Back to Journals » Journal of Pain Research » Volume 18
Exploring the Multifactorial Predictors of Pain in Chronic Musculoskeletal Pain: A Regression-Based Study
Authors González-de-la-Flor A , Bravo-Aguilar M , Almazán-Polo J , García-Pérez-de-Sevilla G , Martínez-Lozano P , Romero-Morales C
Received 12 October 2024
Accepted for publication 31 January 2025
Published 16 April 2025 Volume 2025:18 Pages 2081—2091
DOI https://doi.org/10.2147/JPR.S500636
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Giuliano Lo Bianco
Angel González-de-la-Flor, María Bravo-Aguilar, Jaime Almazán-Polo, Guillermo García-Pérez-de-Sevilla, Pedro Martínez-Lozano, Carlos Romero-Morales
Department of Physiotherapy, Faculty of Medicine, Health and Sport, European University of Madrid, Villaviciosa de Odón, Madrid, Spain
Correspondence: Angel González-de-la-Flor, Department of Physiotherapy, Faculty of Medicine Health and Sport, Universidad Europea de Madrid, Calle Tajo s/N, 28670, Villaviciosa de Odón, Madrid, Spain, Email [email protected]
Objective: This study aimed to assess differences in pain neurophysiology knowledge between individuals with chronic musculoskeletal pain (CMP) and those without CMP, and to explore associations between pain knowledge, pain intensity, and demographic and lifestyle factors.
Methods: A cross-sectional study was conducted with 171 participants, including 120 with CMP and 51 without CMP. Sociodemographic, pain-related, and lifestyle data were collected. Pain knowledge was assessed using the Revised Neurophysiology of Pain Questionnaire (R-NPQ). Statistical analyses included t-tests, chi-squared tests, Pearson’s correlation, and stepwise regression models to identify predictors of pain intensity and CMP presence.
Results: Significant differences were found between participants with and without CMP in BMI (p< 0.001), physical activity (p=0.023), education level (p=0.002), and alcohol consumption (p=0.017). Participants with CMP scored lower on the R-NPQ (mean 4.40 ± 2.1) than those without CMP (mean 6.31 ± 2.03; p< 0.001). Pain intensity was negatively associated with R-NPQ scores (r=− 0.315; p< 0.001), physical activity (r=− 0.199; p=0.030), and education level (rho=0.236; p=0.010). Stepwise regression analysis revealed that R-NPQ scores (20.7%), BMI (6.7%), education level (3.9%), and physical activity (2.6%) collectively explained 33.9% of the variance in pain intensity (adjusted R²=0.339). Binary logistic regression identified BMI, R-NPQ scores, and education level as significant predictors of CMP presence, with higher BMI and lower R-NPQ scores increasing the odds of CMP, while higher education levels and physical activity were predictive factors.
Conclusion: Individuals with CMP exhibited lower knowledge of pain neurophysiology, higher BMI, reduced physical activity levels, and lower educational attainment, all of which were associated with increased pain intensity and a greater likelihood of CMP presence.
Keywords: chronic pain, pain perception, educational status, musculoskeletal pain, body mass index
Introduction
Pain perception is not solely determined by tissue damage and nociceptive input but is influenced by various factors, including an individual’s understanding of their condition.1 In chronic pain, alterations in spinal and cortical nociceptive networks can disrupt the typical relationship between tissue injury, nociceptive signals, and pain perception.2 Chronic pain patients, influenced by healthcare providers, often adopt rigid biomedical beliefs that attribute their pain exclusively to tissue damage.3,4 Misconceptions about pain can contribute to its persistence, as outlined in conceptual frameworks like the fear-avoidance model, which links catastrophic thoughts to fear, avoidance behaviors, disability, and a self-perpetuating cycle of chronic pain.5 Therefore, a patient’s comprehension of the biological mechanisms underlying their pain can be crucial in modulating their pain experience.6
Chronic pain significantly impacts patients’ daily activities, social interactions, workplace productivity, and personal identity.7,8 These patients face challenges such as stigma, bias, and limited access to healthcare resources.9 Prevalence rates of chronic pain range from 19.0% to 46.4%, with low back pain being the most common type.10–12 Chronic pain is often associated with central sensitization, characterized by increased excitability and lowered pain thresholds, which can hinder rehabilitation efforts.13 Misconceptions about pain, such as viewing it as inherently threatening, catastrophic thinking, and maladaptive coping strategies, are common among uninformed or misinformed patients.14
Emerging evidence suggests that unaddressed chronic pain may also impair neurocognitive functions, including memory, attention, and executive function, while exacerbating emotional distress and reducing self-regulation capacity. Contextual factors, such as environmental cues, therapeutic settings, and clinician-patient interactions, further influence pain perception, motor performance, and treatment outcomes in musculoskeletal pain.
Chronic musculoskeletal pain (CMP) is influenced by a complex interplay of cognitive, emotional, and contextual factors. Unaddressed chronic pain may impair neurocognitive functions, such as memory, attention, and executive function, while exacerbating emotional distress and reducing self-regulation capacity.15 Additionally, contextual elements like therapeutic settings and clinician-patient interactions shape pain perception,16 motor performance,17 and treatment outcomes.18–23 In addition, factors such as body mass index (BMI), physical activity, and education level have also been suggested as contributors to CMP.24–27 The contribution of these factors to the development and intensity of CMP remains unclear, warranting further investigation to clarify their roles.
One such approach is Pain Neurophysiology Education (PNE), which has been proposed as a method to address misconceptions and enhance patients’ understanding of pain. PNE interventions aim to reduce the perceived threat of pain by educating patients about its neurophysiological basis, thereby promoting adaptive coping mechanisms and recovery.28,29 Studies have demonstrated improvements in pain levels, functional outcomes, and reduced fear-avoidance behaviors following PNE.30–32 However, despite its effectiveness, the variability in patients’ and healthcare providers’ baseline knowledge about pain neurophysiology remains a challenge. For example, physiotherapy students, for instance, generally exhibit higher knowledge levels compared to medical students, yet their understanding may still be insufficient to optimize patient care.33,34
This study aimed to address these gaps by examining the neurophysiology of pain knowledge among individuals with and without CMP using the Spanish Revised Neurophysiology of Pain Questionnaire (R-NPQ), a validated and sensitive tool.35 Specifically, the study investigated the associations between pain knowledge and demographic factors, pain characteristics, and lifestyle behaviors. Furthermore, a linear stepwise regression model was employed to identify variables explaining the variance in pain intensity, while a binary logistic regression model was used to evaluate the predictive capacity of several factors for the presence of CMP.
We hypothesized that sociodemographic and clinical characteristics will differ significantly between individuals with and without CMP. Pain intensity will be significantly associated with these factors, emerging as significant predictors of pain intensity in individuals with CMP. Furthermore, these factors will also serve as significant predictors of the presence of CMP.
Methods
Study Design
A cross-sectional study was carried out in participants with and without chronic musculoskeletal-related pain. The study followed the Strengthening the Reporting of Observation studies in Epidemiology (STROBE) guidelines and checklist for cross-sectional studies,36 was supervised and approved by the Clinical Ethics Committee of Universidad Europea de Madrid (CI:23.231) and all the recommendations stated in the Declaration of Helsinki were followed.
Participants
Participants were screened and enrolled for potential eligibility in the Universidad Europea de Madrid from January 2023 to February 2024. Announcements were posted on social media to inform potential participants during the recruitment process.
General inclusion criteria applicable for both groups were adults over the age of 18, to have sufficient oral and reading Spanish comprehension capacity and to sign and read the written informed consent. General exclusion criteria were previous surgery or traumatic injury related to their pain or presence of red flags (malignancy processes, infection, or bone fractures).
Specific criteria for people with non-specific chronic CMP were: 1) a Standard International Association for the Study of Pain (IASP) criteria will be used for CMP screening. Two screening questions were used: 1) whether the respondents had pain in joint, bone, muscle vertebral column or tendon; and 2) a question about pain duration. Participants were defined as having CMP if they answered positively to the first question and had a pain duration of >3 months.37 In addition, people without CMP can complete the survey indicating all non-pain-related sociodemographic characteristics.
Sociodemographic Variables
The sociodemographic (non-pain related characteristics) variables collected were age, stratified into four age groups: 18–30, 31–45, 46–65, and above 65 years. The sex was recorded as male or female. Height was measured in meters (m) and weight in kilograms (kg). Body Mass Index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2).38 Specifically focused on smoking or alcohol use, participants were asked to report their smoking status (current smoker or never smoked) and alcohol consumption habits (regular, occasional, non-drinker). The education level was categorized as no education, primary education, secondary education, pre-university or professional education, and university degree. The marital Status was classified into Single, Married or on civil union, Divorced or separated, Widowed) and the professional status into Full time worker, employee, Full-time worker-self-employed, Partial time worker, student, unemployed, house worker or domestic worker, Retired.
The Global Physical Activity Questionnaire (GPAQ), developed by the World Health Organization (WHO), serves as a comprehensive tool to assess physical activity across three key domains: work-related activity, transportation, and leisure time, alongside sedentary behavior, in adults over 18 years old. This questionnaire, encompassing 16 items, quantifies physical activities in terms of their frequency and duration, providing insights into both vigorous and moderate activities as well as sedentary time spent sitting or reclining on a typical day. By categorizing physical activity levels into low, moderate, and high intensities based on WHO guidelines, the GPAQ facilitates the analysis of physical activity patterns and sedentary behaviors.39
Pain-Related Characteristics
Regarding the pain-related characteristics, the pain location (Head, Neck, Thoracic, Low Back, Shoulder, arms, Wrist/hand, pelvis, hip, knee or ankle/foot), pain intensity was measured using the Numeric Pain Rating Scale (NPRS). Furthermore, evidence endorses its efficacy as the most sensitive scale for discerning variations in pain intensity, including gender-based differences. This involves computing the average of three distinct pain intensity assessments: the highest and lowest levels of pain experienced in the past week, and the pain level at the moment of assessment. The NPRS scores ranged from 0, indicating no pain, to 10, denoting the most severe pain conceivable. Based on these scores, pain intensity can be classified as mild (scores 0–3), moderate (scores 4–6), or severe (scores 7–10).40,41 Averaging three distinct measurements allows for a more accurate and consistent estimation of the actual pain intensity, thereby minimizing the impact of any random fluctuations.
Pain management strategies were evaluated based on the frequency of physician visits (whether via the National Health Service or private practice) as categorized into ‘never’, ‘monthly’, ‘quarterly’, or ‘semi-annually’, the nature of the treatment received (including prescriptions for anti-inflammatory or analgesic medication, physical therapy, or the absence of treatment) and the instances of self-medication (specifying the type of medication used and the extent of improvement observed). The extent of improvement achieved through medical treatment was measured by a numerical rating scale.42
Revised Neurophysiology of Pain Questionnaire (R-NPQ)
The Spanish R-NPQ questionnaire, features 13 items focused on the neurophysiology of pain, aiming to evaluate an individual’s comprehension of the biological and physiological aspects of pain as per the latest research in pain science. Respondents can mark each item as ‘true’, ‘false’, or ‘undecided’, with a point awarded for each item correctly identified, leading to a possible score range from 0, indicating limited knowledge, up to 13, denoting extensive understanding. Analysis through the Rasch model for its psychometric attributes has affirmed the tool’s suitability for measuring one’s knowledge of pain mechanisms. Furthermore, the questionnaire’s internal consistency is solid, as shown by a Cronbach’s alpha of 0.90 (0.87–0.92), and it also demonstrates reliable test-retest stability, evidenced by an Intraclass Correlation Coefficient (ICC) of 0.82 (0.73–0.88).35
Sample Size Calculation
A power analysis was performed using G*Power software, with a significance level (α) set at 0.05, a power (1-β) of 0.80, and an expected medium effect size (d=0.5) in the primary outcome. The results indicated that a minimum of 64 participants per group was required, totaling 128 participants for a two-tailed t-test for independent means. Our study included 171 participants (120 with chronic CMP and 51 without CMP), which exceeds this requirement and provides sufficient power to detect significant differences between the groups. Therefore, the sample size of 171 participants is well-justified, supporting the study’s objectives and the validity of its results.
Statistical Analysis
All statistical analyses for this study were performed using SPSS software v.29 for Windows (IBM, Armonk, NY, USA). The normality of the data distribution was verified using the Kolmogorov–Smirnov test and histograms. The Levene test was employed to analyze the homogeneity of variances. Variables with p-values <0.05 were considered non-normally distributed, while those with p-values >0.05 were considered normally distributed. A descriptive analysis was conducted to characterize the sample, with central tendency and dispersion data reported as mean and standard deviation for normally distributed variables, or as median and interquartile range for non-normally distributed variables.
To assess differences between participants with and without CMP, the Student’s t-test for independent samples was used for quantitative data, and the chi-squared test was used for qualitative data, with the significance level set at p<0.05. A one-way ANOVA was conducted to examine differences in R-NPQ and pain intensity across education levels in individuals with CMP. The significance level was adjusted to p < 0.0125 for multiple comparisons.
Associations between variables were calculated using Pearson’s correlation coefficients (r). Multicollinearity and shared variance were identified with r>0.80 to avoid bias and overestimation in the regression model calculations.43,44 The relationship between education level and pain intensity was assessed using Spearman’s rank correlation (rho) coefficient.
A multivariate linear stepwise regression model was calculated to identify predictors of pain intensity, including variables that showed the strongest correlation, no shared variance, and statistical significance (p < 0.05). The stepwise regression model included R-NPQ scores, education level, and physical activity as predictors of pain intensity. These variables were selected because they demonstrated statistically significant correlations with the outcome (p < 0.05). The critical F value significance criterion was set at p < 0.05, and changes in adjusted variance (adj R2) were reported to determine the individual contribution of each variable. Multicollinearity among predictors was assessed using the variance inflation factor (VIF). A threshold of VIF < 5 was used to indicate no significant multicollinearity issues between the independent variables, ensuring that the regression coefficients were interpretable and not inflated by shared variance. Residual analysis was conducted to verify the assumptions of normality, homoscedasticity, and independence of errors. Normality was assessed using the Kolmogorov–Smirnov test and visual inspection of Q-Q plots and histograms of residuals. Homoscedasticity and linearity were evaluated by plotting standardized predicted values against standardized residuals. The independence of errors was tested using the Durbin-Watson statistic, with values close to 2 indicating no significant autocorrelation in the residuals.
A binary logistic regression analysis was performed to examine the predictors of CMP. The dependent variable was CMP presence (1 = Yes, 0 = No), and the predictors included BMI, neurophysiology of pain knowledge (R-NPQ), physical activity, and education level. Education level was entered as a categorical variable, coded into three dummy variables (primary, secondary, and pre-university, with university level as the reference group). The model was assessed using the −2 log-likelihood ratio, Cox and Snell R², and Nagelkerke R² to evaluate goodness-of-fit. Odds ratios (Exp(B)) were used to interpret the strength and direction of relationships between predictors and the outcome. A significance level of p < 0.05 was applied.
Results
Descriptive Data and Differences Between Groups
A total of 171 participants (68.4% female) with and without CMP (n = 120 with CMP and n = 51 without CMP) were included in the study. Sociodemographic variables are shown in Table 1 by total sample and pain condition. Significant differences were observed in weight (p = 0.023), BMI (p < 0.001), physical activity (p = 0.023), education level (p = 0.002) and alcohol consumption habits (p = 0.017) and no differences were found in age, height, marital, professional and smoking status and (p > 0.05) between those participants with and without CMP (See Table 1).
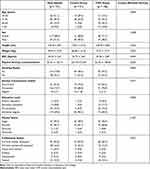 |
Table 1 Sociodemographic Characteristics of the Total Sample, People With and Without Musculoskeletal Chronic Pain |
Regarding the neurophysiology of pain knowledge, participants with CMP showed less score in the R-NPQ than participants without CMP (p < 0.001). Participants with CMP had a mean of 4.40 ± 2.1 points and those without CMP a mean of 6.31 ± 2.03 points with a mean difference of 1.91 points (95% confidence interval: 1.22; 2.60).
Pain-related features, including pain intensity, duration, frequency of physician visits, treatments received, self-medication practices, and the degree of improvement with self-medication, are presented in Table 2. Patients with CMP showed moderate pain intensity, with a mean of 5.23 ± 2.17, while no significant differences in neurophysiology of pain knowledge were observed across education levels (F = 2.259; p = 0.083).
 |
Table 2 Pain-Related Characteristics of the Participants With Musculoskeletal Chronic Pain |
In participants with CMP, pain intensity differed significantly across educational levels, as indicated by the ANOVA results (F= 3.055, p = 0.031). Participants with a university degree reported the lowest mean pain intensity (4.79 ± 1.99), followed by those with secondary education (5.43 ± 1.91). Participants with pre-university education (6.04 ± 2.06) and primary education (6.20 ± 2.74) reported the highest levels of pain intensity.
Bivariate Correlation Analysis
Pain intensity was significant negatively associated with BMI (r = 0.307; p < 0.001), R-NPQ scores (r = −0.315; p < 0.001), physical activity level (r = −0.199; p =0.030) and education level (rho=0.236; p = 0.010). All the rest of variables analyzed were not significantly associated (p>0.05).
Multiple Regression Analysis
Stepwise regression analyses revealed that R-NPQ scores (contributing 20.7%), BMI (6.7%), education level (3.9%), and physical activity (2.6%) significantly predicted pain intensity. Combined, these variables explained 33.9% of the variance in pain intensity (adjusted R²: 0.339). Multicollinearity diagnostics revealed no issues (VIF < 1.1). Residual analysis confirmed that assumptions of normality and homoscedasticity were met (Table 3).
 |
Table 3 Summary of the Regression Analyses to Determine Predictors of Pain Intensity |
Binary Logistic Regression
The binary logistic regression model was statistically significant (χ² (6) = 55.179, p < 0.001) and explained between 27.6% (Cox and Snell R²) and 39.2% (Nagelkerke R²) of the variance in CMP presence.
The significant predictors of CMP included BMI, where each unit increase in BMI was associated with an 18.8% increase in the odds of CMP (Exp(B) = 1.188, p = 0.004). Higher neurophysiology of pain knowledge (R-NPQ) scores were linked to a 35.7% decrease in the odds of CMP (Exp(B) = 0.643, p < 0.001). Regarding education level, participants with pre-university education had 14 times higher odds of CMP compared to those with university education (Exp(B) = 14.056, p = 0.025), while secondary and primary education levels did not show statistical significance. Finally, each additional unit of physical activity (measured in minutes per week) reduced the odds of CMP by 0.3% (Exp(B) = 0.997, p = 0.035) (Table 4).
 |
Table 4 Binary Logistic Regression Results Predicting the Presence of Chronic Musculoskeletal Pain |
Discussion
This cross-sectional study examined the relationship between sociodemographic factors, physical activity, lifestyle behaviors and pain neurophysiology knowledge with CMP in a sample of 171 participants. The study found significant sociodemographic differences between individuals with and without CMP, particularly in BMI, physical activity, education level, alcohol consumption habits, and R-NPQ scores. Lower R-NPQ scores were associated with individuals with CMP, supporting the hypothesis that understanding pain neurophysiology may play a role in chronic pain perception. Linear regression revealed that R-NPQ scores, BMI, education level, and physical activity significantly predicted pain intensity, collectively explaining 33.9% of its variance. Furthermore, binary logistic regression identified the same variables as significant predictors of CMP presence, explaining the variance between 27.6% and 39.2% of the variance.
Consistent with prior studies, individuals with CMP demonstrated lower scores in pain neurophysiology knowledge, as measured by the R-NPQ. This aligns with the biopsychosocial model, which emphasizes the role of cognitive and psychosocial factors in chronic pain.1–5 The findings of this study align with existing literature on the multifactorial influences of BMI, physical activity, and education level on CMP.24–27 These variables emerged as significant predictors in both the linear regression and binary logistic regression analyses, reinforcing their critical role in understanding pain intensity and the likelihood of CMP. BMI was identified as a significant predictor of pain intensity and CMP presence, consistent with previous evidence suggesting that elevated BMI increases the risk of CMP.24,45 Obesity is frequently associated with chronic pain, particularly musculoskeletal pain. The relationship is complex and multidirectional, involving various physiological mechanisms at the neurological and metabolic levels. Obesity can exacerbate pain and increase the consumption of opioids.24 Additionally, pain can act as a barrier to physical activity in obese individuals, creating a vicious cycle that perpetuates both conditions. Excess BMI contributes to mechanical stress on joints and promotes systemic inflammation, which exacerbate pain conditions, particularly in weight-bearing joints.24
Similarly, physical activity demonstrated a predictive role in CMP and pain perception. Regular physical activity was negatively associated with pain intensity and reduced the odds of CMP in our analyses. This aligns with previous findings, suggesting that physical activity mitigates pain by enhancing musculoskeletal health, reducing inflammation, and improving pain modulation mechanisms.24–26 Conversely, inactivity may increase vulnerability to chronic pain, reflecting the observed association between lower activity levels and greater pain intensity.25,26 Physical exercise is an effective, inexpensive, and safe therapeutic option for managing chronic musculoskeletal pain, with non-adverse effects associated with pharmacological treatments or invasive techniques. Exercise has a broad analgesic capacity, improving sleep quality, daily activities, quality of life, physical function, and emotional well-being.24
Educational attainment emerged as a critical factor influencing both pain intensity and the presence of CMP in our study. Participants with lower education levels, particularly those with pre-university education, had significantly higher odds of CMP compared to individuals with a university degree. This aligns with prior evidence linking lower educational attainment to limited health literacy, reduced access to healthcare resources, and less effective pain management strategies.27,46,47 Individuals with higher education levels may possess a greater ability to navigate healthcare systems, adopt healthier lifestyles, and better understand pain mechanisms, potentially mitigating the impact of CMP.46,47 Furthermore, the association between lower educational levels and higher pain intensity observed in our cohort highlights the complex interplay between socioeconomic factors and pain perception.46 This finding emphasizes the need for educational interventions targeting pain neurophysiology, as improving understanding of pain among individuals with lower educational levels could enhance their capacity for self-management and reduce the burden of chronic pain.
Strengths and Implications for Practice
This study has several strengths, including an adequate sample size with a balanced comparison of individuals with and without CMP, allowing for robust statistical analyses. The use of the R-NPQ, ensures the reliability of the data on pain knowledge. The findings of this study emphasize the interconnected roles of pain neurophysiology knowledge, physical activity, BMI, and educational attainment in understanding and addressing CMP. These factors not only influence pain intensity and the likelihood of CMP but also highlight the need for tailored interventions. Improving knowledge of pain neurophysiology through educational programs could help dispel misconceptions, enhance coping strategies, and empower patients to manage their condition more effectively. Promoting physical activity as part of pain management plans is essential, with an emphasis on individualized approaches to account for variability in response, particularly among patients with CMP. Addressing elevated BMI through weight management programs could mitigate the mechanical stress and systemic inflammation that contribute to pain severity, while enhancing health literacy among individuals with lower educational attainment could improve access to resources, foster healthier behaviors, and optimize self-management. Future research should focus on longitudinal studies to explore causal relationships between these factors and CMP, investigate variability in exercise responses, and examine the broader social determinants of health to develop holistic and equitable interventions for chronic pain management.
Limitations
This study has several limitations that should be considered. While the lack of significant differences in variables such as age, height, marital status, and professional status suggests a degree of comparability between groups, this does not entirely eliminate the potential for biases. The cross-sectional design represents a key limitation, as it prevents establishing causal relationships between chronic musculoskeletal pain (CMP) prevalence and neurophysiology of pain knowledge. It remains unclear whether a lack of knowledge contributes to the development of chronic pain or if the presence of chronic pain results in reduced understanding of neurophysiological mechanisms. Longitudinal studies with pain-free cohorts, differing in their baseline knowledge of pain neurophysiology, and extended follow-ups could provide more definitive insights into these relationships. Another limitation is the reliance on self-reported data, which is subject to recall and reporting biases, potentially affecting the accuracy of the findings. Furthermore, the study did not account for different types and severities of chronic pain, which may have varying impacts on pain neurophysiology knowledge. Similarly, variations in physical activity levels were not explored in detail, despite their established influence on pain perception. Finally, the generalizability of the findings is limited to Spanish-speaking populations, as cultural and linguistic differences could influence both neurophysiological knowledge and the experience of pain. Future research should aim to address these limitations by incorporating longitudinal designs, objective measures of physical activity, differentiation between pain types and severities, and cross-cultural validations to enhance the applicability of the findings.
Conclusions
This study showed significant sociodemographic differences were observed between participants with and without CMP, particularly in BMI, physical activity, education level, alcohol consumption habits, and R-NPQ scores. Pain intensity among individuals with CMP varied significantly across educational levels, with higher educational attainment associated with lower pain intensity.
R-NPQ scores, BMI, education level, and physical activity were significant predictors of pain intensity, collectively explaining 33.9% of its variance. Additionally, the binary logistic regression model identified BMI, R-NPQ scores, education level, and physical activity as significant predictors of CMP presence, explaining the variance between 27.6% and 39.2% of the variance.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author, Angel González-de-la-Flor, angel.gonzalez @universidadeuropea.es. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Garland EL. Pain processing in the human nervous system: a selective review of nociceptive and biobehavioral pathways. Prim Care. 2012;39(3):561–571. doi:10.1016/j.pop.2012.06.013
2. Yang S, Chang MC. Chronic pain: structural and functional changes in brain structures and associated negative affective states. Int J Mol Sci. 2019;20(13):3130.
3. Bunzli S, Watkins R, Smith A, Schütze R, O’Sullivan P. Lives on hold: a qualitative synthesis exploring the experience of chronic low-back pain. Clin J Pain. 2013;29(10):907–916. doi:10.1097/AJP.0b013e31827a6dd8
4. Darlow B, Fullen BM, Dean S, Hurley DA, Baxter GD, Dowell A. The association between health care professional attitudes and beliefs and the attitudes and beliefs, clinical management, and outcomes of patients with low back pain: a systematic review. Eur J Pain. 2012;16(1):3–17. doi:10.1016/j.ejpain.2011.06.006
5. King R, Robinson V, Elliott-Button HL, Watson JA, Ryan CG, Martin DJ. Pain reconceptualisation after pain neurophysiology education in adults with chronic low back pain: a qualitative study. Pain Res Manag. 2018;2018. doi:10.1155/2018/3745651
6. Catley MJ, O’Connell NE, Moseley GL. How good is the neurophysiology of pain questionnaire? A Rasch analysis of psychometric properties. J Pain. 2013;14(8):818–827. doi:10.1016/j.jpain.2013.02.008
7. Dueñas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016;9:457. doi:10.2147/JPR.S105892
8. Mankelow J, Ryan C, Taylor P, Martin D. The effect of pain neurophysiology education on healthcare students’ knowledge, attitudes and behaviours towards pain: a mixed-methods randomised controlled trial. Musculoskelet Sci Pract. 2020;50. doi:10.1016/j.msksp.2020.102249
9. Driscoll MA, Knobf MT, Higgins DM, Heapy A, Lee A, Haskell S. Patient experiences navigating chronic pain management in an integrated health care system: a qualitative investigation of women and men. Pain Med. 2018;19(suppl_1):S19–29. doi:10.1093/pm/pny139
10. Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. 2022;163(2):E328–32. doi:10.1097/j.pain.0000000000002291
11. Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6(6). doi:10.1136/bmjopen-2015-010364
12. El-Metwally A, Shaikh Q, Aldiab A, et al. The prevalence of chronic pain and its associated factors among Saudi Al-Kharj population; a cross sectional study. BMC Musculoskelet Disord. 2019;20(1). doi:10.1186/s12891-019-2555-7.
13. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895.
14. Jackson T, Pope L, Nagasaka T, Fritch A, Iezzi T, Chen H. The impact of threatening information about pain on coping and pain tolerance. Br J Health Psychol. 2005;10(Pt 3):441–451. doi:10.1348/135910705X27587
15. Higgins DM, Martin AM, Baker DG, Vasterling JJ, Risbrough V. The relationship between chronic pain and neurocognitive function: a systematic review. Clin J Pain. 2018;34(3):262–275. doi:10.1097/AJP.0000000000000536
16. Rossettini G, Campaci F, Bialosky J, Huysmans E, Vase L, Carlino E. The biology of placebo and nocebo effects on experimental and chronic pain: state of the art. J Clin Med. 2023;12(12):4113. doi:10.3390/jcm12124113
17. Rossettini G, Emadi Andani M, Dalla Negra F, Testa M, Tinazzi M, Fiorio M. The placebo effect in the motor domain is differently modulated by the external and internal focus of attention. Sci Rep. 2018;8(1):1–14. doi:10.1038/s41598-018-30228-9
18. Nijs J, Paul van Wilgen C, Van Oosterwijck J, van Ittersum M, Meeus M. How to explain central sensitization to patients with “unexplained” chronic musculoskeletal pain: practice guidelines. Man Ther. 2011;16(5):413–418. doi:10.1016/j.math.2011.04.005
19. Louw A, Diener I, Butler DS, Puentedura EJ. The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain. Arch Phys Med Rehabil. 2011;92(12):2041–2056. doi:10.1016/j.apmr.2011.07.198
20. Mikamo Y, Takasaki H. Pain neurophysiology knowledge enhances attitudes toward biopsychosocial management of low back pain among japanese physical therapists. Prog Rehabil Med. 2021;6. doi:10.2490/prm.20210039
21. Adillón C, È L, Salvat I. Comparison of pain neurophysiology knowledge among health sciences students: a cross-sectional study. BMC Res Notes. 2015;8(1). doi:10.1186/s13104-015-1585-y
22. Ezzatvar Y, Dueñas L, Balasch-Bernat M, Lluch-Girbés E, Rossettini G. Which portion of physiotherapy treatments’ effect is not attributable to the specific effects in people with musculoskeletal pain? A meta-analysis of randomized placebo-controlled trials. J Orthop Sports Phys Ther. 2024;54(6):391–399. doi:10.2519/jospt.2024.12126
23. Rossettini G, Palese A, Geri T, Mirandola M, Tortella F, Testa M. The knowledge of contextual factors as triggers of placebo and nocebo effects in patients with musculoskeletal pain: findings from a national survey. Front Psychiatry. 2019;10(JULY). doi:10.3389/fpsyt.2019.00478
24. la Corte-Rodriguez H D, Roman-Belmonte JM, Resino-Luis C, Madrid-Gonzalez J, Rodriguez-Merchan EC. The role of physical exercise in chronic musculoskeletal pain: best medicine—A narrative review. Healthcare. 2024;12(2):242. doi:10.3390/healthcare12020242
25. Nascimento Leite M, Kamper SJ, O’Connell NE, et al. Physical activity and education about physical activity for chronic musculoskeletal pain in children and adolescents. Cochrane Database Syst Rev. 2023;2023(7):1.
26. Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of cochrane reviews. Cochrane Database Syst Rev. 2017;2020(2). doi:10.1002/14651858.CD011279.pub3
27. Hansen J, Hansen H, Nilsson C, Ekholm O, Molsted S. Association between educational level and self-reported musculoskeletal pain and physical functioning in Danes 60–70 years old from 2010 to 2017: a longitudinal analysis of trends over time on data from the Danish Health and Morbidity Survey. BMJ Open. 2023;13(11):e073523. doi:10.1136/bmjopen-2023-073523
28. Cox T, Louw A, Puentedura EJ. An abbreviated therapeutic neuroscience education session improves pain knowledge in first-year physical therapy students but does not change attitudes or beliefs. J Man Manip Ther. 2017;25(1):11–21. doi:10.1080/10669817.2015.1122308
29. Colleary G, O’Sullivan K, Griffin D, Ryan CG, Martin DJ. Effect of pain neurophysiology education on physiotherapy students’ understanding of chronic pain, clinical recommendations and attitudes towards people with chronic pain: a randomised controlled trial. Physiotherapy. 2017;103(4):423–429. doi:10.1016/j.physio.2017.01.006
30. Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109(1):5–12. doi:10.1152/jn.00457.2012
31. Moseley GL, Butler DS. Fifteen years of explaining pain: the past, present, and future. J Pain. 2015;16(9):807–813. doi:10.1016/j.jpain.2015.05.005
32. Ziegler AM, Minkalis AL, Langdon ER, Vining R. Learning the neurobiology of pain: a scoping review of pain education from an instructional design perspective. Patient Educ Couns. 2022;105(6):1379–1401. doi:10.1016/j.pec.2021.09.021
33. Watson JA, Ryan CG, Cooper L, et al. Pain neuroscience education for adults with chronic musculoskeletal pain: a mixed-methods systematic review and meta-analysis. J Pain. 2019;20(10):
34. Wood L, Hendrick PA. A systematic review and meta-analysis of pain neuroscience education for chronic low back pain: short-and long-term outcomes of pain and disability. Eur J Pain. 2019;23(2):234–249. doi:10.1002/ejp.1314
35. Torres-Lacomba M, Navarro-Brazález B, Bailón-Cerezo J, Vergara-Pérez F, de la Rosa-Díaz I, Prieto-Gómez V. Assessment tools of patient competences: the spanish version of the R-NPQ and three practical cases in women with breast cancer and persistent pain. Int J Environ Res Public Health. 2021;18(9):4463.
36. von EE, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):1623–1627.
37. Perrot S, Cohen M, Barke A, Korwisi B, Rief W, Treede RD. The IASP classification of chronic pain for ICD-11: chronic secondary musculoskeletal pain. Pain. 2019;160(1):77–82. doi:10.1097/j.pain.0000000000001389
38. Garrow JS. Quetelet index as indicator of obesity. Lancet. 1986;1(8491):1219. doi:10.1016/S0140-6736(86)91207-9
39. Wei Min L, Gutiérrez Cayo H. Efectividad del cuestionario global e internacional de actividad física comparado con evaluaciones prácticas. Revista Cubana de Investigaciones Biomédicas. 2020;39:1–9.
40. Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399–2404. doi:10.1016/j.pain.2011.07.005
41. Boonstra AM, Stewart RE, Köke AJA, et al. Cut-off points for mild, moderate, and severe pain on the numeric rating scale for pain in patients with chronic musculoskeletal pain: variability and influence of sex and catastrophizing. Front Psychol. 2016;7. doi:10.3389/fpsyg.2016.01466
42. Adams P, Murnane EL, Elfenbein M, Wethington E, Gay G. Supporting the self-management of chronic pain conditions with tailored momentary self-assessments. In:
43. Hazra A, Gogtay N. Biostatistics series module 6: correlation and linear regression. Indian J Dermatol. 2016;61(6):593. doi:10.4103/0019-5154.193662
44. Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med. 2018;18(3):91–93. doi:10.1016/j.tjem.2018.08.001
45. Abplanalp SJ, Fulford D. Physical effort exertion and pain: links with trait-based risk for psychopathology. Psychiatry Res. 2019;271:46–51. doi:10.1016/j.psychres.2018.10.010
46. Zajacova A, Rogers RG, Grodsky E, Grol-Prokopczyk H. the relationship between education and pain among adults aged 30–49 in the United States. J Pain. 2020;21(11–12):1270–1280. doi:10.1016/j.jpain.2020.03.005
47. Fentazi D, Pester BD, Yamin JB, Jamison RN, Edwards RR, Meints SM. Why is low educational attainment linked to worse pain and function in fibromyalgia? J Pain. 2025;27:104764. doi:10.1016/j.jpain.2024.104764
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

