarcoma. Molecular studies demonstrate loss of Rb1 loss in dedifferentiated liposarcoma and highlight the importance of this mutation in tumorigenesis and disease progression.60 Dedifferentiated liposarcoma is associated with an important metastatic potential which is strongly related to the several aspects, such as tumour location, necrosis and proliferative rate.61 Although some researchers suggest that tumour location is an independent prognostic factor, due to impossibility of achieving surgical margins in lesions of the retroperitoneum, disease progression and overall survival can be variable.62 Although immunohistochemical and molecular analysis of dedifferentiated liposarcoma is characterized by MDM2 and CDK4 amplification, some papers also note mutant TP53 phenotype and p16 hyperexpression can be identified.62,63 Patients with dedifferentiated liposarcoma usually receive systemic therapy, combining agents such as doxorubicin and ifosfamide or gemcitabine and docetaxel.64,65 The tumour shows a low response rate to trabectedin, eribulin and pazopanib, therefore multiple clinical trials are carried out, in order to investigate response to CDK4, MDM2 and immune check-point inhibitors.65–68 In this matter, Vanni S. et al conducted a study including patients with well differentiated and dedifferentiated liposarcoma in order to investigate effects of CDK 4 inhibitor palbociclib in association with conventional chemotherapy and levatinib.66 Results showed a notable reduction in tumour growth with administration of palbociclib sequential therapy followed by doxorubicin and levatinib.66 The study highlights the prognostic significance of CDK 4 as a therapeutic target for sarcomas, especially dedifferentiated liposarcoma.66 Myxoid liposarcoma is regarded as a radiosensitive malignancy, with subsequent improvement of surgical procedures.68 With the aim to provide tailored treatment and improve the prognosis, multiple studies upon pathogenesis and possible therapeutic targets in myxoid liposarcoma have been carried out.69 This malignancy is associated with FUS-DDIT3 rearrangement and immunohistochemical expression of the corresponding marker.70 To interrogate the response of neoplastic cells in myxoid liposarcoma to JAK1/2 inhibitors, some papers focused on understanding the link between FUS-DDIT3 fusion and JAK-stat signaling.70 Additionally, CD44 was recently validated as a potential stem cell marker for liposarcoma.70 Personalized therapeutic options for patients with pleomorphic liposarcoma are also limited at the moment, considering the rarity of this tumour and the lack of specific criteria for diagnosis and risk evaluation.71 Data upon pathogenesis, immunohistochemical and molecular features of pleomorphic liposarcoma continues to be scarce, due to the limited number of cases and variability regarding the location, histomorphology and clinical outcome of the malignancy.15,72 Treatment usually comprises wide surgical resection and chemotherapy, as the benefit of preoperative radiotherapy is still under debate.72,73 Although some studies revealed TP53 mutation and expression of FABP4/a2 marker of adipocytic differentiation in pleomorphic liposarcoma, there is no specific genetic abnormality related to this tumour.74 In addition, genetic mutations such as NTRK, ROS 1 or ALK gene rearrangements in liposarcomas are currently investigated, and trials using larotrectinib are carried out.75
Conclusions
Dedifferentiated and pleomorphic liposarcoma exhibit aggressive behavior and poor outcome. However, there are differences in the clinical evolution of tumours in patients with the same diagnosis. Risk evaluation of each case is mandatory in order to provide an adequate therapeutic approach and assess the overall prognosis of the disease. Our study addressed the most important clinical and histopathological features of liposarcomas, in correlation with disease progression and survival. We demonstrated that the only adverse prognosis factors are the histological subtype, higher mitotic index, and the presence of tumour necrosis for both PFS and overall survival. Furthermore, this is the first study to provide a new algorithm for risk assessment in liposarcoma. Classification of liposarcomas into low-grade and high-grade lesions is particularly important for a diligent prognostic evaluation and for achieving tailored therapeutic strategies.
Limitations of the study consist of the following: the small number of cases and difficulty achieving follow-up of the patients. Liposarcomas are rare tumours, therefore very few patients get diagnosed with this malignancy in our hospitals. In this respect, we collected cases diagnosed over a long period of time and acquired an adequate number of patients for determining statistically significant parameters. However, larger study groups are desirable for further investigations upon prognostic factors for liposarcoma. Follow-up of the patients is difficult because the healthcare system in our country does not provide an integrative digital system with all the clinical data assessing progressive disease and death of the patients. To avoid any errors, we investigated medical records of the patients and cumulated data available in other departments such as radiology, surgery, orthopedics and oncology for evaluation of disease prognosis. We also interrogated the national health insurance system for documenting the survival of patients during follow-up. Advanced digital systems rigorously providing medical history data for each patient should be implemented for accurate statistical analysis.
Funding
The study was funded by “Carol Davila” University of Medicine and Pharmacy Bucharest, Romania, by the program “Publish not Perish”.
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO Classification of Tumours Editorial Board. Soft tissue and bone tumours [Internet] [cited 2024 06 13]. Lyon (France): International Agency for Research on Cancer; 2020. (WHO classification of tumours series, 5th ed.; vol. 3). Available from: https://tumourclassification.iarc.who.int/chapters/33.
2. Creytens D. What’s new in adipocytic neoplasia? Virchows Arch. 2020;476(1):29–39.PMID: 31501988. doi:10.1007/s00428-019-02652-3
3. Thway K. Well-differentiated liposarcoma and dedifferentiated liposarcoma: an updated review. Semin Diagn Pathol. 2019;36(2):112–121.PMID: 30852045. doi:10.1053/j.semdp.2019.02.006
4. Crago AM, Dickson MA. Liposarcoma: multimodality Management and Future Targeted Therapies. Surg Oncol Clin N Am. 2016;25(4):761–773.PMID: 27591497; PMCID: PMC5010855. doi:10.1016/j.soc.2016.05.007
5. Osuna-Soto J, Caro Cuenca T, Sanz-Zorrilla A, Torrecilla-Martínez A, Ortega Salas R, Leiva-Cepas F. Prognosis and survival of patients diagnosed with well-differentiated and dedifferentiated retroperitoneal liposarcoma. Cir Esp. 2022;100(10):622–628.PMID: 35753575. doi:10.1016/j.cireng.2022.06.034
6. Ciongariu AM, Dumitru AV, Cîrstoiu C, et al. The conundrum of dedifferentiation in a liposarcoma at a peculiar location: a case report and literature review. Medicina (Kaunas). 2023;59(5):967.PMID: 37241198; PMCID: PMC10224154. doi:10.3390/medicina59050967
7. Ho TP. Myxoid liposarcoma: how to stage and follow. Curr Treat Options Oncol. 2023;24(4):292–299.PMID: 36867390. doi:10.1007/s11864-023-01064-5
8. Wan L, Tu C, Qi L, Li Z. Survivorship and prognostic factors for pleomorphic liposarcoma: a population-based study. J Orthop Surg Res. 2021. 16(1):175. Erratum in. J Orthop Surg Res. 2021;16(1):228. PMID: 33663547; PMCID: PMC7931523.doi:10.1186/s13018-021-02327-3.
9. Siew CCH, Apte SS, Baia M, Gyorki DE, Ford S, van Houdt WJ. Retroperit oneal and mesenteric liposarcomas. Surg Oncol Clin N Am. 2022;31(3):399–417.PMID: 35715141. doi:10.1016/j.soc.2022.03.005
10. Bagaria SP, Gabriel E, Mann GN. Multiply recurrent retroperitoneal liposarcoma. J Surg Oncol. 2018;117(1):62–68.PMID: 29266232. doi:10.1002/jso.24929
11. Suster DI, Suster S. Liposarcomas of the med iastinum. Mediastinum. 2020;4:27.PMID: 35118295; PMCID: PMC8794306. doi:10.21037/med-20-42
12. Dermawan JK. Myxoid Pleomorphic Liposarcoma. Surg Pathol Clin. 2024;17(1):25–29.PMID: 38278605. doi:10.1016/j.path.2023.06.005
13. Graham DS, Qorbani A, Eckardt MA, et al. Does “low-grade” dedifferentiated liposarcoma exist? The role of mitotic index in separating dedifferentiated liposarcoma from cellular well-differentiated liposarcoma. Am J Surg Pathol. 2023;47(6):649–660.PMID: 37057834. doi:10.1097/PAS.0000000000002037
14. Terunuma Y, Takahashi K, Doi M, et al. Primary pleomorphic liposarcoma of the liver: a case report and literature review. Surg Case Rep. 2021;7(1):244.PMID: 34797454; PMCID: PMC8603980. doi:10.1186/s40792-021-01322-4
15. Al-Attar M, Jnawali A, Yang M. Rare pleomorphic liposarcoma presented as jejunal obstruction. Case Rep Pathol. 2023;2023:8040232.PMID: 37409099; PMCID: PMC10319459. doi:10.1155/2023/8040232
16. Yu ZY, Gao JW, Liu N, Zhou SX, Zhao XD, Li PY. Predictive factors and a novel nomogram for recurrence of primary retroperitoneal liposarcoma: comprehensive analysis of 128 cases. Oncol Lett. 2023;25(6):257.PMID: 37485421; PMCID: PMC10360145. doi:10.3892/ol.2023.13843
17. Kaiser D, Schelm M, Gerber C, Brown ML, Müller DA. The effect of preoperative radiotherapy on surgical resectability, tumor volume and the necrosis rate of soft tissue sarcomas: a retrospective single-center analysis. Surg Oncol. 2021;39:101668.PMID: 34653769. doi:10.1016/j.suronc.2021.101668
18. Sun P, Ma R, Liu G, Wang L, Chang H, Li Y. Pathological prognostic factors of retroperitoneal liposarcoma: comprehensive clinicopathological analysis of 124 cases. Ann Transl Med. 2021;9(7):574.PMID: 33987272; PMCID: PMC8105808. doi:10.21037/atm-21-972
19. Yu Z, Zhao X, Gao J, Zhou S, Li P, Liu N. Correlation analysis between demographic, surgical, and pathological characteristics with local recurrence-free survival for surgical resected retroperitoneal liposarcoma. World J Surg. 2023;47(8):1946–1955.PMID: 37071133. doi:10.1007/s00268-023-07009-1
20. Fitzgerald K, Slama EM, Bernescu I. Dedifferentiated Liposarcoma of the Transverse Colon. Am Surg. 2022;88(4):790–792.PMID: 34727707. doi:10.1177/00031348211054524
21. Xia H, Fang F, Yuan H, Tu Y. Survival of a patient with multiple-recurrent giant retroperitoneal dedifferentiated liposarcoma for 15 years: a case report. Front Surg. 2022;9:916802.PMID: 36420404; PMCID: PMC9677108. doi:10.3389/fsurg.2022.916802
22. Jagosky MH, Anderson CJ, Symanowski JT, et al. Genomic alterations and clinical outcomes in patients with dedifferentiated liposa rcoma. Cancer Med. 2023;12(6):7029–7038.PMID: 36464833; PMCID: PMC10067084. doi:10.1002/cam4.5502
23. Machhada A, Emam A, Colavitti G, et al. Liposarcoma subtype recurrence and survival: a UK regional cohort study. J Plast Reconstr Aesthet Surg. 2022;75(7):2098–2107.PMID: 35337758. doi:10.1016/j.bjps.2022.02.023
24. Oliveira AM, Nascimento AG. Pleomorphic liposarcoma. Semin Diagn Pathol. 2001;18(4):274–285. PMID: 11757868.
25. Gjorgova Gjeorgjievski S, Thway K, Dermawan JK, et al. Pleomorphic liposarcoma: a series of 120 cases with emphasis on morphologic variants. Am J Surg Pathol. 2022;46(12):1700–1705.PMID: 36006773. doi:10.1097/PAS.0000000000001962
26. Tfayli Y, Baydoun A, Naja AS, Saghieh S. Management of myxoid liposarcoma of the extremity. Oncol Lett. 2021;22(2):596.PMID: 34188698; PMCID: PMC8228380. doi:10.3892/ol.2021.12857
27. Nassif EF, Keung EZ, Thirasastr P, Somaiah N. Myxoid Liposarcomas: systemic Treatment Options. Curr Treat Options Oncol. 2023;24(4):274–291.PMID: 36853469. doi:10.1007/s11864-023-01057-4
28. Wang GY, Lucas DR. Dedifferentiated liposarcoma with myofibroblastic differentiation. Arch Pathol Lab Med. 2018;142(10):1159–1163.PMID: 30281365. doi:10.5858/arpa.2018-0205-RA
29. A MS, C K, Bhargavan RV, Somanathan T, Subhadradevi L. An overview on liposarcoma subtypes: genetic alterations and recent advances in therapeutic strategies. J Mol Histol. 2024;55(3):227–240.PMID: 38696048. doi:10.1007/s10735-024-10195-4
30. Kunisada T, Nakata E, Fujiwara T, et al. Soft-tissue sarcoma in adolescents and young adults. Int J Clin Oncol. 2023;28(1):1–11.PMID: 35084598. doi:10.1007/s10147-022-02119-7
31. Greto D, Saieva C, Loi M, et al. Influence of age and subtype in outcome of operable liposarcoma. Radiol Med. 2019;124(4):290–300.PMID: 30421387. doi:10.1007/s11547-018-0958-4
32. Li Y, Wu G, Zhang Y, et al. Development and validation of a prognostic model to predict the prognosis of patients with retroperitoneal liposarcoma: a large international population-based cohort study. Front Oncol. 2022;12:857827.PMID: 35719991; PMCID: PMC9201285. doi:10.3389/fonc.2022.857827
33. Xiao J, Liu J, Chen M, Liu W, He X. Diagnosis and prognosis of retroperitoneal liposarcoma: a single asian center cohort of 57 cases. J Oncol. 2021;2021:7594027.PMID: 34035812; PMCID: PMC8116140. doi:10.1155/2021/7594027
34. Vijay A, Ram L. Retroperitoneal liposarcoma: a comprehensive review. Am J Clin Oncol. 2015;38(2):213–219.PMID: 24136142. doi:10.1097/COC.0b013e31829b5667
35. Mendoza-Moreno F, Matías-García B, Quiroga-Valcárcel A, et al. Malignant adipocytic tumours: a 20‑year single‑centre retrospective study. Oncol Lett. 2023;25(6):247.PMID: 37153046; PMCID: PMC10161324. doi:10.3892/ol.2023.13833
36. Ishtiaq R, Naeem A, Ratnani I. Thoracic liposarcoma in an end stage renal disease patient. J Ayub Med Coll Abbottabad. 2019;31(2):286–289. PMID: 31094134.
37. Zhang Q, Li JM, Liu JL, Wei YZ. Evaluation of prognostic factors for liposarcoma. Zhonghua Zhong Liu Za Zhi. 2019. 41(12):943–948. Chinese. PMID: 31874553.doi:10.3760/cma.j.issn.0253-3766.2019.12.011.
38. Lee HG, Aurit S, Silberstein P, Gootee J. Primary anatomical site as a prognostic factor for pleomorphic liposarcoma. J Cancer Res Clin Oncol. 2020;146(6):1501–1508.PMID: 32248301. doi:10.1007/s00432-020-03204-y
39. Baudo A, Piccinelli ML, Incesu RB, et al. Surgically treated pelvic liposarcoma and leiomyosarcoma: the effect of tumor size on cancer-specific survival. Surg Oncol. 2024;54:102074.PMID: 38615387. doi:10.1016/j.suronc.2024.102074
40. Tirumani SH, Tirumani H, Jagannathan JP, et al. Metastasis in dedifferentiated liposarcoma: predictors and outcome in 148 patients. Eur J Surg Oncol. 2015;41(7):899–904.PMID: 25659772. doi:10.1016/j.ejso.2015.01.012
41. Masunaga T, Tsukamoto S, Honoki K, et al. Comparison of pre-operative and post-operative radiotherapy in patients with localized myxoid liposarcoma. Jpn J Clin Oncol. 2023;53(12):1153–1161.PMID: 37814462. doi:10.1093/jjco/hyad119
42. Chamberlain F, Benson C, Thway K, Huang P, Jones RL, Gennatas S. Pharmacotherapy for liposarcoma: current and emerging synthetic treatments. Future Oncol. 2021;17(20):2659–2670.PMID: 33880964. doi:10.2217/fon-2020-1092
43. Makise N, Yoshida A, Komiyama M, et al. Dedifferentiated liposarcoma with epithelioid/epithelial features. Am J Surg Pathol. 2017;41(11):1523–1531.PMID: 28719466. doi:10.1097/PAS.0000000000000910
44. Kilpatrick SE. Dedifferentiated liposarcoma: a comprehensive historical review with proposed evidence-based guidelines regarding a diagnosis in need of further clarification. Adv Anat Pathol. 2021;28(6):426–438.PMID: 34326285. doi:10.1097/PAP.0000000000000314
45. Bourgeau M, Gandhi JS, Deeb KK, Bahrami A. Superficial dedifferentiated liposarcoma: a clinicopathologic study. Hum Pathol. 2024;145:63–70.PMID: 38423223. doi:10.1016/j.humpath.2024.02.008
46. Tyler R, Wanigasooriya K, Taniere P, et al. A review of retroperitoneal liposarcoma genomics. Cancer Treat Rev. 2020;86:102013.PMID: 32278233. doi:10.1016/j.ctrv.2020.102013
47. Khan Y, Iqbal S, Fatimi S. Pleomorphic liposarcoma of chest wall: a rare entity with challenging management. Asian Cardiovasc Thorac Ann. 2019;27(4):310–312.PMID: 30808192. doi:10.1177/0218492319834824
48. Akiyama T, Yoshimatsu Y, Noguchi R, et al. Establishment and characterization of NCC-PLPS2-C1: a novel cell line of pleomorphic liposarcoma. Hum Cell. 2023;36(1):468–475.PMID: 36436139. doi:10.1007/s13577-022-00828-9
49. Anderson WJ, Jo VY. Pleomorphic liposarcoma: updates and current differential diagnosis. Semin Diagn Pathol. 2019;36(2):122–128.PMID: 30852046. doi:10.1053/j.semdp.2019.02.007
50. Dry SM. Dedifferentiation in bone and soft tissue sarcomas: how do we define it? What is prognostically relevant? Hum Pathol. 2024;147:139–147.PMID: 38311185. doi:10.1016/j.humpath.2024.02.001
51. VanArsdale T, Boshoff C, Arndt KT, Abraham RT. Molecular Pathways: targeting the Cyclin D-CDK4/6 Axis for Cancer Treatment. Clin Cancer Res. 2015;21(13):2905–2910.PMID: 25941111. doi:10.1158/1078-0432.CCR-14-0816
52. Kovatcheva M, Liu DD, Dickson MA, et al. MDM2 turnover and expression of ATRX determine the choice between quiescence and senescence in response to CDK4 inhibition. Oncotarget. 2015;6(10):8226–8243.PMID: 25803170; PMCID: PMC4480747. doi:10.18632/oncotarget.3364
53. Lin X, Davion S, Bertsch EC, Omar I, Nayar R, Laskin WB. Federation nationale des centers de lutte contre le cancer grading of soft tissue sarcomas on needle core biopsies using surrogate markers. Hum Pathol. 2016;56:147–154.PMID: 27346575. doi:10.1016/j.humpath.2016.06.008
54. Dantey K, Schoedel K, Yergiyev O, Bartlett D, Rao UNM. Correlation of histological grade of dedifferentiation with clinical outcome in 55 patients with dedifferentiated liposarcomas. Hum Pathol. 2017;66:86–92.PMID: 28300575. doi:10.1016/j.humpath.2017.02.015
55. Yıldırım S, Çiftdemir M, Ustabaşıoğlu FE, Üstün F, Usta U. Evaluation of the factors affecting survival and local recurrence in thigh soft tissue sarcomas. Jt Dis Relat Surg. 2024;35(1):130–137.PMID: 38108174; PMCID: PMC10746889. doi:10.52312/jdrs.2023.1289
56. Lee ATJ, Thway K, Huang PH, Jones RL. Clinical and molecular spectrum of liposarcoma. J Clin Oncol. 2018;36(2):151–159.PMID: 29220294; PMCID: PMC5759315. doi:10.1200/JCO.2017.74.9598
57. Kammerer-Jacquet SF, Thierry S, Cabillic F, et al. Differential diagnosis of atypical lipomatous tumor/well-differentiated liposarcoma and dedifferentiated liposarcoma: utility of p16 in combination with MDM2 and CDK4 immunohistochemistry. Hum Pathol. 2017;59:34–40.PMID: 27597521. doi:10.1016/j.humpath.2016.08.009
58. Briski LM, Jorns JM. Primary breast atypical lipomatous tumor/ well-differentiated liposarcoma and dedifferentiated liposarcoma. Arch Pathol Lab Med. 2018;142(2):268–274.PMID: 29372852. doi:10.5858/arpa.2016-0380-RSR2
59. Zhu H, Sun J, Wei S, Wang D, Brandwein M. Well-differentiated laryngeal/hypopharyngeal liposarcoma in the MDM2 era report of three cases and literature review. Head Neck Pathol. 2017;11(2):146–151.PMID: 27492446; PMCID: PMC5429270. doi:10.1007/s12105-016-0747-0
60. Jebastin JAS, Perry KD, Chitale DA, et al. Atypical lipomatous tumor/well-differentiated liposarcoma with features mimicking spindle cell lipoma. Int J Surg Pathol. 2020;28(3):336–340.PMID: 31672072. doi:10.1177/1066896919884648
61. Thway K, Jones RL, Noujaim J, Zaidi S, Miah AB, Fisher C. Dedifferentiated liposarcoma: updates on morphology, genetics, and therapeutic strategies. Adv Anat Pathol. 2016;23(1):30–40.PMID: 26645460. doi:10.1097/PAP.0000000000000101
62. Dubois-Silva A, Barbagelata-Lopez C. Retroperitoneal dedifferentiated liposarcoma. Intern Emerg Med. 2019;14(4):619–620.PMID: 30519920. doi:10.1007/s11739-018-2004-x
63. Gong LH, Liu WF, Ding Y, et al. Dedifferentiated liposarcoma of extremities: a clinicopathologic analysis. Zhonghua Bing Li Xue Za Zhi. 2018. 47(7):511–516. Chinese. PMID: 29996315.doi:10.3760/cma.j.issn.0529-5807.2018.07.006.
64. Zhou MY, Bui NQ, Charville GW, Ganjoo KN, Pan M. Treatment of de-differentiated liposarcoma in the era of immunotherapy. Int J mol Sci. 2023;24(11):9571.PMID: 37298520; PMCID: PMC10253599. doi:10.3390/ijms24119571
65. Schöffski P. Established and experimental systemic treatment options for advanced liposarcoma. Oncol Res Treat. 2022;45(9):525–543.PMID: 35609512. doi:10.1159/000524939
66. Vanni S, Miserocchi G, Gallo G, et al. Role of CDK4 as prognostic biomarker in Soft Tissue Sarcoma and synergistic effect of its inhibition in dedifferentiated liposarcoma sequential treatment. Exp Hematol Oncol. 2024;13(1):74.PMID: 39103896; PMCID: PMC11299298. doi:10.1186/s40164-024-00540-4
67. Saponara M, Stacchiotti S, Gronchi A. Pharmacological therapies for Liposarcoma. Expert Rev Clin Pharmacol. 2017;10(4):361–377.PMID: 28135854. doi:10.1080/17512433.2017.1289086
68. Wang S, Zhou Y, Wang H, Ling J. Survival analysis and treatment strategies for limb liposarcoma patients with metastasis at presentation. Medicine (Baltimore). 2021;100(13):e25296.PMID: 33787618; PMCID: PMC8021344. doi:10.1097/MD.0000000000025296
69. Redroban L, Montalvo N. Vulvar myxoid liposarcoma, an extremely rare diagnosis: a case report and review of literature. Int J Gynecol Pathol. 2019;38(1):17–20.PMID: 29019868. doi:10.1097/PGP.0000000000000460
70. Dolatabadi S, Jonasson E, Andersson L, et al. FUS-DDIT3 fusion oncoprotein expression affects JAK-STAT signaling in myxoid liposarcoma. Front Oncol. 2022;12:816894.PMID: 35186752; PMCID: PMC8851354. doi:10.3389/fonc.2022.816894
71. Hadjimichael AC, Bekos A, Tsukamoto S, et al. Pleomorphic liposarcoma revisited. Orthopedics. 2023;46(2):e72–e80.PMID: 35876778. doi:10.3928/01477447-20220719-05
72. Pe W Jr, Wangsiricharoen S, Ali SZ. Pleomorphic liposarcoma: a clinicopathologic study of 20 FNA cases. Cancer Cytopathol. 2022;130(9):705–713.PMID: 35447010. doi:10.1002/cncy.22580
73. Assi T, Ngo C, Faron M, et al. Systemic therapy in advanced pleomorphic liposarcoma: a comprehensive review. Curr Treat Options Oncol. 2023;24(11):1598–1613.PMID: 37843627. doi:10.1007/s11864-023-01139-3
74. Tiemeier GL, Brown JM, Pratap SE, et al. Pleomorphic liposarcoma of bone: a rare primary malignant bone tumour. Clin Sarcoma Res. 2018;8(1):2. PMID: 29449935; PMCID: PMC5807841.doi:10.1186/s13569-018-0089-7
75. Valenciaga A, Iwenofu OH, Tinoco G. Larotrectinib in a patient with advanced pleomorphic liposarcoma of the uterus. J Natl Compr Canc Netw. 2021;19(7):775–779. doi:10.6004/jnccn.2021.7039.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Establishment and Validation of a Predictive Nomogram for Postoperative Survival of Stage I Non-Small Cell Lung Cancer
Wang ZH, Deng L
International Journal of General Medicine 2022, 15:7287-7298
Published Date: 14 September 2022
Low Pre-ChemoradiotherapyPan-Immune-Inflammation Value (PIV) Measures Predict Better Survival Outcomes in Locally Advanced Pancreatic Adenocarcinomas
Topkan E, Selek U, Kucuk A, Pehlivan B
Journal of Inflammation Research 2022, 15:5413-5423
Published Date: 18 September 2022
Treatment Outcomes and Prognostic Factors in 66 Patients with Chronic Myelomonocytic Leukemia (CMML) in a Single Center
Wang C, Wang Z, Meng F, Luo L, Liu X, Shi J, Huang L
International Journal of General Medicine 2022, 15:7843-7854
Published Date: 18 October 2022
Prognostic Factors of Adrenocortical Carcinoma: Experience from a Regional Medical Center in Eastern China
Li P, Su X, Zhang X, Sun L, Zhang G
International Journal of General Medicine 2023, 16:453-465
Published Date: 3 February 2023
Preoperative Systemic Immune-Inflammation Index is a Potential Biomarker in Adult Patients with High-Grade Gliomas Undergoing Radical Resection
Jiang YT, Wang TC, Zhang W
Journal of Inflammation Research 2023, 16:3479-3490
Published Date: 16 August 2023