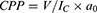Back to Journals » International Journal of Nanomedicine » Volume 19
Functional Evaluation of Niosomes Utilizing Surfactants in Nanomedicine Applications
Authors Gao S , Sui Z, Jiang Q , Jiang Y
Received 30 May 2024
Accepted for publication 15 September 2024
Published 10 October 2024 Volume 2024:19 Pages 10283—10305
DOI https://doi.org/10.2147/IJN.S480639
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Eng San Thian
Shuqi Gao,1,2,* Zhe Sui,3,* Qian Jiang,1,2 Yueyao Jiang1
1Department of Pharmacy, China–Japan Union Hospital of Jilin University, Changchun, Jilin Province, 130033, People’s Republic of China; 2School of Pharmacy, Jilin University, Changchun, Jilin Province, 130021, People’s Republic of China; 3Department of Radiology, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, 130033, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yueyao Jiang, Department of Pharmacy, China–Japan Union Hospital of Jilin University, Changchun, Jilin Province, 130033, People’s Republic of China, Tel +86 13844817007, Email [email protected]
Abstract: Niosomes are key nanocarriers composed of bilayer vesicles formed by non-ionic surfactants and cholesterol, offering advantages such as high physicochemical stability, biodegradability, cost-effectiveness, and low toxicity. This review discusses their significant role in drug delivery, including applications in anticancer therapy and vaccine delivery. It also highlights the impact of non-ionic surfactants on niosome formation, drug delivery pathways, and protein corona formation—a relatively underexplored topic. Furthermore, the application of artificial intelligence in optimizing niosome design and functionality is examined. Future research directions include enhancing formulation techniques, expanding application scopes, and integrating advanced technologies. This review provides comprehensive insights and practical guidance for advancing niosome-based drug delivery systems.
Keywords: niosomes, non-ionic surfactant, drug delivery systems, niosome functionalization
Graphical Abstract:

Introductions
The development of novel drug delivery systems has drawn significant attention, owing to their potential to improve treatment efficacy, reduce drug toxicity and side effects, enhance patient compliance, and drive drug development.1,2 Ideal novel drug delivery systems must accomplish drug delivery at a predetermined rate and release the therapeutically effective amount of the drug at the target site.2 Meeting these requirements with the traditional dosage forms is challenging, whereas vesicular systems excel at addressing them.3
Niosomes are a type of vesicular system and have emerged as a promising tool for drug delivery.4,5 Current research on niosomes mainly focuses on the following areas: (a) Drug delivery systems using niosomes as drug delivery carriers, thus enhancing the bioavailability and controlled release of therapeutic agents. Modifying niosomes allows for targeted delivery.6,7 (b) Vaccine delivery systems using niosomes as carriers for vaccines, thus improving stability, boosting efficacy, and providing long-term immune protection.8,9 (c) Cosmetics and skincare using niosomes for enhanced penetration of active ingredients (such as vitamins, antioxidants) into the skin barrier.10,11 (d) Diagnostics and imaging using niosomes as a versatile platform for enhancing the efficacy and specificity of diagnostic and imaging technologies.12–15(e) Food and agricultural industries using niosomes as food additives, as well as for controlling pesticide release, reducing environmental impact.16
These vesicular structures, composed of non-ionic surfactants with or without cholesterol, mimic the lipid bilayers of liposomes, offering distinct advantages such as enhanced stability and versatility (Figure 1).17 Non-ionic surfactants are amphiphilic molecules with polar heads and non-polar tails that do not have specific, ionic-charged groups in their structure.18 They are pharmacologically non-toxic and inert. They exhibit low hemolysis, cause less irritation to the cell surface, and their pH tends to remain near that of physiological solutions.19 Therefore, non-ionic surfactants are well suited for incorporation into pharmaceutical formulations. The inclusion of a surfactant influences the particle size, polydispersity index (PDI), drug loading, zeta potential, and correlation with the apparent physical stability of nanoparticles (NPs).20 The successful application of NPs in therapeutic targets requires an effective cellular uptake, with the effective interaction between NPs and cell membranes being a key step before cellular uptake.21 This biological interaction is highly dependent on the surface phenomena of NPs and therefore relies on surfactants.21 Consequently, further research on the role of non-ionic surfactants in niosomes and drug therapy is essential.
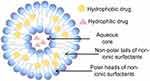 |
Figure 1 Schematic representation of niosomes. (By Figdraw). |
The unique composition of niosomes enables the encapsulation of a wide range of therapeutic agents, including hydrophilic and hydrophobic drugs.22–24 This highlights their significant potential for future therapeutic applications and their broad applicability in treating various diseases. Owing to their tunable properties and biocompatibility, niosomes can be administered via various routes, including the standard oral or parenteral methods, as well as by ocular, intranasal, transdermal, or vaginal methods, as well as inhalation.25–29 The applications of niosomes in various drug administration routes are systematically explored in this review, with a particular focus on the impact of non-ionic surfactants on niosome formation and functionality. The ways non-ionic surfactants influence the formation of niosomes and their roles in the different drug delivery pathways are also presented, highlighting their contributions to optimizing drug delivery efficiency and targeting. Additionally, the effects of non-ionic surfactants on protein corona formation are addressed, elucidating their potential in enhancing drug bioavailability and reducing immune responses. Through this review, we seek to provide a comprehensive understanding and forward-looking insights into the use of niosomes and their modification technologies in the field of drug delivery.
Preparation of Niosomes
Niosomes can be classified into three categories based on their number of bilayers and size: small unilamellar vesicles (SUVs, one bilayer, 10–100 nm), large unilamellar vesicles (LUVs, one bilayer, 100–3000 nm), and multilamellar vesicles (MLV, more than one bilayer, ≥ 5 μm).30,31 Various preparation methods for niosomes are reported in the literature (Table 1), with the types of niosomes produced varying depending on the method used (Figure 2).
 |
Table 1 Types of Niosomes Prepared by Different Methods and Their Advantages and Disadvantages |
The thin-film hydration (TFH) method involves dissolving lipids in an organic solvent, evaporating the solvent under reduced pressure, and then hydrating the lipid film with water at an elevated temperature to form vesicles. The TFH method generally forms MLVs, which can be produced using appropriate cut-off size membranes or ultrasonic treatment to produce small-sized niosomes.32,33 Preparation of niosomes loaded with nintedanib involves the TFH method. Dynamic light scattering analysis of niosome formulations revealed that an increase in cholesterol concentration results in a reduction in vesicle size. According to the polydispersity index (PDI) and evaluation of the particle size distribution, the PDI values of all formulations were within the range of 0.02–0.2, indicating that all developed formulations were uniform, narrowly dispersed, and free of agglomeration. The zeta potential or surface charge of the vesicles influences their biological characteristics, such as the ways they are taken up and internalized by cells. The amount of drug encapsulated in the formulation was measured by lysing the formulation, revealing that the vesicles had a relatively high encapsulation efficiency.29 The trans-membrane pH gradient method involves dissolution of the surfactant and cholesterol in chloroform, evaporation of the solvent to form a lipid film, and hydration of this film with an acidic solution. After forming multilamellar vesicles (MLVs) through freeze-thaw cycles and sonication, an aqueous drug solution is added, and the pH is adjusted to allow the drug to be encapsulated within the vesicles by exploiting the pH gradient.34,35 To prepare niosomes from proniosomes, a proniosome powder—comprising a surfactant-coated water-soluble carrier—is rehydrated in hot water upon agitation. This rehydration forms MLVs, which can be further processed to produce SUVs through high-energy methods or coacervation phase separation. Proniosomes offer advantages in terms of stability, transportation, and scalability.36–39
The microfluidization method affords niosomes via the use of precisely controlled microchannels to mix lipids and aqueous streams at high velocities, facilitating the formation of uniformly small vesicles (< 150 nm) with a low PDI by the self-assembly of surfactant molecules.40–42 Sonication involves mixing cholesterol and a non-ionic surfactant with a drug-containing buffer, followed by ultrasonic treatment. This process initially forms multilamellar vesicles, which can be further sonicated to produce unilamellar vesicles, resulting in small and uniform niosomes.36,43,44 The multiple membrane extrusion technique affords niosomes by forming a thin film comprising the surfactant, cholesterol, and diacetyl phosphate in chloroform, followed by hydration with an aqueous drug solution and extrusion of the suspension through polycarbonate membranes to control the vesicle size.31,45,46
The ether injection method involves slowly injecting a mixture of cholesterol and surfactant dissolved in ether into a preheated aqueous drug solution, leading to the formation of vesicles as the solvent evaporates. This process creates heterogeneous vesicles with variable sizes, however, it exhibits slow rates and residual ether in the suspension.47–49 The “bubble” technique affords niosomes via dispersion of cholesterol and surfactants in a phosphate buffer at 70 °C, followed by homogenization of the mixture and bubbling nitrogen gas through it. The resulting LUVs can be further reduced in size if needed.2,50 The reverse phase evaporation method affords niosomes by dissolving cholesterol and surfactant in a mixture of ether and chloroform, followed by the addition of an aqueous drug phase and sonication. After forming an emulsion, the organic solvent is evaporated, and the resulting viscous suspension is diluted and heated to produce niosomes with a high aqueous space and encapsulation efficiency.1,31,51,52 The lipid injection method prepares niosomes by melting a mixture of lipids and surfactants and injecting it into a hot, agitated aqueous solution containing the drug, without using organic solvents.1,53 The supercritical CO2 (scCO2) method for preparing niosomes involves dissolving surfactants, cholesterol, PBS, and ethanol in a glass view cell, which is then pressurized with CO2 at 200 bar and 60 °C. After 30 min of stirring, niosomes are formed, and the pressure is released, affording uniformly sized LUV niosomes without the need for toxic organic solvents.7,54 The heating method involves dissolution of cholesterol in a liquid heated to approximately 120 °C, followed by cooling to 60 °C and addition of other components while stirring. After preparation, the niosomes are left at room temperature for 30 min, followed by storage in a refrigerator at 4–5 °C under a nitrogen atmosphere. This approach avoids the use of harmful, volatile organic solvents and is a straightforward, one-step procedure.50,55,56
Recent Studies on the Role of Non-Ionic Surfactants
Surfactants contain hydrophobic groups (tail) and hydrophilic groups (head), exhibiting amphiphilicity.57 They can reduce surface tension and interfacial tension at the interfaces of solids, liquids, and gases, and can be used as emulsifiers, foaming agents, corrosion inhibitors, antistatic agents, dispersants, wetting agents, and detergents.20,57–59 Surfactants are typically classified based on the nature of their hydrophilic groups and primarily include anionic surfactants, cationic surfactants, non-ionic surfactants, and amphoteric surfactants. The robustness of non-ionic surfactants against pH and electrolytes gives them an advantage over ionic and other surfactants for pharmaceutical applications.60 As summarized in Table 2, recent studies have highlighted the crucial role of non-ionic surfactants in pharmaceutical applications, especially in enhancing drug delivery systems, stabilizing proteins, and improving the performance of nanoparticles.
 |
Table 2 Summary of Recent Studies on the Effects of Non-Ionic Surfactants |
Non-ionic surfactants are widely used in the preparation of nanocarriers (such as niosomes, bilosomes, and micelles) to enhance drug bioavailability.8,65,66 For example, non-ionic surfactants self-assemble into micelles in aqueous phases and are able to encapsulate hydrophobic drug molecules. Mixed micelles composed of pluronic F127 and cremophor EL can effectively deliver norfloxacin, offering controlled release and good antimicrobial activity against various strains.61 Non-ionic surfactants can protect biologic therapeutic proteins and antibodies from the effects of various solid-liquid interfaces, such as cycloolefin-copolymer and model hydrophobic interfaces. Polysorbate 80 and 20, Poloxamer 188, and Brij 35 offer different levels of protection against these interfaces, highlighting the importance of the optimization of surfactant formulations to stabilize antibodies.62
Non-ionic surfactants have been shown to protect proteins from freezing and surface-induced denaturation. Polysorbate 20 effectively prevents lysozyme aggregation during freeze and thaw, while poloxamer 188 can interact with lysozyme and prevent aggregation at high concentrations; however, some aggregation occurs during freezing but reverses upon thawing.63
Non-ionic surfactants play a crucial role in the production of polymer NPs through emulsion-based methods. They significantly reduce the droplet size in emulsions and enhance the biocompatibility and size tunability of NPs. Additionally, these surfactants not only stabilize the dispersion of NPs but also have extensive effects on pharmacokinetics.20
Non-ionic surfactants enhance the ability of NPs to cross biological barriers. A study reveals that the passage of NPs across the blood-brain barrier (BBB) is primarily influenced by the type of surfactant employed during their fabrication. Specifically, non-ionic surfactants (such as Poloxamer 188, Brij 35, Tween 80, Tween 20, and Lutensol AT80) and cationic surfactants (dextran) facilitate BBB penetration, whereas anionic surfactants, including sodium dodecyl sulfate (SDS), impede it. The particle size and zeta potential do not affect BBB permeability.67 Polysorbate 80 reduced the expression of intestinal mucus barrier and mucosal barrier-related proteins (mucus protein mucin-2, tight junction proteins claudin-1 and occludin), altered the integrity of intestinal epithelial cells, and increased the intestinal epithelial mucosal permeability.64
Impact of Non-Ionic Surfactants on Niosomes
Non-ionic surfactants are the fundamental elements of niosomes, and understanding their properties is crucial for the preparation of the desired niosomes. Common surfactants include alkyl ethers, alkyl glyceryl ethers, sorbitan fatty acid esters, polyoxyethylene fatty acid esters, and poloxamers. The hydrophilic-lipophilic balance (HLB), critical packing parameter (CPP), and gel-liquid transition temperature (TC) are essential for the selection of the non-ionic surfactant.
Hydrophilic-Lipophilic Balance (HLB)
The balance between the hydrophilic and lipophilic groups of non-ionic surfactants is represented by the hydrophilic-lipophilic balance (HLB) value, ranging from 0 to 20.68 The HLB value affects the ability to form niosomes, as well as their particle size, distribution, and encapsulation efficiency (EE). Generally, surfactants with HLB values of 14–17 are not suitable for the formation of niosomal vesicles,7,50,69 because high HLB values demand increased cholesterol concentrations. Cholesterol forms hydrogen bonds with the hydrophilic heads of the surfactants to compensate for the impact of bulky head groups on the critical packing parameters.25,70,71 Pronosomes made from Brij 35 (high HLB) and different proportions of cholesterol were hydrated with hot water to produce niosomes. Analysis of their encapsulation efficiency revealed that niosomes with a 50% cholesterol ratio have higher encapsulation efficiency compared to those with a 20% cholesterol ratio.72
As the HLB of the surfactants increases, the length of the alkyl chain and the vesicular size of the noisomes also increase. Niosomes generated using sorbitan laurate (Span 20 hLB: 8.6) were larger than those generated using sorbitan oleate (Span 80 hLB: 4.3).73 Vesicle sizes prepared with Span 40 (HLB: 6.7) are generally smaller than those prepared with Brij 35 (HLB: 16.9).72 Furthermore, the size distribution of niosomes created with Span 40 was notably larger compared to the vesicles produced with Span 60 (HLB: 4.7).74 Similar results have been reported in other studies.75,76 Such a finding can be attributed to the increase in surface energy as the number of hydrophilic groups increases, thus increasing the particle dimensions. The HLB of a surfactant affects the entrapment efficiency of niosomes. According to a previous study, the entrapment efficiency of ibuprofen decreased when high-HLB surfactants were used, while the entrapment efficiency was higher with a lower HLB.77 By mixing different amounts of Span 40 and Span 60 to achieve varying HLB values and using these surfactants to prepare niosomes, it was confirmed that an increase in the proportion of Span 60 (lower HLB) leads to improved encapsulation efficiency.78 This may have occurred because hydrophilic surfactants, which are highly soluble in water, do not exhibit suitable vesicular structure formation in aqueous environments. Conversely, surfactants that are more lipophilic in nature and have lower HLB values can form vesicles and effectively encapsulate lipophilic and amphiphilic drugs.
Critical Packing Parameter (CPP)
CPP is a dimensionless scale for surfactants and plays a significant role in their assortment.1 The following equation is used to determine the CPP values, where V represents the volume of the nonpolar group, Ic is the length of the critical nonpolar group, and a0 is the area of the polar head group.
CPP values help predict the types of vesicles formed by surfactants. A CPP value below 1/3 indicates the formation of spherical micelles; a value ranging from 1/3 to 1/2 suggests the presence of cylindrical micelles; and a value between 1/2 and 1 indicates the transformation into bilayer vesicles. A CPP value exceeding 1 indicates the formation of inverted micelles.69,79,80
Gel-Liquid Transition Temperature (TC)
The gel–liquid transition temperature (TC) is a crucial factor that directly affects the entrapment efficacy, which is affected by the alkyl-chain length of the surfactant and impacts the fluidity of the vesicles formed.1 The temperature should remain consistently higher than the gel-to-liquid phase transition temperature of the system. At TC, the niosome bilayer undergoes a transition from an ordered gel phase to a disordered liquid phase.81 When the temperature is below the Tc value, the surfactant molecules are tightly packed, forming a rigidly ordered gel phase. As the temperature approaches Tc, the tightly packed surfactants begin to relax, leading to a less ordered liquid phase.82 Generally, an increase in the carbon count in the alkyl chain leads to a higher Tc, consequently leading to a higher entrapment efficiency.7,83
Mixed Non-Ionic Surfactants
Mixed surfactants exert a synergistic effect. Dejan Ćirin et al investigated the interactions between various surfactants at the interface using Rosen’s model for mixed monolayers. Synergism was also observed in the Brij S20/poloxamer 407 mixture.84 Compared to Brij-35 or Poloxamer 407 alone, oral niosomes loaded with tacrolimus (TLM) prepared using a mixture of Brij-35 and Poloxamer 407 as surfactants exhibited a synergistic effect on the dissolution of TLM. They also exhibited greater drug solubility and higher encapsulation efficiency.85
Role of Non-Ionic Surfactants in Niosome Drug Delivery Pathways
The route of administration plays a crucial role in determining drug pharmacokinetics and the appropriate concentration at the target site.86 Niosomes can be administered via almost all delivery routes, such as oral (eg, tacrolimus, atorvastatin),85,87 transdermal (eg, sulfadiazine sodium salt, curcumin),39,88 as well as pulmonary, parenteral, intranasal, and ocular (Table 3).
 |
Table 3 The Mechanism and Application of Niosomes Utilizing Surfactants in Delivering Drugs |
Oral Delivery
Among the different methods of drug administration, the oral route has gained significant attention owing to its distinctive benefits, including adaptability, safety, and patient adherence.124 The oral route is non-invasive, convenient, and cost-effective.125 However, this route of drug administration is also subject to poor or erratic bioavailability owing to a variety of reasons, such as poor water solubility, efflux by gut wall transporters, and first-pass metabolism,126 especially for drugs that belong to the class II biopharmaceutical classification system (BCS II) category.85,94 To overcome these problems, various nanotechnology-based drug delivery systems have been investigated, including polymers,127,128 polysaccharides,129,130 solid nanodispersion,131 solid lipid nanoparticles,132 self-nanoemulsifying drug delivery systems,133,134 nanocrystals,135,136 and vesicular drug delivery systems (VDDS).137 Among these approaches, niosomes belonging to the VDDS have been widely investigated owing to their biocompatibility, non-immunogenicity, high chemical stability, and low cost.55,87
Mechanism of Non-Ionic Surfactants in Niosomes Oral Drug Delivery
Non-ionic surfactants, which are the primary constituents of niosomes, can solubilize poorly soluble drugs owing to their amphiphilic properties.7 Following ingestion, the small size of niosomes enables a substantial interfacial surface area that is conducive to intestinal absorption.94 In addition, niosomes achieve sustained release of drugs and prolong their duration of action by encapsulating them within the lipophilic bilayers. Surfactant elements can also reduce P-glycoprotein (P-gp)-mediated effluxes (Figure 3a). The efflux membrane transporter P-gp is extensively distributed throughout the body and functions as a physical barrier by expelling foreign substances and poisons from cells.138,139 P-gp belongs to a family of ATP-dependent membrane transport proteins responsible for expelling substrates from cells via an ATP-driven process.140 These ATP-dependent transporters interact with numerous substrates, most of which are hydrophobic, such as antitumor medications, therapeutic agents targeting central nervous system (CNS) and the cardiovascular system, as well as antibiotics.141–143 The efflux properties of P-gp are important factors that contribute to the low bioavailability of therapeutic substrates. Therefore, it is crucial to investigate P-gp inhibitors to overcome the low bioavailability of drugs. Notably, certain non-ionic surfactants demonstrate inhibitory properties against P-gp.19,144 Their inert, non-toxic, uncharged nature and rapid access to the cytosolic lipid membrane (site of interaction with the P-gp efflux protein) enable them to function more efficiently as P-gp inhibitors. The observed inhibitory effects have been linked to the modulation of membrane fluidity, suppression of the ATP-binding cassette of the transporter P-glycoprotein, and attachment of surfactants to the drug-binding domain from the cytoplasmic leaflet.19,145,146 Zhao et al demonstrated that the underlying mechanisms behind the inhibition of P-gp include changes in fluidity of the intestinal membrane and suppression of the P-gp ATPase function by two polyoxyethylene alkyl ethers (polyoxyethylene 10-oleyl ether and polyoxyethylene 9-lauryl ether).147
 |
Figure 3 The potential mechanisms of niosomes for improving the oral (a), transdermal (b), ocular (c), intranasal (d), pulmonary (e) bioavailability. (By Figdraw). |
Application of Niosomes in Oral Drug Delivery
Antibacterial Activity
The application of niosomes enhances antibacterial activity. Improper use of antibiotics can lead to drug resistance; thus, widely used antibiotics require strategic and optimal methods to avoid drug resistance. Niosomal formulations loaded with ciprofloxacin induce the inhibition of biofilm formation in Escherichia coli and Staphylococcus aureus.89 The effectiveness of streptomycin sulfate-loaded niosomes against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa surpassed that of the free drug, demonstrating significantly increased antimicrobial and anti-biofilm activities. Moreover, the minimum inhibitory concentration values decreased by a factor ranging from 4 to 8.90 Imran et al synthesized a novel sugar-based twin-tailed surfactant (bergenin-based non-ionic surfactant) for the preparation of niosomal vesicles, leading to the improved oral bioavailability of levofloxacin.91 Compared with plain drug solutions and commercially available suspensions, cefdinir oral niosomes prepared via sonication using Span-60, cholesterol, and soy lecithin as raw materials improved the permeability of cefdinir across goat intestinal membranes. These findings suggest that niosomes exhibit better oral absorption. Studies on antimicrobial activity have shown that niosomal formulations augment the antibacterial activity of cefdinir compared to commercially available suspensions.92 Hanif et al employed niosomal encapsulation to enhance the solubility of tacrolimus and improve its oral bioavailability.85 Griseofulvin, which is an antifungal agent, exhibits low water solubility and limited absorption via the oral route. Consequently, conventional oral administration often fails to yield effective plasma drug profiles. Niosomes prepared from Span 60 have exhibited rapid absorption in vivo, facilitating faster attainment of peak plasma concentrations and prolonged maintenance of high levels of griseofulvin.93
Niosomes are also extensively used to treat chronic diseases. Owing to the frequent and long-term treatment requirements, oral administration is an important route for managing chronic conditions. This mode of administration facilitates patient compliance and enhances treatment compliance while providing a steady and sustained drug concentration.
Anti-Hyperglycemic Effect
Nateglinide exhibits poor water solubility and lipophilicity, and its degree of ionization depends on the pH of the absorption sites, which results in a narrow absorption window. Compared with simple solutions of nateglinide, niosomal dispersions offer a quicker onset of action and higher oral bioavailability. This is attributed to niosomes enhancing the dissolution of nateglinide and increasing its absorption via paracellular pathways, potentially bypassing systemic pre-metabolism.95 Recombinant human insulin embedded in multilamellar niosomes comprising polyoxyethylene alkyl ether surfactants (Brij 52 and Brij 92), sorbitan monostearate (Span 60), and cholesterol exhibited good stability in bile salt solutions. Furthermore, compared to a free insulin solution, the vesicles demonstrated significant protection against proteolytic enzymes.96 Using the thin-film hydration technique, niosomes encapsulating glimepiride demonstrated a seven-fold increase in oral bioavailability, along with a prolonged duration of action. This approach allows for reduced dosage and dosing frequency, potentially minimizing drug-related adverse effects.97
Anti-Hypertensive Effect
Niosomes containing telmisartan were formulated to improve its antihypertensive activity, significantly attenuating the elevated mRNA and protein levels of the angiotensin II type-1 receptor (AT1R) gene.98 Carvedilol has a limited systemic availability owing to its high metabolism, and nanoniosomes are promising as stable carriers for its oral delivery.99 Compared with drug suspensions, niosomes enhance the oral bioavailability of carvedilol by twofold. In addition, bile salt-enriched (sodium cholate or sodium taurocholate) niosomes exhibit stronger intestinal absorption than conventional niosomes.100 Candesartan cilexetil-loaded niosomes prepared using a combination of surfactants, Span 60/Pluronic p85, demonstrated enhanced drug release and stability.101
Anti-Hyperlipidemic Effect
Atorvastatin poses challenges in terms of its biopharmaceutical properties, including a low dissolution rate and extensive pre-systemic disposition, leading to decreased oral bioavailability, thus requiring higher doses and resulting in undesired side effects. Niosomes containing Span 60, cholesterol, and dicetyl-phosphate have been shown to enhance the anti-hyperlipidemic effects of atorvastatin. Further improvement in drug action was achieved by encapsulating niosomes with chitosan.87
Vaccine
The niosomal system may be a potential candidate for oral vaccine delivery. Niosomes encapsulating inactivated vaccines offer a safe approach owing to their inability to replicate, mitigating the risk of disease generation, which are often associated with viral delivery systems. Moreover, compared to free, inactivated vaccines, niosomes that encapsulate these vaccines elicit a more potent immune response.148 Tetanus toxoid (TT) antigens encapsulated in niosomes were evaluated for their immunostimulatory effects after oral administration by measuring serum IgG antibody levels, demonstrating better humoral reactions compared with their ordinary counterparts.102
Transdermal Delivery
Mechanism of Non-Ionic Surfactants in Niosomes Transdermal Drug Delivery
Transdermal drug administration involves the regulated dispensation of medications via the skin to achieve consistent therapeutic concentrations in the body. This route is less invasive and prevents the drug from degrading in the extreme acidity of the stomach, enabling stable transportation of drugs.149 However, there are biological barriers in the human body that are vital for the operation of numerous human organs and serve as shields from physical, chemical, and biological harm.150 Medications must cross these barriers to reach the target site and exert their efficacy. The objective of a transdermal delivery system is to traverse the epidermis, primarily the stratum corneum (SC), and penetrate the dermal capillaries before entering the systemic blood circulation. SC serves as the primary barrier and consists of densely packed and heavily keratinized deceased cells.151 A transdermal drug delivery route focuses on ensuring optimal drug penetration.149 However, SC restricts drug penetration, making it difficult for most drugs to penetrate the skin.105 The application of niosomes simplifies the encapsulation of various bioactive compounds, enhances their physicochemical stability, reduces severe side effects and skin irritation, and improves transdermal absorption, as well as the accumulation of payload at the delivery site.106 Niosomes can engage with SC via fusion, aggregation, and adhesion processes, leading to a significant thermodynamic activity gradient of the drug at the vesicle-skin interface, thus inducing drug penetration.152,153 The skin permeation of niosomes is influenced by the particle size, type of surfactant, elasticity, surface charge, and the concentration of cholesterol.
Surfactants enhance drug permeability via SC by two primary means. First, their surface tension-reducing properties enhance fluidity and enable the solubilization and extraction of lipids from the epidermis, consequently leading to corneocyte disruption by binding and interacting with keratin filaments.154 Second, the adhesion of niosomes to the skin surface induces changes in the SC properties by reducing epidermal water loss, consequently increasing skin hydration and loosening the tightly packed cellular structures (Figure 3b).150
In addition, the stability and skin permeability of niosomes can be improved by incorporating additives into their structure. For example, the use of SDS to enhance the charge within the niosomal system or the introduction of essential oils as potential niosomal fluidizers alters the fluidity of the vesicle membrane.74,107
Application of Niosomes in Transdermal Drug Delivery
Niosomes are lipid-based vesicles that enhance the penetration of drugs via the skin. They can act as reservoirs for the sustained release of active compounds in the skin. When non-ionic surfactants are incorporated into niosomes, the skin tends to tolerate them more effectively compared to when they are used in an emulsion, especially in the context of the local mucosal irritation caused by many anti-inflammatory drugs.27 Diclofenac sodium is a potent nonsteroidal anti-inflammatory drug with significant analgesic effects requiring frequent administration owing to its short half-life; its long-term use can cause adverse gastrointestinal reactions, such as ulcers, bleeding, or intestinal wall perforation. Therefore, controlled transdermal administration is considered a more appropriate administration mode for diclofenac sodium. Niosomes prepared using span20, tween20, and cholesterol and loaded with diclofenac sodium gel demonstrated greater permeability of the skin layer and anti-inflammatory activity than a regular gel with diclofenac sodium.108 Niosomes containing diacerein prepared using the thin film hydration method also demonstrated this superior effect.109 Auda et al prepared niosomes containing celecoxib using a thin-film hydration method with various surfactants. The anti-inflammatory effects of different niosomal gel formulations were evaluated using the carrageenan-induced rat paw edema method. Their findings revealed that the poloxamer niosomal gel exhibited significant anti-inflammatory effects against rat paw edema.110 Besides anti-inflammatory drugs, many other drugs are also suitable for transdermal administration. Niosomes loaded with loratadine (LRD) were incorporated into transdermal patches for the treatment of allergies. Compared with control patches, niosomal patches demonstrated improved drug release and permeability, with the niosomal surfactants acting as penetration enhancers.103 Another study prepared niosomes using Span 40, cholesterol, and SDS for transdermal delivery of salidroside. The inclusion of SDS significantly enhanced the zeta potential and stability of niosomes, with an optimal concentration of 0.1% SDS leading to the highest transdermal flux.74 The niosomal gel for transdermal delivery of propofol addresses the issues of hypersensitivity and pain related to intravenous administration. Compared with the control gel, the niosomal gel demonstrated significantly improved propofol release and bioavailability in rats, indicating its potential as a non-invasive alternative for procedural sedation, particularly in pediatrics.104
Ocular Delivery
Owing to the complex anatomical structure and physiological barriers of the eye, drug delivery to the intraocular tissues is highly challenging.155 The ocular bioavailability of drugs administered in the conventional form is typically less than 5%.156 Research indicates that niosomes are promising as carriers for drug delivery to ocular tissues. Primarily, their nano-scale size enables them to withstand the drainage caused by reflex tearing and blinking. Additionally, niosomes have demonstrated improved retention on the ocular surface compared to alternative carriers (Figure 3c).18 Niosomes encapsulating pilocarpine hydrochloride offer greater permeability, longer ocular retention time, and drug metabolism protection compared to traditional formulations. Additionally, Tween 60 formulations provide more uniform dispersion, optimal ocular delivery particle size, and better entrapment efficiency compared to Span 60 formulations.44 A study developed a niosome-in-gel system using latanoprost for sustained ocular delivery. Niosomes were optimized for drug encapsulation achieving a maximum efficiency of 98%. When incorporated into a Pluronic F127 gel, the formulation showed effective and prolonged intraocular pressure reduction in rabbits, without irritation, suggesting its potential for improving glaucoma treatment adherence compared to traditional eye drops.52 Zeng et al developed niosomes coated with mucoadhesive hyaluronic acid (HA) that improved the tacrolimus precorneal retention time, aqueous humor pharmacokinetics, and corneal permeability. Compared to suspensions or non-coated niosomes, the ocular bioavailability of tacrolimus increased by 2.3- or 1.2-fold, respectively.111 Another study aimed to enhance the anti-inflammatory activity of flurbiprofen (FBP)-prepared niosome gel systems using the non-ionic surfactant Span 60. The gel prolonged the contact time of FBP in ocular tissues, exhibiting a rapid anti-inflammatory action and higher bioavailability in inflamed rabbit eyes.112 Allam et al loaded niosomes by encapsulating betaxolol into pH-responsive in situ-forming gels to prolong the precorneal retention of the drug. This gel exhibited a more sustained in-vitro drug release compared to commercially available eye drops, leading to a prolonged reduction in the intraocular pressure in both normal and glaucomatous rabbits, along with a significant improvement in the relative bioavailability of betaxolol.113 In another study, niosomes were prepared and evaluated for the ocular delivery of hydrochloride naltrexone (NTX) using Span 60. In-vitro transcorneal permeation studies indicated that niosomes control NTX permeation and enhance corneal permeability.114
Injection Administration
The routes of injection administration include subcutaneous injection, intramuscular injection, intravenous injection, and intradermal injection, which can quickly deliver drugs into the body. Intravenous administration enables drugs to directly enter the systemic circulation, leading to a rapid onset of action and high bioavailability. Niosomes can be also delivered via intravascular pathways. Niosomes improve drug stability and extend their presence in the bloodstream. The administration of paclitaxel-loaded niosomes prepared using Span 40 significantly extended the elimination half-life of paclitaxel and delayed its excretion from plasma.115 In addition, targeted delivery to specific sites can be achieved with certain modifications. Tan et al developed a targeted transferrin receptor 6-O-palmitoyl ascorbic acid-based niosome for the intravenous injection of tocotrienols (T3) in breast cancer. They conjugated transferrin to the surface of niosomes using chemical linkers. Mice treated in vivo with tumor-targeted niosomes exhibited an average tumor volume 12 times lower than that of the untreated group.116 He et al formulated PEGylated niosomes containing paeonol to enhance its stability, bioavailability, and prolong cellular uptake. The formulated paeonol exhibited superior cytotoxicity compared to the free drug in HepG2 cells.117 Niosomes can also serve as vaccine carriers, overcoming the limitations associated with the instability of pristine antigens, RNA and DNA molecules, improving efficacy and providing long-term immune protection.8,9 Furthermore, some vaccines using lipid NPs for the prevention of COVID-19 have been approved by the Food and Drug Administration (FDA) or are in clinical trials.9
Intranasal Delivery
Intranasal delivery is suggested as a non-invasive approach for administering therapeutic agents to the brain. The transportation of drugs from the nasal cavity to the brain can be accomplished by two methods: direct transport via the olfactory and trigeminal neuronal pathways or indirect transport via systemic absorption.157 The direct route from the nose to the brain through the olfactory pathway offers excellent brain targeting efficacy (Figure 3d).158 Niosomes composed of non-ionic surfactants can interact with the nasal mucosa, owing to their lipophilicity, thereby enhancing permeability.119 Nefopam hydrochloride (NF) is an analgesic drug with low bioavailability (approximately 36%) due to first-pass metabolism. Nasal delivery of NF-loaded niosomes bypasses first-pass metabolism, enhances drug permeation through the nasal mucosa, and prolongs release time.118 Reports on niosomal systems for bromocriptine to enhance brain delivery via the nasal route, reveal significant improvements in brain targeting and pharmacodynamics. The optimized formulation demonstrated superior brain bioavailability and safety, with a nearly 6.47-fold increase in brain availability compared to the oral equivalent, indicating it as a promising alternative to oral delivery for Parkinson’s Disease management.119 Rinaldi et al prepared and characterized chitosan glutamate-coated niosomes loaded with pentamidine. These formulations showed promising mucosal adhesion and stability, with effective drug embedding and interaction with mucins.26 Olanzapine (OL) is an atypical antipsychotic drug that exhibits enhanced bioavailability when formulated in niosomes. Compared to the intranasal drug solution, the optimized nasal niosomes coated with OL afforded a three-fold higher concentration of OL in the brain.120 A niosomal in situ nasal gel loaded with buprenorphine hydrochloride was developed for treating anxiety, with in vitro permeation studies through sheep nasal mucosa revealing 83.49% drug permeation after 8 h.121
Pulmonary Delivery
Pulmonary administration is generally the preferred route of drug delivery for treating respiratory-related diseases such as pulmonary infections, asthma, and lung cancer. Delivering niosomal drugs via the pulmonary route presents several benefits, including enhanced mucus penetration, prolonged drug release, targeted delivery, and improved therapeutic outcomes (Figure 3e).7 Niosomes encapsulating salbutamol sulfate (SS) were prepared using a reverse-phase evaporation method and formulated into inhalable dosage forms. The SS-loaded niosomes effectively reduced the clearance rate of the drug, improving the deposition and retention of water-soluble SS in the lungs.122 In another study, cilomilast, a phosphodiesterase-4 inhibitor that is used to treat inflammatory lung diseases, was encapsulated within PEGylated phosphatidylcholine-rich niosomes to improve pulmonary delivery via the strong binding of niosomes to the pulmonary surfactant film. A twofold improvement in lung uptake was revealed as well as fewer adverse effects after encapsulation.123 Niosomes loaded with nintedanib for inhalable delivery against non-small cell lung cancer (NSCLC) showed increased drug encapsulation, optimal vesicle size, and size distribution due to cationic modification, resulting in enhanced internalization and significant cytotoxic effects on NSCLC cells.29 Gemcitabine (Gem) and cisplatin (Cis) are used for lung cancer treatment, however, they are highly toxic at high doses. Saimi et al developed a niosome formulation containing low-dosage Gem and Cis and optimized it using a simple heating method, revealing a high aerosol output (96.22%) and controlled drug release over 24 h. Cytotoxicity studies indicated that niosomes significantly reduced Gem and Cis toxicity compared to free drugs, suggesting their potential as a promising aerosolized treatment for lung cancer.56
Other Applications
Niosomes also play a crucial role in diagnostics and imaging. Various imaging tests, such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound imaging, utilize imaging agents or contrast agents to improve contrast and image visualization.159–162 Niosomes can be engineered to deliver imaging agents or contrast agents specifically to target tissues or cells, enhancing the precision of diagnostic imaging and minimizing side effects. Niosomes modified with transferrin (Tf) containing integrated magnetic iron oxide NPs (MIONs) and quantum dots (QDs) were formulated for effective imaging of gliomas, supported by magnetic and active targeting. The developed niosomes exhibited a high potential for cell specific dual targeting (active targeting and magnetic targeting) and dual imaging (magnetic resonance imaging and fluorescence imaging) in gliomas.12 Niosomes labeled with the radioactive technetium-99m isotope and prepared via the thin-film hydration method demonstrated good in-vitro stability and high cancer-cell incorporation capacity.163 Furthermore, niosomes can be designed to carry both diagnostic and therapeutic agents, enabling simultaneous imaging and therapy, which is particularly useful in tracking the progress of treatment. Theranostic pH-responsive niosome preparations for doxorubicin delivery and bio-imaging of breast cancer exhibited good anti-cancer activity at low concentrations.13 Theranostic niosomes used for direct intratumoral injection exhibited significantly enhanced tumor retention and anti-cancer effects.14 In addition, niosomes can be used to deliver probes or biomarkers that bind specifically to disease markers, facilitating early detection and monitoring of diseases. Niosomes comprising Tween 20 and Tween 21 used as colloidal nanocarriers for delivering molecular probes and therapeutic agents were detected with high sensitivity and accuracy using HPLC-FLD devices.15
Role of Non-Ionic Surfactants in Protein Coronas
Protein coronas influence the biodistribution, trafficking, and interactions of nanoparticles (NPs) with cell receptors. After NPs are administered by different means, they are promptly exposed to high protein levels in the bloodstream and quickly bind to proteins on their surface, resulting in the formation of complex protein coronas.164 Two types of distinct corona layers are found on the surface of NPs: hard and soft. The hard corona is the initial tightly bound layer of proteins, whereas the soft corona refers to the outer layer of proteins (not directly bound to the NPs).165 The presence of protein coronas may trigger immune responses, leading to the rapid degradation of the macrophage phagocytic system and non-specific cellular uptake, further affecting drug efficacy and targeting.166,167
Non-ionic surfactants can reduce the nonspecific adsorption of proteins onto nanoparticles, thereby decreasing the formation of protein coronas, which may be owing to the formation of hydrophilic shells on the surface of the nanoparticles, resulting in reduced protein-binding affinity. Additionally, when surfactants strongly bind with nanoparticles, it is difficult for proteins to displace them.168 PEGylation is the conventional method used to reduce protein adsorption. PEGylated drugs and nanocarriers exhibit extended half-lives in the blood and reduced nonspecific cellular uptake compared with unmodified drugs.169,170 However, PEG, which is a nonbiodegradable polyether, may accumulate in the body upon prolonged use of drug-PEG conjugates, leading to potential adverse effects.171,172 Therefore, finding alternatives to PEG is crucial, and non-ionic surfactants have gained attention owing to their non-toxicity and biocompatibility. Mueller et al employed the non-ionic surfactant polyphosphoester (PPE) to coat nanoparticles, offering a simplified approach for modulating protein coronas and their biological effects. After incubation with plasma, protein adsorption on the nanoparticles coated with PPE decreased, and the uptake by the macrophages was reduced.168
In addition, non-ionic surfactants may influence the composition and structure of the protein corona, thereby altering the interactions of nanoparticles with cells or other biomolecules (Figure 4). The specific effects depend on factors such as the type and concentration of non-ionic surfactants, as well as the characteristics of the nanoparticles. The type and relative abundance of proteins influence the intracellular uptake and regulate the internalization of nanoparticles. Among the numerous proteins adsorbed onto nanoparticle surfaces, only a fraction can bind to cells. Less than 27% of the protein coronas can undergo antibody binding owing to steric hindrance.173 Furthermore, only a limited number of cell receptors bind to the different serum proteins present on the surface of nanoparticles for uptake.174 If the protein corona can be personalized to promote preferential internalization of the protein corona nanoparticle by diseased cells rather than by healthy cells, the therapeutic efficacy of the drug may be enhanced. For example, the formulation of niosomes with different surfactants and their varying proportions may influence the characteristics of protein adsorption, thereby affecting the in-vivo targeting performance of nanoparticles. Upon comparing niosomes synthesized from different Tween derivatives (Tween20, Tween21, and Tween80), formulations containing both Tween20 and Tween21 were found to have a relatively higher content of absorbed C1QC protein in the protein coronas and exhibited a higher tendency for internalization by cancer cell lines (HeLa cells for cervical cancer).175
 |
Figure 4 The impact of protein coronas on the characteristics and behavior of niosomes. (By Figdraw). |
Applications of Artificial Intelligence
Artificial intelligence (AI) and nanomedicine play crucial roles in advancing personalized medicine. AI has been used in several notable areas of nanomedicine, including for predicting the structure-activity relationships, composition, safety, and efficacy of niosomes, as well as for pharmacokinetics (Figure 5).176 Complex functions or data can be interpreted, managed, and analyzed more precisely using AI algorithms.177
 |
Figure 5 Machine learning optimization of niosomes. (By Figdraw). |
Various artificial intelligence techniques, including machine learning are currently available to assist in the preparation of niosomes. For example, a machine learning approach was employed to optimize niosome drug formulations. By screening literature with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) system, 114 niosome formulations were analyzed. Eleven properties influencing the particle size and drug entrapment were identified and used to train a neural network model with a hyperbolic tangent sigmoid transfer function and the Levenberg-Marquardt backpropagation algorithm. The model achieved a high prediction accuracy for drug entrapment and particle size. Sensitivity analysis highlighted the drug/lipid ratio and cholesterol/surfactant ratio as key factors. The model’s accuracy was validated through the preparation of donepezil hydrochloride niosome batches, demonstrating a prediction accuracy of over 97%. The study concluded that the global artificial neural network outperforms the local response surface methodology for niosome formulations.178 Machine-learning algorithms can be used to optimize the composition, structure, and properties of niosomes, providing predictive information regarding their stability, drug loading ability, and release, leading to better drug release and absorption effects at a lower cost and time.178,179 These artificial intelligence methods offer more efficient and accurate approaches for preparing and improving the performance of niosomes than traditional methods. However, experimental validations are necessary when applying these technologies to ensure their feasibility and safety.
Conclusion
Niosomes offer multiple advantages as drug delivery systems. Comprising non-ionic surfactants and cholesterol, they demonstrate excellent biocompatibility, minimal toxicity, and fewer adverse effects in vivo. The presence of non-ionic surfactants contributes to the formation of stable structures, playing a crucial role in various administration routes for niosome-mediated drug delivery and enhancing drug permeability and absorption in tissues. Based on current research, we believe that niosomes hold significant potential as multifunctional and efficient drug delivery carriers, and continued investigation is essential to unlocking their full therapeutic potential. Expanding the application of non-ionic surfactants in this area could lead to substantial advancements in drug efficacy and patient outcomes. Furthermore, future developments in niosome technology could benefit from the integration of artificial intelligence to further optimize their design and functionality. This would not only improve therapeutic outcomes but also pave the way for broader clinical applications across various medical fields, including cancer therapy, gene delivery, and vaccine management. Overall, niosomes exhibit high controllability and are promising as significant therapeutic tools in the future of medicine. To fully harness their potential, continued and rigorous research into their key components, especially non-ionic surfactants, is essential for advancing niosome technology and expanding its clinical applications.
Acknowledgments
This work was supported by the Natural Science Foundation of Jilin Province (No. YDZJ202401236ZYTS).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Witika BA, Bassey KE, Demana PH, Siwe-Noundou X, Poka MS. Current advances in specialised niosomal drug delivery: manufacture, characterization and drug delivery applications. Int J Mol Sci. 2022;23(17):9668. doi:10.3390/ijms23179668
2. Rajera R, Nagpal K, Singh SK, Mishra DN. Niosomes: a controlled and novel drug delivery system. Review. Biol Pharm Bull. 2011;34(7):945–953. doi:10.1248/bpb.34.945
3. Laffleur F, Keckeis V. Advances in drug delivery systems: work in progress still needed? Article. Int J Pharm. 2020;590119912. doi:10.1016/j.ijpharm.2020.119912
4. Mahale NB, Thakkar PD, Mali RG, Walunj DR, Chaudhari SR. Niosomes: novel sustained release nonionic stable vesicular systems - an overview. Article. Adv Colloid Interface Sci. 2012;183:46–54. doi:10.1016/j.cis.2012.08.002
5. Ray SK, Bano N, Shukla T, Upmanyu N, Pandey SP, Parkhe G. Noisomes: as novel vesicular drug delivery system. J Drug Delivery Ther. 2018;8(6):335–341. doi:10.22270/jddt.v8i6.2029
6. Abdelkader H, Alani AWG, Alany RG. Recent advances in non-ionic surfactant vesicles (niosomes): self-assembly, fabrication, characterization, drug delivery applications and limitations. Drug Delivery. 2014;21(2):87–100. doi:10.3109/10717544.2013.838077
7. Bhardwaj P, Tripathi P, Gupta R, Pandey S. Niosomes: a review on niosomal research in the last decade. J Drug Delivery Sci Technol. 2020;56:101581. doi:10.1016/j.jddst.2020.101581
8. Riccardi D, Baldino L, Reverchon E. Liposomes, transfersomes and niosomes: production methods and their applications in the vaccinal field. Review. J Transl Med. 2024;22(1):339. doi:10.1186/s12967-024-05160-4
9. Thai Thanh Hoang T, Suys EJA, Lee JS, Nguyen DH, Park KD, Truong NP. Lipid-based nanoparticles in the clinic and clinical trials: from cancer nanomedicine to COVID-19 vaccines. Review. Vaccines. 2021;9(4):359. doi:10.3390/vaccines9040359
10. Abu-Huwaij R, Alkarawi A, Salman D, Alkarawi F. Exploring the use of niosomes in cosmetics for efficient dermal drug delivery. Review. Pharmaceutical Dev Tech. 2023;28(7):708–718. doi:10.1080/10837450.2023.2233613
11. Mawazi SM, Ann TJ, Widodo RT. Application of niosomes in cosmetics: a systematic review. Review. Cosmetics. 2022;9(6):127. doi:10.3390/cosmetics9060127
12. Ag Seleci D, Maurer V, Barlas FB, et al. Transferrin-decorated niosomes with integrated InP/ZnS Quantum dots and magnetic iron oxide nanoparticles: dual targeting and imaging of glioma. Int J Mol Sci. 2021;22(9):4556. doi:10.3390/ijms22094556
13. Saharkhiz S, Zarepour A, Zarrabi A. A new theranostic pH-responsive niosome formulation for doxorubicin delivery and bio-imaging against breast cancer. Int J Pharm. 2023;637:122845. doi:10.1016/j.ijpharm.2023.122845
14. Nowroozi F, Dadashzadeh S, Soleimanjahi H, et al. Theranostic niosomes for direct intratumoral injection: marked enhancement in tumor retention and anticancer efficacy. Nanomedicine. 2018;13(17):2201–2219. doi:10.2217/nnm-2018-0091
15. Primavera R, Di Francesco M, De Cola A, et al. HPLC-FLD and spectrofluorometer apparatus: how to best detect fluorescent probe-loaded niosomes in biological samples. Article. Colloids Surf B Biointerfaces. 2015;135:575–580. doi:10.1016/j.colsurfb.2015.08.006
16. Rezvani M, Hesari J, Peighambardoust SH, Manconi M, Hamishehkar H, Escribano-Ferrer E. Potential application of nanovesicles (niosomes and liposomes) for fortification of functional beverages with Isoleucine-Proline-Proline: a comparative study with central composite design approach. Food Chem. 2019;293:368–377. doi:10.1016/j.foodchem.2019.05.015
17. Bartelds R, Nematollahi MH, Pols T, et al. Niosomes, an alternative for liposomal delivery. Article. PLoS One. 2018;13(4):e0194179. doi:10.1371/journal.pone.0194179
18. Chen S, Hanning S, Falconer J, Locke M, Wen J. Recent advances in non-ionic surfactant vesicles (niosomes): fabrication, characterization, pharmaceutical and cosmetic applications. Eur J Pharm Biopharm. 2019;144:18–39. doi:10.1016/j.ejpb.2019.08.015
19. Rathod S, Desai H, Patil R, Sarolia J. Non-ionic surfactants as a P-Glycoprotein(P-gp) efflux inhibitor for optimal drug delivery—a concise outlook. AAPS Pharm Sci Tech. 2022;23(1):55. doi:10.1208/s12249-022-02211-1
20. Cortés H, Hernández-Parra H, Bernal-Chávez SA, et al. Non-ionic surfactants for stabilization of polymeric nanoparticles for biomedical uses. Materials. 2021;14(12):3197. doi:10.3390/ma14123197
21. Salatin S, Dizaj SM, Khosroushahi AY. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Review. Cell Biol. Int. 2015;39(8):881–890. doi:10.1002/cbin.10459
22. Momekova DB, Gugleva VE, Petrov PD. Nanoarchitectonics of multifunctional niosomes for advanced drug delivery. Review. Acs Omega. 2021;6(49):33265–33273. doi:10.1021/acsomega.1c05083
23. Srinivasan N, Murali R. Niosomes: a promising novel drug delivery systems for phytoconstituents. Review. Ann Phytomed-an I J. 2023;12(1):72–78. doi:10.54085/ap.2023.12.1.18
24. Purohit SJ, Tharmavaram M, Rawtani D, Prajapati P, Pandya H, Dey A. Niosomes as cutting edge nanocarrier for controlled and targeted delivery of essential oils and biomolecules. Review. J Drug Delivery Sci Technol. 2022;73103438. doi:10.1016/j.jddst.2022.103438
25. Verma A, Tiwari A, Saraf S, Panda PK, Jain A, Jain SK. Emerging potential of niosomes in ocular delivery. Review. Expert Opin Drug Delivery. 2021;18(1):55–71. doi:10.1080/17425247.2020.1822322
26. Rinaldi F, Hanieh PN, Chan LKN, et al. Chitosan glutamate-coated niosomes: a proposal for nose-to-brain delivery. Article. Pharmaceutics. 2018;10(2):38. doi:10.3390/pharmaceutics10020038
27. Chatur VM, Dhole SN. Niosomes: a promising drug delivery system in transdermal drug delivery (TDDS). Review. J Pharm Res Int. 2021;33(48B):6–17. doi:10.9734/JPRI/2021/v33i48B33254
28. El-Ridy MS, Abdelbary A, Essam T, Abd El-Salam RM, Kassem AAA. Niosomes as a potential drug delivery system for increasing the efficacy and safety of nystatin. Article. Drug Dev Ind Pharm. 2011;37(12):1491–1508. doi:10.3109/03639045.2011.587431
29. Shukla SK, Nguyen V, Goyal M, Gupta V. Cationically modified inhalable nintedanib niosomes: enhancing therapeutic activity against non-small-cell lung cancer. Article. Nanomedicine. 2022;17(13):935–958. doi:10.2217/nnm-2022-0045
30. Durak S, Esmaeili Rad M, Alp Yetisgin A, et al. Niosomal drug delivery systems for ocular disease—recent advances and future prospects. Nanomaterials. 2020;10(6):1191. doi:10.3390/nano10061191
31. Liga S, Paul C, Moacă E-A, Péter F. Niosomes: composition, formulation techniques, and recent progress as delivery systems in cancer therapy. Pharmaceutics. 2024;16(2):223. doi:10.3390/pharmaceutics16020223
32. Yeo LK, Chaw CS, Elkordy AA. The effects of hydration parameters and co-surfactants on methylene blue-loaded niosomes prepared by the thin film hydration method. Article. Pharmaceuticals. 2019;12(2):46. doi:10.3390/ph12020046
33. Thabet Y, Elsabahy M, Eissa NG. Methods for preparation of niosomes: a focus on thin-film hydration method. Methods. 2022;199:9–15. doi:10.1016/j.ymeth.2021.05.004
34. Asaithambi K, Muthukumar J, Chandrasekaran R, Ekambaram N, Roopan M. Synthesis and characterization of turmeric oil loaded non-ionic surfactant vesicles (niosomes) and its enhanced larvicidal activity against mosquito vectors. Article. Biocatal Agric Biotechnol. 2020;29101737. doi:10.1016/j.bcab.2020.101737
35. Chi L, Wu D, Li Z, et al. Modified release and improved stability of unstable bcs II drug by using cyclodextrin complex as carrier to remotely load drug into niosomes. Article. Mol Pharmaceut. 2016;13(1):113–124. doi:10.1021/acs.molpharmaceut.5b00566
36. Mawazi SM, Ann TJ, Widodo RT. Exploring the evolution of niosomes: from past techniques to future advances in preparation methods-a comprehensive review. Review. Bionanoscience. 2024;14(2):1854–1875. doi:10.1007/s12668-024-01395-z
37. Yuksel N, Bayindir ZS, Aksakal E, Ozcelikay AT. In situ niosome forming maltodextrin proniosomes of candesartan cilexetil: in vitro and in vivo evaluations. Article. Int J Biol Macromol. 2016;82:453–463. doi:10.1016/j.ijbiomac.2015.10.019
38. Shehata TM, Abdallah MH, Ibrahim MM. Proniosomal oral tablets for controlled delivery and enhanced pharmacokinetic properties of acemetacin. Article. AAPS Pharm Sci Tech. 2015;16(2):375–383. doi:10.1208/s12249-014-0233-5
39. Shehata TM, Ibrahim MM, Elsewedy HS. Curcumin niosomes prepared from proniosomal gels: in vitro skin permeability, kinetic and in vivo studies. Article. Polymers. 2021;13(5):791. doi:10.3390/polym13050791
40. Yeo LK, Olusanya TOB, Chaw CS, Elkordy AA. Brief effect of a small hydrophobic drug (Cinnarizine) on the physicochemical characterisation of niosomes produced by thin-film hydration and microfluidic methods. Pharmaceutics. 2018;10(4):185. doi:10.3390/pharmaceutics10040185
41. Al-Kofahi T, Altrad B, Amawi H, Aljabali AA, Abul-Haija YM, Obeid MA. Paclitaxel-loaded niosomes in combination with metformin: development, characterization and anticancer potentials. Article. Therapeutic Delivery. 2024;15(2):109–118. doi:10.4155/tde-2023-0089
42. Obeid MA, Haifawi S, Khadra I. The impact of solvent selection on the characteristics of niosome nanoparticles prepared by microfluidic mixing. Article. Int J Pharmaceutics-X. 2023;5100168. doi:10.1016/j.ijpx.2023.100168
43. Khan DH, Bashir S, Khan MI, Figueiredo P, Santos HA, Peltonen L. Formulation optimization and in vitro characterization of rifampicin and ceftriaxone dual drug loaded niosomes with high energy probe sonication technique. Article. J Drug Delivery Sci Technol. 2020;58101763. doi:10.1016/j.jddst.2020.101763
44. Owodeha-Ashaka K, Ilomuanya M, Iyire A. Evaluation of sonication on stability-indicating properties of optimized pilocarpine hydrochloride-loaded niosomes in ocular drug delivery. Article. Progress Biomater. 2021;10(3):207–220. doi:10.1007/s40204-021-00164-5
45. Moghtaderi M, Sedaghatnia K, Bourbour M, et al. Niosomes: a novel targeted drug delivery system for cancer. Review. Med Oncol. 2022;39(12):240. doi:10.1007/s12032-022-01836-3
46. Gugleva V, Titeva S, Rangelov S, Momekova D. Design and in vitro evaluation of doxycycline hyclate niosomes as a potential ocular delivery system. Int J Pharm. 2019;567:118431. doi:10.1016/j.ijpharm.2019.06.022
47. Negi P, Aggarwal M, Sharma G, et al. Niosome-based hydrogel of resveratrol for topical applications: an effective therapy for pain related disorder(s). Article. Biomed Pharmacother. 2017;88:480–487. doi:10.1016/j.biopha.2017.01.083
48. Moghassemi S, Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. Review. J Control Release. 2014;185:22–36. doi:10.1016/j.jconrel.2014.04.015
49. Patel P, Barot T, Kulkarni P. Formulation, characterization and in-vitro and in-vivo evaluation of capecitabine loaded niosomes. Article. Current Drug Delivery. 2020;17(3):257–268. doi:10.2174/1567201817666200214111815
50. Yasamineh S, Yasamineh P, Ghafouri Kalajahi H, et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int J Pharm. 2022;624:121878. doi:10.1016/j.ijpharm.2022.121878
51. Weng H, Liu X, Ren Y, Li Y, Li X. Fingolimod loaded niosomes attenuates sevoflurane induced cognitive impairments. Article. Biomed Microdevices. 2022;24(1):5. doi:10.1007/s10544-021-00603-x
52. Fathalla D, Fouad EA, Soliman GM. Latanoprost niosomes as a sustained release ocular delivery system for the management of glaucoma. Article. Drug Dev Ind Pharm. 2020;46(5):806–813. doi:10.1080/03639045.2020.1755305
53. Izhar MP, Hafeez A, Kushwaha P, Simrah. Drug delivery through niosomes: a comprehensive review with therapeutic Applications. Review. J Cluster Sci. 2023;34(5):2257–2273. doi:10.1007/s10876-023-02423-w
54. Baldino L, Reverchon E. Niosomes formation using a continuous supercritical CO2 assisted process. Article. J CO2 Util. 2021;52101669. doi:10.1016/j.jcou.2021.101669
55. Seleci DA, Seleci M, Walter J-G, Stahl F, Scheper T. Niosomes as nanoparticular drug carriers: fundamentals and recent applications. Review. J Nanomater. 2016;2016:7372306. doi:10.1155/2016/7372306
56. Mohamad Saimi NI, Salim N, Ahmad N, Abdulmalek E, Abdul Rahman MB. Aerosolized niosome formulation containing Gemcitabine and cisplatin for lung cancer treatment: optimization, characterization and in vitro evaluation. Article. Pharmaceutics. 2021;13(1):59. doi:10.3390/pharmaceutics13010059
57. Veeramanoharan A, Kim SC. A comprehensive review on sustainable surfactants from CNSL: chemistry, key applications and research perspectives. RSC Adv. 2024;14(35):25429–25471. doi:10.1039/d4ra04684f
58. Pokhrel DR, Sah MK, Gautam B, Basak HK, Bhattarai A, Chatterjee A. A recent overview of surfactant-drug interactions and their importance. Review. RSC Adv. 2023;13(26):17685–17704. doi:10.1039/d3ra02883f
59. Zheng Y, Dou J, Wang Y, et al. Sustained release of a polymeric wetting agent from a silicone-hydrogel contact lens material. ACS Omega. 2022;7(33):29223–29230. doi:10.1021/acsomega.2c03310
60. Kaur G, Mehta SK. Developments of polysorbate (Tween) based microemulsions: preclinical drug delivery, toxicity and antimicrobial applications. Int J Pharm. 2017;529(1):134–160. doi:10.1016/j.ijpharm.2017.06.059
61. Tănase MA, Raducan A, Oancea P, et al. Mixed pluronic—cremophor polymeric micelles as nanocarriers for poorly soluble antibiotics—the influence on the antibacterial activity. Pharmaceutics. 2021;13(4):435. doi:10.3390/pharmaceutics13040435
62. Zürcher D, Caduff S, Aurand L, Capasso Palmiero U, Wuchner K, Arosio P. Comparison of the protective effect of polysorbates, poloxamer and Brij on antibody stability against different interfaces. J Pharm Sci. 2023;112(11):2853–2862. doi:10.1016/j.xphs.2023.06.004
63. Yuan X, Krueger S, Shalaev E. Protein-surfactant and protein-protein interactions during freeze and thaw: a small-angle neutron scattering study of lysozyme solutions with polysorbate and poloxamer. Article. J Pharmaceut Sci. 2023;112(1):76–82. doi:10.1016/j.xphs.2022.08.017
64. Zhu Y-T, Yuan Y-Z, Feng Q-P, et al. Food emulsifier polysorbate 80 promotes the intestinal absorption of mono-2-ethylhexyl phthalate by disturbing intestinal barrier. Toxicol Appl Pharmacol. 2021;414:115411. doi:10.1016/j.taap.2021.115411
65. Zarenezhad E, Marzi M, Abdulabbas HT, et al. Bilosomes as nanocarriers for the drug and vaccine delivery against gastrointestinal infections: opportunities and challenges. J Funct Biomater. 2023;14(9):453. doi:10.3390/jfb14090453
66. Oktay AN, Polli JE. Comparison of a single pharmaceutical surfactant versus intestinal biorelevant media for etravirine dissolution: role and impact of micelle diffusivity. Article. Int J Pharm. 2022;624122015. doi:10.1016/j.ijpharm.2022.122015
67. Voigt N, Henrich-Noack P, Kockentiedt S, Hintz W, Tomas J, Sabel BA. Surfactants, not size or zeta-potential influence blood-brain barrier passage of polymeric nanoparticles. Article. Eur J Pharm Biopharm. 2014;87(1):19–29. doi:10.1016/j.ejpb.2014.02.013
68. Hong IK, Kim SI, Lee SB. Effects of HLB value on oil-in-water emulsions: droplet size, rheological behavior, zeta-potential, and creaming index. Article. J Ind Eng Chem. 2018;67:123–131. doi:10.1016/j.jiec.2018.06.022
69. Aparajay P, Dev A. Functionalized niosomes as a smart delivery device in cancer and fungal infection. Eur J Pharm Sci. 2022;168:106052. doi:10.1016/j.ejps.2021.106052
70. Shah N, Prajapati R, Gohil D, Sadhu P, Patel S. Niosomes: a promising novel nano carrier for drug delivery. Review. J Pharm Res Int. 2021;33(48B):53–66. doi:10.9734/JPRI/2021/v33i48B33260
71. Arslanov VV, Ermakova EV, Krylov DI, Popova OO. On the relationship between the properties of planar structures of non-ionic surfactants and their vesicular analogues - niosomes. Article. J Colloid Interface Sci. 2023;640:281–295. doi:10.1016/j.jcis.2023.02.110
72. Teaima MH, Yasser M, El-Nabarawi MA, Helal DA. Proniosomal telmisartan tablets: formulation, in vitro evaluation and in vivo comparative pharmacokinetic study in rabbits. Article. Drug Des Devel Ther. 2020;14:1319–1331. doi:10.2147/dddt.S245013
73. Kamboj S, Saini V, Bala S. Formulation and characterization of drug loaded nonionic surfactant vesicles (niosomes) for oral bioavailability enhancement. Sci World J. 2014;2014:959741. doi:10.1155/2014/959741
74. Zhang Y, Jing Q, Hu H, et al. Sodium dodecyl sulfate improved stability and transdermal delivery of salidroside-encapsulated niosomes via effects on zeta potential. Int J Pharm. 2020;580:119183. doi:10.1016/j.ijpharm.2020.119183
75. Saeting K, Mitrevej A, Leuenberger H, Sinchaipanid N. Development of alendronate niosomal delivery system for gastrointestinal permeability improvement. J Drug Delivery Sci Technol. 2022;67:102885. doi:10.1016/j.jddst.2021.102885
76. Alyami H, Abdelaziz K, Dahmash EZ, Iyire A. Nonionic surfactant vesicles (niosomes) for ocular drug delivery: development, evaluation and toxicological profiling. J Drug Delivery Sci Technol. 2020;60:102069. doi:10.1016/j.jddst.2020.102069
77. Ghanbarzadeh S, Khorrami A, Arami S. Nonionic surfactant-based vesicular system for transdermal drug delivery. Article. Drug Delivery. 2015;22(8):1071–1077. doi:10.3109/10717544.2013.873837
78. Abdel-Rashid RS, Abd Allah FI, Hassan AA, Hashim FM. Design, optimization, and in-vivo hypoglycaemic effect of nanosized glibenclamide for inhalation delivery. Article. J Liposome Res. 2021;31(3):291–303. doi:10.1080/08982104.2020.1806874
79. Kobierski J, Wnetrzak A, Chachaj-Brekiesz A, Dynarowicz-Latka P. Predicting the packing parameter for lipids in monolayers with the use of molecular dynamics. Article. Colloids Surf B Biointerfaces. 2022;211112298. doi:10.1016/j.colsurfb.2021.112298
80. Khalil RA, Zarari A-H. Theoretical estimation of the critical packing parameter of amphiphilic self-assembled aggregates. Appl Surf Sci. 2014;318:85–89. doi:10.1016/j.apsusc.2014.01.046
81. Sharma VK, Mamontov E, Anunciado DB, O’Neill H, Urban V. Nanoscopic dynamics of phospholipid in unilamellar vesicles: effect of gel to fluid phase transition. Article. J Phys Chem B. 2015;119(12):4460–4470. doi:10.1021/acs.jpcb.5b00220
82. Damera DP, Nag A. Tuning the phase transition temperature of hybrid Span60-L64 thermoresponsive niosomes: insights from fluorescence and Raman spectroscopy. J Mol Liq. 2021;340:117110. doi:10.1016/j.molliq.2021.117110
83. Mokhtar M, Sammour OA, Hammad MA, Megrab NA. Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Article. Int J Pharm. 2008;361(1–2):104–111. doi:10.1016/j.ijpharm.2008.05.031
84. Cirin D, Krstonosic V, Sazdanic D. Synergism and antagonism in mixed monolayers: Brij S20/poloxamer 407 and Triton X-100/poloxamer 407 mixtures. Fluid Phase Equilibria. 2018;473:220–225. doi:10.1016/j.fluid.2018.06.009
85. Hanif R, Khan MI, Madni A, et al. Polyoxyethylene lauryl ether (Brij-35) and poloxamer 407-based non-ionic surfactant vesicles for dissolution enhancement of tacrolimus. Article; early access. J Pharm Innovation. 2023;18(3):1487–1499. doi:10.1007/s12247-023-09737-2
86. Parodi A, Buzaeva P, Nigovora D, et al. Nanomedicine for increasing the oral bioavailability of cancer treatments. J Nanobiotechnol. 2021;19(1):354. doi:10.1186/s12951-021-01100-2
87. Fayed ND, Goda AE, Essa EA, El Maghraby GM. Chitosan-encapsulated niosomes for enhanced oral delivery of atorvastatin. J Drug Delivery Sci Technol. 2021;66:102866. doi:10.1016/j.jddst.2021.102866
88. Muzzalupo R, Tavano L, Cassano R, Trombino S, Ferrarelli T, Picci N. A new approach for the evaluation of niosomes as effective transdermal drug delivery systems. Article. Eur J Pharm Biopharm. 2011;79(1):28–35. doi:10.1016/j.ejpb.2011.01.020
89. Maurizi L, Forte J, Ammendolia MG, et al. Effect of ciprofloxacin-loaded niosomes on Escherichia coli and Staphylococcus aureus biofilm formation. Article. Pharmaceutics. 2022;14(12):2662. doi:10.3390/pharmaceutics14122662
90. Mansouri M, Khayam N, Jamshidifar E, et al. Streptomycin sulfate-loaded niosomes enables increased antimicrobial and anti-biofilm activities. Article. Front Bioeng Biotechnol. 2021:9745099. doi:10.3389/fbioe.2021.745099
91. Imran M, Shah MR, Ullah F, et al. Sugar-based novel niosomal nanocarrier system for enhanced oral bioavailability of levofloxacin. Article. Drug Delivery. 2016;23(9):3653–3664. doi:10.1080/10717544.2016.1214991
92. Bansal S, Aggarwal G, Chandel P, Harikumar SL. Design and development of cefdinir niosomes for oral delivery. J Pharm Bioallied Sci. 2013;5(4):318–325. doi:10.4103/0975-7406.120080
93. Jadon PS, Gajbhiye V, Jadon RS, Gajbhiye KR, Ganesh N. Enhanced oral bioavailability of griseofulvin via niosomes. AAPS Pharm Sci Tech. 2009;10(4):1186–1192. doi:10.1208/s12249-009-9325-z
94. Yaghoobian M, Haeri A, Bolourchian N, Shahhosseni S, Dadashzadeh S. The impact of surfactant composition and surface charge of niosomes on the oral absorption of repaglinide as a BCS II model drug. Article. Int j Nanomed. 2020;15:8767–8781. doi:10.2147/ijn.S261932
95. Sultan AA, El-Gizawy SA, Osman MA, El Maghraby GM. Niosomes for oral delivery of nateglinide: in situ-in vivo correlation. Article. J Liposome Res. 2018;28(3):209–217. doi:10.1080/08982104.2017.1343835
96. Pardakhty A, Moazeni E, Varshosaz J, Hajhashemi V, Rouholamini Najafabadi A. Pharmacokinetic study of niosome-loaded insulin in diabetic rats. Daru. 2011;19(6):404–411.
97. Mohsen AM, AbouSamra MM, ElShebiney SA. Enhanced oral bioavailability and sustained delivery of glimepiride via niosomal encapsulation: in-vitro characterization and in-vivo evaluation. Drug Dev Ind Pharm. 2017;43(8):1254–1264. doi:10.1080/03639045.2017.1310224
98. Ahad A, Raish M, Al-Jenoobi FI, Al-Mohizea AM. Sorbitane monostearate and cholesterol based niosomes for oral delivery of telmisartan. Article. Curr Drug Del. 2018;15(2):260–266. doi:10.2174/1567201814666170518131934
99. Taymouri S, Varshosaz J. Effect of different types of surfactants on the physical properties and stability of carvedilol nano-niosomes. Adv Biomed Res. 2016;5(1):48. doi:10.4103/2277-9175.178781
100. Arzani G, Haeri A, Daeihamed M, Bakhtiari-Kaboutaraki H, Dadashzadeh S. Niosomal carriers enhance oral bioavailability of carvedilol: effects of bile salt-enriched vesicles and carrier surface charge. Article. Int j Nanomed. 2015;10:4797–4813. doi:10.2147/ijn.S84703
101. Sezgin-Bayindir Z, Antep MN, Yuksel N. Development and characterization of mixed niosomes for oral delivery using candesartan cilexetil as a model poorly water-soluble drug. Article. AAPS Pharm Sci Tech. 2015;16(1):108–117. doi:10.1208/s12249-014-0213-9
102. Katare R, Gupta PN, Mahor S, et al. Development of polysaccharide-capped niosomes for oral immunization of tetanus toxoid. Article. J Drug Delivery Sci Technol. 2006;16(3):167–172. doi:10.1016/s1773-2247(06)50031-0
103. Sailaja C, Bhupalam PK. A box Behnken design optimized nano vesicular transdermal patch for allergies. Article. Int J Pharm Invest. 2024;14(1):68–75. doi:10.5530/ijpi.14.1.10
104. Zhang W, Zhao X, Yu G, Suo M. Optimization of propofol loaded niosomal gel for transdermal delivery. Article. J Biomater Sci-Polym Ed. 2021;32(7):858–873. doi:10.1080/09205063.2021.1877064
105. Zaid Alkilani A, Musleh B, Hamed R, Swellmeen L, Basheer HA. Preparation and characterization of patch loaded with clarithromycin nanovesicles for transdermal drug delivery. J Func Biomat. 2023;14(2):57. doi:10.3390/jfb14020057
106. Manosroi A, Jantrawut P, Akazawa H, Akihisa T, Manosroi W, Manosroi J. Transdermal absorption enhancement of gel containing elastic niosomes loaded with gallic acid from Terminalia chebula galls. Article. Pharm Biol. 2011;49(6):553–562. doi:10.3109/13880209.2010.528432
107. Eid RK, Essa EA, El Maghraby GM. Essential oils in niosomes for enhanced transdermal delivery of felodipine. Article. Pharmaceutical Dev Tech. 2019;24(2):157–165. doi:10.1080/10837450.2018.1441302
108. Akbari J, Saeedi M, Morteza-Semnani K, et al. Innovative topical niosomal gel formulation containing diclofenac sodium (niofenac). Article. J Drug Targeting. 2022;30(1):108–117. doi:10.1080/1061186x.2021.1941060
109. Aziz DE, Abdelbary AA, Elassasy AI. Investigating superiority of novel bilosomes over niosomes in the transdermal delivery of diacerein: in vitro characterization, ex vivo permeation and in vivo skin deposition study. Article. J Liposome Res. 2019;29(1):73–85. doi:10.1080/08982104.2018.1430831
110. Auda SH, Fathalla D, Fetih G, El-Badry M, Shakeel F. Niosomes as transdermal drug delivery system for celecoxib: in vitro and in vivo studies. Polym Bull. 2016;73(5):1229–1245. doi:10.1007/s00289-015-1544-8
111. Zeng W, Li Q, Wan T, et al. Hyaluronic acid-coated niosomes facilitate tacrolimus ocular delivery: mucoadhesion, precorneal retention, aqueous humor pharmacokinetics, and transcorneal permeability. Article. Colloids Surf B Biointer. 2016;141:28–35. doi:10.1016/j.colsurfb.2016.01.014
112. El-Sayed MM, Hussein AK, Sarhan HA, Mansour HF. Flurbiprofen-loaded niosomes-in-gel system improves the ocular bioavailability of flurbiprofen in the aqueous humor. Article. Drug Dev Ind Pharm. 2017;43(6):902–910. doi:10.1080/03639045.2016.1272120
113. Allam A, Elsabahy M, El Badry M, Eleraky NE. Betaxolol‐loaded niosomes integrated within pH‐sensitive in situ forming gel for management of glaucoma. Int J Pharm. 2021;598:120380. doi:10.1016/j.ijpharm.2021.120380
114. Abdelkader H, Ismail S, Kamal A, Alany RG. Design and evaluation of controlled-release niosomes and discomes for naltrexone hydrochloride ocular delivery. Article. J Pharmaceut Sci. 2011;100(5):1833–1846. doi:10.1002/jps.22422
115. Sezgin Bayindir Z, Besikci A, Yuksel N. Paclitaxel-loaded niosomes for intravenous administration: pharmacokinetics and tissue distribution in rats. Article. Turk J Med Sci. 2015;45(6):1403–1412. doi:10.3906/sag-1408-129
116. Tan DM-Y, J-Y F, Wong F-S, H-M E, Chen Y-S, Nesaretnam K. Tumor regression and modulation of gene expression via tumor-targeted tocotrienol niosomes. Article. Nanomedicine. 2017;12(20):2487–2502. doi:10.2217/nnm-2017-0182
117. R-X H, Ye X, Li R, et al. PEGylated niosomes-mediated drug delivery systems for paeonol: preparation, pharmacokinetics studies and synergistic anti-tumor effects with 5-FU. Review. J Liposome Res. 2017;27(2):161–170. doi:10.1080/08982104.2016.1191021
118. Abou-Taleb HA, Khallaf RA, Abdel-Aleem JA. Intranasal niosomes of nefopam with improved bioavailability: preparation, optimization, and in-vivo evaluation. Article. Drug Des Devel Ther. 2018;12:3501–3516. doi:10.2147/dddt.S177746
119. Sita VG, Jadhav D, Vavia P. Niosomes for nose-to-brain delivery of bromocriptine: formulation development, efficacy evaluation and toxicity profiling. Article. J Drug Delivery Sci Technol. 2020;58101791. doi:10.1016/j.jddst.2020.101791
120. Khallaf RA, Aboud HM, Sayed OM. Surface modified niosomes of olanzapine for brain targeting via nasal route; preparation, optimization, and in vivo evaluation. Article. J Liposome Res. 2020;30(2):163–173. doi:10.1080/08982104.2019.1610435
121. Mathure D, Madan JR, Gujar KN, Tupsamundre A, Ranpise HA, Dua K. Formulation and evaluation of niosomal in situ nasal gel of a serotonin receptor agonist, buspirone hydrochloride for the brain delivery via intranasal route. Pharma nanotech. 2018;6(1):69–78. doi:10.2174/2211738506666180130105919
122. Arafa MG, Ayoub BM. Nano-vesicles of salbutamol sulphate in metered dose inhalers: formulation, characterization and in vitro evaluation. Int J Appl Pharm. 2017;9(6):100–105. doi:10.22159/ijap.2017v9i6.22448
123. Liu F-C, H-P Y, Lin C-Y, Elzoghby AO, Hwang T-L, Fang J-Y. Use of cilomilast-loaded phosphatiosomes to suppress neutrophilic inflammation for attenuating acute lung injury: the effect of nanovesicular surface charge. Article. J Nanobiotechnol. 2018;1635. doi:10.1186/s12951-018-0364-z
124. Yousaf R, Khan MI, Akhtar MF, et al. Development and in-vitro evaluation of chitosan and glyceryl monostearate based matrix lipid polymer hybrid nanoparticles (LPHNPs) for oral delivery of itraconazole. Article; early access. Heliyon. 2023;9(3):e14281. doi:10.1016/j.heliyon.2023.e14281
125. Parvez S, Yadagiri G, Gedda MR, et al. Modified solid lipid nanoparticles encapsulated with amphotericin B and paromomycin: an effective oral combination against experimental murine visceral leishmaniasis. Article. Sci Rep. 2020;10(1):12243. doi:10.1038/s41598-020-69276-5
126. Darwich AS, Neuhoff S, Jamei M, Rostami-Hodjegan A. Interplay of metabolism and transport in determining oral drug absorption and gut wall metabolism: a simulation assessment using the “advanced dissolution, absorption, metabolism (ADAM)”. model. Review. Current Drug Metabol. 2010;11(9):716–729. doi:10.2174/138920010794328913
127. Kou L, Yao Q, Sun M, et al. Cotransporting ion is a trigger for cellular endocytosis of transporter-targeting nanoparticles: a case study of high-efficiency SLC22A5 (OCTN2)-mediated carnitine-conjugated nanoparticles for oral delivery of therapeutic drugs. Article. Adv Healthcare Mater. 2017;6(17):1700165. doi:10.1002/adhm.201700165
128. Uhl P, Grundmann C, Sauter M, et al. Coating of PLA-nanoparticles with cyclic, arginine-rich cell penetrating peptides enables oral delivery of liraglutide. Article. Nanomed Nanotechnol Biol Med. 2020:24102132. doi:10.1016/j.nano.2019.102132
129. Sumaila M, Marimuthu T, Kumar P, Choonara YE. Lipopolysaccharide nanosystems for the enhancement of oral bioavailability. Review. AAPS Pharm Sci Tech. 2021;22(7):242. doi:10.1208/s12249-021-02124-5
130. Zhang L, Shen Y, Qiu L. Loading docetaxel in β-cyclodextrin-based micelles for enhanced oral chemotherapy through inhibition of P-glycoprotein mediated efflux transport. Article. RSC Adv. 2017;7(42):26161–26169. doi:10.1039/c7ra03180g
131. Zhang X, Li J, Rong R, et al. Enhancing the oral bioavailability of poorly water-soluble amisupiride with solid nanodispersion. Article; early access. J Drug Delivery Sci Technol. 2023:86104635. doi:10.1016/j.jddst.2023.104635
132. Banerjee S, Pillai J. Solid lipid matrix mediated nanoarchitectonics for improved oral bioavailability of drugs. Review; early access. Expert Opin Drug Metab Toxicol. 2019;15(6):499–515. doi:10.1080/17425255.2019.1621289
133. Bravo-Alfaro DA, Ochoa-Rodriguez LR, Villasenor-Ortega F, Luna-Barcenas G, Garcia HS. Self-nanoemulsifying drug delivery system (SNEDDS) improves the oral bioavailability of betulinic acid. Article; early access. J Mol Liq. 2022;364:119946. doi:10.1016/j.molliq.2022.119946
134. Shukla M, Jaiswal S, Sharma A, et al. A combination of complexation and self-nanoemulsifying drug delivery system for enhancing oral bioavailability and anticancer efficacy of curcumin. Article. Drug Dev Ind Pharm. 2017;43(5):847–861. doi:10.1080/03639045.2016.1239732
135. Fu Q, Ma M, Li M, et al. Improvement of oral bioavailability for nisoldipine using nanocrystals. Article. Powder Technol. 2017;305:757–763. doi:10.1016/j.powtec.2016.10.068
136. Shen B, Shen C, Zhu W, Yuan H. The contribution of absorption of integral nanocrystals to enhancement of oral bioavailability of quercetin. Article. Acta Pharmaceutica Sinica B. 2021;11(4):978–988. doi:10.1016/j.apsb.2021.02.015
137. Ren Y, Nie L, Zhu S, Zhang X. Nanovesicles-mediated drug delivery for oral bioavailability enhancement. Review. Int j Nanomed. 2022;17:4861–4877. doi:10.2147/ijn.S382192
138. Husain A, Makadia V, Valicherla GR, Riyazuddin M, Gayen JR. Approaches to minimize the effects of P-glycoprotein in drug transport: a review. Drug Dev Res. 2022;83(4):825–841. doi:10.1002/ddr.21918
139. Sharom FJ. Complex interplay between the P-glycoprotein multidrug efflux pump and the membrane: its role in modulating protein function.; Review. Front Oncol. 2014;4:41. doi:10.3389/fonc.2014.00041
140. Abdallah HM, Al-Abd AM, El-Dine RS, El-Halawany AM. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: a review. J Adv Res. 2015;6(1):45–62. doi:10.1016/j.jare.2014.11.008
141. Mu Y, Fu Y, Li J, et al. Multifunctional quercetin conjugated chitosan nano- micelles with P-gp inhibition and permeation enhancement of anticancer drug. Article. Carbohydr Polym. 2019;203:10–18. doi:10.1016/j.carbpol.2018.09.020
142. Mar PL, Gopinathannair R, Gengler BE, et al. Drug interactions affecting oral anticoagulant use. Review. Circ-Arrhythmia Electrophysiol. 2022;15(6):e007956. doi:10.1161/circep.121.007956
143. Sajid A, Raju N, Lusvarghi S, Vahedi S, Swenson RE, Ambudkar SV. Synthesis and characterization of Bodipy-FL-cyclosporine A as a substrate for multidrug resistance-linked P-glycoprotein (ABCB1). Article. Drug Metab Dispos 2019;47(10):1013–1023. doi:10.1124/dmd.119.087734
144. Al-Ali AAA, Steffansen B, Holm R, Nielsen CU. Nonionic surfactants increase digoxin absorption in Caco-2 and MDCKII MDR1 cells: impact on P-glycoprotein inhibition, barrier function, and repeated cellular exposure. Article. Int J Pharm. 2018;551(1–2):270–280. doi:10.1016/j.ijpharm.2018.09.039
145. Moesgaard L, Reinholdt P, Nielsen CU, Kongsted J. Mechanism behind polysorbates’ inhibitory effect on P-glycoprotein. Mol Pharmaceut. 2022;19(7):2248–2253. doi:10.1021/acs.molpharmaceut.2c00074
146. Rege BD, Kao JPY, Polli JE. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Article. Eur J Pharm Sci. 2002;16(4–5):237–246. doi:10.1016/s0928-0987(02)00055-6
147. Zhao W, Alama T, Kusamori K, Katsumi H, Sakane T, Yamamoto A. Effects of 2 polyoxyethylene alkyl ethers on the function of intestinal P-glycoprotein and their inhibitory mechanisms. Article. J Pharmaceut Sci. 2016;105(12):3668–3679. doi:10.1016/j.xphs.2016.09.002
148. Obeid MA, Teeravatcharoenchai T, Connell D, et al. Examination of the effect of niosome preparation methods in encapsulating model antigens on the vesicle characteristics and their ability to induce immune responses. Article. J Liposome Res. 2021;31(2):195–202. doi:10.1080/08982104.2020.1768110
149. Naves L, Dhand C, Almeida L, Rajamani L, Ramakrishna S, Soares G. Poly(lactic-co-glycolic) acid drug delivery systems through transdermal pathway: an overview. Progress Biomater. 2017;6(1):1–11. doi:10.1007/s40204-017-0063-0
150. Gaynanova G, Vasileva L, Kashapov R, et al. Self-assembling drug formulations with tunable permeability and biodegradability. Molecules. 2021;26(22):6786. doi:10.3390/molecules26226786
151. Li J, Xiang H, Zhang Q, Miao X. Polysaccharide-based transdermal drug delivery. Pharmaceuticals. 2022;15(5):602. doi:10.3390/ph15050602
152. Kassem AA, El-Alim SH A, Asfour MH. Enhancement of 8-methoxypsoralen topical delivery via nanosized niosomal vesicles: formulation development, in vitro and in vivo evaluation of skin deposition. Article. Int J Pharm. 2017;517(1–2):256–268. doi:10.1016/j.ijpharm.2016.12.018
153. Mali N, Darandale S, Vavia P. Niosomes as a vesicular carrier for topical administration of minoxidil: formulation and in vitro assessment. Article. Drug Delivery Transl Res. 2013;3(6):587–592. doi:10.1007/s13346-012-0083-1
154. Nene S, Shah S, Rangaraj N, Mehra NK, Singh PK, Srivastava S. Lipid based nanocarriers: a novel paradigm for topical antifungal therapy. J Drug Delivery Sci Technol. 2021;62:102397. doi:10.1016/j.jddst.2021.102397
155. Gorantla S, Rapalli VK, Waghule T, et al. Nanocarriers for ocular drug delivery: current status and translational opportunity. Review. RSC Adv. 2020;10(46):27835–27855. doi:10.1039/d0ra04971a
156. Wang Q, Zhang A, Zhu L, Yang X, Fang G, Tang B. Cyclodextrin-based ocular drug delivery systems: a comprehensive review. Coord Chem Rev. 2023;476:214919. doi:10.1016/j.ccr.2022.214919
157. Jayoush ARA, Hassan HAFM, Asiri H, et al. Niosomes for nose-to-brain delivery: a non-invasive versatile carrier system for drug delivery in neurodegenerative diseases. Review. J Drug Delivery Sci Technol. 2023:89105007. doi:10.1016/j.jddst.2023.105007
158. Mahajan HS, Mahajan MS, Nerkar PP, Agrawal A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Article. Drug Delivery. 2014;21(2):148–154. doi:10.3109/10717544.2013.838014
159. Yi M, Ma L, Zhao W, Zhao J, Fan Q, Hao J. Amphiphilic Au nanoclusters modulated by magnetic gemini surfactants as a cysteine chemosensor and an MRI contrast agent. Article. Langmuir. 2021;37(10):3130–3138. doi:10.1021/acs.langmuir.0c03618
160. Illert P, Waengler B, Waengler C, et al. Functionalizable composite nanoparticles as a dual magnetic resonance imaging/computed tomography contrast agent for medical imaging. Article. J Appl Polym Sci. 2019;136(19):47571. doi:10.1002/app.47571
161. Xu K, Wang M, Tang W, Ding Y, Hu A. Flash nanoprecipitation with Gd(III)-based metallosurfactants to fabricate polylactic acid nanoparticles as highly efficient contrast agents for magnetic resonance imaging. Article. Chem-Asian J. 2020;15(16):2475–2479. doi:10.1002/asia.202000624
162. Wheatley MA, Forsberg F, Dube N, Patel M, Oeffinger BE. Surfactant-stabilized contrast agent on the nanoscale for diagnostic ultrasound imaging. Article. Ultrasound Med Biol. 2006;32(1):83–93. doi:10.1016/j.ultrasmedbio.2005.08.009
163. Ekinci M, Caliskan EE, Cakar B, Ilem-Ozdemir D, Uyanikgil Y, Uyanikgil EOC. [99m Tc]Technetium-labeled niosomes: radiolabeling, quality control, and in vitro evaluation. Acs Omega. 2023;8(7):6279–6288. doi:10.1021/acsomega.2c06179
164. Ge X, Wei M, He S, Yuan W-E. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics. 2019;11(2):55. doi:10.3390/pharmaceutics11020055
165. Milani S, Bombelli FB, Pitek AS, Dawson KA, Rädler J. Reversible versus irreversible binding of transferrin to polystyrene nanoparticles: soft and hard Corona. ACS Nano. 2012;6(3):2532–2541. doi:10.1021/nn204951s
166. Foteini P, Pippa N, Naziris N, Demetzos C. Physicochemical study of the protein-liposome interactions: influence of liposome composition and concentration on protein binding. Article. J Liposome Res. 2019;29(4):313–321. doi:10.1080/08982104.2018.1468774
167. Ke PC, Lin S, Parak WJ, Davis TP, Caruso F. A decade of the protein Corona. Article. Acs Nano. 2017;11(12):11773–11776. doi:10.1021/acsnano.7b08008
168. Mueller J, Bauer KN, Prozeller D, et al. Coating nanoparticles with tunable surfactants facilitates control over the protein Corona. Article. Biomaterials. 2017;115:1–8. doi:10.1016/j.biomaterials.2016.11.015
169. Ginn C, Khalili H, Lever R, Brocchini S. PEGylation and its impact on the design of new protein-based medicines. Review. Future Med Chem. 2014;6(16):1829–1846. doi:10.4155/fmc.14.125
170. Cao Z, Adnan NNM, Wang G, et al. Enhanced colloidal stability and protein resistance of layered double hydroxide nanoparticles with phosphonic acid-terminated PEG coating for drug delivery. Article. J Colloid Interface Sci. 2018;521:242–251. doi:10.1016/j.jcis.2018.03.006
171. Pelegri-O’Day EM, Lin E-W, Maynard HD. Therapeutic protein-polymer conjugates: advancing beyond PEGylation. Article. J Am Chem Soc. 2014;136(41):14323–14332. doi:10.1021/ja504390x
172. Schöttler S, Becker G, Winzen S, et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nature Nanotechnol. 2016;11(4):372–377. doi:10.1038/nnano.2015.330
173. Zhang Y, Wu JLY, Lazarovits J, Chan WCW. An analysis of the binding function and structural organization of the protein Corona. Article. J Am Chem Soc. 2020;142(19):8827–8836. doi:10.1021/jacs.0c01853
174. Ngo W, JLY W, Lin ZP, et al. Identifying cell receptors for the nanoparticle protein Corona using genome screens. Nature Cheml Biol. 2022;18(9):1023–1031. doi:10.1038/s41589-022-01093-5
175. Imperlini E, Celia C, Cevenini A, et al. Nano-bio interface between human plasma and niosomes with different formulations indicates protein Corona patterns for nanoparticle cell targeting and uptake. Article. Nanoscale. 2021;13(10):5251–5269. doi:10.1039/d0nr07229j
176. Greenberg ZF, Graim KS, He M. Towards artificial intelligence-enabled extracellular vesicle precision drug delivery. Adv Drug Delivery Rev. 2023;199:114974. doi:10.1016/j.addr.2023.114974
177. Soltani M, Moradi Kashkooli F, Souri M, et al. Enhancing clinical translation of cancer using nanoinformatics. Cancers. 2021;13(10):2481. doi:10.3390/cancers13102481
178. Shahiwala AF, Qawoogha SS, Faruqui N. Designing optimum drug delivery systems using machine learning approaches: a prototype study of niosomes. AAPS Pharm Sci Tech. 2023;24(4):94. doi:10.1208/s12249-023-02547-2
179. Kashani-Asadi-Jafari F, Aftab A, Ghaemmaghami S. A machine learning framework for predicting entrapment efficiency in niosomal particles. Int J Pharm. 2022;627:122203. doi:10.1016/j.ijpharm.2022.122203
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Stealth-Engineered Albumin-Coated Nanoparticles for Targeted Therapy: Effective Drug Delivery and Tumor Suppression in Xenograft-Zebrafish Model

Bozzer S, Grimaldi MC, De Maso L, Manfredi M, Toffoli G, Dal Bo M, Sblattero D, Macor P
International Journal of Nanomedicine 2024, 19:13267-13286
Published Date: 10 December 2024