Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
Feasibility of Serum Peroxiredoxin 2 as a Biochemical Indicator for Reflecting Severity and Prognosticating Stroke-Associated Pneumonia, Early Neurological Deterioration and Poor Neurological Outcomes in Acute Supratentorial Intracerebral Hemorrhage: An Observational Analytical Clinical Study
Authors Zhang C, Zhang G, Ye Z, Hu G, Ge S, Luo K, Li Z
Received 8 November 2024
Accepted for publication 11 March 2025
Published 20 March 2025 Volume 2025:21 Pages 621—640
DOI https://doi.org/10.2147/NDT.S505346
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rakesh Kumar
Cheng Zhang, Guohai Zhang, Zhehao Ye, Guojie Hu, Shengsheng Ge, Kai Luo, Zhao Li
Department of Neurosurgery, Shengzhou Hospital of Traditional Chinese Medicine, Shengzhou, Zhejiang Province, People’s Republic of China
Correspondence: Zhao Li, Email [email protected]
Background: Peroxiredoxin 2 (Prdx2) functions as an antioxidant and may be involved in acute brain injury. This study aimed to investigate whether Prdx2 can act as a serological marker for assessing the severity and forecasting stroke-associated pneumonia (SAP), early neurological deterioration (END), and neurological outcomes in acute intracerebral hemorrhage (ICH).
Methods: A collective of 167 patients with ICH and 61 controls underwent quantifications for serum Prdx2 levels. In addition, 61 of them allowed Prdx2 measurements on days 1, 3, 5, 7, 10, and 14 post-ICH. Admission National Institutes of Health Stroke Scale (NIHSS) score and hematoma size were documented, and the modified Rankin Scale (mRS) at six-month mark following ICH was registered. Correlations between serum Prdx2 and END, SAP, and poor prognosis (mRS scores of 3– 6) were determined using multivariate models.
Results: Serum Prdx2 levels of patients rapidly increased after stroke, with the highest levels on day 3, and were substantially higher during the initial 14 days than those of controls. Prdx2 levels were closely related to NIHSS scores, hematoma size, and mRS scores, were linearly relevant to likelihoods of SAP, END, and poor prognosis, and were independently predictive of SAP, END, and poor prognosis. These associations were not markedly affected by age, sex, hypertension, or other factors using subgroup analysis. Moreover, the possibilities of END, SAP, and poor prognosis were efficiently distinguished by Prdx2 levels. Its discrimination efficiency was similar to those of the NIHSS scores and hematoma size. The combination of the three variables displayed a higher predictive ability than the combination of NIHSS scores and hematoma volume for prognosis prediction.
Conclusion: A marked increase in serum Prdx2 levels after ICH may accurately mirror hemorrhagic severity and effectively predict END, SAP, and poor neurological outcomes, solidifying serum Prdx2 as a prognosticator of ICH.
Keywords: peroxiredoxin 2, intracerebral hemorrhage, outcome, severity, biomarkers
Introduction
Spontaneous intracerebral hemorrhage (ICH) is unquestionably admitted as one of the most fatal cerebrovascular accidents and is characterized by a lower incidence but higher mortality and disability rates than acute ischemic stroke.1 Following hemorrhagic injury to brain tissues, a plethora of adverse molecular events occur, including intense inflammatory responses, excessive oxidative stress, necrosis imbalance, and acceleration of apoptosis.2 Severity evaluation using the National Institutes of Health Stroke Scale (NIHSS) or hematoma volume is a widely accepted approach for discriminating the likelihood of adverse prognosis following ICH.3 In addition, the modified Rankin Scale (mRS) has been strongly recognized as a clinical metric for assessing neurological functional outcomes in patients.4 Early neurological deterioration (END) and stroke-associated pneumonia (SAP), both of which frequently occur post-ICH, are strongly associated with poor patients.5,6 Owing to the easy obtainability and usability of blood in clinical practice, blood biomarkers have attracted a great deal of attention because of their excellent performance in severity assessment and precise outcome prediction of ICH.7–9
Peroxiredoxin 2 (Prdx2) is an antioxidant enzyme that is extensively distributed in various cell types including vascular smooth muscle cells, red blood cells, and neurons.10 Intracellular Prdx2 exerts pivotal antioxidant effects under pathological conditions such as abdominal aortic aneurysm, rheumatoid arthritis, and sepsis.11–13 However, extracellular Prdx2, a damage-associated molecule, can function as an inflammatory mediator by enhancing the production of various pro-inflammatory factors.14–16 Prdx2 has been gradually studied in some central nervous system diseases such as experimental ICH, Alzheimer’s disease, ischemic stroke, and subarachnoid hemorrhage.17 Similarly, Prdx2 can potently catalyze hydrogen peroxide and diminish the generation of reactive oxygen species, with simultaneous participation in the regulation of numerous signaling pathways in neurons, thereby defending neurons against injuries caused by oxidative stress and inflammation.18–20 In contrast, spillage of Prdx2 from damaged neurons following brain injury can strongly promote the release of inflammatory mediators from glial cells, possibly via activation of the Toll-like receptor 4 (TLR4) /nuclear factor-kappa B (NF-κB) signaling pathway.21–23 Notably, Prdx2 expression in neurons is significantly upregulated in the cerebral cortex, hippocampus, cerebellum, basal ganglia, and substantia nigra of animals after neurodegenerative disorders.24,25 Prdx2 levels are dramatically elevated in cerebrospinal fluid samples of human patients with aneurysmal subarachnoid hemorrhage or traumatic brain injury.26 Overall, Prdx2 may serve as a biochemical marker for brain injury. Herein, the implications of serum Prdx2 levels as predictors of END, SAP, and poor prognosis were revealed in a cohort of patients with ICH.
Materials and Methods
Study Design, Subject Enlistments and Ethical Acquirements
This observational analytical clinical study was conducted at the Shengzhou Hospital of Traditional Chinese Medicine (Shengzhou, China) from March 2020 to March 2023. Theoretically, this study comprises two parts. The first was a cross-sectional study, with the objective of unveiling the evolutionary trajectory of post-ICH serum Prdx2 levels. The second was a prospective cohort study with the purpose of unraveling the significance of admission serum Prdx2 as a serological marker in assessing the severity and prediction of END, SAP and six-month poor neurological functional outcomes following ICH. In accordance with the study type and enrollment criteria shown in Figure 1, patients with ICH and control subjects were recruited to achieve the two study goals. Here, all patients consented for supplying blood specimens at admission and some of them also allowed to provide blood samples on days 1, 3, 5, 7, 10 and 14 after ICH. This study was conducted in compliance with local and institutional ethical and lawful regulations, as well as the guidelines set forth in the Declaration of Helsinki and its follow-up amendments. The study protocol was in advance granted with permission by the Ethics Committee at the Shengzhou Hospital of Traditional Chinese Medicine (Shengzhou, China) (opinion number: SZZYK-2022-005). The patients’ legal representatives and controls were notified of the study contents, and subsequently signed consent forms were garnered from them separately.
Data Collection and Registration
Recordable data, which could be immediately obtained upon arrival of patients at the emergency center, included age, sex, smoking habits, alcohol consumption, medical history of hypertension, diabetes, dyslipidemia, chronic obstructive pulmonary disease, ischemic heart disease, and hyperuricemia, as well as premorbid use of statins, anticoagulants, and antiplatelet agents. Alternatively, the time of arrival at the hospital since the onset of stroke symptoms and interval between blood collection and stroke occurrence were recorded. Non-invasive systolic arterial blood pressure and diastolic arterial blood pressure were read as baseline data at the patients’ entrance to the emergency center. Vomiting was recorded, and dysphagia was diagnosed using a swallowing test. Head computed tomography (CT) was performed according to neuroradiological guidelines. Based on the initial CT imaging, the amount of bleeding was calculated using a previously established equation as follows: 07×a×b×c,27 bleeding sites were classified into two categories (superficial and deep), and blood clots were observed for possible entry into the intraventricular or subarachnoid cavity. The NIHSS score at admission was estimated.28 Patients who had an enhancement of at least 4 points in the NIHSS scores or died within 24 h after hospital admission were considered to have END.29 In compliance with the guidelines set forth by the consensus group, SAP was defined as the appearance of infections that influence the lower airways within the first 7 days post-ICH.30 Assessment of neurological functional conditions by mRS was completed six months after ICH via telephone visits by applying structured inquiry. The follow-up results were dichotomized into good prognosis (scores 0–2) and poor prognosis (scores 3–6).31
Blood Drawing, Sample Processing and Immune Analysis
At the emergency center, 5 mL of venous blood was drawn via venipuncture in the antecubital area of each patient, and venous blood samples were extracted on days 1, 3, 5, 7, 10, and 14 from a fraction of the patients who were allowed for blood collection at various time points after the onset of ICH. In addition, the controls underwent blood drawing by applying the same approaches as the patients at their entrance into our clinical investigation. Blood specimens were placed into 5 mL gel-refilled biochemistry tubes. When blood clots were observed, the blood samples were centrifuged at 2000 × g for ten minutes. The next step was to quickly suck the supernatants and then transfer them into Eppendorf tubes for further preservation at −80 °C for later measurements. To avoid protein decomposition, a batch of serum samples, which were obtained in a quarter, was melted to quantify the serum Prdx2 levels. Enzyme-Linked Immunosorbent Assay (ELISA) was used for detection. The kit was obtained from Shanghai Zeye Biotechnology Co., Ltd. (Catalogue no. ZY72367H). The detection range was 0.312–20 ng/mL, with intra- and inter-assay coefficients of variation of less than 10%. Optical density was measured at 450 nm using an ELISA reader (Biobase Meihua Trading Co., Ltd., China). All ELISA measurements were performed in duplicate by an identical professional technician who did not permit access to the study contents.
Statistical Analysis
The SPSS (version 23.0; SPSS Inc., Chicago, IL, USA) was used for conventional data processing. Numbers (proportions) are displayed to represent categorical variables. The Kolmogorov–Smirnov test was performed to determine whether the quantitative variables were normally distributed. At the base of normality patterns of data distribution, measurement variables were presented as either means (standard deviations, SDs) or medians (percentiles 25th-75th) where appropriate. Statistical methods for comparing differences between two groups were the chi-square test and Fisher’s exact test for categorical variables and the independent-sample Student’s t-test and Mann–Whitney U-test for continuous variables. The Kruskal–Wallis test was used to ascertain the differences in serum Prdx2 levels among multiple subgroups. The outcome variables of interest in the current study were SAP, END, and poor six-month prognosis. The exploratory strategy was to consolidate all significant parameters in the univariate analyses into binary multivariable logistic regression models to discern independent predictors of the preceding outcome variables. Bivariate correlation assessments were performed using the Spearman’s test. With the assistance of R 3.5.1 (https://www.r-project.org), restricted cubic splines (RCSs) were painted to outline the linearity association, and forest plots for subgroup analysis were drawn to delineate interactional effects. Discrimination effectiveness was determined within the framework of receiver operating characteristic (ROC) curve analysis using MedCalc 20 (MedCalc Software, Ltd., Ostend, Belgium). ROC curves, scatter plots, and violin plots were generated using GraphPad Prism 7.01 (GraphPad Software, Inc., San Diego, California, USA). In the current study, all patients agreed with blood-drawings at admission and some of them also agreed to proffer blood specimens on days 1, 3, 5, 7, 10, and 14 after ICH. Only the univariate analysis was performed for analyzing change of serum Prdx2 levels in those patients permitting blood-drawings at multiple time points. Univariate analysis and subsequent multivariate analysis were carried out for assessing prognosis-related data of all patients. Differences with a two-tailed P value less than 0.05, signified statistically noteworthy differences.
Results
Study Populations and Baseline Features
In light of the inclusion criteria in Figure 1, 216 patients, who were diagnosed with primary supratentorial intraparenchymal hemorrhage, were consecutively enlisted, and a subsequent elimination of forty-nine subjects was actualized on the basis of the exclusion requirements in Figure 1. The final selection of 167 patients was completed to conduct a range of epidemiological studies. The baseline characteristics of the all patients are shown in Table 1. In compliance with voluntariness principles, a cumulative of sixty-one patients allowed various time-interval blood drawings. These 61 patients presented with similar basic features as all 167 patients (all P>0.05; Table 1). In addition, the enrollment of 61 controls was finalized in accordance with the eligibility requirements in Figure 1. The controls were aged from 44 to 77 years (median, 58 years; percentiles 25th-75th, 49–67 years), comprised of 33 males and 28 females, and consisted of 22 tobacco smokers and 23 alcohol consumers. Statistically, 61 controls had negligible distinctions over those 61 patients in terms of age, sex ratio, and the percentages of smokers and consumers (all P>0.05).
Temporal Alteration of Serum Prdx2 Levels and Its Relation to ICH Severity
As displayed in Figure 2, there were non-statistically significant disparities in serum Prdx2 levels at admission between all 167 patients and those 61 patients consenting for blood-samplings at multiple time points (P>0.05); among those 61 patients, serum Prdx2 levels were markedly increased shortly following ICH, had subsequent promotion at day 1, reached the highest status on day 3, and afterwards, displayed slow reduction until day 14. Serum Prdx2 levels of those 61 patients maintained an extremely higher state during 14 days than those of 61 controls (P<0.001). As shown in Table 2, the admission NIHSS scores and hematoma volumes of those 61 patients were significantly positively correlated with their serum Prdx2 levels at admission and on days 1, 3, 5, 7, 10, and 14 after ICH (all P<0.05). Among all 167 patients, admission NIHSS scores and hematoma volumes were also substantially positively related to admission serum Prdx2 levels (both P<0.001; Figures 3 and 4).
Serum Prdx2 Levels and Post-ICH Six-month Poor Prognosis
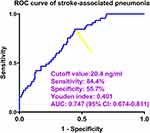
The mRS scores at six months after ICH varied from 0 to 6 (median, 2; lower-upper quartiles, 2–4) among all 167 patients. The scores ranged from 0 to 6 (median, 3; lower-upper quartiles, 1–5) among those 61 patients agreeing with blood-collections at several time points. The difference in mRS scores was not significant between all 167 patients and those 61 patients (P=0.835). The post-ICH mRS scores of those 61 patients were positively correlated with serum Prdx2 levels at all seven time points (all P<0.01; Table 2). Moreover, serum Prdx2 levels at admission were profoundly increased in the order of mRS scores from 0 to 6 in all 167 patients (P<0.001; Figure 5). Among those 61 patients, there were thirty-one patients with development of poor prognosis at six-month mark following ICH. As shown in Figure 6, serum Prdx2 levels at all seven time points in those 61 patients were markedly higher in patients with poor prognosis than in those with good prognosis (all P<0.05). As listed in Table 3, serum Prdx2 levels at admission displayed a similar area under the ROC curve compared to those at the remaining six time points among those 61 patients (all P>0.05). Of all 167 patients, 81 had poor prognosis. Compared with patients with a good prognosis, those with a poor prognosis had markedly higher chances of age ≥ 65 years, diabetes mellitus, chronic obstructive pulmonary disease, dysphasia, and intraventricular expansion of hematoma, as well as dramatically higher NIHSS scores, hematoma volume, blood glucose levels, and serum Prdx2 levels (all P<0.05; Table 1). With the entry of the above nine significantly distinct variables into the binary logistic regression model, NIHSS scores [odds ratio (OR), 1.279; 95% confidence interval (CI), 1.104–1.481; P=0.001], hematoma volume (OR, 1.066; 95% CI, 1.013–1.122; P=0.015), and serum Prdx2 levels (OR, 1.030; 95% CI, 1.014–1.067; P=0.017) were independent predictors of poor prognosis at six months post-ICH. In addition, no significant interaction effects were found between serum Prdx2 levels and other traditional variables (all P >0.05; Table 4). Alternatively, serum Prdx2 levels effectively discriminated the likelihood of poor prognosis, and an appropriate criterion was identified using the Youden method, which was distinguishable from poor prognosis with medium-to-high sensitivity and specificity (Figure 7). Serum Prdx2 levels were linearly linked to the possibility of poor prognosis in the context of RCS assessment (P for nonlinear >0.05; Figure 8). In the ROC curve, the predictive ability of serum Prdx2 levels resembled that of hematoma volume and NIHSS scores (both P>0.05; Figure 9), and the predictive ability of serum Prdx2 levels combined with hematoma volume and NIHSS scores substantially surpassed that of serum Prdx2 levels, NIHSS scores, hematoma volume, and NIHSS scores combined with hematoma volume alone (all P<0.05; Figure 9).
 |
Table 4 Intersubgroup Interaction Analysis Between Serum Peroxiredoxin 2 Levels and 6-month Poor Prognosis After Acute Intracerebral Hemorrhage |
Serum Prdx2 Levels and SAP After ICH
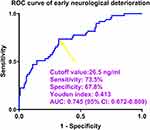
Among those 61 patients willing to supply blood-drawings at many time points, eighteen cases were subjected to SAP after ICH. As shown in Figure 10, serum Prdx2 levels at all prespecified time points were significantly higher in patients with SAP than in those without SAP (all P<0.05). As shown in Table 3, the area under the ROC curve of serum Prdx2 levels at admission was equivalent to those at the remaining six time-points of those 61 patients (all P>0.05). Of all 167 patients, 45 developed SAP. In contrast to patients without SAP, those with SAP had substantially higher opportunities for age ≥ 65 years, diabetes mellitus, dysphasia, and intraventricular hematoma expansion, as well as significantly higher NIHSS scores, hematoma volume, blood glucose levels, and serum Prdx2 levels (all P<0.05; Table 5). When the eight substantially different factors were included in the binary logistic regression model, NIHSS scores (OR, 1.217; 95% CI, 1.074–1.379; P=0.011), hematoma volume (OR, 1.085; 95% CI, 1.029–1.145; P=0.017), and serum Prdx2 levels (OR, 1.019; 95% CI, 1.004–1.035; P=0.022) were independently associated with the occurrence of SAP. In addition, serum Prdx2 levels did not significantly interact with the other conventional parameters (all P >0.05; Table 6). Alternatively, serum Prdx2 levels efficiently distinguished patients at risk of SAP, and by applying the Youden method, an optimal value was generated to efficiently predict the appearance of SAP post-ICH (Figure 11). A linear relationship was observed between serum Prdx2 levels and SAP probability in the background of RCS assessment (P for nonlinear >0.05; Figure 12). Within the framework of ROC curve analysis, the discrimination efficiency of serum Prdx2 levels was similar to that of hematoma volume and NIHSS scores (both P>0.05; Figure 13), and the predictive power of serum Prdx2 levels consolidated with hematoma volume and NIHSS scores profoundly exceeded that of serum Prdx2 levels, NIHSS scores, and hematoma volume alone (all P<0.05; Figure 13), but was not significantly higher than that of NIHSS scores combined with hematoma volume (P>0.05; Figure 13).
 |
Table 5 Factors in Relation to Stroke-Associated Pneumonia and Early Neurological Deterioration After Acute Intracerebral Hemorrhage |
 |
Table 6 Intersubgroup Interaction Analysis Between Serum Peroxiredoxin 2 Levels and Stroke-Associated Pneumonia After Acute Intracerebral Hemorrhage |
Serum Prdx2 Levels and END Following ICH
Of those 61 patients, who voluntarily continued to offer blood-samples at several time points, nineteen individuals had END after ICH. As outlined in Figure 14, serum Prdx2 levels at all predefined seven points were significantly higher in patients with END than in the remaining patients (all P<0.05). Table 3 shows that the area under the ROC curve of admission serum Prdx2 levels was equal to those at the remaining six time-points of those 61 patients (all P>0.05). Forty-nine of all 167 patients experienced END following ICH. Compared to patients without END, those presenting with END tended to have extremely elevated percentages of age ≥ 65 years, diabetes mellitus, and intraventricular hematoma expansion, as well as markedly increased NIHSS scores, hematoma volume, blood glucose levels, and serum Prdx2 levels (all P<0.05; Table 5). When the above-mentioned seven variables of significant disparity were added into the binary logistic regression model, NIHSS scores (OR, 1.203; 95% CI, 1.066–1.357; P=0.012), hematoma volume (OR, 1.076; 95% CI, 1.003–1.155; P=0.040) and serum Prdx2 levels (OR, 1.021; 95% CI, 1.006–1.037; P=0.017) remained independently related to END emergence. Statistically, serum Prdx2 levels showed no significant interaction with the other conventional variables (all P interactions >0.05; Table 7). Alternatively, serum Prdx2 levels powerfully differentiated END probability, and with the assistance of the Youden method, an applicable value was produced for the accurate identification of END occurring post-ICH (Figure 15). A linear relationship was found between serum Prdx2 levels and END possibility based on RCS assessment (P for nonlinearity >0.05; Figure 16). In the ROC curve analysis, the predictive power of serum Prdx2 levels was in the range of hematoma volume and NIHSS scores (both P>0.05; Figure 17), and the distinguishable performance of serum Prdx2 levels merged with hematoma volume and NIHSS scores was obviously higher than that of serum Prdx2 levels, NIHSS scores, and hematoma volume alone (all P<0.05; Figure 17), but was not substantially superior to that of NIHSS scores combined with hematoma volume (P>0.05; Figure 17).
 |
Table 7 Intersubgroup Interaction Analysis Between Serum Peroxiredoxin 2 Levels and Early Neurological Deterioration After Acute Intracerebral Hemorrhage |
Discussion
To the best of our knowledge, no data are available regarding blood Prdx2 levels in patients with brain injury, and we proceeded with the exploration of temporal variations in serum Prdx2 levels following ICH. To accomplish this clinical investigation, a fraction of patients were willing to draw blood at many time points after ICH. This portion of the patients, with analogous baseline features as all patients, held admission serum Prdx2 levels similar to all patients, signifying that this portion of patients could represent the whole group of current patients to some extent from a statistical perspective. Here, serum Prdx2 levels were evidently increased during the early phase after ICH, continued to ascend, reached a peak value, and thereafter slowly decreased until day 14 post-ICH, and were still substantially higher during 14 days than those of controls. These results offer scientific evidence to support the hypothesis that blood Prdx2 levels may be elevated in patients with ICH.
The protective or detrimental effects of Prdx2 principally rely on its intracellular or extracellular.17 Intracellular Prdx2 may display protective functions via antioxidation,18–20 whereas extracellular Prdx2 derived from the spillage of damaged cells may confer detrimental properties by facilitating inflammation.21–23 In the central nervous system, Prdx2 has become a therapeutic target for diseases such as cerebral infarction, traumatic brain injury, ICH, and Alzheimer’s disease.17 A succession of evidence has accumulated regarding the abundant expression of Prdx2 in neurons.24,25 Moreover, there has been a plethora of data showing that Prdx2 expression in neurons could be drastically increased in response to experimental brain injury attributable to trauma, hemorrhage, or ischemia.21–23 Prdx2 can be found in the cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage or traumatic brain injury, and its levels are decisively enhanced in such a state.26 Prdx2 can spill from damaged neurons secondary to brain injury.17 Clearly, blood brain barrier permeability is disrupted by acute ICH.32 Thus, it is possible that Prdx2 leakage into the peripheral blood system via the damaged blood-brain barrier may in part contribute to the elevation of blood Prdx2 levels subsequent to ICH.
Peripheral cells such as vascular and red blood cells are rich in Prdx2.10 Reportedly, Prdx2 may be released from erythrocyte lysate following aneurysmal subarachnoid hemorrhage.33 Systemic inflammatory response syndrome specifically mirrors the resultant systemic injury after ICH, in which the immune system becomes overactive, thereby incurring excessive release of cytokines.34–36 This type of anomalous response frequently leads to cell destruction, tissue damage, and organ dysfunction.34–36 Thus, it is reasonable to assume that a portion of Prdx2 in peripheral blood may originate from peripheral cells.
Extracellular Prdx2 acts as a damage-associated molecule, potentiating it as a ligand to promote macrophages and glial cells to generate a cascade of inflammatory cytokines.21–23 The molecular mechanisms underlying this condition may be related to the activation of the TLR4/NF-κB signaling pathway.21–23 Our finding of an intensive elevation in serum Prdx2 levels in response to acute ICH indicates whether spilling of Prdx2 from injured neurons or other peripheral cells or leakage of Prdx2 to the peripheral system from the central nervous system may strongly enhance the release of inflammatory mediators from glial cells or macrophages, thereby aggravating neuroinflammation and even systemic inflammation. Overall, Prdx2 may be a crucial component of the mechanisms involved in secondary brain injury following ICH.
The clinical application of the NIHSS score and hematoma volume in the assessment of ICH severity is well acknowledged.37–39 In the current study, baseline NIHSS scores and hematoma volume were strongly correlated with serum Prdx2 levels at all seven pre-specified time points in those 61 patients with ICH, and the two baseline indicators were highly related to admission serum Prdx2 levels in all 167 patients. Therefore, serum Prdx2 levels may reflect ICH severity. The mRS is an excellent metric of neurological functional status.40,41 The mRS scores at six months post-ICH were highly correlated with serum Prdx2 levels at all seven predefined time intervals in those 61 patients with ICH. Additionally, serum Prdx2 levels at admission significantly increased in the order of mRS scores. Thus, it is reasonable to assume that serum Prdx2 levels may mirror the clinical outcome of ICH.
In our study, a mRS scores of 3–6 indicated a poor prognosis. Poor prognosis, along with two other adverse effects of ICH, END and SAP, were selected as the three outcome variables of interest. Among those 61 patients, serum Prdx2 levels at admission possessed analogous predictive capability under the ROC curve for poor prognosis, END, and SAP as those on days 1, 3, 5, 7, 10, and 14 after ICH. Therefore, serum Prdx2 level at admission should be a powerful tool for predicting the clinical outcomes of ICH. Statistically, linear correlations were verified between admission serum Prdx2 levels and the preceding outcome variables in all 167 patients diagnosed with ICH. Furthermore, their independent associations were demonstrated with admission serum Prdx2 levels. Up to now, there has been a paucity of data available as regards the mechanisms of Prdx2’s relevance to SAP and END. However, occurrences of SAP and END are powerfully related to heightened inflammation,42,43 and Prdx2, upon spillage from damaged cells, can exert deleterious effects on cells via promoting inflammation.21–23 Thus, it is reasonably believed that links between Prdx2, SAP and END may be interpreted by inflammatory activation. Nevertheless, such a hypothesis should be confirmed in future.
Given that the NIHSS score and hematoma volume are the two strong prognostic determinants of ICH,37–39 they were considered as two comparative objects. Admission serum Prdx2 levels efficiently discriminated the risks of poor prognosis, END, and SAP as well as had similar predictive ability as NIHSS scores and hematoma volume. Alternatively, NIHSS scores and hematoma volumes were merged to form a combination model. In addition, these two approaches, together with serum Prdx2 levels at admission, were combined to build a consolidation model. By comparing the two models, it is possible that serum Prdx2 levels were more advantageous in predicting poor prognosis than in forecasting END and SAP. In summary, serum Prdx2 levels may confer high merits for predicting clinical outcomes after acute ICH.
This study has several strengths and disadvantages. The strengths are that (1) this may be the first series to discern the relationship between serum Prdx2 levels and clinical outcomes of acute brain injury diseases; subsequently, serum Prdx2 as a prognostic candidate of ICH was verified; and (2) for the sake of determining prognostic implications of serum Prdx2 in prognosticating clinical outcomes, three outcome variables, that is, SAP, END, and poor prognosis, were chosen together, all associations were verified by aidance of multivariate analysis, and ROC curve analysis and restricted cubic spline were applied. Therefore, this study is considered to hold acceptable conclusions. The disadvantages are as follows: (1) although a dynamic variation in serum Prdx2 levels was discovered here, the sample size of those 61 patients consenting for suppling blood samples at several time points may not be sufficient as expected; thus, increasing the patient number is a worthily recommended mode; and (2) a collective of 167 cases may be statistically fully accepted to be adequate for clinical analysis, but a larger cohort study is conventionally warranted to further validate the conclusions for generalization. Moreover, as reported in this study, SAP and END had relatively low incidence, so, comparatively speaking, the total patient number should be increased to obtain stronger statistical power.
Conclusions
There is a dynamic alteration in serum Prdx2 levels within 14 days after acute ICH, with the highest levels observed on day 3. Admission serum Prdx2 levels, in firm relation to NIHSS scores and hematoma volume, are independently predictive of post-ICH SAP, END, and six-month poor prognosis, with excellent predictive efficiency for worse clinical outcomes. In summary, serum Prdx2 may be a powerful link to ICH severity and adverse clinical outcomes, solidifying serum Prdx2 as a prognostic biomarker with good prospects during the management of ICH.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available because they are personal data, but are available from the corresponding author upon reasonable request.
Funding
This study was financially supported by a grant from the Zhejiang Province Medical and Health Science and Technology Plan Project (2023KY1280).
Disclosure
The authors declare no potential conflicts of interest in this work.
References
1. Sheth KN. Spontaneous intracerebral hemorrhage. N Engl J Med. 2022;387(17):1589–1596. doi:10.1056/NEJMra2201449
2. Magid-Bernstein J, Girard R, Polster S, et al. Cerebral hemorrhage: pathophysiology, treatment, and future Directions. Circ Res. 2022;130(8):1204–1229. doi:10.1161/CIRCRESAHA.121.319949
3. Thabet AM, Kottapally M, Hemphill JC 3rd. Management of intracerebral hemorrhage. Handb Clin Neurol. 2017;140:177–194. doi:10.1016/B978-0-444-63600-3.00011-8
4. Shu J, Wang W, Ye R, et al. Risk factors of prognosis for spontaneous cerebellar hemorrhage: a systematic review and meta-analysis. Acta Neurochir. 2024;166(1):291. doi:10.1007/s00701-024-06174-z
5. Amer HA, El-Jaafary SIM, Hmae S, Fouad AM, Mohammed SS. Clinical and paraclinical predictors of early neurological deterioration and poor outcome in spontaneous intracerebral hemorrhage. Egypt J Neurol Psychiatr Neurosurg. 2023;59(1):74. doi:10.1186/s41983-023-00675-x
6. Guo R, Yan S, Li Y, et al. A novel machine learning model for predicting stroke-associated pneumonia after spontaneous intracerebral hemorrhage. World Neurosurg. 2024;189:e141–e152. doi:10.1016/j.wneu.2024.06.001
7. Brea D, Sobrino T, Blanco M, et al. Temporal profile and clinical significance of serum neuron-specific enolase and S100 in ischemic and hemorrhagic stroke. Clin Chem Lab Med. 2009;47(12):1513–1518. doi:10.1515/CCLM.2009.337
8. Yan T, Wang ZF, Wu XY, et al. Plasma SIRT3 as a biomarker of severity and prognosis after acute intracerebral hemorrhage: a prospective cohort study. Neuropsychiatr Dis Treat. 2022;18:2199–2210. doi:10.2147/NDT.S376717
9. Wu X, Yan T, Wang Z, et al. Role of plasma Apo-J as a biomarker of severity and outcome after intracerebral hemorrhage: a prospective and cohort study. Clin Chim Acta. 2022;533:148–155. doi:10.1016/j.cca.2022.06.018
10. Balasubramanian P, Vijayarangam V, Deviparasakthi MKG, et al. Implications and progression of peroxiredoxin 2 (PRDX2) in various human diseases. Pathol Res Pract. 2024;254:155080. doi:10.1016/j.prp.2023.155080
11. Jeong SJ, Cho MJ, Ko NY, et al. Deficiency of peroxiredoxin 2 exacerbates angiotensin II-induced abdominal aortic aneurysm. Exp Mol Med. 2020;52(9):1587–1601. doi:10.1038/s12276-020-00498-3
12. Szabó-Taylor KÉ, Eggleton P, Turner CA, et al. Lymphocytes from rheumatoid arthritis patients have elevated levels of intracellular peroxiredoxin 2, and a greater frequency of cells with exofacial peroxiredoxin 2, compared with healthy human lymphocytes. Int J Biochem Cell Biol. 2012;44(8):1223–1231. doi:10.1016/j.biocel.2012.04.016
13. Aki T, Unuma K, Uemura K. The Role of peroxiredoxins in the regulation of sepsis. Antioxidants. 2022;11(1):126. doi:10.3390/antiox11010126
14. Shichita T, Hasegawa E, Kimura A, et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med. 2012;18(6):911–917. doi:10.1038/nm.2749
15. Salzano S, Checconi P, Hanschmann EM, et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc Natl Acad Sci U S A. 2014;111(33):12157–12162. doi:10.1073/pnas.1401712111
16. Garcia-Bonilla L, Iadecola C. Peroxiredoxin sets the brain on fire after stroke. Nat Med. 2012;18(6):858–859. doi:10.1038/nm.2797
17. Liu J, Su G, Gao J, Tian Y, Liu X, Zhang Z. Effects of peroxiredoxin 2 in neurological disorders: a review of its molecular mechanisms. Neurochem Res. 2020;45(4):720–730. doi:10.1007/s11064-020-02971-x
18. Gan Y, Ji X, Hu X, et al. Transgenic overexpression of peroxiredoxin-2 attenuates ischemic neuronal injury via suppression of a redox-sensitive pro-death signaling pathway. Antioxid Redox Signal. 2012;17(5):719–732. doi:10.1089/ars.2011.4298
19. Lu Y, Zhang XS, Zhou XM, et al. Peroxiredoxin 1/2 protects brain against H2O2-induced apoptosis after subarachnoid hemorrhage. FASEB J. 2019;33(2):3051–3062. doi:10.1096/fj.201801150R
20. Kim SH, Fountoulakis M, Cairns N, Lubec G. Protein levels of human peroxiredoxin subtypes in brains of patients with Alzheimer’s disease and Down syndrome. J Neural Transm Suppl. 2001;61:223–235. doi:10.1007/978-3-7091-6262-0_18
21. Bian L, Zhang J, Wang M, Keep RF, Xi G, Hua Y. Intracerebral hemorrhage-induced brain injury in rats: the role of extracellular peroxiredoxin 2. Transl Stroke Res. 2020;11(2):288–295. doi:10.1007/s12975-019-00714-x
22. Du Y, Wang J, Zhang J, et al. Intracerebral hemorrhage-induced brain injury in mice: the role of peroxiredoxin 2-Toll-like receptor 4 inflammatory axis. CNS Neurosci Ther. 2024;30(3):e14681. doi:10.1111/cns.14681
23. Lu Y, Zhang XS, Zhang ZH, et al. Peroxiredoxin 2 activates microglia by interacting with Toll-like receptor 4 after subarachnoid hemorrhage. J Neuroinflamm. 2018;15(1):87. doi:10.1186/s12974-018-1118-4
24. Sunico CR, Sultan A, Nakamura T, et al. Role of sulfiredoxin as a peroxiredoxin-2 denitrosylase in human iPSC-derived dopaminergic neurons. Proc Natl Acad Sci U S A. 2016;113(47):E7564–E7571. doi:10.1073/pnas.1608784113
25. Goemaere J, Knoops B. Peroxiredoxin distribution in the mouse brain with emphasis on neuronal populations affected in neurodegenerative disorders. J Comp Neurol. 2012;520(2):258–280. doi:10.1002/cne.22689
26. De C Jr, Chaitanya GV, Chittiboina P, et al. Variations in the cerebrospinal fluid proteome following traumatic brain injury and subarachnoid hemorrhage. Pathophysiology. 2017;24(3):169–183. doi:10.1016/j.pathophys.2017.04.003
27. Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304–1305. doi:10.1161/01.str.27.8.1304
28. Kwah LK, Diong J. National Institutes of Health Stroke Scale (NIHSS). J Physiother. 2014;60(1):61. doi:10.1016/j.jphys.2013.12.012
29. Zhu W, Zhou J, Ma B, Fan C. Predictors of early neurological deterioration in patients with intracerebral hemorrhage: a systematic review and meta-analysis. J Neurol. 2024;271(6):2980–2991. doi:10.1007/s00415-024-12230-6
30. Smith CJ, Kishore AK, Vail A, et al. Diagnosis of stroke-associated pneumonia: recommendations from the Pneumonia in Stroke Consensus Group. Stroke. 2015;46(8):2335–2340. doi:10.1161/STROKEAHA.115.009617
31. Hanley DF, Thompson RE, Rosenblum M, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint Phase 3 trial. Lancet. 2019;393(10175):1021–1032. doi:10.1016/S0140-6736(19)30195-3
32. Carhuapoma L, Murthy S, Shah VA. Outcome trajectories after intracerebral hemorrhage. Semin Neurol. 2024;44:298–307. doi:10.1055/s-0044-1787104
33. Zhang ZH, Han YL, Wang CX, et al. The effect of subarachnoid erythrocyte lysate on brain injury: a preliminary study. Biosci Rep. 2016;36(4):e00359. doi:10.1042/BSR20160100
34. Boehme AK, Hays AN, Kicielinski KP, et al. Systemic inflammatory response syndrome and outcomes in intracerebral hemorrhage. Neurocrit Care. 2016;25(1):133–140. doi:10.1007/s12028-016-0255-9
35. Tapia-Pérez JH, Karagianis D, Zilke R, Koufuglou V, Bondar I, Schneider T. Assessment of systemic cellular inflammatory response after spontaneous intracerebral hemorrhage. Clin Neurol Neurosurg. 2016;150:72–79. doi:10.1016/j.clineuro.2016.07.010
36. Boehme AK, Comeau ME, Langefeld CD, et al. Systemic inflammatory response syndrome, infection, and outcome in intracerebral hemorrhage. Neurol Neuroimmunol Neuroinflamm. 2017;5(2):e428. doi:10.1212/NXI.0000000000000428
37. Khan OUR, Farooqi HA, Nabi R, Hasan H. Advancements in prognostic markers and predictive models for intracerebral hemorrhage: from serum biomarkers to artificial intelligence models. Neurosurg Rev. 2024;47(1):382. doi:10.1007/s10143-024-02635-2
38. Takahashi J, Sakai K, Sato T, et al. Serum arachidonic acid levels is a predictor of poor functional outcome in acute intracerebral hemorrhage. Clin Biochem. 2021;98:42–47. doi:10.1016/j.clinbiochem.2021.09.012
39. Hviid CVB, Gyldenholm T, Lauridsen SV, Hjort N, Hvas AM, Parkner T. Plasma neurofilament light chain is associated with mortality after spontaneous intracerebral hemorrhage. Clin Chem Lab Med. 2020;58(2):261–267. doi:10.1515/cclm-2019-0532
40. Baker WL, Sharma M, Cohen A, et al. Using 30-day modified rankin scale score to predict 90-day score in patients with intracranial hemorrhage: derivation and validation of prediction model. PLoS One. 2024;19(5):e0303757. doi:10.1371/journal.pone.0303757
41. Geng Z, Yang C, Zhao Z, et al. Development and validation of a machine learning-based predictive model for assessing the 90-day prognostic outcome of patients with spontaneous intracerebral hemorrhage. J Transl Med. 2024;22(1):236. doi:10.1186/s12967-024-04896-3
42. Zhao G, Chen Y, Gu Y, Xia X. The clinical value of nutritional and inflammatory indicators in predicting pneumonia among patients with intracerebral hemorrhage. Sci Rep. 2024;14(1):16171. doi:10.1038/s41598-024-67227-y
43. Leira R, Dávalos A, Silva Y, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63(3):461–467. doi:10.1212/01.wnl.0000133204.81153.ac
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.