Back to Journals » International Journal of Nanomedicine » Volume 20
Harnessing the Power of Traditional Chinese Medicine in Cancer Treatment: The Role of Nanocarriers
Authors Fu Z, Wang S, Zhou X , Ouyang L, Chen Z, Deng G
Received 22 October 2024
Accepted for publication 24 February 2025
Published 13 March 2025 Volume 2025:20 Pages 3147—3174
DOI https://doi.org/10.2147/IJN.S502104
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Xing Zhang
Ziyu Fu,1 Shengmei Wang,1 Xin Zhou,1 Linqi Ouyang,1 Zhen Chen,1 Guiming Deng1,2
1The First Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, 410007, People’s Republic of China; 2The second Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, 410005, People’s Republic of China
Correspondence: Zhen Chen; Guiming Deng, Email [email protected]; [email protected]
Abstract: For centuries, traditional Chinese medicine (TCM) has had certain advantages in the treatment of tumors. However, due to their poor water solubility, low bioavailability and potential toxicity, their effective delivery to target sites can be a major challenge. Nanocarriers based on the active ingredients of TCM, such as liposomes, polymer nanoparticles, inorganic nanoparticles, and organic/inorganic nanohybrids, are a promising strategy to improve the delivery of TCM, resulting in higher therapeutic outcomes and fewer side effects. Therefore, this article intends to review the application of Chinese medicine nano preparation in tumor. Firstly, we introduce the classification and synthesis of nanometer preparations of Chinese medicine. The second part mainly introduces the different responses of TCM nano-preparations in the course of treatment to introduce how TCM nano-preparations play a role in anti-tumor therapy. The third part focuses on Different response modes of Chinese medicine nano preparations in tumor therapy. The fourth part elucidates the application of Chinese medicine nano preparations in the treatment of cancer. Finally, the research direction to be explored in related fields is put forward.
Keywords: traditional Chinese medicine, tumor, nanodelivery system, tumor therapy
Introduction
Traditional Chinese medicine (TCM) has long exerted a significant influence on the health of Asian populations and, increasingly, on a global scale. In recent years, particularly during the management of coronavirus disease 2019 (COVID-19) in China, there has been a growing interest in the potential of TCM. Its popularity is on the rise, and it plays a crucial role in health care.1 TCM primarily derives from natural sources, including plants, animals, and minerals, and is generally classified as natural medicine. Through extensive clinical practice, these remedies are recognized for their relatively mild medicinal properties and low side effects. They exhibit characteristics of multi-target action and overall regulation, leading to the development of various dosage forms. One such advancement is nanotechnology, which involves the manipulation and control of matter at atomic, molecular, and supramolecular scales. The National Nanotechnology Program defines nanotechnology as the processing of substances that range from 1 to 100 nm in at least one dimension. Nanomedicine preparation refers to a pharmaceutical technology that employs nanocarrier systems to convert raw drugs into nanoparticles, attaching the drug to the carrier’s surface or encapsulating it within the carrier using covalent or non-covalent methods, including electrostatic adsorption. Nanoparticles offer numerous advantages, such as a large specific surface area, enhanced targeting capabilities, and effective sustained release. They can improve the solubility and chemical stability of encapsulated drugs, extend the circulation time of drugs in the bloodstream, enhance cellular uptake efficiency, and facilitate targeted drug delivery to tumor sites through mechanisms such as the enhanced permeability and retention (EPR) effect or targeting ligand conjugation.
The application of TCM in cancer treatment has made significant progress and has gradually become an integral part of comprehensive tumor therapy.2,3 In cancer treatment, TCM is not only utilized as an adjuvant therapy but also demonstrates unique advantages in anti-tumor effects and in mitigating the toxic side effects associated with radiotherapy and chemotherapy. As scientific research continues to advance, the mechanisms and clinical applications of TCM in tumor prevention and treatment have been further elucidated and validated. Studies indicate that TCM is widely employed in tumor therapy.4 It has played a positive role in disease prevention and treatment, enhancing bodily resilience, improving anti-cancer efficacy, increasing therapeutic efficiency, reducing toxicity, differentiating diseases and syndromes, and addressing root causes. This widespread acceptance and utilization underscore the clear advantages of TCM in anti-tumor treatment. This article will explore the application of nanotechnology within TCM, highlighting its significance as a direction for reforming TCM dosage forms. It will cover the classification and synthesis of nanopreparations. Based on the varying compositions of TCM nanopreparations, they will be categorized into single nanopreparations, compound nanopreparations, and combinations of two drugs with nanopreparations, discussing the characteristics and applications of each type.
This article introduces the application of nanotechnology in TCM and emphasizes its significance as a reform direction for TCM dosage forms. It reviews the classification and synthesis of nanopreparations, categorizing them into single nanopreparations, compound nanopreparations, and combinations of two drugs with nanopreparations based on their different compositions. The characteristics and applications of various nano-preparations are discussed in detail. Furthermore, the article explores the role of nanopreparations in tumor treatment, including their use in conjunction with radiotherapy, chemotherapy, immunotherapy, and adjuvant therapies. It summarizes the potential and challenges associated with TCM nanopreparations in oncological applications and outlines future research directions and application prospects (Figure 1).
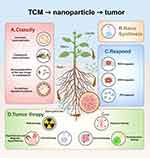 |
Figure 1 (A) Classification of Nanopreparations. (B) Synthesis methods of nanopreparations. (C) Different ways of responding to nanoformulation. (D) Nanopreparations combined with different traditional tumor treatment modalities. Created in BioRender. Deng, G. (2025) https://BioRender.com/r27b934.. |
Nano Particle Formulation
Background
In recent years, nanotechnology has garnered increasing attention, and as research progresses, its fields of application have expanded significantly. Particularly within TCM, the active development of nanotechnology has emerged as a key reform direction for dosage forms. However, TCM has historically faced several limitations, including poor water solubility of many active ingredients, rapid metabolism, low oral absorption rates, low bioavailability, weak targeting capabilities, and a limited range of administration methods. The nanoparticle drug delivery system (NDDS) utilizing nanoparticles presents an effective solution to overcome these barriers in anti-cancer drug delivery,5 enabling precise targeting and site-specific delivery:6 ① TCM nanopreparations can reduce the damage of drugs to normal cells by controlling the release rate and pathway of drugs, thereby reducing toxic and side effects. Charlie-Silva I et al concluded that polymeric nanoparticles are submicron colloidal structures that, depending on their composition, can increase the therapeutic value of artemisinin (ART) and reduce its systemic toxicity.7 ② TCM nano-preparations can increase the solubility and bioavailability of drugs, thereby improving drug efficacy. Wang et al used high-pressure homogenization technology to prepare Berberine nanosuspension (Ber-ns) composed of Berberine (Ber) and D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS). And the hypoglycemic effect of Ber-ns was compared with that of high-dose Ber. Ber-ns was stronger. Compared with the same dose of Ber and metformin (Met, 300 mg/kg), oral administration of Ber-ns (50 mg/kg) The 8-week blood glucose and total cholesterol (TC) and weight loss effects were better.8 ③ TCM nano-preparations can extend the shelf life of drugs, reduce the decomposition and degradation of drugs, and enhance their stability. Ginsenosides in Panax notoginseng are amphipathic molecules, with the C-3 position being a glycosyl hydrophilic group and the C-17 position being A hydrophobic group that has the potential function of acting as a carrier to stabilize the phospholipid bilayer. Some ginsenosides have a steroidal core structure similar to cholesterol. Several studies have proven that cholesterol, as one of the ideal components of liposomes, can improve the efficacy of liposomes and the stability of liposome membranes.9,10 ④ TCM nanopreparations can be made into various dosage forms, such as oral liquids, capsules, tablets, etc. for the convenience of patients. In addition, TCM nanopreparations can also be administered through multiple routes such as oral administration, injection, and external use to meet the needs of different patients. Oral nanomedicine: such as, Ber nanoparticles, Panax notoginseng powder nanoparticles,11 etc. Injection of nanomedicines: such as, ART nanoparticles,12 tanshinone nanoparticles, etc. External nanomedicines: such as, transdermal absorption of astragalus polysaccharide nanoparticles, angelica polysaccharide nanoparticles in the form of patches, etc.13 Targeted nanomedicines: such as, antibody-drug conjugate (ADC) nanoparticles based on tumor cell surface antigens, magnetic nanoparticles based on the tumor microenvironment (TME), etc. This article next lists many TCM nanopreparations to introduce their synthesis methods, types, and active ingredients (Table 1).
 |
Table 1 Application of TCM Nanopreparations in Cancer |
Classification and Synthesis of TCM Nano Preparations
Currently, numerous diseases pose significant threats to human health and life worldwide, with cancer remaining one of the most prevalent and devastating conditions.45 In nearly 100 countries, irrespective of their level of development, cancer is among the most common malignant diseases and serves as the leading cause of morbidity and mortality. It is projected that cancer will become the primary cause of death in every country during the 21st century, representing a major obstacle to increasing life expectancy.46 Chemotherapy drugs have been utilized for cancer treatment for an extended period; however, these drugs are not completely delivered to the tumor during the administration process. Their low specificity and high incidence of side effects significantly limit their effectiveness. NDDS plays a crucial role in integrating TCM with chemotherapy agents. Additionally, various formulations of TCM nanopreparations have been rapidly developed. This article will categorize these preparations into three groups: monomeric nanopreparations, compound nanopreparations, and two-drug combination nanopreparations (Figure 2).
 |
Figure 2 (A) Classification of nano preparations (B) Synthesis methods corresponding to different nanoparticles. (C) Different synthesis methods improve the therapeutic efficacy of TCM nanopreparations. Created in BioRender. Deng, G. (2025) https://BioRender.com/u01j778. |
Monomer Nanoformulations
Monomeric nanopreparations refer to nanopreparations containing only one drug. Such nanopreparations can improve bioavailability, improve the stability and targeting of a single drug, etc. Some common nanopreparations of TCM monomers can often exert anti-tumor effects, such as schisandrin B (Sch B), β-elemene (β-ELE), betulinic acid (BA), quercetin (QUE), curcumin (CUR), etc. These common nanopreparations of TCM ingredients include: ① Polymer nanoparticles: Polymer nanoparticles are nanoparticles that encapsulate drugs in polymer materials. Compared with TCM, polymer nanoparticles have stronger drug-loading capacity, relatively stable structure, non-toxicity, and can overcome resistance. Multi-drug, promote targeted delivery of anti-tumor drugs, etc.47 Wang et al developed multifunctional nanoparticles composed of two functional polymers: pH-sensitive PLGA-PEG-folic acid and cell-penetrating peptide-modified PLGA-PEG (R7).14 The incorporation of PLGA-PEG-folate imparts pH sensitivity to the nanoparticles, enabling tumor-specific targeting through folate conjugation. Additionally, this modification facilitates the escape from P-glycoprotein (p-gp)-mediated drug efflux, thus enhancing cellular uptake efficiency and tumor targeting in tumor-bearing mice. ② Liposomes: Due to the special structure of the lipid bilayer, water-soluble drugs can be coated within the core, and fat-soluble drugs can be coated between the lipid bilayers.48 This characteristic allows it to carry two types of drugs, hydrophilic drugs and hydrophobic drugs. Liposomes are one of the few nanoformulations used in clinical treatments.49 The amphipathic, biocompatibility and biodegradability properties of liposomes have important application value for the delivery of TCM. Liposome-based triptolide delivery is one of the most mature nano-TCM delivery carriers.50 These carriers have improved therapeutic efficacy and safety, improved bioavailability, sustained release, and topical delivery.51 For example, the combination of β-elemene and cisplatin in chemotherapy represents one of the most significant treatment options for lung cancer in China. Cao M et al developed a liposome system co-loaded with both β-elemene and cisplatin, which effectively targets lung cancer.15 These co-loaded liposomes exhibited greater cytotoxicity towards cancer cells compared to the combination of single-loaded liposomes, due to their ability to facilitate simultaneous drug internalization and release. Furthermore, they demonstrated promising therapeutic outcomes in both cell-derived and patient-derived xenografts. ③ Inorganic nanoparticles: Inorganic nanoparticles are defined as nano-scale particles composed of inorganic materials. Their advantages include controllable size adjustment, strong functionality, a relatively stable structure, and a high specific surface area with enhanced surface activity. Common inorganic nanomaterials encompass silica, titanium dioxide, aluminum oxide, calcium carbonate, and aluminum nitride. Cao et al were the first to prepare hollow mesoporous silica nanoparticles (HMSNs) using a Na2CO3 solution as the dissolution medium.16 Due to their favorable linear relationship with in vitro dissolution in Na2CO3 solution and in vivo absorption, HMSNs represent a promising model for the sustained release of water-soluble drugs. The researchers conducted in vitro experiments using the water-soluble compound silybin polyaminoamine as a model drug, demonstrating that HMSNs possess sustained-release properties, significantly enhance bioavailability, and mitigate the poor solubility and limited permeability of silymarin to some extent, addressing the challenges posed by extensive presystemic metabolism and short half-life. ④ Metal-organic framework materials (MOFs): MOFs are a specific class of inorganic metal-organic frameworks composed of inorganic metal centers, such as metal ions or metal clusters, and bridging organic ligands formed through self-assembly. These components interconnect to create a crystalline porous material characterized by a periodic network structure. MOFs exhibit several notable properties, including porosity, a large specific surface area, and the potential for surface modification. Compared to traditional nanocarriers, MOFs possess unique advantages. For instance, certain iron-based nano-MOFs demonstrate antibacterial properties, which, in conjunction with their drug cargo, aid in combating intracellular infections.52 Additionally, they contribute to improved radiation efficiency.53 Each component of MOFs plays a significant role in tumor treatment, either through radiation therapy or in the management of severe infections.54 These properties position MOFs as promising candidates for anti-tumor therapy. Programmed cell death protein 1 (PD-1) inhibitors are the most prevalent immune checkpoint inhibitors and are regarded as promising agents for the treatment of hepatocellular carcinoma (HCC). However, clinical observations reveal that HCC patients exhibit low objective response rates (15%-20%) due to insufficient levels and activity of tumor-infiltrating T lymphocytes (TILs). The concurrent administration of oxymatrine (Om) and astragaloside IV (As) can enhance TIL levels by inhibiting the activation of cancer-associated fibroblasts (CAFs) and to improve TIL activity by augmenting their mitochondrial function. Hong Guo et al developed a MOF-based nanoplatform, which incorporates a platelet membrane (Pm) coating (pmMN@OM & As) to facilitate the simultaneous delivery of OM and As to the HCC microenvironment. Their results indicate that PmMN@OM & As demonstrated a higher total drug loading capacity (33.77 wt%) and effective immune evasion.17 Furthermore, it can target liver cancer tissue under the influence of a magnetic field, thereby exerting prolonged therapeutic effects. ⑤ Biomimetic nanosystems: It is noteworthy that the biological molecules released by cells, known as extracellular vesicles (EVs), represent an emerging class of highly complex drug carriers.55 EVs not only exhibit superior biodistribution characteristics compared to synthetic carriers but also offer the advantage of harnessing cellular processes for drug loading and surface modification.56 Meng Cao et al isolated a novel type of EV-like ginseng-derived nanoparticles (GDNPs) from ginseng.18 These nanoparticles significantly promoted the polarization of M2 to M1 macrophages and increased the production of reactive oxygen species (ROS). Furthermore, treatment with GDNPs notably inhibited melanoma growth in tumor-bearing mice, with an observed increase in M1 macrophages within the tumor tissue, leading to enhanced apoptosis in mouse melanoma cells. The findings suggest that GDNPs can effectively alter M2 polarization in both in vivo and in vitro settings, thereby promoting anti-tumor responses.
Compound Nanopreparations
TCM is a distinctive method of diagnosis and treatment that has been utilized for thousands of years and remains widely practiced in China. Traditional herbal medicines are primarily employed in clinical oncology through compound prescriptions, including oral herbal medicines, granules or capsules, and injections.57 Clinical studies have demonstrated that Sini Decoction can effectively treat breast cancer (BC) by reducing blood vessel and lymphangiogenesis in conjunction with adjuvant chemotherapy.58 Other clinical treatments for BC include Taohong Siwu Decoction, Yanghe Decoction, and Shugan Liangxue Decoction, among others.59–61 For instance, Yi Zhang et al found that Shugan Liangxue Decoction acts as a selective estrogen enzyme regulator by downregulating STS expression in MCF-7 cells, thereby achieving anti-tumor effects.61 Compound Phyllanthus urinaria (CP) is a traditional Chinese herbal medicine that is widely utilized in the clinical treatment of HCC and exhibits significant anti-cancer effects. Lenvatinib is an approved drug for the initial treatment of advanced unresectable liver cancer; however, patient survival is ultimately constrained by the gradual emergence of drug resistance. Mianmian Liao et al proposed the combined use of Compound Phyllanthus and Lenvatinib to investigate their mechanisms of action in liver cancer treatment at the molecular level.19 Their research identified novel autophagy-related miRNAs shared between HepG2-derived exosomes and HepG2 cells. These miRNAs are implicated in the progression of HCC and were identified through the application of Compound Phyllanthus and Lenvatinib. In conclusion, the findings suggest that the combined application of Compound Phyllanthus and Lenvatinib can effectively inhibit liver cancer by promoting exosome-mediated autophagy inhibition. This innovative treatment option demonstrates high efficacy and durability, positioning it as a promising therapeutic strategy for liver cancer. Furthermore, the differentially expressed and related miRNAs identified in this study, associated with exosome-mediated autophagy, may serve as potential targets for the clinical treatment of HCC. Biejiajian Pills (BJJP) are a widely used compound formula in China, consisting of various herbs and primarily utilized for the treatment of liver cancer. Previous studies have demonstrated that BJJP inhibits the growth of liver cancer cells both in vivo and in vitro by exerting direct cytotoxic effects on tumor cells. Research conducted by Xuemei Yang et al revealed that, in addition to its direct cytotoxic effects, BJJP promotes the infiltration of CD8+ T cells into the tumors of H22 tumor-bearing mice, thereby further inhibiting tumor growth.62,63 Additionally, Biejiawan, a classic formula summarized in “golden chamber synopsis”, comprises 23 components, some of which have been developed at the nanoparticle level. Scutellaria baicalensis is one of its components. Baicalein is an active polyphenolic compound isolated from Scutellaria baicalensis and plays a significant role in the treatment of various diseases. It exhibits anticancer activity by inhibiting inflammation and cell proliferation. Furthermore, the anticancer potential of baicalein is primarily mediated through the modulation of several cell signaling pathways, including the induction of apoptosis, autophagy, and cell cycle arrest, as well as the inhibition of angiogenesis and the modulation of the STAT3 and PI3K/Akt pathways, alongside other molecular targets.64 Zheng F et al developed a mPEG-PLGA nanoparticle loaded with baicalein (PM-Ba) designed to target the TME. The results indicate that PM-Ba can inhibit the transforming growth factor β (TGF-β) signaling pathway, thereby preventing the activation of CAFs and subsequently influencing the stromal microenvironment of the tumor.20 Additionally, this agent promotes increased infiltration of cytotoxic T cells and enhances the activation of the tumor immune microenvironment. In a mouse model of BC, intravenous injection of PM-Ba in combination with doxorubicin nanoparticles (PMs-ADM) significantly improved the anti-tumor efficacy. These findings suggest that baicalein encapsulated in nanoparticles may represent a promising strategy for modulating the TME and enhancing adjuvant chemotherapy, indicating that potential TME-reshaping nanoformulations can improve the antitumor effectiveness of nanotherapeutics.
Thus, the application of TCM compounds in anti-tumor treatment is indeed present, serving as an adjunct therapy to enhance therapeutic efficacy and mitigate side effects through the synergistic effects of multiple ingredients. However, it should be noted that although TCM compounds can be used as adjuvant anti-tumor treatments, they cannot cure tumors. It usually needs to be used in combination with other anti-tumor treatments such as chemotherapy, radiotherapy or surgery. But it is undeniable that nano-preparations of Chinese herbal medicine compounds also have good prospects in anti-tumor.65
Combination of Two Drugs Nanoformulations
In clinical practice, cancer patients may develop resistance to a single chemotherapy drug, resulting in reduced subsequent efficacy66 Multidrug resistance (MDR) was once considered to be the main cause of chemotherapy failure and may also promote tumor metastasis and recurrence. According to recent statistics from the American Cancer Society, 90% of cancer patients die from varying degrees of MDR.67
Therefore, combination therapy gradually replaced the initial monotherapy. The combined use of two or more anti-tumor active ingredients to exert complementary and synergistic effects has become the preferred option for tumor treatment. It is worth noting that the combination of TCM and chemotherapy drugs and multi-drug combination therapy with multiple targets and multiple signaling pathways have improved efficacy compared with single-molecule target drugs, and have become new strategies for tumor treatment in recent years.68 Two-drug combination nano-preparations have broad potential in anti-tumor cancer treatment. The main chemical components of many TCMs play a vital role in tumor treatment. For example: ① Enhance the therapeutic effect of chemotherapy drugs - Since the combined effect of CUR and cisplatin (CDDP) was measured in vitro and in vivo, experimental results showed that CUR can enhance the anti-tumor effect of CDDP in A549 cells in vitro.69 In addition, research results show that the combination of ART/metformin loaded PLGA-PEG nanoparticles in the treatment of BC can significantly improve the therapeutic effect21,22 ② Synergistic Therapeutic Effect - Docetaxel (DTX) is currently the only effective drug that prolongs survival and enhances the quality of life in patients with metastatic castration-resistant prostate cancer (mCRPC). Yan J et al developed lipid-polymer hybrid nanoparticles (LPN) that co-encapsulate DTX and CUR to achieve a synergistic therapeutic effect, thereby maximizing efficacy and overcoming challenges associated with multi-drug resistance in nanodrug delivery systems.23 The particle size, zeta potential, drug encapsulation, and delivery of DTX-CUR-LPNs were thoroughly evaluated. The results indicated that DTX-CUR-LPNs exhibit high cytotoxicity and synergistic effects on tumor cells in vitro. ③ Reverse drug resistance - The MDR associated with paclitaxel (PTX) limits its efficacy in anti-tumor chemotherapy, often resulting in treatment failure. Zou et al employed a one-step nanoprecipitation method to co-load PTX and borneol (BNL) into polyethylene glycol-polyamide-amine-acrylamide (PEG-PAMAM) nanoparticles, yielding drug-loaded nanoparticles with enhanced efficiency, a narrower particle size distribution, and a reduced hemolysis rate.24 In comparison to free P/NPs + BNL, the PB/NPs significantly inhibited tumor growth in A2780/PTX tumor-bearing mice. These findings suggest that the co-delivery of PTX and BNL via nanoparticles offers advantages in overcoming MDR. The co-loaded nanosystem, comprising PEG-PAMAM polymer along with PTX and BNL, presents a promising candidate for MDR treatment. ④ Reduce drug dosage. - Isoliquiritigenin (ISL), a compound derived from TCM, demonstrates potential in the treatment of BC. To address the issue of ISL’s low bioavailability due to its limited water solubility, Sun et al developed an asymmetric organic molecule, TBPI, which induces the production of ROS in mitochondria.25 They combined TBPI with ISL and encapsulated this complex in DSPE-PEG-RGD nanoparticles, resulting in the formulation of IT-PEG-RGD nanoparticles, which exhibit a high affinity for BC. These IT-PEG-RGD nanoparticles demonstrated excellent drug delivery capabilities in 4T1 BC cell lines and 4T1 tumor-bearing mouse models, leading to a synergistic anti-tumor effect, enhanced tumor-killing potency, and a reduction in both drug dosage and associated side effects. ⑤ Prolonged survival - As2O3 combined with ginsenoside Rg3 significantly inhibits the proliferation of NCIH1299 cells, prolongs the survival time of tumor-bearing nude mice, and demonstrates a substantial effect on lung cancer treatment.26 ⑥ Reducing drug toxicity - Doxorubicin (DOX) is one of the most widely used anti-tumor drugs for prostate cancer (pCa),70 yet its side effects are unavoidable.62 Consequently, many scientists are striving to mitigate drug side effects by modifying dosage forms or by combining different drugs. Qingfei Zhang et al discovered that particle shape plays a significant role in biodistribution and cellular uptake in drug release applications.71,72 They employed combined drugs to diminish the toxicity of DOX. This approach is frequently observed in nano-preparations of TCM. Tanshinone, an active extract from the TCM Salvia miltiorrhiza, has been shown to possess extensive anticancer properties.73,74 Sun G et al combined pH-sensitive nanoparticles with lipid nanoparticles to prepare lipid nanoparticles loaded with DOX and Tanshinone through emulsification and solvent diffusion, resulting in a PN-DOX/TAN nanoparticle delivery system.27 This system is designed to adapt to the acidic environment of tumor tissue (pH 6.5) and allows for a more complete release of the drug within the tumor area.
Co-Delivery Nanoformulations
Co-delivery nanoformulation is an advanced drug delivery system that involves the co-encapsulation of two or more drugs within the same nanoscale carrier. This strategy aims to ensure that these components are released and function simultaneously in the body, thereby optimizing therapeutic effects. In the realm of TCM, this concept has been further developed into TCM co-delivery nano-preparations, which integrate multiple active ingredients from TCM into a nanocarrier to fully leverage the multi-component synergy inherent in TCM. Typically, TCM comprises a variety of active ingredients with distinct pharmacological effects. These ingredients may exert synergistic effects during the treatment process, prolong their duration of action in the body, and assist in overcoming the challenge of multidrug resistance, ultimately enhancing the therapeutic effect.75 Co-delivery nanoformulation can be achieved through straightforward physical encapsulation methods, such as utilizing liposomes to load two water-soluble drugs within their hydrophilic core, or employing polymeric nanoparticles to simultaneously encapsulate two fat-soluble drugs in their hydrophobic core. Jinming Zhang et al successfully developed an innovative co-delivery system utilizing pH-sensitive nanoparticles composed of amphiphilic poly(β-aminoester) copolymers to deliver the pro-apoptotic drug Dox alongside the potent anti-angiogenic drug CUR.28 By optimizing the drug ratio, Dox and Cur co-loaded nanoparticles ((D + C)/NPs) were prepared. This method of encapsulating the pro-apoptotic drug Dox and the anti-angiogenic agent Cur in pH-sensitive NPs represents an effective and synergistic strategy for inhibiting HCC, marking a significant breakthrough in the field of cancer treatment. Tianyu Chen et al achieved remarkable results in their research on KLA-modified liposomes (KLA-5-FU/PTX Lps) containing 5-fluorouracil (5-FU) and PTX for the treatment of triple-negative BC (TNBC).29 The activity of these liposomes was evaluated in depth. Experimental results indicate that KLA-5-FU/PTX Lps significantly enhances cytotoxic activity against BC cells (MDA-MB-231), improves drug delivery to mitochondria, and induces mitochondria-mediated apoptosis. Additionally, this modified liposome demonstrates a strong ability to target tumors and mitochondria, exhibiting excellent anti-tumor activity in vivo. Therefore, KLA-5-FU/PTX Lps is considered a promising system, providing a novel approach for the targeted delivery of anti-tumor drugs to mitochondria in the treatment of TNBC. Co-delivery nanoformulations are designed to ensure that these components are released and function simultaneously within the body. This system not only improves the bioavailability of drugs during anti-tumor treatment but also enhances their targeting capabilities.
Synthesis of Nanoformulations
Due to the complex texture of TCM ingredients, the application of nanotechnology in this field is closely linked to various synthesis methods. Nanotechnology facilitates the transformation of drugs into nanoparticles. Different synthesis methods can address the inherent limitations of TCM. One significant advantage of nanoparticles is their ability to enhance the permeation of drugs through biological membranes, thereby improving transdermal absorption and intracellular drug efficacy. This article will introduce several straightforward nanosynthesis methods.
Spray Drying Method
Spray Drying: This method provides an efficient means of converting liquid ingredients into dry powder. Spray drying is employed to generate drug particles by rapidly evaporating the solvent, resulting in nano-sized drug particles. This technique allows for the control of particle size and shape through the adjustment of spray parameters, and it can complete the drying process within a few seconds. It is particularly suitable for heat-sensitive drugs and offers advantages such as reduced drying time and consistent product quality. These benefits enhance the solubility, bioavailability, and efficacy of TCM, making it appropriate for large-scale industrial production. Resveratrol, a naturally occurring polyphenol, exhibits cardioprotective and cancer-preventive properties; however, its biological activity is constrained by low bioavailability when administered orally. Rebeca Peñalva et al prepared casein nanoparticles through spray drying purification and drying, subsequently employing the coagulation method to assess the efficacy of these nanoparticles as an oral carrier for resveratrol. In vitro experiments demonstrated that the oral bioavailability of resveratrol encapsulated in casein nanoparticles is 26.5%, which is ten times higher than that of oral polyphenols.30 Photodynamic therapy (PDT) represents a promising approach to cancer treatment; however, reports on its use in lung applications are limited, largely due to the fact that most photosensitizers are given intravenously. To tackle this challenge, researchers have developed nanoparticles that incorporate CUR, a component derived from TCM, functioning as a photosensitizer. In vitro tests involving human lung epithelial cancer cells (A549) revealed that these nanoparticles demonstrated selective cytotoxic effects when activated by a device equipped with LED irradiation. Furthermore, the photocytotoxic effects of CUR nanoparticles were found to be dose-dependent, with the IC50 values showing a direct relationship to the radiation flux used. Subsequently, Baghdan E et al spray-dried these nanoparticles with mannitol as a stabilizing agent, resulting in particles with favorable aerodynamic characteristics for efficient lung deposition.31 The results of this research suggest that spray-dried nanoparticles exhibit good compatibility with lung surfactant and function as effective carriers for drugs in PDT targeting lung cancer. Nonetheless, Ermakova NP et al employed the nano spray desiccant B-90 spray drying method to prepare paclitaxel-loaded poly(3- hydroxybutyrate (PHB) particles loaded with the anti-tumor cell inhibitory drug PTX.32 The resulting paclitaxel biopolymer formulation (PBF) underwent comprehensive studies assessing cytotoxicity (on bone marrow stem cells), as well as acute and chronic toxicity, sensitization, and pyrogenicity. Additionally, histological observations were conducted on mice, rats, and rabbits. Acute toxicity studies indicated that when mice and rats were intraperitoneally injected with PBF, the toxicity of PBF at the same PTX content dose was significantly lower than that of conventional PTX formulations. However, chronic toxicity studies revealed that intraperitoneal injection of PBF exhibited notable cumulative properties and toxic effects, which preclude clinical trials of PBF in its current formulation. Consequently, the parameters of PBF obtained through spray drying require modification for the further development of pharmaceutical formulations. This illustrates the challenges faced by spray drying: drug toxicity, the high viscosity, strong hygroscopicity, and poor fluidity of TCM extracts present challenges in pharmaceutical production, particularly regarding wall adhesion and caking issues during spray drying.76
High-Pressure Homogenization
High-pressure homogenization: Mixing polymer solutions and drugs through high shear force to form nanoscale drug carriers, which is suitable for the nanonization of a variety of drugs and excipients. It can produce very uniform nanoparticles and improve drug dispersion. Through the action of high pressure, large particles can be quickly broken into small nanoparticles. This method is often used to prepare nanoparticles such as polymer-drug conjugates. High-pressure homogenization technology can nanonize TCM raw materials and increase the surface area of the medicine, thereby improving the dissolution rate and absorption efficiency of poorly soluble ingredients.
For example, the optimized Baicalin-Ber composite nanocrystals were developed through high-pressure homogenization. Pharmacokinetic results indicate that, compared to the composite crude suspension, these nanocrystals improve the dissolution rate, solubility, and oral bioavailability, while also promoting the co-absorption of the drug prescriptions Baicalin and Ber.33 Concurrently, a CUR O/W emulsion was prepared using high-pressure homogenization, which effectively protects CUR, resulting in enhanced bioaccessibility and bioavailability.34 Zhai B et al utilized ethanol injection and high-pressure microjet homogenization to develop an amino-terminal fragment (ATF) peptide-targeted liposome carrying β-ELE (ATF24-PEG-lipo-β-E), aimed at targeted delivery to bladder cancer cells that overexpress the urokinase plasminogen activator receptor. This formulation was combined with CDDP for the treatment of bladder cancer.35,36 In comparison to polyethylene glycol-modified β-ELE liposomes (PEG-lipo-β-E), ATF24-PEG-lipo-β-E demonstrated superior targeting efficiency and increased cytotoxicity. Furthermore, PEG-Lipo-β-E has been established as a novel preparation characterized by simple synthesis, high encapsulation efficiency (EE), good stability, enhanced bioavailability, and anti-tumor effects. The targeted delivery of β-ELE presents an effective strategy for bladder cancer treatment and offers a combined approach for managing this disease.
Nano Precipitation Method
The nano-precipitation technique, alternatively known as the solvent displacement approach, involves dissolving traditional Chinese medicinal extracts in a suitable solvent, followed by the rapid addition of a non-solvent under stirring conditions to precipitate the drug into nano-sized particles. Characterized by its straightforward and controllable preparation process, this method is adept at achieving high drug loading, particularly for the formulation of nano-drugs for drugs with poor water solubility. For example, Zeng YY et al have utilized precipitation microfiltration and microfiltration centrifugation technologies to successfully develop a nanomedicine targeting paclitaxel-resistant lung adenocarcinoma, effectively addressing the challenges associated with PTX resistance.37 They selected cabazitaxel and β-ELE, both of which exhibit significant drug resistance, and successfully formulated cabazitaxel liposomes, β-ELE liposomes, and flexible cabazitaxel-β-ELE complex liposomes. Through the application of precipitation microfiltration and microfiltration centrifugation, the research team assessed the encapsulation efficiency of cabazitaxel and β-ELE within these liposomes, with results indicating that the encapsulation efficiency exceeded 95%. The flexible cabazitaxel and β-ELE liposomes exhibited markedly enhanced efficacy in overcoming PTX resistance in lung adenocarcinoma. Notably, as a flexible composite liposome, the dosage of cabazitaxel can be reduced to 25% of the standard cabazitaxel injection dosage while still achieving comparable therapeutic effects. This significant finding suggests that β-ELE has the potential to partially substitute cabazitaxel, thereby decreasing the required dosage of cabazitaxel and mitigating the toxicity associated with related drugs. Rahimnia et al investigated the loading and coupling of CUR onto oleic acid (OA) and citric acid (CA) functionalized iron oxide nanoparticles, focusing on its potential to enhance MRI contrast.38 They synthesized magnetic iron oxide nanoparticles (Fe3O4, MNPs) using a co-precipitation method and assessed the cytotoxicity of the nanosystem (NS) via the MTT assay. The findings indicated that concentrations exceeding 80 μg/mL (CNS ≥ 80 μg/mL) could induce cancer cell death. Furthermore, Qianqian Mao et al have crafted a self-assembling nano-drug, termed LNT-UA, utilizing the natural bioactive constituents ursolic acid (UA) and lentinan (LNT), through the nano-precipitation method, eschewing the need for additional carriers. Within this nano-drug formulation, UA triggers immunogenic cell death (ICD), while LNT augments the maturation of dendritic cells (DCs) and induces a phenotypic shift in tumor-associated macrophages from a pro-tumoral M2 state to an anti-tumoral M1 phenotype, thereby enhancing the immunotherapeutic efficacy against colorectal cancer.39 Camptotheca acuminata Decne, a member of the Nyssaceae family and the Camptotheca genus, is a plant whose roots, fruits, bark, branches, and leaves are all harvested for their medicinal properties. Within the paradigm of TCM, this plant is recognized for its capabilities to combat cancer, clear heat, and expel parasites. An alkaloid extracted therefrom, camptothecin, is noted for its potent anticancer effects. However, the drug’s marked hydrophobicity may impede its encapsulation efficiency due to issues such as premature precipitation, which can result in rapid crystallization, suboptimal drug loading, and significant loss of active pharmaceutical ingredients, thus circumscribing its widespread clinical use.77 Zhang L et al adeptly employed a nano-precipitation technique to encapsulate 10-hydroxycamptothecin-10,20-diisobutyl dicarbonate (HCPT-1) within PCLLA-PEG-PCLLA triblock copolymer nanoparticles.40 This method was augmented by a suite of advanced analytical techniques, including dynamic light scattering (DLS), transmission electron microscopy (TEM), and atomic force microscopy (AFM), to meticulously characterize the nanoparticles. The in vitro release studies of these nanoparticles evinced a sustained release profile, modulated by the particle dimensions and the chemical composition of the copolymer. Furthermore, the biodistribution analysis in murine models indicated that HCPT-1, when encapsulated, exhibits an enhanced persistence in the circulatory system, with its tissue distribution being significantly influenced by the size of the nanoparticles. Collectively, these observations underscore the considerable potential and distinct advantages of PCLLA-PEG-PCLLA nanoparticles as a carrier system for the delivery of poorly soluble anticancer agents or their derivatives, heralding a significant advancement in the realm of nanomedicine.
Self Assembly Method
The self-assembly method primarily entails the spontaneous organization of drug molecules into ordered nanostructures through the utilization of interaction forces between them, including hydrogen bonding and van der Waals forces. The self-assembly method does not necessitate the input of external energy, and the resulting nanostructures exhibit high stability, which enables the realization of both slow and targeted drug release. A considerable number of active ingredients found in TCMs possess self-assembly properties. These include alkaloids, organic acids, flavonoids, terpenoids, polysaccharides, and proteins of natural plant origin.78 The self-assembly method can enhance the stability, solubility, and bioavailability of drugs, facilitating their formation into nanoparticles through the action of various noncovalent forces. For example, Weiwei Wang et al integrated targeted small molecules and NDDS with a regular structure and perfect targeting ability to develop a precursor drug self-assembled nanoplatform, 2-glucosamine-fluorescein-5(6)-isothiocyanate-glutamic acid-paclitaxel (2DA-FITC-PTX NPs) which have demonstrated excellent targeting ability, anticancer activity, and minimal side effects in vitro and in vivo.41 Furthermore, Zuo S et al reported a combinatorial strategy utilizing self-assembled engineered nanomedicine, PC ND, composed of PTX and CUR, for effective and safe chemotherapy in TNBC.42 Compared to free PTX and a simple PTX/Cur mixture, PC ND demonstrated higher treatment efficiency and improved prognosis, while the metastasis rate was significantly lower than that observed in the PTX or PTX/Cur mixture groups. Therefore, self-assembled engineered PC NDs may represent a promising nanomedicine for the chemotherapy of TNBC. Many active components in TCM possess self-assembling properties, enabling them to form nanoparticles through various non-covalent forces. These self-assembled nanoparticles also exist in herbal decoctions and are closely related to the therapeutic effects of the medicines.78 In the field of nanotechnology research, self-assembled nanoparticles are gaining popularity due to their simplicity, eco-friendliness, enhanced biodegradability, and biocompatibility, offering significant advantages over traditional nanofabrication methods. The self-assembly of active components in TCM exhibits antitumor effects or can be combined with other anticancer drugs, generating substantial interest in the field of cancer treatment. These natural small molecules (NSMs) contain modifiable groups, multiple action sites, hydrophobic side chains, and a rigid backbone with self-assembling characteristics. As a result, they can be utilized to construct self-assembled nanoparticles that offer superior therapeutic effects compared to their individual components. By developing self-assembled nano delivery systems, the strong hydrophobicity and poor in vivo stability of NSMs can be effectively overcome, significantly enhancing their bioavailability and strengthening their antitumor efficacy.79
Emulsification Evaporation Method
The herbal extracts were combined with suitable solvents and emulsifiers to create emulsions, which were then subjected to evaporation to produce nanoparticles. This process was employed to enhance the bioavailability, stability, and efficacy of the drugs in question. The emulsification and evaporation process allows for the rapid preparation of nanoemulsions with optimal oil-water compatibility, and the release rate of the drug can be precisely controlled by adjusting the emulsification conditions. This method is applicable to mixed formulations of oil-soluble and water-soluble drugs with hydrophobic drugs. Luo et al initially proposed Chinese yam polysaccharide (CYP) in PLGA via double emulsion solvent evaporation and subsequently investigated the optimal preparation process of CYP-PLGA nanoparticles (CYPP) using response surface methodology (RSM).43 The in vitro release experiments of CYP and CYPP demonstrated that the release rate of CYPP was 53%. The proliferation and flow cytometry results indicated that CYPP was more effective than free CYP and blank PLGA nanoparticles in promoting lymphocyte proliferation and conversion of T lymphocytes to Th cells. The proliferation and flow cytometry results demonstrated that CYPP was more effective in promoting lymphocyte proliferation and facilitating the conversion of T lymphocytes into Th cells in comparison to free CYP and blank PLGA nanoparticles. This evidence supports the assertion that yam polysaccharides also have considerable potential in anti-tumor immunotherapy.80 Furthermore, the early diagnosis of pancreatic cancer is of paramount importance to improve its prognosis. However, the clinical application of many diagnostic methods is limited by the lack of specificity and sensitivity.81 Lu et al developed a novel tumor-targeting molecular probe for pancreatic cancer imaging. They prepared a CKaAKN peptide-conjugated amphiphilic polymer (CKaAKN-PEG-PLGA) composed of poly(lactic acid-hydroxyacetic acid)-poly(ethylene glycol).44 A tumor-targeting delivery system for a magnetic resonance imaging (MRI) contrast agent, ultrasmall superparamagnetic iron oxide (USPIO), was prepared by emulsion solvent evaporation. This system consisted of USPIO polymeric magnetic nanoparticles (USPIO@CKaAKN-PEG-PLGA). The USPIO@CKaAKN-PEG-PLGA nanoparticles markedly enhanced the tumor specificity of protease inhibitors in CKaAKN-positive pancreatic cancer cells (BxPC-3). They are a promising candidate for MRI-enhanced early diagnosis of pancreatic cancer.
In addition to the aforementioned nanosynthesis methods, there are also electrospraying, emulsification polymerization, supercritical fluid technology, freeze-drying, and microfluidic technology. These diverse nanosynthesis techniques have been selected based on the unique composition and properties of TCM. Each method has been tailored to different nanomaterials in accordance with the specific characteristics of TCM, thereby facilitating controlled release, enhancing bioavailability and efficacy, and improving targeting. Nonetheless, the challenge of integrating various chemical compositions with different nanosynthesis methods presents both a difficulty and an opportunity for the development of novel dosage forms for TCM.
Conclusion
Nanotechnology offers a solution to many limitations of TCM, including its single administration method, low solubility, poor stability, short biological half-life, weak targeting effect, susceptibility to metabolism, and rapid elimination. This advancement significantly influences the modern development of TCM. The application of nanotechnology can substantially enhance bioavailability, cellular absorption, and the protection of active ingredients. Consequently, TCM nano-delivery systems hold great potential in this field.
Application of TCM Nanopreparations in Tumors
TCM plays an indispensable role in tumor treatment. Its multi-target effects and ability to regulate the body’s immune function significantly contribute to anti-tumor therapies. The integration of nanotechnology enhances these benefits in the realm of anti-tumor treatment by altering the natural properties of TCM at the nanoscale. For instance, while coarse realgar exhibits slight cytotoxicity against cancer cells, realgar nanoparticles with diameters ranging from 100 to 200 nanometers demonstrate markedly higher efficacy against cancer.82 This increase in cytotoxicity not only minimizes the toxicity of TCM to normal cells but also enhances the targeting and therapeutic effects of these treatments in anti-tumor applications. Consequently, TCM nanoformulations possess considerable potential in the fight against tumors. This article will explore how nanopreparations of TCM contribute to anti-tumor treatment through the distinct responses elicited during the treatment process (Figure 3).
 |
Figure 3 The different reactions caused by nano preparations of traditional Chinese medicine to exert anti-tumor effects. (A) Different traditional Chinese medicine nano-preparations induce ROS response and GSH response in different ways to achieve anti-tumor therapeutic effects. (B) AuNRs carrying curcumin release the drug in the high GSH concentration and acidic environment of tumors and induce immunity in the body Created in BioRender. Deng, G. (2025) https://BioRender.com/o98y508.. |
ROS Response
ROS response refers to the cellular reaction to reactive oxygen species, which are highly reactive molecules that include superoxide anions, hydrogen peroxide, and hydroxyl radicals. These molecules play significant roles in signaling and regulating cellular functions. However, an excess of ROS can result in oxidative stress, potentially leading to various diseases, including cancer, cardiovascular diseases, and neurodegenerative disorders. Numerous ingredients in TCM have demonstrated the ability to exert anti-tumor effects by modulating ROS levels. ① QUE, when combined with PTX, can significantly inhibit cell proliferation, enhance apoptosis, block the G2/M phase of the cell cycle, inhibit cell migration, induce endoplasmic reticulum stress, and increase the production of ROS.83 To address the challenge of MDR encountered during chemotherapy for ovarian cancer, Lu Q et al developed PTX-ATO-QUE nanoparticles (PAQNPs) utilizing a PLGA-PEG nanoplatform. These nanoparticles can encapsulate the mitochondrial oxidative phosphorylation (OXPHOS) inhibitor atovaquone (ATO), the glycolysis inhibitor QUE, and the chemotherapy agent PTX, thereby reversing MDR by inhibiting energy metabolism through multiple pathways.84 PAQNPs effectively lower intracellular ATP levels in tumor cells, and this energy depletion significantly inhibits cell proliferation, reduces P-glycoprotein (P-gp) activity (Drug efflux caused by P-gp reduces the intracellular accumulation of chemotherapy drugs.), and enhances the accumulation of PTX within the cells. Moreover, the increased accumulation of PTX correlates with elevated levels of intracellular reactive oxygen species (ROS), which subsequently leads to apoptosis in chemotherapy-resistant cells. Additionally, PAQNPs markedly inhibited tumor growth in A2780/paclitaxel tumor-bearing NCG mice. Immunohistochemical (IHC) analysis of tumor tissue revealed a reduction in P-gp expression, indicating that PAQNPs effectively reverse MDR in tumors by inducing energy depletion. ② The combined use of TCM and traditional chemotherapy drugs after nanotechnology can effectively increase ROS levels. Traditional chemotherapy drugs, such as CDDP, are not ideal for treating liver cancer due to poor response rates, significant toxicity and side effects, and pronounced drug resistance. Cheng et al found that CUR can enhance the anti-tumor effects of cisplatin.85 This enhancement is associated with ROS, with ROS levels positively correlating to the inhibition of cell proliferation. CUR improves the sensitivity of liver cancer to chemotherapy by regulating multiple signaling pathways. The authors employed an inverse microemulsion-film dispersion method to prepare CDDP/CUR co-loaded liposomes (CDDP/CUR-Lip), which effectively delivered and released both CDDP and CUR to HCC cells. This approach aims to address the unsatisfactory clinical outcomes associated with CDDP monotherapy. Furthermore, CDDP/CUR-Lip increased intracellular ROS levels during HCC cell treatment, presenting an attractive potential strategy for the synergistic treatment of liver cancer using CDDP and CUR. ③ Resveratrol (RSV) is an active ingredient in traditional Chinese medicine known for its anti-tumor effects, and it can also augment the efficacy of conventional chemotherapy drugs. Seyung S. Chung et al have demonstrated that the combined treatment of RSV and 5-FU enhances the anti-proliferative effects on colorectal cancer cells (HCT116 and DLD1), induces cell cycle arrest, and increases S-phase apoptosis.86 This combination inhibits pAkt and pSTAT3 signaling pathways, thereby reducing telomerase activity. The anti-tumor effects of the active ingredients in TCM can also be enhanced by elevating ROS levels. Yasin Öztürk et al validated the anti-tumor properties of RSV, demonstrating that it activates the TRPM2 channel in DBTRG glioblastoma cells.87 By increasing cellular ROS levels, RSV induces mitochondrial dysfunction and oxidative stress, thereby promoting paclitaxel-induced apoptosis. After nanonization, RSV exhibits therapeutic potential as an anticancer agent that induces ferroptosis. Ferroptosis, a novel form of programmed cell death characterized by iron-dependent lipid peroxidation, presents a promising strategy for cancer treatment. Zhang et al were the first to demonstrate that RSV, effectively inhibits the growth of colon cancer cells via the ROS-dependent ferroptosis pathway. Mechanistically, RSV promotes the accumulation of reactive oxygen species and lipid peroxidation in colorectal cancer cells, ultimately triggering ferroptosis.88 They developed a biomimetic nanocarrier by coating RSV-loaded poly(ε-caprolactone)-polyethylene glycol (PCL-PEG) nanoparticles with red blood cell membranes (designated as RSV-NPs@RBCm). This formulation offers the potential to evade macrophage phagocytosis and enhances circulation time. Furthermore, when conjugated with the tumor-penetrating peptide iRGD, RSV-NPs@RBCm can significantly improve tumor-specific tissue penetration and delivery. This study highlights that nano-preparations derived from TCM can effectively inhibit cancer cell growth through the ROS-dependent ferroptosis pathway, presenting a viable therapeutic modality. ④ Triptolide (TPL) is a naturally derived anti-cancer agent that can induce ROS responses through various modalities, including immunotherapy and PDT, to achieve anti-tumor therapeutic effects.89 Current cancer immunotherapies often fall short due to the low immunogenicity of tumor cells. To address this limitation, Wang et al constructed a bovine serum albumin-folic acid (BSA-FA) functionalized iron-containing metal-organic framework (TPL@TFBF), which induced ferroptosis and pyroptosis in tumor cells.90 This approach resulted in the release of a substantial number of damage-associated molecular patterns (DAMPs), thereby enhancing immunogenicity and triggering an effective systemic anti-tumor immune response. The TPL@TFBF demonstrated excellent efficacy against melanoma lung metastasis in vivo. The nanoparticles specifically target melanoma cells through FA modification, facilitating the release of TPL, Fe3+, and tannic acid (TA). TA reduces Fe3+ to Fe2+, thereby triggering the Fenton reaction and generating ROS. Additionally, TPL enhances intracellular ROS production by inhibiting the expression of nuclear factor erythroid 2-related factor 2 (Nrf2). This concurrent increase in intracellular ROS levels induces ferroptosis and pyroptosis in the cells, leading to the release of significant amounts of DAMPs. Consequently, this process stimulates antigen presentation by dendritic cells and promotes the proliferation of cytotoxic T lymphocytes (CD4+/CD8+ T cells), ultimately inhibiting tumor growth and lung metastasis.
In addition, TPL demonstrated enhanced antitumor effects when combined with the photosensitizer chlorine e6 (Ce6). Huang et al synthesized a pH/ROS dual-responsive mPEG-TK-PBAE copolymer, which incorporates pH-sensitive PBAE fragments and ROS-sensitive thione (TK) chains.91 Through the self-assembly process, TPL and Ce6 were successfully co-loaded into mPEG-TK-PBAE nanoparticles, which will hereafter be referred to as TPL/Ce6 NPs. Upon exposure to acidic pH and elevated levels of ROS, the payload within TPL/Ce6 NPs is released rapidly. Notably, the substantial amount of ROS generated by Ce6 upon laser irradiation further accelerates the degradation of the nanosystem, thereby enhancing the tumor microenvironment-responsive drug release and improving the anti-cancer effect. ROS-responsive nanomedicine carriers demonstrate considerable advantages in tumor treatment, including enhanced drug targeting, controlled drug release, and improved therapeutic efficacy. However, they also encounter challenges such as technical complexity, stability concerns, and potential toxicity. Future research should focus on optimizing the design of ROS-responsive nanomaterials while enhancing their stability and safety to facilitate better clinical applications.
GSH Response
GSH-responsive systems refer to drug delivery systems (DDS) or nanoformulations that are engineered to exploit the high concentrations of GSH found in TME. These systems are capable of responding to alterations in the redox status both inside and outside tumor cells. Tumor tissue exhibits distinct physiological characteristics in comparison to normal tissue, one of which is an imbalance in redox status, characterized by elevated levels of ROS and increased concentrations of GSH. This unique microenvironment can be leveraged to design responsive DDS that facilitate more precise drug release and enhance therapeutic efficacy. Therefore, in the process of cancer treatment, we can use the characteristics of the TME to design TCM nano-preparations. ① Xue Liu et al synthesized a liver-targeting nanocarrier material derived from angelica polysaccharide. The micelle preparation process was found to significantly inhibit glutathione (GSH) content while increasing ferroptosis in solid tumor selectively.92 ② GSH responses often coexist with ROS responses. Realgar is a significant mineral source of arsenic that exhibits greater biosafety compared to arsenic oxide and can effectively induce apoptosis in tumor cells.93,94 Yihan Wang et al developed a multifunctional nano-realgar hydrogel designed as an anti-tumor agent and a “sustainable ROS generator” to inhibit the conversion of glutathione disulfide (GSSG) to GSH.95 This inhibition reduces the concentration of GSH in tumor cells, leading to the accumulation of ROS, which ultimately enhances the effectiveness of radiotherapy. ③ Cinnamaldehyde (CA), extracted from the cinnamon plant, has been extensively studied as a potential chemotherapy agent for various cancers, including colon cancer,96 melanoma,97 and BC.98 Similarly, β-Phenylethyl isothiocyanate (PEITC), derived from cruciferous vegetables, exhibits significant anti-tumor effects against human osteosarcoma,99 ovarian cancer,100 and liver cancer.101 Qiuxing Liu et al synthesized a HA-CA/PEITC nanoparticle.102 In vitro and in vivo experimental results demonstrate that this nanoparticle effectively enhances oxidative stress in tumor cells by generating ROS and depleting GSH, ultimately resulting in remarkable anti-tumor effects. ④ At the same time, nanocarriers that are sensitive to GSH levels can also release the active ingredients of TCM on demand. Zhanxia Zhang et al developed a folic acid targeted delivery system and GSH responsive nanomedicine for the delivery of PTX aimed at treating lung cancer.103 Unlike other stimulus-responsive nanomedicines, this nanocarrier is not only sensitive to biorelevant GSH, allowing for on-demand drug release, but it also degrades into biocompatible by-products after completing its delivery task. Consequently, targeting the delivery of TCM ingredients through nano-delivery systems represents a viable anti-tumor strategy by regulating GSH concentration.
pH Response
Microenvironment-responsive DDS enable targeted drug release, reduce side effects, and enhance drug efficacy. The pH levels in diseased tissues, such as those affected by bacterial infections and inflammation in TME, differ from the physiological pH of 7.4.104 This discrepancy allows DDS to release encapsulated drugs specifically within these diseased tissues. Among the various types of DDS, pH-responsive systems have gained significant attention, leading to the development of multiple synthetic methods to create pH-sensitive DDS. These methods often involve the incorporation of pH-sensitive chemical bonds or protonated/deprotonated functional groups. Numerous nano-DDS have been investigated for their pH-responsive properties, including liposomes, micelles, hydrogels, dendrimers, and organic-inorganic hybrid nanoparticles, as well as micron-sized microspheres.105 After being nanosized, the active ingredients of TCM can be used as sensitizers to promote the absorption of chemotherapy drugs in acidic environments. Yun Wang et al reported a GSH detonated and pH-responsive gold nanorod nanocluster (AuNRs) designed to treat MCF-7/ADR cells in conjunction with the chemotherapeutic agent DOX and the pre-chemotherapy sensitizer polycurcumin.106 This system boasts advantages such as higher DOX loading capacity due to the nanoclusters and improved water solubility and stability at physiological pH compared to single AuNRs. When the intracellular GSH concentration reaches 5 mm, the individual AuNRs detonate, triggering the release of DOX at acidic pH levels (pH 6.8 or 5.0). This mechanism effectively enhances the cellular absorption of DOX, thereby achieving a superior anti-tumor effect.
Combination of TCM Nano Preparations with Various Treatment Methods (Figure 4)
 |
Figure 4 (A) Nanoparticles increase radiotherapy targeting. (B) Nanoparticles increase chemotherapy efficacy. (C) Nanoparticles activate body immunity. (D) Traditional Chinese medicine nanoparticles for adjuvant anti-tumor treatment. (E) Nano photosensitizers improve the targeting of cancer therapeutics. (F) The drug is targeted to kill cancer cells through hyperthermia and magnetic hyperthermia. Created in BioRender. Deng, G. (2025)https://BioRender.com/x31c617.. |
Traditional cancer treatments, including surgery, radiotherapy, chemotherapy, and immunotherapy, have notable limitations such as low targeting accuracy, significant side effects, considerable damage to healthy cells, and a risk of recurrence. In contrast, TCM has been extensively utilized in China for anti-tumor therapy. Recent years have seen the application of TCM compounds and active ingredients to control tumor growth, alleviate patient suffering, enhance quality of life, and prolong the lifespan of cancer patients. To address the limitations of conventional tumor treatments, TCM is frequently integrated with these methods through the use of nanotechnology. This integration can occur alongside radiotherapy and chemotherapy or serve as an adjunctive approach to bolster the immune response elicited by anti-cancer vaccines. Notably, in the immunosuppressive TME, TCM can exert anti-tumor effects by stimulating immune responses.107 Furthermore, TCM nanopreparations can enhance drug solubility, targeting, stability, and bioavailability, while simultaneously reducing side effects through nanotechnology. The synergistic application of TCM nanoformulations alongside traditional treatments in cancer therapy signifies the onset of a new era, marked by improved specificity, efficacy, and tolerability in cancer treatments.108
Radiotherapy
Radiation therapy is a method for treating cancer that employs powerful energy beams to destroy the genetic material of cancer cells. X-rays are commonly utilized in this treatment. However, radiation therapy lacks specificity, resulting in damage to both diseased and healthy cells. Despite efforts to minimize harm to healthy tissue during treatment, there are instances where whole-body radiation therapy is necessary. TCM is extensively utilized in China. The compounds and active ingredients derived from TCM have been employed to regulate tumor growth and, when combined with conventional radiotherapy treatments, can serve as sensitizers to amplify the effects of radiotherapy.109 Concurrently, the rapid advancement and application of emerging nanomaterials in the biomedical field offer promising opportunities to enhance the efficacy of radiotherapy and facilitate its development.110 Nanomaterials have garnered significant interest as radiosensitizers, which can amplify the radiation response and counteract radiation resistance. Numerous nanoparticles have been engineered to improve the deposition of radiation energy within cells. Tumors develop mechanisms to target ROS, thereby enhancing their sensitivity to radiotherapy.111 QUE has been shown to increase tumor radiosensitivity through various mechanisms. However, its poor localization within tumor tissue and low water solubility hinder its effectiveness in cancer treatment. To address these limitations, Chunyu Huang et al designed a novel drug delivery system comprising quercetin-loaded mesoporous silica nanoparticles (MSNs) encased in a cancer cell membrane (CM).112 This innovative nanoplatform exhibits a strong anti-cancer effect and favorable QUE loading characteristics under X-ray irradiation. QUE is specifically concentrated in drug delivery system, which, in conjunction with radiotherapy, extends blood circulation, enhances tumor targeting, and promotes apoptosis in tumor cells. This platform facilitates targeted drug delivery and radiotherapy sensitization, offering new insights into the integration of TCM nanopreparations with conventional radiotherapy treatments.
Chemotherapy
Chemotherapy, a long-established method for cancer treatment, involves the use of drugs to kill cancer cells or inhibit their growth and division. These drugs can be administered orally, via injection, through intravenous infusion, or implanted within the body. They typically circulate throughout the body via the bloodstream, allowing them to target cancer cells located in various regions. Chemotherapy drugs exert their effects on multiple stages of cell division, particularly during DNA replication and cell division. Because cancer cells generally divide more rapidly than normal cells, they are more vulnerable to the effects of these drugs. However, a significant challenge in chemotherapy is the incomplete delivery of the drug to the tumor, which can lead to serious side effects. The integration of TCM nanoformulations with chemotherapy drugs may represent a promising anti-tumor strategy, capable of inhibiting tumor growth, overcoming multidrug resistance, and minimizing the side effects associated with chemotherapy.113 In order to improve the therapeutic effect on tumors and overcome the limitations that TCM ingredients often have, such as low dissolution rate and poor bioavailability, these ingredients are formulated into nano-preparations and combined with chemotherapy to significantly improve treatment results. Realgar, historically regarded as a poison, is notably used as a first-line treatment for leukemia, a situation attributed to the development of realgar nano-preparations.114 As previously mentioned, Yihan Wang et al synthesized nano-realgar quantum dots (QDs) coupled with 6-aminonaphthalene (6-AN) molecules and incorporated them into a hyaluronic acid-coated, pH-sensitive dextran hydrogel carrier (DEX-HA gel) to enhance bioavailability, ultimately resulting in the formation of a multifunctional nano-realgar hydrogel (NRA@DH gel).95 This formulation effectively inhibits the proliferation and migration of tumor cells in tumor-bearing mice through the synergistic effects of chemotherapy and radiotherapy, thereby inhibiting tumor growth, improving movement coordination, and prolonging survival. The combination of chemotherapy with TCM nano-preparation represents an emerging strategy for cancer treatment, effectively addressing the challenges of realgar toxicity, low dissolution rate, and low bioavailability in anti-tumor therapy.115 The combination of TCM nano-preparations with chemotherapy drugs not only enhances therapeutic efficacy but also mitigates side effects. Furthermore, certain active ingredients found in TCM can induce alterations in the immunosuppressive state of the TME. Recent studies have indicated that the induction of ICD is associated with certain chemotherapy drugs, leading to increased interest in evaluating these drugs as potential ICD inducers. The ICD mechanism not only facilitates the direct killing of cancer cells but also induces anti-tumor immune responses against a broad spectrum of solid tumors116 Consequently, Dandan Sun et al developed a folic acid (FA)-targeted polyethylene glycol (PEG)-modified amphiphilic cyclic dipeptide nanoparticle (NP) for the co-delivery of ginsenoside Rg3 and QUE.117 They employed both in vitro and in vivo experimental methods to validate the potential of ginsenoside Rg3 as an ICD inducer in colorectal cancer cells. The resulting nanoformulation (CD-PEG-FA.Rg3.QTN) significantly prolonged blood circulation and enhanced tumor targeting in an orthotopic colorectal cancer mouse model, resulting in the transformation of the immunosuppressive TME.
Immunotherapy
The development of tumors is closely linked to the immune system of individuals, and immunotherapy has been established as an effective treatment for various types of cancer, widely adopted in clinical practice. A substantial number of TCMs are utilized in clinical anti-tumor applications, often in the form of compound recipes, with many ingredients demonstrating significant tumor-inhibitory effects.107 The combination of these two treatment modalities represents an innovative cancer treatment strategy that harnesses the benefits of modern medical immunotherapy alongside TCM. Immunotherapy targets tumor cells by activating or enhancing the patient’s own immune response. The anti-tumor immune activity can be further augmented by selectively inhibiting inhibitory pathways within the TME at different stages of tumor development. This specific objective can be achieved through the use of nanoscale drug delivery systems that are both specific and biocompatible. Moreover, nanoformulations incorporating TCM ingredients can offer additional anti-cancer properties, protecting immunotherapy agents from physiological degradation while ensuring their effective delivery to the target site, prolonging circulation time, and facilitating controlled release. This synergistic approach may enhance the immune response and improve therapeutic outcomes. Additionally, numerous TCM ingredients possess anti-inflammatory and antioxidant properties.118 When administered post-surgery, they can mitigate inflammatory responses, promote wound healing, enhance surgical success rates, shorten recovery times, and alleviate immunity-related side effects associated with immunotherapy. Certain TCM ingredients exhibit immunomodulatory effects and can synergistically enhance immunotherapy, thereby further activating or regulating the patient’s immune system to more effectively target tumor cells. Chen et al reported a multifunctional nanomodulator designed to counteract immunosuppressive agents in a targeted manner while enhancing the infiltration of tumor-infiltrating T cells.119 Their biocompatible nanocage, in conjunction with gemcitabine delivery therapy, mitigated the immune suppression associated with regulatory T cells and myeloid-derived suppressor cells, as well as the immunosuppressive effects of M2 macrophages. This strategy falls under immune checkpoint inhibition, a category of immunotherapy that functions by blocking specific proteins, such as PD-L1, on cancer cells that typically interact with PD-1 on T cells, a type of immune cell. This protein binding inhibits T cell activity.120 Immune checkpoint inhibitors can disrupt this binding, enabling T cells to attack cancer cells more effectively. TCM nanopreparations not only inhibit immunosuppressants but also have the potential to reshape the TME and enhance the effects of immunotherapy when combined with traditional chemotherapy drugs. DOX, a conventional chemotherapy agent, is known to induce ICD and is widely utilized in the treatment of HCC. However, its immunogenicity is relatively weak, and HCC often exhibits resistance to both chemotherapy and immunotherapy.121 In studies involving mouse Hepa1-6 and human Huh7 hCC cells, icariin was shown to induce autophagy and apoptosis, thereby facilitating ICD. The optimal molar ratio for combining ICD with DOX is 1:2, which can synergistically enhance ICD induction. Consequently, Yu et al employed a strategy utilizing the immunomodulatory drug icariin to improve the efficacy of ICD in HCC. They developed polylactic acid-glycolic acid (PLGA)-polyethylene glycol (PEG)-aminoethylanisamide (AEAA) nanoparticles for the targeted co-delivery of icaritin and DOX.122 This approach reshaped the immunosuppressive TME, triggered a robust immune memory response, and significantly improved the early anti-HCC effects in mouse liver cancer models. Their findings revealed the anti-HCC mechanism of icariin related to mitophagy and provided an effective immunotherapy strategy for HCC. Furthermore, nanoparticles are also associated with various mechanisms, including nanoparticle-mediated antigen presentation, modulation of cytotoxic T lymphocytes, enhancement of CD4+ cell responses, and regulation of tumor-associated immunosuppressive cells, all of which can be integrated into immunotherapy treatments.123
Adjuvant Therapy
Adjuvant treatment refers to a therapeutic approach that employs additional treatment modalities to enhance the primary treatment’s effectiveness, mitigate side effects, prolong survival, or improve the quality of life. This is particularly relevant in cancer treatment, where the complexity and multifactorial nature of the disease often render a single treatment method insufficient to achieve the desired therapeutic outcome. Adjuvant therapy enables the development of personalized treatment plans tailored to the specific stage of cancer and the individual patient’s conditions, thereby enhancing both treatment efficacy and quality of life. TCM is frequently utilized as an adjuvant treatment for cancer,109 and the integration of its active ingredients with modern nanotechnology has been shown to modify the immune microenvironment of tumors. Recent studies indicate that the combination of TCM with various cancer therapies can produce a synergistic effect.124 Dai L et al developed a novel type of “nano-ginseng” with a well-defined composition, specifically ginsenoside Rb1/protopanaxadiol nanoparticles (Rb1/PPD NPs), which is entirely derived from protopanaxadiol-type extracts.125 The optimized nanoformulation exhibits an appropriate size (~110 nm), high drug loading efficiency (~96.8%), substantial drug loading capacity (~27.9% wt %), and an extended residence time in systemic circulation, being nine times longer than free PPD. It demonstrates significant anti-tumor effects both in vitro and in vivo, shows high accumulation at tumor sites, and inflicts minimal damage to normal tissues. Notably, the production process for this “nano-ginseng” is characterized as simple, scalable, and environmentally friendly. Furthermore, Han S et al developed a nanoformulation using poly(D,L-lactic-co-glycolic acid) (PLGA)-based nanoparticles to co-encapsulate Plumbagin (PLB), DIH, and NH4HCO3 (a pH-sensitive adjuvant).126 This combination was utilized alongside immunotherapy in the treatment of liver cancer, confirming the role of PLB, a compound derived from Plumbago zeylanica L, as an inducer of ICD in HCC cells. The study illustrates that remodeling the immunosuppressive TME in liver cancer is a promising strategy. TCM nanopreparations have been employed as adjuvant treatments in various cancers, including lung cancer,109,127 colon cancer,39 and bladder cancer (colorectal cancer).128 These nanopreparations leverage the characteristics of TCM to enhance drug bioavailability, reduce side effects, and modulate immune responses, while also integrating personalized and comprehensive approaches to auxiliary treatment. The synergy between adjuvant therapy and TCM nanopreparations enhances anti-tumor and anti-cancer effects, alters the tumor immune microenvironment, and improves the efficacy of radiotherapy and overall treatment. This represents a promising therapeutic strategy in cancer management.
Photodynamic Therapy
PDT is a promising method for anti-cancer treatment. The principle of PDT involves the accumulation of photosensitizers, light activation, the generation of ROS, and subsequent cell death.129 This approach exhibits relatively rapid and precise tumor-killing properties.130,131 PDT employs a series of chemical reactions resulting from the interaction between photosensitizers, light irradiation, and oxygen to target tumors. Photosensitizers can be activated by laser irradiation to swiftly produce free ROS, alleviate the hypoxic environment, and ultimately induce tumor cell death.132,133 Beyond direct cytotoxic effects, PDT has the potential to stimulate a robust anti-tumor immune response.134–136 TCM nano-preparations can also demonstrate their advantages in conjunction with photodynamic therapy in several ways. Certain ingredients from TCM have been identified as possessing photosensitizing activities, making them suitable for use as photosensitizers in photodynamic therapy. For instance, compounds such as CUR137 and psoralen138 exhibit photosensitizing properties and can be effectively utilized in this therapeutic approach. Moreover, the combination of immunotherapy with TCM and photodynamic therapy represents an effective anti-tumor strategy. This combined therapy can significantly activate natural killer (NK) cells and inhibit the proliferation of tumor cells in vitro, while also promoting immune cell infiltration into tumors in vivo. Xiaoli Wu et al developed an immunotherapy combining the innate immune activator astragaloside III (As) and the PDT reagent chloride e6 (Ce6) ((As + Ce6)@MSN-PEG).139 This method is utilized in the treatment of colon cancer, where it not only inhibits tumor cell proliferation but also effectively targets the tumor site, induces immune cell infiltration, and enhances the cytotoxicity of natural killer cells and CD8+ T cells. As an effective and safe platform, this approach can be combined with nano-TCM (nano-TCM) and PDT to offer a novel treatment strategy for colon cancer. To further enhance the anti-tumor effect of PDT, TCM and photosensitizers are often used together and can exert a certain synergistic effect. Yu et al designed a photoactivated liposome (TP/Ce6-lp), which integrates the photosensitizers Ce6 and triptolide and leverages the controlled release properties of liposomes to synergistically treat HCC.140 The integration of TCM with photodynamic therapy offers novel strategies and methodologies for tumor treatment. By harnessing the biological activity and photosensitivity inherent in TCM, the efficacy of photodynamic therapy can be significantly enhanced, demonstrating considerable potential for clinical application.
Hyperthermia and Magnetic Hyperthermia
Hyperthermia is a technique that employs elevated temperatures to treat tumors, which can be achieved through either local or whole-body heating. The primary mechanism involves enhancing the effectiveness of other treatments, such as radiotherapy and chemotherapy, by raising the temperature in the tumor area while minimizing damage to surrounding normal tissues. TCM nano-preparations also show promise in hyperthermia, as they can improve drug targeting and efficacy to some extent. Parthenolide (PTL), a germacrane sesquiterpene lactone extracted from the “Yin” Chinese traditional herb feverfew, has garnered significant attention within TCM due to its cytotoxic effects on tumor cells and its various pharmacological properties. To address the challenges of low bioavailability, non-specific distribution, and uncontrolled release of PTL, Anshuo Li et al developed a dual-responsive PTL liposome-chitosan-gold nanoshell (PTL-Lips@CS@GNS) composite system.141 This innovative system harnesses the thermotherapeutic effects generated by the localized surface plasmon resonance of gold nanoshells in the near-infrared region to facilitate photothermal therapy, while simultaneously enhancing the availability of PTL by transitioning the liposome from a gel state to a liquid crystal state under specific conditions for controlled release. Furthermore, in the acidic tumor microenvironment, the composite system demonstrates pH-responsive release characteristics. In addition, there are methods of applying magnetic fields to tumor treatment, which are usually combined with hyperthermia and are called “magnetic field-assisted therapy” or “magnetic hyperthermia”. CUR is recognized as a promising chemotherapeutic agent due to its anticancer properties and favorable biosafety profile.142 However, to address its limitations of poor bioavailability and inadequate therapeutic efficacy, Xing H et al developed Janus magnetic mesoporous silica nanoparticles (Janus M-MSNs) designed for magnetic targeting and thermally enhanced CUR therapy for liver cancer.143 This approach integrates magnetic field and heat therapy, allowing for the targeted delivery of drugs to specific sites, thereby supporting magnetic heat therapy. CUR is incorporated into the silica component of Janus M-MSNs through surface-modified pH-sensitive groups. The Janus M-MSN-Cur exhibits excellent superparamagnetic characteristics, a high CUR loading capacity, and a tumor microenvironment-responsive release pattern for CUR. Furthermore, the application of an external magnetic field enhances the anti-tumor efficacy of Janus M-MSNs-Cur by increasing cellular internalization. Additionally, magnetic heat therapy synergistically augments the effects of chemotherapy. The combination of TCM nano-preparations and thermomagnetic therapy not only enhances the therapeutic efficiency of the drug in vitro and in vivo, but also demonstrates good biological safety.
Dilemma
Control of Quantity and Efficacy
Despite the growing awareness of the potential benefits of nanomedicine and nanodelivery systems, their actual impact on the healthcare system, particularly in cancer treatment and diagnosis, remains limited. This limitation can be attributed to the relatively short history of research in this field, which spans only 20 years. Moreover, TCM nano-preparations encounter various challenges, including the dose-effect relationship, the safety and stability of long-term use, and other factors that necessitate further investigation and validation. Among these issues, the precise control of dosage and efficacy in TCM is particularly crucial. In the absence of a unified and clear standard for assessing the dosage of nano-preparations, there is a risk that the toxic and side effects of nano-carriers or preparations may adversely affect human health. Arsenic trioxide is a primary component of a TCM known as arsenic. It serves as one of the first-line chemotherapy agents for the treatment of acute promyelocytic leukemia.144 Its anti-cancer effects on various human tumor diseases have been extensively studied. However, the clinical application of arsenic trioxide (ATO) in solid tumors remains limited due to significant systemic toxicity, low bioavailability, and rapid renal elimination prior to reaching target sites. Although not without challenges, efforts have been made to enhance the bioavailability of ATO in solid tumors without increasing the dosage. Research indicates that nanomedicines provide several advantages in drug delivery compared to conventional drugs, including improved bioavailability, efficacy, dose response, targeting capabilities, and safety.145 Thus, there is a critical need for preclinical and clinical studies on ATO, particularly those focused on nanosystems, which may offer cancer patients a new combination therapy that is efficient, highly bioavailable, and low in toxicity. Based on the above, we recognize controlling the dosage and efficacy of nanopreparations is particularly crucial. However, the complex composition of TCM, along with the challenges associated with extracting and purifying its active ingredients, directly impacts the quality and efficacy control of nano-preparations. This presents a significant dilemma for TCM nano-delivery systems. Furthermore, the application of nanotechnology may increase the risk of toxic side effects from drugs, especially when the behavior and metabolic mechanisms of nanoparticles within the body remain inadequately understood. In certain tissues, particularly in the liver and spleen, nanoparticles are degraded by lysosomes in mononuclear phagocyte system (MPS) cells, leading to undesirable drug release and subsequent tissue toxicity.146 Similar to the effects observed with TCM, long-term exposure to arsenic can lead to liver toxicity. Andrographolide (AG), a principal diterpene lactone derived from Andrographis paniculata, possesses a variety of physiological functions, including hepatoprotection. However, the therapeutic application of AG is significantly hindered by its insolubility, low bioavailability, and short plasma half-life. Das et al investigated the protective effects of polylactide-glycolide (PLGA) nanocapsule-encapsulated andrographolide (NA) against arsenic-induced liver injury in mice.147 NA, with an average particle size of 65.8 nm and an encapsulation efficiency of 64%, was formulated. Treatment with 40 mg/L sodium arsenite markedly elevated the serum levels of liver function indicators, such as AST, ALT, and ALP, in mice, resulting in arsenic accumulation in the liver and the production of ROS, although no lethality was observed over a 30-day period. These findings indicate that NA may offer protective benefits against arsenic-induced hepatotoxicity. Consequently, sometimes it is essential not only to regulate the dosage but also to mitigate drug toxicity by appropriately incorporating additional TCM nanoparticles. Currently, numerous challenges arise in the toxicological evaluation of nanocarriers.148 Furthermore, large-scale production of nano-preparations presents a significant issue that needs to be addressed, as it directly impacts production costs and the scope of market applications. Nanotechnology offers a promising avenue for the modernization of TCM. However, during the development and application processes, TCM nano-preparations inevitably face various difficulties and challenges. The control of dosage and efficacy of these nano-preparations necessitates further research and validation.
Difficulty in Achieving Compound Nano Preparations
In clinical practice, TCM compounds are a common pharmaceutical dosage form. However, ①These compounds are typically composed of various Chinese medicinal materials, each containing multiple active ingredients with intricate interactions. The absorption, distribution, metabolism, and excretion (ADME) processes of nanoparticles in the body, as well as the drug release mechanisms, remain unclear,149 making it challenging to control the stability and efficacy of nanoformulations, thereby complicating quality control. ②The active ingredients in TCM compounds must be extracted and purified while preserving their biological activity. However, the effective isolates obtained during separation and purification do not fully represent the ingredients present in the compound, nor do they adequately explain the mechanisms of action of these ingredients, which poses a significant challenge.78 ③Converting TCM compounds into nano-preparations requires advanced nanotechnology, including nanoparticle preparation, surface modification, drug loading efficiency, and release control. Compared to the active ingredients of TCM alone, these technologies present additional difficulties for TCM compounds. ④From the perspectives of pharmacokinetics and pharmacodynamics, the ADME processes of active ingredients in TCM compounds during anti-tumor treatment are complex. The diversity of ingredients in these compounds results in varied release and action mechanisms of nano-preparations within the body, which is highly unfavorable for tumor treatment. ⑤Different active ingredients in TCM compounds may exhibit varying levels of toxicity and side effects at the nanoscale. Additionally, the nanomaterials themselves may introduce new safety and toxicity concerns, necessitating extensive experimentation prior to clinical application. ⑥As a novel category of drug, TCM compound nano-preparations must undergo a rigorous regulatory approval process. However, the current regulatory framework may not be entirely suitable for such complex, multi-component nanoformulations, thereby complicating the development and marketing processes.
In summary, numerous challenges remain to be addressed in the anti-tumor treatment using TCM compound nanopreparations. However, the very existence of these challenges—including their complex composition, intricate preparation technologies, and unclear mechanisms of action—may enhance their efficacy. This suggests that TCM compound nanopreparations hold significant potential for future anti-tumor therapies. To overcome these obstacles, multidisciplinary collaboration and sustained investment in scientific research are essential.
Difficulty in Drug Combination and Co-Delivery
Active ingredients of TCM have been utilized in anti-tumor treatments for some time; however, drug delivery through nanosystems remains in the early stages of development. An in-depth exploration of the mechanisms of action of these active ingredients, in conjunction with traditional chemotherapy drugs within the body, is crucial for advancing anti-tumor treatment strategies. The complex mechanisms of action associated with TCM, along with potential toxicity issues arising from nanocarriers, have garnered increasing attention. Consequently, the combination and co-delivery systems of these drug classes face significant challenges. Firstly, determining the optimal ratio of active ingredients from TCM to traditional chemotherapy drugs is critical. Most existing research relies on in vitro cell experiments, making it challenging to ascertain the optimal ratio of these active ingredients post-nanosizing, which is a pivotal step toward their clinical application. Secondly, selecting an appropriate nanocarrier for encapsulating the active ingredients from TCM and traditional chemotherapy drugs is essential. An ideal nanocarrier should maintain the stability of the drug ratio while ensuring precise delivery to tumor tissue. Thirdly, the sequential and precise release of drugs within the body is another key factor influencing drug synergy. Although numerous studies have investigated the drug release behavior of nanodrug delivery systems using pH buffer models that simulate the in vivo microenvironment, these models often fail to accurately reflect the actual drug release conditions within the body.113 Finally, the potential toxicity and side effects of combining the active ingredients of TCM with chemotherapy drugs require substantial support from extensive clinical trials and toxicological experimental data. This presents a significant challenge for the development of TCM nano-preparations. Nevertheless, considerable progress has been made in the combination and co-delivery of these active ingredients with chemotherapy agents. As advancements in nanotechnology continue, an increasing variety of dosage forms are emerging, expanding the application scenarios for TCM and demonstrating broad prospects for its use.
Conclusion
TCM has a rich history of clinical application in China, with thousands of years of clinical practice contributing to its diagnostic foundations. Concurrently, research on nanopreparations has advanced significantly, achieving considerable results in just over two decades. The introduction of nanopreparations in TCM has established a novel drug delivery system that enhances drug solubility and stability, improves targeted delivery and controlled release, facilitates drug penetration through biological barriers, and enhances the multifunctionality of drugs. This innovation has substantially improved the efficacy and bioavailability of TCM in tumor treatment, allowing for safer, more effective, and stable clinical applications across various domains. However, despite the promising potential of TCM nanopreparations, further research is necessary to identify active ingredients with anti-tumor effects and to elucidate their mechanisms of action. In particular, the anti-tumor mechanisms of compound preparations and potential toxic reactions remain unclear,150 and some unprocessed or even processed TCMs retain significant toxicity, raising concerns about the safety of their clinical applications. These issues limit the market viability of TCM nanopreparations. Additionally, challenges such as large-scale production, cost, regulatory compliance, toxicity standards, market acceptance, and the complexities of research and development must be addressed for TCM nanopreparations to achieve widespread clinical use. This article presents an overview of the classification and synthesis of TCM nanopreparations, examining the various responses elicited by these nanopreparations during the treatment process and their role in anti-tumor therapies. It also explores the synergistic effects of TCM and conventional treatment methods in tumor management. These insights may offer potential solutions to the challenges faced by TCM nanopreparations in oncological applications. The integration of TCM with nanotechnology may represent a significant breakthrough in cancer treatment.
Acknowledgments
This work was supported by The “Academician Liang Liu Workstation” guidance project of Hunan University of Chinese Medicine in 2023, 23YS002. Hunan University of Traditional Chinese Medicine discipline construction “open list” project, Pharmacy A2 list project, 22JBZ021.
Disclosure
The authors declare no conflicts of interest.
References
1. Zhao Z, Li Y, Zhou L, et al. Prevention and treatment of COVID-19 using traditional Chinese medicine: a review. Phytomedicine. 2021;85:153308. doi:10.1016/j.phymed.2020.153308
2. Li Z, Feiyue Z, Gaofeng L. Traditional Chinese medicine and lung cancer--From theory to practice. Biomed Pharmacother. 2021;137:111381. doi:10.1016/j.biopha.2021.111381
3. Xu W, Li B, Xu M, Yang T, Hao X. Traditional Chinese medicine for precancerous lesions of gastric cancer: a review. Biomed Pharmacother. 2022;146:112542. doi:10.1016/j.biopha.2021.112542
4. Xiang Y, Guo Z, Zhu P, Chen J, Huang Y. Traditional Chinese medicine as a cancer treatment: modern perspectives of ancient but advanced science. Cancer Med. 2019;8(5):1958–1975. doi:10.1002/cam4.2108
5. Fu S, Li G, Zang W, Zhou X, Shi K, Zhai Y. Pure drug nano-assemblies: a facile carrier-free nanoplatform for efficient cancer therapy. Acta Pharm Sin B. 2022;12(1):92–106. doi:10.1016/j.apsb.2021.08.012
6. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. doi:10.1186/s12951-018-0392-8
7. Charlie-Silva I, Fraceto LF, de Melo N. Progress in nano-drug delivery of artemisinin and its derivatives: towards to use in immunomodulatory approaches. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S611–S620. doi:10.1080/21691401.2018.1505739
8. Wang Z, Wu J, Zhou Q, Wang Y, Chen T. Berberine nanosuspension enhances hypoglycemic efficacy on streptozotocin induced diabetic C57BL/6 mice. Evid Based Complement Alternat Med. 2015;2015:239749.
9. Briuglia ML, Rotella C, McFarlane A, Lamprou DA. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv Transl Res. 2015;5(3):231–242.
10. Wang H, Zheng Y, Sun Q, et al. Ginsenosides emerging as both bifunctional drugs and nanocarriers for enhanced antitumor therapies. J Nanobiotechnology. 2021;19(1):322. doi:10.1186/s12951-021-01062-5
11. Fu W, Liang Y, Xie Z, Wu H, Zhang Z, Lv H. Preparation and evaluation of lecithin/zein hybrid nanoparticles for the oral delivery of Panax notoginseng saponins. Eur J Pharm Sci. 2021;164:105882. doi:10.1016/j.ejps.2021.105882
12. Alven S, Aderibigbe BA. Nanoparticles formulations of artemisinin and derivatives as potential therapeutics for the treatment of cancer, leishmaniasis and malaria. Pharmaceutics. 2020;12(8):748. doi:10.3390/pharmaceutics12080748
13. Wan X, Yin Y, Zhou C, et al. Polysaccharides derived from Chinese medicinal herbs: a promising choice of vaccine adjuvants. Carbohydr Polym. 2022;276:118739. doi:10.1016/j.carbpol.2021.118739
14. Wang Y, Dou L, He H, Zhang Y, Shen Q. Multifunctional nanoparticles as nanocarrier for vincristine sulfate delivery to overcome tumor multidrug resistance. Mol Pharm. 2014;11(3):885–894. doi:10.1021/mp400547u
15. Cao M, Long M, Chen Q, et al. Development of β-elemene and cisplatin co-loaded liposomes for effective lung cancer therapy and evaluation in patient-derived tumor xenografts. Pharm Res. 2019;36(8):121. doi:10.1007/s11095-019-2656-x
16. Cao X, Deng WW, Fu M, et al. In vitro release and in vitro-in vivo correlation for silybin meglumine incorporated into hollow-type mesoporous silica nanoparticles. Int J Nanomed. 2012;7:753–762. doi:10.2147/IJN.S28348
17. Guo H, Liu Y, Li X, et al. Magnetic metal-organic framework-based nanoplatform with platelet membrane coating as a synergistic programmed cell death protein 1 inhibitor against hepatocellular carcinoma. ACS Nano. 2023;17(23):23829–23849. doi:10.1021/acsnano.3c07885
18. Cao M, Yan H, Han X, et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer. 2019;7(1):326. doi:10.1186/s40425-019-0817-4
19. Liao M, Qin M, Liu L, et al. Exosomal microRNA profiling revealed enhanced autophagy suppression and anti-tumor effects of a combination of compound Phyllanthus urinaria and lenvatinib in hepatocellular carcinoma. Phytomedicine. 2024;122:155091. doi:10.1016/j.phymed.2023.155091
20. Zheng F, Luo Y, Liu Y, Gao Y, Chen W, Wei K. Nano-baicalein facilitates chemotherapy in breast cancer by targeting tumor microenvironment. Int J Pharm. 2023;635:122778. doi:10.1016/j.ijpharm.2023.122778
21. Hassani N, Jafari-Gharabaghlou D, Dadashpour M, Zarghami N. The effect of dual bioactive compounds artemisinin and metformin co-loaded in PLGA-PEG nano-particles on breast cancer cell lines: potential apoptotic and anti-proliferative action. Appl Biochem Biotechnol. 2022;194(10):4930–4945. doi:10.1007/s12010-022-04000-9
22. Zhang X, Li N, Zhang G, et al. Nano strategies for artemisinin derivatives to enhance reverse efficiency of multidrug resistance in breast cancer. Curr Pharm Des. 2023;29(43):3458–3466. doi:10.2174/0113816128282248231205105408
23. Yan J, Wang Y, Zhang X, Liu S, Tian C, Wang H. Targeted nanomedicine for prostate cancer therapy: docetaxel and curcumin co-encapsulated lipid-polymer hybrid nanoparticles for the enhanced anti-tumor activity in vitro and in vivo. Drug Deliv. 2016;23(5):1757–1762. doi:10.3109/10717544.2015.1069423
24. Zou L, Wang D, Hu Y, et al. Drug resistance reversal in ovarian cancer cells of paclitaxel and borneol combination therapy mediated by PEG-PAMAM nanoparticles. Oncotarget. 2017;8(36):60453–60468. doi:10.18632/oncotarget.19728
25. Sun F, Shen H, Liu Q, et al. Powerful synergy of traditional Chinese medicine and aggregation-induced emission-active photosensitizer in photodynamic therapy. ACS Nano. 2023;17(19):18952–18964. doi:10.1021/acsnano.3c04342
26. Che JB, Liu ZH, Ma HB, et al. Influence of As2O3 combined with ginsenosides Rg3 on inhibition of lung cancer NCI-H1299 cells and on subsistence of nude mice bearing hepatoma. Asian Pac J Trop Med. 2014;7(10):772–775. doi:10.1016/S1995-7645(14)60134-6
27. Sun G, Sun K, Sun J. Combination prostate cancer therapy: prostate-specific membranes antigen targeted, pH-sensitive nanoparticles loaded with doxorubicin and tanshinone. Drug Deliv. 2021;28(1):1132–1140. doi:10.1080/10717544.2021.1931559
28. Zhang J, Li J, Shi Z, et al. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017;58:349–364. doi:10.1016/j.actbio.2017.04.029
29. Chen T, Chen H, Jiang Y, Yan Q, Zheng S, Wu M. Co-delivery of 5-fluorouracil and paclitaxel in mitochondria-targeted KLA-modified liposomes to improve triple-negative breast cancer treatment. Pharmaceuticals. 2022;15(7):881. doi:10.3390/ph15070881
30. Peñalva R, Morales J, González-Navarro CJ, et al. Increased oral bioavailability of resveratrol by its encapsulation in casein nanoparticles. Int J mol Sci. 2018;19(9):2816. doi:10.3390/ijms19092816
31. Baghdan E, Duse L, Schüer JJ, et al. Development of inhalable curcumin loaded nano-in-microparticles for bronchoscopic photodynamic therapy. Eur J Pharm Sci. 2019;132:63–71. doi:10.1016/j.ejps.2019.02.025
32. Ermakova NP, Bonartsev AP, Zernov AL, et al. Preclinical toxicity of paclitaxel biopolymer formulation. Anticancer Agents Med Chem. 2017;17(12):1661–1668. doi:10.2174/1871520616666160817104529
33. Li Z, Liu Y, Wang J, et al. Baicalin-berberine complex nanocrystals orally promote the co-absorption of two components. Drug Deliv Transl Res. 2022;12(12):3017–3028. doi:10.1007/s13346-022-01167-w
34. Zhang G, Zhang Q, Wang L, et al. Preparation and optimization of O/W emulsions stabilized by triglycerol monolaurate for curcumin encapsulation. Molecules. 2022;27(24):8861. doi:10.3390/molecules27248861
35. Zhai B, Wu Q, Wang W, et al. Preparation, characterization, pharmacokinetics and anticancer effects of PEGylated β-elemene liposomes. Cancer Biol Med. 2020;17(1):60–75. doi:10.20892/j.issn.2095-3941.2019.0156
36. Zhai B, Chen P, Wang W, et al. An ATF(24) peptide-functionalized β-elemene-nanostructured lipid carrier combined with cisplatin for bladder cancer treatment. Cancer Biol Med. 2020;17(3):676–692. doi:10.20892/j.issn.2095-3941.2020.0454
37. Zeng YY, Zeng YJ, Zhang NN, Li CX, Xie T, Zeng ZW. The preparation, determination of a flexible complex liposome co-loaded with cabazitaxel and β-elemene, and animal pharmacodynamics on paclitaxel-resistant lung adenocarcinoma. Molecules. 2019;24(9):1697. doi:10.3390/molecules24091697
38. Rahimnia R, Salehi Z, Shafiee Ardestani M, Doosthoseini H. SPION conjugated curcumin nano-imaging probe: synthesis and bio-physical evaluation. Iran J Pharm Res. 2019;18(1):183–197.
39. Mao Q, Min J, Zeng R, et al. Self-assembled traditional Chinese nanomedicine modulating tumor immunosuppressive microenvironment for colorectal cancer immunotherapy. Theranostics. 2022;12(14):6088–6105. doi:10.7150/thno.72509
40. Zhang L, Hu Y, Jiang X, Yang C, Lu W, Yang YH. Camptothecin derivative-loaded poly(caprolactone-co-lactide)-b-PEG-b-poly(caprolactone-co-lactide) nanoparticles and their biodistribution in mice. J Control Release. 2004;96(1):135–148. doi:10.1016/j.jconrel.2004.01.010
41. Wang W, Fan J, Zhu G, et al. Targeted prodrug-based self-assembled nanoparticles for cancer therapy. Int J Nanomed. 2020;15:2921–2933. doi:10.2147/IJN.S247443
42. Zuo S, Wang Z, An X, et al. Self-assembly engineering nanodrugs composed of paclitaxel and curcumin for the combined treatment of triple negative breast cancer. Front Bioeng Biotechnol. 2021;9:747637. doi:10.3389/fbioe.2021.747637
43. Luo L, Zheng S, Huang Y, et al. Preparation and characterization of Chinese yam polysaccharide PLGA nanoparticles and their immunological activity. Int J Pharm. 2016;511(1):140–150. doi:10.1016/j.ijpharm.2016.06.130
44. Lu L, Jie L, Zhou Y, et al. Preparation and characterization of PLGA-based magnetic polymer nanoparticles for targeting pancreatic adenocarcinoma. Curr Pharm Des. 2023;29(9):686–696. doi:10.2174/1381612829666230324091555
45. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. doi:10.3322/caac.21820
46. Sarisozen C, Dhokai S, Tsikudo EG, Luther E, Rachman IM, Torchilin VP. Nanomedicine based curcumin and doxorubicin combination treatment of glioblastoma with scFv-targeted micelles: in vitro evaluation on 2D and 3D tumor models. Eur J Pharm Biopharm. 2016;108:54–67. doi:10.1016/j.ejpb.2016.08.013
47. Liu Y, Feng N. Nanocarriers for the delivery of active ingredients and fractions extracted from natural products used in traditional Chinese medicine (TCM). Adv Colloid Interface Sci. 2015;221:60–76. doi:10.1016/j.cis.2015.04.006
48. Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–17015. doi:10.1021/acsnano.1c04996
49. Torchilin V. Antibody-modified liposomes for cancer chemotherapy. Expert Opin Drug Deliv. 2008;5(9):1003–1025. doi:10.1517/17425247.5.9.1003
50. Sun R, Dai J, Ling M, Yu L, Yu Z, Tang L. Delivery of triptolide: a combination of traditional Chinese medicine and nanomedicine. J Nanobiotechnology. 2022;20(1):194. doi:10.1186/s12951-022-01389-7
51. El-Samaligy MS, Afifi NN, Mahmoud EA. Evaluation of hybrid liposomes-encapsulated silymarin regarding physical stability and in vivo performance. Int J Pharm. 2006;319(1–2):121–129. doi:10.1016/j.ijpharm.2006.04.023
52. Agostoni V, Chalati T, Horcajada P, et al. Towards an improved anti-HIV activity of NRTI via metal-organic frameworks nanoparticles. Adv Healthc Mater. 2013;2(12):1630–1637. doi:10.1002/adhm.201200454
53. Li X, Porcel E, Menendez-Miranda M, et al. Highly porous hybrid metal-organic nanoparticles loaded with gemcitabine monophosphate: a multimodal approach to improve chemo- and radiotherapy. ChemMedChem. 2020;15(3):274–283. doi:10.1002/cmdc.201900596
54. He S, Wu L, Li X, et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm Sin B. 2021;11(8):2362–2395. doi:10.1016/j.apsb.2021.03.019
55. Witwer KW, Wolfram J. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat Rev Mater. 2021;6(2):103–106. doi:10.1038/s41578-020-00277-6
56. Walker S, Busatto S, Pham A, et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9(26):8001–8017. doi:10.7150/thno.37097
57. Liu J, Mao JJ, Wang XS, Lin H. Evaluation of traditional Chinese medicine herbs in oncology clinical trials. Cancer J. 2019;25(5):367–371. doi:10.1097/PPO.0000000000000404
58. Jiang H, Li M, Du K, et al. Traditional Chinese medicine for adjuvant treatment of breast cancer: Taohong Siwu decoction. Chin Med. 2021;16(1):129. doi:10.1186/s13020-021-00539-7
59. Zeng L, Yang K. Exploring the pharmacological mechanism of Yanghe Decoction on HER2-positive breast cancer by a network pharmacology approach. J Ethnopharmacol. 2017;199:68–85. doi:10.1016/j.jep.2017.01.045
60. Dai Y, Qiang W, Yu X, et al. Guizhi Fuling Decoction inhibiting the PI3K and MAPK pathways in breast cancer cells revealed by HTS(2) technology and systems pharmacology. Comput Struct Biotechnol J. 2020;18:1121–1136. doi:10.1016/j.csbj.2020.05.004
61. Zhang Y, Li PP. Shu-Gan-Liang-Xue Decoction, a Chinese herbal formula, down-regulates the expression of steroid sulfatase genes in human breast carcinoma MCF-7 cells. J Ethnopharmacol. 2010;127(3):620–624. doi:10.1016/j.jep.2009.12.014
62. Sun K, Wu L, Wang S, Deng W. Antitumor effects of Chinese herbal medicine compounds and their nano-formulations on regulating the immune system microenvironment. Front Oncol. 2022;12:949332. doi:10.3389/fonc.2022.949332
63. Yang X, Sun J, Wen B, et al. Biejiajian Pill Promotes the Infiltration of CD8(+) T cells in hepatocellular carcinoma by regulating the expression of CCL5. Front Pharmacol. 2021;12:771046. doi:10.3389/fphar.2021.771046
64. Rahmani AH, Almatroudi A, Khan AA, Babiker AY, Alanezi M, Allemailem KS. The multifaceted role of baicalein in cancer management through modulation of cell signalling pathways. Molecules. 2022;27(22):8023. doi:10.3390/molecules27228023
65. Wang S, Long S, Deng Z, Wu W. Positive role of Chinese herbal medicine in cancer immune regulation. Am J Chin Med. 2020;48(7):1577–1592. doi:10.1142/S0192415X20500780
66. Ma Z, Fan Y, Wu Y, et al. Traditional Chinese medicine-combination therapies utilizing nanotechnology-based targeted delivery systems: a new strategy for antitumor treatment. Int J Nanomed. 2019;14:2029–2053. doi:10.2147/IJN.S197889
67. Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;347(2):159–166. doi:10.1016/j.canlet.2014.03.013
68. Li S, Zhang B, Jiang D, Wei Y, Zhang N. Herb network construction and co-module analysis for uncovering the combination rule of traditional Chinese herbal formulae. BMC Bioinf. 2010;11 Suppl 11(Suppl 11):S6. doi:10.1186/1471-2105-11-S11-S6
69. Zhang W, Shi H, Chen C, et al. Curcumin enhances cisplatin sensitivity of human NSCLC cell lines through influencing Cu-Sp1-CTR1 regulatory loop. Phytomedicine. 2018;48:51–61. doi:10.1016/j.phymed.2018.04.058
70. Sinibaldi VJ, Carducci MA, Moore-Cooper S, Laufer M, Zahurak M, Eisenberger MA. Phase II evaluation of docetaxel plus one-day oral estramustine phosphate in the treatment of patients with androgen independent prostate carcinoma. Cancer. 2002;94(5):1457–1465. doi:10.1002/cncr.10350
71. Zhang Q, He S, Kuang G, et al. Morphology tunable and acid-sensitive dextran-doxorubicin conjugate assemblies for targeted cancer therapy. J Mater Chem B. 2020;8(31):6898–6904. doi:10.1039/D0TB00746C
72. Kuang G, Zhang Q, He S, Wu Y, Huang Y. Reduction-responsive disulfide linkage core-cross-linked polymeric micelles for site-specific drug delivery. Polymer ChemistryPolym Chem. 2020;11(44):7078–7086. doi:10.1039/D0PY00987C
73. Zhou J, Jiang YY, Chen H, Wu YC, Zhang L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020;53(2):e12739. doi:10.1111/cpr.12739
74. Guan Z, Chen J, Li X, Dong N. Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation. Biosci Rep. 2020;40(8):BSR20201807. doi:10.1042/BSR20201807
75. Gao Q, Feng J, Liu W, et al. Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Adv Drug Deliv Rev. 2022;188:114445. doi:10.1016/j.addr.2022.114445
76. Tang PY, Wang XC, Wang XH, et al. Application prospect of reaction engineering approach in studying drying behavior of single droplet during spray drying of traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi. 2023;48(22):6011–6020.
77. Koliqi R, Dimchevska S, Geskovski N, et al. PEO-PPO-PEO/Poly(DL-lactide-co-caprolactone) nanoparticles as carriers for SN-38: design, optimization and nano-bio interface interactions. Curr Drug Deliv. 2016;13(3):339–352. doi:10.2174/1567201813666151130221806
78. Huang J, Zhu Y, Xiao H, et al. Formation of a traditional Chinese medicine self-assembly nanostrategy and its application in cancer: a promising treatment. Chin Med. 2023;18(1):66. doi:10.1186/s13020-023-00764-2
79. Li Q, Lianghao Y, Shijie G, et al. Self-assembled nanodrug delivery systems for anti-cancer drugs from traditional Chinese medicine. Biomater Sci. 2024;12(7):1662–1692. doi:10.1039/D3BM01451G
80. Zhang Y, Jiao L, Wu Z, et al. Fabrication and characterization of Chinese yam polysaccharides PLGA nanoparticles stabilized Pickering emulsion as an efficient adjuvant. Int J Biol Macromol. 2022;209(Pt A):513–524. doi:10.1016/j.ijbiomac.2022.04.043
81. Thomas RM, Jobin C. Microbiota in pancreatic health and disease: the next frontier in microbiome research. Nat Rev Gastroenterol Hepatol. 2020;17(1):53–64. doi:10.1038/s41575-019-0242-7
82. Zhao W, Lu X, Yuan Y, et al. Effect of size and processing method on the cytotoxicity of realgar nanoparticles in cancer cell lines. Int J Nanomed. 2011;6:1569–1577. doi:10.2147/IJN.S21373
83. Zhang X, Huang J, Yu C, et al. Quercetin enhanced paclitaxel therapeutic effects towards PC-3 prostate cancer through ER stress induction and ROS production. Onco Targets Ther. 2020;13:513–523. doi:10.2147/OTT.S228453
84. Lu Q, Gao W, Chen Z, et al. Co-delivery of Paclitaxel/Atovaquone/Quercetin to regulate energy metabolism to reverse multidrug resistance in ovarian cancer by PLGA-PEG nanoparticles. Int J Pharm. 2024;655:124028. doi:10.1016/j.ijpharm.2024.124028
85. Cheng Y, Zhao P, Wu S, et al. Cisplatin and curcumin co-loaded nano-liposomes for the treatment of hepatocellular carcinoma. Int J Pharm. 2018;545(1–2):261–273. doi:10.1016/j.ijpharm.2018.05.007
86. Chung SS, Dutta P, Austin D, Wang P, Awad A, Vadgama JV. Combination of resveratrol and 5-flurouracil enhanced anti-telomerase activity and apoptosis by inhibiting STAT3 and Akt signaling pathways in human colorectal cancer cells. Oncotarget. 2018;9(68):32943–32957. doi:10.18632/oncotarget.25993
87. Öztürk Y, Günaydın C, Yalçın F, Nazıroğlu M, Braidy N. Resveratrol enhances apoptotic and oxidant effects of paclitaxel through TRPM2 channel activation in DBTRG glioblastoma cells. Oxid Med Cell Longev. 2019;2019:4619865. doi:10.1155/2019/4619865
88. Zhang Z, Ji Y, Hu N, et al. Ferroptosis-induced anticancer effect of resveratrol with a biomimetic nano-delivery system in colorectal cancer treatment. Asian J Pharm Sci. 2022;17(5):751–766. doi:10.1016/j.ajps.2022.07.006
89. Girdhani S, Bhosle SM, Thulsidas SA, Kumar A, Mishra KP. Potential of radiosensitizing agents in cancer chemo-radiotherapy. J Cancer Res Ther. 2005;1(3):129–131. doi:10.4103/0973-1482.19585
90. Wang S, Guo Q, Xu R, Lin P, Deng G, Xia X. Combination of ferroptosis and pyroptosis dual induction by triptolide nano-MOFs for immunotherapy of Melanoma. J Nanobiotechnology. 2023;21(1):383. doi:10.1186/s12951-023-02146-0
91. Huang Y, Wu S, Li J, et al. Self-amplified pH/ROS dual-responsive co-delivery nano-system with chemo-photodynamic combination therapy in hepatic carcinoma treatment. Int J Nanomed. 2024;19:3737–3751. doi:10.2147/IJN.S453199
92. Liu X, Wu Z, Guo C, et al. Hypoxia responsive nano-drug delivery system based on angelica polysaccharide for liver cancer therapy. Drug Deliv. 2022;29(1):138–148. doi:10.1080/10717544.2021.2021324
93. Wang T, Meng J, Wang C, et al. Inhibition of murine breast cancer metastases by hydrophilic As(4)S(4) nanoparticles is associated with decreased ROS and HIF-1α downregulation. Front Oncol. 2019;9:333. doi:10.3389/fonc.2019.00333
94. Zhu HH, Wu DP, Jin J, et al. Oral tetra-arsenic tetra-sulfide formula versus intravenous arsenic trioxide as first-line treatment of acute promyelocytic leukemia: a multicenter randomized controlled trial. J Clin Oncol. 2013;31(33):4215–4221. doi:10.1200/JCO.2013.48.8312
95. Wang Y, Wei Y, Wu Y, et al. Multifunctional nano-realgar hydrogel for enhanced glioblastoma synergistic chemotherapy and radiotherapy: a new paradigm of an old drug. Int J Nanomed. 2023;18:743–763. doi:10.2147/IJN.S394377
96. Zhao C, Cao W, Zheng H, et al. Acid-responsive nanoparticles as a novel oxidative stress-inducing anticancer therapeutic agent for colon cancer. Int J Nanomed. 2019;14:1597–1618. doi:10.2147/IJN.S189923
97. Zhang W, Gao J, Cheng C, et al. Cinnamaldehyde enhances antimelanoma activity through covalently binding ENO1 and exhibits a promoting effect with dacarbazine. Cancers. 2020;12(2):311. doi:10.3390/cancers12020311
98. Yu W, He X, Yang Z, et al. Sequentially responsive biomimetic nanoparticles with optimal size in combination with checkpoint blockade for cascade synergetic treatment of breast cancer and lung metastasis. Biomaterials. 2019;217:119309. doi:10.1016/j.biomaterials.2019.119309
99. Lv H, Zhen C, Liu J, Shang P. β-phenethyl isothiocyanate induces cell death in human osteosarcoma through altering iron metabolism, disturbing the redox balance, and activating the MAPK signaling pathway. Oxid Med Cell Longev. 2020;2020:5021983. doi:10.1155/2020/5021983
100. Hong YH, Uddin MH, Jo U, et al. ROS accumulation by PEITC selectively kills ovarian cancer cells via UPR-mediated apoptosis. Front Oncol. 2015;5:167. doi:10.3389/fonc.2015.00167
101. Rose P, Armstrong JS, Chua YL, Ong CN, Whiteman M. Beta-phenylethyl isothiocyanate mediated apoptosis; contribution of Bax and the mitochondrial death pathway. Int J Biochem Cell Biol. 2005;37(1):100–119. doi:10.1016/j.biocel.2004.05.018
102. Liu Q, Ding X, Xu X, et al. Tumor-targeted hyaluronic acid-based oxidative stress nanoamplifier with ROS generation and GSH depletion for antitumor therapy. Int J Biol Macromol. 2022;207:771–783. doi:10.1016/j.ijbiomac.2022.03.139
103. Yao W, Yao J, Qian F, et al. Paclitaxel-loaded and folic acid-modified PLGA nanomedicine with glutathione response for the treatment of lung cancer. Acta Biochim Biophys Sin. 2021;53(8):1027–1036. doi:10.1093/abbs/gmab073
104. Yang L, Li A, Wang Y, Zhang Y. Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy. Signal Transduct Target Ther. 2023;8(1):35. doi:10.1038/s41392-022-01304-4
105. Ding H, Tan P, Fu S, et al. Preparation and application of pH-responsive drug delivery systems. J Control Release. 2022;348:206–238. doi:10.1016/j.jconrel.2022.05.056
106. Wang Y, Wang F, Liu Y, et al. Glutathione detonated and pH responsive nano-clusters of Au nanorods with a high dose of DOX for treatment of multidrug resistant cancer. Acta Biomater. 2018;75:334–345. doi:10.1016/j.actbio.2018.06.012
107. Zhang Y, Lou Y, Wang J, Yu C, Shen W. Research status and molecular mechanism of the traditional Chinese medicine and antitumor therapy combined strategy based on tumor microenvironment. Front Immunol. 2020;11:609705. doi:10.3389/fimmu.2020.609705
108. Xu M, Han X, Xiong H, et al. Cancer nanomedicine: emerging strategies and therapeutic potentials. Molecules. 2023;28(13):5145.
109. Zhang X, Qiu H, Li C, Cai P, Qi F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci Trends. 2021;15(5):283–298. doi:10.5582/bst.2021.01318
110. Song X, Sun Z, Li L, Zhou L, Yuan S. Application of nanomedicine in radiotherapy sensitization. Front Oncol. 2023;13:1088878. doi:10.3389/fonc.2023.1088878
111. Wen S, Ovais M, Li X, et al. Tailoring bismuth-based nanoparticles for enhanced radiosensitivity in cancer therapy. Nanoscale. 2022;14(23):8245–8254. doi:10.1039/D2NR01500E
112. Huang C, Chen T, Zhu D, Huang Q. Enhanced tumor targeting and radiotherapy by quercetin loaded biomimetic nanoparticles. Front Chem. 2020;8:225. doi:10.3389/fchem.2020.00225
113. Li B, Shao H, Gao L, Li H, Sheng H, Zhu L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: a review. Drug Deliv. 2022;29(1):2130–2161. doi:10.1080/10717544.2022.2094498
114. Dilda PJ, Hogg PJ. Arsenical-based cancer drugs. Cancer Treat Rev. 2007;33(6):542–564. doi:10.1016/j.ctrv.2007.05.001
115. Wu J, Shao Y, Liu J, Chen G, Ho PC. The medicinal use of realgar (As₄S₄) and its recent development as an anticancer agent. J Ethnopharmacol. 2011;135(3):595–602. doi:10.1016/j.jep.2011.03.071
116. Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed Engl. 2019;58(3):670–680. doi:10.1002/anie.201804882
117. Sun D, Zou Y, Song L, et al. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm Sin B. 2022;12(1):378–393. doi:10.1016/j.apsb.2021.06.005
118. Zhang L, Wei W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol Ther. 2020;207:107452. doi:10.1016/j.pharmthera.2019.107452
119. Chen C, Li A, Sun P, et al. Efficiently restoring the tumoricidal immunity against resistant malignancies via an immune nanomodulator. J Control Release. 2020;324:574–585. doi:10.1016/j.jconrel.2020.05.039
120. Tang Q, Chen Y, Li X, et al. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. 2022;13:964442. doi:10.3389/fimmu.2022.964442
121. Xian L, Zhao P, Chen X, et al. Heterogeneity, inherent and acquired drug resistance in patient-derived organoid models of primary liver cancer. Cell Oncol Dordr. 2022;45(5):1019–1036. doi:10.1007/s13402-022-00707-3
122. Yu Z, Guo J, Hu M, Gao Y, Huang L. Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma. ACS Nano. 2020;14(4):4816–4828. doi:10.1021/acsnano.0c00708
123. Bai Y, Wang Y, Zhang X, et al. Potential applications of nanoparticles for tumor microenvironment remodeling to ameliorate cancer immunotherapy. Int J Pharm. 2019;570:118636. doi:10.1016/j.ijpharm.2019.118636
124. Zheng H, Wang G, Liu M, Cheng H. Traditional Chinese medicine inhibits PD-1/PD-L1 axis to sensitize cancer immunotherapy: a literature review. Front Oncol. 2023;13:1168226. doi:10.3389/fonc.2023.1168226
125. Dai L, Zhu W, Si C, Lei J. ”nano-ginseng” for enhanced cytotoxicity against cancer cells. Int J mol Sci. 2018;19(2):627. doi:10.3390/ijms19020627
126. Han S, Bi S, Guo T, et al. Nano co-delivery of Plumbagin and Dihydrotanshinone I reverses immunosuppressive TME of liver cancer. J Control Release. 2022;348:250–263. doi:10.1016/j.jconrel.2022.05.057
127. Su XL, Wang JW, Che H, et al. Clinical application and mechanism of traditional Chinese medicine in treatment of lung cancer. Chin Med J. 2020;133(24):2987–2997. doi:10.1097/CM9.0000000000001141
128. Li F, Zheng Z, Chen W, et al. Regulation of cisplatin resistance in bladder cancer by epigenetic mechanisms. Drug Resist Updat. 2023;68:100938. doi:10.1016/j.drup.2023.100938
129. Grimm JB, Tkachuk AN, Xie L, et al. A general method to optimize and functionalize red-shifted rhodamine dyes. Nat Methods. 2020;17(8):815–821. doi:10.1038/s41592-020-0909-6
130. Zhou TJ, Xing L, Fan YT, Cui PF, Jiang HL. Light triggered oxygen-affording engines for repeated hypoxia-resistant photodynamic therapy. J Control Release. 2019;307:44–54. doi:10.1016/j.jconrel.2019.06.016
131. Shi X, Zhang CY, Gao J, Wang Z. Recent advances in photodynamic therapy for cancer and infectious diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(5):e1560. doi:10.1002/wnan.1560
132. Guo Z, Zhou X, Hou C, et al. A chloroplast-inspired nanoplatform for targeting cancer and synergistic photodynamic/photothermal therapy. Biomater Sci. 2019;7(9):3886–3897. doi:10.1039/C9BM00762H
133. Wang M, Zhai Y, Ye H, et al. High co-loading capacity and stimuli-responsive release based on cascade reaction of self-destructive polymer for improved chemo-photodynamic therapy. ACS Nano. 2019;13(6):7010–7023. doi:10.1021/acsnano.9b02096
134. Hwang HS, Shin H, Han J, Na K. Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J Pharm Investig. 2018;48(2):143–151. doi:10.1007/s40005-017-0377-x
135. Duan X, Chan C, Guo N, Han W, Weichselbaum RR, Lin W. Photodynamic therapy mediated by nontoxic core-shell nanoparticles synergizes with immune checkpoint blockade to elicit antitumor immunity and antimetastatic effect on breast cancer. J Am Chem Soc. 2016;138(51):16686–16695. doi:10.1021/jacs.6b09538
136. Yang G, Xu L, Chao Y, et al. Hollow MnO(2) as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun. 2017;8(1):902. doi:10.1038/s41467-017-01050-0
137. Ailioaie LM, Ailioaie C, Litscher G. Latest innovations and nanotechnologies with curcumin as a nature-inspired photosensitizer applied in the photodynamic therapy of cancer. Pharmaceutics. 2021;13(10):1562. doi:10.3390/pharmaceutics13101562
138. Aekrungrueangkit C, Wangngae S, Kamkaew A, et al. Novel psoralen derivatives as anti-breast cancer agents and their light-activated cytotoxicity against HER2 positive breast cancer cells. Sci Rep. 2022;12(1):13487. doi:10.1038/s41598-022-17625-x
139. Wu X, Yang H, Chen X, et al. Nano-herb medicine and PDT induced synergistic immunotherapy for colon cancer treatment. Biomaterials. 2021;269:120654. doi:10.1016/j.biomaterials.2021.120654
140. Yu L, Wang Z, Mo Z, et al. Synergetic delivery of triptolide and Ce6 with light-activatable liposomes for efficient hepatocellular carcinoma therapy. Acta Pharm Sin B. 2021;11(7):2004–2015. doi:10.1016/j.apsb.2021.02.001
141. Li A, Gao W, Zhang X, et al. A dual-responsive “Yin-Yang” photothermal delivery system to accelerate Parthenolide anti-tumor efficacy. Biomater Adv. 2022;138:212935. doi:10.1016/j.bioadv.2022.212935
142. Tomeh MA, Hadianamrei R, Zhao X. A review of curcumin and its derivatives as anticancer agents. Int J mol Sci. 2019;20(5):1033. doi:10.3390/ijms20051033
143. Xing H, Wang Z, Shao D, et al. Janus nanocarriers for magnetically targeted and hyperthermia-enhanced curcumin therapy of liver cancer. RSC Adv. 2018;8(53):30448–30454. doi:10.1039/C8RA05694C
144. Kutny MA, Alonzo TA, Abla O, et al. Assessment of arsenic trioxide and all-trans retinoic acid for the treatment of pediatric acute promyelocytic leukemia: a report from the children’s oncology group AAML1331 trial. JAMA Oncol. 2022;8(1):79–87. doi:10.1001/jamaoncol.2021.5206
145. Yu M, Zhang Y, Fang M, Jehan S, Zhou W. Current advances of nanomedicines delivering arsenic trioxide for enhanced tumor therapy. Pharmaceutics. 2022;14(4):743. doi:10.3390/pharmaceutics14040743
146. Donahue ND, Acar H, Wilhelm S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv Drug Deliv Rev. 2019;143:68–96. doi:10.1016/j.addr.2019.04.008
147. Das S, Pradhan GK, Das S, Nath D, Das Saha K. Enhanced protective activity of nano formulated andrographolide against arsenic induced liver damage. Chem Biol Interact. 2015;242:281–289. doi:10.1016/j.cbi.2015.10.011
148. Ahmad A, Imran M, Sharma N. Precision nanotoxicology in drug development: current trends and challenges in safety and toxicity implications of customized multifunctional nanocarriers for drug-delivery applications. Pharmaceutics. 2022;14(11):2463. doi:10.3390/pharmaceutics14112463
149. Zhang A, Meng K, Liu Y, et al. Absorption, distribution, metabolism, and excretion of nanocarriers in vivo and their influences. Adv Colloid Interface Sci. 2020;284:102261. doi:10.1016/j.cis.2020.102261
150. Liu R, Li X, Huang N, Fan M, Sun R. Toxicity of traditional Chinese medicine herbal and mineral products. Adv Pharmacol. 2020;87:301–346.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

