Back to Journals » Infection and Drug Resistance » Volume 18
Epidemiology, Clinical Characteristics and Treatment Outcomes of Acinetobacter baumannii Infection at a Regional Hospital in Thailand
Authors Sakulkonkij P , Bruminhent J
Received 10 September 2024
Accepted for publication 15 January 2025
Published 25 January 2025 Volume 2025:18 Pages 473—482
DOI https://doi.org/10.2147/IDR.S494712
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Parichart Sakulkonkij,1 Jackrapong Bruminhent2
1Division of Infectious Diseases, Internal Medicine Department, Lampang Hospital, Lampang, Thailand; 2Division of Infectious Diseases, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Parichart Sakulkonkij, Division of Infectious Diseases, Internal Medicine Department, Lampang Hospital, Lampang, Thailand, Email [email protected]
Objective: This retrospective cohort study evaluated the treatment outcome of Acinetobacter baumannii infection.
Methods: In this retrospective cohort study, 476 patients with Acinetobacter baumannii (A. baumannii) infection who were admitted to the internal medicine ward at Lampang Hospital, Lampang, Thailand, from 1 January 2020 to 31 December 2020 were enrolled. Medical records were reviewed.
Results: A total of 476 patients with A. baumannii infection were enrolled. Of these, 204 (43%) survived, while 272 (57%) died. Extensively drug-resistant (XDR) A. baumannii with hospital-acquired pneumonia was the most common presentation. Risk factors for acquiring multidrug-resistant (MDR) pathogens included previous hospitalization or antibiotic use and the presence of an indwelling urinary catheter, which was common in both survived and deceased groups. The survival group was significantly more likely to have received appropriate antibiotic therapy compared to the deceased group (71% vs 51%; p< 0.001), particularly with colistin monotherapy (34% vs 18%; p< 0.001). Additionally, multivariate analysis showed that predictors of unfavorable outcomes, such as multiorgan failure, hypoalbuminemia, hematologic malignancy, and healthcare-associated pneumonia. The survival group had a significantly longer hospital stay compared to the deceased group (15 days vs 7 days; p< 0.001) and also showed an increased microbiological cure rate (49% vs 26%; p< 0.001).
Conclusion: XDR A. baumannii leads to serious nosocomial infections. Understanding the risk factors for XDR A. baumannii infections could enhance colistin prescription prior to the antimicrobial susceptibility testing results.
Keywords: Acinetobacter baumannii infection, multidrug-resistant pathogens, clinical outcomes, microbiological outcomes
Introduction
Acinetobacter baumannii has become an important nosocomial pathogen that is responsible for a broad range of severe infections with significant contact with the health care system.1 Carbapenem-resistant A. baumannii has been prioritized as critical of the need for new antibiotics in recent years.2 Approximately 45% of global isolates are MDR A. baumannii, with more than 60% in the United States,3 while Turkey and Greece reported MDR exceeding 90%.4 XDR strains had a significantly higher mortality rate compared with susceptible strains (70% vs 25%)5 Predisposing factors for Acinetobacter infections are the length of stay, invasive procedures, previous antibiotic exposure, and severe illness.6 Antibiotic therapy is complicated by antibiotic resistance, including carbapenem resistance. Receiving appropriate antibiotic therapy decreases in 30-day mortality (39.5% appropriate therapy vs 65% inappropriate therapy; p=0.011).7 Colistin is an important antibiotic for the treatment of CRAB infections.8 A previous study reported a cure rate for colistin 57–77% among severely ill patients with MDR A. baumannii.9 Numerous studies have described outcome of patients with A. baumannii infection who were treated with nebulized polymyxins. In one study, use of inhaled colistin increased microbial eradication in ventilated patients compared to systemic therapy.10 Another study reported no difference in mortality between systemic colistin and combination of systemic and inhaled colistin therapy.11 In addition, combination therapy had no benefit to prevent emergence of resistance and could not conclude impact on treatment outcome due to the difference of clinical characteristics12 A recent study showed no difference in clinical outcome and microbiological cure between colistin monotherapy and combination therapy with colistin and meropenem.13–15 The current guideline recommends ampicillin-sulbactam for sulbactam susceptible CRAB infection. For severe, high-risk sulbactam-resistant CRAB infection, a combination of in vitro active antibiotics including polymyxin, high-dose tigecycline, ampicillin-sulbactam, aminoglycoside and high-dose extended-infusion meropenem may be considered if a meropenem MIC < 8 µg/mL, and avoid colistin-meropenem or colistin–rifampicin combination therapy.16 In addition, colistin–meropenem combination or other regimens did not result in better outcomes compared with colistin monotherapy because CRAB isolates are almost significantly higher MICs of meropenem (higher than 8 µg/mL), so it is unlikely to offer the advantage of colistin–meropenem combination therapy.17 Difficulties in treating CRAB infections still challenge physicians in optimizing antibiotic therapy in the setting of highly resistant pathogens. To provide timely and proper antibiotic therapy, it is important to know the risk factors for carbapenem-resistant A. baumannii acquisition and the characteristics of A. baumannii infection. Therefore, this study was to investigate treatment outcomes by comparing the appropriateness of antibiotic therapy between the survived and deceased groups. Secondary outcomes included the comparison of 4-week all-cause mortality, length of hospital stay, and microbiological outcome.
Material and Methods
Study Design
The study was conducted at Lampang Hospital. Data were collected from patients with A. baumannii infections who were admitted internal medicine ward between January 1, 2020, and December 31, 2020. Antibiotic susceptibility tests were performed by broth microdilution method, BD Phoenix M50, according to the Clinical and Laboratory Standard Institutes (CLSI) 2019–2020.18 The study protocol was approved by the Lampang Ethics Committees (code 008/66).
The primary outcome is the treatment outcome of A. baumannii infection. The secondary outcomes are the length of hospital stay, microbiological outcomes, and 4-week all-cause mortality.
Definition
Infection was defined as a clinically suspected infection in a patient with a documented source, assessed based on available clinical data and microbiological results, along with the administration of antibiotics by an attending physician. Community-acquired infection was defined as a clinically suspected infection with a positive A. baumannii culture from any site obtained within ≤48 hours of hospitalization.19 Nosocomial infection was defined as a clinically suspected infection with a positive A. baumannii culture obtained >48 hours after hospitalization or within 2 days of hospital discharge.20 Healthcare-associated infection was defined as a clinically suspected infection with a positive A. baumannii culture obtained within ≤48 hours of hospitalization and significant recent exposure to the healthcare system.19–21 MDR A. baumannii was defined as non-susceptibility to at least one agent in three classes of antibiotics approved for the treatment of A. baumannii infection: aminoglycosides, antipseudomonal penicillin-β-lactamase inhibitors, antipseudomonal carbapenems, antipseudomonal fluoroquinolones, trimethoprim-sulfamethoxazole, cephalosporins, ampicillin-sulbactam, polymyxins, or tetracyclines. XDR A. baumannii was defined as susceptibility only to polymyxins and tigecycline.22
Microbiological eradication was defined as the time of negative culture after starting treatment with antibiotics (as most recent cultures on or close to the end of antibiotic treatment from any site of baseline infection were negative).23 Persistence was defined as the same causative organism as in the initial episode was still detected in culture from any site of baseline infection at the end of treatment.23
Superinfection was defined as clinical failure or improvement and isolation of a pathogen not present at baseline was assessed based on available clinical data and microbiological results, and the physician agreed that the patient requires antibiotic therapy.24
Appropriate specific antibiotic therapy was considered based on susceptibility results according to CLSI 2019–2020 and compatible with the clinical course.
Previous antibiotic was defined as antibiotic treatment within 90 days before admission without concern about antibiotic duration.
Previous hospitalization was defined as hospitalization without regard to the cause of admission within 90 days before admission.
Time of presentation was defined as time of suspected infection with any site cultures were taken, which is the index date (day 0).
Time to appropriate antibiotic therapy was defined as the time of any site culture collection to time of the most specific antibiotic therapy in consideration of AST results. If a patient was already on antibiotics at the time of culture collection, the time to receipt of antibiotics was zero.
Statistical Analyses
Continuous variables were presented as mean ± standard deviation (SD) or median and interquartile range (IQR), categorical variables were presented as number (percentage). Student’s t-test was used to examine continuous variables, and Chi-square test or Fisher exact was used to examine categorical variables. Comparison of time to appropriate antibiotic therapy between groups was performed using paired sample t-test. Nelson-Aalen estimator of the cumulative hazard function to evaluate antibiotic therapy. Kaplan–Meier survival curve estimation and Log rank testing was used to compare survival between colistin monotherapy and colistin–meropenem combination therapy. Univariable generalized linear mixed regression analysis was included in the multivariable analysis for risk factors associated with 4 weeks of All-cause Mortality. Stepwise backward elimination was performed until risk factors in the multivariate generalized linear mixed regression model had a p-value <0.05. Statistical analysis was carried out using Stata version 16.0, with a significance p <0.001.
Results
Demographics and Clinical Characteristics
A total of 476 patients were included, 204 (43%) patients were in the survival group and 272 (57%) patients were in the deceased group. Baseline characteristics, medical history and severity of illness are presented in Table 1. Almost of the isolations were XDR A. baumannii strains, more than 90% were resistant to meropenem (MICs of meropenem >4 µg/mL), 185 (91%) in the survived group and 248 (91%) in the deceased group. Respiratory tract infections were the most common site of infections in both groups. Risk factors for MDR pathogens acquisition included previous hospitalization or antibiotic use within 3 months, indwelling Foley catheters were presented in most of the patients in both groups. The severity of illness included septic shock, high Pitt bacteremia score or SOFA Score and multiorgan failure were statistically significant in the deceased group (p < 0.001).
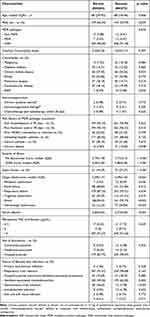 |
Table 1 Characteristics of Patients With A. baumannii Infection |
Antibiotic Therapy and Treatment Outcomes of Patients With A. baumannii Infection
Appropriate antibiotic therapy in the survived group was 71%, compared to 51% in the deceased group (p < 0.001) (Table 2). Figure 1 shows cumulative hazard of antibiotic therapy. A significantly longer length of hospital stay was shown in patients receiving appropriate antibiotic therapy, compared to patients receiving inappropriate antibiotic therapy (15 days vs 7 days; p < 0.001). Figure 2 shows survival probability among patients receiving colistin monotherapy and colistin-meropenem combination therapy. No survival benefit with colistin–meropenem combination therapy compared with colistin monotherapy but were underpowered to assess significant differences (p = 0.064). We found colistin monotherapy was used 34% in the survival group and 18% in the deceased group (p< 0.001). Microbiological eradication was presented 49% in the survival group and 26% in the deceased group; (p<0.001), superinfections were also significantly demonstrated in the survival group compared to the deceased group (26.47% vs 16.54%; p=0.008).
 |
Table 2 Antibiotic Therapy and Treatment Outcomes of Patients With A. baumannii Infection |
 |
Figure 1 Cumulative Hazard of Survival Probability among Patients with A. baumannii Infections Receiving Appropriate Antibiotic Therapy (red) and Inappropriate Antibiotic Therapy (black). |
 |
Figure 2 Cumulative Hazard of Survival Probability among Patients with A. baumannii Infections Receiving colistin monotherapy (black) and colistin plus meropenem (red). |
Factor Associated With All-Cause Mortality
Multivariate analysis revealed independent risk factors for unfavorable outcomes, including hematologic malignancy (AHR = 4.33, 95% CI = 1.53–12.23), multiorgan failure (AHR = 2.58, 95% CI = 1.75–3.80), low serum albumin (AHR = 1.75, 95% CI = 1.23–2.50) and healthcare-associated pneumonia (HCAP) (AHR = 2.10, 95% CI = 1.11–3.96) were significantly demonstrated in deceased group (Table 3).
 |
Table 3 Univariate and Multivariate Cox Regression Analyses of Variables Associated With 4 weeks All-Cause Mortality |
Discussion
This study aimed to assess the treatment outcomes of A. baumannii infection. Most of the patients acquired XDR A. baumannii with hospital-acquired pneumonia. Our study identified similar risk factors for MDR pathogens as previous studies, including previous hospitalization or antibiotic use within 3 months and the presence of an indwelling Foley catheter.6 Recognizing these factors could improve infection control practices and promote antibiotic stewardship according to local epidemiology to proper empirical antibiotics, which optimal infection control and appropriate antibiotic therapy may consequently lower rates of MDR pathogen transmission and optimize outcomes. We compared clinical outcomes between colistin monotherapy and colistin-based combination therapy (mostly with meropenem). Colistin–meropenem combination therapy did not demonstrate a greater survival benefit compared to colistin monotherapy, consistent with previous studies that provided strong evidence against carbapenem-colistin use for the treatment of CRAB infections.14,15 This suggests that there is no evidence of a synergistic effect between colistin and meropenem when the meropenem MIC exceeds 4 µg/mL. According to the IDSA 2024 guidance, most CRAB isolates exhibit highly elevated meropenem MICs, making it unlikely that colistin–meropenem combination therapy would offer any advantage.17
Thus, colistin remains an antibiotic option for XDR A. baumannii infection8,9 and parenteral colistin therapy before AST results may be needed based on local epidemiological data. Additionally, clinical success in the survived group may be related to the lower severity of illness observed. Interestingly, significant clinical responses and microbiological cures were shown in the survived group, superinfections were also more common in the survival group compared to the deceased group. This may be due to the delay in appropriate antibiotic therapy, which impacted bacterial eradication and led to biofilm formation, making infections more difficult to treat.25,26 Our findings showed a high 4-week mortality rate of 87.5%, which is similar to the mortality rate of XDR A. baumannii VAP reported in a previous study (85.3%).27 Using multivariable analysis, HCAP, hematologic malignancy, and severe illness (organ dysfunction ≥4 and hypoalbuminemia) were identified as poor prognostic factors. This suggests that disease severity and malignancy28 are associated with 4-week mortality. However, pathogen-specific factors (eg, A. baumannii resistance) and other clinical factors should also be considered, even though they were not found to be significant in this study. Therefore, early recognition of risk factors for MDR pathogen acquisition, along with measures to prevent the spread and acquisition of drug-resistant organisms, is essential. Additionally, local epidemiological data and rapid AST results are crucial to guide physicians in making decisions aligned with current practice guidelines, enabling the prescription of appropriate antibiotics and improving clinical outcomes. Microbiological eradication should not be the goal of antibiotic treatment, but rather achieving a good clinical response should be prioritized.
This study has several limitations. The retrospective study design lacks randomization and involves a retrospective chart review, which limits data accessibility. Second, more data are needed on the pharmacokinetics, pharmacodynamics, and appropriate dosing of colistin. Third, treatment options are severely limited; to our knowledge, carbapenems and colistin remain the agents of choice for most MDR infections. The role of other agents and combination therapies remains unclear. Due to these limitations, further studies on other antibiotic regimens and the role of antibiotic combinations are essential.
In conclusion, XDR A. baumannii infections have become significant nosocomial pathogens. Colistin–meropenem combination therapy, even with highly elevated meropenem MICs, has not demonstrated a greater survival benefit compared to colistin monotherapy. Identifying risk factors for MDR pathogens, utilizing local epidemiological data, and obtaining rapid AST results are essential for appropriate antibiotic prescriptions and improved clinical outcomes.
Abbreviations
A. baumannii, Acinetobacter baumannii; CRAB, Carbapenem resistant Acinetobacter baumannii; AST, Antibiotic susceptibility testing; MIC: Minimum inhibitory concentration; MDR, Multidrug-resistant; XDR, Extensively drug-resistant; CLSI, Clinical Laboratory Standards Institute; HAP, Hospital-acquired pneumonia; VAP, Ventilator-associated pneumonia; VAT, Ventilator-associated tracheobronchitis; HCAP, Healthcare-associated pneumonia; BSI, Bloodstream infections.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethics Approval
Lampang Clinical Research Ethics Committee. All data was handled confidentially.
Consent to Participate
Not applicable. Confidentially signifies the obligation to keep personal information private and secure following legal and ethical principles.
Consent Statement
The need for written informed consent was waived by the Clinical Research Ethics Committee due to the retrospective nature of the study.
Acknowledgment
Special thanks are given to Medical Technology at Lampang Hospital, Lampang, Thailand.
Chalongpon Santong, Cancer Unit, Faculty of Medicine, Khon Kaen University, Thailand
Funding
This research did not receive any specific grant from funding agencies in the public, Commercial, or not-for-profit sectors.
Disclosure
The authors declare no competing interests in this work.
References
1. Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med. 2008;358(12):1271–1281. doi:10.1056/NEJMra070741
2. Shrivastava SR, Shrivastava PS, Ramasamy J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J Med Soc. 2018;32(1):76–77. doi:10.4103/jms.jms_25_17
3. Control CfD. Prevention. Antibiotic Resistance Threats in the United States. US Department of Health and Human Services, Centres for Disease Control and Prevention; 2019.
4. Xie R, Zhang XD, Zhao Q, Peng B, Zheng J. Analysis of global prevalence of antibiotic resistance in Acinetobacter baumannii infections disclosed a faster increase in OECD countries. Emerging Microbes Infect. 2018;7(1):1–10.
5. Lee H-Y, Chen C-L, Wu S-R, Huang C-W, Chiu C-H. Risk factors and outcome analysis of Acinetobacter baumannii complex bacteremia in critical patients. Crit Care Med. 2014;42(5):1081–1088. doi:10.1097/CCM.0000000000000125
6. Fournier PE, Richet H, Weinstein RA. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clinl Infect Dis. 2006;42(5):692–699. doi:10.1086/500202
7. Erbay A, Idil A, Gözel MG, İ M, Balaban N. Impact of early appropriate antimicrobial therapy on survival in Acinetobacter baumannii bloodstream infections. Int J Antimicrob Agents. 2009;34(6):575–579. doi:10.1016/j.ijantimicag.2009.07.006
8. Jung S, Chung EK, Jun MS, Son ES, Rhie SJ. Differences in colistin administration and bacterial and treatment outcomes in critically ill patients. Sci Rep. 2019;9(1):8781. doi:10.1038/s41598-019-44965-y
9. Eliopoulos GM, Maragakis LL. Antimicrobial Resistance: Acinetobacter baumannii: epidemiology, Antimicrobial Resistance, and Treatment Options. Clinl Infect Dis. 2008;46(8):1254–1263. doi:10.1086/529198
10. Kuo S-C, Lee Y-T, Yang S-P, et al. Eradication of multidrug-resistant Acinetobacter baumannii from the respiratory tract with inhaled colistin methanesulfonate: a matched case-control study. Clin Microbiol Infect. 2012;18(9):870–876. doi:10.1111/j.1469-0691.2011.03682.x
11. Demirdal T, Sari US, Nemli SA. Is inhaled colistin beneficial in ventilator associated pneumonia or nosocomial pneumonia caused by Acinetobacter baumannii? Ann clin microb antimicrobi. 2016;15:1–6.
12. Poulikakos P, Tansarli G, Falagas M. Combination antibiotic treatment versus monotherapy for multidrug-resistant, extensively drug-resistant, and pandrug-resistant Acinetobacter infections: a systematic review. Eur J Clin Microbiol Infect Dis. 2014;33:1675–1685. doi:10.1007/s10096-014-2124-9
13. Kaye KS, Marchaim D, Thamlikitkul V, et al. Colistin monotherapy versus combination therapy for carbapenem-resistant organisms. NEJM Evidence. 2022;2(1):EVIDoa2200131.
14. Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391–400. doi:10.1016/S1473-3099(18)30099-9
15. Kaye K, Marchaim D, Thamlikitkul V, et al. Results From the OVERCOME Trial: Colistin Monotherapy Versus Combination Therapy for the Treatment of Pneumonia or Bloodstream Infection Due to Extensively Drug Resistant Gram-Negative Bacilli. 31st European Congress of Clinical Microbiology & Infectious Diseases; 2021.
16. Paul M, Carrara E, Retamar P, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect. 2022;28(4):521–547. doi:10.1016/j.cmi.2021.11.025
17. Tamma PD, Heil EL, Justo JA, Mathers AJ, Satlin MJ, Bonomo RA. Infectious Diseases Society of America 2024 guidance on the treatment of antimicrobial-resistant gram-negative infections. Clinl Infect Dis. 2024;ciae403. doi:10.1093/cid/ciae403
18. Wayne P. Clinical and laboratory standards institute. Perf Std Antimicrob Susc Test. 2011.
19. Friedman ND, Kaye KS, Stout JE, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Internal Med. 2002;137(10):791–797. doi:10.7326/0003-4819-137-10-200211190-00007
20. Laupland KB, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev. 2014;27(4):647–664. doi:10.1128/CMR.00002-14
21. Vallés J, Calbo E, Anoro E, et al. Bloodstream infections in adults: importance of healthcare-associated infections. J Infect. 2008;56(1):27–34. doi:10.1016/j.jinf.2007.10.001
22. Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
23. Promsen P, Arrayasillapaotorn N, Anutrakulchai S, Chetchotisakd P, Anunnatsiri S, Anutrakulchai S. Factors associated with microbiological outcomes to colistin in patients with multidrug-resistant gram-negative bacterial infections. 2021.
24. Hagel S, Fiedler S, Hohn A, et al. Therapeutic drug monitoring-based dose optimisation of piperacillin/tazobactam to improve outcome in patients with sepsis (TARGET): a prospective, multi-centre, randomised controlled trial. Trials. 2019;20:1–10. doi:10.1186/s13063-019-3437-x
25. Kiem S, Schentag JJ. Correlations between microbiological outcomes and clinical responses in patients with severe pneumonia. Infection & Chemother. 2013;45(3):283–291. doi:10.3947/ic.2013.45.3.283
26. Rasamiravaka T, Labtani Q, Duez P, El Jaziri M. The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. Biomed Res Int. 2015;2015(1):759348. doi:10.1155/2015/759348
27. Özgür ES, Horasan ES, Karaca K, Ersöz G, Atış SN, Kaya A. Ventilator-associated pneumonia due to extensive drug-resistant Acinetobacter baumannii: risk factors, clinical features, and outcomes. Am J Infect Control. 2014;42(2):206–208. doi:10.1016/j.ajic.2013.09.003
28. Leu H-S, Kaiser DL, Mori M, Woolson RF, Wenzel RP. Hospital-acquired pneumonia: attributable mortality and morbidity. American j epid. 1989;129(6):1258–1267. doi:10.1093/oxfordjournals.aje.a115245
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

