Back to Journals » International Journal of Nanomedicine » Volume 20
Extensive Review of Nanomedicine Strategies Targeting the Tumor Microenvironment in PDAC
Authors Liu X , Shao Y, Li Y, Chen Z, Shi T, Tong Q, Zou X , Ju L, Pan J, Zhuang R, Pan X
Received 18 November 2024
Accepted for publication 17 February 2025
Published 17 March 2025 Volume 2025:20 Pages 3379—3406
DOI https://doi.org/10.2147/IJN.S504503
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Xing Liu,1 Yidan Shao,2 Yunjiang Li,3 Zuhua Chen,3 Tingting Shi,2 Qiao Tong,2 Xi Zou,2 Liping Ju,2 Jinming Pan,2 Rangxiao Zhuang,2,* Xuwang Pan2,*
1School of Pharmaceutical Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 311400, People’s Republic of China; 2Department of Pharmaceutical Preparation, Affiliated Hangzhou Xixi Hospital, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310023, People’s Republic of China; 3Radiology Department, Affiliated Hangzhou Xixi Hospital, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310023, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Rangxiao Zhuang; Xuwang Pan, Department of Pharmaceutical Preparation, Affiliated Hangzhou Xixi Hospital, Zhejiang Chinese Medical University, 2, Hengbu Road, Hangzhou, Zhejiang, 310023, People’s Republic of China, Tel +86-571-8648-1609, Email [email protected]; [email protected]
Abstract: Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest cancers in the world, mainly because of its powerful pro-connective tissue proliferation matrix and immunosuppressive tumor microenvironment (TME), which promote tumor progression and metastasis. In addition, the extracellular matrix leads to vascular collapse, increased interstitial fluid pressure, and obstruction of lymphatic return, thereby hindering effective drug delivery, deep penetration, and immune cell infiltration. Therefore, reshaping the TME to enhance tumor perfusion, increase deep drug penetration, and reverse immune suppression has become a key therapeutic strategy. Traditional therapies for PDAC, including surgery, radiation, and chemotherapy, face significant limitations. Surgery is challenging due to tumor location and growth, while chemotherapy and radiation are hindered by the dense extracellular matrix and immunosuppressive TME. In recent years, the advancement of nanotechnology has provided new opportunities to improve drug efficacy. Nanoscale drug delivery systems (NDDSs) provide several advantages, including improved drug stability in vivo, enhanced tumor penetration, and reduced systemic toxicity. However, the clinical translation of nanotechnology in PDAC therapy faces several challenges. These include the need for precise targeting and control over drug release, potential immune responses to the nanocarriers, and the scalability and cost-effectiveness of production. This article provides an overview of the latest nanobased methods for achieving better therapeutic outcomes and overcoming drug resistance. We pay special attention to TME-targeted therapy in the context of PDAC, discuss the advantages and limitations of current strategies, and emphasize promising new developments. By emphasizing the enormous potential of NDDSs in improving the treatment outcomes of patients with PDAC, while critically discussing the limitations of traditional therapies and the challenges faced by nanotechnology in achieving clinical breakthroughs, our review paves the way for future research in this rapidly developing field.
Keywords: nanoscale, drug delivery systems, pancreatic ductal adenocarcinoma, tumor microenvironment, deep penetration
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a malignancy with a notably high mortality rate, with increasing incidence and mortality rates on a global scale. In the United States, the incidence rates of PDAC increased annually by 0.6% to 1% between 2015 and 2019.1 Projections for 2024 anticipate the diagnosis of approximately 66,440 new cases in the United States, with an estimated 51,750 resultant fatalities.1 According to the GLOBOCAN 2022 statistics, among all malignant tumors worldwide, there were 510,566 new cases and 467,005 deaths from PDAC in 2022. PDAC ranks 12th in terms of global cancer incidence but 6th in terms of cancer-related deaths, highlighting its high fatality rate.2 It is accepted as the ‘king of cancers’, and its 5-year about adaptation amount is actual low. As of now, the limited number of chemotherapies and targeted therapies approved by the US Food and Drug Administration (FDA) have led to an improvement in the 5-year survival rate of pancreatic cancer patients, rising from roughly 2% a decade ago to 11% in 2022.3
The initial signs of PDAC are generally non-obvious, and there is a scarcity of diagnostic biomarkers, resulting in the majority of patients being diagnosed at a later stage where surgical removal is seldom possible.4 Approximately 80% of pancreatic cancer patients are ineligible for curative surgery at the time of diagnosis and can only receive palliative treatment. Even those who qualify for surgery frequently suffer from aggressive metastasis, and these metastatic tumors exhibit high resistance to standard chemotherapy and radiotherapy treatments. Consequently, the postoperative five-year survival rate is only approximately 20%.5 For patients who cannot be treated surgically, the effects of chemotherapy and radiotherapy are particularly critical. However, these traditional treatment methods have limited effectiveness and significant side effects. Radiotherapy is challenging because of the deep location of the pancreas and its proximity to vital organs, making it difficult to eradicate the tumor completely without damaging normal tissue.6,7 Chemotherapeutic agents such as gemcitabine (GEM) and FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) are standard treatments for advanced pancreatic cancer; however, they only slightly prolong survival and are often accompanied by drug resistance and significant side effects.8–10 The prognosis of patients with PDAC is extremely poor, with a median survival of less than 12 months following diagnosis.1 By 2030, PDAC is projected to become the second most common cause of cancer-related death in the United States.11 The clinical characteristics of patients with PDAC are summarized in Figure 1, highlighting the urgent need for more effective therapeutic strategies.
 |
Figure 1 The pathological characteristics, clinical characteristics and clinical treatment status of PDAC. |
 |
Figure 2 Timeline for the development of nanoparticle drug delivery systems. Reprinted with permission from Shi J, Votruba AR, Farokhzad OC, et al. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10(9):3223-30.12, |
Liposomes were the first discovered nanoscale drug delivery systems (NDDSs) and were used as carriers for drugs and proteins in the 1960s.13 Since then, an increasing variety of materials have been fabricated into nanoparticles (NPs) and utilized as NDDSs (Figure 2).12 In recent years, the research and development of nanotechnology-based drug delivery systems (DDSs) have offered fresh optimism for the management of PDAC.14–16 Nanocarriers can improve the druggability of chemotherapy drugs, increase their water solubility, and reduce the degradation and damage of drugs by enzymes in the blood due to their encapsulation effect.17 More importantly, nanocarriers loaded with chemotherapy drugs can effectively improve the in vivo distribution of drugs, increase drug enrichment in tumor tissues, reduce drug distribution in normal organs, and exert synergistic and attenuating effects.18 Approval has been granted for several nanomedicines to treat PDAC. (Table 1). In PDAC therapy, liposomes and lipid-based NPs are the most commonly utilized categories of NDDS,19,20 followed by polymeric NPs,21 protein-drug conjugate NPs,22 vaccines (particularly mRNA vaccines),23 inorganic NPs,24 and NPs based on extracellular vesicles.25 At present, several novel NDDSs are undergoing assessment in clinical trials (Table 2).
 |
Table 1 Approved and Marketed Nano-Based Drugs for the Treatment of Pancreatic Cancer |
 |
Table 2 Ongoing Clinical Trials Involving Nanocarriers for the Treatment of Pancreatic Cancer |
Onivyde, a liposomal nanocarrier of irinotecan, gained FDA approval in 2015 and was used in combination with 5-FU and leucovorin in patients with metastatic PDAC, extending the average survival time from 4.2 months with fluorouracil and folic acid alone to 6.1 months.26 However, compared with that of patients treated with a combination of 5-FU and leucovorin, the survival period of patients treated with irinotecan liposomes alone did not improve. The main reason is that the tumor microenvironment (TME) of PDAC impedes the effective delivery of nanodrugs, thus affecting their efficacy.27,28
A prominent characteristic of PDAC is the abundance of tumor stroma, comprising over 70% of the overall tumor mass.28,29 The tumor stroma encompasses a variety of cellular elements like fibroblasts, immune cells, astrocytes, pericytes, and endothelial cells, as well as noncellular constituents such as collagen, hyaluronic acid, fibronectin, growth factors, and cytokines. Additionally, it features biophysical factors including a low pH level, oxygen deprivation, and elevated tumor interstitial pressure.30–32 These components greatly hinder the deep delivery of nanomedicine to tumor tissues. The stroma is not only poorly vascularized but also characterized by relatively non-leaky vessels due to extensive pericyte coverage. This high pericyte coverage hinders the extravasation of both small-molecule chemotherapeutic agents and NPs to the site of the PDAC tumor.33,34 The deposition of collagen and hyaluronic acid in the stroma leads to vascular and lymphatic collapse, compromising blood flow and limiting drug delivery.35,36 Furthermore, the collapse of blood vessels in PDAC results in an elevation of interstitial hydraulic pressure that surpasses both the static water pressure of the collecting vessels and the osmotic pressure of the vessel wall, thereby impeding the penetration of nanomedicines.37 These components engage in various interactions that facilitate the advancement of PDAC and the spread of tumor metastasis, which is also the main reason for drug resistance and chemotherapy failure in patients with PDAC (Figure 3).28,38 Furthermore, the fibrotic microenvironment of PDAC comprises inhibitory elements of both the innate and adaptive immune systems, hence also known as immune “cold” tumors, with reduced numbers of natural killer cells and cytotoxic CD8+ T cells and increased numbers of tumor-associated macrophages (TAMs), cancer-associated fibroblasts (CAFs), N2 neutrophils, myeloid-derived suppressor cells, and T-regulatory cells at the PDAC tumor site.19,39,40 Taken together, the dense stroma of PDAC, coupled with immune suppression, adversely affects drug delivery, resulting in resistance to most therapeutics, including two first-generation nanocarriers designed for chemotherapy delivery.41 Therefore, considering the key issues of the PDAC microenvironment and nanodrug delivery, more efficient NDDSs are needed to improve the therapeutic effect. Nanocarriers come in a wide array of compositions, shapes, and surface modifications, each with its unique properties that can influence how they interact with the TME. For instance, some nanocarriers might be more stable at low pH levels or better able to navigate through dense stroma due to their size and shape. Moreover, recent advancements in nanotechnology have led to the development of smart nanocarriers that can respond to specific stimuli within the TME, such as pH changes or the presence of specific enzymes. These advanced designs have the potential to overcome some of the challenges outlined in the passage more effectively than traditional nanocarriers. The proposed workflow for the development of actively targeted nanocarriers is illustrated in Figure 4.42
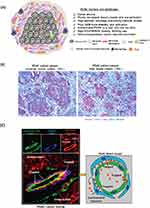 |
Figure 3 (A) Schematic to show the barriers and challenges that are responsible for failed chemotherapy in PDAC, including as a result of an abundant dysplastic stroma, which serves as a physical and biological barrier, including the immunosuppressive tumor microenvironment. (B) Trichrome staining of PDAC tissue sections. Moderate (~50%) and high (N70%) stroma content PDAC tumors were shown. Blue: stroma, eg collagen. Purple: tumor cells. (C).Figure 1C demonstrates that PDAC tumor develops a dense stromal barrier, which blocks the vascular access of IV–injected red fluorescent liposomes. Higher level magnification was provided to show the localization of the liposomes in the tumor in relation to endothelial cells (CD31) and pericyte (NG2) fluorescent markers. The fluorescence microscopy image obtained from PDAC tumor site showed a region of a blood vessel where pericytes were trapping some liposomes just beyond their point of egress from the vascular fenestrations. Reprinted from Advanced Drug Delivery Reviews, Meng H, Nel AE. Use of nano engineered approaches to overcome the stromal barrier in pancreatic cancer. Adv Drug Deliv Rev. 130:50–57. Copyright 2018, with permission from Elsevier.28 |
 |
Figure 4 Assembly of drug-loaded liposomes and their predicted effects on pancreatic cancer cells. Reprinted with permission from Rosenblum D, Joshi N, Tao W, et al. Progress and challenges toward targeted delivery of cancer therapeutics. Nat Commun. 2018;9(1):1410. Creative Commons.42 Liposomes were generated using a film-ultrasonic dispersion method; DFO and YC1 were encapsulated into the hydrophilic and hydrophobic layers, respectively. The surface of the nanoparticles was decorated with transferrin (TF) via chemical cross-linking. When injected intravenously into mice, nanoparticle-encapsulated DFOs (TNP-DFO-YC1) exhibit a much longer circulation half-life than free DFO (I) and accumulate in tumor tissue through the EPR effect (II). TNP-DFO-YC1 were then selectively taken up by cancer cells, which express high levels of TFR1 on their surface (III). After internalization of TNP-DFO-YC1, the drugs are released inside the cell where they exert their antitumor effects (IV). |
Targeting Pancreatic Ductal Adenocarcinoma Cells
On the basis of the highly expressed receptors on the surface of PDAC cells, a nanodelivery system targeting PDAC cells was constructed via ligand modification technology. The nanotargeted delivery system can increase the concentration of drugs in PDAC tissues, effectively deliver drugs to PDAC cells through ligand‒receptor-mediated endocytosis, increase the concentration of drugs in cells, enhance their efficacy, and reduce their toxicity to normal tissues. Many receptors, such as the transferrin receptor (TfR), epidermal growth factor receptor (EGFR), integrin receptor, and urokinase plasminogen activator receptor (uPAR), on the surface of PDCA cells have been confirmed.
Transferrin Receptor
Iron serves as a vital element for cell proliferation and energy metabolism, intricately linked to oxygen transport and electron transfer processes within the respiratory chain, and holds a pivotal role in tumor growth. Transferrin (Tf) serves as the primary hydrophilic transport protein for iron in the plasma, facilitating the movement of iron from liver tissue to cells located in various other tissues. The molecular weight of Tf is approximately 7.7 kDa.43 Tf can bind to TfRs on the cell surface, form a complex, and then enter the cell through endocytosis.44 TfR is a transmembrane glycoprotein that mediates the absorption of iron by cells by binding to Tf. It is the main protein receptor for iron metabolism in the body. Currently, two types of TfRs, TfR1 and TfR2, have been discovered. TfR is expressed in both normal and malignant tissues, with studies revealing that cancer cells exhibit an expression level of TfR that is approximately 100 times higher compared to normal cells.45 This is due to the rapid growth of cancer cells, which significantly elevates their demand for iron during processes such as growth, DNA synthesis, differentiation, and regeneration. Consequently, to adapt to increased iron demand and maintain rapid cell division, TfR expression is upregulated in many malignant tumor tissues.46,47 More than 90% of malignant pancreatic cancer cells overexpress TfR.48,49 Hence, Tf can function as a targeted ligand in drug delivery systems, enabling it to bind with pertinent drugs and efficiently transport them to tumor cells.50
Wang and colleagues developed tFNA nanocarriers adorned with tTR14 for the delivery of CEBPA-saRNA.51 In vitro studies showed successful delivery of CEBPA-saRNA into cells, leading to significant activation of the tumor suppressor genes CEBPA and its downstream effector P21, which notably inhibited the proliferation of PDAC cells. Additionally, in a mouse model of PDAC, the use of tTR14-modified tFNA for delivering CEBPA-saRNA effectively increased the expression of CEBPA and P21 genes, resulting in suppression of tumor growth. McCarthy et al assessed the effectiveness of the 3DNA® Nanocarrier (3DNA) platform in delivering therapeutics directly to PDAC tumors in vivo.52 Compared to unconjugated 3DNA treatment, transferrin-conjugated 3DNA significantly enhanced the targeted delivery of DNA to tumors. For the first time, Bano and colleagues introduced a platform for triple-receptor-targeted photoimmuno-nanoconjugates (TR-PINs).53 The functionalization of TR-PIN with cetuximab, holo-transferrin, and trastuzumab respectively imparted specificity towards EGFR, TfR, and human epidermal growth factor receptor 2 (HER-2). The results demonstrate that TR-PINs exhibited a significantly higher degree of destruction compared to monotargeted Cet-PINs in 3D in vitro heterocellular models of heterogeneous pancreatic ductal adenocarcinoma (PDAC; MIA PaCa-2 cells) and heterogeneous head and neck squamous cell carcinoma (HNSCC; SCC9 cells), which included low-EGFR-expressing T47D cells (high TfR) or SKOV-3 cells (high HER-2). Prabhuraj et al synthesized curcumin (Cur) loaded mesoporous silica nanoparticles (MSNs), subsequently coated them with polyethylene glycol (PEG), and conjugated them with Tf to specifically target human PC cells.54 Confocal microscopy demonstrated that, compared with that of free curcumin, the curcumin uptake of MSN-NH2-Cur-PEG-Tf was seven times greater. An in vitro cytotoxicity study on MIA PaCa-2 cells revealed that MSN-NH2-Cur-PEG-Tf exhibited threefold greater cytotoxicity than free curcumin. Preclinical evaluation of antitumor effectiveness in the MIA PaCa-2 subcutaneous xenograft model demonstrated that both MSN-NH2-Cur-PEG and MSN-NH2-Cur-PEG-Tf were capable of suppressing tumor growth and reducing distant metastasis to vital organ sites. Lang et al utilized TfR1-targeted liposomes (TNP-DFO-YC1) to simultaneously deliver DFO and YC1 to pancreatic tumors in a mouse model. By encapsulating DFO, its circulation time was extended, leading to enhanced accumulation in tumor tissues through the enhanced permeability and retention (EPR) effect and facilitating efficient internalization by cancer cells expressing high levels of TfR1 (Figure 5).55
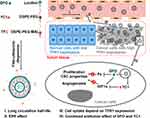 |
Figure 5 Schematic illustration of the proposed workflow in the development of actively targeted nanocarriers. Reprinted with permission from Lang J, Zhao X, Wang X, et al. Targeted co-delivery of the iron chelator deferoxamine and a HIF1α inhibitor impairs pancreatic tumor growth. ACS Nano. 2019;13(2):2176–2189. Copyright 2019, American Chemical Society.55 |
Epidermal Growth Factor Receptor
EGFR, which is also referred to as ERBB1 or HER1, is a transmembrane receptor of the tyrosine kinase type, belonging to the ERBB family of cell surface receptor tyrosine kinases.56 When EGF binds to EGFR, it stimulates the receptor to dimerize with other ERBB family members, specifically HER2, forming homodimers or heterodimers. The receptor phosphorylates and activates downstream effector factors such as MAPK and PI3K, which are closely related to cell proliferation, differentiation, angiogenesis, and other functions.57,58 Research shows that approximately 30% of epidermal-derived tumors experience excessive activation and dysregulation of EGFR function. EGFR is overexpressed in up to 90% of PDAC cells. The increased expression of EGFR and its ligand in PDAC tissue indicates poor prognosis. Therefore, EGFR has become one of the most important targets for tumor-targeted therapy.59
Mondal et al reported that GE11 peptide (YHWYGYTPQNVI)-modified and GEM-loaded polymer micelles targeting EGFR on the surface of pancreatic cancer cells improved delivery efficiency.60 The clinical application of full-length EGFR ligands is hindered by their high mitogenic and proangiogenic properties. The GE11 peptide (YHWYGYTPQNVI), identified through a phage display peptide library, exhibits specific binding to EGFR with reduced mitogenic activity. The findings indicated that, 24 hours after systemic administration, GE11-linked mixed micelles predominantly accumulated in orthotopic pancreatic tumors and the tumor vasculature. In contrast to HW12-linked mixed micelles, both unmodified mPEG-b-PCC-g-GEM-g-DC micelles and free GEM formulations showed the ability to inhibit the growth of orthotopic pancreatic tumors. Mondal et al codelivered GEM and miR-205 to target EGFR on the surface of pancreatic cancer cells through cetuximab-modified polymer micelles and treated pancreatic cancer through a combination of chemotherapy and gene therapy.61 The tumor-targeting efficiency of the cetuximab-modified micelles was twice that of the unmodified micelles, and compared with the single-loaded GEM or miR-205 micelles, the cetuximab-modified micelles loaded with GEM and miR-205 had significantly improved therapeutic effects, with an inhibition rate of 80%.
Integrin Receptor
Integrins constitute a family of cell surface receptors responsible for facilitating adhesion to the extracellular matrix (ECM) and establishing links with the cytoskeleton.62,63 Integrins not only serve as structural connectors but also function as bidirectional signaling hubs, transmitting biochemical and biomechanical signaling pathways to regulate cell adhesion and influence a variety of phenotypic responses.64,65 The activation of integrins and/or the binding of ligands results in the assembly of protein complexes localized to the plasma membrane,63,66,67 serving as mechanosensitive molecular clutches that relay forces between the ECM and the cytoskeleton.68 All integrins can bind the arginine-glycine-aspartate (RGD) tripeptide.69 Therefore, RGDs are often used as ligands for integrin receptors. Integrin αvβ3 receptors are internalized by many viruses and have been shown to be highly expressed in diseased tissues such as malignant tumors.70–73
According to Huang et al, cRGD peptide-modified bioresponsive chimeric polymersomes (cRGD-BCPs) facilitate highly efficient delivery of siKRAS to PANC-1 tumors, resulting in potent silencing of KRAS G12D mRNA in tumor cells and effective inhibition of PC tumor growth in mice.74 The cellular uptake and silencing efficiency of cRGD-BCP-siKRAS in PANC-1 cells were significantly influenced by the cRGD density. Specifically, a cRGD density of 15.7 mol.% was found to be optimal, resulting in a 3.7-fold increase in internalization and a 3.6-fold enhancement in gene silencing compared to nontargeted BCP-siKRAS. Interestingly, cRGD-BCP-siKRAS significantly boosted the delivery of siKRAS to PANC-1 tumors. At a dose of 3 mg/kg, it successfully reduced KRAS G12D gene expression by 90%, leading to potent tumor suppression and remarkable survival benefits. Specifically, the median survival time was extended to 101 days, compared to 38 days in the PBS group and 59 days in the BCP-siKRAS group. Notably, 40% of the mice treated with cRGD-BCP-siKRAS achieved complete tumor regression. It seems that the nanodelivery of siKRAS mediated by cRGD offers a promising treatment option for pancreatic cancer. Khosravani et al developed RGD-modified nanoliposomes (NLPs) encapsulating Erlotinib (Erlo) and Arsenic Trioxide (ATO) (NLPs-ATO-Erlo-RGD) to enhance the solubility and mitigate the toxicity of both drugs for use in cancer therapy.75 In the MTT experiment, NLPs-ATO-Erlo-RGD exhibited significantly greater toxicity against the αvβ3-overexpressing PC3 cell line compared to NLPs-ATO-Erlo. Furthermore, apoptosis assays conducted using Annexin V/PI staining revealed that NLPs-ATO-Erlo-RGD induced the highest apoptotic rates in PC3 cells, with 59.9% in early apoptosis and 23% in late apoptosis. This was compared to NLPs-ATO-Erlo, which resulted in 35.8% early apoptosis and 10.9% late apoptosis, and the combination of free ATO and Erlo, which led to 14.2% early apoptosis and 16.3% late apoptosis. Ma et al formulated RGD-human serum albumin (HSA)-conjugated gemcitabine (GEM)/curcumin (CUR) nanoparticles (RGD-HSA-GEM/CUR NPs).76 Among the RGD-HSA-GEM/CUR NP, HSA-GEM/CUR NP, and free GEM/CUR groups, the RGD-HSA-GEM/CUR NP group exhibited the highest cytotoxicity in vitro. In vivo results demonstrated that the RGD-HSA-GEM/CUR NP treatment was consistently more effective in controlling tumor growth compared to the other treatments, with a tumor volume of 354.6 ± 39.8 mm3, versus 687.3 ± 59.4 mm3 for the free GEM/CUR group and 545.7 ± 57.2 mm3 for the HSA-GEM/CUR NP group throughout the study period.
Urokinase Plasminogen Activator Receptor
Urokinase plasminogen activator (uPA) is a member of the proteolytic enzyme family that can bind to fibrinolytic enzymes to dissolve the cell basement membrane and ECM, creating conditions for tumor cell metastasis and invasion.77 uPAR interacts with uPA to regulate multiple pathways.78–81 uPAR is typically expressed at low levels in the majority of normal tissues and organs, whereas it is overexpressed in the majority of tumor tissues; uPAR is closely associated with the invasion and metastasis of malignant tumors, playing crucial roles in the degradation of the ECM, tumor angiogenesis, cell proliferation, and apoptosis. Additionally, it is linked to MDR in tumor cells, which is significant in determining the malignancy and prognosis of tumors.82–84 Approximately 86% to 90% of pancreatic cancer tissues exhibit high expression of uPAR, with the mRNA level of uPAR in these tissues being 9.6 times higher than that in adjacent normal tissues.85,86
 |
Figure 6 (A) The comparative study of interventional photothermal therapy and.87 I interstitial brachytherapy. BLI: biolumines-cence imaging. (B). Evaluation of uPAR expression, in vitro cellular uptake and cytotoxicity. a) uPAR expression is relatively high in SW1990 cells and PANC-1 cells, but low in normal human pancreatic ductal epithelial cells HPDE6-C7 by immunofluorescence labeling. Controls in each cell line were only incubated with secondary antibody. The merged images of control groups were listed right. Scale bar is 50 µm. b) SW1990, PANC-1, and HPDE6-C7 cells were pre-incubated with uIGNs, IGNs, and GNs (at 50 µg mL−1) for 24 h. ICP-MS was performed to detect Au element entered in SW1990, PANC-1 and HPDE6-C7 cells. c) The viability of SW1990 cells incubated with uIGNs, IGNs, ICG, and GNs at different concentrations for 24 h and treated with NIR laser irradiation (λ = 808 nm; 2.0 W cm−2) for 5 min. Data represent mean ± SD of triplicate experiments. ns: P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001. Reprinted from Hu Y, Chi C, Wang S, et al. A comparative study of clinical intervention and interventional photothermal therapy for pancreatic cancer. Adv Mater. 2017;29(33). © 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.88 |
Hu et al prepared gold NPs modified with uPAR antibodies and investigated the efficacy of interventional photothermal therapy (IPTT) in animal models.88 Compared to iodine-125 (125I) interstitial brachytherapy (IBT-125I), IPTT resulting in complete ablation through a single intervention has a median survival rate that is 25% higher. (Figure 6). Gao et al utilized uPAR-targeted magnetic iron oxide nanoparticles (IONPs) for the chemotherapy of pancreatic cancer via intraperitoneal administration.89 The results indicated that IP delivery of IONPs resulted in the accumulation of 17% of the total injected NPs within the tumor in an orthotopic mouse model of pancreatic cancer. This was three times higher than the accumulation achieved through intravenous delivery. When the IONPs are loaded with the chemical drug cisplatin, the tumor inhibition rate can reach 40%, whereas that of free cisplatin is only 15%, indicating a significant increase in the inhibition rate. Li et al reported a novel gold nanocluster-based platform modified with the active targeting ligand U11 peptide for confocal laser endomicroscopy-guided PTT and photodynamic therapy (PDT) for PDAC.90 In this system, labeling the nanoclusters with the U11 peptide significantly enhances their affinity and accelerates their uptake by pancreatic cancer cells. Cell apoptosis staining revealed that when incorporating the uPAR-targeted unit, the antitumor efficacy of the CTSE-sensitive nanocluster AuS-U11 was notably superior to that of the nontargeted nanocluster AuS-PEG and the insensitive nanocluster AuC-PEG. Both in vivo and ex vivo optical imaging confirmed the high accumulation of AuS-U11 in the orthotopic pancreatic tumor model. Table 3 outlines various instances of intelligent NDDSs that are engineered to focus on both noncellular and cellular elements within the TME.
 |
Table 3 NDDSs Targeting PDAC Cells |
Targeting the Tumor Microenvironment
The pancreatic cancer’s compact tumor matrix creates a nearly impervious physical barrier, hindering effective drug delivery and penetration, which leads to PDAC’s poor response to treatment.91 Hence, focusing on acellular stromal components to facilitate deeper penetration of NDDSs into tumor tissue is regarded as a potential approach for treating PDAC.92
Targeting Noncellular Stroma Components
Hyaluronic Acid
Hyaluronic acid (HA), a significant macromolecule in the ECM, consists of a linear chain of repeating disaccharides: (β, 1-4)-linked glucuronic acid and (β, 1-3)-linked N-acetylglucosamine.93 The deposition of HA in the tumor stroma increases, forming a nonflowing gel liquid phase, causing vascular collapse and increasing interstitial fluid pressure. In addition to increasing tissue hardness, it also compresses the blood vessels in the tumor, leading to low perfusion and hypoxia in the tumor focus. Low perfusion can significantly reduce the delivery of chemotherapy drugs to tumors, thereby diminishing their therapeutic effectiveness. Hypoxia can give tumor cells a survival advantage, induce the activation of related tumor pathways, and enhance their invasion, infiltration, and metastasis abilities.94 In recent times, there has been a growing trend in experimental designs to utilize HAase for degrading intratumoral HA in order to verify its antitumor activity in preclinical and early-phase clinical studies.
Chen et al developed an intelligent GEM@nanogel system, termed GEM@NGH, which comprises a reduction-sensitive core, encapsulating GEM payloads, and a corona of HAase arranged on its cationic surface.95 The GEM@NGH system exhibited remarkable ECM eradication capabilities and demonstrated robust solid tumor penetration ability both in vitro and in vivo. Upon reaching the TME, HAase efficiently cleaved HA accelerating ECM degradation. Simultaneously, the nanogel disintegrated due to the exposure of disulfide bonds to elevated glutathione levels, resulting in the release of GEM. Yu et al constructed a resveratrol and HAase coloaded nano-DDS (PDA@Res@HAase) and employed a three-dimensional (3D) tumor sphere model to assess its capacity to enhance the deep penetration of resveratrol within the TME.96 The results indicated that the migration ratio of the group treated with PDA@Res@HAase was significantly reduced compared to the group treated with free resveratrol, hinting at the potential role of PDA@Res@HAase in the HA-rich TME. (Figure 7). Song et al created a pH-sensitive biomimetic nanosystem designed to carry a high load of HAase, effectively target tumors, and release its contents in a controlled manner.97 The high loading rate of HAase, reaching 19.47%, was achieved due to the precise matching of pore size and the cationic surface charge of the nanosystem. The nanosystem possesses the capability to deeply penetrate PDAC, resulting in a tumor growth inhibition ratio of 80.46%. This demonstrates its significant potential for clinical application and transformation.
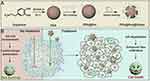 |
Figure 7 (A) Preparation of PDA@Res@HAase. (B) Schematic Representation of PDA@Res@HAase to Enhance the Res Cytotoxicity by Degradation of HA. Reprinted with permission from Yu S, Zhang L, Yang Y, et al. Polydopamine-based resveratrol-hyaluronidase nanomedicine inhibited pancreatic cancer cell invasive phenotype in hyaluronic acid enrichment tumor sphere model. ACS Pharmacol Transl Sci. 2024;7(4):1013–1022. Copyright 2024, American Chemical Society.96 |
Collagen
Collagen, which is the most abundant protein found in the ECM, plays a crucial role in determining the transport of drugs and other molecules between cells within a tumor.98 Eliminating existing collagen components can destroy dense matrices, and the most direct method is to use enzymes that can degrade collagen. Collagenase is highly effective at degrading collagen. Multiple researches have shown that collagenase can reduce the collagen density of the tumor ECM and increase the transport of macromolecules or nanomedicines to tumors.99–102 Liu et al created a collagenase scaffold integrated with metal-organic frameworks (MOFs) through host-guest interactions between protein-metal-organic ligands, termed PMOCol.The purpose of this novel construct is to regulate the ECM in deep tumor areas, with the goal of improving and optimizing immunotherapy results.99 The degree of collagenase encapsulation of PMOCol was high (80.3%, w/w) and surpassed that of previously reported protein-carrying MOFs. PMOCol exhibits a high sensitivity to acidic conditions in the TME, allowing it to disintegrate and release active collagenase. Collagen decomposition facilitated by collagenase accelerated the penetration of therapeutic agents into avascular regions, triggering immunogenic cell death in deep tissues and exposing immunogenicity. This, in turn, enhanced immunocyte infiltration and promoted tumor regression. Li et al engineered nanodrug-bacteria conjugates capable of penetrating the ECM and delivering surface-conjugated protein cages, composed of collagenases and anti-programmed death-ligand 1 (PD-L1) antibodies, directly to the parenchyma of PDAC tumors.100 The results indicated that reactive oxygen species (ROS) present in the PDAC microenvironment stimulate the release of collagenase, leading to the degradation of oncogenic collagen and the inhibition of the integrin α3β1-FAK signaling pathway. This, in turn, alleviates immunosuppression and potentiates the efficacy of anti-PD-L1 immunotherapy. Qiu et al synthesized a range of cationic mesoporous silica nanoparticles (CMSNs) characterized by large and customizable pore sizes, and CMSN4.5 was the best for collagenase.101 After loading with doxorubicin (DOX), the DOX/CMSNs4.5-NH2-Coll nanodrug demonstrated the highest efficacy in tumor therapy, achieving a tumor growth inhibition ratio of 86.1%. Qi et al designed and prepared dual drug-loaded lipid NPs, named AT-CCs, which were decorated with both collagen-binding peptide (CBP) and collagenase-I, specifically for the treatment of pancreatic fibrosis.102 AT-CCs are capable of targeting the fibrotic pancreas through the CBP and degrading excessive collagen with the grafted collagenase I. This allows for the effective delivery of all-trans-retinoic acid and ammonium tetra-thiomolybdate into the pancreas. The results indicate that AT-CC represents a safe and effective collagen-targeted drug delivery system for reversing pancreatic fibrosis through degradation. Yang et al developed a collagenase-functionalized biomimetic drug-loaded Au nanoplatform, named Col-M@AuNCs/Dox, which integrates multiple functionalities into a single system. These include the degradation of the ECM, active targeting, immune evasion, near-infrared light-triggered drug release, and the synergistic provision of antitumor therapy and diagnosis.103 As the Col-M@AuNCs/Dox nanoplatform penetrates into tumor tissue, the dense ECM in PDAC tissues is gradually degraded by the collagenase. This results in a looser ECM structure, enabling deeper penetration within the tumor parenchyma. Yu et al created two types of polymeric nanocarriers based on methoxy PEG-b-poly(caprolactone) (mPEG-PCL). These nanocarriers were designed to encapsulate halofuginone (HF), resulting in HKS NPs, and paclitaxel (PTX), resulting in PKS NPs.104 HF, a naturally occurring low-molecular-weight alkaloid, possesses potent inhibitory effects on collagen. Pretreatment with HF nanomedicine helped restore stromal homeostasis and significantly improved the distribution and penetration of PTX nanomedicine into carcinoma cells. This led to a positive modulation in the infiltration of cytotoxic T cells and resulted in significant tumor growth regression in two models of PDAC. Wang et al designed collagenase-conjugated transcytosis nanoparticles (Col-TNPs) that, in response to tumor acidity, dissociate into collagenase and cationized gold NPs. These NPs have the ability to manipulate the ECM and facilitate active transcytosis within tumors.105 By disrupting the ECM, the active transcytosis of cationized NPs into deep tumor tissues is further enhanced. Additionally, this disruption improves the radiosensitization efficacy of these NPs in PDAC. Luo et al successfully synthesized collagenase-loaded hollow TiO2 (Col-H-TiO2) NPs. These NPs are capable of degrading stromal barriers and generating sufficient ROS production.106 In a patient-derived xenograft model, after administering the NPs (NPs), ultrasonic irradiation caused the release of collagenase. This collagenase degraded the tumor matrix fibers, resulting in a decrease in intratumoral IFP and an increase in the penetration and retention of the NPs within the tumor tissues. Zinger et al designed a 100-nm liposome encapsulating collagenase, known as a collagozome. This formulation was intended to protect collagenase from premature deactivation and prolong its release rate at the target site.107 Pretreatment with collagozome allowed for increased penetration of drugs into the pancreas, which led to improved treatment of PDAC. When PDAC tumors were pretreated with collagozome followed by paclitaxel micelles, the tumors were 87% smaller compared to tumors that were pretreated with empty liposomes followed by paclitaxel micelles. Degrading the ECM did not result in an increase in the number of circulating tumor cells or the degree of metastasis.
Fibronectin
Fibronectin, a key component of the PDAC stroma, plays a role in the remodeling of the extracellular matrix and fibrosis.108 It facilitates various interactions between cells and extracellular molecules, thereby modulating cell adhesion and proliferation. It possesses multiple binding domains for integrins, growth factors, and components of the extracellular matrix, which can become accessible through conformational alterations or proteolytic cleavage.109 Therefore, fibronectin can serve as a target protein for PDAC.
Zhao et al created a nanosystem capable of altering its size, consisting of PEG-PLGA nanospheres encapsulated within liposomes for the co-delivery of vactosertib (VAC) and paclitaxel (TAX). The surface of this nanosystem is modified with APTEDB, and is referred to as NS-TAX@Lipo-VAC.110 The nanosystem can attach to abundant fibronectin associated with tumors in the stroma of PDAC (Figure 8). Upon the liposomes collapsing, it reduces in size by releasing the encapsulated nanospheres loaded with TAX and VAC. By inhibiting ECM hyperplasia, VAC enables TAX to more easily reach cancer cells, especially due to the nanosystem’s small size. Consequently, this approach results in more effective shrinkage of pancreatic tumor xenografts compared to a combination of the free drugs.
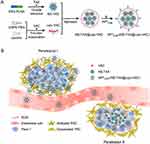 |
Figure 8 Scheme of Penetration Cascade of Size Switchable Nanosystem in Pancreatic Desmoplastic Stroma. (A) Illustration of synthesis of NS-TAX@Lipo-VAC. (B) Schematic illustration of overcoming biological barriers by APTEDB-(NS-TAX@Lipo-VAC) in stromal-enriched orthotopic pancreatic tumor model: penetration I, change in size of nanosystem mediated penetration; penetration II, enhanced penetration due to ECM reduction. Reprinted with permission from Zhao X, Yang X, Wang X, et al. Penetration cascade of size switchable nanosystem in desmoplastic stroma for improved pancreatic cancer therapy. ACS Nano. 2021;15(9):14149–14161. Copyright 2021, American Chemical Society.110 |
Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) are key extracellular enzymes involved in cancer. Increased levels of MMPs are linked to tumor progression and invasiveness, making them widely targeted in cancer therapy.111 Recently, MMPs have been explored as potent tumor microenvironmental stimuli for “smart” drug delivery systems and tumor targeting that are responsive to MMPs. These systems have demonstrated significant potential in cancer diagnosis and treatment.112
Cao et al constructed a TM33 peptide-modified gelatin/oleic acid nanoparticle loaded with tanshinone IIA (TNA) (TM33-GON/TNA) to disrupt tumor vascular endothelial barriers by inhibiting the transformation of resting platelets to activated platelets.113 TM33-GON/TNA can attach to activated platelets by specifically binding to their surface P-selectin and, under the stimulation of MMP-2, release TNA into the extracellular space, resulting in a high local concentration of TNA. Treatment with TM33-GON/TNA led to a 3.2-fold increase in the tumor permeation of Evans blue (a macromolecule marker), a 4.0-fold increase in the tumor permeation of small-sized Nab-PTX (10 nm), and an 11.2-fold increase in the tumor permeation of large-sized DOX-Lip (100 nm), all without enhancing drug delivery to normal tissues. In a murine pancreatic cancer model, the combination of TM33-GON/TNA and Nab-PTX demonstrated superior antitumor efficacy with minimal side effects. Yin et al prepared GEM and ERL coloaded NPs on the surface of which a nonsubstrate MT1-MMP binding peptide was decorated (M-M GEM/ERL NPs).114 The M-M GEM/ERL NPs exhibited the highest uptake ability, with a value of 67.65± 2.87%, the longest half-life, the largest area under the curve, and the best tumor inhibition efficiency, which was 69.81±4.13%. Cun et al created a multifunctional, size-switchable nanoparticle called DGL/GEM@PP/GA, which is designed for targeted TAF regulation and enhanced deep tumor penetration.115 Once accumulated at the tumor site, in response to the overexpression of MMP-2 in the TME, the GEM-conjugated small nanoparticles (DGL/GEM) are released from the DGL/GEM@PP/GA multifunctional size-switchable nanoparticle, leaving behind large NPs loaded with 18β-glycyrrhetinic acid (GA). The released DGL/GEM can penetrate deep into the tumor region and release GEM intracellularly to kill tumor cells. Dong et al presented a protein-free collagen depletion strategy for drug delivery into solid tumors on the basis of the activation of endogenous MMP-1 and −2 via nitric oxide (NO).116 MSNs were loaded with DOX and an NO donor (S-nitrosothiol) to create DN@MSNs. The loaded NO activated MMPs, which in turn degraded collagen within the tumor ECM. The administration of DN@MSNs led to an enhanced penetration of both the nanovehicle and DOX into the tumor, resulting in significantly improved antitumor efficacy. Importantly, no overt toxicity was observed.
Targeting the Cellular Stroma Components
Cancer-Associated Fibroblasts
CAFs, which are the most abundant stromal cells within the TME, stimulate a desmoplastic reaction, synthesize ECM proteins and cytokines, and exert a direct impact on the biological characteristics of cancer cells. The complexity of these various effects poses challenges in eliminating tumor cells from the body, leading to a growing focus on therapeutic strategies that target CAFs in recent years.117
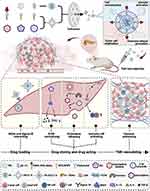 |
Figure 9 Preparation, characterization, and mechanism of action of PI/JGC/L-A nanoparticles in targeting CAFs and enhancing antitumor efficacy. PI/JGC/L-A nanoparticles are formulated by combining JGC/L-A, composed of CHEMS, DOPE, SPC, and DSPE-PEG-AEAA, with PI, composed of BPEI and pIL-12. These nanoparticles are designed to encapsulate three drugs, namely, JQ1, C18 ceramide, and gemcitabine elaidate. Intravenous administration of PI/JGC/L-A allows its delivery to the TME of PDAC via the circulatory system. Within the TME, the DSPE-PEG-AEAA surface modification of PI/JGC/L-A enables its targeting of CAFs by binding to the Sigma 1R receptor present on their surface. Subsequently, the nanoparticles enter the CAFs and release the loaded drugs. JQ1 facilitates the formation of drug-loaded “barrels” and normalizes CAFs by promoting ECM remodeling. Under the influence of C18, gemcitabine elaidate is converted to gemcitabine, which is then loaded onto CAF exosomes and released, leading to cancer cell death within the “barrel”. Moreover, pIL-12 expression results in the secretion of IL-12, stimulating immune cell activity and proliferation within the “barrel”. The synergistic effect of these active ingredients promotes TME remodeling, while the combination of chemotherapy and immunotherapy achieves a superior antitumor effect. Reprinted from Journal of National Cancer Center, 3, 4, Su X, Wang Z, Duan S. Targeted drug delivery systems for pancreatic ductal adenocarcinoma: overcoming tumor microenvironment challenges with CAF-specific nanoparticles, 306–309. Copyright 2023 with permission from Elsevier.118 Abbreviations: CAFs, cancer-associated fibroblasts; CHEMS, cholesteryl hemisuccinate; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DSPE-PEG-AEAA, 1,2-distearoyl-sn-glycero-3-phosphoe-thanolamine-poly(ethylene glycol)-aminoethyl anisamide; ECM, extracellular matrix; PDAC, pancreatic ductal adenocarcinoma; PI, cationic inner core; pIL-12, IL-12 plasmid; SPC, soybean lecithin; TME, tumor microenvironment. |
Zhou et al created EVs loaded with miR-138-5p and the antifibrotic drug pirfenidone (PFD). They further modified the surface of these EVs with integrin α5-targeting peptides, resulting in a novel formulation named IEVs-PFD/138. This formulation aims to reprogram CAFs and inhibit their tumor-promoting effects.119 IEVs-PFD/138 successfully reprogrammed CAFs and remodeled the TME, leading to a reduction in tumor pressure. This, in turn, enhanced GEM perfusion, alleviated tumor hypoxia, and increased the sensitivity of cancer cells to chemotherapy. Su et al prepared PI/JGC/L-A NPs specifically designed to target CAFs and enhance their antitumor effectiveness. These NPs are composed of a combination of JGC/L-A, which incorporates CHEMS, DOPE, SPC, and DSPE-PEG-AEAA, and PI, which is made up of BPEI and pIL-12. Encapsulated within these NPs are three therapeutic agents: JQ1, C18 ceramide, and GEM elaidate. The DSPE-PEG-AEAA surface decoration on PI/JGC/L-A facilitates targeting of CAFs by binding to the Sigma‒Aldrich 1R receptor present on their surface. Once inside the CAFs, the NPs release the encapsulated drugs (Figure 9).118 Zhao et al developed a DDS specifically designed to target CAFs, utilizing RBC vesicle partial protection technology, termed RBC-Fn-NPs. They evaluated the capacity of this DDS to reprogram the stromal environment, enhance tumor penetration, and boost antitumor efficacy in PDAC.120 The FnBPA5 peptide, when exposed, demonstrated a strong binding affinity not only for CAFs but also for key components of the ECM in PDAC, including collagen I and relaxed fibronectin. The strategy utilizing RBC vesicle-mediated protection against FnBPA5 targeting and RA -induced protein reduction has been verified to effectively reprogram the dense stroma in PDAC. This, in turn, enhances the penetration of DOX into the tumor tissue. Transforming growth factor-β (TGF-β) plays an indispensable role in promoting CAF activation and proliferation, and CAFs present major physical barriers for chemotherapeutic drug delivery. Herein, Feng et al created CAF-targeting polymer NPs known as CRE-NP(α-M), which were coated with the CREKA peptide and encapsulated the TCM α-mangostin (α-M). These NPs were designed to modulate the TME by interrupting the TGF-β/Smad signaling pathway.121 CRE-NPs (α-Ms) successfully deactivated CAFs, decreased ECM production, facilitated tumor vascular normalization, and improved blood flow to the tumor site. After pretreatment with CRE-NP(α-M), the sequentially targeted drug delivery agent CRP-MC(Trip) exhibited potent tumor growth inhibition in an orthotopic tumor model. Li et al developed a novel nanoparticle that reacts to the membrane biomarker FAP-α found on CAFs and is activated by near-infrared laser irradiation.122 A small-sized albumin nanoparticle containing paclitaxel (HSA-PTX) was encapsulated within CAP-modified thermosensitive liposomes (CAP-TSLs), where CAP is a FAP-α responsive cleavable amphiphilic peptide. Furthermore, the photothermal agent IR-780 was incorporated into CAP-TSL to create CAP-ITSL. HSA-PTX@CAP-ITSL enhanced the retention of HSA-PTX in solid tumors, and release of HSA-PTX was triggered by FAP-α, which is specifically expressed on CAFs. Chen et al prepared captopril-loaded CAF-targeted liposomes (FH-Lip-Cap).123 The targeted delivery of captopril effectively reduced ECM deposition by inhibiting the TGF-β1-Smad2 signaling pathway, thereby enhancing the penetration of subsequently administered liposome-encapsulated GEM. This strategy holds promise as a means of overcoming the stromal barrier in pancreatic cancer treatment.
Endothelial Cells
Cilengitide-activated thermosensitive liposomes loaded with DOX (MC-T-DOX) are modified with membrane type 1-matrix metalloproteinase (MT1-MMP) at an optimal low density. When administered intravenously at a low cilengitide dose to mice with hypoperfused pancreatic tumors, MC-T-DOX may be activated by MT1-MMP on tumor endothelial cells, leading to the release of cilengitide. This, in turn, promotes endothelial cell migration and angiogenesis, enhancing the accumulation and broader distribution of MC-T-DOX at the tumor site. In the tumor interstitium, heat-induced release of DOX from MC-T-DOX further increases its bioavailability and antitumor efficacy.124
Cancer Stem Cells
There is a type of embryonic-like cell in tumor tissue called cancer stem cells (CSCs), or cancer-initiating cells, which have the ability to self-renew and differentiate and are closely related to the initiation and proliferation of tumors.125 Recently, CSCs have drawn much interest as possible treatments for pancreatic cancer because of their ability to locate tumors.87 Pancreatic CSCs express CD44, CD24, and ESA simultaneously.126 Hence, the eradication of CD44+ cells could be crucial for treating pancreatic cancer effectively.127
Li et al designed and created a micelle system, MP@HA, featuring in vivo targeting and responsive drug release capabilities, co-loading GEM monophosphate and the STAT3 inhibitor silibinin.128 The HA on the micelle’s surface specifically binds to the CD44 molecule on tumor cells, facilitating micelle accumulation at the tumor site. The nitroimidazole-modified polymeric backbone triggers micellar collapse in response to hypoxic conditions in the tumor environment, releasing silibinin to broadly regulate STAT3 molecules within the PDAC microenvironment. Additionally, the polymer fragment attached to GEM monophosphate enables deep penetration into PDAC tumors, achieving thorough chemotherapy. Bartkowski et al investigated a novel nanocarrier composed of carbon nano-onions (CNOs) and assessed its capacity to selectively target cancer cells overexpressing the HA receptor CD44.129 Their findings revealed that the CNO-based nanocarrier, when conjugated with HA as the targeting moiety, was efficiently internalized by CD44+ PANC-1 and MIA PaCa-2 cells, while sparing CD44-negative Capan-1 cells. Notably, upon successful loading of the CNO-based nanocarrier with chemotherapeutic 4-(N)-acyl-sidechain-containing prodrugs derived from GEM, it exhibited enhanced efficacy in eradicating PDAC cells, particularly those that are resistant to GEM and express CD44. Jangid et al created dual-targeting polymersomes (DTPSs) loaded with piperlongumine (PL) for effective treatment of pancreatic cancer. In this study, HA was modified with DSPEPEG-NH2, PEG, and phenylboronic acid (PBA) groups (Figure 10).130 The DTPSs were designed to selectively recognize CD44 and sialic acid (SA) receptors, and deliver PL to MIA PaCa-2 pancreatic cancer cells through interactions involving HA-CD44 and PBA-SA. The DTPSs facilitated increased cellular uptake mediated by both SA and CD44, whereas single-targeted polymersomes only utilized CD44-mediated uptake. When loaded with PL, the DTPSs were efficiently internalized by MIA PaCa-2 cancer cells overexpressing SA and CD44, resulting in up to 80% inhibition of cell growth, a reduction in cell spheroidal volume, and a 58.3% increase in the number of dead cells. Navarro-Marchal et al constructed olive oil liquid nanocapsules (O2LNCs) functionalized through covalent attachment of an anti-CD44-fluorescein isothiocyanate antibody (αCD44).131 Their findings demonstrated significant targeted uptake of αCD44-O2LNCs and a fourfold increase in antitumor efficacy of paclitaxel-loaded αCD44-O2LNCs compared to free paclitaxel in pancreatic cancer stem cells (PCSCs). Furthermore, αCD44-O2LNCs were capable of selectively targeting PCSCs in an orthotopic xenotransplant in vivo model.
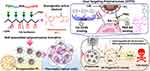 |
Figure 10 A scheme showing the structure of the synthesized PBA PEG-HA PEG-Lipid bioconjugate and piperlongumine (PL) as an alkaloid-based anticancer molecule. PL-loaded dual-targeted polymersomes (PL@DTPS) were prepared via self-assembly hydrophobic encapsulation. Phenylboronic acid (PBA) moiety present on the PL@DTPS surface periphery can bind to sialic acid (SA) and the hyaluronic acid (HA) backbone can bind with CD44 ligands, facilitating anticancer activity of PL@DTPS against MIA PaCa-2 pancreatic cancer cells. Reprinted from International Journal of Biological Macromolecules, 8, 18, Wang Y, Gao Z, Du X, et al. Co-inhibition of the TGF-β pathway and the PD-L1 checkpoint by pH-responsive clustered nanoparticles for pancreatic cancer microenvironment regulation and anti-tumor immunotherapy, 1208415121–5132. Copyright 2020 with permission from Elsevier.130 |
Stellate Cells
Pancreatic stellate cells (PSCs) account for more than 50% of the stromal components in PDAC tumor tissue. PSCs induce connective tissue proliferation by secreting ECM and promote tumor cell proliferation and invasion by secreting various cytokines and miRNAs.132 Modulation of the activation or function of PSCs through the use of new targeted materials or the blockade of relevant signaling can enable the regulation of the developmental process of pancreatic cancer.
Wang et al designed a polyamino acid-based nanodrug, Cal/siR-NP, which carries the PSC activation inhibitor calcipotriol and anti-CXCL12 siRNA.133 These Cal/siR-NPs have the ability to enter pancreatic tumors, inactivate PSCs, and decrease CXCL12 levels. In vivo studies of orthotopic pancreatic tumor treatment revealed that the simultaneous delivery of calcipotriol and anti-CXCL12 siRNA altered the TME of PDAC, causing a reduction in the ECM and a decrease in the number of immunosuppressive T cells. Ultimately, this led to an increase in the infiltration of cytotoxic T cells, making immune checkpoint blockade (ICB) therapy a feasible option for immunologically “cold” pancreatic tumors. Zhao et al developed targeted ATO-loaded nanoparticles (scAb-ATO-NPs) using poly (D, L-lactide) and poly (ethylene glycol) (PEG-PDLLA) and decorated them with single-chain antibody against FAP-α (scAbFAP-α) to boost ATO delivery to PSCs and enhance GEM’s antitumor effects.134 These scAb-ATO-NPs not only converted aPSCs to qPSCs, reducing ECM production, but also aided GEM’s penetration into tumor tissue after stroma disruption. Combined with GEM, this therapy significantly inhibited tumor growth in desmoplastic pancreatic cancer.
Targeting Stromal and Pancreatic Cancer Cells
Yu et al crafted a multitarget, pH-sensitive liposome (ATF@Pt Lps) encapsulating cisplatin (Pt) in its aqueous core, which exhibits a high affinity for uPAR in pancreatic cancer cells, TAMs, and CAFs.135 Administration of ATF@Pt Lps systemically allowed for the penetration of the central stromal-cellular barrier and efficient drug delivery to tumor cells, yielding a robust therapeutic outcome in a Panc02 cell-derived mouse tumor model. However, the varied distribution of TAMs and tumor cells within tumors poses a challenge for dual targeting. To enhance chemoimmunotherapy outcomes in pancreatic cancer patients by repolarizing TAMs, Li et al developed a TME-responsive micellar system co-loaded with GEM and the PI3K inhibitor wortmannin (Wtmn), targeting both TAMs and tumor cells.136 They created GEM-conjugated dendritic polylysine DGL nanoparticles (GDs) and linked them to polycaprolactone-polyethylene glycol micelles encapsulating Wtmn (PP/Wtmn) via a cathepsin B (CTSB) substrate peptide, forming GD@PP/Wtmn micelles. In the TME, the high expression of CTSB triggered the release of GD, allowing it to overcome the stromal barrier and deeply penetrate the tumor. Meanwhile, PP/Wtmn remained in the perivascular area rich in TAMs. By inhibiting the PI3K pathway, M2-like TAMs were repolarized into M1-like TAMs, activating antitumor immunity and synergizing with GEM to suppress tumor growth. Table 4 summarizes examples of smart NDDSs specifically designed to target the TME.
 |
Table 4 NDDSs Targeting TME in PDAC |
Targeting the Immunosuppressive Tumor Microenvironment
Targeting Cancer-Associated Fibroblast Signaling
Xie et al introduced cholesterol-modified polymeric CXCR4 antagonist NPs (blocking cancer-stroma interactions) for delivering anti-miR-210 and siKRASG12D, aiming to deactivate stroma-generating PSCs and eliminate pancreatic cancer cells.137 The CXCR4/CXCL12 axis plays a role in cancer’s ability to evade the immune system. Overexpression of miR-210 aids cancer cells in adapting to hypoxic environments. Additionally, KRAS mutations are pivotal in the initiation, progression, and survival of tumors.138 By inhibiting CXCR4 and reducing the levels of miR-210/KRASG12D, triple-acting NPs (CXCR4/miR-210/siKRASG12D) effectively modulated the desmoplastic TME by deactivating PSCs and enhancing the infiltration of cytotoxic T cells. Compared to individual therapies, the combined therapy exhibited superior therapeutic outcomes, evidenced by a slowdown in tumor growth, depletion of stromal tissue, decreased immunosuppression, inhibition of metastasis, and an extension of survival time. PDAC features a dense ECM surrounding tumor cells, which sequesters infiltrating CD8+ T cells and hinders drug penetration. A viable strategy to enhance T-cell infiltration and cytotoxicity involves the concurrent inhibition of both the TGF-β pathway and the PD-1/PD-L1 checkpoint. Wang et al utilized acidic tumor extracellular pH (pHe)-responsive clustered NPs (LYiClustersiPD-L1) to deliver a TGF-β receptor inhibitor (LY2157299) and siRNA targeting PD-L1 (siPD-L1), aiming to regulate the PDAC stroma microenvironment and enhance antitumor immunotherapy.139 Encapsulated within the hydrophobic core of a nanoparticle, LY2157299 effectively inhibits the activation of PSCs, leading to a reduction in type I collagen. The surface of LYiClustersiPD-L1 is adsorbed with siPD-L1, which is released along with small-sized poly(amidoamine) (PAMAM) at pHe. This allows the siRNA to penetrate into the tumors and silence PD-L1 gene expression in tumor cells. Compared to monotherapy, LYiClustersiPD-L1 significantly boosts the number of tumor-infiltrating CD8+ T cells and triggers antitumor immunity, synergistically suppressing tumor growth in both a subcutaneous Panc02 xenograft model and an orthotopic tumor model (Figure 11).
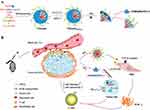 |
Figure 11 (A) Self-assembly and structural features of LYiClustersiPD-L1. The TGF-β receptor inhibitor LY2157299 was encapsulated in the core, while siPD-L1 was adsorbed on the surface through electrostatic interactions with positively charged PAMAM. At acidic tumor pHe, siPD-L1 can be released with PAMAM. (B) LYiClustersiPD-L1 accumulated in the tumor site and was restricted to the stroma. LY2157299 was delivered into PSCs at the stroma, while PAMAM/siPD-L1 was released at the pHe and penetrated into tumors for tumor cell targeting. On synergistic therapy of both, enhanced CD8+ T cell infiltration and cytotoxicity were expected for cancer therapy. Used with permission of Royal Society of Chemistry. Wang Y, Gao Z, Du X, et al. Co-inhibition of the TGF-β pathway and the PD-L1 checkpoint by pH-responsive clustered nanoparticles for pancreatic cancer microenvironment regulation and anti-tumor immunotherapy. Biomater Sci. 2020;8(18):5121–5132. Permission conveyed through Copyright Clearance, Inc.139 |
Tumor-Associated Macrophages
TAMs originate from circulating monocytes and constitute the main population of immune cells in solid tumors.140 TAMs can promote cancer cell proliferation and stimulate tumor angiogenesis and extracellular matrix breakdown, thereby enhancing tumor invasion and metastasis. TAMs can also suppress immune responses, preventing tumor cells from being attacked by natural killer cells and T cells during tumor development and after recovery from chemotherapy or immunotherapy.141
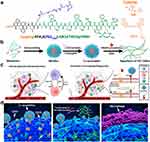 |
Figure 12 Design and proposed mechanism of PAC-SABIs. (a) Peptide molecular structure design of Pep-PEG. (b) Schematic illustration of PAC-SABIs formation process including peptide self-assembling, antibody modification, and ALP catalysis. (c) Schematic illustration of immune quiescent microenvironment and in vivo construction of PAC-SABIs. Figure was created with BioRender.com and released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 international license. (d) Proposed mechanism of PAC-SABIs-mediated activation of macrophage phagocytosis against cancer cell.Reprinted from Zhang W, Zeng Y, Xiao Q, et al. An in-situ peptide-antibody self-assembly to block CD47 and CD24 signaling enhances macrophage-mediated phagocytosis and anti-tumor immune responses. Nat Commun. 2024;15(1):5670.142 |
Zhang et al developed peptide‒antibody combo-supramolecular in situ-assembled nanofibers to bidargetly inhibit CD47 and CD24 (PAC-SABI).142 By concurrently inhibiting CD47 and CD24 signaling, PAC-SABI augments the phagocytic capacity of macrophages both in vitro and in vivo, fostering antitumor responses in mouse models of pancreatic cancer. Furthermore, due to PAC-SABI-induced macrophage repolarization and enhanced CD8+ T-cell tumor infiltration, subsequent anti-PD-1 therapy further curbs the progression of 4T1 tumors, thereby prolonging survival rates (Figure 12). Chen et al developed a nanoparticle system featuring β-glucan-functionalized zinc and doxorubicin (βGlus‒ZnD NPs), which is capable of being administered orally.143 After administration, βGlus-ZnD NPs are phagocytosed by endogenous macrophages (βGlus-ZnD@MΦ). Due to the tumor-homing and “stealth” properties imparted by endogenous MΦ, these NPs ultimately accumulate in the tumor tissue. Meanwhile, the MΦ that carry βGlus-ZnD NPs become activated, producing matrix metalloproteinases that break down the desmoplastic stromal barrier. These MΦ differentiate toward the M1-like phenotype, modulating the TME and recruiting effector T cells, which ultimately leads to the apoptosis of the tumor cells by encapsulating bacterial-derived cyclic dimeric adenosine monophosphate (CDA) within nanoscale coordination polymers.144 ZnCDA specifically targets TAMs to regulate antigen processing and presentation, which in turn stimulates an antitumor T-cell response. ZnCDA has been shown to enhance the antitumor effectiveness of both radiotherapy and immune checkpoint inhibitors in immunologically “cold” pancreatic and glioma tumor models. This suggests a promising combined treatment approach for difficult-to-treat human cancers. Gao et al created an injectable, thermosensitive chitosan hydrogel (CHG) that can be administered in situ and is loaded with lipid-encapsulated immune regulatory factor 5 mRNA and C-C chemokine ligand 5 (CCL5) siRNA nanoparticle complexes (LPR@CHG). This hydrogel is designed to reprogram the antitumor immune microenvironment.145 The LPR@CHG hydrogel upregulated immune regulatory factor 5, downregulated CCL5 secretion, increased the number of M1 phenotype macrophages, and finally initiated T-cell-mediated immune responses. The LPR@CHG hydrogel holds promise as an immunotherapy platform that can alter the immunosuppressive TME and bolster the effectiveness of existing pancreatic immunotherapies, while also reducing systemic toxicity.
Antigen-Presenting Cells
Immunotherapeutic methods have had limited success in treating PDAC due to its weak immunogenicity and highly immunosuppressive TME. This TME is characterized by an abundance of dysfunctional and immunosuppressed antigen-presenting cells (APCs). Lorkowski Gao et al created a highly effective immunostimulatory nanoparticle (immuno-NP) to activate and expand APCs within tumors and induce local secretion of interferon β.146 The potency of the immuno-NP is derived from its loading of two synergistic immune modulators: an agonist of the stimulator of interferon genes (STING) pathway and an agonist of the Toll-like receptor 4 (TLR4) pathway. By adjusting the ratio of these two agonists in the immuno-NP, the functional synergy can be fine-tuned, resulting in an 11-fold increase in interferon β production compared to any single agonist variant. In the orthotopic murine Panc02 model of PDAC, systemic administration of the immuno-NPs led to their deposition in the perivascular regions of the tumor, which align with APC-rich areas. Consequently, the immuno-NPs were predominantly taken up by APCs, with a significant portion of tumor dendritic cells (>56%) effectively absorbing them. This led to a substantial expansion of APCs, resulting in an 11.5-fold increase in the number of dendritic cells and lymphocyte infiltration throughout the pancreatic tumor compared to untreated animals. Chibaya et al devised an immunotherapy strategy that integrates the administration of STING and TLR4 innate immune agonists, encapsulated together within lipid-based NPs, alongside senescence-inducing RAS-targeted therapies. This approach aims to restructure the immunosuppressive TME of PDAC through the senescence-associated secretory phenotype.147 Administering these treatment regimens to both transplanted and autologous mouse models of PDAC enhances the cellular uptake of NPs within the PDAC TME. This leads to the induction of type I interferon and other proinflammatory signaling, boosts antigen presentation by tumor cells and APCs, and subsequently activates both innate and adaptive immune responses. This dual strategy has demonstrated robust T-cell-mediated and type I interferon-driven tumor regression, resulting in long-term survival in preclinical PDAC models, contingent on the activation of STING in both tumor and host cells. Furthermore, STING and TLR-4-induced type I interferon signaling are linked to the immune potentiation of natural killer cells and CD8+ T cells in human PDAC samples. Consequently, combining local delivery of immune agonists with systemic tumor-targeted therapy orchestrates innate and adaptive immune responses driven by type I interferon, providing sustained antitumor efficacy against PDAC. Yao et al synthesized the mesoporous nanomaterial Cu2MoS4 (CMS) coated with PEG and loaded with the PD-L1 inhibitor BMS-1 and the CXCR4 inhibitor Plerixafor, resulting in the nanodrug CMS/PEG-B-P.148 CMS/PEG-B-P exhibits a more potent suppressive effect on PD-L1 and CXCR4 expression, promoting apoptosis and inhibiting proliferation of KPC pancreatic cancer cells. Furthermore, it activates mouse immune cells, including dendritic cells and RAW264.7 macrophages. In C57BL/6 mice, CMS/PEG-B-P inhibits tumor growth and remodels the tumor immune microenvironment by enhancing the infiltration of CD4+ and CD8+ T cells, polarizing macrophages, and reducing immunosuppressive cells. Additionally, it modulates cytokine release within the tumor immune microenvironment, increasing immunostimulatory cytokines INF-γ and IL-12 while decreasing IL-6, IL-10, and IFN-α levels created a novel antigen delivery system utilizing polysaccharide-coated gold nanoparticles (AuNPs) specifically targeted to APCs expressing Dectin-1.149 The AuNPs were functionalized with mucin 4 and a tumor-associated carbohydrate antigen, and were tested for their ability to bind to dectin-1, undergo APC processing and presentation in vitro, and elicit immune responses in mice. The findings demonstrated that these particles elicited a robust in vivo immune response, characterized by high-titer antibody production and the activation of antigen-recognizing T cells. Additional analysis showed a beneficial antitumor cytokine balance, with minimal expression of the immunosuppressive cytokine IL-10. Table 5 provides an overview of smart NDDSs aimed at addressing the immunosuppressive TME in pancreatic cancer.
 |
Table 5 NDDSs Targeting the Immunosuppressive TME in PDAC |
Conclusion
Although there have been significant advances in the treatment of PDAC in recent years, it encounters substantial obstacles in terms of achieving curability, as it is a typical case of poor response to standard therapies and a considerable chance of recurrence. NDDSs can target pancreatic cancer cells, the TME, and the immunosuppressive TME, showing great potential and application prospects in the treatment of PDAC. NDDSs, through their unique physical and chemical properties, can achieve targeted delivery and deep penetration of drugs, elevate drug concentrations within tumor sites, and reduce systemic toxicity. Different types of NDDSs, such as lipid NPs, polymer NPs, and inorganic NPs, have each characteristic and have made significant research progress in the treatment of PDAC. In the context of PDAC treatment, one type that stands out due to its ability to address the unique challenges of PDAC is the hybrid cell membrane-based nano drug delivery system. This type of NDDS combines the advantages of different cell membranes to create a functional and biocompatible nano carrier. It can enhanced biocompatibility and immune evasion, targeted drug delivery, improved drug penetration, stimuli-responsive drug release, personalized medicine. In conclusion, hybrid cell membrane-based nano drug delivery systems represent a promising approach for the treatment of PDAC.
In nanodrug delivery systems, gene therapy and RNA interference have been applied, and the effectiveness of immunotherapy has also increased. In the future, intelligent nanomedicine delivery systems, multifunctional nanomedicine delivery systems, and personalized therapies will become the focus of research. While this approach holds significant potential, it is vital to design safe nanodelivery systems and guarantee the avoidance of adverse health impacts and environmental hazards. Despite many challenges, with the continuous progress and innovation of technology, NDDSs have broad application prospects in the treatment of PDAC, which is expected to bring new treatment hope and a better survival prognosis for pancreatic cancer patients.
Funding
This work was supported by the Public Welfare Technology Application Projects supported by the Science Technology Department of Zhejiang Province (LGF22H290003), the National Natural Science Foundation of China (82104699), the Major Projects of Hangzhou Health Science and Technology Plan (Z20230014), and the Zhejiang Provincial Medical and Health Science and Technology Plan (2025706913).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. doi:10.3322/caac.21820
2. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
3. Wood LD, Canto MI, Jaffee EM, et al. Pancreatic Cancer: pathogenesis, screening, diagnosis, and treatment. Gastroenterology. 2022;163(2):386–402.e1. doi:10.1053/j.gastro.2022.03.056
4. Singhi AD, Koay EJ, Chari ST, et al. Early detection of pancreatic cancer: opportunities and challenges. Gastroenterology. 2019;156(7):2024–2040. doi:10.1053/j.gastro.2019.01.259
5. Schizas D, Charalampakis N, Kole C, et al. Immunotherapy for pancreatic cancer: a 2020 update. Cancer Treat Rev. 2020;86:102016. doi:10.1016/j.ctrv.2020.102016
6. Hessmann E, Buchholz SM, Demir IE, et al. Microenvironmental determinants of pancreatic cancer. Physiol Rev. 2020;100(4):1707–1751. doi:10.1152/physrev.00042.2019
7. Del Chiaro M, Sugawara T, Karam SD, et al. Advances in the management of pancreatic cancer. BMJ. 2023;383:e073995. doi:10.1136/bmj-2022-073995
8. Hu ZI, O’Reilly EM. Therapeutic developments in pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2024;21(1):7–24. doi:10.1038/s41575-023-00840-w
9. Halbrook CJ, Lyssiotis CA, Pasca di magliano M, et al. Pancreatic cancer: advances and challenges. Cell. 2023;186(8):1729–1754. doi:10.1016/j.cell.2023.02.014
10. Chapa-González C, López K, Lomelí KM, et al. A Review on the Efficacy and Safety of Nab-Paclitaxel with Gemcitabine in Combination with Other Therapeutic Agents as New Treatment Strategies in Pancreatic Cancer. Life. 2022;12(3):327.
11. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi:10.1158/0008-5472.CAN-14-0155
12. Shi J, Votruba AR, Farokhzad OC, et al. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10(9):3223–3230. doi:10.1021/nl102184c
13. Gregoriadis G, Ryman BE. Liposomes as carriers of enzymes or drugs: a new approach to the treatment of storage diseases. Biochem J. 1971;124(5):58. doi:10.1042/bj1240058P
14. Zhu L, Mao H, Yang L. Advanced iron oxide nanotheranostics for multimodal and precision treatment of pancreatic ductal adenocarcinoma. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;14(4):e1793. doi:10.1002/wnan.1793
15. Hou W, Yang B, Zhu H. Nanoparticle-based therapeutic strategies for enhanced pancreatic ductal adenocarcinoma immunotherapy. Pharmaceutics. 2022;14(10):2033. doi:10.3390/pharmaceutics14102033
16. Redruello P, Perazzoli G, Cepero A, et al. Nanomedicine in pancreatic cancer: a new hope for treatment. Curr Drug Targets. 2020;21(15):1580–1592. doi:10.2174/1389450121666200703195229
17. Valenzuela Villela KS, Alvarado Araujo KV, Garcia Casillas PE, et al. Protective Encapsulation of a Bioactive Compound in Starch-Polyethylene Glycol-Modified Microparticles: degradation Analysis with Enzymes. Polymers (Basel). 2024;16(14):2075. doi:10.3390/polym16142075
18. Roacho-Pérez JA, Garza-Treviño EN, Delgado-Gonzalez P, et al. Target Nanoparticles against Pancreatic Cancer: fewer Side Effects in Therapy. Life. 2021;11(11):1187. doi:10.3390/life11111187
19. Rbanova M, Cihova M, Buocikova V, et al. Nanomedicine and epigenetics: new alliances to increase the odds in pancreatic cancer survival. Biomed Pharmacother. 2023;165:115179. doi:10.1016/j.biopha.2023.115179
20. Raza F, Evans L, Motallebi M, et al. Liposome-based diagnostic and therapeutic applications for pancreatic cancer. Acta Biomater. 2023;157:1–23. doi:10.1016/j.actbio.2022.12.013
21. Su T, Yang B, Gao T, et al. Polymer nanoparticle-assisted chemotherapy of pancreatic cancer. Ther Adv Med Oncol. 2020;12:1758835920915978. doi:10.1177/1758835920915978
22. Cazes A, Betancourt O, Esparza E, et al. A MET targeting antibody‒drug conjugate overcomes gemcitabine resistance in pancreatic cancer. Clin Cancer Res. 2021;27(7):2100–2110. doi:10.1158/1078-0432.CCR-20-3210
23. Huang X, Zhang G, Tang TY, et al. Personalized pancreatic cancer therapy: from the perspective of mRNA vaccine. Mil Med Res. 2022;9(1):53. doi:10.1186/s40779-022-00416-w
24. Sun M, Wang T, Li L, et al. The application of inorganic nanoparticles in molecular targeted cancer therapy: EGFR targeting. Front Pharmacol. 2021;12:702445. doi:10.3389/fphar.2021.702445
25. Papadakos SP, Dedes N, Pergaris A, et al. Exosomes in the treatment of pancreatic cancer: a moonshot to PDAC treatment? Int J mol Sci. 2022;23(7):3620. doi:10.3390/ijms23073620
26. Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomized, open-label, Phase 3 trial. Lancet. 2016;387(10018):545–557. doi:10.1016/S0140-6736(15)00986-1
27. Ho WJ, Jaffee EM, Zheng L. The tumor microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17(9):527–540. doi:10.1038/s41571-020-0363-5
28. Meng H, Nel AE. Use of nano engineered approaches to overcome the stromal barrier in pancreatic cancer. Adv Drug Deliv Rev. 2018;130:50–57. doi:10.1016/j.addr.2018.06.014
29. Tonini V, Zanni M. Pancreatic cancer in 2021: what you need to know to win. World J Gastroenterol. 2021;27(35):5851–5889. doi:10.3748/wjg.v27.i35.5851
30. Apte MV, Xu Z, Pothula S, et al. Pancreatic cancer: the microenvironment needs attention too! Pancreatology. 2015;15(4 Suppl):S32–38. doi:10.1016/j.pan.2015.02.013
31. Pothula SP, Xu Z, Goldstein D, et al. Key role of pancreatic stellate cells in pancreatic cancer. Cancer Lett. 2016;381(1):194–200. doi:10.1016/j.canlet.2015.10.035
32. Zeng S, Pöttler M, Lan B, et al. Chemoresistance in pancreatic cancer. Int J mol Sci. 2019;20(18):4504. doi:10.3390/ijms20184504
33. Dimou A, Syrigos KN, Saif MW. Overcoming the stromal barrier: technologies to optimize drug delivery in pancreatic cancer. Ther Adv Med Oncol. 2012;4(5):271–279. doi:10.1177/1758834012446008
34. Meng H, Zhao Y, Dong J, et al. Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS Nano. 2013;7(11):10048–10065. doi:10.1021/nn404083m
35. Chen IM, Willumsen N, Dehlendorff C, et al. Clinical value of serum hyaluronan and propeptide of type III collagen in patients with pancreatic cancer. Int, J, Cancer. 2020;146(10):2913–2922. doi:10.1002/ijc.32751
36. Nieskoski MD, Marra K, Gunn JR, et al. Collagen complexity spatially defines microregions of total tissue pressure in pancreatic cancer. Sci Rep. 2017;7(1):10093. doi:10.1038/s41598-017-10671-w
37. DuFort CC, DelGiorno KE, Hingorani SR. Mounting pressure in the microenvironment: fluids, solids, and cells in pancreatic ductal adenocarcinoma. Gastroenterology. 2016;150(7):1545–1557.e2. doi:10.1053/j.gastro.2016.03.040
38. Sherman MH, Beatty GL. Tumor microenvironment in pancreatic cancer pathogenesis and therapeutic resistance. Annu Rev Pathol. 2023;18(1):123–148. doi:10.1146/annurev-pathmechdis-031621-024600
39. Jiang S, Fagman JB, Ma Y, et al. A comprehensive review of pancreatic cancer and its therapeutic challenges. Aging. 2022;14(18):7635–7649. doi:10.18632/aging.204310
40. Park W, Chawla A, O’Reilly EM. Pancreatic cancer: a review. JAMA. 2021;326(9):851–862. doi:10.1001/jama.2021.13027
41. Kota J, Hancock J, Kwon J, et al. Pancreatic cancer a review: stroma and its current and emerging targeted therapies. Cancer Lett. 2017;391:38–49. doi:10.1016/j.canlet.2016.12.035
42. Rosenblum D, Joshi N, Tao W, et al. Progress and challenges toward targeted delivery of cancer therapeutics. Nat Commun. 2018;9(1):1410. doi:10.1038/s41467-018-03705-y
43. Zhang Y, Sun T, Jiang C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm Sin B. 2018;8(1):34–50. doi:10.1016/j.apsb.2017.11.005
44. Qian ZM, Li H, Sun H, et al. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54(4):561–587. doi:10.1124/pr.54.4.561
45. Choudhury H, Pandey M, Chin PX, et al. Transferrin receptors-targeting nanocarriers for efficient targeted delivery and transcytosis of drugs into the brain tumors: a review of recent advancements and emerging trends. Drug Deliv Transl Res. 2018;8(5):1545–1563. doi:10.1007/s13346-018-0552-2
46. Jhaveri A, Luther E, Torchilin V. The effect of transferrin-targeted, resveratrol-loaded liposomes on neurosphere cultures of glioblastoma: implications for targeting tumor-initiating cells. J Drug Target. 2019;27(5–6):601–613. doi:10.1080/1061186X.2018.1550647
47. Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18(16):4266–4276. doi:10.1158/1078-0432.CCR-11-3114
48. Ryschich E, Huszty G, Knaebel HP, et al. Transferrin receptor is a marker of malignant phenotype in human pancreatic cancer and in neuroendocrine carcinoma of the pancreas. Eur J Cancer. 2004;40(9):1418–1422. doi:10.1016/j.ejca.2004.01.036
49. Jeong SM, Hwang S, Seong RH. Transferrin receptor regulates pancreatic cancer growth by modulating mitochondrial respiration and ROS generation. Biochem Biophys Res Commun. 2016;471(3):373–379. doi:10.1016/j.bbrc.2016.02.023
50. Muddineti OS, Kumari P, Ghosh B, et al. Transferrin-modified vitamin-e/lipid based polymeric micelles for improved tumor targeting and anticancer effect of curcumin. Pharm Res. 2018;35(5):97. doi:10.1007/s11095-018-2382-9
51. Wang L, Yao Q, Guo X, et al. Targeted delivery of CEBPA-saRNA for the treatment of pancreatic ductal adenocarcinoma by transferrin receptor aptamer decorated tetrahedral framework nucleic acid. J Nanobiotechnology. 2024;22(1):392. doi:10.1186/s12951-024-02665-4
52. McCarthy GA, Jain A, Di Niro R, et al. A novel 3DNA® nanocarrier effectively delivers payloads to pancreatic tumors. Transl Oncol. 2023;32:101662. doi:10.1016/j.tranon.2023.101662
53. Bano S, Obaid G, Swain JWR, et al. NIR photodynamic destruction of PDAC and HNSCC nodules using triple-receptor-targeted photoimmuno-nanoconjugates: targeting heterogeneity in cancer. J Clin Med. 2020;9(8):2390. doi:10.3390/jcm9082390
54. P RS, Mal A, Valvi SK, et al. Noninvasive preclinical evaluation of targeted nanoparticles for the delivery of curcumin in treating pancreatic cancer. ACS Appl Bio Mater. 2020;3(7):4643–4654. doi:10.1021/acsabm.0c00515
55. Lang J, Zhao X, Wang X, et al. Targeted co-delivery of the iron chelator deferoxamine and a HIF1α inhibitor impairs pancreatic tumor growth. ACS Nano. 2019;13(2):2176–2189.
56. Yamaoka T, Ohba M, Ohmori T. Molecular-targeted therapies for epidermal growth factor receptor and its resistance mechanisms. Int J mol Sci. 2017;18(11):2420. doi:10.3390/ijms18112420
57. Rajaram P, Chandra P, Ticku S, et al. Epidermal growth factor receptor: role in human cancer. Indian J Dent Res. 2017;28(6):687–694. doi:10.4103/ijdr.IJDR_534_16
58. Mitchell RA, Luwor RB, Burgess AW. Epidermal growth factor receptor: structure‒function informing the design of anticancer therapeutics. Exp Cell Res. 2018;371(1):1–19. doi:10.1016/j.yexcr.2018.08.009
59. Sabbah DA, Hajjo R, Sweidan K. Review on epidermal growth factor receptor (EGFR) structure, signaling pathways, interactions, and recent updates of EGFR inhibitors. Curr Top Med Chem. 2020;20(10):815–834. doi:10.2174/1568026620666200303123102
60. Mondal G, Kumar V, Shukla SK, et al. EGFR-targeted polymeric mixed micelles carrying gemcitabine for treating pancreatic cancer. Biomacromolecules. 2016;17(1):301–313. doi:10.1021/acs.biomac.5b01419
61. Mondal G, Almawash S, Chaudhary AK, et al. EGFR-targeted cationic polymeric mixed micelles for codelivery of gemcitabine and miR-205 for treating advanced pancreatic cancer. Mol Pharm. 2017;14(9):3121–3133. doi:10.1021/acs.molpharmaceut.7b00355
62. Bachmann M, Kukkurainen S, Hytönen VP, et al. Cell adhesion by integrins. Physiol Rev. 2019;99(4):1655–1699. doi:10.1152/physrev.00036.2018
63. Mishra YG, Manavathi B. Focal adhesion dynamics in cellular function and disease. Cell Signal. 2021;85:110046.
64. Humphries JD, Chastney MR, Askari JA, et al. Signal transduction via integrin adhesion complexes. Curr Opin Cell Biol. 2019;56:14–21. doi:10.1016/j.ceb.2018.08.004
65. Humphries JD, Zha J, Burns J, et al. Pancreatic ductal adenocarcinoma cells employ integrin α6β4 to form hemidesmosomes and regulate cell proliferation. Matrix Biol. 2022;110:16–39. doi:10.1016/j.matbio.2022.03.010
66. Green HJ, Brown NH. Integrin intracellular machinery in action. Exp Cell Res. 2019;378(2):226–231. doi:10.1016/j.yexcr.2019.03.011
67. Chastney MR, Conway JRW, Ivaska J. Integrin adhesion complexes. Curr Biol. 2021;31(10):R536–42. doi:10.1016/j.cub.2021.01.038
68. Oria R, Wiegand T, Escribano J, et al. Force loading explains spatial sensing of ligands by cells. Nature. 2017;552(7684):219–224. doi:10.1038/nature24662
69. D’Amore VM, Donati G, Lenci E, et al. Molecular view on the iRGD peptide binding mechanism: implications for integrin activity and selectivity profiles. J Chem Inf Model. 2023;63(20):6302–6315. doi:10.1021/acs.jcim.3c01071
70. Davis PJ, Lin HY, Hercbergs A, et al. Coronaviruses and integrin αvβ3: does thyroid hormone modify the relationship? Endocr Res. 2020;45(3):210–215. doi:10.1080/07435800.2020.1767127
71. Li C, Su M, Yin B, et al. Integrin αvβ3 enhances replication of porcine epidemic diarrhea virus on Vero E6 and porcine intestinal epithelial cells. Vet Microbiol. 2019;237:108400. doi:10.1016/j.vetmic.2019.108400
72. Turaga RC, Sharma M, Mishra F, et al. Modulation of cancer-associated fibrotic stroma by an integrin αvβ3 targeting protein for pancreatic cancer treatment. Cell Mol Gastroenterol Hepatol. 2021;11(1):161–179. doi:10.1016/j.jcmgh.2020.08.004
73. Cheng TM, Chang WJ, Chu HY, et al. Nano-Strategies targeting the integrin αvβ3 network for cancer therapy. Cells. 2021;2021(7):1684. doi:10.3390/cells10071684
74. Huang R, Du H, Cheng L, et al. Targeted nanodelivery of siRNA against KRAS G12D inhibits pancreatic cancer. Acta Biomater. 2023;168:529–539. doi:10.1016/j.actbio.2023.07.008
75. Khosravani F, Mir H, Mirzaei A, et al. Arsenic trioxide and Erlotinib loaded in RGD-modified nanoliposomes for targeted combination delivery to PC3 and PANC-1 cell lines. Biotechnol Appl Biochem. 2023;70(2):811–823.
76. Ma T, Jiang JL, Qi WX, et al. A novel delivery system of RGD-HSA loaded GEM/CUR nanoparticles for the treatment of pancreatic cancer therapy. Drug Des Devel Ther. 2022;16:2395–2406. doi:10.2147/DDDT.S366558
77. Ai C, Zhang J, Lian S, et al. FOXM1 functions collaboratively with PLAU to promote gastric cancer progression. J Cancer. 2020;11(4):788–794. doi:10.7150/jca.37323
78. Zhai BT, Tian H, Sun J, et al. Urokinase-type plasminogen activator receptor (uPAR) as a therapeutic target in cancer. J Transl Med. 2022;20(1):135. doi:10.1186/s12967-022-03329-3
79. Cheng Y, Hall TR, Xu X, et al. Targeting uPA-uPAR interaction to improve intestinal epithelial barrier integrity in inflammatory bowel disease. EBioMed. 2022;75:103758. doi:10.1016/j.ebiom.2021.103758
80. Kanno Y. The uPA/uPAR system orchestrates the inflammatory response, vascular homeostasis, and immune system in fibrosis progression. Int J mol Sci. 2023;24(2):1796. doi:10.3390/ijms24021796
81. Lv T, Zhao Y, Jiang X, et al. uPAR: an essential factor for tumor development. J Cancer. 2021;12(23):7026–7040. doi:10.7150/jca.62281
82. Yu S, Huang G, Yuan R, et al. A uPAR targeted nanoplatform with an NIR laser-responsive drug release property for tri-modal imaging and synergistic photothermal-chemotherapy of triple-negative breast cancer. Biomater Sci. 2020;8(2):720–738. doi:10.1039/C9BM01495K
83. Zhu W, Yu H, Jia M, et al. Multitargeting liposomal codelivery of cisplatin and rapamycin inhibits pancreatic cancer growth and metastasis through stromal modulation. Int J Pharm. 2023;644:123316. doi:10.1016/j.ijpharm.2023.123316
84. Kimura S, D’Andrea D, Iwata T, et al. Expression of urokinase-type plasminogen activator system in nonmetastatic prostate cancer. World J Urol. 2020;38(10):2501–2511. doi:10.1007/s00345-019-03038-5
85. Kumar AA, Buckley BJ, Ranson M. The urokinase plasminogen activation system in pancreatic cancer: prospective diagnostic and therapeutic targets. Biomolecules. 2022;12(2):152. doi:10.3390/biom12020152
86. Xue A, Scarlett CJ, Jackson CJ, et al. Prognostic significance of growth factors and the urokinase-type plasminogen activator system in pancreatic ductal adenocarcinoma. Pancreas. 2008;36(2):160–167. doi:10.1097/MPA.0b013e31815750f0
87. Mahadiuzzaman ASM, Dain Md Opo FA, Alkarim S. Stem cell-based targeted therapy in pancreatic cancer: current approaches and future prospects. Tissue Cell. 2024;89:102449. doi:10.1016/j.tice.2024.102449
88. Hu Y, Chi C, Wang S, et al. A comparative study of clinical intervention and interventional photothermal therapy for pancreatic cancer. Adv Mater. 2017;29(33). doi:10.1002/adma.201700448.
89. Gao N, Bozeman EN, Qian W, et al. Tumor penetrating theranostic nanoparticles for enhancement of targeted and image-guided drug delivery into peritoneal tumors following intraperitoneal delivery. Theranostics. 2017;7(6):1689–1704. doi:10.7150/thno.18125
90. Li H, Wang P, Deng Y, et al. Combination of active targeting, enzyme-triggered release and fluorescent dye into gold nanoclusters for endomicroscopy-guided photothermal/photodynamic therapy to pancreatic ductal adenocarcinoma. Biomaterials. 2017;139:30–38. doi:10.1016/j.biomaterials.2017.05.030
91. Munir MU. Nanomedicine penetration to tumor: challenges, and advanced strategies to tackle this issue. Cancers. 2022;14(12):2904. doi:10.3390/cancers14122904
92. Jiang B, Zhou L, Lu J, et al. Stroma-targeting therapy in pancreatic cancer: one coin with two sides? Front Oncol. 2020;10:576399. doi:10.3389/fonc.2020.576399
93. Caon I, Bartolini B, Parnigoni A, et al. Revisiting the hallmarks of cancer: the role of hyaluronan. Semin Cancer Biol. 2020;62:9–19. doi:10.1016/j.semcancer.2019.07.007
94. Hakim N, Patel R, Devoe C, et al. Why HALO 301 failed and implications for treatment of pancreatic cancer. Pancreas. 2019;3(1):e1–4. doi:10.17140/POJ-3-e010
95. Chen D, Zhu X, Tao W, et al. Regulation of pancreatic cancer microenvironment by an intelligent gemcitabine@nanogel system via in vitro 3D model for promoting therapeutic efficiency. J Control Release. 2020;324:545–559. doi:10.1016/j.jconrel.2020.06.001
96. Yu S, Zhang L, Yang Y, et al. Polydopamine-based resveratrol-hyaluronidase nanomedicine inhibited pancreatic cancer cell invasive phenotype in hyaluronic acid enrichment tumor sphere model. ACS Pharmacol Transl Sci. 2024;7(4):1013–1022. doi:10.1021/acsptsci.3c00304
97. Song X, Li CL, Qiu N, et al. pH-sensitive biomimetic nanosystem based on large-pore mesoporous silica nanoparticles with high hyaluronidase loading for tumor deep penetration. ACS Appl Mater Interfaces. 2023;15(32):38294–38308. doi:10.1021/acsami.3c06909
98. Hauge A, Rofstad EK. Antifibrotic therapy to normalize the tumor microenvironment. J Transl Med. 2020;18(1):207. doi:10.1186/s12967-020-02376-y
99. Liu Q, Wang L, Su Y, et al. Ultrahigh enzyme loading metal-organic frameworks for deep tissue pancreatic cancer photoimmunotherapy. Small. 2024;20(10):e2305131. doi:10.1002/smll.202305131
100. Li Z, Mo F, Guo K, et al. Nanodrug-bacteria conjugates-mediated oncogenic collagen depletion enhances immune checkpoint blockade therapy against pancreatic cancer. Med. 2024;5(4):348–67.e7. doi:10.1016/j.medj.2024.02.012
101. Qiu N, Lv QY, Li CL, et al. Optimization and mechanisms of proteolytic enzyme immobilization onto large-pore mesoporous silica nanoparticles: enhanced tumor penetration. Int J Biol Macromol. 2024;271(Pt 1):132626. doi:10.1016/j.ijbiomac.2024.132626
102. Qi L, Han H, Han MM, et al. Remodeling of imbalanced extracellular matrix homeostasis for reversal of pancreatic fibrosis. Biomaterials. 2023;292:121945. doi:10.1016/j.biomaterials.2022.121945
103. Yang XY, Zhang JG, Zhou QM, et al. Extracellular matrix modulating enzyme functionalized biomimetic Au nanoplatform-mediated enhanced tumor penetration and synergistic antitumor therapy for pancreatic cancer. J Nanobiotechnology. 2022;20(1):524. doi:10.1186/s12951-022-01738-6
104. Yu N, Zhang X, Zhong H, et al. Stromal homeostasis-restoring nanomedicine enhances pancreatic cancer chemotherapy. Nano Lett. 2022;22(21):8744–8754. doi:10.1021/acs.nanolett.2c03663
105. Wang L, Dou J, Jiang W, et al. Enhanced intracellular transcytosis of nanoparticles by degrading extracellular matrix for deep tissue radiotherapy of pancreatic adenocarcinoma. Nano Lett. 2022;22(17):6877–6887. doi:10.1021/acs.nanolett.2c01005
106. Luo J, Cao J, Ma G, et al. Collagenase-loaded H-TiO2 nanoparticles enhance ultrasound imaging-guided sonodynamic therapy in a pancreatic carcinoma xenograft model via digesting stromal barriers. ACS Appl Mater Interfaces. 2022;14(36):40535–40545. doi:10.1021/acsami.2c08951
107. Zinger A, Koren L, Adir O, et al. Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS Nano. 2019;13(10):11008–11021. doi:10.1021/acsnano.9b02395
108. Jiang H, Torphy RJ, Steiger K, et al. Pancreatic ductal adenocarcinoma progression is restrained by stromal matrix. J Clin Invest. 2020;130(9):4704–4709. doi:10.1172/JCI136760
109. Lin TC, Yang CH, Cheng LH, et al. Fibronectin in Cancer: friend or Foe. Cells. 2019;9(1):27. doi:10.3390/cells9010027
110. Zhao X, Yang X, Wang X, et al. Penetration cascade of size switchable nanosystem in desmoplastic stroma for improved pancreatic cancer therapy. ACS Nano. 2021;15(9):14149–14161. doi:10.1021/acsnano.0c08860
111. Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog mol Biol Transl Sci. 2017;147:1–73.
112. Yao Q, Kou L, Tu Y, et al. MMP-responsive ‘Smart’ drug delivery and tumor targeting. Trends Pharmacol Sci. 2018;39(8):766–781. doi:10.1016/j.tips.2018.06.003
113. Cao J, Yang P, Wang P, et al. ‘Adhesion and release’ nanoparticle-mediated efficient inhibition of platelet activation disrupts endothelial barriers for enhanced drug delivery in tumors. Biomaterials. 2021;269:120620. doi:10.1016/j.biomaterials.2020.120620
114. Yin N, Yu H, Zhang X, et al. Enhancement of pancreatic cancer therapy efficacy by type-1 matrix metalloproteinase-functionalized nanoparticles for the selective delivery of gemcitabine and erlotinib. Drug Des Devel Ther. 2020;14:4465–4475. doi:10.2147/DDDT.S270303
115. Cun X, Chen J, Li M, et al. Tumor-associated fibroblast-targeted regulation and deep tumor delivery of chemotherapeutic drugs with a multifunctional size-switchable nanoparticle. ACS Appl Mater Interfaces. 2019;11(43):39545–39559. doi:10.1021/acsami.9b13957
116. Dong X, Liu HJ, Feng HY, et al. Enhanced drug delivery by nanoscale integration of a nitric oxide donor to induce tumor collagen depletion. Nano Lett. 2019;19(2):997–1008. doi:10.1021/acs.nanolett.8b04236
117. Qin Q, Yu R, Eriksson JE, et al. Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma therapy: challenges and opportunities. Cancer Lett. 2024;591:216859. doi:10.1016/j.canlet.2024.216859
118. Su X, Wang Z, Duan S. Targeted drug delivery systems for pancreatic ductal adenocarcinoma: overcoming tumor microenvironment challenges with CAF-specific nanoparticles. J Natl Cancer Cent. 2023;3(4):306–309. doi:10.1016/j.jncc.2023.10.001
119. Zhou P, Du X, Jia W, et al. Engineered extracellular vesicles for targeted reprogramming of cancer-associated fibroblasts to potentiate therapy of pancreatic cancer. Signal Transduct Target Ther. 2024;9(1):151. doi:10.1038/s41392-024-01872-7
120. Zhao T, Zhang R, He Q, et al. Partial ligand shielding nanoparticles improve pancreatic ductal adenocarcinoma treatment via a multifunctional paradigm for tumor stroma reprogramming. Acta Biomater. 2022;145:122–134. doi:10.1016/j.actbio.2022.03.050
121. Feng J, Xu M, Wang J, et al. Sequential delivery of nanoformulated α-mangostin and triptolide overcomes permeation obstacles and improves therapeutic effects in pancreatic cancer. Biomaterials. 2020;241:119907. doi:10.1016/j.biomaterials.2020.119907
122. Li M, Zhang Z, He Q. Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy. J Control Release. 2020;321:564–575. doi:10.1016/j.jconrel.2020.02.040
123. Chen X, Jia F, Huang Y, et al. Cancer-associated fibroblast-targeted delivery of captopril to overcome penetration obstacles for enhanced pancreatic cancer therapy. ACS Appl Bio Mater. 2022;5(7):3544–3553. doi:10.1021/acsabm.2c00486
124. Wei Y, Song S, Duan N, et al. MT1-MMP-activated liposomes to improve tumor blood perfusion and drug delivery for enhanced pancreatic cancer therapy. Adv Sci. 2020;7(17):1902746. doi:10.1002/advs.201902746
125. Marhaba R, Klingbeil P, Nuebel T, et al. CD44 and EpCAM: cancer-initiating cell markers. Curr Mol Med. 2008;8(8):784–804. doi:10.2174/156652408786733667
126. Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi:10.1158/0008-5472.CAN-06-2030
127. Kesharwani P, Chadar R, Sheikh A, et al. CD44-targeted nanocarrier for cancer therapy. Front Pharmacol. 2022;12:800481. doi:10.3389/fphar.2021.800481
128. Li C, Chen Q, Jiang C. Intelligent micelles for on-demand drug delivery targeting extracellular matrix of pancreatic cancer. J Control Release. 2024;373:879–889. doi:10.1016/j.jconrel.2024.07.058
129. Bartkowski M, Bincoletto V, Salaroglio IC, et al. Enhancing pancreatic ductal adenocarcinoma (PDAC) therapy with targeted carbon nano-onion (CNO)-mediated delivery of gemcitabine (GEM)-derived prodrugs. J Colloid Interface Sci. 2024;659:339–354. doi:10.1016/j.jcis.2023.12.166
130. Jangid AK, Kim S, Kim K. Delivery of piperlongumine via hyaluronic acid/phenylboronic acid-mediated dual targetable polymersome for enhanced anticancer functionality against pancreatic tumor. Int J Biol Macromol. 2024;275(Pt 2):133738. doi:10.1016/j.ijbiomac.2024.133738
131. Navarro-Marchal SA, Griñán-Lisón C, Entrena JM, et al. Anti-CD44-conjugated olive oil liquid nanocapsules for targeting pancreatic cancer stem cells. Biomacromolecules. 2021;22(4):1374–1388. doi:10.1021/acs.biomac.0c01546
132. Schnittert J, Bansal R, Prakash J. Targeting pancreatic stellate cells in cancer. Trends Cancer. 2019;5(2):128–142. doi:10.1016/j.trecan.2019.01.001
133. Wang R, Hong K, Zhang Q, et al. A nanodrug simultaneously inhibits pancreatic stellate cell activation and regulatory T-cell infiltration to promote the immunotherapy of pancreatic cancer. Acta Biomater. 2023;169:451–463. doi:10.1016/j.actbio.2023.08.007
134. Zhao Y, Yao H, Yang K, et al. Arsenic trioxide-loaded nanoparticles enhance the chemosensitivity of gemcitabine in pancreatic cancer via the reversal of pancreatic stellate cell desmoplasia by targeting the AP4/galectin-1 pathway. Biomater Sci. 2022;10(20):5989–6002. doi:10.1039/D2BM01039A
135. Yu H, Zhu W, Lin C, et al. Stromal and tumor immune microenvironment reprogramming through multifunctional cisplatin-based liposomes boosts the efficacy of anti-PD-1 immunotherapy in pancreatic cancer. Biomater Sci. 2023;12(1):116–133. doi:10.1039/D3BM01118F
136. Li T, Chen D, Liu H, et al. Spatially targeting and regulating tumor-associated macrophages using a raspberry-like micellar system sensitizes pancreatic cancer chemoimmunotherapy. Nanoscale. 2022;14(36):13098–13112. doi:10.1039/D2NR03053E
137. Xie Y, Hang Y, Wang Y, et al. Stromal modulation and treatment of metastatic pancreatic cancer with local intraperitoneal triple miRNA/siRNA nanotherapy. ACS Nano. 2020;14(1):255–271. doi:10.1021/acsnano.9b03978
138. Malik S, Westcott JM, Brekken RA, et al. CXCL12 in pancreatic cancer: its Function and potential as a therapeutic drug target. Cancers. 2021;4(1):86. doi:10.3390/cancers14010086
139. Wang Y, Gao Z, Du X, et al. Co-inhibition of the TGF-β pathway and the PD-L1 checkpoint by pH-responsive clustered nanoparticles for pancreatic cancer microenvironment regulation and anti-tumor immunotherapy. Biomater Sci. 2020;8(18):5121–5132. doi:10.1039/D0BM00916D
140. Iyer S, Enman M, Sahay P, et al. Novel therapeutics to treat chronic pancreatitis: targeting pancreatic stellate cells and macrophages. Expert Rev Gastroenterol Hepatol. 2024;18(4–5):171–183. doi:10.1080/17474124.2024.2355969
141. Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–291. doi:10.1126/science.1232227
142. Zhang W, Zeng Y, Xiao Q, et al. An in-situ peptide-antibody self-assembly to block CD47 and CD24 signaling enhances macrophage-mediated phagocytosis and anti-tumor immune responses. Nat Commun. 2024;15(1):5670. doi:10.1038/s41467-024-49825-6
143. Chen KH, Nguyen N, Huang TY, et al. Macrophage-hitchhiked orally administered β-glucans-functionalized nanoparticles as ”precision-guided stealth missiles” for targeted pancreatic cancer therapy. Adv Mater. 2023;35(40):e2304735. doi:10.1002/adma.202304735
144. Yang K, Han W, Jiang X, et al. Zinc cyclic di-AMP nanoparticles target and suppress tumors via endothelial STING activation and tumor-associated macrophage reinvigoration. Nat Nanotechnol. 2022;17(12):1322–1331. doi:10.1038/s41565-022-01225-x
145. Gao C, Cheng K, Li Y, et al. Injectable immunotherapeutic hydrogel containing RNA-loaded lipid nanoparticles reshapes tumor microenvironment for pancreatic cancer therapy. Nano Lett. 2022;22(22):8801–8809. doi:10.1021/acs.nanolett.2c01994
146. Lorkowski ME, Atukorale PU, Bielecki PA, et al. Immunostimulatory nanoparticle incorporating two immune agonists for the treatment of pancreatic tumors. J Control Release. 2021;330:1095–1105. doi:10.1016/j.jconrel.2020.11.014
147. Chibaya L, DeMarco KD, Lusi CF, et al. Nanoparticle delivery of innate immune agonists combined with senescence-inducing agents promotes T-cell control of pancreatic cancer. Sci Transl Med. 2024;16(762):eadj9366. doi:10.1126/scitranslmed.adj9366
148. Yao Z, Qi C, Zhang F, et al. Hollow Cu2MoS4 nanoparticles loaded with immune checkpoint inhibitors reshape the tumor microenvironment to enhance immunotherapy for pancreatic cancer. Acta Biomater. 2024;173:365–377. doi:10.1016/j.actbio.2023.10.024
149. Trabbic KR, Kleski KA, Barchi Jr JJ. A stable gold nanoparticle-based vaccine for the targeted delivery of tumor-associated glycopeptide antigens. ACS Bio Med Chem Au. 2021;1(1):31–43. doi:10.1021/acsbiomedchemau.1c00021
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

