Back to Journals » Cancer Management and Research » Volume 16
Expression Analysis of FANCD2 in Endometrial Carcinoma
Authors Zhang L, Chang J, Wu X
Received 29 August 2024
Accepted for publication 25 November 2024
Published 5 December 2024 Volume 2024:16 Pages 1715—1725
DOI https://doi.org/10.2147/CMAR.S488275
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Li Zhang,1 Juan Chang,2 Xiuwei Wu2
1Department of Obstetrics and Gynecology, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, 442000, People’s Republic of China; 2Department of Hematology, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, 442000, People’s Republic of China
Correspondence: Xiuwei Wu, Department of Hematology, Taihe Hospital, Hubei University of Medicine, Renmin Southern 32, Shiyan, Hubei, 442000, People’s Republic of China, Email [email protected]
Background: Cisplatin is a major chemotherapy drug in the treatment of Uterine Corpus Endometrial carcinoma (UCEC), and drug resistance often limits its efficacy. Studying the mechanism of cisplatin resistance in endometrial carcinoma is of great clinical importance. This study aims to analyze the expression and value of FANCD2 in UCEC.
Methods: The expression of FANCD2, prognosis, and relationship between FANCD2 and immune cell infiltration in UCEC were analyzed by using bioinformatics. The expression levels of FANCD2 in 62 cases of endometrial carcinoma and 28 cases of normal endometrial tissues were detected by RT-PCR, and the relationship between FANCD2 expression and clinicopathological features was analyzed. A FANCD2 knockdown plasmid was constructed and transfected into Ishikawa cells to detect the levels of GSH and MDA in the presence of different concentrations of cisplatin.
Results: Bioinformatics analysis showed that FANCD2 was highly expressed in UCEC tissues, and patients with high expression had poor prognosis. Immune infiltration analysis revealed that (B cell, CD8 T cell, macrophage, neutrophil, dendritic cell) infiltration was negatively correlated with FANCD2 expression. Compared with those in Ishikawa-Vector, the levels of GSH significantly decreased and those of MDA significantly increased in Ishikawa-FANCD2KD treated with different concentrations of cisplatin.
Conclusion: FANCD2 was highly expressed in UCEC, and the down-regulation of FANCD2 affected the levels of GSH and MDA to increase the cisplatin sensitivity of Ishikawa cells.
Keywords: endometrial carcinoma, FANCD2, cisplatin, CD8 T cells
Introduction
UCEC is a common gynecological malignancy that primarily affects women aged 50 years and above. In 2020, approximately 417,000 new cases and 97,000 deaths were attributed to UCEC.1 The incidence of UCEC has increased by 1% annually.2 UCEC is divided into two types in accordance with its pathogenesis and biological behavioral characteristics: Type I, which is estrogen-dependent and highly prevalent, and type II, which is nonestrogen-dependent and typically occurs in older women. Type I patients, who are generally younger than type II patients, often exhibit molecular events, such as PTEN inactivation or microsatellite instability, resulting in a favorable prognosis. On the other hand, type II patients have poor differentiation and a high malignancy degree and frequently have TP53 mutations and HER2 overexpression, leading to poor prognosis. The role of FANCD2, a protein involved in the DNA repair pathway, in the development of osteosarcoma,3 ovarian cancer,4 hepatocellular carcinoma.5 In this study, the expression of FANCD2 in UCEC tissues and cells was analyzed to explore the possible mechanism of FANCD2 as a potential therapeutic target of cisplatin.
Methods
Patients and Specimens
Bioinformatics Analysis
The clinical data of UCEC were downloaded from the HPA, TCGA, and GTEx databases to analyze the expression of the FANCD2 gene/protein in UCEC. The relationship between the expression of FANCD2 and overall survival (OS) in patients with UCEC was analyzed by using the K–M online tool. The RNAseq data of the STAR process of the TCGA-UCEC project were downloaded and organized from the TCGA database, and we extracted data with TPM format. Immune cell infiltration, the correlation between FANCD2 expression and immune cell infiltration, and the relationship between immune cell infiltration and PTEN/TP 53 mutation in UCEC were analyzed by using R language. The immune infiltration algorithm was based on the ssGSEA algorithm provided in R-GSVA, which used 24 kinds of immune cell markers to calculate the immune infiltration situation of the corresponding cloud data.
Patients and Specimens
Our institution procured 62 fresh UCEC specimens (46 cases of endometrioid carcinoma, 10 cases of serous carcinoma, and 6 cases of mixed carcinoma) and 28 normal endometrial tissues from uterine fibroids from January 2021 to December 2022. Of the specimens, 30 were highly differentiated, 19 were moderately differentiated, and 13 were poorly differentiated. On the basis of FIGO stage, 25 cases were stage I, 24 cases were stage II, and 13 cases were stages III and IV. Inclusion criteria: 1) clear diagnosis by pathology; 2) complete clinical data and follow-up data; 3) patients with endometrial cancer surgery; 4) patients who did not received postoperative adjuvant therapy (including chemotherapy, radiotherapy or endocrine therapy); 5) did not have malignant tumors at a second site. Exclusion criteria: 1) The patient did not follow the standard surgical treatment; 2) Patients with other malignancies; 3) Patients who had received chemotherapy or radiotherapy before surgery; 4) malignant tumors at other sites; 5) severe liver and kidney dysfunction. All fresh tissues were rinsed with 0.9% saline, cleared of any surface blood, and stored in Trizol at 4 °C overnight. They were subsequently preserved in liquid nitrogen. All patients underwent pathological diagnosis and had comprehensive clinical data. In accordance with the Declaration of Helsinki, all protocols were approved by the Ethics Committee of Taihe Hospital (Reference Number: 2024KS54). The patients in this study were aware and signed the informed consent form, which was approved by the ethics committee.
Cell Culture and Treatment
Ishikawa cells (Procell, CL-0283) were cultured in DMEM containing 10% fetal bovine serum and 1% penicillin–streptomycin at 37 °C and 5% CO2. Different concentrations of cisplatin (0, 5, 10, and 20 μM) were prepared and administered when the cells had fused to 90% confluence. Blank vector (Gene Create, China) and FANCD2 knockdown plasmid vector were transfected by using Lipofectamine 3000 in accordance with instructions and treated with cisplatin. Two oligonucleotide sequences used to knockdown FANCD2 in Ishikawa cells were 5′-GCG GCA GAC AGA AGU GAA UTT-3′ and 5′-AUU CAC UUC UGU CUG CCG CTT-3′.
Real-Time PCR Analysis
Total RNA was extracted from all fresh samples (Trizol Reagent, Thermo Fisher) and then reverse-transcribed into cDNA for amplification (Reverse Transcription System A3500, Thermo Fisher). The primers were used for PCR as follows: GAPDH, 5′-GAA GGT GAA GGT CGG AGT C-3′ and 5′- GAA GAT GGT GAT GGG ATT TC-3′; FANCD2, 5′- TCT TCC GTG TGA TGA TGG CTG AAC-3′ and 5′- ATG AAT CTG CTG CGA GTC TGC TG-3′. The amplification system comprised 2.4 μL of Mg2+, 2 µL each of forward and reverse primers, 0.3 µL of Taq enzyme, 3 µL of 10× buffer, 1.5 µL of 2 mmol/L dNTPs, 1 µL of 10× SYBR-Green I, and 5 µL of cDNA. It was brought to a volume of 30 µL by using sterile water. The amplification conditions were 96 °C for 4 min, 94 °C for 30s, 60 °C for 30s, and 72 °C for 1 min with 40 cycles. Dissociation curve analysis was performed at the end point of 40 PCR cycles. The mRNA expression levels of FANCD2 in each sample were normalized to those of GAPDH and were determined by the 2−∆∆Ct method.
Western Blot Analysis
Proteins separated by SDS-PAGE were electrophoretically transferred to polyvinylidene difluoride membranes. The blots were incubated in 1X PBS containing 5% non-fat dry milk for 2.5 h. The blot was incubated with primary antibody (ZSGBBIO, TA309656) overnight at 4°C. After washing, the blots were incubated with 1:1000 dilution of horse radish peroxidase-conjugated secondary antibodies (ZSGBBIO, ZB-5301) for 1 h at room temperature. After extensive washed with high and low salt buffers, the immunoreactive proteins were visualized using enhanced chemiluminescence detection reagents. Two leader were used in this study (Thermofisher, 26637 and Beyotime P0075). GAPDH was supplied by Proteintech Group (Cat No. 10494-1-AP). Densitometry was quantitated with Total Lab 1.11 software.
Detection of GSH and MDA Levels
The cells were inoculated into a six-well culture plate at the cell density of 5 × 104/well and incubated with different concentrations of cisplatin for 12 h when they had fused to 90% confluence. The treated cells were collected and tested in accordance with the instructions of GSH (Beyotime, S0053) and MDA (Beyotime, S0131S) assay kits. All cell experiments were repeated six times.
Statistical Analysis
Independent sample t test was used to analyze differences, and the K–M method was used for survival analysis. P < 0.05 indicated that the difference was statistically significant.
Results
Expression and Prognosis of FANCD2 in Different Databases
First, the expression of FANCD2 protein in normal and UCEC tissues from the HPA and Ualcan databases was analyzed. Cells in normal tissues were not stained, whereas those in tumor tissues were deeply stained brown by HPA (Figure 1A). The expression of FANCD2 protein in tumor tissues was significantly higher than that in normal tissues from Ualcan (Figure 1B). Data from the TCGA and GTEx databases were downloaded to analyze the expression of FANCD2 in 174 tumor tissues and 91 normal tissues. The data showed that the expression in tumor tissues was significantly higher than that in normal tissues (Figure 1C). In the paired samples, the expression level in cancer tissue was also higher than that in normal tissue (Figure 1D). A statistically significant difference between expression levels in samples of different histological types and grades was also found (Figure 1E). An online tool was also used to analyze the prognosis of patients with different FANCD2 expression levels. This tool showed that patients with low expression had better OS than those without (Figure 1F), and in patients with stage I, stages I and II, and endometrial-type UCEC, the high expression of FANCD2 resulted in poor prognosis. (Figure 1G).
Analysis of Immune Cell Infiltration
Tumor-related immune cell infiltration has attracted increasing attention. The analysis of the relationship between FANCD2 expression and immune cell infiltration showed that FANCD2 expression was negatively correlated with the infiltration of B cells, CD8 T cells, neutrophils, macrophages, and dendritic cells (Figure 2A–C). The relationship between the mutation of PTEN and TP53, two common genes in UCEC, and the infiltration of immune cells was also analyzed. PTEN mutation was positively correlated with the infiltration of CD8T cells, macrophages, and dendritic cells, whereas that of TP53 was negatively correlated with the infiltration of CD8T cells, macrophages, and dendritic cells (Figure 2D and E).
 |
Figure 2 Analysis of immune cell infiltration in UCEC. (A) TIMER analysis; (B) and (C) R language analysis; (D) PTEN mutation; (E) TP53 mutation. *P<0.05, **P<0.01, ***P<0.001. |
The mutation rate of TP53 in UCEC was 37.1%. UCEC cases were divided into the mutant and wild groups in accordance with whether TP53 was mutated or not. The expression of FANCD2 was analyzed in different groups by using the Timer online tool, and the expression of FANCD2 in the mutant group was significantly higher than that in the wild group (Figure 3A and B). The mutation rate of PTEN in UCEC was 62.9%. However, no statistically significant difference in the expression of FANCD2 was found between the mutant and wild groups (Figure 3C and D).
Analysis of Prognostic Factors of UCEC
The Cox regression analysis of the prognostic factors of UCEC showed that clinical stage, age, histological type, histologic grade, tumor invasion, and FANCD2 expression were associated with prognosis (Figure 4).
 |
Figure 4 Cox regression analysis of prognostic factors in UCEC.*P<0.05, **P<0.01, ***P<0.001. |
Relationship Between FANCD2 Expression and Clinicopathological Features
Data were downloaded from the TCGA database to analyze the correlation between FANCD2 expression and clinicopathological features through the chi-square test. The results showed that FANCD2 expression was correlated with age, histological type, histologic grade, and clinical stage (Table 1). FANCD2 expression was 0.56 ± 0.16 in 62 UCEC tissues and 0.29 ± 0.11 in 28 normal tissues with a statistically significant difference. At the same time, the relationship between FANCD2 expression and clinicopathological features (age, body mass index, menopausal status, diabetes status, clinical stage, differentiation degree, tissue type, etc). was analyzed. The results showed that FANCD2 expression was highest in low differentiation, second-highest in middle differentiation, and lowest in high differentiation. The difference between different differentiation degrees was statistically significant. The expression of FANCD2 in endometrioid carcinoma was significantly lower than that in serous and mixed carcinomas, whereas the difference in expression levels among other clinical features was not statistically significant. A total of 62 UCEC cases were divided into types I and II in accordance with pathological results. FANCD2 expression in type II patients was significantly higher than that in type I patients (Figure 5).
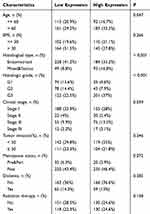 |
Table 1 Baseline Charactetistics of Study Population |
 |
Figure 5 Relationship between FANCD2 expression and clinicopathological features. *P<0.05, **P<0.01, ***P<0.001. |
Analysis of FANCD2 Expression, GSH and MDA Levels in Ishikawa Cells
The levels of FANCD2, GSH, and MDA were detected after treatment with different concentrations of cisplatin. The results showed that FANCD2 and GSH levels gradually decreased and MDA levels gradually increased in Ishikawa cells after treatment with different concentrations of cisplatin (Figure 6A–C).
FANCD2 knockdown plasmid was constructed and transfected into Ishikawa cells, and the expression level of FANCD2 was detected by Western blotting to verify the transfection efficiency. The protein expression level in the FANCD2 knockdown group was significantly lower than that in the blank vector group, indicating successful transfection (Figure 6D). Compared with the Vector treatment, treatment with different concentrations of cisplatin further decreased GSH levels and increased MDA levels in Ishikawa-FANCD2KD cells (Figure 6E and F).
Discussion
UCEC is a common malignant tumor in the female reproductive system. Its incidence is steadily increasing, and its age of onset is decreasing. Given the numerous factors that affect UCEC prognosis, particularly the poor results in some type II patients, investigating the pathogenesis of UCEC and identifying suitable therapeutic targets for precision medicine are crucial. This article presents a preliminary investigation into the potential of FANCD2 as a target for chemotherapy drugs.
Bioinformatics analysis integrating diverse omics data provides novel approaches to identify oncogenes or tumor suppressor genes, develop tumor diagnostic and prognostic models, and select therapeutic targets. This study initiated a bioinformatics analysis of FANCD2 expression in UCEC. The analysis revealed significantly higher expression levels in tumor tissues than in normal tissues. Additionally, low FANCD2 expression was associated with long OS. Regression analysis showed that clinical stage, histological type, and FANCD2 expression were prognostic factors, and in patients with stage I, stages I & II, and endometrial-type UCEC, high FANCD2 expression resulted in poor prognosis.
In liver cancer, the expression of FANCD2 and progression of HBV-associated hepatocellular carcinoma are positively correlated.6 In colorectal cancer, high FANCD2 expression can serve as a prognostic marker and assist in guiding personalized treatment.7 Furthermore, the analysis of clinical samples demonstrated an increase in FANCD2 expression in serous and mixed types, as well as in patients with low differentiation and type II cancer. Research conducted by international scholars on DNA repair proteins in UCEC showed that the positive expression of type I FANCD2 is associated with tumor grading and staging. Moreover, compared with other patients, type II patients with positive TP53 or FANCD2 expression are more prone to relapse, exhibiting lower 5-year relapse-free survival and OS rates.8
Tumor cell immunity plays a crucial role in immune response and escape mechanisms and is characterized by the diverse effects of various types of immune cells at different stages of tumor cell immunity. The incorporation of immunotherapy into the initial treatment for advanced and metastatic endometrial cancer brings about a substantial improvement in oncologic outcomes. This treatment is currently a focal point of investigation for evaluating the potential of chemotherapy-free regimens. The meta-analysis substantiated the adoption of chemotherapy alongside immunotherapy, revealing a significant improvement in progression-free survival compared to chemotherapy alone across all patient groups.9
The bioinformatics analyses of immune cell infiltration has revealed that the expression of FANCD2 in UCEC tumor tissues is negatively correlated with the infiltration of common immune cells (B cell, CD8 T cell, macrophage, neutrophil and dendritic cell). Endometrial tissue consists of a substantial number of leukocytes-the quantity and phenotype of which vary throughout the menstrual cycle-potentially associated with the immune protection required during endometrial rupture.10 Consequently, the tumor immune response may be specifically enhanced in UCEC cells. Irrespective of the UCEC subtype, high OS and disease-free survival are associated with the high intratumoral quantity of CD8+ T cells.11 In UCEC, the immunomodulatory molecule IDO1 plays a critical role in promoting tumor immune suppression by converting L-tryptophan into the immunosuppressive metabolite L-kynurenine, thus facilitating the differentiation of regulatory T cells and inducing tolerance in dendritic and myeloid-derived suppressor cells.12,13
Furthermore, the expression of regulatory molecules is associated with a reduction in the number of intratumoral CD8+ T and natural killer cells and progression-free survival. Similarly, in lung adenocarcinoma, FANCD2 expression levels are associated with tumor-infiltrating immune cells and their corresponding gene characteristics, and FANCD2 overexpression is strongly correlated with low survival rates in patients with LUAD.14 In adrenocortical carcinoma, FANCD2 is highly associated with prognosis, the expression of immune regulatory factors in the tumor microenvironment, and the effectiveness of immunotherapy.15 In patients diagnosed with Fanconi anemia, FANCD2 may participate in the maturation of immature B and T cells.16 Interestingly, an analysis of common gene mutations in UCEC and their correlation with immune cell infiltration revealed that PTEN mutations are positively associated with CD8+ T cell, macrophage, and dendritic cell infiltration, whereas TP53 mutations exhibit a negative association that aligns with the poor prognosis observed in patients with type II UCEC
Cell experiments have demonstrated that the high expression of FANCD2 in Ishikawa cells treated with varying concentrations of cisplatin leads to a decrease in the levels of FANCD2 and GSH, whereas MDA levels increase. In Ishikawa cells, when FANCD2 is knocked down, GSH levels further decrease and MDA levels further increase, indicating that the reduced expression of FANCD2 can result in decreased GSH levels and increased MDA levels. Additionally, the down-regulation of FANCD2 expression may enhance the sensitivity of Ishikawa cells to cisplatin through a cell death mechanism known as ferroptosis. Ferroptosis, which is characterized by GSH depletion and lipid peroxidation, is different from apoptosis. FANCD2 serves as an inhibitor of ferroptosis while also participating in DNA damage repair, thereby preventing iron accumulation and lipid peroxidation in cells. However, further investigation is required to fully understand the specific mechanisms of FANCD2 in UCEC.17
Common ferroptosis inducers act directly on GPX4 and inhibit its activity, further affecting the synthesis of GSH, reducing cell antioxidant capacity, and accumulating reactive oxygen species (ROS), ultimately leading to oxidative damage and ferroptosis.18 Studies have shown that simvastatin can affect the intracellular ROS levels of Ishikawa cells by up-regulating MDA levels and down-regulating GSH levels. Moreover, it can inhibit Ishikawa cell proliferation, promote cell oxidation and ferroptosis by participating in the RAS/MAPK signaling pathway.19 However, whether the infiltration of immune cells is also involved in this mechanism needs further analysis and research. Furthermore, knockdown of FANCD2 expression in UCEC cell lines inhibited malignant proliferation and migration ability. Down-regulated FANCD2 confers sensitivity of UCEC cells to interstrand crosslinking agents. This study provides evidence for the malignant progression and prognostic value of FANCD2 in UCEC.20
In summary, the high expression of FANCD2 is a reason for the poor prognosis of patients with UCEC and may be related to the infiltration of immune cells and ferroptosis. Moreover, the down-regulation of FANCD2 may increase the sensitivity of Ishikawa cells to cisplatin through affecting the level of GSH and MDA, indicating that FANCD2 has certain potential as a therapeutic target of cisplatin. However, the specific mechanism involved needs further study. In subsequent experiments, we will include additional cell lines to analyze further the pathways and related mechanisms involved in ferroptosis and mediated by FANCD2 in cisplatin resistance in UCEC. By using this approach in combination with animal experiments and in vitro/vivo systematic studies, we will provide a theoretical basis for FANCD2 as a therapeutic target.
Conclusion
In summary, bioinformatics analysis combined with clinical sample validation confirmed that FANCD2 was highly expressed in UCEC. Meanwhile, it was found in cell analysis that the down-regulation of FANCD2 affected the levels of GSH and MDA to increase the cisplatin sensitivity of Ishikawa cells. This study provides a theoretical basis for FANCD2 as a therapeutic target for endometrial cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Laversanne M, Sung H. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
2. Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
3. Li X, Liu J. FANCD2 inhibits ferroptosis by regulating the JAK2/STAT3 pathway in osteosarcoma. BMC Cancer. 2023;23(1):179. doi:10.1186/s12885-023-10626-7
4. Joshi S, Campbell S, Lim JY, et al. Subcellular localization of FANCD2 is associated with survival in ovarian carcinoma. Oncotarget. 2020;11(8):775–783. doi:10.18632/oncotarget.27437
5. Yang Z, Song Y, Li Y, et al. Integrative analyses of prognosis, tumor immunity, and ceRNA network of the ferroptosis-associated gene FANCD2 in hepatocellular carcinoma. Front Genet. 2022;13:955225. doi:10.3389/fgene.2022.955225
6. Su H, Liu Y, Huang J. Ferroptosis-Related Gene SLC1A5 Is a Novel Prognostic Biomarker and Correlates with Immune Microenvironment in HBV-Related HCC. J Clin Med. 2023;12(5):1715. doi:10.3390/jcm12051715
7. Shi WK, Liu YX, Qiu XY, et al. Construction and validation of a novel Ferroptosis-related gene signature predictive model in rectal Cancer. BMC Genomics. 2022;23(1):764. doi:10.1186/s12864-022-08996-6
8. Mhawech-Fauceglia P, Wang D, Kim G, et al. Expression of DNA repair proteins in endometrial cancer predicts disease outcome. Gynecol Oncol. 2014;132(3):593–598. doi:10.1016/j.ygyno.2014.02.002
9. Bogani G, Monk BJ, Powell MA, et al. Adding immunotherapy to first-line treatment of advanced and metastatic endometrial cancer. Ann Oncol. 2024;35(5):414–428. doi:10.1016/j.annonc.2024.02.006
10. Laine A, Gonzalez-Lopez AM, Hasan U, et al. Immune Environment and Immunotherapy in Endometrial Carcinoma and Cervical Tumors. Cancers. 2023;15(7):2042. doi:10.3390/cancers15072042
11. Pakish JB, Zhang Q, Chen Z, et al. Immune Microenvironment in Microsatellite-Instable Endometrial Cancers: hereditary or Sporadic Origin Matters. Clin Cancer Res. 2017;23(15):4473–4481. doi:10.1158/1078-0432.CCR-16-2655
12. Prendergast GC, Malachowski WJ, Mondal A, et al. Indoleamine 2,3-Dioxygenase and Its Therapeutic Inhibition in Cancer. Int Rev Cell Mol Biol. 2018;336:175–203. doi:10.1016/bs.ircmb.2017.07.004
13. Salminen A. Role of indoleamine 2,3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process. Ageing Res Rev. 2022;75:101573. doi:10.1016/j.arr.2022.101573
14. Zhang J, Wang D, Chen X, et al. Upregulation of Ferroptosis-Related Fanconi Anemia Group D2 is a Poor Prognostic Factor and an Indicator of Tumor Immune Cell Infiltration in Lung Adenocarcinoma. Front Genet. 2022;13:825685. doi:10.3389/fgene.2022.825685
15. Shen C, Wang Y. Ferroptosis Biomarkers for Predicting Prognosis and Immunotherapy Efficacy in Adrenocortical Carcinoma. Arch Med Res. 2023;54(1):45–55. doi:10.1016/j.arcmed.2022.12.003
16. Deniskin R, Sasa GS, Nandiwada SL, et al. Lymphopenia With Clinical and Laboratory Features of Combined Immune Deficiency in an 11-Year-Old Female With FANCD2 Variants and Fanconi Anemia. Front Pediatr. 2018;6:390. doi:10.3389/fped.2018.00390
17. Song X, Xie Y, Kang R, et al. FANCD2 protects against bone marrow injury from ferroptosis. Biochem Biophys Res Commun. 2016;480(3):443–449. doi:10.1016/j.bbrc.2016.10.068
18. Zhou D, Wu Q, Qiu H, et al. Simvastatin Inhibits Endometrial Cancer Malignant Behaviors by Suppressing RAS/Mitogen-Activated Protein Kinase (MAPK) Pathway-Mediated Reactive Oxygen Species (ROS) and Ferroptosis. Evid Based Complement Alternat Med. 2022;2022:6177477. doi:10.1155/2022/6177477
19. Zheng C, Ren Z, Chen H, et al. FANCD2 promotes the malignant behavior of endometrial cancer cells and its prognostic value. Exp Cell Res. 2022;421(2):113388. doi:10.1016/j.yexcr.2022.113388
20. Liu P, Zhang Z, Cai Y, et al. Ferroptosis: mechanisms and role in diabetes mellitus and its complications. Ageing Res Rev. 2024;94:102201. doi:10.1016/j.arr.2024.102201
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




