Back to Journals » Journal of Pain Research » Volume 18
Evaluating Triptan Safety in Pediatric Migraine Management: A Comprehensive Pharmacovigilance Analysis
Authors Chen J, Huang S, Chen Y, Luo C, Li Y
Received 6 March 2025
Accepted for publication 12 June 2025
Published 27 June 2025 Volume 2025:18 Pages 3185—3205
DOI https://doi.org/10.2147/JPR.S524809
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Giorgio Veneziano
Junchen Chen, Shunqiu Huang, Yashi Chen, Cheng Luo, Yong Li
Department of Neurosurgery, the First Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong, People’s Republic of China
Correspondence: Yong Li, Email [email protected]
Introduction: Triptans are the only FDA-approved migraine-specific treatment for pediatric patients, yet comprehensive real-world safety data remains limited, particularly regarding rare adverse events and age-specific safety profiles.
Methods and Materials: We conducted a pharmacovigilance analysis using the FDA Adverse Event Reporting System (FAERS) database from 2004– 2024, focusing on adverse events associated with sumatriptan, rizatriptan, zolmitriptan, and almotriptan in patients aged 6– 18 years. After systematic deduplication following FDA guidelines, disproportionality analysis was performed using reporting odds ratios (ROR) and risk-signal detection ratios (RSDR). Subgroup analyses compared safety signals between children (6– 11 years) and adolescents (12– 18 years) at both Preferred Terms and System Organ Class levels.
Results: Among 19,557 triptan-related cases in FAERS, 375 (1.9%) were pediatric cases, predominantly female (70.7%) and aged 12– 18 years (91.2%). Sumatriptan was most frequently reported (77.3%), followed by rizatriptan (17.9%). Significant safety signals included posterior reversible encephalopathy syndrome with sumatriptan (ROR=86.69, 95% CI=26.6– 282.54), acute respiratory failure with rizatriptan (ROR=98.12, 95% CI=40.17– 239.64), and renal infarction with zolmitriptan (ROR=2231.93, 95% CI=667.65– 7461.24). Age-stratified analysis revealed distinct profiles: younger children (6– 11 years) showed higher risks for gastric emptying impairment (ROR=331.24) and throat tightness (ROR=77.14), while adolescents (12– 18 years) experienced more diverse adverse events, notably pharyngeal swelling (ROR=133.81) and chest discomfort (ROR=19.05).
Conclusion: Real-world triptan safety profiles reveal age-specific risks in pediatric populations, emphasizing the need for tailored monitoring strategies and age-appropriate safety protocols.
Keywords: triptans, faers, migraine, pediatric, pharmacovigilance, adverse events
Introduction
Migraine is a widespread issue that poses a significant burden on people across various age ranges and populations.1 In childhood, migraine is the most frequent primary headache, with its prevalence increasing with age and mainly changing in connection with puberty, occurring between 8–14 years for girls and 9–15 years for boys.2,3 Triptans are considered the standard of care for acute migraine treatment, with 5-Hydroxytryptamine 1 (5-HT1) receptor agonists remaining a mainstream of migraine therapy and research.4 Triptans are a crucial tool in the acute treatment of pediatric migraine headaches, as they are the only FDA-approved migraine-specific treatment available for children and adolescents.5–9
The safety profile of triptans in pediatric populations has been well established through systematic reviews and meta-analyses, consistently demonstrating that triptans are generally well-tolerated in children and adolescents, with mild and transient adverse events such as fatigue, dizziness, debility, dry mouth, nausea/vomiting, and taste disturbance or nasal symptoms.10–12 These side effects are self-limiting and rarely lead to treatment discontinuation, with no significant differences observed between age groups or formulations. Importantly, no serious adverse events have been reported in pediatric trials, and while triptans have vasoconstrictive properties via 5-HT1B receptors, cardiovascular risks have not been a significant concern in this population, though caution is advised for those with cardiovascular risk factors.13,14
However, despite the established safety profile of triptans in pediatric migraine treatment, several critical knowledge gaps remain. Unlike adults, children and adolescents have different pharmacokinetic and pharmacodynamic profiles, which may influence drug efficacy and safety. While systematic reviews and meta-analyses have provided valuable insights into common adverse events, they are limited in detecting rare but potentially serious adverse reactions due to their inherent methodological constraints and relatively small sample sizes.10,11 Additionally, there is insufficient evidence comparing safety profiles across different age groups (6–11 versus 12–18 years) and among various triptan formulations in pediatric populations.12 Furthermore, the real-world safety surveillance data for pediatric triptan use remains inadequate, particularly regarding the detection and assessment of rare adverse events.13 Clinical trial data on triptan use in the pediatric population remain limited, resulting in uncertainties about optimal dosing and monitoring. This gap raises concerns about potential adverse events, some of which may be rare but clinically significant in this vulnerable group. Therefore, predicting rare adverse events in pediatric patients treated with triptans is critically important. Early identification of these events can guide clinicians to balance the benefits of migraine relief with safety considerations, ultimately improving treatment outcomes and reducing risk. Understanding these risks is essential to optimizing pediatric migraine care and supports the rationale for our study focusing on safety surveillance in this population. The FDA Adverse Event Reporting System (FAERS) database, with its large-scale post-marketing surveillance data, offers a unique opportunity to address these knowledge gaps through comprehensive pharmacovigilance signal detection analysis, enabling the identification of potential safety signals across different age groups and triptan formulations, while also capturing rare adverse events that may not be detected in traditional clinical trials.15,16
Therefore, this study aimed to conduct a comprehensive pharmacovigilance analysis using the FAERS database to address these knowledge gaps. We focused on four commonly prescribed triptans (sumatriptan, rizatriptan, zolmitriptan, and almotriptan) in pediatric populations aged 6–18 years. Through disproportionality analysis of adverse event reports, we sought to: (1) evaluate the safety signals of triptans in pediatric populations; (2) compare safety profiles between different age subgroups (6–11 versus 12–18 years); and (3) identify potential rare but serious adverse events. This large-scale pharmacovigilance study represents the first systematic effort to leverage real-world post-marketing surveillance data for comprehensive safety signal detection in pediatric triptan use, providing valuable insights for clinical decision-making and risk management in pediatric migraine treatment.
Methods and Materials
Study Design and Data Source
We performed a pharmacovigilance analysis of adverse events linked to four triptans (Sumatriptan, Rizatriptan, Zolmitriptan, and Almotriptan) that have been used in children aged 6 to 12, utilizing the FAERS database. FAERS, a vital public database managed by the FDA, is used for collecting reports on adverse events and medication errors linked to approved medications.16 The study made use of OpenVigil 2.1 software to extract adverse event report data for four triptans from the FAERS database, ranging from the first quarter of 2004 to the third quarter of 2024. This study is a secondary analysis of publicly available, anonymized data from the FAERS. Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements.
Data Extraction and Descriptive Analysis
The FAERS database captures data through spontaneous reporting, which inherently carries the risk of duplicate entries or withdrawn reports.15 To address these limitations, the FDA has issued an official guidance document detailing deduplication protocols and criteria for identifying reports to be excluded. In alignment with these directives, this study rigorously adhered to the FDA’s recommendations for data refinement, as outlined on its official website. The refinement process involved the systematic removal of duplicate entries using an FDA-endorsed methodology. Specifically, the fields Primary ID(PRIMARYID), Case ID(CASEID), and FDA Date(FDA_DT) were extracted from the Demographics(DEMO) table, and the data were organized hierarchically by CASEID, FDA_DT, and PRIMARYID. Where multiple reports shared the same CASEID, the entry with the most recent FDA_DT value was retained. Similarly, in cases where both CASEID and FDA_DT were identical, the report with the highest PRIMARYID value was preserved. Furthermore, beginning in the first quarter of 2004, each quarterly data set included an appended list of reports flagged for deletion. After deduplication, the reports were removed based on the CASEID listings in the deletion report log. The deduplication and data refinement were carried out using FDA-approved methods.17
Statistical Analysis
Disproportionality analysis was used in data mining to assess the link between triptans and specific adverse events in the PT category. To identify signals indicating a possible increased risk of drug-associated adverse events, the reporting odds ratio (ROR) from the 2×2 crosstab (Supplementary Table 1) was employed. The risk-signal detection ratio (RSR) is calculated as the proportion of a risk signal to all PT reports for each drug. Supplementary Table 2 illustrates the calculation of ROR and the criteria for a significant signal. A potential risk signal was deemed significant if it had at least three reported cases, a ROR of 2 or more, a lower 95% confidence interval limit of at least 1, and a Chi-square test value of 4 or higher.18
Subgroup Analysis
Risk signal results for patients in each age group will be assessed according to the SOC level in the subgroup analysis. Subsequently, the results for each age group were organized by the number of cases, and the top 10 adverse events for triptans were compared across age groups. We evaluated these four triptans in two subgroups, ages 6–11 and ages 12–18, based on the pediatric age ranges indicated in FDA drug labels.
Results
Characteristics of the Population Data for Triptans
The details of patients and adverse events can be found in Table 1. In the FAERS database, a total of 19,557 cases involving triptan use were reported, of which 375 (1.9%) were pediatric cases. Among these pediatric cases, the majority were female (265 cases, 70.7%), while males accounted for 102 cases (27.2%), and gender was unknown in 8 cases (2.1%). The age distribution showed that most pediatric patients were aged 12–18 years (342 cases, 91.2%), with a smaller proportion aged 6–11 years (33 cases, 8.8%). The United States reported the most pediatric cases with 154 (41.1%), followed by the United Kingdom with 131 cases (34.9%), Japan with 11 cases (2.9%), and both Germany and the Netherlands with 10 cases each (2.7%). Regarding the specific triptans used, sumatriptan was the most frequently reported drug (290 cases, 77.3%), followed by rizatriptan (67 cases, 17.9%), zolmitriptan (15 cases, 4.0%), and almotriptan (3 cases, 0.8%). Clinical outcomes revealed that 83 pediatric cases (22.1%) resulted in hospitalization, while 14 cases (3.7%) were classified as life-threatening. Disability was reported in 18 cases (4.8%), and there were 2 deaths (0.5%).
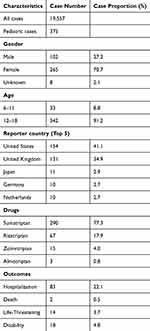 |
Table 1 Clinical Characteristics of Pediatric Patients Using Triptans |
Disproportionality Analysis Characteristics for Triptans
We identified AE signals for four triptans in pediatric patients. Figure 1 highlights the distribution of adverse event cases across System Organ Class (SOC) for pediatric patients using triptans, with the majority of cases reported in the 12–18 years age group compared to the 6–11 years group. The most frequently affected SOCs were General Disorders and Administration Site Conditions (274 cases), Nervous System Disorders (201 cases), and Respiratory, Thoracic, and Mediastinal Disorders (116 cases), with the older age group consistently contributing the majority of cases in each category.
The distribution of Preferred Terms (PTs) linked to triptans across SOCs showed notable differences between the general and pediatric populations (Supplementary Table 3). In the general population, the most frequently reported SOCs were General Disorders and Administration Site Conditions (83.48%, 15,523 cases), Nervous System Disorders (79.04%, 5,031 cases), and Product Issues (98.49%, 8,196 cases). In the pediatric population, the leading SOCs were General Disorders and Administration Site Conditions (74.45%, 204 cases), Nervous System Disorders (73.13%, 147 cases), and Respiratory, Thoracic, and Mediastinal Disorders (86.21%, 100 cases).
Several SOCs showed substantial differences between groups. Respiratory, Thoracic, and Mediastinal Disorders were more prevalent in children (86.21%) than in the general population (33.57%). Similarly, Musculoskeletal and Connective Tissue Disorders occurred in 80.95% of pediatric cases compared to 38.78% in adults. Conversely, Product Issues dominated in the general population (98.49%) but were less frequent in pediatric cases (60%). Other notable findings include Metabolism and Nutrition Disorders, comprising 83.33% of pediatric cases but only 2% in adults. Immune System Disorders were also less prevalent in children (57.14%) than in the general population (91.28%).
Figure 2 compares the Risk-Signal Detection Ratios (RSDRs) of four triptans between the general and pediatric populations. In the pediatric population, sumatriptan exhibited the highest RSDR at 28.09%, while rizatriptan had the highest RSDR in the general population at 17.99%. In contrast, Almotriptan had the lowest RSDR in both groups, with 11.65% in the general population and 11.11% in the pediatric group.
Supplementary Table 4 presents the disproportionality analysis of adverse events (Risk PTs) associated with four triptans in the general and pediatric populations. Sumatriptan showed the highest proportion of Risk PTs in both populations, with 20.06% in the general population and a significantly higher 39.05% in the pediatric population (p < 0.01), indicating a statistically significant difference. While rizatriptan, zolmitriptan and almotriptan showed comparable proportions of Risk PTs between the two populations, with no statistically significant differences.
Risk Signal Distribution in Pediatric Patients
System Organ Class-Specific Safety Signals
Pharmacovigilance analysis identified statistically significant safety signals and negative signals across various system organ classes (SOCs)(Table 2). In nervous system disorders, posterior reversible encephalopathy syndrome (PRES) was a significant positive signal associated with sumatriptan, with 3 cases reported (ROR=86.69, 95% CI=26.6–282.54), while somnolence also exhibited a positive signal with sumatriptan, based on 8 cases (ROR=3.13, 95% CI=1.96–4.99). Conversely, medication overuse headache was identified as a negative signal due to insufficient case numbers (N=1, ROR=3936.38, 95% CI=500.54–30,956.77). For respiratory disorders, acute respiratory failure linked to rizatriptan showed a strong positive signal, with 5 cases reported (ROR=98.12, 95% CI=40.17–239.64), and pharyngeal swelling was a notable positive signal for almotriptan, with 8 cases reported (ROR=197.85, 95% CI=121.03–323.44). In contrast, dyspnoea with zolmitriptan represented a negative signal (N=3, ROR=1.84, 95% CI=0.46–7.39).
 |
Table 2 The ROR of the AE Reports in Pediatrics |
For renal and urinary disorders, renal infarction associated with zolmitriptan was a strong positive signal, with 3 cases reported (ROR=2231.93, 95% CI=667.65–7461.24), and prerenal failure linked to almotriptan was another strong signal, with 2 cases (ROR=5160.68, 95% CI=1132.64–23,513.76). Conversely, acute kidney injury with sumatriptan was a negative signal, with 3 cases reported (ROR=1.41, 95% CI=0.45–4.39). For psychiatric disorders, positive signals included visual hallucinations with zolmitriptan, based on 1 case (ROR=40.11, 95% CI=5.49–292.79), and euphoric mood with rizatriptan, also with 1 case (ROR=127.16, 95% CI=17.38–930.17).
Drug-Specific Risk Profiles
Distinct safety profiles were observed for each triptan based on this pharmacovigilance analysis(Table 2). For sumatriptan, a strong positive signal was identified for posterior reversible encephalopathy syndrome (PRES) with 3 cases reported (ROR=86.69, 95% CI=26.6–282.54) and somnolence with 8 cases (ROR=3.13, 95% CI=1.96–4.99). However, a negative signal was noted for thrombocytopenia with 3 cases (ROR=1.43, 95% CI=0.46–4.45). Rizatriptan showed a strong positive signal for acute respiratory failure, based on 5 cases (ROR=98.12, 95% CI=40.17–239.64), and for hyperacusis, with 3 cases (ROR=35.48, 95% CI=11.29–111.49). A negative signal was identified for tachycardia with 3 cases (ROR=1.07, 95% CI=0.34–3.32). Zolmitriptan was associated with significant positive signals for renal infarction, with 3 cases (ROR=2231.93, 95% CI=667.65–7461.24), and dysarthria, with 9 cases (ROR=37.62, 95% CI=16.67–84.93), but headache was a negative signal with 8 cases (ROR=0.83, 95% CI=0.41–1.66). Lastly, almotriptan demonstrated strong positive signals for prerenal failure, based on 2 cases (ROR=5160.68, 95% CI=1132.64–23,513.76), and pharyngeal swelling, with 8 cases (ROR=197.85, 95% CI=121.03–323.44), while vomiting, with 2 cases, was identified as a negative signal (ROR=12.64, 95% CI=2.85–56.02).
Age-Stratified Subgroup Analysis
The age-stratified subgroup analysis revealed distinct adverse event profiles between the younger age group (6–11 years) and the older age group (12–18 years) for triptan use(Table 3). In the younger group (n=33), dyspnoea was the most frequently reported adverse event, with 4 cases (12.12%) and a significant ROR of 11.06 (95% CI: 4.03–30.33). Other notable signals included nausea (3 cases, 9.09%, ROR=5.95, 95% CI: 1.87–18.9), arthralgia (3 cases, 9.09%, ROR=14, 95% CI: 4.4–44.53), and impaired gastric emptying, which showed an exceptionally high ROR of 331.24 (95% CI: 78.62–1395.66) based on 2 cases (6.06%). Additionally, throat tightness (2 cases, 6.06%, ROR=77.14, 95% CI: 18.78–316.94) and chest pain (2 cases, 6.06%, ROR=13.92, 95% CI: 3.41–56.81) were significant signals, while vomiting (2 cases, 6.06%, ROR=1.98, 95% CI: 0.49–8.08) and headache (2 cases, 6.06%, ROR=2.56, 95% CI: 0.63–10.43) were less prominent. In contrast, the older group (n=342) exhibited a broader range of adverse events, with dyspnoea remaining a significant signal (33 cases, 9.65%, ROR=5.84, 95% CI: 4.13–8.27). Other frequently reported events included chest discomfort (25 cases, 7.31%, ROR=19.05, 95% CI: 12.77–28.43), application site erythema (24 cases, 7.02%, ROR=67.77, 95% CI: 44.64–102.91), and migraine (24 cases, 7.02%, ROR=18.2, 95% CI: 12.1–27.38). Additionally, pharyngeal swelling showed an extremely high ROR of 133.81 (95% CI: 81.54–219.58) with 18 cases (5.26%), while vomiting (19 cases, 5.56%, ROR=1.61, 95% CI: 1.03–2.54) and nausea (19 cases, 5.56%, ROR=1.67, 95% CI: 1.06–2.63) were less pronounced. These findings highlight age-related differences in adverse event profiles, with younger children showing higher risks for rare but severe events such as impaired gastric emptying and throat tightness, while older adolescents experienced a wider variety of adverse events, including pharyngeal swelling and chest discomfort.
 |
Table 3 Subgroup Analysis of the Top 10 Specific Risk Signals in the Younger Age Group (6–11 Years) and Older Age Group (12–18 Years) for Triptans |
Discussion
Our pharmacovigilance analysis using FAERS data revealed significant discrepancies between clinical trial findings and real-world safety profiles of triptans in pediatric populations. Previous clinical trials and systematic reviews have consistently characterized triptans as well-tolerated medications with primarily mild adverse events.13 For instance, a randomized, double-blind trial of sumatriptan nasal spray reported mainly mild adverse events such as taste disturbance (26%) and nausea (9%).6 Similarly, clinical trials of rizatriptan and zolmitriptan demonstrated generally favorable safety profiles with predominantly mild and self-limiting adverse events.7,9However, our real-world data analysis uncovered several previously unreported serious adverse events that warrant attention. Most notably, we identified significant safety signals for severe neurological complications such as PRES associated with sumatriptan. We also found concerning signals for organ-specific adverse events, including renal infarction with zolmitriptan, acute respiratory failure with rizatriptan, and prerenal failure with almotriptan.10,11 These serious adverse events were not detected in previous clinical trials, highlighting the limitations of pre-marketing safety assessments.10,11
The disparity between clinical trial data and our real-world findings can be attributed to several factors. First, clinical trials typically employ strict inclusion/exclusion criteria and relatively small sample sizes, which may limit their ability to detect rare adverse events.13 Second, the controlled environment of clinical trials does not fully capture the complexities of real-world clinical practice, where factors such as comorbidities, concomitant medications, and varying adherence patterns can significantly influence safety outcomes.14 Third, the short duration and limited sample size of clinical trials make it challenging to identify rare but serious adverse events that may only become apparent with widespread use.12
Previous studies on triptan safety in pediatric populations have primarily focused on overall safety profiles without detailed age-stratified analyses.10,11 While some clinical trials have included both children and adolescents, the age-specific safety differences have not been systematically evaluated.6,7 A recent systematic review highlighted the need for age-specific safety assessments but was limited by the small sample sizes and restricted age ranges in available clinical trials.10
Our analysis of the FAERS database revealed a range of commonly reported side effects associated with triptan use in the pediatric population, as detailed in Table 3. These frequently reported events include neurological symptoms such as dizziness, fatigue, somnolence, and paresthesia, along with gastrointestinal complaints like nausea and vomiting. Although these adverse events are generally transient and non-life-threatening, they may affect patient comfort and treatment adherence. These findings correspond well with pediatric clinical trial data, where similar side effects have been consistently documented and are generally well tolerated.6,19 Recognizing and counseling patients and caregivers about these side effects is critical for managing expectations and supporting adherence to therapy. Therefore, a comprehensive safety assessment that encompasses both common and rare adverse events offers a more complete understanding of triptan tolerability in pediatric migraine treatment, ultimately facilitating informed clinical decision-making and optimizing therapeutic outcomes.
In our study, there were two reported deaths associated with triptan use in the pediatric population. While such fatal outcomes are deeply concerning, currently there is a lack of published evidence, particularly in children and adolescents, describing deaths directly attributable to triptan use. Most safety data on serious outcomes including death have been derived from adult populations. For instance, a large population-based cohort study in adults by Ghanshani et al found no significant association between triptan exposure and increased risk of acute myocardial infarction, heart failure, or all-cause death.20 This study, which included over 130,000 triptan users with a mean follow-up of nearly six years, provides reassuring evidence regarding the cardiovascular and overall safety of triptans in migraine treatment. The adjusted hazard ratio for all-cause mortality was 1.00 (95% CI 0.93–1.08), indicating that triptans do not elevate mortality risk in adults. While these findings provide reassurance about the cardiovascular and overall safety of triptans in adult populations, pediatric data remain limited. Nevertheless, given the potential severity, clinicians should exercise caution especially in children with underlying cardiovascular risk factors or other comorbidities. Further pharmacoepidemiologic studies specifically focused on pediatric populations are warranted to better characterize these rare but critical safety events.
Regarding specific adverse events, sumatriptan showed a significantly higher proportion of Risk PTs in pediatric patients (39.05%) compared to the general population (20.06%, p<0.01), particularly for neurological adverse events such as PRES. Our pharmacovigilance analysis also revealed distinct safety profiles between younger children (6–11 years) and adolescents (12–18 years). In the younger age group, we identified significant signals for respiratory and gastrointestinal adverse events, with notably high risks for dyspnoea and impaired gastric emptying. This finding aligns with recent research suggesting that younger children may have increased sensitivity to respiratory and gastrointestinal effects of medications due to developmental differences in organ systems.21 In contrast, adolescents exhibited a broader spectrum of adverse events, with prominent signals for pharyngeal swelling and chest discomfort, suggesting potentially different mechanisms of drug response in this age group.
These age-related differences in safety profiles can be attributed to several factors identified in recent literature. First, developmental changes in neurotransmitter systems and receptor expression patterns may influence drug responses across different age groups.22 The distribution and density of 5-HT receptors, particularly those involved in pain modulation and neurotransmitter regulation, may differ significantly between children and adolescents.23,24 Second, age-related differences in drug metabolism and clearance may contribute to varying safety profiles, as highlighted by recent pharmacokinetic studies in pediatric populations.25,26 Third, it may be attributed to age-related differences in blood-brain barrier(BBB) permeability and neurovascular regulation. The immature BBB may allow greater drug penetration into the central nervous system, potentially increasing the risk of neurological adverse events.27,28 Furthermore, emerging evidence suggests that pubertal status may influence drug responses, particularly for medications affecting neurovascular systems.29,30 These findings underscore the importance of age-specific dosing strategies and monitoring protocols in pediatric triptan use.
Our analysis identified PRES and renal infarction as critical safety signals associated with triptan use in pediatric populations, with ROR of 86.69 and 2231.93 respectively. These findings carry substantial clinical implications given the potential for irreversible organ damage. PRES, characterized by vasogenic edema in posterior cerebral regions, may manifest with seizures (60% −75% cases) or visual disturbances (33%).31 The pathophysiology behind the association between triptans and PRES is thought to involve vasoconstriction and hypoperfusion, which can lead to endothelial dysfunction and subsequent vasogenic edema.32,33 Previous case reports demonstrate temporal correlations between triptan use and acute renal infarction, supported by imaging evidence of wedge-shaped perfusion defects and arterial thrombosis.34,35 The association between triptans and renal infarction, though rare, arises from their vasoconstrictive properties via 5-HT₁B/₁D receptor activation in renal arteries, exacerbated in children by immature vasculature with heightened receptor density (2.3×adults) and reduced endothelial nitric oxide synthase activity.36–38
At the SOC level, several notable differences emerged. Respiratory, Thoracic, and Mediastinal Disorders showed substantially higher prevalence in pediatric patients (86.21%) compared to the general population (33.57%), suggesting increased respiratory sensitivity in children. Similarly, Musculoskeletal and Connective Tissue Disorders were more frequent in pediatric cases (80.95%) than in adults (38.78%). These differences may reflect developmental variations in organ system maturity and drug response patterns.39,40
Each triptan demonstrated characteristic safety signals, reflecting their distinct pharmacological properties. Sumatriptan showed significant signals for neurological events, while rizatriptan was associated with respiratory complications. Zolmitriptan exhibited strong signals for renal events and almotriptan showed significant signals for pharyngeal swelling. These differences may reflect variations in receptor selectivity and tissue distribution patterns among triptans.41,42 Our analysis revealed distinct patterns in adverse event reporting among different triptans. Sumatriptan was the most frequently reported triptan in pediatric cases. This higher reporting frequency for sumatriptan may be attributed to its broader usage pattern and longer market presence, allowing for more comprehensive safety data collection.43,44 Additionally, sumatriptan’s diverse formulations (oral, nasal, subcutaneous) may contribute to its higher reporting rate, as different administration routes can present unique safety profiles.45 Nasal formulations showed higher associations with local adverse events such as pharyngeal swelling and respiratory symptoms, while oral formulations were more commonly associated with gastrointestinal events.46 Subcutaneous formulations demonstrated unique injection-site reactions and a higher frequency of systemic effects, possibly due to rapid absorption and higher bioavailability.47 These findings suggest that formulation choice should be guided by individual patient characteristics and risk factors.
Our pharmacovigilance analysis provides crucial insights for optimizing triptan selection and monitoring strategies in pediatric populations. The distinct safety profiles observed between age groups suggest the need for age-specific treatment approaches. In younger children, the high risk of respiratory and gastrointestinal adverse events, particularly dyspnea and impaired gastric emptying, necessitates careful monitoring of these systems during treatment. For adolescents, the broader spectrum of adverse events, including pharyngeal swelling and chest discomfort, requires comprehensive monitoring protocols. The identification of high-risk subgroups warrants special attention. Patients with pre-existing respiratory conditions may be particularly vulnerable to adverse events, given the high prevalence of respiratory complications in pediatric cases. Based on these findings, we recommend implementing a systematic monitoring strategy: (1) comprehensive baseline assessment focusing on respiratory, cardiovascular, and renal function before initiating triptan therapy; (2) regular monitoring of organ-specific adverse events, particularly in high-risk patients; and (3) age-appropriate follow-up protocols with enhanced vigilance for rare but serious complications such as PRES. These measures, combined with individualized risk assessment, can help optimize the safety of triptan therapy in pediatric populations.
Several limitations of this study should be acknowledged. First, as with all FAERS-based studies, the data relies on spontaneous reporting, which may lead to underreporting of adverse events and reporting bias.48 The actual incidence of adverse events cannot be calculated due to the lack of denominator data regarding the total number of pediatric patients exposed to triptans.49 Second, reports from non-healthcare professionals may introduce diagnostic bias, and the database may contain incomplete or inaccurate information regarding patient characteristics, medical history, and concurrent medications.50 Additionally, the causality between triptans and reported adverse events cannot be definitively established through this type of observational study. Third, while our analysis revealed important age-specific safety signals, the relatively small number of pediatric cases, particularly in the 6–11 age group (33 cases, 8.8%), may limit the statistical power for detecting rare adverse events in this population.51 The uneven distribution of cases across age groups may also affect the generalizability of our findings. Fourth, the potential impact of confounding factors, such as comorbidities, concomitant medications, and migraine severity, could not be fully assessed due to the inherent limitations of spontaneous reporting systems.52 Furthermore, the lack of standardized follow-up information makes it challenging to evaluate the long-term outcomes of reported adverse events. Finally, our analysis may be affected by various forms of bias inherent to pharmacovigilance studies, including selective reporting of more severe cases, stimulated reporting due to safety alerts, and the Weber effect.53 These factors should be considered when interpreting our findings, and further validation through prospective clinical studies is warranted.
Conclusion
In conclusion, this comprehensive pharmacovigilance analysis of triptan safety in pediatric populations reveals important differences between real-world safety profiles and clinical trial findings, highlighting the need for age-specific safety monitoring approaches. The identification of previously unreported serious adverse events, particularly PRES with sumatriptan and renal complications with zolmitriptan, emphasizes the value of post-marketing surveillance in detecting rare but significant safety signals. The distinct safety profiles observed between younger children and adolescents underscore the importance of age-tailored treatment strategies and monitoring protocols. While triptans remain valuable tools in pediatric migraine management, our findings suggest the need for enhanced vigilance, particularly in high-risk subgroups and specific organ systems. Future research should focus on prospective studies to validate these safety signals, investigate the mechanisms underlying age-specific adverse events, and develop targeted risk mitigation strategies. Additionally, the development of pediatric-specific formulations and dosing regimens that account for developmental differences in drug response and metabolism could help optimize the benefit-risk profile of triptans in children. Finally, the establishment of dedicated pediatric pharmacovigilance networks could improve the detection and characterization of age-specific safety signals in real-world settings.
Abbreviations
5-HT1, 5-Hydroxytryptamine 1; BBB, blood-brain barrier; CASEID, Case ID; DEMO, Demographics; FAERS, FDA Adverse Event Reporting System; FDA_DT, FDA Date; PRES, posterior reversible encephalopathy syndrome; PRIMARYID, Primary ID; PTs, Preferred Terms; RSDRs, Risk-Signal Detection Ratios; ROR, reporting odds ratio.
Ethic Statement
According to item 1 and 2 of Article 32 of the Measures for Ethical Review of Life Science and Medical Research Involving Human Subjects dated February 18, 2023, China, research projects meeting one of the following conditions may apply for exemption from ethical review: research conducted using legally obtained publicly available data, or research using anonymized data that cannot identify specific individuals and does not involve personal privacy or commercial interests.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yonker ME, McVige J, Zeitlin L, Visser H. A multicenter, randomized, double-blind, placebo-controlled, crossover trial to evaluate the efficacy and safety of zolmitriptan nasal spray for the acute treatment of migraine in patients aged 6 to 11 years, with an open-label extension. Headache. 2022;62(9):1207–1217. doi:10.1111/head.14391
2. Ursitti F, Valeriani M. Migraine in childhood: gender differences. Eur J Paediatr Neurol. 2023;42:122–125.
3. Mortimer MJ, Kay J, Jaron A. Epidemiology of headache and childhood migraine in an urban general practice using Ad Hoc, Vahlquist and IHS criteria. Dev Med Child Neurol. 1992;34(12):1095–1101.
4. Tanaka M, Török N, Vécsei L. Are 5-HT(1) receptor agonists effective anti-migraine drugs. Expert Opin Pharmacother. 2021;22(10):1221–1225.
5. Chatterjee JH, Blume HK. Triptans in the acute migraine management of children and adolescents: an update. Curr Pain Headache Rep. 2024;28(7):641–649.
6. Winner P, Rothner AD, Saper J, et al. A randomized, double-blind, placebo-controlled study of sumatriptan nasal spray in the treatment of acute migraine in adolescents. Pediatrics. 2000;106(5):989–997.
7. Ahonen K, Hämäläinen ML, Eerola M, Hoppu K. A randomized trial of rizatriptan in migraine attacks in children. Neurology. 2006;67(7):1135–1140.
8. Gelfand AA, Goadsby PJ. Treatment of pediatric migraine in the emergency room. Pediatr Neurol. 2012;47(4):233–241.
9. Lewis DW, Winner P, Hershey AD, Wasiewski WW, Committee AMS. Efficacy of zolmitriptan nasal spray in adolescent migraine. Pediatrics. 2007;120(2):390–396.
10. Evers S. The efficacy of triptans in childhood and adolescence migraine. Curr Pain Headache Rep. 2013;17(7):342.
11. Richer L, Billinghurst L, Linsdell MA, et al. Drugs for the acute treatment of migraine in children and adolescents. Cochrane Database Syst Rev. 2016;4(4):CD005220.
12. Wöber-Bingöl Ç. Pharmacological treatment of acute migraine in adolescents and children. Paediatr Drugs. 2013;15(3):235–246.
13. Chanchlani R, Agrawal A, Janjua D, Hafsa SN. The efficacy of different triptans for the treatment of acute headache in pediatric migraine: a systematic review. Indian Pediatr. 2023;60(8):663–671.
14. Dodick D, Lipton RB, Martin V, et al. Consensus statement: cardiovascular safety profile of triptans (5-HT agonists) in the acute treatment of migraine. Headache. 2004;44(5):414–425.
15. Wang G, Su L, Liu Y, et al. Cardiovascular toxicity of tisagenlecleucel in children and adolescents: analysis of spontaneous reports submitted to FAERS. Front Immunol. 2025;16:1499143.
16. Zhang Z, He J, Liang Y, et al. Adverse events associated with azithromycin and clarithromycin in adults aged ≥65: a disproportionality analysis of the FDA Adverse Event Reporting System (FAERS) database. Expert Opin Drug Saf. 2024;1–8.
17. Fusaroli M, Isgrò V, Cutroneo PM, et al. Post-marketing surveillance of car-t-cell therapies: analysis of the Fda adverse event reporting system (FAERS) Database. Drug Saf. 2022;45(8):891–908.
18. Xue Y, Feng S, Li G, Zhang C. Safety profile of vascular endothelial growth factor receptor tyrosine-kinase inhibitors in pediatrics: a pharmacovigilance disproportionality analysis. Front Pharmacol. 2023;14:1160117.
19. Winner P, Rothner AD, Wooten JD, Webster C, Ames M. Sumatriptan nasal spray in adolescent migraineurs: a randomized, double-blind, placebo-controlled, acute study. Headache. 2006;46(2):212–222. doi:10.1111/j.1526-4610.2006.00339.x
20. Ghanshani S, Chen C, Lin B, Duan L, Shen YA, Lee MS. Risk of acute myocardial infarction, heart failure, and death in migraine patients treated with triptans. Headache. 2020;60(10):2166–2175. doi:10.1111/head.13959
21. Johnson TN, Rostami-Hodjegan A. Resurgence in the use of physiologically based pharmacokinetic models in pediatric clinical pharmacology: parallel shift in incorporating the knowledge of biological elements and increased applicability to drug development and clinical practice. Paediatr Anaesth. 2011;21(3):291–301.
22. McLaughlin MJ, Wagner J, Shakhnovich V, Carleton B, Leeder JS. Considerations for Implementing Precision Therapeutics for Children. Clin Transl Sci. 2019;12(2):140–150.
23. Eide PK, Hole K. The role of 5-hydroxytryptamine (5-HT) receptor subtypes and plasticity in the 5-HT systems in the regulation of nociceptive sensitivity. Cephalalgia. 1993;13(2):75–85.
24. Fink KB, Göthert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59(4):360–417.
25. Barnett S, Errington J, Sludden J, et al. Pharmacokinetics and pharmacogenetics of cyclophosphamide in a neonate and infant childhood cancer patient population. Pharmaceuticals. 2021;14(3):272.
26. Zou P, Leil TA. Exposure matching-based pediatric dose selection for drugs with renal excretion - lessons learned from pediatric development of direct oral anticoagulants. Clin Pharmacol Ther. 2024;116(5):1174–1187.
27. Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front Pharmacol. 2012;3:46.
28. Moretti R, Pansiot J, Bettati D, et al. Blood-brain barrier dysfunction in disorders of the developing brain. Front Neurosci. 2015;9:40.
29. Friemel CM, Spanagel R, Schneider M. Reward sensitivity for a palatable food reward peaks during pubertal developmental in rats. Front Behav Neurosci. 2010;4:39.
30. Waxman DJ, Celenza JL. Sexual dimorphism of hepatic gene expression: novel biological role of KRAB zinc finger repressors revealed. Genes Dev. 2003;17(21):2607–2613.
31. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14(9):914–925.
32. Magaña SM, Matiello M, Pittock SJ, et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology. 2009;72(8):712–717.
33. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043–1049.
34. Fulton JA, Kahn J, Nelson LS, Hoffman RS. Renal infarction during the use of rizatriptan and zolmitriptan: two case reports. Clin Toxicol. 2006;44(2):177–180.
35. Abramovitz B, Leonberg-Yoo A, Bahrainwala JZ, Litt H, Rudnick MR. Bilateral renal infarctions during the use of sumatriptan. Kidney Int Rep. 2018;3(5):1233–1236.
36. Pauwels PJ, Tardif S, Palmier C, Wurch T, Colpaert FC. How efficacious are 5-HT1B/D receptor ligands: an answer from GTP gamma S binding studies with stably transfected C6-glial cell lines. Neuropharmacology. 1997;36(4–5):499–512.
37. Watts SW, Thompson JM. Characterization of the contractile 5-hydroxytryptamine receptor in the renal artery of the normotensive rat. J Pharmacol Exp Ther. 2004;309(1):165–172.
38. Tadipatri S, van Heuven-Nolsen D, Feniuk W, Saxena PR. Analysis of the 5-HT receptors mediating contractions in the rabbit isolated renal artery. Br J Pharmacol. 1991;104(4):887–894.
39. Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167.
40. van den Anker J, Reed MD, Allegaert K, Kearns GL. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J Clin Pharmacol. 2018;58(10):S10–S25.
41. Cameron C, Kelly S, Hsieh SC, et al. Triptans in the acute treatment of migraine: a systematic review and network meta-analysis. Headache. 2015;55(4):221–235.
42. Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, MaassenVanDenBrink A. Is selective 5-HT(1F) receptor agonism an entity apart from that of the triptans in antimigraine therapy. Pharmacol Ther. 2018;186:88–97.
43. Dodick DW. Triptan nonresponder studies: implications for clinical practice. Headache. 2005;45(2):156–162.
44. Thorlund K, Mills EJ, Wu P, et al. Comparative efficacy of triptans for the abortive treatment of migraine: a multiple treatment comparison meta-analysis. Cephalalgia. 2014;34(4):258–267.
45. Derry CJ, Derry S, sMoore RA. Sumatriptan (all routes of administration) for acute migraine attacks in adults - overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;2014(5):CD009108.
46. Rapoport AM, Tepper SJ, Sheftell FD, Kung E, Bigal ME. Which triptan for which patient. Neurol Sci. 2006;27 Suppl 2:S123–9.
47. Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001;358(9294):1668–1675.
48. Alves C, Macedo AF, Marques FB. Sources of information used by regulatory agencies on the generation of drug safety alerts. Eur J Clin Pharmacol. 2013;69(12):2083–2094.
49. Star K, Edwards IR. Pharmacovigilance for children’s sake. Drug Saf. 2014;37(2):91–98.
50. Hauben M, Aronson JK. Defining ‘signal’ and its subtypes in pharmacovigilance based on a systematic review of previous definitions. Drug Saf. 2009;32(2):99–110.
51. Blake KV, Zaccaria C, Domergue F, La Mache E, Saint-Raymond A, Hidalgo-Simon A. Comparison between paediatric and adult suspected adverse drug reactions reported to the European medicines agency: implications for pharmacovigilance. Paediatr Drugs. 2014;16(4):309–319.
52. Osokogu OU, Fregonese F, Ferrajolo C, et al. Pediatric drug safety signal detection: a new drug-event reference set for performance testing of data-mining methods and systems. Drug Saf. 2015;38(2):207–217.
53. Pariente A, Gregoire F, Fourrier-Reglat A, Haramburu F, Moore N. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: the notoriety bias. Drug Saf. 2007;30(10):891–898.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
A Real-World Disproportionality Analysis of Olaparib: Data Mining of the Public Version of FDA Adverse Event Reporting System
Shu Y, He X, Liu Y, Wu P, Zhang Q
Clinical Epidemiology 2022, 14:789-802
Published Date: 28 June 2022
Association of Clopidogrel with Interstitial Lung Disease: Gaining Insight Through the Japanese Pharmacovigilance Database
Kozaru M, Kambara H, Higuchi A, Kagatsume T, Hosohata K
Vascular Health and Risk Management 2024, 20:415-420
Published Date: 2 September 2024
Unveiling the Hidden Risks: An Update Decade-Long Analysis of Abraxane-Related Adverse Events from the FAERS Database
Zhao YC, Li X, Wang CQ, Jiao Y, Shen YN, Wang TJ, Zhang CH
International Journal of Nanomedicine 2024, 19:11847-11858
Published Date: 14 November 2024
Exploring the Top 50 Drugs Associated with Restless Legs Syndrome Based on the FDA Data from 2004 to 2024
Wei S, Song X, Chen R, Chen S, Lu B
Nature and Science of Sleep 2025, 17:929-946
Published Date: 15 May 2025