Back to Journals » Infection and Drug Resistance » Volume 18
Helicobacter Pylori Infection as an Independent Risk Factor for Kidney Stone Formation in China: A Cross-Sectional Study
Authors Gong R , Ling Y, Wang S, Wang W, Meng L, Zeng J, Lin R, Xu S
Received 11 March 2025
Accepted for publication 20 May 2025
Published 5 June 2025 Volume 2025:18 Pages 2881—2888
DOI https://doi.org/10.2147/IDR.S523335
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Rui Gong,1,* Yan Ling,1,* Shi Wang,2,* Weijun Wang,3 Lingjun Meng,3 Junchao Zeng,1 Rong Lin,3 Sanping Xu1
1Health Management Center Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 2Department of Thyroid and Breast, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 3Department of Gastroenterology, Union Hospital, Tongji Medical college, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Rong Lin, Department of Gastroenterology, Union Hospital, Tongji Medical college, 1277 Jiefang Avenue, Jianghan District, Wuhan, Hubei Province, 430022, People’s Republic of China, Email [email protected] Sanping Xu, Health Management Center Union Hospital, Tongji Medical college, Huazhong University of Science and Technology, 1277 Jiefang Avenue, Jianghan District, Wuhan, Hubei Province, 430022, People’s Republic of China, Email [email protected]
Introduction: This cross-sectional study aimed to explore the relationship between Helicobacter pylori (Hp) infection and kidney stone formation in China, given the high incidence of Hp infection and its potential to cause damage to multiple systems.
Methods: Conducted at the Department of Health Management of Wuhan Union Hospital, the study included 48,294 hp-negative and 28,455 hp-positive individuals. Hp infection was detected using the 13C urea breath test (13C-UBT), and kidney stones were identified via urinary Doppler ultrasonography.
Results: Results showed that Hp-negative (Hp-) individuals had higher levels of blood urea nitrogen (BUN), serum creatinine (Scr), and uric acid compared to Hp-positive (Hp+) individuals, while Hp- patients had lower urine pH (P < 0.001). The prevalence of kidney stones was significantly higher in the Hp+ group. Univariate and multivariate regression analyses indicated that Hp infection is an independent risk factor for kidney stones (OR: 1.275, 95% CI: 1.219– 1.333, P < 0.001) after adjusting for confounding factors such as age, body mass index (BMI), blood pressure, and lipid profiles.
Conclusion: In conclusion, Helicobacter pylori infection is an independent risk factor for kidney stone development in China.
Keywords: Helicobacter pylori, kidney stone, urine acid, urine pH, risk factor
Introduction
Kidney stones may cause urinary tract obstruction, infection, and damage to renal function, which may lead to irreversible organ damage in some severe cases. In the past 40 years, the incidence of kidney stones in Europe, America, and parts of Southeast Asia has significantly increased.1 Similarly, a meta-analysis reported a significant increase in the incidence of kidney stones in China, from 5.85% in the 1990s to 10.86% in the 2010s.2 The treatment of kidney stones costs a lot, which brings a heavy burden to the health care system every year.3 Therefore, it is necessary to further study the risk factors of the development of kidney stones, so as to provide new strategies for the primary prevention of kidney stones.
The etiology of kidney stones is very complex and multifactorial.4 It has been shown that the formation of kidney stones is affected by many factors, such as genetic, dietary, environmental factors and so on.5 In recent years, studies have shown that gastrointestinal flora play a potential role in stone formation.6 Specifically, certain bacterial infections and alterations in the gut microbiome can influence the metabolic pathways involved in stone formation.
In addition to Helicobacter pylori (Hp), other bacterial infections have also been implicated in the formation of kidney stones. For example, urinary tract infections (UTIs) caused by bacteria such as Escherichia coli and Proteus mirabilis can lead to the formation of struvite stones, also known as “infection stones”.7 These bacteria produce urease, an enzyme that hydrolyzes urea to produce ammonia and carbonate, leading to an increase in urine pH and the precipitation of magnesium ammonium phosphate (struvite) crystals.8 Gastric factors, including Hp infection, can also influence kidney stone formation through several mechanisms. Hp infection has been shown to induce systemic inflammation and metabolic changes, which may contribute to the development of kidney stones.9 For instance, Hp infection can lead to alterations in lipid metabolism, resulting in hyperlipidemia, which is a known risk factor for kidney stone formation10 Additionally, Hp infection can cause changes in gastric acid secretion, which may indirectly affect the urinary excretion of stone-forming substances.11 When the injury invaded the urinary system, a kidney stone formed.
Since Hp was first isolated from the gastric mucosa of patients with chronic gastritis and peptic ulcer in 1982, it has been demonstrated that Hp infection is not only associated with gastric diseases but can also lead to damage to multiple systems.12 Specifically, Hp infection has been identified as a potential factor associated with stone formation,13 although the specific mechanism remains unclear. Studies have shown that the incidence rate of gallstones in Hp positive patients is significantly higher than that in Hp negative patients; however, after Hp eradication, the incidence rate of gallstones inclined.14 However, studies on the correlation between Hp infection and kidney stones are rare. Only one retrospective study with a small sample size showed that the incidence of kidney stones was higher in Hp+ patients.9 No cross-sectional study has been conducted on the association between Hp infection and kidney stones. Therefore, this study aimed to explore the relationship between Hp infection and kidney stones.
Methods
Study Population
This cross-sectional study included healthy adults who underwent comprehensive physical examinations at the Health Management Center of Wuhan Union Hospital between January 2015 and December 2019. Participants who underwent renal ultrasound (US) for the detection of kidney stones and 13C urea breath test (13C-UBT) for the detection of H. pylori infection were included (n = 122,763). Patients who had undergone nephrectomy and those with congenital renal dysplasia were excluded. Additionally, patients with a history of recent Hp treatment (within the past 6 months) were also excluded to avoid potential confounding effects of prior medication on the current Hp infection status. Duplicate cases were excluded from this study. Finally, 76749 patients were included in the study, included 48294 hp+ and 28455 hp- patients.
Data Collection
Clinical laboratory tests, including measurements of sex, age, body mass index (BMI), blood pressure (BP), fasting blood glucose (FBG), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), total cholesterol (TC), triacylglycerol (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGT), blood urea nitrogen (BUN), serum creatinine (Scr), uric acid, urine pH were performed and the results were collected as baseline data.
The Diagnose of Hp Infection and Kidney Stones
13C-UBT was used to detect Hp infection. The urinary doppler ultrasound was applicated to detect kidney stones.
Statistical Analysis
Spss26.0 software was used for statistical analysis. Continuous variables are expressed as mean ±SD. The T test was used to compare continuous variables between the two groups. The chi-square test was used to analyze categorical variables. Multivariate regression analysis was used to explore the relationship between the Hp levels and kidney stones. P < 0.05.
Result
The Characteristics of the Subjects
A total of 76749 subjects which including 45,862 men (59.8%) and 30,887 (40.2%) women with a mean age of 45.30±12.88 years old comprised the primary population (Table 1). The incidence of kidney stones was 12.1%(n = 9280). All individuals were divided into two groups according to Hp infection status. A total of 48,294 individuals were Hp+ whereas 28,455 were Hp-. The proportion of hypertension patients and the FBG in Hp+ group was significantly higher than that in Hp- group, and compared with Hp - group, the Hp+ group had a more adverse lipid profiles, such as higher levels of TC (P < 0.001), TG (P < 0.001) and LDL-C (P < 0.001), and lower levels of HDL-C (P < 0.001). Furthermore, the levels of blood urea nitrogen (BUN) and serum creatinine concentration (Scr) were higher in Hp-negative (Hp-) individuals compared to Hp-positive (Hp+) individuals. In contrast, Hp+ individuals had higher levels of uric acid and lower urine pH compared to Hp- individuals (P < 0.001).
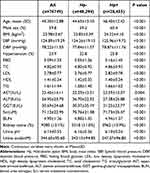 |
Table 1 The Baseline of the Patients |
Specifically, the mean urine pH was significantly lower in Hp+ patients compared to Hp- patients (P < 0.001). Additionally, the mean uric acid level was significantly higher in Hp+ patients compared to Hp- patients (P < 0.001).
Continuous variables are shown as the mean±SD. Hp, Helicobacter pylori; BMI, body mass index; SBP, Systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; TC, total cholesterol; TG, triacylglycerol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase; BUN, blood urea nitrogen; Scr, serum creatinine concentration.
The Incidence Rate of Kidney Stones Was Significantly Higher in Hp+ Individuals
The prevalence of kidney stones according to different Hp infection statuses is shown in Figure 1. The incidence of kidney stones in Hp+ group was 13.9%, which was significantly higher than that in the control group (11.0%; P < 0.001). Further analysis showed that the incidence rate of kidney stones in male with Hp infection was 17.4%, whereas that in Hp- group was 14.1% (P < 0.001). The prevalence of kidney stones in female with Hp infection was also significantly higher than in the control group (8.5% vs 6.6%, P < 0.001).
 |
Figure 1 The prevalence of kidney stones according to different Hp infection status in all the individuals (A), male (B), female (C). ***P < 0.001. |
Univariate and Multivariate Analysis to Evaluate the Risk Factors for Kidney Stones
The association of the 16 continuous and two categorized variables with the prevalence of kidney stones was analyzed using univariate and multivariate logistic regression (Table 2). Univariate analysis revealed that all variables were associated with the prevalence of kidney stones (P < 0.001). Multivariate analysis revealed that Hp infection, age, sex, BMI, DBP, FBG, LDL-C, HDL-C, urine pH, and urine acid were significantly associated with kidney stones (P < 0.05), whereas AST, ALT, GGT, SBP, TC, TG, BUN, and Scr were not.
 |
Table 2 The Risk of Kidney Stone in the Univariate and Multivariate Analyses |
As shown in Table 3, Hp infection was associated with an increased risk of kidney stones (OR = 1.221, 95%Cl:1.157–1.288, P < 0.001). After adjusting for age and sex (model 1), the results demonstrated that H. pylori infection increased the risk of kidney stones (OR = 1.258, 95%Cl:1.203–1.315, P < 0.001)). After adjusting for BMI, SBP, and DBP (Model 2), the same conclusion was drawn (OR = 1.242, 95%Cl:1.184–1.303, P < 0.001). Meanwhile, models 3 (OR = 1.287, 95%Cl:1.228–1.349, P < 0.001) and 4 (OR = 1.275, 95%Cl:1.219–1.333, P < 0.001) also demonstrated that Hp infection could promote the formation of kidney stones.
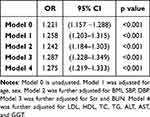 |
Table 3 The Risk of Kidney Stones According to the Infection of Hp |
Model 0 is unadjusted. Model 1 was adjusted for age and gender. Model 2 was further adjusted for BMI, SBP, DBP. Model 3 was further adjusted for serum creatinine and BUN. Model 4 was further adjusted for the LDL, HDL, TC, TG, ALT, AST, and GGT levels.
As shown in Table 4, after adjusting for BMI and blood pressure (Model 2) (OR = 0.940, 95%Cl: 0.899–0.983, P = 0.007), Scr and BUN (Model 3) (OR = 0.949, 95%Cl: 0.910–0.990, P = 0.016), lower urine pH increased the risk of kidney stones.
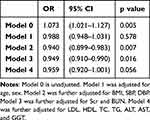 |
Table 4 The Risk of Kidney Stones According to the Urine pH |
In addition, Table 5 shows that in subgroups stratified by sex, age, BMI, hypertension, diabetes, and dyslipidemia, no significant correlation between Hp infection and an increased risk of kidney stones was detected.
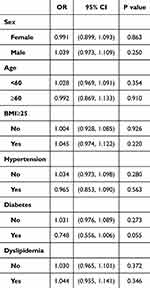 |
Table 5 Association Between Hp Infection and Kidney Stoned in Clinically Relevant Subgroups |
Model 0 is unadjusted. Model 1 was adjusted for age and gender. Model 2 was further adjusted for BMI, SBP, DBP. Model 3 was further adjusted for serum creatinine and BUN. Model 4 was further adjusted for the LDL, HDL, TC, TG, ALT, AST, and GGT levels.
Discussion
Our cross-sectional study involving more than 70 thousand patients showed that the incidence rate of kidney stones was higher in Hp+ patients, and Hp infection was an independent risk factor for kidney stones. The findings are consistent with the study’s objectives and contribute to the broader context of research on kidney stone formation.
Hp can cause many gastric diseases such as atrophic gastritis, peptic ulcer, gastric cancer, and mucosa-associated lymphoid tissue lymphoma.15,16 Recently, it was reported that Hp is associated with extragastric diseases such as thyroid nodules, metabolic syndrome, and autoimmune diseases.17 Additionally, some studies have shown that Hp specific DNA can be detected in bile duct tissues and stone samples from patients,18 and cross-sectional studies have shown that Hp is an important factor leading to gallstone occurrence.19
The potential mechanisms linking Hp infection to kidney stone formation are multifaceted. Hp infection induces a shift in intestinal flora, which may lead to metabolic changes that contribute to stone formation.20 For example, Hp infection can impair the function of Oxalobacter formigenesis, a bacterium that consumes intestinal oxalate and reduces the risk of stone formation.21 This furthermore, changes in gastric acidity can affect the absorption and metabolism of various nutrients and minerals, potentially influencing the urinary excretion of stone-forming substances [32].
Previous studies have shown that BMI, diabetes, hyperlipidemia, and age are closely related to the occurrence of kidney stones,22,23 and our study also proves this point. The mechanism of stone formation in obese patients appears to be related to insulin resistance, improper diet, and other metabolic factors, which can lead to an increase in stones in the urinary system.24
Bacterial infections, particularly those involving the urinary tract, can play a significant role in stone formation.25 For example, certain bacteria such as Escherichia coli and Proteus mirabilis produce urease, an enzyme that hydrolyzes urea to produce ammonia and carbonate, leading to an increase in urine pH and the precipitation of magnesium ammonium phosphate (struvite) crystals.26,27 These infection-induced stones, also known as “struvite stones”, are a common cause of upper urinary tract infections and can lead to significant morbidity if not treated promptly.
Gastric acidity plays a crucial role in the pathogenesis of Hp infection.28 The bacterium thrives in the acidic environment of the stomach, where it can cause chronic inflammation and damage to the gastric mucosa.29 This chronic inflammation can lead to systemic effects, including alterations in lipid metabolism and increased levels of inflammatory cytokines, which may contribute to the formation of kidney stones.30 Furthermore, changes in gastric acidity can affect the absorption and metabolism of various nutrients and minerals, potentially influencing the urinary excretion of stone-forming substances.31
The study also had some limitations; for example, due to the limitations of the cross-sectional study, it cannot explain the causal relationship between Hp infection and kidney stones. Therefore, we are planning a prospective study to prove whether the eradication of Hp leads to a decline in the incidence of kidney stones after the exclusion of confounding factors. Meanwhile, we are exploring the mechanism of Hp induced kidney stones in detail, which will be helpful in clarifying the key points of disease prevention.
In summary, our study demonstrates that Hp infection is an independent risk factor for kidney stone formation in China. The findings highlight the potential mechanisms linking Hp infection to kidney stone formation, including alterations in gastric acidity, systemic inflammation, and metabolic changes. Future research should focus on elucidating these mechanisms in more detail and exploring the potential benefits of Hp eradication in reducing the incidence of kidney stones.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology ([2022] No. 0422). Informed consent was obtained from all the participants. All the procedures were performed in accordance with the principles of the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 81770539, 81974068, and 81900580).
Disclosure
The authors declared that they have no conflicts of interest regarding this work.
References
1. Kachkoul R, Touimi GB, Mouhri GE, Habbani RE, Mohim M, Lahrichi A. Urolithiasis: history, epidemiology, aetiologic factors and management. Malays J Pathol. 2023;45:333–352.
2. Wang W, Fan J, Huang G, et al. Prevalence of kidney stones in mainland China: a systematic review. Scientific Reports. 2017;7:41630. doi:10.1038/srep41630
3. Li Y, Di X, Liu M, Wei J, Li T, Liao B. Association between daily sitting time and kidney stones based on the national health and nutrition examination survey (NHANES) 2007-2016: a cross-sectional study. Int J Surg. 2024;110:4624–4632. doi:10.1097/JS9.0000000000001560
4. Thongprayoon C, Krambeck AE, Rule AD. Determining the true burden of kidney stone disease. Nat Rev Nephrol. 2020;16:736–746. doi:10.1038/s41581-020-0320-7
5. Liu M, Wu J, Gao M, et al. Lifestyle factors, serum parameters, metabolic comorbidities, and the risk of kidney stones: a Mendelian randomization study. Front Endocrinol. 2023;14:1240171. doi:10.3389/fendo.2023.1240171
6. Lemberger U, Pjevac P, Hausmann B, et al. The microbiome of kidney stones and urine of patients with nephrolithiasis. Urolithiasis. 2023;51:27. doi:10.1007/s00240-022-01403-5
7. Ansaldi Y, Martinez de Tejada Weber B. Urinary tract infections in pregnancy. Clin Microbiol Infect. 2023;29:1249–1253. doi:10.1016/j.cmi.2022.08.015
8. Wu M, Liu M, Zhang Y, et al. Serum HDL partially mediates the association between exposure to volatile organic compounds and kidney stones: a nationally representative cross-sectional study from NHANES. Sci Total Environ. 2024;907:167915. doi:10.1016/j.scitotenv.2023.167915
9. Verit A, Guner ND. Helicobacter pylori and urinary system stones: endoluminal damage as sub-hypothesis to support the current stone theory. Med Hypotheses. 2014;83:677–680. doi:10.1016/j.mehy.2014.09.016
10. Giulioni C, Castellani D, Traxer O, et al. Experimental and clinical applications and outcomes of using different forms of suction in retrograde intrarenal surgery. Results from a systematic review. Actas Urol Esp. 2024;48:57–70. doi:10.1016/j.acuro.2023.02.008
11. Mir C, Rodriguez A, Rodrigo D, et al. Analysis of urine composition from split 24-h samples: use of 12-h overnight samples to evaluate risk factors for calcium stones in healthy and stone-forming children. J Pediatr Urol. 2020;16:371e371–371e377. doi:10.1016/j.jpurol.2020.02.011
12. Kamboj AK, Cotter TG, Oxentenko AS. Helicobacter pylori: the past, present, and future in management. Mayo Clin Proc. 2017;92:599–604. doi:10.1016/j.mayocp.2016.11.017
13. Tessadori F, Duran K, Knapp K, et al. Recurrent de novo missense variants across multiple histone H4 genes underlie a neurodevelopmental syndrome. Am J Hum Genet. 2022;109:750–758. doi:10.1016/j.ajhg.2022.02.003
14. Zhang FM, Yu CH, Chen HT, et al. Helicobacter pylori infection is associated with gallstones: epidemiological survey in China. World J Gastroenterol. 2015;21:8912–8919. doi:10.3748/wjg.v21.i29.8912
15. Salar A. Gastric MALT lymphoma and Helicobacter pylori. Med Clin. 2019;152:65–71. doi:10.1016/j.medcli.2018.09.006
16. Reshetnyak VI, Reshetnyak TM. Significance of dormant forms of Helicobacter pylori in ulcerogenesis. World J Gastroenterol. 2017;23:4867–4878. doi:10.3748/wjg.v23.i27.4867
17. Gravina AG, Zagari RM, De Musis C, Romano L, Loguercio C, Romano M. Helicobacter pylori and extragastric diseases: a review. World J Gastroenterol. 2018;24:3204–3221. doi:10.3748/wjg.v24.i29.3204
18. Misra V, Misra SP, Dwivedi M, Shouche Y, Dharne M, Singh PA. Helicobacter pylori in areas of gastric metaplasia in the gallbladder and isolation of H. pylori DNA from gallstones. Pathology. 2007;39:419–424. doi:10.1080/00313020701444473
19. Xu MY, Ma JH, Yuan BS, Yin J, Liu L, Lu QB. Association between Helicobacter pylori infection and gallbladder diseases: a retrospective study. J Gastroenterol Hepatol. 2018;33:1207–1212. doi:10.1111/jgh.14054
20. Grigor’eva IN, Romanova TI. Gallstone Disease and Microbiome. Microorganisms. 2020;8:835. doi:10.3390/microorganisms8060835
21. Kharlamb V, Schelker J, Francois F, Jiang J, Holmes RP, Goldfarb DS. Oral antibiotic treatment of Helicobacter pylori leads to persistently reduced intestinal colonization rates with Oxalobacter formigenes. J Endourol. 2011;25:1781–1785. doi:10.1089/end.2011.0243
22. Poore W, Boyd CJ, Singh NP, Wood K, Gower B, Assimos DG. Obesity and Its Impact on Kidney Stone Formation. Rev Urol. 2020;22:17–23.
23. Francis S, Saavedra-Rodriguez K, Perera R, Paine M, Black WCT, Delgoda R. Insecticide resistance to permethrin and malathion and associated mechanisms in Aedes aegypti mosquitoes from St. Andrew Jamaica. PLoS One. 2017;12:e0179673. doi:10.1371/journal.pone.0179673
24. Singh NP, Boyd CJ, Poore W, Wood K, Assimos DG. Obesity and kidney stone procedures. Rev Urol. 2020;22:24–29.
25. Labis V, Gaiduk I, Bazikyan E, et al. The role of metal nanoparticles in the pathogenesis of stone formation. Int J Mol Sci. 2024;25. doi:10.3390/ijms25179609
26. Wu X, Xie R, Ding J, et al. Recovery of phosphate and ammonium nitrogen as struvite from aqueous solutions using a magnesium-air cell system. Sci Total Environ. 2022;819:152006. doi:10.1016/j.scitotenv.2021.152006
27. Uttamamul N, Jitpean S, Lulitanond A, et al. Risk factors for canine magnesium ammonium phosphate urolithiasis associated with bacterial infection. J Vet Sci. 2022;23:e6. doi:10.4142/jvs.21040
28. Dascalu RI, Bolocan A, Paduaru DN, et al. Multidrug resistance in Helicobacter pylori infection. Front Microbiol. 2023;14:1128497. doi:10.3389/fmicb.2023.1128497
29. Haghighi FH, Farsiani H. Is Lactococcus lactis a suitable candidate for use as a vaccine delivery system against Helicobacter pylori? Curr Microbiol. 2024;82:30. doi:10.1007/s00284-024-03994-1
30. Yao L, Yang P. Relationship between remnant cholesterol and risk of kidney stones in U.S. Adults: a 2007-2016 NHANES analysis. Ann Med. 2024;56:2319749. doi:10.1080/07853890.2024.2319749
31. Noonin C, Thongboonkerd V. Beneficial roles of gastrointestinal and urinary microbiomes in kidney stone prevention via their oxalate-degrading ability and beyond. Microbiol Res. 2024;282:127663. doi:10.1016/j.micres.2024.127663
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

