Back to Journals » Drug Design, Development and Therapy » Volume 19
Exploring the Mechanism of 2ʹ-Hydroxychalcone Improving Copper Sulfate-Induced Inflammation in Zebrafish Through Network Pharmacology
Authors Shen Y , Yao YD, Li H, Zhang Q, Wang CL , Hu L, Hu YC , Chen MH
Received 4 December 2024
Accepted for publication 28 May 2025
Published 4 June 2025 Volume 2025:19 Pages 4809—4834
DOI https://doi.org/10.2147/DDDT.S510195
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Tamer Ibrahim
YuZhou Shen,1,* Yan Dong Yao,1,* Haili Li,1,* Qian Zhang,1,2,* Cheng Lin Wang,1,* Li Hu,1 Ying Chun Hu,1 Mu Hu Chen1
1Department of Emergency Medicine, The Affiliated Hospital of Southwest Medical University, Lu Zhou, People’s Republic of China; 2Department of Infectious Diseases Department, The Affiliated Hospital of Southwest Medical University, Lu Zhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mu Hu Chen, Email [email protected] Ying Chun Hu, Email [email protected]
Introduction: 2ʹ-Hydroxychalcone is universally acknowledged as a Chinese medicine monomer featured by aromatic properties, exhibiting anti-inflammatory and antioxidant effects. As a consequence, the study emphasis was placed on the anti-inflammatory, anti-oxidative and exercise capacity reinforcement effects of 2ʹ-Hydroxychalcone on Danio rerio young fish under the action of CuSO4. Simultaneously, research endeavors were made to delve into how functional changes of target affect the inflammation and exercise capacity of Danio rerio young fish.
Methods: Upon mating breeding, mature transgenic zebrafish and type AB zebrafish expressing red fluorescent macrophages T g (mpeg1:m Cherry) were cultured for 72 h and exposed to 12.5, 6.25, 3.14 and 0uM 2ʹ-Hydroxychalcone, respectively, for three hours of pretreatment, which were subsequently incubated in CuSO4 at 20uM concentration for 12 h. A diverse array of test indexes was hereby utilized, encompassing the migration of red fluorescent-labeled macrophages, levels of inflammatory cytokines, zebrafish behavioral motility, and gene expression patterns correlated with oxidative stress and mitochondrial biogenesis, to assess the drugs’ efficacy in alleviating inflammation.
Results: 2ʹ-Hydroxychalcone anti-inflammatory target protein was found by adopting the bioinformatics method. Its effect on zebrafish behavior ability and the change trend of oxidative stress index were explored by changing the functional state of the target, such as changing the functional activity of the target by micro-injection technology. As indicated by the results, 2ʹ-Hydroxychalcone could hinder the migration of macrophages and the mitochondrial function of CuSO4. Apart from that, 2ʹ-Hydroxychalcone could lessen the level of inflammatory factors and oxidative stress. In addition, 2ʹ-Hydroxychalcone conspicuously hindered the expression of interleukin-1β and interleukin-TNF-α, and lowered the expression of COX2. An augment in the levels of the target protein TRPV1 was observed during inflammation.
Discussion: The experimental findings validated the anti-inflammatory and anti-oxidant activities of 2ʹ-Hydroxychalcone and preliminarily confirmed the effect of the target on the behavior and oxidative stress level of zebrafish.
Keywords: 2ʹ-hydroxychalcone, CUSO4, mitochondrial biogenesis, oxidative stress reaction, behavior analysis system, ROS
Introduction
Chalcones, a distinctive class of organic compounds featured by their α, β-unsaturated carbonyl system, have captured enormous attention in the field of pharmacology attributable to their broad spectrum of biological activities. It is particularly noteworthy that these distinctive compounds serve as essential precursors in the biosynthetic pathway of flavonoids within plants. More importantly, their synthetic derivatives have been the focal point of extensive laboratory studies, aiming to explore and utilize their therapeutic potential across a vast spectrum of medical applications. The intricate association between the chemical structure of different chalcone derivatives and their interaction with biological targets is a realm of research that continues to captivate scientists worldwide.
Among a multitude of pharmacological activities attributed to chalcones, their potential as anti-inflammatory agents stand out prominently. These compounds primarily exert their anti-inflammatory effects by specifically targeting and inhibiting the key mediators of the inflammatory response, such as cyclooxygenase, prostaglandin E2, inducible nitric oxide synthase, and nuclear factor κB. Researchers have meticulously probed into the structure–activity relationships (SARs) of chalcone derivatives to optimize and reinforce their efficacy as anti-inflammatory agents. Cutting-edge computational methods have been instrumental in determining the optimal orientation of chalcone scaffolds against critical enzymes like cyclooxygenase-1 and cyclooxygenase-2, which are paramount for the formation of stable and effective enzyme-inhibitor complexes.1
In the realm of cancer research, chalcones have emerged as promising candidates for anti-cancer agents. For instance, through meticulous chemical modifications, 2-hydroxy-4-alkoxy chalcone derivatives have been synthesized and subsequently assessed for their cytotoxic activity against a wide spectrum of human tumor cell lines. These derivatives have demonstrated the ability to induce apoptosis through the mitochondrial pathway, which evidently underlines their enormous potential as potent anticancer agents. Quantitative structure–activity relationship (QSAR) studies have offered golden insights, illustrating that the presence of the α, β-unsaturated carbonyl double bond and the planar structure of chalcones are pivotal for their biological activity.2
On top of that, chalcones have been investigated for their potential role in overcoming drug resistance, which is a significant challenge in cancer therapy. They have been identified as inhibitors of P-glycoprotein, a multi-drug resistance efflux transporter that is normally responsible for the lessened effectiveness of chemotherapy drugs. Relevant scholars have synthesized revolutionary chalcone derivatives and probed deep into their ability to hinder the efflux of daunomycin, a common chemotherapy agent, thereby demonstrating remarkable activity in this context. The pharmacophoric features that influence the inhibitory activity against P-GP include hydrogen bond acceptors, methoxy groups, and hydrophobic groups, which conduct a crucial role in the design of more effective chalcone-based inhibitors.3
One of the principal mechanisms by which chalcones exert their anti-inflammatory effects involves the inhibition of cyclooxygenase (COX) enzymes, with a particular emphasis on COX-2, which serves as a pivotal player in the inflammatory process. As correlated studies have demonstrate, chalcone derivatives can selectively hinder COX-2 activity, thereby reducing the production of pro-inflammatory prostaglandins.4 In particular, chalcones have been reported to suppress the expression of inducible nitric oxide synthase (INOS) and the production of nitric oxide (NO), both of which are involved in the inflammatory response.5
Another pivotal pathway targeted by chalcones is the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway. NF-κB is a transcription factor that regulates the expression of multifarious pro-inflammatory cytokines and enzymes. Chalcones have been demonstrated to hinder the activation of NF-κB, thereby lowering the expression of inflammatory mediators such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β).6,7 This inhibitory effect is frequently attained by suppressing upstream kinases and impeding the nuclear translocation of NF-κB.
Aside from that, chalcones have been found to possess antioxidant properties, which contribute to their anti-inflammatory effects. By lowering oxidative stress, chalcones can further mitigate inflammation and protect tissues from damage.8 The combination of these mechanisms makes chalcones versatile agents in the management of inflammatory conditions.
As a consequence of its unique advantages and versatility, the zebrafish (Danio rerio) has emerged as a crucial model organism in numerous fields of biomedical research. Zebrafish have been extensively utilized, primarily in the realm of vertebrate development research, with a particular focus on the cardiovascular system. The ability to monitor heart contraction and cytosolic calcium oscillations in zebrafish has offered innovative insights into cardiac physiology and pharmacology. For instance, relevant studies have suggested that dopamine and verapamil can alter calcium signaling and muscle contraction in anesthetized zebrafish, highlighting the model’s utility in studying cardio-pharmacological responses.9
Other than cardiovascular research, zebrafish are favourable to facilitate the study of neurodevelopmental disorders. The model’s genetic tractability and the ability to recapitulate human disease phenotypes render it an exceptional tool for understanding the genetic underpinnings of these disorders. Recent advancements have fixed their attention on modeling neurodevelopmental disorders in zebrafish, exploring early brain development, gene expression modulation, and phenotype characterization. This research holds immense significance for the development and evaluation of potential therapeutic compounds.10
Zebrafish constitute a highly potent model for investigating the metabolism of both endogenous compounds and xenobiotics. The conservation of enzymes involved in these metabolic processes between zebrafish and humans makes them an ideal system for investigating the physiological, pharmacological, and toxicological roles of cytosolic sulfotransferases (SULTs). This exploration lays a robust foundation for further investigating SULTs’ involvement in homeostasis and detoxification processes.11
Moreover, zebrafish have been adopted to dig into the role of autophagy in skeletal development and disease. The zebrafish model, with its advantages such as high fecundity and optical transparency, tremendously facilitates the examination of developmental processes and genetic mutations. Research in this domain is directed towards identifying potential therapeutic targets for skeletal disorders, thereby underscoring the zebrafish’s significance in the fields of developmental biology and disease research.12
Altogether, the zebrafish model system continues to be a valuable tool in a diverse array of research domains, offering insights into vertebrate biology and disease mechanisms. Its applicability in high-throughput screening and drug discovery further highlights its enormous potential in translational research.
In some sense, the anti-inflammatory effects of chalcone derivatives are associated with the release inhibition of inflammatory mediators, such as nitric oxide, TNF-α, and interleukin-1beta, all of which are generated through lipopolysaccharide-stimulated macrophages.13 The effect may stem from the concurrent suppression of multifarious inflammatory mediators or the activation of transcription factors,14 such as NFKB, AP-1, which regulate the inflammatory response by direct inhibition.15,16 Nowadays, chalcone has been proven to activate endogenous cytoprotective pathways and amplify its anti-inflammatory effects.17,18 Likewise, polyphenolic compounds such as curcumin and caffeic acid benzene exhibit cytoprotective properties. The ethyl ester of these compounds, which induces HO-1 and requires the activation of the transcription factor Nrf2, further contributes to cytoprotecting.19,20
This research is dedicated to probing into the anti-inflammatory, anti-oxidative and oxidative effects of CuSO4-induced 2ʹ-Hydroxychalcone on zebrafish, and to investigating factors influencing the behavior and oxidative stress of zebrafish.
Materials
2ʹ-Hydroxychalcone and copper sulfate were sourced from Shanghai Tao Shu Biotechnology Co., Ltd. (CAS 1214477); RAW264.7 cells were purchased from Shanghai Ming jin Biotechnology Co., Ltd. Sigma-Aldrich 7631. Rapid RNA extraction kit was provided by Chengdu Dongsheng Science and Technology Co., Ltd. (NB304-2). Reverse transcription reagent and fluorescence quantitative reagent were sourced from Seq-Hunt biotechnology CA01-100 and AF01-5; IL-1β. TNF-α enzyme-linked immunosorbent assay kit was provided by Beijing Hua Bo Biotechnology Co., Ltd., Beijing, HBDY-91117O2, HBDY-2212O2. The TRPV1 agonists and inhibitors were purchased from Shanghai Tao Shu Biotechnology Co., Ltd. (CAS 16561298, CAS 331395), DMSO and 3-Aminobenzoate Methanesulfonic Acid Co., Ltd. All the aforementioned reagents were stored at −20°C, and the working solutions were prepared by diluting with 0.1% DMSO on the day of the experiment.
Equipment
The hereby-used apparatus included an SZX16, Olympus, Tokyo, Japan; 6-well plate, a BIOFIT, Guangzhou, China; a Centrifuge 5425 R. Germany, USA; a zebra fish behavioral analysis system (DANIOVISION, GHAMHER, Tokyo, Japan); Zebrafish micro-injection system (SZX10, Olympus, Tokyo, Japan). Zebrafish incubators, etc.
Experimental Methods
Selection of Experimental Animals
In line with the ARRIVE guidelines, the key aspects concerning animal models for in vivo experiments include:
- Ethical Statement: In accordance with the European Union’s animal protection laws, zebrafish embryos and larvae within five days of birth are not considered animals and can thus be employed as substitutes for mammalian testing, adhering to the 3R principles (Replacement, Reduction, Refinement);
- Study Design: Cultivation is conducted in aquariums with freshwater and ventilation, with the water temperature maintained at 28°C, and a light cycle of 14 hours of illumination followed by 10 hours of darkness. Zebrafish larvae are fed twice daily. Prior to further experimentation, juvenile fish are typically examined under a microscope to ensure normal morphological development.21 The night prior to when experimental embryos are required, one or two mature males are paired with two females in individual tanks, to facilitate mating and ensure spawning takes place the following day. The resulting embryos are initially quality-checked under a stereomicroscope, with only those of acceptable quality retained for further culture in an aquarium for 72 hours.
- Description of experimental animals: This study utilizes larvae (within 5 days post-fertilization) derived from matings of mature AB wild-type zebrafish and T g (mpeg1:m Cherry) transgenic zebrafish (over 6 months post-fertilization). These animals were provided by the zebrafish experimental center at the Cheng bei campus of Southwest Medical University.
Screening of CuSO4 Concentration Effects
Zebrafish larvae were subjected to a range of CuSO4 solution concentrations in their embryo culture water for a duration of for 6 hours to induce inflammation. Untreated and treated larvae were used as control (n = 30) and experimental (n = 30) groups, severally.22
Zebrafish larvae were initially exposed to dissimilar concentrations of 2ʹ-Hydroxychalcone solution for 6 hours to screen for early toxic doses, followed by co-incubation with CuSO4 to determine long-term concentrations. Untreated and treated larvae were categorized as control (n = 30) and experimental (n = 30) groups, respectively.
Bioinformatics Analysis
Prediction of 2ʹ-Hydroxychalcone Target Genes
The Swiss Target Prediction database (http://www.swisstargetprediction.ch/)23 and the SUPERPRED database (https://prediction.charite.de/index.php)24 were utilized to identify the target genes of the 2ʹ-Hydroxychalcone monomeric compounds. The conditions were set as follows: targeting Homo sapiens, and duplicate entries were removed to facilitate the generation of a final list.
Prediction of Inflammation-Related Genes
Online Mendelian Inheritance in Man (OMIM) (https://www.omim.org/) was employed for this experiment25 Disease Gene Interaction (DISGENET) (https://www.disgenet.org/)26 and GENECARDS (http://www.genecards.org/)27 inflammatory-related genes were screened. The criteria were established with “inflammation” as the principal keyword, and any redundant genes were subsequently eliminated.
Crossover of Targets Between Active Ingredient and Disease
By using Venny (https://bioinfogp.cnb.csic.es)28 potential target genes associated with 2ʹ-Hydroxychalcone and inflammation were analyzed to identify overlapping targets between the bioactive components and the disease.
Protein–Protein Interaction (Protein–Protein Interaction, PPI)
STRING (https://string-db.org/)21 was employed to investigate the overlapping associations between 2ʹ-Hydroxychalcone target genes and inflammation. The conditions were set for Homo sapiens, with a confidence score cutoff of ≥0.400 (medium confidence) as the threshold for association.
Target Network Construction
The compound target network was constructed by employing the visualization software CYTOSCAPE (https://cytoscape.org/)29 to more effectively illustrate the correlations between 2ʹ-Hydroxychalcone and inflammation. The common target genes of 2ʹ-Hydroxychalcone and inflammation were employed as network nodes. Subsequently, the connections among these nodes were among these nodes were depicted by drawing lines to construct an interconnected target network.
Analysis of Gene Ontology and Pathway Enrichment
GO and KEGG analyses were conducted by adopting annotations, visualizations, and the Database30 for Annotation, Visualization, and Integrated Discovery (DAVID) (https://david.ncifcrf.gov/home.jsp)31 The software displayed the top 30 entries determined through computational analysis. A P-value of less than 0.05 was used to indicate statistical significance.
Molecular Docking
The 2D structure of the small molecular ligand was retrieved from the PubChem database (http://pubchem.ncbi.nlm.nih.gov/)32 and the 2D structure was input into the Chem Office 20.0 software to form the 3D structure, which was saved as a mol2 file. Following the above steps, the crystal structure of TRPV1 protein constructed by AlphaFold was selected as the molecular docking receptor by employing UNIPROT database. Molecular Operating Environment 2019 software was adopted to minimize the compound energy, pre-treat the target protein, and spot the active pocket. At last, molecular docking was performed by utilizing MOE 2019, with the docking protocol configured to execute 50 iterations. The binding affinity of the interactions was assessed on the basis of the calculated binding energies. The outcomes were visualized by utilizing PyMOL2.6.0 and Discovery studio 2019 software.
Molecular Dynamics Simulation
Small molecules were prepared by adopting AmberTools22 to implement the GAFF force field, while Gaussian 16 was utilized for hydrogenation and RESP charge calculation. The potential data derived were incorporated into the topology file for molecular dynamics simulations, which were performed at a static temperature of 300 K and atmospheric pressure (1 bar). On top of that, the force field was Amber99sb-ildn, the solvent was water molecules (Tip3p water model), and the total charge of the simulation system was neutralized by adding the appropriate number of Na+ ions. The molecular dynamics simulation system initially minimized the energy by employing the steepest descending method. Subsequently, equilibrium simulations were carried out using the isothermal-isobaric ensemble (NVT) and the isothermal-isobaric ensemble (NPT), with a coupling constant of 0.1ps and a duration of 100ps. In the end, the free molecular dynamics simulation was carried out in 5,000,000 steps, with each step having a length of 2 fs, resulting in a total simulation time of 100 ns. Subsequent to the simulation, the trajectory analysis was performed by adopting the software’s proprietary tools to calculate the root mean square variance (RMSD), root mean square fluctuation (RMSF), and the radius of gyration for each amino acid. These calculations were complemented with MMGBSA free energy estimates and free energy landscape data.33
Cell Culture
RAW264.7 cells were cultivated for culture in complete medium enriched with 10% fetal bovine serum and incubated at 37°C in an atmosphere containing 5% carbon dioxide. Following a period of 72 hours, the medium was refreshed, and the cells were gently detached when the cells reached 70–80% confluence. They were subsequently suspended and evenly distributed into a 96-well plate for another 24 hours of culture, so as to assess whether the cell count could satisfy the experimental requirements. Cells in the inflammatory group were cultured in medium containing LPS (100 ng/mL) without penicillin-streptomycin solution for 6 hours, while at the same time point, the medium of the control cells was replaced without any further treatment. In the inflammatory treatment group, the cells were treated with drug for 4 h, and the cell supernatant was subsequently collected after 6 hours of inflammatory stimulation with LPS.
Screening of the Medicine Monomer 2ʹ-Hydroxychalcone
By adopting RAW264.7 macrophages, an anti-inflammatory TCM monomer library (MCE, Shanghai, China) was purchased and screened for monomeric anti-inflammatory drugs through the construction of an inflammatory model. The level of TNF-α in the cell culture supernatant was detected by employing a double-antibody sandwich method. Afterwards, the specimen was added to microplate wells coated with TNF-α antibodies, then combined with a detection antibody labeled with horseradish peroxidase, forming an antibody-antigen-enzyme-labeled antibody complex. After thoroughly washing, the TMB chromogenic agent was added to facilitate color development. The TMB-ELISA substrate system turns blue under the catalysis of HRP enzyme and then turns yellow under the action of acid. The intensity of the color is directly proportional to the concentration of TNF-α in the sample. The absorbance is measured at a wavelength of 450 nm by employing a microplate reader, and the content of TNF-α in the sample is calculated in accordance with the standard curve. The intensity of the color demonstrates positive association with the TNF-α in the sample.34
Induction of Inflammatory Changes by CuSO4 Under Drug Influence
Statistical Changes in Transgenic Fluorescent Macrophages
Transgenic fry was distributed in 6-well plates (divided into 5 groups: control group (PBS), model group (20 µmol/mL CuSO4), and three drug groups (CuSO4 + 12.5 µmol/L 2ʹ-Hydroxychalcone, CuSO4 + 6.25 µmol/L 2ʹ-Hydroxychalcone, CuSO4 + 3.13 µmol/mL 2ʹ-Hydroxychalcone). Each group comprises 30 juvenile fish. Additionally, a minimum of three parallel repeat experiments are conducted. The duration of the pre-treatment remains consistent with previous procedures. In order to facilitate the statistical analysis of macrophage changes, CuSO4 is introduced to induce a 12-hour period prior to data collection.
Quantitative Analysis of Reactive Oxygen Species (ROS)
In this experimental study, we utilized a ROS (Reactive Oxygen Species) detection kit to measure the ROS levels in zebrafish belonging to the control group, model group, and treatment group. The experimental procedure was carried out as follows: ① First and foremost, the three groups of zebrafish—control, model, and treatment—were exposed to 2ʹ-hydroxychalcone solution for a duration of 24 hours. With regard to post-treatment, the zebrafish were incubated with 10μM DCFH-DA (2ʹ,7ʹ-dichlorofluorescein diacetate) in the dark for 30 minutes, enabling DCFH-DA to enter the zebrafish and undergo oxidation into DCF (dichlorofluorescein), thereby marking the internal ROS.② Subsequent to incubation, the surviving zebrafish were rinsed three times with PBS (phosphate-buffered saline) to remove unincorporated DCFH-DA, with strict light avoidance during the process to prevent degradation of DCFH-DA and DCF under light exposure. ③ Afterwards, they were anesthetized with 0.16% M%-222, a commonly used zebrafish anesthetic, so as to maintain the stability of the zebrafish during subsequent imaging. ④ After anesthetizing and stabilizing the zebrafish, a fluorescence microscope was utilized to capture images of each larvae’s side. ⑤ These images documented the fluorescence signals of ROS production within the zebrafish. ⑥ The Image-Pro Plus software was utilized to perform quantitative analysis of fluorescence intensity. ⑦This software enabled us to extract fluorescence intensity data from the collected images, allowing us to compare the differences in ROS levels among the control, model, and treatment groups. ⑧ Consequently, we assessed the impact of 2ʹ-hydroxychalcone on ROS production in zebrafish and its potential therapeutic effects in the treatment group.
Changes of Motor Behavior of Larvae Upon 2ʹ-Hydroxychalcone Exposure
The grouping of zebrafish and the number of individuals in each group were as previously specified. To gain a clearer understanding of the impact of 2ʹ-Hydroxychalcone on the motor behavior of zebrafish, we conducted alternating light-dark experiments within a 24-hour period following CuSO4 induction.
Expression of Inflammatory Factors
Determination of Inflammatory Factor Expression
In this section, zebrafish grouping and quantity remained unchanged in line with the instructions of the tissue extraction kit. To induce the “inflammatory storm phenomenon” in early zebrafish, juvenile fish were pretreated with varying concentrations of 2ʹ-Hydroxychalcone, followed by CuSO4 induction for 12 hours, after which total RNA was extracted. Gene primers were designed and compared through the NCBI website and synthesized by Beijing Gene Chem Co., Ltd., with primer sequences detailed in Supplementary material 1. The PCR reaction procedure was meticulously performed in accordance with the step-by-step instructions provided by the reagent manufacturer.
The changes of inflammatory proteins and oxidative stress in zebrafish were measured in a systematic manner.
The approach entails pre-treating juvenile zebrafish with dissimilar concentrations of 2ʹ-Hydroxychalcone, followed by a 12-hour induction with CuSO4, after which the fish are homogenized in cold saline. The additional details remain consistent with the procedures previously outlined. The homogenate is subsequently centrifuged at 4°C at 5000 rpm for 10 minutes to collect the supernatant, which is subsequently utilized for the following analyses. Inflammatory cytokines such as IL-1β and TNF-α, as well as redox indicators encompassing GR, GST, GPX, and COX2, are measured in line with the kit instructions. The assessment of inflammatory mediators is conducted by evaluating their gene expression levels.
Determination of the Damage Effect of CUSO4 on the Functional Morphology of Mitochondria
① The zebrafish grouping and treatment methods (quantity) will still coincide with the previous setup, which was actualized by utilizing the expression of the ATP synthase subunit 1β gene to reflect the basic functional state of the mitochondria.
② Changes were observed in mitochondrial morphology by utilizing transmission electron microscopy.
Wild-type zebrafish were distributed in 6-well culture plates (divided into 5 groups), which comprise a control group (PBS), a model group (CuSO4), and three drug groups (CuSO4 + 12.5 uM 2ʹ-Hydroxychalcone), (CuSO4 + 6.25 uM 2ʹ-Hydroxychalcone), (CuSO4 + 3.13 uM 2ʹ-Hydroxychalcone). Each group consisted of 30 juvenile fish. It is noteworthy that the experiments were conducted in parallel and repeated for a minimum of three times.
Specific operational steps for transmission electron microscopy are detailed in Supplementary material 2.
The Function Alteration of Target TRPV1 on Zebrafish Behavior, ROS, and Macrophage Migration
① Wild-type larvae were incubated to 72 hours post-fertilization and then divided into five groups of 30 larvae each. TRPV1 agonist PMA was prepared at concentrations of 0, 10, 50, 100, and 200 uM, separately. The solution was administered into the yolk sacs of the larvae through micro-injection, with an injection volume of approximately 2–3 nl. Subsequent to injection, each group of larvae was immersed in embryo culture water, and 24 hours later, a behavioral screening experiment was conducted. (To eliminate any errors arising from the injection process, the embryos were carefully screened post-injection, and only healthy larvae were selected for inclusion in the experiment) ② Likewise, the concentrations of the TRPV1 inhibitor CA were selected as 0, 10, 20, 50, and 100 uM, severally. These were injected into the yolk sacs of juvenile fish to observe behavioral changes. Quality control checks were also conducted on the injected juvenile fish.
③ To identify for the optimal drug concentration, we subjected ten juvenile fish, randomly selected from each of the aforementioned groups, to both ROS staining and macrophage migration analysis. This allowed us to assess how alterations in TRPV1 function affect ROS levels. Each experimental group was tested no fewer than three times.
Probing deep into how the interaction between 2ʹ-Hydroxychalcone and its target affect inflammatory changes
The study is designed to delve into how 2ʹ-Hydroxychalcone affect target interactions in the context of inflammatory changes. To ascertain the expression of target genes under inflammatory conditions, juvenile fish were categorized into five distinct groups (control injection group, model injection group, and three groups treated with different drug concentrations). The control group was injected with PBS as a reference. Each group consisted of 30 fish. Micro-injection was performed on the yolk sac by utilizing the selected concentrations of agonists or inhibitors as reference quantities. After a 24-hour period, macrophage migration and ROS staining were observed under a microscope, with the experiments being conducted in triplicate at a minimum to ensure reproducibility.
Statistical Analysis
Data statistical analysis was conducted by employing GraphPad Prism 9 (La Jolla, USA, www.graphpad.com). Afterwards, the research findings are presented as mean ± SD unless otherwise specified. Normal distribution was first analyzed by employing the Shapiro–Wilk test, followed by the Bartlett or Levene test for homogeneity of variances. Statistical significance was evaluated by adopting two-tailed unpaired Student’s t-tests or one-way ANOVA. When the data deviate from a normal distribution, the non-parametric Kruskal-Walli’s test is applied, followed by paired Mann–Whitney U-tests. In this experiment, there is one control group and multiple treatment groups. When comparing each treatment group to the control group, the preferred approach to controlling experimental error rates is the application of the DMRT test. Statistical significance is established in all analyses when the p-value is less than 0.05 (indicated by: *p < 0.05, **p < 0.01, ***p < 0.0001).
Results
2ʹ-Hydroxychalcone Drug Concentration Screening
Elisa kit was employed to measure the concentration of TNF-α inflammatory factors in serum, serving as an indirect indicator of the anti-inflammatory properties of traditional Chinese medicine monomer. As demonstrated in Figure 1, the expression of LPS TNF-α exhibited a marked augment, demonstrating conspicuous up-regulation in contrast to the control group (P < 0.0001).
 |
Figure 1 The expression of TNF-α protein in control group, model group and drug group were detected by Elisa kit. **** Denotes P < 0.0001. |
Biological information analysis section (2ʹ-Hydroxychalcone and inflammatory) is uploaded as Supplementary material 3.
138 target genes for 2ʹ-Hydroxychalcone were identified in Supplementary material 4.
Screen for the Concentration of Action of 2ʹ-Hydroxychalcone
The concentration of 2ʹ-Hydroxychalcone was determined grounded in the lethal curve of zebrafish, during which the MTC of 2ʹ-Hydroxychalcone was found to below 25 uM (Figure 2A). At a concentration of 25 uM, 2ʹ-Hydroxychalcone and the CuSO4 group showed approximately 90% mortality after 48 hours, while the CuSO4 model group had about 75% mortality. The mortality rate was approximately 30% at a concentration of 12.5 uM, around 50% at 6.25 uM, and nearly 70% at 3.13 uM, whereas the CuSO4 model group consistently exhibited a mortality rate of about 75% (Figure 2B).
The Effect of 2ʹ-Hydroxychalcone on the Accumulation of
Inflammatory Cells and Oxidative Stress Resulted from CuSO4
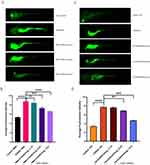
As suggested in Figure 3A, the majority of macrophages remained in the ventral trunk in the control group. As exhibited in Figure 3B, the macrophages in zebrafish larvae exposed to CuSO4 for 6 hours actively migrated from the ventral trunk to the ventrolateral area. The number of ventrolateral fluorescent-labeled macrophages was counted subsequent to the above steps. It was determined that the inflammation inhibition rate achieved with 12.5 uM of 2ʹ-Hydroxychalcone could reach up to 60% (Figure 3C). Upon comparing the fluorescence intensity between the control group and different drug groups, it was noted that the control group displayed the faintest green fluorescence, while the CuSO4 group showed the most intense fluorescence. The fluorescence intensity lowered as the concentration of 2ʹ-Hydroxychalcone increased, as suggested in Figure 3D. Statistical analysis by adopting ImageJ software demonstrated that the oxidative stress ROS levels in zebrafish revealed a progressive abatement with the augment of drug concentration (Figure 3E).
The Effect of 2ʹ-Hydroxychalcone on the Expression Levels of Inflammatory Cytokine mRNAs and Proteins
As demonstrated in Figure 4A and B, after induction by CuSO4 (20 uM), the expression levels of IL-1β and TNF-α were dramatically higher than those of the control group (P < 0.05). Under the influence of different concentrations of 2ʹ-Hydroxychalcone, the expression levels of IL-1β and TNF-α mRNAs were strikingly lower than those of the CuSO4 group (P < 0.05). As illustrated in the results of Figure 4C, compared with the control group, the protein concentration of IL-1β in the CuSO4-exposed samples was noticeably increased (P < 0.05). In different exposure concentrations of 2ʹ-Hydroxychalcone, the protein levels of IL-1β stemmed from CuSO4 were all lessened, with 12.5 uM 2ʹ-Hydroxychalcone remarkably decreasing the CuSO4-induced protein levels of IL-1β. Similarly, as depicted in Figure 4D, the protein concentration of TNF-α in the CuSO4-exposed samples was strikingly higher in comparison with the control group (P < 0.05). In the groups exposed to 6.25 uM and 12.5 uM of 2ʹ-Hydroxychalcone, there was a noticeable abatement in CuSO4-induced TNF-α expression (P < 0.05).
Effect of 2ʹ-Hydroxychalcone on the Behavior of Zebrafish Spawned from CuSO4
The observed abnormal behavioral activities encompassed cell sac opening or closure, yolk absorption, arrhythmia, and other such manifestations. For this reason, the changes of mean velocity, mean distance of motion and rotation angle of larvae under light and dark stimulation were hereby analyzed to dig into the effect of 2ʹ-Hydroxychalcone exposure on light and dark sensitivity. In Figure 5A and B, the mean velocity of larvae exposed to 20uM CuSO4 was substantially lowered in response to stimulation at high and low velocities at light-dark rhythm in comparison with the control group (P < 0.05). Simultaneously, the average speed of larvae was strikingly legated when treated with 6.25uM and 12.5uM 2ʹ-Hydroxychalcone (P < 0.05). These observations jointly illustrated that CuSO4 exposure hindered the sensitivity of larvae to light, suggesting the emergence of a hysteresis reaction. The zebrafish larvae exhibited a recovery in their light-dark responses, accompanied by an augment in movement distance (P < 0.05). Aside from that, under the influence of CuSO4, the rotation angle of the zebrafish was observed to be in a decreasing trend, as demonstrated in Figure 5C. Nevertheless, the rotation angle was recovered under the action of different concentrations of 2ʹ-Hydroxychalcone. Figure 5D and E display heat maps and trajectory maps through the zebrafish behavioral system, which illustrates substantial distinctions in performance under drug action.
Effect of 2ʹ-Hydroxychalcone on Oxidative Stress in Zebrafish
In this section, the expression levels of GRs, GSTs, GPXs and COX2s were assessed and compared to look into the effect of 2ʹ-Hydroxychalcone on oxidative stress in zebrafish. Figure 6A–D present the effect of 2ʹ-Hydroxychalcone on the gene levels of GR, GST, GPX and COX subsequent to CuSO4 induction. As exhibited in the Fig. s, the levels of GR, GST and GPX in the 20uM CuSO4 model decreased while those of COX increased (P < 0.05). The levels of GRs, GSTs and GPXs in 6.25uM and 12.5uM 2ʹ-Hydroxychalcone groups were dramatically increased, while those of COXs were noticeably lessened (P < 0.05).
Effects of CuSO4 on Mitochondrial Morphology, Mitochondrial ATP Synthase Beta and Target TRPV1
As illustrated in Figure 7A, transmission electron microscopy reveals a homogeneous distribution of cytoplasm within the muscle tissue sections of zebrafish sections, characterized by fractured and dissolved myofibrils. The light-dark band structures are distinct, myofilaments are densely packed, and sarcomeres exhibit symmetry. Mitochondria (M, green) exhibit intact membranes, slightly deeper matrix density, well-defined cristae, and scattered distribution. Sarcoplasmic reticulum (Spr) demonstrates no expansion. Z-lines (Z) and H-bands (H) are fractured and misaligned. On the contrary, Figure 7B exhibits slightly paler cytoplasm in muscle tissue, discontinuous myofibril structures, partially visible light-dark band structures, and dense myofilaments. Mitochondria (M, green) retain intact membranes, yet their matrix appears sparse and dissolved, with numerous fractured cristae remaining scattered. Sarcoplasmic reticulum (Spr) is notably expanded, and Z-lines (Z) and H-bands are fractured. In Figure 8A and B, the expression levels of the ATP synthase β gene are utilized to indirectly reflect changes in mitochondrial biogenesis function and the expression levels of TRPV1 under inflammatory conditions. In contrast to the control group, the model group treated with 20 µmol/mL CuSO4 revealed a remarkable abatement in ATP synthase β levels and a striking augment in TRPV1 levels (P < 0.05).
Effect of 2ʹ-Hydroxychalcone-Related Target TRPV1 on Zebrafish Behavior, ROS and Macrophage Migration
As demonstrated in Figure 9A and B, at 120 h, compared with the control group, the moving distance and moving speed reached the optimal state (P > 0.05) when the concentration of PMA was 50uM, which did not have a great adverse effect on zebrafish themselves, and was suitable for subsequent experimental concentration. Similarly, the concentration of inhibitor CA was set to (0, 10, 50 and 100uM), and the treatment was the same as before, as depicted in Figure 9C and D.
Green fluorescence intensity in the presence of PMA50uM and CA100uM was detected after ROS staining, as revealed in Figure 10A and B, with no effect on zebrafish themselves at this agonist concentration and inhibitor concentration in contrast to the control group (P > 0.05). Similarly, in Figure 10C and D, macrophage migration numbers did not differ remarkably between groups (P > 0.05).
Changes of Target TRPV1 Functional State
Changes of 2ʹ-Hydroxychalcone Inflammatory State Stemmed from CuSO4
TRPV1 agonist PMA was utilized to enhance its function, and the change level of ROS was detected by grouping, as depicted in Figure 11A and B. In comparison to the control group, the fluorescence intensity of CuSO4 group exhibited a remarkable augment (P < 0.05), while the value of drug group demonstrated a decrease as the concentration increased. As demonstrated in Figure 11C and D, the same inhibitor CA was divided into groups to detect the ROS level upon micro-injection of 100uM. In contrast to the control group, the ROS level in the model group increased (P < 0.05), and the drug group markedly decreased with the augment of the concentration (P < 0.05).
Transgenic macrophages were employed to monitor cell migration. Under agonist intervention, as suggested in Figure 12A and B, the number of macrophage migration in the model group increased in contrast to the control group, while that of macrophage migration in the drug group gradually decreased with the augment of drug concentration. Under the effect of the inhibitor, as illustrated in Figure 12C and D, the model group exhibited a conspicuous augment in macrophage migration in contrast to the control group (P < 0.05). Conversely, the macrophage migration gradually decreased in the drug group with the elevation of drug concentration (P < 0.05).
Possible Mechanisms of Inhibition of CUSO4-Induced Inflammatory Response by 2ʹ-Hydroxychalcone
Excessive intake of CuSO4 gave rise to mitochondrial damage in tissues and organs, ultimately triggering an oxidative stress response that resulted in the generation of a substantial amount of ROS. Hence, a large amount of inflammatory factors IL-1β and TNF-α were finally released by inducing the continuous production of NLRP3. As an anti-inflammatory Chinese medicine monomer, the presence of 2ʹ-Hydroxychalcone not only hindered the production of ROS but also lessened the degree of oxidative stress response, which could also exert certain influence on the functional change of the binding membrane protein target TRPV1 to facilitate the occurrence and development of the inflammatory state in Figure 13.
Discussion
As a naturally occurring compound, 2ʹ-Hydroxychalcone displays anti-inflammatory and antioxidant effects, which can not only activate protective cellular pathways but also can serve as potential therapeutic strategies. Antioxidant, free radical scavenging, and anti-inflammatory effects of 2ʹ-Hydroxychalcone have been established across a wide spectrum of experimental models.28,35 It is highly ubiquitous in natural sources such as hops, plants and broccoli.36 The unique chemical structure of the compound potently stimulates the Phase II detoxification enzyme family, thereby contributing to its potential as an anticancer agent.37
In practical terms, as already demonstrated by several research findings, pre-treatment of animals with phase II enzyme inducers mitigates the risk of chemically induced cancer. Interestingly, day-taking of dry broccoli buds alleviates oxidative stress, hypertension and inflammation in rats,38 demonstrating the benefits and diversity of specific plant constituents. The anti-inflammatory properties of chalcone derivatives have undergone extensive investigation, and cell experiments have suggested that their effects appear to be associated with the suppression of inflammatory mediators, such as nitric oxide (NOs) and tumor necrosis factor a (TNF-α) produced by lipopolysaccharide (LPS) stimulated macrophages.39 Afterwards, CuSO4 was employed to stimulate the inflammatory response in zebrafish, which in turn generated TNF-α and IL-1β. 2ʹ-Hydroxychalcone was employed to ameliorate the inflammatory state, mitigate the massive release of inflammatory factors and lower the oxidative stress response (Figures 4–6).
Subsequently, 51 potential anti-inflammatory targets of 2ʹ-hydroxychalcone monomer compounds were identified by conducting network pharmacological analysis. Network maps revealed TRPV1 and 5HTR as two pivotal targets, potentially serving as target genes. As an anti-inflammatory monomer, its consistently comprises a single component, thereby eliminating the interference of other components and offering guidance for uncovering its subsequent mechanism of action. The results of the KEGG pathway enrichment analysis showed that aside from enriching pathways that affected the metabolism of organisms, the 2ʹ-hydroxychalcone monomer compounds also targeted TRPs family-related pathways tightly correlated with the development of inflammation (Supplementary material 3).
TRPV1 was initially cloned from the transient receptor potential channel family, and its expression was validated by adopting calcium imaging-based assays.40 It is particularly noteworthy that TRPV1 not only distributes in neuronal cells,41 but its functional TRPV1 channels also exist in some non-neuronal tissues, such as human skin epidermal keratinocytes,42 gastric epithelial cells,43 urinary tract epithelial cells and smooth muscle epithelial cells.44 Its functional channel comprises the homo- or isomers of four single subunits.45 As a crucial ion channel, it is involved in the perception of pain and plays a pivotal role in the progression of inflammatory hyperalgesia.46,47 Studies have demonstrated that knockout of the TRPV1 gene in mice prevents the development of thermal hyperalgesia during inflammation. On top of that, pharmacological investigations with capsaicin and potent TRPV1 antagonists have consistently confirmed comparable effects. As a result, the real significance of TRPV1 was the potential target of new analgesics and anti-inflammatory drugs. In particular, the analysis of bioinformatics technology revealed that the anti-inflammatory Chinese medicine monomer 2ʹ-Hydroxychalcone and through the application of molecular docking technology, the target TRPV1 exhibited a strong correlation and demonstrated a low binding energy. Simultaneously, the findings derived from the molecular dynamics simulation results demonstrated that the anti-inflammatory Chinese medicine monomer 2ʹ-Hydroxychalcone and the target TRPV1 had a stable effect between TRPV1 and the small molecule 2ʹ-Hydroxychalcone as well as a tight structure (Supplementary material 3). Apart from that, there was a potential target for anti-inflammatory drugs, which could be utilized to delve into how changing the functional state of the molecule affects the physiological and biochemical factors of zebrafish in the inflammatory state.
Excessive copper exposure has been proven to bring about oxidative stress, inflammation and liver injury.48,49 It is pivotal to note that there is a vicious cycle of oxidative stress and inflammation in hepatotoxicity.50 Oxidative stress, normally spawned from direct copper exposure51 and augmented ROS release on account of phagocyte activation, can give rise to DNA damage and cellular injury, thereby exacerbating tissue damage. The AP-1 and NF-κB signaling pathways up-regulate inflammatory cytokines and chemokines by releasing increased ROS bringing about inflammation. Oxidative stress and inflammation are crucial components of copper-induced toxicity. For this reason, the discovery of novel, safe therapeutics to address CuSO4-mediated hepatic injury stands as a paramount research priority. In addition, mortality rate of 20uM reached 70% and above upon exposure to CuSO4 for 72 h. The concentration of 2ʹ-Hydroxychalcone was 3.13, 6.25 and 12.5 μg/mL, respectively, which had a protective effect on CuSO4 damage (Figures 2–3).
In the course of this study, it was discovered that 2ʹ-Hydroxychalcone exerted a protective effect on zebrafish from CuSO4-induced death. Aside from that, 2ʹ-Hydroxychalcone could alleviate the inflammation stemmed from CuSO4 in zebrafish. This study marked the first attempt to assess the protective effect of 2ʹ-Hydroxychalcone on CuSO4-induced inflammation and oxidative stress in zebrafish.
Inflammation in the body triggers cellular changes and immune responses, ultimately resulting in tissue repair and cell proliferation of damaged tissues. Nevertheless, should inflammation remain unresolved or regulatory mechanisms undergo malfunction, it can give rise to chronic inflammation and a spectrum of diseases.52–54 There is a mounting amount of evidence suggesting that inflammation conducts a paramount role as a toxicological mechanism associated with copper. Oxidative stress and redox signaling facilitate the release of inflammatory factors. Furthermore, the process of “reinduced inflammation” is believed to be the underlying cause driving the progression of most chronic diseases. As already suggested by the experimental findings, CuSO4 increases the levels of inflammatory cytokines IL-1β, IL-2, IL-12, TNF-α, IFN-γ and anti-inflammatory cytokines IL-4 mRNAs in the spleen of mice, which evidently demonstrates that excessive Cu can give rise to inflammation in the spleen of mice. In this study, the results corroborate literature findings that copper exposure not only up-regulates IL-1β and TNF-α at both the gene and protein levels but also reinforces oxidative stress in zebrafish larvae.49,55 On top of that, prior studies have suggested that treatment with CuSO4 can trigger inflammation in the liver and lungs of mice.56,57 For this reason, the anti-inflammatory monomer 2ʹ-Hydroxychalcone derived from Chinese medicine was hereby selected as the focal point of the research, and CUSO4 was employed to establish the acute inflammation model of zebrafish. The objective was to characterize the anti-inflammatory properties of the species and elucidate the specific mechanisms by which it exerts its anti-inflammatory effects, thereby providing valuable insights and reference. As our research findings have already illustrated, 2ʹ-Hydroxychalcone could alleviate the inflammation of zebrafish stemmed from CuSO4 by anti-inflammation and anti-oxidation. Further exploration was carried out on the protective effect of 2ʹ-Hydroxychalcone on inflammation and oxidative stress resulted from CuSO4 in zebrafish.
Mitochondrial biogenesis is typically triggered to facilitate the synthesis of new organelle components, which thereby is advantageous for the replacement of dysfunctional mitochondria.58 F-ATP synthase converts the energy of the H+ gradient to ATP with astonishing efficiency attributable to the considerably low permeability of the intima to H+ and charged substances.59,60 Nonetheless, mitochondria can undergo an elevation in Ca2+ dependent permeability, which detrimentally impacts energy conservation. The expression of ATPase beta, a biomarker of MBs, in mouse cells functions as an indicator of MBs efficiency.61 When impaired, mitochondrial biogenesis may be activated to produce new organelle components, which subsequently incorporate proteins and lipids into existing mitochondria.62 Nevertheless, mitochondria can display an augment in Ca2+ dependent permeability that affects the structure. In this study, the level of gene expression was quantified to provide insight into the alterations occurring in the biological process of the organism. For instance, the content of ATP synthase beta in zebrafish treated with CuSO4 suggested a dramatic decline, as demonstrated in Figure 8A and B. On top of that, under electron microscope, the model group exhibited a limited number of mitochondria compared to the control group, with intact membranes, a sparse and dissolved matrix, fractured cristae, and reduced structural damage to the mitochondria (Figure 7A and B).
Afterwards, antioxidant and oxidative gene expression in zebrafish was further explored to understand the modulation of ROS levels by 2ʹ-Hydroxychalcone at the molecular level. Herein, the expression levels of the antioxidant genes GR, GPX and GST were quantified, and a comparison was made between the gene expression in larvae treated with CuSO4 and those treated 2ʹ-Hydroxychalcone. Simultaneously, the expression levels of oxidoreductive indices GR, GST, GPX and COX mRNAs were determined to verify the potential of 2ʹ-Hydroxychalcone in ultimately improving the body’s oxidative stress status and repairing the damage stemmed from CuSO4 (Figure 6). The antioxidant gene GR exerts a protective effect on organisms against oxidative stress.63 The Gpx1 gene, widely recognized as GPX, is an enzyme that conducts a crucial role in maintaining the homeostasis of the intracellular environment and lessening the degree of lipid peroxidation. GST enzymes can diminish lipid hydroperoxide levels and detoxify metabolites by leveraging their intrinsic peroxidase activity, thereby effectively mitigating oxidative stress. As apparently demonstrated by the research findings, compared with the control, the expression levels of the antioxidant genes GR, GPX and GST were substantially decreased upon treatment with CuSO4 for 6 h, while the expression levels of these genes were conspicuously augmented by treatment with 2ʹ-Hydroxychalcone 12.5 μM. COX2 catalyzes the conversion of arginine into prostaglandins, lipid mediators synthesized by inflammatory cells, which comprise macrophages.
As a calcium-permeable non-selective cation channel, TRPV1 acts as the exclusive receptor for capsaicin, which is a component of vanillin. Mice deficient in TRPV1 fails to demonstrate pain-related behaviors in response to capsaicin.64 In zebrafish, TRPV1 is not activated through the same mechanisms as in mammals. Nonetheless, enormous evidence suggests that akin to TRPV1 in other vertebrates, zebrafish TRPV1 can be activated by acidic pH, 2-APB and PMA. For this reason, PMA was hereby selected as an agonist for zebrafish TRPV1 in the present study, where its efficacy has helped it be employed as a protein kinase C activator.65,66 In particular, there are a multitude of associated targets on TRPV1 that may be phosphorylated by protein kinases. For instance, several residues on TRPV1, S 502, T 704, S 744, S 800 and S 820 were found in rat studies to be phosphorylated by protein kinase C.67 CA,68 an inhibitor of TRPV1, has been proven to ameliorate zebrafish obesity (caffeic acid) by modulating the peroxisome proliferative factor-activated receptor gamma. These two drugs exert an influence on the permeability of calcium ions through TRPV1, thereby modulating the intracellular calcium ion concentration and eliciting distinct physiological and biochemical responses. In this study, the alteration of TRPV1 functional state was manifested through changes in zebrafish behavior. On the contrary, the inhibitor presented the opposite effect. As evidenced by the analysis results, both the agonist and inhibitor elicited the most pronounced behavioral changes upon reaching a concentration of 50 µmol/mL. It was speculated that the maximum possible “occupancy” of the binding site of TRPV1 resulted in that neither the increment of agonist concentration nor the inhibitor concentration could change its maximum state. As a consequence, the concentration of 50uM was selected as the research focus. As associated studies have already demonstrated, TRPV1 activation can influence apoptosis in a spectrum of cell types through multiple mechanisms.
Figures 11 and 12 present changes in ROS and macrophage migration detected in inflammatory states while changing the functional state of TRPV1 with agonists and inhibitors. When an agonist was applied, the model group exhibited a higher number of ROS and migratory macrophages in comparison with the control group, whereas the drug group suggested an augment in the number of migratory macrophages. Regarding the inhibitor, there was no remarkable difference between the model group and the control group, nor between the drug group and the control group. As the results of Figure 8D indicated, the expression of TRPV1 was increased in the inflammatory group. As already evidenced by relevant studies, inflammatory mediators can impose a diverse range of effects on TRPV1 and can make it “sensitized”. Certain secreted inflammatory factors, such as protons and certain lipid mediators, can directly activate TRPV1. A potential mechanism involves the modulation of TRPV1 channel gating by protein kinases, phosphatases, and/or lipid mediators derived from receptor-coupled intracellular signaling pathways. On top of that, factors such as NGF, insulin and IGF-1 may enhance the density of TRPV1 channels on the nociceptor membrane69–71 and up-regulate TRPV1 expression levels.72–74
Altogether, the existing investigation that 2ʹ-Hydroxychalcone possesses anti-inflammatory and antioxidant activities, which can ameliorate CuSO4-induced oxidative stress. It also modulates the TRPV1 channel, reducing ROS production and macrophage migration in zebrafish. These experimental findings suggest the potential of 2ʹ-Hydroxychalcone as an emerging anti-inflammatory and immunomodulatory agent.
Data Sharing Statement
Data is contained within the article.
Approval Statement
Ethics Committee: Southwest Medical University Clinical Trial Ethics Committee; This study complies with relevant ethical standards and supports this clinical research. The ethical review document should be uploaded as a separate file (PDF version).
Acknowledgments
Thank you to the Zebrafish Experimental Center of Southwest Medical University for providing the zebrafish.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Sichuan Provincial Key Clinical Specialty Construction Project. Alpha-defensin expression levels as a factor inducing coronary heart disease in hyperlipidemia patients (00030729/02). The Collaborative Research Project between Luzhou City and Southwest Medical University (2024LZXNYDJ038).
Disclosure
All authors state no conflict of interest.
References
1. Ur Rashid H, Xu Y, Ahmad N, Muhammad Y, Wang L. Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E2, inducible NO synthase and nuclear factor κb. Activities Bioorg Chem. 2019; 87:335–365.
2. Marquina S, Maldonado-Santiago M, Sánchez-Carranza JN, et al. Design, synthesis and QSAR study of 2ʹ-hydroxy-4’-alkoxy chalcone derivatives that exert cytotoxic activity by the mitochondrial apoptotic pathway. Bioorg Med Chem. 2019;27(1):43–54.
3. Parveen Z, Brunhofer G, Jabeen I, Erker T, Chiba P, Ecker GF. Synthesis, biological evaluation and 3D-QSAR studies of new chalcone derivatives as inhibitors of human P-glycoprotein. Bioorg Med Chem. 2014;22(7):2311–2319.
4. Fu ZY, Jin QH, Qu YL, Guan LP. Chalcone derivatives bearing chromen or benzo[f]chromen moieties: design, synthesis, and evaluations of anti-inflammatory, analgesic, selective COX-2 inhibitory activities. Bioorg Med Chem Lett. 2019;29(15):1909–1912. doi:10.1016/j.bmcl.2019.05.051
5. Mohd Faudzi SM, Abdullah MA, Abdull Manap MR, et al. Inhibition of nitric oxide and prostaglandin E2 production by pyrrolylated-chalcones: synthesis, biological activity, crystal structure analysis, and molecular docking. Studies Bioorg Chem. 2020;94:103376. doi:10.1016/j.bioorg.2019.103376
6. Pham TH, Kim MS, Le MQ, et al. Fargesin exerts anti-inflammatory effects in THP-1 monocytes by suppressing PKC-dependent AP-1 and NF-ĸB signaling. Phytomedicine. 2017;24:96–103. doi:10.1016/j.phymed.2016.11.014
7. Gan FF, Zhang R, Ng HL, et al. Novel dual-targeting anti-proliferative dihydrotriazine-chalcone derivatives display suppression of cancer cell invasion and inflammation by inhibiting the NF-κB signaling pathway. Food Chem Toxicol. 2018;116(Pt B):238–248. doi:10.1016/j.fct.2018.04.003
8. HL Y, TY Y, Gowrisankar YV, et al. Suppression of LPS-induced inflammation by chalcone flavokawain a through activation of Nrf2/ARE-mediated antioxidant genes and inhibition of ROS/NFκB signaling pathways in primary splenocytes. Oxid Med Cell Longev. 2020;2020:3476212.
9. Muntean BS, Horvat CM, Behler JH, et al. A comparative study of embedded and anesthetized zebrafish in vivo on myocardiac calcium oscillation and heart muscle contraction. Front Pharmacol. 2010;1:139.
10. Vaz R, Hofmeister W, Lindstrand A. Zebrafish models of neurodevelopmental disorders: limitations and benefits of current tools and techniques. Int J Mol Sci. 2019;20(6):1296.
11. Kurogi K, Liu TA, Sakakibara Y, Suiko M, Liu MC. The use of zebrafish as a model system for investigating the role of the SULTs in the metabolism of endogenous compounds and xenobiotics. Drug Metab Rev. 2013;45(4):431–440.
12. Moss JJ, Hammond CL, Lane JD. Zebrafish as a model to study autophagy and its role in skeletal development and disease. Histochem Cell Biol. 2020;154(5):549–564.
13. Herencia F, López-García MP, Ubeda A, et al. Nitric oxide-scavenging properties of some chalcone derivatives. Nitric Oxide-Biol Ch. 2002;6(2):242–246.
14. Madan B, Batra S, Ghosh B. 2ʹ-hydroxychalcone inhibits nuclear factor-kappab and blocks tumor necrosis factor-alpha- and lipopolysaccharide-induced adhesion of neutrophils to human umbilical vein endothelial cells. Mol Pharmacol. 2000;58(3):526–534.
15. Foresti R, Green CJ, Motterlini R. Generation of bile pigments by Haem oxygenase: a refined cellular strategy in response to stressful insults. Biochem Soc Symp. 2004;71:177–192. doi:10.1042/bss0710177
16. Foresti R, Motterlini R. The heme oxygenase pathway and its interaction with nitric oxide in the control of cellular homeostasis. Free Radical Res. 1999;31(6):459–475. doi:10.1080/10715769900301031
17. Balogun E, Hoque M, Gong P, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371(Pt 3):887–895. doi:10.1042/bj20021619
18. Motterlini R, Foresti R, Bassi R, et al. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radical Bio Med. 2000;28(8):1303–1312. doi:10.1016/S0891-5849(00)00294-X
19. Scapagnini G, Foresti R, Calabrese V, et al. Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol Pharmacol. 2002;61(3):554–561. doi:10.1016/S0026-895X(24)12118-9
20. Lyu J, Wang K, Ye M. Modification-free approaches to screen drug targets at proteome level. Trac-Trend Anal Chem. 2020;124115574.
21. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi:10.1093/nar/gky1131
22. Li W, Luo F, Wu X, et al. Anti-Inflammatory effects and mechanisms of dandelion in RAW264.7 macrophages and zebrafish larvae. Front Pharmacol. 2022;13:906927. doi:10.3389/fphar.2022.906927
23. Middleton E, Drzewiecki G. Flavonoid inhibition of human basophil histamine release stimulated by various agents. Biochem Pharmacol. 1984;33(21):3333–3338. doi:10.1016/0006-2952(84)90102-3
24. Batt DG, Goodman R, Jones DG, et al. 2ʹ-substituted chalcone derivatives as inhibitors of interleukin-1 biosynthesis. J Med Chem. 1993;36(10):1434–1442. doi:10.1021/jm00062a016
25. Kim YP, Ban HS, Lim SS, et al. Inhibition of prostaglandin E2 production by 2ʹ-hydroxychalcone derivatives and the mechanism of action. J Pharm Pharmacol. 2001;53(9):1295–1302. doi:10.1211/0022357011776595
26. Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi:10.1126/science.288.5464.306
27. Davis JB, Gray J, Gunthorpe MJ, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–187. doi:10.1038/35012076
28. Ballesteros JF, Sanz MJ, Ubeda A, et al. Synthesis and pharmacological evaluation of 2ʹ-hydroxychalcones and flavones as inhibitors of inflammatory mediators generation. J Med Chem. 1995;38(14):2794–2797. doi:10.1021/jm00014a032
29. Holmås S, Puig RR, Acencio ML, Mironov V, Kuiper M. The Cytoscape BioGateway app: explorative network building from the biogateway triple store. Bioinformatics. 2019;36(6):1966–1967. (). doi:10.1093/bioinformatics/btz835
30. Yang L, Zhang YH, Huang F, Li Z, Huang T, Cai YD. Identification of protein-protein interaction associated functions based on gene ontology and KEGG pathway. Front Genet. 2022;13:1011659.
31. Waterston T. The life and contribution of Professor David Sanders through his publications. Arch Dis Child. 2020;105(4):406–407. doi:10.1136/archdischild-2019-318330
32. Wang Y, Bryant SH, Cheng T, et al. PubChem bioassay: 2017 update. Nucleic Acids Res. 2017;45(D1):D955–D963. doi:10.1093/nar/gkw1118
33. Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ. GROMACS: fast, flexible, and free. J. Comput Chem. 2005;26(16):1701–1718. doi:10.1002/jcc.20291
34. Gudbrandsdottir S, Hasselbalch HC, Nielsen CH. Activated platelets enhance IL-10 secretion and reduce TNF-α secretion by monocytes. J Immunol. 2013;191(8):4059–4067.
35. Nakamura Y, Watanabe S, Miyake N, et al. Dihydrochalcones: evaluation as novel radical scavenging antioxidants. J Agricul Food Chem. 2003;51(11):3309–3312. doi:10.1021/jf0341060
36. Miranda CL, Stevens JF, Ivanov V, et al. Antioxidant and prooxidant actions of prenylated and nonprenylated chalcones and flavanones in vitro. J Agricul Food Chem. 2000;48(9):3876–3884. doi:10.1021/jf0002995
37. Ponce MA, Scervino JM, Erra-Balsells R, et al. Flavonoids from shoots, roots and roots exudates of Brassica alba. Phytochemistry. 2004;65(23):3131–3134. doi:10.1016/j.phytochem.2004.08.031
38. Dinkova-Kostova AT, Massiah MA, Bozak RE, et al. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci USA. 2001;98(6):3404–3409. doi:10.1073/pnas.051632198
39. Wu L, Noyan Ashraf MH, Facci M, et al. Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proc Natl Acad Sci U S A. 2004;101(18):7094–7099. doi:10.1073/pnas.0402004101
40. Parvez S, Malik KA, Ah Kang S, et al. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol. 2006;100(6):1171–1185. doi:10.1111/j.1365-2672.2006.02963.x
41. Helliwell RJ, McLatchie LM, Clarke M, et al. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett. 1998;250(3):177–180. doi:10.1016/S0304-3940(98)00475-3
42. Southall MD, Li T, Gharibova LS, et al. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J Pharmacol Exp Ther. 2003;304(1):217–222. doi:10.1124/jpet.102.040675
43. Kato S, Aihara E, Nakamura A, et al. Expression of vanilloid receptors in rat gastric epithelial cells: role in cellular protection. Biochem Pharmacol. 2003;66(6):1115–1121. doi:10.1016/S0006-2952(03)00461-1
44. Birder LA, Kanai AJ, de Groat WC, et al. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci U S A. 2001;98(23):13396–13401. doi:10.1073/pnas.231243698
45. Kedei N, Szabo T, Lile JD, et al. Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem. 2001;276(30):28613–28619. doi:10.1074/jbc.M103272200
46. Di Marzo V, Bisogno T, Melck D, et al. Interactions between synthetic vanilloids and the endogenous cannabinoid system. FEBS Lett. 1998;436(3):449–454. doi:10.1016/S0014-5793(98)01175-2
47. Dinh QT, Groneberg DA, Mingomataj E, et al. Expression of substance P and vanilloid receptor (VR1) in trigeminal sensory neurons projecting to the mouse nasal mucosa. Neuropeptides. 2003;37(4):245–250. doi:10.1016/S0143-4179(03)00065-9
48. Hernandez PP, Undurraga C, Gallardo VE, et al. Sublethal concentrations of waterborne copper induce cellular stress and cell death in zebrafish embryos and larvae. Biol Res. 2011;44(1):7–15. doi:10.4067/S0716-97602011000100002
49. Leite CE, Maboni LO, Cruz FF, et al. Involvement of purinergic system in inflammation and toxicity induced by copper in zebrafish larvae. Toxicol Appl Pharm. 2013;272(3):681–689. doi:10.1016/j.taap.2013.08.001
50. Liu J, Zhao H, Wang Y, et al. Alterations of antioxidant indexes and inflammatory cytokine expression aggravated hepatocellular apoptosis through mitochondrial and death receptor-dependent pathways in Gallus gallus exposed to arsenic and copper. Environ Sci Pollut R. 2018;25(16):15462–15473. doi:10.1007/s11356-018-1757-0
51. Hayyan M, Hashim MA, AlNashef IM. Superoxide ion: generation and chemical implications. Chem Rev. 2016;116(5):3029–3085. doi:10.1021/acs.chemrev.5b00407
52. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025
53. Singh N, Baby D, Rajguru JP, et al. Inflammation and cancer. Ann Afr Med. 2019;18(3):121–126. doi:10.4103/aam.aam_56_18
54. He G, Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2010;21(1):159–168. doi:10.1038/cr.2010.183
55. Pereira TC, Campos MM, Bogo MR. Copper toxicology, oxidative stress and inflammation using zebrafish as experimental model. J Appl Toxicol. 2016;36(7):876–885. doi:10.1002/jat.3303
56. Jian Z, Guo H, Liu H, et al. Oxidative stress, apoptosis and inflammatory responses involved in copper-induced pulmonary toxicity in mice. Aging. 2020;12(17):16867–16886. doi:10.18632/aging.103585
57. Wang L, Wang Y, Zhou S, et al. Imbalance between glutamate and GABA in Fmr1 knockout astrocytes influences neuronal development. Genes. 2016;7(8):45. doi:10.3390/genes7080045
58. watt IN, Montgomery MG, Runswick MJ, et al. Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc Natl Acad Sci U S A. 2010;107(39):16823–16827. doi:10.1073/pnas.1011099107
59. Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim Biophys Acta. 2011;1807(12):1507–1538. doi:10.1016/j.bbabio.2011.09.018
60. Rehman H, Krishnasamy Y, Haque K, et al. Green tea polyphenols stimulate mitochondrial biogenesis and improve renal function after chronic cyclosporin a treatment in rats. PLoS One. 2013;8(6):e65029. doi:10.1371/journal.pone.0065029
61. Peterson YK, Cameron RB, Wills LP, et al. β2-Adrenoceptor agonists in the regulation of mitochondrial biogenesis. Bioorg Med Chem Lett. 2013;23(19):5376–5381. doi:10.1016/j.bmcl.2013.07.052
62. Kumar A, Dubey AK, Kumar V, et al. Overexpression of rice glutaredoxin genes LOC_Os02g40500 and LOC_Os01g27140 regulate plant responses to drought stress. Ecotox Environ Safe. 2020;200110721.
63. Amantini C, Mosca M, Lucciarini R, et al. Distinct thymocyte subsets express the vanilloid receptor VR1 that mediates capsaicin-induced apoptotic cell death. Cell Death Differ. 2004;11(12):1342–1356. doi:10.1038/sj.cdd.4401506
64. Kamakura T, Ishida Y, Nakamura Y, et al. Functional expression of TRPV1 and TRPA1 in rat vestibular ganglia. Neurosci Lett. 2013;552. doi:10.1016/j.neulet.2013.07.019
65. Tominaga M, Caterina MJ, Malmberg AB, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–543. doi:10.1016/S0896-6273(00)80564-4
66. Hu HZ, Gu Q, Wang C, et al. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279(34):35741–35748. doi:10.1074/jbc.M404164200
67. Numazaki M, Tominaga T, Toyooka H, et al. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277(16):13375–13378. doi:10.1074/jbc.C200104200
68. Kamakura T, Ishida Y, Nakamura Y, et al. Functional expression of TRPV1 and TRPA1 in rat vestibular ganglia. Neurosci Lett. 2013;552:92–97.
69. Morenilla-Palao C, Planells-Cases R, García-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem. 2004;279(24):25665–25672. doi:10.1074/jbc.M311515200
70. Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. 2005;1:17. doi:10.1186/1744-8069-1-17
71. Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24(24):4211–4223.
72. Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A. 2000;97(14):8134–8139. doi:10.1073/pnas.100129497
73. Puntambekar P, Mukherjea D, Jajoo S, Ramkumar V. Essential role of Rac1/NADPH oxidase in nerve growth factor induction of TRPV1 expression. J Neurochem. 2005;95(6):1689–1703. doi:10.1111/j.1471-4159.2005.03518x
74. Noji H, Yasuda R, Yoshida M, et al. Direct observation of the rotation of F1-ATPase. Nature. 1997;386(6622):299–302. doi:10.1038/386299a0
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.