Back to Journals » International Journal of Nanomedicine » Volume 19
Exploring and Anticipating the Applications of Organic Room-Temperature Phosphorescent Materials in Biomedicine and Dentistry
Authors Zhang Y, Zhu Y, Deng T, Du Y
Received 10 September 2024
Accepted for publication 28 November 2024
Published 8 December 2024 Volume 2024:19 Pages 13201—13216
DOI https://doi.org/10.2147/IJN.S492759
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sachin Mali
Yao Zhang,* Yeyuhan Zhu,* Tian Deng, Yangge Du
State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Key Laboratory of Oral Biomedicine Ministry of Education, Hubei Key Laboratory of Stomatology, School & Hospital of Stomatology; Medical Research Institute, School of Medicine, Wuhan University, Wuhan, 430071, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tian Deng; Yangge Du, The State Key Laboratory Breeding Base of Basic Science of Stomatology(Hubei MOST) & Key Laboratory of Oral Biomedicine Ministry of Education School & Hospital of Stomatology, Wuhan University, Wuhan, 430079, People’s Republic of China, Email [email protected]; [email protected]
Abstract: As popular materials, organic room-temperature phosphorescent (RTP) materials have been studied and developed in many fields. RTP materials have the characteristics of a high signal-to-noise ratio (SNR) and high reactive oxygen species (ROS) quantum yield, which can achieve clear bioimaging and efficient ability of anti-tumor and antibacterial, and have received extensive attention from researchers for imaging, tumor therapy, and antibacterial treatment. Moreover, owing to their flexible molecular structures and various synthesis systems and methods, it may be possible to design and synthesize materials according to individual physiologic environments of patients in medical applications, making bioimaging more accurate and greatly improving tumor and bacterial killing rates. So they have great development potential in the medical field. On the basis of introducing the mechanism of RTP materials that emit phosphorescence and generate ROS, this review summarizes the medical applications of RTP materials from three aspects—bioimaging, tumor treatment and antibacterial treatment—to provide a basis for their application in the field of stomatology.
Keywords: RTP materials, bioimaging, anti-tumor, antibacterial, ROS
Introduction
Bioimaging, tumor therapy and antimicrobial therapy are three important and correlated areas of medical development. Conventional bioimaging commonly utilizes inorganic materials containing Au,1,2 Ir (III),3 Fe2O34 and other metallic materials as probes, which also has the ability of killing tumors. Although the materials can achieve targeted bioimaging of tumors in the body, they have the shortcomings of low tumor clearance rate and high toxicity,5 which cannot achieve tumor therapy. Therefore, people turn their attention to organic materials, such as diamond,6 graphene,5 carbon dots,7,8 which, due to their low toxicity, have been investigated extensively. However, current studies have shown that these materials have not yet led to the removal of tumors and bacteria, thereby limiting their application. Zhang et al prepared a carbon dot-embedded epitope imprinted polymer (C-MIP), and achieved targeted bioimaging of EGFR-overexpressing tumors in tumor-bearing mice by the specific recognition ability for the epidermal growth factor receptor (EGFR) of the material. However, C-MIP can only possess the capacity as a drug carrier and lacks the ability to eliminate tumor cells. In contrast, RTP materials possess dual bioimaging capabilities for both tumor and bacteria,9 as well as dual therapeutic abilities for anti-tumor and antibacterial treatments.10,11 At the same time, its side effects on the body are comparable to those of other organic materials, and it has a more comprehensive clinical application potential.
RTP materials are a class of materials that can sense, store, analyze, and respond to the surrounding environment or information.12 Room temperature phosphorescent materials emerged in the 20th century. However, due to technical limitations, RTP materials are mainly derived from polymers such as proteins and aromatic compounds.13–15 Therefore, there are problems such as difficult detection of phosphorescence and short emission time,16 which limit application of RTP materials to detection of protein structure and drug concentration by phosphorescence emission.17 With the development of various disciplines such as chemistry and physics, as well as advancements in nanomaterials technology, the synthesis methods of RTP materials have been continuously improved, and the material structure has been continuously optimized and combined with nanotechnology, so that RTP materials have a long emission life and high exciton utilization rate, which has attracted the attention of researchers in the fields of electronic information,18 optics19,20 and medicine.
RTP materials include a variety of materials with different properties, which are mainly divided into four types: inorganic compounds, organic-inorganic hybrid compounds, pure organic crystalline compounds and polymer compounds.21 Inorganic materials and hybrid materials generally contain metals such as silver and chromium, which have the disadvantages of high toxicity, high cost and complex synthesis process;22 polymer materials have the disadvantages of limited types of phosphors and limited topological structure of matrix materials,23 which have great limitations in future application and development. In contrast, RTP materials have great advantages in the application and development of bioimaging, tumor therapy and antibacterial fields due to their low toxicity, diverse structures, especially their high SNR and high ROS yield. In recent years, researchers have successfully achieved efficient room-temperature phosphorescence emission and ROS generation by regulating the structures and composition of RTP materials. Currently, the reported RTP materials have a variety of structures, such as carbazole,24 phenothiazine,25 phenylpyrrole,26 polycyclic aromatic hydrocarbon,27 triphenylamine,28 boron difluoride β-diketone,29 naphthalimide and chromone derivative structures,30–32 which paves the way for enhancing properties and biomedical applications of RTP materials. (Figure 1)
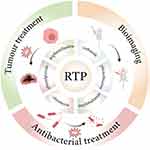 |
Figure 1 The applications of RTP materials and related structures. Notes: Created in BioRender. Zhang, y. (2024) https://BioRender.com/g40o406 |
However, due to the poor stability and poor tissue penetration of RTP materials, there may be some problems in the clinical application of stomatology, such as the unclear imaging of deep oral tumors and low tumor cell clearance rate because of poor tissue penetration and material decomposition which are caused by oral saliva PH, lysozyme content and other factors. Room-temperature phosphorescent (RTP) materials have long emission lifetimes and high exciton utilization and have attracted much attention in the fields of bioimaging,33 tumor treatment and antibacterial treatment. However, RTP materials are still in the stage of clinical research and development, and applications in the diagnosis and treatment of diseases in the field of stomatology are relatively rare. Therefore, this review summarizes the principles and research progress in RTP materials for use in bioimaging, tumor treatment and antibacterial treatment, and discusses their potential and problems regarding diagnosis and treatment in fields related to stomatology.
Mechanism
Unlike traditional fluorescence signals, RTP originates from the slow radiative transition of triplet excitons after excitation is stopped; thus, RTP has the unique characteristics of a longer lifetime and a greater Stokes shift.34 The Jablonski diagram indicates that after RTP molecules absorb light energy, their electrons transition from the ground state (S0) to the singlet excited state (Sn).35,36 Next, the electrons have two main radiative transition paths. One involves vibrational relaxation (VR) or internal conversion (IC) from the singlet excited state (Sn) to the lowest singlet excited state (S1),37–39 followed by the emission of a photon upon the transition to the ground state (S0) and thus the generation of phosphorescence;40,41 the other path involves the intersystem crossing (ISC) transition from S1 to the triplet excited state (Tn) and then a transition from Tn to the lowest triplet excited state (T1) with the emission of a photon, resulting in phosphorescence.42,43
Based on the two phosphorescence paths, there are two mechanisms for generating ROS. In the first type of mechanism, electrons are channelled from the Sn intersystem to T1 to produce intermediates. The released energy is absorbed by O2 to produce superoxide anion radicals, and ROS are produced by a chain reaction. In the second type of mechanism, an electron crosses from the Sn system to T1, and the IC energy is transferred to the ground-state oxygen molecule (triplet oxygen molecule) such that it overcomes the spin-forbidden state and converts into singlet oxygen (1O2).44–46
After ROS are produced by the material, they mainly clear or kill cells and bacteria through penetration. Their ability to kill cells can be attributed to three related mechanisms: direct cytotoxicity to cells, damage to the cellular vascular system, and induction of a strong inflammatory response in the body.47 These three mechanisms eventually cause the body’s immune response to be triggered, resulting in cell death.44 To kill bacteria, ROS mainly rely on strong oxidation to destroy the bacterial cell membrane, increase the permeability of the bacterial cell membrane, and cause outflow of intracellular substances, eventually leading to bacterial inactivation.48–50 Moreover, ROS destroy bacterial nucleotide repair enzymes and genetic materials,42 affect bacterial biosynthesis, and subsequently inhibit and kill bacteria. (Figure 2)
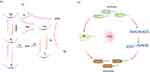 |
Figure 2 Mechanism of bioimaging and generation of ROS. (a). The Jablonski diagram, which is the mechanism of bioimaging. (b).Antibacterial mechanism of ROS. |
Application of RTP Materials in Medicine
Application of RTP Materials in Bioimaging
Currently, conventional bioimaging techniques used in clinical practice include Magnetic Resonance Imaging (MRI), Computed Tomography (CT), and Positron Emission Tomography (scan) (PET).51 MRI is primarily used for soft tissues imaging, but it has poor sensitivity when it comes to examining the lungs and gastrointestinal tract, and the examination process is relatively time-consuming.52 CT offers higher resolution in soft tissue imaging, but there is a certain degree of ionizing radiation, which is harmful to the human body.53 PET is more focused on functional imaging, but it offers relatively low resolution.54 In the field of materials science, the research of bioimaging applications has also made great progress, such as quantum dots,55 metal nanoparticles and so on.56 Quantum dots also have large Stokes shift, which can be used for single molecule imaging, super-resolution imaging and three-dimensional imaging. However, compared with RTP materials, some quantum dots, especially those containing lead and chromium, are toxic to living cells and cause damage to organisms and the environment.57 In addition, the long-life characteristics of RTP materials eliminate the interference of biological autofluorescence which occurs in traditional optical bioimaging, allowing in vivo imaging without real-time external excitation. Therefore, the application of RTP materials in bioimaging can enhance the sensitivity and specificity of in vivo imaging, and provides higher imaging SNR, which results in higher spatial and temporal resolution as well as imaging efficiency. To further meet the needs of in vivo biological imaging, the key to RTP materials research is developing substances with appropriate excitation wavelengths, long lifetimes, and effective red light emission. Gao et al summarized two methods to improve the phosphorescence properties of RTP materials:
(1) Heteroatoms, heavy atoms or carbonyl groups with lone pair electrons are introduced to increase the spin‒orbit coupling (SOC) and promote the ISC process.58
(2) The nonradiative relaxation pathway is suppressed by polymer-assisted methods, host–guest combination, etc., thereby stabilizing triplet excitons in a rigid environment.58
In the past five years, through the unremitting efforts of researchers, remarkable progress has been made in this field. For example, Xu et al successfully developed a series of ultralong organic phosphorescent (UOP)-based photosensitizers (PSs) with long-lived triplet excited states for bacterial imaging and photodynamic therapy (PDT);59 the pseudorotaxane polymer constructed by Zhou et al through host‒guest interactions between cucurbit[n]urils(CBs) and hyaluronic acid (HA)-4-(4-bromophenyl)pyridin-1-ium bromide (BrBP) exhibited an ultralong RTP lifetime and an efficient phosphorescence quantum yield in aqueous solution.60 In addition, many studies have shown that pure organic RTP materials can be used for bioimaging in living mice (Table 1). For example, Xiao et al first obtained organic room-temperature phosphorescent materials with long wavelengths and long lifetimes through a host‒guest doping strategy, which showed good tissue penetration ability for bioimaging and enabled successful realization of tumor imaging in living mice.27
 |
Table 1 The Experimental Progress and Research of RTP Materials in the Field of Bioimaging |
However, in practical biomedical applications, RTP materials still face other problems that need to be solved. First, owing to the relatively short excitation wavelength of RTP materials, deep tissue penetration is still a key challenge. In other words, obtaining high-efficiency phosphorescence under visible light excitation, especially near-infrared (NIR) light excitation, is still a key challenge. Therefore, Zhao et al proposed a practical method for constructing organic RTP materials with near-infrared wavelengths and ultralong lifetimes and successfully prepared several organic RTP materials with near-infrared wavelength emission and significantly long lifetimes.11 In addition, since the microenvironment inside a tumor is highly hypoxic in the organism, tumor cells grow in a disorderly manner. Therefore, achieving accurate RTP hypoxia imaging, which can help effectively locate hypoxic tumors, can make great contributions to the diagnosis and treatment of tumors. For example, Zhang et al synthesized a J-aggregation-induced second near-infrared phosphorescent material based on 5.15-bis(2,6-bis(dodecyloxy)phenyl)-porphyrin platinum(II) (PpyPt), which has good biological hypoxia-sensing potential.61 Therefore, RTP materials are expected to show superior potential in deep intraoral tumor imaging.
Application of RTP Materials in Tumor Therapy
Cancer has become an important disease which threatens people’s life and health. Traditional treatment methods mainly include radiotherapy, chemotherapy, and surgical resection, which may cause great damage to body cells and may not be able to cure the tumor and prevent recurrence. To overcome the disadvantages of traditional treatment methods, nano-targeted delivery system, photothermal therapy (aPTT) and photodynamic therapy (aPDT) of nanomaterials have become new research directions. nano-targeted delivery system mainly uses polymer-based nanocarriers and lipid-based nanocarriers to transport drugs to targeted sites and release drugs,62,63 which achieves drug aggregation at the tumor site and reduces systemic side effects caused by traditional chemotherapeutic drugs.64 However, this treatment method has problems such as premature and uncontrollable drug release,65 resulting in a greatly reduced effect of cancer treatment. The aPTT therapy operates by converting light energy into heat energy through materials, and releasing heat energy in local tissues to achieve tumor cell damage.66 The aPDT therapy operates by releasing ROS through materials, which gets rid of the limitation of drug therapy. At the same time, the controllable ROS release of materials can achieve precise treatment of cancer.67,68
At present, the nanomaterials used for aPTT and aPDT therapy mainly include gold nanoparticles, graphene oxide, carbon dots, etc.69–72 Gold nanoparticles are commonly used in aPTT therapy, which have a low incidence of complications, and there are various methods for the synthesis of gold nanoparticles.73,74 However, the materials have high cytotoxicity and are easy to deposit in human body,75 making it difficult to achieve wide clinical application. Graphene oxide and carbon dots are commonly used in aPDT therapy, which have the characteristics of high ROS production rate and low toxicity. However, the limited synthesis method of materials leads to a single structure and poor stability, and there may be individual differences in clinical treatment effects.76–78 In recent years, RTP materials have been continuously developed. In addition to achieving high ROS production and precise targeting capabilities and combining the basic characteristics of low toxicity and high stability, RTP materials also have characteristics of diverse structures and flexible synthesis methods. It may be possible to synthesize the corresponding molecules according to the physical and chemical environment of patient ‘s body, which may greatly improve the anti-cancer effect of the materials and have great development potential in tumor treatment.
In the early stage of the development of RTP materials, people mainly focused on inorganic metal RTP materials. Alemayehu et al used corroles (H3[p/mTCPC]), gold and their complexes to prepare Au[p/mTCPC]. After coincubation of the material with a rat model of bladder cancer for 24 h, the tumor tissue was examined under 435 nm light excitation irradiation. The mortality rate of the tumor cells was as high as 50%, but the surrounding healthy tissues were affected to some extent.79 Zhao et al obtained the nanoprobe Gd[PC]@ZIF-8 by using a host–guest system. Through detection of the PDT performance, the researchers found that its 1O2 quantum yield was as high as 0.88. After coincubation with HeLa cells, the survival rate of HeLa cells was reduced to 30%, which provided a new idea for PDT for tumor treatment.80
Since inorganic metal RTP materials may have certain toxicity, the development of organic RTP materials has further advanced PDT in the field of tumor therapy.(Table 2) Miao et al prepared N-doped carbon dot (CND)-CY3.5 nanocomposites via a hydrothermal method using triethylenetetraminehexaacetic acid (TTHA) as the raw material and successfully achieved a high 1O2 quantum yield of 0.63 in a water environment. In in vitro tumor experiments, CNDs killed 99.9% of HeLa cells; at the same time, after the material was coincubated with the tumor model mice, obvious necrosis was detected in the tumor tissue of the mice, whereas the surrounding tissue was not damaged, indicating that this material was good and had a specific tumor clearance ability.46 However, traditional excitation is commonly excited by UV, which may lead to gene mutation and cell damage after patients exposure. So the proposal of near-infrared two-photon excitation(TPE) avoids cells damage caused by excitation light, improves the tissue penetration depth of excitation light, and achieves the removal of deep tumors.81 Xu et al designed BF2DCz-bovine serum albumin (BSA) nanoparticles (NPs) based on TPE-PDT with an organic RTP material. After TPE, the diffusion depth of the NPs reached 100 nm, and the quantum efficiency of 1O2 reached 90.3%, leading to successful and accurate killing of tumor cells in deep tissue.81 In tumor therapy, organic RTP materials have the advantages of high efficiency, low damage and low toxicity, which are not achievable with inorganic RTP materials. Moreover, because of the more diverse design of organic RTP materials, they can be improved according to different physical and chemical environments of cancer patients themselves, so that the materials are more in line with patients’ needs and further improve the accuracy of tumor diagnosis and clearance rate of tumors. Therefore, with the continuous development of RTP materials, their application in tumor treatment has gradually become possible in both the oral field and the clinical field.
 |
Table 2 The Experimental Progress and Research of RTP Materials in Tumor Therapy |
Application of RTP Materials for Antibacterial Treatment
Antibiotics are the main means for people to inhibit bacteria and prevent infection.82 However, with the use of antibiotics, the emergence of antibiotic-resistant bacteria has become a major obstacle to their further use.83–85 Therefore, the development of new antibacterial methods is highly important for anti-infection and disease treatment. In recent years, related antibacterial materials such as LL37 peptide86 and titanium dioxide nanoparticles87 have achieved the clearance of drug-resistant bacteria and broadened the spectrum of antibacterial materials. At the same time, with the development of nanomaterials, it provides various new ideas in antibacterial infection and prevention of infection complications, which is of great significance.
According to related research, nano-targeted delivery system and aPDT therapy are two feasible methods in the field of nanomaterial-based antibacterial research.88–90 Due to the dispersion of bacteria, more nanocarriers may be needed to achieve bacteria clearance. Meanwhile, the antibacterial function of nanocarriers is mainly achieved by drugs, which may lead to failure due to bacterial resistance.91,92 But aPDT therapy of nanomaterials ensures the antibacterial effect through generation of ROS rather than traditional drugs, which can not only clear bacteria but also avoid the side effects of traditional drugs.93 At the same time, aPDT therapy can clear more bacteria with little amount of material via the excellent diffusion ability of ROS. At present, most of the nanomaterials used in aPDT therapy are porphyrin-based nanomaterials. This material employs porphyrin as a photosensitizer (PS), utilizing precious metals, metal oxides, and carbon materials as carriers for the porphyrin. And through encapsulation and photoexcitation, it achieves the release of ROS.73 In contrast with porphyrin-based nanomaterials, in addition to its basic characteristics such as low toxicity, RTP materials have unique targeted antibacterial capabilities.31,42 Due to the high flexibility of RTP materials, it may be possible to specifically target the corresponding bacteria by improving the molecular structure and combining with antibacterial peptides94 to achieve accurate clear of the target bacteria, reducing the likelihood of a diminished antibacterial effect and minimizing damage to healthy flora, which may be caused by the widespread diffusion of ROS.
In the past ten years, RTP material antibacterial therapy has achieved promising results in the oral field.(Table 3) Klepac-Ceraj et al prepared methylene blue (MB)-loaded polylactic acid-glycolic acid (PLGA) copolymers, namely MB-PLGA NPs, which were cocultured with oral flora culture medium and irradiated with 665 nm red light. The results revealed that the biofilm inhibition rate reached 50%, which provides a basis for the treatment of periodontitis and other diseases with RTP materials.95 Golmohamadpour et al synthesized three different metal organic framework (MOF)-ICG nanomaterials. Through coculture of Enterococcus faecalis and PDT experiments, the antibacterial ability of Al-101-ICG was found to reach 89%, which provides a strong basis for the use of RTP materials for the treatment of oral periapical periodontitis.96
 |
Table 3 The Experimental Progress and Research of RTP Materials for Antibacterial Treatments |
However, there are three main shortcomings of RTP materials antibacterial therapy, which are easy aggregation, poor water solubility and unstable chemical properties. At present, most RTP materials need to be carried by water phase, so poor water solubility may lead to a decrease in their availability. At the same time, due to the shortcomings of easy aggregation and instability of materials, RTP particles in the solution may aggregate into large particles and precipitate, and even directly decompose due to changes in physical and chemical environment such as temperature, which ultimately leads to a large amount of material loss and a great decrease in antibacterial effect. So researchers have proposed several improvement strategies for overcoming the limitations of RTP materials as PSs,32,47 which lay a foundation for the application of RTP materials in the field of antibacterial.
(1) RTP materials are combined with nanomaterials to improve their water solubility, hinder their aggregation, and promote the production of ROS to improve their antibacterial ability.97
(2) The triplet exciton decay of RTP materials is regulated to prolong the generation of ROS and improve their antibacterial ability.98
(3) The degree of aggregation of an RTP material is adjusted to hinder the quenching caused by aggregation, prolong the generation and action time of ROS, and improve its antibacterial ability.83,99
(4) The positive and negative electrostatic interactions of materials are utilized to increase their ability to bind with bacteria, thereby improving their antibacterial ability.100,101(Figure 3)
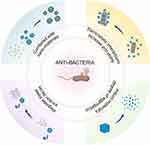 |
Figure 3 Four improvement strategies for overcoming three limitations of RTP materials as PSs. Notes: Created in BioRender. Zhang, y. (2024) https://BioRender.com/e59q768. |
According to the strategies, Akbari et al prepared nano-graphene oxide (NGO)-indocyanine green (ICG), improved the photodynamic characteristics of ICG, and cocultured the material with faecal cocci. After light irradiation, the number of bacteria detected decreased by 90.6%, and the biofilm formation rate decreased to 0.06%.102
Owing to the gradual improvement in people’s living standards, the incidence of oral diseases such as dental caries and periapical periodontitis has gradually increased. Moreover, in the process of treating oral diseases, bacteria such as Enterococcus faecalis and Porphyromonas gingivalis have important impacts on the development of diseases.103–105 Therefore, the ability of RTP materials to inhibit such bacteria provides a new direction for clinical treatment, and the realization of efficient inhibition of various oral bacteria is highly important for the control and treatment of oral periodontitis and other diseases.
Summary and Outlook
In recent years, RTP materials have made significant progress in biomedical fields such as in vivo bioimaging, tumor therapy, and antibacterial. Based on these results, we believe that RTP materials also have great application potential in the oral field. First of all, RTP material has excellent optical properties, which can provide high SNR and good tissue penetration. At the same time, with the unique targeting ability of the material to tumor cells, RTP materials can be used for accurate positioning and diagnosis of oral tumors and judgment of the development stage of periodontitis inflammation. At the same time, RTP material has good tumor killing ability, and due to its various synthesis methods, its molecule can be designed according to individual differences of patients, making the material more suitable for the individual microenvironment of patients, and further improving the detection rate and tumor killing rate of oral cancer. Secondly, in terms of antibacterial properties, RTP materials have good antibacterial properties and can effectively inhibit oral bacterial infection. We hope that it can be used for the sterilization of dental related instruments, which can reduce the problems of long time and high energy consumption of traditional instruments sterilization. It can also accurately target specific bacteria and clear them through different molecular structures, such as the removal of oral pathogenic bacteria in patients with periodontitis and pulpitis, so as to improve the therapeutic effect and quality of life of patients.
However, RTP materials may also have some problems in the treatment of these diseases. For example, in oral tumor bioimaging, the resolution of RTP may be affected by material concentration, so when the resolution is reduced due to the decrease of material concentration, the accuracy of detection and diagnosis of subtle lesions may decrease. In addition, in the complex oral environment, it is necessary to determine whether RTP materials can effectively kill deep tumor cells and reduce tumor recurrence rate. Besides, the antibacterial effect of RTP materials may have the problem of poor durability. So more materials will be needed in sterilization of instruments and treatment of periodontitis and dental caries, resulting in high prices and waste of medical resources. Therefore, there are two urgent problems to be solved in the clinical application of RTP materials. On the one hand, RTP materials show concentration dependence when used for tumor imaging, tumor therapy and antibacterial purposes, and the effect windows of different materials are significantly different. So in clinical applications, the utilization of RTP materials requires precise control of the concentration and effect duration. On the other hand, the three major applications of RTP materials in medicine are often excited by ultraviolet or infrared light, so even a short time of irradiation may have adverse effects on the human body.
Based on the above problems, we expect that it is necessary to develop RTP materials that can be excited by visible light and can be used sustainably to improve the safety and comfort of treatment. From a clinical point of view, this not only avoids the side effects of excitation light like ultraviolet and infrared light, but also reduces waste of material resources due to the poor treatment effect caused by decomposability of the materials. From an economic point of view, for oral diseases that require repeated use of materials for antibacterial purposes, such as periodontitis, dental caries, etc., reducing the cost of RTP materials is also crucial for future development, which can be achieved by sustainable and visible-light-excited RTP materials. This can not only reduce the economic burden of patients, but also increase the accessibility and application range of materials. So RTP materials can give full play to their potential, provide innovative solutions for oral cancer and antimicrobial therapy, and ultimately contribute to improving human health and well-being.
Abbreviations
RTP, room-temperature phosphorescent; SNR, signal-to-noise ratio; ROS, reactive oxygen species; S0, ground state; Sn, singlet excited state; VR, vibrational relaxation; IC, internal conversion; ISC, intersystem crossing; Tn, triplet excited state; singlet oxygen, 1O2; MRI, magnetic resonance imaging; CT, computed tomography; PET, positron emission tomography; SOC, spin‒orbit coupling; UOP, ultralong organic phosphorescent; PSs, photosensitizers; PDT, photodynamic therapy; NIR, near-infrared; NPs, nanoparticles.
Acknowledgments
This work was supported by the Youth Fund of National Natural Science Foundation of China, under grant no. 82101021.
Disclosure
The authors declare that they have no competing interest in this work.
References
1. Palmal S, Jana NR. Gold nanoclusters with enhanced tunable fluorescence as bioimaging probes. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(1):102–110. doi:10.1002/wnan.1245
2. H E, M D. Gold nanoparticles and quantum dots for bioimaging. Microsc Res Tech. 2011;74(7). doi:10.1002/jemt.20928
3. You Y. Phosphorescence bioimaging using cyclometalated Ir(III) complexes. Curr Opin Chem Biol. 2013;17(4):699–707. doi:10.1016/j.cbpa.2013.05.023
4. Erathodiyil N, Ying JY. Functionalization of inorganic nanoparticles for bioimaging applications. Acc Chem Res. 2011;44(10):925–935. doi:10.1021/ar2000327
5. Lu H, Li W, Dong H, Wei M. Graphene quantum dots for optical bioimaging. Small. 2019;15(36):e1902136. doi:10.1002/smll.201902136
6. Wang X, Sang D, Zou L, et al. Multiple bioimaging applications based on the excellent properties of nanodiamond: a review. Molecules. 2023;28(10):4063. doi:10.3390/molecules28104063
7. Wolfbeis OS. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem Soc Rev. 2015;44(14):4743–4768. doi:10.1039/c4cs00392f
8. Wang C, Chen L, Tan R, et al. Carbon dots and composite materials with excellent performances in cancer-targeted bioimaging and killing: a review. Nanomedicine. 2023;18(25):1875–1901. doi:10.2217/nnm-2023-0216
9. Wang Y, Gao H, Yang J, et al. High performance of simple organic phosphorescence host-guest materials and their application in time-resolved bioimaging. Adv Mater. 2021;33(18):e2007811. doi:10.1002/adma.202007811
10. Wang S, Xu M, Huang K, et al. Biocompatible metal-free organic phosphorescent nanoparticles for efficiently multidrug-resistant bacteria eradication. Sci China Mater. 2020;63(2):316–324. doi:10.1007/s40843-019-1191-9
11. Zhao Y, Yang J, Liang C, et al. Fused‐ring pyrrole‐based near‐infrared emissive organic RTP material for persistent afterglow bioimaging. Angew Chem Int Ed. 2024;63(5):e202317431. doi:10.1002/anie.202317431
12. Xu X, Wang J, Yan B. Facile fabrication of luminescent Eu(III) functionalized HOF hydrogel film with multifunctionailities: quinolones fluorescent sensor and anticounterfeiting platform. Adv Funct Mater 2021;31(37):2103321. doi:10.1002/adfm.202103321
13. Dinh TV, Yen EL, Winefordner JD. Room-temperature phosphorescence of several polyaromatic hydrocarbons. Talanta. 1977;24(2):146–8.
14. Hurtubise J Tjioe SW. The solid-matrix room-temperature luminescence detection and characterization of polyaromatic hydrocarbons without a heavy atom. Talanta. 1994;41(4):595–8.
15. Schauerte JA, Steel DG, Gafni A. Time-resolved room temperature tryptophan phosphorescence in proteins. Methods Enzymol. 1997;278:49–71. doi:10.1016/s0076-6879(97)78006-6
16. Papp SA, Vanderkooi JM. Tryptophan phosphorescence at room temperature as a tool to study protein structure and dynamics. Photochem Photobiol. 1989;49(6):775–84.
17. Segura Carretero A, Cruces Blanco C, Cañabate Díaz B, Fernández Gutiérrez A. Room-temperature phosphorimetric method for the determination of the drug naphazoline in pharmaceutical preparations. Analyst. 1998;123(5):1069–1071. doi:10.1039/a708672e
18. Gan N, Zou X, Zhang Y, Gu L, An Z. Recent advances in multicolor organic room-temperature phosphorescence. Appl Phys Rev 2023;10(2):021313. doi:10.1063/5.0140824
19. Weinberger R, Rembish K, Cline Love LJ Comparison of techniques for generating room temperature phosphorescence in fluid solution.
20. Campiglia AD, Perry LM, Winefordner JD. Automatic sampling system employing a nebulizer for solid-surface room-temperature phosphorescence analysis. Appl Spectrosc AS. 1989;43(8):1341–1343. doi:10.1366/0003702894204434
21. Xu X, Yan B. Recent advances in room temperature phosphorescence materials: design strategies, internal mechanisms and intelligent optical applications. Phys Chem Chem Phys. 2023;25(3):1457–1475. doi:10.1039/d2cp05063c
22. Jia J, Lu W, Gao Y, Li L, Dong C, Shuang S. Recent advances in synthesis and applications of room temperature phosphorescence carbon dots. Talanta. 2021;231:122350. doi:10.1016/j.talanta.2021.122350
23. Wang J, Lou XY, Wang Y, Tang J, Yang YW. Recent advances of polymer-based pure organic room temperature phosphorescent materials. Macromol Rapid Commun. 2021;42(9):e2100021. doi:10.1002/marc.202100021
24. Zhen X, Tao Y, An Z, et al. Ultralong phosphorescence of water-soluble organic nanoparticles for in vivo afterglow imaging. Adv Mater. 2017;29(33). doi:10.1002/adma.201606665
25. Yang J, Gao H, Wang Y, et al. The odd–even effect of alkyl chain in organic room temperature phosphorescence luminogens and the corresponding in vivo imaging. Mater Chem Front. 2019;3(7):1391–1397. doi:10.1039/C9QM00108E
26. Yang J, Zhang Y, Wu X, et al. Rational design of pyrrole derivatives with aggregation-induced phosphorescence characteristics for time-resolved and two-photon luminescence imaging. Nat Commun. 2021;12(1):4883. doi:10.1038/s41467-021-25174-6
27. Xiao F, Gao H, Lei Y, et al. Guest-host doped strategy for constructing ultralong-lifetime near-infrared organic phosphorescence materials for bioimaging. Nat Commun. 2022;13(1):186. doi:10.1038/s41467-021-27914-0
28. Dang Q, Jiang Y, Wang J, et al. Room-temperature phosphorescence resonance energy transfer for construction of near-infrared afterglow imaging agents. Adv Mater. 2020;32(52):e2006752. doi:10.1002/adma.202006752
29. Wang XF, Xiao H, Chen PZ, et al. Pure organic room temperature phosphorescence from excited dimers in self-assembled nanoparticles under visible and near-infrared irradiation in water. J Am Chem Soc. 2019;141(12):5045–5050. doi:10.1021/jacs.9b00859
30. Huang Q, Gao H, Yang S, Ding D, Lin Z, Ling Q. Ultrastable and colorful afterglow from organic luminophores in amorphous nanocomposites: advanced anti-counterfeiting and in vivo imaging application. Nano Res. 2020;13(4):1035–1043. doi:10.1007/s12274-020-2740-x
31. Datta S, Xu J. Recent advances in organic molecular-to-supramolecular self-assembled room-temperature phosphorescent materials for biomedical applications. ACS Appl Bio Mater. 2023;6(11):4572–4585. doi:10.1021/acsabm.3c00677
32. Wang X. Organic phosphorescent nanoscintillator for low-dose X-ray-induced photodynamic therapy. Nat Commun. 2022;13(1):
33. Chang B, Chen J, Bao J, Sun T, Cheng Z. Molecularly engineered room-temperature phosphorescence for biomedical application: from the visible toward second near-infrared window. Chem Rev. 2023;123(24):13966–14037. doi:10.1021/acs.chemrev.3c00401
34. Dai X, Liu Z, Ge Y, Wei P. Ultralong aqueous organic room-temperature phosphorescent probes for in vivo time-resolved bioimaging. TrAC Trends Analy Chem. 2023;168:117339. doi:10.1016/j.trac.2023.117339
35. Ni AY, Zhang BL, Zhang PP, et al. Water friendly room temperature phosphorescence doped materials prepared via metal organic framework matrix transformation. Dyes Pigm 2023;210:110959. doi:10.1016/j.dyepig.2022.110959
36. Zhu Y, Guan Y, Niu Y, et al. Ultralong polymeric room temperature phosphorescence materials fabricated by multiple hydrogen bondings resistant to temperature and humidity. Adv Opt Mater 2021;9(21):2100782. doi:10.1002/adom.202100782
37. Zhou Y, Ma J, Zhang P, Liu Z, Chi Z, Liang G. Deep-blue ultralong room-temperature phosphorescence from halogen-free organic materials through cage effect for various applications. Adv Opt Mater 2021;9(20):2100959. doi:10.1002/adom.202100959
38. Panda SK, De A, Banerjee S. Room-temperature phosphorescence from organic materials in aqueous media. Photochem Photobiol. 2024;100(4):796–829. doi:10.1111/php.13956
39. Liu Y, Zhan G, Liu ZW, Bian ZQ, Huang CH. Room-temperature phosphorescence from purely organic materials. Chin Chem Lett 2016;27(8):1231–1240. doi:10.1016/j.cclet.2016.06.029
40. Zhou WL, Lin W, Chen Y, Liu Y. Supramolecular assembly confined purely organic room temperature phosphorescence and its biological imaging. Chem Sci. 2022;13(27):7976–7989. doi:10.1039/D2SC01770A
41. Zhen X, Qu R, Chen W, Wu W, Jiang X. The development of phosphorescent probes for in vitro and in vivo bioimaging. Biomater Sci. 2021;9(2):285–300. doi:10.1039/D0BM00819B
42. Zhi J, Zhou Q, Shi H, An Z, Huang W. Organic room temperature phosphorescence materials for biomedical applications. Chem Asian J. 2020;15(7):947–957. doi:10.1002/asia.201901658
43. Lv Z, Zou L, Wei H, Liu S, Huang W, Zhao Q. Phosphorescent starburst Pt(II) porphyrins as bifunctional therapeutic agents for tumor hypoxia imaging and photodynamic therapy. ACS Appl Mater Interfaces. 2018;10(23):19523–19533. doi:10.1021/acsami.8b05944
44. Sun B, Bte Rahmat JN, Zhang Y. Advanced techniques for performing photodynamic therapy in deep-seated tissues. Biomaterials. 2022;291:121875. doi:10.1016/j.biomaterials.2022.121875
45. Ji B, Wei M, Yang B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics. 2022;12(1):434–458. doi:10.7150/thno.67300
46. Miao Y, Zhang X, Li J, Yang W, Huang X, Lv J. Preparation and photodynamic antibacterial/anticancer effects of ultralong-lifetime room-temperature phosphorescent N-doped carbon dots. RSC Adv. 2022;12(32):20481–20491. doi:10.1039/D2RA02251F
47. Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. Ca a Cancer J Clinicians. 2011;61(4):250–281. doi:10.3322/caac.20114
48. Bhatta AK, Keyal U, Wang X, Gellén E. A review of the mechanism of action of lasers and photodynamic therapy for onychomycosis. Lasers Med Sci. 2017;32(2):469–474. (). doi:10.1007/s10103-016-2110-9
49. Varaprasad GL, Varaprasad GL, Gupta VK, et al. Recent advances and future perspectives in the therapeutics of prostate cancer. Exp Hemato Oncol. 2023;12(1):80.
50. Liao W, Shi X, Gang ZL, et al. Comparison and mechanism study of antibacterial activity of cationic and neutral oligo-thiophene-ethynylene. ACS Appl Mater Interfaces. 2021; 13(34):41012–20. doi:10.1021/acsami.1c02474
51. Zhang Y, Li H, Yang M, et al. Organic room-temperature phosphorescence materials for bioimaging. Chem Commun. 2023;59(36):5329–5342. doi:10.1039/D3CC00923H
52. Wald LL. Ultimate MRI. J Magn Reson. 2019;306:139–144. doi:10.1016/j.jmr.2019.07.016
53. Decazes P, Hinault P, Veresezan O, Thureau S, Gouel P, Vera P. Trimodality PET/CT/MRI and Radiotherapy: a Mini-Review. Front Oncol. 2020;10:614008. doi:10.3389/fonc.2020.614008
54. Fonti R, Conson M, Del Vecchio S. PET/CT in radiation oncology. Semin Oncol. 2019;46(3):202–209. doi:10.1053/j.seminoncol.2019.07.001
55. Sobhanan J, Rival JV, Anas A, Sidharth Shibu E, Takano Y, Biju V. Luminescent quantum dots: synthesis, optical properties, bioimaging and toxicity. Adv Drug Delivery Rev 2023;197:114830. doi:10.1016/j.addr.2023.114830
56. Tan P, Li H, Wang J, Gopinath SCB. Silver nanoparticle in biosensor and bioimaging: clinical perspectives. Biotechnol Appl Biochem 2021;68(6):1236–1242. doi:10.1002/bab.2045
57. Fan JW, Vankayala R, Chang CL, Chang CH, Chiang CS, Hwang KC. Preparation, cytotoxicity and in vivo bioimaging of highly luminescent water-soluble silicon quantum dots. Nanotechnology. 2015;26(21):215703. doi:10.1088/0957-4484/26/21/215703
58. Gao H, Gao Z, Jiao D, et al. Boosting room temperature phosphorescence performance by alkyl modification for intravital orthotopic lung tumor imaging. Small. 2021;17(22):2005449. doi:10.1002/smll.202005449
59. Xu L, Zhou K, Ma H, et al. Ultralong organic phosphorescent nanocrystals with long-lived triplet excited states for afterglow imaging and photodynamic therapy. ACS Appl Mater Interfaces. 2020;12(16):18385–18394. doi:10.1021/acsami.0c04005
60. Zhou WL, Chen Y, Yu Q, et al. Ultralong purely organic aqueous phosphorescence supramolecular polymer for targeted tumor cell imaging. Nat Commun. 2020;11(1):4655. doi:10.1038/s41467-020-18520-7
61. Zhang W, Chen S, Sun P, et al. NIR‐II J‐aggregated Pt(II)‐porphyrin‐based phosphorescent probe for tumor‐hypoxia imaging. Adv Healthcare Mat. 2022;11(15):2200467. doi:10.1002/adhm.202200467
62. Kahraman E, Güngör S, Özsoy Y. Potential enhancement and targeting strategies of polymeric and lipid-based nanocarriers in dermal drug delivery. Therap Deliv. 2017;8(11):967–985. doi:10.4155/tde-2017-0075
63. Ahmad J, Amin S, Rahman M, et al. Solid matrix based lipidic nanoparticles in oral cancer chemotherapy: applications and pharmacokinetics. Curr Drug Metab. 2015;16(8):633–644. doi:10.2174/1389200216666150812122128
64. Estanqueiro M, Amaral MH, Conceição J, Sousa Lobo JM. Nanotechnological carriers for cancer chemotherapy: the state of the art. Colloids Surf B. 2015;126:631–648. doi:10.1016/j.colsurfb.2014.12.041
65. Bourbour S, Darbandi A, Bostanghadiri N, Ghanavati R, Taheri B, Bahador A. Effects of antimicrobial photosensitizers of photodynamic therapy (PDT) to treat periodontitis. Curr Pharm Biotechnol. 2024;25(10):1209–1229. doi:10.2174/1389201024666230720104516
66. Li X, Wang Y, Liu T, Zhang Y, Wang C, Xie B. Ultrasmall graphene oxide for combination of enhanced chemotherapy and photothermal therapy of breast cancer. Colloids Surf B. 2023;225:113288. doi:10.1016/j.colsurfb.2023.113288
67. Li Y, Bai G, Zeng S, Hao J. Theranostic carbon dots with innovative NIR-II emission for in vivo renal-excreted optical imaging and photothermal therapy. ACS Appl Mater Interfaces. 2019;11(5):4737–4744. doi:10.1021/acsami.8b14877
68. Liang G, Wang H, Shi H, et al. Recent progress in the development of upconversion nanomaterials in bioimaging and disease treatment. J Nanobiotechnol. 2020;18(1):154. doi:10.1186/s12951-020-00713-3
69. Mauro N, Utzeri MA, Varvarà P, Cavallaro G. Functionalization of metal and carbon nanoparticles with potential in cancer theranostics. Molecules. 2021;26(11):3085. doi:10.3390/molecules26113085
70. Báez DF. Graphene-based nanomaterials for photothermal therapy in cancer treatment. Pharmaceutics. 2023;15(9):2286. doi:10.3390/pharmaceutics15092286
71. Liu Y, Crawford BM, Vo-Dinh T. Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy. 2018;10(13):1175–1188. doi:10.2217/imt-2018-0029
72. Chu X, Zhang P, Liu Y, et al. A multifunctional carbon dot-based nanoplatform for bioimaging and quaternary ammonium salt/photothermal synergistic antibacterial therapy. J Mater Chem B. 2022;10(15):2865–2874. doi:10.1039/d1tb02717d
73. Chen S, Huang B, Tian J, Zhang W. Advancements of porphyrin-derived nanomaterials for antibacterial photodynamic therapy and biofilm eradication. Adv Healthcare Mater. 2024;13(27):2401211. doi:10.1002/adhm.202401211
74. Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275(2):496–502. doi:10.1016/j.jcis.2004.03.003
75. Singh P, Pandit S, Mokkapati V, Garg A, Ravikumar V, Mijakovic I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci. 2018;19(7):1979. doi:10.3390/ijms19071979
76. Liao C, Li Y, Tjong SC. Graphene nanomaterials: synthesis, biocompatibility, and cytotoxicity. Int J Mol Sci. 2018;19(11):3564. doi:10.3390/ijms19113564
77. Shafiee A, Iravani S, Varma RS. Graphene and graphene oxide with anticancer applications: challenges and future perspectives. MedComm. 2022;3(1):e118. doi:10.1002/mco2.118
78. Xu J, Ning J, Wang Y, Xu M, Yi C, Yan F. Carbon dots as a promising therapeutic approach for combating cancer. Bioorg Med Chem 2022;72:116987. doi:10.1016/j.bmc.2022.116987
79. Alemayehu AB, Day NU, Mani T, et al. Gold Tris(carboxyphenyl)corroles as multifunctional materials: room temperature near-IR phosphorescence and applications to photodynamic therapy and dye-sensitized solar cells. ACS Appl Mater Interfaces. 2016;8(29):18935–18942. doi:10.1021/acsami.6b04269
80. Zhao Z, Ru J, Zhou P, et al. A smart nanoprobe based on a gadolinium complex encapsulated by ZIF-8 with enhanced room temperature phosphorescence for synchronous oxygen sensing and photodynamic therapy. Dalton Trans. 2019;48(45):16952–16960. doi:10.1039/C9DT03955D
81. Xu Z, Jiang Y, Shen Y, et al. A biocompatible photosensitizer with a high intersystem crossing efficiency for precise two-photon photodynamic therapy. Mater Horiz. 2022;9(4):1283–1292. doi:10.1039/D1MH01869H
82. Blair JMA, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJV. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13(1):42–51. doi:10.1038/nrmicro3380
83. Vickers ML, Dulhunty JM, Ballard E, et al. Risk factors for multidrug‐resistant
84. Lachiewicz AM, Hauck CG, Weber DJ, Cairns BA, Van Duin D. Bacterial infections after burn injuries: impact of multidrug resistance. Clinl Infect Dis. 2017;65(12):2130–2136. doi:10.1093/cid/cix682
85. Van Duin D, Strassle PD, DiBiase LM, et al. Timeline of health care–associated infections and pathogens after burn injuries. Am J Infect Control. 2016;44(12):1511–1516. doi:10.1016/j.ajic.2016.07.027
86. Comune M, Rai A, Palma P, TondaTuro C, Ferreira L. Antimicrobial and pro-angiogenic properties of soluble and nanoparticle-immobilized LL37 peptides. Biomater Sci. 2021;9(24):8153–8159. doi:10.1039/D1BM01034D
87. Mansoor A, Khurshid Z, Khan MT, et al. Medical and dental applications of titania nanoparticles: an overview. Nanomaterials. 2022;12(20):3670. doi:10.3390/nano12203670
88. Diogo P, Amparo F. Faustino M, Palma PJ, Rai A, Graça P. M. S. Neves M, Miguel Santos J. May carriers at nanoscale improve the Endodontic’s future? Adv Drug Delivery Rev 2023;195:114731. doi:10.1016/j.addr.2023.114731
89. Diogo P, Faustino MAF, MGPMS N, et al. An insight into advanced approaches for photosensitizer optimization in endodontics—A critical review. J Funct Biomat. 2019;10(4):44. doi:10.3390/jfb10040044
90. Diogo P, Gonçalves T, Palma P, Santos JM. Photodynamic antimicrobial chemotherapy for root canal system asepsis: a narrative literature review. Int J Dent. 2015;2015:269205. doi:10.1155/2015/269205
91. Rai A, Prabhune A, Perry CC. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J Mater Chem. 2010;20(32):6789–6798. doi:10.1039/C0JM00817F
92. Diogo P, Mota M, Fernandes C, et al. Is the chlorophyll derivative Zn(II)e6Me a good photosensitizer to be used in root canal disinfection? Photodiag Photodyn Ther. 2018;22:205–211. doi:10.1016/j.pdpdt.2018.04.009
93. Rai A, Ferrão R, Palma P, et al. Antimicrobial peptide-based materials: opportunities and challenges. J Mater Chem B. 2022;10(14):2384–2429. doi:10.1039/D1TB02617H
94. Theodoro LH, Lopes AB, Nuernberg MAA, et al. Comparison of repeated applications of aPDT with amoxicillin and metronidazole in the treatment of chronic periodontitis: a short-term study. J Photochem Photobiol B. 2017;174:364–369. doi:10.1016/j.jphotobiol.2017.08.012
95. Klepac-Ceraj V, Patel N, Song X, et al. Photodynamic effects of methylene blue-loaded polymeric nanoparticles on dental plaque bacteria: photodestruction of oral bacteria. Lasers Surg Med. 2011;43(7):600–606. doi:10.1002/lsm.21069
96. Golmohamadpour A, Bahramian B, Khoobi M, Pourhajibagher M, Barikani HR, Bahador A. Antimicrobial photodynamic therapy assessment of three indocyanine green-loaded metal-organic frameworks against Enterococcus faecalis. Photodiagn Photodyn Ther. 2018;23:331–338. doi:10.1016/j.pdpdt.2018.08.004
97. Qi M, Chi M, Sun X, et al. Novel nanomaterial-based antibacterial photodynamic therapies to combat oral bacterial biofilms and infectious diseases. IJN. 2019;14:6937–6956. doi:10.2147/IJN.S212807
98. Chao C, Kang L, Dai W, et al. Microfluidic-based modulation of triplet exciton decay in organic phosphorescent nanoparticles for size-assisted photodynamic antibacterial therapy. J Mater Chem B. 2023;11(14):3106–3112. doi:10.1039/D2TB02662G
99. Zhao H, Xu J, Wang Y, et al. A photosensitizer discretely loaded nanoaggregate with robust photodynamic effect for local treatment triggers systemic antitumor responses. ACS Nano. 2022;16(2):3070–3080. doi:10.1021/acsnano.1c10590
100. Zhang X, Cheng Y, You J, Zhang J, Yin C, Zhang J. Ultralong phosphorescence cellulose with excellent anti-bacterial, water-resistant and ease-to-process performance. Nat Commun. 2022;13(1):1117. doi:10.1038/s41467-022-28759-x
101. Sun X, Luo S, Zhang L, Miao Y, Yan G. Photodynamic antibacterial activity of oxidase-like nanozyme based on long-lived room-temperature phosphorescent carbon dots. Food Chem. 2024;434:137541. doi:10.1016/j.foodchem.2023.137541
102. Akbari T, Pourhajibagher M, Hosseini F, et al. The effect of indocyanine green loaded on a novel nano-graphene oxide for high performance of photodynamic therapy against Enterococcus faecalis. Photodiagn Photodyn Ther. 2017;20:148–153. doi:10.1016/j.pdpdt.2017.08.017
103. Mombelli A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol 2000. 2018;76(1):85–96. doi:10.1111/prd.12147
104. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi:10.1016/S0140-6736(05)67728-8
105. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. doi:10.1038/nrdp.2017.38
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

