Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Frequency and Sex-Specific Associations of Metabolic Syndrome in Rwandans Seeking Outpatient Care: A Cross Sectional, Single Centre Study
Authors Gafirita J , Musarurwa C , Ntaganda E, Uwimana M, Hirwa AD, Mukahigiro M, Twizelimana L, Nshimirimana ML, Rulisa S , Bavuma C, Ivan E, Tumusiime DK
Received 9 May 2024
Accepted for publication 12 October 2024
Published 17 October 2024 Volume 2024:17 Pages 3803—3816
DOI https://doi.org/10.2147/DMSO.S477481
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Rebecca Conway
James Gafirita,1,* Cuthbert Musarurwa,1,* Evariste Ntaganda,2,* Marguerite Uwimana,3,* Aime Dieudonne Hirwa,4,* Mediatrice Mukahigiro,5,* Laetitia Twizelimana,6,* Marie Louise Nshimirimana,6,* Stephen Rulisa,7,8 Charlotte Bavuma,8,9,* Emile Ivan,10 David K Tumusiime11,*
1Department of Biomedical Laboratory Sciences College of Medicine and Health Sciences, University of Rwanda, Kigali, Rwanda; 2Division of Non-Communicable Diseases, Rwanda Biomedical Center (RBC), Ministry of Health, Kigali, Rwanda; 3Department of NCD, Ruhengeri Provincial Hospital, Musanze, Northern Province, Rwanda; 4Department of Surgery, Ruhengeri Provincial Hospital, Musanze, Northern Province, Rwanda; 5Department of Laboratory Diagnosis, Ruhengeri Provincial Hospital, Musanze, Northern Province, Rwanda; 6Department of Internal Medicine, Ruhengeri Provincial Hospital, Musanze, Northern Province, Rwanda; 7Department of Obstetrics and Gynecology, Kigali University Teaching Hospital, Kigali, Rwanda; 8Department of Obstetrics and Gynecology School of Medicine and Pharmacy, College of Medicine and Health Science, University of Rwanda, Kigali, Rwanda; 9Department of Internal Medicine, Kigali University Teaching Hospital, Kigali, Rwanda; 10Department of Drugs Assessment and Registration, Division of Human Medicine and Device Registration, Rwanda Food and Drugs Authority, Kigali, Rwanda; 11Department of Physiotherapy School of Health Sciences, College of Medicine and Health Sciences, University of Rwanda, Kigali, Rwanda
*These authors contributed equally to this work
Correspondence: James Gafirita, Email [email protected]
Background: The prevalence of cardiometabolic diseases is escalating in sub-Saharan Africa (SSA) alongside the prevailing high burden of communicable diseases. Although many countries in SSA, including Rwanda, have existing data on the prevalence of individual components of the MetS, many SSA countries have insufficient data to guide policy makers on the magnitude of MetS. This study sought to determine the magnitude of MetS and its associated risk factors by sex at a referral teaching hospital in Rwanda.
Methods: A cross-sectional, study was conducted among adults aged 35 to 65 years presenting at Ruhengeri Referral Teaching Hospital, Rwanda. We collected socio-clinicodemographic data using the World Health Organization (WHO) STEPwise tool for non-communicable diseases. We used the National Cholesterol Education Program Adult Treatment Panel III criteria for MetS.
Results: Overall, 99 (23.5%) males and 322 (76.5%) female participants with mean ± SD age 47.5 ± 8.2 years were enrolled. The overall frequency of MetS was 51.9% (95% CI: 47.0– 56.8) and was significantly higher (p < 0.001) in females 193 (59.4%) compared to males 26 (26.3%). Significant differences by sex were also noted in the proportions of visceral obesity; 70.4% vs 7.1% (p < 0.001), hypoalphalipoproteinaemia 36.1% vs 69.7% (p < 0.0001), type 2 diabetes mellitus; 18.4% vs 31.6% (p = 0.020) and body mass index 25.9 ± 15.6 vs 28.2 ± 6.4 (p = 0.032). On multivariate logistic regression, older age (odds ratio (OR) 1.05; 95% confidence interval ((CI) 1.01– 1.10)), higher body weight (OR 1.06; 95% CI 1.04– 1.08) and higher total cholesterol (1.25; 95% CI 1.05 − 1.74) were significantly associated with MetS in females; whereas only higher body weight (OR1.10; 95% CI 1.04– 1.18) was significantly associated with MetS in males.
Conclusion: A high frequency of MetS was observed in the present study, which was higher among females. Our findings emphasize the need for tailored prevention and intervention strategies to mitigate the long-term impact of MetS.
Keywords: frequency, metabolic syndrome, non-communicable diseases, Rwanda
Background
The metabolic syndrome (MetS) first reported by Reaven in 1988 as syndrome X is a cluster of five risk factors that increase the probability of developing type 2 diabetes mellitus (T2D) and cardiovascular diseases (CVD).1,2 Cardiovascular diseases are a major cause of death globally,3 with premature deaths globally being increasingly associated with cardiometabolic diseases in young adults.4 Several studies have reported MetS to be highly prevalent in many countries in Africa, although there is a paucity of specific estimates of MetS prevalence in African populations.5 The prevalence of MetS often mirrors the prevalence of T2D and obesity. Obesity is, however, no longer exclusively associated with affluence and its prevalence is reported to be on the increase even in poorly resourced countries.6
The World Health Organization (WHO) broadly stratifies non-communicable diseases (NCDs) risk factors into behavioral and metabolic risk factors.7 Behavioral risk factors consist of physical inactivity, excessive use of alcohol, inappropriate dietary habits, and use of tobacco. Metabolic risk factors include overweight and obesity, hypertension (HT), fasting hyperglycemia, and dyslipidemia.8 The escalation in the prevalence of NCDs has been associated with globalization, urbanization, and socioeconomic transformations that have led to widespread changes in population level NCD risk factor profiles.8,9
The 2018 WHO-NCD country profile reported that NCDs were estimated to account for ~44% of all deaths in Rwanda, of which 14% were CVD’s.10 Previous studies on NCDs in Rwanda have primarily focused only on determining the prevalence of individual MetS components. A study by Ntaganda et al (2022) reported a high prevalence of undiagnosed and uncontrolled hypertension in rural Rwanda,11 and a study that determined the prevalence of central obesity and its association with cardiovascular risk factors among women of reproductive age reported a prevalence of 48.5%.12 Another study conducted on rural and urban dwelling Rwandans aged 15 to 64 years reported that 1:6 Rwandans had hyperglycemia,13 while a cross-sectional secondary data analysis by Mukabutera et al (2016) reported an increasing prevalence of overweight and obesity among Rwandan women, with the rise more pronounced in women living urban areas.14 Despite the increasing burden of NCDs in SSA and Rwanda in particular, no study has holistically explored the prevalence of MetS in the Rwandan population. Furthermore, understanding the prevalence of the MetS and possible gender disparities in prevalence in this low-income setting can provide insights into unique risk factors and health behaviors that predispose to NCDs thus allowing the development of gender-specific health promotion and disease prevention strategies. The current study therefore determined the prevalence of MetS in a diverse Rwandan population and explored sex differences in the clustering of MetS components.
Methods
Study Design and Setting
A single centre-based cross-sectional study was conducted at the Outpatient Department of the Ruhengeri Referral Teaching Hospital, Musanze District, Northern Province, in Rwanda. Data collection commenced in February 2023 and ended in April 2023. The hospital is estimated to serve a population of 400 000, from 18 health centers and has a bed capacity of 400.
Study Population
The study design leveraged on the Rwandan Ministry of Health policy on NCDs screening that targets members of the population aged 35 years and above. Male and female participants attending the Outpatient Department at Ruhengeri Referral Teaching Hospital were recruited. A minimum sample size of 385 participants was calculated using Cochran’s formula for cross-sectional studies based on an assumed prevalence of 50%, precision of 5% and 95% confidence interval. The sample size was inflated by a factor of 10% to cater for possible missing data. Thus, a minimum sample size of 422 participants was required.
Inclusion and Exclusion Criteria
Consenting participants who had fasted for 10–14 hours and were aged 35–65 years were eligible for recruitment. We excluded participants below 35 and above 65 years of age, pregnant or lactating women, the severely ill, those attending the renal outpatient clinic and those who failed to give written informed consent.
Data Collection Procedure
Data collection adopted the WHO stepwise approach to NCD surveillance comprising three steps. The first step involved completion of an interviewer-assisted questionnaire to collect information on sociodemographic characteristics including marital status, level of education, possession of health insurance, and occupation.15 Lifestyle assessment was also conducted based on determining key modifiable risk factors of NCDs that included tobacco use, alcohol consumption, fruits and vegetable consumption, frequency of physical activity, and history of pre-existing NCDs such as high blood pressure and diabetes mellitus (DM). Diagnosis of these NCDs was confirmed using defined criteria or by ascertaining if participants were taking prescription drugs for the treatment of the respective conditions. A diagnosis of new DM cases was confirmed using fasting plasma glucose and glycated hemoglobin (HbA1c) levels.
For physical measurements assessments, height was measured in metres (m) using a stadiometer (Seca, Hamburg, Germany) with the participant standing in an upright position without wearing shoes. Weight was measured in kilograms (kg) using a weight measuring scale (Seca, Hamburg, Germany) with participants wearing minimal clothing. Waist circumference (WC) was determined in centimeters (cm) using normal tension measuring tape midway applied between the inferior angle of the ribs and the suprailiac crest. Three blood pressure (BP) measurements were taken in mmHg using an Omron digital automated blood pressure machine (Omron Healthcare co., Ltd, Koto, Japan) after participants had been allowed to rest for at least 5 minutes. The first blood pressure reading was discarded, and the mean value of the final two measurements was recorded as the participant’s blood pressure reading. A total of 10 millilitres (mls) of whole blood was collected by venipuncture from each participant after an overnight fast (10–14 hours) and aliquoted as follows: 3mls into grey top tube, 4mls into plain tube (red top) and 4mls into purple top (EDTA) for determination of plasma glucose, lipid profiles and HbA1c, respectively. Plasma and serum were harvested from the grey top tube and the red top tube, respectively, after centrifugation at 3000rpm for 5–10 minutes within 30 minutes of venipuncture. All laboratory assays, fasting plasma glucose (grey top), lipid profiles (red top) and HbA1c were carried out on the COBAS C311 auto analyzer (Roche Diagnostics, Rotkreuz, Switzerland) in accordance with the manufacturer’s protocols and following the principles of good clinical laboratory practice.
Socioeconomic Category and Education Level
Study participants were stratified into five socioeconomic categories (SEC) as defined by the Rwandan government, with category 5 being the wealthiest and category 1 being the poorest.16 The study participants were also stratified by educational level as primary/elementary school level (first 6 years of formal school starting at 7 years of age) followed by secondary/high school for another 6 years before enrolling for tertiary/college education.
Metabolic Syndrome Criteria
The definition of the MetS was adopted from the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (NCEP ATP III) guidelines, which consider MetS as the presence of any three among the following five risk factors:17
- Abdominal obesity: WC >102 cm for men and >88 cm for women.
- Elevated serum triglycerides (TG): >1.7 mmol/L or specific treatment for hypertriglyceridemia.
- Decreased HDL-C: <1.03 mmol/L for men or <1.29 mmol/L for women or treatment for hypoalphalipoproteinemia.
- Elevated Blood Pressure (BP) >130/85 mmHg or treatment of previously diagnosed hypertension.
- Elevated fasting glucose >5.6 mmol/L or treatment of previously diagnosed diabetes mellitus.
Data Management and Analysis
Participant data were entered into a password protected excel database and cleaned before coding and analysis using Stata statistical software version 15 (StataCorp, College Station, Texas, USA). Categorical data were summarized as count and proportion n (%) whilst parametric continuous data were summarized as mean± standard deviation (SD). Non-parametric data were summarized as median and interquartile range (IQR). The Student’s t-test for independent samples was used to compare means derived from two independent groups. Two group median comparisons of non-parametric data were done using the Wilcoxon rank sum test. Logistic regression analysis was used to evaluate the determinants of the metabolic syndrome. Bivariate logistic regression was used in the first instance to identify putative risk factors. A multivariate logistic regression model was fit incorporating variables identified from bivariate logistic regression as having a p-value of 0.25 or less. Logistic regression analysis results were reported as odds ratios and their 95% confidence intervals [OR (95% CI)] and p-values. In all cases of statistical comparisons, the level of significance was set at p = 0.05.
Ethical Approval
The study protocol was reviewed and approved by the Rwanda National Ethics Committee (32/RNEC/2022), following the Declaration of Helsinki. Each participant gave written informed consent prior to enrolment. Participant data were anonymized by assignment of unique participant identifiers.
Results
Demographic Characteristics
Out of the 432 participants enrolled, 422 had complete data on all the essential variables from all the three steps of data collection. The study participants comprised 99 (23.5%) males and 323 (76.5%) females with an overall mean age of 47.5 ± 8.2 years and an age range of 35 to 65 years. There were no statistically significant differences according to sex observed for the various demographic variables presented in Table 1 (p > 0.05). The majority of the participants 41.1% (n = 172) were below 44 years of age whilst 267 (63.3%) resided in urban areas.
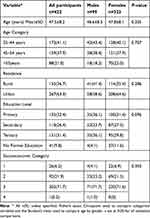 |
Table 1 Participants Demographic Data According to Gender |
The overall educational level was not significantly different according to gender, although more female participants; 37 (11.6%) lacked formal education compared to male participants; 4 (4.1%) (p = 0.028). In addition, there were no significant differences according to gender in the socioeconomic categories (Table 1).
Prevalence of Metabolic Syndrome
The prevalence of the MetS overall and according to sex are presented in Table 2 together with behavioral factors that are commonly associated with increased risk of MetS.
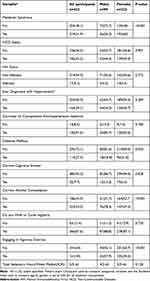 |
Table 2 Summary of Participant Socioclinical Data |
Overall, 219 (51.1%) study participants met the diagnostic criteria of the MetS and there was a statistically significant difference in the frequency of MetS according to sex (p < 0.001) with more female participants; 60% (n = 193) compared to 26.3% (n = 26) male participants. There was a significant difference in the prevalence of DM by sex (p = 0.020) with 96 (31.0%) female participants diagnosed as diabetic compared to 18 (18.4%) males. In terms of behavioural risk factors, of the overall study participants, 13 (13.3%) male participants smoked cigarettes compared to 19 (6.0%) female participants; (p = 0.028) whilst 76 (78.3%) males drank alcohol compared to 147 (47.3%) women (p < 0.001). Significant differences were also observed in the proportions of individuals who undertook vigorous exercise according to sex, with significantly more males partaking in vigorous exercise compared to females (p < 0.001).
Prevalence of Individual Components of the MetS According to the NCEP ATP III Guidelines
The frequencies of the individual components of the MetS were determined and compared according to sex. The results are presented in Figure 1.
 |
Figure 1 Prevalence of individual components of the MetS. |
Hypoalphalipoproteinemia was the single overall most prevalent component of the metabolic syndrome at 61.9% (n = 257) and its prevalence was also significantly higher in females compared to males (p < 0.001) The overall single least prevalent component of the MetS was hypertriglyceridemia 31.4% (n = 130) and the prevalences were not significantly different by sex (p = 0.540). The overall prevalence of abdominal obesity was 55.6% (n = 233) with the prevalence was significantly higher in females (70.4%) compared to males (7.1%, p < 0.001).
Anthropometrics, Blood Pressure and Laboratory Findings
The overall mean ± SD BMI was 27.7 ± 9.4 and females had significantly higher BMI (28.2 ± 6.4) compared to males (25.9 ± 15.6, p = 0.032). Similarly, females had significantly higher mean ± SD weight (95.5 ± 15.0) compared to males (87.8 ± 10.1, p = 0.038) and significantly higher mean ± SD WC (95.5 ± 15.0) compared to males (87.7 ± 10.1) (p < 0.001). Among the serum lipid profile parameters, total cholesterol (4.78 ± 1.27 vs 4.47 ± 1.05, p = 0.030), LDL-cholesterol (2.79 ± 0.91 vs 2.45 ± 0.70, p < 0.001), non-HDL cholesterol (3.65 ± 1.32 vs 3.28 ± 1.03, p = 0.013) and the LDL-C/HDL-C ratio (2.60 ± 1.43 vs 2.25 ± 0.90 (p = 0.022) were significantly higher in females compared to males. No significant differences were observed in glycaemic indices, blood pressure measurements and the remainder of the serum lipid parameters. A summary of anthropometric, blood pressure and laboratory measurements of the overall study participants are presented in Table 3.
 |
Table 3 Anthropometrics, Blood Pressure and Laboratory Findings by Gender |
Factors Associated with Metabolic Syndrome: Bivariate Logistic Regression Analysis
The impact of factors associated with MetS was further investigated using logistic regression analysis. The results are presented in Tables 4 and 5.
 |
Table 4 Factors Associated with the MetS Overall and According to Sex: Bivariate Logistic Analysis |
 |
Table 5 Factors Associated with Metabolic Syndrome Overall and According to Sex: Multivariable Logistic Regression Analysis |
For the overall study population in bivariate logistic regression analysis longer sedentary hours (OR 1.13) higher weight (1.05), higher LDLC (OR1.01), higher total cholesterol (OR 1.47) and increasing age (OR 1.04) were associated with the MetS. On further bivariate logistic analysis by sex, for males only increasing weight (OR 1.06) was significantly associated with the MetS, whereas in females higher socioeconomic status (OR 1.67), longer sedentary hours (OR1.13), increasing weight (OR 1.05), increasing LDLC (OR 1.01), increasing total cholesterol (OR 1.51), and increasing age (1.06) were associated with higher prevalence odds (67%, 13%, 5%, 1%, 51% and 6%) of developing MetS respectively.
Multivariate Logistic Regression Analysis
The risk factors that were significantly associated with MetS in bivariate logistic regression analysis were fit into a multivariable logistic regression model. The results are summarised in Table 5.
On multivariate logistic regression analysis for the overall population, age (aOR1.05), weight (aOR 1.07), and total cholesterol (aOR 1.24) were significantly associated with the MetS; whereas in females, age (aOR 1.05), weight (aOR 1.06) and total cholesterol (1.25) were significantly associated with the MetS; whereas only increasing weight (aOR1.10) tended to be associated with MetS in males.
Discussion
The current study determined the prevalence of MetS among the adult outpatient population presenting at a provincial hospital in Northern Rwanda. The study showed a high magnitude of MetS prevalence in this Rwandan population when compared to a previous study done on Rwandan patients seeking care at a neuropsychiatric hospital in Kigali, Rwanda.18 Furthermore, significant differences were also observed in the prevalence of MetS according to sex.
The previous prospective study carried out in Rwanda, evaluated MetS incidence among patients with epilepsy after a 1-year follow-up period. This study reported an incidence of MetS of 2.4%.18 The present study also revealed a higher burden of MetS compared to published data derived from the region. A study from rural Uganda reported a prevalence of MetS of 19%,19 whilst a study from Kenya on MetS and its predictors in an urban population reported a prevalence of 35%.20 Furthermore, another study from Tanzania that explored gender-related differences in the prevalence of CVD risk factors and their correlates in an urban Tanzanian population reported a prevalence of 38%.21 Elsewhere, in Qatar, the prevalence of MetS was 48.8% with a higher prevalence observed in males (56.7%) compared to females (42.5%).22 The possible explanations for the discrepancies reported by the different studies might include different cultural behaviors, lifestyles, targeted population, geographical factors and possibly different study periods. For instance, the present study targeted outpatient participants within the 35–65 years age range and staff at the study site, while other studies from the region focused on participants aged 18 years and older. In addition, the other studies enrolled community-based participants.
Advancing age is a well-documented risk factor for MetS,23 due to the various biochemical changes that occur during the aging process that affect the normal physiological processes. The current study that enrolled older participants would therefore be expected to show a higher prevalence of MetS compared to studies that enrolled younger participants.
The findings from the current study also showed a significantly higher prevalence of the MetS according to sex (p < 0.001) with females having a higher frequency compared to males. This finding is consistent with reports from other studies that were carried out in Africa and elsewhere,24–27 that reported higher prevalences of MetS in women compared to men. The differences observed in the magnitude of MetS prevalence according to sex could be attributed in part to the higher rates of abdominal obesity, BMI and low-HDL which were significantly higher in females compared to males in the present study (Figure 1). Similarly, from the serum lipid profile parameters, mean total cholesterol (p = 0.030), LDL-C (p = 0.0009), non-HDL-C (0.013) and the mean LDL-C/HDLC ratio (p = 0.022) were significantly higher in females compared to males in the present study (Table 3). On the other hand, in contrast to these findings, significantly higher frequencies of MetS were observed in males compared to females in studies done on Chinese and Qatari populations.22,28 The underlying causes of such disparities in the sex-specific prevalences of MetS have largely remained unclear, but such discordance could be attributed to ethnic differences in circulating hormone levels, population diversity, lipid metabolism, lifestyle habits, cultural behaviors, and the use of different MetS definition criteria.28
The WHO stratifies NCDs risk factors into behavioral and metabolic risk categories.7 Behavioral risk factors consist of such factors as physical inactivity, excessive use of alcohol, inappropriate diet, and tobacco use. Metabolic risk factors include overweight and obesity, raised blood pressure, hyperglycemia, and hyperlipidemia.8 A recent study that examined the impact of different dietary patterns and nutrients on MetS onset and severity revealed that healthy dietary habits such as the Mediterranean diet could reduce the burden of MetS and generate health benefits in the young and adult population.29
The current study observed low HDL-C as the single overall most prevalent component of the MetS (61.9%) and the prevalence of this hypoalphalipoproteinemia was significantly higher in females compared to males (p < 0.0001). Females are physiologically expected to have higher serum HDL-C levels compared to males due to the effects of oestrogen,30 but in the present study, there was no significant difference observed in the mean serum HDL-C concentrations according to sex. We speculate that the observed discordance could have been a result of significantly higher prevalence of DM and obesity in females compared to males. In addition, the proportion of women who regularly undertook physical exercises was significantly lower than that in males. These three factors are known to modulate serum HDL-C levels leading to lower concentrations.31 Furthermore, moderate alcohol consumption has been reported to be associated with elevations of serum HDL-C levels. In the present study, significant more males used alcohol compared to females. This might have further contributed to the disparities observed in the proportions of hypoalphalipoproteinemia according to sex.
The second most prevalent single MetS risk component was abdominal obesity, whose prevalence was significantly higher in female participants compared to males (p < 0.001). This gender disparity was further reflected in the mean BMI, with females recording a significantly higher mean (28.2 ± 6.4) compared to males (25.9 ± 15.6, p = 0.032). Obesity is associated with a high prevalence of impaired glucose tolerance (IGT), and it is also an independent risk factor for type 2 diabetes (T2D).32,33 The findings from the present study were in concordance with this observation, since we observed a significant difference in the prevalence of DM according to gender (p = 0.020) with 31.0% among female participants diagnosed with DM compared to 18.4% among males.
The differences in the distribution of MetS risk factors according to sex observed in the present study suggests that males possibly lead a healthier life style compared to females. This assertion is supported by findings from this study (Table 5). It is, however, noteworthy that the current study enrolled adult participants aged 35 years and above, suggesting that hormonal factors such as postmenopausal weight gain might explain the higher prevalence of MetS metabolic risk factors in women compared to men.34 In concordance with findings from the present study, other investigators also reported higher prevalences of central obesity in females compared to males.19,35–37 The visceral obesity and overweight differences by sex could also be attributed to the higher rates of sedentarism in females compared to males.38 Cultural behaviors, such as the practice of resting and fattening girls in preparation for engagement and marriage and restricting mothers indoors during postpartum period,21,39,40 as well as the African male’s preferences for plump partners41 could also explain the propensity of females to abdominal obesity.
Findings from the present study indicate that significantly more men drank alcohol and smoked cigarettes compared to females (p = 0.028) and (p > 0.001), respectively. Similar results have been reported in studies conducted in other sub-Saharan African countries such as, Uganda,19 Tanzania,21,42 Benin,43 and SSA in general.44 On the other hand, other researchers previously reported an association between alcohol consumption and a lower prevalence of MetS,45–48 as well as a protective effect of moderate alcohol use on coronary heart disease.49 However, according to recent WHO recommendations, no level of alcohol consumption should be considered safe.50 This calls for all countries to re-invest in mass awareness campaigns on the risks and harms associated with drinking any amount of alcohol. On the other hand, this recommendation by the WHO, however, defies the recognized beneficial effects of moderate alcohol consumption on plasma HDL cholesterol concentration.51
The current study has numerous strengths. First, it has generated updated prevalence of MetS in a health setting in a low-income country which, similar to many other low- and middle-income countries, is undergoing an epidemiological transition. The study therefore provides important baseline data for future research in similar settings. Furthermore, this is only the second study that has explored the prevalence and risk factors of MetS in Rwanda. Third, the application of a mixture of assessments (biochemical, anthropometric, and self-reported sociodemographic data) using accredited laboratory methods gives credence to the findings from the present study. Finally, the current study explored extensively the putative risk factors associated with the MetS except for only a few that were beyond the scope of our study. Such unexplored factors include possible genetic factors, polycystic ovary disease and insulin resistance although aspects of the latter could be reflected by the glycemic indices evaluated.
The study, however, also has some limitations that include the cross-sectional nature of the study design which precludes drawing of causal associations between study variables. Furthermore, we recruited participants aged at least 35 years from one referral health facility and this might curtail the generalizability of findings. This focus on an outpatient population might have introduced selection bias, as individuals seeking healthcare might differ from the general population. While this bias might have led to an overestimation of MetS prevalence due to the inclusion of individuals with underlying health concerns, it is also possible that our study participants included health-conscious individuals who are more proactive in seeking preventive care. Future studies should consider these factors when interpreting our findings and aim to recruit participants from diverse settings.
In addition, the sample size of male participants was smaller than that of the female participants. However, although small, the sub sample size was still sufficiently large to meet underlying assumptions for the statistical tests that were employed during analysis.
Conclusion
In conclusion, our study reveals an alarmingly high prevalence of MetS at 51.9%, with a significant gender disparity favoring females. This highlights the urgent need for targeted interventions focusing on behavioral modifications, particularly in women. Leveraging the emerging field of precision lifestyle medicine, we advocate for a comprehensive approach that integrates genetic profiling, environmental factors, and lifestyle habits to tackle the multifaceted risk factors of MetS. A concerted effort between clinical and public health strategies is crucial to mitigate the burden of MetS.
Abbreviations
MetS, Metabolic syndrome; BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NCDs, non-communicable diseases; SBP, systolic blood pressure; WC, waist circumference; WHO, World Health Organization; NCEP ATP III, National Cholesterol Education Program-Adult Treatment Panel III; TG, Triglycerides; HDL, High Density Lipoprotein.
Data Sharing Statement
All the necessary data and materials are within this manuscript. In case any more data or materials are needed, they are readily are available on request from the corresponding author guided by the Rwanda Ministry of Health guidelines.
Acknowledgments
The authors would like to express deepest gratitude to all study participants, data collectors, Ruhengeri provincial hospital, University of Rwanda through CEBHA+ project for participating in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lemieux I, Després JP. Metabolic syndrome: past, present and future. Nutrients. 2020;12(11):3501. doi:10.3390/nu12113501
2. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi:10.1016/j.jacc.2010.05.034
3. WHO. Global status report on NCDs. 2014. Available from: http://www.who.int/ncd.
4. Chong B, Kong G, Shankar K, et al. The global syndemic of metabolic diseases in the young adult population: a consortium of trends and projections from the Global Burden of Disease 2000-2019. Metabolism. 2023;141:155402. doi:10.1016/j.metabol.2023.155402
5. Bowo-Ngandji A, Kenmoe S, Ebogo-Belobo JT, et al. Prevalence of the metabolic syndrome in African populations: a systematic review and meta-analysis. PLoS One. 2023;18(7):e0289155. doi:10.1371/journal.pone.0289155
6. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. doi:10.1007/s11906-018-0812-z
7. Roberts B, Williams HA, Angell S. Noncommunicable Diseases. In: Health in Humanitarian Emergencies: Principles and Practice for Public Health and Healthcare Practitioners. Cambridge University Press; 2018:460–473. doi:10.1017/9781107477261.032
8. Esmailnasab N, Moradi G, Delaveri A. Risk factors of non-communicable diseases and metabolic syndrome. Iran J Public Health. 2012;41(7):77–85.
9. Sliwa K, Acquah L, Gersh BJ, Mocumbi AO. Impact of socioeconomic status, ethnicity, and urbanization on risk factor profiles of cardiovascular disease in Africa. Circulation. 2016;133(12):1199–1208. doi:10.1161/CIRCULATIONAHA.114.008730
10. WHO. The 2018 WHO-NCD country profile. Available from: https://cdn.who.int/media/docs/default-source/country-profiles/ncds/rwa_en.pdf?sfvrsn=20fc2fdf_41&download=true.
11. Ntaganda E, Mugeni R, Harerimana E, et al. High rates of undiagnosed and uncontrolled hypertension upon a screening campaign in rural Rwanda: a cross-sectional study. BMC Cardiovasc Disord. 2022;22(1):197. doi:10.1186/s12872-022-02606-9
12. Kantarama E, Uwizeye D, Uwineza A, Muvunyi CM. Prevalence of central obesity and its association with cardiovascular risk factors among women of reproductive age in Rwanda. African J Biomed Res. 2023;26(1):37–43. doi:10.4314/ajbr.v26i1.5
13. Bavuma CM, Niyibizi JB, Bitunguhari L, Musafiri S, McQuillan R, Wild S. Prevalence and characteristics associated with diabetes mellitus and impaired fasting glucose among people aged 15 to 64 years in rural and urban Rwanda: secondary data analysis of World Health Organization surveillance data. Pan Afr Med J. 2022;41:115. doi:10.11604/pamj.2022.41.115.30682
14. Mukabutera A, Nsereko E, Aline U, Umwungerimwiza YD, Cyprien M. Overweight or obesity prevalence, trends and risk factors among women in Rwanda: a cross-sectional study using the Rwanda demographic and health surveys, 2000–2010. Rwanda J. 2016;3(1):14–20. doi:10.4314/rj.v3i1.3F
15. WHO. WHO STEPS Surveillance Manual Last Updated: 26 January 2017. 2017. Available from: https://www.who.int/docs/default-source/ncds/ncd-surveillance/steps/steps-manual.pdf.
16. Afi. Enhancing Financial Inclusion in Rwanda. The Role of Social Protection Programs. 2019. Available from: https://www.afi-global.org/wp-content/uploads/2020/07/AFI_MS_Rwanda_AW_digital.pdf.
17. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi:10.1161/CIRCULATIONAHA.109.192644
18. Ndayambaje FX, Gahutu JB, Rugera SP. Incidence of the metabolic syndrome among patients with epilepsy attending a neuropsychiatric hospital in Kigali, Rwanda. Int J Curr Sci Res Rev. 2021;04(05):101–109. doi:10.47191/ijcsrr/V4-i5-03
19. Ben-Yacov L, Ainembabazi P, Stark AH, Kizito S, Bahendeka S. Prevalence and sex-specific patterns of metabolic syndrome in rural Uganda. BMJ Nutr Prev Health. 2020;3(1):11–17. doi:10.1136/bmjnph-2019-000050
20. Omuse G, Maina D, Hoffman M, et al. Metabolic syndrome and its predictors in an urban population in Kenya: a cross sectional study. BMC Endocr Disord. 2017;17(1):1. doi:10.1186/s12902-017-0188-0
21. Njelekela MA, Mpembeni R, Muhihi A, et al. Gender-related differences in the prevalence of cardiovascular disease risk factors and their correlates in urban Tanzania. BMC Cardiovasc Disord. 2009;9(1):30. doi:10.1186/1471-2261-9-30
22. Syed MA, Al Nuaimi AS, Latif Zainel AJA, A/Qotba HA. Prevalence of metabolic syndrome in primary health settings in Qatar: a cross sectional study. BMC Public Health. 2020;20(1):611. doi:10.1186/s12889-020-08609-5
23. Stout MB, Justice JN, Nicklas BJ, Kirkland JL. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology. 2017;32(1):9–19. doi:10.1152/physiol.00012.2016
24. Gebreegziabiher G, Belachew T, Mehari K, Tamiru D. Magnitude and associated factors of metabolic syndrome among adult urban dwellers of Northern Ethiopia. Diabetes Metab Syndr Obes. 2021;14:589–600. doi:10.2147/DMSO.S287281
25. Liang X, Or B, Tsoi MF, Cheung CL, Cheung BMY. Prevalence of metabolic syndrome in the United States National Health and Nutrition Examination Survey 2011-18. Postgrad Med J. 2023;99(1175):985–992. doi:10.1093/postmj/qgad008
26. Jahangiry L, Khosravi-Far L, Sarbakhsh P, Kousha A, EntezarMahdi R, Ponnet K. Prevalence of metabolic syndrome and its determinants among Iranian adults: evidence of IraPEN survey on a bi-ethnic population. Sci Rep. 2019;9(1):7937. doi:10.1038/s41598-019-44486-8
27. Zhao Y, Yan H, Yang R, Li Q, Dang S, Wang Y. Prevalence and determinants of metabolic syndrome among adults in a rural area of Northwest China. PLoS One. 2014;9(3):e91578. doi:10.1371/journal.pone.0091578
28. Ye Y, Zhou Q, Dai W, et al. Gender differences in metabolic syndrome and its components in southern China using a healthy lifestyle index: a cross-sectional study. BMC Public Health. 2023;23(1):686. doi:10.1186/s12889-023-15584-0
29. Angelico F, Baratta F, Coronati M, Ferro D, Del Ben M. Diet and metabolic syndrome: a narrative review. Intern Emerg Med. 2023;18(4):1007–1017. doi:10.1007/s11739-023-03226-7
30. Žitňanová I, Šiarnik P, Füllöp M, et al. Gender differences in LDL- and HDL-cholesterol subfractions in patients after the acute ischemic stroke and their association with oxidative stress markers. J Clin Biochem Nutr. 2018;63(2):144–148. doi:10.3164/jcbn.17-105
31. Zhang Y, Yang J, Ye J, et al. Separate and combined associations of physical activity and obesity with lipid-related indices in non-diabetic and diabetic patients. Lipids Health Dis. 2019;18(1):49. doi:10.1186/s12944-019-0987-6
32. La Sala L, Pontiroli AE. Prevention of diabetes and cardiovascular disease in obesity. Int J Mol Sci. 2020;21(21):8178. doi:10.3390/ijms21218178
33. Chandrasekaran P, Weiskirchen R. The role of obesity in type 2 diabetes mellitus-an overview. Int J Mol Sci. 2024;25(3):1882. doi:10.3390/ijms25031882
34. Johari SM, Shahar S. Metabolic syndrome: the association of obesity and unhealthy lifestyle among Malaysian elderly people. Arch Gerontol Geriatr. 2014;59(2):360–366. doi:10.1016/j.archger.2014.04.003
35. López-Sobaler AM, Aparicio A, Aranceta-Bartrina J, et al. Overweight and general and abdominal obesity in a representative sample of Spanish adults: findings from the ANIBES study. Biomed Res Int. 2016;2016:8341487. doi:10.1155/2016/8341487
36. Hassapidou M, Papadopoulou SK, Vlahavas G, et al. Association of physical activity and sedentary lifestyle patterns with obesity and cardiometabolic comorbidities in Greek adults: data from the National Epidemiological Survey. Hormones (Athens). 2013;12(2):265–274. doi:10.14310/horm.2002.1410
37. Munyogwa MJ, Ntalima KS, Kapalata SN. Setting–based prevalence and correlates of central obesity: findings from a cross-sectional study among formal sector employees in Dodoma City, Central Tanzania. BMC Public Health. 2021;21(1):1–8. doi:10.1186/s12889-020-10142-4
38. Ekpenyong CE, Akpan UP, Ibu JO, Nyebuk DE. Gender and age specific prevalence and associated risk factors of type 2 diabetes mellitus in Uyo metropolis, South Eastern Nigeria. Diabetologia Croatica. 2012;41(1).
39. Maletnlema TN. A Tanzanian perspective on the nutrition transition and its implications for health. Public Health Nutr. 2002;5(1A):163–168. doi:10.1079/phn2001289
40. Kanter R, Caballero B. Global gender disparities in obesity: a review. Adv Nutr. 2012;3(4):491–498. doi:10.3945/an.112.002063
41. Brink PJ. The fattening room among the Annang of Nigeria. Med Anthropol. 1989;12(1):131–143. doi:10.1080/01459740.1989.9966016
42. Munyogwa MJ, Mtumwa AH. The prevalence of abdominal obesity and its correlates among the adults in Dodoma region, Tanzania: a community-based cross-sectional study. Adv Med. 2018;2018:6123156. doi:10.1155/2018/6123156
43. Sodjinou R, Agueh V, Fayomi B, Delisle H. Obesity and cardio-metabolic risk factors in urban adults of Benin: relationship with socio-economic status, urbanisation, and lifestyle patterns. BMC Public Health. 2008;8(1):84. doi:10.1186/1471-2458-8-84
44. Sreeramareddy CT, Pradhan PM, Sin S. Prevalence, distribution, and social determinants of tobacco use in 30 sub-Saharan African countries. BMC Med. 2014;12(1):243. doi:10.1186/s12916-014-0243-x
45. Agarwal DP. Cardioprotective effects of light-moderate consumption of alcohol: a review of putative mechanisms. Alcohol Alcohol. 2002;37(5):409–415. doi:10.1093/alcalc/37.5.409
46. Clerc O, Nanchen D, Cornuz J, et al. Alcohol drinking, the metabolic syndrome and diabetes in a population with high mean alcohol consumption. Diabet Med. 2010;27(11):1241–1249. doi:10.1111/j.1464-5491.2010.03094.x
47. Husemoen LL, Jørgensen T, Borch-Johnsen K, Hansen T, Pedersen O, Linneberg A. The association of alcohol and alcohol metabolizing gene variants with diabetes and coronary heart disease risk factors in a white population. PLoS One. 2010;5(8):e11735. doi:10.1371/journal.pone.0011735
48. Alkerwi A, Boutsen M, Vaillant M, et al. Alcohol consumption and the prevalence of metabolic syndrome: a meta-analysis of observational studies. Atherosclerosis. 2009;204(2):624–635. doi:10.1016/j.atherosclerosis.2008.10.036
49. Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342. doi:10.1136/bmj.d636
50. World Health Organization. ”No level of alcohol consumption is safe for our health.” 2023. Available from: https://www.who.int/europe/news/item/04-01-2023-no-level-of-alcohol-consumption-is-safe-for-our-health.
51. Wilkens TL, Sørensen H, Jensen MK, Furtado JD, Dragsted LO, Mukamal KJ. Associations between alcohol consumption and HDL subspecies defined by ApoC3, ApoE and ApoJ: the cardiovascular health study. Curr Probl Cardiol. 2023;48(1):101395. doi:10.1016/j.cpcardiol.2022.101395
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

