Back to Journals » Research Reports in Clinical Cardiology » Volume 16
Flow Mediated Dilation and Its Correlation with Coronary and Systemic Atherosclerosis in Patients Scheduled for Surgical Myocardial Revascularization
Authors Boieriu AM, Luca CD, Tint D
Received 28 July 2024
Accepted for publication 12 January 2025
Published 4 February 2025 Volume 2025:16 Pages 1—7
DOI https://doi.org/10.2147/RRCC.S489002
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Kones
Alexandra Maria Boieriu,1,2 Cezar Dumitrel Luca,1,3 Diana Tint1,4
1Faculty of Medicine, “Transylvania” University, Braşov, Romania; 2Cardiology Department, County Emergency Hospital, Braşov, Romania; 3Cardiology Department, Cardiovascular Rehabilitation Hospital, Covasna, Romania; 4Cardiology Department, ICCO Clinics, Braşov, Romania
Correspondence: Alexandra Maria Boieriu, Faculty of Medicine, “Transylvania” University, Eroilorst, Nr 29, Braşov, 500036, Romania, Email [email protected]
Purpose: The study aimed to evaluate endothelial dysfunction (ED), assessed through flow mediated dilation (FMD), in individuals with severe coronary artery disease (CAD) scheduled for coronary artery bypass graft surgery (CABG). Additionally, we examined the association between the severity of EDevaluated throughFMD and the extent of atherosclerosis at the coronary arteries level (SYNTAX I score), carotid arteries and peripheral arteries.
Methods: The study included 84 participants recruited between January 2020 and June 2021. Doppler ultrasonography was used to assess both FMD and the degree of carotid artery stenosis. SYNTAX I score was calculated based on coronary angiography, and peripheral artery disease (PAD) was evaluated using computed tomography.
Results: FMD was used as a surrogate for assessing ED. We found no statistical difference between men and women [4.46 (1.03– 6.75) in women versus 2.55 (0.95– 6.13 in men; p=0.21). A significant association was observed between FMD and SYNTAX score (p < 0.001). A lower FMD corresponded to a higher SYNTAX score, reflecting an extensive burden of coronary atherosclerosis. Furthermore, ED, as measured by FMD, was indicative of carotid atherosclerosis and PAD, as evidenced by lower FMD in patients with severe carotid plaques (p < 0.001) and severe PAD (p < 0.01).
Conclusion: FMD might be a reliable tool to assess systemic atherosclerosis, ED in the brachial artery being related to carotid artery plaque, CAD and peripheral lower limb ischemia.
Keywords: flow mediated dilation, SYNTAX I score, carotid stenosis, peripheral artery disease, coronary artery by-pass graft, coronary artery disease
Introduction
Endothelial dysfunction (ED)encompasses various aspects, ranging from vascular tonus alteration [reduced nitric oxide (NO) vasodilator response] to proinflammatory and prothrombotic status, reduced antioxidant activity, excess synthesis of adhesion molecules and a pro-proliferative status in smooth vascular muscle cells. ED was identified in all stages of ischemic cardiomyopathy: it is present before the formation of atherosclerotic plaque and persists throughout the disease, including acute coronary syndromes.1 The impairment of ED is implicated in the origin and development of atherosclerotic cardiovascular disease (CVD).2
ED emerges early in the progression of cardiovascular disease. Previous large-scale studies involving healthy populations with cardiovascular risk factors have shown that certain individual risk factors are associated with reduced FMD (flow-mediated dilation).3,4 Endothelial function is especially altered in subjects diagnosed with diabetes mellitus.5,6
Previous research established significant correlations between FMD, as a tool for ED assessment, and the severity of CAD (based on the SYNTAX score).7,8 Furthermore, FMD appears to be associated with carotid artery atherosclerosis9,10 and PAD.11
The aim of the present study was to assess ED, as measured by FMD, in patients with severe CAD undergoing CABG. We also assessed the severity of CAD using the SYNTAX I score and conducted screenings for carotid artery plaque and PAD. Finally, we tested the hypothesis that FMD was associated with CAD and systemic atherosclerotic burden (as identified in the carotid and lower limb territories).
Material and Methods
We prospectively included 84 patients with chronic coronary disease and severe coronary lesions requiring CABG, between January 2020 and June 2021. The study protocol obtained ethical clearance from the Ethical Committee of Transylvania University and adhered to the principles outlined in the Helsinki Declaration and the Code for Good Clinical Practice. A written Informed Consent was obtained from all patients.
Patients with acute coronary syndromes, subclavian artery stenosis, associated significant valvular disease, severe hepatic/renal failure, recent/active bleeding, coagulation disorders, and active malignancy were excluded from the study.
Hypertension was defined as systolic blood pressure values greater than 140/90 mmHg according to the 2018 European Society of Cardiology (ESC)/European Society of Hypertension (ESH) guidelines.12 The criteria for diagnosing diabetes included a fasting blood glucose level >7.0 mmol/L, a 2-hour plasma glucose value >11.1 mmol/L during a 75-g oral glucose tolerance test, glycated hemoglobin (HbA1c) >6.5%, or, in patients with classic symptoms of hyperglycemia or a hyperglycemic crisis, a random plasma glucose >11.1 mmol/L.13 For ease of analysis, we included patients with a total plasma cholesterol level>5.2 mmol/l or high-density lipoprotein plasma concentration <1 mmol/l in the dyslipidemia group.14
To diagnose CAD and evaluate its severity according to the SYNTAX I score, coronary angiogram was performed. The SYNTAX I score was determined using the number of diseased arteries, the location, and the aspect of atherosclerotic plaques (https://syntaxscore.org/). Carotid anatomy was evaluated using Doppler ultrasonography. For practical purposes, we documented the stenosis of the internal carotid artery. Carotid artery stenosis was defined as mild (<50%), moderate (50–69%), and severe (70–99%).15
PAD was diagnosed based on symptoms, clinical signs, Doppler ultrasonography, and its severity was evaluated using computed tomography angiography. PAD was classified in 6 groups (0-no PAD; 1-mild stenosis <25%; 2-moderate stenosis 25–50%; 3-moderate to severe stenosis 50–75%; 4-critical stenosis 75–99%; 5-occlusion) based on computed tomography angiography.16,17
Prior to the FMD measurement, the subjects had to fast for at least six hours, refrain from physical exertion, and not consume caffeine or smoke for the previous twenty-four hours.18–22 For a minimum of ten minutes, patients were kept in a quiet room in the supine posture. The vascular linear probe operating in 2D mode at 7.5–12 MHz was used to measure FMD. To create reactive hyperemia, the occlusion cuff was wrapped around the forearm and inflated for five minutes to a pressure 50 mmHg higher than the systolic blood pressure. During reactive hyperemia, the diameter change was continuously assessed. The diameter of the brachial artery was measured basally (before the cuff was inflated), 3 minutes after the cuff was deflated, 60–90 seconds after maximal reactive hyperemia, and 3–10 cm above the antecubital fossa. FMD was calculated as percentage index according to current guidelines.23 Flow-mediated dilation is usually expressed as the percentage increase in post cuff-release vessel diameter relative to the baseline diameter. There is ongoing discussion on the normal reference levels. In accordance with recent research, we considered a value <6.5% as indicative for ED.24
Statistics
Data were analyzed using Microsoft Office Excel 2019 and JASP 0.19 software. Patient demographics and characteristics were compared using the T-test, Chi-square and Mann–Whitney U-tests. To ascertain correlations in the analyzed data, 2-tailed Pearson (for normally distributed variables), Spearman (for skewed distribution) and one way ANOVA tests were employed, with statistical significance set at p <0.05.
Results
We included patients aged 46–88 years, 79.76% of the studied population being male. The mean age was 64.25 ± 7.24 for males and 68.41 ± 10.44 for females. The studied population was composed of overweight patient, with a mean BMI (body mass index) of 28.07 kg/m2 for males and 29.48 kg/m2 for females. Arterial hypertension and dyslipidemia were the most common cardiovascular risk factors identified. Smoking was reported in 22.38% of males and 11.76% of females, while type 2 diabetes mellitus was present in 38.8% of males and 41.17% of females. Clinical characteristics of the subjects, based on their gender, are displayed in Table 1.
 |
Table 1 Baseline characteristics of the subjects according to gender |
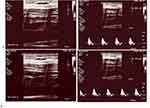
An example of FMD measurement is presented in Figure 1 (Figure 1a: the basal diameter and velocity of the brachial artery and Figure 1b: peak diameter and velocity of the brachial artery measured during maximal reactive hyperemia).
We observed a significant correlation between FMD and SYNTAXI score (r=0.898; p<0.001), indicating that a low FMD corresponds to severe coronary atherosclerosis (Figure 2).
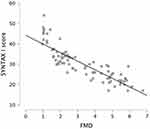 |
Figure 2 The correlation between SYNTAX score and FMD. |
To evaluate the correlation between FMD and the presence and severity of carotid atherosclerotic disease, patients were divided into three groups according to the severity of carotid artery stenosis assessed using carotid ultrasound examination: group 1 [20 patients, 23.81% with mild carotid artery stenosis (<50%)], group 2[34 patients,40.47% with moderate carotid artery stenosis (50–69%)], and group 3[30 patients,35.71% included patients with severe carotid artery atherosclerosis (70–99%)]. Figure 3 depicts the relation between FMD and the severity of carotid atheroma.
 |
Figure 3 FMD and the severity of carotid artery stenosis (p<0.001; p value according to One-Way ANOVA). |
Lower limb PAD was diagnosed in 11 (16.41%) of the male population. The female population included in the study did not have associated PAD. PAD was classified in 6 groups (0-no PAD; 1-mild stenosis <25%; 2-moderate stenosis 25–50%; 3-moderate to severe stenosis 50–75%; 4-critical stenosis, 75–99%; 5-occlusion) based on computed tomography angiography. Out of the 84 patients enrolled 73 did not have associated PAD, 4 patients had moderate lower limb PAD (25–50%), 4 patients had moderate-to-severe PAD (50–75%), and 3 patients associated critical stenosis (75–99%) in the lower limbs. The difference between mean FMD for patients with PAD and those without diagnosed PAD was statistically significant at a p value of 0.001. The relation between FMD and PAD is presented in Figure 4.
 |
Figure 4 The relation between FMD and PAD (p<0.001; p value according to One-Way ANOVA). |
Discussion
There were no statistically significant differences between males and females in terms of the clinical characteristics of the studied population (p values are mentioned in Table 1). Both groups exhibited FMD values below the 6.5% (the median was 2.55% in the male group and 4.46% in the female group), which represents the cutoff identified in previous studies as a marker for acute cardiovascular events.23,24 Although the median for FMD in males was lower than in females (2.55% versus 4.46%), the result was not statistically significant (p=0.214). As the study involved mainly postmenopausal women (mean age 68.41±10.44 years), our result is in accordance with previous research showing similar FMD values in older men and women.25
Furthermore, FMD values correlated with the SYNTAX I score (r=0.898; p<0.001), underlining that vasodilator response is diminished in patients with severe CAD.Previous trials have proven that FMD and SYNTAX I score are associated in patients with a low cardiovascular risk, without established coronary artery disease or vascular atherosclerotic disease.7,8,26,27 However, little research was carried out on high-risk patients. To our knowledge, the present study is the first to measure brachial FMD in patients with severe CAD scheduled for CABG, and to prove that an important association persists between FMD values and SYNTAX score.
Endothelial dysfunction assessed by FMD is also associated with the presence and severity of carotid plaques. Our study showed that a low FMD value, indicating severe ED, was correlated with severe atherosclerosis in the carotid territory, as well.9,28
To our knowledge, FMD measurement is less commonly used in the field of vascular surgery. However, it has proven to be an important tool for evaluating endothelial function in lower limb artery disease.11 Our study found that a low brachial FMD was associated with severe PAD.
Advancements in the standardization of FMD methodology and analysis have made it a valuable tool for evaluating therapeutic interventions. FMD assessment could also be a useful tool for evaluating ED in patients undergoing CABG in which procedures are conducted to improve endothelial function (for example: antioxidant medication; endogenous release of nitric oxide after ischemic preconditioning). As it is a non-invasive method, FMD can be used multiple times, with virtually no side effects, and it might indicate the effect of experimental therapies earlier, guiding the next steps of researchers (proceeding/discontinuing the study).
Several limitations need mentioning. First, FMD measurement is affected by multiple factors, some of them being corrected before the investigation was carried out (subjects had to fast for at least six hours, refrain from physical exertion, not consume caffeine or smoke for the previous twenty-four hours, maintaining supine posture for at least 10 minutes in a quiet room prior to the examination). A limitation of this study is the small sample size of patients diagnosed with PAD, as only 11 patients were identified with associated lower limb atherosclerosis based on the criteria used. This limited number of PAD cases may affect the findings related to lower limb artery disease and its relationship with endothelial function. Larger studies on PAD patients are needed to confirm and expand upon these results. Further research is needed to validate the results obtained for this category.
Conclusion
First, in patients with severe coronary artery disease scheduled for CABG surgery, endothelial dysfunction, estimated by FMD, is impaired. Second, FMD values correlated with SYNTAX I score, carotid artery plaque and PAD, reflecting the burden of systemic atherosclerosis.
Disclosure
The author(s) report no conflicts of interest in this work.
References
1. Gutierrez E, Flammer AJ, Lerman LO, et al. Endothelial dysfunction over the course of coronary artery disease. Eur Heart J. 2013;34(41):3175–3181. doi:10.1093/eurheartj/eht351
2. Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi:10.1056/NEJM199901143400207
3. Yang PT, Yuan H, Wang YQ, et al. Correlations between brachial endothelial function and cardiovascular risk factors: a survey of 2,511 Chinese subjects. J Thorac Dis. 2014;6(10):1441–1451. doi:10.3978/j.issn.2072-1439.2014.08.04
4. Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24(6):1468–1474. doi:10.1016/0735-1097(94)90141-4
5. Dubsky M, Veleba J, Sojakova D, et al. Endothelial Dysfunction in Diabetes Mellitus: new Insights. Int J mol Sci. 2023;24(13):10705. doi:10.3390/ijms241310705
6. Cutruzzolà A, Parise M, Scavelli FB, et al. Time in Range Does Not Associate With Carotid Artery Wall Thickness and Endothelial Function in Type 1 Diabetes. J Diabetes Sci Technol. 2022;16(4):904–911. doi:10.1177/1932296821993178
7. Sancheti S, Shah P, Phalgune DS. Correlation of endothelial dysfunction measured by flow-mediated vasodilatation to severity of coronary artery disease. Indian Heart J. 2018;70(5):622–626. doi:10.1016/j.ihj.2018.01.008
8. Manganaro A, Ciracì L, Andrè L, et al. Endothelial Dysfunction in Patients With Coronary Artery Disease: insights From a Flow-Mediated Dilation Study. Clin App ThrombHemost. 2014;20(6):583–588. doi:10.1177/1076029614524620
9. Rundek T, Hundle R, Ratchford E, et al. Endothelial dysfunction is associated with carotid plaque: a cross-sectional study from the population based Northern Manhattan Study. BMC Cardiovasc Disord. 2006;6:35. doi:10.1186/1471-2261-6-35
10. Mahdavi-Roshan M, Salari A, Nasrollah ZJ, Doostdar-Sanaye M. Brachial endothelial function and carotid intima-media thickness in patients with coronary artery disease. Arch Adv Biosci. 2015;6(4):15–19. doi:10.22037/jps.v6i4.10622
11. Bellamkonda K, Williams M, Handa A, Lee R. Flow Mediated Dilatation as a Biomarker in Vascular Surgery Research. J Atheroscler Thromb. 2017;24:779–787.
12. Williams B, Mancia G, Spiering W, et al. ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. Erratum in: Eur Heart J. 2019; 40(5):475. doi: 10.1093/eurheartj/ehy686. doi:10.1093/eurheartj/ehy339.
13. Marx N, Federici M, Schütt K, et al. ESC Scientific Document Group, 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes: developed by the task force on the management of cardiovascular disease in patients with diabetes of the European Society of Cardiology (ESC. Eur Heart J. 2023;44(39):4043–4140. doi:10.1093/eurheartj/ehad192
14. Mach F, Baigent C, Catapano AL, et al. ESC Scientific Document Group, 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41(1):111–188. doi:10.1093/eurheartj/ehz455
15. NASCET Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325(7):445–453. doi:10.1056/NEJM199108153250701
16. Ofer A, Nitecki SS, Linn S, et al. Multidetector CT Angiography of Peripheral Vascular Disease: aProspective Comparison with Intraarterial Digital SubtractionAngiography. AJR. 2003;180(3):719–7240361–803X/03/1803–719. doi:10.2214/ajr.180.3.1800719
17. Met R, Bipat S, Legemate DA, Reekers JA, Koelemay MJ. Diagnostic performance of computed tomography angiography in peripheral arterial disease: a systematic review and meta-analysis. JAMA. 2009;301(4):415–424. doi:10.1001/jama.301.4.415
18. Thijssen DHJ, Bruno R, van Mil ACCM, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. 2019;40(30):2534–2547. doi:10.1093/eurheartj/ehz350
19. Shechter M, Shalmon G, Scheinowitz M, et al. Impact of acute caffeine ingestion on endothelial function in subjects with and without coronary artery disease. Am J Cardiol. 2011;107(9):1255–1261. doi:10.1016/j.amjcard.2010.12.035
20. Bau PFD, Bau CHD, Naujorks AA, Rosito GA. Early and late effects of alcohol ingestion on blood pressure and endothelial function. Alcohol. 2005;37(1):53–58. doi:10.1016/j.alcohol.2005.10.034
21. Dawson EA, Green DJ, Cable NT, Thijssen DHJ. Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol. 2013;115(11):1589–1598. doi:10.1152/japplphysiol.00450.2013
22. Ghiadoni L, Donald AE, Cropley M, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102(20):2473–2478. doi:10.1161/01.CIR.102.20.2473
23. Maruhashi T, Kajikawa M, Kishimoto S, et al. Diagnostic criteria of flow-mediated vasodilation for normal endothelial function and nitroglycerin-induced vasodilation for normal vascular smooth muscle function of the brachial artery. J Am Heart Assoc. 2020;9(2):e013915. doi:10.1161/JAHA.119.013915
24. Heiss C, Rodriguez-Mateos A, Bapir M, Ss S, Sies H, Kelm M. Flow-mediated dilation reference values for evaluation of endothelial function and cardiovascular health. Cardiovasc Res. 2023;119(1):283–293. doi:10.1093/cvr/cvac095
25. Holder SM, Brislane A, Dawson EA, et al. Relationship Between Endothelial Function and the Eliciting Shear Stress Stimulus in Women: changes Across the Lifespan Differ to Men. J Am Heart Assoc. 2019;8(4):e010994. doi:10.1161/JAHA.118.010994
26. Broxterman RM, Witman MA, Trinity JD, et al. Strong Relationship Between Vascular Function in the Coronary and Brachial Arteries. Hypertension. 2019;74(1):208–215. doi:10.1161/HYPERTENSIONAHA.119.12881
27. Maruhashi T, Soga J, Fujimura N, et al. Relationship between flow-mediated vasodilation and cardiovascular risk factors in a large community-based study. Heart. 2013;99(24):1837–1842. doi:10.1136/heartjnl-2013-304739
28. Shirokane K, Tamaki T, Kim K, Tsuchiya M, Yamazaki M, Morita A. Relationship between Flow-mediated Endothelial Vasodilation and the Pulse Wave Velocity, and Cervical Carotid Artery Stenosis. Neurol Med Chir. 2020;60(6):293–298. doi:10.2176/nmc.oa.2019-0193
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.