Back to Journals » International Journal of Nanomedicine » Volume 20
Green Synthesis of Gold Nanoparticles Using Clerodendrum trichotomum Thunberg for Antibacterial and Anticancer Applications
Authors Shakoor A, Ferdous UT, Khan SA , Gulzar MM
Received 28 October 2024
Accepted for publication 14 February 2025
Published 3 March 2025 Volume 2025:20 Pages 2645—2658
DOI https://doi.org/10.2147/IJN.S503254
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Adnan Shakoor,1,2 Umme Tamanna Ferdous,2 Shakeel Ahmad Khan,3 Muhammad Majid Gulzar1,4
1Department of Control & Instrumentation Engineering, King Fahd University of Petroleum & Minerals, Dhahran, 31261, Saudi Arabia; 2Center for Bio Systems and Machines, King Fahd University of Petroleum & Minerals, Dhahran, 31261, Saudi Arabia; 3Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Kowloon, 999077, Hong Kong; 4Interdisciplinary Research Center for Sustainable Energy Systems (IRC-SES), King Fahd University of Petroleum & Minerals, Dhahran, 31261, Saudi Arabia
Correspondence: Shakeel Ahmad Khan, Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Kowloon, 999077, Hong Kong, Email [email protected]
Purpose: The potential use of gold nanoparticles (AuNPs) in healthcare research has increased dramatically in recent years, owing to advancements in their synthesis techniques, including green synthesis. Although Clerodendrum trichotomum is a popular medicinal plant that harbors many bioactive phytochemicals in various parts of the world, the green synthesis of AuNPs using this precious plant is still under investigation. Therefore, this study aimed to explore the green synthesis of AuNPs from C. trichotomum Thunberg leaves (CTT-AuNPs) and their antibacterial and cytotoxic properties.
Methods: AuNPs were synthesized from Clerodendrum trichotomum Thunberg and characterized using various microscopic and spectroscopic techniques. A serial dilution technique was used to determine the antibacterial activity of the synthesized CTT-AuNPs against two bacterial species, Klebsiella pneumoniae and Staphylococcus aureus. The cytotoxicity assay was carried out in the breast cancer cell line (MCF-7), whereas the biocompatibility test was performed in the kidney cell line (293T).
Results: Multiple spectroscopic characterization techniques confirmed the successful synthesis of CTT-AuNPs. The average size of CTT-AuNPs was 19.1± 2.2 nm. The results of this study revealed that CTT-AuNPs showed strong antibacterial activity against S. aureus and K. pneumoniae, which was further confirmed using a live/dead dual staining assay. CTT-AuNPs also significantly reduced the proliferation of MCF-7 breast cancer cells to 32.67% at 120 μg/mL after 24 hours of incubation. These green-synthesized CTT-AuNPs exhibited excellent cytobiocompatibility with 293T kidney cells. The dual staining method further confirmed the cytotoxicity and biocompatibility of CTT-AuNPs compared with chemically synthesized AuNPs.
Conclusion: This work will pave the way for the production of biocompatible AuNPs from C. trichotomum Thunberg that can be used in different disease treatments.
Keywords: antibacterial, biocompatibility, Clerodendrum trichotomum Thunberg, cytotoxicity, gold nanoparticles
Introduction
Since cancer and antimicrobial resistance (AMR) are the two biggest causes of death and their incidence is increasing every year, they represent a threat to global health. AMR was related to 4.71 million deaths in 2021, an increase every year, and it has been predicted that the AMR-associated death toll will increase to more than 8 million by 2050.1 The improper use of antibiotics is the leading cause of AMR, prompting researchers to search for new anti-microbial drugs.2 In contrast, more than 9 million new cancer-related deaths are recorded in 2022.3 Resistance to chemotherapeutic drugs may cause more than 80% cancer-related death.4 To combat growing health concerns related to drug resistance, researchers are now looking for alternative treatments. Since ancient times, plant-based medicines have been used to treat various diseases in humans. With advancements in the purification and characterization of plant-based phytochemicals, new approaches have been introduced for the discovery of cancer drugs from natural sources. More than 3500 plants exhibit promising cytotoxic activity against different cancer cells. More than 50% of all licensed anticancer medications have inextricable links with natural sources.5
In recent years, nanobiotechnology, which exploits nanoparticles to address various medical challenges, has received increasing attention in healthcare research. Nanoparticles considerably improve the safety and effectiveness of anticancer drugs in terms of safety and effectiveness.6 Among the different metallic nanoparticles, AuNPs have several benefits for use in cancer therapy, including distinctive physiochemical characteristics such as stability, biocompatibility, high thermal activity, optical and electrical properties, high surface area-to-volume ratio, and surface chemistry, and they are easily fabricated into a variety of shapes and sizes.7,8 Their small size offers more surface area relative to the total mass for interaction with biomolecules and allows easy penetration into cells, which is attributable to their strong cytotoxicity.9 They can also be used to design antibacterial drugs because of their enhanced solubility, specificity, and reduced side effects.10,11
Green synthesis of AuNPs using medicinal plants is a sustainable process that maximizes therapeutic efficacy, specific binding, and targeted delivery. This may also reduce toxicity, which is a major concern in the discovery of novel anticancer drugs.12 Several studies have reported minimal toxicity of AuNPs against non-cancerous cells. AuNP concentration, diverse sizes, shapes, and capping agents majorly affect their cytotoxic activity.13 Apoptosis is induced in cancer cells via ROS-mediated mitochondrial dysfunction. In addition, AuNPs can inhibit cell cycle progression in the G0/G1 phase.14 AuNPs also exhibit antibacterial activity by attacking the bacterial cell walls and DNA. Apart from their antibacterial activity, their antioxidant capacity also accounts for their faster wound-healing properties.15
In this study, the ornamental plant Clerodendrum trichotomum Thunberg was used for green synthesis of AuNPs. C. trichotomum Thunberg has long been used in Chinese folk medicine. This plant has also been found in Japan and South Korea. The biological activities of the different plant parts were explored. Several studies have reported the cytotoxic activity of this plant against human cancers, including gastric adenocarcinoma and nasopharyngeal, liver, blood, cervical, lung, and kidney cancer. This plant possesses many bioactive phytochemicals responsible for its cytotoxic properties. Phenylpropanoids, diterpenoids, and steroids are the major compounds found in this plant that show significant anticancer activity against different cancer cells. The antibacterial properties of various solvent extracts of this plant have been reported.16
The biosynthesis of nanoparticles was performed using different Clerodendrum species. AgNPs synthesized from C. inerme, C. infortunatum, C. phlomidis, C. splendens, C. viscosum, and C. glandulosum have been reported to exhibit antimicrobial, antioxidant, cytotoxic, and antiparasitic activity.17–22 Aluminum and zinc oxide nanoparticles from C. phlomidis, C. heterophyllum, and C. infortunatum have antibacterial activities.23–25 In the case of AuNPs, only C. infortunatum and C. inerme have been investigated, and their antibacterial, antioxidant, and antiproliferative capacities have been documented.20,26 The phytochemicals in plant extracts reduce metal ions to nanoparticles, which may depend on their reduction potential and the functional groups in their structures. It has been reported that flavonoids and phenolic compounds in Artemisia capillaris extract synthesize AuNPs. The hydroxyl and amino groups in the compound structures play crucial roles in the reduction process. Au-ion reduction involves the oxidation of an aldehyde group to a carboxyl group.27
As mentioned earlier, C. trichotomum Thunberg is frequently used in Chinese traditional medicine. Dried leaves and stems of this plant are used to treat hypertension and inflammatory diseases.28 However, there is a lack of data on nanoparticle synthesis from C. trichotomum Thunberg, despite its excellent bioactive properties. Therefore, this study aimed to investigate the synthesis of gold nanoparticles (CTT-AuNPs) from C. trichotomum and to evaluate their antibacterial and cytotoxic effects. To the best of our knowledge, this is the first report of synthesizing nanoparticles from the medicinal plant C. trichotomum Thunberg which may further improve their biomedical functions.
Methodology
Plant Material Collection
Clerodendrum trichotomum Thunberg was collected from a wild area native to tropical and subtropical regions. Its identification was performed by Dr. Zaheer (Department of Botany, Punjab University, Lahore, Pakistan). A voucher specimen of Clerodendrum trichotomum Thunberg was deposited at the herbarium of the Department of Botany, GC University, Lahore, Pakistan (CTT: GC. Herb. Bot. 240).
Plant Extraction
Plant extraction was performed using a previously described protocol20 with slight modifications (Figure 1). Fresh leaves from C. trichotomum Thunberg (CTT) were harvested from C. trichotomum. After cleaning with deionized water (dH2O), the leaves were oven-dried at 60 °C and finely crushed to obtain a powder. Fifteen grams of the powdered leaves were combined with 100 mL of dH2O water and heated at 50–60 °C for 15 min. The mixture was filtered to obtain the plant extracts. The plant extract obtained from this process was stored at 4 °C in an airtight bottle until further use.
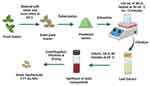 |
Figure 1 The leaf extract preparation and green synthesis of gold nanoparticles using C. trichotomum Thunberg (Created in BioRender.com). |
Green Synthesis of Gold Nanoparticles
Green synthesis of AuNPs using CTT was performed following a previously described protocol20 with slight modifications (Figure 1). Briefly, 10.20 g HAuCl4 was added to 100 mL of plant extract for the green synthesis of AuNPs (concentration of Au3+ in the mixture was 0.3001 M), and the mixture was agitated for 80 min at 65 ◦C. Following this, it was noticed that the color of the mixed solution changed to ruby red because of surface plasmon resonance, which showed that the necessary gold nanoparticles had formed. After centrifuging the nanoparticles twice at 3000 rpm, they were filtered and washed thrice with a solution of deionized water and ethanol. Subsequently, the gold nanoparticles were subjected to a 40 ◦C oven-drying process and a 3-hour muffle furnace calcined at 500 °C. The produced NPs were preserved in an airtight container for biological applications and characterization.
Characterization of CTT-AuNPs
Powder X-ray diffraction (XRD) with a wavelength (λ) of 0.154 nm over the 2θ range of 20°–80° was used to assess the crystallinity and purity of the CTT-AuNPs in powder form. Morphological characterization of the CTT-AuNP powder was performed using Transmission Electron Microscopy (TEM). Nano particle size analyzer was used to measure the size of the nanoparticles. A composition analysis was performed using Energy-Dispersive X-ray (EDX) spectroscopy.
Antibacterial Assay
The antibacterial activity of CTT-AuNPs was assessed using a serial dilution method, as described previously.29 The Gram-positive bacterium Staphylococcus aureus ATCC® 23235TM and gram-negative bacterium Klebsiella pneumoniae (ATCC® 13,883) were used in this experiment. Briefly, each bacterium (5×105 CFU/mL) was cultured overnight on agar plates at 37 °C. After incubation, bacterial colonies were diluted in PBS and adjusted to 1×107 CFU/mL. The bacterial culture (10 µL) was mixed with 1 mL of Muller-Hinton broth and added to each well of a 24-well plate at a final concentration of 1×105 CFU/mL. Next, 50 µL of CTT-AuNPs (250 µg/mL) were added to each well and incubated at 37 °C for 24 h. The serial dilution plate counting method was used to determine the bacterial count in each well, and the following formula was used to calculate the log10 reduction:29
Where 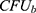 and
and 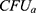 are the number of bacterial colonies before and after incubation, respectively.
are the number of bacterial colonies before and after incubation, respectively.
Live/Dead Bacteria Staining Assay
A confocal laser scanning microscope (CLSM, FV-1200, Olympus, Tokyo, Japan) was used to determine the bacterial cell viability using a previously described protocol. Each bacterium, at a confluency of 105–106 was inoculated into a 24-well microtiter plate and incubated for one hour. After incubation, the suspended cells were discarded and rinsed three times with saline water. Samples (250 µg/mL) were inoculated and incubated overnight. A live/dead bacterial viability kit was used, in which two fluorescent dyes, DAPI and propidium iodide (PI), were used to stain live (blue) and dead (red) bacteria, respectively.
Anticancer Activity
Cell Culture
Breast cancer cells (MCF-7 cells, commercially purchased from Sigma-Aldrich, Germany) were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) in a humidified atmosphere containing 5% CO2 and 95% air at 37 °C. The cells were cultured in DMEM for 24 h in a 96-well plate to obtain a cell confluence of 5×108 cells/well.
MTT Assay
The cytotoxicity of CTT-AuNPs was tested against MCF-7 cells using a previously described protocol.29 CTT-AuNPs at a concentration of 120 µg/mL were applied to cancer cells and incubated for 24 h at 37 °C. Doxorubicin was used as a positive control. After incubation, the medium was discarded, and the cells were washed with phosphate-buffered saline. Each well was filled with 15 µL of MTT reagent (0.5 mg/mL), and the plate was incubated in the dark for 4 h at 37 °C. After 4 h, 150 µL of DMSO was added to each well and the absorbance was measured at 570 nm. The percentage of viable cells was calculated using the following equation:29
Live/Dead Cell Staining Assay
Fluorescent staining was used to further investigate the cytotoxicity of CTT-AuNPs using DAPI and PI staining. The same technique described for bacterial cell staining was used, except for the sample amount. CTT-AuNPs (10 µL, 120 µg/mL) were used in the assay. Each well was treated with 4 µg/mL of staining solution and incubated for 20 min. Images were taken at excitation wavelength of 488/545 nm using a confocal laser scanning microscope.
Biocompatibility Assay
Biocompatibility tests for CTT-AuNPs were performed using the same protocol as described in the previous section on the non-cancerous 293T kidney cell line. Chemically synthesized AuNPs (Chemi-AuNPs) were used to compare the biocompatibility of the CTT-AuNPs.
Results
Nanoparticle Characterization
The green-synthesized CTT-AuNPs were characterized by XRD, which revealed three diffraction peaks at 2θ= 37.78°, 44.02°, and 64.58° corresponding to the (111), (200), and (220) planes, respectively (Figure 2a). The nanoparticle size analysis revealed that the green-synthesized CTT-AuNPs had an average size of 19.1 ± 2.2 nm (Figure 2b). The EDX analysis further confirmed that the nanoparticles were mainly composed of gold (Au). The different peaks of C, N, and O in the spectra may be attributed to phytochemicals from the C. trichotomum Thunberg extracts coated on the nanoparticles (Figure 2c). TEM analysis revealed that the green-synthesized CTT-AuNPs were spherical and uniformly distributed (Figure 2d).
 |
Figure 2 (a) XRD analysis; (b) size distribution; (c) EDX analysis and (d) TEM analysis of the green synthesized CTT-AuNPs. |
Antibacterial Assay
The antibacterial activity of CTT-AuNPs was tested against two bacteria, S. aureus and K. pneumoniae, along with the plant extract and chemically synthesized NPs. The data obtained from this study showed that green-synthesized CTT-AuNPs reduced bacterial growth better than the plant extract and chemically synthesized NPs (Chemi-AuNPs) (Figure 3). This study revealed that CTT-AuNPs exhibit bactericidal activity (log10 CFU/mL) against both K. pneumoniae (4.1 log10 CFU/mL) and S. aureus. (4.35 log10 CFU/mL) where CTT extract alone showed only bacteriostatic effect and (2.15 log10 CFU/mL) on K. pneumoniae and bactericidal effect (3.25 log10 CFU/mL) on S. aureus. In contrast, chemi-AuNPs exhibited better bactericidal effects than the plant extracts against both K. pneumoniae (3.1 log10 CFU/mL) and S. aureus. (3.45 log10 CFU/mL, respectively).
The DAPI/PI dual staining method was used to confirm the antibacterial activity of the green-synthesized CTT-AuNPs (Figure 4a–d). The results showed that most S. aureus and K. pneumoniae cells developed red fluorescence after treatment with green-synthesized CTT-AuNPs, confirming the presence of dead bacterial cells (Figure 4b and d). In the control, bacterial cells emitted blue fluorescence, confirming the presence of live cells (Figure 4a and c).
 |
Figure 4 CLSM image of live/dead bacteria. (a and c) control and (b and d) Treated bacteria with green synthesized CTT-AuNPs. |
Cytotoxicity Assay
The cytotoxic effect of the green-synthesized CTT-AuNPs was evaluated against MCF-7 cells, along with that of the C. trichotomum Thunberg extract and chemically synthesized AuNPs. The results demonstrated that the green-synthesized CTT-AuNPs exhibited the strongest cytotoxicity against MCF-7 cells compared to the plant extract or chemi-AuNPs. CTT-AuNPs reduced MCF-7 cell viability to 32.67%, whereas the plant extract alone reduced cell viability to 68.33% at the same concentration after 24 h of incubation. Chemi-AuNPs reduced the cell viability to 50% after the same incubation period (Figure 5).
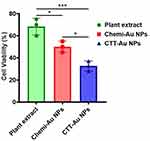 |
Figure 5 Cytotoxic activity of green synthesized CTT-AuNPs against MCF-7 cells after 24 h of incubation (*** and * denote p < 0.005 and p < 0.05, respectively). |
A dual-staining method using a fluorescent dye was used to confirm the cytotoxic effects (Figure 6a–d). The results showed the cytotoxic effect of the green-synthesized CTT-AuNPs, where most of the cells were red-stained, indicating dead cells (Figure 6d). In contrast, the plant extract and chemi-AuNPs showed less toxicity against MCF-7 cells (Figure 6b and c), which is represented in the image showing more blue-stained cells that represent live cells.
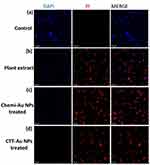 |
Figure 6 CLSM image of live/dead MCF-7 cells. (a) control; (b) plant extract, (c) cells treated with Chemi-AuNPs, and (d) cells treated with green synthesized CTT-AuNPs. |
Biocompatibility Assay
The cytocompatibility of green-synthesized CTT-AuNPs was assessed in 293T kidney epithelial cells and compared with that of Chemi-AuNPs. Figure 7a–c show the cytocompatibility results. The data showed that CTT-AuNPs exhibited fewer toxic effects on 293T cells than Chemi-AuNPs. The results showed that Chemi-AuNP-treated cells were more red-stained (Figure 7b) than CTT-AuNP-treated cells (Figure 7c), indicating that CTT-AuNPs were more biocompatible.
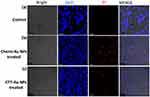 |
Figure 7 CLSM image of live/dead 293T cells. (a) control; (b) cells treated with chemically synthesized gold nanoparticles, and (c) cells treated with green synthesized CTT-AuNPs. |
Discussion
It has been reported that the leaf extract of C. trichotomum Thunberg contains a diverse array of biologically active phytochemicals, including flavonoids, phenolics, alkaloids, terpenoids, anthraquinones, carbohydrates, saponins, and tannins.16 In the present synthesis process, these compounds play a crucial role in facilitating the reduction and stabilization of the nanoparticles. The proposed mechanism (Figure 8) for the green synthesis of CTT-AuNPs using plant extracts involves a synergistic reduction and stabilization process mediated by phytochemicals, such as polyphenols, flavonoids, alkaloids, and saponins. The reduction of gold ions (Au3+) to elemental gold (Au0) occurs via a redox reaction facilitated by the electron-donating functional groups in phytochemicals, particularly the hydroxyl (-OH) groups in polyphenols and flavonoids. During this process, the reducing agents are oxidized, transitioning from their enol form to stable keto derivatives. Concurrently, the synthesized gold nanoparticles are capped and stabilized by various phytochemicals, including alkaloids, proteins, and saponins, which interact with the nanoparticle surface through functional groups such as hydroxyl, amine (–NH2), and carbonyl (–C=O) groups. This capping process prevented nanoparticle aggregation and ensured stability and uniformity. The resulting nanoparticles are monodisperse and biologically compatible owing to the dual role of phytochemicals as reducing and capping agents. This eco-friendly, green synthesis method eliminates the need for hazardous chemical reductants or stabilizers and provides a sustainable approach for the production of AuNPs with potential applications in nanomedicine, catalysis, and environmental science.
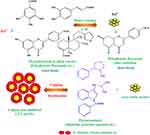 |
Figure 8 The schematic illustration depicts the proposed mechanism for the green synthesis of CTT-AuNPs utilizing the leaf extract of C. trichotomum Thunberg (Adapted from Khan et al20 under Creative Commons Attribution (CC BY) license). |
This study aimed to synthesize AuNPs from a popular medicinal plant, C. trichotomum Thunberg, and to evaluate their antibacterial and anticancer properties. The green-synthesized CTT-AuNPs showed good bactericidal activity, with a log10 reduction of more than 3 log10 CFU/mL against both the bacterial species (Table 1). Generally, the bacteriostatic and bactericidal effects of an antibacterial agent are defined as <3 or ≥3 log10 CFU/mL, respectively.30 In addition, CTT-AuNPs showed better antibacterial activity against the gram-positive bacterium S. aureus than against Gram-negative K. pneumoniae, which is in line with the results of many previous studies.18,20,29 This growth inhibition mechanism can be attributed to the cell wall structure, composition, and overall surface charge of the gram-positive bacteria. Gram-positive bacteria have a thicker cell wall layer, consisting of peptidoglycan and teichoic acid, than gram-negative bacteria. Both materials have a high negative charge on their surfaces, attracting positively charged metal nanoparticles. However, Gram-positive bacteria may have a higher negative charge than Gram-negative bacteria. A recent study showed that Bacillus subtilis (gram-positive) has a higher negative surface charge than E. coli (Gram-negative).31 Although green-synthesized AuNPs exhibit increased bactericidal activity against gram-positive bacteria, some studies have shown that Gram-negative AuNPs are more sensitive to AuNPs.32 In contrast, Ag NPs also showed greater sensitivity to gram-negative bacteria than to Gram-positive bacteria.33 The generation of reactive oxygen species (ROS), which help to impair bacterial cells, may be one of the reasons for its antibacterial activity.
 |
Table 1 Comparison of Antibacterial Activity of CTT-AuNPs With Other AuNPs Biosynthesized From Different Plants |
Based on previously reported studies, we proposed an antibacterial mechanism of green-synthesized CTT-AuNPs, as shown in Figure 9. They may exert antibacterial effects through a multifaceted mechanism. They penetrate the bacterial cell wall and disrupt membrane integrity via electrostatic interactions and oxidative stress, resulting in increased permeability.38 Inside the cell, CTT-AuNPs may catalyze the formation of ROS, which induce DNA damage through strand breaks and nucleotide oxidation, inhibit protein synthesis by disrupting ribosomal function, and cause enzymatic inactivation by oxidizing functional groups or directly binding to active sites.20 Furthermore, CTT-AuNPs impaired bacterial respiration by disrupting the electron transport chain, leading to energy depletion and metabolic collapse. Despite the upregulation of stress-related genes such as sodA, rpoS, and grxA,40 cumulative oxidative stress and metabolic disruption overwhelm bacterial defense mechanisms. This cascade of damage culminates in cell death, highlighting the robust antibacterial mechanism of CTT-AuNPs and their potential against multidrug-resistant bacteria.
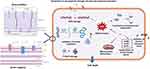 |
Figure 9 Effect of nanoparticles inside bacterial cells (Created in Biorender.com). |
The DAPI/PI dual staining method confirmed the antibacterial activity of green-synthesized CTT-AuNPs. DAPI stains nucleic acids, whereas PI can only be detected in membrane-impaired bacterial cells, thus confirming cell death. Dead cells showed red fluorescence when the PI dye entered the damaged membrane. In contrast, live cells emit blue fluorescence under a microscope, confirming that the cells are intact and deter the PI dye from entering the cells.41
These CTT-AuNPs exhibited excellent cytotoxicity on MCF-7 cells but less compared to non-cancerous 293T cells, which was further confirmed by a dual staining assay. The physicochemical state of AuNPs, including their morphology (size and shape) and surface area, may play a crucial role in exerting high cytotoxicity toward cancer cells.42 Small AuNPs can provide more surfaces to interact with bioactive molecules depending on their volume.43 However, several reports have revealed that AuNPs synthesized from different Clerodendrum species have good cytotoxic effects on cancer cells (Table 2). The leaf extract of C. infortunatum, which is rich in clerodin, showed good cytotoxicity against leukemic cell lines.26 C. inerme leaf extract significantly reduced the viability of MCF-7 cells after 24 hr.20 Phytochemicals present in Clerodendrum trichotomum are also good sources of anticancer agents, as mentioned earlier.16
 |
Table 2 Comparison of the Cytotoxicity of CTT-AuNPs With Other Biosynthesized AuNPs From Different Plants |
Moreover, the presence of CTT phytochemicals could be attributed to their enhanced biocompatibility. The increasing demand for AuNPs in the biomedical field has raised concerns regarding biosafety. Safe interactions between the host and AuNPs may confirm their safety and nullify the adverse effects on host immunity.43 Therefore, it is crucial to test the cytobiocompatibility of green-synthesized AuNPs. Several previous studies have reported the biocompatibility of green-synthesized AuNPs.20,47,48
Conclusion
This study reports the successful synthesis of AuNPs from C. trichotomum Thunberg using an eco-friendly and green approach. The CTT-synthesized AuNPs were successfully characterized using TEM, SEM, XRD, and EDX. The synthesized AuNPs demonstrated a better log10 reduction in the growth of both Gram-positive and Gram-negative bacteria compared to the plant extract alone and commercial nanoparticles. Moreover, these green-synthesized particles showed excellent cytotoxic effects on breast cancer cells, while showing less detrimental effects on non-cancerous kidney cells (293T) than chemically synthesized gold nanoparticles, which proves their enhanced biocompatibility. These excellent antibacterial and cytotoxic properties of the biosynthesized AuNPs are due to the synergistic effect of bioactive phytochemicals present in the CTT extract. Therefore, this study demonstrated that using the C. trichotomum Thunberg leaf extract to produce CTT-AuNPs in an environmentally friendly manner with increased biological functions is a cost-effective and practical alternative to traditional chemical techniques.
Acknowledgments
Author(s) at KFUPM would like to acknowledge the support received from Center for Biosystems and Machines at KFUPM under research Funded Grant #INBS2501. The author at Hong Kong Polytechnic University acknowledge the support received from PolyU Distinguished Postdoctoral Fellowship Scheme”.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas, took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This publication is based on work supported by the King Fahd University of Petroleum & Minerals under research grant fund #INBS2501, and the author at KFUPM acknowledges this support.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Naghavi M, Vollset SE, Ikuta KS, et al. Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050. Lancet. 2024;404(10459):1199–1226. doi:10.1016/S0140-6736(24)01867-1
2. Alara JA, Alara OR. An overview of the global alarming increase of multiple drug resistant: a major challenge in clinical diagnosis. Infect Disord Drug Targets. 2024;24(3):e250723219043. doi:10.2174/1871526523666230725103902
3. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
4. Dhanyamraju PK. Drug resistance mechanisms in cancers: execution of pro-survival strategies. J Biomed Res. 2024;38(2):95–121. doi:10.7555/JBR.37.20230248
5. Chandra S, Gahlot M, Choudhary AN, et al. Scientific evidences of anticancer potential of medicinal plants. Food Chem Adv. 2023;2:100239. doi:10.1016/j.focha.2023.100239
6. Das CGA, Kumar VG, Dhas TS, Karthick V, Kumar CMV. Nanomaterials in anticancer applications and their mechanism of action - A review. Nanomed Nanotechnol Biol Med. 2023;47:102613. doi:10.1016/j.nano.2022.102613
7. Lee KX, Shameli K, Yew YP, et al. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int J Nanomed. 2020;15:275–300. doi:10.2147/IJN.S233789
8. Al Saqr A, Khafagy E-S, Alalaiwe A, et al. Synthesis of gold nanoparticles by using green machinery: characterization and in vitro toxicity. Nanomaterials. 2021;11(3):808. doi:10.3390/nano11030808
9. Adewale OB, Davids H, Cairncross L, Roux S. Toxicological behavior of gold nanoparticles on various models: influence of physicochemical properties and other factors. Int J Toxicol. 2019;38(5):357–384. doi:10.1177/1091581819863130
10. Liew KB, Janakiraman AK, Sundarapandian R, et al. A review and revisit of nanoparticles for antimicrobial drug delivery. J Med Life. 2022;2022(3):328–335. doi:10.25122/jml-2021-0097
11. Piktel E, Suprewicz Ł, Depciuch J, et al. Varied-shaped gold nanoparticles with nanogram killing efficiency as potential antimicrobial surface coatings for the medical devices. Sci Rep. 2021;11(1):1–20. doi:10.1038/s41598-021-91847-3
12. Khan T, Ullah N, Khan MA, Mashwani ZUR, Nadhman A. Plant-based gold nanoparticles; a comprehensive review of the decade-long research on synthesis, mechanistic aspects and diverse applications. Adv Colloid Interface Sci. 2019;272. doi:10.1016/j.cis.2019.102017
13. Azooz EA, Abduladheem NA, El Abbadi NK. An overview of the green synthesis of coinage metallic nanoparticles: protocols, challenges, and cancer treatments. Nano Life. 2025;15(05):2430011. doi:10.1142/S1793984424300115
14. Bharadwaj KK, Rabha B, Pati S, et al. Green synthesis of gold nanoparticles using plant extracts as beneficial prospect for cancer theranostics. Molecules. 2021;26(21). doi:10.3390/molecules26216389
15. Hamed SH, Azooz EA, Al-mulla EAJ. Nanoparticles-assisted wound healing: a review. Nano Biomed Eng. 2023;15(4):425–435. doi:10.26599/NBE.2023.9290039
16. Gomulski J, Grzegorczyk-Karolak I. Clerodendrum trichotomum Thunberg—an ornamental shrub with medical properties. Molecules. 2024;29(14):3272. doi:10.3390/molecules29143272
17. Dhara M, Khatun R, Mondal A, et al. Preparation of biofunctionalized silver nanoparticles using Clerodendrum glandulosum leaf extract for evaluation of its antibacterial efficacy. Chem Pap. 2024;78:4875–4890. doi:10.1007/s11696-024-03437-y
18. Jayakumar A, Vedhaiyan RK. Rapid synthesis of phytogenic silver nanoparticles using Clerodendrum splendens: its antibacterial and antioxidant activities. Korean J Chem Eng. 2019;36(11):1869–1881. doi:10.1007/s11814-019-0389-5
19. Sriranjani R, Srinithya B, Vellingiri V, et al. Silver nanoparticle synthesis using Clerodendrum phlomidis leaf extract and preliminary investigation of its antioxidant and anticancer activities. J Mol Liq. 2016;220:926–930. doi:10.1016/j.molliq.2016.05.042
20. Khan SA, Shahid S, Lee CS. Green synthesis of gold and silver nanoparticles using leaf extract of Clerodendrum inerme; characterization, antimicrobial, and antioxidant activities. Biomolecules. 2020;10(6):835. doi:10.3390/biom10060835
21. Nahar K, Yang DC, Rupa EJ, Khatun K, Al-Reza SM. Eco-friendly synthesis of silver nanoparticles from Clerodendrum viscosum leaf extract and its antibacterial potential. Nanomedicine Res J. 2020;5(3):276–287. doi:10.22034/NMRJ.2020.03.008
22. Sahoo RK, Tamuli KJ, Narzary B, et al. Clerodendrum viscosum Vent leaf extract supported nanosilver particles: characterization, antiplasmodial and anticancer activity. Chem Phys Lett. 2020;738:136893. doi:10.1016/j.cplett.2019.136893
23. Kirubakaran D, Selvam K, Lavanya M, Shivaswamy MS, Sivasakthi V, BaigBaig AA. Eco-friendly synthesis of zinc oxide nanoparticles by Clerodendrum heterophyllum leaf extract and their biological applications. BioNanoSci. 2023;13:2252–2264. doi:10.1007/s12668-023-01222-x
24. Kumar S, Bithel N, Kumar S, Kishan, Sen M, Banerjee C. Phyto-mediated synthesis of zinc oxide nanoparticles from Clerodendrum infortunatum L. leaf extract and evaluation of antibacterial potential. South Afr J Bot. 2024;164:146–151. doi:10.1016/j.sajb.2023.11.029
25. Thanaraj S, Mitthun ANK, Geetha Sravanthy P, Carmelin DS, Surya M, Saravanan M. Green synthesis of aluminum oxide nanoparticles using Clerodendrum phlomidis and their antibacterial, anti-inflammatory, and antioxidant activities. Cureus. 2024;16(1):1–12. doi:10.7759/cureus.52279
26. Nagaraj B, Musthafa SA, Muhammad S, et al. Anti-microbial and anti-cancer activity of gold nanoparticles phytofabricated using clerodin enriched clerodendrum ethanolic leaf extract. J King Saud Univ Sci. 2022;34(4):101989. doi:10.1016/j.jksus.2022.101989
27. Timoszyk A, Grochowalska R. Mechanism and antibacterial activity of gold nanoparticles (AuNPs) functionalized with natural compounds from plants. Pharmaceutics. 2022;14(12):1–30. doi:10.3390/pharmaceutics14122599
28. Lee JY, Lee JG, Sim SS, Whang WK, Kim CJ. Anti-asthmatic effects of phenylpropanoid glycosides from Clerodendron trichotomum leaves and Rumex gmelini herbes in conscious Guinea-pigs challenged with aerosolized ovalbumin. Phytomedicine. 2011;18(2–3):134–142. doi:10.1016/j.phymed.2010.06.014
29. Sellami H, Khan SA, Ahmad I, Alarfaj AA, Hirad AH, Al-sabri AE. Green synthesis of silver nanoparticles using Olea europaea leaf extract for their enhanced antibacterial, antioxidant, cytotoxic and biocompatibility applications. Int J mol Sci. 2021;22:12562. doi:10.3390/ijms222212562
30. Eze EM, Okolo JC, Ogu GI, Orjiakor PI. In vitro antibacterial efficacy of velvet tamarind (Dialium guineense Wild) root extract. Int J Adv Acad Res. 2018;4(3):46–58.
31. Cremin K, Jones BA, Teahan J, et al. Scanning ion conductance microscopy reveals differences in the ionic environments of gram-positive and negative bacteria. Anal Chem. 2020;92(24):16024–16032. doi:10.1021/acs.analchem.0c03653
32. Senthilkumar S, Kashinath L, Ashok M, Rajendran A. Antibacterial properties and mechanism of gold nanoparticles obtained from pergularia daemia leaf extract. J Nanomed Res. 2017;6(1):1–4. doi:10.15406/jnmr.2017.06.00146
33. Singh A, Gautam PK, Verma A, et al. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: a review. Biotechnol Rep. 2020;25:e00427. doi:10.1016/j.btre.2020.e00427
34. Arief S, Nasution FW, Zulhadjri, Labanni A. High antibacterial properties of green synthesized gold nanoparticles using Uncaria gambir Roxb. leaf extract and triethanolamine. J Appl Pharm Sci. 2020;10(8):124–130. doi:10.7324/JAPS.2020.10814
35. Kamala Nalini SP, Vijayaraghavan K. Green synthesis of silver and gold nanoparticles using aloe vera gel and determining its antimicrobial properties on nanoparticle impregnated cotton fabric. J Nanotechnol Res. 2020;2(3):42–50. doi:10.26502/jnr.2688-85210015
36. Moustafa NE, Alomari AA. Green synthesis and bactericidal activities of isotropic and anisotropic spherical gold nanoparticles produced using Peganum harmala L leaf and seed extracts. Biotechnol Appl Biochem. 2019;66(4):664–672. doi:10.1002/bab.1782
37. Folorunso A, Akintelu S, Oyebamiji AK, et al. Biosynthesis, characterization and antimicrobial activity of gold nanoparticles from leaf extracts of Annona muricata. J Nanostruct Chem. 2019;9(2):111–117. doi:10.1007/s40097-019-0301-1
38. Balasubramanian S, Kala SMJ, Pushparaj TL. Biogenic synthesis of gold nanoparticles using Jasminum auriculatum leaf extract and their catalytic, antimicrobial and anticancer activities. J Drug Deliv Sci Technol. 2020;57:101620. doi:10.1016/j.jddst.2020.101620
39. Rajan A, Vilas V, Philip D. Studies on catalytic, antioxidant, antibacterial and anticancer activities of biogenic gold nanoparticles. J Mol Liq. 2015;212:331–339. doi:10.1016/j.molliq.2015.09.013
40. Paesa M, Remirez de Ganuza C, Alejo T, et al. Elucidating the mechanisms of action of antibiotic-like ionic gold and biogenic gold nanoparticles against bacteria. J Colloid Interface Sci. 2023;633:786–799. doi:10.1016/j.jcis.2022.11.138
41. Pizzo E, Zanfardino A, Di Giuseppe AMA, et al. A new active antimicrobial peptide from PD-L4, a type 1 ribosome inactivating protein of Phytolacca dioica L.: a new function of RIPs for plant defence? FEBS Lett. 2015;589(19):2812–2818. doi:10.1016/j.febslet.2015.08.018
42. Steckiewicz KP, Barcinska E, Malankowska A, et al. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J Mater Sci Mater Med. 2019;30(2):1–15. doi:10.1007/s10856-019-6221-2
43. Kus‐liśkiewicz M, Fickers P, Ben Tahar I. Biocompatibility and cytotoxicity of gold nanoparticles: recent advances in methodologies and regulations. Int J Mol Sci. 2021;22(20):10952. doi:10.3390/ijms222010952
44. Adewale OB, Anadozie SO, Potts-Johnson SS, et al. Investigation of bioactive compounds in Crassocephalum rubens leaf and in vitro anticancer activity of its biosynthesized gold nanoparticles. Biotechnol Rep. 2020;28:e00560. doi:10.1016/j.btre.2020.e00560
45. Dorosti N, Jamshidi F. Plant-mediated gold nanoparticles by Dracocephalum kotschyi as anticholinesterase agent: synthesis, characterization, and evaluation of anticancer and antibacterial activity. J Appl Biomed. 2016;14(3):235–245. doi:10.1016/j.jab.2016.03.001
46. Khandanlou R, Murthy V, Saranath D, Damani H. Synthesis and characterization of gold-conjugated Backhousia citriodora nanoparticles and their anticancer activity against MCF-7 breast and HepG2 liver cancer cell lines. J Mater Sci. 2018;53(5):3106–3118. doi:10.1007/s10853-017-1756-4
47. Dehghani F, Mosleh-Shirazi S, Shafiee M, Kasaee SR, Amani AM. Antiviral and antioxidant properties of green synthesized gold nanoparticles using Glaucium flavum leaf extract. Appl Nanosci. 2023;13(6):4395–4405. doi:10.1007/s13204-022-02705-1
48. Gurunathan S, Han JW, Park JH, Kim JH. A green chemistry approach for synthesizing biocompatible gold nanoparticles. Nanoscale Res Lett. 2014;9(1):1–11. doi:10.1186/1556-276X-9-248
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Synthesis of Biogenic Gold Nanoparticles by Using Sericin Protein from Bombyx mori Silk Cocoon and Investigation of Its Wound Healing, Antioxidant, and Antibacterial Potentials
Das G, Seo S, Yang IJ, Nguyen LTH, Shin HS, Patra JK
International Journal of Nanomedicine 2023, 18:17-34
Published Date: 4 January 2023
Sericin-Assisted Green Synthesis of Gold Nanoparticles as Broad-Spectrum Antimicrobial and Biofilm-Disrupting Agents for Therapy of Bacterial Infection
Cai R, Cheng Q, Zhao J, Zhou P, Wu Z, Ma X, Hu Y, Wang H, Lan X, Zhou J, Tao G
International Journal of Nanomedicine 2025, 20:3559-3574
Published Date: 19 March 2025