Back to Journals » Infection and Drug Resistance » Volume 18
Epidermolysis Bullosa with Esophageal Complications and Co-Infection with Helicobacter pylori: A Case Report
Authors Lin Y, Kong W, Li S, Wang M
Received 22 September 2024
Accepted for publication 22 January 2025
Published 28 February 2025 Volume 2025:18 Pages 1215—1222
DOI https://doi.org/10.2147/IDR.S497443
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Yingmin Lin,1 Wei Kong,2 Shuying Li,2 Min Wang3,4
1Department of General Practice, Qilu Hospital of Shandong University, Jinan, Shandong Province, 250012, People’s Republic of China; 2Cheeloo College of Medicine, Shandong University, Jinan, Shandong Province, 250012, People’s Republic of China; 3Department of Geriatric, Gastroenterology, Qilu Hospital of Shandong University, Jinan, Shandong Province, 250012, People’s Republic of China; 4Shandong Key Laboratory of Cardiovascular Proteomics, Jinan, Shandong Province, 250012, People’s Republic of China
Correspondence: Min Wang, Department of Geriatric, Gastroenterology, Qilu Hospital of Shandong University, Jinan, Shandong Province, People’s Republic of China, Email [email protected]
Abstract: Epidermolysis bullosa (EB) is a group of rare genetic skin disorders that are hereditary and heterogeneous, characterized by skin and mucosal fragility and blister formation, often induced by minimal trauma. Esophageal complications represent a significant extracutaneous manifestation of EB. The lack of standardized diagnostic and therapeutic guidelines of EB with esophageal complications contributes to inconsistent management and a higher susceptibility to recurrence. For patients with EB experiencing digestive tract symptoms, there are few reports that specifically address the follow-up and continuity of mucosal repair treatment. To date, EB with esophageal complications and co-infection with Helicobacter pylori (H. pylori) has been rarely reported. The impact of H. pylori infection on EB remains unclear. Here, we report a case of a 26-year-old man diagnosed with EB and esophageal complications. The patient presented with post-sternal pain, dysphagia, esophageal obstruction, and vomiting. Gastroscopy revealed scattered flake erosions on the esophageal mucosa. The pathological examination revealed inflammatory granulation tissue with necrosis and focal squamous epithelium showing mild atypical hyperplasia. Significant improvement in symptoms was observed after long-term mucosal repair therapy. After being lost to follow-up, the patient developed symptomatic exacerbation and co-infection with H. pylori. The patient’s condition improved after the eradication of H. pylori, combined with ongoing treatment for esophageal complications and regular follow-up. Patients with EB who have esophageal complications require long-term mucosal repair treatment and regular follow-up. Co-infection with H. pylori may be an important factor in disease recurrence.
Keywords: epidermolysis bullosa, esophageal complications, Barrett’s esophagus, Helicobacter pylori, case report
Introduction
Epidermolysis bullosa (EB) is a rare hereditary disease that affects multiple systems, with a reported prevalence and incidence of 11.7 per one million people.1 The gastrointestinal tract is one of the primary areas affected in these patients.2 Extracutaneous injury in inherited EB is associated with complications such as dysphagia, gastroesophageal reflux disease (GERD), esophageal perforation, and stricture. These complications may impair oral intake, leading to malnutrition. Hiatal hernia and Barrett’s esophagus (BE) are clinically rare in this context. Co-infection with H. pylori in these patients has been rarely reported previously. We report a recent case of EB with concurrent esophageal complications and co-infection with H. pylori. In this case, the recovery from the disease was closely associated with consistent mucosal repair treatment and standardized follow-up, as well as the eradication of H. pylori.
Case Report
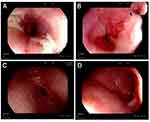
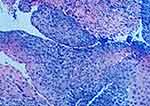
A 26-year-old man presented to the gastroenterology clinic with post-sternal pain, dysphagia, esophageal obstruction, and vomiting during the past 1 month. The patient has been experiencing recurrent skin ulcerations and blisters since the age of 1, primarily located on the joints of the hands and feet. According to his medical history, he was diagnosed with and treated for EB in the dermatology department. He was referred by his dermatologist after developing digestive symptoms. The patient was emaciated and malnourished upon admission. He denied having any family history of EB, esophageal diseases, or any other gastrointestinal diseases. The body mass index (BMI) was 18.05 kg/m2. Blisters were discovered on the joints of the hands and feet. Nail dysplasia was present in both the hands and feet (Figure 1). Physical examination revealed a soft abdomen with no tenderness or rebound tenderness. Bowel sounds were normal. The gastroscopy revealed scattered flake erosions on the esophageal mucosa, which was covered with white patches (Figure 2A). Four biopsy samples were taken, and these specimens were firm and prone to bleeding. The submucosal vessels had a smooth texture, and normal contraction and peristalsis were observed. Congestion and edema were revealed on the antral mucosa (Figure 2C) while cardia and duodenal bulb were not affected (Figure 2B and D). The rapid urease test (RUT) result was negative. Histopathology revealed inflammatory granulation tissue with necrosis and focal squamous epithelium exhibiting mild atypical hyperplasia (Figure 3). The electrocardiogram, chest X-ray, and cardiac ultrasound showed no apparent abnormalities.
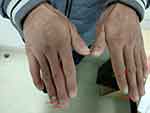 |
Figure 1 Nail dysplasia was observed in this adult with EB when first presented to the gastroenterology clinic in 2017. The red arrow indicates nail dysplasia and skin scarring. |
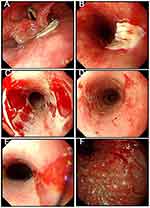
The patient declined multi-gene panel testing for EB due to financial constraints and preferred a pharmaceutical treatment approach. Rabeprazole (10 mg every 12 hours) and sucralfate (5 mL every 12 hours) were administered. The patient was instructed to follow a semi-liquid diet. After continuous treatment, the patient’s symptoms improved significantly. He underwent a gastroscopy (3 months after the first admission), which revealed that the esophageal mucosa was smooth and soft, with a clear texture and good dilatation. An orange-colored lingual mucosa was visible above the dentate line, exhibiting surface hyperemia (Figure 4A and B). Barrett’s esophagus, hiatal hernia, chronic atrophic gastritis with hyperemia were diagnosed (Figure 4A–F), and the RUT result was negative. Therefore, the treatment plan was adjusted. Rabeprazole (10 mg every 24 hours) and glutamine (0.5 g every 8 hours) were administered. Chinese proprietary medicine (Weifuchun Tablets (WFC), 1.44 g every 8 hours) was administered as adjuvant treatment. It has been widely used in treating a variety of chronic stomach disorders including Chronic atrophic gastritis and Gastric precancerous lesions in China clinically.3–5 The active ingredients were Radix Ginseng Rubra (red ginseng), Rabdosia amethystoides H. Hara, and fried Fructus Aurantii. The patient was allowed to consume soft food. After one year of treatment, the patient’s symptoms completely disappeared, and his appetite returned to normal. Subsequently, Rabeprazole (10 mg every week) was administered. The patient gradually transitioned to a regular diet, and his condition remained stable. We recommended that he return regularly to reinforce the treatment. However, the patient was lost to follow-up after that, until 2019 (18 months after the first admission and 3 months after the last subsequent visit), when he was admitted with worsening symptoms. He then underwent a gastroscopy at the local hospital, which revealed flake erosions in the pharynx and esophagus (Figure 5A–E), as well as severe chronic atrophic gastritis (Figure 5F). The RUT showed a positive result for H. pylori infection.
To eradicate H. pylori, the patient was prescribed rabeprazole (10 mg every 12 hours), colloidal pectin bismuth (200 mg every 12 hours), amoxicillin (1000 mg every 12 hours), and clarithromycin (500 mg every 12 hours) for a two-week period. Subsequently, rabeprazole (10 mg every 12 hours), glutamine (0.5 g every 8 hours) and sucralfate (5 mL every 12 hours) were administered to facilitate mucosal repair. After more than five months of treatment, the patient’s symptoms had significantly improved. The treatment plan was then adjusted to Rabeprazole (10 mg every 24 hours), teprenone (50 mg every 8 hours) and WFC (1.44 g every 8 hours). The patient received regular follow-up appointments and prescriptions for medication. At 7 years postoperatively, the patient remained asymptomatic. During this period, the patient got married and had a healthy child.
Discussion
In 1886, the German dermatologist Heinrich Koebner proposed the concept of epidermolysis bullosa (EB).6,7 EB is a rare genetic disorder characterized by predominant lesions in the skin, such as skin blisters, erosions, milia, deformities, or absence of fingernails and toenails, scarring, and extensive granulation tissue.8 These lesions can also occur in extracutaneous sites, including the eye, nose, ear, upper airway, genitourinary tract, and gastrointestinal tract.9 The gastrointestinal tract is commonly affected in various subtypes of EB, with the upper esophagus being the most frequently involved area.2 Patients typically present with symptoms of dysphagia and malnutrition. Gastroesophageal reflux can result in mucosal blisters, which can lead to esophageal stenosis.10 As reported, approximately 1% of patients with EB experienced complications with hiatal hernia and BE.2 Given the rarity of the disease, reporting on the gastroscopic features of this patient diagnosed with EB contributes to the expanding body of knowledge on EB with gastrointestinal complications.
Furthermore, we report the co-infection of H. pylori in this patient. After the patient was lost to follow-up, the disease recurred, with H. pylori infection leading to severe chronic atrophic gastritis. The patient was living alone at the time of diagnosis for H. pylori infection, and the risk of family clustering is relatively low. However, since China is a country with a high prevalence of H. pylori infection, with the latest statistics showing an infection rate of 46.7%,11 the patient has clear environmental factors for H. pylori infection. Some studies suggest that H. pylori may be associated with skin diseases such as Rosacea, Psoriasis, Chronic Urticaria, Alopecia areata, and Autoimmune bullous diseases (AIBD) including pemphigus, pemphigoid, dermatitis herpetiformis, epidermolysis bullosa acquisita, and linear Ig A disease.12 Mortazavi et al demonstrates that the prevalence of H. pylori infection is significantly greater in untreated patients with pemphigus vulgaris (PV) (79.3%) in comparison to healthy controls (59.5%), suggesting a possible pathogenic role of H. pylori in AIBD.13 The precise mechanism through which H. pylori influences skin diseases remains unclear. H. pylori has the capacity to induce various inflammatory mediators, including interleukins (IL-1, IL-2, IL-6, IL-8, IL-10), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ). This induction may contribute to chronic low-level systemic inflammation within the human body. Furthermore, H. pylori antigens exhibit structural similarities to components of the host’s own antigens, which can lead to molecular mimicry and cross-reactivity, potentially resulting in autoimmune responses. These mechanisms may play a role in the development of systemic diseases beyond the gastrointestinal tract that are associated with H. pylori infection.14 From this perspective, the cessation of H. pylori treatment may yield a beneficial effect on skin diseases. However, to the best of our knowledge, the association between EB and H. pylori infection has rarely been described, and there is little clinical experience regarding whether eradicating H. pylori is beneficial for patients with EB and esophageal complications. Some studies have provided evidence that H. pylori infection is inversely associated with BE.15 In our case, on the contrary, after eradicating H. pylori and treating with a proton pump inhibitor, the patient achieved satisfactory symptom control. This suggests that eradicating H. pylori may be one of the key factors in improving the patient’s condition and promoting mucosal repair in EB patients.
Significant progress has been made in treating patients with EB using a variety of approaches. Over the last decade, various therapies such as stem cell therapy, protein replacement, and gene therapies have been investigated.16–18 However, the current approaches are not yet curative for EB. In the absence of a specific treatment to cure EB, the management approach primarily focuses on addressing symptoms. For patients with EB and digestive tract symptoms, previous case reports have mainly focused on subsequent surgical interventions, such as esophageal replacement surgery,19 interventional therapy in the colon,20 and esophageal dilatation surgery.21,22 Nutritional support is a vital component of the non-operative treatment strategy for most patients with malnutrition. Treatment with an H2 antagonist or proton pump inhibitor appears to be effective,1 but there is still a lack of standardized treatment protocols. Surgical treatment is a difficult decision when conditions worsen.
Our patient exhibited clear symptoms in the digestive tract. After the treatment, the patient’s symptoms were effectively controlled, leading to a more successful cure. This indicates that EB combined with digestive tract symptoms should not be overlooked. Early and prolonged intervention is crucial to facilitate optimal healing of the digestive tract mucosa. Treatment should commence with dietary modifications.1 Proton pump inhibitors are indispensable for treatment, and the dosage can be adjusted according to symptom changes. Gastric mucosal protectants, such as glutamine, sucralfate, and teprenone, are vital adjunctive medications. Nutritional supplementation aids in alleviating malnutrition, thereby enhancing recovery.
Furthermore, in the management of the patient, the Chinese proprietary medicine WFC exhibited significant efficacy in enhancing gastric mucosal lesions and addressing H. pylori infection. WFC is frequently utilized in the treatment of atrophic gastritis and intestinal metaplasia. It mainly contains Radix Ginseng Rubra (red ginseng), Rabdosia amethystoides H. Hara, and fried Fructus Aurantii.23 Red ginseng is known to contain ginsenosides, which exhibit anti-tumor, neuroprotective, and antioxidant properties, in addition to enhancing gastrointestinal motility and regulating immune responses.24 R. amethystoides demonstrates a range of activities, including anti-tumor, antioxidant, anticoagulant, antibacterial, anti-complement, and antipyretic effects, and has the capacity to repair gastric mucosa while promoting gastric mucosal hyperplasia.25 Furthermore, Fructus Aurantii has been shown to enhance gastrointestinal motility and facilitate the regeneration of gastric mucosal glands.26 Several clinical studies have demonstrated that both monotherapy with WFC and combination treatments incorporating WFC exhibit relatively favorable therapeutic effects on precancerous lesions associated with gastric cancer.27 The combination therapy utilizing WFC has demonstrated an enhanced H. pylori eradication rate in patients with H. pylori -positive atrophic gastritis when compared to conventional triple or quadruple therapy.28 Concerning the management of gastrointestinal complications associated with EB and the concurrent presence of H. pylori, the potential definitive role of WFC remains ambiguous. Nevertheless, our efforts contribute novel insights and practical evidence for the exploration of treatment options for EB.
In addition, severe esophageal strictures and malnutrition are common in patients with EB who experience concurrent esophageal complications, possibly due to inadequate attention during the pre-disease period and insufficient long-term mucosal repair treatment. Surgical and interventional procedures are necessary when esophageal strictures cannot be reversed and are causing increased suffering for the patient. Continued treatment with regular follow-up is essential to consolidate the effects of treatment and reduce the recurrence and exacerbation of the disease, potentially decreasing the likelihood of later esophageal strictures that may require surgical intervention.
Our case has several limitations. Firstly, the patient was diagnosed with EB in the dermatology department, as indicated in the medical records. Unfortunately, the pathology of the EB diagnosis could not be traced. Secondly, the patient declined genetic testing for himself and his family due to financial constraints, and as a result, we were unable to assess his family’s genetic predisposition. Thirdly, the association between EB and H. pylori is not conclusive and requires confirmation through additional studies. However, this case serves as a reminder that H. pylori need to be taken seriously in patients with EB. It also demonstrates the importance of consistent mucosal repair treatment and standardized follow-up to prevent more severe complications.
Conclusion
In conclusion, we presented a case of an EB patient with esophageal complications, such as hiatal hernia and BE, who was also co-infected with H. pylori, a rare phenomenon in clinical practice. For patients with EB and esophageal complications, it is crucial to ensure continuity in mucosal repair treatment and adhere to standardized protocols for comprehensive treatment and prevention of disease recurrence. Eradicating H. pylori may facilitate mucosal repair and alleviate symptoms.
Ethics Approval and Informed Consent
Ethical approval was obtained from the Ethical Review Board of Qilu Hospital of Shandong University (Ethical No. 2021248). The details of the case have received approval for publication from the hospital’s ethics committee and have successfully undergone the research ethics review process.
Consent for Publication
Informed written consent was obtained from the patient for the publication of this report and any accompanying images.
Acknowledgments
We thank the patient and his family for their cooperation.
Funding
This work was supported by the Natural Science Foundation of Shandong Province, China (ZR2020MH239).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bardhan A, Bruckner-Tuderman L, Chapple ILC, et al. Epidermolysis bullosa. Nature Reviews Disease Primers. 2020;6(1):78. doi:10.1038/s41572-020-0210-0
2. Fine JD, Johnson LB, Weiner M, Suchindran C. Gastrointestinal complications of inherited epidermolysis bullosa: cumulative experience of the National Epidermolysis Bullosa Registry. J Pediatr Gastroenterol Nutr. 2008;46(2):147–158. doi:10.1097/MPG.0b013e31812f5667
3. Ma L, Hu X, Zhang W, Qi D, Chen L, Yin M. Weifuchun suppresses the malignancy of gastric cancer cells by targeting KPNA2 through miR-26a-5p-mediated destabilization and the deactivation of the MAPK signaling pathway. J Ethnopharmacol. 2024;334:118538. doi:10.1016/j.jep.2024.118538
4. Xie D, Wu C, Wang D, et al. Wei-fu-chun tablet halted gastric intestinal metaplasia and dysplasia associated with inflammation by regulating the NF-κB pathway. J Ethnopharmacol. 2024;318(Pt B):117020. doi:10.1016/j.jep.2023.117020
5. Wang B, Zhou W, Zhang H, Wang W, Zhang B, Li S. Exploring the effect of Weifuchun capsule on the toll-like receptor pathway mediated HES6 and immune regulation against chronic atrophic gastritis. J Ethnopharmacol. 2023;303:115930. doi:10.1016/j.jep.2022.115930
6. Fine JD, Eady RAJ, Bauer EA, et al. The classification of inherited epidermolysis bullosa (EB): report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol. 2008;58(6):931–950. doi:10.1016/j.jaad.2008.02.004
7. Köbner H. Hereditäre Anlage zur Blasenbildung (Epidermolysis bullosa hereditaria). Aus der Poliklinik. 2009;12(02):21–22. doi:10.1055/s-0028-1139665
8. Kotalevskaya YY, Stepanov VA. Syndromic epidermolysis bullosa simplex subtype due to mutations in the KLHL24 gene: series of case reports in Russian families. Front Med. 2024;11:1418239. doi:10.3389/fmed.2024.1418239
9. Intong LRA, Murrell DF. Inherited epidermolysis bullosa: new diagnostic criteria and classification. Clin Dermatol. 2012;30(1):70–77. doi:10.1016/j.clindermatol.2011.03.012
10. Tishler JM, Han SY, Helman CA. Esophageal involvement in epidermolysis bullosa dystrophica. American Journal of Roentgenology. 1983;141(6):1283–1286. doi:10.2214/ajr.141.6.1283
11. Ding SZ, Du YQ, Lu H, et al. Chinese consensus report on family-based Helicobacter pylori infection control and management (2021 Edition). Gut. 2022;71(2):238–253. doi:10.1136/gutjnl-2021-325630
12. Gravina AG, Priadko K, Ciamarra P, et al. Extra-gastric manifestations of Helicobacter pylori infection. J Clin Med. 2020;9(12):3887. doi:10.3390/jcm9123887
13. Mortazavi H, Hejazi P, Khamesipour A, et al. Frequency of seropositivity against infectious agents amongst pemphigus vulgaris patients: a case-control study on Strongyloides stercoralis, Helicobacter pylori, Toxoplasma gondii, Leishmania major, and Epstein-Barr virus. Int J Dermatol. 2015;54(11):e458–465. doi:10.1111/ijd.12869
14. He J, Liu Y, Ouyang Q, et al. Helicobacter pylori and unignorable extragastric diseases: mechanism and implications. Front Microbiol. 2022;13:972777. doi:10.3389/fmicb.2022.972777
15. Wang Z, Shaheen NJ, Whiteman DC, et al. Helicobacter pylori infection is associated with reduced risk of Barrett’s Esophagus: an analysis of the Barrett’s and Esophageal Adenocarcinoma Consortium. Am J Gastroenterol. 2018;113(8):1148–1155. doi:10.1038/s41395-018-0070-3
16. Hou PC, Wang HT, Abhee S, Tu WT, McGrath JA, Hsu CK. Investigational Treatments for Epidermolysis Bullosa. Am J Clin Dermatol. 2021;22(6):801–817. doi:10.1007/s40257-021-00626-3
17. Bischof J, Hierl M, Koller U. Emerging gene therapeutics for Epidermolysis Bullosa under development. Int J mol Sci. 2024;25(4):2243. doi:10.3390/ijms25042243
18. Niti A, Koliakos G, Michopoulou A. Stem cell therapies for Epidermolysis Bullosa treatment. Bioengineering. 2023;10(4):422. doi:10.3390/bioengineering10040422
19. Sehhat S, Amirie SA. Oesophageal reconstruction for complete stenosis due to dystrophic epidermolysis bullosa. Thorax. 1977;32(6):697–699. doi:10.1136/thx.32.6.697
20. Elton C, Marshall RE, Hibbert J, Cameron R, Mason RC. Pharyngogastric colonic interposition for total oesophageal occlusion in epidermolysis bullosa. Diseases of the Esophagus. 2000;13(2):175–177. doi:10.1046/j.1442-2050.2000.00109.x
21. Pawar SV, Mohite AR, Surude RG, Rathi PM, Nayak CS. Epidermolysis bullosa acquisita associated with dysphagia and stricture of esophagus. Indian Journal of Dermatology, Venereology and Leprology. 2016;82(6):717–719. doi:10.4103/0378-6323.190846
22. Kargar S, Shiryazdi SM, Neamatzadeh H, Ramazani V. Kindler syndrome: the case of two Iranian sisters. Giornale Italiano Di Dermatologia E Venereologia: organo Ufficiale. Societa Italiana Di Dermatologia E Sifilografia. 2018;153(1):111–114. doi:10.23736/S0392-0488.16.04887-2
23. Gu Z, Ling J, Cong J, Li D. A review of therapeutic effects and the pharmacological molecular mechanisms of Chinese Medicine Weifuchun in treating precancerous gastric conditions. Integr Cancer Ther. 2020;19:1534735420953215. doi:10.1177/1534735420953215
24. Jin Y, Tian T, Ma Y, Xu H, Du Y. Simultaneous determination of ginsenoside Rb1, naringin, ginsenoside Rb2 and oridonin in rat plasma by LC-MS/MS and its application to a pharmacokinetic study after oral administration of Weifuchun tablet. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1000:112–119. doi:10.1016/j.jchromb.2015.06.027
25. Dang Y, Liu T, Yan J, et al. Gastric cancer proliferation and invasion is reduced by macrocalyxin C via activation of the miR-212-3p/Sox6 Pathway. Cellular Signalling. 2020;66:109430. doi:10.1016/j.cellsig.2019.109430
26. Wang S, Bao YR, Li TJ, et al. Mechanism of Fructus Aurantii Flavonoids Promoting Gastrointestinal Motility: from organic and inorganic endogenous substances combination point of view. Pharmacogn Mag. 2017;13(51):372–377. doi:10.4103/pm.pm_179_16
27. Li HZ. Treatment of gastric precancerous lesions with Weiansan. WJG. 2006;12(33):5389. doi:10.3748/wjg.v12.i33.5389
28. Jiang Z, Deng B, Zhang Y, et al. Efficacy and safety of seven Chinese patent medicines combined with conventional triple/quadruple therapy for Helicobacter pylori-positive peptic ulcers: a systematic review and network meta-analysis. BMJ Open. 2024;14(4):e074188. doi:10.1136/bmjopen-2023-074188
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.