Back to Journals » Infection and Drug Resistance » Volume 18
Explore the Application Value of Metagenomic Next-Generation Sequencing of Bronchoalveolar Lavage Fluid in the Early Diagnosis of Pulmonary Tuberculosis
Authors Lu Y, Zhang C, Wu J, Xu X, Lu A, Huang H, Chen M
Received 8 January 2025
Accepted for publication 7 April 2025
Published 11 April 2025 Volume 2025:18 Pages 1837—1845
DOI https://doi.org/10.2147/IDR.S512047
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Yangni Lu,1,* Chunlan Zhang,1,* Jianlin Wu,1 Xianli Xu,1 Ailian Lu,1 Huiya Huang,2 Maowei Chen1
1Department of Infectious Diseases, Wuming Hospital Affiliated to Guangxi Medical University, Nanning, Guangxi, People’s Republic of China; 2Department of General Medicine, Wuming Hospital Affiliated to Guangxi Medical University, Nanning, Guangxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Maowei Chen, Email [email protected] Jianlin Wu, Email [email protected]
Objective: Compare the diagnostic efficacy of bronchoalveolar lavage fluid (BALF) metagenomic next-generation sequencing (mNGS) with that of four traditional methods in the diagnosis of pulmonary tuberculosis (PTB), and explore the application value of BALF mNGS in the early diagnosis of PTB.
Methods: A retrospective analysis was performed on 102 patients with suspected PTB in Wuming Hospital Affiliated with Guangxi Medical University from January 2021 to August 2024, among which 61 cases were PTB and 41 cases were non - PTB. Diagnostic performance metrics (sensitivity, specificity, positive/negative predictive value [PPV/NPV], accuracy) were calculated for BALF mNGS, sputum TB-DNA, sputum acid-fast bacilli (AFB) smear, BALF AFB smear, and BALF TB-DNA, using clinical diagnosis as the reference standard.
Results: BALF mNGS demonstrated a sensitivity of 75.41% (46/61), specificity of 87.80% (36/41), PPV of 90.20% (46/51), NPV of 70.59% (36/51), and accuracy of 80.39% (82/102). Its accuracy was significantly higher than sputum-based methods (53.03– 58.82%, P < 0.0125) and second only to BALF TB-DNA (84.13%, P > 0.0125). BALF mNGS exhibited superior sensitivity compared to sputum TB-DNA (38.00%), sputum AFB smear (41.67%), and BALF AFB smear (41.50%) (P < 0.0125). While specificity and PPV showed no significant differences among methods, BALF mNGS had a higher NPV than sputum-based assays (53.03– 54.17%) but lower than BALF TB-DNA (82.53%, P < 0.0125). Both BALF mNGS (Kappa = 0.608, P < 0.001) and BALF TB-DNA (Kappa = 0.670, P < 0.001) showed strong concordance with clinical diagnosis.
Conclusion: BALF mNGS demonstrates high sensitivity and accuracy for PTB diagnosis, outperforming conventional sputum-based methods. Although BALF TB-DNA achieved the highest specificity and NPV, BALF mNGS serves as a robust supplementary tool, particularly for early-stage or paucibacillary PTB. Integration of these molecular techniques may optimize diagnostic workflows in high-TB-burden settings.
Keywords: pulmonary tuberculosis, metagenomics next-generation sequencing, bronchoalveolar lavage fluid, diagnostic efficiency
Introduction
Tuberculosis (TB) remains a leading cause of morbidity and mortality worldwide, with an estimated 10.6 million new cases and 1.6 million deaths reported in 2021.1,2 Pulmonary tuberculosis (PTB), accounting for over 80% of TB cases,2 poses unique diagnostic challenges due to its nonspecific early symptoms, prolonged latency, and high transmissibility.3 Early and accurate diagnosis is critical not only for timely treatment initiation but also for interrupting community transmission.
Conventional diagnostic methods for PTB, including sputum acid-fast bacilli (AFB) smear microscopy and Mycobacterium tuberculosis (MTB) culture, face well-documented limitations. While AFB smear microscopy is rapid and cost-effective, its sensitivity is suboptimal (50–60%) and heavily dependent on bacterial load, leading to frequent false-negative results in paucibacillary or early-stage infections.4 Although MTB culture remains the gold standard with high specificity (>99%), its prolonged turnaround time (2–8 weeks) delays treatment decisions.5 These limitations underscore the urgent need for novel diagnostic tools capable of balancing speed, sensitivity, and practicality.5,6
Bronchoalveolar lavage fluid (BALF), enriched with pathogen-derived nucleic acids from lower respiratory tract infections, has emerged as a promising specimen for molecular diagnostics.7,8 Metagenomic next-generation sequencing (mNGS), an unbiased high-throughput sequencing technology, enables simultaneous detection of all microbial genomes within a sample, offering advantages in diagnosing complex or atypical infections. Recent studies have validated its utility in bloodstream infections and meningitis,9,10 bloodstream infections,11 meningitis, and encephalitis.12 However, at present, there are still relatively few studies on the efficacy of mNGS in BALF for the diagnosis of PTB.
To address this gap, we conducted a retrospective comparative study evaluating the diagnostic efficacy of BALF mNGS against four traditional methods (sputum TB-DNA, sputum AFB smear, BALF AFB smear, and BALF TB-DNA) in suspected PTB patients. Our findings aim to clarify whether BALF mNGS can serve as a reliable adjunct or alternative to conventional approaches, particularly in early-stage or paucibacillary PTB cases.
Materials and Methods
Study Design
This study was a retrospective case-control study conducted at Wuming Hospital Affiliated with Guangxi Medical University from January 2021 to March 2024. A total of 102 consecutive patients with suspected pulmonary tuberculosis (PTB), presenting with clinical symptoms such as cough, night sweats, weight loss, and chest CT findings indicative of tuberculosis, were enrolled. Based on the following diagnostic criteria, patients were categorized into the PTB group (61 cases) and the non-PTB control group (41 cases).
Individuals suspected of having tuberculosis (TB) must meet at least one of the following criteria: 1) a history of exposure to TB; 2) manifestations of TB infection, including cough, fever, night sweats, or weight loss, with extrapulmonary TB presenting its own set of symptoms; 3) radiological signs indicative of tuberculosis. The definitive clinical diagnostic criteria for tuberculosis comprise: 1) detection of pathogens, such as through acid-fast staining smears or culture of specimens; 2) imaging results and clinical signs that rule out alternative conditions while confirming histopathological evidence of tuberculosis lesions; 3) a notable reduction or complete resolution of tuberculosis symptoms after three months of anti-tuberculosis treatment.
Patient Recruitment and Data Collection
Patient sociodemographic and clinical data were extracted from electronic medical records, including age, sex, body mass index (BMI), comorbidities (eg, diabetes), and laboratory results. Data anonymization was performed to ensure confidentiality. Ethical approval was obtained from the Institutional Review Board of Wuming Hospital (No. WM-2022(068)), and informed consent was waived due to the retrospective nature of the study.
Sample Collection and Processing
Sputum specimens were collected from patients in a well-ventilated, isolated room to minimize aerosol transmission risks. After overnight fasting and oral rinsing with water, patients were instructed to cough deeply to expectorate lower respiratory tract sputum, avoiding contamination with saliva or food residues. A minimum volume of 2 mL was collected into sterile containers, immediately transported to the laboratory, and stored at 2–8°C for processing within 5 days. Purulent or caseous portions were prioritized for testing; saliva-dominated samples were rejected. For quality control, sputum was liquefied with 4% NaOH (1:1–2 volume ratio), vortexed for 30 seconds, and incubated at room temperature for 15 minutes before further analysis.
BALF was obtained via bronchoscopy under local anesthesia. The bronchoscope was advanced to the target lung segment (typically the lobe with significant radiographic abnormalities), followed by the instillation of 20–50 mL sterile 37°C saline. Fluid was immediately aspirated under 50–100 mmHg negative pressure, achieving a minimum recovery rate of ≥40%. The aspirate was filtered through double-layered sterile gauze, centrifuged (3000 ×g, 15 minutes), and the pellet was either processed immediately or stored at −80°C/liquid nitrogen. Strict aseptic techniques were maintained throughout to prevent contamination by upper respiratory flora.
Laboratory Testing Methods
Acid-Fast Bacilli (AFB) Smear Microscopy: Sputum or BALF pellets were smeared onto frosted glass slides (10×20 mm oval area) and air-dried. Slides were heat-fixed and stained using the Ziehl-Neelsen method: carbol fuchsin was applied, heated to steaming for 5 minutes, rinsed, decolorized with 5% hydrochloric acid-alcohol until colorless, and counterstained with 0.06% methylene blue for 30 seconds. Stained slides were examined under 100× oil immersion microscopy. Acid-fast bacilli appeared as bright red rods against a blue background. Results were graded as 1+ (1–8 bacilli/300 fields), 2+ (1–9 bacilli/100 fields), 3+ (1–9 bacilli/10 fields), or 4+ (≥10 bacilli/1 field).
Mycobacterium tuberculosis DNA Detection (TB-DNA): DNA was extracted from sputum or BALF pellets (200 μL) using a magnetic bead-based kit (eg, QIAamp DNA Mini Kit). PCR amplification targeted MTB-specific sequences (IS6110 or 16S rRNA) with TaqMan probes. Reactions included initial denaturation (95°C, 5 minutes), followed by 45 cycles of denaturation (95°C, 15 seconds) and annealing/extension (60°C, 60 seconds). Results were interpreted using Ct values: ≤38 indicated positivity, >38 negativity. Internal controls (negative/positive) were included to ensure assay validity.
Bronchoalveolar Lavage Fluid Metagenomic Next-Generation Sequencing (BALF mNGS): Total DNA was extracted from BALF pellets via mechanical lysis (glass bead vortexing) and proteinase K digestion. Libraries were prepared by fragmenting DNA (Covaris ultrasonication), end-repairing, adapter ligation, and PCR amplification (Illumina-compatible primers). Sequencing was performed on the Illumina NovaSeq 6000 platform (2×150 bp), generating ≥20 million reads per sample. The bioinformatics analysis included human sequence removal (alignment to GRCh38) and microbial identification (NCBI NT/NR databases). MTB was confirmed if ≥3 unique reads were mapped to MTB-specific regions with ≥1% genome coverage. Strict contamination controls (negative: sterile water; positive: MTB H37Rv) were enforced.
Statistical Analysis
Data analysis was performed using SPSS 23.0 statistical software. Measurement data were expressed as mean ± standard deviation; count data were expressed as rates. The chi-square test was used for inter-group comparisons. For pairwise comparisons of multiple groups of rates, the Bonferroni method was used for correction. The significance level was set as the original level divided by the number of comparisons, and a P < 0.0125 was considered statistically significant. The Kappa test was used to evaluate the consistency between the results of BALF mNGS, sputum TB - DNA, BALF TB-DNA, AFB smear in sputum, AFB search in BALF in the diagnosis of PTB and the clinical diagnosis results. A Kappa value < 0.4 indicated poor consistency, a value between 0.4 and less than 0.75 indicated moderate consistency, and ≥0.75 indicated good consistency. A P < 0.05 was considered statistically significant.
This study adopted a case-control group design. First, the research subjects were divided into the experimental group and the control group according to the diagnosis. The general data of the two groups were compared, and the value of BALF mNGS, BALF TB-DNA, sputum TB-DNA, AFB smear in sputum, and AFB search in BALF in the diagnosis of PTB was calculated and analyzed. Taking the clinical diagnosis result as the gold standard, the value of BALF mNGS, BALF TB-DNA, sputum TB-DNA, AFB smear in sputum, and AFB search in BALF in the diagnosis of PTB was analyzed. Sensitivity = (number of true positive cases / (number of true positive + false negative cases)) × 100%; negative predictive value = (number of true negative cases / (number of false negative + true negative cases)) × 100%; accuracy = ((number of true positive + true negative cases) / total number of cases) × 100%; positive predictive value = (number of true positive cases / (number of true positive + false positive cases)) × 100%; specificity = (number of true negative cases / (number of false positive + true negative cases)) × 100%. The consistency between the diagnosis results of BALF mNGS, BALF TB-DNA, sputum TB - DNA, AFB smear in sputum, and AFB search in BALF in PTB and the clinical diagnosis results were analyzed.
Results
Patient Characteristics
Among the 61 patients in the PTB group, the mean age was 58.9 ± 2.1 years, and males accounted for 67.21%. Among the 41 non-PTB patients, the average age was 55.12 ± 3.00 years old, and males accounted for 68.30%. The body mass index (BMI) in the PTB group was 19.90 ± 0.37 (kg/m²), which was significantly lower than that in the non -PTB group, and the difference was statistically significant (P < 0.05). The proportion of patients with PTB complicated with diabetes was higher than that in the non - PTB group, and the difference was statistically significant (P < 0.05). Among them, the BMI of the PTB patients complicated with diabetes was 21.49 ± 0.84 (kg/m²), while the BMI of the PTB patients without diabetes was 19.24 ± 0.51 (kg/m²)(Table 1).
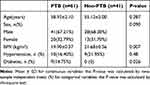 |
Table 1 Comparison of General Conditions of Patients |
Diagnostic Results of Different Detection Methods
When BALF mNGS was utilized for the diagnosis of PTB, among the 61 PTB patients, 46 showed positive test results; among the 41 non - PTB patients, 36 had negative test results (Table 2).
 |
Table 2 Diagnostic Results of BALF mNGS |
In the case of using sputum TB-DNA to diagnose PTB, among 50 PTB patients, 19 had positive test results, and among 36 non - PTB patients, 35 had negative test results (Table 3).
 |
Table 3 Diagnosis Results of Using Sputum TB - DNA |
When a sputum smear for AFB was employed for the diagnosis of PTB, among 60 PTB patients, 25 had positive test results, and among 41 non - PTB patients, 41 had negative test results (Table 4).
 |
Table 4 Diagnostic Results of Sputum Smear for AFB |
When AFB in BALF was adopted for the diagnosis of PTB, among 59 PTB patients, 19 had positive test results, and among 40 non - PTB patients, 39 had negative test results (Table 5).
 |
Table 5 Diagnosis Results of AFB in BALF |
When TB-DNA in BALF was adopted for the diagnosis of PTB, among 27 PTB patients, 20 had positive test results, and among 36 non - PTB patients, 33 had negative test results (Table 6).
 |
Table 6 Diagnosis Results of Using BALF TB - DNA |
Comparison of the Diagnostic Value of BALF mNGS and Other Detection Methods in the Diagnosis of Pulmonary Tuberculosis
This study evaluated the performance metrics of BALF mNGS in the diagnosis of PTB. The results demonstrated that its sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 75.41% (46/61), 87.80% (36/41), 90.20% (46/51), 70.59% (36/51), and 80.39% (82/102), respectively. Compared to other diagnostic methods, the accuracy of BALF mNGS (80.39%) was second only to BALF TB-DNA (84.13%) and significantly higher than other methods (P < 0.0125). In terms of sensitivity, BALF mNGS (75.41%) was significantly higher than sputum TB-DNA (38.00%), sputum smear AFB (41.67%), and BALF smear AFB (41.50%) (P < 0.0125). In terms of specificity and PPV, there was no statistically significant difference between BALF mNGS (87.80%) and the other four diagnostic methods (P > 0.0125). For negative predictive value, BALF mNGS (70.59%) was higher than sputum TB-DNA (53.03%), sputum smear (53.95%), and BALF smear (54.17%) but lower than BALF TB-DNA (82.53%) (P < 0.0125). Furthermore, BALF mNGS (Kappa = 0.608, P < 0.001) and BALF TB-DNA (Kappa = 0.670, P < 0.001) showed good agreement with clinical diagnostic results in PTB diagnosis, indicating that both methods have high reliability in PTB diagnosis (Table 7).
 |
Table 7 Comparison of the Value of Various Examination Methods for Diagnose PTB |
Discussion
This study aimed to evaluate the diagnostic value of BALF mNGS in the early diagnosis of PTB. The results demonstrated that BALF mNGS exhibited significantly higher sensitivity and accuracy in diagnosing PTB compared to sputum TB-DNA, sputum AFB smear, and BALF AFB smear, although no significant difference was observed when compared to BALF TB-DNA. In terms of NPV, BALF mNGS was significantly lower than BALF TB-DNA but still outperformed the other three diagnostic methods. Furthermore, both BALF mNGS (Kappa = 0.608, P < 0.001) and BALF TB-DNA (Kappa = 0.670, P < 0.001) showed good agreement with clinical diagnosis, indicating their high reliability in PTB diagnosis.
China is a country with a high burden of TB globally. According to the WHO Global TB Report 2022, although the epidemic has declined in recent years, China has a large population base. The diagnosis and effective treatment of early-stage TB cases are crucial for TB control.13,14 Delayed diagnosis and treatment of TB cases can spread the disease in the community, increase the severity of the disease, and are associated with a higher risk of death.15,16 Currently, the diagnosis of active PTB is mainly based on a comprehensive assessment of the patient’s clinical symptoms and signs, chest CT examination, immunology, etiology, and other indicators.17,18
With the development of molecular biology techniques, the use of BALF mNGS to detect pathogens in pulmonary infections has gradually become more widely applied and has gradually shown its advantages. This study confirms that the sensitivity (75.41%) and accuracy (80.39%) of BALF mNGS in the diagnosis of PTB are significantly higher than those of sputum TB-DNA (38.00%; 53.03%), AFB smear (41.67%; 58.82%), and BALF AFB detection (41.50%; 54.17%), which was similar to the studies by Zhou’s team and Shi’s team.19,20 Compared to conventional diagnostic approaches, mNGS demonstrates a substantial reduction in the risk of false-negative results, particularly in cases such as PTB where sputum smears yield negative results but cultures are positive.21 This is achieved through the unbiased capture of all nucleic acid sequences within the sample. Furthermore, mNGS effectively mitigates false-positive outcomes attributed to non-specific amplification by leveraging its high-resolution sequencing capabilities. This dual advantage underscores the enhanced diagnostic reliability of mNGS in clinical settings, offering a more robust and accurate approach to pathogen detection. Its high sensitivity is particularly suitable for diagnosing early-stage infections or patients with low bacterial loads, while its high accuracy provides a reliable basis for clinical decision-making, especially in terms of precise medication and epidemic control. This, in turn, helps reduce the misuse of broad-spectrum antibiotics and the risk of drug resistance.22 Furthermore, the multi-pathogen detection capability of mNGS demonstrates its core value in immunocompromised patients or those with complex infections. Although there is no significant difference in sensitivity (75.41% vs 74.07%, P > 0.0125) or accuracy (80.39% vs 84.13%, P > 0.0125) between BALF mNGS and BALF TB-DNA, their technical characteristics and clinical applicability are fundamentally distinct. BALF TB-DNA, based on targeted PCR amplification of Mycobacterium tuberculosis (MTB)-specific genes such as IS6110 or 16S rRNA, offers high specificity (91.67%) and a rapid turnaround time (typically <6 hours), making it the preferred tool for rapid MTB diagnosis in resource-limited settings.23 Additionally, its reliance on live bacterial DNA reduces the risk of interference from environmental mycobacteria, particularly Mycobacterium gordonae. In contrast, BALF mNGS utilizes unbiased whole-genome sequencing, enabling the detection of MTB as well as the simultaneous identification of co-infecting pathogens such as bacteria, fungi, and viruses.24 Its high sensitivity (75.41%) offers significant advantages in patients with poor-quality sputum or extremely low bacterial loads. BALF TB-DNA, however, cannot detect non-target pathogens or novel MTB variants, such as strains lacking IS6110, and only confirms the presence of MTB without providing drug resistance gene information, necessitating additional testing, such as Xpert MTB/RIF. However, the advantage of GeneXpert lies in its rapid detection of the Mycobacterium tuberculosis complex and rifampin resistance genes. Its targeted design, nevertheless, limits its ability to identify mixed infections or atypical strains. Notably, the diagnostic sensitivity of GeneXpert in BALF for PTB ranges from 70% to 80%,25,26 which is comparable to that of BALF mNGS (75.41%). In contrast, BALF mNGS, utilizing whole-genome sequencing technology, enables simultaneous detection of MTB, co-existing pathogens, and drug resistance gene profiles, providing more comprehensive diagnostic and therapeutic information for complex cases. BALF mNGS may generate false-positive signals due to laboratory contamination, such as mycobacterial DNA from reagents or the operating environment, residual DNA from non-viable bacteria, or environmental commensal organisms.19 It relies on high-quality databases and algorithms to distinguish pathogenic bacteria from background microorganisms; otherwise, misinterpretation of results may occur. In summary, mNGS should be prioritized for broad-spectrum screening in cases of suspected mixed infections or immunocompromised patients. For cases where mNGS results are positive but lack sufficient clinical evidence, BALF TB-DNA can serve as a confirmatory tool. Additionally, the drug-resistance gene detection capability of mNGS can be leveraged to guide personalized treatment strategies.
The World Health Organization’s TB Report 2023 estimates that among the newly diagnosed TB patients worldwide in 2022, 2.2 million cases were attributed to malnutrition.3 In this study, the BMI of the PTB group was 19.90 ± 0.37 (kg/m²), which was significantly lower than that of the non - PTB group. Moreover, the BMI of the PTB patients combined with diabetes was 21.49 ± 0.84 (kg/m²), lower than the overweight level. This result was similar to the study by Peng Lu.27 This phenomenon reminds us that when diabetic patients have a BMI level lower than the overweight state, attention should be paid to screening for tuberculosis infection. It also indicates that if PTB patients are not treated on time, they are prone to develop nutrition-related diseases, such as protein-energy malnutrition, drug-induced liver injury, immunodeficiency, and pulmonary infections, thereby increasing the risk of anti-tuberculosis treatment failure.28 Early diagnosis and treatment are of great importance in tuberculosis control.
Studies have found that25,26,29 the sensitivity of sputum smear acid-fast staining ranges from 8% to 55%. In this study, the sensitivity of sputum smear for AFB was 41.67% (25/60), which was also relatively low. This may be caused by multiple factors. For example, the quality of sputum specimens varies. If the patient’s sputum-expectoration method is incorrect or the sputum collection time is inappropriate, it may lead to an insufficient number of MTB in the specimens. Although the sensitivity of sputum smear AFB and BALF AFB tests is relatively low, their widespread clinical application makes such comparisons still meaningful in practice. The findings of this study demonstrate that BALF mNGS can effectively address the limitations of traditional methods, particularly in scenarios where high-quality sputum samples are difficult to obtain or when there is a need to rapidly rule out non-tuberculosis infections.
Compared with other detection methods, BALF mNGS has a relatively good consistency with the clinical diagnosis results in the diagnosis of PTB (Kappa = 0.608, P < 0.001). This further proves the reliability of BALF mNGS in the diagnosis of PTB. Its results are in good agreement with the comprehensive clinical diagnosis results and can provide strong diagnostic evidence for clinicians. However, other detection methods, such as sputum TB - DNA, sputum smear for AFB, and BALF for AFB detection, have relatively poor consistency with the clinical diagnosis results (lower Kappa values), which indicates that these methods have certain limitations in diagnostic accuracy and may not be able to provide accurate enough results during the diagnosis process of PTB. Given this, BALF mNGS is expected to become an important supplementary examination method for the early diagnosis of PTB, providing strong support for the early detection and accurate diagnosis of PTB.
This retrospective analysis in this study shows that although BALF mNGS has a relatively high diagnostic value in the early diagnosis of PTB has obvious advantages in terms of accuracy and sensitivity compared with traditional detection methods, and has a good consistency with clinical diagnosis results, this study has the limitation of a relatively small number of included cases. This limitation may affect the representativeness and stability of the research results and needs to be further improved in future research and clinical applications. For example, expanding the sample size for more in-depth research to further verify the value of BALF mNGS in the diagnosis of PTB. Meanwhile, this study did not directly compare the diagnostic performance of BALF mNGS with GeneXpert MTB/RIF. Future research should expand the sample size and integrate molecular testing with drug resistance analysis to further clarify the clinical positioning of different technologies.
Conclusion
The study results show that BALF mNGS demonstrates relatively high accuracy and sensitivity in the early diagnosis of PTB, showing good diagnostic efficacy. Given the limitations of traditional detection methods, BALF mNGS may serve as a valuable adjunct for the early diagnosis of PTB, thereby increasing the early detection rate of PTB. To validate the findings of this study, larger-scale and more meticulously designed prospective studies are needed.
Abbreviations
PTB, Pulmonary tuberculosis; BALF, Bronchoalveolar lavage fluid; mNGS, Metagenomic next-generation sequencing; BMI, Body mass index; TB-DNA, Tuberculosis deoxyribonucleic acid; AFB, Acid - fast bacilli; NTM, Non - tuberculous mycobacteria; NPV, Negative predictive value; PPV, Positive predictive value; MTB, Mycobacterium tuberculosis.
Data Sharing Statement
Please contact the corresponding author for data requests.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the principles of the Declaration of Helsinki. Ethical approval was obtained from the Institutional Review Board of Wuming Hospital Affiliated with Guangxi Medical University (No. WM-2022(068)). The Institutional Review Board confirmed that the data had been anonymized or kept confidential. As this is a low-risk retrospective medical record review study, the Institutional Review Board of Wuming Hospital Affiliated with Guangxi Medical University determined that obtaining consent to participate was not necessary and waived it.
Acknowledgments
We would like to thank all members who contributed to the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by self-raised funds from the Health Commission of Guangxi, China (grant No. Z-A20220775).
Disclosure
All authors declare that they have no competing interests in this work.
References
1. Luies L, du Preez I. The echo of pulmonary tuberculosis: mechanisms of clinical symptoms and other disease-induced systemic complications. Clin Microbiol Rev. 2020;33(4). doi:10.1128/CMR.00036-20
2. Cox H, Nicol MP. Tuberculosis eradication: renewed commitment and global investment required. Lancet Infect Dis. 2018;18(3):228–229. doi:10.1016/S1473-3099(17)30692-8
3. Bagcchi S. WHO’s global tuberculosis report 2022. Lancet Microbe. 2023;4(1):e20. doi:10.1016/S2666-5247(22)00359-7
4. Sossen B, Richards AS, Heinsohn T, et al. The natural history of untreated pulmonary tuberculosis in adults: a systematic review and meta-analysis. Lancet Respir Med. 2023;11(4):367–379. doi:10.1016/S2213-2600(23)00097-8
5. Deshwal H, Avasarala SK, Ghosh S, et al. Forbearance with bronchoscopy: a review of gratuitous indications. Chest. 2019;155(4):834–847. doi:10.1016/j.chest.2018.08.1035
6. Young DB, Perkins MD, Duncan K, et al. Confronting the scientific obstacles to global control of tuberculosis. J Clin Invest. 2008;118(4):1255–1265. doi:10.1172/JCI34614
7. Lee HS, Kee SJ, Shin JH, et al. Xpert MTB/RIF assay as a substitute for smear microscopy in an intermediate-burden setting. Am J Respir Crit Care Med. 2019;199(6):784–794. doi:10.1164/rccm.201804-0654OC
8. Batyrshina YR, Schwartz YS. Modeling of mycobacterium tuberculosis dormancy in bacterial cultures. Tuberculosis. 2019;117:7–17. doi:10.1016/j.tube.2019.05.005
9. Deng W, Xu H, Wu Y, et al. Diagnostic value of bronchoalveolar lavage fluid metagenomic next-generation sequencing in pediatric pneumonia. Front Cell Infect Microbiol. 2022;12:950531. doi:10.3389/fcimb.2022.950531
10. Qin W, Guo T, You T, et al. Metagenomic next generation sequencing of bronchoalveolar lavage fluids for the identification of pathogens in patients with pulmonary infection: a retrospective study. Diagn Microbiol Infect Dis. 2024;110(1):116402. doi:10.1016/j.diagmicrobio.2024.116402
11. Qin C, Zhang S, Zhao Y, et al. Diagnostic value of metagenomic next-generation sequencing in sepsis and bloodstream infection. Front Cell Infect Microbiol. 2023;13(1117987). doi:10.3389/fcimb.2023.1117987
12. Shangguan L, Xue L, Shang J, et al. The application value of metagenomic next-generation sequencing in community-acquired purulent meningitis after antibiotic intervention. Bmc Infect Dis. 2023;23(1):683. doi:10.1186/s12879-023-08672-4
13. Floyd K, Glaziou P, Zumla A, et al. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med. 2018;6(4):299–314. doi:10.1016/S2213-2600(18)30057-2
14. Chakaya J, Khan M, Ntoumi F, et al. Global tuberculosis report 2020 - reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. 2021;1(Suppl 1):S7–s12. doi:10.1016/j.ijid.2021.02.107
15. Lee CH, Wang JY, Lin HC, et al. Treatment delay and fatal outcomes of pulmonary tuberculosis in advanced age: a retrospective nationwide cohort study. Bmc Infect Dis. 2017;17(1):449. doi:10.1186/s12879-017-2554-y
16. Kim SH, Min J, Cho JY, et al. Clinical profiles and outcomes of pulmonary tuberculosis patients with delayed treatment at a tertiary hospital in South Korea. Ann Palliat Med. 2021;10(3):2948–2957. doi:10.21037/apm-20-1521
17. Wetscherek MTA, Sadler TJ, Lee JYJ, et al. Active pulmonary tuberculosis: something old, something new, something borrowed, something blue. Insights Imaging. 2022;13(1):3. doi:10.1186/s13244-021-01138-8
18. Goletti D, Delogu G, Matteelli A, et al. The role of IGRA in the diagnosis of tuberculosis infection, differentiating from active tuberculosis, and decision making for initiating treatment or preventive therapy of tuberculosis infection. Int J Infect Dis. 2022;124(Suppl 1):S12–s19. doi:10.1016/j.ijid.2022.02.047
19. Shi CL, Han P, Tang PJ, et al. Clinical metagenomic sequencing for diagnosis of pulmonary tuberculosis. J Infect. 2020;81(4):567–574. doi:10.1016/j.jinf.2020.08.004
20. Zhou X, Wu H, Ruan Q, et al. Clinical evaluation of diagnosis efficacy of active mycobacterium tuberculosis complex infection via metagenomic next-generation sequencing of direct clinical samples. Front Cell Infect Microbiol. 2019;9:351. doi:10.3389/fcimb.2019.00351
21. Liu X, Chen Y, Ouyang H, et al. Tuberculosis diagnosis by metagenomic next-generation sequencing on bronchoalveolar lavage fluid: a cross-sectional analysis. Int J Infect Dis. 2021;104:50–57. doi:10.1016/j.ijid.2020.12.063
22. Li Y, Jiao M, Liu Y, et al. Application of metagenomic next-generation sequencing in mycobacterium tuberculosis infection. Front Med. 2022;9:802719.
23. Wang HY, Lu JJ, Chang CY, et al. Development of a high sensitivity TaqMan-based PCR assay for the specific detection of mycobacterium tuberculosis complex in both pulmonary and extrapulmonary specimens. Sci Rep. 2019;9(1):113. doi:10.1038/s41598-018-33804-1
24. Zou X, Zhu Y, Qin Y, et al. Value analysis of next-generation sequencing combined with xpert in early precise diagnosis of pulmonary tuberculosis. Diagn Microbiol Infect Dis. 2023;107(1):115921. doi:10.1016/j.diagmicrobio.2023.115921
25. Uddin MKM, Ather MF, Akter S, et al. Diagnostic yield of xpert MTB/RIF assay using bronchoalveolar lavage fluid in detecting mycobacterium tuberculosis among the sputum-scarce suspected pulmonary TB patients. Diagnostics. 2022;12(7):1676. doi:10.3390/diagnostics12071676
26. Zhang H, Li H, Tan M, et al. GeneXpert MTB/RIF combined with conventional methods for tuberculosis in Shanghai Regional medical center: a retrospective diagnostic study. Ann Transl Med. 2022;10(10):575. doi:10.21037/atm-22-1374
27. Lu P, Zhang Y, Liu Q, et al. Association of BMI, diabetes, and risk of tuberculosis: a population-based prospective cohort. Int J Infect Dis. 2021;109:168–173. doi:10.1016/j.ijid.2021.06.053
28. Xu X, Zhu H, Cai L, et al. Malnutrition is associated with an increased risk of death in hospitalized patients with active pulmonary tuberculosis: a propensity score matched retrospective cohort study. Infect Drug Resist. 2022;15:6155–6164. doi:10.2147/IDR.S382587
29. Qiu X, Zheng S, Yang J, et al. Comparing mycobacterium tuberculosis RNA accuracy in various respiratory specimens for the rapid diagnosis of pulmonary tuberculosis. Infect Drug Resist. 2022;15:4195–4202. doi:10.2147/IDR.S374826
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

