Back to Journals » Infection and Drug Resistance » Volume 18
Etiology of Hospital-Acquired Pneumonia (HAP) and Ventilator-Associated Pneumonia (VAP) in Tertiary-Care Hospitals in Thailand: A Multicenter, Retrospective Cohort Study
Authors Rongrungruang Y, Plongla R , Pleumkanitkul S, Hantrakun V , Khawcharoenporn T
Received 20 August 2024
Accepted for publication 25 December 2024
Published 20 January 2025 Volume 2025:18 Pages 351—361
DOI https://doi.org/10.2147/IDR.S492299
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yong Rongrungruang,1 Rongpong Plongla,2 Suwapan Pleumkanitkul,3 Viriya Hantrakun,4 Thana Khawcharoenporn5
1Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; 2Division of Infectious Diseases, Department of Medicine and Center of Excellence in Antimicrobial Resistance and Stewardship, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand; 3Samitivej Hospital Sukhumvit, Samitivej Public Co., Ltd., Bangkok, Thailand; 4Real World Solutions APAC, IQVIA Inc., Bangkok, Thailand; 5Infectious Diseases Unit, Department of Internal Medicine, Faculty of Medicine, Thammasat University, Pathumthani, Thailand
Correspondence: Thana Khawcharoenporn, Infectious Diseases Unit, Department of Internal Medicine, Faculty of Medicine, Thammasat University, Pathumthani, Thailand, 12120, Email [email protected]
Purpose: To describe the top three causative organisms of hospital acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) in Thailand.
Patients and Methods: This multi-center retrospective cohort study included HAP/VAP patients hospitalized in 2019 in three university-affiliated hospitals and a private hospital in Bangkok, Thailand. Medical records of patients with a documented diagnosis of nosocomial pneumonia (NP) were systematically reviewed to collect data on demographic, clinical, microbiological, and 30-day readmission due to NP.
Results: A total of 240 patients were included in the study, comprises patients with VAP (62.9%), HAP (36.7%), and ventilated HAP (vHAP) (0.4%). All of the patients had late-onset NP, occurring after five days of hospitalization with median time to NP of 13 days (interquartile range [IQR] 6– 25 days) from admission. The top three causative pathogens of NP were Acinetobacter baumannii (44.2%), Pseudomonas aeruginosa (34.6%), and Klebsiella pneumoniae (28.3%). A high rate of carbapenem resistance (CR) in A. baumannii (92.5%) was observed. Lower rates of CR were observed in K. pneumoniae (20.6%) and P. aeruginosa isolates (16.9%). Readmission rate due to NP within 30 days after discharge was less than 2% with median time of 4 days (IQR 3– 20 days) after discharge. After diagnosis of NP, 19 patients were transferred to intensive care units with median length of stays of 11 days (IQR 3– 24 days). Fifty-one percent of HAP patients received mechanical ventilation support after the diagnosis of NP with median length of mechanical ventilation use of 12 days (IQR 6– 22 days).
Conclusion: A. baumannii, with its significant carbapenem resistance, presents a major HAP/VAP pathogens and imposes a substantial burden on healthcare resources in this study. Implementation of regular surveillance for causative organisms of NP and their susceptibility profiles are critical for the success of HAP/VAP management, and reducing the related burden of healthcare resources.
Keywords: nosocomial pneumonia, antimicrobial susceptibility, healthcare-resource burdens, etiologic agents, Thailand
Introduction
Nosocomial pneumonia (NP), including hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP), constitutes a significant healthcare-associated challenge for patients in tertiary-care hospitals, particularly in low- and middle-income countries (LMICs). Bacterial pathogens, including extensively antimicrobial-resistant strains prevalent in hospital settings, are the primary etiological agents of NP.1,2 HAP and VAP, caused by antibiotic-resistant organisms, impose substantial morbidity, mortality, and economic burdens, leading to prolonged stays in intensive care units (ICUs) and hospitals, thereby escalating healthcare costs.2,3 Updated data on the epidemiology and burden of NP predominantly originate from high-income countries (HICs) due to systematic surveillance systems.4 The incidence of NP is notably higher in ICUs and among patients reliant on mechanical ventilator support.1,5 In Asian hospitals, the incidence rates vary, ranging from 1 to 21 cases per 1000 admissions for HAP and from 3.5 to 46 cases per 1000 ventilator days for VAP.5 The associated case fatality rate spans 25% to 54.5%.5 A systematic review of nosocomial infections in some Southeast Asian (SEA) countries has estimated the pooled incidence density of VAP at 14.7 per 1000 ventilator-days, with mortality rate of 46% and excess length of hospital stay of up to 21 days.3 However, data on nosocomial infections from SEA countries remain scarce. Consequently, the burden of NP is substantially underestimated, and epidemiological insights remains elusive.3
The distribution and burden of causative organisms in NP can vary across countries, geographical regions, and economic statuses. Gram-negative bacteria such as Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Acinetobacter species, and antimicrobial-resistant Gram-positive bacteria (such as methicillin-resistant Staphylococcus aureus or MRSA), are recognized as significant causes of NP in HICs.2,6 However, in Asia, the incidences of MRSA-caused NP is lower, while carbapenem-resistant A. baumannii and Enterobacterales-caused NP are more prevalent compared to HICs.3,7 Notably, Acinetobacter spp. account for a higher proportion of NP cases (ranging from 26.9% to 66%) than P. aeruginosa (16.7% to 22%) in countries like Malaysia, Thailand, Pakistan, and India.3,7,8
In Thailand, the etiology and antibiotic resistance patterns of NP have gradually changed over time.9 A large multi-center point prevalence study highlighted P. aeruginosa was the predominant pathogen in hospital-acquired infections (HAIs) in 2001, with lower respiratory infections accounting a third of HAIs.10 However, subsequent data spanning the years 2008–2009 and 2015–2017 have revealed that A. baumannii has become the most common pathogen in nosocomial infections, particularly in cases of HAP and VAP.11–13 To address the evolving landscape of NP, up-to-date information on epidemiology, bacteriology, and healthcare-resource utilization is crucial. This data informs appropriate antimicrobial therapy and effective prevention and control strategies. The investigators aimed to characterize the predominant pathogens, assess 30-day rehospitalization rate, and evaluate healthcare resource utilization related to NP in Thailand. Additionally, given the emerging antimicrobial resistance of P. aeruginosa, the characteristics of patients with P. aeruginosa-related NP are presented in detail in this study.
Materials and Methods
Study Design and Settings
This is a multi-center, retrospective cohort study conducted in four hospitals in Bangkok, Thailand. The study sites were tertiary-care hospitals with capacities ranging from 700 to 2000 beds. Three of the hospitals were university-affiliated: Thammasat University Hospital (site 01), Siriraj Hospital (site 02), and King Chulalongkorn Memorial Hospital (site 03). The fourth was a private hospital, Samitivej Hospital (site 04).
Eligible participants included adult patients (aged 18 and over) who were hospitalized in inpatient units and/or ICUs from January to December 2019. These patients had chest radiographic evidence of pneumonia and a documented diagnosis of HAP or VAP with a diagnosis code according to the International Classification of Diseases, Tenth Revision (ICD-10) codes J15 and J18. In addition, we identified and included only patients with a positive bacterial culture for at least one pathogenic organism, while patients whose bacterial cultures were negative, or who had NP caused by other infections and non-infectious causes were not identified.
Terms and Definitions
The definitions and classifications of NP (HAP and VAP) used were in accordance with the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) 2016 criteria.1 HAP was defined as pneumonia not incubating at the time of hospital admission and occurring 48 hours or more after admission.1 VAP was defined as a pneumonia occurring 48 hours or more after endotracheal intubation.1 Additionally, pneumonia in patients with severe HAP who require mechanical ventilation was classified as a ventilated HAP (vHAP).6
Polymicrobial infection was defined as the presence of more than one causative organism identified from clinical specimens. Carbapenem resistance (CR) was defined as bacterial isolates that were resistant to imipenem and/or meropenem. Given that ertapenem and doripenem were not consistently tested against all isolates during routine antimicrobial susceptibility testing across the four study sites, they were not included in the definition of CR. Prolonged hospitalization was defined as a hospital stay exceeding five days. Thirty-day rehospitalization was defined as a hospitalization due to NP that occurred within 30 days after discharge from the index admission.
Data Collection
Medical records of patients who met the inclusion criteria were reviewed and recorded in the case report form. Data collected included demographic information, hospital admission details, clinical signs and symptoms of respiratory infections, microbiological and radiological findings related to the diagnosis of pneumonia, and data on identification and antimicrobial susceptibility of causative agents. All study hospitals conducted routine antimicrobial susceptibility testing (AST) in accordance with Thailand’s microbiological laboratory standards, interpreting results based on guidelines from either the Clinical and Laboratory Standards Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing (EUCAST).14 Antibiotic susceptibility test results include categories of susceptible, intermediate, and resistant. However, only data pertaining to the susceptible category for the tested antibiotics was collected. Clinical outcomes, such as in-hospital mortality at the index hospitalization, were not collected in this study.
Additionally, the length of ICU stays, the durations of mechanical ventilation, and 30-day rehospitalization rates due to NP were recorded. Due to administrative challenges, the data on 30-day rehospitalization were collected from three of four study hospitals (Site 02, Site 03, and Site 04). Data on the predefined comorbidities and risk factors of patients who had NP due to P. aeruginosa were collected. These data were not collected for patients who had NP with other organisms.
Ethical Considerations
Considering the study’s retrospective design, the ethics committees approved the waiver of informed consent. We conducted the study in accordance with the Declaration of Helsinki. The study utilized anonymized datasets, ensuring the protection of patient confidentiality. This study was approved by the Human Research Ethics Committee of Thammasat University (Medicine) (Reference number: 155/2564), the Siriraj Institutional Review Board (Reference number: 783/2021), Institutional Review Board, Faculty of Medicine, Chulalongkorn University (Reference number: 1255/2021), and Bangkok Hospital Institutional Review Board (Reference number: 2021–49).
Statistical Analysis
Descriptive statistics were utilized to characterize the distribution of patient characteristics and microbiological data. Continuous variables were summarized using the median, interquartile range (IQR), and the overall range (minimum to maximum). The Chi-square test or Fisher’s exact test was used to compare proportions between groups as appropriate. A P-value of less than 0.05 was deemed to indicate statistical significance. All statistical analyses were conducted using SAS version SAS® Release 9.4 (SAS Institute Inc., Cary, NC).
Results
A total of 240 NP patients were retrospectively included from the four study hospitals. The majority of the patients (215/240 [89.5%]) were included from the three university-affiliated hospitals, particularly from site 02 (n = 98) (Figure 1).
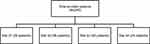 |
Figure 1 Distribution of hospital acquired pneumonia and ventilator-associated pneumonia patients by study sites. |
Patient Characteristics
The median age of patients at the time of initial admission was 73 years (IQR 62–82 years, range 20–102 years) (Table 1). There were slightly more male patients enrolled in the study (127/240 [52.9%]) than female patients. Three frequent (reported in ≥60% patients) signs and symptoms of NP were a new onset or worsening cough, dyspnea, or tachypnea (95.4%); body temperature >38°C (85.0%); and a new onset of purulent sputum, change in character of sputum, increased respiratory secretion, or increased suctioning requirement (67.1%). Ninety-seven percent of the patients had a new or progressive persistent infiltrate as confirmed by chest radiography (Table 1).
 |
Table 1 Demographic and Baseline Clinical Characteristics |
Overall, 62.9% (151/240) were diagnosed with VAP, 36.7% (88/240) with HAP, and 0.4% (1/240) with vHAP. The median time to NP was 13 days (IQR 6–25 days; range 2–200 days) from admission date, and it was similar between HAP and VAP patients (Table 2).
 |
Table 2 Causative Pathogens of Nosocomial Pneumonia During the Index Admission |
Among the 83 patients who had P. aeruginosa NP, the most frequently observed underlying conditions were moderate to severe renal or liver diseases (38.6%), diabetes (32.5%), and solid tumors (21.6%) (Table 3). More than 80% of them had a history of prolonged hospitalization (86% [72/83]), use of invasive devices (90.4% [75/83]), and prior intravenous antibiotic use within the last 90 days (86.8% [72/83]).
 |
Table 3 Factors Associated with Nosocomial Pneumonia Due to P. aeruginosa |
Causative Organisms
The overall top three most common isolated NP pathogens were A. baumannii (44.2%), P. aeruginosa (34.6%), and K. pneumoniae (28.3%) (Table 2), which were similar to the top three pathogens observed in VAP patients, while the leading pathogen for HAP was P. aeruginosa, followed by K. pneumoniae, and A. baumannii. Other isolates included Stenotrophomonas maltophilia (17.9%), Escherichia coli (10%), and Staphylococcus aureus (MSSA) (3.3%).
Among all NP patients, the most common site of pathogen isolation was endotracheal aspirates (73.8%), followed by the bloodstream (68.3%), and sputum (26.3%). This trend was similar to that observed among VAP patients. However, the most common site of isolation for HAP patients was sputum, followed by the bloodstream and endotracheal aspirates in HAP patients. Of all patients, 17 (7.1%) had positive blood and respiratory specimen cultures for any organisms (data not shown) and 16 of them (94%) had concordant blood and respiratory specimens which were culture positive for the same organism (A. baumannii [10/16], K. pneumoniae [5/16], and P. aeruginosa [3/16]). Interestingly, 13 of 16 patients had polymicrobial infections indicating that blood and/ or respiratory specimens had culture positive for more than one causative organism (Supplementary Table 1). Of all patients, two patients had only blood cultures that were tested positive for bacteria. Both patients tested positive for E. coli, and additionally, one patient had A. baumannii and the other had K. pneumoniae.
Antimicrobial-Susceptible Organisms
The majority of P. aeruginosa, K. pneumoniae, and E. coli isolates (>90%) were susceptible to amikacin, while only about 20% of A. baumannii isolates were (Supplementary Table 2). Less than half of A. baumannii isolates were susceptible to the tested antibiotics, with 47.2% being susceptible to colistin. Susceptibility to colistin in A. baumannii was lower in HAP patients (25%) compared to VAP patients (52.3%). Over 80% of P. aeruginosa isolates were susceptible to key antibiotics like gentamicin, ciprofloxacin, ceftazidime, piperacillin/tazobactam, and meropenem. For K. pneumoniae, 41.2–77.9% were susceptible to these antibiotics. Overall, the proportion of P. aeruginosa and K. pneumoniae which were susceptible to these four antibiotics were similar between HAP and VAP patients.
Carbapenem Resistance (CR)
A high rate of CR (92.5%) was observed among A. baumannii isolates (HAP: 85.0%; VAP: 94.2%). A lower CR rate was observed in K. pneumoniae (20.6% [HAP: 29.2%; VAP: 15.9%]) and P. aeruginosa isolates (16.9% [HAP: 12.9%; VAP: 19.6%]) (Table 4). Across the four study sites, CR was observed predominantly in A. baumannii (50.0–100%), followed by K. pneumoniae (5.6–36.0%) and P. aeruginosa (6.3–33.3%) (Table 5).
 |
Table 4 Carbapenem Resistance Rates of the Top Three Organisms Causing Nosocomial Pneumonia |
 |
Table 5 Distribution of the Three Major Organisms and Their Carbapenem Resistant Rates Stratified by Study Hospitals |
30-Day Rehospitalization Due to NP
Due to operational challenges, the NP-related 30-day rehospitalization rates were determined among patients admitted to three out of four study sites. Less than 2% of the patients (3/181 [1.7%]) were rehospitalized with NP within 30 days after discharge. Two patients had VAP and one patient had HAP. The median time of readmission after discharge was 4 days (IQR 3–20 days; range 3–20 days). The causative pathogens isolated for all three patients at readmission were A. baumannii (3), P. aeruginosa (1), and S. maltophilia (1), and these were isolated from endotracheal aspirates (2), bloodstream, bronchoalveolar lavage (BAL), and sputum (one each). Two out of three patients who were readmitted had the same causative organisms as those identified during their initial admission, specifically A. baumannii and P. aeruginosa. The one other patient suffered from NP caused by a different organism compared to what was observed during their first admission.
Healthcare Resource Utilization
After the diagnosis of NP, 19 patients were transferred to ICUs with the median length of stay (LOS) of 11 days (IQR 3–24 days, range 1–53 days). LOS was longer for HAP patients compared to VAP patients (12.5 vs 7 days). Approximately half of HAP patients (45/88 [51%]) received mechanical ventilation support following the diagnosis of NP, and the median length of mechanical ventilation use was 12 days (IQR 6–22 days, range 2–103 days). Among the three patients who had 30-day readmission, their median LOS during rehospitalization was 33 days (IQR and range 19–79 days).
Discussion
A. baumannii, P. aeruginosa, and K. pneumoniae were significant causes of both HAP and VAP in the public and private hospitals in this study. These organisms are also widely recognized as major nosocomial pathogens in Thailand and in Asia.13,15 Our research aligns with studies from Northeastern and Central Thailand, which found that three pathogens are frequently responsible for HAP and VAP.11,12,15,16 Similar reports of these three causative organisms have been noted in other Asian regions.7,8,17,18 Altogether, these suggest the persistent burden of these bacteria causing NP in our region and continent for the last decades.
Consistent with previous findings,17,18 we demonstrated that A. baumannii poses a significant threat to hospitalized patients receiving mechanical ventilation, as more than half of the VAP cases in our study were caused by this organism. This can be attributed to its biological niche and its ability to acquire antibiotic resistance, which includes resistance to desiccation, and persistence in hospital environment, such as on materials and medical devices.19,20 This indicates that the efforts of infection control program could be dedicated to effective infection control measures, including hand hygiene, contact isolation, oral and environmental decontamination, aspiration prevention, and assessment for weaning from the ventilator and avoiding reintubation, in preventing VAP caused by A. baumannii and other organisms.21,22
P. aeruginosa is an important cause of opportunistic infections in individuals with significant underlying diseases and in mechanically ventilated patients.23,24 Similar to previous findings, patients in our study with P. aeruginosa NP often have comorbidities such as renal or liver diseases, diabetes, and solid tumors.24,25 They also frequently have risk factors including prolonged hospitalization, use of invasive devices, and recent antibiotic treatment.24,25 However, our data show P. aeruginosa infection is slightly more prevalent in patients with HAP than in those with VAP.
The resistance to carbapenems (imipenem and meropenem) varies among three pathogens across different study hospitals. While the factors contributing to the differences in CR rates among tertiary-care hospitals remain unclear, several potential factors could be taken into consideration. These include patient-related factors, the hospital ward environment, the use of broad-spectrum antimicrobials, and infection control programs.26,27
Comparing the CR rates of A. baumannii, P. aeruginosa, and K. pneumoniae observed in our study with national data reported by the National Antimicrobial Resistance Surveillance Centre, Thailand (NARST) in 2019 and a recent large nationwide surveillance study (October 2017-December 2019),9,28 we found notable differences. The CR rate of A. baumannii in this study (92.5%) was substantially higher than both published reports (68.1% and 77%).9,28 Meanwhile, P. aeruginosa in this study had lower CR rate (16.9%) than the two nationwide reports (19.2% and 22.3%).9,28 The differences in CR rate of K. pneumoniae (20.6%) in this study compared to those reported (10.8% and 32%) were not definitive.9,28 One possible explanation for these differences lies in the composition of isolates included in the surveillance reports. Both NARST and the nationwide study included isolates from various clinical sources (such as blood, respiratory samples, and urine) and could represent both community-acquired and hospital-acquired cases.
In contrast to previous reports, a high proportion of bacteremic NP was observed in our study: 10% vs 68%, respectively.29,30 However, understanding the underlying reasons for this discrepancy remains challenging and cannot be determined from the study data. Several potential factors could be considered, including the local bacteriology of causative organisms, for example, A. baumannii with biofilm associated with bacteremic VAP,31 alternative infections,29 and/ or study selection criteria (included only patients with positive culture).
It has been demonstrated that hospitalized patients who experienced NP have an increased risk of rehospitalization after discharge.32 A recent database study in Europe reported that 12.6% of NP patients experienced a 30-day readmission, half of which were due to recurrent pneumonia.33 In our present study, only two percent of patients were rehospitalized due to A. baumannii NP within a median of four days after discharge. Several factors may contribute to the observed low 30-day readmission rate. First, the retrospective design that relied on data available in medical records, which may not capture all relevant information. For example, patients transferred to other hospitals or those who passed away during or after their index admission might not be fully accounted for. Some patients may have sought medical attention at different facilities after discharge, leading to underestimation of readmission rates. To gain a comprehensive understanding of NP’s impact on healthcare resources, future research should include robust readmission data and explore additional risk factors.
The findings of this study should be considered in light of several important limitations. First, due to the retrospective nature of this study, there may be selection bias. Medical records were selected based on the completeness of information required by the protocol, limited to patients with the diagnostic code of J15/J18 (bacterial pneumonia). Additionally, we did not consider the time window between HAP/VAP diagnosis and specimen collection to distinguish between HAP/VAP and HAI as recommended by the Centers for Disease Control’s National Healthcare Safety Network (CDC/NHSN).34 While the top three observed pathogens align with published literature, their burden might still be underestimated or overestimated due to the influence of HAI pathogens. Second, the present study involved tertiary-care hospitals, generalizability to other level of care hospitals is limited. Third, apart from carbapenem resistance, the study was designed to collect data on isolates that were susceptible to the tested antibiotics, not the classical susceptibility test results (susceptible, intermediate, and resistant). Therefore, the susceptibility findings should be interpreted with caution. Fourth, the study results are limited to the causative bacteria of HAP/VAP and do not include other types of pathogens, such as fungi and viruses. Fifth, the results of this study do not reflect the clinical burden due to the lack of mortality outcomes. Acknowledging these limitations, we suggest that future studies on nosocomial pneumonia report comprehensive microbiological data (including all identified pathogens and complete antibiotic susceptibility patterns), clinical data (including reasons for hospitalization), treatment information, and outcomes. Despite these limitations, our study systematically and retrospectively included HAP and VAP cases in 2019, providing valuable real-world data from four tertiary-care centers in urban Bangkok and its vicinity, Thailand. To the best of our knowledge, this represents the first multi-center study to investigate the 30-day rehospitalization rates for HAP and VAP in Thailand.
Conclusion
This real-world study in Thailand highlights the significant burden of A. baumannii, P. aeruginosa, and K. pneumoniae in HAP and VAP. A. baumannii and its high carbapenem resistance posed the highest health threat to hospitalized patients and the largest burden on health care resources. Understanding of the causative organisms and their susceptibility profiles is critical for the success of HAP and VAP antimicrobial management. Therefore, the implementation of regular review or surveillance for NP may substantially benefit patient outcomes and management of health care resources.
Abbreviations
AST, antimicrobial susceptibility testing; BAL, bronchoalveolar lavage; CI, confidence interval; CR, carbapenem resistance; CRF, case report form; ESBL, extended-spectrum beta-lactamases; ET, endotracheal; HAP, hospital-acquired pneumonia; HRU, healthcare resource utilization; ICU, intensive care unit; IQR, interquartile range; IMI, imipenem; MEM, meropenem; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; NARST, national antimicrobial resistance surveillance centre; NP, nosocomial pneumonia; SD, standard deviation; SMX, sulfamethoxazole; TMP, trimethoprim; VAP, ventilator-associated pneumonia; vHAP, ventilated hospital acquired pneumonia; WBC, white blood cell.
Acknowledgments
We would like to acknowledge the contributions provided by the registered nurse (RN) and study team members at Thammasat University Hospital (Sirinporn Sajak [RN], Chutikarn Intasorn, and Chankawee Komaratat), and at Siriraj Hospital (Arunee Singhachat [RN], Kwanruan Chalee [RN]) for their support in research conduct, data collection, and clinical and administrative work. We thank Pornsawan Permtermsin (MSD) and Nontakan Sricharoen (IQVIA) for project management oversight, Submuang Pimolchave (MSD) for project administrative support, Nakrob Pongumpai (MSD) for manuscript coordination, and Dr. Ee Min Tan (IQVIA) for the medical writing review.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors (Y.R., R.P., S.P., and T.K.) report no conflicts of interest in this work. VH is a full-time employee of IQVIA Inc. that was commissioned to conduct of this study. This study was financially supported by MSD (Thailand) Co. Ltd. The funder of the investigators and study had no role in data collection, data analysis, data interpretation, or writing of the manuscript.
References
1. Kalil AC, Metersky ML, Klompas M. et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin Infect Dis. 2016;63(5):e61–e111. doi:10.1093/cid/ciw353
2. Antoni T, Michael SN, Jean C, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respi J. 2017;50(3):1700582. doi:10.1183/13993003.00582-2017
3. Ling ML, Apisarnthanarak A, Madriaga G. The burden of healthcare-associated infections in Southeast Asia: a systematic literature review and meta-analysis. Clin Infect Dis. 2015;60(11):1690–1699. doi:10.1093/cid/civ095
4. Saleem Z, Godman B, Hassali MA, Hashmi FK, Azhar F, Rehman IU. Point prevalence surveys of health-care-associated infections: a systematic review. Pathog Glob Health. 2019;113(4):191–205. doi:10.1080/20477724.2019.1632070
5. Chawla R. Epidemiology, etiology, and diagnosis of hospital-acquired pneumonia and ventilator-associated pneumonia in Asian countries. Am J Infect Control. 2008;36(4 Suppl):S93–S100. doi:10.1016/j.ajic.2007.05.011
6. American Thoracic Society and Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi:10.1164/rccm.200405-644ST.
7. Kharel S, Bist A, Mishra SK. Ventilator-associated pneumonia among ICU patients in WHO Southeast Asian region: a systematic review. PLoS One. 2021;16(3):e0247832. doi:10.1371/journal.pone.0247832
8. Yin Y, Zhao C, Li H, et al. Clinical and microbiological characteristics of adults with hospital-acquired pneumonia: a 10-year prospective observational study in China. Eur J Clin Microbiol Infect Dis. 2021;40(4):683–690. doi:10.1007/s10096-020-04046-9
9. National antimicrobial resistant surveillance center Thailand. antimicrobial resistance 2000-2022. Available from: http://narst.dmsc.moph.go.th/data/AMR%202000-2022-12M.pdf.
10. Danchaivijitrmd S, Dhiraputra C, Santiprasitkul S, Judaeng T. Prevalence and impacts of nosocomial infection in Thailand 2001. J Med Assoc Thai. 2005;88(Suppl 10):S1–S9.
11. Arayasukawat P, So-ngern A, Reechaipichitkul W, et al. Microorganisms and clinical outcomes of early- and late-onset ventilator-associated pneumonia at Srinagarind Hospital, a tertiary center in Northeastern Thailand. BMC Pulm Med. 2021;21(1):47. doi:10.1186/s12890-021-01415-8
12. Werarak P, Kiratisin P, Thamlikitkul V. Hospital-acquired pneumonia and ventilator-associated pneumonia in adults at Siriraj Hospital: etiology, clinical outcomes, and impact of antimicrobial resistance. J Med Assoc Thai. 2010;93(Suppl 1):S126–S138.
13. Werarak P, Waiwarawut J, Tharavichitkul P, et al. Acinetobacter baumannii nosocomial pneumonia in tertiary care hospitals in Thailand. J Med Assoc Thai. 2012;95(Suppl 2):S23–S33.
14. Thai National Institute of Health, Department of medical science, ministry of public health, Thailand. Manual of microbiological laboratory standards; 2017. Available from: http://narst.dmsc.moph.go.th/manuals/standard_manual_2560.pdf.
15. Reechaipichitkul W, Phondongnok S, Bourpoern J, Chaimanee P. Causative agents and resistance among hospital-acquired and ventilator-associated pneumonia patients at Srinagarind Hospital, northeastern Thailand. Southeast Asian J Trop Med Public Health. 2013;44(3):490–502.
16. Poovieng J, Sakboonyarat B, Nasomsong W. Bacterial etiology and mortality rate in community-acquired pneumonia, healthcare-associated pneumonia and hospital-acquired pneumonia in Thai university hospital. Sci Rep. 2022;12(1):9004. doi:10.1038/s41598-022-12904-z
17. Feng DY, Zhou YQ, Zou XL, et al. Differences in microbial etiology between hospital-acquired pneumonia and ventilator-associated pneumonia: a single-center retrospective study in Guang Zhou. Infect Drug Resist. 2019;12:993–1000. doi:10.2147/idr.S204671
18. Dongol S, Kayastha G, Maharjan N, et al. Epidemiology, etiology, and diagnosis of health care acquired pneumonia including ventilator-associated pneumonia in Nepal. PLoS One. 2021;16(11):e0259634. doi:10.1371/journal.pone.0259634
19. Villegas MV, Hartstein AI. Acinetobacter outbreaks, 1977-2000. Infect Control Hosp Epidemiol. 2003;24(4):284–295. doi:10.1086/502205
20. McConnell MJ, Actis L, Pachón J. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev. 2013;37(2):130–155. doi:10.1111/j.1574-6976.2012.00344.x
21. Maselli DJ, Restrepo MI. Strategies in the prevention of ventilator-associated pneumonia. Ther Adv Respir Dis. 2011;5(2):131–141. doi:10.1177/1753465810395655
22. Berenholtz SM, Branson R, Cawcutt K, et al. Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 Update. Infect Control Hosp Epidemiol. 2022;43(6):687–713. doi:10.1017/ice.2022.88
23. Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171(11):1209–1223. doi:10.1164/rccm.200408-1044SO
24. Bouza E, Guillen-Zabala H, Rojas A, et al. Comparative study of the etiology of nosocomial bacteremic pneumonia in ventilated and non-ventilated patients: a 10-year experience in an institution. Microbiol Spectr. 2023;11(6):e01517–e01523. doi:10.1128/spectrum.01517-23
25. Rello J, Borgatta B, Lisboa T. Risk factors for Pseudomonas aeruginosa pneumonia in the early twenty-first century. Intensive Care Med. 2013;39(12):2204–2206. doi:10.1007/s00134-013-3046-1
26. Doron S, Davidson LE. Antimicrobial stewardship. Mayo Clin Proc. 2011;86(11):1113–1123. doi:10.4065/mcp.2011.0358
27. Aiesh BM, Nazzal MA, Abdelhaq AI, Abutaha SA, Zyoud SH, Sabateen A. Impact of an antibiotic stewardship program on antibiotic utilization, bacterial susceptibilities, and cost of antibiotics. Sci Rep. 2023;13(1):5040. doi:10.1038/s41598-023-32329-6
28. Yungyuen T, Chatsuwan T, Plongla R, et al. Nationwide surveillance and molecular characterization of critically drug-resistant gram-negative bacteria: results of the research university network Thailand study. Antimicrob Agents Chemother. 2021;65(9):e0067521. doi:10.1128/aac.00675-21
29. Zhang D, Yang D, Makam AN. Utility of blood cultures in pneumonia. Am J Med. 2019;132(10):1233–1238. doi:10.1016/j.amjmed.2019.03.025
30. Prapasiri P, Jareinpituk S, Keawpan A, et al. Epidemiology of radiographically-confirmed and bacteremic pneumonia in rural Thailand. Southeast Asian J Trop Med Public Health. 2008;39(4):706–718.
31. Chukamnerd A, Saipetch N, Singkhamanan K, et al. Association of biofilm formation, antimicrobial resistance, clinical characteristics, and clinical outcomes among Acinetobacter baumannii isolates from patients with ventilator-associated pneumonia. Clin Respir J. 2024;18(1):e13732. doi:10.1111/crj.13732
32. Balane JAL, Yap CDD, Villanueva CAG, Palileo-Villanueva LAM, Tamondong-Lachica DR. Predictors of readmission in a medical department of a tertiary university hospital in the Philippines. BMC Health Serv Res. 2023;23(1):617. doi:10.1186/s12913-023-09608-z
33. Kam Sing H, Jacqueline S, James S. Thirty-day readmission among patients with non-ventilator hospital acquired pneumonia and effects on outcomes. Eur Respir J. 2019;54(suppl 63):PA2916. doi:10.1183/13993003.congress-2019.PA2916
34. Centers for Disease Control and National Healthcare Safety Network. CDC/NHSN surveillance definitions for specific types of infections; 2024. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

