Back to Journals » International Journal of Nanomedicine » Volume 19
Gelatin Methacrylic Acid Hydrogel-Based Nerve Growth Factors Enhances Neural Stem Cell Growth and Differentiation to Promote Repair of Spinal Cord Injury
Authors Shen M, Wang L , Li K, Tan J, Tang Z, Wang X, Yang H
Received 29 May 2024
Accepted for publication 14 October 2024
Published 19 October 2024 Volume 2024:19 Pages 10589—10604
DOI https://doi.org/10.2147/IJN.S480484
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sachin Mali
Mingkui Shen,1,* Lulu Wang,2,* Kuankuan Li,1 Jun Tan,1,3 Zhongxin Tang,1 Xiaohu Wang,4 Hejun Yang1
1Department of Mini-Invasive Spinal Surgery, The Third People’s Hospital of Henan Province, Zhengzhou, Henan, 450006, People’s Republic of China; 2Department of Plastic Surgery, The Third People’s Hospital of Henan Province, Zhengzhou, Henan, 450006, People’s Republic of China; 3Department of Clinical Medicine, Zhengzhou University, Zhengzhou, 450001, People’s Republic of China; 4Department of Orthopedics, Zhengzhou Central Hospital, Zhengzhou, 450007, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hejun Yang, Department of Mini-invasive Spinal Surgery, The Third People’s Hospital of Henan Province, Intersection of Zhengguang Road and Minsheng Road, Jinshui District, Zhengzhou, Henan, 450006, People’s Republic of China, Email [email protected]
Background: The challenge in treating irreversible nerve tissue damage has resulted in suboptimal outcomes for spinal cord injuries (SCI), underscoring the critical need for innovative treatment strategies to offer hope to patients.
Methods: In this study, gelatin methacrylic acid hydrogel scaffolds loaded with nerve growth factors (GMNF) were prepared and used to verify the performance of SCI. The physicochemical and biological properties of the GMNF were tested. The effect of GMNF on activity of neuronal progenitor cells (NPCs) was investigated in vitro. Histological staining and motor ability was carried out to assess the ability of SCI repair in SCI animal models.
Results: Achieving nerve growth factors sustained release, GMNF had good biocompatibility and could effectively penetrate into the cells with good targeting permeability. GMNF could better enhance the activity of NPCs and promote their directional differentiation into mature neuronal cells in vitro, which could exert a good neural repair function. In vivo, SCI mice treated with GMNF recovered their motor abilities more effectively and showed better wound healing by macroscopic observation of the coronal surface of their SCI area. Meanwhile, the immunohistochemistry demonstrated that the GMNF scaffolds effectively promoted SCI repair by better promoting the colonization and proliferation of neural stem cells (NSCs) in the SCI region and targeted differentiation into mature neurons.
Conclusion: The application of GMNF composite scaffolds shows great potential in SCI treatment, which are anticipated to be a potential therapeutic bioactive material for clinical application in repairing SCI in the future.
Keywords: hydrogel scaffold, nerve growth factor, spinal cord injury, neuronal regeneration
Introduction
As a major nerve pathway connecting the brain to the rest of the body, abnormal damage to the structure and function of the spinal cord due to external trauma can lead to muscle movement dysfunction, loss of sensation and physiological dysfunction in patients.1 In addition, the mental health problems associated with spinal cord injury (SCI), such as depression and anxiety, coupled with social isolation and medical financial pressures, have an overall negative impact on patients’ lives.2 Currently, the treatment of SCI consists mainly of the use of steroid hormones and surgical decompression in the acute phase, and prolonged physical and rehabilitation therapy in the later phase.3,4 However, the difficulty of treating irreversible damage to nerve tissue has led to unsatisfactory outcomes for SCI, and new treatment options are urgently needed to bring hope to patients.
The course of SCI is complex and one of the main difficulties in rehabilitation is the creation of an inhibitory microenvironment. Inflammatory responses are common after SCI, which can lead to immune cell infiltration and the release of inflammatory mediators, creating an environment unfavorable to nerve regeneration.5 At the same time, hypoxia and destruction of the extracellular matrix also exacerbate the formation of an inhibitory microenvironment, further hindering nerve regeneration.6 In addition, the depletion of endogenous nerve growth factors (NGFs) is a key difficulty in SCI rehabilitation: in the early stages of SCI, damage to neurons and supporting cells results in the release of large amounts of NGFs.7 However, when trophic factors are depleted, repair of nerve tissue at the site of injury may be impeded.8 Therefore, intervening in the inhibitory microenvironment and promoting NGF replenishment are key points in the treatment of SCI.
With the advancement of biotechnology, a variety of new scaffold materials have been used in the medical field, including alveolar bone repair, drug carriers, and so on.9,10 Among them, hydrogel scaffolds with good biocompatibility, biomimicry, injectability and mechanical properties similar to those of spinal cord tissues show potential applications in SCI repair, which is conducive to providing a suitable tissue microenvironment for neuronal regeneration, as well as appropriate structure and support for the injury site.11,12 Gelatin methacrylic acid is a photosensitive bio-hydrogel material prepared by methacrylate anhydride (MA) and gelatin (Gelatin), which has excellent biocompatibility. This synthetic macromolecule material can be stimulated by ultraviolet light or visible light curing reaction to form a three-dimensional structure suitable for cell growth and differentiation with certain strength for tissue regeneration and repair.13–15 Meanwhile, as an important factor in neural stem cell differentiation and axon growth, the use of exogenous nerve growth factor (NGFs) can effectively promote SCI repair.16 NGFs which are demonstrated as biological macromolecules can promote the growth, development, differentiation, and maturation of central and peripheral neurons, maintain the normal function of the nervous system, and accelerate the repair of the nervous system after injury.17,18 However, the multiple problems faced in use NGFs, such as short half-life, easy degradation, poor biological activity, and poor targeting of delivery, limit the sustained effect on nerve cells.19–21 Due to the good drug-carrying capacity of hydrogel scaffolds, they have been used in several studies to carry growth factors, drugs or other therapeutic agents to promote local tissue repair or anti-inflammatory, anti-infective and other functions.22,23 Therefore, if NGFs can be combined with hydrogel scaffolds, they may be able to improve the efficacy and feasibility of exogenous NGFs in SCI repair.
Currently, there are more studies on SCI repair, but fewer studies have combined gel scaffolds with NGF in the treatment of SCI, and it is not clear whether this will affect the sustained release of NGF, the directionality of nerve regeneration, and the biocompatibility of the new composite scaffolds. Therefore, we aimed to investigate the efficacy of gel/NGF scaffolds in the treatment of SCI to promote long-term repair of SCI.
Materials and Methods
GMNF Preparation
According to the previous study,24 6% (w/w) gelatin methacrylic acid (GelMA, molecular weight = 150,000) and 8% (w/w) polyethylene oxide (PEO, molecular weight = 100,000) were mixed in a volume ratio of 1:1 and gently pipetted 10 times. The mixture was then rapidly lyophilized. The lyophilized GelMA mixture was redissolved with 0.25% (w/v) phenyl-2, 4, 6-trimethylbenzoyl- phosphonate lithium solution (LAP) and stirred at 37°C for 1 hour at a speed of 600 rpm. After these steps, the scaffold structure of GelMA was ready for use. Subsequently, 5 µg of NGF (molecular weight = 13.4 kDa) was added to 1 mL GelMA solution and gently stirred at 4°C for 30 min. After this step, the NGF was successfully loaded into the inner hole of GelMA, forming the final complex GMNF. Finally, the GMNF complex was cross-linked under the blue light (405 nm) for 30s and stored at −20°C for further use. The workflow was showed as Figure 1. All reagents used were purchased from Sigma-Aldrich, USA.
Cell Culture
To culture the neural progenitor cells (NPCs), the brain of C57BL/6 mice at E12 were isolated in Hank’s Buffered Salt Solution (Gibco, USA) on ice. The cerebral cortex of the mice was rapidly dissected and the meningeal tissue on the cortex was removed under a stereomicroscope. The cortical tissues were cut with scissors and 150 µL Collagenase type II (Sigma-Aldrich, USA) were added to digest the tissues at 37°C for 15 min. The cells were resuspended by centrifugation at 1000 rpm for 10 min and incubated in NSC medium. The medium was DMEM/F-12 (Thermo Fisher, USA) supplemented with 2% B-27 (Gibco, USA), 1% penicillin/streptomycin (P/S) (Thermo Fisher, USA), 20 ng/mL epidermal growth factor (Thermo Fisher, USA) and 20 ng/mL fibroblast growth factor (Gibco, USA). Cells passaging between 2 ~ 4 were prepared for further experiments. When differentiating neurons, the culture medium in NPC was replaced to neurobasal (Gibco, USA) supplemented with 2% B-27, GlutaMAX (Gibco, USA) and 1% P/S solution.
Physicochemical Properties of GMNF
The compressive modulus test was performed on the prepared hydrogels (GelMA, GMNF). The infinite compression test was conducted using a mechanical tester (ZwickRoell, Germany) equipped with a 250 g force sensor. The stress-strain curve was obtained using axial compression with a speed ratio of 0.01 mm s−1. Calculate the compressive modulus within the strain range of 0.2 mm compression range, ensuring that the linear determination coefficient is greater than 0.99.
The ultrastructure of GMNF was imaged by scanning electron microscopy (SEM) (FEI-F50, USA). To test the degradation of GMNF, PBS or PBS-elastase mixture (simulating the biological process of GMNF degradation in vivo) was dripped into GMNF, and then placed in a 37°C incubator at a shaking speed of 100 rpm. Every other day, the PBS was aspirated from the mixture and the remaining GMNF was weighed to calculate the percentage of remaining weight. To test the releasing efficiency of NGF in GMNF, NGF was coupled with fluorescein isothiocyanate (FITC, Thermo fisher, USA) in PBS with a weight ratio of 1:9 and stirred at 4°C for 12 hours. Then GMNF-FITC was produced following the protocol of GMNF production and the fluorescence intensity of FITC was measured by a microplate reader (Lonza, Switzerland) after collecting certain volume mixture in GMNF-FITC solution at the designed time point, including 0, 3, 6, 9, 12, 15, 18, and 21 days.
Biological Properties of GMNF
To assess the foreign body response of GMNF in vivo, we injected GMNF into the spinal cord of wide-type mice to evaluate the activated status of macrophages around the injection site, and we labelled the macrophages with anti-CD68 antibody. To assess the cytotoxicity of GMNF, we added GMNF to cultured neurons to evaluate the growth pattern and living status of the neurons. To evaluate the infiltration ability of GMNF, we incubate GMNF-FITC with cultured neurons at 37°C for 1 hour and image the fluorescence signal in the soma for the infiltration assessment. The pictures were taken by confocal microscopy (Leica, Germany).
Live/Dead Assay
Live/Dead Viability/Cytotoxicity Kit was applied to test the live status of cultured cells. Configure the working solution according to the manual instructions, and adding the working solution into the cultured cells after washing the cells with PBS for three times. Using fluorescence microscopy for imaging.
Immunofluorescence Staining for Cultured Cell
Cultured cells were fixed with 4% PFA after being washed with PBS. Then cells were incubated with 0.3% Triton X-100 (Sigma-Aldrich, USA), 3% bovine serum albumin (BSA) (Sigma-Aldrich, USA), and 5% goat serum (Solarbio, China) at room temperature for 2 hours. PBS containing 0.3% Triton X-100 and primary antibody was added to the cells and incubated for 24 hours. The primary antibodies used in this study were shown as Table 1. PBS containing 0.1% Triton X-100, DAPI (Solarbio, China), and secondary antibody was added to the cells and incubated at room temperature for 2 hours. The information of secondary antibodies was shown in Table 1. Using fluorescence microscopy and confocal microscopy for imaging.
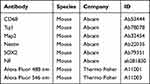 |
Table 1 Antibody Used in This Research |
SCI Animal Model Establishment
12-week-old female C57BL/6 mice (Jackson lab) weighing 20–22 g were selected. All the mice were randomly divided into control (wide type), SCI, NGF (treated with single NGF), and GMNF (treated with GMNF) groups. Animal ethics committee approval was obtained from the Animal Experimental Center of Zhengzhou University and followed by the Laboratory Animal Guide for Ethical Review of Animal Welfare for all animal experiments. For SCI modeling, mice were anesthetized with 3% pentobarbital sodium, until they exhibited silent but regular breathing. Then a NYU MASCIS Impactor (W.M. Keck Center for Collaborative Neuroscience, USA) was used to cause the SCI. After a laminectomy was performed at the T9-T10 level, a 10-gram weight from a height of 25 mm dropped to cause the injury. Post-surgery, the mice- exhibited hindlimb immobility, indicating a state of paraplegia. All the animal received penicillin injection for 7 days to prevent infection.
Motor Ability Assessment
To evaluate the motor ability of mice after SCI, here Basso, Beattie, and Bresnahan (BBB) open field locomotor tests were used to assess locomotor ability before 2 days and 2, 7, 14, 21, 28, 35, and 42 days after SCI by recording the locomotor behavior using videotape. Hind limb motor function was assessed, which was divided into three stages, early recovery (1–7 points), intermediate recovery (8–13 points) and late recovery (14–21 points).25 A higher score reflected a better recovery of the hind limb motor function. Besides, footprint test was also applied to evaluate the injury severity, including scaling stride length and sway distance to evaluate the walking status of SCI mice. The motor evoked potential (MEP) was recorded to evaluate the nerve conduction which also reflected the injury severity of SCI model. The schematic of SCI model and motor ability assessment were shown as Figure 2.
Immunofluorescence Staining for Tissue
The spinal cord slices were incubated with PBS containing 0.3% Triton X-100, 3% BSA and 5% goat serum for 2 hours. Then the slices were transferred to PBS solution with 0.3% Triton X-100 and primary antibody and incubated for 24 hours or 48 hours. The primary antibodies used in this study were shown in Table 1. The slices were transferred to PBS containing 0.1% Triton X-100, DAPI and secondary antibodies, and incubated at room temperature for 2 hours. The secondary antibodies were shown as Table 1. Images were captured using a confocal microscopy.
Statistical Analysis
All quantitative data were expressed as mean and standard deviation, and GraphPad Prism software (version 8.0.1; La Jolla, CA, USA) were used for statistical analyses. The statistical significance of differences between two groups was evaluated using Student’s t-test, and comparison among multiple groups was made by using one-way analysis of variance (ANOVA). In all tests, a p-value less than 0.05 was considered a statistical difference.
Results
Phenotype and Evaluation of SCI Model
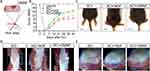
In normal mice, the BBB score remained stable at 21, indicating a healthy state of locomotion. But in SCI mice, the locomotor ability was completely lost in the first two days, indicating complete paralysis of the SCI mice. The SCI mice then recovered slightly, with a score of less than half that of normal mice (Figure 3A). The footprint text suggested a decreased stride length and an increased sway distance with hind limb dragging, suggesting weak locomotor behavior and uncoordinated gait (Figure 3B and C). A prolonged MEP latency in SCI mice reflected slow nerve conduction after SCI (Figure 3D). Observing the macroscopic phenotype of the SCI mice, it was evident that the hind limbs were completely stretched and weak, unable to support the entire body weight, whereas the limbs of healthy mice were able to dominate their bodies (Figure 3E and F). After the spinal cord isolation, injury sites in SCI mice were easily identified with bleeding, swelling and uneven surfaces (Figure 3G and H). All these images and parameters suggest a successful SCI model in mice.
Physicochemical and Biological Properties of GMNF
The stress-strain curve of the scaffold showed (Supplementary materials Figure S1) that the GMNF hydrogel was similar to the GelMA hydrogel scaffold, with stress increasing with strain (Supplementary materials Figure S1A). In addition, the compressive modulus of GelMA and GMNF hydrogels were respectively 3.24 ± 0.08 kPa and 3.09 ± 0.05 kPa, with no statistically significant difference (Supplementary materials Figure S1B). The results showed that these scaffolds had sufficient mechanical properties to maintain the spatial structure of non-weight-bearing areas.
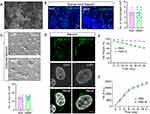
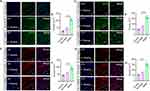
The ultrastructure of GMNF under scanning electron microscopy showed a sparse porous structure, and the pores are closely adjacent to each other, which is convenient for loading related drugs (Figure 4A). After injection of GMNF into normal spinal cord tissue, there was no significant increase in the number of macrophages around the injection site, and the foreign body reaction was low compared with the single injection of NGF, avoiding secondary injury to the injection site (Figure 4B). In cultured mature neurons (DIV13), GMNF was able to maintain normal neuron survival and less cell toxicity was caused to the cultured mature neurons (Figure 4C). After coupling FITC with NGF in GMNF, bright green fluorescence was detected in the soma of cultured neurons, suggesting an enrichment of GMNF molecules accumulated in the soma. These data demonstrate the high infiltration ability of GMNF into target neurons, which is necessary for its function at the target sites (Figure 4D). To test the degradation of GMNF, PBS or PBS-elastase mixture (simulating the biological process of GMNF degradation in vivo) was added to GMNF, and it was found that GMNF degraded slowly until almost 50 days, which is beneficial for long-term function in the body (Figure 4E). The NGF releasing activity kept more than 21 days during which NGF increased rapidly to a high level at the first nine days and then maintains a relatively balanced release rate in both PBS and PBS-elastase mixture, which may be caused by a greater concentration difference between GMNF and the target site at the beginning (Figure 4F). Hence, GMNF has stable properties and high releasing efficiency, less cell and tissue toxicity and high infiltration ability to support its function efficiently.
GMNF Enhances Cultured NPCs Growth and Survival
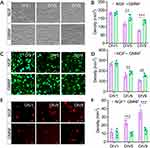
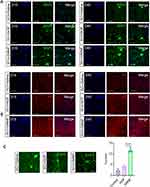
When GMNF was added to the cultured NPCs from the beginning, the density of NPCs remained more stable, whereas NPCs added with single NGF showed a moderate cell loss at DIV9. This phenotype could be detected as early as DIV5 (Figure 5A and B). In cultured NPCs, the live/dead assay showed more live cells and fewer dead cells in NPCs added with GMNF. On the first day of culture, NPCs treated with NGF and GMNF showed a similar live status. But at DIV5, more NPCs were dead in the NGF group compared to GMNF group. The phenotype was even more severe at DIV9 (Figure 5C–F), suggesting that GMNF kept more NPC alive and growing.
GMNF Promotes Cultured NPCs Differentiation and Neuronal Development
During the differentiation period of NPCs into neurons, it was clearly observed that NPCs gradually took on the morphological characteristics of neurons with gradually elongated neurites and altered cell bodies. However, NPCs had an enhanced differentiation process after treatment with GMNF, and most NPCs showed neuron-like morphology with elongated neurites and larger soma at DIV60 (Figure 6A), and the density of neuron-like cells was higher compared with NGF-treated NPCs during the differentiation process (Figure 6B). Using anti-Tuj1 staining, neurite outgrowth was clearly observed throughout the differentiation process, including DIV 40 (Figure 6C and D), DIV 50 (Figure 6E and F) and DIV 60 (Figure 6G and H). GMNF could significantly promote neuronal maturation from NPCs with better differentiated neurites, including more complex and enriched branches, quantified by the number of neurites around the soma (Figure 6D, 6F–H) and more easily detectable neurite connections (Figure 6G). We again confirmed the successful differentiation of NPCs into neurons with anti-Map2 staining (Figure 6I and J), which also showed a longer and more complex structure of neurites. Finally, we imaged the morphology of single neurons with AAV-EGFP and clearly observed the neurites extending from the soma of a single neuron and confirmed the better differentiation status of these neurons with GMNF (Figure 6K and 6L). Figure 6 Continued.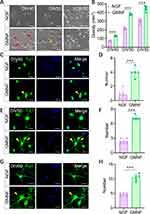
GMNF Effectively Rescues the Behavior and Promote Wound Recovery in SCI Mice
After injection of GMNF into the SCI injury area (Figure 7A), we can clearly observe the effective locomotion recovery of these mice, especially during the first three weeks, even compared to the mice with pure NGF injection by evaluating the BBB score (Figure 7B). The hind limb of the SCI mice injected with GMNF showed obvious recovery, becoming stronger, more controllable and better able to support the whole body, while the SCI mice injected with NGF could still show some paralysis phenotype (Figure 7C). After exposing the spinal cord of the injured area, it was easily observed that the wound of SCI mice injected with GMNF healed better, with a smoother surface and less scarring (Figure 7D). In the coronal section of the spinal cord, we could find a more complete shape and fewer defects in this section (Figure 7E). Thus, GMNF effectively improved the recovery of SCI mice and promoted the locomotion ability of these mice.
GMNF Promotes the Neural Stem Cell Proliferation in SCI Area
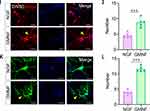
To find out the cellular mechanism of GMNF’s functional influence on SCI mice, the injury area was isolated from these mice with different treatments and neural stem cell-related markers, including anti-Nestin and anti-SOX2, were used to image the neural stem cells in the injury area. We found that the spinal cord tissue at the injury site exhibited more Nestin+ and SOX2+ neural stem cells at the early injury period (the 4th day after injury) and an increased number at the middle stage of the recovery process (the 14th day after injury) in SCI mice treated with GMNF (Figure 8A–C, 8E–G), with a higher density in the injury site of SCI mice treated with GMNF compared to NGF treatment (Figure 8B–D, 8F–H). These cellular results highlighted the key role of neural stem cells in SCI recovery after GMNF treatment.
GMNF Promotes Differentiation of Neural Stem Cells into Neurons in the SCI Area
As the neural stem cells differentiated into neurons, we observed the developmental status of the dendrites and axons in these cells using anti-Map2 and anti-NF. As a result, the dendrites marked with anti-Map2 and the axons marked with anti-NF were denser in the SCI areas injected with GMNF, whether in the early or late stage of differentiation (Figure 9A and B). After single neuron imaging with AAV-EGFP, we found that the neurites of differentiating neurons in SCI areas injected with GMNF were more complex with an increased number of processes and branching structure, suggesting that GMNF could promote neuronal development during neural stem cell differentiation into neurons (Figure 9C).
Discussion
In this study, we innovatively constructed a nanocomplex to achieve more effective repair after spinal cord injury. Through in vitro and in vivo experiments, we found that this biomaterial can effectively deliver NGF to tissues and cells, and it can promote the proliferation and differentiation of neural stem cells and the growth and development of neurons more efficiently, thus accelerating the recovery process of spinal cord injury.
GMNF Has a High and Sustained Drug Release Capacity and Good Biocompatibility
By studying the physicochemical and biological properties of GMNF, we found that this complex can penetrate cells well and release the drugs they carry continuously and stably for 3–4 weeks, which is very necessary for spinal cord repair. The findings of our study revealed compelling evidence regarding the performance of the GelMA hydrogel as a delivery system for NGFs. The GelMA hydrogel demonstrated a remarkably high and sustained drug release capacity throughout the experimental duration. When it comes to nerve regeneration, this characteristic is of paramount importance, as NGF releases must be controlled and prolonged in order to optimize neural stem cell growth and differentiation.26 According to our study, the GelMA hydrogel provides a continuous and stable supply of NGFs to the surrounding neural microenvironment due to its sustained drug release. It is possible to attribute GelMA hydrogel’s unique chemical and structural properties to its controlled release kinetics of NGFs. By incorporating methacrylic acid into the gelatin matrix, the hydrogel is likely to exhibit controlled release, allowing growth factors to gradually diffuse throughout.15,27 In order to promote neural tissue regeneration, this feature mimics the spatial and temporal dynamics of neurotrophic factor gradients in vivo. Continuous drug release may improve neural stem cell differentiation during critical repair periods, leading to more complete neuronal connections.
Additionally, the system is highly compatible with the human body and has a very low likelihood of causing a foreign body reaction. A further benefit of GelMA hydrogel is its excellent biocompatibility. When considering materials for in vivo applications, biocompatibility is a critical factor, especially in the delicate and complicated spinal cord environment.28,29 Within the GelMA hydrogel matrix, neural stem cells grew and differentiated vigorously, indicating its compatibility with stem cells.30,31 Gelatine and methacrylic acid contribute to GelMA’s biocompatibility, which reduces the risk of adverse effects on cellular viability. GelMA hydrogel’s ability to support neural stem cells and its lack of cytotoxic effects make it an ideal scaffold for neural tissue engineering, so there is no need to worry about artificial stimulation and damage caused by the complex material itself on the wound, and it can better play the efficacy of the drugs it carries.
Thence, our study suggests that GelMA hydrogel has great potential when it comes to delivering NGFs to injured spinal cords. As a result of its high and sustained drug release capacity and excellent biocompatibility, it can be an important tool for creating an optimal microenvironment for the regeneration of neural tissue. For this GelMA-based system to be used in the treatment of spinal cord injuries, further research is necessary, including in vivo investigations.
GMNF Better Promotes NPCs Proliferation and Differentiation
GMNF can better enhance the growth and survival of NPCs compared to NGF and promotes their differentiation into mature neuronal cells. The reasons considered are that GMNF composite scaffolds, as a new type of biomaterial, have good biocompatibility and adjustability, and their ECM-like porous structure can effectively mimic the microenvironment of NPC growth in vivo, providing a good ECM for cell proliferation and differentiation.32 Meanwhile, GMNF has good adhesion and affinity for cells, which can provide an effective three-dimensional support structure for cell growth and differentiation, thus further increasing their survival rate on the scaffold.33 In addition, GMFN effectively solves the problems of short half-life, easy degradation and inactivation, and poor targeting of NGFs, and achieves their gradual and controllable release,34,35 which is able to stimulate the proliferation and differentiation of NPCs, and promote their development in the direction of neurons. Yao et al36 also demonstrated that hydrogel scaffolds supported axonal regeneration of neural stem cells (NSCs) within their channels, favoring NSCs infiltration and local neuronal differentiation.
The results of this study indicate a significantly enhanced proliferation of NPCs in the presence of GMNF. This heightened cell proliferation is of particular interest as it suggests that the GelMA hydrogel, serving as a carrier for nerve growth factors, creates a microenvironment conducive to robust cellular replication.37,38 The controlled release of growth factors from the GelMA hydrogel is likely a key factor in this observed effect, providing a sustained and optimal supply to NPCs over time. The specific mechanisms underlying the enhanced proliferation warrant further exploration. It is conceivable that the unique properties of the GelMA hydrogel, including its biocompatibility and structural characteristics, play a role in facilitating cell adhesion, migration, and mitosis. The promotion of NPCs proliferation by GMNF may have significant implications for therapeutic strategies aiming at replenishing damaged neural tissue in conditions such as spinal cord injuries.
Additionally, equally compelling is the observation that GMNF not only facilitates cell proliferation but also promotes the differentiation of NPCs into more specialized neural cell types. The increased propensity of NPCs to differentiate into neurons, astrocytes, or oligodendrocytes in the presence of GMNF underscores its potential as a modulator of neural lineage commitment. This finding is crucial for the regeneration of functional neural tissue, as diverse cell types are necessary for rebuilding complex neural networks.39,40 The molecular pathways and signaling cascades driving the observed effects on NPCs differentiation merit further investigation. Understanding how GMNF orchestrates the fate determination of NPCs could provide valuable insights into the design of targeted therapies for neurological disorders and injuries.
The ability of GMNF to simultaneously enhance cell proliferation and guide NPCs towards specific neural lineages positions it as a promising candidate for regenerative medicine applications. Harnessing the regenerative potential of GMNF may open new avenues for the development of therapies aimed at repairing and restoring function to damaged neural tissue. Thus, GMNF helps to better mimic the microenvironment of neural regeneration in vivo under in vitro conditions, providing new possibilities for neural tissue engineering and repair.
GMNF Better Improves Behavior in SCI Mouse Models by Promoting NSCs Proliferation and Differentiation
To further confirm the efficacy of GMNF in SCI treatment, we created SCI models and conducted animal experiments. The results confirmed that GMNF could better promote the repair of SCI area in mice models as well as the proliferation and differentiation of NSCs, thus restoring nerve conduction and improving their behavioral ability. The reasons maybe that GMNF provides a good three-dimensional support structure and, furthermore, its tunability allows its physical and chemical properties to be optimized to better mimic the physiological environment of the SCI region.41,42 Thus, this helps to promote the colonization and proliferation of NSCs in the injury region. Meanwhile, the introduction of NGFs further enhances the biological activity of the injured area.43 Hu et al44 demonstrated that NGFs had the effect of stimulating the growth and differentiation of neuronal cells, and by loading NGFs into the GelMA gel scaffolds enabling their gradual release; they provide continuous neural stimulation and guide the development of NSCs towards neuronal differentiation. In addition, it has been shown that hydrogels, through their natural bioactive molecules, are able to attract and activate endogenous stem cells in the surrounding tissues,45 thereby inducing their decolonization in the SCI area, which in turn enhances the potential for nerve regeneration. Thus, GMNF creates a microenvironment more favorable for NSCs growth and differentiation, which better facilitates their biological response in the SCI region.
Despite the excellent potential of GMNF in SCI models, there are some shortcomings in the study. We have not conducted an in-depth study of the extent to which GelMA gel scaffolds alleviate the inflammatory state in the SCI region, which may limit our understanding of the full effect of GelMA. Later studies should focus on the role of GelMA in the inflammatory microenvironment to better understand its mechanism in providing suitable biological conditions and promoting NSC colonization. This will help provide more comprehensive and actionable information for SCI treatment and advance the field of neural tissue engineering.
Conclusion
In summary, GMNF composite scaffolds have good biocompatibility and can effectively prolong the half-life of NGFs, allowing them to better play a scaffolding role in the SCI region, and their porous structure promotes the proliferation and colonization of NPCs and facilitates their differentiation and maturation. In addition, animal experiments further demonstrated that GMNF could more markedly promote SCI repair in vivo and promote NSCs proliferation and differentiation, thereby restoring nerve conduction and improving behavioral ability in SCI models. It is a fascinating and potentially impactful area of research, as spinal cord injuries often result in severe and lasting consequences. Using hydrogels for controlled release of nerve growth factors can be a promising strategy to create a supportive environment for neural regeneration. Neural stem cells play a crucial role in this process as they have the potential to differentiate into various neural cell types. Therefore, the application of GMNF composite scaffolds shows great potential in SCI treatment.
Acknowledgment
This work was supported by the Key Research and Development Project of Henan Province (No. 241111313800), the Medical Science and Technology Research Project of Henan Province (No. LHGJ20230670), Zhengzhou Medical and Health Science and Technology Innovation Guidance Plan Project (No. 2024YLZDJH046, No. 2024YLZDJH047, and No. 2024YLZDJH048), and the National Natural Science Foundation of China (Yu Wei Medical Letter [2023] No.30).
Disclosure
The authors declare that they have no competing interests.
References
1. Eli I, Lerner DP, Ghogawala Z. Acute Traumatic Spinal Cord Injury. Neurol Clinics. 2021;39(2):471–488. doi:10.1016/j.ncl.2021.02.004
2. Megía García A, Serrano-Muñoz D, Taylor J, Avendaño-Coy J, Gómez-Soriano J. Transcutaneous Spinal Cord Stimulation and Motor Rehabilitation in Spinal Cord Injury: a Systematic Review. Neurorehabil Neural Repair. 2020;34(1):3–12. doi:10.1177/1545968319893298
3. Huang L, Fu C, Xiong F, He C, Wei Q. Stem Cell Therapy for Spinal Cord Injury. Cell Transplan. 2021;30:963689721989266. doi:10.1177/0963689721989266
4. Lima R, Monteiro A, Salgado AJ, Monteiro S, Silva NA. Pathophysiology and Therapeutic Approaches for Spinal Cord Injury. Int J Mol Sci. 2022;23(22). doi:10.3390/ijms232213833
5. Freyermuth-Trujillo X, Segura-Uribe JJ, Salgado-Ceballos H, Orozco-Barrios CE, Coyoy-Salgado A. Inflammation: a Target for Treatment in Spinal Cord Injury. Cells. 2022;11(17). doi:10.3390/cells11172692
6. Amo-Aparicio J, Martínez-Muriana A, Sánchez-Fernández A, López-Vales R. Neuroinflammation Quantification for Spinal Cord Injury. Current proto immun. 2018;123(1):e57. doi:10.1002/cpim.57
7. Ni J, Suzuki T, Karnup SV, Gu B, Yoshimura N. Nerve growth factor-mediated Na(+) channel plasticity of bladder afferent neurons in mice with spinal cord injury. Life Sci. 2022;298:120524. doi:10.1016/j.lfs.2022.120524
8. Xia N, Gao Z, Hu H, et al. Nerve growth factor loaded macrophage-derived nanovesicles for inhibiting neuronal apoptosis after spinal cord injury. J biomat appli. 2021;36(2):276–288. doi:10.1177/08853282211025912
9. Liu S, Jia X, Hao J, et al. Tissue Engineering of JAK Inhibitor-Loaded Hierarchically Biomimetic Nanostructural Scaffold Targeting Cellular Senescence for Aged Bone Defect Repair and Bone Remolding. Adv Healthcare Mater. 2023;12(30):e2301798. doi:10.1002/adhm.202301798
10. Babaei M, Ghaee A, Nourmohammadi J. Poly (sodium 4-styrene sulfonate)-modified hydroxyapatite nanoparticles in zein-based scaffold as a drug carrier for vancomycin. Mater Sci Eng C Mater Biol Appl. 2019;100:874–885. doi:10.1016/j.msec.2019.03.055
11. Tran KA, Jin Y, Bouyer J, et al. Magnetic alignment of injectable hydrogel scaffolds for spinal cord injury repair. Biomater Sci. 2022;10(9):2237–2247. doi:10.1039/D1BM01590G
12. Cai M, Chen L, Wang T, et al. Hydrogel scaffolds in the treatment of spinal cord injury: a review. Front Neurosci. 2023;17:1211066. doi:10.3389/fnins.2023.1211066
13. Kurian AG, Singh RK, Patel KD, Lee JH, Kim HW. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact Mater. 2022;8:267–295.
14. Shen M, Wang L, Gao Y, et al. 3D bioprinting of in situ vascularized tissue engineered bone for repairing large segmental bone defects. Mater Today Bio. 2022;16:100382. doi:10.1016/j.mtbio.2022.100382
15. Wang L, Shen M, Hou Q, Wu Z, Xu J, Wang L. 3D printing of reduced glutathione grafted gelatine methacrylate hydrogel scaffold promotes diabetic bone regeneration by activating PI3K/Akt signaling pathway. Int J Biol Macromol. 2022;222(Pt A):1175–1191. doi:10.1016/j.ijbiomac.2022.09.236
16. Song Z, Wang Z, Shen J, Xu S, Hu Z. Nerve growth factor delivery by ultrasound-mediated nanobubble destruction as a treatment for acute spinal cord injury in rats. Int j Nanomed. 2017;12:1717–1729. doi:10.2147/IJN.S128848
17. Rocco ML, Soligo M, Manni L, Aloe L. Nerve Growth Factor: early Studies and Recent Clinical Trials. Curr Neuropharm. 2018;16(10):1455–1465. doi:10.2174/1570159X16666180412092859
18. Aloe L, Rocco ML, Balzamino BO, Micera A. Nerve Growth Factor: a Focus on Neuroscience and Therapy. Curr Neuropharm. 2015;13(3):294–303. doi:10.2174/1570159X13666150403231920
19. Li D, Yang T, Shao C, Cao Z, Zhang H. LncRNA MIAT activates vascular endothelial growth factor A through RAD21 to promote nerve injury repair in acute spinal cord injury. Molec Cellular Endocrin. 2021;528:111244. doi:10.1016/j.mce.2021.111244
20. Yamanaka K, Eldeiry M, Aftab M, et al. Synergetic Induction of NGF With Diazoxide and Erythropoietin Attenuates Spinal Cord Ischemic Injury. J Surg Res. 2019;233:124–131. doi:10.1016/j.jss.2018.07.021
21. Ho-Shui-Ling A, Bolander J, Rustom LE, Johnson AW, Luyten FP, Picart C. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143–162. doi:10.1016/j.biomaterials.2018.07.017
22. Lazaridou M, Bikiaris DN, Lamprou DA. 3D Bioprinted Chitosan-Based Hydrogel Scaffolds in Tissue Engineering and Localised Drug Delivery. Pharmaceutics. 2022;14(9):1978. doi:10.3390/pharmaceutics14091978
23. Li H, Ji Q, Chen X, et al. Accelerated bony defect healing based on chitosan thermosensitive hydrogel scaffolds embedded with chitosan nanoparticles for the delivery of BMP2 plasmid DNA. J Biomed Mater Res Part A. 2017;105(1):265–273. doi:10.1002/jbm.a.35900
24. Anirudhan TS, Mohan AM. Novel pH sensitive dual drug loaded-gelatin methacrylate/methacrylic acid hydrogel for the controlled release of antibiotics. Int J Biol Macromol. 2018;110:167–178. doi:10.1016/j.ijbiomac.2018.01.220
25. Fan H, Tang HB, Shan LQ, et al. Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats. J Neuroinflamm. 2019;16(1):206. doi:10.1186/s12974-019-1613-2
26. Ghosh S, Ghosh S, Sharma H, Bhaskar R, Han SS, Sinha JK. Harnessing the power of biological macromolecules in hydrogels for controlled drug release in the central nervous system: a review. Int J Biol Macromol. 2023;254(Pt 1):127708. doi:10.1016/j.ijbiomac.2023.127708
27. Zhu W, Dong Y, Xu P, et al. A composite hydrogel containing resveratrol-laden nanoparticles and platelet-derived extracellular vesicles promotes wound healing in diabetic mice. Acta Biomater. 2022;154:212–230. doi:10.1016/j.actbio.2022.10.038
28. Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi:10.1016/j.biomaterials.2015.08.045
29. Klotz BJ, Gawlitta D, Rosenberg A, Malda J, Melchels FPW. Gelatin-Methacryloyl Hydrogels: towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016;34(5):394–407. doi:10.1016/j.tibtech.2016.01.002
30. Cheng J, Chen Z, Liu C, et al. Bone mesenchymal stem cell-derived exosome-loaded injectable hydrogel for minimally invasive treatment of spinal cord injury. Nanomedici. 2021;16(18):1567–1579. doi:10.2217/nnm-2021-0025
31. Shi M, Xu Q, Ding L, et al. Cell Infiltrative Inner Connected Porous Hydrogel Improves Neural Stem Cell Migration and Differentiation for Functional Repair of Spinal Cord Injury. ACS Biomater Sci Eng. 2022;8(12):5307–5318. doi:10.1021/acsbiomaterials.2c01127
32. Huang C, Liu Y, Ding J, et al. Thermosensitive quaternized chitosan hydrogel scaffolds promote neural differentiation in bone marrow mesenchymal stem cells and functional recovery in a rat spinal cord injury model. Cell Tissue Res. 2021;385(1):65–85. doi:10.1007/s00441-021-03430-x
33. Li Y, Cheng S, Wen H, et al. Coaxial 3D printing of hierarchical structured hydrogel scaffolds for on-demand repair of spinal cord injury. Acta Biomater. 2023;168:400–415. doi:10.1016/j.actbio.2023.07.020
34. Ji H, Gu J, Song X, et al. A nerve growth factor persistent delivery scaffold seeded with neurally differentiated bone marrow mesenchymal stem cells promoted the functional recovery of spinal cord injury in rats. Am J Transl Res. 2021;13(4):2127–2142.
35. Chen H, Li J, Yan H. The transplantation of human urine stem cells combined with chondroitinase ABC promotes brain-derived neurotrophic factor and nerve growth factor following spinal cord injury in rats. Int J Clin Exp Pathol. 2018;11(8):3858–3866.
36. Yao Z, Yuan W, Xu J, et al. Magnesium-Encapsulated Injectable Hydrogel and 3D-Engineered Polycaprolactone Conduit Facilitate Peripheral Nerve Regeneration. Adv scie. 2022;9(21):e2202102. doi:10.1002/advs.202202102
37. Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31(21):5536–5544. doi:10.1016/j.biomaterials.2010.03.064
38. Li X, Li X, Yang J, et al. Living and Injectable Porous Hydrogel Microsphere with Paracrine Activity for Cartilage Regeneration. Small. 2023;19(17):e2207211. doi:10.1002/smll.202207211
39. Liu X, Huang H, Snutch TP, Cao P, Wang L, Wang F. The Superior Colliculus: cell Types, Connectivity, and Behavior. Neuroscience Bulletin. 2022;38(12):1519–1540. doi:10.1007/s12264-022-00858-1
40. Cadwell CR, Bhaduri A, Mostajo-Radji MA, Keefe MG, Nowakowski TJ. Development and Arealization of the Cerebral Cortex. Neuron. 2019;103(6):980–1004.
41. Siddiqui AM, Thiele F, Stewart RN, et al. Open-Spaced Ridged Hydrogel Scaffolds Containing TiO(2)-Self-Assembled Monolayer of Phosphonates Promote Regeneration and Recovery Following Spinal Cord Injury. Int J Mol Sci. 2023;24(12). doi:10.3390/ijms241210250
42. Song S, Li Y, Huang J, Cheng S, Zhang Z. Inhibited astrocytic differentiation in neural stem cell-laden 3D bioprinted conductive composite hydrogel scaffolds for repair of spinal cord injury. Biomate advan. 2023;148:213385. doi:10.1016/j.bioadv.2023.213385
43. Wang L, Gu S, Gan J, et al. Neural Stem Cells Overexpressing Nerve Growth Factor Improve Functional Recovery in Rats Following Spinal Cord Injury via Modulating Microenvironment and Enhancing Endogenous Neurogenesis. Front Cell Neurosci. 2021;15:773375. doi:10.3389/fncel.2021.773375
44. Hu X, Li R, Wu Y, et al. Thermosensitive heparin-poloxamer hydrogel encapsulated bFGF and NGF to treat spinal cord injury. J Cell & Mol Med. 2020;24(14):8166–8178. doi:10.1111/jcmm.15478
45. Xu Y, Zhou J, Liu C, et al. Understanding the role of tissue-specific decellularized spinal cord matrix hydrogel for neural stem/progenitor cell microenvironment reconstruction and spinal cord injury. Biomaterials. 2021;268:120596. doi:10.1016/j.biomaterials.2020.120596
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.