Back to Journals » International Journal of Nanomedicine » Volume 19
Exosomes Induce Crosstalk Between Multiple Types of Cells and Cardiac Fibroblasts: Therapeutic Potential for Remodeling After Myocardial Infarction
Authors Feng Y, Wang Y, Li L, Yang Y, Tan X, Chen T
Received 6 May 2024
Accepted for publication 9 October 2024
Published 19 October 2024 Volume 2024:19 Pages 10605—10621
DOI https://doi.org/10.2147/IJN.S476995
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Krishna Nune
Yijuan Feng,1,* Yan Wang,1,* Li Li,1,* Yan Yang,1 Xiaoqiu Tan,1– 3 Tangting Chen1,2
1Key Laboratory of Medical Electrophysiology of the Ministry of Education, Medical Electrophysiological Key Laboratory of Sichuan Province, Institute of Cardiovascular Research, Southwest Medical University, Luzhou, Sichuan, People’s Republic of China; 2Department of Cardiology, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, People’s Republic of China; 3Department of Physiology, School of Basic Medical Sciences, Southwest Medical University, Luzhou, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tangting Chen; Xiaoqiu Tan, Key Laboratory of Medical Electrophysiology of the Ministry of Education, Medical Electrophysiological Key Laboratory of Sichuan Province, Institute of Cardiovascular Research, Southwest Medical University, Luzhou, Sichuan, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Recanalization therapy can significantly improve the prognosis of patients with acute myocardial infarction (AMI). However, infarction or reperfusion-induced cardiomyocyte death, immune cell infiltration, fibroblast proliferation, and scarring formation lead to cardiac remodeling and gradually progress to heart failure or arrhythmia, resulting in a high mortality rate. Due to the inability of cardiomyocytes to regenerate, the healing of infarcted myocardium mainly relies on the formation of scars. Cardiac fibroblasts, as the main effector cells involved in repair and scar formation, play a crucial role in maintaining the structural integrity of the heart after MI. Recent studies have revealed that exosome-mediated intercellular communication plays a huge role in myocardial repair and signaling transduction after myocardial infarction (MI). Exosomes can regulate the biological behavior of fibroblasts by activating or inhibiting the intracellular signaling pathways through their contents, which are derived from cardiomyocytes, immune cells, endothelial cells, mesenchymal cells, and others. Understanding the interactions between fibroblasts and other cell types during cardiac remodeling will be the key to breakthrough therapies. This review examines the role of exosomes from different sources in the repair process after MI injury, especially the impacts on fibroblasts during myocardial remodeling, and explores the use of exosomes in the treatment of myocardial remodeling after MI.
Keywords: exosomes, myocardial infarction, myocardial fibrosis, cardiac fibroblasts
Introduction
Myocardial infarction (MI) is the most serious manifestation of coronary artery disease and a major cause of related deaths.1 The main clinical treatment for MI aims to unblock the coronary artery, restore blood flow, and minimize post-infarction myocardial remodeling.2 Adult myocardial cells cannot regenerate after necrosis, so the maintenance of normal cardiac structure after MI mainly relies on the proliferation of fibroblasts.3 However, cardiac fibrosis and remodeling caused by continuous fibroblast activation4 still requires the further search for new therapeutic strategies. Studies on extracellular vesicles have exponentially increased in the past decade, from basic researches to clinical translational applications. Due to the endogenous, biocompatible and multifunctional properties, the extracellular vesicles may become a novel treatment for various cardiac diseases in the future.5,6
Exosomes being paracrine is a key pathway for intercellular information exchange, transporting critical proteins7 and genetic material (such as miRNA,8 mRNA,9 and DNA10), mediating the transfer of various biomolecules between cells, which is an important component of various physiological and pathological processes.11 Recent studies have shown that various types of cells, including myocardial cells,12,13 endothelial cells (ECs),14 immune cells,15 and stem cells,16 can secrete extracellular vesicles to participate in the process of myocardial remodeling. Certain extracellular vesicles exhibit significant advantages in myocardial remodeling after heart attack, while others aggravate the progression of myocardial remodeling. Although there are literature reviews on the effects of exosomes on cardiovascular diseases, as well as how the contents of exosomes (microRNA (miRNA), non-coding RNAs (ncRNA), long non-coding RNAs (lncRNA) et al) affect cardiac fibrosis or myocardial remodeling processes after MI,17–19 there is currently no literature that specifically explains how exosomes and their contents derived from different cell sources act on fibroblasts and their mechanisms after MI. This would provide a detailed understanding of the role of fibroblasts in the myocardial remodeling process following MI.
Thus, this review focuses on the dual role of fibroblasts in the pathological process of myocardial repair, and provides an overview of the role of exosome-mediated intercellular crosstalk from various cell types targeting fibroblasts in post-MI fibrosis. This review aims to illustrate the potential therapeutic significance of exosomes in the treatment of myocardial remodeling after MI.
Myocardial Remodeling After MI
The pathological manifestations of MI primarily encompass the apoptosis and necrosis of cardiomyocytes, the activation of inflammatory factors, the phagocytosis and elimination of macrophages, the activation of fibroblasts, the secretion of extracellular matrix (ECM), and the process of angiogenesis.20–22
Cardiac fibroblasts (CFs) serve as the main effector cells in the repair process. After the clearance of dead and damaged tissues by phagocytes, the inflammatory response diminishes. At this stage, pro-fibrotic factors, including transforming growth factor-b (TGF-β), endothelin-1, reactive oxygen species (ROS), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF),23–26 work in conjunction with neuroendocrine signals and mechanical stress to activate CF proliferation and transformation into myofibroblasts.27 A variety of signaling pathways have been demonstrated to be involved in the repair process, among which the main pathways including the TGF-β1/Smad-2/3 pathway,28 and other pathways such as matrix metalloproteinases (MMPs), tissue inhibitor of matrix metalloproteinases (TIMPs), and nuclear factor κB (NF-κB),29,30 which regulate the intensity and duration of pro-fibrotic signals, and induce myofibroblasts to secret collagen and ECM to repair the areas loss of cardiomyocytes. These processes play a protective role in the early stage of MI, maintaining the structural integrity of the injured myocardium,31 which is called substitute fibrosis. At the same time, myofibroblasts express actin and myosin, which are required for contraction, and compensate for part of the contractile function.32 In the late stage of injury repair, the gradual decrease of pro-fibrotic factors, collagen cross-linking, and apoptosis of myofibroblasts indicates the cessation of the repair process. However, if the stimulus persists, the activation of myofibroblasts will lead to excessive deposition of ECM, which triggers diffuse interstitial fibrosis and perivascular fibrosis, thus leading to cardiac stiffness and severely affecting the systolic and diastolic functions.33,34 Excessive myocardial fibrosis will also lead to electrophysiological remodeling of the heart, which will induce ventricular arrhythmias.35 Therefore, controlling the proliferation and activation of fibroblasts to achieve a dynamic balance between pro-fibrosis and anti-fibrosis is important for post-MI repair.
Exosomes
Exosomes (Exos) are extracellular vesicular bodies that are characterized by their bilayer membrane structure and range in diameter 30–150 nm.36 These Exos can be secreted by a variety of cells. They are structurally stable and small in size, containing RNAs, proteins, and other molecules involved in metabolic and growth processes.37 Additionally, they express a variety of antigens and antibodies in their membrane structures, which can mediate the interaction and communications between different cells.38 Exosomogenesis encompasses the process of plasma membrane invagination, the encapsulation of components in close proximity to the membrane, and the subsequent formation of early endosomes (Figure 1). Early endosomes undergo a gradual maturation process, leading to formation of late endosomes. Late endosomes emerge through budding from the endosome membrane, leading to subsequent formation of polyvesicles.39,40 The formation of intracellular vesicles can be accomplished via the endosomal sorting complex required for transport (ESCRT)-dependent pathway for the sorting and transporting of ubiquitin proteins,41 and ceramide enrichment induced budding is another route.42 The precise mechanism underlying the transport and fusion of intracellular polyvesicles to the plasma membrane is still unclear, potentially implicating the involvement of the small Rab GTPases protein family.43 Currently, researchers posit that exosomes represent a novel mechanism of intercellular communication, and potentially a new therapeutic target capable of modulating the occurrence and development of diseases. Exos are released in both physiological and pathological conditions.44 The substances in exosomes depend on the cell state, and the identification of different exosomes is achieved through the analysis of their surface markers.45 Presently, there are several confirmed markers of exosomes: four transmembrane family proteins (CD9, CD63, CD81, CD82, CD89, etc.),46 heat shock proteins (HSP27, HSC70, HSP90, etc.),47 proteins related to membrane vesicles formation (Alix, TSG101, etc.),48,49 integrins, major histocompatibility complex (MHC) class I/II molecules,50 and some biological enzymes.51 When exosomes are released into the extracellular environment, they not only act on neighboring cells through parasecretion, but also play a remote regulatory role via the circulation of body fluids. Intercellular communication can be realized through various ways. For example, recipient cells can internalize Exos cargo through uptake pathways of endocytosis and membrane fusion, thus obtaining information substances from the donor cells.52 Exos in the plasma of patients with coronary heart disease contain circular RNA circ_0001785, which “sponges” the expression of miR-513a-5p in the endothelial cells. This interaction leads to increased downstream expression of TGFBR3, which promotes endothelial cell proliferation and migration while inhibiting apoptosis, thereby delaying the onset of atherosclerosis.53 Secondly, Exos mediate intercellular communication and molecular transport by facilitating the transfer of various bioactive molecules, including nucleic acids, proteins, and lipids from donor cells to recipient cells.54 Macrophage-derived Exos can penetrate the blood–brain barrier and deliver a cargo protein (BDNF, brain-derived neurotrophic factor) to the brain to play a therapeutic role in central nervous system diseases.55 In addition, Exos can also be in the form of receptor-ligand (receptor membrane protein-exosome membrane protein), which directly regulates the signaling pathways in the recipient cells. Exos can also be used as drug delivery systems for disease treatment.56,57
Effect of Exosomes from Different Cell Sources on Injury Repair After MI
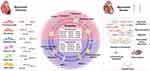
Exos, serving as the “mailman” for exchanging biological information between cells, have great potential and value in the treatment of various diseases, including cancer, kidney diseases, inflammatory diseases, and neurodegenerative diseases.22,58–61At the same time, more and more cardiovascular studies are focusing on the contribution of exosomes, especially in various stages after MI.62 The repair response post-MI is necessary for the injured myocardium, and CFs are the major bearers of myocardial remodeling. When activated, CFs transform into functional myofibroblasts, and this transformation is regulated by a variety of factors.63 After being captured by CFs, exosomes derived from different cells can regulate the repair and fibrosis after MI by acting on the phenotype, secretion, proliferation, activation and other processes of CFs.64 Understanding the mechanisms is important for the development of new methods for targeted treatment of adverse remodeling caused by excessive fibrosis after MI. The effects of exosomes from different sources on cardiac remodeling will be discussed in detail.
Cardiomyocyte-Derived Exosomes
Cardiomyocytes (CMs) are the most abundant cells of the heart which cannot regenerate,65 and CMs in the region of infarction are in a state of ischemia and hypoxia due to reduced blood supply. In recent years, researchers have demonstrated that CMs can release exosomes containing a variety of cytokines to enhance information transfer with other cells, reduce vascular endothelial damage, attenuate fibrosis and play a certain cardioprotective role in repair after MI.66,67 Recently, exosomes released in cardiospheres by CMs and progenitor cells are referred to as cardiosomes, which mediate cell-cell/matrix communications at the infarct zone. Cardiosomes cargo transfers cardiomyocyte-specific miR-195 to fibroblasts, promoting the activation of myofibroblasts.68 Research has demonstrated that the expression of miR-222 has been shown a significant increase in exosomes secreted by ischemic CMs, which positively affects angiogenesis after MI by stimulating cellular tubulogenesis and outgrowth, promoting endothelial cell growth and proliferation, as well as angiogenesis.69 Simultaneously, the interaction between CMs and fibroblasts becomes increasingly pronounced. The miR-92a in cardiosomes stimulates the transformation of fibroblast into myofibroblast by downregulating Smad7-mediated transcriptional repression of α-smooth muscle actin (α-SMA).70 The exosomal miR-217 secreted by CMs enhances the fibroblast proliferation by regulating phosphatase and tensin homologue (PTEN).71 The aforementioned studies indicate that cardiosomes have some positive effects during the pathophysiological process of MI by promoting vascular regeneration and activating fibroblasts involved in cardiac repair.
However, CMs also secrete exosomes containing factors that negatively regulate myocardial remodeling. The exosomal miR-19a-3p in cardiosomes inhibits the proliferation of endothelial cells and decreases angiogenesis after MI by targeting hypoxia inducible factor-1α (HIF-1α).72 Exosoma lncRNA AK139128, secreted by hypoxic CMs, obviously suppresses fibroblast growth and accelerates fibroblast apoptosis when co-cultured with fibroblasts, thereby playing an important role in slowing down the procession of myocardial fibrosis.73
Fibroblast-Derived Exosomes
As a part of non-myocardial cells in the normal cardiac architecture, CFs play an important role in myocardial remodeling.74 After MI, fibroblasts migrate to the damaged area under the regulation of a series of bioactive factors. They transform and proliferate, secrete large amounts of collagen, and participate in the repair of injury heart.75 However, myofibroblasts play a dual role in ventricular remodeling. On one hand, they contribute to the formation of scar tissue and the maintenance of cardiac integrity by regulating the levels of extracellular matrix and various proteins. On the other hand, when a substantial number of myofibroblasts are activated, excessive deposition of extracellular matrix and proteins can disrupt cardiac rhythm and lead to arrhythmia.76 Previous studies have displayed some cardioprotective effects of exosomes partially derived from CFs. A study has shown that cardiac fibroblasts secreted exosomes (CFs-Exo) can enhance ventricular conductivity and reduce the risk of RA after cold ischemia/reperfusion (I/R). At the same time, cardiac fibroblasts containing sevoflurane increase the relative expression of Cx43 and enhance the activity of myocardial cells after hypoxia/reoxygenation (H/R). Sevoflurane-treated cardiac fibroblasts exosomes (Sev-CFs-Exo) increase the expression of Cx43, reduce it and improve the area of myocardial infarction.77 H/R-induced exosomal secretion from fibroblasts confers protection to H9C2 cells against H/R injury and enhances cell survival rates. Following ischemic post-treatment, the secretion of fibroblast-derived exosomal miR-423-3p is elevated, and the targeted modulation of Ras-related protein Rap-2c (RAP2C) can mitigate cellular apoptosis and ameliorate myocardial injury.78 MiR-133a, derived from CF exosomes under H/R conditions, regulates the expression of embryonic lethal abnormal vision like 1 (ELAVL1), thereby inhibiting cardiomyocyte apoptosis, reducing cardiac injury, and promoting tissue repair and fibrosis.79 Furthermore, exosomes containing miR-133a also facilitate the differentiation of fibroblasts into CM-like cells.80 However, some studies have indicated that not all exosomes secreted by fibroblasts exert a positive inhibitory effect on cardiac fibrosis following MI.81 For instance, Exos containing miR-223 act as potent pro-fibrotic factors, promoting the proliferation, migration, and differentiation of CFs. Consequently, inhibiting miR-223 after MI may prevent malignant cardiac fibrosis and arrhythmias.82 Fibroblasts also secrete miR-21-3p, which can induce cardiomyocyte hypertrophy and accelerate ventricular remodeling.83 In conclusion, exosomes derived from fibroblasts not only act on cardiomyocytes but also affect the signaling pathways of the fibroblasts themselves, thereby influencing myocardial remodeling after MI. Targeted regulation of exosomes derived from fibroblasts can help to maintain ventricular stability, reduce myocardial fibrosis and alleviate myocardial remodeling after MI.
Endotheliocyte-Derived Exosomes
Endotheliocytes (ECs) serve as the communication link between blood and matrix. After MI, blood flow occlusion and tissue ischemia cause damage to endothelial cells, driving them to synthesize and secrete exosomes containing biologically active substances.84 Carter et al demonstrated that exosomes derived from ECs promoted angiogenesis, thereby attenuating repair adverse effects.85 Additionally, inhibition of miRNA-126 also reduced area of MI and preserved cardiac function.86
Endothelial progenitor cells (EPCs) are the precursors of vascular ECs. Under the stimulation of various factors, EPCs can be mobilized from the bone marrow to the peripheral blood to participate in injury repair, which play a paracrine role through the release of various cytokines.87 Accumulating data have proved that exosomes derived from endothelial progenitor cells (EPCs-Exos) can promote mesenchymal to endothelial transition (MEndoT) engaging them in angiogenesis in cardiac injury areas, thus reversing fibrosis. CFs treated with EPCs-Exos shows up-regulated expression of EC-specific markers, such as vascular endothelial growth factor receptor-2, and down-regulated expression of fibrosis-related markers, such as α-SMA, collagen I, TGF-β, and high mobility group box 1 (HMGB1). These results suggest that EPCs-Exos can promote the CF proliferation and angiogenesis by increasing MEndoT and inhibiting HMGB1 expression.88 Upregulation of miR-218-5p or miR-363-3p in EPC-Exos after MI inhibits myocardial fibrosis through MEndoT elevation mediated by targeting the p53/JMY signaling pathway.89 In addition, enrichment of miR-1246 or miR-1290 in EPC-Exos induces upregulation of E74-like factor-5 (ELF5) and specificity protein-1 (SP1), which also achieves the same effect of improving myocardial fibrosis after MI.90 Similarly, YBX-1-mediated miR-133 sorting into H/R-induced EPCs-Exos promotes angiogenesis and MEndoT of CFs and inhibits fibrosis in vitro.91 All these results indicate that EPC-Exos have the potential roles for promoting MEndoT and improving myocardial fibrosis.
Immune Cell-Derived Exosomes
Although CFs are the executor of cardiac repair after MI, the importance of the immune system as the initiator of this process is undeniable.92 Following the occurrence of MI, resident and infiltrating immune cells are rapidly recruited and activated, initiating a complex immune response.93 When the inflammatory stage gradually transitions to the repair stage, it is necessary to timely inhibit the inflammatory process and activate the repair response through the transformation of immunosuppressive lymphocytes and pro-inflammatory/anti-inflammatory immune cells.94–96 Exos secreted by immune cells, play an important role in mediating intercellular crosstalk with CFs and participating in the repair of injured myocardium.97 M1-macrophages-derived exosomes (M1-Exos) inhibit cardiac repair. Previous studies have investigated the upregulation of miR-155 in exosomes secreted by M1 macrophages after MI, which can down-regulate multiple target genes of ECs, including the small GTPase rac1 (RAC1), p21-activated kinase 2 (PAK2), Sirtuin 1 (Sirt1) and Adenosine5’-monophosphate-activated protein kinase α 2 (AMPKα 2), thereby reducing the angiogenesis and inhibiting cardiac healing.98 M2 macrophages promote cardiac repair. A long non-coding RNA (lncRNA-ASLNCS5088) is enriched in M2 macrophage-derived exosomes (M2-Exos) treated with TGF-β1. This lncRNA could be transferred to fibroblasts with high efficiency and act as an endogenous sponge to absorb miR-200c-3p, resulting in increased activation of CF and synthesis of collagen I, collagen III, glutaminase, and α-SMA.99 Moreover, upregulation of HuR (human antigen R) in M-Exos, exposed to high glucose, could also mediate intercellular crosstalk with CFs, significantly increasing the fibrotic response both in vitro and in vivo.100 Some M2-Exos promote cardiac repair by inhibiting CM apoptosis. Some studies have shown that M2-Exos carry miR-1271-5p in the acute myocardial infarction (AMI) mouse model, which reduces cardiomyocyte apoptosis and promotes cardiac repair by down-regulating SOX6.101 M2-Exos containing circUbe3a promote the proliferation, migration, and phenotypic transformation of CFs by targeting the miR-138-5p/RhoC axis, which may exacerbate myocardial fibrosis following AMI.102 In addition, dendritic cells (DCs) were also involved, that MI-induced DCS-derived exosomes (DCS-Exos) were found to directly activate CD4+ cells in the spleen. Activated CD4+ T cells could then migrate to the infarcted myocardium and play a crucial role in improving myocardial healing.103 The activated CD4+ T cell-derived exosomes (CD4-Exos) enriched with miR-142-3p, which activate the WNT signaling, induce pro-fibrotic effects of CFs, mediate the proliferation, migration and differentiation of CFs, promote cardiac fibrosis, and accelerate cardiac remodeling after ischemia.104 It can be seen from the above that, due to the complexity of the inflammatory process and the diversity of immune cell types after MI, the regulatory mechanisms of the Exos crosstalk with fibroblasts are also completely different. It is also confirmed that intervening the immune response in the course of MI to control the fibrosis process and make the injured myocardium reach the appropriate repair state can be a promising treatment in the future.
Adipose Tissue-Derived Exosomes
Adipose tissue mainly consists of adipocytes (ADs) and adipose stem cells (ADSCs), which are considered to be an endocrine organ of the body. Both ADs and ADSCs can release exosomes into the circulating plasma under stress to regulate cell behavior and metabolism, in which exosome cargo acts as an important mediator.105,106 Exosomes derived from adipose tissue (Ads-Exos) primarily play a role in mediating local inflammatory responses and regulating metabolic processes. Ads-Exos produced under palmitate stress protects the heart from I/R injury, demonstrating a significant reduction in fibrosis following I/R. However, the study did not determine whether the most pronounced effects of Ads-Exos are on the initial infarct size or on subsequent remodeling.107 In addition, the secretion of inducible nitric oxide synthase (iNOS) in the exosomes is also increased, thus promoting cardiac fibrosis and dysfunction.108 These publications imply the existence of a likely adipose-to-fibroblastic paracrine mechanism.
Numerous studies have demonstrated exosomes derived from ADSCs participate in the activation of CFs and promote repair through a variety of pathways, such as Wnt/β-catenin, p-Akt/Akt and TGF-β1.109–111 It have beneficial effects on adverse remodeling after MI. Down-regulation of miR-196a-5p in ADSCs-Exos effectively reverses myofibroblast activation and reduces collagen expression after MI in rats.112 ADSCs-Exos carrying miRNA-671 reduces myocardial fibrosis and inflammation by targeting TGFBR2/Smad2 axis, thereby alleviating MI injury in vivo and in vitro.113 In addition, miR-126 enrichment in ADSCs-Exos also reduces the expression of fibrosis-related proteins in H9c2 cells under hypoxic conditions.114
Cardiac Progenitor Cell-Derived Exosomes
Anversa et al first proposed cardiac progenitor cells (CPCs) in 1998,115 including cardiac sphere-derived cells (CDCs).116 CPCs are a special class of cardiac progenitor cells capable of forming clusters in suspension that can differentiate into CMs and some mesenchymal cells under certain conditions.117 CPCs implantation in the ischemic heart promotes the recovery of cardiac function and ameliorates arrhythmia in AMI/DCM models in rats, dogs and pigs, suggesting that CPCs exhibit a strong protective effect in the process of chronic ischemic myocardial remodeling.118–121 Exosomes derived from CPCs (CPCs-Exos) play a key role in this process. Human cardiac progenitor cell-derived exosomes (hCDC-Exos) can not only inhibit the proliferation of fibroblasts but also reprogram them to undergo phenotypic transformation.122 Hypoxia-induced CPCs-Exos reduce TGF-β-mediated profibrotic gene expression in fibroblasts and enhance endothelial tube formation. Microarray analysis identifies 11 up-regulated miRNAs that are relative to normoxic conditions, suggesting their potential involvement.123 Another study demonstrated that cardiac sphere cell-derived exosomes (CDCs-Exos) altered the CF phenotype and secretome in a beneficial positive feedback loop both in vivo and in vitro. This alteration included the secretion of higher levels of stromal cell-derived factor 1 (SDF-1) and vascular endothelial growth factor (VEGF).124 In addition, miR-146a-5p is enriched in CDCs-Exos, which may alleviate myocardial fibrosis in dilated cardiomyopathy.125 Apart from exosomes, certain proteins secreted by CPCs also decrease the degree of cardiac remodeling after MI. Soluble endothelial glycoprotein secreted by cardiac spheres injected into the infarct area reduces scar formation and collagen density, and enhances ventricular function by inhibiting the TGF-β1/Smad signaling pathway.126 These results indicate that CPCs-Exos have significant potential in the treatment of myocardial remodeling after infarction.
Despite the beneficial effects of CDC-Exos on the injured heart, clinical application of exosomes is facing great challenges. CDC-Exos with cardiac homing peptide (CHP) modification (exosomes bind to CHP via Doxorubicin-N-hydroxysuccinimide (DOX-NHS) linker) preferentially target the heart, and reduces the degree of fibrosis and scar area, increases cell proliferation and angiogenesis, and significantly improves its therapeutic effect on MI.127
Exosomes Derived from Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs), a kind of pluripotent stem cells with multi-directional differentiation potential, have been utilized in the replacement therapy of infarcted myocardium.128 However, the retention and survival rate of MSCs in the infarcted myocardium after transplantation are very low, which poses challenges for their clinical application.129 While MSCs-derived exosomes (MSCs-Exos) provide the possible opportunity and play an important role in ameliorating excessive fibrosis after MI.
It is well known that bone marrow mesenchymal stem cells (BMSCs) can promote myocardial repair. Numerous studies have shown that exosomes secreted by BMSCs (BMSCs-EXos) can exert therapeutic effects by promoting angiogenesis and M2 macrophage transformation to reduce inflammation and promote anti-apoptosis in CMs.130,131 Meanwhile, BMSCs-Exos also have great potential in anti-myocardial fibrosis. MiR-24 and miR-29 are key players in a variety of fibrotic diseases and are highly expressed in BMSCs-Exos, which are internalized by local fibroblasts, reducing fibroblast proliferation and transformation through reprogramming.132 MiR-29b-3p, as a member of the miR-29 family, is down-regulated in the myocardium of rats with MI. However, upregulated miR-29b-3p delivered by BMSC-Exos improves myocardial angiogenesis and ventricular remodeling, and reduces myocardial fibrosis by targeting a disintegrin and metalloproteinase with thrombospondin 16 (ADAMTS16).133 Interestingly, the expression of miR-212-5p is low in the clinicopathological samples and animal models of cardiac fibrosis caused by MI, while its expression is abundant in BMSCs-Exos. MiR-212-5p from BMSCs-Exos has the potential to alleviate myocardial fibrosis by inhibiting the NLRC5/VEGF/TGF-β1/Smad axis after MI.134 Furthermore, miR-129-5p of BMSCs-Exos also alleviates fibrosis by inhibiting inflammation in MI mice through the targeting of HMGB1.135
Compared to other MSC-Exos, exosomes derived from human umbilical cord mesenchymal stem cells (hucMSC-Exos) are widely used in the treatment of various diseases due to their non-invasive collection method and higher proliferation rate. And there are two-sided effects in the role of hucMSC-Exos in cardiac remodeling after MI. On the one hand, hucMSC-Exos can be used as an effective carrier for specific miRNAs and proteins to reduce myocardial fibrosis. HucMSC-Exos loaded with miR-29b mimics are internalized by CFs in vitro and down-regulate the expression of fibrosis-related proteins. After implantation into the infarcted hearts of mice, hucMSC-Exos reduce fibrosis and improve cardiac function in vivo.136 In addition, overexpressing TIMP2 in hucMSC-Exos reduces ECM (eg, MMP2, MMP9 and α-SMA) and collagen deposition, and inhibits TGFβ-induced secretion of CFs, thereby reducing MI-induced cardiac remodeling. The potential mechanism may be mediated through the Akt/Sfrp2 pathway.137 On the other hand, hucMSC-Exos can also repair the damaged myocardium by promoting fibrosis. Hypoxia leads to the down-regulation of Smad7 and up-regulation of miRNA-125b-5p both in vitro and in vivo, which are reversed after treatment with hucMSC-EXO. The results show that hucMSC-exosomes may promote the expression of Smad7 by downregulating miR-125b-5p, thereby enhancing myocardial fibrosis.138 Intraoperative injection of hucMSC-Exos in rats with MI increases the density of myofibroblasts and decreases the apoptosis of myocardial cells in the inflammatory stage of the infarction area after operation. This indicates that hucMSC-Exos promotes the differentiation of fibroblasts into myofibroblasts in the inflammatory environment, thereby promoting cardiac repair.139
Human Induced Pluripotent Stem Cell-Derived Exosomes
Human induced pluripotent stem cells (hiPSCs) can be obtained by transforming certain differentiated human cells into hiPSCs through genetic reprogramming technology.140 They also possess totipotency, do no present ethical concerns, and have abundant sources, making them a common choice in regenerative medicine. Currently, numerous of studies have confirmed the application value of hiPSCs in MI,141 and clinical trials are gradually carried out.142 HiPSCs-derived exosomes (hiPSCs-Exos) have complex components and maintain the glycan characteristics of hiPSCs-Exos.143 Systematic proteomics and miRNA profiling of hipSCs-Exos show that the unique miRNAs of hiPSCs-Exos regulate mTOR signaling, cell senescence, retinol metabolism, and tumor necrosis factor (TNF) signaling.144 Meanwhile, the abundant cargo of hiPSCs-Exos mediates therapeutic effects in myocardial repair after MI. Recent studies have shown that large myocardial patches designed from hiPSCs-derived CMs can improve the recovery of infarcted myocardium in pigs.145 HiPSCs-Exos can improve the survival rate of CMs and reduce fibrosis by regulating the production and autophagic flux of hypoxic CMs.146 Furthermore, exosomes isolated from hiPSCs-EC containing miR-199b-5p also alleviate ischemic injury by promoting angiogenesis.147 However, it should be noted that hiPSCs-Exos induced by special treatments may actually promote fibrosis. For example, hiPSCs can differentiate into MSCs after being stimulated by Ang II or TGF-β1, which promotes the secretion and mRNA expression of collagen I, collagen III and elastin in fibroblasts.148
Cortical Bone Stem Cell-Derived Exosomes
Cortical bone stem cell-derived exosomes (CBSCs-Exos) can also mediate the crosstalk with fibroblasts, thereby playing a protective role in the remodeling stage of infarct-related myocardium. The reduction in scar size and improvement in cardiac function are observed after CBSCs-Exos treatment in both MI and I/R models. Further studies suggest that CBSsC-Exos alter fibroblast activation through the hereto-unknown mechanism of decreasing small nucleolar RNA (snoRNA) signaling within CFs. Those findings demonstrate that CBSCs-Exos induce alterations in cardiac remodeling via decreasing fibroblast activation, leading to long-term reductions in cardiac scarring, and improvements in cardiac repair and function.149
Circulating Exosomes in Plasma
Circulating exosomes are released by almost all cells. After MI, the cargo and quantity of circulating exosomes are significantly changed,150 while free miRNAs can also be packaged in exosomes, stably exist in the blood circulation, and reach target cells through the blood circulation to rapidly regulate intracellular signals.151 Some circulating exosomes are anti-myocardial fibrosis, such as those rich in miR-133, miR-135, and miR-29.152 Up-regulating miR-29a in the plasma significantly reduces interstitial fibrosis in the left ventricle border region in MI.153 Up-regulation of miR-133a in the blood of patients with AMI inhibits myocardial fibrosis.154,155 Conversely, circulating Exos can also promote fibrosis. miR-21 mimic-loaded Exos resulted in the down-regulation of phosphatase and tensin homologue (PTEN), increased expression levels of Smad7 and MMP2, and increased myocardial fibrosis in MI mice.156 Down-regulation of miR-425 and miR-744 in plasma Exos of patients with heart failure (HF) attenuates the inhibitory effects on TGF-β1, resulting in the overexpression of collagen I and α-SMA to promote fibrosis.157 In addition, it is interesting to note that the isolation and detection of different subtypes of circulating exosomes can reflect the pathophysiological changes of heart diseases and can be used as a diagnostic tool for cardiovascular disease in the future.158,159
The mechanisms of action of exosomes from all these sources on post-MI fibrosis or fibroblasts are summarized in Table 1 and Figure 2
 |
Table 1 Specifies the Biological Effects, Acting Factors and Mechanisms of Exosomes from Different Cell Sources in the Model |
The Use of Exosomal Therapy
Exosome therapy represents an innovative therapeutic strategy that has attracted considerable attention and application within the cardiovascular disease in recent years.160
It has been proved in animal models that minimally invasive gel injections, gelatin microneedles, microneedle patches,102,136,161 and various exosome treatments effectively modulate cellular functions and facilitate tissue repair. For example, bioengineered CPCs-Exos transfected with the pro-angiogenic miR-322 (CPCexo-322), can enhance therapeutic efficacy in a mouse model of MI. The engineering of CPCs-Exos through miRNA programming can promote angiogenesis, making it a potentially effective therapy for ischemic cardiovascular diseases.162 The delivery system (GelMA-MN@3D-Exo) was constructed using microneedles topical delivery technology, MSC exosomes (3D-MSC-Exo) 3D culture, and biocompatible gelatin methacryloyl (GelMA) employed used to construct for the delivery of for (GelMA-MN@3D-Exo). The (GelMA-MN@3D-Exo) microneedles microneedle can mitigate attenuated ischemia-reperfusion cell damage in the middle cerebral artery occlusion (MCAO) model.161 A gelatin-based biocompatible microneedles (MN) patch loaded with exosomes containing microRNA-29b (miR-29b) mimics was fabricated, which possessed anti-fibrotic activity and prevented excessive cardiac fibrosis after MI.136 These results show that Exos involved in cardiac repair by activating signal transduction that promotes cell survival, regulating autophagy, reducing cardiac oxidative stress levels, regulating immune cell polarization, and cytokine secretion.79,132,163 Although these experiments have confirmed the therapeutic efficacy of Exos and cargos, the specific application of Exos in humans as a treatment for MI or cardiovascular diseases needs to be further verified. Several studies involving patients with acute coronary artery disease have observed changes in exosomal load; however, the application of exosomal therapy in patients with heart disease has not yet been implemented.164 Consequently, the future efficacy of Exos application in the treatment of cardiovascular diseases remains to be determined.
Conclusions and Prospects
The damage of the heart caused by MI varies depending on the degree of repair, but the permanent loss of CMs and the formation of scar tissue are common features. Fibroblasts are major contributors to scar tissue, and there is increasing evidence that exosomes play an important regulatory role in them. The corresponding pathophysiological changes in both in situ and distal cells lead to variations in the exosome-carrying cargoes they produce, and the intercellular exosome crosstalk with CFs has a dual effect on myocardial remodeling after MI. On the one hand, the proliferation and activation of CFs are inhibited, the expression of fibrosis-related proteins is down-regulated, and the phenotype and secretion of CFs are changed, thus achieving anti-fibrosis. On the contrary, it promotes fibrosis by activation of CFs. In-depth study of exosome cargo and signaling pathways of different cellular origin not only helps to provide molecular mechanisms for complex damage repair after MI, but also guides the delivery of effective exosome cargo through the carrier for targeted therapy of infarcted myocardium. At present, there have been relevant studies, but they have not been used in clinical treatment of heart disease, which will become a new direction of clinical research on exosomes in cardiovascular diseases. Despite this, much is still unknown and more research is needed to fully understand the mechanisms of cell crosstalk in cardiac repair to optimize cell-free therapy.
Acknowledgments
Preparation of this manuscript was supported by the National Natural Science Foundation of China (82270334, 82470323), the Science and Technology Department in Sichuan province of China (2022YFS0607, 2022NSFSC1602, 2023ZYD0093, 2024JDHJ0051), Office of Science and Technology of Luzhou City (2023JYJ004). All figures were drawn by Figdraw.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jinawong K, Apaijai N, Chattipakorn N, et al. Cognitive impairment in myocardial infarction and heart failure. Acta Physiol. 2021;232(1):e13642. doi:10.1111/apha.13642
2. Saito Y, Kobayashi Y. Percutaneous coronary intervention strategies in patients with acute myocardial infarction and multivessel disease: completeness, timing, lesion assessment, and patient status. J Cardiol. 2019;74(2):95–101. doi:10.1016/j.jjcc.2019.04.001
3. Guo QY, Yang JQ, Feng XX, et al. Regeneration of the heart: from molecular mechanisms to clinical therapeutics. Mil Med Res. 2023;10(1):18. doi:10.1186/s40779-023-00452-0
4. Yang P, Luo Q, Wang X, et al. Comprehensive analysis of fibroblast activation protein expression in interstitial lung diseases. Am J Respir Crit Care Med. 2023;207(2):160–172. doi:10.1164/rccm.202110-2414OC
5. Duan L, Xu L, Xu X, et al. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13(3):1387–1397. doi:10.1039/d0nr07622h
6. Han QF, Li WJ, Hu KS, et al. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol Cancer. 2022;21(1):207. doi:10.1186/s12943-022-01671-0
7. Sun X, Song W, Teng L, et al. MiRNA 24-3p-rich exosomes functionalized DEGMA-modified hyaluronic acid hydrogels for corneal epithelial healing. Bioact Mater. 2022;25:640–656. doi:10.1016/j.bioactmat.2022.07.011
8. Tavasolian F, Lively S, Pastrello C, et al. Proteomic and genomic profiling of plasma exosomes from patients with ankylosing spondylitis. Ann Rheum Dis. 2023;82(11):1429–1443. doi:10.1136/ard-2022-223791
9. Yanatori I, Richardson DR, Dhekne HS, et al. CD63 is regulated by iron via the IRE-IRP system and is important for ferritin secretion by extracellular vesicles. Blood. 2021;138(16):1490–1503. doi:10.1182/blood.2021010995
10. Vaidya M, Smith J, Field M, et al. Analysis of regulatory sequences in exosomal DNA of NANOGP8. PLoS One. 2023;18(1):e0280959. doi:10.1371/journal.pone.0280959
11. Gandham S, Su X, Wood J, et al. Technologies and standardization in research on extracellular vesicles. Trends Biotechnol. 2020;38(10):1066–1098. doi:10.1016/j.tibtech.2020.05.012
12. Femminò S, Penna C, Margarita S, et al. Extracellular vesicles and cardiovascular system: biomarkers and cardioprotective effectors. Vasc Pharmacol. 2020:135106790. doi:10.1016/j.vph.2020.106790
13. Yuan Y, Mei Z, Qu Z, et al. Exosomes secreted from cardiomyocytes suppress the sensitivity of tumor ferroptosis in ischemic heart failure. Signal Transduct Target Ther. 2023;8(1):121. doi:10.1038/s41392-023-01336-4
14. Li H, Wang L, Ma T, et al. Exosomes secreted by endothelial cells derived from human induced pluripotent stem cells improve recovery from myocardial infarction in mice. Stem Cell Res Ther. 2023;14(1):278. doi:10.1186/s13287-023-03462-w
15. Xiong YY, Gong ZT, Tang RJ, et al. The pivotal roles of exosomes derived from endogenous immune cells and exogenous stem cells in myocardial repair after acute myocardial infarction. Theranostics. 2021;11(3):1046–1058. doi:10.7150/thno.53326
16. Gao L, Qiu F, Cao H, et al. Therapeutic delivery of microRNA-125a-5p oligonucleotides improves recovery from myocardial ischemia/reperfusion injury in mice and swine. Theranostics. 2023;13(2):685–703. doi:10.7150/thno.73568
17. Fan J, Ren M, He Y. Diagnostic and therapeutic properties of exosomes in cardiac fibrosis. Front Cell Dev Biol. 2022;10:931082. doi:10.3389/fcell.2022.931082
18. Tang X, Leng M, Tang W, et al. The roles of exosome-derived microRNAs in cardiac fibrosis. Molecules. 2024;29(6):1199. doi:10.3390/molecules29061199
19. Liu Y, Lyu X, Tan S, et al. Research progress of exosomal non-coding RNAs in cardiac remodeling. Int J Med Sci. 2023;20(11):1469–1478. doi:10.7150/ijms.83808
20. Frantz S, Hundertmark MJ, Schulz-Menger J, et al. Left ventricular remodelling post-myocardial infarction: pathophysiology, imaging, and novel therapies. Eur Heart J. 2022;43(27):2549–2561. doi:10.1093/eurheartj/ehac223
21. Zhang Q, Chen L, Huang L, et al. CD44 promotes angiogenesis in myocardial infarction through regulating plasma exosome uptake and further enhancing FGFR2 signaling transduction. Mol Med. 2022;28(1):145. doi:10.1186/s10020-022-00575-5
22. Zhang Q, Wang L, Wang S, et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct Target Ther. 2022;7(1):78. doi:10.1038/s41392-022-00925-z
23. Zhang Y, Wen W, Liu H. The role of immune cells in cardiac remodeling after myocardial infarction. J Cardiovasc Pharm. 2020;76(4):407–413. doi:10.1097/FJC.0000000000000876
24. Sun Y. Oxidative stress and cardiac repair/remodeling following infarction. Am J Med Sci. 2007;334(3):197–205. doi:10.1097/MAJ.0b013e318157388f
25. Liu Y, Xu J, Wu M, et al. The effector cells and cellular mediators of immune system involved in cardiac inflammation and fibrosis after myocardial infarction. J Cell Physiol. 2020;235(12):8996–9004. doi:10.1002/jcp.29732
26. Fan D, Takawale A, Lee J, et al. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5(1):15. doi:10.1186/1755-1536-5-15
27. Frangogiannis NG. Pathophysiology of myocardial infarction. Compr Physiol. 2015;5. doi:10.1002/cphy.c150006
28. Guo Y, Gupte M, Umbarkar P, et al. Entanglement of GSK-3β, β-catenin and TGF-β1 signaling network to regulate myocardial fibrosis. J Mol Cell Cardiol. 2017;110:109–120. doi:10.1016/j.yjmcc.2017.07.011
29. Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res. 2000;46(2):214–224. doi:10.1016/s0008-6363(00)00003-1
30. Vellaichamy E, Khurana ML, Fink J, et al. Involvement of the NF-kappa B/matrix metalloproteinase pathway in cardiac fibrosis of mice lacking guanylyl cyclase/natriuretic peptide receptor A. J Biol Chem. 2005;280(19):19230–19242. doi:10.1074/jbc.M411373200
31. Burke RM, Burgos Villar KN, Small EM. Fibroblast contributions to ischemic cardiac remodeling. Cell Signal. 2020;77:109824. doi:10.1016/j.cellsig.2020.109824
32. Small EM. The actin-MRTF-SRF gene regulatory axis and myofibroblast differentiation. J Cardiovasc Transl Res. 2012;5(6):794–804. doi:10.1007/s12265-012-9397-0
33. Ushakov A, Ivanchenko V, Gagarina A. Regulation of myocardial extracellular matrix dynamic changes in myocardial infarction and postinfarct remodeling. Curr Cardiol Rev. 2020;16(1):11–24. doi:10.2174/1573403X15666190509090832
34. Talman V, Ruskoaho H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016;365(3):563–581. doi:10.1007/s00441-016-2431-9
35. Nattel S, Maguy A, Le Bouter S, et al. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87(2):425–456. doi:10.1152/physrev.00014.2006
36. Yang D, Zhang W, Zhang H, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684–3707. doi:10.7150/thno.41580
37. Malekian F, Shamsian A, Kodam SP, et al. Exosome engineering for efficient and targeted drug delivery: current status and future perspective. J Physiol. 2023;601(22):4853–4872. doi:10.1113/JP282799
38. Wei H, Chen Q, Lin L, et al. Regulation of exosome production and cargo sorting. Int J Biol Sci. 2021;17(1):163–177. doi:10.7150/ijbs.53671
39. Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30(17):3481–3500. doi:10.1038/emboj.2011.286
40. Lai JJ, Chau ZL, Chen SY, et al. Exosome processing and characterization approaches for research and technology development. Adv Sci. 2022;9(15):e2103222. doi:10.1002/advs.202103222
41. Colombo M, Moita C, van Niel G, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–5565. doi:10.1242/jcs.128868
42. Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi:10.1126/science.1153124
43. Blanc L, Vidal M. New insights into the function of rab GTPases in the context of exosomal secretion. Small GTPases. 2017;9(1–2):95–106. doi:10.1080/21541248.2016.1264352
44. Zhu L, Sun HT, Wang S, et al. Isolation and characterization of exosomes for cancer research. J Hematol Oncol. 2020;13(1):152. doi:10.1186/s13045-020-00987-y
45. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Bio. 2018;19(4):213–228. doi:10.1038/nrm.2017.125
46. Zhou Z, Yang Z, Zhou L, et al. The versatile roles of testrapanins in cancer from intracellular signaling to cell-cell communication: cell membrane proteins without ligands. Cell Biosci. 2023;13(1):59. doi:10.1186/s13578-023-00995-8
47. Clayton A, Turkes A, Navabi H, et al. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118(Pt 16):3631–3638. doi:10.1242/jcs.02494
48. Ferreira JV, da Rosa Soares A, Ramalho J, et al. LAMP2A regulates the loading of proteins into exosomes. Sci Adv. 2022;8(12):eabm1140. doi:10.1126/sciadv.abm1140
49. Yan C, Tian X, Li J, et al. A high-fat diet attenuates AMPK α1 in adipocytes to induce exosome shedding and nonalcoholic fatty liver development in vivo. Diabetes. 2020;70(2):577–588. doi:10.2337/db20-0146
50. Cardeñes B, Clares I, Toribio V, et al. Cellular integrin α5β1 and exosomal ADAM17 mediate the binding and uptake of exosomes produced by colorectal carcinoma cells. Int J Mol Sci. 2021;22(18). doi:10.3390/ijms22189938
51. Zhang M, Xie Y, Li S, et al. Proteomics analysis of exosomes from patients with active tuberculosis reveals infection profiles and potential biomarkers. Front Microbiol. 2022;12:800807. doi:10.3389/fmicb.2021.800807
52. Mathieu M, Martin-Jaular L, Lavieu G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi:10.1038/s41556-018-0250-9
53. Tong X, Dang X, Liu D, et al. Exosome-derived circ_0001785 delays atherogenesis through the ceRNA network mechanism of miR-513a-5p/TGFBR3. J Nanobiotechnology. 2023;21(1):362. doi:10.1186/s12951-023-02076-x
54. McKelvey K, Powell K, Ashton A, et al. Exosomes: mechanisms of uptake. J Circ Biomark. 2015;4(1). doi:10.33393/jcb.2015.2057
55. Yuan D, Zhao Y, Banks WA, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. doi:10.1016/j.biomaterials.2017.07.011
56. Wang C, Li Z, Liu Y, et al. Exosomes in atherosclerosis: performers, bystanders, biomarkers, and therapeutic targets. Theranostics. 2021;11(8):3996–4010. doi:10.7150/thno.56035
57. Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. doi:10.3402/jev.v3.24641
58. Thongboonkerd V. Roles for exosome in various kidney diseases and disorders. Front Pharmacol. 2020;10:1655. doi:10.3389/fphar.2019.01655
59. Yu W, Hurley J, Roberts D, et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32(4):466–477. doi:10.1016/j.annonc.2021.01.074
60. Ma X, Liu B, Fan L, et al. Native and engineered exosomes for inflammatory disease. Nano Res. 2022;16(5):6991–7006. doi:10.1007/s12274-022-5275-5
61. Pulliam L, Sun B, Mustapic M, et al. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J Neurovirol. 2019;25(5):702–709. doi:10.1007/s13365-018-0695-4
62. Patil M, Henderson J, Luong H, et al. The art of intercellular wireless communications: exosomes in heart disease and therapy. Front Cell Dev Biol. 2019;7:315. doi:10.3389/fcell.2019.00315
63. Daseke MJ, Tenkorang MAA, Chalise U, et al. Cardiac fibroblast activation during myocardial infarction wound healing: fibroblast polarization after MI. Matrix Biol. 2020:91–92109–116. doi:10.1016/j.matbio.2020.03.010
64. Hohn J, Tan W, Carver A, et al. Roles of exosomes in cardiac fibroblast activation and fibrosis. Cells. 2021;10(11). doi:10.3390/cells10112933
65. Karbassi E, Fenix A, Marchiano S, et al. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat Rev Cardiol. 2020;17(6):341–359. doi:10.1038/s41569-019-0331-x
66. Kuo HF, Hsieh CC, Wang SC, et al. Simvastatin attenuates cardiac fibrosis via regulation of cardiomyocyte-derived exosome secretion. J Clin Med. 2019;8(6). doi:10.3390/jcm8060794
67. Liao Z, Chen Y, Duan C, et al. Cardiac telocytes inhibit cardiac microvascular endothelial cell apoptosis through exosomal miRNA-21-5p-targeted cdip1 silencing to improve angiogenesis following myocardial infarction. Theranostics. 2021;11(1):268–291. doi:10.7150/thno.47021
68. Morelli MB, Shu J. Sardu C, et al.Cardiosomal microRNAs are essential in post-infarction myofibroblast phenoconversion. Int J Mol Sci. 2019;21(1). doi:10.3390/ijms21010201
69. Ribeiro-Rodrigues TM, Laundos TL, Pereira-Carvalho R, et al. Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovasc Res. 2017;113(11):1338–1350. doi:10.1093/cvr/cvx118
70. Wang X, Morelli MB, Matarese A, et al. Cardiomyocyte-derived exosomal microRNA-92a mediates post-ischemic myofibroblast activation both in vitro and ex vivo. ESC Heart Fail. 2020;7(1):284–288. doi:10.1002/ehf2.12584
71. Nie X, Fan J, Li H, et al. MiR-217 promotes cardiac hypertrophy and dysfunction by targeting PTEN. Mol Ther Nucleic Acids. 2018;12:254–266. doi:10.1016/j.omtn.2018.05.013
72. Gou L, Xue C, Tang X, et al. Inhibition of Exo-miR-19a-3p derived from cardiomyocytes promotes angiogenesis and improves heart function in mice with myocardial infarction via targeting HIF-1α. Aging. 2020;12(23):23609–23618. doi:10.18632/aging.103563
73. Wang L, Zhang J. Exosomal lncRNA AK139128 derived from hypoxic cardiomyocytes promotes apoptosis and inhibits cell proliferation in cardiac fibroblasts. Int J Nanomed. 2020;15:3363–3376. doi:10.2147/IJN.S240660
74. Pinto AR, Ilinykh A, Ivey MJ, et al. Revisiting cardiac cellular composition. Circ Res. 2015;118(3):400–409. doi:10.1161/CIRCRESAHA.115.307778
75. Sun Y, Ma M, Cao D, et al. Inhibition of fap promotes cardiac repair by stabilizing BNP. Circ Res. 2023;132(5):586–600. doi:10.1161/CIRCRESAHA.122.320781
76. Liu M, López de Juan Abad B, Cheng K. Cardiac fibrosis: myofibroblast-mediated pathological regulation and drug delivery strategies. Adv Drug Deliv Rev. 2021;173504. doi:10.1016/j.addr.2021.03.021
77. Ma Y, Cao Y, Gao H, et al. Sevoflurane improves ventricular conduction by exosomes derived from rat cardiac fibroblasts after hypothermic global ischemia-reperfusion injury. Drug Des Devel Ther. 2023;17:1719–1732. doi:10.2147/DDDT.S408595
78. Luo H, Li X, Li T, et al. MicroRNA-423-3p exosomes derived from cardiac fibroblasts mediates the cardioprotective effects of ischaemic post-conditioning. Cardiovasc Res. 2019;115(7):1189–1204. doi:10.1093/cvr/cvy231
79. Liu N, Xie L, Xiao P, et al. Cardiac fibroblasts secrete exosome microRNA to suppress cardiomyocyte pyroptosis in myocardial ischemia/reperfusion injury. Mol Cell Biochem. 2022;477(4):1249–1260. doi:10.1007/s11010-021-04343-7
80. Yaping XU, Guotian Y, Dandan J, et al. Fibroblast-derived exosomal miRNA-133 promotes cardiomyocyte-like differentiation. Acta Histochem. 2022;124(6):151931. doi:10.1016/j.acthis.2022.151931
81. Ranjan P, Kumari R, Goswami SK, et al. Myofibroblast-derived exosome induce cardiac endothelial cell dysfunction. Front Cardiovasc Med. 2021;8:676267. doi:10.3389/fcvm.2021.676267
82. Liu X, Xu Y, Deng Y, et al. MicroRNA-223 regulates cardiac fibrosis after myocardial infarction by targeting RASA1. Cell Physiol Biochem. 2018;46(4):1439–1454. doi:10.1159/000489185
83. Bang C, Batkai S, Dangwal S, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124(5):2136–2146. doi:10.1172/JCI70577
84. Mathiesen A, Hamilton T, Carter N, et al. Endothelial extracellular vesicles: from keepers of health to messengers of disease. Int J Mol Sci. 2021;22(9). doi:10.3390/ijms22094640
85. Carter N, Mathiesen AH, Miller N, et al. Endothelial cell-derived extracellular vesicles impair the angiogenic response of coronary artery endothelial cells. Front Cardiovasc Med. 2022;9:923081. doi:10.3389/fcvm.2022.923081
86. Akbar N, Braithwaite AT, Corr EM, et al. Rapid neutrophil mobilization by VCAM-1+ endothelial cell-derived extracellular vesicles. Cardiovasc Res. 2023;119(1):236–251. doi:10.1093/cvr/cvac012
87. Sen S, McDonald SP, Coates PT, et al. Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clin Sci. 2011;120(7):263–283. doi:10.1042/CS20100429
88. Ke X, Yang D, Liang J, et al. Human endothelial progenitor cell-derived exosomes increase proliferation and angiogenesis in cardiac fibroblasts by promoting the mesenchymal-endothelial transition and reducing high mobility group Box 1 protein B1 expression. DNA Cell Biol. 2017;36(11):1018–1028. doi:10.1089/dna.2017.3836
89. Ke X, Yang R, Wu F, et al. Exosomal miR-218-5p/miR-363-3p from endothelial progenitor cells ameliorate myocardial infarction by targeting the p53/JMY signaling pathway. Oxid Med Cell Longev. 2021;2021:5529430. doi:10.1155/2021/5529430
90. Huang Y, Chen L, Feng Z, et al. EPC-Derived exosomal miR-1246 and miR-1290 regulate phenotypic changes of fibroblasts to endothelial cells to exert protective effects on myocardial infarction by targeting ELF5 and SP1. Front Cell Dev Biol. 2021;9:647763. doi:10.3389/fcell.2021.647763
91. Lin F, Zeng Z, Song Y, et al. YBX-1 mediated sorting of miR-133 into hypoxia/reoxygenation-induced EPC-derived exosomes to increase fibroblast angiogenesis and MEndoT. Stem Cell Res Ther. 2019;10(1):263. doi:10.1186/s13287-019-1377-8
92. Chalise U, Becirovic-Agic M, Lindsey ML. The cardiac wound healing response to myocardial infarction. WIREs Mech Dis. 2022;15(1):e1584. doi:10.1002/wsbm.1584
93. Farache Trajano L, Smart N. Immunomodulation for optimal cardiac regeneration: insights from comparative analyses. NPJ Regen Med. 2021;6(1):8. doi:10.1038/s41536-021-00118-2
94. Vermeren S, Elks PM, Ellett F. Editorial: neutrophil functions in host immunity, inflammation and tissue repair. Front Immunol. 2021;12:810346. doi:10.3389/fimmu.2021.810346
95. Li T, Yan Z, Fan Y, et al. Cardiac repair after myocardial infarction: a two-sided role of inflammation-mediated. Front Cardiovasc Med. 2023;9:1077290. doi:10.3389/fcvm.2022.1077290
96. Yap J, Cabrera-Fuentes HA, Irei J, et al. Role of macrophages in cardioprotection. Int J Mol Sci. 2019;20(10). doi:10.3390/ijms20102474
97. Wen H, Peng L, Chen Y. The effect of immune cell-derived exosomes in the cardiac tissue repair after myocardial infarction: molecular mechanisms and pre-clinical evidence. J Cell Mol Med. 2021;25(14):6500–6510. doi:10.1111/jcmm.16686
98. Liu S, Chen J, Shi J, et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res Cardiol. 2020;115(2):22. doi:10.1007/s00395-020-0781-7
99. Chen J, Zhou R, Liang Y, et al. Blockade of lncRNA-ASLNCS5088-enriched exosome generation in M2 macrophages by GW4869 dampens the effect of M2 macrophages on orchestrating fibroblast activation. FASEB J. 2019;33(11):12200–12212. doi:10.1096/fj.201901610
100. Govindappa PK, Patil M, Garikipati VNS, et al. Targeting exosome-associated human antigen R attenuates fibrosis and inflammation in diabetic heart. FASEB J. 2020;34(2):2238–2251. doi:10.1096/fj.201901995R
101. Long R, Gao L, Li Y, et al. M2 macrophage-derived exosomes carry miR-1271-5p to alleviate cardiac injury in acute myocardial infarction through down-regulating SOX6. Mol Immunol. 2021:13626. doi:10.1016/j.molimm.2021.05.006
102. Wang Y, Li C, Zhao R, et al. CircUbe3a from M2 macrophage-derived small extracellular vesicles mediates myocardial fibrosis after acute myocardial infarction. Theranostics. 2021;11(13):6315–6333. doi:10.7150/thno.52843
103. Zhao X, Wang J, He J, et al. Effects of activated CD4+ T cell-derived exosomes on cardiac remodeling after myocardial infarction. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33(11):1332–1336. doi:10.3760/cma.j.cn121430-20210709-01038
104. Cai L, Chao G, Li W, et al. Activated CD4+ T cells-derived exosomal miR-142-3p boosts post-ischemic ventricular remodeling by activating myofibroblast. Aging. 2020;12(8):7380–7396. doi:10.18632/aging.103084
105. Kita S, Maeda N, Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J Clin Invest. 2019;129(10):4041–4049. doi:10.1172/JCI129193
106. Mei R, Qin W, Zheng Y, et al. Role of adipose tissue derived exosomes in metabolic disease. Front Endocrinol. 2022;13:873865. doi:10.3389/fendo.2022.873865
107. Crewe C, Funcke JB, Li S, et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 2021;33(9):1853–1868.e11. doi:10.1016/j.cmet.2021.08.002
108. Lin JR, Ding LL, Xu L, et al. Brown adipocyte ADRB3 mediates cardioprotection via suppressing exosomal iNOS. Circ Res. 2022;131(2):133–147. doi:10.1161/CIRCRESAHA.121.320470
109. He L, Zhu C, Jia J, et al. ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/β-catenin pathway. Biosci Rep. 2020;40(5). doi:10.1042/BSR20192549
110. Zhang W, Bai X, Zhao B, et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res. 2018;370(2):333–342. doi:10.1016/j.yexcr.2018.06.035
111. Liu J, Li F, Liu B, et al. Adipose-derived mesenchymal stem cell exosomes inhibit transforming growth factor-β1-induced collagen synthesis in oral mucosal fibroblasts. Exp Ther Med. 2021;22(6):1419. doi:10.3892/etm.2021.10854
112. De almeida oliveira NC, Neri EA, Silva CM, et al. Multicellular regulation of miR-196a-5p and miR-425-5 from adipose stem cell-derived exosomes and cardiac repair. CLIN SCI. 2022;136(17):1281–1301. doi:10.1042/CS20220216
113. Wang X, Zhu Y, Wu C, et al. Adipose-derived mesenchymal stem cells-derived exosomes carry microRNA-671 to alleviate myocardial infarction through inactivating the TGFBR2/Smad2 axis. Inflammation. 2021;44(5):1815–1830. doi:10.1007/s10753-021-01460-9
114. Luo Q, Guo D, Liu G, et al. Exosomes from MiR-126-overexpressing adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem. 2017;44(6):2105–2116. doi:10.1159/000485949
115. Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83(1):1–14. doi:10.1161/01.res.83.1.1
116. Marbán E, Liao K. On the cellular origin of cardiosphere-derived cells (CDCs). Basic Res Cardiol. 2022;117(1):12. doi:10.1007/s00395-022-00914-x
117. Amini H, Rezaie J, Vosoughi A, et al. Cardiac progenitor cells application in cardiovascular disease. J Cardiovasc Thorac. 2017;9(3):127–132. doi:10.15171/jcvtr.2017.22
118. Wang S, Chen W, Ma L, et al. Infant cardiosphere-derived cells exhibit non-durable heart protection in dilated cardiomyopathy rats. Cytotechnology. 2019;71(6):1043–1052. doi:10.1007/s10616-019-00328-z
119. Hensley MT, de Andrade J, Keene B, et al. Cardiac regenerative potential of cardiosphere-derived cells from adult dog hearts. J Cell Mol Med. 2015;19(8):1805–1813. doi:10.1111/jcmm.12585
120. Dawkins JF, Ehdaie A, Rogers R, et al. Biological substrate modification suppresses ventricular arrhythmias in a porcine model of chronic ischaemic cardiomyopathy. Eur Heart J. 2022;43(22):2139–2156. doi:10.1093/eurheartj/ehac042
121. Gallet R, Dawkins J, Valle J, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38(3):201–211. doi:10.1093/eurheartj/ehw240
122. Lang JK, Young RF, Ashraf H, et al. Inhibiting extracellular vesicle release from human cardiosphere derived cells with lentiviral knockdown of nsmase2 differentially effects proliferation and apoptosis in cardiomyocytes, fibroblasts and endothelial cells in vitro. PLoS One. 2016;11(11):e0165926. doi:10.1371/journal.pone.0165926
123. Gray WD, French KM, Ghosh-Choudhary S, et al. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2014;116(2):255–263. doi:10.1161/CIRCRESAHA.116.304360
124. Tseliou E, Fouad J, Reich H, et al. Fibroblasts rendered antifibrotic, antiapoptotic, and angiogenic by priming with cardiosphere-derived extracellular membrane vesicles. J Am Coll Cardiol. 2015;66(6):599–611. doi:10.1016/j.jacc.2015.05.068
125. Hirai K, Ousaka D, Fukushima Y, et al. Cardiosphere-derived exosomal microRNAs for myocardial repair in pediatric dilated cardiomyopathy. Sci Transl Med. 2020;12(573). doi:10.1126/scitranslmed.abb3336
126. Tseliou E, Reich H, de Couto G, et al. Cardiospheres reverse adverse remodeling in chronic rat myocardial infarction: roles of soluble endoglin and Tgf-β signaling. Basic Res Cardiol. 2014;109(6):443. doi:10.1007/s00395-014-0443-8
127. Vandergriff A, Huang K, Shen D, et al. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics. 2018;8(7):1869–1878. doi:10.7150/thno.20524
128. Przybyt E, Harmsen MC. Mesenchymal stem cells: promising for myocardial regeneration? Curr Stem Cell Res T. 2013;8(4):270–277. doi:10.2174/1574888x11308040002
129. Li L, Chen X, Wang WE, et al. How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cells Int. 2015;2016:9682757. doi:10.1155/2016/9682757
130. Wang X, Bai L, Liu X, et al. Cardiac microvascular functions improved by MSC-derived exosomes attenuate cardiac fibrosis after ischemia-reperfusion via PDGFR-β modulation. Int J Cardiol. 2021;344:13–24. doi:10.1016/j.ijcard.2021.09.017
131. Xu R, Zhang F, Chai R, et al. Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. J Cell Mol Med. 2019;23(11):7617–7631. doi:10.1111/jcmm.14635
132. Shao L, Zhang Y, Lan B, et al. MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. Biomed Res Int. 2017;2017:4150705. doi:10.1155/2017/4150705
133. Zheng J, Zhang X, Cai W, et al. Bone marrow mesenchymal stem cell-derived exosomal microRNA-29b-3p promotes angiogenesis and ventricular remodeling in rats with myocardial infarction by targeting ADAMTS16. Cardiovasc Toxicol. 2022;22(8):689–700. doi:10.1007/s12012-022-09745-7
134. Wu Y, Peng W, Fang M, et al. MSCs-derived extracellular vesicles carrying miR-212-5p alleviate myocardial infarction-induced cardiac fibrosis via NLRC5/VEGF/TGF-β1/SMAD axis. J Cardiovasc Transl. 2021;15(2):302–316. doi:10.1007/s12265-021-10156-2
135. Wang S, Dong J, Li L, et al. Exosomes derived from miR-129-5p modified bone marrow mesenchymal stem cells represses ventricular remolding of mice with myocardial infarction. J Tissue Eng Regen M. 2021;16(2):177–187. doi:10.1002/term.3268
136. Yuan J, Yang H, Liu C, et al. Microneedle patch loaded with exosomes containing microRNA-29b prevents cardiac fibrosis after myocardial infarction. Adv Healthc Mater. 2023;12(13):e2202959. doi:10.1002/adhm.202202959
137. Ni J, Liu X, Yin Y, et al. Exosomes derived from TIMP2-modified human umbilical cord mesenchymal stem cells enhance the repair effect in rat model with myocardial infarction possibly by the Akt/Sfrp2 pathway. Oxid Med Cell Longev. 2019;2019:1958941. doi:10.1155/2019/1958941
138. Wang XL, Zhao YY, Sun L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells improve myocardial repair via upregulation of Smad7. Int J Mol Med. 2018;41(5):3063–3072. doi:10.3892/ijmm.2018.3496
139. Shi Y, Yang Y, Guo Q, et al. Exosomes derived from human umbilical cord mesenchymal stem cells promote fibroblast-to-myofibroblast differentiation in inflammatory environments and benefit cardioprotective effects. Stem Cells Dev. 2019;28(12):799–811. doi:10.1089/scd.2018.0242
140. Parameswaran S, Balasubramanian S, Rao MS, et al. Concise review: non-cell autonomous reprogramming: a nucleic acid-free approach to induction of pluripotency. Stem Cells. 2011;29(7):1013–1020. doi:10.1002/stem.655
141. Barad L, Schick R, Zeevi-Levin N, et al. Human embryonic stem cells vs human induced pluripotent stem cells for cardiac repair. Can J Cardiol. 2014;30(11):1279–1287. doi:10.1016/j.cjca.2014.06.023
142. Osada H, Kawatou M, Fujita D, et al. Therapeutic potential of clinical-grade human induced pluripotent stem cell-derived cardiac tissues. JTCVS Open. 2021;8:359–374. doi:10.1016/j.xjon.2021.09.038
143. Saito S, Hiemori K, Kiyoi K, et al. Glycome analysis of extracellular vesicles derived from human induced pluripotent stem cells using lectin microarray. Sci Rep. 2018;8(1):3997. doi:10.1038/s41598-018-22450-2
144. Bi Y, Qiao X, Liu Q, et al. Systemic proteomics and miRNA profile analysis of exosomes derived from human pluripotent stem cells. Stem Cell Res Ther. 2022;13(1):449. doi:10.1186/s13287-022-03142-1
145. Ye L, Chang YH, Xiong Q, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15(6):750–761. doi:10.1016/j.stem.2014.11.009
146. Santoso MR, Ikeda G, Tada Y, et al. Exosomes from induced pluripotent stem cell-derived cardiomyocytes promote autophagy for myocardial repair. J Am Heart Assoc. 2020;9(6):e014345. doi:10.1161/JAHA.119.014345
147. Ye M, Ni Q, Qi H, et al. Exosomes derived from human induced pluripotent stem cells-endothelia cells promotes postnatal angiogenesis in mice bearing ischemic limbs. Int J Biol Sci. 2019;15(1):158–168. doi:10.7150/ijbs.28392
148. Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. doi:10.1186/s12967-015-0417-0
149. Schena GJ, Murray EK, Hildebrand AN, et al. Cortical bone stem cell-derived exosomes’ therapeutic effect on myocardial ischemia-reperfusion and cardiac remodeling. Am J Physiol-Heart C. 2021;321(6):H1014–H1029. doi:10.1152/ajpheart.00197.2021
150. Bei Y, Chen T, Banciu DD, et al. Circulating exosomes in cardiovascular diseases. Adv Exp Med Biol. 2017;998:255–269. doi:10.1007/978-981-10-4397-0_17
151. Dykes IM. Exosomes in cardiovascular medicine. Cardiol Ther. 2017;6(2):225–237. doi:10.1007/s40119-017-0091-9
152. Parikh M, Pierce GN. A brief review on the biology and effects of cellular and circulating microRNAs on cardiac remodeling after infarction. Int J Mol Sci. 2021;22(9). doi:10.3390/ijms22094995
153. Yamaguchi T, Izumi Y, Nakamura Y, et al. Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol. 2014:178239. doi:10.1016/j.ijcard.2014.10.144
154. D’Alessandra Y, Devanna P, Limana F, et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J. 2010;31(22):2765–2773. doi:10.1093/eurheartj/ehq167
155. Xiao Y, Zhao J, Tuazon JP, et al. MicroRNA-133a and myocardial infarction. Cell Transplant. 2019;28(7):831–838. doi:10.1177/0963689719843806
156. Kang JY, Park H, Kim H, et al. Human peripheral blood‑derived exosomes for microRNA delivery. Int J Mol Med. 2019;43(6):2319–2328. doi:10.3892/ijmm.2019.4150
157. Wang L, Liu J, Xu B, et al. Reduced exosome miR-425 and miR-744 in the plasma represents the progression of fibrosis and heart failure. Kaohsiung J Med Sci. 2018;34(11):626–633. doi:10.1016/j.kjms.2018.05.008
158. Neves KB, Rios FJ, Sevilla-Montero J, et al. Exosomes and the cardiovascular system: role in cardiovascular health and disease. J Physiol-London. 2022;601(22):4923–4936. doi:10.1113/JP282054
159. Khalyfa A, Gozal D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J Transl Med. 2014;12:162. doi:10.1186/1479-5876-12-162
160. Han C, Yang J, Sun J, et al. Extracellular vesicles in cardiovascular disease: biological functions and therapeutic implications. Pharmacol Ther. 2022;233:108025. doi:10.1016/j.pharmthera.2021.108025
161. Zhang Q, Liu T, Li Y, et al. Gelatin methacryloyl microneedle loaded with 3D-MSC-exosomes for the protection of ischemia-reperfusion. Int J Biol Macromol. 2024;275(Pt 1):133336. doi:10.1016/j.ijbiomac.2024.133336
162. Youn SW, LI Y, Kim YM, et al. Modification of cardiac progenitor cell-derived exosomes by miR-322 provides protection against myocardial infarction through Nox2-dependent angiogenesis. Antioxidants. 2019;8(1). doi:10.3390/antiox8010018
163. Sun XH, Wang X, Zhang Y, et al. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res. 2019;177:23–32. doi:10.1016/j.thromres.2019.02.002
164. Silva-Palacios A, Arroyo-Campuzano M, Flores-García M, et al. Citicoline modifies the expression of specific miRNAs related to cardioprotection in patients with ST-segment elevation myocardial infarction subjected to coronary angioplasty. Pharmaceuticals. 2022;15(8). doi:10.3390/ph15080925
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.