Back to Journals » International Journal of Nanomedicine » Volume 19
Enteral Route Nanomedicine for Cancer Therapy
Authors Zhang LZ, Du RJ, Wang D, Qin J , Yu C, Zhang L, Zhu HD
Received 11 June 2024
Accepted for publication 3 September 2024
Published 25 September 2024 Volume 2024:19 Pages 9889—9919
DOI https://doi.org/10.2147/IJN.S482329
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Lin-Zhu Zhang,* Rui-Jie Du,* Duo Wang,* Juan Qin, Chao Yu, Lei Zhang, Hai-Dong Zhu
Center of Interventional Radiology & Vascular Surgery, Department of Radiology, Nurturing Center of Jiangsu Province for State Laboratory of AI Imaging & Interventional Radiology (Southeast University), Basic Medicine Research and Innovation Center of Ministry of Education, Zhongda Hospital, Medical School, Southeast University, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Zhang; Hai-Dong Zhu, Center of Interventional Radiology & Vascular Surgery, Department of Radiology, Nurturing Center of Jiangsu Province for State Laboratory of AI Imaging & Interventional Radiology (Southeast University), Basic Medicine Research and Innovation Center of Ministry of Education, Zhongda Hospital, Medical School, Southeast University, Nanjing, 210009, People’s Republic of China, Email [email protected]; [email protected]
Abstract: With the in-depth knowledge of the pathological and physiological characteristics of the intestinal barrier–portal vein/intestinal lymphatic vessels–systemic circulation axis, oral targeted drug delivery is frequently being renewed. With many advantages, such as high safety, convenient administration, and good patient compliance, many researchers have begun to explore targeted drug delivery from intravenous injections to oral administration. Over the past few decades, the fields of materials science and nanomedicine have produced various drug delivery platforms that hold great potential in overcoming the multiple barriers associated with oral drug delivery. However, the oral transport of particles into the systemic circulation is extremely difficult due to immune rejection and biochemical invasion in the intestine, which limits absorption and entry into the bloodstream. The feasibility of the oral delivery of targeted drugs to sites outside the gastrointestinal tract (GIT) is unknown. This article reviews the biological barriers to drug absorption, the in vivo fate and transport mechanisms of drug carriers, the theoretical basis for oral administration, and the impact of carrier structural evolution on oral administration to achieve this goal. Finally, this article reviews the characteristics of different nano-delivery systems that can enhance the bioavailability of oral therapeutics and highlights their applications in the efficient creation of oral anticancer nanomedicines.
Keywords: oral nanomedicine, oral targeted drug delivery, nanoparticles, cancer treatment, biological barriers
Graphical Abstract:

Introduction
The route of drug administration is the most important factor in determining drug concentration and residence time at the target site.1,2 Despite well-known drawbacks such as poor drug bioavailability and rapid degradation rates and metabolism in the intestine and liver, oral therapy is considered the most ideal and convenient route of drug delivery for both systemic and local administration drug delivery.3 It is essential for patients to perceive oral drug delivery positively, particularly when compared to infusions or other parenteral methods, which are common in cancer treatments. Additionally, the economic benefits of reducing hospital stays for patients should also be considered.4
The gastrointestinal tract (GIT) encompasses a surface area of approximately 300 square meters and is enveloped with a sticky mucosal layer that aids in the adhesion and absorption of drugs.5 Notwithstanding, oral drug delivery poses a daunting task owing to the intricate nature of the GIT and the impediments to drug delivery, such as substandard solubility, stability, and permeability. In addition, an unpleasant taste, gastric irritation, and susceptibility to intestinal and liver barrier metabolism are common drawbacks of oral medications.6,7 However, targeted delivery to specific locations in the GIT can be achieved through appropriate drug design or delivery vehicles.8 The residence time of digested food in the duodenum is relatively brief, and the pH is lower than that of the remainder of the small intestine. This indicates that the retention of drugs in the duodenum can be successfully avoided by increasing the pKa value of the delivery vehicle. For instance, Lozoya-Agullo et al employed poly(lactic-co-glycolic) acid (PLGA) nanoparticles for colonic delivery, demonstrating that drug release in the duodenum could be significantly avoided due to insufficient ambient pH.9
Drug delivery systems (DDSs) are frequently utilized to improve the stability of drugs, regulate drug release, and accumulate drugs at the lesion site, thus improving drug effectiveness.10–13 In 1906, Ehrlich first proposed the “magic bullet” hypothesis, which introduced the idea of targeted drugs.14,15 Since rituximab, the first targeted treatment was approved by the FDA in 1997, targeted drug delivery systems (TDDSs) have emerged, leading the way for targeted therapy.14
Some researchers advocate using oral targeted drug delivery systems (OTDDSs) in biomedical applications, citing their promising potential.16 Oral administration involves the administration of drugs or therapeutic agents that are transported into the systemic circulation and accumulate at target sites beyond the GIT. The proposed OTDDS is considered to have various potential benefits, such as enhanced compliance, improved convenience of medication usage, and lowered expenses for production and healthcare. Historically, oral administration has proven to be more effective for treatments based on small molecules. Due to the diverse and heterogeneous nature of the biological barrier in the GIT, most new biotherapeutics and high-molecular-weight molecules are not ideal for delivery using this route.1
Recent advancements in materials science are expanding the options for cancer treatments.17 Specifically, nanomedicines and their bulk carriers demonstrate significant potential in improving the delivery of small molecules and macromolecules.18–21 Wang et al demonstrated that tartaric-acid-modified mesoporous silica nanoparticles exhibit excellent mucus penetration, mucosal adhesion, cellular uptake, intestinal transport, and gastrointestinal retention during continuous oral absorption. The nanoparticles are efficiently absorbed into the bloodstream during oral delivery, exhibiting absorption rates that are 1.72–2.05 times higher than those of other nanocarriers. They also contribute to a more efficient intestinal transit of loaded doxorubicin (DOX), with absorption rates that are 2.32–27.03 times higher than those of other samples. Furthermore, the nanoparticles exhibit satisfactory bioavailability (449.73%) and a stronger anti-tumor effect (up to 95.43%).22 The oral delivery of nanocarriers can both promote regulated drug release and protect the drug payload from adverse biological and chemical conditions in the GIT. In addition, by avoiding first-pass metabolism, oral nanocarriers enhance targeting to specific GIT cell morphologies, penetrate the mucus barrier, and improve drug bioavailability.22 The drug delivery community has shown great interest in the search for novel approaches and materials that facilitate the transition from intravenous to oral drug delivery.23,24 The viability of OTDDSs must be taken into account when considering oral targeted therapy. This includes the consideration of transport mechanisms, intestinal and cellular distribution, absorption pathways, and the structural progression of the carriers in the body. The mucosal mucus layer of the intestine has a large surface area (greater than 300 m2), which facilitates the attachment of drugs and subsequent absorption.25 Given the abundance of enterocytes throughout the intestine, particularly in the microfold cells (M cells) lining the lymphatic area of the small intestine. M cells are specialized cells present in the intestinal epithelium and are part of the gut-associated lymphoid tissue. M cells are morphologically different from intestinal epithelial cells. On the surface of the intestine, M cells have short and irregular microvilli, which are different from the highly organized and uniformly and closely arranged microvilli of intestinal epithelial cells. Therefore, M cells have higher permeability than other intestinal epithelial cells and can be used as a channel for nanoparticles to cross the epithelium. After ingestion, the drug carrier will immediately enter the lymphatic system without loss.26,27 Other factors that contribute to reduced drug efficiency due to mechanical degradation within the gastrointestinal tract are osmotic pressure along the gastrointestinal tract, the peristalsis of the gastrointestinal muscles, and shear stress generated by gastric fluid flow rates within the gastric lumen.28 Furthermore, the flow of gastric juice may also reduce the contact time between drug molecules and the epithelial layer, thereby hindering drug absorption.29 Enveloped biologics, such as viruses, vaccines, and cells, are frequently the primary components susceptible to mechanical disruption. In a study by Valon et al, the potential effects of mechanical stress on various cell types were investigated. The findings indicated that shear stress and compaction may result in cell apoptosis and death.30 Furthermore, Choi et al demonstrated that high osmotic pressure can compromise the integrity of viruses in acidic environments.28
This review provides a detailed summary of nanotechnologies for oral chemotherapy, highlighting their unique properties and the challenges they face in overcoming intestinal biological barriers. It emphasizes the many advantages of oral administration over injection: avoidance of discomfort, trauma, infection, and complications. A major challenge with oral drug delivery is the relatively low bioavailability of the drug. Nanotechnology can significantly improve the bioactivity and availability of oral drugs. The applications of organic and inorganic nanomaterials in oral nanomedicines are reviewed, demonstrating their respective advantages and application prospects. Specific challenges and potential opportunities for the future development of oral nanomedicines are presented and analyzed. The main physiological barriers facing oral drugs are described, and insights into how nanotechnology can overcome these barriers through a range of mechanisms of action are provided. Research on oral nanomedicines based on nanomaterials and specific application cases in cancer treatment are discussed. Challenges and opportunities encountered in future clinical translation and industrial production are proposed, and coping strategies are emphasized. The review calls for more solutions with innovative designs and applications to promote the clinical transformation and standardized production of oral nanomedicines. The ultimate goal is to benefit patients and improve their quality of life through innovative nanotechnology designs and applications. We also highlight their potential use in the treatment of cancer (Table 1).
 |
Table 1 Oral Nanomedicine for Cancer Drug Delivery |
Biological Barriers to OTDDSs
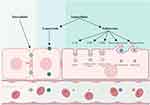
Biological barriers to the transport of drugs or carriers hinder the effective accumulation of nanoparticles at sites of disease, impeding the swift targeting of diseased areas. While the transportation of materials within the GIT is highly efficient, biological defense mechanisms have evolved to prevent foreign objects—such as synthetic nanoparticles—from being harmful. In a similar vein, the defense system acts as a built-in impediment to nanoparticle absorption. Orally administered nanoparticles may face challenges in achieving systemic circulation due to resistance and destruction. Multiple barriers must be overcome for nanoparticles to complete their intended tasks. In general, physical and biochemical barriers form an absorption barrier that prevents nanoparticles from traveling from the gastrointestinal lumen to the liver (Figure 1).27
Physical barriers restrict the penetration of particles, including mucus, tight junctions (TJs), and cell membranes. The mucus layer is the primary barrier that nanoparticles must overcome to enter into the circulatory system. It has been emphasized that mucus, which safeguards epithelial surfaces, acts as a significant obstruction to nanoparticle penetration.62,63 As materials traverse, mucus is continuously released from the mucus layer to wash away pathogens and lubricate the epithelium as substances pass through. The shortening of the nanoparticle residence time in mucus turnover results in their incapability to penetrate the loosely adherent layer.64 To overcome these barriers, Wang et al developed a strategy to modify the nanocarrier surface with cationic cell-penetrating peptides, which are hidden by a hydrophilic succinylated casein layer. Succinylated casein is a mucus-inert natural material that degrades specifically in the intestine, thereby protecting nanocarriers from the harsh gastric environment. This degradation also promotes mucus penetration and induces cell-penetrating peptide exposure upon degradation, which, in turn, facilitates efficient transepithelial transport.51 During the breakdown of the mucus layer, the nanoparticles encounter cellular barriers, such as TJs and cell membranes. These TJs between adjacent epithelial cells permit the passage of only small water-soluble molecules while limiting the movement of lipophilic compounds, macromolecules, and particles.65 Li et al prepared nanocarriers with variable physical properties through the self-assembly of hydrolyzed α-lactalbumin peptide fragments. The nanotubes can instantaneously and reversibly open the TJs between cells, thereby facilitating the entry of mangiferin into the blood circulation and enhancing its bioavailability.50 The confinement of macromolecules or aggregates at TJs obstructs the potential uptake pathway of nanoparticles, which is referred to as the paracellular route. Similarly, cell membranes either favorably or unfavorably allow foreign particles to pass through them. The direct penetration of cell membranes by nanoparticles is impracticable. Therefore, the primary absorption mechanism is membrane transport.66 Biochemical barriers present additional factors that substantially impede nanoparticle uptake and transport. Harsh gastrointestinal conditions, including immune cells, efflux pumps, variable pH, and digestive enzymes, combined with hepatic clearance, result in significant transport barriers.66 For oral targeted nanoparticles to enter the circulation, they must successfully avoid chemical destruction and metabolic enzymes, as well as physiological efflux immune phagocytosis, and hepatic first-pass effects. Meeting these requirements necessitates a careful overall design of the nanoparticles.14 A novel delivery system has been developed, comprising polymeric liposomes modified with N-succinic anhydride and D-fructose-conjugated chitosan. This system has been designed to achieve the delivery of paclitaxel by targeting the dual transporters of monocarboxylic acid transporters and glucose transporters.56 The system is therefore capable of efficient targeted delivery.
The oral bioavailability of these drugs can be significantly enhanced by the use of nanocarriers, which can increase drug solubility, prevent drug degradation by gastrointestinal enzymes, and facilitate drug passage through mucus gel layers and absorptive membranes. The aforementioned properties of nanocarriers, including self-emulsifying drug delivery systems, solid lipid nanoparticles, nanostructured lipid carriers, liposomes, polymeric nanoparticles, inorganic nanoparticles, and polymeric micelles, are largely dependent on their surface chemistry. In particular, the following determined by the surface chemistry of the nanocarrier: interactions with food, digestive enzymes, bile salts, and electrolytes; diffusion behavior across the mucus gel layer; and fate on the absorptive membrane. Bioinert surfaces that limit interactions with gastrointestinal fluids and contents and mucus, adhesive surfaces that provide intimate contact with the gastrointestinal mucosa, and absorption-enhancing surfaces can be designed. Furthermore, a charge conversion surface is capable of converting its zeta potential from negative to positive directly at the absorbing membrane, thereby providing a surface for targeted drug release, which is advantageous. In addition to the aforementioned passive surfaces, it is even possible to create active surfaces that cleave mucus glycoproteins during their passage through the mucus gel layer.
Paracellular and Transcellular Transport Pathways and Mechanisms of OTDDSs
DDS carriers are typically fashioned using functional nanoparticles, which are commonly referred to as nanocarriers for delivering therapeutic drugs. These molecular aggregates, known as nanocarriers, are larger and heavier than the molecular threshold that separates the gap between the TJs and the cell membrane pores. The likelihood of nanoparticles penetrating through the cell membrane or intercellular space directly is nearly non-existent. Many nanocarriers are engineered to undergo transepithelial transport in a self-regulated manner, such as briefly opening TJs and inducing membrane deformation. Based on this, Lamson et al reported that a negatively charged silica nanoparticle can indirectly cause TJ disruption by binding to integrins, thereby enhancing the paracellular delivery of protein drugs.67 Nevertheless, two primary pathways, the paracellular and transcellular pathways, are responsible for the movement of particulate matter throughout the GIT (Figure 2).
The TJ gap spans from 10 to 30 and 50 angstrom, implying that entities with molecular radii surpassing 151 angstrom (around 3.5 kDa) would be barred from accessing this absorption pathway.68 Paracellular transport’s passive mode necessitates that particles possess sufficient smallness or deformability to cross the intercellular space. Nanocarriers smaller than 5 nm are delivered to the basolateral blood or lymphatic capillaries beneath the GIT wall via paracellular pathways that follow concentration gradients. For example, 5 nm ionic gold nanoparticles have been found to preferentially penetrate epithelial Caco-2 cell monolayers via the paracellular pathway.69 Paracellular transport via endothelial leakage induced by nanoparticles has recently been demonstrated, which is likely to also occur within the intestinal epithelium.70 Additionally, the size distribution of artificial nanoparticles frequently exhibits inhomogeneity and polydispersity. A small proportion of ultrafine nanoparticles consistently exists within the system. These minute nanoparticles can be carried into the microcirculation via paracellular pathways. However, compared to overall uptake, the quantity transported is minimal. Moreover, by momentarily opening the TJ pathway, carefully engineered nanoparticles can pass through the intestinal epithelium and reach the underlying microcirculation.71–73 By mimicking the natural mechanism of pathogen interaction with intestinal epithelial cells, lectin-modified methacrylic acid-based NPs were used to encapsulate a small bacterial fragment, C-CPE, which was able to interact with the tight junction protein Claudin-4, leading to the opening of tight junctions.74 Although it is not nanoparticles’ primary means of transportation, they can evade lysis due to their inability to enter cells. Enzymatic digestion is advantageous for the realization of OTDDSs. The transcellular route through endocytosis remains the primary transport pathway for most nanocarriers. The uptake of oral nanoparticles primarily involves membrane mobile transport. When nanoparticles approach cells, the interactions between the particles and the cell membrane arise from various sources, resulting in the deformation of the membrane to enclose the nanoparticles, which are then taken up by the cell.75
Endocytic and Transcytotic Transport Pathways and Mechanisms of OTDDSs
Endocytosis is a cellular physiological process whereby cells absorb molecules via the phagocytosis of extracellular molecules through cell membrane movement.76 Particle uptake is significantly influenced by endocytosis, especially carrier-mediated endocytosis. Specifically, phagocytes—monocytes, dendritic cells, neutrophils, and macrophages—are the targets of phagocytosis. Intestinal immune cells carry out most phagocytosis that occurs in the intestine.77 The primary function of phagocytosis is to eliminate dead cells, pathogens, and cellular debris. However, its involvement in intestinal particle uptake remains unclear. Pinocytosis is present in almost all eukaryotic cells and serves as an essential method for capturing external fluids and soft particles.78 Due to its capacity to rapidly engulf large quantities of droplets, pinocytosis is also known as macropinocytosis. Pinocytosis is typically a non-selective method of uptake.79 In the case of liquids or soft particles, macropinocytosis serves as the primary endocytic pathway. It is now generally accepted that actin regulates the endocytic process of pinocytosis, which is not directly triggered by cargo or receptor molecules.80 Compared to other endocytic vesicles, macropinosomes are larger and lack cytoplasmic membranes. They are produced by the process of macropinocytosis. Their size and shape vary a lot, ranging from 0.5 to 10 mm. Consequently, pinocytosis plays a role in transporting large, delicate nanostructures in the GIT.81
The main mechanism of particle uptake is thought to be carrier-mediated endocytosis, as opposed to phagocytosis and macropinocytosis. However, its efficiency and speed are not yet fully understood. Certain proteins or receptors on the cell membrane enable the selective process known as carrier-mediated endocytosis.82 Utilizing specialized biological macromolecules, this form of transport is responsible for transporting particles into the cytoplasm. The two types of biological macromolecules are receptors and transporters. Different transmembrane proteins, called transporters, allow extracellular materials to be selectively transported across biological membranes.83 Receptors are biological macromolecules that respond to particular ligands. The carrier proteins located within the cell membrane typically assist in receptor-mediated endocytosis. During membrane transport, carrier proteins—typically self-assembling proteins—cover transport vesicles. They have the ability to assemble into polyhedral lattices that aid receptor-mediated endocytosis by organizing and sorting essential membrane proteins.84 A mechanism for the selective uptake of various endogenous and exogenous particles is provided by carrier-mediated endocytosis. After endocytosis, molecular aggregates or particles first bind to specific cell surface receptors, resulting in the formation of carrier protein-coated pits. Technical terms are clarified when they are initially used. These pits subsequently emerge from the membrane, generating small vesicles that possess the carrier and its ligands. The presently recognized carrier proteins contain clathrin, caveolin, RhoA, CDC42, flotillin, and ARF6.85 Among the proteins involved in carrier-mediated endocytosis, clathrin and caveolin have been identified as the key proteins responsible, for diverse endocytotic functions. These three main modes of carrier-mediated endocytosis are outlined below.
In mammalian cells, the main endocytic pathway is called clathrin-mediated endocytosis (CME). It regulates the recycling of transporters and transmembrane receptors to change the composition of the plasma membrane in response to external stimuli and to control cell surface signaling.86 Particles initially adhere to the cell membrane during clathrin-mediated endocytosis through non-specific electrostatic or hydrophobic interactions, or by recognizing specific receptors. This causes the cell membrane to invaginate and form endocytic pits.87 Extracellular particles are encapsulated in clathrin-coated vesicles at the neck of the pore through the fusion of cell membranes. After merging with early endosomes, the contents of these vesicles undergo sorting for either recycling back into the plasma membrane or transportation to lysosomes. Endocytic particles are predominantly degraded within lysosomes. However, there are occasional instances whereby these particles evade degradation and successfully exit the endocytosis/lysosome pathway, thus permitting their subcellular delivery.88
Particles are internalized via caveolae-mediated endocytosis (CvME) in the plasma membrane, leading to the creation of small, cup-shaped invaginations known as caveolae. These invaginations possess a unique caveolae protein shell, which measures 50–100 nm in diameter, and they are abundant in the lipid rafts of cholesterol and sphingolipids.89 They are involved in many different transport processes, such as endocytosis, and caveolin is regarded as one of the most significant membrane proteins because it helps move different cargoes.90,91 Studies have indicated that intestinal epithelial cells demonstrate significant activity in CvME.82,92 In contrast to CME, CvME has the potential to allow micro-/nano-particles to directly enter the cytoplasm, avoiding endo-/lyso-somal compartments.93 This could have a beneficial impact on targeted oral drug delivery. As a result of caveolae’s small size, the CvME internalization of large particles (>100 nm) is challenging. Research has indicated that the size of the particle alone (excluding ligands) determines its entry route.94 The CvME pathway establishes an approximate 60 nm limit that mainly internalizes the particle, with negligible entry via CME.95
In cells lacking clathrin and caveolin, endocytosis occurs in a manner that is independent of these proteins.96 In the absence of fluids and cytokines that are similar to clathrin and caveolin, cells take up various cargos via this pathway. This pathway requires specific lipid components, predominantly cholesterol. Because only a small number of particles are capable of inducing this type of endocytosis, the endocytic pathway is unique. Occasionally, bacteria and viruses hijack target particles and enter host cells through this pathway. Vesicles or pits of around 90 nm in diameter are formed through clathrin-/caveolin-independent endocytosis, with the internalized particles progressing into early and late endosomes.97 Folate-modified nanoparticles serve as a notable instance of internalization pathway employment via this mechanism.98
Transcytosis is a form of transcellular transportation utilized for larger molecules or particles. It serves as a mechanism for carrying cargo through the cell interior and into neighboring cells through a series of coordinated endocytosis and exocytosis.99 The cargo penetrates the membrane of the cell through endocytosis from one side and is subsequently transported to the opposing side through exocytosis. Epithelial cells, particularly secretory cells, are the most frequent sites of transcytosis. Transcytosis serves as a convenient means for pathogens to invade tissues and, thus, is essential for the transmembrane transportation of nanoparticles before reaching the portal vein. It is the main mechanism for transporting nanoparticles across cells among a variety of transcellular transport mechanisms. Despite the ability of nanoparticles, especially ligand-modified nanoparticles, to be internalized into cells, there exists an “easy entry but difficult transcytosis” phenomenon.100 For instance, researchers investigated intestinal mucins in a Caco-2/HT29 coculture cell model.101 Their goal was to promote endocytosis while limiting the transcytosis of nanoparticles across enterocytes. Notably, zwitterionic nanoparticles demonstrated significant basolateral exocytosis.102 However, in contrast to transcytosis, nanoparticles become trapped inside the cell and are unable to leave the cell and enter the bloodstream.
Fate of OTDDS in the GIT
OTDDS carriers are typically composed of micro-/nano-particles that feature functional ligands.103 By improving absorption and distribution, they can control the pharmacokinetics of administered drugs, which can lead to attenuation and synergy. Nonetheless, these carriers undergo a sequence of biological processes prior to entering the systemic circulation, which results in substantial uncertainty regarding their effectiveness in targeted oral delivery.104 To investigate the practicality of OTDDSs, an understanding of the mechanism of OTDDS vectors as they journey through the GIT and into the systemic circulation is crucial.
OTDDS carriers undergo immediate physiological and/or biochemical processing upon exposure to harsh gastrointestinal conditions. This processing may include depolymerization, digestion, degradation, and excretion in feces.105 During this process, some carriers appear as intact particles, while others undergo deformation prior to absorption. Depolymerization is associated with the disintegration of carriers (molecular aggregates). When nanoparticles, such as small-molecule micelles, self-assemble from amphiphiles with a high critical micelle concentration, this phenomenon is easily observed in physiological settings.106 The biotransformation of nanomaterials by gastric acid or gastrointestinal enzymes is known as “digestive degradation”. While degradation refers to the breaking down of large molecules that shorten the polymer’s molecular chain, digestion primarily refers to the breaking down of large particles into small particles or conversion into small molecular substances.107 In the discoid bodies of enterocytes, certain degradation products of lipid carriers can be reconstituted into novel nanostructures or form chylomicrons for subsequent transport.108,109 In addition to dissociation, digestion, and degradation, intestinal peristalsis is also responsible for the removal of certain particles from the body.
Previous information relates to the fate transition of foregut cells to absorptive epithelial cells in OTDDS vectors.110 Upon reaching the absorptive epithelium, trans-epithelial transport takes place alongside intestinal epithelial post-transformation, which leads to the further disposal of the OTDDS vector in the intracellular environment. When orally consumed, drug carriers may undergo digestion either in the lumen of the GIT or in the cytoplasm. The post-disposal of OTDDS vectors in intestinal epithelial cells mainly involves two aspects: apical exocytosis and lysosomal degradation through efflux pumps.111 The OTDDS carrier collapses as a result of intracellular digestion by lysosomes, blocking the particles’ ability to transcytose into capillaries intertwined with the intestinal endothelium.112 In addition, apical exocytosis increases the likelihood of rectal excretion and decreases vector internalization. The OTDDS carrier is extracted and eliminated by the liver after successfully crossing the intestinal epithelium and entering the portal vein, further reducing the risk of systemic circulation.113
After being orally administered, most OTDDS carriers undergo the biological processes mentioned above. OTDDS vectors generally follow a predictable path from the gastrointestinal lumen to the liver, although this can vary depending on the specific vector.114 While some OTDDS vectors are excreted or digested before reaching the portal vein, others can cross the oral barrier and enter the bloodstream. The efficacy of OTDDSs is determined by their ability to survive the liver clearance process, internalize into intestinal epithelial cells, penetrate the mucus layer on the intestinal epithelium, and remain intact during gastrointestinal transit.115 It is therefore crucial to investigate the ability of OTDDSs to perform these tasks.
The structural evolution of OTDDS vectors has a significant impact on oral administration. The OTDDS carrier will be exposed to the harsh environment of the GIT. Oral targeted delivery can have unpredictable consequences due to the potential for structural and morphological changes in OTDDS carriers during intestinal lumen and transmembrane transport.116 Premature drug release often occurs, even when nanocarriers remain intact in gastrointestinal fluids.117 Variations in transport routes also lead to variations in the biological disposal of the vehicle. This is particularly important given the high probability of lysosomal degradation during transcellular transport. The carrier may degrade or break down, causing the payload and ligand to detach from the carrier. It can be expected that, for environmentally and enzymatically unstable vectors, off-target effects will occur in vivo. It is imperative that the OTDDS delivery vehicle retains its structural integrity both before and after absorption to facilitate the flow of payload and ligands into the circulatory system.118
The destabilization of oral OTDDSs is facilitated by gastrointestinal pH, enzymes, and digestive aids (including bile salts, lecithin, and bicarbonate), as well as intracellular lysosomal enzymes.119,120 Fragile nanostructures may deteriorate or disintegrate during transport across membranes. The inefficacy of oral insulin utilizing different nanocarriers suggests that the stability of the nanostructures determines their systemic absorption. Furthermore, the stability and integrity of the OTDDS vector have a significant impact on how it enters the systemic circulation and subsequently affects biological processes. Any alteration to the microstructure of the vehicle makes it impossible to address the concept of “aiming”. Therefore, when designing OTDDSs, drug developers should focus on the structural evolution of the carrier both before and after intestinal absorption.
Oral Targeted Drug Delivery Strategies
For patients, oral administration is still the preferred route, with over two-thirds of clinically used drugs being delivered orally. The unique advantages of oral drug delivery, such as high compliance, low manufacturing costs, and low sterility requirements, make it ideal for chronic disease management and long-term medication.121 Therefore, researchers are increasingly focusing on developing OTDDSs. The hallmark of OTDDSs is their ability to target distant sites beyond the GIT. This allows therapeutic drugs to penetrate the biological barrier of the GIT and enter the systemic circulation, enabling them to concentrate on the desired target area.122 Nevertheless, administering nanomedicines orally and reducing off-target effects are complex tasks. The varying and distinct physiology of the entire GIT, including variable pH, digestive enzymes, mucin turnover, and efflux pumps, generates a formidable obstacle to nanocarriers entering the systemic circulation, confronting them with several limitations to overcome.25,123 It has been reported that over four-fifths of nanoparticles administered orally are not absorbed by the GIT.124 It is evident that OTDDSs still encounter numerous obstacles. For instance, the lipids and surfactants present in oral lipid drug carriers may act as substrates for gastrointestinal lipases. The levels of these enzymes, in conjunction with pH and bile secretion, are crucial parameters that determine the fate of lipid formulations and the dispersion, dissolution, and absorption of lipophilic drugs in the gastrointestinal tract. It is therefore essential to have a basic understanding of lipase, pH, and bile acid levels in vivo in order to develop relevant in vitro models.125 Furthermore, these parameters and their changes in healthy subjects are now well documented. However, in vivo data for specific populations (age groups, patients with various diseases, patients receiving treatments affecting gastrointestinal parameters, etc.) are rare, and obtaining these data from clinical studies is sometimes difficult due to ethical restrictions. Therefore, it is highly desirable to gain a better understanding of the biological fate, absorption, and transport properties of nanoparticles to expedite the rational design of OTDDSs.
Generally, OTDDSs refer to delivery systems that focus on gastrointestinal tissues located locally or remote tissues outside of the GIT. Colon-specific drug delivery systems are primarily included in the former category, as they are more concerned with particles’ local activities than their systemic absorption. For the purpose of this discussion, we concentrate on the systemic targeting outcomes of oral administration and do not elaborate further on the nanocarriers used. Several requirements must be met to achieve targeted oral drug delivery. Several biological barriers prevent nanotherapeutics from effectively entering the bloodstream and accumulating at diseased sites.7 The idea is to preserve the full composition of the drug delivery systems that can be designed after passing through the hepatic and gastrointestinal absorptive epithelium. Two necessary conditions are required to achieve oral targeted medication administration. Firstly, once the drug escapes hepatic extraction through the portal vein and the absorptive epithelium. OTDDS must maintain structural integrity during delivery to withstand both extracellular and intracellular biodegradation, ensuring the drug and ligand smoothly reach the tumor site.
In the latter scenario, the delivery system can undergo destruction within the gastrointestinal lumen. However, the resultant components can be reassembled into fresh and targeted nanostructures once the GIT has evolved. Qin et al reported a method to achieve specific release and activation of the prodrug 5-fluorocytosine (5-FC) in the tumor microenvironment by utilizing the tumor tropism of yeast and the extracellular hyaluronidase level. The enzyme cytosine deamination on the surface of yeast can catalyze the conversion of 5-FC into cytotoxic 5-fluorouracil (5-FU), thereby effectively inhibiting tumor growth and prolonging the survival of tumor-bearing mice.126 To maintain a stable low-energy state, molecules naturally organize themselves into stable structures, usually micro-/nano-particles, through the action of van der Waals forces, hydrogen bonding, hydrophobic effects, and electrostatic interactions.127 Even after digestion in the gastrointestinal tract, targeted drug carriers can still combine with ligand materials to form new nanostructures, regardless of whether endogenous chemicals such as bile salts are present.128,129 It has been demonstrated that self-assembled peptides with β-sheet motifs are capable of forming nanofiber structures. These structures are stabilized by hydrophobic packing in the core of the fiber and a network of hydrogen bonds along the long axis. By modulating electrostatic interactions between the peptide and the pH and salt composition of the solvent, the length of the nanofibers can be significantly extended, leading to fiber entanglement and the formation of hydrogels. Furthermore, the nanofibers can be customized with extensive modifications to enable the delivery of small molecules, proteins, and cells.130 These nanostructures include mixed micelles and vesicles, and they can emerge due to physicochemical interactions after remodeling during the evolution of the GIT. Once OTDDSs have surpassed gastrointestinal and hepatic barriers and entered the systemic circulation, they can act as intravascular vehicles for the targeted delivery of payloads to disease sites. To overcome the strong mucus and villus barrier, a polymeric micelle has been synthesized that can rapidly penetrate mucus and be absorbed by villi, effectively delivering paclitaxel to tumors. The therapeutic effect of this polymeric micelle on hepatocellular carcinoma and triple-negative breast cancer is even more pronounced than that of an intravenous polyethylene glycol-based micelle. The alcohol-free counterpart of PTX, or free PTX, is more effective.39
The development of effective oral cancer nanomedicines necessitates overcoming a series of physiological and anatomical obstacles, including those encountered along the gastrointestinal tract and mesenteric capillaries, as well as those within the tumor itself.8,131 The first barrier to nanomedicines reaching intestinal villus cells is the intestinal mucus on epithelial cells.132 Polyethylene glycol (PEG)-modified self-emulsifying drug delivery systems can improve the hydrophobicity of the carrier surface. When highly lipid-soluble drugs are administered orally, they can enhance the carrier’s mucus layer penetration and cellular internalization, effectively increasing intracellular drug concentrations, which provides a promising method for improving the bioavailability of oral drugs.133 It is now evident that a non-fouling surface, ie, not bound to any biomacromolecules in the intestinal mucus, is of paramount importance for nanomedicine penetration into the viscous mucus.134 For instance, nanoparticles coated with dense hydrophilic polymers, such as polyethylene glycol, are capable of rapidly penetrating physiological mucus secretions and are therefore designated as mucus-penetrating particles.135 However, such nanoparticles are invisible to cells, which renders them difficult to internalize, even in villous cells.40,136 A zwitterionic betaine polymer has recently been identified as a specific interactor with proton-assisted amino acid transporter 1 (PAT1), which is overexpressed in epithelial cells. This binding enables the deep penetration of intestinal mucus and efficient transepithelial absorption for insulin delivery.73
OTDDSs are different from vascular targeted drug delivery systems (VTDDSs), which are involved in the selective concentration and localization of drugs to specific sites, such as organs, tissues, cells, subcellular organelles, and structures. This is achieved through vascular delivery pathways utilizing carriers, ligands, or antibodies. In contrast, VTDDSs use functional vectors containing payloads that are delivered to target sites through systemic circulation via affinity and contact with specific cells.27 In the previous several decades, VTDDSs have made significant progress in the field of anti-tumor treatment via intravascular administration.137,138 However, intravenous drug administration is an invasive method that causes considerable inconvenience.
Similar to VTDDSs, OTDDSs require various nanocarriers, such as metal–organic hybrid nanocarriers, organic nanocarriers, and inorganic nanocarriers. Combined diagnostics and treatment frequently use inorganic carriers. Nanotubes, quantum dots, silicon/carbon/selenium nanoparticles, and gold nanoparticles are commonly used to provide targeted delivery. Due to their excellent biocompatibility and biodegradability, the nanocarriers commonly employed in drug delivery are primarily made of organic biological materials, such as cell-originated exosomes, which are used as carriers; liposomes; nanoemulsions; micelles; nanovesicles; nanogels; lipid nanoparticles; and polymer nanoparticles. It is imperative to use carriers that maintain the physiological environment of the body while delivering drugs to targeted tissues, which these organic nanocarriers provide. Metal–organic frameworks have also been adequately examined as hybrid nanoparticles for specific drug delivery.139 Zhou et al designed a pH-triggered self-unfolding capsule that encapsulates zwitterionic hydrogel-coated metal–organic framework (MOF) nanoparticles. MOF nanoparticles exhibit a high loading capacity for exendin-4, while the zwitterionic hydrogel layer confers unique transmucosal penetration capabilities and the effective internalization of nanocarriers by epithelial cells.42 The ligands utilized to modify nanocarriers may include vitamins, sugars, antibodies, aptamers, oligopeptides, biomimetic cell membranes, lectins, transferrin, and lactoferrin.140,141 OTDDSs with ligand-specific biomarkers can be abundantly expressed in lesions but less so in normal tissues.142 The influence of endogenous and exogenous factors, including pH, enzymes, light, temperature, and magnetism, enables OTDDSs to achieve the specific delivery and controlled release of their cargo.143,144 In order to achieve the systemic administration of OTDDSs, it is necessary to consider a number of obstacles, including digestion, absorption, and transportation. As long as the target particles remain in circulation for an extended period, they can be concentrated at the target site.
OTDDSs for Cancer Treatment
In contrast to the wide range of research applications for VTDDSs, there have been few advances in OTDDSs. Recent research suggests that the primary determinants of oral carrier absorption and transport are particle size, carrier type (material), and the mode of transport.145 OTDDSs are delivered by a variety of carriers, including liposomes, micro-/nano-emulsions, micelles, polymer/composite nanoparticles, quantum dots, and yeast microcapsules.146 These carriers can be classified into two groups: gastrointestinal digestible carriers and non-digestible carriers (although they biodegrade in the body). Bioimaging has strongly supported the explanation of the transmembrane transport of various vectors.147,148 It is clear that both digestive and dyspeptic vectors can be transported across intestinal epithelial cells as a group.149,150 However, the transport of intact nanoparticles depends on their size.151 Particles larger than 200 nm, such as those measuring 500, 550, 600, 1000, and 2000 nm, are unable to effectively cross the intestinal membrane and primarily attach to the villous surface.152,153 In addition, bioimaging has shown that most of the digestion of the digestive vehicle takes place in the GIT, especially for lipid-containing formulations.154–156 Only a small proportion of nanoparticles are able to withstand lipolysis in the GIT, traverse the intestinal epithelium, and migrate to the liver or systemic circulation. Transepithelial absorption is severely restricted for carriers that are poorly digested, such as silicon- and polymer-based nanoparticles, even those with particle sizes smaller than 200 nm.157,158 Fluorescence is a sign that smaller polymer carriers, such as micelles smaller than 50 nm, can be fully absorbed in the liver and blood.159 Nevertheless, the overall absorption of nanoparticles is relatively limited in comparison to oral dosages, especially when administered through the enterocyte pathway. Several oral nanomedicines currently in clinical trials are listed in Table 2.
 |
Table 2 Recent Clinical Trials on Nanoparticle Formulations for OTDDSs |
Polymers
Polylactic acid (PLA) and polylactic acid-co-glycolic acid (PLGA) offer excellent biocompatibility and resistance to the gastrointestinal environment.160 They have recently been shown to increase the stability of liposomal formulations and are widely used to stabilize oral pharmaceutical formulations, such as tablets and capsules.161 As such, they could improve the oral delivery properties of other materials. However, due to the high cost of processing, synthesis, and subsequent large-scale production, PLGA nanoparticles pose significant challenges for real-world applications.8 The commonly used polymer PLGA has been shown to effectively target the Na+-coupled organic cation/carnitine transporter 2 (OCTN2) expressed in the lumen of the small intestine, enhancing paclitaxel delivery (Figure 3A).31 Studies have demonstrated that the lymphatic system can absorb most PLA through pathway caveolin-mediated transport, indicating a high level of biocompatibility, safety, and sustained drug release capabilities.162 However, low gastrointestinal absorption and rapid elimination are evident when administered orally. Raloxifene hydrochloride that has been PEGylated and encapsulated in PLA nanoparticles can have enhanced bioavailability, and it has been demonstrated to be useful in treating breast cancer.34,163 Since the gastrointestinal epithelium is rich in folate receptors, the pharmacokinetic properties of hydrophobic chemotherapeutic drugs such as paclitaxel can be improved via the modification of PLA particles with folate (Figure 3B).164 Folic acid stimulates the internalization of particles by gastrointestinal epithelial cells and improves their diffusion within the mucus layer. In a previous study, D-alpha-tocopheryl polyethylene glycol (PEG) succinate was added to the delivery system, resulting in a decrease in the release rate of paclitaxel and an increase in the loading rate of the drug.36 The system’s safety and efficacy were successfully tested in a rat lung cancer model (Figure 3C).36 Polycaprolactone (PCL) is a polymer commonly utilized to create biocompatible nanoparticles for delivering various chemotherapeutic drugs. These drugs include docetaxel,165 cisplatin,166 methotrexate,166 and paclitaxel.167 Ellagic acid, an anticancer drug, has been shown to have improved oral bioavailability when delivered via PCL nanoparticles. Furthermore, the PCL nanoencapsulation of ellagic acid has been found to increase its hydrophilicity and uptake by M cells in the lymphatic system, resulting in more than a threefold increase in bioavailability.38
In recent years, Eudragit® polymers, synthesized from esters of acrylic and methacrylic acids, have demonstrated significant potential in developing pH-sensitive and innovative drug delivery systems capable of binding various therapeutic agents, including proteins, vitamins, hormones, vaccines, and genes. Utilizing Eudragit® EPO (EEP) as a carrier for curcumin can substantially enhance its serum concentration.168 Additionally, coating nanoparticles with Eudragit® S100 allows for pH-dependent drug solubility, increased gastrointestinal stability, and enhanced cellular uptake of protein drugs.169,170 Amorphous solid dispersions of Eudragit E neutralized with hydrochloric acid (Eudragit E/HCl) have been shown to optimize the solubility of trans-resveratrol, achieving an oral bioavailability of 40% in rat studies.171 Different grades of Eudragit® are tailored to target therapeutic agents to specific sites via oral delivery, such as stomach-specific and colon-specific delivery.172 Existing research underscores the unique ability of Eudragit® polymers to precisely target incorporated drugs to specific sites. Eudragit S100 (ES)-coated doxorubicin hydrochloride (DOX) nanoparticles are increasingly recognized for their precision in colon cancer treatment and reduced systemic distribution.173,174 Various nanoformulation technologies leveraging Eudragit® polymers enhance drug solubility, stability, and bioavailability. The application of Eudragit® polymers in drug formulation holds significant research value and broad potential, particularly in improving the oral absorption of poorly soluble drugs, developing colon-targeted drug delivery systems, and achieving controlled and targeted drug release.
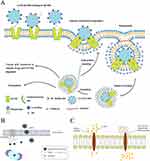 |
Figure 3 Therapeutic role of PLA and PLGA-loaded drugs in tumors. (A) LC-PLGA NPs and Na+ bind to the specific sites of OCTN2, and OCTN2 changes its confirmation from outward-facing to an occluded state, inducing the following membrane invagination and endocytosis. In this process, multipoint binding could increase the interaction and accelerate NP absorption. Additionally, Na+ is also essential in this process. Adapted with permission from Kou L, Yao Q, Sun M, et al. Cotransporting ion is a trigger for cellular endocytosis of transporter-targeting nanoparticles: a case study of high-efficiency SLC22A5 (OCTN2)-mediated carnitine-conjugated nanoparticles for oral delivery of therapeutic drugs. Adv Healthc Mater. 2017;6(17). Copyright © 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.31 (B) Schematic diagram of the possible inhibiting mechanism of the P-gp efflux transport of paclitaxel-loaded TPGS mixed polymersome. Reprinted from International Journal of BiologicalMacromolecules, 139, Xiao Qian Pan, Yan Chun Gong, ZiLing Li, Yu Ping Li, Xiang Yuan Xiong, Folate-conjugatedpluronic/polylactic acidpolymersomes for oral delivery ofpaclitaxel, 377-386, Copyright 2019, with permission from Elsevier.164 (C) Schematic illustration of PTX-TP-M inhibiting the efflux system and contributing to absorption. Adapted with permission from Hou J, Sun E, Zhang ZH, et al. Improved oral absorption and anti-lung cancer activity of paclitaxel-loaded mixed micelles. Drug Deliv. 2017;24(1):261–269. Copyright © 2017 Taylor & Francis.36 |
Inorganic Materials
Currently, oral drugs loaded with inorganic nanoparticles are more stable under the acidic conditions of the intestine, although select materials do completely dissolve at low pH values. Silica nanoparticles, which possess favorable biocompatibility, have undergone significant investigation as a means of improving the oral delivery of therapeutic drugs.175 These nanoparticles may be produced in a porous form to accommodate diverse payloads.176 Silica has been approved as a safe food and drug additive by the US FDA and European FSA.1 Its oral ingestion is deemed safe, although amorphous silica dissolves slowly at a low pH and quicker at a higher pH. Thus, exploiting GIT pH gradients with amorphous silica is a lucrative tool. Additionally, silica nanoparticles, porous and adjustable, can hold various payloads (Figure 4),175 including biologics, and they can protect them from digestive enzymes after encapsulation. Engineered mesoporous silica particles encapsulate biologics, safeguarding them from the digestive enzymes pancreatic alpha-amylase and lipase.177 Recently, there has been talk of using silica nanostructures to create floating drug delivery systems that allow for prolonged gastric retention in the presence of plant polymers and sodium bicarbonate.178 Utilizing similar technology based on aluminum silicate aims to enhance the delivery of the antineoplastic drug methotrexate, which has a particularly short half-life.179 Moreover, organometallic silicate nanocomposites decorated with gold nanoparticles (AC-Au), whose surface coating is pH-sensitive, can significantly enhance the oral delivery of methotrexate for colorectal cancer treatment.180 Additionally, selenium is another element that can be employed in oral nanomedicines due to its high anticancer potential.43 However, the clinical utilization of selenium is associated with notable adverse effects that restrict its application.44,181 Therefore, certain scholars have generated synthetic biological selenium nanoparticles via Bacillus licheniformis bacteria, which exhibit low toxicity.44
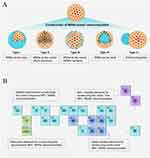 |
Figure 4 MSNs-based nanocomposites developed in the biomedical field. (A) Various nanostructured MSNs-based nanocomposites. Depending on the assembly process, the functional nanostructures can be introduced as the shell (Type I) or core (Type IV), can be loaded in the pore channels (Type II) or surface (Type III), and can form Janus-type hierarchical structure (Type V). (B) Typical elements used for constructing various types of MSNs-based nanocomposites. There are four main categories of nanocomposites based on the elemental type, including noble metal NPs/MSNs, metal compound NPs/MSNs, upconversion NPs/MSNs, and metal-free NPs/MSNs nanocomposites. MSNs: Mesoporous silica nanoparticles. Adapted with permission from Xu B, Li S, Shi R, Liu H. Multifunctional mesoporous silica nanoparticles for biomedical applications. Signal Transduct Target Ther. 2023;8(1):435. Copyright © 2023, The Author(s).175 |
Chitosan
Polysaccharides are amphiphilic molecules with the innate capability to enclose anti-tumor therapeutics and exhibit favorable wetting properties to overcome the mucus barrier. These molecules are obtained from various biological sources, such as chitosan from animals, alginates from algae, pectins from plants, and glucans from bacteria.182 They exhibit remarkable biocompatibility and are easy to formulate into nanoparticles, finding wide usage in multiple fields. Polysaccharide nanoparticles, such as other nanoformulations, have been found to inhibit efflux pump activity and can be selectively taken up by M cells in certain cases (Figure 5A).45,183 Chitosan is considered a benchmark biomaterial due to its ability to promote the relaxation of TJs and enhance paracellular uptake.180,184,185 This occurrence is most notable at lower pH levels, where chitosan becomes protonated, resulting in the destabilization of the linkage.186 However, chemical adjustments such as methylation can expand these traits and enhance chitosan’s potential to operate over a broader pH spectrum, increasing its solubility within.187 Because chitosan is partially soluble in water, hydrogel formulations can contain a variety of payloads, including biologics such as proteins and siRNA.47 The most promising material for oral nanomedicine may be chitosan because of its chemical modification and hybridization with other materials. Adding arginine and acrylonitrile groups to chitosan nanoparticles has been shown to increase the bioavailability of curcumin (Figure 5B).188 Acrylonitrile induces the self-assembly of nanoparticles and provides the hydrophobic structure needed to host hydrophobic therapeutics. By increasing solubility, promoting cell surface interactions, and prolonging the GIT residence time, arginine regulates drug release.188 Cyclodextrin, another polysaccharide widely utilized to develop oral nanomedicines, effectively encapsulates the hydrophobic drug docetaxel and can impede efflux pump activity.189 In a previous study, when given orally, cyclodextrin nanoparticles considerably enhanced the bioavailability of paclitaxel. The drug was observed in the bloodstream 24 hours after administration, demonstrating encouraging outcomes in the treatment of mouse sarcoma models.190,191 The therapeutic efficacy of celecoxib in the treatment of colorectal cancer has been demonstrated in clinical trials. Segale et al prepared a chitosan-coated NP microsphere of celecoxib, which can avoid the rapid release of celecoxib when encountering an acidic environment and enhance its release in the intestinal lumen.192 Furthermore, Sinha et al developed a novel biopolymer composite multi-system comprising chitosan, succinate, and alginate, which is employed to wrap capecitabine for the treatment of colorectal cancer. The study also examined the efficacy of the biopolymer composite multi-joint system in the treatment of rectal cancer. The results demonstrated that the multi-system exhibited the greatest degree of swelling in an intestinal environment with a pH of 7.4, with minimal swelling observed in an acidic environment. This results in the protection of the drug and the effective targeting of the colon.193 A layer-by-layer self-assembly approach was also employed to develop chitosan nanoparticles for oral delivery, with the objective of treating colorectal cancer.194 The layer-by-layer self-assembly method employed polycaprolactone (PCL, 95% w/w) as the substrate, which markedly enhanced the high loading efficiency of 5-fluorouracil (5-FU). Moreover, the outermost layer was functionalized with folic acid, thereby conferring selective tumor lesion binding and targeting capabilities. The findings demonstrated that 5-FU exhibited remarkable stability and a pronounced cytotoxic effect on colon cancer cell lines.
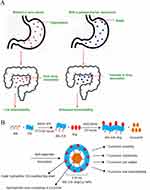 |
Figure 5 (A) Enhancement of drug absorption through polysaccharide nanocarriers. Reprinted from International Journal of Biological Macromolecules, 130, Madhumita Dey, Madhurima Das, Anindita Chowhan, TapanKumar Giri, Breaking the barricade of oral chemotherapy through polysaccharide nanocarrier, 34-49, Copyright 2019, with permission from Elsevier.183 (B) Schematic drawing of self-assembled cur-encapsulated AN–CS–Arg NPs. Adapted with permission from Raja MA, Zeenat S, Arif M, Liu C. Self-assembled nanoparticles based on amphiphilic chitosan derivative and arginine for oral curcumin delivery. Int J Nanomed. 2016;11:4397–4412. Copyright © 2016 Dove Medical Press.188 |
In order to enhance the pH-dependent properties of chitosan NPs, Zhang et al developed an automated oral insulin delivery system comprising ion attraction between polyglycolic acid (PGLA), chitosan, and alginate. This approach has been shown to enhance the in vitro efficacy of chitosan NPs against gastric acid erosion.195 Furthermore, studies have been conducted to enhance the stability of chitosan NPs in acidic environments via the addition of gelatin.196 Consequently, the exploration of more effective and non-toxic pH-stable materials represents a crucial avenue for the advancement of chitosan NPs as cancer-specific treatments. The resulting GIT presents a series of physical, chemical, and enzymatic obstacles that hinder oral drug delivery and stability. These obstacles are considered to be some of the major issues to overcome. At present, the application of chitosan NPs is still in the verification stage of in vitro and animal experiments. There is still a considerable distance to travel before chitosan NPs can be considered a viable option for oral therapy and intestinal disease treatment.
Protein
In addition, protein carriers made from proteins exhibit hydrophilic and lipophilic properties, rendering them suitable for accommodating drugs with various chemical and physical characteristics. Moreover, protein nanoparticles can traverse the M cells found in the GIT.48 Previous research has demonstrated that the disintegration of protein nanoparticles in the GIT can be prevented through the use of protease inhibitors.197 The methodology for producing protein nanoparticles influences their susceptibility to enzymatic degradation. Protein carriers generated by desolvation have a greater sensitivity to degradation by pepsin than particles synthesized via emulsification, potentially due to dissimilarities in the cleaved peptide bonds of pepsin.198 Furthermore, protein stability in the GIT may be improved by integrating proteins with other materials.199 For instance, the incorporation of the carbohydrate pectin can guard the particles while increasing curcumin loading efficiency when applied to casein/zein nanoformulations.52 The surface of the protein carrier allows for chemical modification at multiple sites, which promotes particle accumulation at the intestinal epithelial level.200 The protein carrier’s cellular specificity is also a notable feature. Interestingly, the accumulation of doxorubicin in liver cancer is effectively increased after the oral administration of nanoparticles composed of apotransferrin and lactoferrin. However, further examination is required to determine its absorption mechanism.53 Bovine casein nanoparticles can be efficiently loaded with resveratrol through hydrogen and hydrophobic bonds.201,202 Research has illustrated that resveratrol is exceptionally proficient at encasing casein nanoparticles, and these particles can be released in a regulated fashion in both gastrointestinal fluids.203 Moreover, this casein nanoparticle showcases superb interactions, which consequently lead to the enhanced bioavailability of resveratrol within the body.
Lipid nanoparticles improve the solubility of hydrophobic drugs when encapsulated.204 They are often mixed with hybrid formulations for oral delivery to improve their stability in the gastrointestinal tract. However, some research suggests that liposomes may interact with bile salts to form vesicles and micelles that are transcytosally absorbed in the upper gastrointestinal tract.205,206 Mixed lipid–polymer nanoparticles have been formulated to enhance the oral bioavailability of cabazitaxel, which is affected by the common low solubility and high metabolism problems of taxanes (Figure 6).55 The ε-caprolactone polymeric structure can safeguard it from an acidic environment, and triglyceride can improve the drug-loading capacity. Finally, surface modification with positively charged octadecyl amine and neutrally charged poly (ethylene oxide) improves mucosal penetration and cellular uptake. Due to the known lymphatic transport of cabazitaxel, M cells may be more likely to take up these particles. This technique has the potential to significantly increase the oral bioavailability and efficacy of the chemotherapy drug.55
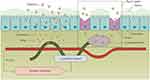 |
Figure 6 Polymer–lipid hybrid nanoparticles are able to efficiently orally deliver the anticancer drug cabazitaxel into the systemic circulation and can achieve the desired oral anticancer effect. Reprinted from Journal of Controlled Release, 269, Tianyang Ren, Qian Wang, Ying Xu, Lin Cong, Jingxin Gou, Xiaoguang Tao, Yu Zhang, Haibing He, Tian Yin, Haotian Zhang, Yan Zhang, Xing Tang, Enhanced oral absorption and anticancer efficacy of cabazitaxel by overcoming intestinal mucus and epithelium barriers using surface polyethylene oxide (PEO) decorated positively charged polymer-lipid hybrid nanoparticles, 423-438, Copyright 2018, with permission from Elsevier.55 |
Lipid
Furthermore, a study conducted by a team of researchers revealed that berberine, which has anti-tumor properties, can have improved oral bioavailability when administered using PEGylated PLGA-stabilized lipid nanoparticles.207 Although oral medications are advantageous for clinical purposes, the GIT can degrade the particles, leading to their failure.208 In a previous study, a hybrid lipid–PLGA system with PEGylation was developed to improve particulate drug encapsulation, stability, and interaction with the gastrointestinal epithelium.209 PEG surface modification is necessary to cross the mucus barrier, as demonstrated by the authors’ finding of increased in vivo intestinal absorption.207 Other research teams have also applied a coating of N-carboxymethyl chitosan onto solid lipid nanoparticles, which are known to enhance the solubility of hydrophobic drugs and steadily release payloads at intestinal pH levels.58,210 This process also assists in absorption through the lymphatic system, thereby circumventing first-pass metabolism by the liver.211 The payload is safeguarded in the stomach’s acidic environment and absorbed into the mesenteric lymph nodes thanks to the N-carboxymethyl chitosan coating.212 Short interfering RNA and long interfering RNA are biological agents that are well suited for oral administration but are sensitive to the aggressive gastric surroundings and are unable to cross the GIT epithelium.213 The lipid blend that makes up the particles includes amphiphilic lipids that can form complexes with RNA, cholesterol, DSPC, and PEGylated lipids, thereby increasing particle stability and the permeability of short interfering RNA through mucus.60 In a previous study, the authors discovered that the optimal concentration of PEG is crucial for short interfering RNA to effectively traverse the mucus barrier, confirming the significance of optimizing surface modification density.60 Although the in vitro delivery efficiency is high, pepsin and bile salts impact the stability of these particles, which leads to their aggregation and degradation, respectively. It is crucial to consider all factors that contribute to the gastrointestinal environment because pepsin, the protease, can still have an impact on the therapeutic effectiveness of lipid nanoparticles.60,214 The results emphasize the need for a comprehensive analysis of gastrointestinal conditions to ensure the success of lipid nanoparticle-based therapies. The concentration of pepsin in the stomach varies greatly in vivo before and after meals and decreases during fasting.60 It also readily accumulates in significant amounts in intestinal crypts, where the particles can transfer short interfering RNA to immune cells. This finding highlights the importance of the timing of the dose and diet in the management of gastrointestinal disorders.
Biologics and Others
In recent years, various biologics have been explored and applied for the treatment of cancer, including the delivery of small molecule drugs, nucleic acids, peptides, and proteins.215,216 Utilizing different nanoparticles, microparticles, hydrogels, or their combinations significantly enhances bioavailability and targeting specificity within the intestine. Biologic nanoparticles possess features such as robust absorption, immune cell stimulation, pH responsiveness, and attributes of biocompatibility, biodegradability, non-toxicity, selectivity, and specificity, marking them as exceptional carriers or ingredients for oral drug delivery.141,217,218 The self-assembly of nanoparticles based on lipid bilayers plays a pivotal role in biological systems.219 For instance, hydrogel materials that can mimic bacterial flagellar movement can be used to develop semi-intelligent microrobots akin to drug carriers.220 Additionally, hydrogel-based pH-responsive biologics can astutely release drugs in accordance with the pH conditions of living cells. The properties of hydrogels can be tailored to match the specific pH environment of the target site.221
There are multiple types of bacterial biologics employed in oral drug delivery systems, including bacterial ghosts, nanoparticle-enhanced bacteria, and recombinant bacteria.222–224 Bacterial ghosts are non-viable pseudobacterial constructs devoid of genetic material. Extracted from bacteriophages, they possess an innate capability to target immune cells and boost immunity for disease treatment. Examples include the use of Salmonella enteritidis and Vibrio cholera in the preparation of biologic vaccines.225,226 Similarly, live attenuated Salmonella bacteria have been coated with DNA-condensed cationic polymer nanoparticles to produce oral cancer vaccines (Figure 7A).32 The goal of the system is to deliver the immunogenic drug to the cytoplasm while avoiding phagosome entrapment and remaining stable at a low ph. E. coli or Vibrio cholera ghosts have been utilized to facilitate the delivery of therapeutic agents such as plasmid DNA, hepatitis B virus core protein, and doxorubicin drugs. In a notable development, Tang et al engineered a nanoparticle-coated, attenuated Salmonella vector for oral DNA vaccine delivery as a cancer treatment strategy.32 Similarly, Fan et al innovatively merged nanoparticle-based photothermal conversion with thermosensitive plasmids to create oral nanocarriers designed for anti-tumor drug delivery.227 These nanoparticle-enhanced bacterial systems are frequently referred to as microrobots. Surface-functionalized with nanoparticles and loaded with therapeutic drugs, microrobots are adept at targeted cellular delivery.218 Generally, this advanced delivery system facilitates the transport of proteins or genes to specific target sites and proves useful in deploying therapeutic drugs within hypoxic tumor regions. Additionally, bioinspired helical microrobots present potential for drug localization and diagnostic purposes, while biological hybrid vectors embedded with magnesium can adjust stomach pH levels. Nevertheless, the issues of bioavailability and toxicity related to these materials persist as significant concerns.
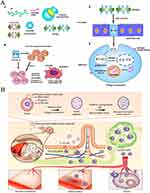 |
Figure 7 (A) Schematic illustration of cationic nanoparticle-coated attenuated salmonellae for improved antigen expression and tumor-targeting immune response activation. (1) Engineering of polyplex nanoparticle-coated Salmonellae. (2) Oral DNA vaccine delivery mediated by nanoparticle-coated Salmonellae. (3) Intracellular trafficking of nanoparticle-coated Salmonellae and antigen expression. (4) Activation of antitumor immune response. Adapted with permission from Hu Q, Wu M, Fang C, et al. Engineering nanoparticle-coated bacteria as oral DNA vaccines for cancer immunotherapy. Nano Lett. 2015;15(4):2732–2739. Copyright © 2015 American Chemical Society.32 (B) Schematic diagram of yeast capsule-mediated oral delivery of nanoparticles to inflammation-associated disease sites distant from the gastrointestinal tract. Adapted with permission fromZhou X, Zhang X, Han S, et al. Yeast microcapsule-mediated targeted delivery of diverse nanoparticles for imaging and therapy via the oral route. Nano Lett. 2017;17(2):1056–1064. Copyright © 2017 American Chemical Society.61 |
Viral mimic systems resemble viruses in structure, yet they differ in function or genetic attributes. The primary vectors employed in gene delivery include adenoviruses, retroviruses, and lentiviruses.228 These vectors possess capabilities such as replication, drug binding, cellular penetration, and system stability. According to Takamura et al, plasmid DNA can be effectively encapsulated within the hepatitis E virus in vitro, forming a biological agent that successfully targets the intestinal mucosa.229 Furthermore, virosomes, circular carriers, and monolayer/bilayer phospholipid membranes have been utilized for the oral delivery of therapeutic agents. The core cavity of the virosome encapsulates DNA, RNA, proteins, or pharmaceuticals. The viral exterior safeguards its contents from proteolytic degradation and the acidic environment of the stomach, effectively delivering therapeutic compounds into the cytoplasm while bypassing endosomal degradation. Nonetheless, the potential for immunogenicity restricts the application of virosomes in drug delivery systems. Strategic modification and employment of carriers offer promising prospects for future biomedical applications.230
Various derivatives extracted from yeast cells exhibit promising drug delivery attributes due to their size, morphology, drug loading efficiency, and targeting capabilities. These derivatives are apt for oral administration and M cell targeting; M cells, notably, lack microvilli and mucus layers, facilitating the entry of microparticles into the immune system. Zhou et al demonstrated a cisplatin-derived nanotransmitter administered orally via yeast microcapsules for tumor therapy.231 Post-oral administration, yeast microparticles traverse to the intestine and are channeled to the tumor site through M cells. The yeast’s outer layer and cytoplasm are chemically removed and substituted with therapeutic nanoparticles via electrostatic forces. M cells facilitate the movement of yeast-carrying nanoparticles to the lymphatic system, where macrophages identify and transport these particles to tumors, maintaining their potent anti-tumor effects (Figure 7B).61
Milk-derived exosomes have the potential to produce safe oral nanomedicines,33,49 as they form a part of the everyday diet, are easily obtainable, and remain stable at a low pH.57,232 In a previous study, it was found that, in vitro, milk exosomes demonstrated high efficacy and permitted the regulated delivery of the paclitaxel drug in gastric juice.37 In vivo, the encapsulated exosomes displayed substantial tumor eradication effects in a subcutaneous lung cancer model without any adverse reactions associated with the drug or carrier.233 A slight inhibition of tumor growth was observed upon exosome administration. This effect is possibly attributable to the presence of potentially anti-tumorous molecules, such as complex α-lactalbumin and oleic acid, derived from human milk, contained within the exosomes.234 The absorption mechanism remains unclear, although a prior report has demonstrated that the oral administration of exosomes can target several organs, notably the liver, spleen, kidneys, and pancreas.235 Research in this area is currently highly active, and a range of foods such as grapes may serve as feasible sources of exosomes, thereby broadening the selection of oral nanomedicine platforms.236
Cancer represents a significant global public health concern. The intricate immune microenvironment of malignant tumors renders single treatment methods, such as surgery, radiotherapy, and chemotherapy, inadequate for preventing tumor proliferation and recurrence. Nevertheless, nanotechnology can combine two or more therapeutic methods to achieve synergistic effects in cancer treatment. These include, but are not limited to, improving the solubility and local drug concentration of hydrophobic drugs, overcoming various biological barriers, prolonging the circulation of drug systems, and preventing rapid systemic clearance.237 Consequently, nanotechnology continues to be developed and used in cancer treatment. For instance, it can be employed to enhance the solubility and stability of drugs, as well as to improve the bioavailability and targeting of drugs. Nevertheless, oral nanomedicines also encounter certain limitations and challenges in the treatment of malignant tumors.
Firstly, one of the primary challenges faced by OTDDSs is the issue of biocompatibility. Given that OTDDSs must enter the body via the gastrointestinal tract, it is of paramount importance that they exhibit stability and biocompatibility in the gastric acid and gastrointestinal tract environment. Some nanomaterials may cause gastrointestinal irritation or toxic reactions, and they may even have adverse effects on intestinal flora, thereby affecting the absorption and efficacy of drugs. Furthermore, nanomedicines may induce adverse reactions or allergic reactions, thereby limiting their clinical applicability. Secondly, another challenge faced by OTDDSs is that of supervision. Due to the distinctive physicochemical properties of nanomedicines, their safety and effectiveness assessment methodologies must differ from those employed for traditional drugs. It is therefore incumbent upon regulatory agencies to formulate corresponding evaluation standards and regulatory policies to ensure the safety and effectiveness of OTDDSs. Finally, the clinical translation of OTDDSs represents another significant challenge. Currently, clinical research on OTDDSs is still in its infancy, and there is a paucity of large-scale clinical trial data to support its development. Furthermore, the preparation technology and production process of OTDDSs also require further improvement to ensure their stability and consistency in clinical applications. In conclusion, although OTDDSs have a wide range of potential applications, they still face significant challenges, including issues related to biocompatibility, regulatory, and clinical obstacles. It is recommended that future research and regulatory work be intensified to facilitate the development and application transformation of oral nanomedicines.
Future Perspectives
Oral drug delivery technology has advanced considerably, from basic tablets to advanced nanoparticle systems. This progress has been facilitated by an improved understanding of the intestinal barrier and potential access points to the systemic circulation through the portal vein and intestinal lymphatic vessels. The extensive literature pertaining to the delivery of small and large molecules across the intestinal barrier has only instilled confidence in unorthodox delivery methods. It is vital to acknowledge that, while delivery technology holds as much significance as the active pharmaceutical ingredient itself, a single technology is not applicable to all cases. To ensure a seamless transfer, it is crucial to strike a balance between innovation and concomitant risks. The main objective is to optimize the minimum effective therapeutic concentration through the use of delivery technology. It should be relatively easy to repurpose existing medication through unconventional oral delivery strategies, as we already possess the knowledge of pharmacology and safety profiles, which can significantly diminish the likelihood of attrition during clinical stage drug development. He et al reported the development of an oral polyphenol-armored nanomedicine coated with chitosan and tannic acid on the surface of the nanomedicine. This coating renders the nanomedicine resistant to the harsh environment of the gastrointestinal tract and enables targeted adherence to specific parts of the colon.238 Researchers believe that understanding the in vivo metabolism of nanomaterials is critical to understanding the safety and effectiveness (endpoints) of nanomedicines. By clarifying these processes, we can connect the carrier’s (structural design) features with the endpoints (efficacy and/or safety) of nanomedicines. Nanomedicine presents a burgeoning frontier for advancing oral drug delivery, and it currently offers a promising option for enhancing the delivery of biological agents and chemotherapy. The scientific community has identified chitosan, PLGA, and casein as the best starting materials for success in this field due to their numerous and affordable synthesis options. Current research highlights the benefits of nanomedicine in improving the bioavailability of drugs, but it ignores the possibility of carriers increasing the concentration of drugs at tumor sites. More research is needed to develop methods that overcome the limitations of gastrointestinal epithelial cells, facilitate drug release at their interface, and improve tumor targeting. Ma et al constructed a high-performance Pluronic F127 (P127)-modified gold shell (AuS)-polymer core nano-oral therapeutic drug loaded with curcumin (CUR) (P127-AuS@CUR). P127-AuS@CUR generates brief, mild photothermal effects under near-infrared irradiation, which facilitates nano-penetration of colonic mucus and facilitates cellular internalization, lysosomal escape, and controlled CUR release.239
OTDD treatment is a non-invasive treatment method that is more popular with patients and has lower time and economic costs. However, poor gastrointestinal barriers (gastric acid barrier and intestinal mucosal barrier) have a significant impact on the bioavailability of oral drugs. With the continuous advancement of nanotechnology, numerous researchers have discovered that, in the process of applying nanotechnology to treat and/or diagnose various diseases, engineered oral nanomedicines are capable of overcoming the gastric acid barrier and effectively crossing the intestinal mucus and epithelial barriers. This process improves the solubility, safety, targeting, and half-life of oral drugs and successfully reaches the delivery area. Consequently, the bioavailability of OTDDS is enhanced, the controlled release of OTDDSs is attained, and the residence time of OTDDSs in the lesion area is prolonged.
Despite the encouraging results that have been achieved thus far, there are still some unresolved issues in the practical applications of OTDDSs that require further attention. Firstly, the current primary method for enhancing the therapeutic efficacy of OTDDSs is to optimize their bioavailability through nanotechnology. However, there are a few notable instances where this approach has been employed to improve the overall performance of OTDDSs. Secondly, the majority of the current research on OTDDSs focuses on organs or tissues, with research on cells or organelles still in its infancy. Thirdly, there is a paucity of nanomaterial design and development strategies based on biomimetic engineering, including OTDDSs based on inorganic and organic nanoparticles. Fourthly, OTDDSs will undoubtedly bring great hope and convenience to the treatment of patients with various diseases in the future. However, they still face many inevitable challenges before clinical transformation and industrialization. For instance, current studies engineer bio-interface interactions (from crossing biological barriers to systemic clearance) between OTDDSs and human tissues by modulating some key physicochemical parameters, such as morphology, surface chemistry, and elasticity. However, biological barriers possess intrinsic dynamic systems that may lead to inaccurate analysis and characterization, thereby affecting the delivery fate of OTDDSs at the target site.240,241 Moreover, a significant number of experiments remain at the proof-of-concept stage in terms of the ambiguous biotoxicity and pharmacokinetics of OTDDSs in animals and humans.242
In recent years, nanocellulose has emerged as a versatile and sustainable nanomaterial. Analogous to dietary fibers, nanocellulose resists digestion in the human gastrointestinal tract, demonstrating substantial potential in the delivery of biological agents. Additionally, the significance of gut microbiota species cannot be overstated; integrating microbial informatics methods into drug development and clinical practice is anticipated to enhance drug delivery efficiency while mitigating adverse effects on the host and microbial communities. Similarly, a comprehensive understanding of the survival strategies employed by human-associated microbial communities in response to various carrier compounds is essential for the rational design of carrier-targeted microecological regulations of small-molecule drugs. Future research should elucidate the mechanistic basis of carrier-microbe-host interactions, investigate innovative carriers, and translate scientific discoveries into clinical applications. Moreover, pH-sensitive Eudragit polymers combined with other nanozymes offer notable advantages in protein drug delivery. Recently, 3D printing technology has also gained significant attention. 3D-printed carrier nanomedicines can integrate diagnosis, detection, and treatment, enabling multifunctional clinical applications. Some of the latest advancements suggest that smart nanorobots can serve as advanced therapeutic platforms, offering strong targeting capabilities in these intelligent carrier systems.
Finally, the repeatability and controllability of OTDDSs are unstable, and issues such as the manufacturing cost of nanomaterials, regulatory approvals from relevant departments, and the standardization of industrial production must be considered by researchers, experts, and technicians.242 The following suggestions are put forth for the advancement of OTDDSs: (1) Oral administration is a dosage form that is readily accepted by patients and does not unduly encumber their daily lives. Consequently, it is possible to identify a multitude of pharmaceutical resources from animals, plants, microorganisms, and other sources present in our daily diet. These can be utilized through nanotechnology to respond to common diseases through the diet, thereby reducing the economic cost of drugs and the time cost of treatment. It is recommended that treatments be administered simultaneously. Furthermore, OTDDSs are of significant importance for the early detection and screening of diseases. For instance, the use of artificial intelligence, such as nanorobots, or the design of new sophisticated nanomaterials with multiple functions, such as nuclear magnetic resonance and fluorescence imaging, can facilitate the elimination of human diseases in their initial stages through oral administration. (2) In order to achieve effective and real-time control of these diseases, the maximum dose and frequency of oral administration should not be too high. Consequently, in the context of animal models and preclinical trials, it is necessary to adapt the parameters of the nanomedicine system in order to optimize the effective dose and frequency of OTDDSs, thereby facilitating better compliance with treatment and greater comfort for patients. In conclusion, although there are still some issues and challenges to be overcome in the future development of OTDDS, there are also significant potential benefits and opportunities. Once these issues have been resolved, the potential applications of OTDDSs will be numerous and promising.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the National Natural Science Foundation of China (82302330, 82372067, 82072039), Postdoctoral Fellowship Program of CPSF under Grant Number BX20230068, and Research Personnel Cultivation Program of Zhongda Hospital Southeast University (CZXM-GSP-RC81).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Parodi A, Buzaeva P, Nigovora D, et al. Nanomedicine for increasing the oral bioavailability of cancer treatments. J Nanobiotechnology. 2021;19(1):354. doi:10.1186/s12951-021-01100-2
2. Wang D, Qiu G, Zhu X, et al. Macrophage-inherited exosome excise tumor immunosuppression to expedite immune-activated ferroptosis. J Immunother Cancer. 2023;11(5). doi:10.1136/jitc-2022-006516
3. Stielow M, Witczyńska A, Kubryń N, Fijałkowski Ł, Nowaczyk J, Nowaczyk A. The bioavailability of drugs-the current state of knowledge. Molecules. 2023;28(24). doi:10.3390/molecules28248038
4. Eii MN, Walpole S, Aldridge C. Sustainable practice: prescribing oral over intravenous medications. BMJ. 2023;383:e075297. doi:10.1136/bmj-2023-075297
5. Gao S, Bell EC, Zhang Y, Liang D. Racial disparity in drug disposition in the digestive tract. Int J Mol Sci. 2021;22(3). doi:10.3390/ijms22031038
6. Shreya AB, Raut SY, Managuli RS, Udupa N, Mutalik S. Active targeting of drugs and bioactive molecules via oral administration by ligand-conjugated lipidic nanocarriers: recent advances. AAPS Pharm Sci Tech. 2018;20(1):15. doi:10.1208/s12249-018-1262-2
7. Homayun B, Lin X, Choi HJ. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics. 2019;11(3). doi:10.3390/pharmaceutics11030129
8. El Moukhtari SH, Rodríguez-Nogales C, Blanco-Prieto MJ. Oral lipid nanomedicines: current status and future perspectives in cancer treatment. Adv Drug Deliv Rev. 2021;173:238–251. doi:10.1016/j.addr.2021.03.004
9. Lozoya-Agullo I, Araújo F, González-álvarez I, et al. PLGA nanoparticles are effective to control the colonic release and absorption on ibuprofen. Eur J Pharm Sci. 2018;115:119–125. doi:10.1016/j.ejps.2017.12.009
10. Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–951. doi:10.1038/nbt.3330
11. Huang P, Wang X, Liang X, et al. Nano-, micro-, and macroscale drug delivery systems for cancer immunotherapy. Acta Biomater. 2019;85:1–26. doi:10.1016/j.actbio.2018.12.028
12. Wang D, Nie T, Huang C, et al. Metal-cyclic dinucleotide nanomodulator-stimulated STING signaling for strengthened radioimmunotherapy of large tumor. Small. 2022;18(41):e2203227. doi:10.1002/smll.202203227
13. Ding Y, Wang Y, Hu Q. Recent advances in overcoming barriers to cell-based delivery systems for cancer immunotherapy. Exploration. 2022;2(3):20210106. doi:10.1002/exp.20210106
14. Traynor K. Targeted drug therapy remains a challenge. Am J Health Syst Pharm. 2011;68(24):2320–2324. doi:10.2146/news110084
15. Wang D, Zhou J, Fang W, et al. A multifunctional nanotheranostic agent potentiates erlotinib to EGFR wild-type non-small cell lung cancer. Bioact Mater. 2022;13:312–323. doi:10.1016/j.bioactmat.2021.10.046
16. Xu Y, Shrestha N, Préat V, Beloqui A. Overcoming the intestinal barrier: a look into targeting approaches for improved oral drug delivery systems. J Control Release. 2020;322:486–508. doi:10.1016/j.jconrel.2020.04.006
17. Chen C, Beloqui A, Xu Y. Oral nanomedicine biointeractions in the gastrointestinal tract in health and disease. Adv Drug Deliv Rev. 2023;203:115117. doi:10.1016/j.addr.2023.115117
18. Wang D, Zhang M, Zhang Y, et al. Intraparticle double-scattering-decoded sonogenetics for augmenting immune checkpoint blockade and CAR-T therapy. Adv Sci. 2022;9(32):e2203106. doi:10.1002/advs.202203106
19. Wang D, Zhang M, Qiu G, et al. Extracellular matrix viscosity reprogramming by in situ au bioreactor-boosted microwavegenetics disables tumor escape in CAR-T Immunotherapy. ACS Nano. 2023;17(6):5503–5516. doi:10.1021/acsnano.2c10845
20. Wang D, Feng C, Xiao Z, et al. Therapeutic hydrogel for enhanced immunotherapy: a powerful combination of MnO2 nanosheets and vascular disruption. Nano Today. 2022;47:101673. doi:10.1016/j.nantod.2022.101673
21. Wang D, Zhu X, Wang X, et al. Multichannel sonocatalysis amplifiers target IDH1-mutated tumor plasticity and attenuate ros tolerance to repress malignant cholangiocarcinoma. Adv. Funct. Mater. 2023;33(48):2303869. doi:10.1002/adfm.202303869
22. Wang Y, Zhao L, Dai Y, et al. Enantioselective oral absorption of molecular chiral mesoporous silica nanoparticles. Adv Mater. 2023:e2307900. doi:10.1002/adma.202307900
23. Su H, Wang Y, Liu S, et al. Emerging transporter-targeted nanoparticulate drug delivery systems. Acta Pharm Sin B. 2019;9(1):49–58. doi:10.1016/j.apsb.2018.10.005
24. He H, Lu Y, Qi J, Zhu Q, Chen Z, Wu W. Adapting liposomes for oral drug delivery. Acta Pharm Sin B. 2019;9(1):36–48. doi:10.1016/j.apsb.2018.06.005
25. Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13(9):655–672. doi:10.1038/nrd4363
26. Ma S, Wang L, Huang X, et al. Oral recombinant Lactobacillus vaccine targeting the intestinal microfold cells and dendritic cells for delivering the core neutralizing epitope of porcine epidemic diarrhea virus. Microb Cell Fact. 2018;17(1):20. doi:10.1186/s12934-018-0861-7
27. Ren Y, Wu W, Zhang X. The feasibility of oral targeted drug delivery: gut immune to particulates? Acta Pharm Sin B. 2023;13(6):2544–2558. doi:10.1016/j.apsb.2022.10.020
28. Choi HJ, Kim MC, Kang SM, Montemagno CD. The osmotic stress response of split influenza vaccine particles in an acidic environment. Arch Pharm Res. 2014;37(12):1607–1616. doi:10.1007/s12272-013-0257-5
29. O’Neill MJ, Bourre L, Melgar S, O’Driscoll CM. Intestinal delivery of non-viral gene therapeutics: physiological barriers and preclinical models. Drug Discov Today. 2011;16(5–6):203–218. doi:10.1016/j.drudis.2011.01.003
30. Valon L, Levayer R. Dying under pressure: cellular characterisation and in vivo functions of cell death induced by compaction. Biol Cell. 2019;111(3):51–66. doi:10.1111/boc.201800075
31. Kou L, Yao Q, Sun M, et al. Cotransporting ion is a trigger for cellular endocytosis of transporter-targeting nanoparticles: a case study of high-efficiency SLC22A5 (OCTN2)-mediated carnitine-conjugated nanoparticles for oral delivery of therapeutic drugs. Adv Healthc Mater. 2017;6(17). doi:10.1002/adhm.201700165
32. Hu Q, Wu M, Fang C, et al. Engineering nanoparticle-coated bacteria as oral DNA vaccines for cancer immunotherapy. Nano Lett. 2015;15(4):2732–2739. doi:10.1021/acs.nanolett.5b00570
33. Betker JL, Angle BM, Graner MW, Anchordoquy TJ. The potential of exosomes from cow milk for oral delivery. J Pharm Sci. 2019;108(4):1496–1505. doi:10.1016/j.xphs.2018.11.022
34. Provinciali N, Suen C, Dunn BK, DeCensi A. Raloxifene hydrochloride for breast cancer risk reduction in postmenopausal women. Expert Rev Clin Pharmacol. 2016;9(10):1263–1272. doi:10.1080/17512433.2016.1231575
35. Kala SG, Chinni S. Development of raloxifene hydrochloride loaded mPEG-PLA nanoparticles for oral delivery. Indian J Pharm Educ Res. 2021;2021:55.
36. Hou J, Sun E, Zhang ZH, et al. Improved oral absorption and anti-lung cancer activity of paclitaxel-loaded mixed micelles. Drug Deliv. 2017;24(1):261–269. doi:10.1080/10717544.2016.1245370
37. Agrawal AK, Aqil F, Jeyabalan J, et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine. 2017;13(5):1627–1636. doi:10.1016/j.nano.2017.03.001
38. Mady FM, Shaker MA. Enhanced anticancer activity and oral bioavailability of ellagic acid through encapsulation in biodegradable polymeric nanoparticles. Int J Nanomed. 2017;12:7405–7417. doi:10.2147/ijn.S147740
39. Fan W, Wei Q, Xiang J, et al. Mucus penetrating and cell-binding polyzwitterionic micelles as potent oral nanomedicine for cancer drug delivery. Adv Mater. 2022;34(16):e2109189. doi:10.1002/adma.202109189
40. Qin JJ, Wang W, Sarkar S, Zhang R. Oral delivery of anti-MDM2 inhibitor SP141-loaded FcRn-targeted nanoparticles to treat breast cancer and metastasis. J Control Release. 2016;237:101–114. doi:10.1016/j.jconrel.2016.07.008
41. Kolluru LP, Chandran T, Shastri PN, Rizvi SAA, D’Souza MJ. Development and evaluation of polycaprolactone based docetaxel nanoparticle formulation for targeted breast cancer therapy. J Nanopart Res. 2020;22(12):372. doi:10.1007/s11051-020-05096-y
42. Zhou Y, Chen Z, Zhao D, Li D, He C, Chen X. A pH-triggered self-unpacking capsule containing zwitterionic hydrogel-coated MOF nanoparticles for efficient oral exendin-4 delivery. Adv. Mater. 2021;33(32):2102044. doi:10.1002/adma.202102044
43. Tan HW, Mo HY, Lau ATY, Xu YM. Selenium Species: current Status and Potentials in Cancer Prevention and Therapy. Int J Mol Sci. 2018;20(1). doi:10.3390/ijms20010075
44. Sonkusre P. Specificity of biogenic selenium nanoparticles for prostate cancer therapy with reduced risk of toxicity: an in vitro and in vivo study. Front Oncol. 2019;9:1541. doi:10.3389/fonc.2019.01541
45. Mandracchia D, Trapani A, Tripodo G, et al. In vitro evaluation of glycol chitosan based formulations as oral delivery systems for efflux pump inhibition. Carbohydr Polym. 2017;166:73–82. doi:10.1016/j.carbpol.2017.02.096
46. Biswas S, Chattopadhyay M, Sen K, Saha MK, Maji H. Structure-toxicity relationship of chemically modified chitosan as an oral protein drug delivery carrier. Journal of Pharmaceutical Sciences and Pharmacology. 2014;1. doi:10.1166/jpsp.2014.1016
47. Li S, Zhang H, Chen K, et al. Application of chitosan/alginate nanoparticle in oral drug delivery systems: prospects and challenges. Drug Deliv. 2022;29(1):1142–1149. doi:10.1080/10717544.2022.2058646
48. Clark MA, Hirst BH, Jepson MA. Lectin-mediated mucosal delivery of drugs and microparticles. Adv Drug Deliv Rev. 2000;43(2–3):207–223. doi:10.1016/s0169-409x(00)00070-3
49. Pozo-Acebo L D, Hazas MLL, Tomé-Carneiro J, et al. Bovine milk-derived exosomes as a drug delivery vehicle for miRNA-based therapy. Int J Mol Sci. 2021;22(3). doi:10.3390/ijms22031105
50. Li X, Jafari SM, Zhou F, et al. The intracellular fate and transport mechanism of shape, size and rigidity varied nanocarriers for understanding their oral delivery efficiency. Biomaterials. 2023;294:121995. doi:10.1016/j.biomaterials.2023.121995
51. Wang Y, Zhao Y, Cui Y, et al. Overcoming multiple gastrointestinal barriers by bilayer modified hollow mesoporous silica nanocarriers. Acta Biomater. 2018;65:405–416. doi:10.1016/j.actbio.2017.10.025
52. Chang C, Wang T, Hu Q, Zhou M, Xue J, Luo Y. Pectin coating improves physicochemical properties of caseinate/zein nanoparticles as oral delivery vehicles for curcumin. Food Hydrocoll. 2017;70:143–151. doi:10.1016/j.foodhyd.2017.03.033
53. Golla K, Bhaskar C, Ahmed F, Kondapi AK. A target-specific oral formulation of Doxorubicin-protein nanoparticles: efficacy and safety in hepatocellular cancer. J Cancer. 2013;4(8):644–652. doi:10.7150/jca.7093
54. Planas JM, Alfaras I, Colom H, Juan ME. The bioavailability and distribution of trans-resveratrol are constrained by ABC transporters. Arch Biochem Biophys. 2012;527(2):67–73. doi:10.1016/j.abb.2012.06.004
55. Ren T, Wang Q, Xu Y, et al. Enhanced oral absorption and anticancer efficacy of cabazitaxel by overcoming intestinal mucus and epithelium barriers using surface polyethylene oxide (PEO) decorated positively charged polymer-lipid hybrid nanoparticles. J Control Release. 2018;269:423–438. doi:10.1016/j.jconrel.2017.11.015
56. Xing Y, Lian X, Zhang Y, Zhang Y, Guo X. Polymeric liposomes targeting dual transporters for highly efficient oral delivery of paclitaxel. Carbohydr Polym. 2024;334:121989. doi:10.1016/j.carbpol.2024.121989
57. Izumi H, Kosaka N, Shimizu T, Sekine K, Ochiya T, Takase M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J Dairy Sci. 2012;95(9):4831–4841. doi:10.3168/jds.2012-5489
58. Madan J, Pandey RS, Jain V, Katare OP, Chandra R, Katyal A. Poly (ethylene)-glycol conjugated solid lipid nanoparticles of noscapine improve biological half-life, brain delivery and efficacy in glioblastoma cells. Nanomedicine. 2013;9(4):492–503. doi:10.1016/j.nano.2012.10.003
59. Jellbauer S, Panthel K, Hetrodt JH, Rüssmann H. CD8 T-cell induction against vascular endothelial growth factor receptor 2 by Salmonella for vaccination purposes against a murine melanoma. PLoS One. 2012;7(4):e34214. doi:10.1371/journal.pone.0034214
60. Ball RL, Bajaj P, Whitehead KA. Oral delivery of siRNA lipid nanoparticles: fate in the GI tract. Sci Rep. 2018;8(1):2178. doi:10.1038/s41598-018-20632-6
61. Zhou X, Zhang X, Han S, et al. Yeast microcapsule-mediated targeted delivery of diverse nanoparticles for imaging and therapy via the oral route. Nano Lett. 2017;17(2):1056–1064. doi:10.1021/acs.nanolett.6b04523
62. Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv. Drug Delivery Rev. 2012;64(6):557–570. doi:10.1016/j.addr.2011.12.009
63. Wang D, Jiang Q, Dong Z, et al. Nanocarriers transport across the gastrointestinal barriers: the contribution to oral bioavailability via blood circulation and lymphatic pathway. Adv Drug Deliv Rev. 2023;203:115130. doi:10.1016/j.addr.2023.115130
64. Martínez-López AL, González-Navarro CJ, Aranaz P, Vizmanos JL, Irache JM. In vivo testing of mucus-permeating nanoparticles for oral insulin delivery using Caenorhabditis elegans as a model under hyperglycemic conditions. Acta Pharmaceutica Sinica B. 2021;11(4):989–1002. doi:10.1016/j.apsb.2021.02.020
65. Horowitz A, Chanez-Paredes SD, Haest X, Turner JR. Paracellular permeability and tight junction regulation in gut health and disease. Nat Rev Gastroenterol Hepatol. 2023;20(7):417–432. doi:10.1038/s41575-023-00766-3
66. Ju Y, Guo H, Edman M, Hamm-Alvarez SF. Application of advances in endocytosis and membrane trafficking to drug delivery. Adv. Drug Delivery Rev. 2020;157:118–141. doi:10.1016/j.addr.2020.07.026
67. Lamson NG, Berger A, Fein KC, Whitehead KA. Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat Biomed Eng. 2020;4(1):84–96. doi:10.1038/s41551-019-0465-5
68. Salama NN, Eddington ND, Fasano A. Tight junction modulation and its relationship to drug delivery. Adv. Drug Delivery Rev. 2006;58(1):15–28. doi:10.1016/j.addr.2006.01.003
69. Lin IC, Liang M, Liu T-Y, Monteiro MJ, Toth I. Cellular transport pathways of polymer coated gold nanoparticles. Nanomed Nanotechnol Biol Med. 2012;8(1):8–11. doi:10.1016/j.nano.2011.09.014
70. Lee M, Ni N, Tang H, et al. A framework of paracellular transport via nanoparticles-induced endothelial leakiness. Adv. Sci. 2021;8(21):2102519. doi:10.1002/advs.202102519
71. Jiang L, Li X, Liu L, Zhang Q. Thiolated chitosan-modified PLA-PCL-TPGS nanoparticles for oral chemotherapy of lung cancer. Nanoscale Res Lett. 2013;8(1):66. doi:10.1186/1556-276X-8-66
72. Twarog C, Fattah S, Heade J, Maher S, Fattal E, Brayden DJ. Intestinal permeation enhancers for oral delivery of macromolecules: a comparison between salcaprozate sodium (SNAC) and Sodium Caprate (C10). Pharmaceutics. 2019;11(2):78.
73. Han X, Lu Y, Xie J, et al. Zwitterionic micelles efficiently deliver oral insulin without opening tight junctions. Nature Nanotechnol. 2020;15(7):605–614. doi:10.1038/s41565-020-0693-6
74. Ramirez-Velez I, Namjoshi AA, Effiong UM, Peppas NA, Belardi B. Paracellular delivery of protein drugs with smart enteropatho nanoparticles. ACS Nano. 2024;18(32):21038–21051. doi:10.1021/acsnano.4c02116
75. Zhang S, Gao H, Bao G. Physical principles of nanoparticle cellular endocytosis. ACS Nano. 2015;9(9):8655–8671. doi:10.1021/acsnano.5b03184
76. Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77(3):759–803. doi:10.1152/physrev.1997.77.3.759
77. Snoeck V, Goddeeris B, Cox E. The role of enterocytes in the intestinal barrier function and antigen uptake. Microb Infect. 2005;7(7):997–1004. doi:10.1016/j.micinf.2005.04.003
78. King JS, Kay RR. The origins and evolution of macropinocytosis. Philos Trans R Soc B. 2019;374(1765):20180158. doi:10.1098/rstb.2018.0158
79. Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89(8):836–843. doi:10.1038/icb.2011.20
80. Kerr MC, Teasdale RD. Defining Macropinocytosis. Traffic. 2009;10(4):364–371. doi:10.1111/j.1600-0854.2009.00878.x
81. Ejazi SA, Louisthelmy R, Maisel K. Mechanisms of nanoparticle transport across intestinal tissue: an oral delivery perspective. ACS Nano. 2023;17(14):13044–13061. doi:10.1021/acsnano.3c02403
82. Yameen B, Choi WI, Vilos C, Swami A, Shi J, Farokhzad OC. Insight into nanoparticle cellular uptake and intracellular targeting. J Control Release. 2014;190:485–499. doi:10.1016/j.jconrel.2014.06.038
83. Diallinas G, Martzoukou O. Transporter membrane traffic and function: lessons from a mould. FEBS J. 2019;286(24):4861–4875. doi:10.1111/febs.15078
84. Sochacki KA, Dickey AM, Strub M-P, Taraska JW. Endocytic proteins are partitioned at the edge of the clathrin lattice in mammalian cells. Nat Cell Biol. 2017;19(4):352–361. doi:10.1038/ncb3498
85. Canton I, Battaglia G. Endocytosis at the nanoscale. Chem Soc Rev. 2012;41(7):2718–2739. doi:10.1039/c2cs15309b
86. Mettlen M, Chen P-H, Srinivasan S, Danuser G, Schmid SL. Regulation of clathrin-mediated endocytosis. Annu. Rev. Biochem. 2018;87(1):871–896. doi:10.1146/annurev-biochem-062917-012644
87. Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19(5):313–326. doi:10.1038/nrm.2017.132
88. Lu H, He S, Zhang Q, et al. Dual-sensitive dual-prodrug nanoparticles with light-controlled endo/lysosomal escape for synergistic photoactivated chemotherapy. 10.1039/D1BM01154E. Biomater. Sci. 2021;9(21):7115–7123. doi:10.1039/D1BM01154E
89. Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol. 2003;23(7):1161–1168. doi:10.1161/01.Atv.0000070546.16946.3a
90. Wang M, Zhang Y, Feng J, et al. Preparation, characterization, and in vitro and in vivo investigation of chitosan-coated poly (d,l-lactide-co-glycolide) nanoparticles for intestinal delivery of exendin-4. Int J Nanomed. 2013;8:1141–1154. doi:10.2147/ijn.S41457
91. Zhong X, Chen B, Yang Z. Nanocochleates as the potential delivery systems for oral antitumor of hydroxycamptothecin. J Biomed Nanotechnol. 2018;14(7):1339–1346. doi:10.1166/jbn.2018.2572
92. Ravindran S, Tambe JA, Suthar KJ, Chahar SD, Fernandes MJ, Desai V. Nanomedicine: bioavailability, Biotransformation and Biokinetics. Current Drug Metabolism. 2019;20(7):542–555. doi:10.2174/1389200220666190614150708
93. Behzadi S, Serpooshan V, Tao W, et al. Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev. 2017;46(14):4218–4244. doi:10.1039/c6cs00636a
94. Kou L, Sun J, Zhai Y, He Z. The endocytosis and intracellular fate of nanomedicines: implication for rational design. Asian J. Pharm. Sci. 2013;8(1):1–10. doi:10.1016/j.ajps.2013.07.001
95. Sousa de Almeida M, Susnik E, Drasler B, Taladriz-Blanco P, Petri-Fink A, Rothen-Rutishauser B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. 10.1039/D0CS01127D. Chem. Soc. Rev. 2021;50(9):5397–5434. doi:10.1039/D0CS01127D
96. Kirkham M, Fujita A, Chadda R, et al. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005;168(3):465–476. doi:10.1083/jcb.200407078
97. Guo X, Wei X, Chen Z, Zhang X, Yang G, Zhou S. Multifunctional nanoplatforms for subcellular delivery of drugs in cancer therapy. Pro Mater Sci. 2020;107:100599. doi:10.1016/j.pmatsci.2019.100599
98. Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145(3):182–195. doi:10.1016/j.jconrel.2010.01.036
99. Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83(3):871–932. doi:10.1152/physrev.00001.2003
100. Wu L, Bai Y, Liu M, et al. Transport mechanisms of butyrate modified nanoparticles: insight into “easy entry, hard transcytosis” of active targeting system in oral administration. Mol Pharmaceut. 2018;15(9):4273–4283. doi:10.1021/acs.molpharmaceut.8b00713
101. Yang D, Liu D, Qin M, et al. Intestinal mucin induces more endocytosis but less transcytosis of nanoparticles across enterocytes by triggering nanoclustering and strengthening the retrograde pathway. ACS Appl. Mater. Interfaces. 2018;10(14):11443–11456. doi:10.1021/acsami.7b19153
102. Zheng Y, Xing L, Chen L, et al. Tailored elasticity combined with biomimetic surface promotes nanoparticle transcytosis to overcome mucosal epithelial barrier. Biomaterials. 2020;262:120323. doi:10.1016/j.biomaterials.2020.120323
103. Favaro-Trindade CS, de Matos Junior FE, Okuro PK, et al. Encapsulation of active pharmaceutical ingredients in lipid micro/nanoparticles for oral administration by spray-cooling. Pharmaceutics. 2021;13(8). doi:10.3390/pharmaceutics13081186
104. Wu W, Li T, Zheng Y. Editorial of special issue “the biological fate of drug nanocarriers”. Acta Pharmaceutica Sinica B. 2021;11(4):850–851. doi:10.1016/j.apsb.2021.04.004
105. Qi M, Wang X, Chen J, et al. Transformation, absorption and toxicological mechanisms of silver nanoparticles in the gastrointestinal tract following oral exposure. ACS Nano. 2023;17(10):8851–8865. doi:10.1021/acsnano.3c00024
106. Lu Y, Zhang E, Yang J, Cao Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018;11(10):4985–4998. doi:10.1007/s12274-018-2152-3
107. Dima C, Assadpour E, Dima S, Jafari SM. Bioavailability of nutraceuticals: role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr Rev Food Sci Food Saf. 2020;19(3):954–994. doi:10.1111/1541-4337.12547
108. Chaudhary S, Garg T, Murthy RS, Rath G, Goyal AK. Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target. 2014;22(10):871–882. doi:10.3109/1061186x.2014.950664
109. Kim KS, Suzuki K, Cho H, Youn YS, Bae YH. Oral nanoparticles exhibit specific high-efficiency intestinal uptake and lymphatic transport. ACS Nano. 2018;12(9):8893–8900. doi:10.1021/acsnano.8b04315
110. Gossmann R, Fahrländer E, Hummel M, Mulac D, Brockmeyer J, Langer K. Comparative examination of adsorption of serum proteins on HSA- and PLGA-based nanoparticles using SDS-PAGE and LC-MS. Eur J Pharm Biopharm. 2015;93:80–87. doi:10.1016/j.ejpb.2015.03.021
111. Wong CY, Al-Salami H, Dass CR. Cellular assays and applied technologies for characterisation of orally administered protein nanoparticles: a systematic review. J Drug Targeting. 2020;28(6):585–599. doi:10.1080/1061186X.2020.1726356
112. Wang S, Li Z, Aispuro D, et al. Hydroxyl-rich hydrophilic endocytosis-promoting peptide with no positive charge. J Am Chem Soc. 2022;144(44):20288–20297. doi:10.1021/jacs.2c07420
113. Zhang Z, Ma L, Jiang S, et al. A self-assembled nanocarrier loading teniposide improves the oral delivery and drug concentration in tumor. J Control Release. 2013;166(1):30–37. doi:10.1016/j.jconrel.2012.12.018
114. Zhang G, Wang Q, Tao W, et al. Glucosylated nanoparticles for the oral delivery of antibiotics to the proximal small intestine protect mice from gut dysbiosis. Nat Biomed Eng. 2022;6(7):867–881. doi:10.1038/s41551-022-00903-4
115. Pattipeiluhu R, Arias-Alpizar G, Basha G, et al. Anionic lipid nanoparticles preferentially deliver mRNA to the Hepatic reticuloendothelial system. Adv Mater. 2022;34(16):e2201095. doi:10.1002/adma.202201095
116. Xiao B, Viennois E, Chen Q, et al. Silencing of intestinal glycoprotein CD98 by orally targeted nanoparticles enhances chemosensitization of colon cancer. ACS Nano. 2018;12(6):5253–5265. doi:10.1021/acsnano.7b08499
117. Zoya I, He H, Wang L, Qi J, Lu Y, Wu W. The intragastrointestinal fate of paclitaxel-loaded micelles: implications on oral drug delivery. Chin. Chem. Lett. 2021;32(4):1545–1549. doi:10.1016/j.cclet.2020.09.038
118. Truong-Le V, Lovalenti PM, Abdul-Fattah AM. Stabilization challenges and formulation strategies associated with oral biologic drug delivery systems. Adv. Drug Delivery Rev. 2015;93:95–108. doi:10.1016/j.addr.2015.08.001
119. Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obesity. 2002;26(3):S18–S24. doi:10.1038/sj.ijo.0802173
120. Lopes M, Simões S, Veiga F, Seiça R, Ribeiro A. Why most oral insulin formulations do not reach clinical trials. Ther Deliv. 2015;6(8):973–987. doi:10.4155/tde.15.47
121. Date AA, Hanes J, Ensign LM. Nanoparticles for oral delivery: design, evaluation and state-of-The-art. J Control Release. 2016;240:504–526. doi:10.1016/j.jconrel.2016.06.016
122. Sun M, Hu H, Sun L, Fan Z. The application of biomacromolecules to improve oral absorption by enhanced intestinal permeability: a mini-review. Chin Chem Lett. 2020;31(7):1729–1736. doi:10.1016/j.cclet.2020.02.035
123. Abuhelwa AY, Williams DB, Upton RN, Foster DJ. Food, gastrointestinal pH, and models of oral drug absorption. Eur J Pharm Biopharm. 2017;112:234–248. doi:10.1016/j.ejpb.2016.11.034
124. Lalatsa A, Garrett NL, Ferrarelli T, Moger J, Schätzlein AG, Uchegbu IF. Delivery of peptides to the blood and brain after oral uptake of quaternary ammonium palmitoyl glycol chitosan nanoparticles. Mol Pharmaceut. 2012;9(6):1764–1774. doi:10.1021/mp300068j
125. Amara S, Bourlieu C, Humbert L, Rainteau D, Carrière F. Variations in gastrointestinal lipases, pH and bile acid levels with food intake, age and diseases: possible impact on oral lipid-based drug delivery systems. Adv Drug Deliv Rev. 2019;142:3–15. doi:10.1016/j.addr.2019.03.005
126. Qin Y-T, Liu X, An J-X, et al. Oral saccharomyces cerevisiae -Guided enzyme prodrug therapy combined with immunotherapy for the treatment of orthotopic colorectal cancer. ACS Nano. 2024;18(34):23497–23507. doi:10.1021/acsnano.4c07115
127. Habibi N, Kamaly N, Memic A, Shafiee H. Self-assembled peptide-based nanostructures: smart nanomaterials toward targeted drug delivery. Nano Today. 2016;11(1):41–60. doi:10.1016/j.nantod.2016.02.004
128. Porter CJH, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–248. doi:10.1038/nrd2197
129. Williams HD, Ford L, Igonin A, et al. Unlocking the full potential of lipid-based formulations using lipophilic salt/ionic liquid forms. Adv Drug Deliv Rev. 2019;142:75–90. doi:10.1016/j.addr.2019.05.008
130. Moore AN, Hartgerink JD. Self-assembling multidomain peptide nanofibers for delivery of bioactive molecules and tissue regeneration. Acc Chem Res. 2017;50(4):714–722. doi:10.1021/acs.accounts.6b00553
131. Xiao Y, Tang Z, Wang J, et al. Oral insulin delivery platforms: strategies to address the biological barriers. Angew Chem Int Ed Engl. 2020;59(45):19787–19795. doi:10.1002/anie.202008879
132. Bansil R, Turner BS. The biology of mucus: composition, synthesis and organization. Adv Drug Deliv Rev. 2018;124:3–15. doi:10.1016/j.addr.2017.09.023
133. Sandmeier M, Hoeng J, Skov Jensen S, et al. Oral formulations for highly lipophilic drugs: impact of surface decoration on the efficacy of self-emulsifying drug delivery systems. J Colloid Interface Sci. 2024;677(Pt A):1108–1119. doi:10.1016/j.jcis.2024.07.233
134. Huckaby JT, Lai SK. PEGylation for enhancing nanoparticle diffusion in mucus. Adv Drug Deliv Rev. 2018;124:125–139. doi:10.1016/j.addr.2017.08.010
135. Huang X, Chisholm J, Zhuang J, et al. Protein nanocages that penetrate airway mucus and tumor tissue. Proc Natl Acad Sci U S A. 2017;114(32):E6595–e6602. doi:10.1073/pnas.1705407114
136. Hama S, Itakura S, Nakai M, et al. Overcoming the polyethylene glycol dilemma via pathological environment-sensitive change of the surface property of nanoparticles for cellular entry. J Control Release. 2015;206:67–74. doi:10.1016/j.jconrel.2015.03.011
137. Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9(1):1410. doi:10.1038/s41467-018-03705-y
138. Liu P, Gao C, Chen H, et al. Receptor-mediated targeted drug delivery systems for treatment of inflammatory bowel disease: opportunities and emerging strategies. Acta Pharmaceutica Sinica B. 2021;11(9):2798–2818. doi:10.1016/j.apsb.2020.11.003
139. He S, Wu L, Li X, et al. Metal-organic frameworks for advanced drug delivery. Acta Pharmaceutica Sinica B. 2021;11(8):2362–2395. doi:10.1016/j.apsb.2021.03.019
140. Zhang X, Wu W. Ligand-mediated active targeting for enhanced oral absorption. Drug Discovery Today. 2014;19(7):898–904. doi:10.1016/j.drudis.2014.03.001
141. Gagliardi M. Biomimetic and bioinspired nanoparticles for targeted drug delivery. Therapeutic Delivery. 2017;8(5):289–299. doi:10.4155/tde-2017-0013
142. Duan H, Liu Y, Gao Z, Huang W. Recent advances in drug delivery systems for targeting cancer stem cells. Acta Pharm Sin B. 2021;11(1):55–70. doi:10.1016/j.apsb.2020.09.016
143. Layek B, Mandal S. Natural polysaccharides for controlled delivery of oral therapeutics: a recent update. Carbohydr Polym. 2020;230:115617. doi:10.1016/j.carbpol.2019.115617
144. Zhang M, Gao S, Yang D, et al. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharmaceutica Sinica B. 2021;11(8):2265–2285. doi:10.1016/j.apsb.2021.03.033
145. Wang Y, Li H, Rasool A, Wang H, Manzoor R, Zhang G. Polymeric nanoparticles (PNPs) for oral delivery of insulin. J Nanobiotechnology. 2024;22(1):1. doi:10.1186/s12951-023-02253-y
146. Cao Y, Rewatkar P, Wang R, Hasnain SZ, Popat A, Kumeria T. Nanocarriers for oral delivery of biologics: small carriers for big payloads. Trends Pharmacol Sci. 2021;42(11):957–972. doi:10.1016/j.tips.2021.08.005
147. Zalmi GA, Jadhav RW, Mirgane HA, Bhosale SV. Recent advances in aggregation-induced emission active materials for sensing of biologically important molecules and drug delivery system. Molecules. 2022;27(1):150.
148. Zhang Z, Liu C, Lu Y, et al. In vivo fluorescence imaging of nanocarriers in near-infrared window II based on aggregation-caused quenching. J Nanobiotechnology. 2024;22(1):488. doi:10.1186/s12951-024-02761-5
149. Xia F, Fan W, Jiang S, et al. Size-dependent translocation of nanoemulsions via oral delivery. ACS Appl. Mater. Interfaces. 2017;9(26):21660–21672. doi:10.1021/acsami.7b04916
150. He H, Xie Y, Lv Y, et al. Bioimaging of intact polycaprolactone nanoparticles using aggregation-caused quenching probes: size-dependent translocation via oral delivery. Adv. Healthcare Mater. 2018;7(22):1800711. doi:10.1002/adhm.201800711
151. Hui Y, Yi X, Wibowo D, et al. Nanoparticle elasticity regulates phagocytosis and cancer cell uptake. Sci Adv. 2020;6(16):eaaz4316. doi:10.1126/sciadv.aaz4316
152. Wang R, Zhou L, Wang W, Li X, Zhang F. In vivo gastrointestinal drug-release monitoring through second near-infrared window fluorescent bioimaging with orally delivered microcarriers. Nat Commun. 2017;8:14702. doi:10.1038/ncomms14702
153. Des Rieux A, Pourcelle V, Cani PD, Marchand-Brynaert J, Préat V. Targeted nanoparticles with novel non-peptidic ligands for oral delivery. Adv Drug Deliv Rev. 2013;65(6):833–844. doi:10.1016/j.addr.2013.01.002
154. Pham AC, Nguyen T-H, Nowell CJ, Graham B, Boyd BJ. Examining the gastrointestinal transit of lipid-based liquid crystalline systems using whole-animal imaging. Drug Delivery Transl Res. 2015;5(6):566–574. doi:10.1007/s13346-015-0253-z
155. Xia F, Chen Z, Zhu Q, et al. Gastrointestinal lipolysis and trans-epithelial transport of SMEDDS via oral route. Acta Pharmaceutica Sinica B. 2021;11(4):1010–1020. doi:10.1016/j.apsb.2021.03.006
156. Qi J, Zhuang J, Lu Y, Dong X, Zhao W, Wu W. In vivo fate of lipid-based nanoparticles. Drug Discovery Today. 2017;22(1):166–172. doi:10.1016/j.drudis.2016.09.024
157. Kou L, Sun R, Xiao S, et al. OCTN2-targeted nanoparticles for oral delivery of paclitaxel: differential impact of the polyethylene glycol linker size on drug delivery in vitro, in situ, and in vivo. Drug Delivery. 2020;27(1):170–179. doi:10.1080/10717544.2019.1710623
158. Skotland T, Iversen TG, Llorente A, Sandvig K. Biodistribution, pharmacokinetics and excretion studies of intravenously injected nanoparticles and extracellular vesicles: possibilities and challenges. Adv Drug Deliv Rev. 2022;186:114326. doi:10.1016/j.addr.2022.114326
159. He H, Wang L, Ma Y, et al. The biological fate of orally administered mPEG-PDLLA polymeric micelles. J Control Release. 2020;327:725–736. doi:10.1016/j.jconrel.2020.09.024
160. Ranakoti L, Gangil B, Bhandari P, et al. Promising role of polylactic acid as an ingenious biomaterial in scaffolds, drug delivery, tissue engineering, and medical implants: research developments, and prospective applications. Molecules. 2023;28(2). doi:10.3390/molecules28020485
161. Fluksman A, Lafuente A, Braunstein R, et al. Modular drug-loaded nanocapsules with metal dome layers as a platform for obtaining synergistic therapeutic biological activities. ACS Appl Mater Interfaces. 2023;15(43):50330–50343. doi:10.1021/acsami.3c07188
162. Uhl P, Grundmann C, Sauter M, et al. Coating of PLA-nanoparticles with cyclic, arginine-rich cell penetrating peptides enables oral delivery of liraglutide. Nanomedicine. 2020;24:102132. doi:10.1016/j.nano.2019.102132
163. Kala SG, Chinni S. Development and characterization of venetoclax nanocrystals for oral bioavailability enhancement. AAPS Pharm Sci Tech. 2021;22(3):92. doi:10.1208/s12249-021-01968-1
164. Pan XQ, Gong YC, Li ZL, Li YP, Xiong XY. Folate-conjugated pluronic/polylactic acid polymersomes for oral delivery of paclitaxel. Int J Biol Macromol. 2019;139:377–386. doi:10.1016/j.ijbiomac.2019.07.224
165. Varan C, Bilensoy E. Cationic PEGylated polycaprolactone nanoparticles carrying post-operation docetaxel for glioma treatment. Beilstein J Nanotechnol. 2017;8:1446–1456. doi:10.3762/bjnano.8.144
166. Akbari E, Mousazadeh H, Hanifehpour Y, et al. Co-loading of cisplatin and methotrexate in nanoparticle-based PCL-PEG system enhances lung cancer chemotherapy effects. Journal of Cluster Science. 2022;33(4):1751–1762. doi:10.1007/s10876-021-02101-9
167. Witt S, Scheper T, Walter JG. Production of polycaprolactone nanoparticles with hydrodynamic diameters below 100 nm. Eng Life Sci. 2019;19(10):658–665. doi:10.1002/elsc.201800214
168. Zhao Y, Xu X, Dai A, Jia Y, Wang W. Enhanced dissolution and bioavailability of curcumin nanocrystals prepared by hot melt extrusion technology. Int J Nanomed. 2024;19:5721–5737. doi:10.2147/ijn.S463918
169. Kim GL, Song JG, Han HK. Enhanced oral efficacy of semaglutide via an ionic nanocomplex with organometallic phyllosilicate in type 2 diabetic rats. Pharmaceutics. 2024;16(7). doi:10.3390/pharmaceutics16070886
170. Lee SH, Back SY, Song JG, Han HK. Enhanced oral delivery of insulin via the colon-targeted nanocomposite system of organoclay/glycol chitosan/Eudragit(®)S100. J Nanobiotechnology. 2020;18(1):104. doi:10.1186/s12951-020-00662-x
171. Ha ES, Choi DH, Baek IH, Park H, Kim MS. Enhanced oral bioavailability of resveratrol by using neutralized eudragit e solid dispersion prepared via spray drying. Antioxidants. 2021;10(1). doi:10.3390/antiox10010090
172. Andrés Real D, Gagliano A, Sonsini N, et al. Design and optimization of pH-sensitive Eudragit nanoparticles for improved oral delivery of triclabendazole. Int J Pharm. 2022;617:121594. doi:10.1016/j.ijpharm.2022.121594
173. Su Y, Pan H, Wang J, Liu D, Pan W. Eudragit S100 coated nanodiamond-based nanoparticles as an oral chemo-photothermal delivery system for local treatment of colon cancer. Colloids Surf B Biointerfaces. 2024;237:113849. doi:10.1016/j.colsurfb.2024.113849
174. Wathoni N, Nguyen AN, Rusdin A, et al. Enteric-coated strategies in colorectal cancer nanoparticle drug delivery system. Drug Des Devel Ther. 2020;14:4387–4405. doi:10.2147/dddt.S273612
175. Xu B, Li S, Shi R, Liu H. Multifunctional mesoporous silica nanoparticles for biomedical applications. Signal Transduct Target Ther. 2023;8(1):435. doi:10.1038/s41392-023-01654-7
176. Wu H, Li CS, Tang XR, et al. Impact of calcium ions at physiological concentrations on the adsorption behavior of proteins on silica nanoparticles. J Colloid Interface Sci. 2023;656:35–46. doi:10.1016/j.jcis.2023.11.058
177. Waara ER, Iqbal MN, Robert-Nicoud G, et al. Entrapping digestive enzymes with engineered mesoporous silica particles reduces metabolic risk factors in humans. Adv Healthc Mater. 2020;9(11):e2000057. doi:10.1002/adhm.202000057
178. Abbaraju PL, Meka AK, Jambhrunkar S, et al. Floating tablets from mesoporous silica nanoparticles. J Mater Chem B. 2014;2(47):8298–8302. doi:10.1039/c4tb01337a
179. Carino IS, Pasqua L, Testa F, et al. Silica-based mesoporous materials as drug delivery system for methotrexate release. Drug Deliv. 2007;14(8):491–495. doi:10.1080/10717540701606244
180. Bajracharya R, Baral KC, Lee SH, Song JG, Han HK. Organometallic phyllosilicate-gold nanocomplex: an effective oral delivery system of methotrexate for enhanced in vivo efficacy against colorectal cancer. Int J Nanomed. 2023;18:7257–7266. doi:10.2147/ijn.S437860
181. Aldosary BM, Sutter ME, Schwartz M, Morgan BW. Case series of selenium toxicity from a nutritional supplement. Clin Toxicol. 2012;50(1):57–64. doi:10.3109/15563650.2011.641560
182. Zhu Y, Li H, Peng C, et al. Application of protein/polysaccharide aerogels in drug delivery system: a review. Int J Biol Macromol. 2023;247:125727. doi:10.1016/j.ijbiomac.2023.125727
183. Dey M, Das M, Chowhan A, Giri TK. Breaking the barricade of oral chemotherapy through polysaccharide nanocarrier. Int J Biol Macromol. 2019;130:34–49. doi:10.1016/j.ijbiomac.2019.02.094
184. Hong SC, Yoo SY, Kim H, Lee J. Chitosan-based multifunctional platforms for local delivery of therapeutics. Mar Drugs. 2017;15(3). doi:10.3390/md15030060
185. Xiong W, Xiong SH, Chen QL, et al. Brij-functionalized chitosan nanocarrier system enhances the intestinal permeability of P-glycoprotein substrate-like drugs. Carbohydr Polym. 2021;266:118112. doi:10.1016/j.carbpol.2021.118112
186. Rezaei N, Zarkesh I, Fotouhi A, Alikhani HK, Hassan M, Vosough M. Chitosan-coated nanoparticles in innovative cancer bio-medicine. Drug Dev Res. 2024;85(3):e22189. doi:10.1002/ddr.22189
187. El-Meligy MA, Abd El-Monaem EM, Eltaweil AS, et al. Recent advancements in metallic au- and ag-based chitosan nanocomposite derivatives for enhanced anticancer drug delivery. Molecules. 2024;29(10). doi:10.3390/molecules29102393
188. Raja MA, Zeenat S, Arif M, Liu C. Self-assembled nanoparticles based on amphiphilic chitosan derivative and arginine for oral curcumin delivery. Int J Nanomed. 2016;11:4397–4412. doi:10.2147/ijn.S106116
189. Zhang L, Shen Y, Qiu L. Loading docetaxel in β-cyclodextrin-based micelles for enhanced oral chemotherapy through inhibition of P-glycoprotein mediated efflux transport. RSC Adv. 2017;7(42):26161–26169. doi:10.1039/C7RA03180G
190. Zheng X, Fang Z, Huang W, et al. Ionic co-aggregates (ICAs) based oral drug delivery: solubilization and permeability improvement. Acta Pharm Sin B. 2022;12(10):3972–3985. doi:10.1016/j.apsb.2022.04.011
191. Nasr M, Hashem F, Teiama M, Tantawy N, Abdelmoniem R. Folic acid grafted mixed polymeric micelles as a targeted delivery strategy for tamoxifen citrate in treatment of breast cancer. Drug Deliv Transl Res. 2024;14(4):945–958. doi:10.1007/s13346-023-01443-3
192. Sanchez-Ballester NM, Soulairol I, Bataille B, Sharkawi T. Flexible heteroionic calcium-magnesium alginate beads for controlled drug release. Carbohydr Polym. 2019;207:224–229. doi:10.1016/j.carbpol.2018.11.096
193. Sinha P, Udhumansha U, Rathnam G, Ganesh M, Jang HT. Capecitabine encapsulated chitosan succinate-sodium alginate macromolecular complex beads for colon cancer targeted delivery: in vitro evaluation. Int J Biol Macromol. 2018;117:840–850. doi:10.1016/j.ijbiomac.2018.05.181
194. Janardhanam LSL, Indukuri VV, Verma P, Dusane AC, Venuganti VVK. Functionalized layer-by-layer assembled film with directional 5-fluorouracil release to target colon cancer. Mater Sci Eng C Mater Biol Appl. 2020;115:111118. doi:10.1016/j.msec.2020.111118
195. Zhang L, Qin H, Li J, et al. Preparation and characterization of layer-by-layer hypoglycemic nanoparticles with pH-sensitivity for oral insulin delivery. J Mater Chem B. 2018;6(45):7451–7461. doi:10.1039/c8tb02113a
196. Ceylan O, Karakus H, Cicek H. Design and in vitro antibiofilm activity of propolis diffusion-controlled biopolymers. Biotechnol Appl Biochem. 2021;68(4):789–800. doi:10.1002/bab.1991
197. McGinn BJ, Morrison JD. Investigations into the absorption of insulin and insulin derivatives from the small intestine of the anaesthetised rat. J Control Release. 2016;232:120–130. doi:10.1016/j.jconrel.2016.04.002
198. Tan YL, Ho HK. Navigating albumin-based nanoparticles through various drug delivery routes. Drug Discov Today. 2018;23(5):1108–1114. doi:10.1016/j.drudis.2018.01.051
199. Morsbach S, Gonella G, Mailänder V, et al. Engineering proteins at interfaces: from complementary characterization to material surfaces with designed functions. Angew Chem Int Ed Engl. 2018;57(39):12626–12648. doi:10.1002/anie.201712448
200. Gueta O, Amiram M. Expanding the chemical repertoire of protein-based polymers for drug-delivery applications. Adv Drug Deliv Rev. 2022;190:114460. doi:10.1016/j.addr.2022.114460
201. Yin X, Cheng H, Wusigale dong H, Huang W, Liang L. Resveratrol stabilization and loss by sodium caseinate, whey and soy protein isolates: loading, antioxidant activity, oxidability. Antioxidants. 2022;11(4). doi:10.3390/antiox11040647
202. Acipreste Hudson E, de Paula HM C, Coelho YL, et al. The kinetics of formation of resveratrol-β-cyclodextrin-NH(2) and resveratrol analog-β-cyclodextrin-NH(2) supramolecular complexes. Food Chem. 2022;366:130612. doi:10.1016/j.foodchem.2021.130612
203. Peñalva R, Morales J, González-Navarro CJ, et al. Increased oral bioavailability of resveratrol by its encapsulation in casein nanoparticles. Int J Mol Sci. 2018;19(9). doi:10.3390/ijms19092816
204. Ban C, Jo M, Lim S, Choi YJ. Control of the gastrointestinal digestion of solid lipid nanoparticles using PEGylated emulsifiers. Food Chem. 2018;239:442–452. doi:10.1016/j.foodchem.2017.06.137
205. Wang T, Luo Y. Biological fate of ingested lipid-based nanoparticles: current understanding and future directions. Nanoscale. 2019;11(23):11048–11063. doi:10.1039/c9nr03025e
206. Walther B, Lett AM, Bordoni A, et al. GutSelf: interindividual variability in the processing of dietary compounds by the human gastrointestinal tract. Mol Nutr Food Res. 2019;63(21):e1900677. doi:10.1002/mnfr.201900677
207. Yu F, Ao M, Zheng X, et al. PEG-lipid-PLGA hybrid nanoparticles loaded with berberine-phospholipid complex to facilitate the oral delivery efficiency. Drug Deliv. 2017;24(1):825–833. doi:10.1080/10717544.2017.1321062
208. Pund S, Borade G, Rasve G. Improvement of anti-inflammatory and anti-angiogenic activity of berberine by novel rapid dissolving nanoemulsifying technique. Phytomedicine. 2014;21(3):307–314. doi:10.1016/j.phymed.2013.09.013
209. Hu Y, Hoerle R, Ehrich M, Zhang C. Engineering the lipid layer of lipid-PLGA hybrid nanoparticles for enhanced in vitro cellular uptake and improved stability. Acta Biomater. 2015;28:149–159. doi:10.1016/j.actbio.2015.09.032
210. Venishetty VK, Chede R, Komuravelli R, Adepu L, Sistla R, Diwan PV. Design and evaluation of polymer coated carvedilol loaded solid lipid nanoparticles to improve the oral bioavailability: a novel strategy to avoid intraduodenal administration. Colloids Surf B Biointerfaces. 2012;95:1–9. doi:10.1016/j.colsurfb.2012.01.001
211. Managuli RS, Raut SY, Reddy MS, Mutalik S. Targeting the intestinal lymphatic system: a versatile path for enhanced oral bioavailability of drugs. Expert Opin Drug Deliv. 2018;15(8):787–804. doi:10.1080/17425247.2018.1503249
212. Baek JS, Cho CW. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur J Pharm Biopharm. 2017;117:132–140. doi:10.1016/j.ejpb.2017.04.013
213. Han L, Tang C, Yin C. Oral delivery of shRNA and siRNA via multifunctional polymeric nanoparticles for synergistic cancer therapy. Biomaterials. 2014;35(15):4589–4600. doi:10.1016/j.biomaterials.2014.02.027
214. Madureira AR, Campos DA, Oliveira A, Sarmento B, Pintado MM, Gomes AM. Insights into the protective role of solid lipid nanoparticles on rosmarinic acid bioactivity during exposure to simulated gastrointestinal conditions. Colloids Surf B Biointerfaces. 2016;139:277–284. doi:10.1016/j.colsurfb.2015.11.039
215. Hu X, Yang G, Chen S, Luo S, Zhang J. Biomimetic and bioinspired strategies for oral drug delivery. Biomater Sci. 2020;8(4):1020–1044. doi:10.1039/c9bm01378d
216. Chen Z, Wang Z, Gu Z. Bioinspired and biomimetic nanomedicines. Acc Chem Res. 2019;52(5):1255–1264. doi:10.1021/acs.accounts.9b00079
217. Wang L, Liu J. Dopamine polymerization-mediated surface functionalization toward advanced bacterial therapeutics. Acc Chem Res. 2024;57(6):945–956. doi:10.1021/acs.accounts.3c00798
218. Sabu C, Rejo C, Kotta S, Pramod K. Bioinspired and biomimetic systems for advanced drug and gene delivery. J Control Release. 2018;287:142–155. doi:10.1016/j.jconrel.2018.08.033
219. Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat Mater. 2009;8(1):15–23. doi:10.1038/nmat2344
220. Laganenka L, López ME, Colin R, Sourjik V. Flagellum-mediated mechanosensing and RflP control motility state of pathogenic Escherichia coli. mBio. 2020;11(2). doi:10.1128/mBio.02269-19
221. Naeem A, Yu C, Zang Z, Zhu W, Deng X, Guan Y. Synthesis and evaluation of rutin-hydroxypropyl β-cyclodextrin inclusion complexes embedded in xanthan gum-based (HPMC-g-AMPS) hydrogels for oral controlled drug delivery. Antioxidants. 2023;12(3). doi:10.3390/antiox12030552
222. Jalava K, Eko FO, Riedmann E, Lubitz W. Bacterial ghosts as carrier and targeting systems for mucosal antigen delivery. Expert Rev Vaccines. 2003;2(1):45–51. doi:10.1586/14760584.2.1.45
223. Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10(7):521–535. doi:10.1038/nrd3499
224. da Silva AJ, Zangirolami TC, Novo-Mansur MT, Giordano Rde C, Martins EA. Live bacterial vaccine vectors: an overview. Braz J Microbiol. 2014;45(4):1117–1129. doi:10.1590/s1517-83822014000400001
225. Kudela P, Koller VJ, Mayr UB, Nepp J, Lubitz W, Barisani-Asenbauer T. Bacterial Ghosts as antigen and drug delivery system for ocular surface diseases: effective internalization of Bacterial Ghosts by human conjunctival epithelial cells. J Biotechnol. 2011;153(3–4):167–175. doi:10.1016/j.jbiotec.2011.03.022
226. Ali RH, Ali ME, Samir R. Production and characterization of bacterial ghost vaccine against Neisseria meningitidis. Vaccines (Basel). 2022;11(1). doi:10.3390/vaccines11010037
227. Fan JX, Li ZH, Liu XH, Zheng DW, Chen Y, Zhang XZ. Bacteria-mediated tumor therapy utilizing photothermally-controlled TNF-α expression via oral administration. Nano Lett. 2018;18(4):2373–2380. doi:10.1021/acs.nanolett.7b05323
228. Zhang X, Gong C, Akakuru OU, Su Z, Wu A, Wei G. The design and biomedical applications of self-assembled two-dimensional organic biomaterials. Chem Soc Rev. 2019;48(23):5564–5595. doi:10.1039/c8cs01003j
229. Takamura S, Niikura M, Li TC, et al. DNA vaccine-encapsulated virus-like particles derived from an orally transmissible virus stimulate mucosal and systemic immune responses by oral administration. Gene Ther. 2004;11(7):628–635. doi:10.1038/sj.gt.3302193
230. Xie J, Xu R, Chapter 5 - Trends in orally viral vector gene delivery and therapy. In: Nanostructures for Oral Medicine. Andronescu E, Grumezescu AM editors. Elsevier; 2017:123–146.
231. Zhou X, Ling K, Liu M, et al. Targeted delivery of cisplatin-derived nanoprecursors via a biomimetic yeast microcapsule for tumor therapy by the oral route. Theranostics. 2019;9(22):6568–6586. doi:10.7150/thno.35353
232. Vaswani K, Mitchell MD, Holland OJ, et al. A method for the isolation of exosomes from human and bovine milk. J Nutrit Metabo. 2019;2019:5764740. doi:10.1155/2019/5764740
233. Aqil F, Munagala R, Jeyabalan J, et al. Milk exosomes - Natural nanoparticles for siRNA delivery. Cancer Lett. 2019;449:186–195. doi:10.1016/j.canlet.2019.02.011
234. Pei W, Cai L, Gong X, et al. Drug-loaded oleic-acid grafted mesoporous silica nanoparticles conjugated with α-lactalbumin resembling BAMLET-like anticancer agent with improved biocompatibility and therapeutic efficacy. Mater Today Bio. 2022;15:100272. doi:10.1016/j.mtbio.2022.100272
235. Timofeeva AM, Paramonik AP, Sedykh SS, Nevinsky GA. Milk exosomes: next-generation agents for delivery of anticancer drugs and therapeutic nucleic acids. Int J Mol Sci. 2023;24(12). doi:10.3390/ijms241210194
236. Rahimi Ghiasi M, Rahimi E, Amirkhani Z, Salehi R. Leucine-rich repeat-containing g-protein coupled receptor 5 gene overexpression of the rat small intestinal progenitor cells in response to orally administered grape exosome-like nanovesicles. Adv Biomed Res. 2018;7:125. doi:10.4103/abr.abr_114_18
237. Zhu YX, Jia HR, Jiang YW, et al. A red blood cell-derived bionic microrobot capable of hierarchically adapting to five critical stages in systemic drug delivery. Exploration. 2024;4(2):20230105. doi:10.1002/exp.20230105
238. He H, Qin Q, Xu F, et al. Oral polyphenol-armored nanomedicine for targeted modulation of gut microbiota-brain interactions in colitis. Sci Adv. 2023;9(21):eadf3887. doi:10.1126/sciadv.adf3887
239. Ma Y, Gou S, Zhu Z, et al. Transient mild photothermia improves therapeutic performance of oral nanomedicines with enhanced accumulation in the colitis mucosa. Adv Mater. 2024;36(14):e2309516. doi:10.1002/adma.202309516
240. Ahadian S, Finbloom JA, Mofidfar M, et al. Micro and nanoscale technologies in oral drug delivery. Adv Drug Deliv Rev. 2020;157:37–62. doi:10.1016/j.addr.2020.07.012
241. Nie D, Liu C, Yu M, Jiang X, Wang N, Gan Y. Elasticity regulates nanomaterial transport as delivery vehicles: design, characterization, mechanisms and state of the art. Biomaterials. 2022;291:121879. doi:10.1016/j.biomaterials.2022.121879
242. Deng B, Liu S, Wang Y, et al. Oral nanomedicine: challenges and opportunities. Adv. Mater. 2024;36(6). doi:10.1002/adma.202306081
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.