Back to Journals » International Journal of General Medicine » Volume 18
Geriatric Nutritional Risk Index (GNRI) and Prognostic Nutritional Index (PNI) Before Treatment as the Predictive Indicators for Bone Metastasis in Prostate Cancer Patients
Authors Chen L, Rao H, Chen N, Li R, Chen D, Jiang H
Received 10 January 2025
Accepted for publication 18 May 2025
Published 24 May 2025 Volume 2025:18 Pages 2703—2713
DOI https://doi.org/10.2147/IJGM.S516768
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Leonardo Reis
Libo Chen,1,* Hui Rao,2,* Nanhui Chen,1 Renyuan Li,3 Dan Chen,4 Huiming Jiang1
1Department of Urology, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China; 2Department of Laboratory Medicine, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China; 3Data Center, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China; 4Surgical Center, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huiming Jiang, Department of Urology, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China, Email [email protected]
Objective: Inflammation and nutritional status are involved in the occurrence and progression of cancer. The purpose of this study was to investigate the relationship of nutritional status indices (geriatric nutritional risk index (GNRI), neutrophil to albumin ratio (NAR), prognostic nutritional index (PNI)), and comprehensive inflammatory indices (pan-immune inflammation value (PIV), systemic immune inflammation index (SII), and system inflammation response index (SIRI)) and bone metastasis of prostate cancer.
Methods: A retrospective analysis was performed on 888 prostate cancer patients treated in Meizhou People’s Hospital from November 2017 to December 2022. Clinical characteristics were collected, including age, body mass index (BMI), bone metastasis, and GNRI, NAR, PNI, PIV, SII, and SIRI levels. The optimal cutoff values of these indices were calculated by receiver operating characteristic (ROC) curve, and the relationship between these indices and bone metastasis was analyzed.
Results: There were 836 (94.1%) cases were ≥ 60 years old, indicating that the majority of prostate cancer patients were elderly men. There were 640 (72.1%) patients without bone metastasis and 248 (27.9%) patients with bone metastasis. The levels of GNRI and PNI in patients with bone metastasis were significantly lower than those without, while NAR, PIV, SII, and SIRI were not statistically significant. And the levels of GNRI and PNI in patients with multiple bone metastasis were significantly lower than those with single bone metastasis. When bone metastasis was taken as the endpoint of GNRI and PNI, the critical value of GNRI was 97.05 (sensitivity 55.2%, specificity 67.5%, area under the ROC curve (AUC) = 0.639), the PNI cutoff value was 44.925 (sensitivity 51.2%, specificity 67.2%, AUC = 0.634), and the AUC of GNRI plus PNI was 0.647.
Conclusion: Prostate cancer is more common in older men; about a quarter of patients have bone metastasis. GNRI and PNI have predictive efficacy in bone metastasis and multiple bone metastasis of prostate cancer, but NAR, PIV, SII, and SIRI do not.
Keywords: prostate cancer, bone metastasis, geriatric nutritional risk index, prognostic nutritional index
Introduction
Prostate cancer is an epithelial malignant tumor occurring in the prostate and is the most common malignant tumor of the male genitourinary system.1 Prostate cancer has become the second most common cancer in men worldwide after lung cancer.2 Both the incidence and mortality of prostate cancer are on the rise in China.3–5 With the intensification of global population aging, the incidence of prostate cancer will increase.6 The onset of prostate cancer is occultic, and most of the patients have no obvious symptoms in the early stage, and some of them are already in the advanced stage when they are found.7 In some patients, tumor metastasis is found at first visit, leading to a decrease in quality of life.8
For the treatment of prostate cancer, radical treatment has a relatively high effectiveness and focal therapy has fewer side effects.9 Tumor metastasis is a thorny issue that has to be faced in the treatment of prostate cancer. Approximately 54% of patients have distant metastases at the time of initial diagnosis, among which about 80% are bone metastasis.10 Bone metastasis of prostate cancer often cause skeletal related events (SREs) and a series of other complications, which are the main causes of quality of life decline and death in patients with metastatic prostate cancer.11 SREs caused by bone metastasis of prostate cancer include pathological fracture, spinal cord compression, bone surgery, and bone radiation therapy.12 Effective prediction of the risk of bone metastasis in prostate cancer patients is beneficial to the treatment and prognosis of patients. Magnetic resonance imaging (MRI) has high sensitivity and specificity in the diagnosis of bone metastases in prostate cancer, but it is limited in the diagnosis of long limbs and cortical metastases.13 Positron emission computed tomography (PET) can find smaller and more bone marrow lesions, but the detection is time-consuming and the patient tolerance is poor, and the clinical application value needs to be further analyzed.14 Bone biopsy usually produces less tumor tissue, and it is difficult to carry out widely as an invasive examination.15 The detection of peripheral blood markers has potential value in the prediction of bone metastasis of prostate cancer due to its convenient specimen acquisition and simple detection method.
Inflammation and nutritional status are involved in the occurrence and development of some diseases16,17 and are now receiving extensive attention.18 Nutritional deficiency can disrupt the regulation of prostate hormones, induce oxidative stress and inflammation, alter growth factor signaling and lipid metabolism, and promote the occurrence and progression of prostate cancer.19 At present, some comprehensive indices based on serum albumin levels have gradually gained recognition in the assessment of disease progression and prognosis. Geriatric nutritional risk index (GNRI), which is used to assess an individual’s nutritional status, has been shown to be associated with a number of diseases and is considered a potentially valuable indicator in tumors.20,21 Neutrophil to albumin ratio (NAR) is an important index that comprehensively reflects the level of systemic immunity and nutritional status and has been proved to be closely related to tumor and cardiovascular and cerebrovascular diseases by many studies.22,23 Prognostic nutritional index (PNI) which can reflect the immune and nutritional status of the host and serve as an effective prognostic factor for a variety of tumors.24,25
The occurrence and development of many human diseases are more or less involved in the process of immune inflammatory response.26,27 Immunoinflammatory response is also involved in the development and progression of tumors.28,29 The tumor microenvironment (TME) is an important site for the growth, proliferation, and metastasis of tumor cells, among which the infiltration of inflammatory cells is one of its significant characteristics.30 The inflammatory response regulates the expression of a series of genes related to cell proliferation, survival, invasion and metastasis, thereby promoting the development of cancer.31 In addition, inflammation can also support the growth and metastasis of tumors by inducing angiogenesis, providing sufficient nutrition and oxygen to tumor cells.32 In recent years, some comprehensive inflammatory indices (pan-immune inflammation value (PIV), systemic immune inflammation index (SII), and system inflammation response index (SIRI)) have received widespread attention. SII is a comprehensive index of neutrophils, platelets and lymphocytes, and has been proven to predict the prognosis of liver cancer, pancreatic cancer, cervical cancer and other cancers.33,34 The SIRI index, an indicator of overall lymphocytes, monocytes, and neutrophils levels, has been linked to the prognosis of pancreatic, gastric, and breast cancers.35–37 However, the predictive value of GNRI, NAR, PNI, PIV, SII, and SIRI in prostate cancer bone metastasis is unclear. This study evaluated the relationship between these indicators and bone metastasis in patients with prostate cancer.
Materials and Methods
Subjects
The prostate cancer patients, who were hospitalized in Meizhou People’s Hospital from November 2017 to December 2022, were included in this study. Inclusion criteria: (1) prostate cancer patients were confirmed by histopathology examination; (2) whole-body bone scans are performed using emission computed tomography (ECT), computed tomography (CT), magnetic resonance imaging (MRI), or nuclide bone imaging; (3) medical records were complete; and (4) serum albumin, and peripheral blood cell analysis was performed at least once before surgery, and there were no inflammation-related factors affecting blood indexes. Exclusion criteria: (1) patients with other malignant tumor diseases; (2) patients with diseases that affect relevant peripheral blood inflammatory indicators, such as severe infections, autoimmune diseases, and so on; (3) patients with dysfunction of important organs; and (4) medical records were incomplete. The study was approved by the Ethics Committee of Medicine, Meizhou People’s Hospital (Clearance No.: 2024-C-241).
Data Collection
Clinical characteristics of the patients were collected from the medical records system of our hospital, including age, body mass index (BMI), and bone metastasis. Blood routine test data were collected at admission and before treatment. The blood cell analysis was tested by the Sysmex XE-2100 haematology analyzer (Sysmex Corporation, Japan) and serum albumin was measured by Roche automatic biochemical analyzer, according to standard operating procedures (SOP).
Data Processing and Statistical Analysis
The inflammation index GNRI, NAR, PNI, PIV, SII, and SIRI were calculated according to the following formula:
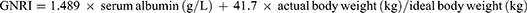

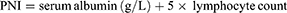
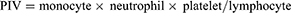


SPSS statistical software version 26.0 (IBM Inc., USA) and GraphPad Prism 8.0 were used for data analysis and mapping, and continuous data were compared using t-test or Mann–Whitney U-test. The categorical variables were compared using by Chi-square test or Fisher’s exact test. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cutoff values of GNRI, NAR, PNI, PIV, SII, and SIRI to distinguish bone metastasis of prostate cancer. p<0.05 was set as statistically significant.
Results
Clinical Characteristics of Prostate Cancer Patients
There were 888 patients with prostate cancer, 52 (5.9%) cases were <60 years old and 836 (94.1%) cases were ≥60 years old, indicating that the majority of prostate cancer patients were elderly men. There were 94 (10.6%) cases with BMI <18.5 kg/m2, 515 (58.0%) cases with BMI 18.5–23.9 kg/m2, and 279 (31.4%) cases with BMI ≥24.0 kg/m2 (Table 1).
 |
Table 1 Characteristics and Laboratory Parameters of Prostate Cancer Patients with and without Bone Metastasis |
In this study, there were 640 (640/888, 72.1%) patients without bone metastasis and 248 (248/888, 27.9%) patients with bone metastasis. The levels of albumin and lymphocyte count in bone metastasis group were lower than those in non-bone metastasis group (p<0.001). The differences of levels of neutrophil count, monocytes count, and platelet count were not statistically significant between the two groups (Table 1).
Comparison of GNRI, NAR, PNI, PIV, SII, and SIRI in Prostate Cancer Patients with or without Bone Metastasis
The levels of GNRI (96.15 (88.38, 103.15) vs 100.90 (94.80, 107.30), p<0.001) and PNI (44.80 (40.36, 48.88) vs 47.55 (43.75, 51.60), p<0.001) in patients with bone metastasis were significantly lower than those in patients without bone metastasis. The differences of levels of NAR, PIV, SII, and SIRI were not statistically significant between patients with and without bone metastasis (Figure 1).
Comparison of GNRI, NAR, PNI, PIV, SII, and SIRI in Prostate Cancer Patients with Non-Bone Metastasis, Single Bone Metastasis, and Multiple Bone Metastasis
The levels of GNRI (95.00 (87.90, 102.70) vs 100.90 (94.80, 107.30), p<0.001) and PNI (44.10 (40.25, 48.35) vs 47.55 (43.75, 51.60), p<0.001) in patients with multiple bone metastasis were significantly lower than those in patients without bone metastasis. The levels of GNRI (95.00 (87.90, 102.70) vs 100.20 (96.00, 106.50), p<0.001) and PNI (44.10 (40.25, 48.35) vs 48.50 (45.20, 51.10), p<0.001) in patients with multiple bone metastasis were significantly lower than those in patients with single bone metastasis (Figure 2A).
The levels of SII (702.73 (396.84, 1209.59) vs 594.68 (403.49, 916.32), p=0.042) and SIRI (1.540 (0.955, 3.100) vs 1.400 (0.883, 2.278), p=0.020) in patients with multiple bone metastasis were significantly higher than those in patients without bone metastasis (Figure 2B).
ROC Analysis of GNRI, and PNI Used in the Differential Diagnosis of Bone Metastasis in Prostate Cancer
When bone metastasis was taken as the endpoint of GNRI and PNI, the critical value of GNRI was 97.05 (sensitivity 55.2%, specificity 67.5%, area under the ROC curve (AUC) = 0.639), the PNI cutoff value was 44.925 (sensitivity 51.2%, specificity 67.2%, AUC = 0.634), and the AUC of GNRI plus PNI was 0.647 (Figure 3A).
When multiple bone metastasis was taken as the endpoint (single bone metastasis as control) of GNRI and PNI, the critical value of GNRI was 95.95 (sensitivity 53.1%, specificity 76.9%, AUC = 0.649), the PNI cutoff value was 44.575 (sensitivity 53.6%, specificity 82.1%, AUC = 0.683), and the AUC of GNRI plus PNI was 0.687 (Figure 3B).
Discussion
Inflammation and nutritional status related to malignant tumors have been one of the hot issues in recent years.38 Tumor burden is not the only reason affecting the prognosis of tumor patients. Based on the differences in individual factors of patients with malignant tumors and the various biological characteristics of tumor cells themselves, there is still a large gap in the overall survival rate of patients at the same stage or using similar treatment programs.39,40 Therefore, while continuing to focus on the characteristics of the tumor itself, constantly exploring the mechanism of tumor progression and updating treatment strategies,41,42 more and more clinicians and researchers have paid attention to the study on the changes of TME43 and the internal inflammation,44 immunity, and nutritional status.38 GNRI45 and PNI46 are new tumor prognostic indicators emerging in recent years.
Low GNRI was associated with poorer overall survival (OS)47,48 and cancer-specific survival (CSS),47,49 and disease-free survival (DFS)48 in cancer patients. GNRI has been poorly studied in prostate cancer. Miao et al found that lower GNRI levels were associated with a higher risk of prostate cancer.50 Shu et al revealed that GNRI was associated with postoperative complications of prostate cancer.51 Some studies suggested that low GNRI was an independent risk of prostate cancer and poorer OS52–54 and PFS.53 In this study, GNRI is considered to be a potential predictor of prostate cancer bone metastasis, and this study enriches the data on GNRI in prostate cancer metastasis.
PNI was a risk predictor of tumor metastasis of some cancers.55–59 PNI was also associated with survival in patients with gastric cancer,60,61 colorectal cancer,62,63 cervical cancer,64 NSCLC,65 esophageal cancer,25 pancreatic cancer,59 and renal cell carcinoma.66 Studies of PNI in prostate cancer are rarely reported. Some studies showed that PNI was a predictive marker of OS,67–69 PFS,67,69,70 and CSS68,69 in prostate cancer patients. A lower PNI was a risk factor for a shorter recurrence time in prostate cancer patients after prostatectomy.71 In this study, PNI may be a potential predictive indicator of prostate cancer bone metastasis.
Tumor metastasis depends on tumor angiogenesis and invasion of immune cells.72 The site of tumor metastasis was rich in activated immune cells.73 General speaking, the tumor microenvironment composed of the above cells is in a relatively stable state, and once the above homeostatic state is broken, tumor cell immune escape and cancer progression may occur. Tumor cells induce immune cells to promote the occurrence and development of bone metastasis, and immune cells may be potential therapeutic targets for bone metastasis.74 Wang et al have established a prostate cancer bone metastasis prediction model based on multiple immune-inflammatory parameters.75 However, in this study, PIV, SII, and SIRI were not effective in predicting the risk of bone metastasis of prostate cancer.
Immunoinflammatory response is involved in the development of tumor by changing the tumor microenvironment, affecting gene homeostasis, inducing tumor cell invasion and metastasis.28,29 Lymphocytes and platelets are involved in tumor progression, while neutrophils can increase cancer cell invasion, proliferation, and metastasis, helping cancer cells evade immune surveillance.76 Increased monocyte counts are associated with reduced overall survival from malignant tumors.77,78 At present, the study of immunoinflammatory indices in prostate cancer mainly focuses on the relationship between these indices and prostate cancer risk and prognosis. A meta-analysis suggested that high SII may be associated with poorer OS and PFS.79 Another meta-analysis showed that high SII was significantly associated with poorer OS; however, high SII level was not significantly associated with lymph node metastasis,80 contrary to another study.81 Some studies showed that high SII values were associated with an increased risk of prostate cancer.82,83 The inflammatory response of prostate cancer is closely related to age, which may be associated with the common characteristics of cancer progression and aging, such as genomic instability, cellular senescence and chronic inflammation.84 In addition, the interaction between aging and oxidative stress also plays an important role in the progression of tumors.85 Furthermore, the results of this study show that the proportion of underweight (BMI<18.5) patients with bone metastasis than without. Generally speaking, overweight patients have higher levels of inflammation and may have a higher susceptibility to cancer. The opposite results obtained in this study may be related to the different sample sizes included and the differences among the populations.
The role of PIV, SII, and SIRI in prostate cancer metastasis has not been reported, while some studies have been reported in some other cancer types. High level of SII was associated with distant metastasis of colorectal cancer,86 and renal cell carcinoma.87 SII was associated with lymph node metastasis in patients with papillary thyroid cancer,88,89 gastric cancer,90–92 breast cancer,93,94 and esophageal cancer.95 However, there was no significant correlation between SII and lymph node metastasis of pancreatic cancer,96 prostate cancer,80 upper tract urothelial carcinoma.97 This study found that PIV, SII, and SIRI had limited predictive efficacy in prostate cancer bone metastasis.
Among a panel of inflammation and nutritional markers, the GNRI and PNI performed best as a predictive factor of bone metastasis in patients with prostate cancer. However, this study still has the following limitations. First, as a retrospective study from a single medical institution, this research may have some methodological limitations, such as the problem of missing or incomplete patient information affecting the quality and completeness of the data, the inability to control the factors included in the analysis, which may affect the accuracy of the research results, and the restrictions on the selection of the included research subjects leading to the non-representativeness of the research subjects. Second, this study did not conduct stratified analysis of the correlation between the severity of bone metastasis, the number of metastasis and these indicators of prostate cancer. Third, the results of ROC curve analysis in this study showed that GNRI and PNI only had moderate predictive efficacy. The combination of GNRI and PNI with other indicators to comprehensively evaluate the risk of bone metastasis of prostate cancer may have better predictive efficacy. Finally, the molecular mechanism of these indicators in bone metastasis of prostate cancer patients was not thoroughly studied in this paper, and further research in animal experiments and clinical studies is needed.
Conclusions
In summary, prostate cancer is more common in older men, and about a quarter of patients have bone metastasis. Nutritional indicators (GNRI and PNI) based on albumin may have a good predictive effect on bone metastasis and multiple bone metastasis of prostate cancer. Of course, the true influence and role of GNRI and PNI in bone metastasis of prostate cancer require more research to reveal, especially the conclusions drawn from randomized controlled trials, systematic reviews, and meta-analyses. Furthermore, GNRI and PNI need to be combined with other indicators to enhance their clinical value.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of Medicine, Meizhou People’s Hospital. All participants signed informed consent in accordance with the Declaration of Helsinki.
Acknowledgments
The author would like to thank other colleagues whom were not listed in the authorship of Department of Urology, Meizhou People’s Hospital for their helpful comments on the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Project of Medical and Health Scientific Research of Meizhou City (Grant No.: 2025-B-31).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Sekhoacha M, Riet K, Motloung P, Gumenku L, Adegoke A. Prostate cancer review: genetics, diagnosis, treatment options, and alternative approaches. Molecules. 2022;27(17):5730. doi:10.3390/molecules27175730
2. Sung H, Ferlay J, Siegel RL. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–590. doi:10.1097/CM9.0000000000002108
4. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
5. Wang Y, Yan Q, Fan C, et al. Overview and countermeasures of cancer burden in China. Sci China Life Sci. 2023;66(11):2515–2526.
6. Feng DC, Li DX, Wu RC, et al. Global burden and cross-country inequalities in urinary tumors from 1990 to 2021 and predicted incidence changes to 2046. Mil Med Res. 2025;12(1):12. doi:10.1186/s40779-025-00599-y
7. Lowrance W, Dreicer R, Jarrard DF, et al. Updates to advanced prostate cancer: AUA/SUO guideline (2023). J Urol. 2023;209(6):1082–1090. doi:10.1097/JU.0000000000003452
8. Ravi Kumar AS, Lawrentschuk N, Hofman MS. Prostate-specific membrane antigen PET/computed tomography for staging prostate cancer. Curr Opin Urol. 2020;30(5):628–634. doi:10.1097/MOU.0000000000000799
9. Feng D, Li D, Xiao Y, Wu R, Wang J, Zhang C. Focal ablation therapy presents promising results for selectively localized prostate cancer patients. Chin J Cancer Res. 2023;35(4):424–430. doi:10.21147/j.issn.1000-9604.2023.04.08
10. Isheden G, Grassmann F, Czene K, Humphreys K. Lymph node metastases in breast cancer: investigating associations with tumor characteristics, molecular subtypes and polygenic risk score using a continuous growth model. Int J Cancer. 2021;149(6):1348–1357. doi:10.1002/ijc.33704
11. Parry MG, Cowling TE, Sujenthiran A, et al. Identifying skeletal-related events for prostate cancer patients in routinely collected hospital data. Cancer Epidemiol. 2019;63:101628. doi:10.1016/j.canep.2019.101628
12. Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. doi:10.1016/S0140-6736(10)62344-6
13. Nakanishi K, Tanaka J, Nakaya Y, et al. Whole-body MRI: detecting bone metastases from prostate cancer. Jpn J Radiol. 2022;40(3):229–244. doi:10.1007/s11604-021-01205-6
14. Mohseninia N, Zamani-Siahkali N, Harsini S, Divband G, Pirich C, Beheshti M. Bone metastasis in prostate cancer: bone scan versus PET imaging. Semin Nucl Med. 2024;54(1):97–118. doi:10.1053/j.semnuclmed.2023.07.004
15. Sailer V, Schiffman MH, Kossai M, et al. Bone biopsy protocol for advanced prostate cancer in the era of precision medicine. Cancer. 2018;124(5):1008–1015. doi:10.1002/cncr.31173
16. Eckart A, Struja T, Kutz A, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. 2020;133(6):713–722.e717. doi:10.1016/j.amjmed.2019.10.031
17. Yao J, Chen X, Meng F, Cao H, Shu X. Combined influence of nutritional and inflammatory status and depressive symptoms on mortality among US cancer survivors: findings from the NHANES. Brain Behav Immun. 2024;115:109–117. doi:10.1016/j.bbi.2023.10.002
18. Stumpf F, Keller B. Inflammation and nutrition: friend or foe? Nutrients. 2023;15(5):1159. doi:10.3390/nu15051159
19. Oczkowski M, Dziendzikowska K. Dietary factors and prostate cancer development, progression, and reduction. Nutrients. 2021;13(2):496. doi:10.3390/nu13020496
20. Yiu CY, Liu CC. Efficacy of the geriatric nutritional risk index for predicting overall survival in patients with head and neck cancer: a meta-analysis. Nutrients. 2023;15(20):4348. doi:10.3390/nu15204348
21. Shi T, Wang Y, Peng Y, et al. Advanced lung cancer inflammation index combined with geriatric nutritional risk index predict all-cause mortality in heart failure patients. BMC Cardiovasc Disord. 2023;23(1):565. doi:10.1186/s12872-023-03608-x
22. Varim C, Celik FD, Sunu C, et al. The role of neutrophil albumin ratio in predicting the stage of non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2022;26(8):2900–2905. doi:10.26355/eurrev_202204_28621
23. Çekmen B, Bildik B, Atiş ŞE, Güven H. The role of neutrophil-albumin ratio in the diagnosis of acute appendicitis and its efficacy in predicting perforation. Ulus Travma Acil Cerrahi Derg. 2022;29(1):52–58. doi:10.14744/tjtes.2022.56570
24. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai zasshi. 1984;85(9):1001–1005. PMID: 6438478.
25. Liao G, Zhao Z, Yang H, Chen M, Li X. Can prognostic nutritional index be a prediction factor in esophageal cancer?: A meta-analysis. Nutr Cancer. 2020;72(2):187–193. doi:10.1080/01635581.2019.1631859
26. Betrains A, Staels F, Schrijvers R, et al. Systemic autoinflammatory disease in adults. Autoimmun Rev. 2021;20(4):102774. doi:10.1016/j.autrev.2021.102774
27. Ma H, Liu M, Fu R, et al. Phase separation in innate immune response and inflammation-related diseases. Front Immunol. 2023;14:1086192. doi:10.3389/fimmu.2023.1086192
28. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. doi:10.1186/s12199-018-0740-1
29. Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation - have anti-inflammatory therapies come of age? Nat Rev Clin Oncol. 2021;18(5):261–279. doi:10.1038/s41571-020-00459-9
30. Zhou H, He Q, Li C, et al. Focus on the tumor microenvironment: a seedbed for neuroendocrine prostate cancer. Front Cell Dev Biol. 2022;10:955669. doi:10.3389/fcell.2022.955669
31. Gong Y, Wang L, Yu H, et al. Prostate cancer in world trade center responders demonstrates evidence of an inflammatory cascade. Mol Cancer Res. 2019;17(8):1605–1612. doi:10.1158/1541-7786.MCR-19-0115
32. Ciummo SL, Sorrentino C, Fieni C, Di Carlo E. Interleukin-30 subverts prostate cancer-endothelium crosstalk by fostering angiogenesis and activating immunoregulatory and oncogenic signaling pathways. J Exp Clin Cancer Res. 2023;42(1):336. doi:10.1186/s13046-023-02902-y
33. Huang H, Liu Q, Zhu L, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. 2019;9(1):3284. doi:10.1038/s41598-019-39150-0
34. Han R, Tian Z, Jiang Y, et al. Prognostic significance of the systemic immune inflammation index in patients with metastatic and unresectable pancreatic cancer. Front Surg. 2022;9:915599. doi:10.3389/fsurg.2022.915599
35. Li S, Lan X, Gao H, et al. Systemic Inflammation Response Index (SIRI), cancer stem cells and survival of localised gastric adenocarcinoma after curative resection. J Cancer Res Clin Oncol. 2017;143(12):2455–2468. doi:10.1007/s00432-017-2506-3
36. Kim JS, Choi M, Kim SH, Hwang HK, Lee WJ, Kang CM. Systemic inflammation response index correlates with survival and predicts oncological outcome of resected pancreatic cancer following neoadjuvant chemotherapy. Pancreatology. 2022;22(7):987–993. doi:10.1016/j.pan.2022.08.009
37. Zhu M, Chen L, Kong X, et al. The systemic inflammation response index as an independent predictor of survival in breast cancer patients: a retrospective study. Front Mol Biosci. 2022;9:856064. doi:10.3389/fmolb.2022.856064
38. Zhang X, Pang L, Sharma SV, Li R, Nyitray AG, Edwards BJ. Malnutrition and overall survival in older patients with cancer. Clin Nutr. 2021;40(3):966–977. doi:10.1016/j.clnu.2020.06.026
39. Wang D, Guo D, Shi F, et al. The predictive effect of the systemic immune-inflammation index for patients with small-cell lung cancer. Future Oncol. 2019;15(29):3367–3379. doi:10.2217/fon-2019-0288
40. Luo T, Hu J. Predicting survival in patients with neuroendocrine prostate cancer: a SEER-based comprehensive study. World J Mens Health. 2025;43(2):415–427. doi:10.5534/wjmh.240061
41. Wu Y, Peng S, Cheng B, et al. FOXA1-dependent PUS1 regulates EIF3b stability in a non-enzymatic pathway mediating prostate cancer bone metastasis. Int J Biol Sci. 2024;20(11):4566–4584. doi:10.7150/ijbs.100905
42. Cheng B, He H, Chen B, et al. Assessment of treatment outcomes: cytoreductive surgery compared to radiotherapy in oligometastatic prostate cancer - an in-depth quantitative evaluation and retrospective cohort analysis. Int J Surg. 2024;110(6):3190–3202. doi:10.1097/JS9.0000000000002047
43. Li J, Guan Y, Zhu R, Wang Y, Zhu H, Wang X. Identification of metabolic genes for the prediction of prognosis and tumor microenvironment infiltration in early-stage non-small cell lung cancer. Open Life Sci. 2022;17(1):881–892. doi:10.1515/biol-2022-0091
44. Wu Q, Liu Z, Li B, Liu YE, Wang P. Immunoregulation in cancer-associated cachexia. J Adv Res. 2024;58:45–62. doi:10.1016/j.jare.2023.04.018
45. Tsai YT, Kuo LT, Wang YT. Prognostic utility of the geriatric nutritional risk index for head and neck cancer: systematic review and meta-analysis. Head Neck. 2024;46(8):2086–2097. doi:10.1002/hed.27842
46. Zheng Y, Wang K, Ou Y, et al. Prognostic value of a baseline prognostic nutritional index for patients with prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2024;27(4):604–613. doi:10.1038/s41391-023-00689-9
47. He L, Li Y, Qu L, Zhang F. Prognostic and clinicopathological value of the geriatric nutritional risk index in gastric cancer: a meta-analysis of 5,834 patients. Front Surg. 2022;9:1087298. doi:10.3389/fsurg.2022.1087298
48. An S, Eo W, Lee S. Comparison of the clinical value of the geriatric nutritional risk index and prognostic nutritional index as determinants of survival outcome in patients with gastric cancer. J Cancer. 2022;13(12):3348–3357. doi:10.7150/jca.77397
49. Matsunaga T, Saito H, Osaki T, et al. Impact of geriatric nutritional risk index on outcomes after gastrectomy in elderly patients with gastric cancer: a retrospective multicenter study in Japan. BMC Cancer. 2022;22(1):540. doi:10.1186/s12885-022-09638-6
50. Miao S, Bao C, Zhang Y, et al. Associations of the geriatric nutritional risk index with high risk for prostate cancer: a cross-sectional study. Nutrition. 2023;115:112164. doi:10.1016/j.nut.2023.112164
51. Shu W, Tao W, Chunyan H, et al. Preoperative nutritional evaluation of prostate cancer patients undergoing laparoscopic radical prostatectomy. PLoS One. 2022;17(2):e0262630. doi:10.1371/journal.pone.0262630
52. Naiki T, Takahara K. The geriatric nutritional risk index predicts prognosis in Japanese patients with LATITUDE high-risk metastatic hormone-sensitive prostate cancer: a multi-center study. Cancers. 2023;15(22):5333. doi:10.3390/cancers15225333
53. Chang LW, Hung SC, Li JR, et al. Geriatric nutritional risk index as a prognostic marker for patients with metastatic castration-resistant prostate cancer receiving docetaxel. Front Pharmacol. 2020;11:601513. doi:10.3389/fphar.2020.601513
54. Okamoto T, Hatakeyama S. Impact of nutritional status on the prognosis of patients with metastatic hormone-naïve prostate cancer: a multicenter retrospective cohort study in Japan. World J Urol. 2019;37(9):1827–1835. doi:10.1007/s00345-018-2590-2
55. Liu J, Sun R, Cai K, Xu Y, Yuan W. A nomogram combining neutrophil to lymphocyte ratio (NLR) and prognostic nutritional index (PNI) to predict distant metastasis in gastric cancer. Sci Rep. 2024;14(1):15391. doi:10.1038/s41598-024-65307-7
56. Kosuga T, Konishi T, Kubota T, et al. Value of prognostic nutritional index as a predictor of lymph node metastasis in gastric cancer. Anticancer Res. 2019;39(12):6843–6849. doi:10.21873/anticanres.13901
57. Zhang J, Gong J, Liu H, Zhou W, Cai M, Zhang C. Prognostic nutritional index predicts lateral lymph node metastasis and recurrence free survival in papillary thyroid carcinoma. BMC Cancer. 2024;24(1):1039. doi:10.1186/s12885-024-12801-w
58. Li C, Yin Y, Yang Z, Zhang Q, Wang W, Liu J. Prognostic effect of the pretreatment prognostic nutritional index in cervical, ovarian, and endometrial cancer: a meta-analysis. BMC Women’s Health. 2024;24(1):464. doi:10.1186/s12905-024-03310-w
59. Huang JC, Pan B, Jiang T, Zhang XX, Lyu SC, Lang R. Effect of the preoperative prognostic nutritional index on the long-term prognosis in patients with borderline resectable pancreatic cancer after pancreaticoduodenectomy. Front Oncol. 2023;13:1098459. doi:10.3389/fonc.2023.1098459
60. Ishiguro T, Aoyama T, Ju M, et al. Prognostic nutritional index as a predictor of prognosis in postoperative patients with gastric cancer. In Vivo. 2023;37(3):1290–1296. doi:10.21873/invivo.13207
61. Xishan Z, Ye Z, Feiyan M, Liang X, Shikai W. The role of prognostic nutritional index for clinical outcomes of gastric cancer after total gastrectomy. Sci Rep. 2020;10(1):17373. doi:10.1038/s41598-020-74525-8
62. Keskinkilic M, Semiz HS. The prognostic value of immune-nutritional status in metastatic colorectal cancer: Prognostic Nutritional Index (PNI). Support Care Cancer. 2024;32(6):374. doi:10.1007/s00520-024-08572-6
63. Xu YS, Liu G, Zhao C, et al. Prognostic value of combined preoperative carcinoembryonic antigen and prognostic nutritional index in patients with Stage II-III colon cancer. Front Surg. 2021;8:667154. doi:10.3389/fsurg.2021.667154
64. Niu Z, Yan B. Prognostic and clinicopathological effect of the prognostic nutritional index (PNI) in patients with cervical cancer: a meta-analysis. Ann Med. 2023;55(2):2288705. doi:10.1080/07853890.2023.2288705
65. Xu S, Cao S. High prognostic nutritional index (PNI) as a positive prognostic indicator for non-small cell lung cancer patients with bone metastasis. Clin Respir J. 2021;15(2):225–231. doi:10.1111/crj.13288
66. Hu X, Wang YH, Lia T, et al. Prognostic value of preoperative prognostic nutritional index in patients with renal cell carcinoma after nephrectomy. Clin Chim Acta. 2020;509:210–216. doi:10.1016/j.cca.2020.06.025
67. Tobing E, Tansol C, Tania C, Sihombing AT. Prognostic Nutritional Index (PNI) as independent predictor of poor survival in prostate cancer: a systematic review and meta-analysis. Clin Genitourin Cancer. 2024;22(5):102142. doi:10.1016/j.clgc.2024.102142
68. Ellez HI, Keskinkilic M, Semiz HS, Arayici ME. The Prognostic Nutritional Index (PNI): a new biomarker for determining prognosis in metastatic castration-sensitive prostate carcinoma. J Clin Med. 2023;12(17):5434. doi:10.3390/jcm12175434
69. Li B, Lu Z, Wang S, et al. Pretreatment elevated prognostic nutritional index predicts a favorable prognosis in patients with prostate cancer. BMC Cancer. 2020;20(1):361. doi:10.1186/s12885-020-06879-1
70. Li SY, Wan LL, Liu YF, Li YW, Huang X, Liu RJ. Prognostic value of three clinical nutrition scoring system (NRI, PNI, and CONUT) in elderly patients with prostate cancer. Front Nutr. 2024;11:1436063. doi:10.3389/fnut.2024.1436063
71. Yang F, Pan M, Nie J, Xiao F, Zhang Y. Evaluation of the prognostic nutritional index for the prognosis of Chinese patients with high/extremely high-risk prostate cancer after radical prostatectomy. World J Clin Cases. 2022;10(25):8863–8871. doi:10.12998/wjcc.v10.i25.8863
72. Delprat V, Michiels C. A bi-directional dialog between vascular cells and monocytes/macrophages regulates tumor progression. Cancer Metastasis Rev. 2021;40(2):477–500. doi:10.1007/s10555-021-09958-2
73. Cunha LL, Nonogaki S, Soares FA, Vassallo J, Ward LS. Immune escape mechanism is impaired in the microenvironment of thyroid lymph node metastasis. Endocr Pathol. 2017;28(4):369–372. doi:10.1007/s12022-017-9495-2
74. Luo G, He Y, Zhao Q, Yu X. Immune cells act as promising targets for the treatment of bone metastasis. Recent Pat Anticancer Drug Discov. 2017;12(3):221–233. doi:10.2174/1574892812666170606123113
75. Wang Z, Sun Y, Ren W, et al. Establishment and validation of a predictive model for bone metastasis in prostate cancer patients based on multiple immune inflammatory parameters. Am J Transl Res. 2023;15(2):1502–1509. PMID: 36915765.
76. Karakousis G, Yang R, Xu X. Circulating melanoma cells as a predictive biomarker. J Invest Dermatol. 2013;133(6):1460–1462. doi:10.1038/jid.2013.34
77. Cassetta L, Fragkogianni S, Sims AH, et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. 2019;35(4):588–602.e510. doi:10.1016/j.ccell.2019.02.009
78. Liu M, Zhou J, Liu X, et al. Targeting monocyte-intrinsic enhancer reprogramming improves immunotherapy efficacy in hepatocellular carcinoma. Gut. 2020;69(2):365–379. doi:10.1136/gutjnl-2018-317257
79. Meng L, Yang Y, Hu X, Zhang R, Li X. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: a systematic review and meta-analysis. J Transl Med. 2023;21(1):79. doi:10.1186/s12967-023-03924-y
80. Zhang B, Xu T. Prognostic significance of pretreatment systemic immune-inflammation index in patients with prostate cancer: a meta-analysis. World J Surg Oncol. 2023;21(1):2. doi:10.1186/s12957-022-02878-7
81. Qi W, Zhou Y, Liu Z, et al. Revealing the prognostic and clinicopathological significance of systemic immune-inflammation index in patients with different stage prostate cancer: a systematic review and meta-analysis. Front Med. 2022;9:1052943. doi:10.3389/fmed.2022.1052943
82. Luo Z, Wang W, Xiang L, Jin T. Association between the systemic immune-inflammation index and prostate cancer. Nutr Cancer. 2023;75(10):1918–1925. doi:10.1080/01635581.2023.2272800
83. Wang S, Ji Y, Chen Y, et al. The values of systemic immune-inflammation index and neutrophil-lymphocyte ratio in the localized prostate cancer and benign prostate hyperplasia: a retrospective clinical study. Front Oncol. 2021;11:812319. doi:10.3389/fonc.2021.812319
84. Wang J, Shao F, Yu QX, et al. The common hallmarks and interconnected pathways of aging, circadian rhythms, and cancer: implications for therapeutic strategies. Research. 2025;8:0612. doi:10.34133/research.0612
85. Li D, Yu Q, Wu R, et al. Interactions between oxidative stress and senescence in cancer: mechanisms, therapeutic implications, and future perspectives. Redox Biol. 2024;73:103208. doi:10.1016/j.redox.2024.103208
86. Dong M, Shi Y. Prognostic and clinicopathological significance of systemic immune-inflammation index in colorectal cancer: a meta-analysis. Ther Adv Med Oncol. 2020;12:1758835920937425. doi:10.1177/1758835920937425
87. Xu J, Song J, Yang Z, et al. Pre-treatment systemic immune-inflammation index as a non-invasive biomarker for predicting clinical outcomes in patients with renal cell carcinoma: a meta-analysis of 20 studies. Biomarkers. 2023;28(3):249–262. doi:10.1080/1354750X.2023.2164906
88. Zhang Z, Xia F, Wang W, Huang Y, Li X. The systemic immune-inflammation index-based model is an effective biomarker on predicting central lymph node metastasis in clinically nodal-negative papillary thyroid carcinoma. Gland Surg. 2021;10(4):1368–1373. doi:10.21037/gs-20-666
89. Zhao L, Zhou T, Zhang W, et al. Blood immune indexes can predict lateral lymph node metastasis of thyroid papillary carcinoma. Front Endocrinol. 2022;13:995630. doi:10.3389/fendo.2022.995630
90. Qiu Y, Zhang Z, Chen Y. Prognostic value of pretreatment systemic immune-inflammation index in gastric cancer: a meta-analysis. Front Oncol. 2021;11:537140. doi:10.3389/fonc.2021.537140
91. Huang L, Liu S, Lei Y, et al. Systemic immune-inflammation index, thymidine phosphorylase and survival of localized gastric cancer patients after curative resection. Oncotarget. 2016;7(28):44185–44193. doi:10.18632/oncotarget.9923
92. Zhang J, Zhang L, Duan S, Li Z, Li G, Yu H. Single and combined use of the platelet-lymphocyte ratio, neutrophil-lymphocyte ratio, and systemic immune-inflammation index in gastric cancer diagnosis. Front Oncol. 2023;13:1143154. doi:10.3389/fonc.2023.1143154
93. Zhang Y, Sun Y. Prognostic value of the systemic immune-inflammation index in patients with breast cancer: a meta-analysis. Cancer Cell Int. 2020;20:224. doi:10.1186/s12935-020-01308-6
94. Tong L, Wang S, Zhang R, Wu Y, Xu D, Chen L. High Levels of SII and PIV are the risk factors of axillary lymph node metastases in breast cancer: a retrospective study. Int J Gen Med. 2023;16:2211–2218. doi:10.2147/IJGM.S411592
95. Zhang Y, Xiao G, Wang R. Clinical significance of systemic immune-inflammation index (SII) and C-reactive protein-to-albumin ratio (CAR) in patients with esophageal cancer: a meta-analysis. Cancer Manag Res. 2019;11:4185–4200. doi:10.2147/CMAR.S190006
96. Shui Y, Li M, Su J, Chen M, Gu X, Guo W. Prognostic and clinicopathological significance of systemic immune-inflammation index in pancreatic cancer: a meta-analysis of 2,365 patients. Aging. 2021;13(16):20585–20597. doi:10.18632/aging.203449
97. Jan HC, Wu KY, Tai TY, Weng HY, Yang WH, Ou CH. The Systemic Immune-Inflammation Index (SII) increases the prognostic significance of lymphovascular invasion in upper tract urothelial carcinoma after radical nephroureterectomy. Cancer Manag Res. 2022;14:3139–3149. doi:10.2147/CMAR.S378768
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Exosome-Derived miRNAs as Potential Biomarkers for Prostate Bone Metastasis
Lu Z, Hou J, Li X, Zhou J, Luo B, Liang S, Lo RK, Wong TM, Kuang GM
International Journal of General Medicine 2022, 15:5369-5383
Published Date: 1 June 2022
Prostate Cancer Bone Metastasis: Molecular Mechanisms of Tumor and Bone Microenvironment
Jiang H
Cancer Management and Research 2025, 17:219-237
Published Date: 1 February 2025




