Back to Journals » Journal of Inflammation Research » Volume 18
Guillain–Barré Syndrome During the Outbreak of Omicron in Southern China: A Multicenter Case-Control Study
Authors Gui M, Fu P, Luo L, Liu Q, Chen J, Han Z, Chang L, Chen H, Gong D, Chen J, Liu Y, Zhang R , Zhang M, Xiang M, Yang X, Lin J, Bu B, Li Z
Received 28 October 2024
Accepted for publication 29 April 2025
Published 22 May 2025 Volume 2025:18 Pages 6543—6555
DOI https://doi.org/10.2147/JIR.S503263
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Xiaoyu Liu
Mengcui Gui,1,2 Peicai Fu,1,2 Lijun Luo,3 Qunhui Liu,4 Jun Chen,5 Zhongmou Han,6 Liying Chang,7 Hui Chen,8 Daokai Gong,9 Juan Chen,10 Yafang Liu,11 Rong Zhang,12 Ming Zhang,13 Mingqing Xiang,14 Xiaohua Yang,15 Jing Lin,1,2 Bitao Bu,1,2 Zhijun Li1,2
1Department of Neurology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030, People’s Republic of China; 2Hubei Key Laboratory of Neural Injury and Functional Reconstruction, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China; 3Department of Neurology, Wuhan Hospital of Traditional Chinese and Western Medicine, Wuhan, Hubei, 430022, People’s Republic of China; 4Department of Neurology, the Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, Enshi Clinical College of Wuhan University, Enshi, Hubei, 445099, People’s Republic of China; 5Department of Neurology, Taihe Hospital, Affiliated Hospital of Hubei University of Medicine, Shiyan, Hubei, 442099, People’s Republic of China; 6Department of Neurology, Yichang Central People’s Hospital, 1st Affiliated Hospital of China Three Gorges University, Yichang, Hubei, 443002, People’s Republic of China; 7Department of Neurology, Xiangyang Central Hospital, Hubei University of Arts and Science, Xiangyang, Hubei, 441106, People’s Republic of China; 8Department of Neurology, the People’s Hospital of Laifeng County, Enshi, Hubei, 445799, People’s Republic of China; 9Department of Neurology, Jingzhou Central Hospital, Yangtze University, Jingzhou, Hubei, 441021, People’s Republic of China; 10Department of Neurology, Minda Hospital of Hubei Minzu University, Enshi, Hubei, 445099, People’s Republic of China; 11Department of Neurology, Huangshi Central Hospital, Hubei Polytechnic University, Huangshi, Hubei, 435000, People’s Republic of China; 12Department of Neurology, Hubei Aerospace Hospital, Xiaogan, Hubei, 432009, People’s Republic of China; 13Department of Neurology, Jingmen Hospital of Traditional Chinese Medicine, Jingmen, Hubei, 448001, People’s Republic of China; 14Department of Neurology, the First People’s Hospital of Jingzhou, 1st Affiliated Hospital of Yangtze University, Jingzhou, Hubei, 434000, People’s Republic of China; 15Department of Neurology, the First People’s Hospital of Tianmen, Affiliated Hospital of Hubei University of Science and Technology, Tianmen, Hubei, 431701, People’s Republic of China
Correspondence: Zhijun Li, Department of Neurology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030, People’s Republic of China, Email [email protected]
Purpose: The largest nationwide outbreak of Omicron, a severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) variant, occurred between December 2022 and February 2023 in China. This multicenter case-control study investigated the clinical features of GBS during this period.
Methods: The clinical characteristics of patients diagnosed with GBS associated with SARS-CoV-2 were assessed during an Omicron outbreak at 14 referral hospitals in Hubei Province, Southern China. In the case-control study, patients with GBS were identified and diagnosed between 2021 and 2022 at Tongji Hospital in Wuhan, Hubei province.
Results: Forty-one patients were diagnosed with GBS during the Omicron outbreak. The median patient age was 57.5 years, and 51.2% were male. The median period between the preceding infection and onset of neurological symptoms was 10 days. The majority of the patients (38 cases [92.7%]) presented with classic sensorimotor neuropathy, with the lower limbs involved more often; 17 cases (41.5%) were accompanied by cranial neuropathies, which was most observed with the bilateral or unilateral facial paralysis (13 cases [31.7%]). Albuminocytologic dissociation was observed in 27 patients (71.1%), and mild pleocytosis was found in five patients (12.2%), with a maximum of 22 cells/mm3. Thirty-two patients finished the electrophysiological studies, and axonal variants were confirmed in 21 cases predominantly as acute motor-sensory axonal neuropathy (40.6%) or acute motor axonal neuropathy (25.0%). Anti-ganglioside antibodies were detected in 19 patients (46.3%). Intravenous immunoglobulin administration improved the patients’ symptoms.
Conclusion: The characteristics of SARS-CoV-2–associated GBS during the Omicron outbreak appear clinically as sensorimotor neuropathy, with a predominant electrophysiological axonal form. A mainly classic post-infectious immune-mediated mechanism may be involved in this process, such as a temporal profile of clinical symptoms, axon-associated autoantibodies, and improvement by immunotherapy.
Keywords: Guillain–Barré syndrome, omicron, sensorimotor neuropathy, axonal variants, post-infectious, immune-mediated mechanism
Introduction
Coronavirus disease-2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Although SARS-CoV-2 primarily involves in the respiratory system, increasing evidence suggests that its pathology may extensively involve in multiple other organs, including the central and peripheral nervous system.1,2 In the peripheral nervous system, COVID-19 is associated with smell and taste dysfunction, muscle injury, Guillain-Barré syndrome (GBS), and its variants.2
GBS is a rare immune-mediated disease of the peripheral nerves and roots that manifests as acute flaccid paralysis and anomalous sensory symptoms but potentially leads to severe disability and fatality.3 The global incidence of GBS varies, with most countries reporting an incidence of about 1.1–1.8 per 100,000 person-years.4,5 A recent national survey reported that the incidence of GBS was 0.233 in children and 0.829 in adults per 100,000 person-years in China as of 2021.6 Approximately two-thirds of patients who developed GBS reported symptoms of an infection in the 6 weeks preceding disease onset, notably Campylobacter jejuni, cytomegalovirus (CMV), Epstein-Barr virus, influenza, and Zika virus.3 Since the COVID-19 outbreak in January 2020, numerous case reports of GBS have been published.7–9 The clinical and electrodiagnostic patterns of COVID-19–associated GBS in the early pandemic experience are similar to those of classic post-infectious GBS.10 However, the strength of the association between GBS and SARS-CoV-2 infection remains unclear.
Omicron is a variant of SARS-CoV-2 with high infectivity and antibody evasion ability.11,12 A nationwide outbreak of Omicron occurred in China from December 2022 to February 2023. According to the information from the Virus Disease Institute at the Chinese Center for Disease Control and Prevention, a total of 1142 cases have been fully sequenced nationwide from December 1st to December 26th in 2022 in China. The results revealed that the BA.5.2 and BF.7 variants of the Omicron strain were the predominant strains nationwide. Given the initial threat of the original COVID-19 strain to the nervous system and the increasing reports of GBS, it is important to examine the impact of the Omicron variant on the peripheral nervous system. However, whether the population characteristics, clinical and electrodiagnostic feature of COVID-19–associated GBS are different from other post-infection GBS is remains unclear. This multicenter case-control study aimed to investigate the characteristics of GBS during this period.
Methods
Study Design and Participants
Fourteen centers participated in this case-control study in Hubei Province, southern China. Patients were diagnosed with GBS that occurred following SARS-CoV-2 infection during the outbreak of the Omicron pandemic between December 2022 and February 2023 were enrolled as S-GBS, while patients with GBS with other types of preceding infections diagnosed between 2021 and 2022 at Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China, were included as case-controls (C-GBS). In the C-GBS group, SARS-CoV-2 testing was performed regularly during the hospital stay (on admission and then every 7 days during hospitalization); therefore, SARS-CoV-2 infection could be ruled out. All patients (aged > 18 years) participated in this study were diagnosed with GBS by neurologists based on the Brighton Collaboration GBS Working Group criteria and classified according to the Brighton Criteria, ranging from high to low diagnostic certainty.13 The exclusion criteria were peripheral neuropathies with other factors, undiagnosed cases or cases with incomplete clinical data, and COVID-19 vaccination or COVID-19 infection within 3 months.
Electronic questionnaires were distributed to the neurologists at each center. The patients’ clinical and demographic data were collected from medical records obtained during the hospital stay. Demographic data, including sex, age at onset, medical history and comorbidities, clinical signs and symptoms of both COVID-19 and GBS, illness duration and severity, cerebrospinal fluid (CSF) examination results, electrophysiological examination results, radiographic test results, treatment received, and clinical prognosis information were collected. The GBS Disability Scale grading system (Hughes scale) was used to measure disability degree.14 The scale ranges from 0 to 6, with lower scores indicating more preserved function. Cases were classified as severe (Hughes grade ≥ 3) or mild (Hughes grade < 3). The Medical Research Council (MRC) scale was used to evaluate muscle strength in the 12 different muscle groups.15 The scores range from 0 to 60, with higher scores indicating greater muscle strength preservation. Two trained neurologists were responsible for the final data collection: one recorded all data and the other reviewed each patient’s final diagnosis.
SARS-Cov-2 Detection
A combination of ribonucleic acid (RNA) and/or specific antigen (nucleocapsid protein) detection of SARS-CoV-2 was performed as described previously.16 Respiratory specimens were collected using nasopharyngeal and pharyngeal swabs. The rapid and accurate detection of SARS-CoV-2 nucleic acids is enabled by real-time reverse transcription polymerase chain reaction.
The SARS-CoV-2-specific antigen is detected by a lateral-flow immunochromatographic assay using gold nanoparticles and a colorimetric label to provide a rapid platform for point-of-contact detection. This was detected by calorimetry. The assay was completed within 20 min with approximately 90% accuracy. It can only identify acute or early infections if the virus is actively replicating.
Electrophysiological Examination
Electrophysiological assessments were performed using standard electromyography techniques, including motor and sensory nerve conduction studies, H-reflexes, and F-waves of the peripheral nerves (median and ulnar nerves in the upper limbs as well as peroneal, tibial, and sural nerves in the lower limbs). The measured parameters included motor nerve velocity, distal motor latency (DML), minimum F-wave latency, minimum H-reflex latency, compound muscle action potential (CMAP) amplitude, presence of a conduction block, sensory nerve velocity, and sensory nerve action potential amplitude.
Ganglioside Antibody Detection
The ganglioside antibodies were tested by a qualified commercial company. A dot immunoblotting assay (MyBiotech, China) was used to assess patients’ serum and CSF samples. Autoantibodies were tested for reactivity to gangliosides, such as GM1, GM2, GM3, GM4, GD1a, GD1b, GD2, GD3, GT1a, GT1b, GQ1b, and sulfatides.
Statistical Analysis
GraphPad Prism 9.2.0 (GraphPad Software, USA) was used for the statistical analysis and picture drafting. A descriptive analysis was conducted to describe the patients’ demographic and clinical characteristics. Based on the distribution of values, continuous data are expressed mean ± standard deviation or as median and interquartile range (IQR). We applied the t test or the Mann–Whitney U-test to test nonparametric continuous data and the χ2 or Fisher’s exact test to compare proportions. Differences were considered statistically significant at p<0.05.
Results
Clinical Characteristics of COVID-19
Forty-one patients were diagnosed with GBS during the study period. The clinical features of the COVID-19 patients are summarized in Table 1.
 |
Table 1 Clinical Manifestations of COVID-19 Preceding Guillain–Barré Syndrome (n=41) |
The median patient age was 57.5 years, and the population included 21 men (51.2%) and 20 women (48.8%). The most common medical history was hypertension. Thirty-eight patients (92.7%) had received one or more doses of COVID-19 vaccine at least 3 months prior to the outbreak. All COVID-19 vaccines were confirmed by the authorities as inactivated, adenoviral, or mRNA. All the patients had close contact with confirmed COVID-19 cases.
The median persistence of COVID-19 symptoms in the 41 patients was 5 days. The patients primarily reported symptoms of acute upper respiratory tract infection, mainly having a moderate to high fever (above 38.5°C), along with a prominent dry cough and sore throat, which significantly hindered swallowing. Fatigue, headache, and muscle pain were also prevalent. Anosmia and/or ageusia were observed in 10 cases. Only 3 patients experienced gastrointestinal symptoms such as diarrhea and reduced appetite at the same time. Dyspnea was less frequent, occurring in three patients. And other respiratory pathogens were screened negative based on the electronic medical records.
All the cases were confirmed COVID-19 by the detection of RNA and/or specific antigen of SARS-CoV-2. Twenty-eight patients finished the pulmonary computed tomography (CT), half of which showed typical radiological features of viral infection. Representative radiological changes on a chest CT presented as bilateral pulmonary parenchymal ground-glass and consolidated pulmonary opacities with a peripheral or posterior distribution due to inflammatory responses16,17 were helpful in increasing the veracity of a definitive COVID-19 diagnosis, especially in the conditions of negative tests for RNA and/or a specific antigen. Due to the concentrated infections of a large population in a short period of time, the close contact history with a confirmed case of COVID-19 and the tight medical resources, 9 (22.0%) patients who were positive of antigen detection did not take additional RNA testing in our retrospective study.
Despite no generally proven effective therapies, some treatments have shown benefits in certain subpopulations of COVID-19 patients.18 Thirty-four patients (82.9%) received symptomatic treatment for the COVID-19. Other treatments were also supplied as well for the patients according to different conditions (as shown in Table 1).
Neurological Findings of GBS Spectrum in S-GBS Group
The neurological findings and laboratory features of patients in the S-GBS group are summarized in Table 2.
 |
Table 2 Neurological Features of Guillain–Barré Syndrome Spectrum (n, % or Median, IQR) |
In the S-GBS group, the median time between SARS-CoV-2 infection and the onset of neurological symptoms was 10 days, while the period between onset and nadir was 9 days. The most common neurological manifestation at nadir was flaccid paralysis of the limbs (36 cases [87.8%]) accompanied by sensory disturbances simultaneously or separately (27 cases [65.9%]). The cranial nerves were involved in 17 patients (41.5%), with bilateral or unilateral facial palsy being the most common (13 cases [31.7%]). On neurological examination at the nadir of the GBS, the median grade of the Hughes scale was 2 (IQR, 1–5) (Figure 1A). The percentage of patients with a Hughes scale score ≥ 3 was 46% (19 cases) (Figure 1B). The median MRC score was 48 (IQR, 0–60), and the MRC scores in the lower extremities (median, 24; IQR, 0–30) were lower than those in the upper limbs (median, 26; IQR, 0–30) (p=0.0385) (Figure 1C). Six patients (14.6%) developed respiratory failure at nadir and received assisted ventilation. The median duration of hospital stay was 14 days (IQR, 6–60 days).
A CSF examination was completed in 38 patients (92.7%). Albuminocytologic dissociation was observed in 27 patients (71.1%). Five patients (13.2%) had slight pleocytosis with a maximum of 22 cells/mm3, indicating a mild inflammatory phenomenon. Nerve conduction studies and electromyography were performed in 32 patients (78.0%). The electrophysiological characteristics predominantly involved axonal damage patterns in the peripheral nerves (21 cases [65.6%]), classified mainly as 13 cases (40.6%) of AMSAN, 8 cases (25.0%) of AMAN, 6 cases (18.8%) of AIDP, and 3 cases (9.4%) of GBS variants. Two patients (6.3%) underwent equivocal studies that did not allow a subtype classification (Supplementary Table 1). In analyses of ganglioside antibodies obtained from 41 patients, 19 (46.3%) exhibited a positive autoimmune response to the gangliosides, including four cases in the CSF simultaneously or separately. Twelve patients (29.2%) tested positive for only one type of autoantibody, while seven (17.1%) demonstrated overlapping antibodies. In addition, among all ganglioside antibodies, immunoglobulin G–anti-GM1 antibody in the serum was mostly observed in patients with S-GBS (seven cases [17.1%]) (Table 3).
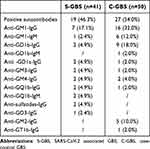 |
Table 3 Gangliosides Antibodies Spectrum in SARS-CoV-2 Associated GBS (n, %) |
Forty-one patients were classified according to the Brighton Criteria, ranging from high to low of diagnostic certainty: as 20 patients (48.8%) met level 1, 17 cases (41.5%) met level 2, and four cases (9.8%) met level 3 (Table 2).
Clinical Features of S-GBS Versus C-GBS
Fifty cases of GBS were included in the C-GBS group. According to medical records, all patients complained of infective symptoms preceding GBS, such as fever, influenza-like symptoms, and diarrhea. Detailed pathogenicity data were not recorded. The clinical findings of C-GBS are summarized in Table 2.
The clinical characteristics of S-GBS and C-GBS were similar in all aspects (post-infection pattern, temporal profile, limb weakness distribution, axonal damage pattern, detection of anti-ganglioside antibodies, and CSF findings) except for different electrophysiological subtype distributions such as dominant AMSAN in S-GBS and AMAN in C-GBS (Figure 2A–C) (Tables 2 and 3).
Treatment for and Short-Term Prognosis of GBS
In S-GBS group, a total of 33 patients (80.5%) had received IVIG within 2 weeks after symptom onset, while 3 patients (7.3%) were treated with plasma exchange. Twenty-five patients (61%) were treated with glucocorticoids simultaneously or separately to reduce the inflammatory reaction triggered by COVID-19 (Table 2). Five out of the 6 patients underwent assisted ventilation when the disease progressed improved thereafter. Only one elderly female (90 years of age) in the S-GBS group, who had a history of hypertension and was unvaccinated against COVID-19, died. She experienced rapid progressive bulbar paralysis and quadriparalysis after the COVID-19 infection, which persistently deteriorated despite treatment with IVIG and glucocorticoids, and ultimately died of respiratory failure. At discharge, the Hughes scale (median, 1; IQR, 0–6) decreased significantly, compared with that at the nadir (p=0.0028) (Figure 1A). The proportion of patients with a Hughes scale score ≥ 3 decreased from 46% to 22% (p=0.02), suggesting a good short-term prognosis (Figure 1B). The MRC score (median, 26; IQR, 0–30) of the lower limbs also increased significantly after treatment compared with that at the nadir (p=0.018). Good improvement was observed in the MRC sum score (median, 56; IQR, 0–60) and upper limb score (median, 30; IQR, 0–30) at discharge, but the value was comparable with that at the nadir (p=0.063 and p=0.202, respectively) (Figure 1C).
Patients in the C-GBS group also received treatment with IVIG (34 [68.0%]) or plasma exchange (seven [14.0%]). The symptoms were significantly improved at discharge, with the Hughes scale score decreasing sharply (median, 2; IQR, 0–4) and the MRC sum score increasing significantly (median, 48; IQR, 26–60) compared to those at the nadir (p<0.0001 and p=0.0003, respectively). However, these values in the C-GBS group were comparable to those in the S-GBS group (p=0.326 and p=0.308, respectively) (Figure 2B and C).
Discussion
In our study, the most patients were mild cases of COVID-19 and the older were more often affected.4 As most of the exposures were concentrated in December 2022 in our study, it was challenging to distinguish the distinct viral variants of COVID-19 within this investigation. However, according to the sampling survey from the Virus Disease Institute at the Chinese Center for Disease Control and Prevention, the BA.5.2 and BF.7 variants of the Omicron strain were the predominant strains nationwide from December 1st to December 26th in 2022 in China, accounting for 80% of the total prevalence. Before the outbreak of Omicron, a very strict dynamic COVID-zero strategy was implemented in Hubei compared to other provinces. We infer that Omicron strain might have had a higher proportion in Hubei than in other regions in the short period following the relaxation of COVID-zero and isolation policy. A recent study found that the replication of Omicron variants was less efficient (more than 10 times lower) in human lung tissue than in the prime strain [27], indicative of less severe disease associated with the lower respiratory tract. This hypothesis was further supported by growing real-world data showing that the risk of progression to severe clinical outcomes, severity of illness hospitalization, and deaths due to the Omicron outbreak was lower than that in the delta (B.1.617.2) variant.19–21 Moreover, the symptoms of Omicron infection are milder and shorter, especially among vaccinated individuals, than those of the delta variant.20
As for the neurological findings in the COVID-19 group, the sensorimotor form was dominant, and the distribution of weakness was more often involved in the lower limbs as previously reported.5,7 The Hughes scale, a well-known disability scale ranging from 0 to 6, was used to assess clinical disability. A Hughes scale score ≥ 3 generally indicates serious motor dysfunction and a poor prognosis.14 Mechanistic ventilation is required in severe cases of disease progression. In some previous studies,5,22 the clinical findings seemed more serious; approximately one-third of patients required mechanical ventilation and autonomic dysfunction was more frequent, which might be related to different variants of SARS-CoV-2 in different local regions.
In our study, the clinical characteristics of S-GBS and C-GBS are similar in terms of post-infection pattern, temporal profile, limb weakness distribution, axonal damage pattern, detection of anti-ganglioside antibodies, CSF findings, and good short-term prognosis, but different electrophysiological subtype distributions dominate (AMSAN in S-GBS and AMAN in C-GBS). Axonal GBS, redefined as a new subtype of GBS in Northern China in 1993,23 remains the most common variant in Eastern and Northern China,24,25 similar to the predominant variant in our case-control group. Our findings suggest that the characteristics of SARS-CoV-2–associated GBS resemble those of other classic post-infectious GBS cases, particularly in China, where the axonal variant is common. Two additional GBS groups with a similar viral pandemic background further support a post-infectious mechanism, such as the Zika-associated GBS cohort in French Polynesia26 and the Japanese encephalitis–associated GBS cohort in China.10 Both GBS cohorts share some common features with S-GBS, including a post-infectious process, axonal variants, and positive anti-ganglioside antibody testing. Therefore, we speculate a mainly post-infectious mechanism may have been involved in COVID-19-associated GBS in our study. However, a strong causal relationship between COVID-19 and GBS has not been established.
An international prospective cohort study recently reported that SARS-CoV-2 patients frequently had a sensorimotor phenotype with facial palsy and they significantly more often had a demyelinating subtype, which differs from our findings.27 Meanwhile, a great majority of SARS-CoV-2 patients were electrophysiologically diagnosed with AIDP,5,7,8,28 suggesting that demyelinating nerve conducting study results might be a specific feature of GBS following SARS-CoV-2 infection at the population level.27 Additionally, bilateral facial paralysis and facial palsy with distal paresthesia as well as the AIDP subtype are the main clinical manifestations of vaccine-associated GBS that have been reported mostly within a 6-week risk window of the adenoviral vector vaccine for COVID-19.29–33 In our study, there are still certain differences in the clinical profiles compared to other GBS cases related to COVID-19, which might due to differences in evolutionary changes in the virus, host-dependent factors, antigenic target differences, and social and psychological factors during a pandemic. Censi and colleagues found that various time points (the first wave of the pandemic and subsequent years) as well as regional differences have a notable impact on GBS following the COVID-19 pandemic.34,35 Palaiodimou and colleagues conducted the first meta-analysis on this topic.36 However, it is limited by inadequate statistical analyses and outdated data.
In addition, technical errors in electrophysiological testing need to be taken into account. Caution should be exercised when making an electrophysiological diagnosis of AIDP in the early phase of GBS. The electrodiagnostic subtypes of a significant number of GBS patients were changed during follow-up, and 22–38% of patients switched from AIDP or equivocal subtypes to axonal GBS after a series electrodiagnostic study.37 Therefore, electrodiagnostic studies are more reliable when performed 3–6 weeks versus 1–2 weeks after GBS onset.38 Considering the limitations in clinical practice, some patients completed only one electrophysiological examination, as in most of our cases, and deviations are inevitable in the electrophysiological classification. Although the GBS subtype diagnosis currently does not impact treatment, we believe that it is important to understand its underlying pathophysiology and prognosis.37
We identified an axonal damage pattern in the S-GBS group, frequently accompanied by anti-ganglioside antibodies, particularly anti-GM1 antibodies. A specific mechanistic link was observed between certain anti-ganglioside antibodies and distinct phenotypic characteristics of infection-associated GBS. For example, GM1 and GD1a antibody levels are frequently elevated in patients with AMAN or AMSAN.39 CMV-associated GBS with anti-GM2 antibodies presents mainly as the AMSAN variant.40,41 In our case-control group, anti-GM2 antibodies were detected, though their association with CMV infection remains uncertain. Interestingly, Zika virus–associated GBS initially manifested as AMAN with anti-GA1 antibodies and later, at follow-up 3 months later, involved multiple antibodies, including anti-GM1 antibodies.26
Whether the “molecular mimicry” mechanism still works in SARS-CoV-2–associated GBS remains to be clarified, although anti-ganglioside antibodies are found in up to 60% of GBS cases.42–44 Keddie et al44 inferred that molecular mimicry causation is less likely identified when searching for homology of the axonal or myelin surface proteins and glycoproteins between any SARS-CoV2 proteins and human nerves and obtaining nothing. In addition, the concept of molecular mimicry between SARS-CoV-2 and various human organs and tissues may serve as a potential trigger for multi-organ autoimmunity in COVID-19,45 such as through the lymphocytic recognition of self-antigens, molecular mimicry,7 or human heat shock proteins.46 Another possibility is that post-translational modifications of viral proteins result in the generation of immunogenic surface glycomolecules47 that might participate in the autoimmune response. Considering the close relationship between anti-ganglioside antibodies and axonal neuropathies in infection-associated GBS, a complicated hyperreactive autoimmune response targeting the peripheral nervous system may be involved in GBS development regardless of the initial triggers.
In another Zika virus–associated GBS cohort in Colombia, approximately half of the patients had a rapid onset of neurological symptoms during viremia, the so-called para-infectious onset, reflecting a variable clinical phenotype that differs from the known post-infectious mechanisms. The presence of viruses in the CSF of three patients in this Zika virus–associated GBS cohort suggests a possible neuroinvasive process.48 A few COVID-19-associated GBS cases also seemed to have a para-infectious profile, with the virus presenting in the CSF.9,43 In our study, we could not confirm the mechanism of para-infection. However, mild pleocytosis was observed in five patients in our study with a maximum of 22 cells/mm3, as observed in another study,7 indicating a slight inflammatory reaction possibly caused by viremia. Moreover, owing to the inflammatory mechanism of SARS-CoV-2, it actively contributes to the increase in the inflammatory response and triggers a lack of control in the immune system, which can result in GBS-associated neurological illnesses.8
As COVID-19 variants continue evolving worldwide, more studies are necessary to investigate the possible neurological effects of SARS-CoV-2 infection.
Limitations
Our study is limited by its retrospective nature. First, we collected cases that occurred during the Omicron variant outbreak in a narrow time window in a local area in southern China, which was not representative of all COVID-19-associated GBS cases during the pandemic. Second, most patients were followed up for less than 2 months; therefore, possible acute onset- chronic inflammatory demyelinating polyneuropathy (A-CIDP) could not be completely ruled out. In addition, as a multicenter study, it was difficult to achieve complete unity in the electrophysiological studies, such as cutoff values and interpretation of the final results. Most patients underwent the electrophysiological examination only once within 3 weeks of disease onset; therefore, some AMAN cases might have been unrecognized. However, this did not change the final judgment as axonal variants were the predominant forms in our study. In addition, the false-positive rate for anti-ganglioside antibodies should be considered. Finally, although we excluded COVID-19 infection during the hospital stay, the pathogens of the case-control GBS could not be ascertained because of the lack of relevant data recorded, and we confirmed the preceding infections by medical record review and clinical symptoms.
Conclusion
Based on this multicenter retrospective study of 41 cases diagnosed during the outbreak of Omicron variants in southern China, we report that the clinical features of SARS-CoV-2- associated GBS appears to be classic sensorimotor neuropathy with a predominant electrophysiological axonal form, which differs from other COVID-19-associated GBS cases reported previously. Furthermore, the temporal profile of neurological symptoms, presence of axon-associated antibodies, and improvement with immunotherapy suggest a post-infectious immune-mediated mechanism.
Abbreviations
SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; GBS, Guillain–Barré Syndrome; COVID-19, Coronavirus disease-2019; AIDP, acute inflammatory demyelinating polyradiculoneuropathy; AMAN, cute motor axonal neuropathy; AMSAN, acute axonal sensory and motor neuropathy; CMV, cytomegalovirus; S-GBS, SARS-CoV-2 infection; C-GBS, case controls-GBS; CSF, cerebrospinal fluid; MRC, Medical Research Council; RNA, ribonucleic acid; DML, distal motor latency; CMAP, compound muscle action potential; IQR, interquartile range; CT, computed tomography; IVIG, intravenous injection of immunoglobulin; A-CIDP, acute onset- chronic inflammatory demyelinating polyneuropathy.
Data Sharing Statement
The original contributions presented in the study are included in the article/ Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Approval and Informed Consent
The study was approved by the Committee of Clinical Investigation at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan China (NO. [2023]-S171), and the protocol was conducted in accordance with the Declaration of Helsinki. Written consent was obtained from all patients or their relatives.
Funding
This work was supported by the National Natural Science Foundation of China (82371411), Natural Science Foundation of Hubei Province (2020CFB744, 2022CFB726), Health Commission of Hubei Province scientific research project (WJ2021M119).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Khan S, Gomes J. Neuropathogenesis of SARS-CoV-2 infection. Elife. 2020;9. doi:10.7554/eLife.59136.
2. Koralnik IJ, Tyler KL. COVID-19: a global threat to the nervous system. Ann Neurol. 2020;88(1):1–11. doi:10.1002/ana.25807
3. Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain-Barre syndrome in ten steps. Nat Rev Neurol. 2019;15(11):671–683. doi:10.1038/s41582-019-0250-9
4. McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barre syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32(2):150–163. doi:10.1159/000184748
5. Filosto M, Cotti Piccinelli S, Gazzina S, et al. Guillain-Barre syndrome and COVID-19: a 1-year observational multicenter study. Eur J Neurol. 2022;29(11):3358–3367. doi:10.1111/ene.15497
6. Zheng P, Tian DC, Xiu Y, Wang Y, Shi FD. Incidence of Guillain-Barre syndrome (GBS) in China: a national population-based study. Lancet Reg Health West Pac. 2022;18:100302. doi:10.1016/j.lanwpc.2021.100302
7. Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain-Barre syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol Apr. 2021;268(4):1133–1170. doi:10.1007/s00415-020-10124-x
8. Pimentel V, Luchsinger VW, Carvalho GL, et al. Guillain-Barre syndrome associated with COVID-19: a systematic review. Brain Behav Immun Health. 2023;28:100578. doi:10.1016/j.bbih.2022.100578
9. Araujo NM, Ferreira LC, Dantas DP, et al. First report of SARS-CoV-2 detection in cerebrospinal fluid in a child with Guillain-Barre syndrome. Pediatr Infect Dis J. 2021;40(7):e274–e276. doi:10.1097/INF.0000000000003146
10. Wang G, Li H, Yang X, et al. Guillain-Barre syndrome associated with JEV infection. N Engl J Med. 2020;383(12):1188–1190. doi:10.1056/NEJMc1916977
11. Shrestha LB, Foster C, Rawlinson W, Tedla N, Bull RA. Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: implications for immune escape and transmission. Rev Med Virol. 2022;32(5):e2381. doi:10.1002/rmv.2381
12. Sun C, Xie C, Bu GL, Zhong LY, Zeng MS. Molecular characteristics, immune evasion, and impact of SARS-CoV-2 variants. Signal Transduct Target Ther. 2022;7(1):202. doi:10.1038/s41392-022-01039-2
13. Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain-Barre syndrome and fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29(3):599–612. doi:10.1016/j.vaccine.2010.06.003
14. Hughes RA, Newsom-Davis JM, Perkin GD, Pierce JM. Controlled trial prednisolone in acute polyneuropathy. Lancet. 1978;2(8093):750–753. doi:10.1016/s0140-6736(78)92644-2
15. Kleyweg RP, van der Meche FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barre syndrome. Muscle Nerve. 1991;14(11):1103–1109. doi:10.1002/mus.880141111
16. Kevadiya BD, Machhi J, Herskovitz J, et al. Diagnostics for SARS-CoV-2 infections. Nat Mater. 2021;20(5):593–605. doi:10.1038/s41563-020-00906-z
17. Hosseiny M, Kooraki S, Gholamrezanezhad A, Reddy S, Myers L. Radiology perspective of coronavirus disease 2019 (COVID-19): lessons from severe acute respiratory syndrome and middle east respiratory syndrome. AJR Am J Roentgenol. 2020;214(5):1078–1082. doi:10.2214/AJR.20.22969
18. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. doi:10.1038/s41579-020-00459-7
19. Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437–446. doi:10.1016/S0140-6736(22)00017-4
20. Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399(10335):1618–1624. doi:10.1016/S0140-6736(22)00327-0
21. Lewnard JA, Hong PMM VX, Kahn R, Lipsitch M, Tartof SY, Tartof SY. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat Med. 2022;28(9):1933–1943. doi:10.1038/s41591-022-01887-z
22. Caress JB, Castoro RJ, Simmons Z, et al. COVID-19-associated Guillain-Barre syndrome: the early pandemic experience. Muscle Nerve. 2020;62(4):485–491. doi:10.1002/mus.27024
23. McKhann GM, Cornblath DR, Griffin JW, et al. Acute motor axonal neuropathy: a frequent cause of acute flaccid paralysis in China. Ann Neurol. 1993;33(4):333–342. doi:10.1002/ana.410330402
24. Song Y, Zhang Y, Yuki N, et al. Guillain-Barre syndrome in Eastern China: a study of 595 patients. Eur J Neurol. 2021;28(8):2727–2735. doi:10.1111/ene.14898
25. Tian J, Cao C, Li T, et al. Electrophysiological subtypes and prognostic factors of guillain-barre syndrome in Northern China. Front Neurol. 2019;10:714. doi:10.3389/fneur.2019.00714
26. Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387(10027):1531–1539. doi:10.1016/S0140-6736(16)00562-6
27. Luijten LWG, Leonhard SE, van der Eijk AA, et al. Guillain-Barre syndrome after SARS-CoV-2 infection in an international prospective cohort study. Brain. 2021;144(11):3392–3404. doi:10.1093/brain/awab279
28. Uncini A, Foresti C, Frigeni B, et al. Electrophysiological features of acute inflammatory demyelinating polyneuropathy associated with SARS-CoV-2 infection. Neurophysiol Clin. 2021;51(2):183–191. doi:10.1016/j.neucli.2021.02.001
29. Hanson KE, Goddard K, Lewis N, et al. Incidence of Guillain-Barre syndrome after COVID-19 vaccination in the vaccine safety datalink. JAMA Network Open. 2022;5(4):e228879. doi:10.1001/jamanetworkopen.2022.8879
30. Keh RYS, Scanlon S, Datta-Nemdharry P, et al. COVID-19 vaccination and Guillain-Barre syndrome: analyses using the national immunoglobulin database. Brain. 2023;146(2):739–748. doi:10.1093/brain/awac067
31. Andreozzi V, D’Arco B, Pagliano P, Toriello A, Barone P. Bilateral facial palsy after COVID-19 vaccination. Neurol Sci. 2022;43(7):4069–4079. doi:10.1007/s10072-022-05982-4
32. Castiglione JI, Crespo JM, Lecchini L, et al. Bilateral facial palsy with paresthesias, variant of Guillain-Barre syndrome following COVID-19 vaccine: a case series of 9 patients. Neuromuscul Disord. 2022;32(7):572–574. doi:10.1016/j.nmd.2022.05.003
33. Yu M, Nie S, Qiao Y, Ma Y. Guillain-Barre syndrome following COVID-19 vaccines: a review of literature. Front Immunol. 2023;14:1078197. doi:10.3389/fimmu.2023.1078197
34. Censi S, Bisaccia G, Gallina S, Tomassini V, Uncini A. Guillain-Barre syndrome and SARS-CoV-2 infection: a systematic review and meta-analysis on a debated issue and evidence for the ‘Italian factor’. Eur J Neurol. 2024;31(2):e16094. doi:10.1111/ene.16094
35. Censi S, Bisaccia G, Gallina S, Tomassini V, Uncini A. Guillain-Barre syndrome and COVID-19 vaccination: a systematic review and meta-analysis. J Neurol. 2024;271(3):1063–1071. doi:10.1007/s00415-024-12186-7
36. Palaiodimou L, Stefanou MI, Katsanos AH, et al. Prevalence, clinical characteristics and outcomes of Guillain-Barre syndrome spectrum associated with COVID-19: a systematic review and meta-analysis. Eur J Neurol. 2021;28(10):3517–3529. doi:10.1111/ene.14860
37. Uncini A, Kuwabara S. The electrodiagnosis of Guillain-Barre syndrome subtypes: where do we stand? Clin Neurophysiol. 2018;129(12):2586–2593. doi:10.1016/j.clinph.2018.09.025
38. Rajabally YA, Durand MC, Mitchell J, Orlikowski D, Nicolas G. Electrophysiological diagnosis of Guillain-Barre syndrome subtype: could a single study suffice? J Neurol Neurosurg Psychiatry. 2015;86(1):115–119. doi:10.1136/jnnp-2014-307815
39. Yuki N, Hartung HP. Guillain-Barre syndrome. N Engl J Med. 2012;366(24):2294–2304. doi:10.1056/NEJMra1114525
40. Spagnoli C, Iodice A, Salerno GG, et al. CMV-associated axonal sensory-motor Guillain-Barre syndrome in a child: case report and review of the literature. Eur J Paediatr Neurol. 2016;20(1):168–175. doi:10.1016/j.ejpn.2015.11.004
41. Dourado Junior MET, Sousa BF, Costa N, Jeronimo SMB. Cytomegalovirus infection in Guillain-Barre syndrome: a retrospective study in Brazil. Arq Neuropsiquiatr. 2021;79(7):607–611. doi:10.1590/0004-282X-ANP-2020-0464
42. Dalakas MC. Guillain-Barre syndrome: the first documented COVID-19-triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm. 2020;7(5). doi:10.1212/NXI.0000000000000781
43. Khan F, Sharma P, Pandey S, et al. COVID-19-associated Guillain-Barre syndrome: postinfectious alone or neuroinvasive too? J Med Virol. 2021;93(10):6045–6049. doi:10.1002/jmv.27159
44. Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barre syndrome. Brain. 2021;144(2):682–693. doi:10.1093/brain/awaa433
45. Angileri F, Legare S, Marino Gammazza A, Conway de Macario E, Jl Macario A, Cappello F. Molecular mimicry may explain multi-organ damage in COVID-19. Autoimmun Rev. 2020;19(8):102591. doi:10.1016/j.autrev.2020.102591
46. Lucchese G, Floel A. SARS-CoV-2 and Guillain-Barre syndrome: molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress Chaperones. 2020;25(5):731–735. doi:10.1007/s12192-020-01145-6
47. Mary B, Maurya S, Arumugam S, Kumar V, Jayandharan GR. Post-translational modifications in capsid proteins of recombinant adeno-associated virus (AAV) 1-rh10 serotypes. FEBS J. 2019;286(24):4964–4981. doi:10.1111/febs.15013
48. Parra B, Lizarazo J, Jimenez-Arango JA, et al. Guillain-barre syndrome associated with Zika virus infection in Colombia. N Engl J Med. 2016;375(16):1513–1523. doi:10.1056/NEJMoa1605564
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



