Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 20
Frequency of Exacerbations of Chronic Obstructive Pulmonary Disease Associated with the Long-Term Exposure to Air Pollution in the AIREPOC Cohort
Authors Herrera Lopez AB , Torres-Duque CA , Casas Herrera A, Arbeláez MP, Riojas-Rodríguez H, Texcalac-Sangrador JL, Rojas NY, Rodriguez-Villamizar LA
Received 25 October 2024
Accepted for publication 1 February 2025
Published 22 February 2025 Volume 2025:20 Pages 425—435
DOI https://doi.org/10.2147/COPD.S498437
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Astrid Berena Herrera Lopez,1 Carlos A Torres-Duque,2 Alejandro Casas Herrera,3 María Patricia Arbeláez,4 Horacio Riojas-Rodríguez,5 José Luis Texcalac-Sangrador,6 Néstor Y Rojas,7 Laura Andrea Rodriguez-Villamizar8
1Facultad de Medicina, Universidad de los Andes, Bogotá D.C, Colombia, Universidad de Antioquia, Medellín, Antioquia, Colombia; 2CINEUMO, Fundación Neumológica Colombiana, Bogotá, Colombia; 3AIREPOC Programa, Fundación Neumológica Colombiana, Bogotá, Colombia; 4Facultad Nacional de Salud Pública, Universidad de Antioquia, Medellín, Antioquia, Colombia; 5Centro de Investigación en Salud Poblacional, Instituto Nacional de Salud Pública, Cuernavaca, Morelos, México; 6Centro de Investigación en Salud Poblacional. Instituto Nacional de Salud Pública, Ciudad de México, México; 7Departamento de Ingeniería Química y Ambiental, Universidad Nacional de Colombia, Bogotá, Colombia; 8Departamento de Salud Pública, Escuela de Medicina, Universidad Industrial de Santander, Bucaramanga, Colombia
Correspondence: Astrid Berena Herrera Lopez, Email [email protected]; [email protected]
Background: Exacerbations of chronic obstructive pulmonary disease (COPD-E) have been associated with levels of air pollution. The occurrence of COPD-E is associated with increased mortality in this population.
Purpose: To determine the association between long-term exposure to PM2.5 and NO2, and the frequency of COPD-E in patients belonging to AIREPOC, an institutional integrated care program for COPD in Bogota, Colombia.
Patients and Methods: Retrospective cohort study included patients with COPD living in Bogotá, between 2018 and 2021, who received health care in the AIREPOC program. Each patient´s home address was geolocated. Information from local air quality network stations was used to estimate daily and annual mean PM2.5 and NO2 exposure level for each patient using the inverse distance squared weighted regression (IDWR) method. The effect of PM2.5 and NO2 concentrations categorized at 15 μg/m3 and 25 μg/m3 respectively on the frequency of COPD-E was estimated using a zero-truncated negative binomial model adjusted for potential confounders. Goodness-of-fit was assessed by residuals.
Results: During the observation period, 580 COPD-E occurred in 722 patients. Significant associations were found between COPD-E and NO2 concentrations ≥ 25 μg/m3 (incidence density ratio, RDI: 1.29, 95% CI: 1.02– 1.67) after adjustment for sun exposure, COPD severity, depression, and ambient humidity. No association was found between the frequency of COPD-E and PM2.5 concentrations ≥ 15μg/m3.
Conclusion: Prolonged exposure to high levels of NO2 increases the frequency of COPD exacerbations in patients residing in Bogotá. These results highlight the importance of strengthening air quality control measures and educating people with COPD to know and interpret the local air quality indices and to follow the recommendations derived from its alterations.
Keywords: air pollution, long-term effect, chronic obstructive pulmonary disease, COPD exacerbations, negative binomial regression truncated at zero
Graphical Abstract:

Introduction
Although the adverse effects of air pollution have been widely recognized and air quality programs have been operating in most countries, it remains a major cause of morbidity and mortality.1 It affects both high-income and low-middle-income countries, where exposure to particulate and gaseous pollutants exceeds the levels recommended by the World Health Organization (WHO).2,3 According to data from the Global Burden of Diseases, injuries, and Risk Factors (GBD) study, air pollution was responsible of 6.67 (95% CI 5.90–7.49) million premature deaths and more than 210 (95% CI 189–240) million disability-adjusted life years (DALYs) in 2019.4 Air pollution is currently the seventh leading risk factor for non-communicable and communicable diseases,4 affecting almost all organic systems of the human body (3). About 18% of deaths from exacerbations of chronic pulmonary disease have been attributed to air pollution.1
The exacerbations of chronic obstructive pulmonary disease (COPD-E) are considered an important negative event during the disease5 and have been linked to air pollution.6–11 COPD-E accelerates lung function decline,12 reduce quality of life,13 decrease survival,5 increase direct costs of care14–16 and increase the risk of all-cause mortality and acute cardiovascular events.17–20 Moderate to severe exacerbations increase the risk of future exacerbations and the risk of death.21 A history of exacerbations is the most important predictor of future exacerbations.17,21 Therefore, identifying patients at risk for COPD-E is important to reduce the burden of the disease and prevent the cycle of lung damage, worsening quality of life, increased disease burden, increased healthcare costs, and death.21
The long-term exposure to air pollution has been associated with the development of COPD.6–11 Overall, these studies have involved urbanized areas in Europe, North America, and Asia, but there is no information on this association in Latin American countries, where it is recognized that air pollutants concentrations are generally higher than in North America and Europe.4,22 Uncertainty therefore remains as to whether the same effect is observed in different geographical areas and what its magnitude is. In addition, the effect of air pollution on COPD in Bogotá, a large city located at high altitude (2640 m) in a tropical country, is poorly documented, with only one published retrospective descriptive study.23
Bogotá is the most populous city in the world located at high altitude (2640 m) and is situated in a tropical region. The air density is lower, and the ventilation is higher to compensate for the lower oxygen pressure It is recognized as a megacity and has experienced positive population growth because of internal migration and a high birth rate. Bogotá D.C. is home to the country’s largest vehicle fleet, with 2,838,874 vehicles registered in 2021.24 Between 2022 and 2023, the vehicle fleet increased by 4.81% (869,662) and has the highest vehicle density (1000), with a vehicle age of 10 years.25 Sixty-one percent of PM2.5 concentration was generated by mobile sources and 39% by stationary sources.26
On the other hand, meteorological conditions resulted in temperature inversions in which a warm atmospheric layer traps other colder ones. This meteorological phenomenon traps and prevents the dispersion of pollutants at ground level, which contributes to the poor air quality in Bogotá.22
Bogota has problems with urban mobility, vehicle fleet volume, and population growth that demand more services and energy consumption, factors that contribute to poor air quality and climate change.
Moreover, little is known about the long-term effects of air pollution on COPD, especially PM2.5 and NO2. To date, the published literature reports the short-term27–35 and long-term32,36–39 effects of air pollution on hospital admissions caused by COPD-E. Very few studies have evaluated the long-term effects of air pollution on the exacerbation frequency spectrum. Furthermore, addressing the COPD-E related to air pollution exposure is relevant given the increasing stationary and mobile sources of air pollution as well as the sustained use of fossil fuels by anthropogenic activities and other energy consumption. This study aimed to determine the association between long-term exposure to PM2.5 and NO2 and the incidence of COPD-E in patients belonging to AIREPOC, an institutional integrated care program for COPD, in Bogota, Colombia, between 2018 and 2021.
Materials and Methods
Study Design and Population
The AIREPOC Program includes patients with COPD of the Fundación Neumológica Colombiana, a specialized tertiary care facility, in the capital district of Bogota, Colombia. All patients have a clinically and spirometry (forced expiratory volume in one second/forced vital capacity ratio [FEV1/FVC] below the lower limit of normal) confirmed diagnosis of COPD and receive a personalized integrated and continuous management (AIREPOC cohort). This is a retrospective longitudinal study of adults aged 32 to 97 years at the time of recruitment between 2018 and 2021. A total of 722 participants with a diagnosis of COPD were identified from the clinical records of the AIREPOC program. For each of them, demographic, risk factors, medical history, spirometry, including Global Initiative on Obstructive Lung Disease (GOLD) classification40 and exacerbation information, were available, and were complemented by information from national health system records, including hospitalizations and COVID-19 infections. COPD exacerbations were verified in patients’ clinical records.
This study was approved by the Ethics Committee of the National School of Public Health of the University of Antioquia (CEI- 21030002-00162) and the ethics committee of the Fundación Neumológica Colombiana. Patients at AIREPOC entry gave written informed consent to use their data in research studies. The present study complies with the ethical principles of human subjects research and the Declaration of Helsinki.
Air Pollution Measurements and Exposure Estimation
Environmental air pollution exposure information was provided by the Environment Secretary of Bogotá based on data from the Air Quality Monitoring Network, which consists of 20 air quality monitoring stations. Data obtained for the stations included the daily average of air pollutants and meteorological variables, for the study period. Daily estimations for air pollutants were calculated when the temporal representativeness ≥75% of the hourly record for each pollutant.
The exposure levels of air pollutants were assigned based on the residential addresses and the location of the air quality monitoring stations that were assumed as centroids of circular buffers of 5 km and 10 km. Daily estimations for each residential address were estimated by using the Inverse Distance Weighted Squared (IDWR) method.40–44 Linkage of environmental data to cohort participants was conducted by using COPD-E dates and locations of participants’ home addresses. For the estimation of long-term exposure to air pollutants, annual means for PM2.5, NO2, and meteorological variables were calculated for each patient in the cohort for the four years of follow-up. Air pollutants were recorded on a 24-hour basis for four years of study from air monitoring stations. The air pollutant concentrations were converted to quartiles of exposure for each pollutant.
Outcome Variable
We determined the frequency of COPD-E during the study period as the main outcome variable. Exacerbations requiring additional pharmacological intervention (steroids and/or antibiotics) or emergency visits or hospitalizations were considered. Information about the date of occurrence and length of hospitalization was obtained from the clinical records of the AIREPOC program. The previous history of COPD-E was determined by the registry of daily counts of hospital admissions or outpatient managed exacerbations during 2017 and verified from clinical records. Information was complemented from the COPD-E identified by the National Health System using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code ICD-10: J44.1, J440. Patients from AIREPOC gave written informed consent to use their data in research studies during their first clinical visit.
Statistical Analysis
Negative binomial regression truncated at zero was used to estimate the coefficient and the 95% confidence interval (95% CI) associated with categories of exposure with cutoffs at 15 µg/m3 for PM2.5 and 25 µg/m3 for NO2, corresponding to intermediate objectives of the WHO 2021 guidelines.3 Associations were evaluated using the Kruskal–Wallis test. The Akaike’s Information Criterion (AIC) was used to select the variables to be included in the models. The goodness-of-fit of the model was assessed by residual analysis. Collinearity was assessed by the Variance Inflation Factor (VIF). All significance tests were conducted with a 95.0% significance. Data were processed using the R statistical package, version 4.3.2 R Core Team, 2023.45
Results
Characteristics of the AIREPOC Cohort Participants
The cohort included 722 participants from the AIREPOC program with an age average of 75.1 years old and confirmed COPD diagnosis. During the four years of follow-up, 580 episodes of COPD-E occurred in 382 (52.9%) participants for a ratio of 1.52 COPD-E/person. Among participants, the mean pre-bronchodilator FEV1 and FVC were 1.37 and 3.14 liters, respectively.
Participants who developed COPD-E were more likely to be married, be classified in groups C and D according to GOLD 2019, with higher depression according to the Brief Patient Health Questionnaire (PHQ-9), more anxiety measured by Beck scale, fewer meters walked according to the 6-minute walk, with lower quality of life according to the Saint George scale, and with higher severity of COPD symptoms by the Assessment Test or CAT scale (Table 1) (Figure S1).
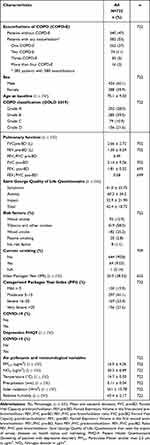 |
Table 1 Characteristics of the Participants of the 2018–2021 AIREPOC Cohort |
Exposure to Air Pollution
The mean air pollutant levels of PM2.5 and NO2 estimated for the cohort patients were 16.02 ± 4.36 μg/m3 and 30.34 ± 6.89 μg/m3, respectively. During the follow-up period, statistically significant decreases in annual PM2.5 and NO2 concentrations were observed. The averages of the meteorological variables were 14.73 ± 0.5 °C for temperature, 161.19 ± 15.78 W/m2 for solar radiation, and 65.44 ± 2.17% for relative humidity over the period (Table 1). Spearman correlation coefficients were strongest between the two study pollutants (rs = 0.62), followed by PM2.5 and temperature (rs = 0.49) and PM2.5 and solar radiation (rs = 0.38). The correlation of PM2.5 and NO2 with the number of COPD-E events was 0.06 and −0.02, respectively. The spatial distribution of exposure to PM2.5 μg/m3 and NO2 μg/m3 for each patient in the AIREPOC program from 2018 to 2021 can be seen in Figure S2.
In the single pollutant models (Table 2), long-term exposure to NO2 was associated with increased COPD-E frequency, adjusted for COPD severity, relative humidity, and solar radiation. In both models, adjusting for the forced expiratory volume in the first-second (FEV1) was not significant. Those patients exposed to concentrations ≥25 µg/m3 compared to those exposed to levels <25 µg/m3 of NO2 increased the frequency of COPD-E by 29% (95% CI 2%–67%). In contrast, there was no statistically significant association between COPD-E and PM2.5 concentrations equal or higher than 15 µg/m3.
 |
Table 2 Variables Associated With Frequency of COPD Exacerbations; Negative Binomial Model Truncated at Zero |
The goodness-of-fit test shows that the predicted values are similar to those observed for all regressions (Table 3). Both models, for PM2.5 and NO2, show that the values of the Variance Inflation Factor (VIF) did not exceed the magnitude of 10; consequently, there was no collinearity (Table 3).
 |
Table 3 Goodness-of-Fit Comparison of Zero-Truncated Negative Binomial Models |
No significant interaction was found for temperature, PM2.5, and NO2. In the stratified analysis by sex, similar associations were found with statistically significant associations between COPD-E and NO2 exposure for males (RDI:1.57, 95% CI:1.05–2.35) and absence of association for women. For PM2.5 neither sex, males and females, showed association with the frequency of COPD-E Tables S1 and S2.
Discussion
An association was observed between long-term air pollution exposure and COPD-E frequency. Individuals with chronic exposure to concentrations of NO2 ≥ 25 µg/m3 had 29% (95% CI: 2%-67%) increase in the incidence rate of COPD-E frequency. However, no such association was observed for concentrations of PM2.5 ≥ 15 µg/m3.
In addition, NO2 exposure was found to be more strongly associated with the frequency of COPD-E in patients classified as GOLD C/D (exacerbator patients), with lower ambient relative humidity, and in people with exposure to higher levels of solar radiation. There was no association with pre-bronchodilator FEV1 value or depression status. This may be partly due to the limited sample size of people with depression.
It was also found that, in the sex-stratified analysis, we found a stronger association for NO2 concentrations among males, with an increased risk in the frequency of COPD-E of 57% (95% CI 5–135%), while no association was evident for NO2 concentrations in females. Absence of associations among females might be partly explained by the limited sample size of COPD-E frequency cases in this group.
In the context of our current knowledge of the known adverse respiratory effects of prolonged exposure to NO2 air pollution,46 our findings are consistent with some studies9,37,47–51 reporting that COPD patients are more likely to suffer COPD-E related to increased levels of PM2.5 and NO2 air pollution. However, no studies were found in the current literature that demonstrated an association between the frequency of COPD-E and chronic exposure to PM2.5 and NO2. Given the scarcity of literature on this topic, these findings may serve as knowledge for future research.
At the time of the study, only the study by Morantes and Fajardo, in 2019 in Bogotá D. C, reported that high concentrations of PM2.5 and PM10 with 48-hour lag (lag 2) were associated with increased sputum volume (OR: 4.74; 95% CI:1.02–21.90), purulence (OR: 6.58;95% CI:2.51–17.20), pleuritic pain (OR:3.62;95% CI:1.27–10.37), antibiotic use (OR:2.87;95% CI:1.17–7.07) and corticosteroids (OR:2.62;95% CI:1.07–6.44).23
A new finding identified the association between the frequency of COPD-E and prolonged exposure to PM2.5 and NO2 in patients with higher solar radiation exposure, worse COPD severity according to GOLD C/D, and lower relative humidity. These variables have not been investigated in previous epidemiological studies of short- and long-term effects. Only two studies from Ferrari et al52 and Huh et al,53 documented the negative effect of solar radiation, temperature, and relative humidity on COPD-E.
Our findings are consistent with current knowledge about biological plausibility connecting air pollutants exposure and COPD. It is known that biological mechanisms may differ between short-term and long-term exposure to air pollution in COPD patients. Short-term exposure increases Th1 and Th17 cytokines while decreasing Th2 cytokines,54 which may trigger acute airway inflammation and exacerbation,55 leading to death,55,56 whereas long-term exposure leads to airway remodeling, fibrosis, and smooth muscle hyperplasia55 resulting in COPD progression57 and disease exacerbation.54 Particulate matter may exacerbate inflammation by inducing epithelial remodeling and dendritic cell dysfunction in COPD patients.54,58
The high solar intensity has been associated with melatonin depletion, which may be responsible for adverse health effects in COPD patients by increasing inflammation and oxidative stress activity in the lung.59 The melatonin in the lung modulates proinflammatory cytokines such as interleukin 1β and 6, and tumor necrosis factor-alpha (TNF-α).59 The intensity of solar radiation can also affect by disrupting the 24-hour circadian rhythm and the physicochemical properties and toxicity of gaseous pollutants.59
In addition, a significant relationship between inflammatory markers and PM2.5 and NO2 has been observed, as evidenced by Squillacioti et al.60 Both in vitro and in vivo animal studies have shown the systemic inflammatory effect mediated by oxidative stress and lung inflammation from both short- and long-term exposure to NO2 and PM2.5.60–63 The main source that generates NO2 is fuel burning by vehicles, in addition to other sources such as power plants and agriculture, its concentration is higher in urban areas and is considered a marker of traffic-related air pollution mixing.64 In Bogota, 69% of PM2.5 emissions were due to resuspension; emissions from on-road mobile sources contribute 89% by NOx and 22% by PM2.5.65 In addition, NOX contributes to the formation of other pollutants such as ozone and other secondary particulate matter. Consequently, an increase in NO2 reacts with other pollutants and dissipates faster than PM2.5, NO2 levels can vary depending on traffic patterns64 and meteorological variables. Evidence suggests that NO2 and PM10, PM2,5 exposure increases acute systemic inflammation in patients with COPD,3,60,66,67 which could lead to the accumulation of disease exacerbations resulting in a long-term increase in the frequency of COPD-E.
NO2 could be pathophysiologically related with COPD exacerbations because it promotes the production of free radicals such as reactive oxygen species (ROS), the which could add to the fact that in people with respiratory diseases the antioxidant function is compromised, making it ineffective against ROS aggressions.60
This study had several limitations. First, the effect of other pollutants was not assessed, because the data did not meet the criterion of temporal representativeness. Second, a measurement bias is possible in the estimation of individual exposure because air pollution data and urban meteorological factors may differ from individual mobility exposure levels and may not represent the total exposure of the cohort; however, participants were older than 75 years, which implies they probably spent most of the time at home. In addition, the potential information bias of exposure misclassification was controlled by geolocating the participants’ addresses more than eight times.
If coding errors occurred, they would be random and should not be a valid argument for non-differential misclassification, which would bias the study results toward the null. Third, records in the AIREPOC program included incomplete data; however, missing values were less than 15% for spirometry parameters (2.77%), dyspnea (1.24%); arterial gases (9.4%), COPD severity classification by GOLD (11.77%); and IPA (13.05%). We used real-world data that allow for external validity and generalizability due to their volume and long follow-up, thus reducing clinical uncertainty.68 However, by selecting a subset of people with COPD participating in a specialized institutional program, it could lead to selection bias. We believe that patients with COPD who are not participants in specialized programs such as AIREPOC are probably in poorer clinical conditions and living in more polluted areas of the city and therefore might be more susceptible to the effects of air pollutants. Thus, the potential selection bias in our cohort is probably underestimating the effect measure of association between air pollutants and frequency of COPD-E in the general population of COPD patients in Bogotá.
The main strengths of our study are related to the following aspects: the use of real-life data that fully capture care throughout the follow-up period and include information for important covariates such as smoking, occupational exposure, validated scales measuring the quality of life, depression, anxiety, history of exacerbation, and occupational exposure. The use of the long-term estimates of air pollutant’s concentration reduces the potential exposure measurement bias compared to short-term studies as the temporal variations in the monthly and annual exposure averages are smaller. Another important strength was the use of a standardized definition of COPD-E validated by GOLD.69
Our results provide evidence that improving air quality in Bogota could reduce the burden of COPD-E frequency, the prognosis of the disease, and improve the COPD control related to air pollution etiotype. For achieving this goal, it is necessary to involve patients and local stakeholders in the management of health risks in the COPD population, provide training for health professionals on the effects and health risks of exposure to air pollution and use different media to communicate the risks to the general population and COPD patients.70
The results of this research highlight the importance of continuing with regulatory efforts to reduce air pollution emissions and environmental education for the susceptible population, as well as for health personnel to learn about evidence-based recommendations on air pollution; the inclusion of a real-time health and environmental epidemiological surveillance system to identify air pollution episodes in a timely and effective manner, promote citizen oversight, and the community should acquire environmental habits such as using public transportation, carpooling, walking or using electric vehicles, performing preventive maintenance on vehicles, using clean energy for cooking and avoiding open burning.71
Conclusion
This cohort study shows evidence of significant associations between air pollution and increased risks of COPD-E frequency among patients participating in a specialized rehabilitation program in Bogotá. Long-term NO2 exposures were associated with higher exacerbation frequency. In addition, this work provides novel findings that prolonged exposure to NO2, together with the effect of high solar intensity, higher severity of COPD, and lower relative humidity, increase the risk of COPD frequency.
Acknowledgments
We would like to thank all the patients involved in this study and the staff of the Fundación Neumologica Colombiana. Authors also want to thank Horacio Riojas-Rodríguez and José Luis Texcalac-Sangrador for their support in the process of exposure estimation. We want to thank the Subdirectorate of Air, Auditory and Visual Quality of the District Secretary of Environment of Bogotá D.C, for providing the data on criteria of air pollutants and meteorological variables.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing financial interests or known personal relationships that could have influenced the investigation reported in this paper. The authors are solely responsible for the content and writing in this article.
References
1. Organización Mundial de la Salud OMS. Contaminación del aire ambiente (exterior). 2021. Available from: https://www.who.int/es/news-room/fact-sheets/detail/ambient-outdoor-air-quality-and-health.
2. Landrigan PJ, Fuller R, Fisher S, et al. Pollution and children’s health. Sci Total Environ. 2019;650:2389–2394. doi:10.1016/j.scitotenv.2018.09.375
3. World Health Organization WHO. WHO global air quality guidelines Particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. 2021. p. 5–300. Available from: https://www.who.int/publications/i/item/9789240034228.
4. Murray CJL; Collaborators G 2019 RF. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1223–1249.
5. Hogea SP, Tudorache E, Fildan AP, Fira-Mladinescu O, Marc M, Oancea C. Risk factors of chronic obstructive pulmonary disease exacerbations. Clin Respir J. 2020;14(3):183–197. doi:10.1111/crj.13129
6. Moore E, Chatzidiakou L, Kuku MO, et al. Global associations between air pollutants and chronic obstructive pulmonary disease hospitalizations: a systematic review. Ann Am Thorac Soc. 2016;13(10):1814–1827. doi:10.1513/AnnalsATS.201601-064OC
7. Evangelopoulos D, Chatzidiakou L, Walton H, et al. Personal exposure to air pollution and respiratory health of COPD patients in London. Eur Respir J. 2021;58(1):2003432. doi:10.1183/13993003.03432-2020
8. Glencross DA, Ho TR, Camiña N, Hawrylowicz CM, Pfeffer PE. Air pollution and its effects on the immune system. Free Radic Biol Med. 2020;151:56–68. doi:10.1016/j.freeradbiomed.2020.01.179
9. Andersen ZJ, Hvidberg M, Jensen SS, et al. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution: a cohort study. Am J Respir Crit Care Med. 2011;183(4):455–461. doi:10.1164/rccm.201006-0937OC
10. Kwon SO, Hong SH, Han YJ, et al. Long-term exposure to PM(10) and NO(2) in relation to lung function and imaging phenotypes in a COPD cohort. Respir Res. 2020;21(1):247. doi:10.1186/s12931-020-01514-w
11. Liu Y, Yan S, Poh K, Liu S, Iyioriobhe E, Sterling DA. Impact of air quality guidelines on COPD sufferers Impact of air quality guidelines on COPD sufferers. Int J Chron Obs Pulmon Dis. 2016;11:839–872. doi:10.2147/COPD.S49378
12. Du W, Zhang W, Hu H, Zhang M, He Y, Li Z. Associations between ambient air pollution and hospitalizations for acute exacerbation of chronic obstructive pulmonary disease in Jinhua, 2019. Chemosphere. 2021;267:128905. doi:10.1016/j.chemosphere.2020.128905
13. Guo J, Chen Y, Zhang W, Tong S, Dong J. Moderate and severe exacerbations have a significant impact on health-related quality of life, utility, and lung function in patients with chronic obstructive pulmonary disease: a meta-analysis. Int J Surg. 2020;78:28–35. doi:10.1016/j.ijsu.2020.04.010
14. Miravitlles M, Jardim JR, Zitto T, Rodrigues JE, López H. Estudio Farmacoeconómico del Tratamiento Antibiótico de las Agudizaciones de la Bronquitis Crónica y la EPOC en Latinoamérica. Arch Bronconeumol. 2003;39(12):549–553. doi:10.1016/S0300-2896(03)75453-2
15. Peña E, Osorio D, Gamboa Ó, et al. Carga de enfermedad atribuible al uso de tabaco en Colombia y potenciales beneficios sanitarios y económicos del aumento del precio del cigarrillo mediante impuestos. Rev Colomb Cancerol. 2019;23(4):135–143. Spanish. doi:10.35509/01239015.31
16. Naranjo L, Torres-Duque CA, Colodenco D, et al. Highlights of an expert advisory board on acute exacerbations of chronic obstructive pulmonary disease (AE-COPD) in Latin America. Int J Chron Obstruct Pulmon Dis. 2020;15:1919–1929. doi:10.2147/COPD.S261258
17. Whittaker H, Rubino A, Müllerová H, et al. Frequency and severity of exacerbations of COPD associated with future risk of exacerbations and mortality: a UK routine health care data study. Int J Chron Obstruct Pulmon Dis. 2022;17:427–437. doi:10.2147/COPD.S346591
18. Müllerová H, Marshall J, de Nigris E, et al. Association of COPD exacerbations and acute cardiovascular events: a systematic review and meta-analysis. Ther Adv Respir Dis. 2022;16:17534666221113648. doi:10.1177/17534666221113647
19. Balbirsingh V, Mohammed AS, Turner AM, Newnham M. Cardiovascular disease in chronic obstructive pulmonary disease: a narrative review. Thorax. 2022;77(9):939–945. doi:10.1136/thoraxjnl-2021-218333
20. Maeda T, Dransfield MT. Chronic obstructive pulmonary disease and cardiovascular disease: mechanistic links and implications for practice. Curr Opin Pulm Med. 2024;30(2):141–149. doi:10.1097/MCP.0000000000001040
21. Hurst JR, Han MK, Singh B, et al. Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res. 2022;23(1):213. doi:10.1186/s12931-022-02123-5
22. IQAir. World air quality report region and city PM2.5 ranking 2023. 2024. p. 45. Available from: https://www.iqair.com/world-most-polluted-cities?srsltid=AfmBOoqlzRDDqGaLlbRCShiiVgco0vZr5P6VaO9otDvjgvHm4Xl7Yt4P.
23. Morantes-Caballero JA, Fajardo Rodriguez HA. Effects of air pollution on acute exacerbation of chronic obstructive pulmonary disease: a descriptive retrospective study (pol-AECOPD). Int J Chron Obstruct Pulmon Dis. 2019;14:1549–1557. doi:10.2147/COPD.S192047
24. Contraloría. La calidad del aire en Bogotá D.C en la vigencia 2022. Bogotá D.C. Spanish; 2023.
25. Centro Virtual de Negocios y Asopartes en Colombia CVN. Datos del parque automotor en Colombia que cualquier empresario del gremio debería saber. 2021. Available from: https://cvn.com.co/datos-del-parque-automotor-en-colombia-que-cualquier-empresario-del-gremio-deberia-saber/.
26. Instituto de Hidrología Meteorología y Estudios Ambientales IDEAM. Emisiones. Available from: http://www.ideam.gov.co/web/siac/emisionesaire.
27. Jin JQ, Han D, Tian Q, et al. Individual exposure to ambient PM2.5 and hospital admissions for COPD in 110 hospitals: a case-crossover study in Guangzhou, China. Environ Sci Pollut Res. 2022;29(8):11699–11706. doi:10.1007/s11356-021-16539-x
28. Zhang H, Niu Y, Yao Y, Chen R, Zhou X, Kan H. The Impact of ambient air pollution on daily hospital visits for various respiratory diseases and the relevant medical expenditures in Shanghai, China. Int J Environ Res Public Heal. 2018;15(15).
29. Santus P, Russo A, Madonini E, et al. How air pollution influences clinical management of respiratory diseases. A case-crossover study in Milan. Respir Res. 2012;13(95). doi:10.1186/1465-9921-13-95
30. Lin C, Li D, Lu J, et al. Short-term associations between ambient fine particulate matter pollution and hospital visits for chronic obstructive pulmonary disease in Yinzhou District, China. Env Sci Pollut Res. 2020;27(17):21647–21653. doi:10.1007/s11356-020-08448-2
31. Dong J, You J, Wang J, Bao H. Association between short-term ambient air pollution and outpatient visits for acute exacerbation of chronic obstructive pulmonary disease in Lanzhou, 2013–19. Environ Geochem Health. 2023;45(5):2495–2509. doi:10.1007/s10653-022-01363-0
32. Devries R, Kriebel D, Sama S. Outdoor air pollution and COPD related emergency department visits, hospital admissions and mortality: a meta-analysis. COPD. 2022;14(2):113–121.
33. Kloog I, Nordio F, Zanobetti A, Coull BA, Koutrakis P, Schwartz JD. Short term effects of particle exposure on hospital admissions in the Mid-Atlantic states: a population estimate. PLoS One. 2014;9(2):e88578. doi:10.1371/journal.pone.0088578
34. Ko FWS, Tam W, Wong TW, et al. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax. 2007;62(9):780–785. doi:10.1136/thx.2006.076166
35. Xie J, Teng J, Fan Y, Xie R, Shen A. The short-term effects of air pollutants on hospitalizations for respiratory disease in Hefei, China. Int J Biometeorol. 2019;63(3):315–326. doi:10.1007/s00484-018-01665-y
36. De Matteis S, Forastiere F, Baldacci S, et al. Issue 1 - “Update on adverse respiratory effects of outdoor air pollution”. Part 1: outdoor air pollution and respiratory diseases: a general update and an Italian perspective. Pulmonology. 2022;28(4):284–296. doi:10.1016/j.pulmoe.2021.12.008
37. Zanobetti A, Bind MAC, Schwartz J. Particulate air pollution and survival in a COPD cohort. Environ Health. 2008;7(1):48. doi:10.1186/1476-069X-7-48
38. Simoni M, Baldacci S, Maio S, Cerrai S, Sarno G, Viegi G. Adverse effects of outdoor pollution in the elderly. J Thorac Dis. 2015;7(1):34–45. doi:10.3978/j.issn.2072-1439.2014.12.10
39. Badida P, Krishnamurthy A, Jayaprakash J. Meta analysis of health effects of ambient air pollution exposure in low- and middle-income countries. Environ Res. 2023;216(Pt 4):114604. doi:10.1016/j.envres.2022.114604
40. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(1900164):1900164. doi:10.1183/13993003.00164-2019
41. Téllez-rojo MM, Rothenberg SJ, Texcalac-sangrador JL, et al. Children’s acute respiratory symptoms associated with PM 2.5 estimates in two sequential representative surveys from the Mexico City Metropolitan Area. Environ Res. 2020;180(September 2019):108868. doi:10.1016/j.envres.2019.108868
42. Cervantes-Martínez K, Stern D, Zamora-Muñoz JS, et al. Air pollution exposure and incidence of type 2 diabetes in women: a prospective analysis from the Mexican Teachers. Cohort Sci Total Environ. 2022;818:151833. doi:10.1016/j.scitotenv.2021.151833
43. Torrico-Lavayen R, Vargas-Alarcón G, Riojas-Rodriguez H, et al. Long-term exposure to ambient fine particulate matter and carotid intima media thickness at bilateral, left and right in adults from Mexico City: results from GEA study. Chemosphere. 2023;335:139009. doi:10.1016/j.chemosphere.2023.139009
44. Ugalde-resano R, Riojas-rodríguez H, Texcalac-sangrador L, Cruz JC, Hurtado-Díaz M. Short term exposure to ambient air pollutants and cardiovascular emergency department visits in Mexico city. Environmental Research. 2022;207(December 2021). doi:10.1016/j.envres.2021.112600
45. The R foundation for statistical C; posit software PBC. R version 4.3.2 (2023-10-31 ucrt). 2023.
46. Marchetti P, Miotti J, Locatelli F, et al. Long-term residential exposure to air pollution and risk of chronic respiratory diseases in Italy: the BIGEPI study. Sci Total Environ. 2023;884:163802. doi:10.1016/j.scitotenv.2023.163802
47. Kang S, Hong YS, Park J, et al. Air pollution and mortality in patients with chronic obstructive pulmonary disease: a cohort study in South Korea. Ther Adv Chronic Dis. 2023;14:20406223231176176. doi:10.1177/20406223231176175
48. Zhang Z, Wang J, Lu W. Exposure to nitrogen dioxide and chronic obstructive pulmonary disease (COPD) in adults: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2018;25(15):15133–15145. doi:10.1007/s11356-018-1629-7
49. Bourbeau J, Doiron D, Biswas S, et al. Ambient air pollution and dysanapsis: associations with lung function and chronic obstructive pulmonary disease in the Canadian cohort obstructive lung disease study. Am J Respir Crit Care Med. 2022;206(1):44–55. doi:10.1164/rccm.202106-1439OC
50. Tran HM, Chen TT, Lu YH, et al. Climate-mediated air pollution associated with COPD severity. Sci Total Environ. 2022;843:156969. doi:10.1016/j.scitotenv.2022.156969
51. Doiron D, Bourbeau J, De Hoogh K, Hansell AL. Ambient air pollution exposure and chronic bronchitis in the Lifelines cohort. Thorax. 2021;76(8):772–779. doi:10.1136/thoraxjnl-2020-216142
52. Ferrari U, Exner T, Wanka ER, et al. Influence of air pressure, humidity, solar radiation, temperature, and wind speed on ambulatory visits due to chronic obstructive pulmonary disease in Bavaria, Germany. Int J Biometeorol. 2012;56(1):137–143. doi:10.1007/s00484-011-0405-x
53. Huh JY, Hong J, Han DW, Park YJ, Jung J, Lee SW. The impact of air pollutants and meteorological factors on chronic obstructive pulmonary disease exacerbations: a nationwide study. Ann Am Thorac Soc. 2022;19(2):214–226. doi:10.1513/AnnalsATS.202103-298OC
54. Wang Q, Liu S. The effects and pathogenesis of PM2.5 and its components on chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2023;18:493–506. doi:10.2147/COPD.S402122
55. Mack SM MA, Madl AK, Pinkerton KE. Respiratory health effects of exposure to ambient particulate matter and bioaerosols. Compr Physiol. 2019;10(1):1–20. doi:10.1002/cphy.c180040
56. Faustini A, Stafoggia M, Colais P, et al. Air pollution and multiple acute respiratory outcomes. Eur Respir J. 2013;42(2):304–313. doi:10.1183/09031936.00128712
57. Wang Y, Xu J, Meng Y, et al. Role of inflammatory cells in airway remodeling in COPD. Int J Chron Obs Pulmon Dis. 2018;13:3341–3348. doi:10.2147/COPD.S176122
58. Paplinska-Goryca M, Misiukiewicz-Stepien P PM, Proboszcz M, et al. Interactions of nasal epithelium with macrophages and dendritic cells variously alter urban PM-induced inflammation in healthy, asthma and COPD. Sci Rep. 2021;11(1):
59. Zilli Vieira CL, Koutrakis P, Liu M, Gottlieb DJ, Garshick E. Intense solar activity reduces urinary 6-sulfatoxymelatonin in patients with COPD. Respir Res. 2023;24(1):91. doi:10.1186/s12931-023-02390-w
60. Squillacioti G, Bellisario V, Ghelli F, et al. Air pollution and oxidative stress in adults suffering from airway diseases. Insights from the Gene Environment Interactions in Respiratory Diseases (GEIRD) multi-case control study. Sci Total Environ. 2024;909:168601. doi:10.1016/j.scitotenv.2023.168601
61. Lederer AM, Fredriksen PM, Nkeh-chungag BN, et al. Environmental Inhalants and Cardiovascular Disease Cardiovascular effects of air pollution: current evidence from animal and human studies. Am J Physiol Hear Circ Physiol. 2021;320(4):1417–1439. doi:10.1152/ajpheart.00706.2020
62. de P SU, Arbex MA, Braga ALF, et al. Environmental air pollution: respiratory effects. J Bras Pneumol. 2021;47(1):e20200267–e20200267. doi:10.36416/1806-3756/e20200267
63. Requia WJ, Adams MD, Arain A, Papatheodorou S, Koutrakis P, Mahmoud M. Global association of air pollution and cardiorespiratory diseases: a systematic review, meta-analysis, and investigation of modifier variables. Am J Public Health. 2018;108(S2):S123–30. doi:10.2105/AJPH.2017.303839
64. Health Effects Institute. Air quality and health in cities: a state of global air report 2022. Boston; 2022.
65. Secretaria Distrital de Ambiente. Inventario de emisiones de Bogotá, contaminantes atmosféricos 2020. Spanish. Bogotá D.C.; 2021.
66. Bălă GP, Râjnoveanu RM, Tudorache E, Motișan R, Oancea C. Air pollution exposure — the (in) visible risk factor for respiratory diseases. Environ Sci Pollut Res. 2021;14.
67. Kravchenko J, Lyerly HK. Objective, method, and inclusion and exclusion criteria. N C Med J. 2018;79(5):289–300. doi:10.18043/ncm.79.5.289
68. Concato J, Stein P, Corrigan-curay J, Pan GJD, Ball R. Randomized, observational, interventional, and real-world—What’s in a name? Pharmacoepidemiol Drug Saf. 2020;11(June):1514–1517. doi:10.1002/pds.5123
69. Gold Science Committee. 2022 GOLD REPORTS 2022 global strategy for prevention. Diagnosis Manag COPD. 2022.
70. Gutierrez MP, Zuidema P, Mirsaeidi M, Campos M, Kumar N. Association between African dust transport and acute exacerbations of COPD in Miami. J Clin Med. 2020;9(8):2496. doi:10.3390/jcm9082496
71. Secretaría Distrital de Ambiente. Documento ejecutivo: plan estratégico para la gestión integral de la calidad del aire de Bogotá 2030– plan Aire 2030 Unidos Por Un Nuevo Aire. Spanish.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
A 4-Year Retrospective Claims Analysis of Oral Corticosteroid Use and Health Conditions in Newly Diagnosed Medicare FFS Patients with COPD
Bazell C, Pollack M, Comellas AP, Sethi S, Alston M, Pyenson B, Hansen D, Caplen M, Staresinic A, Styczynski J, Feigler N
International Journal of Chronic Obstructive Pulmonary Disease 2022, 17:2635-2652
Published Date: 17 October 2022

