Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Feasibility and Outcomes of Vessel-Sparing Surgery for Soft Tissue Sarcoma of the Thigh Adjacent to Femoral Vessels
Authors Yang ZM , Qu H, Wang KY, Wu JD, Liu B, Jin LB, Huang X, Lin N, Tao HM, Ye ZM
Received 26 November 2024
Accepted for publication 11 April 2025
Published 25 April 2025 Volume 2025:21 Pages 553—564
DOI https://doi.org/10.2147/TCRM.S506754
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor De Yun Wang
Zheng-Ming Yang,1– 5,* Hao Qu,1– 5,* Ke-Yi Wang,1– 5 Jia-Dan Wu,1– 5 Bing Liu,1– 5 Li-Bin Jin,1– 5 Xin Huang,1– 5 Nong Lin,1– 5 Hui-Min Tao,1– 5 Zhao-Ming Ye1– 5
1Department of Orthopedics, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, 310000, People’s Republic of China; 2Orthopedics Research Institute, Zhejiang University, Hangzhou, Zhejiang, 310000, People’s Republic of China; 3Clinical Research Center of Motor System Disease of Zhejiang Province, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, 310000, People’s Republic of China; 4Zhejiang Key Laboratory of Motor System Disease Precision Research and Therapy, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, 310000, People’s Republic of China; 5State Key Laboratory of Transvascular Implantation Devices, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, 310009, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zheng-Ming Yang, Department of Orthopedics, The Second Affiliated Hospital of Zhejiang University School of Medicine, No. 1504 of Jiang Hon Road, BinJiang District, Hangzhou, Zhejiang, 310000, People’s Republic of China, Tel +8613758228633, Email [email protected]
Objective: This study aims to investigate the feasibility and oncological outcomes of vessel-sparing surgery for soft tissue sarcomas located near the femoral vessels in the thigh. A comparison was made with cases where the tumor was not adjacent to the femoral vessels, focusing on recurrence rates, survival prognosis, and the viability of vessel-sparing surgical techniques.
Methods: A retrospective analysis was conducted on 211 cases. After well-differentiated liposarcoma were excluded from further analysis, 148 cases involved tumors not adjacent to the femoral vessels, while 22 cases were located near the vessels. Postoperative functional outcomes, survival rates, and local recurrence were evaluated. Due to the imbalance in case numbers between the two groups, propensity score matching was applied at a 1:1 ratio, after which the two datasets were compared and analyzed.
Results: By the last follow-up, 40 had experienced recurrence, 35 patients had died, 15 were surviving with tumors, and 161 were living tumor-free. No statistically significant differences were found between the survival and recurrence-free survival curves for cases with sarcomas adjacent to the femoral vessels compared to those with tumors located elsewhere, both before and after propensity score matching. Tumor grade and size were identified as key factors influencing survival and recurrence outcomes in soft tissue sarcoma of the thigh.
Conclusion: For soft tissue sarcoma of the thigh located adjacent to femoral vessels, vessel-sparing surgery involving vascular sheath removal demonstrates favorable outcomes. Tumor size greater than 10 cm and high pathological grade were significant predictors of survival and recurrence risk.
Keywords: femoral vessels, prognosis, soft tissue sarcoma, thigh, vascular resection
Introduction
Soft tissue sarcoma refers to malignant tumors arising from mesenchymal and neuroectodermal tissues, excluding bone, and represents a relatively rare form of cancer, accounting for approximately 1% of all adult malignancies.1 Surgical intervention is the primary treatment modality for limb soft tissue sarcoma. While limb soft tissue sarcomas infrequently involve major blood vessels, managing cases that do is a significant challenge for surgeons.2
Sub-adventitial dissection of the tumor is regarded as an acceptable technique when the malignancy’s severity is low and the involvement of surrounding blood vessels does not exceed 50%.3,4 However, this approach raises concerns about potential damage to blood vessels and an increased risk of recurrence due to tumor contamination.5
Recent advancements in vascular surgery have improved outcomes in vascular resection and reconstruction, making these procedures safe options for managing soft tissue sarcomas that involve large vessels.6–9 Despite these developments, the complexity of vascular resection and reconstruction techniques and associated complications often deter surgeons from opting for these methods, particularly when the tumor does not invade the vascular wall or completely encircle the blood vessels. Consequently, the appropriate surgical approach for soft tissue sarcomas involving large vessels remains a topic of ongoing debate.
Currently, there are limited studies on the surgical management and prognosis of soft tissue sarcoma involving large blood vessels in the extremities. In this study, we analyzed soft tissue sarcoma of the thigh located adjacent to the femoral vessels, examined the outcomes of vessel-sparing surgery, compared these cases with those in which the sarcoma was not adjacent to the femoral vessels, evaluated the prognosis in terms of recurrence and survival, and explored the feasibility of the vessel-sparing surgical approach.
Methods
Study Participants
A retrospective analysis was conducted on 211 cases that met the inclusion and exclusion criteria, who had been treated at our center between May 2011 and May 2021. Inclusion criteria: (1) tumors located in the thigh, (2) diagnosis of soft tissue sarcoma, (3) patients who underwent surgical treatment, and (4) availability of complete medical records and follow-up data (the follow-up time for surviving patients greater than 2 years). Exclusion criteria: (1) tumors originating from bone that invade the soft tissues of the thigh, (2) metastatic tumors, (3) cases where the tumor invades the vascular wall or completely encircles the blood vessels, (4) patients who declined participation or had incomplete follow-up data.
This study was conducted with informed consent provided by the patients themselves and their authorized family members and was approved by the hospital ethics committee [(2022) Ethics Review Study No. (0503)].
Pathological grading was performed using the Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grading system, which evaluates three parameters: tumor differentiation, necrosis rate, and mitotic count, with each parameter classified as G1, G2, or G3.10 Among the 211 cases, 112 were classified as low-grade and 99 as high-grade.
In this study, adjacency to blood vessels was defined as the absence of a normal tissue layer between the tumor and the blood vessels, excluding cases where the tumor invades the vascular wall or completely encircles the blood vessels. The tumor-vascular relationship was classified according to the Fujiwara classification11 as follows: Type 1: > 5 mm, Type 2: ≤ 5 mm, > 0 mm, Type 3: in direct contact with the blood vessels, and Type 4: encircling the blood vessels. Among the 211 cases analyzed, 184 involved tumors not adjacent to the femoral vessels, while 27 involved tumors adjacent to the femoral vessels, all of which were Fujiwara Type 3.
Given the favorable outcomes associated with marginal resection for well-differentiated liposarcoma, these cases were excluded from further analysis of survival and recurrence. Consequently, 5 cases of well-differentiated liposarcoma were excluded from the 27 patients with tumors adjacent to the femoral vessels, leaving 22 cases for analysis. Similarly, 36 cases of well-differentiated liposarcoma were excluded from the 184 patients with tumors not adjacent to the femoral vessels, leaving 148 cases for further evaluation.
To describe the degree of adjacency of the tumors to the blood vessels, we adopted the angular classification of the perimeter of the tumor-vascular contact range proposed by Holzapfel.12 This classification includes: no contact, ≤ 90°, 91°–180°, 181°–270°, and > 271°. In this study, the contact range for the cases varied from 90° to 270°, with 9 cases falling within the range of 91°–179° and 13 cases within the range of 180°–269° (Figure 1).
 |
Figure 1 Schematic diagram illustrating cases with contact ranges of 91°–179° and 180°–269°. The arrow indicates the extent of the tumor adjacent to the blood vessels. |
Surgical Methods
All patients in this group underwent surgical treatment involving the resection of the tumor, pseudocapsule, and a margin of normal tissue surrounding the tumor, ensuring a clearance of at least 2–3 cm. In some cases, muscle resection was also performed. For soft tissue sarcomas adjacent to the femoral vessels, dissection extended through the normal tissue to reach the vascular sheath, which was then opened down to the vascular wall. The sheath was separated from the vascular wall by creating a fenestration along both sides. The sheath was removed along with the tumor to achieve vascular contextualization (Figure 2).
 |
Figure 2 Schematic illustration of the vascular choroid following vascular sheath resection in cases of soft tissue sarcoma adjacent to the femoral vessels. |
No vascular injuries or surgical complications occurred during the process of separating the blood vessels in this group. After surgery, all dead spaces were closed, and 1–2 drainage tubes were placed, which were removed when the drainage volume decreased to less than 20–30 mL. Perioperatively, first- or second-generation cephalosporin antibiotics were routinely administered to prevent infection.
Follow-Up
All patients were followed up through outpatient re-examinations at 1, 3, 6, and 12 months after surgery, and then every 6 months thereafter. These follow-ups assessed postoperative function and oncological outcomes, including survival status and local recurrence. To evaluate postoperative functional outcomes, the Musculoskeletal Tumor Society (MSTS) functional score was used. This scoring system assesses six dimensions: pain, functional activity, emotional acceptance, external support, walking, and gait, with a total possible score of 30 points. A higher score indicates better functional recovery and overall patient well-being.
Statistical Analysis
Statistical differences in clinicopathological variables between cases with tumors adjacent to the femoral vessels and those not adjacent were analyzed using either the Student’s t-test or Mann–Whitney U-test for quantitative data, and the chi-square test or Fisher’s exact test for qualitative data. Multivariate analysis was conducted using the Cox proportional hazards regression model with stepwise selection to identify potential risk factors for recurrence. Kaplan-Meier analysis was employed to generate overall survival and recurrence-free survival curves, with the Log rank test used for comparison.
To mitigate bias between cases with tumors adjacent to the femoral vessels and those not adjacent, propensity score matching at a 1:1 ratio was applied to balance covariates across the groups, including sex, age, tumor size, tumor grade, diagnosis, chemotherapy, and radiotherapy. All statistical analyses were performed using R (R Foundation for Statistical Computing, Austria, version 2024.04.0-735). A P < 0.05 was considered statistically significant.
Results
Demographic Data
The cohort included 117 males and 94 females, ranging in age from 7 to 88 years (average age of 53 years). The diagnoses were as follows: 89 cases of liposarcoma, 33 cases of undifferentiated high grade pleomorphic sarcoma, 19 cases of myxofibrosarcoma, 12 cases of rhabdomyosarcoma, 12 cases of leiomyosarcoma, 9 cases of synovial sarcoma, 6 cases of solitary fibrous tumor, 4 cases of extraskeletal myxoid chondrosarcoma, 3 cases of malignant peripheral nerve sheath tumor, 3 cases of soft tissue Ewing sarcoma, and 21 cases of other soft tissue tumors.
Among the 22 cases with tumors adjacent to the femoral vessels, there were 13 males and 9 females, with ages ranging from 28 to 87 years (average age of 55.7±14.5 years). The diagnoses included 7 cases of liposarcoma, 4 cases of undifferentiated high grade pleomorphic sarcoma, 2 cases of myxofibrosarcoma, 2 cases of leiomyosarcoma, 1 case of solitary fibrous tumor, 1 case of extraskeletal myxoid chondrosarcoma, 1 case of malignant peripheral nerve sheath tumor, and 4 cases of other soft tissue tumors.
In terms of pathological types, liposarcoma includes well-differentiated liposarcoma, myxoid liposarcoma, and dedifferentiated liposarcoma; rhabdomyosarcoma includes embryonal rhabdomyosarcoma, alveolar rhabdomyosarcoma, and pleomorphic rhabdomyosarcoma.
Overall Oncology Prognosis
A total of 211 cases were followed for an average of (40.6 ± 24.9) months. At the time of the last follow-up, there were 35 recorded deaths, 40 instances of tumor recurrence, 15 patients who survived with tumors, and 161 patients who achieved tumor-free survival. Among the 40 patients who experienced tumor recurrence, 25 were still alive at the last follow-up, while the remaining 15 had succumbed to their condition. Excluding 44 cases for which functional scores could not be obtained due to death or other reasons, the remaining 167 patients had a postoperative MSTS score averaging (26.0 ± 3.2) points, with scores ranging from 9 to 30 points.
Prognostic Analysis of Survival and Recurrence of Thigh Soft Tissue Sarcoma Adjacent to the Femoral Vessels
After excluding well-differentiated liposarcoma cases, there were 22 patients with tumors adjacent to the femoral vessels and 148 patients with tumors that were not adjacent to the femoral vessels. The data from both groups were analyzed and compared (see Table 1). The analysis of survival curves and recurrence-free survival curves indicated no statistical differences between the two groups of soft tissue sarcoma, whether adjacent to or not adjacent to the femoral vessels (see Figure 3A and B).
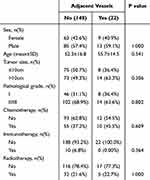 |
Table 1 Comparison of Parameters Between Soft Tissue Sarcomas Adjacent to and Not Adjacent to the Femoral Vessels |
To address the discrepancy in case numbers between the groups, a propensity score matching was conducted at a 1:1 ratio. After matching, the parameters for the 22 cases in each group were found to be largely consistent (see Table 2). Subsequent comparisons and analyses of the data revealed that there continued to be no statistical differences in survival curves and recurrence-free survival curves between the two groups following matching (see Figure 3C and D).
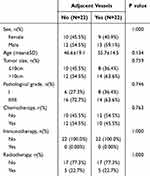 |
Table 2 Comparison of Parameters Between Soft Tissue Sarcomas Adjacent to and Not Adjacent to the Femoral Vessels After Matching |
Analysis of Influencing Factors for Survival and Recurrence of Soft Tissue Sarcoma of the Thigh Before and After Matching
Univariate analysis of survival risk prior to matching indicated that pathological grade was a significant influencing factor (see Table 3). Further multivariate analysis confirmed that pathological grade remained an independent influencing factor, with a hazard ratio (HR) of 8.567 (95% confidence interval [CI]: 1.997–36.752; P = 0.003).
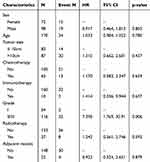 |
Table 3 Univariate Cox Regression Analysis of Survival Risk Prior to Matching |
Similarly, univariate analysis of recurrence risk before matching also identified pathological grade as a significant influencing factor (see Table 4). The multivariate analysis reaffirmed that pathological grade was an independent influencing factor for recurrence risk, yielding an HR of 4.631 (95% CI: 1.816–11.815; P = 0.003). Additionally, while tumor size did not reach statistical significance, it demonstrated a trend towards significance, with an HR of 1.69 (95% CI: 0.931–3.089; P = 0.084).
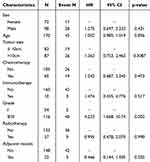 |
Table 4 Univariate Cox Regression Analysis of Recurrence Risk Prior to Matching |
Data analysis following propensity score matching revealed that no statistical variables were identified in the univariate and multivariate analyses of survival risk (see Table 5). In the context of recurrence risk, univariate analysis after matching did not yield any statistical variables (see Table 6). However, multivariate analysis indicated that tumor size was statistically significant in relation to the risk of recurrence, with tumors greater than 10 cm demonstrating a recurrence risk that was 12.88 times higher compared to those measuring less than 10 cm (HR: 12.883; 95% CI: 1.567–105.9; P = 0.017). Furthermore, while pathological grade did not reach statistical significance, it exhibited a trend towards significance, with an HR of 4.968 (95% CI: 0.824–29.94; P = 0.080).
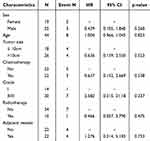 |
Table 5 Univariate Cox Regression Analysis of Survival Risk Following Matching |
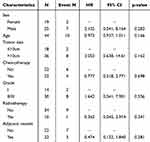 |
Table 6 Univariate Cox Regression Analysis of Recurrence Risk Following Matching |
Discussion
Currently, limb-salvage surgery is the predominant treatment approach for limb soft tissue sarcomas.13 However, managing soft tissue sarcomas that involve large blood vessels poses a significant challenge for surgeons.2 Prior to surgery, it is crucial to assess the feasibility of tumor resection, identify the pathological type, evaluate the location of tumor, and determine the extent of invasion into surrounding tissues, as well as the degree of involvement of adjacent blood vessels and nerves. This assessment informs the surgical approach for resection.
In clinical practice, various imaging modalities such as digital subtraction angiography (DSA), magnetic resonance angiography (MRA), and computed tomography angiography (CTA) are routinely utilized to evaluate the location, characteristics, and vascular invasion associated with tumors. Among these, MRI is recognized as a particularly effective imaging technique for assessing the relationship between large blood vessels and tumors.14–17
According to the Fujiwara classification, the tumor-vascular relationship is categorized as follows:11 Type 1 indicates a distance of more than 5 mm from the blood vessels; Type 2 refers to a distance of 0 to 5 mm; Type 3 indicates proximity to blood vessels; and Type 4 signifies encirclement of the blood vessels. To further characterize the adjacency of tumors to blood vessels, Holzapfel et al introduced an angular classification of the tumor-vascular contact range, which includes categories such as no contact, ≤ 90°, 91°–180°, 181°–270°, and > 271°.12
Despite the prevalence of studies focusing on the efficacy of different surgical modalities, there remains a notable deficiency in detailed descriptions of the extent and degree of involvement of large blood vessels in the selected cases. As a result, a consensus regarding the extent of large vessel involvement and the corresponding treatment strategies is still lacking in the current literature.
With advancements in clinical technology and the adoption of a multidisciplinary approach, limb-salvage surgery involving vascular resection and reconstruction has yielded improved outcomes, with vascular surgeons playing a crucial role in this process. However, the complexity of reconstruction techniques and the potential for significant complications often lead many surgeons to hesitate in prioritizing the resection and reconstruction of major blood vessels.
Furthermore, literature reports frequently lack specific descriptions regarding the degree of tumor invasion into blood vessels, resulting in an absence of standardized guidelines for determining the extent of vascular invasion and the appropriate surgical approach. In cases of soft tissue sarcomas where the tumor has breached the vascular sheath, invaded the vascular wall, or completely encircled the blood vessels, vascular resection and reconstruction are generally accepted by most surgeons. Conversely, for soft tissue sarcomas exhibiting varying degrees of involvement with large vessels, the choice of treatment remains a topic of ongoing debate.
When soft tissue sarcoma involving large blood vessels only invades the vascular sheath and adjacent tissues, the vascular sheath serves as a critical “barrier” that inhibits the tumor’s progression into the blood vessels. Kawaguchi et al from Japan has proposed a relatively detailed method for evaluating margins, designating any tissue that resists tumor invasion as a barrier, with the vascular sheath equated to a protective layer of 2 cm of normal tissue.18
In instances where the adventitia and other structures of the vascular wall within the sheath remain largely intact and free from tumor cell invasion, it is generally acceptable to excise the vascular sheath along with surrounding tissues exhibiting adhesions or invasions. This approach is predicated on the notion that the artery’s exposed adventitial layer can maintain the integrity of the vascular wall. Therefore, in cases where the vascular sheath remains intact, opting for a limb-salvage procedure that involves resecting the vascular sheath while preserving the underlying vessel is considered a reasonable strategy.
Soft tissue sarcomas involving blood vessels are infrequently reported in the literature. While such involvement has been associated with an increased risk of recurrence, it has not been shown to adversely affect survival outcomes.15 However, the literature lacks a clear delineation of the extent of vascular involvement, and various surgical approaches have been employed, including vessel-sparing techniques and vascular resection. Currently, there is no consensus on whether vessel-sparing surgery for soft tissue sarcoma adjacent to blood vessels can achieve the desired therapeutic effects.
Cases of soft tissue sarcomas of the thigh that invade or completely encircle the femoral vessels were specifically excluded in this study, focusing instead on the feasibility of vessel-sparing surgical treatment. In this study, there were 148 tumors which were not adjacent to the femoral vessels and 22 tumors that were adjacent. A comparative analysis of these two groups revealed no statistically significant differences in survival and recurrence-free survival curves. Given the disparity in case numbers between the two groups, propensity score matching was performed at a 1:1 ratio, resulting in matched parameters for 22 cases in each group. Even after matching, there was no statistical difference in survival or recurrence-free survival curve analyses.
These findings suggest that for soft tissue sarcomas of the thigh, which are adjacent to the femoral vessels, the vessel-sparing surgical approach—specifically the removal of the vascular sheath—can yield favorable outcomes.
According to the angular classification of the perimeter of the tumor-vascular contact range proposed by Holzapfel et al, the contact ranges are categorized as follows:12 no contact, ≤ 90°, 91°–180°, 181°–270°, and > 271°. In this study, the extent of invasion observed in the cases ranged from 90° to 270°, with 9 cases falling within the 91°–179° range and 13 cases within the 180°–269° range. Consequently, the data indicate that the cases eligible for limb-salvage vessel-sparing surgery with vascular sheath resection correspond to Fujiwara Type 3, with all instances involving contact of less than 270°.
However, there is currently no corresponding clinical experience documented in this dataset regarding the feasibility of limb-salvage vessel-sparing surgery with vascular sheath resection for cases exhibiting a contact range greater than 270°. This limitation highlights the need for further investigation and discussion on the implications and potential outcomes for such cases.
Soft tissue sarcomas exhibit a wide range of pathological types and individual differences, with numerous factors influencing prognosis. Tumor size and pathological grade are frequently cited in the literature as critical determinants of survival and prognostic risk factors.19–22 In a prospective study, Pisters et al found that the pathological grade of tumors was significantly associated with distant recurrence, survival, and local recurrence, noting that malignant schwannoma or fibrosarcoma had a higher propensity for local recurrence.23 Ezuddin et al conducted a retrospective analysis evaluating the risk of local recurrence in soft tissue sarcoma based on histological type, grade, tumor size, and margin status, confirming that a high pathological grade correlated with increased local recurrence risk.24 Furthermore, a study by Zagars et al at M.D. Anderson Cancer Center, which included 1225 patients with localized primary soft tissue sarcoma, similarly demonstrated that high-grade sarcomas were associated with local recurrence.25
The findings of this study align with the conclusions drawn from previous literature, all emphasizing the significant impact of pathological grade and tumor size on survival and recurrence. Statistical analyses conducted before matching revealed that pathological grade was a key influencing factor on both survival and recurrence risk, as evidenced by both univariate and multivariate analyses. Although the univariate analysis post-matching did not identify any statistical variables regarding recurrence risk, multivariate analysis indicated that tumor size was a statistically significant factor, with patients who had tumors larger than 10 cm having a 12.88-fold increased risk of recurrence compared to those with smaller tumors. Additionally, while the pathological grade did not achieve statistical significance, it exhibited a notable trend concerning survival and recurrence risk.
Consequently, for patients presenting with tumor sizes greater than 10 cm and/or high pathological grades, treatment strategies should be formulated with greater caution, and surgical interventions must be approached with increased thoroughness to optimize outcomes.
This study has several limitations: (1) As a retrospective study, the results may be subject to bias. Additionally, the prolonged follow-up duration could affect the statistical power of the study’s conclusions; (2) The relatively small number of cases involving soft tissue sarcoma of the thigh adjacent to the femoral blood vessels may impact the generalizability of the research findings. However, given the low incidence of soft tissue sarcoma and the challenges associated with clinical research in this area, this study aims to provide a theoretical basis for the vessel-sparing surgical method in select clinical cases. Nevertheless, further case accumulation and additional research are essential; (3) The incidence of soft tissue sarcoma is low, and the pathological diagnosis of the disease is diverse and complex, resulting in variability in diagnoses among different patients.
Conclusion
In summary, for soft tissue sarcoma of the thigh adjacent to the femoral vessels, the vessel-sparing surgical method that involves the removal of the vascular sheath can yield better outcomes. Additionally, tumor size greater than 10 cm and high pathological grade are significant influencing factors for prognosis concerning survival and recurrence risk.
Abbreviations
HR, Hazard ratio; 95% CI, confidence interval.
Data Sharing Statement
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of The Second Affiliated Hospital of Zhejiang University School of Medicine [No. (2022) Ethics Review Study No. (0503)]. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all patients and their authorized family members.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that they have no conflict of interest regarding this work.
References
1. Hui JY. Epidemiology and etiology of sarcomas. Surg Clin North Am. 2016;96(5):901–914. PMID: 27542634. doi:10.1016/j.suc.2016.05.005
2. Ghosh J, Bhowmick A, Baguneid M. Oncovascular surgery. Eur J Surg Oncol. 2011;37(12):1017–1024. PMID: 21917411. doi:10.1016/j.ejso.2011.08.131
3. Fortner JG, Kim DK, Shiu MH. Limb-preserving vascular surgery for malignant tumors of the lower extremity. Arch Surg. 1977;112(4):391–394. PMID: 849146. doi:10.1001/archsurg.1977.01370040043007
4. Wang Z, Guo Z, Li J, Li XD, Sang HX. Functional outcomes and complications of reconstruction of the proximal humerus after intra-articular tumor resection. Orthop Surg. 2010;2(1):19–26. PMID: 22009903; PMCID: PMC6583620. doi:10.1111/j.1757-7861.2009.00058.x
5. Bonardelli S, Nodari F, Maffeis R, et al. Limb salvage in lower-extremity sarcomas and technical details about vascular reconstruction. J Orthop Sci. 2000;5(6):555–560. PMID: 11180918. doi:10.1007/s007760070005
6. Leggon RE, Huber TS, Scarborough MT. Limb salvage surgery with vascular reconstruction. Clin Orthop Relat Res. 2001;387:207–216. PMID: 11400886. doi:10.1097/00003086-200106000-00028
7. Hohenberger P, Allenberg JR, Schlag PM, Reichardt P. Results of surgery and multimodal therapy for patients with soft tissue sarcoma invading to vascular structures. Cancer. 1999;85(2):396–408. PMID: 10023708.
8. Muramatsu K, Ihara K, Miyoshi T, Yoshida K, Taguchi T. Clinical outcome of limb-salvage surgery after wide resection of sarcoma and femoral vessel reconstruction. Ann Vasc Surg. 2011;25(8):1070–1077. PMID: 21831587. doi:10.1016/j.avsg.2011.05.009
9. Okamoto K, Koga A, Tazume H, et al. Early and mid-term outcomes after vascular reconstruction for patients with lower-extremity soft-tissue malignant tumors. Ann Vasc Dis. 2018;11(2):228–232. PMID: 30116416; PMCID: PMC6094028. doi:10.3400/avd.oa.18-00027
10. Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33(1):37–42. PMID: 6693192. doi:10.1002/ijc.2910330108
11. Fujiwara T, Medellin MR, Sambri A, et al. Preoperative surgical risk stratification in osteosarcoma based on the proximity to the major vessels. Bone Joint J. 2019;101-B(8):1024–1031. PMID: 31362545. doi:10.1302/0301-620X.101B8.BJJ-2018-0963.R1
12. Holzapfel K, Regler J, Baum T, et al. Local staging of soft-tissue sarcoma: emphasis on assessment of neurovascular encasement-value of MR imaging in 174 confirmed cases. Radiology. 2015;275(2):501–509. PMID: 25584707. doi:10.1148/radiol.14140510
13. Williard WC, Collin C, Casper ES, Hajdu SI, Brennan MF. The changing role of amputation for soft tissue sarcoma of the extremity in adults. Surg Gynecol Obstet. 1992;175(5):389–396. PMID: 1440165.
14. Ledoux P, Kind M, Le Loarer F, et al. Abnormal vascularization of soft-tissue sarcomas on conventional MRI: diagnostic and prognostic values. Eur J Radiol. 2019;117:112–119. PMID: 31307635. doi:10.1016/j.ejrad.2019.06.007
15. Sambri A, Caldari E, Montanari A, et al. Vascular proximity increases the risk of local recurrence in soft-tissue sarcomas of the thigh—a retrospective MRI study. Cancers. 2021;13(24):6325. PMID: 34944944; PMCID: PMC8699708. doi:10.3390/cancers13246325
16. Abatzoglou S, Turcotte RE, Adoubali A, Isler MH, Roberge D. Local recurrence after initial multidisciplinary management of soft tissue sarcoma: is there a way out? Clin Orthop Relat Res. 2010;468(11):3012–3018. PMID: 20700676; PMCID: PMC2947683. doi:10.1007/s11999-010-1481-7
17. Gronchi A, Lo Vullo S, Colombo C, et al. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg. 2010;251(3):506–511. PMID: 20130465. doi:10.1097/SLA.0b013e3181cf87fa
18. Kawaguchi N, Ahmed AR, Matsumoto S, Manabe J, Matsushita Y. The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:165–172. PMID: 15021149. doi:10.1097/00003086-200402000-00027
19. Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: an update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70(3):200–229. PMID: 32275330. doi:10.3322/caac.21605
20. Weskamp P, Ufton D, Drysch M, et al. Risk Factors for Occurrence and Relapse of Soft Tissue Sarcoma. Cancers. 2022;14(5):1273. PMID: 35267581; PMCID: PMC8909240. doi:10.3390/cancers14051273
21. Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260(3):416–21;discussion421–2. PMID: 25115417; PMCID: PMC4170654. doi:10.1097/SLA.0000000000000869
22. Li RH, Zhou Q, Li AB, Zhang HZ, Lin ZQ. A nomogram to predict metastasis of soft tissue sarcoma of the extremities. Medicine. 2020;99(21):e20165. PMID: 32481285; PMCID: PMC7250057. doi:10.1097/MD.0000000000020165
23. Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14(5):1679–1689. PMID: 8622088. doi:10.1200/JCO.1996.14.5.1679
24. Ezuddin NS, Pretell-Mazzini J, Yechieli RL, Kerr DA, Wilky BA, Subhawong TK. Local recurrence of soft-tissue sarcoma: issues in imaging surveillance strategy. Skeletal Radiol. 2018;47(12):1595–1606. PMID: 29785452. doi:10.1007/s00256-018-2965-x
25. Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer. 2003;97(10):2530–2543. PMID: 12733153. doi:10.1002/cncr.11365
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


