Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Excessive Daytime Sleepiness as a Risk Factor for Impulse Control Disorders in Parkinson’s Disease
Authors Tang X, Liang Q, Li T, Ouyang Y, Huang ZX, Tang X, Jin J, Yu L, Wang X
Received 5 July 2024
Accepted for publication 8 December 2024
Published 13 December 2024 Volume 2024:20 Pages 2517—2527
DOI https://doi.org/10.2147/NDT.S485339
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yu-Ping Ning
Xiaohui Tang,1,2 Qian Liang,1 Tao Li,1 Yetong Ouyang,1 Zhe Xue Huang,1 Xiaoshun Tang,1 Jiayi Jin,1 Lijia Yu,1 Xijin Wang1
1Department of Neurology, Shanghai Tongji Hospital, School of Medicine, Tongji University, Shanghai, People’s Republic of China; 2Department of Neurology, Zhabei Central Hospital, Shanghai, People’s Republic of China
Correspondence: Xijin Wang, Department of Neurology, Shanghai Tongji Hospital, School of Medicine, Tongji University, 389 Xincun Road, Putuo District, Shanghai, 200065, People’s Republic of China, Tel +8613651627382, Email [email protected]
Purpose: Impulse control disorders (ICDs) and excessive daytime sleepiness (EDS) are common symptoms in Parkinson’s disease (PD). Few longitudinal studies have focused on the association between EDS and ICDs. This longitudinal study aimed at assessing association between EDS and ICDs in PD.
Patients and Methods: Patients without ICDs were incorporated from the Parkinson’s Progression Markers Initiative. All patients were followed until the onset of ICDs or the end of 4 years. A total of 260 PD patients were included. Univariable and multivariable logistic regression were used to explore association between EDS and ICDs.
Results: The overall frequency of ICDs at the end of follow-up was 23.8% (62 patients). The mean duration from dopamine replacement therapy to develop ICDs was 3.30 ± 2.42 years. Patients with ICDs had significantly higher Epworth Sleepiness Scale (ESS) score (P = 0.002) and higher proportion of EDS (P = 0.030) when compared to patients without ICDs. The multivariable logistic regression analysis indicated that high ESS (OR = 2.01, 95% CI 1.01– 4.04, p = 0.049) score, high dopamine agonist equivalent daily dose (OR = 2.54, 95% CI 1.37– 4.71, p = 0.003), high Geriatric Depression Scale (OR = 2.33, 95% CI 1.27– 4.28, p = 0.006) score and postural instability (OR = 3.03, 95% CI 1.26– 7.29, p = 0.013) were associated with ICDs occurrence.
Conclusion: Our results indicated that EDS acts as a risk for ICDs occurrence in PD. Clinicians should pay attention to EDS in clinical practice. This may be a promising new approach to better understand and therapy ICDs.
Keywords: excessive daytime sleepiness, impulse control disorders, Parkinson’s disease, risk factors
Introduction
Parkinson’s disease (PD) is recognized as a common neurodegenerative disease. Motor symptoms are the characteristics of PD that cause increasing disability. However, the burden on patients and their caregivers may worsen with the emergence of numerous non-motor symptoms, including impulse control disorders (ICDs) and excessive daytime sleepiness (EDS).
ICDs are repetitive reward-seeking behaviors characterized by an impairment in resisting some temptation. They can lead to severe effects on life safety and disrupt major aspects of daily life.1 The unfortunate aspect is that patients with ICDs often repeat those behaviors without control, even though they are well aware of the negative consequences. Classical ICDs encompass pathologic gambling, binge eating, compulsive shopping and hypersexuality, but other related behaviors have been described, including hobbyism/punding and dopamine dysregulation syndrome.2 Reported frequency of ICDs vary considerably on the basis of sample characteristics in PD. A recent national study reported that ICDs prevalence increased from 19.7% at baseline to 32.8% after 5 years.3
Usage of dopamine agonist is widely considered as an important risk factor for ICDs.4 In addition, early age of disease onset, impulsive personality, depression, anxiety and genetic variants are also associated with the development of ICDs.5 Little attention has been paid to the association of ICDs and daytime sleepiness. Sleep deprivation have been linked to a lack of control over impulsive behavior in healthy adults. PD patients who accompanied by ICDs have an increased prevalence of Restless Legs Syndrome (RLS) symptoms and worse sleep efficiency. Presumably due to a failure of top-down control of prefrontal cortex when experiencing sleep disturbances.6 It is supported by prior neurophysiological studies. For example, increased measures of impulsivity were correlated with GABAergic activation by reducing prefrontal inhibition.7,8 Additionally, glutamate and γ-aminobutyric-acid (GABA) are important neurotransmitters in brain circuitry to control wake and sleep.9,10 However, few longitudinal studies have focused on assessing whether EDS is associated with the development of ICDs.
It is important to profile the role of EDS in developing impulse control disorders. To date, most of the evidence comes from cross-sectional studies.11 In this longitudinal study, we intend to explore the relationship between EDS and the development of ICDs. At the meantime, we intend to find a new approach to better understand and therapy ICDs.
Materials and Methods
Participants
All data used in this longitudinal study were obtained from July 2010 to June 2018 from the Parkinson’s Progression Markers Initiative (PPMI), an international multicenter investigation designed to assess the progression of PD, as previously described in detail.12 We downloaded the dataset on 19th July 2023. The inclusion criteria for patients with PD were the following: (1) received dopamine replacement therapy regularly for at least 3 months, (2) participants who did not develop ICDs were enrolled in our study. All patients were followed until the onset of ICDs or the end of 4 years. In brief, a total of 260 patients were included in this paper. The PPMI study was approved by the institutional review board at each PPMI site, and participants provided written informed consent for research. Since this research only involves informational that legally obtained from public data and does not cause harm to the human body, the Ethics Committee of Shanghai Tongji Hospital, School of Medicine, Tongji University, exempted it from ethical review.
Assessments of Demographic and Clinical Characteristics
PPMI collects a vast body of clinical assessments. For this study, demographic, motor and non-motor symptoms at enrollment were included. These included age at PD onset, age at enrollment, duration of PD, sex, Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) parts 1A, 1B, 2, and 3, Hoehn and Yahr (H&Y) stage, initial symptoms at diagnosis (resting tremor, rigidity, bradykinesia and postural instability), duration of medication. All analyses were performed using the MDS-UPDRS assessment “off” medication, more than 6 hours after the last dose of dopaminergic therapy as defined in the PPMI study protocol. Medication data were used to calculate a total levodopa equivalency daily dose (TLEDD) and dopamine agonist equivalent daily dose (DAED) based on established algorithm.13 The Rapid Eye Movement Behavior Disorder (RBD) Questionnaire (RBDSQ) was used to screen RBD. Patients would be classified to positive for RBD if they scored ≥5.14 Cognition was evaluated by the Montreal Cognitive Assessment (MoCA, score ≥ 26 is the cutoff point for normal cognition). Anxiety was evaluated by State‐Trait Anxiety Inventory scores (STAI, including state anxiety and trait anxiety). Depression was evaluated by Geriatric Depression Scale (GDS-15). Autonomic dysfunction was evaluated by Scale for Outcomes in Parkinson’s disease Autonomic (SCOPA-AUT).
Assessments of ICDs and EDS
All patients completed the Questionnaire for Impulse Control Disorders in Parkinson’s Disease Short Form (QUIP-S) to identify those with possible ICDs. The cutoff point for each ICD was ≥1 affirmative answer to any question.15 All the included patients had a QUIP-S of 0 at enrollment. All patients were followed annually for up to 4 years or until the onset of ICDs. Patients were considered to have ICDs once they provided the first affirmative answer to any question of QUIP-S during the follow-up. The time of development of ICDs was based on their recollection of when the symptom first occurred. At the end of follow-up, patients were divided into two groups: with ICDs (ICDs group) and without ICDs (NICDs group). It was worth noting that we were concerned about four typical ICDs in this study, including pathologic gambling, binge eating, compulsive shopping, and hypersexuality, but did not include hobbyism/punding and dopamine dysregulation syndrome. This is because previous studies suggested that typical ICDs have different risk factors from punding and dopamine dysregulation syndrome.16 Based on the above criteria, we next considered two subgroups in the cohort of patients with ICDs: with only one type of ICDs (Group 1) and with more than one type of ICDs (Group 2). This kind of grouping was used to identify whether there is a difference in clinical characteristics between patients with only one type of ICDs and those with more than one type of ICDs. According to the methods of previous studies,17,18 in the present work, all patients also completed the Epworth Sleepiness Scale (ESS) at enrollment. This is a validated evaluation of EDS with eight items. According to conventions in previous studies, patients were dichotomized as having EDS when ESS ≥ 10.19
Statistical Analysis
Continuous data were compared by independent t test or Mann–Whitney U-test and presented as mean ± standard deviation or median (interquartile range [IQR]), as appropriate. Categorical variables were compared by Pearson’s chi-square or Fisher’s exact test and presented as numbers and percentage, as appropriate. To identify risk factors for ICDs, we conducted univariable logistic regression models for all baseline variables, followed by a forward stepwise variable selection to select variables for the multivariable logistic regression, with the significance level for entry and stay being 0.1 (the default setting) to avoid rejection of potentially important variables due to uncontrolled confounders. For logistic regression analysis, continuous variables were transformed to categorical variables by the corresponding medians. At the meantime, we conducted the multivariable logistic model adjusted age and sex. OR with 95% CI was calculated. We also assessed multicollinearity of the selected variables by using the variance inflation factor (VIF).
A two-sided p value <0.05 was considered to be statistically significant. Statistical analysis was performed using R version 4.3.1 (Foundation for Statistical Computing, Vienna, Austria).
Results
Prevalence and Demographic Characteristics
A total of 260 patients were enrolled into this study. The overall frequency of ICDs at the end of follow-up was 23.8% (62 patients). There was no significant difference between NICDs and ICDs group in age of onset of PD, age at enrollment, duration of PD and sex (Table 1). Among patients with ICDs, 85.5% (53 patients) accompanied by only one type of ICDs and 14.5% (9 patients) accompanied by more than one type of ICDs. Among 53 patients with only one type of ICDs, 54.7% (29 patients) with binge eating, 30.2% (16 patients) with hypersexuality, 13.2% (7 patients) with compulsive shopping, and 1.9% (1 patient) with pathologic gambling. There was also no significant difference between patients with only one type of ICDs and with more than one type of ICDs group in age of onset of PD (P = 0.987), age at enrollment (P = 0.973), duration of PD (P = 0.491) and sex (P = 0.464) (Table 2).
 |
Table 1 Clinical Characteristics of All the Patients and Patients with and without ICDs |
 |
Table 2 Clinical Characteristics of Group 1 and Group 2 |
Clinical Characteristics
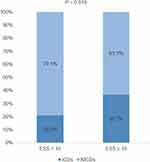
The mean duration from dopamine replacement therapy to the onset of ICDs in this cohort was 3.30 ± 2.42 years. Patients with ICDs had significantly higher DAED (P = 0.012) and significantly higher proportion of using DAED (P = 0.008) than patients without ICDs. However, there was no significant difference in TLEDD between patients with and without ICDs (P = 0.870). In view of motor symptoms that appeared at diagnosis, there was no significant difference in the proportion of tremor (P = 0.156), rigidity (P = 0.310), and bradykinesia (P = 0.858) between patients with and without ICDs. Notably, the proportion of postural instability in patients with ICDs appeared higher than that in those without ICDs, but the difference was not statistically significant (P = 0.073). Patients with ICDs had significantly higher MDS-UPDRS IA than patients without ICDs (P = 0.026). Whereas, there was no difference in MDS-UPDRS IB (P = 0.910), MDS-UPDRS II (P = 0.894), MDS-UPDRS III (P = 0.119) and H&Y (P = 0.971) between these two groups. Patients with ICDs had a significantly higher GDS score (P = 0.006) and an ESS score (P = 0.002) when compared to patients without ICDs. In addition, the proportion of EDS in patients with ICDs was also significantly higher than that in patients without ICDs (P = 0.030). There was no significant difference in the proportion of RBD in patients with and without ICDs (P = 0.090). There was no significant difference in MoCA (P = 0.825), SCOPA (P = 0.484), state anxiety (P = 0.368) and trait anxiety (P = 0.541) between patients with and without ICDs (see Table 1 for details). At the meantime, we found that the proportion of ICDs in patients with EDS was significantly higher than that in patients without EDS (Figure 1). When comparing patients with only one subtype of ICDs and patients with more than one subtype of ICDs, there was no difference in demographic characteristics, motor and non-motor symptoms, excepted for the proportion of postural instability (P = 0.044) (see Table 2 for details).
Risk Factors for Development of ICDs
Firstly, we used univariable logistic regression analysis to find out significant variables to avoid error or miss important variables with a border of P <0.1. Finally, the following variables were incorporated in the multivariable logistic regression analysis: age, sex, DAED, postural instability, RBD, GDS and EDS. The results of the multivariable logistic regression analysis were shown in Table 3. Results of VIF (GDS 1.01, DAED 1.06, EDS 1.01, postural instability 1.06) suggested no multicollinearity among the above-selected variables. Forest plot of the multivariable logistic regression analysis was displayed in Figure 2. We found that EDS was independently associated with the development of ICDs (OR = 2.01, 95% CI 1.01–4.04, p = 0.049). In addition, high DAED (OR = 2.54, 95% CI 1.37–4.71, p = 0.003), high GDS (OR = 2.33, 95% CI 1.27–4.28, p = 0.006) and appearance of postural instability at diagnosis (OR = 3.03, 95% CI 1.26–7.29, p = 0.013) also showed significant correlation with ICDs.
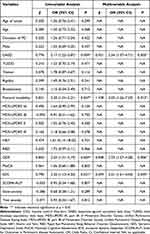 |
Table 3 Logistic Regression Analysis of ICDs (N = 260) |
 |
Figure 2 Forest plot of multivariable logistic regression analysis. Abbreviations: DAED, dopamine agonist equivalent daily dose; GDS, Geriatric Depression Scale; DXPOSION, Postural instability. |
Discussion
This study followed a group of PD patients free of ICDs at baseline for 4.0 years. The prevalence of ICDs at the end of follow-up was 23.8%, which was consistent with prior studies.20 We found that those who were going to develop ICDs had significantly higher proportion of EDS and significantly higher ESS score than those who were not. Moreover, we found EDS was independently associated with development of ICDs. In addition, depression, high dopamine agonist and appearance of postural instability are also risk factors of ICDs.
When compared to patients without ICDs, patients with ICDs had significantly severer depression, higher proportion of postural instability at diagnosis, higher DAED and more serious excessive daytime sleepiness. Patients with ICDs also had significantly severer MDS-UPDRS IA that mainly used to assess disturbances in mood.21 However, we found that the proportion of postural instability at diagnosis was the only variable that is significantly different between patients with only one subtype of ICDs and patients with more than one subtype of ICDs. Those variables may be associated with the development of ICDs but may not be associated with subtype of ICDs, except for postural instability.
The combination of EDS, depression, dopamine agonist and postural instability significantly predicted ICDs, with appearance of EDS, severe depression, high dopamine agonist and appearance of postural instability at diagnosis being associated with occurrence of ICDs. Our results add to clinical evidence suggesting that patients with postural instability at diagnosis of PD have an increased risk of ICDs. There is also a neuroanatomical explanation for their relationship. Inferior frontal gyrus has been classically involved in inhibitory control along with the lateral anterior prefrontal cortex and supplementary motor area. Prior studies found that patients with ICDs showed stronger activation in inferior frontal gyrus.22,23 Additionally, studies found that the bilateral inferior frontal gyrus and superior temporal gyrus are the only regions associated with the presence of postural instability in PD.24 Patients with postural instability and gait disorders showed increased recruitment of inferior frontal gyrus and supplementary motor area when performing the dual-task.25 In addition, the association for ICDs and depression has been noted.3,26 The prevalence of depression in PD patients is high. A previous study found that sleep status, daily use of levodopa, a high H-Y, a high UPDRS II score, and a high UPDRS III score were all risk factors for depression in PD patients.27 Dopaminergic denervation of the mesolimbic pathway would lead patients to experience both depression and ICDs.28,29 However, a previous study showed that PD depression is a state that predisposes to the development of ICDs. They supported that depression could be regarded as a consequence of ICDs, either as a risk factor for developing ICDs.26 Dopamine agonist is widely considered as a risk factor for ICDs.30,31 The point worth emphasizing in this study was that we separated DAED from TLEDD3 and found correlation between ICDs and dopamine agonist. This association was reported for dopamine agonist with a preferential affinity for D2-like receptors,32 and was higher with oral short-lasting dopamine agonist than oral long-lasting dopamine agonist.33 In agreement with a previous longitudinal study,3 we did not find a significant association between TLEDD and ICDs. As some evidence suggested, high TLEDD dose was associated with an increased risk of ICDs to a lesser extent.34
Prior studies found an association between RBD and ICDs.35 Fewer studies explored association of EDS and development of ICDs. In this longitudinal study, we were more concerned with EDS and its influence on the development of ICDs. Consistent with a prior cross-sectional study, ICDs were significantly associated with EDS.11 Our results of logistic regression analysis confirmed the role of EDS in leading to ICDs. This association was strong (OR = 2.01) and may assist clinicians to distinguish patients who might develop ICDs in future. Studies reported that EDS is mainly a consequence of alterations in sleep and wakefulness structures due to neurodegeneration.9 The pedunculopontine nucleus (PPN) as a part of the pathway of arousal was hypothesized relatively early to promote cortical desynchronization through switching the thalamus from a synchronized to a desynchronized mode.36,37 This desynchronization results in disturbance of arousal. In addition, the PPN is mainly comprised of cholinergic neurons, glutamatergic, and GABAergic neurons.38,39 Increased measures of impulsivity were correlated with GABAergic activation.7 Furthermore, as we know, amygdala has an important role in stimulus-reward learning.40 The increased activity in the amygdala that results from suboptimal sleep may contribute to the heightened reward-seeking behavior.41,42 More than that, the dopamine mediated mesolimbic circuitry is not only responsible for rewarding and reinforcement but it is also heavily involved in the regulation of sleep/wake states. The same pathway from the ventral tegmental area to the nucleus accumbens is critically involved in both the attribution of incentive salience to cues and the regulation of sleep-wake states.41 Our results were supported by the above-shared neurobiological mechanisms between sleep and impulsivity. This prospective study may not confirm the causality of the association between EDS and ICDs, but it does suggest that EDS seems to act as a risk factor for the occurrence of ICDs. Thus, clinicians should consider screening for EDS in routine clinical practice. The ESS is a reliable tool to assess self-reported daytime sleepiness.43 It is not time-consuming and easy to understand. Positive results in screening for EDS should attract clinicians’ attention and prompt further evaluation, and possibly lead to therapeutic adjustments. Certainly, it would be better to compare patients with EDS at baseline and patients who developed it during follow-up. In the future studies, we will use more follow-up data to further investigate the relationship between EDS and ICDs.
There are several limitations of this study. First, both ICDs and EDS were evaluated by subjective scales, which might suffer from subjective assessment bias. It is necessary to assess sleepiness objectively by appropriate instruments because there are many potential confounding factors in patients with PD,44 such as polysomnography and quantitative electroencephalogram. However, these scales are reliable, convenient, and practicable for clinicians. Second, the data came from the PPMI study. Most participants were so young and mild that they cannot represent the general population. However, this benefited researchers in exploring the risk factors for ICDs through follow-up. Third, the study sample was small. Further evaluation of the potential role of EDS in ICDs development should be conducted in large sample sizes. Fourth, medication information has only been provided for dopaminergic treatments with TLEDD and DAED that would omit any medications that could potentially cause ICDs. Additionally, it would lead to miss out on discovering new drug adjustment program. It is essential to distinguish different medications in the future studies. Despite these limitations, this was a rarely prospective design with longer duration of follow-up to examine the relationship between ICDs and EDS.
Conclusion
In summary, our results indicated that EDS acts as a risk for ICDs occurrence in PD. Clinicians should pay attention to screening for EDS in clinical practice. Positive results in screening for EDS should prompt further evaluation, and possibly lead to therapeutic adjustments. It may represent a promising new approach to better understand ICDs.
Data Sharing Statement
Data used in this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database. For up-to-date information on the study, visit www.ppmi-info.org.
Code Availability
R codes for data analysis are available upon reasonable request.
Ethics Approval
The PPMI study was approved by the institutional review board at each PPMI site, and participants provided written informed consent for research. The authors have no relevant financial or non-financial interests to disclose.
Acknowledgments
Data used in this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database. For up-to-date information on the study, visit www.ppmi-info.org. PPMI—a public–private partnership—is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including AbbVie, Avid Radiopharmaceuticals, Biogen, Bristol-Myers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramal, Roche, Servier, UCB and Golub Capital.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Key R&D Program of China (2022YFF1202800, 2022YFF1202804), 2022 Research Project of Shanghai Municipal Health Commission (20224Y0151), the National Natural Science Foundation of China (82271227), the National Natural Science Foundation of China (82301339), the National Natural Science Foundation of China (81671273), the National Natural Science Foundation of China (81171204), the Key R&D Program of Shandong Province (2020CXGC011301), the Putuo District Science and Technology Commission of Shanghai (2022–KP08), the Project of Shanghai Tongji Hospital (RCQD2203). We would like to thank all staff involved in data processing.
Disclosure
The author(s) reported no conflicts of interest in this work.
References
1. Erga AH, Alves G, Tysnes OB, Pedersen KF. Impulsive and compulsive behaviors in Parkinson’s disease: impact on quality of and satisfaction with life, and caregiver burden. Parkinsonism Relat Disord. 2020;78:27–30. doi:10.1016/j.parkreldis.2020.07.007
2. Evans AH, Strafella AP, Weintraub D, Stacy M. Impulsive and compulsive behaviors in Parkinson’s disease. Mov Disord. 2009;24(11):1561–1570. doi:10.1002/mds.22505
3. Corvol JC, Artaud F, Cormier-Dequaire F, et al. Longitudinal analysis of impulse control disorders in Parkinson disease. Neurology. 2018;91(3):e189–e201. doi:10.1212/WNL.0000000000005816
4. Biundo R, Weis L, Abbruzzese G, et al. Impulse control disorders in advanced Parkinson’s disease with dyskinesia: the ALTHEA study. Mov Disord. 2017;32(11):1557–1565.
5. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547–555. doi:10.1097/WCO.0000000000000955
6. Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--A prefrontal amygdala disconnect. Curr Biol. 2007;17(20):R877–878. doi:10.1016/j.cub.2007.08.007
7. Trujillo P, Song AK, Hay KR, et al. Dopamine-induced changes to thalamic GABA concentration in impulsive Parkinson disease patients. NPJ Parkinsons Dis. 2022;8(1):37. doi:10.1038/s41531-022-00298-8
8. Mick I, Ramos AC, Myers J, et al. Evidence for GABA-A receptor dysregulation in gambling disorder: correlation with impulsivity. Addict Biol. 2017;22(6):1601–1609. doi:10.1111/adb.12457
9. Perez-Carbonell L, Mignot E, Leschziner G, Dauvilliers Y. Understanding and approaching excessive daytime sleepiness. Lancet. 2022;400(10357):1033–1046. doi:10.1016/S0140-6736(22)01018-2
10. Yu X, Li W, Ma Y, et al. GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat Neurosci. 2019;22(1):106–119. doi:10.1038/s41593-018-0288-9
11. Scullin MK, Sollinger AB, Land J, et al. Sleep and impulsivity in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(11):991–994. doi:10.1016/j.parkreldis.2013.06.018
12. Parkinson Progression Marker I, Jennings D, Lasch S. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011;95(4):629–635. doi:10.1016/j.pneurobio.2011.09.005
13. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–2653. doi:10.1002/mds.23429
14. Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire--A new diagnostic instrument. Mov Disord. 2007;22(16):2386–2393. doi:10.1002/mds.21740
15. Weintraub D, Hoops S, Shea JA, et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease. Mov Disord. 2009;24(10):1461–1467. doi:10.1002/mds.22571
16. Weintraub D, Posavi M, Fontanillas P, et al. Genetic prediction of impulse control disorders in Parkinson’s disease. Ann Clin Transl Neurol. 2022;9(7):936–949. doi:10.1002/acn3.51569
17. Ehgoetz Martens KA, Lukasik EL, Georgiades MJ, et al. Predicting the onset of freezing of gait: a longitudinal study. Mov Disord. 2018;33(1):128–135. doi:10.1002/mds.27208
18. Baig F, Kelly MJ, Lawton MA, et al. Impulse control disorders in Parkinson disease and RBD: a longitudinal study of severity. Neurology. 2019;93(7):e675–e687. doi:10.1212/WNL.0000000000007942
19. Simuni T, Caspell-Garcia C, Coffey C, et al. Correlates of excessive daytime sleepiness in de novo Parkinson’s disease: a case control study. Mov Disord. 2015;30(10):1371–1381. doi:10.1002/mds.26248
20. Scott BM, Eisinger RS, Burns MR, et al. Co-occurrence of apathy and impulse control disorders in Parkinson disease. Neurology. 2020;95(20):e2769–e2780. doi:10.1212/WNL.0000000000010965
21. Saez-Francas N, Marti Andres G, Ramirez N, et al. Clinical and psychopathological factors associated with impulse control disorders in Parkinson’s disease. Neurologia. 2016;31(4):231–238. doi:10.1016/j.nrl.2015.05.002
22. Paz-Alonso PM, Navalpotro-Gomez I, Boddy P, et al. Functional inhibitory control dynamics in impulse control disorders in Parkinson’s disease. Mov Disord. 2020;35(2):316–325. doi:10.1002/mds.27885
23. Esteban-Penalba T, Paz-Alonso PM, Navalpotro-Gomez I, Rodriguez-Oroz MC. Functional correlates of response inhibition in impulse control disorders in Parkinson’s disease. Neuroimage Clin. 2021;32:102822. doi:10.1016/j.nicl.2021.102822
24. Quattrone A, Calomino C, Sarica A, et al. Neuroimaging correlates of postural instability in Parkinson’s disease. J Neurol. 2024;271(4):1910–1920. doi:10.1007/s00415-023-12136-9
25. Sarasso E, Gardoni A, Piramide N, et al. Dual-task clinical and functional MRI correlates in Parkinson’s disease with postural instability and gait disorders. Parkinsonism Relat Disord. 2021;91:88–95. doi:10.1016/j.parkreldis.2021.09.003
26. Marin-Lahoz J, Sampedro F, Martinez-Horta S, Pagonabarraga J, Kulisevsky J. Depression as a risk factor for impulse control disorders in Parkinson disease. Ann Neurol. 2019;86(5):762–769. doi:10.1002/ana.25581
27. Li L, Wang Z, You Z, Huang J. Prevalence and influencing factors of depression in patients with Parkinson’s disease. Alpha Psychiatry. 2023;24(6):234–238. doi:10.5152/alphapsychiatry.2023.231253
28. Aarsland D, Pahlhagen S, Ballard CG, Ehrt U, Svenningsson P. Depression in Parkinson disease--epidemiology, mechanisms and management. Nat Rev Neurol. 2011;8(1):35–47. doi:10.1038/nrneurol.2011.189
29. Vriend C, Pattij T, van der Werf YD, et al. Depression and impulse control disorders in Parkinson’s disease: two sides of the same coin? Neurosci Biobehav Rev. 2014;38:60–71. doi:10.1016/j.neubiorev.2013.11.001
30. Pringsheim T, Day GS, Smith DB, et al. Dopaminergic therapy for motor symptoms in early Parkinson disease practice guideline summary: a report of the AAN guideline subcommittee. Neurology. 2021;97(20):942–957. doi:10.1212/WNL.0000000000012868
31. Fusaroli M, Giunchi V, Battini V, et al. Exploring the underlying mechanisms of drug-induced impulse control disorders: a pharmacovigilance-pharmacodynamic study. Psychiatry Clin Neurosci. 2023;77(3):160–167. doi:10.1111/pcn.13511
32. Weintraub D, Mamikonyan E. Impulse control disorders in Parkinson’s disease. Am J Psychiatry. 2019;176(1):5–11. doi:10.1176/appi.ajp.2018.18040465
33. Rizos A, Sauerbier A, Antonini A, et al. A European multicentre survey of impulse control behaviours in Parkinson’s disease patients treated with short- and long-acting dopamine agonists. Eur J Neurol. 2016;23(8):1255–1261. doi:10.1111/ene.13034
34. Pontieri FE, Assogna F, Pellicano C, et al. Sociodemographic, neuropsychiatric and cognitive characteristics of pathological gambling and impulse control disorders NOS in Parkinson’s disease. Eur Neuropsychopharmacol. 2015;25(1):69–76. doi:10.1016/j.euroneuro.2014.11.006
35. Fantini ML, Figorilli M, Arnulf I, et al. Sleep and REM sleep behaviour disorder in Parkinson’s disease with impulse control disorder. J Neurol Neurosurg Psychiatry. 2018;89(3):305–310. doi:10.1136/jnnp-2017-316576
36. Davin A, Chabardes S, Devergnas A, et al. Excessive daytime sleepiness in a model of Parkinson’s disease improved by low-frequency stimulation of the pedunculopontine nucleus. NPJ Parkinsons Dis. 2023;9(1):9. doi:10.1038/s41531-023-00455-7
37. Steriade M. Arousal: revisiting the reticular activating system. Science. 1996;272(5259):225–226.
38. Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29(2):340–358.
39. Mena-Segovia J, Bolam JP. Rethinking the pedunculopontine nucleus: from cellular organization to function. Neuron. 2017;94(1):7–18.
40. Hu RK, Zuo Y, Ly T, et al. An amygdala-to-hypothalamus circuit for social reward. Nat Neurosci. 2021;24(6):831–842.
41. Ahrens AM, Ahmed OJ. Neural circuits linking sleep and addiction: animal models to understand why select individuals are more vulnerable to substance use disorders after sleep deprivation. Neurosci Biobehav Rev. 2020;108:435–444. doi:10.1016/j.neubiorev.2019.11.007
42. Rihm JS, Menz MM, Schultz H, et al. Sleep deprivation selectively upregulates an amygdala-hypothalamic circuit involved in food reward. J Neurosci. 2019;39(5):888–899. doi:10.1523/JNEUROSCI.0250-18.2018
43. Goncalves MT, Malafaia S, Moutinho Dos Santos J, Roth T, Marques DR. Epworth sleepiness scale: a meta-analytic study on the internal consistency. Sleep Med. 2023;109:261–269. doi:10.1016/j.sleep.2023.07.008
44. Provini F, Ferri R. Excessive daytime sleepiness in Parkinson’s disease: the key is beyond sleep macrostructure. Sleep. 2023;46(4). doi:10.1093/sleep/zsac209
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.