Back to Journals » International Journal of Nanomedicine » Volume 19
Evodiamine: A Extremely Potential Drug Development Candidate of Alkaloids from Evodia rutaecarpa
Authors Lin L, Liu Y, Tang R, Ding S, Lin H, Li H
Received 13 January 2024
Accepted for publication 23 July 2024
Published 23 September 2024 Volume 2024:19 Pages 9843—9870
DOI https://doi.org/10.2147/IJN.S459510
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mian Wang
Longfei Lin,1,* Yuling Liu,1,* Ruying Tang,1 Shilan Ding,1 Hongmei Lin,2,3 Hui Li1,4
1Institute Chinese Materia Medica China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China; 2Beijing Research Institute of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 3National Medical Products Administration Key Laboratory for Research Evaluation of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 4Institute of Traditional Chinese Medicine Health Industry, China Academy of Chinese Medical Sciences, Nanchang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Li, Institute Chinese Materia Medica China Academy of Chinese Medical Sciences, Beijing, 100700, People’s Republic of China, Email [email protected] Hongmei Lin, Beijing Research Institute of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, 100029, People’s Republic of China, Email [email protected]
Abstract: Evodiamine (EVO) is a tryptamine indole alkaloid and the main active ingredient in Evodia rutaecarpa. In recent years, the antitumor, cardioprotective, anti-inflammatory, and anti-Alzheimer’s disease effects of EVO have been reported. EVO exerts antitumor effects by inhibiting tumor cell activity and proliferation, blocking the cell cycle, promoting apoptosis and autophagy, and inhibiting the formation of the tumor microvasculature. However, EVO has poor solubility and low bioavailability. Several derivatives with high antitumor activity have been discovered through the structural optimization of EVO, and new drug delivery systems have been developed to improve the solubility and bioavailability of EVO. Current research found that EVO could have toxic effects, such as hepatotoxicity, nephrotoxicity, and cardiac toxicity. This article reviews the pharmacological activity, derivatives, drug delivery systems, toxicity, and pharmacokinetics of EVO and provides research ideas and references for its further in-depth development and clinical applications.
Keywords: evodiamine, pharmacology, derivatives, drug delivery systems
Introduction
Evodia rutaecarpa is the dried, nearly ripe fruit of Evodia rutaecarpa (Juss). Benth., E. rutaecarpa (Juss). Benth. var. officinalis (Dode) Huang, or E. rutaecarpa (Juss). Benth. var. bodinieri (Dode) Huang, which are plants belonging to the Rutaceae family. Fructus Evodiae is mainly distributed in Shanxi, Hebei, Shandong, Jiangsu, Anhui, Sichuan, Yunnan, northern Guizhou, and Hanzhong in the Shaanxi Province and other places in China. It was first recorded in “Sheng Nong’s herbal classic” and is described in the Compendium of Materia Medica as follows: “Eating it too much will cause eye irritation and hair loss”. “It has small poison, stimulates spleen fire, and should be avoided by those with eye diseases”. It has anti-emetic, anti-ulcer, analgesic, and other effects, and is often used to treat nausea with acid regurgitation, Jueyin headache, and dawn diarrhea.
The chemical components of Fructus Evodiae are alkaloids, terpenoids (including bitter principles), flavonoids, phenylpropanoids, anthraquinones, sterols, and volatile oils, with alkaloids being the main active ingredients. Numerous alkaloids have been isolated, which can be divided into indole quinoline alkaloids, quinolone alkaloids, and other alkaloids according to their chemical structures. Among them, evodiamine (EVO) and rutaecarpine are the main active components. Modern pharmacological research showed that they have antitumor, cardioprotective, anti-gastric ulcer, antibacterial, anti-inflammatory, and analgesic effects. Dehydroevodiamine, which is the symbolic component of Fructus Evodiae, has a vasodilatory effect.
With the widespread use of Evodia rutaecarpa in clinical practice, its toxicity has gradually been exposed. The 2020 edition of the Chinese Pharmacopoeia reported that Evodia rutaecarpa has little toxicity and stipulates that the amount of 2–5 g, and the appropriate amount is for external use. In clinical practice, excessive doses of Evodia rutaecarpa often lead to toxicity, which is mainly manifested as liver toxicity, indicated by significant increases in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Adverse reactions caused by the improper use of Evodia rutaecarpa occur in clinical practice. However, the main toxic components and their mechanism of action are still unclear.
EVO is a tryptamine indole alkaloid appearing as yellow, flaky crystals and having a relative molecular weight of 303.36, molecular formula of C19H17N3O, and melting point of 278 °C. It is insoluble in water but soluble in acetone and other organic solvents. The structure of EVO is unstable when the temperature exceeds 60 °C or when the pH is < 5 or > 9. Under such conditions, EVO is prone to chemical changes. It also contains several reactive groups with the potential for chemical modification. In recent years, EVO has received extensive attention due to its low cytotoxicity, wide bioactivity, and excellent physicochemical properties. Although some reviews on EVO have been published, most are older reviews and focused on its pharmacological effects. Limited current studies have addressed the synthesis, derivatives, and formulation of EVO, which are key information for the application of EVO. This is the first review to comprehensively summarize the pharmacological activity, toxicity, absorption, and metabolism of ECO in vivo; the synthesis, species, and activity of its derivatives; and the preparation, development, and other aspects of EVO to provide ideas and references for its further in-depth research, development, and utilization.
Pharmacological Effects
Studies have shown that EVO has many pharmacological activities, such as anticancer, cardioprotective, anti-atherosclerosis, anti-inflammatory, anti-Alzheimer’s disease (AD), and antibacterial activity (Figure 1), indicating high medicinal value.
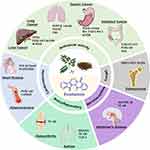 |
Figure 1 The pharmacological activity and mechanism of evodiamine. |
Anticancer Activity
In recent years, studies have found that EVO has potent anticancer activity, can significantly inhibit the proliferation of various cancer cells, and has a curative effect in liver cancer, lung cancer, gastric cancer, and colorectal cancer. Its mechanism of action is mainly related to inducing cancer-cell apoptosis, inhibiting cancer-cell proliferation, and affecting the cancer cell cycle and cell migration (Table 1).
 |
Table 1 The Summary of Anticancer Effect and Mechanism of Evodiamine on Different Cancers |
Liver Cancer
Liver cancer is a common malignant tumor characterized by high malignancy, rapid development, and high mortality. Its incidence rate ranks fifth among all cancers, and the mortality rate in males ranks second.1 Clinical data show that the 5-year survival rate of patients with liver cancer is less than 10%. Surgery in the early stages is the usual treatment; however, it is associated with poor postoperative prognosis and a high recurrence rate.2 Studies in an immunocompetent mouse model of orthotopic intrahepatic xenotransplantation confirmed that EVO could significantly inhibit the growth of cancer cells in model mice.3 Other studies showed that EVO could significantly induce the cell-cycle arrest of hepatocellular carcinoma (HCC) cells in the G2/M phase, upregulate P53 and BAX, and decrease the expression of NOD1, p-P65, p-ERK, p-p28, and p-JNK. The mechanism may be due to the induction of apoptosis in HCC cells by inhibiting the NOD1 signaling pathway in vitro and in vivo.4 Treatment with EVO was reported to increase WWOX expression in a dose-dependent manner in HepG2 and Hepa1-6 tumor-bearing mice. As a new inducer of oxidative stress, EVO exerts its anti-HCC effect by activating the WWOX-dependent pathway.5 EVO can also reduce phospho-protein kinase B (p-Akt) level that is activated by SC79, decrease the activity of tumor-specific growth factors and alpha-fetoprotein, leading to the cleavage of caspase-3 and an increase in Bax/Bcl2, and anti-liver cancer effects.6 Yes-associated protein (YAP)/transcriptional co-activator with PDZ-binding motif (TAZ) is associated with metastasis and cancer formation and progression in various cancers. YAP is a key target in the treatment of HCC. Studies have shown that EVO significantly reduced YAP levels and HCC growth in a dose-dependent manner. It was also shown to exert anti-metastatic effects on Hep3B and Huh-7 cells7,8. EVO combined with vorinostat synergistically inhibited the proliferation of liver cancer cells under hypoxia by downregulating HIF-1α.9 Thus, overall, it can be used as an anti-liver cancer drug.
Lung Cancer
Currently, lung cancer is one of the most malignant and rapidly growing tumors and is associated with high morbidity and mortality. More than 2.2 million new cases of lung cancer and nearly 1.8 million deaths worldwide due to lung cancer were reported in 2020.
EVO decreased the cell viability of A549 and NCI-H522 cells in a dose- and time-dependent manner, induced DNA damage, and arrested the cell cycle in the G2/M phase, significantly inhibiting cell cycle-related proteins.10 It also reduced the expression of pro-caspase-3 protein, indicating that EVO may induce the apoptosis of lung cancer cells via the endogenous apoptosis pathway.11 It significantly reduced the protein levels of epithelial-mesenchymal transition (EMT)-related molecules (N-cadherin, vimentin, and E-cadherin) in cells and increased the expression of E-cadherin (epithelial marker) protein to mediate the inhibition of cell invasion.12,13 Another study found that EVO could induce the release of cytochrome C, activate the cascade reactions of caspase-3 and caspase-9 endogenous pathways and the exogenous apoptosis pathway induced by death receptor 5 and caspase-8, suggesting that EVO may induce apoptosis in lung cancer cells via both endogenous and exogenous induction pathways.14 Besides upregulating the expression of apoptosis-related genes and proteins in tumors, EVO was also shown to activate the endoplasmic reticulum stress pathway and inhibit the invasion and migration of lung cancer cells stimulated by hepatocyte growth factor15 to inhibit proliferation and increase the apoptosis rate of A549 and LLC cells.16 In addition, EVO was reported to activate DNA methyltransferase-induced Notch3 methylation, prevent Notch3 from splitting on the cell surface by γ-exosome complex, and inhibit Notch3 signal pathway conduction, thereby inhibiting cancer cell proliferation and enhancing the anticancer activity of EVO.17,18 EVO significantly inhibited the growth of non-small cell lung cancer (NSCLC) cells, induced apoptosis, and arrested the cell cycle in the G2 phase. The mechanism for these effects may be through increasing CD8+ T cells and downregulating the MUC1-C/PD-L1 axis to inhibit NSCLC.19 Regarding the inhibition of tumor angiogenesis, EVO pretreatment significantly decreased the formation of human umbilical vein endothelial cells (HUVECs) and may regulate the formation of capillaries by downregulating the expression of phosphorylated ERK (extracellularly regulated protein kinase) and vascular endothelial growth factor.20
Gastric Cancer
Gastric cancer is a common malignant tumor of the digestive system and was the fifth most common cancer and the fourth leading cause of cancer deaths worldwide in 2020. In the same year, the total number of new cases of gastric cancer worldwide was approximately 1.1 million, and the death toll was approximately 770,000, accounting for 8% of all cancer-related deaths. Gastric cancer is associated with a higher mortality rate due to its lower rate of early detection.21,22
Studies have shown that EVO can inhibit the proliferation of gastric cancer stem cells (GCSCs), change their activity, and reduce the spheroid-formation ability of gastric cancer cells. EVO downregulated the Wnt/β-catenin pathway by reducing the expression of β-catenin, c-myc, and cyclin D1, thus inhibiting the proliferation and stem-cell self-renewal characteristics of GCSCs and reducing the expression of EMT factors.23 The expression of Survivin decreased in a dose-dependent manner, the expression of caspase-3/8/9 and Bax increased significantly, and the expression of Bcl2 decreased significantly in the EVO-treated SGC7901 gastric cancer cell line, suggesting that EVO induces apoptosis in these cells by downregulating the expression of the anti-apoptosis gene Survivin and upregulating the expression of the pro-apoptosis gene caspase-3, as well as inhibiting the G2/M phase of the cell cycle in these cells. Additionally, EVO inhibits the proliferation of gastric cancer cells by mitotic arrest and subsequent mitotic slippage.24–26 EVO increased the sensitivity of the BGC-823 gastric cancer cell line to radiotherapy and inhibited the growth of xenograft tumors.27 The expression of mTOR, 4E-BP1, and p70S6K genes was significantly inhibited after treatment of SGC7901 cells or human peripheral blood monocyte models with EVO or EVO combined with z-VADfmk, respectively.28 In addition, the inhibitory efficiency of EVO was higher when combined with other cancer-treatment approaches.29
Intestinal Cancer
Intestinal cancer can be mainly divided into colon cancer and rectal cancer. It is a malignant tumor with a high worldwide incidence, and its morbidity and mortality have always been at the forefront of malignant tumors. Tumor metastasis and inflammatory infiltration are the main characteristics of intestinal cancer. Studies have reported the effects of 75 natural compounds on the migration and proliferation of intestinal cancer cells. EVO has been reported to have the most obvious effect in affecting the migration ability of cells with the lowest migration/proliferation value; 10 mg/mL EVO was reported to inhibit cancer cell movement activity by 70% to exert anticancer effects by regulating the Notch and WNT signaling pathways.30,31 EVO can significantly reduce the protein levels of autocrine motility factor in cell supernatants and inhibit PGI to reduce cancer cell migration. It can also induce cell apoptosis by inhibiting the JAK2/STAT3 pathway, regulating the activity of the p53 signaling pathway, and downregulating the expression of recombinant matrix metalloproteinase (MMP)-3.32 EVO significantly inhibited the migration and invasion of HT-116 and HT-29 cells, the sirtuin1-mediated NF-κB pathway, and the migration and invasion of CRC cells.33
EVO inhibited Bcl2 in SW480 colon cancer cells; induced the expression of caspase-3, Bax, LC3II, and Beclin1; and induced the synergistic effect of cell autophagy and apoptosis on cell death.34 EVO prevented the proliferation of human colorectal cancer cells (HCT-116) and colon cancer (LoVo) cells, wherein the expression of IGF-1 was inhibited, PI3K/Akt signal transduction was reduced, and HIF-1α expression was downregulated, thus exerting an inhibitory effect on colon cancer.35 EVO was also reported to reduce the levels of inflammatory factors, such as interleukin (IL)-1β, IL-2, IL-6, IL-15, IL-17, IL-22, tumor necrosis factor (TNF)-α, NF-κB, IKKα+β, and IκBα, in colon cancer models and exert its antitumor effect.36
Other Tumors
In addition to its efficacy in the above cancers, EVO was shown to exert significant pharmacological activity in cancers of the endocrine system (pancreatic cancer,37 thyroid carcinoma,38 renal carcinoma39), urinary system (urothelial cell carcinoma,40 bladder cancer41), reproductive system (ovarian cancer,42 cervical carcinoma,43 breast carcinoma), respiratory system (oral cancer,44 nasopharyngeal carcinoma,45 tongue squamous cell carcinoma46), nervous system (melanoma47), and digestive system (cholangiocarcinoma48).
The anticancer activity of EVO against these cancers involves multiple pathways. EVO inhibits the activation of IL-6-induced STAT3 signaling, upregulates the expression of SHP-2, and plays a role in inhibiting the proliferation of cholangiocarcinoma tumors. In several cancer models, apoptotic bodies were significantly increased after treatment with EVO. At the same time, cell arrest in the G0/G1 phase and the abnormal expression of caspase-related proteins49 were shown to also target the p53-p21-Rb pathway to inhibit the proliferation of breast cancer MCF-7 cells and MDA-MB-231 cells.50 EVO activated Janus kinase and PERK in human ovarian cancer cells to destroy the mitochondrial membrane potential and induce apoptosis in these cells.51 The mechanism of the anti-prostate cancer action of EVO is related to the direct targeting of Src, the reduction of dihydrotestosterone-induced Src activation, and the blockage of the transcriptional activity of androgen receptors (ARs).52 EVO induced M-phase cell-cycle arrest by inactivating CUL4A E3 ligase, thereby blocking the degradation of p53 and the transcriptional activation of p21 and inhibiting the growth of esophageal squamous cell carcinoma (ESCC).53 EVO inhibited the proliferation, invasion, and angiogenesis of oral squamous cell carcinoma (OSCC) by targeting the receptor for advanced glycation end products (RAGE) and affecting the downstream signal transduction system of advanced glycation end products/RAGE.54 In addition, EVO disrupted the heat shock protein (HSP) system by binding to the N-terminal ATP-binding pocket of HSP70 and causing ubiquitin-mediated degradation, thereby acting as an effective HSP70-targeted anticancer drug.55
Disorders of the Cardiovascular and Cerebrovascular Systems
Cardioprotection
EVO can protect the heart by preventing cardiac fibrosis, inhibiting the proliferation of vascular smooth muscle cells, and alleviating myocardial ischemic injury. EVO can block the TGF-β1/Smad signaling pathway, decrease the expression of fibroblast-specific protein α-smooth muscle actin and vimentin, and increase the expression of the interstitial system markers CD31 and CD34, thereby inhibiting endothelial cell migration and cardiac EMT. EVO was shown to significantly prevent the activation of Smad2, Smad3, ERK1/2, and Akt, and the nuclear translocation of Smad4 in cardiac fibroblasts and HUVECs, thus preventing cardiac fibrosis, suggesting the potential therapeutic effect of EVO in myocardial fibrosis.56 EVO also prevented isoproterenol-induced myocardial fibrosis by regulating the interstitial transformation of endothelial cells, and its mechanism was related to blocking the TGF-β1/Smad signal pathway.57 EVO caused cell-cycle arrest in the G0/G1 phase by reducing the expression of proliferating cell nuclear antigen, cyclin-dependent kinase (CDK2/4/6), and cyclin D, which inhibited the proliferation of platelet-derived growth factor-BB (PDGF-BB)-induced vascular smooth muscle cells (VSMCs) by inhibiting the MAPK/ERK signaling pathway and decreasing the production of reactive oxygen species (ROS) in cells.58 β1-AR antagonists are mainly used to treat ischemic heart disease and chronic heart failure. Studies have found that EVO is a potential β1-AR antagonist, and in vivo experiments showed that it can alleviate myocardial ischemic injury by regulating β1-AR.59,60 Additionally, EVO significantly reduced symptoms, such as decreased cardiac blood flow due to cardiac allergy, and protected rats from cardiac allergic injury and myocardial ischemic injury by regulating CGRP release.61,62
Anti-Atherosclerosis Effects
Atherosclerosis is the leading cause of coronary heart disease, cerebral infarction, and peripheral vascular disease, and lipid metabolism disorders are the pathological basis of atherosclerosis. Elevated cholesterol levels are one of the reasons for the development of atherosclerosis. Studies have found that EVO can significantly regulate cholesterol absorption. When combined with berberine, serum cholesterol, triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and liver total cholesterol levels were decreased in hyperlipidemic rats, and the combination significantly promoted serum cholesterol decreases in high-fat diet-induced rats.63 Activation of the capsaicin receptor (TRPV1) improved high-fat diet-induced atherosclerosis.64 The activation of TRPV1 was shown to regulate lipid metabolism, reduce foam cell formation, protect endothelial cells, inhibit smooth muscle cell proliferation, and inhibit inflammation and oxidation.65 After 4 weeks of EVO gavage (10 mg/kg), the atherosclerotic plaques in the aortic sinuses of ApoE−/−TRPV1−/− mice became larger, serum cholesterol levels increased, HDL levels decreased, and TNF-α, MCP-1, IL-6, and MIP-2 levels increased compared to ApoE−/− mice, indicating that EVO reduced serum cholesterol, alleviated hyperlipidemia, and played an anti-atherosclerosis role by mediating the TRPV1 receptor.66 EVO also activated the Ca2+-dependent PI3K/Akt/CaMKII signaling pathway via the TRPV1 receptor, phosphorylated eNOS protein in endothelial cells, increased the synthesis and release of nitric oxide (NO), and exerted an anti-atherosclerosis effects.67
The uncontrolled migration of VSMCs into the intima is a key process in the development of atherosclerosis. EVO (0.1 μmol/L and 0.5 μmol/L) inhibited the PDGF-BB-induced migration of VSMCs by activating peroxisome proliferator-activated receptor-γ and reducing the expression of MMPs, cell adhesion molecules, and osteopontin, thereby exerting an anti-atherosclerosis effect.68
Anti-Inflammatory Activity
At the cellular level, EVO inhibits the secretion of various cell-related inflammatory factors by the following mechanisms. (1) Inhibition of the secretion of macrophage (Mφ) inflammatory factors: EVO can inhibit the secretion of lipopolysaccharide (LPS)-induced prostaglandin (PG)E2 and affect macrophage migration by inhibiting the activities of NF-κB and its mediated cyclooxygenase-2 (COX-2).69 In an LPS-induced macrophage model, EVO enhanced the activation of the NLRP3 inflammasome in a dose-dependent manner, induced an increase in IL-1β and caspase-1 cleavage, and enhanced the activity of inflammasomes by inducing α-tubulin acetylation, thus reducing the inflammatory response.70 (2) Inhibition of the secretion of monocyte inflammatory factors: EVO can inhibit the secretion of inflammatory factors, the expression of numerous chemokine receptors such as CCR1 and CCR2, and the migration of human MOs induced by the lymphotoxin analog (LIGHT).71 (3) Inhibition of ROS production in neutrophilic granulocytes: EVO can reduce the production of ROS and superoxide anions by inhibiting the activity of reduced coenzyme II (NADPH) oxidase in mouse NE.72 (4) Inhibition of the production of proinflammatory factors in microglial cells (MCs): After pretreatment of mouse BV2 cells with LPS, EVO significantly inhibited the synthesis of inducible nitric oxide synthase (iNOS), which, in turn, significantly inhibited the production of proinflammatory factors in MCs.73
In vivo studies found that EVO could significantly reduce the expression of inflammatory cytokines such as TNF-α and IL-4 in mouse models of allergies, regulate the synthesis of cytokines related to allergic reactions in mast cells and basophils, and further alleviate immunoglobulin (Ig)E-induced allergic diseases.74 EVO was shown to reduce intracellular LPS content, rebalance the abundance of Escherichia coli and lactic acid bacteria, alleviate dextran sodium sulfate-induced inflammatory reactions, and maintain the integrity of the intestinal tract and homeostasis of the intestinal flora, thereby showing benefits in treating ulcerative colitis.75 EVO is also effective in treating osteoarthritis. It was shown to decrease the production of NO, IL-6, TNF-α, and PGE2 in mice with osteoarthritis and inhibit the phosphorylation of the NF-κB signaling pathway stimulated by IL-1β, thus exerting an anti-inflammatory effect and inhibiting cartilage degeneration.76 EVO reduced IgE and interferon-γ levels and inflammatory cell infiltration in the lung tissues of asthmatic rats, reduced toll-like receptor (TLR-4), MyD88, NF-κB, and HMGB1 mRNA levels in lung tissues, and decreased airway inflammation and lung tissue remodeling through the HMGB1/NF-κB/TLR-4 pathway.77
EVO has been reported to inhibit HIF-1α by dephosphorylating Akt and p70S6K in a hypoxia model. It also inhibited the expression of COX-2 and iNOS and the release of PGE2, indicating the potential of EVO in treating hypoxic inflammatory diseases.78 The P2X4 receptor (P2X4R) is a subtype of ATP receptors that plays an important role in pain, inflammatory, and immune responses. EVO administration to HUVECs stimulated with a high concentration of glucose in vitro inhibited the ATP receptor P2X4R signaling pathway to downregulate the expression of inflammatory factors NF-κB and TNFR-α, reduce ROS production, promote NO synthesis, exert anti-inflammatory effects, and protect HUVECs from glucose toxicity.79
Alzheimer’s Disease
With the acceleration of population aging in recent years, the incidence of neurodegenerative diseases, such as AD, has increased significantly, and 15 million people are expected to be affected by dementia by 2050.80 Studies have shown the efficacy of EVO in treating AD. A Morris water-maze experiment of AD mice found that EVO could significantly reduce the average latency, significantly improve the spatial cognition and learning ability, significantly reduce the deposition of amyloid plaques in the brain, increase the levels of serum acetylcholine and choline acetyltransferase, and reduce acetylcholinesterase levels in the serum, hypothalamus, and brain.81 EVO reduced the expression of inflammatory cytokines in the brains of mice with AD, increased glucose uptake in the brain tissues, significantly increased glutathione activity and decreased superoxide dismutase activity in the hippocampus, increased Akt/GSK-3β signaling pathway and inhibited NF-κB activity, and significantly inhibited the activation of glial cells and the levels of inflammatory factors (TNF-α, IL-1β, and IL-6) in the hippocampus, suggesting that anti-inflammatory activity was involved in the anti-AD effect of EVO.82,83 In addition, EVO can passively diffuse through the single-cell blood-brain barrier. It showed a concentration-dependent neuroprotective effect on PC12 cells injured by MPP+ or H2O2, indicating its potential as a neuroprotective drug.84
Antibacterial Effects
EVO was shown to enhance NLRP3-mediated IL-1β production and neutrophil recruitment by promoting the LPS-induced acetylation of α-tubulin around microtubule tissue centers in macrophages, which enhanced innate immunity to bacterial infections and resistance to pathogenic infections in the host.70 EVO was reported to interact with arginine 161 and aspartic acid 551 residues of E. coli topoisomerase (Topo)1, inhibit bacterial Topo1, and show a significantly lower minimal inhibitory concentration value of 128 μg/mL compared to antibiotics (> 512 μg/mL) against a clinical isolate of Klebsiella pneumoniae.85 The growth of Helicobacter pylori reference strains and clinical isolates was inhibited by EVO. Its inhibitory mechanisms were attributed to the downregulation of the gene expression of both the replication and transcription machinery of H. pylori.86
Other Activities
In addition to the pharmacological activities described above, research studies are continuously confirming other pharmacological activities of EVO. EVO can upregulate the expression of brain-derived neurotrophic factor and phosphorylated myoprotein-related kinase B and reduce the levels of 5-HT and NA, thus exerting an antidepressant effect.87 EVO restored the MMP3-OPN-MAPK pathway in a zebrafish model of osteoporosis, significantly increasing the calcium and phosphorus content and increasing bone formation, indicating its significant anti-osteoporosis effect.88
Toxicology
China Pharmacopoeia (2020 Edition) reports that E. rutaecarpa has little toxicity, and adverse reactions caused by its improper use may sometimes occur in a clinical setting. Six main alkaloids were isolated from E. rutaecarpa, among which EVO had the most acute toxicity, with an LD50 of 77.8 mg/kg.89 Recent studies found that EVO is toxic to several organs: (1) Hepatotoxicity: EVO can induce an increase in the release of AST, ALT, lactate dehydrogenase (LDH), and alkaline phosphatase; enhance p-p38/p38 expression; and induce mitochondrial swelling, vacuolation, MPT pore opening, and a significant decrease in the mitochondrial potential, leading to ATP depletion and cytochrome C release and ultimately triggering cell death.90 The mechanism of EVO-induced liver injury is likely related to the MAPK signaling pathway (p38).91 The hepatotoxicity of EVO has also been suggested to be related to lipid-peroxidation injury, cell apoptosis, and cholestasis.92 (2) Cardiotoxicity: using a cardiomyocyte model in in vitro studies, the IC50 of cells treated with EVO for 24 h was reported to be 28.44 μg/mL. LDH release and malondialdehyde (MDA) levels of the treated cardiomyocytes increased significantly, whereas the activity of superoxide dismutase decreased. The LC10 of EVO was reported to be 354 ng/mL in a zebrafish heart model. After treatment with EVO, the heart rate and blood circulation in zebrafish changed significantly; the pericardium appeared malformed, and heart failure was induced.93 (3) Nephrotoxicity: Screening of traditional Chinese medicine drugs for nephrotoxicity identified EVO as potentially nephrotoxic. In vitro experiments with different concentrations of EVO showed that EVO could significantly inhibit the activity of HEK293 cells. LDH release increased in treated cells, the morphology of renal cells changed significantly, and the expression of autophagy-related proteins increased, indicating that the renal cytotoxicity of EVO may be related to autophagy-related pathways.94
Pharmacokinetic Studies of EVO
In recent years, scholars have conducted pharmacokinetic studies of EVO isolated from different species and have a good understanding of its mechanism in vivo. EVO was administered to rats via intragastric administration at a dose of 100 mg/kg, and blood concentrations were determined using LC-MS/MS.95 The maximum drug concentration in the blood (Cmax) was determined to be 5.3 ng/mL. It was also administered intravenously to rats at a dose of 2 mg/kg, and the area under the curve (AUC) was determined to be 21 μg·mL·min−1.96 Beagle dogs are commonly used to study the metabolism of drugs in vivo. Some researchers orally administered 10 mg/kg EVO to beagle dogs and established a method for determining blood drug concentrations using LC-MS/MS, where the Cmax was determined to be 30.9 ng/mL.97 In vivo studies of EVO were conducted in rabbits after they were injected via the ear vein.98 EVO had poor absorption and poor bioavailability after oral administration. In addition, many studies have reported the pharmacokinetics of Fructus Evodiae extract and prescriptions containing Fructus Evodiae and analyzed the absorption and metabolism of EVO.99–102 One study analyzed the tissue distribution of EVO. After injecting EVO into the tail veins of mice, the Cmax was attained within 15 min, and the tissue distribution concentration from highest to lowest was in the order of the lungs, kidneys, brain, heart, and liver, indicating lung targeting.103 An MDCK pHaMDR cell monolayer model was used to confirm that EVO could pass through the blood-brain barrier via passive diffusion,84 which is consistent with its tissue distribution.
Metabolism of EVO in vivo: After the oral administration of EVO in rats, 6 metabolites (2 Phase I metabolites and 4 Phase II metabolites) were identified using UPLC-LTQ-Orbitrap, namely, 10-hydroxyevodiamine (M1), 3-hydroxyevodiamine (M2), 10-hydroxyevodiamine sulfate (M3), 3-hydroxyevodiamine sulfate (M4), 10-hydroxyevodiamine glucuronide (M5), and 3-hydroxyevodiamine glucuronide (M6).104
In vitro studies have been conducted on the metabolic characteristics of EVO-conditioned human liver microsomes and liver cells.105 A total of 12 phase I metabolites were detected in human liver microsomes, and 19 metabolites were detected in human liver cells, including 7 phase II metabolites. Among them, 12 metabolites were identified for the first time.106 EVO was orally administered to rats at a dose of 30 mg/kg, and the levels of 4 metabolites, namely, 10-hydroxyevodiamine, 3-hydroxyevodiamine, 10-hydroxyevodiamine glucuronide, and 3-hydroxyevodiamine glucuronide, were determined. The content of 18-hydroxyevodiamine was the highest, with a Cmax of 302.09 ng/mL and an AUC0-t of 3279.39 ng.h/mL.107 Oxidation, N-demethylation, dehydrogenation, glucuronidation, and glutathione (GSH) binding were shown to be the main metabolic pathways of EVO. A study found that EVO inhibited the activity of cytochrome P450 (CYP)1A2, CYP2C9, and CYP2D6, suggesting that its disposition by the body depends on these metabolic pathways.108 The GSH conjugate of EVO is dominated by benzylsulfide adducts at position C-8, and its formation is mainly catalyzed by heterologously expressed recombinant CYP3A4, CYP1A2, and CYP2D6.109 The metabolic pathway of EVO is shown in Figure 2.
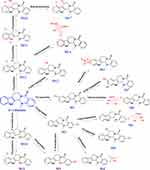 |
Figure 2 Proposed major metabolic pathway of evodiamine in vivo. |
The study also found that EVO could affect the composition of the intestinal flora, increase the abundance of Lactobacillus acidophilus and levels of acetic acid, and exert therapeutic effects in ulcerative colitis.110
Derivatives of EVO
The structural modification of the active ingredients isolated from plants is an effective approach to discovering new drugs.111,112 EVO shows outstanding pharmacological activity because of its special chemical structure, and molecular-targeting studies have found that EVO can serve as a basic skeleton of multitargeted antitumor drugs. In recent years, numerous derivatives have been developed by chemically modifying the structure of EVO to significantly enhance its antitumor activity. The effectiveness of EVO has been gradually improved by structure-activity relationship studies and continuous in-depth research to yield novel EVO derivatives with higher antitumor activity. The structural modification of EVO mainly involves modifications of the A-E rings. The main derivatives of EVO are shown in Figure 3.
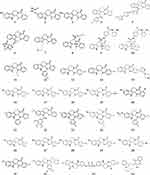 |
Figure 3 Structure of the main derivatives of evodiamine. |
Synthesis of EVO
Currently, 5 synthesis methods are commonly used for EVO, of which 4 are based on N-methylo-aminobenzoic acid(1) as a raw material, and the other is synthesized using a one-pot method. The synthesis route is shown in Figure 4. Method 1: N-methylo-aminobenzoic acid first undergoes acylation and masonry and then undergoes intermediate states (2) and (3) to generate the key intermediate ketone (4), which is then reacted with 3.4-dihydrocarboline to obtain EVO. Method 2: N-methylo-aminobenzoic acid is also used as the raw material and is first reacted with phosgene to generate intermediate (5) and then reacted with 3.4-dihydrocarboline to obtain EVO. Method 3: In this method, N-methylo-aminobenzoic acid is reacted with phosgene (5) to form an amide, which is then substituted with 3-indoleethylamine to obtain the product (7). After cyclization, EVO can be obtained. Method 4: The product is obtained by reacting N-methylo-aminobenzoic acid and 3-indole-ethylamine(8) with triphenyl phosphate to form an amide, which reacts with formaldehyde to cyclize under the action of mercury acetate. Method 5: Tryptophan, N-methylindocyanine anhydride, triethylenediamine, trifluoroacetic anhydride, triethyl orthoformate, and N, N-dimethylacetamide are used as raw materials and are sequentially integrated into a reaction bottle through acylation, decarboxylation, condensation, and Pictet Spengler reactions to achieve a one-pot synthesis of EVO.113,114
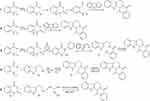 |
Figure 4 The main synthesis routes of evodiamine. |
Modification of the a Ring of EVO
The A ring of EVO can be modified mainly at the C-10 and C-12 positions. The introduction of different substituents at the C-10 position showed the following increasing order of antitumor activity strength: hydroxyl > iodine > fluorine > methoxy > bromine. The hydroxyl-substituted compound 1 obtained by substitution at the C-10 position showed better anticancer activity than camptothecin, topotecan, or irinotecan. The IC50s of A549, MDAMB-435, and HCT116 were < 3 nM,115 and they exerted a good inhibitory effect on the proliferation of certain drug-resistant cells. The mechanism of action was by inhibiting HSP70 function by directly binding and destabilizing HSP70 protein.116 The C-13b position has an asymmetric center with enantiomers, and the (S)-isomer is more active than the (R)-isomer.117
10-Hydroxy EVO has good activity in vitro, but its in vivo antitumor effect is not very potent. A phosphate derivative (compound 2) was designed and synthesized to overcome this obstacle, which was orally active and showed improved antitumor efficacy in vivo in an HCT116 xenograft model (TGI value of 59%).118 Compound 3 obtained by amino substitution at the C-10 position showed enhanced antitumor effects. Its IC50 for H1688 and H446 cells was 1.14 and 5.40 μM, respectively. A significant improvement in bioavailability was noted, and its AUC and Cmax was 7.02 and 4.62 times that of EVO, respectively.119
Modification of the B Ring of EVO
N-13 on the B ring of EVO is the site that is most easily modified. In 2010, Dong et al first discovered that EVO was an effective inhibitor of the antitumor target Topo using molecular docking and reported that it could be used as a lead compound to develop antitumor drugs. They subsequently introduced different substituents at the N-13 position of EVO and found that the introduction of a 4-chlorophenyl moiety led to compound 4 with the highest activity and IC50 values ranging from 0.049 to 2.60 μmol/L.120
The substitution of N-13 with a sulfur-containing group yielded compound 5 with good antitumor effects. Its IC50 values in human leukemia HL-60 and colon epithelial adenocarcinoma Caco-2 cells were 0.58 and 2.02 μM, respectively. Compound 5 could inhibit the cell apoptosis cycle in the G2/M phase and lead to mitochondrial dysfunction in HL-60 cells.121 An NO donor (compound 6) that was synthesized based on the N-13 site modification showed good cytotoxicity and selectivity between tumor and normal liver cells, with an IC50 in BGC-823 cells of 0.07 μM. The mechanism at the molecular level revealed that 13c caused cell-cycle arrest in the S phase and induced apoptosis in Bel-7402 cells through mitochondria-related caspase-dependent pathways.122 Replacing N-13 with ether-containing groups not only improved the antitumor effect but also enhanced the water solubility of EVO. For example, the IC50 of compound 7 for various tumors was in the range of 1–2 µM, indicating its potential to be developed as an antitumor drug.123
Modification of the C Ring of EVO
Enantiomers of EVO can be obtained based on the spatial characteristics of the C ring.124 De Petrocellis et al synthesized a series of enantiomeric EVO derivatives and found that S-EVO derivatives were more effective than R-EVO derivatives in human and rat transient receptor potential TRPV1 in transfected HEK-293 cells. The EC50 of compound 8 was 2 nM.125
A series of derivatives with dual effects of inhibiting histone deacetylase 1 (HDAC1) and Topo2 were synthesized by substituting the C-7 position of the C ring. Compounds 9 and 10 showed good antitumor activities both in vitro and in vivo. In a mouse HCT116 xenograft model, the oral administration of compound 9 at a dose of 150 mg/kg for 15 days led to a tumor inhibition rate of 75.2% without obvious toxicity.126
Modification of the D Ring of EVO
Modification of the D ring of EVO has been performed mainly at the C-5 and N-methyl (N14) sites, with the antitumor activity of carbonylthio substitutions at the C-5 site being stronger than that of carbonyl and methylene (C=S > C=O, CH2). The increasing order of activity of substituents at the N14 site of the D ring of EVO was S > O > N-Me > NH > CN.
A series of N-14 alkyl-substituted EVO derivatives were designed and synthesized. Among them, the N(14)-propyl-substituted derivative (compound 11) showed good inhibitory activity on MGC-803, RKO, and SGC-7901 cells and could significantly inhibit the polymerization of Topo1 and tubulin.127 Substituting the N-14 position of the D ring with 3′-fluorophenyl afforded compound 12 with good antitumor activity, which could inhibit Topo1 at 200 μM and arrest the cell cycle in both HGC-27 and HT-29 cells at the G2/M phase.128
Modification of the E Ring of EVO
The C-3 position on the E ring is the optimal active site. When electron-donating substituents (hydroxyl, amino, and alkyl groups) or weak electron-withdrawing substituents (halogens) are introduced at the C-3 position, the antitumor activity increases. For example, when C-3 was replaced by F or Cl (compounds 13 and 14), their IC50s against A549, MDAMB-435, and HCT116 were all < 3 nM. However, introducing strong electron-withdrawing groups (trifluoromethyl or nitro groups) at the C-3 position did not increase antitumor activity. Compound 15, with substitution at the C-3 position, showed good antitumor activity with a broad spectrum of activity and was an inhibitor of Topo1/Topo2. Molecular docking studies further confirmed that the derivatives acted by the dual inhibition of Topo1 and Topo2.129
Synergistic Modification of EVO
Synergistic Modification of the A and E Rings
Hydroxyl and halogen groups can be substituted at the third position of the E ring on the basis of hydroxyl substitution at the tenth position of the A ring to further optimize the structure of EVO, which can not only retain the antitumor effect but also significantly improve its water solubility, which is very helpful in enhancing the in vivo antitumor effect (compounds 16–20).
Synergistic Modification of the A and D Rings
A series of compounds were synthesized by the synergistic modification of the C-10 site of the A ring and the N-14 site of the D ring. N-phenyl and sulfur atom substitution at the N14 site yielded compounds that were more active than those with oxygen atom substitution. Among them, compound 21 had a methyl substitution at the C-10 position and phenyl substitution at the N-14 position and showed good antitumor effects both in vitro and in vivo.130
Synergistic Modification of the A, B, and E Rings
Researchers have synthesized a series of compounds based on 3,4,5-trimethoxyphenyl substitution at the N-13 position of the B ring, as well as the substitution of functional groups, such as methyl and halogen, on the A and E rings. All synthesized derivatives showed good antitumor activity. Among them, the IC50 of compound 22 (only for A and B) against HGC-27 cells was 3.3 ± 1.5 μM.131
Synergistic Modification of the A, D, and E Rings
N-14 phenyl-substituted EVO derivatives show potential as antitumor drugs, but the preparation of these derivatives usually involves the modification of other rings. Compound 23 prepared by the substitution of N14-3′-fluorophenyl in the D ring combined with methyl substitution at the C-10 position of the A ring and the C-3 position of the E ring, showed good anti-gastric cancer effects in vitro and in vivo with a TGI of 70.12% in a MGC-803 xenograft model. Further studies on the antitumor mechanism showed that compound 23 inhibited Topo1.132 Further modification by N14-3′-fluorophenyl substitution led to derivatives 24 and 25,133 with high antitumor activity and good inhibition against Topo1 and Topo2. Compound 24 inhibited Topo1 and Topo2 by 55.15% and 55.50% at 25 μM, respectively, whereas compound 25 had a tumor inhibition rate of 36.35%.134
Wang et al designed 11 EVO-inspired novel scaffolds and their derivatives, among which compounds 26–30 were based on C-10 hydroxyl substitutions. All synthesized compounds showed excellent in vitro and in vivo antitumor efficacy with good tolerability and low toxicity. 10-Hydroxyl thio-EVO (26) was the most potent antitumor compound, with a tumor growth inhibition rate of 48% in vivo. 3-Chloro-10-hydroxyl thio EVO (28) is the first-in-class triple Topo1/Topo2/tubulin inhibitor.135
Further optimization studies were carried out on this basis, and the synthesized compound 31 showed inhibitory activity in the HCT116 cell line in the low nanomolar range. Further mechanistic studies on the antitumor effect indicated that compound 31 acted by the dual inhibition of Topo1 and tubulin and induced apoptosis by G2 cell-cycle arrest. The quaternary ammonium salt of compound 31 exhibited excellent in vivo antitumor activity (TGI = 66.6%) and low toxicity in an HCT116 xenograft model.136
Other EVO Modifications
Some researchers have made other modifications to EVO and synthesized a series of tetrahydro-β-carboline derivatives by eliminating the D ring of EVO. Among them, compound 32 showed good antitumor effects, with an IC50 < 1 μM against HCT116, A549, and MDA-MB-231 cells.137
The design and in vitro antitumor effects of a series of bis-EVO derivatives have been reported, wherein compound 33 effectively inhibited the proliferation and migration of HCT116 cells. The mechanism of action in HCT116 cells was attributed to the induction of apoptosis and arresting the cell cycle at the G2/M phase.138
In another study, a series of C ring-opened EVO derivatives were synthesized by treatment with TFA, among which, compound 34 showed strong antitumor activity with IC50 values against BGC803 and SW480 cells of 2.53 and 2.84 μM, respectively, and it exhibited improved solubility by nearly 20 times.139
The main structural modification paths and characteristics of EVO are shown in Figure 5, and the antitumor activity of the modified derivatives is shown in Supporting Data. In addition to studying the antitumor activity, modified and transformed EVO derivatives have been evaluated for the treatment of AD140,141 and pulmonary hypertension142 and were also found to exert bacteriostatic143 and insecticidal144 effects.
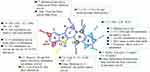 |
Figure 5 Modified derivatives of evodiamine A-E rings and their main characteristics. |
EVO Preparations
EVO has many pharmacological activities, and many new preparations have been developed to improve the solubility and bioavailability of EVO (Figure 6) and also to realize some special functions, such as targeted enrichment, to better improve efficacy or reduce toxic side effects.145–147
 |
Figure 6 Evodiamine preparations. |
Particulate Preparation
Particulate drug delivery systems refer to solid, liquid, semi-solid, or gaseous drug preparations composed of particles with a certain size (in the micron or nanometer range) prepared using a drug or a suitable carrier and a certain dispersion embedding technology. These systems can improve the solubility of insoluble drugs, the bioavailability of drugs, and drug stability, as well as reduce adverse drug reactions, delay drug release, and improve drug targeting.
Liposomes
Liposomes are microcystic carrier preparations with lipid bilayer encapsulation. They can wrap water-soluble or fat-soluble drugs in the vesicles or bilayer membranes formed by the hydrophilic head and have good biocompatibility in vivo. Liposomes have become a hotspot in drug research because of their advantages in increasing drug solubility, delaying drug release, improving the stability of encapsulated drugs, improving drug bioavailability, and reducing the toxicity of encapsulated drugs. A study used soybean lecithin and cholesterol as starting materials to prepare EVO and berberine hydrochloride co-encapsulated liposomes using the film-dispersion method. The particle size was 126 nm, and the average encapsulation efficiency of rutaecarpine liposomes was 99%. Its inhibitory effect on the proliferation of B16 melanoma cells was significantly better than that of the original drug.148 Using phosphatidylcholine and DSPE-mPEG2000 as carrier materials and the film-dispersion method, EVO and the photosensitizer indocyanine green were integrated into a liposomal nanoplatform for noninvasive diagnostic imaging and combinatorial therapy against OSCC. The particle size of the liposomes was about 120 nm, and the drug loading was 87.49% ± 2.79%. The IC50 against CAL 27 and Fadu cells was decreased, and the liposomes had a significant inhibitory effect on in situ tongue tumors compared to the original drug.149
Mesoporous silica nanoparticles (MSNs)@p(NIPAM-co-MA) were constructed for the co-delivery of EVO and BBR to further enrich the functions of the liposomes. The liposomes were temperature-sensitive and pH-sensitive due to N-isopropylacrylamide (NIPAM) and methacrylic acid (MA) modifications and had a sustained release effect due to the DSPE-PEG-2000 coating on the MSNs. The particle size of the nanoparticles was 230 nm, with an encapsulation efficiency of 98% and a drug-loading efficiency of 56%. The IC50 in HUVECs, HepG-2, HCT-8, and HeLa cells was in the range of 7.40 to 16.75 μg/mL, which was much higher than that of the free drug.150
Micelles
Polymer micelles are self-assembled nanoparticles with hydrophobic cores and hydrophilic shells. They have the characteristics of a wide drug-loading range, stable structure, good tissue permeability, and long in vivo retention time, and they can effectively carry a drug to the target. A study used poly(lactic co-glycolic acid) (PLGA) as a polymer material and prepared EVO-PLGA nanoparticles using the solvent evaporation method. The drug loading was 5.8%, the encapsulation efficiency was 63.8%, and the particle size was 157.3 nm. In vitro studies showed that the proliferation of MCF-7 breast cancer cells decreased by > 50% after 48 h of incubation with 16 mM EVO-PLGA nanoparticles, which was obviously better than that of the free drug.151 EVO-glycyrrhizic acid micelles were prepared with glycyrrhizic acid as a functional carrier using the film dispersion method to treat liver fibrosis. The average size of the micelles was 130.8 nm, the encapsulation efficiency was 91.23%, and the drug loading was 8.42%. In the treatment of carbon tetrachloride-induced liver fibrosis in rats, the experimental results showed that its effects in improving liver function and liver fibrosis indices were superior to those of free EVO.152
Targeting is an important characteristic of micelles. Pluronic F108 nanoparticles loaded with folic acid were prepared using solvent evaporation to achieve tumor targeting. The particle size of the micelle was 50.33 ± 3.09 nm, and the entrapment efficiency was 71.30 ± 3.76%. In vitro studies showed that the IC50 for the original EVO drug and nanomicelles in HeLa cells was 45.41 ± 6.50 and 27.86 ± 1.63 μg/mL, respectively.153 Other studies constructed nanomicelles loaded with EVO and the EGF-targeting ligand GE11 and PEG-polyamino acids using fluorescent labeling, which could effectively target colorectal cancer cells. The average particle size was 255 nm, the encapsulation efficiency was 94.1%, and drug loading was 10%. The IC50 of the nanoparticles and free drugs in LoVo cells was 9.732 and 19.51 μg/mL, respectively. Fluorescence imaging studies showed that the nanomicelles aggregated at tumor sites in nude mice, and the antitumor effects in mouse colon cancer models in situ showed that the nanoparticles could inhibit tumor growth efficiently, achieving an inhibitory effect analogous to that of cetuximab.154 Some studies developed mitochondrial-targeted reduction-sensitive micelles that can co-deliver doxorubicin and EVO. The micelles first target mitochondria through triphenylphosphorus ions and then release the drugs by cleaving the disulfide bonds of the micelles by GSH. The particle size of the micelles was about 85 nm, and the drug loading and encapsulation efficiency of EVO were 5.6% and 62%, respectively. The IC50 value of the micelles against 4T1 cells was 1.45 μg/mL, and the tumor inhibition rate in 4T1 tumor model mice was up to 90%, both of which were superior to those of the free drugs.155
Other Nanoparticles
Some EVO nanopreparations have been synthesized using inorganic materials, magnetic materials, and cyclodextrins to achieve targeting and magnetic responses, as well as to improve bioavailability and other functions. A study used graphene quantum dots (GQDs) as a carrier to deliver EVO, functionalized it with folic acid (FA), and synthesized a GQDs-FA-EVO nanocomposite system using a self-assembly method. The particle size was 85–130 nm, and the drug loading was 10%.
In vitro experimental results showed that the nanoparticles inhibited the growth of OSCC cells by > 50%. In tumor-bearing nude mice, GQDs-FA-EVO significantly reduced the tumor volume by 19% after 18 days compared with the original drug.156 Polymer nanoparticles loaded with superparamagnetic iron oxide nanoparticles and EVO were synthesized using Fe3O4 and an MPEG-PLGA copolymer. The hydrodynamic diameter was approximately 261 nm, and the drug loading and encapsulation efficiency were 8.61 ± 0.73% and 40.36 ± 3.42%, respectively. The iron content was about 9.34%, the sustained drug release lasted more than 70 h, and the IC50 of HeLa cell nanocarriers and free EVO was 13.8 and 9.84 g/mL, respectively. Using the H22 tumor ascites mouse model, the tumor inhibition rate of the nanoparticles reached 70% at a dose of 10 mg/kg.157 EVO nanoparticles modified using recombinant human epidermal growth factor were prepared using the solvent evaporation method and carbodiimide chemistry. These nanoparticles can be used to treat gastric mucosal injury by activating the EGFR signaling pathway and the anti-inflammatory effect of EVO. The nanoparticle size was 179.07 nm, and the encapsulation efficiency was 94%. The degree of gastric mucosal injury was significantly reduced in male Sprague–Dawley rats with IND-associated gastric mucosal injury, the serum levels TNF-α and IL-6 were decreased, COX-2 levels in gastric tissue were decreased, and NO levels in gastric tissue were increased after the oral administration of the nanoparticle preparation.158 Phospholipid and hydroxypropyl-β-cyclodextrin were used to construct EVO-loaded nanoparticles using the solvent evaporation method. The synthesized nanoparticles had a size of 183.23 nm and showed better absorption than free EVO in the entire gastrointestinal tract. The relative bioavailability was 4.58 times that of free EVO. The IC50 of nanoparticles (EA) in H446 (human small cell lung cancer) cells was 17.20 μM, 0.78 μM, and 0.91 μM at 24 h, 48 h, and 72 h, respectively, and the inhibition rates were better than those of free EVO (> 30.00 μM, 29.54 μM, and 3.97 μM, respectively). In vivo experiments showed that the nanoparticles inhibited 76% of the H446 cell-transplanted tumors in nude mice and were better than that of free EVO (46%).159
Microemulsions and Nanoemulsions
Microemulsions are composed of a water phase, a surfactant, a cosurfactant, and an oil phase. They have low viscosity, isotropic and stable thermodynamic properties, and are translucent, transparent, or slightly milky. These features of nanoemulsions can improve the bioavailability and permeability of EVO. Studies have used Cremophor® EL and PEG400 as surfactants or co-surfactants to prepare EVO microemulsions with a particle size of 10.7–48.1 nm. In an in vitro transdermal experiment, the skin throughput of the EVO microemulsion was 2.55–11.36 times that of the aqueous suspension. In microemulsion application with in vivo microdialysis, the maximum concentrations of EVO from the microemulsion were 1.52 and 3.06 times that from ointment and tincture, respectively,160 and its transport mechanism was mainly attributed to the intercellular space.161 Water-in-oil EVO nanoemulsions were constructed using soybean phospholipid, Cremophor EL 35, and PEG400. The relative bioavailability of the nanoemulsion was 6.3 times higher, and its effective permeability in the colon was 8.64 times that of free EVO.162 A study used Fructus bruce oil to prepare emulsified nanosystems containing EVO. Fructus bruce oil has 2 excipient properties (oil phase and stabilizer) and a drug-like property (antitumor effect). The particle size of the nanoemulsion was 31.2 nm, and the IC50 against A549 cells was 6.79 µM, 1.95 µM, and 1.55 µM for 12 h, 24 h, and 48 h, respectively. In vivo studies showed that its absorption constant and bioavailability were 8.38 times and 4.6 times higher than those of free EVO, respectively, and its tumor inhibition rate in the subcutaneous tumorigenesis model of the human A549 NSCLC cell line in nude mice was obviously better than that of free EVO.163 The EVO nanoemulsion was prepared using ethyl oleate, Cremophor EL 35, and PEG400 as the oil phase, surfactant, and co-surfactant, respectively. The particle size was about 29 nm, and the IC50 for A549 cells was 31.53 μM, 17.62 μM, and 7.24 μM for 12 h, 24 h, and 48 h, respectively, which were better than those of free EVO. Pharmacokinetic studies showed that the bioavailability (4.7 times that of the original drug) and in situ absorption characteristics (2–14 fold) of the nanoemulsions were significantly better than those of free EVO.164 An EVO-phospholipid complex was prepared using solvent evaporation, and Brucea javanica oil, Cremophor EL 35, and polyethylene glycol 400 were added to prepare water-in-oil emulsions with a particle size of 613.3 nm. The preparation exhibited improved oral absorption and bioavailability of EVO. The absorption rate constant and permeability in different intestinal segments were 3.65–6.76 times that of free EVO and the relative bioavailability was 8.47 times that of free EVO.165
Hydrogels
A hydrogel is a 3-dimensional network structure formed by the cross-linking of hydrophilic polymer chains. Hydrogels have good biocompatibility and can regulate the amount and rate of drug release by swelling and responding to specific stimuli (pH and temperature) to achieve sustained drug release. Using Cremophor EL as a surfactant and ethyl oleate as an emulsifier, a microemulsion was prepared and loaded into a hydrogel with a hyaluronic acid structure to increase the transdermal penetration of EVO by 2.60 times and enhance the analgesic effect in a mouse model of pain.166 Studies have also prepared a self-assembled thermosensitive in situ hydrogel by constructing a berberine hydrochloride/HP-β-CD inclusion complex and EVO/HP-β-CD inclusion complex and then adding PEG8000, p188, and p407 and stirring to achieve the continuous co-delivery of berberine hydrochloride and EVO. After intranasal administration of this hydrogel, the bioavailability of berberine and EVO increased by approximately 135 and 112 times, respectively, compared with the free drugs after intragastric administration.167
Others
In addition to the above preparations, some studies have reported the preparation of EVO in a phospholipid complex as a cyclodextrin-inclusion compound and as a dispersible tablet to improve the solubility and dissolution rate of fat-soluble drugs and further improve their bioavailability. Some studies designed a new type of rutaecarpine-phospholipid complex by the noncovalent complexation of phospholipid and rutaecarpine, with an average particle size of 246.1 nm. Its complexation rate reached 96.5%. Pharmacokinetic studies showed that its relative bioavailability increased significantly to 218.82% compared with free EVO.168 An EVO inclusion complex was prepared using hydroxypropyl-β-cyclodextrin, and its inclusion rate reached 96%, obviously improving drug pharmacokinetics. The relative bioavailability was 256.73% that of free EVO,169 and the inclusion complex significantly enhanced the antitumor effects. The IC50 of EVO and EVO/HP-β-CD in HepG2 cells was 8.516 and 0.977 μM, respectively.170 Solid dispersions of different concentrations of EVO and PVP-K30 were prepared using the solvent method, and pharmacokinetic studies in beagle dogs showed that the Cmax and AUC0-24 h of EVO solid dispersion were 2.66 and 1.50 times higher than those of the physical mixture of samples, respectively, significantly improving the absorption and bioavailability rates.171
Summary
EVO is the main alkaloid in E. rutaecarpa, and it has great potential for development as an anticancer, anti-inflammatory, cardioprotective, and anti-AD agent. Its antitumor effects, especially, appear to be of the most interest, and numerous studies have confirmed the antitumor effects of EVO.172–174 Its mechanism of action is by acting on Topo1, blocking the cell cycle, inhibiting cell proliferation, inducing apoptosis, and inhibiting the invasion and metastasis of tumor cells. The pharmacological effects of this natural and novel anticancer drug are worthy of further exploration in basic research and clinical trials and may bring new options for treating patients with tumors. EVO also exerts anti-inflammatory, cardioprotective, anti-atherosclerosis, and other effects, but its exact curative effect and mechanism of action need elucidation. With the gradual deepening of the research on the main components of Evodia rutaecarpa, the multiple targets and mechanisms of the pharmacological activity of EVO have gradually emerged. However, studies reported that EVO is associated with potential liver, heart, and kidney toxicity, and there are potential risks in its clinical use, which seriously limit its clinical application. Previous studies revealed that the effective-toxic effects were mainly related to administration time and concentration. Long-term, high-dose administration may induce toxicity; therefore, when using EVO or drugs containing EVO to treat diseases, attention should be paid to the administration time and dosage to reduce toxicity and side effects. Further in-depth research is also needed on the toxicological mechanisms, and new clinical strategies need to be sought from the perspectives of toxicity efficacy conversion, efficacy enhancement, and toxicity reduction. In addition, comprehensive systematic exposure and liver exposure studies should be carried out based on the absorption and metabolism of EVO to identify a range for the safe clinical use of EVO. The supervision of the clinical use of Evodia rutaecarpa must be strengthened to control its dosage strictly and reduce the occurrence of adverse reactions.
Prospects
The poor water solubility of EVO limits its wide use in clinical settings. The following 2 pathways are currently used to further develop and utilize EVO: (1) Structural modifications of EVO: Studies on the structural modification of EVO and synthesis of novel EVO derivatives to improve the in vivo biological activity of EVO, increase the in vivo absorption of EVO, improve the bioavailability of EVO, and enhance the curative effect of EVO are useful approaches. Numerous potent EVO derivatives have been synthesized by modifying the A–E rings of EVO. However, further studies and verification are warranted before using these compounds in clinical settings. (2) Studies on EVO nanopreparations: Nanopreparations of EVO have been synthesized to improve the solubility, bioavailability, and therapeutic efficacy of EVO. Microparticle preparations, hydrogel, cyclodextrin-inclusion compounds, and solid dispersions have led to an increase in EVO absorption, bioavailability, and efficacy. Nanopreparations, such as liposomes and micelles, can improve the sustained-release and targeting effects, which can further improve the curative effect of EVO and reduce its toxicity and side effects.
However, current studies on EVO need further corroboration. Future research should focus on the following aspects. (1) Although many studies have reported the pharmacological activities of EVO, they are generally limited to cell or animal (preclinical) studies, and no clinical studies have been conducted. Thus, its efficacy needs to be further confirmed, and its complex mechanism of action needs to be elucidated. (2) Tumor multidrug resistance reversal agents are a current worldwide hot research topic, and subsequent research should focus on whether EVO can reverse drug resistance in tumor cells. (3) Several studies have shown that EVO has a certain toxicity risk. Thus, further studies on the affected sites, mechanism of toxicity, and its “dose-effect toxicity” relationship are necessary. Additional studies on the toxicological mechanisms and detoxification strategies are also warranted to provide guidance for subsequent clinical research. (4) At present, most of the pharmacokinetic studies on EVO have focused on absorption and metabolism, but there are few studies on its distribution in tissues and metabolism in vivo. In addition, most of the previous studies were pharmacokinetic studies conducted in animals. Therefore, an in-depth pharmacokinetic study of EVO in humans is needed to provide a basis for its later clinical translation. (5) EVO derivatives can improve the physicochemical properties and bioavailability of EVO; however, the stability, efficacy, and safety of several derivatives need to be further confirmed. (6) At present, research findings related to EVO preparations indicate that the derivatives have better bioavailability than the parent drug. However, these compounds are mostly at the basic laboratory research stage. A means to further their industrial production is an aspect that should be focused on.
Limitations: This article has many limitations. (1) It is only a summary of the current research on EVO and does not discuss the heterogeneity among studies or variations in experimental methodologies. There may be one-sidedness in some pharmacological effects or therapeutic effects. (2) At present, there is limited research on the toxicity of EVO, and this article cannot fully reveal the potential toxic effects of EVO or the adverse reactions that may occur in clinical practice. (3) Since EVO and its formulations have not yet reached the clinical trial stage, the current study is only based on the summary of preclinical studies.
Acknowledgments
This work was financially sponsored by the Beijing Nova Program (20230484300) and Fundamental Research Funds for the Central Public Welfare Research Institutes (CI2021A04309, ZZ13-YQ-059).
Disclosure
The authors declare that they have no competing financial interests.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Wang H, Lu Z, Zhao X. Tumorigenesis, diagnosis, and therapeutic potential of exosomes in liver cancer. J Hematol Oncol. 2019;12(1):133. doi:10.1186/s13045-019-0806-6
3. Chou ST, Hsiang CY, Lo HY, et al. Exploration of anti-cancer effects and mechanisms of Zuo-Jin-Wan and its alkaloid components in vitro and in orthotopic HepG2 xenograft immunocompetent mice. BMC Complement Altern Med. 2017;17(1):121. doi:10.1186/s12906-017-1586-6
4. Guo XX, Li XP, Zhou P, et al. Evodiamine Induces Apoptosis in SMMC-7721 and HepG2 Cells by Suppressing NOD1 Signal Pathway. Int J Mol Sci. 2018;19(11):3419. doi:10.3390/ijms19113419
5. Hu CY, Wu HT, Su YC, Lin CH, Chang CJ, Wu CL. Evodiamine exerts an anti-hepatocellular carcinoma activity through a WWOX-dependent pathway. Molecules. 2017;22(7). doi:10.3390/molecules22071175
6. Yang F, Shi L, Liang T, et al. Anti-tumor effect of evodiamine by inducing Akt-mediated apoptosis in hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;485(1):54–61. doi:10.1016/j.bbrc.2017.02.017
7. Yun UJ, Bae SJ, Song YR, Kim YW. A Critical YAP in Malignancy of HCC is regulated by evodiamine. Int J Mol Sci. 2022;23(3). doi:10.3390/ijms23031855
8. Liu F, Lou G, Zhang T, et al. Anti-metastasis traditional Chinese medicine monomer screening system based on perinucleolar compartment analysis in hepatocellular carcinoma cells. Am J Transl Res. 2019;11(6):3555–3566.
9. Li YL, Zhang NY, Hu X, et al. Evodiamine induces apoptosis and promotes hepatocellular carcinoma cell death induced by vorinostat via downregulating HIF-1alpha under hypoxia. Biochem Biophys Res Commun. 2018;498(3):481–486. doi:10.1016/j.bbrc.2018.03.004
10. Hong JY, Park SH, Min HY, Park HJ, Lee SK. Anti-proliferative effects of evodiamine in human lung cancer cells. J Cancer Prev. 2014;19(1):7–13. doi:10.15430/JCP.2014.19.1.7
11. Fang C, Zhang J, Qi D, et al. Evodiamine induces G2/M arrest and apoptosis via mitochondrial and endoplasmic reticulum pathways in H446 and H1688 human small-cell lung cancer cells. PLoS One. 2014;9(12):e115204. doi:10.1371/journal.pone.0115204
12. Wei J, Li Z, Yuan F. Evodiamine might inhibit TGF-beta1-induced epithelial-mesenchymal transition in NRK52E cells via Smad and PPAR-gamma pathway. Cell Biol Int. 2014;38(7):875–880. doi:10.1002/cbin.10270
13. Panda M, Biswal BK. Evodiamine inhibits stemness and metastasis by altering the SOX9-beta-catenin axis in non-small-cell lung cancer. J Cell Biochem. 2022;123(9):1454–1466. doi:10.1002/jcb.30304
14. Mohan V, Agarwal R, Singh RP. A novel alkaloid, evodiamine causes nuclear localization of cytochrome-c and induces apoptosis independent of p53 in human lung cancer cells. Biochem Biophys Res Commun. 2016;477(4):1065–1071. doi:10.1016/j.bbrc.2016.07.037
15. Ogasawara M, Suzuki H. Inhibition by evodiamine of hepatocyte growth factor-induced invasion and migration of tumor cells. Biol Pharm Bull. 2004;27(4):578–582. doi:10.1248/bpb.27.578
16. Li Y, Wang Y, Wang X, et al. Evodiamine suppresses the progression of non-small cell lung carcinoma via endoplasmic reticulum stress-mediated apoptosis pathway in vivo and in vitro. Int J Immunopathol Pharmacol. 2022;36:3946320221086079. doi:10.1177/03946320221086079
17. Yang X, Zhang Y, Huang Y, et al. Evodiamine suppresses Notch3 signaling in lung tumorigenesis via direct binding to gamma-secretases. Phytomedicine. 2020;68:153176. doi:10.1016/j.phymed.2020.153176
18. Su T, Yang X, Deng JH, et al. Evodiamine, a novel NOTCH3 methylation stimulator, significantly suppresses lung carcinogenesis in vitro and in vivo. Front Pharmacol. 2018;9:434. doi:10.3389/fphar.2018.00434
19. Jiang ZB, Huang JM, Xie YJ, et al. Evodiamine suppresses non-small cell lung cancer by elevating CD8(+) T cells and downregulating the MUC1-C/PD-L1 axis. J Exp Clin Cancer Res. 2020;39(1):249. doi:10.1186/s13046-020-01741-5
20. Shyu K-G, Lin S, Lee -C-C, et al. Evodiamine inhibits in vitro angiogenesis: implication for antitumorgenicity. Life Sci. 2006;78(19):2234–2243. doi:10.1016/j.lfs.2005.09.027
21. Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. 2023;20(5):338–349. doi:10.1038/s41571-023-00747-0
22. Morgan E, Arnold M, Camargo MC, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: a population-based modelling study. EClinicalMedicine. 2022;47:101404. doi:10.1016/j.eclinm.2022.101404
23. Wen Z, Feng S, Wei L, Wang Z, Hong D, Wang Q. Evodiamine, a novel inhibitor of the Wnt pathway, inhibits the self-renewal of gastric cancer stem cells. Int J Mol Med. 2015;36(6):1657–1663. doi:10.3892/ijmm.2015.2383
24. Shen H, Zhao S, Xu Z, Zhu L, Han Y, Ye J. Evodiamine inhibits proliferation and induces apoptosis in gastric cancer cells. Oncol Lett. 2015;10(1):367–371. doi:10.3892/ol.2015.3153
25. Yang L, Liu X, Wu D, et al. Growth inhibition and induction of apoptosis in SGC‑7901 human gastric cancer cells by evodiamine. Mol Med Rep Apr. 2014;9(4):1147–1152. doi:10.3892/mmr.2014.1924
26. Zhu LH, Bi W, Liu XD, et al. Induction of apoptosis by evodiamine involves both activation of mitotic arrest and mitotic slippage. Oncol Rep. 2011;26(6):1447–1455. doi:10.3892/or.2011.1444
27. Hu C, Gao X, Han Y, et al. Evodiamine sensitizes BGC-823 gastric cancer cells to radiotherapy in vitro and in vivo. Mol Med Rep. 2016;14(1):413–419. doi:10.3892/mmr.2016.5237
28. Liu X, Yang L, Bi Y, Wang LH, Huang H. Effect of evodiamine in inducing apoptosis of gastric cancer SGC-7901 cells through mTOR signal pathway. Zhongguo Zhong Yao Za Zhi. 2015;40(16):3262–3266.
29. Yue G, Wei J, Qian X, et al. Synergistic anticancer effects of polyphyllin I and evodiamine on freshly-removed human gastric tumors. PLoS One. 2013;8(6):e65164. doi:10.1371/journal.pone.0065164
30. Ogasawara M, Matsubara T, Suzuki H. Screening of natural compounds for inhibitory activity on colon cancer cell migration. Biol Pharm Bull. 2001;24(6):720–723. doi:10.1248/bpb.24.720
31. Kim H, Yu Y, Choi S, et al. Evodiamine eliminates colon cancer stem cells via suppressing notch and Wnt signaling. Molecules. 2019;24(24). doi:10.3390/molecules24244520
32. Zhao LC, Li J, Liao K, et al. Evodiamine induces apoptosis and inhibits migration of HCT-116 human colorectal cancer cells. Int J Mol Sci. 2015;16(11):27411–27421. doi:10.3390/ijms161126031
33. Zhou P, Li XP, Jiang R, et al. Evodiamine inhibits migration and invasion by Sirt1-mediated post-translational modulations in colorectal cancer. Anticancer Drugs. 2019;30(6):611–617. doi:10.1097/CAD.0000000000000760
34. Wang D, Ge S, Chen Z, Song Y. Evodiamine exerts anticancer effects via induction of apoptosis and autophagy and suppresses the migration and invasion of human colon cancer cells. J BUON. 2019;24(5):1824–1829.
35. Huang J, Chen ZH, Ren CM, et al. Antiproliferation effect of evodiamine in human colon cancer cells is associated with IGF-1/HIF-1alpha downregulation. Oncol Rep. 2015;34(6):3203–3211. doi:10.3892/or.2015.4309
36. Zhang Y, Zhang Y, Zhao Y, et al. Protection against ulcerative colitis and colorectal cancer by evodiamine via anti‑inflammatory effects. Mol Med Rep. 2022;25(5). doi:10.3892/mmr.2022.12704
37. Hong Z, Wang Z, Zhou B, et al. Effects of evodiamine on PI3K/Akt and MAPK/ERK signaling pathways in pancreatic cancer cells. Int J Oncol. 2020;56(3):783–793. doi:10.3892/ijo.2020.4956
38. Kim SH, Kang JG, Kim CS, Ihm SH, Choi MG, Lee SJ. Evodiamine in combination with histone deacetylase inhibitors has synergistic cytotoxicity in thyroid carcinoma cells. Endocrine. 2019;65(1):110–120. doi:10.1007/s12020-019-01885-1
39. Yuan XL, Zhang P, Liu XM, et al. Cytological assessments and transcriptome profiling demonstrate that evodiamine inhibits growth and induces apoptosis in a renal carcinoma cell line. Sci Rep. 2017;7(1):12572. doi:10.1038/s41598-017-12918-y
40. Shi CS, Li JM, Chin CC, Kuo YH, Lee YR, Huang YC. Evodiamine induces cell growth arrest, apoptosis and suppresses tumorigenesis in human urothelial cell carcinoma cells. Anticancer Res. 2017;37(3):1149–1159.
41. Zhang T, Qu S, Shi Q, He D, Jin X. Evodiamine induces apoptosis and enhances TRAIL-induced apoptosis in human bladder cancer cells through mTOR/S6K1-mediated downregulation of Mcl-1. Int J Mol Sci. 2014;15(2):3154–3171. doi:10.3390/ijms15023154
42. Wei L, Jin X, Cao Z, Li W. Evodiamine induces extrinsic and intrinsic apoptosis of ovarian cancer cells via the mitogen-activated protein kinase/phosphatidylinositol-3-kinase/protein kinase B signaling pathways. J Tradit Chin Med. 2016;36(3):353–359. doi:10.1016/S0254-6272(16)30049-8
43. Yang J, Wu LJ, Tashino S, Onodera S, Ikejima T. Protein tyrosine kinase pathway-derived ROS/NO productions contribute to G2/M cell cycle arrest in evodiamine-treated human cervix carcinoma HeLa cells. Free Radic Res. 2010;44(7):792–802. doi:10.3109/10715762.2010.481302
44. Sachita K, Kim Y, Yu HJ, Cho SD, Lee JS. In vitro assessment of the anticancer potential of evodiamine in human oral cancer cell lines. Phytother Res. 2015;29(8):1145–1151. doi:10.1002/ptr.5359
45. Peng X, Zhang Q, Zeng Y, Li J, Wang L, Ai P. Evodiamine inhibits the migration and invasion of nasopharyngeal carcinoma cells in vitro via repressing MMP-2 expression. Cancer Chemother Pharmacol. 2015;76(6):1173–1184. doi:10.1007/s00280-015-2902-9
46. Wu Y, Wang J, Zhao J, et al. Gene regulation analysis of the effects of evodiamine on tongue squamous cell carcinoma. J Cell Biochem. 2019;120(9):15933–15940. doi:10.1002/jcb.28869
47. Wang C, Li S, Wang MW. Evodiamine-induced human melanoma A375-S2 cell death was mediated by PI3K/Akt/caspase and Fas-L/NF-kappaB signaling pathways and augmented by ubiquitin-proteasome inhibition. Toxicol In Vitro. 2010;24(3):898–904. doi:10.1016/j.tiv.2009.11.019
48. Zhu B, Zhao L, Liu Y, et al. Induction of phosphatase shatterproof 2 by evodiamine suppresses the proliferation and invasion of human cholangiocarcinoma. Int J Biochem Cell Biol. 2019;108:98–110. doi:10.1016/j.biocel.2019.01.012
49. Zhang Y, Zhang QH, Wu LJ, Tashiro S, Onodera S, Ikejima T. Atypical apoptosis in L929 cells induced by evodiamine isolated from Evodia rutaecarpa. J Asian Nat Prod Res. 2004;6(1):19–27. doi:10.1080/1028602031000119772
50. Han S, Woo JK, Jung Y, et al. Evodiamine selectively targets cancer stem-like cells through the p53-p21-Rb pathway. Biochem Biophys Res Commun. 2016;469(4):1153–1158. doi:10.1016/j.bbrc.2015.12.066
51. Chen TC, Chien CC, Wu MS, Chen YC. Evodiamine from Evodia rutaecarpa induces apoptosis via activation of JNK and PERK in human ovarian cancer cells. Phytomedicine. 2016;23(1):68–78. doi:10.1016/j.phymed.2015.12.003
52. Cheng P, Zhang X, Wang X, et al. Identification of evodiamine as a suppressor of prostate cancer progression by reducing AR transcriptional activity via targeting Src. Endocrine. 2022;75(2):635–645. doi:10.1007/s12020-021-02907-7
53. Zhang L, Li L, Chen X, et al. Evodiamine inhibits ESCC by inducing M-phase cell-cycle arrest via CUL4A/p53/p21 axis and activating noxa-dependent intrinsic and DR4-dependent extrinsic apoptosis. Phytomedicine. 2023;108:154493. doi:10.1016/j.phymed.2022.154493
54. Ren L, Lou Y, Sun M. The anti-tumor effects of evodiamine on oral squamous cell carcinoma (OSCC) through regulating advanced glycation end products (AGE) / receptor for advanced glycation end products (RAGE) pathway. Bioengineered. 2021;12(1):5985–5995. doi:10.1080/21655979.2021.1972082
55. Hyun SY, Le HT, Min HY, et al. Evodiamine inhibits both stem cell and non-stem-cell populations in human cancer cells by targeting heat shock protein 70. Theranostics. 2021;11(6):2932–2952. doi:10.7150/thno.49876
56. Wu QQ, Xiao Y, Jiang XH, et al. Evodiamine attenuates TGF-beta1-induced fibroblast activation and endothelial to mesenchymal transition. Mol Cell Biochem. 2017;430(1–2):81–90. doi:10.1007/s11010-017-2956-6
57. Jiang XH, Wu QQ, Xiao Y, et al. Evodiamine prevents isoproterenol-induced cardiac fibrosis by regulating endothelial-to-mesenchymal transition. Planta Med. 2017;83(9):761–769. doi:10.1055/s-0042-124044
58. Ge X, Chen SY, Liu M, Liang TM, Liu C. Evodiamine inhibits PDGF‑BB‑induced proliferation of rat vascular smooth muscle cells through the suppression of cell cycle progression and oxidative stress. Mol Med Rep. 2016;14(5):4551–4558. doi:10.3892/mmr.2016.5798
59. Bacova BS, Radosinska J, Wallukat G, et al. Suppression of beta1-adrenoceptor autoantibodies is involved in the antiarrhythmic effects of omega-3 fatty acids in male and female hypertensive rats. Int J Mol Sci. 2020;21(2). doi:10.3390/ijms21020526
60. Xue H, Cheng Y, Wang X, Yue Y, Zhang W, Li X. Rutaecarpine and evodiamine selected as beta1-AR inhibitor candidates using beta1-AR/CMC-offline-UPLC/MS prevent cardiac ischemia-reperfusion injury via energy modulation. J Pharm Biomed Anal. 2015;115:307–314. doi:10.1016/j.jpba.2015.07.022
61. Rang WQ, Du YH, Hu CP, et al. Protective effects of calcitonin gene-related peptide-mediated evodiamine on Guinea-pig cardiac anaphylaxis. Naunyn Schmiedebergs Arch Pharmacol. 2003;367(3):306–311. doi:10.1007/s00210-002-0677-0
62. Rang WQ, Du YH, Hu CP, et al. Protective effects of evodiamine on myocardial ischemia-reperfusion injury in rats. Planta Med. 2004;70(12):1140–1143. doi:10.1055/s-2004-835841
63. Zhou X, Ren F, Wei H, et al. Combination of berberine and evodiamine inhibits intestinal cholesterol absorption in high fat diet induced hyperlipidemic rats. Lipids Health Dis. 2017;16(1):239. doi:10.1186/s12944-017-0628-x
64. Ma L, Zhong J, Zhao Z, et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc Res. 2011;92(3):504–513. doi:10.1093/cvr/cvr245
65. Zhang C, Ye L, Zhang Q, Wu F, Wang L. The role of TRPV1 channels in atherosclerosis. Channels (Austin). 2020;14(1):141–150. doi:10.1080/19336950.2020.1747803
66. Wei J, Ching LC, Zhao JF, et al. Essential role of transient receptor potential vanilloid type 1 in evodiamine-mediated protection against atherosclerosis. Acta Physiol (Oxf). 2013;207(2):299–307. doi:10.1111/apha.12005
67. Ching LC, Kou YR, Shyue SK, et al. Molecular mechanisms of activation of endothelial nitric oxide synthase mediated by transient receptor potential vanilloid type 1. Cardiovasc Res. 2011;91(3):492–501. doi:10.1093/cvr/cvr104
68. Ge X, Chen S, Liu M, Liang T, Liu C. Evodiamine Attenuates PDGF-BB-induced migration of rat vascular smooth muscle cells through activating PPARgamma. Int J Mol Sci. 2015;16(12):28180–28193. doi:10.3390/ijms161226093
69. Choi YH, Shin EM, Kim YS, Cai XF, Lee JJ, Kim HP. Anti-inflammatory principles from the fruits of Evodia rutaecarpa and their cellular action mechanisms. Arch Pharm Res. 2006;29(4):293–297. doi:10.1007/BF02968573
70. Li CG, Zeng QZ, Chen MY, et al. Evodiamine augments NLRP3 inflammasome activation and anti-bacterial responses through inducing alpha-tubulin acetylation. Front Pharmacol. 2019;10:290. doi:10.3389/fphar.2019.00290
71. Heo SK, Yun HJ, Yi HS, Noh EK, Park SD. Evodiamine and rutaecarpine inhibit migration by LIGHT via suppression of NADPH oxidase activation. J Cell Biochem. 2009;107(1):123–133. doi:10.1002/jcb.22109
72. Ko HC, Wang YH, Liou KT, et al. Anti-inflammatory effects and mechanisms of the ethanol extract of Evodia rutaecarpa and its bioactive components on neutrophils and microglial cells. Eur J Pharmacol. 2007;555(2–3):211–217. doi:10.1016/j.ejphar.2006.10.002
73. Fan X, Zhu J-Y, Sun Y, et al. Evodiamine inhibits zymosan-induced inflammation in vitro and in vivo: inactivation of NF-κB by Inhibiting IκBα phosphorylation. Inflammation. 2017;40(3):1012–1027. doi:10.1007/s10753-017-0546-0
74. Shin YW, Bae EA, Cai XF, Lee JJ, Kim DH. In vitro and in vivo antiallergic effect of the fructus of Evodia rutaecarpa and its constituents. Biol Pharm Bull. 2007;30(1):197–199. doi:10.1248/bpb.30.197
75. Shen P, Zhang Z, Zhu K, et al. Evodiamine prevents dextran sulfate sodium-induced murine experimental colitis via the regulation of NF-kappaB and NLRP3 inflammasome. Biomed Pharmacother. 2019;110:786–795. doi:10.1016/j.biopha.2018.12.033
76. Xian S, Lin Z, Zhou C, Wu X. The Protective Effect of Evodiamine in Osteoarthritis: an In Vitro and In Vivo Study in Mice Model. Front Pharmacol. 2022;13:899108. doi:10.3389/fphar.2022.899108
77. Wang Q, Cui Y, Wu X, Wang J. Evodiamine protects against airway remodelling and inflammation in asthmatic rats by modulating the HMGB1/NF-kappaB/TLR-4 signalling pathway. Pharm Biol. 2021;59(1):192–199. doi:10.1080/13880209.2020.1871374
78. Liu YN, Pan SL, Liao CH, et al. Evodiamine represses hypoxia-induced inflammatory proteins expression and hypoxia-inducible factor 1alpha accumulation in RAW264.7. Shock. 2009;32(3):263–269. doi:10.1097/SHK.0b013e31819940cb
79. Lv Q, Xue Y, Li G, et al. Beneficial effects of evodiamine on P2X(4)-mediated inflammatory injury of human umbilical vein endothelial cells due to high glucose. Int Immunopharmacol. 2015;28(2):1044–1049. doi:10.1016/j.intimp.2015.08.020
80. Nichols E, Steinmetz JD, Vollset SE. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health. 2022;7(2):e105–e125. doi:10.1016/S2468-2667(21)00249-8
81. Zhang Y, Wang J, Wang C, et al. Pharmacological basis for the use of evodiamine in alzheimer’s disease: antioxidation and antiapoptosis. Int J Mol Sci. 2018;19(5):1.
82. Yuan SM, Gao K, Wang DM, et al. Evodiamine improves congnitive abilities in SAMP8 and APP(swe)/PS1(DeltaE9) transgenic mouse models of Alzheimer’s disease. Acta Pharmacol Sin. 2011;32(3):295–302. doi:10.1038/aps.2010.230
83. Wang D, Wang C, Liu L, Li S. Protective effects of evodiamine in experimental paradigm of Alzheimer’s disease. Cogn Neurodyn. 2018;12(3):303–313.
84. Zhang YN, Yang YF, Yang XW. Blood-brain barrier permeability and neuroprotective effects of three main alkaloids from the fruits of Euodia rutaecarpa with MDCK-pHaMDR cell monolayer and PC12 cell line. Biomed Pharmacother. 2018;98:82–87. doi:10.1016/j.biopha.2017.12.017
85. Wu JY, Chang MC, Chen CS, et al. Topoisomerase I inhibitor evodiamine acts as an antibacterial agent against drug-resistant Klebsiella pneumoniae. Planta Med. 2013;79(1):27–29. doi:10.1055/s-0032-1327925
86. Yang JY, Kim JB, Lee P, Kim SH. Evodiamine inhibits helicobacter pylori growth and helicobacter pylori-induced inflammation. Int J Mol Sci. 2021;22(7):1.
87. Jiang ML, Zhang ZX, Li YZ, Wang XH, Yan W, Gong GQ. Antidepressant-like effect of evodiamine on chronic unpredictable mild stress rats. Neurosci Lett. 2015;588:154–158. doi:10.1016/j.neulet.2014.12.038
88. Yin H, Wang J, Wu M, Ma Y, Wang S, Su Q. Preventive effects of evodiamine on dexamethasone-induced osteoporosis in zebrafish. Biomed Res Int. 2019;2019:5859641. doi:10.1155/2019/5859641
89. Yang XW, Zhang H, Li M, Du LJ, Yang Z, Xiao SY. Studies on the alkaloid constituents of Evodia rutaecarpa (Juss) Benth var. bodinaieri (Dode) Huang and their acute toxicity in mice. J Asian Nat Prod Res. 2006;8(8):697–703. doi:10.1080/10286020412331286425
90. Cai Q, Wei J, Zhao W, et al. Toxicity of Evodiae fructus on rat liver mitochondria: the role of oxidative stress and mitochondrial permeability transition. Molecules. 2014;19(12):21168–21182. doi:10.3390/molecules191221168
91. Li F, Dong YZ, Zhang D, Zhang XM, Lin ZJ, Zhang B. Molecular mechanisms involved in drug-induced liver injury caused by urate-lowering Chinese herbs: a network pharmacology study and biology experiments. PLoS One. 2019;14(5):e0216948. doi:10.1371/journal.pone.0216948
92. Gao YD, Zhu A, Li LD, et al. Cytotoxicity and underlying mechanism of evodiamine in HepG2 cells. Beijing Da Xue Xue Bao Yi Xue Ban. 2021;53(6):1107–1114. doi:10.19723/j.issn.1671-167X.2021.06.017
93. Yang W, Ma L, Li S, Cui K, Lei L, Ye Z. Evaluation of the cardiotoxicity of evodiamine in vitro and in vivo. Molecules. 2017;22(6):1.
94. Yan XJ, Yu X, Wang XP, et al. Mitochondria play an important role in the cell proliferation suppressing activity of berberine. Sci Rep. 2017;7:41712. doi:10.1038/srep41712
95. Xu JH, Liu WY, Zheng F, Sun D, Yang Q, Rao J. Determination of evodiamine by high performance liquid chromatography tandem mass spectrometry and pharmacokinetic studies in rats. Chin J Clin Pharmacol Ther. 2007;12(4):427–433.
96. Jeng KF, Lin YH, Lin LC, Chou CJ, Tsai TH, Chen CF. High-performance liquid chromatographic determination of evodiamine in rat plasma: application to pharmacokinetic studies. J Chromatogr B. 1995;668(2):343–345. doi:10.1016/0378-4347(95)00090-6
97. Xia YY, Xu HY, Cai YY, Si DY, Liu CX. Simultaneous determination of evodiamine and evodine in Beagle dog plasma using liquid chromatography tandem mass spectrometry. J Asian Nat Prod Res. 2013;15(3):235–243. doi:10.1080/10286020.2012.762357
98. Lin C, Pan X, Li W, et al. Simultaneous determination of evodiamine and rutecarpine in rabbit plasma by LC-ESI-MS and its application to pharmacokinetics. Pharmazie. 2011;66(12):920–923.
99. Xu S, Peng J, Li Y, et al. Pharmacokinetic comparisons of rutaecarpine and evodiamine after oral administration of Wu-Chu-Yu extracts with different purities to rats. J Ethnopharmacol. 2012;139(2):395–400. doi:10.1016/j.jep.2011.11.023
100. Xu H, Geng Y, Liu R, et al. Qualitative screening of absorbed indoloquinazoline alkaloids and their metabolites in rat plasma after the oral administration of Wu-Zhu-Yu decoction by high-resolution mass spectrometry with multiple data mining algorithms. J Sep Sci. 2016;39(16):3260–3266. doi:10.1002/jssc.201600435
101. Qian P, Zhang YB, Yang YF, Xu W, Yang XW. Pharmacokinetics studies of 12 alkaloids in rat plasma after oral administration of zuojin and fan-zuojin formulas. Molecules. 2017;22(2). doi:10.3390/molecules22020214
102. Hu CQ, Li F, Yang XW. Simultaneous determination and pharmacokinetic analysis of seven alkaloids and two flavonoids from rat plasma by HPLC-DAD after oral administration of Wuzhuyu decoction. J Asian Nat Prod Res. 2012;14(4):370–381. doi:10.1080/10286020.2012.656093
103. Luo J, Wen W, Chen J, Zeng X, Wang P, Xu S. Differences in tissue distribution ability of evodiamine and dehydroevodiamine are due to the dihedral angle of the molecule stereo-structure. Front Pharmacol. 2023;14:1109279. doi:10.3389/fphar.2023.1109279
104. Wu FP, Yang Y, Liu LH, et al. Evodiamine Ultra–performance liquid chromatography with linear ion trap-orbitrap hydroxyevodiamine sulfate hydroxyevodiamine glucuronide metabolites. Trop J Pharm Res. 2016;15(3):623–629. doi:10.4314/tjpr.v15i3.26
105. Sun HZ, Fang ZZ, Cao YF, Sun XY, Hong M. Investigation of the in vitro metabolism of evodiamine: characterization of metabolites and involved cytochrome p450 isoforms. Phytother Res. 2013;27(5):705–712. doi:10.1002/ptr.4766
106. Zhang Z, Fang T, Zhou H, Yuan J, Liu Q. Characterization of the in vitro metabolic profile of evodiamine in human liver microsomes and hepatocytes by UHPLC-Q exactive mass spectrometer. Front Pharmacol. 2018;9:130. doi:10.3389/fphar.2018.00130
107. Wang C, Yue F, Ai G, Yang J. Simultaneous determination of evodiamine and its four metabolites in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. Biomed Chromatogr. 2018;32(7):e4219. doi:10.1002/bmc.4219
108. Zhang YT, Zhang DF, Ge NY, et al. Effect of evodiamine on CYP enzymes in rats by a cocktail method. Pharmacology. 2016;97(5–6):218–223. doi:10.1159/000443178
109. Wen B, Roongta V, Liu L, Moore DJ. Metabolic activation of the indoloquinazoline alkaloids evodiamine and rutaecarpine by human liver microsomes: dehydrogenation and inactivation of cytochrome P450 3A4. Drug Metab Dispos. 2014;42(6):1044–1054. doi:10.1124/dmd.114.057414
110. Wang MX, Lin L, Chen YD, et al. Evodiamine has therapeutic efficacy in ulcerative colitis by increasing Lactobacillus acidophilus levels and acetate production. Pharmacol Res. 2020;159:104978. doi:10.1016/j.phrs.2020.104978
111. Yao H, Liu J, Xu S, Zhu Z, Xu J. The structural modification of natural products for novel drug discovery. Expert Opin Drug Discov. 2017;12(2):121–140. doi:10.1080/17460441.2016.1272757
112. Guo Z. The modification of natural products for medical use. Acta Pharm Sin B. 2017;7(2):119–136. doi:10.1016/j.apsb.2016.06.003
113. Deng JD, Lei S, Jiang Y, et al. A concise synthesis and biological study of evodiamine and its analogues. Chem. Commun. 2019;55(21):3089–3092. doi:10.1039/C9CC00434C
114. Wang ZX, Xiang JC, Wang M, Ma JT, Wu YD, Wu AX. One-pot total synthesis of evodiamine and its analogues through a continuous biscyclization reaction. Organic Letters. 2018;20(20):6380–6383. doi:10.1021/acs.orglett.8b02667
115. Dong G, Wang S, Miao Z, et al. New tricks for an old natural product: discovery of highly potent evodiamine derivatives as novel antitumor agents by systemic structure-activity relationship analysis and biological evaluations. J Med Chem. 2012;55(17):7593–7613. doi:10.1021/jm300605m
116. Min HY, Lim Y, Kwon H, et al. An A-ring substituted evodiamine derivative with potent anticancer activity against human non-small cell lung cancer cells by targeting heat shock protein 70. Biochem Pharmacol. 2023;211:115507. doi:10.1016/j.bcp.2023.115507
117. Li ZG, Dong GQ, Wang SZ, et al. Optical evodiamine derivatives: asymmetric synthesis and antitumor activity. Chin. Chem. Lett. 2015;26(3):267–271. doi:10.1016/j.cclet.2014.11.011
118. Chen S, Bi K, Wu S, et al. Water-soluble derivatives of evodiamine: discovery of evodiamine-10-phosphate as an orally active antitumor lead compound. Eur J Med Chem. 2021;220:113544. doi:10.1016/j.ejmech.2021.113544
119. Wang T, Qi D, Hu X, et al. A novel evodiamine amino derivative as a PI3K/AKT signaling pathway modulator that induces apoptosis in small cell lung cancer cells. Eur J Pharmacol. 2021;906:174215. doi:10.1016/j.ejphar.2021.174215
120. Dong G, Sheng C, Wang S, Miao Z, Yao J, Zhang W. Selection of evodiamine as a novel topoisomerase I inhibitor by structure-based virtual screening and hit optimization of evodiamine derivatives as antitumor agents. J Med Chem. 2010;53(21):7521–7531. doi:10.1021/jm100387d
121. Hu X, Jiao R, Li H, et al. Antiproliferative hydrogen sulfide releasing evodiamine derivatives and their apoptosis inducing properties. Eur J Med Chem. 2018;151:376–388. doi:10.1016/j.ejmech.2018.04.009
122. Zhao N, Tian KT, Cheng KG, et al. Antiproliferative activity and apoptosis inducing effects of nitric oxide donating derivatives of evodiamine. Bioorg Med Chem. 2016;24(13):2971–2978. doi:10.1016/j.bmc.2016.05.001
123. Song S, Chen Z, Li S, Huang Y, Wan Y, Song H. Design, synthesis and evaluation of N13-substituted evodiamine derivatives against human cancer cell lines. Molecules. 2013;18(12):15750–15768. doi:10.3390/molecules181215750
124. Nguyen NV, Lee KR, Lee YJ, et al. Chiral high-performance liquid chromatographic separation of evodiamine enantiomers and rutaecarpine, isolated from Evodiae fructus. J Pharm Biomed Anal. 2013;81-82:151–159. doi:10.1016/j.jpba.2013.04.018
125. De Petrocellis L, Schiano Moriello A, Fontana G, et al. Effect of chirality and lipophilicity in the functional activity of evodiamine and its analogues at TRPV1 channels. Br J Pharmacol. 2014;171(10):2608–2620. doi:10.1111/bph.12320
126. Huang Y, Chen S, Wu S, Dong G, Sheng C. Evodiamine-inspired dual inhibitors of histone deacetylase 1 (HDAC1) and topoisomerase 2 (TOP2) with potent antitumor activity. Acta Pharm Sin B. 2020;10(7):1294–1308. doi:10.1016/j.apsb.2019.11.011
127. Deng J, Long L, Peng X, et al. N(14)-substituted evodiamine derivatives as dual topoisomerase 1/tubulin-Inhibiting anti-gastrointestinal tumor agents. Eur J Med Chem. 2023;255:115366. doi:10.1016/j.ejmech.2023.115366
128. Hao X, Deng J, Zhang H, et al. Design, synthesis and bioactivity evaluation of novel N-phenyl-substituted evodiamine derivatives as potent anti-tumor agents. Bioorg Med Chem. 2021;55:116595. doi:10.1016/j.bmc.2021.116595
129. Fang K, Dong GQ, Gong H, et al. Design, synthesis and biological evaluation of E-ring modified evodiamine derivatives as novel antitumor agents. Chin. Chem. Lett. 2014;25(7):978–982. doi:10.1016/j.cclet.2014.03.043
130. Fan X, Deng J, Shi T, et al. Design, synthesis and bioactivity study of evodiamine derivatives as multifunctional agents for the treatment of hepatocellular carcinoma. Bioorg Chem. 2021;114:105154. doi:10.1016/j.bioorg.2021.105154
131. Peng Y, Xiong R, Li Z, et al. Design, synthesis, and biological evaluation of 3’,4’,5’-trimethoxy evodiamine derivatives as potential antitumor agents. Drug Dev Res. 2021;82(7):1021–1032. doi:10.1002/ddr.21806
132. Liang Z, Lei F, Deng J, et al. Design, synthesis and bioactivity evaluation of novel evodiamine derivatives with excellent potency against gastric cancer. Eur J Med Chem. 2022;228:113960. doi:10.1016/j.ejmech.2021.113960
133. Lei F, Xiong Y, Wang Y, et al. Design, synthesis, and biological evaluation of novel evodiamine derivatives as potential antihepatocellular carcinoma agents. J Med Chem. 2022;65(11):7975–7992. doi:10.1021/acs.jmedchem.2c00520
134. Liang Z, Wang Y, Zhang H, et al. Design, synthesis and bioactivity evaluation of favorable evodiamine derivative scaffold for developing cancer therapy. Eur J Med Chem. 2022;239:114530. doi:10.1016/j.ejmech.2022.114530
135. Wang S, Fang K, Dong G, et al. Scaffold diversity inspired by the natural product evodiamine: discovery of highly potent and multitargeting antitumor agents. J Med Chem. 2015;58(16):6678–6696. doi:10.1021/acs.jmedchem.5b00910
136. Wang L, Fang K, Cheng J, et al. Scaffold hopping of natural product evodiamine: discovery of a novel antitumor scaffold with excellent potency against colon cancer. J Med Chem. 2020;63(2):696–713. doi:10.1021/acs.jmedchem.9b01626
137. Ma Z, Huang Y, Wan K, et al. Structural simplification of evodiamine: discovery of novel tetrahydro-beta-carboline derivatives as potent antitumor agents. Bioorg Med Chem Lett. 2021;40:127954. doi:10.1016/j.bmcl.2021.127954
138. Liang H, Wang W, Zhu F, Chen S, Liu D, Sheng C. Discovery of novel bis-evodiamine derivatives with potent antitumor activity. Bioorg Med Chem. 2022;65:116793. doi:10.1016/j.bmc.2022.116793
139. Guo W, Wang X, Zhang J, Zhang T, Lv H, Zhao C. Synthesis of ring opening of evodiamine derivatives and evaluation on their biological activity. Chem Biol Drug Des. 2022;99(4):535–546. doi:10.1111/cbdd.13996
140. Pang S, Sun C, Gao S, Yang Y, Pan X, Zhang L. Evodiamine derivatives improve cognitive abilities in APP(swe)/PS1(DeltaE9) transgenic mouse models of Alzheimer’s disease. Animal Model Exp Med. 2020;3(2):193–199. doi:10.1002/ame2.12126
141. Pang S, Li S, Cheng H, et al. Discovery of an evodiamine derivative for PI3K/AKT/GSK3beta pathway activation and AD pathology improvement in mouse models. Front Mol Neurosci. 2022;15:1025066. doi:10.3389/fnmol.2022.1025066
142. Zhang T, Lai Z, Yuan S, et al. Discovery of Evodiamine Derivatives as Highly Selective PDE5 Inhibitors Targeting a Unique Allosteric Pocket. J Med Chem. 2020;63(17):9828–9837. doi:10.1021/acs.jmedchem.0c00983
143. Yang CJ, Li HX, Wang JR, et al. Design, synthesis and biological evaluation of novel evodiamine and rutaecarpine derivatives against phytopathogenic fungi. Eur J Med Chem. 2022;227:113937. doi:10.1016/j.ejmech.2021.113937
144. Liu J, Shi Y, Chen S, Li F, Wen W, Wang Y. Discovery of evodiamine derivatives as potent insecticide candidates. Bioorg Med Chem. 2022;62:116727. doi:10.1016/j.bmc.2022.116727
145. Kim D, Javius-Jones K, Mamidi N, Hong S. Dendritic nanoparticles for immune modulation: a potential next-generation nanocarrier for cancer immunotherapy. Nanoscale. 2024;16:10208–10220. doi:10.1039/D4NR00635F
146. Myung JH, Tam KA, Park SJ, Cha A, Hong S. Recent advances in nanotechnology-based detection and separation of circulating tumor cells. Wiley interdisciplinary reviews. Nanomed Nanobiotechnol. 2016;8(2):223–239. doi:10.1002/wnan.1360
147. Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnol. 2007;2(12):751–760. doi:10.1038/nnano.2007.387
148. Lin H, Lin L, Choi Y, Michniak-Kohn B. Development and in-vitro evaluation of co-loaded berberine chloride and evodiamine ethosomes for treatment of melanoma. Int J Pharm. 2020;581:119278. doi:10.1016/j.ijpharm.2020.119278
149. Wei Z, Zou H, Liu G, et al. Peroxidase-mimicking evodiamine/indocyanine green nanoliposomes for multimodal imaging-guided theranostics for oral squamous cell carcinoma. Bioact Mater. 2021;6(7):2144–2157. doi:10.1016/j.bioactmat.2020.12.016
150. Feng Y, Li NX, Yin HL, Chen TY, Yang Q, Wu M. Thermo- and pH-responsive, lipid-coated, mesoporous silica nanoparticle-based dual drug delivery system to improve the antitumor effect of hydrophobic drugs. Mol Pharm. 2019;16(1):422–436. doi:10.1021/acs.molpharmaceut.8b01073
151. Zou L, Chen F, Bao J, et al. Preparation, characterization, and anticancer efficacy of evodiamine-loaded PLGA nanoparticles. Drug Deliv. 2016;23(3):908–916. doi:10.3109/10717544.2014.920936
152. Deng YG, Lyu XL, Zhu YL, et al. Preparation of evodiamine-glycyrrhizic acid micelles with glycyrrhizic acid as carrier and their anti-hepatic fibrosis activity. Zhongguo Zhong Yao Za Zhi. 2020;45(13):3136–3143. doi:10.19540/j.cnki.cjcmm.20200424.304
153. Solanki R, Jangid AK, Jadav M, Kulhari H, Patel S. Folate functionalized and evodiamine-loaded pluronic nanomicelles for augmented cervical cancer cell killing. Macromol Biosci. 2023;23(9):e2300077. doi:10.1002/mabi.202300077
154. Li C, Cai G, Song D, et al. Development of EGFR-targeted evodiamine nanoparticles for the treatment of colorectal cancer. Biomater Sci. 2019;7(9):3627–3639. doi:10.1039/C9BM00613C
155. Tan X, Zhou Y, Shen L, Jia H, Tan X. A mitochondria-targeted delivery system of doxorubicin and evodiamine for the treatment of metastatic breast cancer. RSC Adv. 2019;9(63):37067–37078. doi:10.1039/C9RA07096F
156. Ma Y, Liu Y, Wang YR, Guo YZ. The nanocomposite system comprising folic acid-modified graphene quantum dots loaded with evodiamine in the treatment of oral squamous cell carcinoma. Mater Des. 2022;220(1):110838. doi:10.1016/j.matdes.2022.110838
157. Lv Y, Ding G, Zhai J, Guo Y, Nie G, Xu L. A superparamagnetic Fe3O4-loaded polymeric nanocarrier for targeted delivery of evodiamine with enhanced antitumor efficacy. Colloids Surf B Biointerfaces. 2013;110:411–418. doi:10.1016/j.colsurfb.2013.04.038
158. Wang SL, Y JIN, Zhao Q, et al. Evodiamine-loaded rhEGF-conjugated bovine serum albumin nanoparticles alleviate indomethacin-associated gastric mucosal injury in male SD rats. J Drug Delivery Sci Technol. 2023;82(2021):104345. doi:10.1016/j.jddst.2023.104345
159. Yang J, Fang C, Liu H, et al. Ternary supramolecular nanocomplexes for superior anticancer efficacy of natural medicines. Nanoscale. 2021;13(35):15085–15099. doi:10.1039/D1NR02791C
160. Zhang YT, Zhao JH, Zhang SJ, et al. Enhanced transdermal delivery of evodiamine and rutaecarpine using microemulsion. Int J Nanomed. 2011;6:2469–2482. doi:10.2147/IJN.S25258
161. Zhang YT, Huang ZB, Zhang SJ, et al. In vitro cellular uptake of evodiamine and rutaecarpine using a microemulsion. Int J Nanomed. 2012;7:2465–2472. doi:10.2147/IJN.S30616
162. Hu J, Chen D, Jiang R, Tan Q, Zhu B, Zhang J. Improved absorption and in vivo kinetic characteristics of nanoemulsions containing evodiamine-phospholipid nanocomplex. Int J Nanomed. 2014;9:4411–4420. doi:10.2147/IJN.S59812
163. Zhao J, Liu S, Hu X, et al. Improved delivery of natural alkaloids into lung cancer through woody oil-based emulsive nanosystems. Drug Deliv. 2018;25(1):1426–1437. doi:10.1080/10717544.2018.1474970
164. Liu S, Chen D, Yuan Y, et al. Efficient intracellular delivery makes cancer cells sensitive to nanoemulsive chemodrugs. Oncotarget. 2017;8(39):65042–65055. doi:10.18632/oncotarget.17536
165. Hu J, Sun L, Zhao D, et al. Supermolecular evodiamine loaded water-in-oil nanoemulsions: enhanced physicochemical and biological characteristics. Eur J Pharm Biopharm. 2014;88(2):556–564. doi:10.1016/j.ejpb.2014.06.007
166. Zhang YT, Li Z, Zhang K, et al. Co-delivery of evodiamine and rutaecarpine in a microemulsion-based hyaluronic acid hydrogel for enhanced analgesic effects on mouse pain models. Int J Pharm. 2017;528(1–2):100–106. doi:10.1016/j.ijpharm.2017.05.064
167. Xu D, Qiu C, Wang Y, Qiao T, Cui YL. Intranasal co-delivery of berberine and evodiamine by self-assembled thermosensitive in-situ hydrogels for improving depressive disorder. Int J Pharm. 2021;603:120667. doi:10.1016/j.ijpharm.2021.120667
168. Tan Q, Liu S, Chen X, et al. Design and evaluation of a novel evodiamine-phospholipid complex for improved oral bioavailability. AAPS Pharm Sci Tech. 2012;13(2):534–547. doi:10.1208/s12249-012-9772-9
169. Zhang X, Liu HM, Lei TT, Feng J, Zhang JQ. [A preliminary study of pharmacokinetics of evodiamine hydroxypropyl-beta-cyclodextrin inclusion complex]. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36(4):548–551. Sesotho
170. Qiu C, Gao LN, Yan K, Cui YL, Zhang Y. A promising antitumor activity of evodiamine incorporated in hydroxypropyl-beta-cyclodextrin: pro-apoptotic activity in human hepatoma HepG2 cells. Chem Cent J. 2016;10:46. doi:10.1186/s13065-016-0191-y
171. Xu H, Zhang T, Yang H, et al. Preparation of evodiamine solid dispersions and its pharmacokinetics. Indian J Pharm Sci. 2011;73(3):276–281. doi:10.4103/0250-474X.93511
172. Jiang J, Hu C. Evodiamine: a novel anti-cancer alkaloid from Evodia rutaecarpa. Molecules. 2009;14(5):1852–1859. doi:10.3390/molecules14051852
173. Hu X, Li D, Chu C, et al. Antiproliferative effects of alkaloid evodiamine and its derivatives. Int J Mol Sci. 2018;19(11). doi:10.3390/ijms19113403
174. Panda M, Tripathi SK, Zengin G, Biswal BK. Evodiamine as an anticancer agent: a comprehensive review on its therapeutic application, pharmacokinetic, toxicity, and metabolism in various cancers. Cell Biol Toxicol. 2023;39(1):1–31. doi:10.1007/s10565-022-09772-8
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

