Back to Journals » Drug Design, Development and Therapy » Volume 19
Exploring the Cardioprotective Mechanisms of Ligusticum wallichii in Myocardial Infarction Through Network Pharmacology and Experimental Validation
Authors Yang H, Cao J, Zhou L, Chen J, Tang J, Chen J, Yin L, Xie L , Li J, Luo J
Received 5 June 2024
Accepted for publication 19 December 2024
Published 17 January 2025 Volume 2025:19 Pages 281—302
DOI https://doi.org/10.2147/DDDT.S481499
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Huan Yang,1 Jun Cao,1 Lijie Zhou,1 Jiangchuan Chen,1 Jiaman Tang,1 Jiamei Chen,1 Lengyun Yin,1 Li Xie,2 Jianmin Li,1 Jinwen Luo3
1Department of Pulmonary and Critical Care Medicine, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Clinical Medicine Research Center For Respiratory Rehabilitation in Hunan Province, Changsha, Hunan, People’s Republic of China; 2Department of Cardiovascular Surgery, The Second Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 3Department of Cardio-Thoracic Surgery, Hunan Children’s Hospital, Changsha, Hunan, People’s Republic of China
Correspondence: Jinwen Luo, Department of Cardio-thoracic Surgery, Hunan Children’s Hospital, Changsha, Hunan, People’s Republic of China, Email [email protected] Jianmin Li, Department of Pulmonary and Critical Care Medicine, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, Hunan, People’s Republic of China, Email [email protected]
Background: Myocardial infarction represents a coronary artery ailment with the highest incidence and fatality rates among cardiovascular conditions. However, effective pharmacological interventions remain elusive. This study seeks to elucidate the molecular mechanisms underlying the effects of Ligusticum wallichii on myocardial infarction through network pharmacology and experimental validation.
Methods: Initially, potential targets of Ligusticum wallichii’s active ingredients and myocardial infarction-related targets were retrieved from databases. Subsequently, core targets of Ligusticum wallichii on myocardial infarction were identified via the PPI network analysis and subjected to GO and KEGG pathway enrichment analyses. Molecular docking was employed to validate the binding affinities between the core targets and the bioactive components. The findings from network pharmacology analysis were corroborated through in vitro and in vivo experiments.
Results: Seven active ingredients from Ligusticum wallichii were identified, corresponding to 122 targets. Molecular docking revealed robust binding affinities of Myricanone, Senkyunone, and Sitosterol to key target proteins (EGFR, STAT3, and SRC). In vitro, experiments demonstrated that pretreatment with the active components of Ligusticum wallichii protected myocardial cells from OGD exposure and modulated the expression of their key target genes. In vivo, experiments showed that the active components of Ligusticum wallichii significantly improved myocardial infarction via alleviating myocardial fibrosis and oxidative stress and did not elicit toxic effects in mice.
Conclusion: The collective findings suggest that Ligusticum wallichii shows promising potential for myocardial infarction treatment by regulating key target proteins (EGFR, STAT3, and SRC), which play roles in oxidative stress and myocardial fibrosis.
Keywords: Ligusticum wallichii, myocardial infarction, Molecular docking, network pharmacology, experimental validation
Introduction
The occurrence of myocardial infarction, characterized by necrosis of the heart muscle, significantly contributes to global morbidity and mortality, resulting in approximately 7.4 million deaths annually.1,2 The established pathophysiology involves the formation of a blood clot within a coronary artery, leading to reduced blood flow to the heart muscle. Current therapeutic approaches include pharmacological interventions, percutaneous coronary intervention (PCI), and coronary artery bypass grafting (CABG).3,4 However, reperfusion strategies such as PCI and CABG may cause injury due to ischemia/reperfusion, antiplatelet therapy increases the risk of bleeding, and statin therapy carries the potential for drug-induced liver injury.5–8 Therefore, the identification of effective drugs to enhance myocardial perfusion and mitigate the extent of myocardial damage is urgently required.
Nutraceuticals, particularly those with anti-inflammatory, antioxidant, and vascular-protective properties, have shown significant promise in ameliorating cardiovascular risk factors and mitigating disease progression, complementing conventional therapeutic approaches.9 Ligusticum wallichii (Chuanxiong) is the dried rhizome of Ligusticum chuanxiong Hort., first recorded in the earliest complete Pharmacopoeia of China (Shennong’s Classic of Materia Medica) from Warring States Period to Han Dynasty.10 Numerous clinical, pharmacological, and chemical investigations have demonstrated the anti-inflammatory, antioxidant, anti-fibrotic, and immunomodulatory properties of Ligusticum wallichii.11–13 In this context, Ligusticum wallichii represents a promising nutraceutical for cardiovascular health. Ligusticum wallichii’s antioxidant and anti-inflammatory capabilities align with these findings, suggesting that it may confer additional cardioprotective effects when integrated with current treatments. These properties could be particularly advantageous in reducing reperfusion injury, enhancing vascular function, and limiting myocardial fibrosis, thus contributing to improved clinical outcomes for myocardial infarction patients.
Ligusticum wallichii has been reported to play an important role in the treatment of cardiovascular and cerebrovascular diseases such as coronary heart disease,14 cerebral ischemia,15 diabetes,16 and hypertension.17 Ligusticum wallichii can improve blood fluidity18 attenuate endothelial cell damage,19 and inhibit vascular smooth muscle cell proliferation.20 Unlike conventional treatments that often target single pathways, Ligusticum wallichii contains multiple bioactive compounds, which allows it to modulate various aspects of the myocardial infarction pathophysiology simultaneously. This multi-targeted approach aligns with the principles of Traditional Chinese Medicine, which emphasize a holistic perspective and comprehensive disease management. It has been reported that Ligusticum wallichii-based nanomaterials alleviate bleeding and bacterial infections in the Staphylococcus aureus-infected mouse skin wound model, promoting wound healing.21 This result suggests that Ligusticum wallichii has a great hemostatic effect. Also, Ligusticum wallichii extract was reported to protect against ischemic reperfusion injury and improve myocardial antioxidant status in myocardial ischemic reperfusion rats.22 In addition, Ligusticum wallichii extract prevents liver fibrotic injury and ameliorates methotrexate-induced oxidative liver injury,12,23 suggesting its protective role in improving liver toxicity. These studies indicate that Ligusticum wallichii has important potential to address current limitations in the treatment of myocardial infarction.
Besides, Ligusticum wallichii also affects several known pathways related to inflammation, oxidative stress, and apoptosis, which may be linked to the improvement of myocardial infarction. It has been demonstrated that Ligusticum wallichii inhibits the STAT3 signaling pathway, the key pro-inflammatory pathway in myocardial infarction, decreasing the secretion of inflammatory factors.24,25 Tetramethylpyrazine, the active component of Ligusticum wallichii, was reported to suppress oxidative stress and apoptosis by the miR‑182/mitochondrial apoptotic pathway.26 MiR-182 has been identified as a key factor regulating oxidative stress and apoptosis in ischemic cardiac injury and myocardial infarction.27,28 Thus, these known pathways may be intervention targets for Ligusticum wallichii in the treatment of myocardial infarction. Nevertheless, the comprehensive mechanism of Ligusticum wallichii intervention in myocardial infarction remains unclear, with limited research at the molecular level. Myocardial infarction involves changes in multiple gene expressions, making it challenging to achieve optimal therapeutic outcomes with drugs targeting a single pathway. Network pharmacology offers a systematic method to explore the complex mechanisms underlying drug treatment, utilizing network visualization diagrams based on comprehensive biological data.29–31 This approach is in line with the holistic principles of Traditional Chinese Medicine, which prioritize comprehensive disease management and provide innovative insights and methodologies for understanding intricate disease mechanisms. Molecular docking utilizes computational techniques to investigate the spatial interactions between drug molecules and targets, facilitating the targeted design of effective drugs.32–34 Incorporating Ligusticum wallichii into therapeutic strategies, especially as a complementary nutraceutical, could present an innovative approach to managing myocardial infarction by leveraging its multi-targeted benefits. The present study employed molecular docking and network pharmacology techniques, guided by the holistic perspective of Traditional Chinese Medicine, along with experimental validation to elucidate the target proteins, associated pathways, and underlying mechanisms involved in the intervention of myocardial infarction by Ligusticum wallichii.
Methods
Bioactive Components and Targets of Ligusticum wallichii
The bioactive constituents of Ligusticum wallichii were obtained from the Traditional Chinese Medicine Systems Pharmacology (TCMSP) Database. Given that Ligusticum wallichii is primarily administered orally, we applied drug-likeness (DL) and oral bioavailability (OB) as filtering criteria. Specifically, compounds with DL values equal to or greater than 30% and OB values equal to or greater than 0.18 were retained. After screening the TCMSP database and cross-referencing with relevant literature, we identified the active ingredients of Ligusticum wallichii. Subsequently, we generated the three-dimensional structures of all compounds using 3D Viewer and saved them in mol2 file format for target prediction purposes. Target information was then retrieved from the TCMSP database based on feature data and physicochemical parameters, while SMILES files for these active ingredients were obtained from the PubChem database. These SMILES files were utilized for target prediction through the SwissTargetPrediction website (http://old.swisstargetprediction.ch/). In SwissTargetPrediction, the top 15% of results are considered to have a 75% likelihood of being true targets of the compound. Therefore, the first 15 predictions from SwissTargetPrediction were selected. The network proximity z’-score was calculated based on the mean and standard deviation of random distances. Using the PharmMapper database results, the Z-score, which offers a better confidence interval and statistical relevance compared to the “Fit score”, was used as the primary metric. A Z-score > 0 was considered significant, while a negative Z-score indicated non-significance. Additionally, a higher z’-score suggests a stronger alignment between compound molecules and protein targets. The combined targets identified from both the SwissTargetPrediction and TCMSP databases represent the principal targets of active ingredients in Ligusticum wallichii.
Identification of Myocardial Infarction-Related Targets
Targets related to myocardial infarction were identified through screening of the GeneCards database and OMIM database (https://omim.org/). Initially, a search for “myocardial infarction” was conducted in the OMIM database under the “Gene Map” section, and the search results were downloaded in Excel format. Subsequently, the GeneCards database was utilized to retrieve information on “myocardial infarction”, and the search outcomes were also saved as Excel files. The relevance score serves to evaluate a gene’s association with a particular disease or biological process. In the GeneCards database, each gene is assigned a relevance score between 0 and 100, where higher scores indicate a stronger connection to the disease or biological process. For specific disease-gene pairs, a gene-disease association is established if the gda score is above 0 or the relevance score exceeds 1. Consequently, a relevance score of ≥ 15 in GeneCards was considered a significant association. Finally, employ Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/index.html), an online tool, to import both sets of data tables from Ligusticum wallichii and myocardial infarction-related targets. Subsequently, the intersection targets between Ligusticum wallichii and myocardial infarction were identified.
Construction of Bioactive Component-Target Network
To identify common targets between Ligusticum wallichii and myocardial infarction, we utilized the Venn diagram online tool (https://bioinfogp.cnb.csic.es/tools/venny/). This involved intersecting the targets associated with myocardial infarction and those targeted by active ingredients of Ligusticum wallichii, resulting in the visualization of the shared targets through a Venn diagram. These intersecting genes signify potential targets of Ligusticum wallichii in the treatment of myocardial infarction. Furthermore, we predicted target proteins for active ingredients and myocardial infarction and standardized their naming to gene names. To enhance data reliability, we used PPI data specific to “Homo sapiens” with a confidence score of ≥0.7 (highest confidence: ≥0.9, high: ≥0.7, medium: 0.4–0.7, low: <0.4) to build the PPI network and isolated the largest connected component. Finally, the selected targets related to myocardial infarction were imported into Cytoscape 3.7.1 software to construct an ingredient-target network map.
Construction of the Protein-Protein Interaction (PPI) Network
The intersection target protein was inputted into the STRING database, specifying “Homo sapiens” as the species, to retrieve protein-protein interaction (PPI) information. Subsequently, the resulting data file in Tab Separated Values (TSV) format was exported and imported into Cytoscape 3.8.2 software for visualization processing. Network analysis was conducted using CytoHubba, an integrated plug-in within Cytoscape, employing 11 topology methods derived from shortest-path algorithms. These methods encompass degree, maximum neighborhood component density, edge penetration component, maximum group centrality, and six centralities (closeness, radiality, degree, betweenness, bottleneck, and stress). Notably, the maximum clique centrality (MCC) algorithm has demonstrated superior accuracy in predicting pivotal targets. In this study, key core targets indicative of Ligusticum wallichii’s therapeutic efficacy against myocardial infarction were identified by employing the MCC topology algorithm. Moreover, essential sub-networks of active ingredients from Ligusticum wallichii were delineated within the PPI network, playing a significant role in the therapeutic mechanism.
Enrichment Analysis
GO analysis and KEGG pathway enrichment analysis were conducted using the clusterProfilerGO.R package in R software (https://www.r-project.org/) along with Perl language. An adjusted p-value threshold of <0.05 was applied, and the significant enrichment results were visualized using the ggplot2 package. The identified target genes underwent online analysis via the DAVID database, and were subsequently imported into Omicsbean software with the specified ID type as Gene Symbol and species as “Homo sapiens”. Following this, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and Gene Ontology (GO) enrichment analyses were performed on the target proteins. The resulting data was then imported into an Excel worksheet for further filtering of functions and pathways, with statistical significance set at P < 0.05. Finally, the outcomes were visualized using a bar chart.
Construction of Active Ingredient-Target-Disease Network
The active ingredient-target-disease network was constructed using Cytoscape 3.7.1 software by integrating the shared genes from both active ingredient and disease datasets with the top 20 pathways identified through KEGG analysis.
Molecular Docking
Molecular docking studies were conducted using the identified key targets and their respective ligands, utilizing the docking software AutoDock Vina’ and ‘Discovery Studio 2019’ to assess their interactions. Protein crystal structures were retrieved from the RCSB PDB database (https://www.rcsb.org/), while compounds were sourced from the TCMSP database. AutoDock Tools facilitated the preparation of both the protein and compound structures by isolating the protein from its original ligand, eliminating water molecules, adding charges, and converting the structures to “PDBQT” format. For semi-flexible molecular docking, the Libdock module in Discovery Studio 2019 (DS2019) was employed. This process included removing water molecules from the receptor protein and energy minimization of both the protein and ligand. To ensure accurate ionization and tautomerization of amino acid residues, non-polar hydrogens were merged, and partial atomic charges were assigned using the Gasteiger-Marsili method. The docking points were defined to facilitate the connection. Ultimately, docking results and mapping analyses were derived using Vina docking.
Cell Culture and Treatment
H9C2 cells, procured from the American Type Culture Collection (ATCC), were seeded into 35 mm dishes at a density of 1×106 cells and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The cells were then incubated at 37°C in a humidified atmosphere containing 5% CO2. The cell culture medium was replaced with serum-free and glucose-free DMEM every three days. To establish an in vitro myocardial infarction model, H9C2 cells were washed with PBS and cultured in DMEM. The cells were then incubated under hypoxic conditions (1% O2, 5% CO2, and 94% N2) for 3 h. After the oxygen-glucose deprivation (OGD) treatment, they were placed in a complete culture medium with 5% CO2 and 95% air for varying durations (1, 2, and 3 hours). Control cells were maintained in DMEM under normoxic conditions.
Chemicals and Reagents
Myricanone (CAS No. 32492–74-3, 98% purity) and Sitosterol (CAS No. 5779–62-4, 98% purity) were provided by Baoji Chenguang Biotechnology Co., Ltd (China). Senkyunone (CAS No. 142182–61-4, 98% purity) was procured by Chengdu Pusi Biotechnology Co., Ltd (China). The positive control drug diazoxide (CAS No. 364–98-7, 99% purity) was supplied by Invivochem (Guangzhou, China).
Cell Viability Assay
Cell viability was assessed using a Cell Counting Kit-8 (CCK-8, Cat.No. GK10001) assay kit obtained from GLPBIO, California, United States. H9c2 cells were initially seeded into 96-well plates. Following treatment, each well was incubated with 10 μL of CCK-8 solution for 2 h at 37°C. Subsequently, the absorbance at 450 nm was measured using a microplate reader (Thermo Varioskan LUX, MA, United States).
Reverse Transcription-Quantitative PCR (RT-qPCR)
Trizol, a total RNA extraction reagent, was sourced from Invitrogen, while the qRT-PCR kit and gene-specific primers were acquired from Shanghai Bioengineering Co., Ltd. (China). Total RNA was extracted from the cells using Trizol, followed by a purity assessment. The RNA was then reverse-transcribed into cDNA, using a reaction mixture containing the extracted RNA, 5x RNA buffer, M-MLV reverse transcriptase, RNase inhibitor, deoxyribonucleotide (dNTP), and RNase-free double-distilled water (ddH2O). The reverse transcription reaction was conducted in a PCR machine programmed to 37°C for 5 minutes, 85°C for 5 seconds, and then 4°C to terminate the reaction. Next, cDNA, Taq DNA polymerase, dNTPs, ddH2O, MgCl2, and buffer were amplified in PCR plates. The initial denaturation was set at 94°C for 30s, followed by annealing at 58°C for 15s, and extension at 72°C for 30s. Ct values were recorded for statistical analysis after completing 40 cycles. The primer sequences for EGFR, SRC, and STAT3 utilized in the RT-qPCR analysis are provided in Table 1.
 |
Table 1 qRT-PCR Primer Sequences |
Experimental Animal and Establishment of Myocardial Infarction Model
Adult male C57BL/6 mice (10–14 weeks old, 22–28 g) were obtained from Cavens Lab Animals Co., Ltd (Changzhou, China), with certification under Animal Qualification Certificate No.: SYXK (Xiang) 2020–0002. The mice were housed under controlled conditions (25 ± 2 °C temperature, 60 ± 5% humidity, and a 12-hour light/dark cycle) and allowed a one-week acclimation period before experimentation. This study received approval from the Animal Care Guidelines and Institutional Animal Ethics Committee of Hunan Normal University for the use of experimental animals.
Next, C57BL/6 mice were randomly divided into six groups (n = 6 per group): Sham, myocardial infarction + PBS, myocardial infarction + Myricanone (30 mg/kg), myocardial infarction + Sitosterol (30 mg/kg), myocardial infarction + Senkyunone (15 mg/kg), and myocardial infarction + diazoxide (5 mg/kg). Anesthesia was administered via intraperitoneal injection of sodium pentobarbital (50 mg/kg), and all surgical procedures and subsequent analyses were conducted by a blinded investigator. Myocardial infarction was induced by ligating the proximal left anterior descending coronary artery (LAD), while Sham mice underwent the same surgery without LAD ligation. Medicine or an equal volume of PBS was administered daily via oral gavage for two weeks both before and after the myocardial infarction.
Triphenyltetrazolium Chloride Staining
Heart tissue samples were collected post-euthanasia and cooled in a −80°C freezer for 8 minutes before being sliced into 2–3 mm sections. These sections were then incubated in a 2% triphenyltetrazolium chloride (TTC) solution at 37°C for 30 minutes in the dark, followed by fixation in 4% paraformaldehyde for another 30 minutes. Images were captured using an FSX100 microscope (Olympus, Shenzhen, China), and the sizes of the differently stained areas were analyzed using Image-Pro Plus 6.0 software to calculate the ratio of the infarct area to the left ventricular area.
Serum Biochemistry Assays
Myocardial infarction diagnosis was confirmed by assessing changes in biomarkers indicative of cardiac damage, specifically through measuring serum aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and creatine kinase-isoenzyme MB (CK-MB) activities. Oxidative stress markers, including serum activity levels of catalase (CAT), superoxide dismutase (SOD), and malondialdehyde (MDA), as well as reduced glutathione (GSH) levels, were also evaluated. These assays were performed following the manufacturer’s protocol (Merck, Germany; Beyotime, China) using commercially available kits (AST, Cat.No. MAK467; LDH, Cat.No. MAK066; CK-MB, Cat.No. MABX7621-10KC; CAT, Cat.No. S0051; SOD, Cat. No. 19160; MDA, Cat.No. S0131M; GSH, Cat.No. S0052).
Heart Histopathological Examination
Following sacrifice, myocardial samples were collected and fixed in 4% paraformaldehyde. The heart, liver, lung, spleen, and kidney tissues were then dehydrated, embedded in paraffin, and subjected to hematoxylin-eosin (HE) and/or Masson-trichrome (MT) staining. Images of these stained sections were captured using the FSX100 microscope (Olympus, Shenzhen, China).
Statistical Analysis
The data presented in this study are expressed as mean ± SD from six independent experiments. One-way analysis of variance (ANOVA) followed by post-hoc correction was employed to analyze differences among multiple groups. A significance level of p < 0.05 was used to determine statistical significance in all cases. Statistical analyses were performed using GraphPad software (version 8.0.1) and SPSS 24.0. A p-value < 0.05 was considered statistically significant.
Results
Evaluation of Active Ingredients and Potential Targets of Ligusticum wallichii
Active compounds from Ligusticum wallichii were identified via the TCMSP database, resulting in the retrieval of 7 bioactive compounds by applying the criteria of DL ≥ 0.18 and OB ≥ 30% (Table 2). Subsequently, the TCMSP database was utilized to gather the respective targets associated with these active compounds, yielding a total of 269 corresponding targets following data deduplication and summarization.
 |
Table 2 Active Components in Ligusticum Wallichii |
Acquisition of Myocardial Infarction-Related Targets
The term “myocardial infarction” was queried in both the OMIM and GeneCards databases. Subsequently, the myocardial infarction-related targets identified from these databases were consolidated. Through the screening process, duplicate entries were removed, resulting in a combined total of 1930 targets associated with myocardial infarction.
Construction of the Active Ingredient-Target and PPI Network
The intersection of potential targets of Ligusticum wallichii with those related to myocardial infarction was analyzed, yielding a total of 122 shared targets identified using the Venn diagram tool (Figure 1). Subsequently, the shared targets and active components were input into Cytoscape 3.8.2 for visualization, resulting in the generation of the “active ingredient-target network” (Figure 2). To explore how Ligusticum wallichii shields against myocardial infarction, we initially utilized the String database to analyze the intersection targets. Our criteria comprised selecting Homo sapiens as the organism and setting the confidence level to the highest (0.900). Figure 3A illustrates the network linking drug and disease targets, encompassing 122 nodes and 319 edges, with an average node degree of 5.23 and a local clustering coefficient of 0.900. Next, we imported the network data into Cytoscape 3.8.2 software and employed the NetworkAnalyzer plug-in tool to generate a PPI network diagram (Figure 3B) based on node density values. The size of nodes in the diagram correlated with their degrees, with larger nodes representing genes with higher degrees. Figure 4 presents the top 10 proteins ranked by degree centrality (Degree, Betweenness, and Closeness). Among them, EGFR, SRC, and STAT3 were identified through the intersection of targets obtained via these three methods (Figure 5A and Table 3). Furthermore, Myricanone, Senkyunone, and Sitosterol from Ligusticum wallichii exhibited significant correlations with these core targets (Figure 5B). These proteins play critical roles in PPI and serve as pivotal targets for Ligusticum wallichii’ active ingredients in addressing myocardial infarction.
 |
Table 3 Screening of Key Targets of PPI Network |
 |
Figure 1 The overlap of target genes between the active constituents of Ligusticum wallichii and those associated with myocardial infarction. |
 |
Figure 4 Frequency histograms depicting the top 10 genes according to their degree values in the PPI network. |
Results of Functional Enrichment Analysis
Following the identification of cross-targets, we conducted KEGG and GO enrichment analyses to uncover associated biological functions and metabolic pathways. The set of 122 targets was imported into the Metascape platform for GO functional enrichment analysis, revealing the top 10 significantly enriched entries (Figure 6). The analysis uncovered 65 cellular components (CCs) pathways, including critical functions such as plasma membrane, cytoplasm, cytosol, endoplasmic reticulum membrane, perinuclear region of cytoplasm, and receptor complex. The study identified a total of 128 molecular functions (MF), encompassing various cellular structures such as steroid binding, heme binding, and identical protein binding. Additionally, in terms of biological processes (BPs), the 398 enriched pathways indicated the involvement of diverse processes like inflammatory response, response to lipopolysaccharide, positive regulation of cell migration, and positive regulation of protein kinase B signaling in the therapeutic effects of Ligusticum wallichii on myocardial infarction. Subsequently, the bubble plot results from the KEGG enrichment analysis, depicted in Figure 7, underscored Ligusticum wallichii’ impact on myocardial infarction, revealing 112 pathways. Focusing our comparative analysis on 20 pathways closely linked to lipid and atherosclerosis, PI3K-Akt signaling pathway, EGFR tyrosine kinase inhibitor resistance, HIF-1 signaling pathway, and inflammatory mediator regulation of TRP channels. To visually represent the key pathways and associated targets, a “target-pathway” network diagram was constructed using Cytoscape software (Figure 8). This underscores the significance of these signaling pathways in the potential therapeutic effects of Ligusticum wallichii on myocardial infarction.
 |
Figure 6 GO enrichment analysis. |
 |
Figure 7 Enrichment analysis of KEGG pathways. |
 |
Figure 8 Diagram illustrating the network between targets and pathways. |
Results of Molecular Docking
To explore the interaction between Ligusticum wallichii’ active ingredients and target genes, molecular docking was performed using Pymol and Autodock software. Specifically, molecular docking was conducted for three crucial targets and three active components of Ligusticum wallichii, with corresponding binding energies recorded. Figure 5B demonstrated that the binding energy resulting from molecular docking was notably below −2 kcal/mol, suggesting robust binding capabilities between the target proteins and compounds. Visual representations of the docking results are presented in Figures 9–11. These results indicated that Ligusticum wallichii’ active ingredients have a strong binding affinity to their respective targets.
 |
Figure 9 Molecular docking analysis of active compounds with EGFR. (A) Myricanone-EGFR; (B) Senkyunone-EGFR; (C) Sitosterol-EGFR. |
 |
Figure 10 Molecular docking analysis of active compounds with SRC. (A) Myricanone-SRC; (B) Senkyunone-SRC; (C) Sitosterol-SRC. |
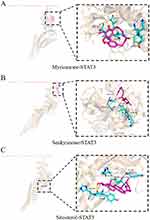 |
Figure 11 Molecular docking analysis of active compounds with STAT3. (A) Myricanone-STAT3; (B) Senkyunone-STAT3; (C) Sitosterol-STAT3. |
Effect of the Active Components of Ligusticum wallichii on OGD-Induced H9c2 Cell Injury
In order to investigate the potential efficacy of Ligusticum wallichii’s active components (chemical structures of Myricanone, Senkyunone, and Sitosterol depicted in Figure 12A) against myocardial infarction, we initially evaluated their cytotoxic effects on H9c2 cell growth. Results indicated that under normal conditions, Myricanone, Senkyunone, and Sitosterol exhibited no cytotoxicity to H9c2 cells at concentrations below 160 μM (Figure 12B). During ischemic conditions, the reduction in glucose and oxygen levels induces damage to cardiomyocytes. Among various in vitro methods for simulating ischemic pathology, OGD is a commonly utilized approach. Thus, to examine the cardioprotective potential of these active components, we established an OGD-induced model in H9c2 cells. H9c2 cells were subjected to OGD insult for specified durations, and cell viability was assessed using a CCK8 assay kit. As depicted in Figure 12C, exposure to OGD for 1, 3, or 6 hours significantly decreased the viability of H9c2 cells, with the most pronounced cell injury observed at the 3-hour mark. Hence, subsequent experimental measurements were conducted after a 3-hour treatment duration. Following this, H9c2 cells were treated with varying doses of Myricanone, Senkyunone, and Sitosterol, along with the positive control drug diazoxide, for 48 hours before OGD exposure. As illustrated in Figure 12D–F, treatment with Myricanone, Senkyunone, and Sitosterol at 80 μM notably protected H9c2 cells against OGD-induced injury. Therefore, we selected 80 μM concentrations of Myricanone, Senkyunone, and Sitosterol for subsequent experiments.
Effect of the Active Components of Ligusticum wallichii on Myocardial Infarction in vivo
To verify the cardioprotective effects of Ligusticum wallichii’s active components following myocardial infarction, in vivo studies were conducted using mice. As shown in Figure 13A and B, infarct size, determined by TTC staining, was notably reduced in the Myricanone, Senkyunone, Sitosterol, and diazoxide groups compared to the myocardial infarction group. Control mice exhibited normal myofibril structure with distinct horizontal striations, minimal necrosis or edema, and maintained continuity between adjacent myofibrils (Figure 13C), with a small presence of collagen fibers (Figure 13D and E). Conversely, myocardial fibers in model mice subjected to LAD ligation showed substantial localized myocardial cell swelling, rupture, and necrosis, accompanied by inflammatory cell infiltration. Myocardial tissue in the myocardial infarction group was largely replaced by collagen, indicating severe myocardial fibrosis due to LAD ligation. Pretreatment with Myricanone, Senkyunone, Sitosterol, and diazoxide notably mitigated these LAD ligation-induced tissue abnormalities, reducing myocardial fibrosis post-infarction (Figures 13C–E). Diagnostic markers, including CK-MB, AST, and LDH, released from the myocardium into the bloodstream upon cellular membrane rupture, serve as indicators of cardiac damage. Mice in the LAD ligation group displayed a significant elevation of these enzyme levels compared to controls. Pretreatment with Myricanone, Senkyunone, Sitosterol, and diazoxide conferred marked protection against these enzyme increases induced by LAD ligation (Figures 13F–H). Cellular defenses against intense oxidative stress rely on antioxidant enzymes. In comparison to the control group, myocardial infarction mice showed a marked elevation in serum MDA levels (Supplementary Figure 1A), alongside reduced serum levels of CAT, SOD, and GSH (Supplementary Figure 1B–D). CAT and SOD help prevent myocardial cell damage induced by free radicals during myocardial infarction. Importantly, pretreatment with the active components of Ligusticum wallichii significantly enhanced these antioxidant enzyme activities. This suggests that the cardioprotective effects of Ligusticum wallichii are partly due to its antioxidative properties. In addition, Ligusticum wallichii’s active components showed no toxic effects in other tissues, including the lung, kidney, liver, and spleen (Supplementary Figure 1E–F). Collectively, these findings suggest that Ligusticum wallichii’s active components significantly improve myocardial infarction outcomes in vivo.
Effect of the Active Components of Ligusticum wallichii on the Modulation of Target Gene Expression
Network pharmacology and molecular docking experiments showed that SRC, EGFR, and STAT3 were the core target genes of Ligusticum wallichii’s active components (Myricanone, Senkyunone, and Sitosterol). Thus, in our next investigation into the influence of active compounds derived from Ligusticum wallichii on the expression of their target genes, we conducted qRT-PCR assays. Results revealed significant decreases in the expression levels of SRC and increases in EGFR and STAT3 expression, upon treatment with Myricanone, Senkyunone, and Sitosterol (Figure 13I–K). Collectively, these findings highlight how the active constituents of Ligusticum wallichii suppress myocardial infarction by modulating target gene expression, corroborating the results obtained from network pharmacology and molecular docking analyses.
Discussion
Myocardial infarction presents a grave health concern with significant morbidity, imposing a substantial burden on both individuals and society.35,36 The multifaceted mechanisms underlying myocardial infarction encompass cell death, mitochondrial dysfunction, inflammatory responses, oxidative stress, and ATP depletion.37 Furthermore, myocardial infarction can precipitate various complications, including arrhythmia, heart failure, and cardiac rupture.38 Traditional Chinese medicine, comprising individual herbs or complex formulas with multiple bioactive compounds, holds promise for pharmacological intervention in myocardial infarction.39,40 Ligusticum wallichii offers a promising complementary approach due to its diverse pharmacological properties, including anti-inflammatory, antioxidant, anti-fibrotic, and immunomodulatory effects.41–43 In this study, we employed a network pharmacology approach complemented by experimental validation and molecular docking analyses to elucidate the pharmacological mechanisms underlying Ligusticum wallichii’s efficacy in myocardial infarction treatment.
Previous studies have documented the cardiovascular disease-modifying properties of the active constituents found in Ligusticum wallichii.44–46 Our investigation unveils the significant intervention potential of Myricanone, Senkyunone, and Sitosterol from Ligusticum wallichii in myocardial infarction. Through molecular docking analysis, we identified these compounds as key mediators in the myocardial infarction intervention process, demonstrating their robust binding affinity to EGFR, SRC, and STAT3. These target genes are integral to the pharmacodynamic effects mediated by the active ingredients of Ligusticum wallichii and play essential roles in the pathogenesis and progression of myocardial infarction. Studies have demonstrated that elevated EGFR levels facilitate cardiac repair and enhance remodeling following myocardial infarction by promoting angiogenesis.47,48 Inhibition of SRC enhances heart function and diminishes fibrosis, edema, and tissue injury after myocardial infarction.49,50 Moreover, activation of STAT3 has been shown to markedly enhance cardiac function and alleviate myocardial hypertrophy and remodeling by inhibiting oxidative stress and inflammation in both in vivo and in vitro models of myocardial infarction.51,52 Of note, our study indicates that Ligusticum wallichii’s active components, particularly Myricanone, Senkyunone, and Sitosterol, show promising potential for myocardial infarction treatment by targeting key proteins such as EGFR, STAT3, and SRC, which play roles in oxidative stress and myocardial fibrosis. These molecular actions suggest that Ligusticum wallichii could serve as a novel adjunctive therapy in myocardial infarction management.
In a clinical context, incorporating Ligusticum wallichii could offer an innovative approach alongside conventional treatments, with its antioxidant and antifibrotic properties potentially enhancing myocardial protection and improving recovery outcomes. For instance, these components could serve as adjunctive therapies to standard interventions such as percutaneous coronary intervention and statins by mitigating post-myocardial infarction complications like oxidative stress and fibrosis, which are central to cardiac remodeling and dysfunction. Conventional treatments, while effective at symptom management and reducing acute damage, often lack these broader protective effects. Antioxidants such as Myricanone, Senkyunone, and Sitosterol components in Ligusticum wallichii demonstrated an enhanced capacity to counter oxidative damage and support cardiac tissue repair, potentially translating into better long-term outcomes for myocardial infarction patients by reducing fibrosis and preserving cardiac function. Our results suggest that Ligusticum wallichii may complement existing therapies by enhancing myocardial resilience and healing. This positions Ligusticum wallichii as a promising adjunct in myocardial infarction therapy, particularly where oxidative stress and inflammation are implicated in recurrent cardiac events. Further clinical trials would be essential to establish safe dosage parameters, evaluate its efficacy when used alongside standard myocardial infarction treatments, and refine administration protocols for optimized patient outcomes. Additionally, given that traditional strategies for treating myocardial infarction may result in ischemia/reperfusion injury, bleeding risk, and the potential for drug-induced liver injury. It is of great significance to perform safety assessment in vivo. Importantly, our results also suggested that Ligusticum wallichii’s active components not only effectively alleviate myocardial infarction in vivo, but also do not elicit side or toxic effects in mice. These findings support the potential of Ligusticum wallichii in clinical settings as a complementary therapeutic option, adding to current pharmacological strategies for myocardial infarction by specifically addressing underlying mechanisms related to myocardial injury and repair. Taken together, by targeting these critical pathways, Ligusticum wallichii holds the potential to address limitations in existing myocardial infarction therapies, offering protection at multiple molecular levels. Through its modulation of core gene expression, Ligusticum wallichii may thus provide a more comprehensive therapeutic strategy for myocardial infarction.
Conclusion
In this study, network pharmacology was employed to analyze the active components and key targets associated with Ligusticum wallichii’s intervention in myocardial infarction. Molecular docking software was utilized to illustrate the robust binding affinity between Ligusticum wallichii’s active ingredients and crucial targets such as EGFR, SRC, and STAT3. In vivo and in vitro experiments validation results indicate that Ligusticum wallichii exerts a protective effect against myocardial infarction by alleviating myocardial fibrosis and oxidative stress. These findings underscore the importance of understanding Ligusticum wallichii’s function and mechanism, offering valuable insights for novel therapeutic approaches to manage myocardial infarction.
Data Sharing Statement
The corresponding author is able to provide access to the datasets utilized and/or analyzed in the present study upon request via a suitable mode of communication.
Acknowledgments
The authors gratefully acknowledge the Natural Science Foundation of Hunan Province, China (Grant Number: 2022JJ70013), National Key Clinical Specialty Construction (Cultivation) Project, and Hunan Provincial Department of Science and Technology Innovation Platform and Talent Program Project (Grant Number: 2023SK4056).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Xu W, Wang L, Zhang R, et al. Diagnosis and prognosis of myocardial infarction on a plasmonic chip. Nat Commun. 2020;11(1):1654. doi:10.1038/s41467-020-15487-3
2. Sagris M, Antonopoulos AS, Theofilis P, et al. Risk factors profile of young and older patients with myocardial infarction. Cardiovasc Res. 2022;118(10):2281–2292. doi:10.1093/cvr/cvab264
3. Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. 2022;327(7):662–675. doi:10.1001/jama.2022.0358
4. Bloch EM, Tobian AAR. Optimizing blood transfusion in patients with acute myocardial infarction. N Engl J Med. 2023;389(26):2483–2485. doi:10.1056/NEJMe2312741
5. Shi HT, Huang ZH, Xu TZ, Sun AJ, Ge JB. New diagnostic and therapeutic strategies for myocardial infarction via nanomaterials. EBioMedicine. 2022;78:103968. doi:10.1016/j.ebiom.2022.103968
6. He J, Liu D, Zhao L, et al. Myocardial ischemia/reperfusion injury: mechanisms of injury and implications for management (review). Exp Ther Med. 2022;23(6):430. doi:10.3892/etm.2022.11357
7. Valgimigli M, Frigoli E, Heg D, et al. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N Engl J Med. 2021;385(18):1643–1655. doi:10.1056/NEJMoa2108749
8. Björnsson ES. Clinical management of patients with drug-induced liver injury (Dili). United Eur Gastroenterol J. 2021;9(7):781–786. doi:10.1002/ueg2.12113
9. Scicchitano P, Cameli M, Maiello M, et al. Nutraceuticals and dyslipidaemia: beyond the common therapeutics. J Funct Foods. 2014;6:11–32. doi:10.1016/j.jff.2013.12.006
10. Zheng Q, Huang YY, Zhu PC, et al. Ligustrazine exerts cardioprotection in animal models of myocardial ischemia/reperfusion injury: preclinical evidence and possible mechanisms. Front Pharmacol. 2018;9:729. doi:10.3389/fphar.2018.00729
11. Wang J, Liu N, Zhang F. Tetramethylpyrazine protects oxidative stability and gelation property of rabbit myofibrillar proteins. Food Sci Anim Resour. 2019;39(4):623–631. doi:10.5851/kosfa.2019.e52
12. Li YJ, Liu RP, Ding MN, et al. Tetramethylpyrazine prevents liver fibrotic injury in mice by targeting hepatocyte-derived and mitochondrial DNA-enriched extracellular vesicles. Acta Pharmacol Sin. 2022;43(8):2026–2041. doi:10.1038/s41401-021-00843-w
13. Li J, Gong X. Tetramethylpyrazine: an active ingredient of Chinese herbal medicine with therapeutic potential in acute kidney injury and renal fibrosis. Front Pharmacol. 2022;13:820071. doi:10.3389/fphar.2022.820071
14. Guo M, Liu Y, Shi D. Cardiovascular actions and therapeutic potential of tetramethylpyrazine (active component isolated from rhizoma chuanxiong): roles and mechanisms. Biomed Res Int. 2016;2016:2430329. doi:10.1155/2016/2430329
15. Chang CY, Wu CC, Pan PH, et al. Tetramethylpyrazine alleviates mitochondrial abnormality in models of cerebral ischemia and oxygen/glucose deprivation reoxygenation. Exp Neurol. 2023;367:114468. doi:10.1016/j.expneurol.2023.114468
16. Zhuang Z, Wang ZH, Huang YY, Zheng Q, Pan XD. Protective effect and possible mechanisms of ligustrazine isolated from Ligusticum wallichii on nephropathy in rats with diabetes: a preclinical systematic review and meta-analysis. J Ethnopharmacol. 2020;252:112568. doi:10.1016/j.jep.2020.112568
17. Kim EY, Rhyu MR. Synergistic vasorelaxant and antihypertensive effects of Ligusticum wallichii and angelica gigas. J Ethnopharmacol. 2010;130(3):545–551. doi:10.1016/j.jep.2010.05.048
18. Wang Y, Zhu H, Tong J, Li Z. Ligustrazine improves blood circulation by suppressing platelet activation in a rat model of allergic asthma. Environ Toxicol Pharmacol. 2016;45:334–339. doi:10.1016/j.etap.2016.06.016
19. Yang B, Li H, Qiao Y, et al. Tetramethylpyrazine attenuates the endotheliotoxicity and the mitochondrial dysfunction by doxorubicin via 14-3-3$\gamma$/Bcl-2. Oxid Med Cell Longev. 2019;2019:5820415. doi:10.1155/2019/5820415
20. Li RW, Yang C, Shan L, et al. Relaxation effect of a novel danshensu/tetramethylpyrazine derivative on rat mesenteric arteries. Eur J Pharmacol. 2015;761:153–160. doi:10.1016/j.ejphar.2015.04.041
21. Zhu X, Zhou Y, Yan S, et al. Herbal medicine-inspired carbon quantum dots with antibiosis and hemostasis effects for promoting wound healing. ACS Appl Mater Interfaces. 2024;16(7):8527–8537. doi:10.1021/acsami.3c18418
22. Zengyong Q, Jiangwei M, Huajin L. Effect of Ligusticum wallichii aqueous extract on oxidative injury and immunity activity in myocardial ischemic reperfusion rats. Int J Mol Sci. 2011;12(3):1991–2006. doi:10.3390/ijms12031991
23. Zhang B, Lu C, Bai M, et al. Tetramethylpyrazine identified by a network pharmacology approach ameliorates methotrexate-induced oxidative organ injury. J Ethnopharmacol. 2015;175:638–647. doi:10.1016/j.jep.2015.09.034
24. Li Y, Zhu J, Tang J. Computational systems pharmacology and molecular docking reveal an anti-apoptosis and anti-inflammatory mechanism of compound angelica ligusticum wallichii granules in the treatment of endometriosis. Drug Des Devel Ther. 2023;17:743–759. doi:10.2147/dddt.S392500
25. Sun JY, Du LJ, Shi XR, et al. An IL-6/STAT3/MR/FGF21 axis mediates heart-liver cross-talk after myocardial infarction. Sci Adv. 2023;9(14):eade4110. doi:10.1126/sciadv.ade4110
26. Li X, Wang Q, Ren Y, et al. Tetramethylpyrazine protects retinal ganglion cells against H2O2‑induced damage via the microRNA‑182/mitochondrial pathway. Int J Mol Med. 2019;44(2):503–512. doi:10.3892/ijmm.2019.4214
27. Yang Y, Johnson J, Troupes CD, et al. miR-182/183-Rasa1 axis induced macrophage polarization and redox regulation promotes repair after ischemic cardiac injury. Redox Biol. 2023;67:102909. doi:10.1016/j.redox.2023.102909
28. Yue R, Lu S, Luo Y, et al. Mesenchymal stem cell-derived exosomal microRNA-182-5p alleviates myocardial ischemia/reperfusion injury by targeting GSDMD in mice. Cell Death Discov. 2022;8(1):202. doi:10.1038/s41420-022-00909-6
29. Nogales C, Mamdouh ZM, List M, Kiel C, Casas AI, Schmidt H. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci. 2022;43(2):136–150. doi:10.1016/j.tips.2021.11.004
30. Zhao L, Zhang H, Li N, et al. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023;309:116306. doi:10.1016/j.jep.2023.116306
31. Noor F, Asif M, Ashfaq UA, Qasim M, Tahir Ul Qamar M. Machine learning for synergistic network pharmacology: a comprehensive overview. Brief Bioinform. 2023;24:3. doi:10.1093/bib/bbad120
32. Pinzi L, Rastelli G. Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci. 2019;20:18. doi:10.3390/ijms20184331
33. Li T, Guo R, Zong Q, Ling G. Application of molecular docking in elaborating molecular mechanisms and interactions of supramolecular cyclodextrin. Carbohydr Polym. 2022;276:118644. doi:10.1016/j.carbpol.2021.118644
34. Crampon K, Giorkallos A, Deldossi M, Baud S, Steffenel LA. Machine-learning methods for ligand-protein molecular docking. Drug Discov Today. 2022;27(1):151–164. doi:10.1016/j.drudis.2021.09.007
35. Salari N, Morddarvanjoghi F, Abdolmaleki A, et al. The global prevalence of myocardial infarction: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2023;23(1):206. doi:10.1186/s12872-023-03231-w
36. Reynolds HR, Smilowitz NR. Myocardial Infarction with nonobstructive coronary arteries. Annu Rev Med. 2023;74:171–188. doi:10.1146/annurev-med-042921-111727
37. Lindahl B, Mills NL. A new clinical classification of acute myocardial infarction. Nat Med. 2023;29(9):2200–2205. doi:10.1038/s41591-023-02513-2
38. Zhang Q, Wang L, Wang S, et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct Target Ther. 2022;7(1):78. doi:10.1038/s41392-022-00925-z
39. Wang Y, Xue Y, Guo HD. Intervention effects of traditional Chinese medicine on stem cell therapy of myocardial infarction. Front Pharmacol. 2022;13:1013740. doi:10.3389/fphar.2022.1013740
40. Zhang J, Weng J, Yuan M, Shen X, Weng Y, Shen X. Effects of traditional Chinese exercises on cardiac rehabilitation in patients with myocardial infarction: a meta-analysis of randomized controlled trials. Front Cardiovasc Med. 2023;10:1223677. doi:10.3389/fcvm.2023.1223677
41. Li D, Long Y, Yu S, et al. Research advances in cardio-cerebrovascular diseases of ligusticum chuanxiong hort. Front Pharmacol. 2021;12:832673. doi:10.3389/fphar.2021.832673
42. Wang J, Wang L, Zhou H. The isolation, structural features and biological activities of polysaccharide from ligusticum chuanxiong: a review. Carbohydr Polym. 2022;285:118971. doi:10.1016/j.carbpol.2021.118971
43. Qin Y, Chen F, Tang Z, et al. Ligusticum chuanxiong hort as a medicinal and edible plant foods: antioxidant, anti-aging and neuroprotective properties in Caenorhabditis elegans. Front Pharmacol. 2022;13:1049890. doi:10.3389/fphar.2022.1049890
44. Lin J, Wang Q, Zhou S, Xu S, Yao K. Tetramethylpyrazine: a review on its mechanisms and functions. Biomed Pharmacother. 2022;150:113005. doi:10.1016/j.biopha.2022.113005
45. Zhang DY, Peng RQ, Wang X, et al. A network pharmacology-based study on the quality control markers of antithrombotic herbs: using salvia miltiorrhiza - ligusticum chuanxiong as an example. J Ethnopharmacol. 2022;292:115197. doi:10.1016/j.jep.2022.115197
46. Tang F, Yan YM, Yan HL, et al. Chuanxiongdiolides R4 and R5, phthalide dimers with a complex polycyclic skeleton from the aerial parts of Ligusticum chuanxiong and their vasodilator activity. Bioorg Chem. 2021;107:104523. doi:10.1016/j.bioorg.2020.104523
47. Howangyin KY, Zlatanova I, Pinto C, et al. Myeloid-epithelial-reproductive receptor tyrosine kinase and milk fat globule epidermal growth factor 8 coordinately improve remodeling after myocardial infarction via local delivery of vascular endothelial growth factor. Circulation. 2016;133(9):826–839. doi:10.1161/circulationaha.115.020857
48. Reichelt ME, O’Brien S, Thomas WG, Headrick JP. Transactivation of the epidermal growth factor receptor in responses to myocardial stress and cardioprotection. Int J Biochem Cell Biol. 2017;83:97–110. doi:10.1016/j.biocel.2016.12.014
49. Weis S, Shintani S, Weber A, et al. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J Clin Invest. 2004;113(6):885–894. doi:10.1172/jci20702
50. Zheng L, Trease AJ, Katsurada K, et al. Inhibition of Pyk2 and Src activity improves Cx43 gap junction intercellular communication. J Mol Cell Cardiol. 2020;149:27–40. doi:10.1016/j.yjmcc.2020.09.004
51. Rao T, Tong H, Li J, Huang J, Yin Y, Zhang J. Exploring the role and mechanism of hyperoside against cardiomyocyte injury in mice with myocardial infarction based on JAK2/STAT3 signaling pathway. Phytomedicine. 2023;128:155319. doi:10.1016/j.phymed.2023.155319
52. Chen ZR, Hong Y, Wen SH, Zhan YQ, Huang WQ. Dexmedetomidine pretreatment protects against myocardial ischemia/reperfusion injury by activating stat3 signaling. Anesth Analg. 2023;137(2):426–439. doi:10.1213/ane.0000000000006487
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






