Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
Exogenous GABA Alleviates Tourette Syndrome-Like Behavior in Sprague-Dawley Rats by Altering Gut Microbiota and Striatum Metabolism
Authors Xu Y, Li LN, He XJ, Wang S, Li X, Feng H, Zhang HF, Song L, Shi HS, Tian XY
Received 21 January 2025
Accepted for publication 27 March 2025
Published 4 April 2025 Volume 2025:21 Pages 711—727
DOI https://doi.org/10.2147/NDT.S512191
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Ying Xu,1,2 Li-Na Li,1,2,* Xiang-Jun He,1,2 Shuang Wang,2 Xincheng Li,2 Hao Feng,2 Hui-Feng Zhang,1 Li Song,2 Hai-Shui Shi,2– 4,* Xiao-Yu Tian1,*
1The Department of Pediatrics, The Second Hospital of Hebei Medical University, Shijiazhuang, 050000, People’s Republic of China; 2Neuroscience Research Center, Institute of Medical and Health Science, Hebei Medical University, Shijiazhuang, 050017, People’s Republic of China; 3Nursing School, Hebei Medical University, Shijiazhuang, 050031, People’s Republic of China; 4Hebei Key laboratory of Neurophysiology, Hebei Medicinal University, Shijiazhuang, 050017, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao-Yu Tian, The Department of Pediatrics, The Second Hospital of Hebei Medical University, Shijiazhuang, 050000, People’s Republic of China, Email [email protected]
Context: Tourette syndrome (TS) is a common chronic neuropsychiatric disorder with a prevalence of approximately 1% in children and adolescents. TS is characterized by sudden involuntary motor tics along with vocal tics. A pathological study on postmortem patients has reported a 50– 60% reduction in striatal gamma-aminobutyric acidergic (GABAergic) interneurons, suggesting a role for GABAergic system imbalances in tic disorder development. However, the effect of exogenous GABA administration on tic alleviation remains unreported.
Objective: In this study, we aim to investigate the therapeutic effects of exogenous GABA on TS-like behaviors in Sprague-Dawley rats and explore its potential mechanisms, including gut microbiota regulation, oxidative stress mitigation, and restoration of GABA-glutamate balance, to provide insights into TS pathogenesis and alternative treatment strategies.
Materials and Methods: A TS model rat was established through intraperitoneal administration of 3,3-Iminodipropionitrile (150 mg/kg/day), followed by GABA (20 mg/kg/day) administration by gavage. 15 minutes of behavioral testing (stereotypical behavior and head twitching behavior) was then conducted. 16S rRNA sequencing identified microbiome changes, and LC-MS assessed striatal metabolite changes.
Results: The results showed that a 4-week GABA treatment alleviated TS-like behavior in rats. GABA treatment led to an increase in Acinetobacter and other beneficial bacteria. GABA also significantly upregulated 15 striatal metabolites compared with TS group. By correlation analysis of striatal metabolites and intestinal bacteria, statistical analysis showed that Clostridium_sensu_stricto_1 was negatively correlated with metabolites on the top 20 differential gut microbiota and metabolites. Moreover, changes in gut microbiota correlated with alterations in striatal metabolites, suggesting a gut-brain axis involvement.
Conclusion: Exogenous GABA alleviated TS-like behavior in rats by reducing harmful gut flora and modulating striatal GABA-glutamate metabolism. Despite challenges like low blood-brain barrier permeability and dose safety in humans, GABA’s therapeutic potential may be realized through prodrug development and optimized dosing. These findings are preliminary and require further clinical validation.
Keywords: Tourette syndrome, GABA, gut microbiota, striatum metabolism
Introduction
Tourette syndrome (TS) is a common neurobehavioral and neuropsychiatric disorder typically occurring in childhood,1 with an estimated prevalence of approximately 1% in children and adolescents of the world.2 TS is characterized by sudden, involuntary motor tics, such as blinking, head shaking, and twitching of the trunk, limbs, and other skeletal muscles, alongside vocal twitching. TS co-occurs with Attention Deficit Hyperactivity Disorder, Obsessive Compulsive Disorder, and other psychological and behavioral disorders.3,4 TS symptoms are recurrent and difficult to manage and significantly impair learning, physical growth, and mental development in children and adolescents.5 The etiology and pathogenesis of TS are comprehensive and unclear, potentially involving a combination of genetic factors, structural abnormalities in the central nervous system, neurotransmitter and immune imbalances, as well as psychological and environmental influences.6 Treatment remains challenging as commonly used medications in children, such as sulpiride and haloperidol, are limited in clinical application owing to adverse extrapyramidal side effects, alongside dizziness and somnolence.7 Therefore, given the significant side effects of current treatments, exploring alternative therapeutic strategies is essential. Gamma-aminobutyric acid (GABA)—a primary inhibitory neurotransmitter—is a nutrient known to play a role in neurological disorders.8 Study shows that its administration reduced depressive phenotype in mice.9,10 GABA exhibits various biological activities, including antioxidant, anti-inflammatory, antimicrobial, and anti-allergic properties. Additionally, reports show that GABA protects the liver, kidney, and intestines against toxin-induced damage.11 The pathophysiologic mechanisms in TS include disruption of GABA conduction, leading to impaired inhibition within the cortical-basal ganglia loop.12 A study shows the reduced GABA concentrations in the SM1 brain region in children with TS, with these levels correlating to the convulsion severity.13 Pathological studies from postmortem examinations of patients with TS reveal a 50–60% reduction in striatal GABAergic and cholinergic interneurons.14 In contrast, studies report that administering GABA-A receptor antagonists to rodents in the striatum and intra-cortex induces twitching symptoms, which can be alleviated by the administration of GABA-A receptor agonists.15 The above suggests that GABAergic system dysregulation plays a significant role in TS pathogenesis and may provide insights into its underlying mechanisms. However, the specific role of GABA in TS and its related mechanisms remains unreported.
For many years, GABA was thought to be synthesized exclusively in the brain. However, recent studies show that GABA is also produced in the gastrointestinal tract, where it plays a crucial role in maintaining gastrointestinal health by regulating the intestinal microbiota, which may alter GABA levels.16–18 With the introduction of the “brain-gut axis” concept, the potential role of microbiome in neuropsychiatric disorders such as depression and autism has been recognized.19 In this study, we investigated the potential therapeutic effects of exogenous GABA on TS-like behavior in Sprague-Dawley (SD) rats. We hypothesize that GABA exerts its effects by modulating gut microbiota, reducing oxidative stress in the striatum, and restoring the GABA-glutamate metabolic balance.
Materials and Methods
Animals
Overall, 24 male SD rats, aged 4 weeks, were obtained from Beijing Hua fu kang Biotechnology Co., Ltd. The rats were housed in a controlled environment with a temperature of 22 ± 2°C, humidity of 50% ± 5%, and a 12-h light/dark cycle (Lights on from 8:00 PM to 8:00 AM), with ad libitum access to food and water. All animal experiments were approved by the Local Committee on Animal Care and Use and Protection at Hebei Medical University and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals guidelines. The animals were divided into three groups—the CON, TS, and GABA groups (each with n = 8). 3,3′-Iminodipropionitrile (IDPN) is a neurotoxic compound commonly used to induce TS models in rats. IDPN-induced rats exhibit spontaneous tics similar to those of human TS, mainly manifested by involuntary movements of the head and stereotyped behavior. 3,3-Iminodipropionitrile (IDPN) was purchased from TCI Co., Ltd. and administered to the TS and GABA groups via intraperitoneal injection at a dosage of 150 mg/kg/day. The CON group received an equivalent volume of 0.9% saline (5 mL/kg/day) for 7 days. Behavior tests were conducted on Day 7 following injection completion. Two observers, unaware of the animal group, recorded stereotypical behaviors and head twitching (HTR) in rats for 15 min. Experiments were conducted in a box (35 cm length × 25 cm width × 25 cm height), with the box wiped clean with alcohol after each observation to remove feces and urine. Upon successful model establishment, GABA (20mg/kg/day) was administered by gavage to the treatment group, and the TS group received the same volume of normal saline for 4 weeks. The rat dose (20 mg/kg) was calculated using the human-equivalent dose conversion formula, resulting in a dosage approximately 6.3 times the human dose, but not exceeding 500 mg per day. The initial weight of animals and weekly weight changes were recorded. At the end of the experiment, striatal and ileal specimens were collected under pentobarbital anesthesia.
Humane euthanasia methods of laboratory animals were employed at the end of the experiments, following institutional, national, and international guidelines to minimize pain and suffering. This study was conducted according to the Guide for the Care and Use of Laboratory Animals and approved by the Research Ethics Committee of the Second Hospital of Hebei Medical University (2023-AE-195). This experiment also complied with ARRIVE guidelines.
Gut Microbiota Analyses
DNA Extraction and Amplification
Total genomic DNA was extracted using MagPure Soil DNA LQ Kit (Magan) following the manufacturer’s instructions (China). DNA concentration and integrity were assessed with a NanoDrop 2000 (Thermo Fisher Scientific, USA) and agarose gel electrophoresis. Extracted DNA was stored at −20°C until further analysis. The extracted DNA served as a template for polymerase chain reaction (PCR) amplification of bacterial 16S rRNA genes using barcoded primers and Takara Ex Taq (Takara). For bacterial diversity analysis, the V3–V4 (or V4–V5) variable regions of the 16S rRNA genes were amplified with universal primers 343F (5′-TACGGRAGGCAGCAG-3′) and 798R (5′-AGGGTATCTAATCCT-3′) 1 for the V3–V4 regions, or 515F (5′-GTGCCAGCMGCCGCGG-3′) and 907R (5′-CCGTCAATTCMTTTRAGTTT-3′) 2 for V4–V5 regions].
Library Construction and Sequencing
Amplicon quality was visualized using agarose gel electrophoresis. PCR products were purified using AMPure XP beads (Agencourt) and amplified for another round of PCR. After a second purification with AMPure XP beads, the final amplicon was quantified using the Qubit dsDNA Assay Kit (Thermo Fisher Scientific, USA), and the concentrations were then adjusted for sequencing. Sequencing was conducted on an Illumina NovaSeq 6000 with 250 bp paired-end reads. (Illumina Inc., San Diego, CA; OE Biotech Company, Shanghai, China).
Liquid Chromatography-Mass Spectrometry Sample Preparation
A 20 mg sample was weighed into a 1.5 mL EP tube, followed by the addition of two small steel beads and 400 μL methanol-water (V:V = 4:1, containing L-2-chlorophenylalanine, 4 μg/mL). The mixture was pre-cooled in the refrigerator at −40 °C for 2 min and ground at 60 Hz for 2 min. Ultrasonic extraction was conducted in an ice-water bath for 10 min and left to stand at −40 °C for 30 min. The sample was centrifuged at 12,000 rpm and 4°C for 10 min. A 300 μL aliquot of the supernatant was transferred to an LC-MS injection vial and evaporated to dryness. The extract was then redissolved in 300 μL of methanol-water (V:V = 1:4), vortexed for 30s, and sonicated for 3 min in an ice-water bath. The solution was allowed to stand at −40°C for 2 h, followed by centrifugation at 12,000 rpm and 4°C for 10 min. A 150-μL aliquot of the supernatant was drawn using a syringe and filtered through a 0.22 μM membrane filter (0.5 μm). The supernatant was filtered using a 0.22 μm organic-phase pinhole filter, transferred to an LC injection vial, and stored at −80°C until LC-MS analysis. Quality control samples were prepared by combining equal volumes of extracts from all samples. Note: All extraction reagents were pre-cooled at −20°C before use. The analytical instrument used in this experiment was a liquid-mass spectrometry system consisting of an ACQUITY UPLC I-Class plus ultra-high-performance liquid chromatography coupled with a QE high-resolution mass spectrometer.
The chromatographic column used was an ACQUITY UPLC HSS T3 (100 mm × 2.1 mm, 1.8 um) with a temperature of 45°C. The mobile phases were A-water (containing 0.1% formic acid) and B-acetonitrile, with a flow rate of 0.35 mL/min and an injection volume of 3 μL. The ion source was ESI, and the signal acquisition of the sample mass spectra was conducted in the positive-negative mode.
Data Reprocessing and Statistical Analysis
Two-way ANOVA was used to record body weight changes during the experiment. T-test and One-way ANOVA were used for behavior test analysis. The statistical analysis results were presented as mean ± SEM. Statistical analyses were conducted using GraphPad Prism 8 (GraphPad Software, San Diego, USA), except for the sequencing data. For sequencing results, Kruskal–Wallis tests were followed by Dunn’s multiple comparison confinement. Based on Bray–Curtis distances, principal coordinates analysis (PCoA) was conducted to compare microbiota community changes between samples. A p-value of < 0.05 was considered statistically significant. Correlations were computed using Spearman correlations with Benjamini–Hochberg correction for multiple comparisons.
Representative sequences for each ASV were selected using the QIIME 2 software package and compared against the Silva (version138) database. Species comparison annotations were generated using the default parameters of the q2-feature-classifier software. The unweighted Unifrac distance matrix was calculated and analyzed using R and unweighted Unifrac PCoA was conducted to assess the beta diversity of the samples. Based on the R package, differences were analyzed using the Kruskal–Wallis and Wilcoxon statistical algorithm.
The original LC-MS data were processed using the software Progenesis QI V2.3 (Nonlinear, Dynamics, Newcastle, UK) for baseline filtering, peak identification, integration, retention time correction, peak alignment, and normalization. The main parameters applied included a 5-ppm precursor tolerance, 10-ppm product tolerance, and a 5% production threshold. Compound identification was based on the precise mass-to-charge ratio, secondary fragments, and isotopic distribution, using databases such as the Human Metabolome Database (HMDB), Lipidmaps (V2.3), Metlin, EMDB, PMDB, and self-built databases for qualitative analysis. The extracted data were then processed by removing peaks with missing values (ion intensity = 0) in > 50% of groups. Zero values were replaced with half of the minimum value and screened based on the qualitative results of the compound. Compounds with scores below 36 (out of 60) points were considered inaccurate and excluded. A combined data matrix from the positive and negative ion data was imported into R for principal component analysis (PCA) to observe the overall distribution of the samples and the stability of the whole analysis process. Orthogonal partial least-squares-discriminant analysis (OPLS-DA) and partial least-squares-discriminant analysis were utilized to distinguish the metabolites that differed between groups. To prevent overfitting, 7-fold cross-validation, and 200 response permutation testing were applied to evaluate model quality. Variable importance of projection (VIP) values obtained from the OPLS-DA model was used to rank the overall contribution of each variable to group discrimination. A two-tailed Student’s T-test was further used to verify the significance of metabolite differences between groups. Differential metabolites were selected based on VIP values > 1.0 and p-values < 0.05.
Results
Exogenous GABA Alleviates Tourette Syndrome-Like Behavior and Growth Impairment in SD Rats
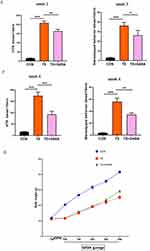
Exogenous GABA alleviates TS-induced growth impairment and TS-like behavior by modulating neurotransmitter balance, reducing neuronal hyperexcitability, and promoting inhibitory signaling in the central nervous system. Figure 1A shows the experimental timeline. In this study, after 7 days of intraperitoneal injection with IDPN and saline, behavioral testing showed that administration of IDPN induced significant TS-like and stereotypical behavior compared to that of the CON group (Figure 1B). Intragastric administration of GABA to the treatment group showed no significant effect on HTR behavior or stereotypical behavior during the first two weeks (Figure 1C and D). After the third week of treatment, the HTR behavior began to improve with statistical differences observed (P < 0.05), though stereotypical behavior remained unchanged (Figure 1E). By the fourth week of treatment, the HTR and stereotypical behavior were alleviated, with statistically significant differences (P < 0.05, Figure 1F). Monitoring body weight revealed that, after a week of IDPN intraperitoneal injection modeling, the TS group showed significant differences in body weight after modeling compared to that of the CON group, despite no differences in initial body weight (P < 0.05). This effect manifested as slow weight growth and significant final weight loss. After 4 weeks of GABA treatment, the treated group gained weight compared to that of the TS group, with a statistically significant difference (P < 0.05), indicating partial restoration of growth and development (Figure 1G). These findings suggest that exogenous GABA treatment alleviates TS-like behavior in SD rats. Figure 1 Continued.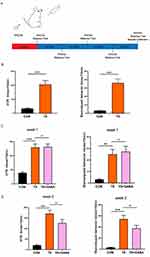
Exogenous GABA Alleviates Tourette Syndrome-Induced Intestinal Flora Imbalance in SD Rats
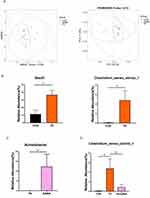
Exogenous GABA may positively influence TS-like behavior by regulating gut microbes. To explore this potential mechanism, we conducted 16S rRNA gene sequencing analysis on rat fecal samples. Beta diversity analysis, including PCoA and non-metric multidimensional scaling (NMDS) analysis, was conducted. PCoA revealed the relative differences in sequence similarity among strains. NMDS, which is based on evolutionary relationships or quantitative distance matrices, was used to compare differences between samples or groups (Figure 2A). Analysis revealed no difference between the CON and TS groups at the phylum level. At the class level, Bacilli was increased, while at the genus level, Clostridium_sensu_stricto_1 showed a significant increase in the TS group (Figure 2B). Exogenous GABA treatment led to an increase in Acinetobacter at the genus level (Figure 2C). Combining the changes in the three groups, at the genus level, Clostridium_sensu_stricto_1 was increased in the TS group compared to the CON group, and GABA treatment alleviated the Clostridium_sensu_stricto_1 abnormality, suggesting a potential mechanism (Figure 2D). These findings suggest that, while TS and GABA treatment alters gut microbiota, GABA treatment may alleviate aberrant alterations in the TS-induced gut microbiota. This modulation of the gut microbiota by GABA could contribute to its therapeutic effects on TS, possibly through the gut-brain axis, by reducing inflammation, restoring microbial balance, and improving gut barrier function. Further studies are needed to elucidate the specific pathways through which GABA influences gut microbiota and how these changes translate into improvements in TS symptoms.
Exogenous GABA Alleviates Tourette Syndrome-Like Behavior in SD Rats by Maintaining Striatal Metabolic Homeostasis
The gut-brain axis is important in maintaining homeostasis,20 particularly as striatal metabolic alterations may contribute to TS progression. To further investigate the role of GABA, we conducted LC-MS analysis to determine changes in striatal metabolites in rats. First, PCA and OPLS-DA showed significant differences between the TS and control groups (Figure 3A). Based on the overall distribution of metabolic differences, the volcano plot analysis showed that TS significantly (p < 0.05) downregulated 71 metabolites (blue color) compared to that of the CON group. This downregulation included L-Glutamine, Glutaconic acid, Glutathione, γ-Glutamylacetamide, γ-Glutamylcysteine, L-Proline, L-Glutamic acid, Ammonia aspartate, and γ-Glutamylcysteinylserine, among others. In addition to abnormal amino acid metabolism, TS induced abnormal lipid metabolism. The altered lipids were primarily glycerophospholipid. These included phosphatidylethanolamine, phosphoserine (PS), phosphatidylcholine, and phosphatidylinositol. Additionally, 54 metabolites were upregulated (shown red, P < 0.05), including Gluten exorphin C and others, whereas no significant change in metabolites is indicated as gray (Figure 3B). Following exogenous GABA administration, a Venn diagram showed that some of the metabolic abnormalities caused by TS were alleviated (Figure 3C). We cross-referenced the downregulated metabolite induced by TS compared to that of the CON group and the upregulated metabolite induced by GABA treatment compared to that of the TS group and observed that 15 metabolites were significantly upregulated after GABA treatment. The upregulated metabolites included Bhos#36, armillaripin, γ-Glutamylcysteine, 19-oxodesacetylcinobuf-agin, chlorophyll, WithaferinA, JP83, L-proline, trans-cinnamic acid (TCA), indoleacrylic acid, ammonia aspartate,[(2s,4R,5R,6R,14S,16R)-14-hydroxy-7,11-dimethyl-6-(2-OXOPYRAN-4-Y-L)-3-oxapentacyclo[8.8.0.02,4.02,7.011,16] octadecan-5-yl] acetate, PGP (22:6(5Z, 8E, 1-0Z, 13Z, 15E, 19Z) −2OH (7s, 17S)/20:4(8Z, 11Z, 14Z, 17Z), PS (21:0/18:0), 3-(4-Met-hyl-3-pentenyl)thiophene (Table 1). These findings suggest that exogenous GABA may ameliorate the striatal metabolic abnormality induced by TS.
 |
Table 1 Relief of TS After Administration of GABA Caused Abnormal Metabolites |
 |
Figure 3 Continued. |
After confirming the changes in striatal metabolites, we conducted a Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis on the differential metabolites and observed that TS induced 36 metabolic pathways to be downregulated and statistically different compared to that of the control (p < 0.05). Abnormalities in glutamate and GABA metabolic pathways—including glutamatergic synapse, glutathione metabolism, GABAergic synapse, D-amino acid metabolism, arginine biosynthesis, alanine, aspartate, and glutamate metabolism—were observed in the top 20 (Figure 3C). However, following exogenous GABA administration, 10 metabolic pathways were upregulated and statistically different (p < 0.05) compared to that of the TS group. Most of these upregulated pathways were downregulated by TS, including arginine biosynthesis, aminoacyl-tRNA biosynthesis, GABAergic synapse, D-amino acid metabolism, central carbon metabolism in cancer, and protein digestion and absorption, among others (Figure 3D). These findings suggest that GABA can mitigate abnormal metabolism in the striatum via pathways such as arginine biosynthesis, aminoacyl-tRNA biosynthesis, GABAergic synapse, D-amino acid metabolism, central carbon metabolism in cancer, and protein digestion and absorption (Figure 3E). Our results suggest that TS rats may have an imbalance between GABA and glutamate, the main excitatory neurotransmitter. GABA may restore the balance of excitatory-inhibitory neurotransmitters and alleviate tic symptoms by inhibiting the release or action of glutamate.
Correlation Between Gut Flora and Striatal Metabolism in the Relief of Tourette Syndrome-Like Behavior by Exogenous GABA
To study the relationship and obtain the correlation coefficient between the striatal metabolites as well as the microbial species abundance, Spearman correlation was employed to rank the two variables and conduct a linear correlation analysis. Spearman correlation was chosen over other statistical methods, such as Pearson’s correlation, because it is non-parametric and does not assume a normal distribution of the data. This makes it more suitable for analyzing potentially non-linear relationships or datasets with outliers, which are common in biological and metabolomic studies. We focused on the top 20 differential gut microbiota and metabolites. We observed that Clostridium_sensu_stricto_1 was negatively correlated with metabolites including WithaferinA, JP83, bhos#36, 19-oxodesacetylcinobufagin, (2s,4R,5R,6R,14S,16R)-14-Hydroxy-7,11-dimethyl-6-(2-OXOPYRAN-4-YL)-3-oxapentacyclo[8.8.0.02,4.02,7.011,16] octadecan-5-yl] acetate. Additionally, bacillus was negatively correlated with dopamine, pyrinuron, and bhos#36, whereas it exhibited a positive correlation with gluten exorphin C. This indicates that changes in striatal metabolites are closely related to gut microbiota (Figures 4A, B and 5).
 |
Figure 5 Mechanisms associated with the alleviation of TS-like behaviour in SD rats by exogenous GABA. |
Discussion
In this study, we employed an IDPN-induced mouse model to simulate tic disorder, targeting TS-like and stereotypic behaviors, a model consistent with that of previous studies.21 GABA alleviates tic-like behaviors through multiple mechanisms, including restoring neurotransmitter balance, modulating the CSTC (cortico-striatal-thalamic-cortical) circuit, reducing neuronal hyperexcitability, interacting with the gut-brain axis, exerting anti-inflammatory effects, and mitigating stress and anxiety. These combined actions help normalize neural activity and reduce the frequency and severity of tics.22,23 Our results show that exogenous administration of GABA alleviates TS-like behavior in SD rats, potentially through a mechanism that may involve reducing harmful flora and mitigating the GABA-Glu homeostasis in the striatum. Previous studies show a link between gut microbiota and behavior.24,25 Given such a basis, we explored the potential mechanisms by which exogenous GABA mitigates tic-like behaviors via 16s rRNA gene sequencing.
Our results showed that Clostridium_sensu_stricto_1 was elevated in the intestines of rats in the TS model, which was subsequently alleviated by exogenous GABA. This finding is consistent with previous study findings, which show that Clostridium_sensu_stricto_1 expression is elevated in mouse models of colitis and is directly associated with acute pancreatitis and necrotizing colitis in rats.26,27 Furthermore, the large amount of Clostridium_sensu_stricto_1 may be the reason for visceral allergy in irritable bowel syndrome.28–30 We hypothesize that TS induces a decrease in intestinal barrier capacity, which allows harmful substances toward the center, and that GABA administration may alleviate this. Additionally, another study demonstrates that prenatal lead exposure and stress impair learning memory involving elevated gut flora Bacilli.31 This also suggests that alterations in Clostridium_sensu_stricto_1 and Bacilli in the gut can modulate intestinal barrier capacity and regulate central alterations. Following GABA administration, we observed an increase in Acinetobacter compared to that of the TS group. A study shows that milk improves sleep in insomniac mice by enhancing intestinal Acinetobacter.32 GABA supplementation’s effects are multifaceted, involving both direct neurotransmission modulation and gut microbiota-mediated pathways, with the dominant mechanism depending on dosage, route of administration, and individual physiological conditions. These findings suggest that exogenous GABA may act centrally through the gut flora. However, how did the center change? Studies show that the gut and brain can influence central nervous function through a bidirectional regulation of nerves, hormones, and immunity.33 Our study shares high similarity with previous studies in reduced microbiota diversity, gut-brain axis action and elevated inflammatory markers, but differ in changes in specific strains and the focus of metabolic pathways.34 The findings on gut microbiota and TS are largely consistent in highlighting the role of dysbiosis, gut-brain axis dysfunction, and neuroinflammation in the pathophysiology of TS. Several studies show that the composition of the gut flora of individuals with TS differs from that of the normal population, particularly in the number and species of certain bacterial strains. Further studies show that the gut flora influences the onset and development of tic disorders via interactions with the striatum.35,36
Following the identification of microbiome changes, we conducted a striatal metabolomics analysis and observed TS-induced abnormalities in striatal metabolism in rats. This imbalance of glutamate and GABA metabolism is obvious, which is consistent with that of previous studies.37,38 Previous studies share many similarities with our study regarding the effects of GABA on gut microbiota and glutamate metabolism, particularly in promoting the growth of GABA-producing bacteria and regulating glutamate-GABA balance.39 Our finding indicates that GABA-glutamate metabolism is indeed in a state of imbalance in the striatum of SD rats in the TS model. This imbalance may be attributed to a decrease in L-Glutamine, Glutaconic acid, L-Glutamic acid, Glutathione, γ-Glutamylacetamide, γ-Glutamylcysteine, L-proline, and γ-Glutamylcysteinylserine. L-proline and γ-Glutamylcysteine—that are part of GABA-glutamate metabolism—can be elevated in the striatum following GABA administration. We observed that L-proline—an analog of GABA—was reduced in the striatum of TS rats. We hypothesize that it may play a role in GABA function that is depleted following exogenous GABA administration but not after GABA administration. This improves the metabolic balance of Glu and GABA, whose effect is replaced by an increase in accumulation. Nevertheless, γ-Glutamylcysteine—a precursor of glutathione—exhibits anti-inflammatory properties.40 Additionally, KEGG enrichment analysis showed that they act via upregulating arginine biosynthesis, aminoacyl-tRNA biosynthesis, GABAergic synapse, and D-amino acid metabolism, all of which were pathways downregulated by TS. We observed that TS induced elevated gluten exorphin C. A study shows elevated gluten exorphin C in urine samples from children with neurodevelopmental disorders, and its elevation is associated with a plethora of CNS symptoms such as autism syndromes, which include social indifference, maladaptive, stereotypical, and repetitive behaviors, increased incidence of epilepsy, and language problems. Conversely, the elevated circulating peptides may be attributed to decreased peptide catabolism in the intestinal mucosa and increased uptake of specific peptides.41 This finding also suggests an effect of striatal metabolites on behavior.
TS-induced abnormalities in other striatal metabolites can also be restored following exogenous GABA administration. This includes upregulation of armillaripin, TCA, Iidoleacrylic acid, WithaferinA, Ammonia aspartate, and JP83, among others. Armillaripin, a proto-IRU-type sesquiterpenoid alcohol aromatic ester isolated from the mycelium of Armillariella species, has demonstrated a broad spectrum of biological activities, such as antitumor, antibacterial, anti-inflammatory, and antivirus, among others.42,43 TCA—that is known to be produced in the large intestine by microbial metabolism of L-phenylalanine and polyphenol compounds in food—is involved in enhancing gut barrier function and regulating the gut microbiota. Additionally, colon-targeted TCA ameliorates colitis in rats by activating GPR109A.44 A study also suggests that cinnamic acid restores striatal fibers and neurotransmitters, as well as improves behavioral functions in MPTP-intoxicated mice. Indoleacrylic acid demonstrates beneficial effects on intestinal epithelial barrier function and reduces the inflammatory response of immune cells.45 These findings suggest that GABA may ameliorate the central inflammatory state induced by TS. Additionally, WithaferinA has metabolites with direct neuroprotective effects.46 These findings suggest that GABA administration can also exhibit a positive effect on central function by increasing the exertion of neuroprotective metabolites. Finally, we conducted a correlation between the gut microbiota and striatal metabolites. We observed a negative correlation between Clostridium_sensu_stricto_1 and WithaferinA, JP83, bhos#36, 19-oxodesacetylcinobufagin, and (2s,4R,5R,6R,14S,16R)-14-Hydroxy-7,11-dimethyl-6-(2-OXOPYRAN-4-YL)-3-oxapentacyclo[8.8.0.02,4.02,7.011,16]octadecan-5-yl] acetate. This correlation corroborates the relationship between the gut microbial and striatal metabolism in our results. In human TS studies, metabolite correlations similar in microbiome to our findings have been observed, particularly regarding reduced SCFAs levels, dysregulation of tryptophan metabolism and abnormal glutamate-GABA balance.47,48
Our findings suggest that exogenous GABA can attenuate TS-like behavior, which can significantly reduce the side effects associated with current pharmacological treatments. GABA administration may relieve TS symptoms by regulating neurotransmitter balance, gut-brain axis, and anti-inflammatory effects, with multiple target advantages and fewer side effects. GABA administration offers a potential novel therapeutic option compared to conventional therapies, but its long-term safety and efficacy still need further investigation.27,49 Moreover, our study has some limitations that should be addressed. First, given the higher incidence of TS in men compared to that in women,50 we exclusively established and explored TS models in male mice without exploring changes in females. However, the role of gender differences in TS cannot be ignored. It has been suggested that sex hormones (such as testosterone and estrogen) may affect the pathological process of TS by regulating the dopaminergic and GABAergic systems.51,52 The effect of gender on the composition and function of the gut microbiota may also contribute to differences in TS symptoms and response to treatment.53 Additionally, we did not evaluate the pathology of the gut nor test for blood markers. However, we exclusively conducted animal studies, which have been confirmed in small clinical trials and our clinical trials. A clinical treatment drug group was not set up as a control in our experiment because it is considered that GABA as a nutrient plays a palliative role and not a therapeutic one.54 This may assist the treatment drug to better control TS-like behaviors. We believe that the underlying mechanisms involved in mitigating TS-like behavior by GABA administration are complex and that the interaction of indicators is not discussed well in our study. Future studies should further investigate the specific role of GABA in TS.
Conclusions
In summary, this study demonstrates the first evidence that exogenous GABA mitigates TS-like behavior in TS model rats by modulating harmful intestinal flora and striatal GABA-glutamate metabolism, central inflammatory state, and directly enhancing neuroprotective metabolites. Furthermore, it offers novel ideas for the clinical management of TS.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article. All data associated with this study has not been deposited into a publicly available repository.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the “14th Five-Year Plan” Clinical Medicine Innovation Research Team of Hebei Medical University, Class B Team Project (2022LCTD-B15), Hebei Provincial Health Commission Project (20230594), Hebei Province Third Batch of Young Top Talents Program, and Natural Science Foundation Program of Hebei Province (Upper-level Project) (H2019206223).
Disclosure
Professor Xiao-Yu Tian reports grants from Clinical Medical Excellence Training Program funded by government (ZF2023112), grants from the “14th Five-Year Plan” Clinical Medicine Innovation Research Team of Hebei Medical University, Class B Team Project (2022LCTD-B15), grants from Hebei Provincial Health Commission Project (20230594), grants from Hebei Province Third Batch of Young Top Talents Program, outside the submitted work. The authors declare that there is no conflict of interest.
References
1. Efron D, Dale RC. Tics and Tourette syndrome. J Paediatr Child Health. 2018;54(10):1148–1153. doi:10.1111/jpc.14165
2. Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA, Ben-Shlomo Y. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov Disord. 2015;30(2):221–228. doi:10.1002/mds.26089
3. Fernandez de la Cruz L, Mataix-Cols D. General health and mortality in Tourette syndrome and chronic tic disorder: a mini-review. Neurosci Biobehav Rev. 2020;119:514–520. doi:10.1016/j.neubiorev.2020.11.005
4. Johnson KA, Worbe Y, Foote KD, Butson CR, Gunduz A, Okun MS. Tourette syndrome: clinical features, pathophysiology, and treatment. Lancet Neurol. 2023;22(2):147–158. doi:10.1016/s1474-4422(22)00303-9
5. Set KK, Warner JN. Tourette syndrome in children: an update. Curr Probl Pediatr Adolesc Health Care. 2021;51(7):101032. doi:10.1016/j.cppeds.2021.101032
6. Kalanithi PSA, Zheng W, Kataoka Y, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci USA. 2005;102(37):13307–13312. doi:10.1073/pnas.0502624102
7. Gong H, Du X, Su A, Du Y. Pharmacological treatment of Tourette’s syndrome: from the past to the future. Neurol Sci. 2024;45(3):941–962. doi:10.1007/s10072-023-07172-2
8. Ren Z, Pribiag H, Jefferson SJ, et al. Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol Psychiatry. 2016;80(6):457–468. doi:10.1016/j.biopsych.2016.02.009
9. Teng J, Zhou W, Zeng Z, Zhao W, Huang Y, Zhang X. Quality components and antidepressant-like effects of GABA green tea. Food Funct. 2017;8(9):3311–3318. doi:10.1039/c7fo01045a
10. Vollenweider I, Smith KS, Keist R, Rudolph U. Antidepressant-like properties of α2-containing GABA(A) receptors. Behav Brain Res. 2011;217(1):77–80. doi:10.1016/j.bbr.2010.10.009
11. Rashmi D, Zanan R, John S, Khandagale K, Nadaf A. Chapter 13 - γ-Aminobutyric Acid (GABA): biosynthesis, role, commercial production, and applications. In: Attaur R, editor. Studies in Natural Products Chemistry. Elsevier; 2018:413–452.
12. Bronfeld M, Belelovsky K, Bar-Gad I. Spatial and temporal properties of tic-related neuronal activity in the cortico-basal ganglia loop. J Neurosci. 2011;31(24):8713–8721. doi:10.1523/jneurosci.0195-11.2011
13. Larsh TR, Huddleston DA, Horn PS, et al. From urges to tics in children with Tourette syndrome: associations with supplementary motor area GABA and right motor cortex physiology. Cereb Cortex. 2023;33(7):3922–3933. doi:10.1093/cercor/bhac316
14. Puts NAJ, Harris AD, Crocetti D, et al. Reduced GABAergic inhibition and abnormal sensory symptoms in children with Tourette syndrome. J Neurophysiol. 2015;114(2):808–817. doi:10.1152/jn.00060.2015
15. Pogorelov V, Xu M, Smith HR, Buchanan GF, Pittenger C. Corticostriatal interactions in the generation of tic-like behaviors after local striatal disinhibition. Exp Neurol. 2015;265:122–128. doi:10.1016/j.expneurol.2015.01.001
16. Pokusaeva K, Johnson C, Luk B, et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil. 2017;29(1). doi:10.1111/nmo.12904
17. Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693(Pt B):128–133. doi:10.1016/j.brainres.2018.03.015
18. Strandwitz P, Kim KH, Terekhova D, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4(3):396–403. doi:10.1038/s41564-018-0307-3
19. Sorboni SG, Moghaddam HS, Jafarzadeh-Esfehani R, Soleimanpour S. A comprehensive review on the role of the gut microbiome in human neurological disorders. Clin Microbiol Rev. 2022;35(1):e00338–20. doi:10.1128/cmr.00338-20
20. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. doi:10.1152/physrev.00018.2018
21. Zhang F, Li A. Dual ameliorative effects of Ningdong granule on dopamine in rat models of Tourette’s syndrome. Sci Rep. 2015;5:7731. doi:10.1038/srep07731
22. Gittis AH, Kreitzer AC. Striatal microcircuitry and movement disorders. Trends Neurosci. 2012;35(9):557–564. doi:10.1016/j.tins.2012.06.008
23. Nuss P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr Dis Treat. 2015;11:165–175. doi:10.2147/ndt.S58841
24. Davidson GL, Cooke AC, Johnson CN, Quinn JL. The gut microbiome as a driver of individual variation in cognition and functional behaviour. Philos Trans R Soc London Ser B. 2018;373(1756):20170286. doi:10.1098/rstb.2017.0286
25. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
26. Martino D, Macerollo A, Leckman JF. Neuroendocrine aspects of Tourette syndrome. Int Rev Neurobiol. 2013;112:239–279. doi:10.1016/b978-0-12-411546-0.00009-3
27. Sharon G, Cruz NJ, Kang DW, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177(6):1600–1618.e17. doi:10.1016/j.cell.2019.05.004
28. Li Y-J, Li J, Dai C. The role of intestinal microbiota and mast cell in a rat model of visceral hypersensitivity. J Neurogastroenterol Motil. 2020;26(4):529–538. doi:10.5056/jnm20004
29. Liu J, Luo M, Qin S, Li B, Huang L, Xia X. Significant succession of intestinal bacterial community and function during the initial 72 hours of acute pancreatitis in rats. Front Cell Infect Microbiol. 2022;12:808991. doi:10.3389/fcimb.2022.808991
30. Yang W-Y, Lee Y, Lu H, Chou C-H, Wang C. Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS One. 2019;14(5):e0205784. doi:10.1371/journal.pone.0205784
31. Socala K, Doboszewska U, Szopa A, et al. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol Res. 2021;172:105840. doi:10.1016/j.phrs.2021.105840
32. Mo L, Jing H, Du X, et al. Goat and cow milk differ in altering the microbiota composition and neurotransmitter levels in insomnia mouse models. Food Funct. 2023;14(14):6526–6540. doi:10.1039/d3fo00797a
33. Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2011:294. doi:10.3389/fphys.2011.00094
34. Vendrik KEW, Ooijevaar RE, de Jong PRC, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. 2020;10:98. doi:10.3389/fcimb.2020.00098
35. Dohnalova L, Lundgren P, Carty JRE, et al. A microbiome-dependent gut-brain pathway regulates motivation for exercise. Nature. 2022;612(7941):739–747. doi:10.1038/s41586-022-05525-z
36. Liao J-F, Cheng Y-F, Li S-W, et al. Lactobacillus plantarum PS128 ameliorates 2,5-Dimethoxy-4-iodoamphetamine-induced tic-like behaviors via its influences on the microbiota-gut-brain-axis. Brain Res Bull. 2019;153:59–73. doi:10.1016/j.brainresbull.2019.07.027
37. Mahone EM, Puts NA, Edden RAE, Ryan M, Singer HS. GABA and glutamate in children with Tourette syndrome: a 1H MR spectroscopy study at 7 T. Psychiatry Research-Neuroimaging. 2018;273:46–53. doi:10.1016/j.pscychresns.2017.12.005
38. Hatano M, Nakajima W, Tani H, et al. Characterization of patients with major psychiatric disorders with AMPA receptor positron emission tomography. mol Psychiatry. 2024. doi:10.1038/s41380-024-02785-1
39. Boonstra E, de Kleijn R, Colzato LS, Alkemade A, Forstmann BU, Nieuwenhuis S. Neurotransmitters as food supplements: the effects of GABA on brain and behavior. Front Psychol. 2015;6:1520. doi:10.3389/fpsyg.2015.01520
40. Yang Y, Li L, Hang Q, et al. γ-glutamylcysteine exhibits anti-inflammatory effects by increasing cellular glutathione level. Redox Biol. 2019;20:157–166. doi:10.1016/j.redox.2018.09.019
41. Bojovic K, Stankovic B, Kotur N, et al. Genetic predictors of celiac disease, lactose intolerance, and vitamin D function and presence of peptide morphins in urine of children with neurodevelopmental disorders. Nutr Neurosci. 2019;22(1):40–50. doi:10.1080/1028415x.2017.1352121
42. Li L, Wang S, Ma L, Liu Q, Zhong B, Zhang H. The active constituents and the medicinal value of the Armillariamellea. J Jilin Med Univ. 2018;39(04):307–310. doi:10.13845/j.cnki.issn1673-2995.2018.04.030
43. Yang JS, Su YL, Wang YL, et al. Chemical constituents of Armillaria mellea mycelium. VI. Isolation and structure of armillaripin. Yao Xue Xue Bao. 1990;25(5):353–356.
44. Kang C, Kim J, Ju S, et al. Colon-targeted trans-cinnamic acid Ameliorates rat colitis by activating GPR109A. Pharmaceutics. 2023;15(1):41. doi:10.3390/pharmaceutics15010041
45. Wlodarska M, Luo C, Kolde R, et al. Indoleacrylic acid produced by commensal Peptostreptococcus species suppresses inflammation. Cell Host Microbe. 2017;22(1):25. doi:10.1016/j.chom.2017.06.007
46. Raziya Banu M, Ibrahim M, Prabhu K, Rajasankar S. Withaferin-A protects the nigral dopamine neuron and recovers motor activity in aged rats. Cells Tissues Organs. 2020;208(1–2):59–65. doi:10.1159/000505183
47. Bao C, Wei M, Pan H, et al. A preliminary study for the clinical effect of one combinational physiotherapy and its potential influence on gut microbial composition in children with Tourette syndrome. Front Nutr. 2023;10:1184311. doi:10.3389/fnut.2023.1184311
48. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. doi:10.1038/nrn3346
49. Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M. Neurobiological substrates of Tourette’s disorder. J Child Adolesc Psychopharmacol. 2010;20(4):237–247. doi:10.1089/cap.2009.0118
50. Robertson MM. Corrections. A personal 35 year perspective on Gilles de la Tourette syndrome: prevalence, phenomenology, comorbidities, and coexistent psychopathologies. Lancet Psychiatry. 2015;2(4):291. doi:10.1016/s2215-0366(15)00132-7
51. Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62(2):155–198. doi:10.1124/pr.109.002071
52. Hara Y, Waters EM, McEwen BS, Morrison JH. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol Rev. 2015;95(3):785–807. doi:10.1152/physrev.00036.2014
53. Jašarević E, Morrison KE, Bale TL. Sex differences in the gut microbiome-brain axis across the lifespan. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150122. doi:10.1098/rstb.2015.0122
54. Hu YKL, Li L. Observation on the efficacy of inosine and γ-aminobutyric acid in the treatment of Tourette’s syndrome. Shandong Medicine. 1995;(7):12.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.