Back to Journals » International Journal of Nanomedicine » Volume 19
Herbal Medicine-Derived Exosome-Like Nanovesicles: A Rising Star in Cancer Therapy
Authors Chu K, Liu J, Zhang X, Wang M, Yu W, Chen Y, Xu L, Yang G, Zhang N, Zhao T
Received 5 June 2024
Accepted for publication 18 July 2024
Published 25 July 2024 Volume 2024:19 Pages 7585—7603
DOI https://doi.org/10.2147/IJN.S477270
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Krishna Nune
Kaifei Chu,1,2,* Jie Liu,2,* Xu Zhang,1,2 Minran Wang,1 Wanping Yu,1 Yuyue Chen,1 Lingling Xu,2 Geng Yang,1 Naru Zhang,1 Tiejun Zhao1,2
1Key Laboratory of Novel Targets and Drug Study for Neural Repair of Zhejiang Province, School of Medicine, Hangzhou City University, Hangzhou, People’s Republic of China; 2College of Life Sciences, Zhejiang Normal University, Jinhua, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tiejun Zhao; Naru Zhang, Key Laboratory of Novel Targets and Drug Study for Neural Repair of Zhejiang Province, School of Medicine, Hangzhou City University, Hangzhou, Zhejiang, 310015, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Plant-derived exosome-like nanovesicles (PDNVs) are small nanoscale vesicles containing lipids, RNAs, proteins and some plant natural products secreted by plant cells. Over the last decade, PDNVs have garnered significant interest due to its exceptional therapeutic benefits in the treatment of various diseases. Herbal medicine, as a medicinal plant, plays an important role in the treatment of diseases including cancer. Especially in recent years, the function of herbal medicine derived exosome-like nanovesicles (HMDNVs) in the treatment of cancer has been widely concerned, and has become a research hotspot of nanomedicine. In this review, the biological characteristics, functions and the therapeutic advantages of PDNVs are reviewed, as well as the recent achievements and research progress of HMDNVs in cancer treatment, demonstrating its enormous promise as a cancer therapy, and new insights are provided for future research and development of anti-tumor drugs.
Keywords: herbal medicine, plant-derived exosome-like nanovesicles, extracellular vesicles, cancer treatment, nanomedicine
Introduction
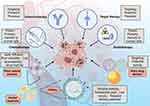
Cancer ranks a leading cause of premature death in most countries around the world,1 its morbidity and mortality rates are still increasing rapidly. According to data from GLOBOCAN, in 2022, there were approximately 20 million new cancer cases and nearly 9.7 million deaths worldwide, and in 2050, there will be an estimated 35 million new cancer cases worldwide.2 As the most important and effective methods for cancer treatment before, radiotherapy and chemotherapy have played a great role in improving the survival rate and quality of life of cancer patients, but they also show great toxicity,3,4 specifically, they not only inhibit or kill rapidly proliferating cancer cells, but also act on rapidly proliferating normal cells, such as bone marrow, hair follicle and gastrointestinal tract cells. The aforementioned strategy, which lacked specificity and was suboptimal, underwent a transformation upon the discovery of cell signaling networks implicated in the regulation of cell proliferation and differentiation. This breakthrough facilitated the development of drugs that selectively target these networks, thereby paving the way for the future implementation of targeted therapy and immunotherapy (Figure 1),5 which aim to inhibit the signaling pathways that facilitate uncontrolled proliferation of malignant cells, or precisely deliver chemotherapy agents and biological antibodies exclusively to cancer cells, thereby minimizing harm to normal cells and mitigating undesirable side effects.6,7
Over the past few decades, with plant natural products extracted from herbal medicines showing safety and efficacy in anticancer activity, holding the promise for improving chemotherapy and being a new drug for cancer treatment, it has attracted wide attention.8 However, it still suffers some limitations, such as poor solubility, low bioavailability, short half-life, etc.9 Although the synthetic nanoparticles and liposomes coated with active substances have been developed to solve these problems well, yielded notable reductions in negative health consequences and improved delivery efficiency due to the enhanced permeability and retention effect (EPR) meanwhile,10,11 the issues pertaining to premature drug release during the synthesis, storage, or circulation of nanoparticles, as well as concerns regarding potential toxicity and the absence of tumor specificity, also emerged (Figure 1).12 As a result, the number of drug-loaded nanoparticles that have received approval for clinical applications remains limited.13
However, PDNVs, plant-derived extracellular vesicles resembling exosomes and characterized by a phospholipid bilayer enclosing RNA, proteins, lipids, and various bioactive compounds, have recently garnered attention as a potential therapeutic approach in the field of oncology owing to their notable stability, substantial yield, low toxicity, targeted delivery potential, and effectiveness (Figure 1). These PDNVs not only capitalize on the advantages of artificial nanoparticles while circumventing their drawbacks, but also exhibit substantial potential and application value in the field of cancer treatment. Moreover, within the PDNVs family, HMDNVs, which inherit therapeutic properties from their herbal plant origins, demonstrate enhanced tumor destruction capabilities, are anticipated to emerge as a new star in cancer treatment.
PDNVs
Definition of Exosomes
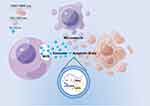
Eukaryotic cells secrete three main types of extracellular vesicles (EVs), each has a unique biogenetic mechanism. The largest of these are known as apoptotic bodies (1000–5000 nm in diameter), which are membrane vesicles released from cells during late programmed cell death. The other two classes of EVs are microvesicles (100–1000 nm in diameter) that bud off directly from the plasma membrane and exosomes (30–150 nm in diameter) that are released from cells after multivesicular bodies (MVBs) fuse with the plasma membrane (Figure 2).14,15 Among them, exosomes are nanovesicles with a phospholipid bilayer structure, containing a variety of proteins, lipids and RNAs that play an important role in intercellular communication.16,17
The Origin of Exosomes
In 1983, cell release vesicles were first observed during in vitro culture of sheep reticulocytes, which could be harvested by centrifugation at 100000g for 90 min,18 and were confirmed and officially referred to as exosomes more than a decade later.19 Since then, mammalian exosomes have ushered in a research peak. In addition, in 2007, it was found that exosomes contain mRNA and miRNA, which can be transferred to another cell and function in this new location. The research on exosomes was further pushed to a climax.20
Plant cells, unlike mammalian cells, have cell walls that may inhibit the creation and release of EVs, hence research into plant exosomes has been put on hold. However, there is growing evidence that plants also secrete similar exosome-like vesicles. Even 16 years earlier than the first mammalian exosomes were observed, as early as 1967, carrot MVBs have been observed to fuse with the plasma membrane and secrete similar exosome-like vesicles outside the cell.21 It was not until 2009 that plant exosomes were first isolated from sunflower seeds by Regente et al.22 Since then, PDNVs have gradually begun to be studied. In recent years, PDNVs have been isolated from fruits, roots, seeds, and leaves of various plants by standardized exosome separation and purification techniques such as ultracentrifugation, and their physicochemical properties (size, shape, surface charge, contents, etc.) and therapeutic activities in different diseases have been extensively studied.23
Physiological Function of PDNVs
Initially, it was thought that exosomes were produced by cells as a way of getting rid of substances they did not need.24 Yet, there is mounting evidence that exosomes play a critical role in cellular communication, mammalian reproduction and development, immune response and infection, and the incidence of a wide range of illnesses.25 In a similar vein, growing research on plant exosomes has revealed that these tiny particles are crucial for sustaining immune defense, intercellular communication, and cell homeostasis (Figure 3).
Maintain Cell Homeostasis
After being attacked by the pathogen, MVB starts to multiply rapidly in infected plant cells and releases exosomes more often. These exosomes, which contain a lot of hydrogen peroxide and callose, build up surrounding the nearby healthy cells, producing protective papillae structures. At the same time, they close off the plasmodesmata, thus preventing the further penetration of fungi and the propagation of apoptotic signals, and finally maintain cell homeostasis.26
Immune Defense
Cai et al discovered that Arabidopsis cells secrete exosome-like extracellular vesicles capable of delivering sRNAs to the fungus Botrytis cinerea Pers. These vesicles containing sRNAs congregate at the site of infection and are taken up by fungal cells to silence fungal genes critical to pathogenicity.27
This suggests that Arabidopsis thaliana has evolved to use exosome mediated cross-kingdom RNAi as a part of its immune response in the arms race against pathogens for a long time.
In addition, plants can rapidly transport intracellular signal peptide-deficient proteins to the cell extranet to combat biological and abiotic stresses via an exosome pathway independent of the classical endoplasmic reticulum - Golgi secretory pathway. On average, the majority of these signal peptide-deficient proteins, which make up about 50% of the secreted proteins, have antibacterial activity.28
Intercellular Communication
Furthermore, several proteins involved in immune signaling were also identified in an analysis of the proteomics of pathogen-infected Arabidopsis exosomes, suggesting that, similar to mammalian exosomes, plant exosomes regulate pathogen recognition by facilitating extracellular transport of key signaling proteins, thus transmitting signals to neighboring cells for enhanced pathogen detection.29
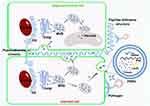
Possible Biogenetic Pathway of PDNVs
The release of exosomes by MVB fusion with the plasma membrane is considered to be the main pathway of exosome production in mammalian cells,30 while MVB-mediated exosome-like vesicles secretion has also been observed in different plant cells of different plant species by transmission electron microscopy (TEM).31 Therefore, it is thought that the same biogenetic pathway may exist in plants. MVBs are late endosomes (LE) containing intraluminal vesicles (ILVs) that are produced by limiting membrane invagination and budding into the lumen. As with mammalian cells, ESCRT complexes (ESCRT-0, I, II, and III) are thought to be involved in the maturation of ILVs and cargo sorting of ILVs in plants. Although the plant has no canonical ESCRT-0 subunit, it contains ubiquitin-bound TOM1-like (TOL) proteins instead. The formation of ESCRT-I and II complexes deforms the membrane into a bud, which then recruits ubiquitin-binding protein TOL, allowing cargo to be transported into the bud. Finally, the ESCRT-III subunit is recruited by the ESCRT-I and II complexes to the bud neck, contract the plasma membrane and cut the bud to form an ILV. Eventually, MVB fuses with the vacuole or lysosome membrane leading to degradation of its contents or fuses with the plasma membrane leading to its release in the form of exosomes rather than ILVs (Figure 3).32,33
In addition, a recent study found that proteins enriched in Catharanthus roseus (L). Don leaves-derived PDNVs contained MVB and ILV associated proteins involved in late endosome maturation or MVB-mediated vesicular trafficking, which provided evidence for the biological origin of the MVB pathway in PDNVs.34
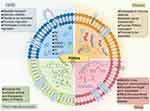
The Contents of PDNVs and Their Functions
PDNVs were found to contain mainly lipids, proteins, RNA, and some plant natural products (Figure 4).35,36
Lipids
The presence of a lipid bilayer structure in PDNVs is crucial for their transport capability and the stability of their contents.37 Therefore, lipids play a very important biological role in PDNVs. Teng et al showed that the exosomal membranes of PDNVs are enriched with phosphatidic acid (PA), phosphatidylcholines (PC), phosphatidylethanolamine (PE), digalactosyldiacylglycerol (DGDG), and monogalactosyldiacylglycerol (MGDG). However, PDNVs sourced from different origins exhibit variations in lipid composition. For instance, ginger and turmeric PDNVs-derived lipids are enriched with PAs, comprising 35.2% and 34.4% of the total lipid content, respectively. In contrast, grapefruit and garlic PDNVs contain significantly lower levels of PAs, representing only 3.5% and 5.5% of the total lipid content, respectively. Instead, the predominant lipid species in grapefruit and garlic PDNVs is PC, accounting for 36.2% and 52.6% of the total lipid content, respectively. Furthermore, it was observed that grape-derived PDNVs exhibited a high percentage of PA at 53.2% and PE at 26.1%.37 On the contrary, PDNVs derived from oranges juice contained PE,∼40%, PC,∼25%, phosphatidylinositol (PI,∼12%) and PA,∼5%,38 which closely resemble the lipid composition of grapefruit-derived PDNVs; in particular, their PE/PA ratio is opposite to that of grapefruit- or ginger-derived PDNVs. These results suggest that Rutaceae plant-derived PDNVs have a similar specific lipid composition.
These distinct lipid ratios found in PDNVs contribute to their specific functions. Teng et al have demonstrated that PDNVs derived from edible plants exhibit a preference for uptake by gut bacteria in a manner dependent on the lipid composition of PDNVs. Specifically, PDNVs enriched with PC lipids, such as those derived from grapefruit, are preferentially taken up by Ruminococcaceae sp. Conversely, PDNVs enriched with PA lipids, such as those derived from ginger, are preferentially taken up by Lactobacillaceae. Furthermore, PA lipids are involved in regulating the duration and quantity of PDNVs accumulation in the gut, PC lipids facilitate the migration of PDNVs from the intestine to the liver.39 Another study revealed that the unsaturated state of PA lipids plays a crucial role in the uptake of ginger-derived PDNVs by pathogens, with PA (34:1) not absorbed by Porphyromonas gingivalis compared to PA (34:2).40 In addition, the substantial presence of ether phospholipids in Catharanthus roseus-derived PDNVs potentially accounts for its exceptional stability and facilitates its involvement in the immunomodulatory mechanisms of host cells.34 The binding of Garlic-derived PDNVs PA (36:4) to microglial cell Brain Acid-Soluble Protein 1 (BASP1) is necessary for the internalization of Garlic-derived PDNVs and the subsequent suppression of indoleamine 2,3-dioxygenase 1 (IDO1) expression.41 Ginseng-derived PDNVs were enriched with DGMG, 59.4%, PE, 16.8% and ceramide (Cer, 13.8%). Different from other PDNVs, this high content of DGMG and unique ceramide may be involved in changing the polarization of macrophages (Figure 4).42
Proteins
Proteins play a crucial role in the execution of gene function and significantly contribute to the functioning of PDNVs. Numerous studies have documented the enrichment of proteins within diverse PDNVs. For instance, a proteomic analysis conducted on Arabidopsis EVs revealed a notable enrichment of proteins associated with biotic and abiotic stress. Particularly, these proteins were involved in signaling and immune responses, indicating a potential specialization of these EVs in immune defense and stress adaptation. Furthermore, the presence of proteins associated with reactive oxygen species (ROS) signaling and membrane transport proteins within EVs suggests their potential role in regulating ROS levels, promoting ROS signaling, and facilitating EVs formation and secretion.28 In a study conducted by Ou et al, a comprehensive analysis of 1843 proteins in Catharanthus roseus (L). Don leaves-derived PDNVs was performed, revealing 1184 differentially expressed proteins compared to the plant proteome. Notably, 471 significantly up-regulated proteins, primarily encompassing transporters and signaling proteins, were identified, potentially contributing to the maturation of Catharanthus roseus (L). Don leaves-derived PDNVs and signaling processes.34
Furthermore, the researchers conducted an experiment wherein M2-like macrophages were treated with PDNVs derived from ginseng that had been digested with DNase I/RNase I. The results indicated that there were no significant differences in the induction of IL-6 and TNF-α compared to the undigested group, and there was no effect on the upregulation of surface markers in M1-like macrophages. Conversely, in the proteinase K digestion treatment group, only a minimal amount of IL-6 and TNF-α was induced, and the upregulation of M1-like macrophage surface markers did not occur. These findings suggest that protein, rather than nucleic acid, is involved in the polarization of macrophages by ginseng PDNVs.42 Similarly, after the elimination of the surface proteins of garlic-derived PDNVs through trypsin digestion, the group subjected to digestion exhibited a reduced level of cellular uptake in comparison to the undigested group. This finding suggests the significant involvement of surface proteins in the internalization process. Subsequent investigations revealed that the mannose-specific binding protein, II lectin, located on the membrane surface of garlic-derived PDNVs, interacted with cluster of differentiation 98 (CD98) on the cell membrane surface, thereby facilitating their uptake by cells (Figure 4).43
RNAs
Microribonucleic acid (miRNAs) is a class of small (18–24 nt) non-coding RNA that plays an important role in post-transcriptional gene regulation in animals and plants.44 Studies have shown that exogenous plant miRNAs are present in the serum and tissues of a variety of animals, that these exogenous plant miRNAs are mainly obtained through food intake, and that the possible carriers of these plant-derived miRNAs are plant extracellular vesicles.45
In fact, several studies have demonstrated the presence of RNA, particularly miRNA, in PDNVs. These miRNAs can be assimilated by host cells and subsequently modulate the expression of target genes, which greatly supports the cross-kingdom regulation of plant-derived miRNAs. For example, a deep sequencing of ginger derived PDNVs by Zhang et al revealed 125 different miRNAs in them, ranging in length from 15 to 27 nucleotides, binding software analysis showed that 124 of these microRNAs may target and regulate human gene expression by binding to their 3’-untranslated regions (3’ -UTRs).35 Moreover, Xiao et al further analyzed the miRNA profile of 11 PDNVs derived from different fruits and vegetables and showed that some of the highly expressed miRNAs could regulate the expression of inflammatory cytokines and cancer-related genes in vitro.46 These results suggest that PDNVs-derived miRNAs can also mediate cross-kingdom communication, regulate the expression of related genes in the recipient host cells, and affect the occurrence and development of a variety of diseases (Figure 4).
Plant Natural Products
With the advancement of research on plant natural products and the enhancement of extraction technology, a new era has emerged in the field of utilizing plant natural products for the prevention and treatment of complex diseases.47 For instance, it has been observed that approximately 49% of cancer drugs approved by the food and drug administration (FDA) between the 1940s and 2014 were either plant natural products or derived directly from them.48 The accumulating evidence indicates that PDNVs not only possess certain characteristics inherited from their source plants but also contain plant natural products that hold potential therapeutic value. For instance, PDNVs derived from cucumbers possess bioactive secondary metabolites, notably cucurbitonin B, which has been documented to impede the progression of leukemia, breast cancer, lung cancer, and liver cancer;49,50 Furthermore, the presence of sulforaphane compounds in broccoli-derived PDNVs has been found to hinder the development of diverse cancers, such as pancreatic, intestinal, leukemia, and prostate cancers. The manipulation of sulforaphane through knock-out and knock-in experiments has demonstrated its significant association with the biological effects of PDNVs.51,52 A separate study conducted an isolation of three distinct sizes of ginger nanovesicles, revealing that 6-gingerol and 6-shogaol are inherent active constituents common among them. Furthermore, their findings demonstrated that PDNVs with elevated concentrations of natural products (5.68 μg/mg 6-gingerol and 2.95 μg/mg 6-shogaol) exhibited a more pronounced therapeutic impact, thereby indicating the significance of natural products in the therapeutic efficacy of ginger-derived PDNVs (Figure 4).35
The intricate and heterogeneous lipids, RNAs, proteins, and natural products found in PDNVs are essential for cellular communication and intercellular material exchange, which may also make PDNVs promising candidates for the treatment of human diseases, especially cancer. Consequently, comprehensive knowledge regarding the constituents and potential functions of each PDNV is crucial for the advancement of novel drugs derived from PDNVs.
Advantages of PDNVs as Therapeutic Agents
PDNVs display a number of clinical characteristics and exceptional therapeutic advantages over mammalian exosomes or synthetic nanoparticles, including ease of mass production, low immunogenicity and toxicity, effective cellular uptake, and excellent stability (Figure 1).
Easy to Mass Produce
The yield of the drug itself is a prerequisite for its large-scale use, and PDNVs come from the plant itself, which is exactly what it is. Li et al showed that it was possible to process up to 3 liters of ginger juice in 1 hour, equivalent to 300 cell petri dishes (150 mm), with a yield ratio 300 times higher than the production of mammalian exosomes, just by using ordinary juicers.53
Low Toxicity and Immunogenicity
In order to realize the potential of PDNVs in nanomedicine, full attention needs to be paid to safety and toxicology issues. Unlike artificial nanoparticles and liposomes, which contain potential toxicity in vivo, or exosomes secreted by mammalian cells, which contain immunogenicity introduced by secretory cells,54,55 PDNVs come mostly from edible plants, so they are low toxic to humans. To support this, in a safety test of cucumber sarcocarp-derived PDNVs by Chen et al, hemolysis tests showed that intravenous these PDNVs did not cause erythrocyte rupture, nor did it cause an increase in serum levels of pro-inflammatory cytokines (including TNF-α and IL-1β) in mice after 7 days of caudal intravenous cucumber sarcocarp-derived PDNVs; In addition, HE staining showed no obvious damage to myocardial, liver, spleen, kidney and other organs of these PDNVs treated mice. These results indicated that cucumber sarcocarp-derived PDNVs was non-toxic and safe in vivo and in vitro.50 What is more, unlike artificial nanoparticles that cross the placental barrier in pregnant mice and cause pregnancy complications, Wang et al injected DiR labeled Ginger-derived PDNVs into the tail vein of pregnant mice and found that these PDNVs did not cross the placental barrier, suggesting that Ginger-derived PDNVs is also safe for the fetus in pregnant women and can be used as delivery vectors for certain drugs in pregnant women.56
Efficient Cellular Uptake
Efficient uptake of nanoparticles by target cells is critical for their use as drug or intracellular drug delivery vehicles.57 Therefore, Wang et al, in order to evaluate the efficient uptake of grapefruit derived PDNVs by different cell types, co-cultured different cell types with PKH26-labeled grapefruit derived PDNVs. The results showed that most cells such as GL26, A549, SW620, CT26 and 4T1 were able to internalize these PDNVs. Moreover, more than 20% of B cells and 14% of T cells took up these PDNVs within 12 hours of co-culture, which is remarkable and exciting because B cells and T cells are the most difficult to be transfected with any commercially available transfection reagent. In addition, in the cellular uptake efficiency experiment of grapefruit derived PDNVs and cationic liposomes, only about 40% of the cationic liposomes entered the cells. However, more than 80% of grapefruit derived PDNVs were taken up by the cells.56
Excellent Stability
In order to ensure patient safety and product efficacy during clinical use, its stability and biocompatibility under the relevant conditions of the target administration route (eg, oral administration, intravenous administration, etc.) are particularly important.58 Therefore, to investigate the stability of PDNVs in vitro, researchers suspended them in different solutions and judged their stability based on their size change, that is, whether they could still maintain nanoscale size. For example, Zhang et al found that ginger derived PDNVs were very stable in simulated solutions of gastric and intestinal juice and were resistant to freeze/thaw cycles.35 Similarly, Liu et al found that turmeric derived PDNVs could maintain nanoscale size in simulated solutions of gastric (pH~2) and intestinal (pH~6.5) fluid, demonstrating their stability, indicating that they can be administered orally;59 Chen et al showed that teaflower derived PDNVs were stable in gastric, small intestine, and colon mock solutions as well as DMEM, indicating that they can be ingested or injected.60
Furthermore, the assessment of PDNV stability during extended storage periods and the investigation of optimal storage conditions are crucial for ensuring the reliability of experimental results and the viability of future applications. Hwang et al demonstrated that PDNVs derived from yam remained functionally active after being stored at −80°C for one year, with no significant differences observed compared to freshly prepared samples, which aligns with the stability exhibited by mammalian exosomes.61 Likewise, other studies have shown that the prepared PDNVs can be stored at −80°C and used in subsequent experiments.42,62 Notably, in a specific study, the authors tested the stability of Dendropanax morbifera leaf-derived PDNVs alone or with preservatives under various temperature conditions. Results indicated that PDNVs using the preservative TMO and stored at 4 ° C showed the most excellent stability and were more resistant to freeze-thaw cycles. Therefore, it is well worth considering adding preservatives to the solvents of PDNVs to improve their stability.63
Role of the HMDNVs in Cancer Therapy
In view of the excellent therapeutic advantages shown by PDNVs, more and more studies have been devoted to exploring the potential of PDNVS in cancer treatment and providing a basis for the development of new drugs for cancer treatment, especially those derived from plants with certain therapeutic activities (Table 1). The antitumor mechanisms of the following various PDNVs are shown in Figure 5.
 |
Table 1 Characterization, Target Cancer and Mechanism of Action of Different CHMDNVs |
Ginseng
Known as the “king of herbs”, the use of ginseng in traditional Chinese medicine can be traced back more than 5000 years to Huangdi Neijing. Ginsenoside, the primary active component of ginseng, has been demonstrated to have a number of advantageous benefits, including anti-inflammatory, antioxidant, and anti-cancer effects. The results of clinical studies have shown that ginseng can improve psychological function, immune function, and diabetes-related conditions.72,73
Cao et al isolated GDNPs from ginseng root by sucrose gradient ultracentrifugation with a hydrodynamic size of about 344.8 nm and a negative zeta potential (− 25.4 mV), which contain various proteins, amino acids, lipids, nucleotides, and ginsenosides Rg3, the active component of ginseng. Moreover, they have shown that ginseng derived PDNVs induce apoptosis in murine melanoma cells by inducing polarization of M1-like macrophages through TLR-4/ MyD88 signaling and enhancing total ROS production.42 In addition, their another further study show that ginseng derived PDNVs increase the release of CCL5 and CXCL9 from tumor-associated macrophages (TAMs) facilitates the recruitment of CD8+ T cells to the tumor site, which has a synergistic effect on PD-1 monoclonal antibody therapy without systemic toxicity detected.64 These two studies provides a basis for ginseng derived PDNVs to be further used as nanomedicine for tumor immunotherapy.
Tea Flower
Tea plant, native to the Yunnan-Guizhou Plateau in southwest China, has a cultivation history of more than 5000 years. In traditional Chinese medicine, its flowers—known as tea flowers—are used for deodorization, skin care, cough alleviation, and phlegm. Tea flowers have a chemical make-up that is comparable to that of tea; they are mostly made of catechins, polysaccharides, proteins, amino acids, and saponins. As a result, it provides a range of health advantages, including antioxidant, anti-inflammatory, anti-tumor, hypoglycemic, and hypolipidemic properties as well as anti-allergic activity.74,75
Chen et al obtained teaflower derived PDNVs by sucrose density gradient centrifuge, derived from bands between 15% and 30% sucrose gradients. These PDNVs has a hydrodynamic size of 131 nm, a negative zeta potential (− 7.6mV), and a high concentration of polyphenols, flavonoids, functional proteins, and lipids. In terms of anticancer activity, teaflower derived PDNVs induced apoptosis of breast cancer cells mainly by inducing oxidative stress, leading to mitochondrial damage and cell cycle arrest in vivo. In vitro, teaflower derived PDNVs preferentially accumulate in breast tumor tissues and their lung metastasis sites, and inhibit breast tumor growth and lung metastasis by producing reactive oxygen species and regulating microbiota.60
Asparagus Cochinchinensis
Asparagus cochinchinensis (Lour). Merr. (A. cochinchinensis), as part of traditional Chinese medicine, A. cochinchinensis first appeared in Shennong’s Classic of Materia Medica), the medicinal value of this plant has been proven by clinical studies over a long period of time. A. cochinchinensis’ dried roots have long been used for treating asthma, cough, constipation, and thrombosis, either alone or in combination with other herbal medicines. To far, more than 90 chemicals from A. cochinchinensis have been isolated and described, with therapeutic actions ranging from anti-asthma to anti-inflammatory, antioxidant, anti-tumor, antidepressant, neuroprotective, and ameliorating Alzheimer’s disease and intestinal illnesses.76
Zhang et al successfully extracted A. cochinchinensis-derived PDNVs by sucrose density gradient ultracentrifuge, which were mainly concentrated in bands between 30% and 45%, with an average size of 119nm, negative zeta potential (− 21mV), containing a variety of lipids, proteins and small RNAs, and showing typical cup-like structure. In addition, the antitumor properties of A. cochinchinensis-derived PDNVs in vitro have been demonstrated in hepatocellular studies, demonstrating their ability to inhibit tumor growth, induce apoptosis, and up-regulate apoptosis-related factors.65
To conclude, as a promising alternative to A. cochinchinensis extracts, A. cochinchinensis-derived PDNVs may achieve satisfactory therapeutic benefits and better drug-like properties over their traditional counterparts.
Bitter Melon
Bitter melon is a vegetable commonly eaten in Asia, especially in India and China, and is distinguished by its bitter taste. In the meanwhile, bitter melon is used as a folk remedy to treat conditions including diabetes, eczema, pneumonia, as well as being an antiviral, anti-malaria, and anti-bacterial agent. In addition, more than 30 medicinal compounds are found in bitter melon, including proteins, peptides, and small compounds, some of which have anti-tumor, anti-diabetic, and anti-HIV properties.77,78
By electrophoresis and dialysis, Yang et al isolated bitter melon derived PDNVs from bitter melon juice, which could be taken up by oral squamous cell carcinoma (OSCC) cells and induce cell apoptosis. Strong anticancer activity was seen, with the impact being 100 times greater than that of bitter melon juice with a similar quantity of proteins and RNAs. Also, it has been demonstrated that bitter melon derived PDNVs can lessen OSCC resistance to 5-fluorouracil and improve the therapeutic impact of the drug.66
In addition, Wang et al also isolated the bands between 30% and 45% sucrose gradients by sucrose density gradient ultracentrifugation and obtained Momordica charantia-derived PDNVs with a size of about 120 nm and rich in multiple miRNAs. Furthermore, they found that these PDNVs inhibited glioma cell proliferation by arresting cell cycle through PI3K/AKT signaling axis, and inhibited U251 glioma cell migration and invasion by accelerating the epithelial-mesenchymal transition and decreasing MMP9 in vitro. Importantly, Momordica charantia-derived PDNVs cross the blood-brain barrier (BBB) and are enriched in tumor tissues, where they inhibit glioma growth and metastasis in vivo.67
Garlic
Garlic (Allium sativum L.) belonging to the family Liliaceae, is one of the oldest cultivated plants in the world, has been used for centuries for food and medicine. The medicinal use of garlic has been documented in ancient medical works of multiple civilizations, and in ancient China, garlic was one of the most commonly used Chinese herbal medicine since 2700 BC and was effective in treating most human diseases such as infections, cancer and heart disease.79 According to modern medical research, garlic contains a number of bioactive compounds, such as organic sulfides, saponins, phenolic compounds, and polysaccharides, which have anti-inflammatory, anti-bacterial, anti-viral, anti-fungal, and anti-tumor properties.80,81
İrem Ozkan et al isolated small garlic-derived PDNVs between 50 and 150nm in diameter by using aqueous two-phase systems. Moreover, they found that garlic-derived PDNVs promoted cancer cell apoptosis by increasing the gene expression level of pro-apoptotic genes such as p53, Bax, Cas3 and Cas9 and decreasing the gene expression level of anti-apoptotic gene Bcl-2, while blocking the cell cycle in S phase to inhibit cancer cell proliferation, yet having little or no toxicity to normal cells.68
Fingerroot
Fingerroot, belonging to the Boesenbergia genera within the Zingiberaceae family, is a herb extensively distributed in Asian countries, where it is frequently employed as a culinary component and in ethnomedicinal formulations. Furthermore, investigations have revealed that fingerroot extracts and isolated compounds exhibit diverse pharmacological properties, including antiviral, anti-inflammatory, and anticancer activities.82
In a study, fingerroot-derived PDNVs were obtained from finger root juice through the implementation of differential centrifugation and size-exclusion techniques. Out of more than 30 fractions, fraction 8 exhibited the highest vesicle count, the most negative zeta potential value (−26.9±6.1mV), and an average particle size of 100.2±10.1 nm. Furthermore, fingerroot-derived PDNVs were found to contain various biomolecules such as proteins, alkaloids, phenols, lipids, and organic compounds. Notably, the presence of naringenin chalcone, pinostrobin, and pinocembrin in these PDNVs was identified, which was the bioactive constituents in finger root with anticancer activity. In addition, the findings of this study demonstrate that fingerroot-derived PDNVs possess a discerning ability to combat colorectal cancer cells by perturbing the equilibrium of intracellular redox processes and promoting programmed cell death. These results imply that fingerroot-derived PDNVs hold promise as an innovative therapeutic approach for individuals diagnosed with colorectal cancer.69
Dendropanax Morbiferus
The utilization of Dendropanax Morbiferus, a flowering plant belonging to the Araceae family and native to southern Asia, as a traditional remedy for diverse ailments has been well-documented. The therapeutic potential of extracts derived from the roots, leaves, and stems of Dendropanax Morbiferus encompasses the treatment of inflammatory conditions, diarrhea, diabetes, cancer, and certain microbial infections.83
To enhance the intracellular delivery efficiency and clinical effectiveness of bioactive compounds present in Dendropanax Morbiferus sap, a series of techniques involving continuous centrifugation and filtration, based on mammalian exosome separation technology, were employed for the isolation and acquisition of Dendropanax Morbiferus sap derived PDNVs with particle sizes between 100 and 200 nm and surface potentials of −25 mV. Additionally, it was observed that PDNVs derived from Dendropanax morbifera sap exhibited a dose-dependent inhibitory impact on cancer-associated fibroblasts (CAFs), pivotal contributors to cancer metastasis. Furthermore, these PDNVs regulated the expression levels of diverse genes, particularly those associated with growth factors and extracellular matrix components such as integrins and collagen. These results imply that PDNVs derived from Dendropanax morbifera sap hold promise as anti-CAF agents for diminishing CAF populations within the tumor microenvironment.70
In addition, their another study determined that the combined administration of Dendropanax morbifera-derived PDNVs and Pinus densiflora-derived PDNVs yielded superior efficacy compared to the sole use of Dendropanax morbifera-derived PDNVs, while also resulting in a reduced incidence of adverse reactions. This combination therapy involving Dendropanax morbifera-derived PDNVs and Pinus densiflora-derived PDNVs holds promise as a potential pharmacological regimen for tumor treatment, and future research endeavors should prioritize investigating the synergistic effects of Dendropanax morbifera-derived PDNVs in conjunction with other PDNVs.84
Moringa Oleifera
Moringa oleifera (MO), commonly referred to as the “tree of life” or “miracle tree”, exhibits widespread growth in nearly all tropical and subtropical areas and is recognized as a significant botanical specimen owing to its extensive therapeutic and non-therapeutic advantages. Historically, this plant has been employed for the treatment of various ailments such as wounds, pain, ulcers, liver and heart diseases, cancer, and inflammation. To date, over one hundred compounds have been identified in MO, demonstrating robust antioxidant, anti-cancer, antihypertensive, hepatoprotective, and nutritional properties.85
Marina Potesta et al utilized MO seed powder as a primary material to produce MO aqueous extract (MOES). The acquired MOES underwent filtration using 0.45 μm and 0.22 μm pore size filters, and the resulting PDNVs were extracted through centrifugation at 13,000 × g for 5 minutes. The majority of the obtained PDNVs exhibited sizes ranging from 240 to 500 nm and contained RNA, protein, and lipids, but showed DNA deletion, indicating a comparable morphology and density to exosomes found in mammalian organisms. In tumor cell lines, treatment with MOES derived PDNVs resulted in a reduction in cell viability and an increase in apoptosis levels, concomitant with a downregulation of B-cell lymphoma 2 protein expression and a decrease in mitochondrial membrane potential. Notably, the effects observed with PDNVs treatment were comparable to those observed with MOES treatment and transfection using a pool of small RNAs isolated from MOES as a control.71
In conclusion, the utilization of MOES derived PDNVs, as an extract of MO, not only exhibits superior efficacy but also demonstrates significantly attenuated adverse effects compared to MO. This conclusion may offer a promising direction for further research and utilization of herbal medicine.
Clinical Trials of HMDNVs or PDNVs Loaded with Natural Active Products of CHM
In view of the excellent performance of HMDNVs as novel therapeutic agents and the promising anticancer effects shown in various studies, putting the potential HMDNVs into clinical use as early as possible is highly desired. However, as the research is still in its infancy, there are very few clinical trials on plant exosomes in progress or completed,86 one of which aimed to see whether ginger or aloe derived exosomes could treat and improve the condition of Polycystic Ovary Syndrome, but was unfortunately withdrawn because no patients were enrolled. In another study, grape exosomes were investigated as an anti-inflammatory agent to reduce oral mucositis during radiotherapy and chemotherapy for head and neck cancer. The remaining two clinical trials related to HMDNVs are shown in Table 2.
 |
Table 2 Two Clinical Trials Related to CHMDNVs |
Conclusions and Perspectives
Although the research on PDNV is still in its infancy, it has attracted wide attention as a new generation of therapeutic agents due to its widespread availability, ease of access, allowing large-scale production, and demonstrating its safety and stability in vivo and in vitro, as well as high cellular internalization rate. However, it is precisely because it is in the preliminary stage, it has to be admitted that there are still some problems in the present research of PDNVs.
Most of the current isolation methods first use a juicer to destroy plant tissues to obtain PDNVs. As a result, both EVs and artificial nanoparticles/microsomes formed by the fusion of ruptured membranes, as well as natural intracellular vesicles are isolated inevitably.87 In addition, ultracentrifugation is a common method for exosome purification. Nevertheless, repeated precipitation of exosomes under high centrifugal force may damage membrane integrity or promote aggregation.88 What’s more, high centrifugal force also results in the formation of non-vesicular contaminants such as protein aggregates, lipoproteins and RNA bound to ribonucleoprotein complexes.89 These problems may lead to errors in the content as well as functional analysis of PDNVs. Although the decreased centrifugal force is efficient in avoiding aggregation between vesicles and eliminating impurities, it will surely reduce the yield somewhat. Therefore, designing a better centrifugal force to achieve a satisfactory balance between yield and purity is worthy of further exploration. Moreover, an innovative study used plant tissue and cell culture techniques to obtain pure and authentic ginseng derived EVs,90 which avoided tissue fragmentation and thus the generation of artificial nanoparticles/microsomes and intracellular vesicles. Of fact, this strategy also gives up the original simplicity and cost advantages of traditional methods, and additional study is needed to establish the practicality of this method.
According to the guidelines from Minimal information for studies of extracellular vesicles 2018 (MISEV2018).91 Western blotting of exosomes’ marker proteins is an essential step in identifying mammalian exosomes. Based on the origin and biogenesis of exosomes, membrane transport and fusion proteins, tetraspanin proteins, Heat shock proteins and vesicle-forming proteins are taken as its marker proteins, such as GTPases, CD9, CD81, HSP90, and TSG101.92 For plant EVs, including PDNVs, there are not any mature or trustworthy marker proteins, though. In spite of Marcela Pinedo et al identifying three common protein families in plant EVs and considering three potential candidate marker proteins in 2021,87 it was not universally regarded as accurate. Therefore, more plant EV proteomic analyses are still required to identify a set of plant EV protein markers that are conserved among plants, reliable, and widely recognized. Moreover, there are no commercially available antibodies against these candidate markers at present.
Another prominent issue is the confusion regarding the nomenclature of PDNVs, which, as was mentioned before, varies throughout the literature. For example, turmeric derived exosome-like nanovesicles, ginger derived nanoparticles, A. cochinchinensis derived nanovesicles, bitter melon derived extracellular vesicles, garlic-derived SEVs, etc. In this review, in order to highlight their similarities with mammalian exosomes in morphological structure (phospholipid bilayer nanovesicles), contents (lipids, proteins, RNA), and function (intercellular communication, immune defense), we have named them plant-derived exosome-like nanovesicles.
It is worth mentioning that given their excellent stability, safety, biocompatibility, and ability to facilitate large-scale manufacture, PDNVs are also exploited as drug delivery vectors. For maximum economic benefit, these vectors are usually made from relatively inexpensive plants, such as grapefruit, ginger, cabbage,93 and lemon.94 Among them, grapefruit and ginger derived PDNVs are the most widely studied delivery vectors. Due to its outstanding delivery ability, the former has been studied for the delivery of doxorubicin plus heparin,95 curcumin plus doxorubicin,96 miRNA97 and siRNA plus paclitaxel as well as JSI-124 (Cucurbitacin I)98 to treat glioma, colon cancer, breast cancer and other diseases. Ginger derived PDNVs have been investigated for the delivery of doxorubicin99 and siRNA53,100 to treat colon cancer due to their delivery ability and own therapeutic properties, which often produce synergistic effects to enhance the therapeutic effect. In addition, PDNVs have been modified to enhance their targeting ability or loading capacity, which is equally an attractive aspect of PDNVs and a popular area for investigation.
Since HMDNV frequently inherits certain features of the original herbal medicine, we recommend that future research on HMDNV begin with a focus on the previous pharmacological effects of the selected herbal medicine and the therapeutic activities of the natural active products contained in it, and then go on to the corresponding illness type for investigation, or vice versa. This is not an inherently uninnovative model; rather, it is a vital step toward developing novel uses of herbal medicine and their natural active components in the treatment of illnesses and malignancies, as well as an efficient strategy to address the bottleneck in their current research. Of course, it would be preferable to discover new therapeutic activities of HMDNVs in a novel manner. What’s more, there is growing evidence that the therapeutic efficacy of HMDNV is frequently superior to that of the same dose of the natural active substance itself and the herbal juice containing the same dose. For example, cucumber sarcocarp-derived PDNVs containing the same dose of CuB exhibited more potent anticancer activity than free CuB;50 In comparison with bitter melon juice containing the same concentration of proteins and RNAs, bitter melon derived PDNVs showed 100 times greater anticancer activity.66 This might be because of the vesicular structure, which improves the stability and bioavailability of the active compounds, or because the complex and varied components in PDNVs synergistically boost anticancer action. This also raises a new issue: the components in PDNVs are unknown and complicated, potentially posing safety issues. As a result, even though the majority of PDNVs are generated from edible plants, each member of the PDNVs family should undergo safety testing.
Herbal medicine has been enduring for thousands of years, with its unique effect on some difficult and complicated diseases. Nonetheless, there is still a great deal of room for the development of HMDNVs and its use in the treatment of cancer as the present study on HMDNVs is relatively scant. Only in China, it is reported that there are more than 10,000 prescriptions of Chinese herbal medicine recorded in various ancient Chinese medical works,101 and according to the previous national natural resources survey, there are a total of 12807 kinds of Chinese herbal medicine,102 which will be a huge treasure trove full of surprises for the development of new anticancer nanomedicine.
Abbreviations
PDNVs, Plant-derived exosome-like nanovesicles; HMDNVs, herbal medicine derived exosome-like nanovesicles; EPR, enhanced permeability and retention effect; EVs, extracellular vesicles; MVBs, Multivesicular bodies; TEM, transmission electron microscopy; LEs, late endosomes; ILVs, intraluminal vesicles; ESCRT, endosomal sorting complexes required for transport; TOL, TOM1-like; PA, phosphatidic acid; PC, phosphatidylcholines; PE, phosphatidylethanolamine; DGDG, digalactosyldiacylglycerol; MGDG, monogalactosyldiacylglycerol; PI, phosphatidylinositol; BASP1, Brain Acid-Soluble Protein 1; IDO1, indoleamine 2.3-dioxygenase 1; Cer, ceramide; ROS, reactive oxygen species; CD98, cluster of differentiation 98; miRNA, microribonucleic acid; 3’ -UTRs, 3’-untranslated regions; FDA, food and drug administration; TAMs, tumor-associated macrophages; OSCC, oral squamous cell carcinoma; BBB, blood-brain barrier; CAFs, cancer-associated fibroblasts; MO, Moringa oleifera; MISEV2018, Minimal information for studies of extracellular vesicles 2018.
Acknowledgments
The authors wish to thank Jinjian Lu (University of Macau) for comments on the draft manuscript. We also thank the drawing support provided by the Figdraw platform (www.figdraw.com).
Funding
This work was supported by a grant from the National Natural Science Foundation of China to T.Z. (No. 32370147), a grant from the Special Support Program for High-level Talents in Zhejiang Province to T.Z. (No. 2023R5242).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Soerjomataram I, Bray F. Planning for tomorrow: global cancer incidence and the role of prevention 2020-2070. Nat Rev Clin Oncol. 2021;18(10):663–672. doi:10.1038/s41571-021-00514-z
2. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2024;2024. doi:10.3322/caac.21834
3. Thompson MK, Poortmans P, Chalmers AJ, et al. Practice-changing radiation therapy trials for the treatment of cancer: where are we 150 years after the birth of Marie Curie? Br J Cancer. 2018;119(4):389–407. doi:10.1038/s41416-018-0201-z
4. Furue H. Kako rokujuunenkan no kagakuryouhou ni yoru gan chiryou [過去六十年間の化学療法による癌治療] [Chemotherapy cancer treatment during the past sixty years]. Gan To Kagaku Ryoho. Japanese. 2003;30(10):1404–1411.
5. Chabner BA, Roberts TG. Timeline: chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5(1):65–72. doi:10.1038/nrc1529
6. Joo WD, Visintin I, Mor G. Targeted cancer therapy--are the days of systemic chemotherapy numbered? Maturitas. 2013;76(4):308–314. doi:10.1016/j.maturitas.2013.09.008
7. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2022;99(19):12293–12297. doi:10.1073/pnas.192461099
8. Luo H, Vong CT, Chen H, et al. Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. ChinMed. 2019;14:48. doi:10.1186/s13020-019-0270-9
9. Wei D, Yang H, Zhang Y, et al. Nano-traditional Chinese medicine: a promising strategy and its recent advances. 10.1039/D2TB00225F. J Mat Chem B. 2022;10(16):2973–2994. doi:10.1039/D2TB00225F
10. Ngoune R, Peters A, von Elverfeldt D, Winkler K, Pütz G. Accumulating nanoparticles by EPR: a route of no return. J Control Release. 2016;238:58–70. doi:10.1016/j.jconrel.2016.07.028
11. Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi:10.1146/annurev-med-040210-162544
12. Zheng Y, Wang Y, Xia M, et al. The combination of nanotechnology and traditional Chinese medicine (TCM) inspires the modernization of TCM: review on nanotechnology in TCM-based drug delivery systems. Drug Delivery Transl Res. 2022;12(6):1306–1325. doi:10.1007/s13346-021-01029-x
13. Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24(12):1504–1534. doi:10.1002/adma.201104763
14. Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113(1):1–11. doi:10.1007/s11060-013-1084-8
15. György B, Szabó TG, Pásztói M, et al. Membrane vesicles, current state-of-The-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667–2688. doi:10.1007/s00018-011-0689-3
16. Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8(7):E727. doi:10.3390/cells8070727
17. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi:10.1083/jcb.201211138
18. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasm a membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–9420.
19. Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi:10.1084/jem.183.3.1161
20. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi:10.1038/ncb1596
21. Halperin W, Jensen WA. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J Ultrastruct Res. 1967;18(3):428–443. doi:10.1016/s0022-5320(67)80128-x
22. Regente M, Corti-Monzón G, Maldonado AM, Pinedo M, Jorrín J, de la Canal L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009;583(20):3363–3366. doi:10.1016/j.febslet.2009.09.041
23. Karamanidou T, Tsouknidas A. Plant-Derived Extracellular Vesicles as Therapeutic Nanocarriers. Int J Mol Sci. 2021;23(1):191. doi:10.3390/ijms23010191
24. Yáñez-Mó M, Siljander PRM, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi:10.3402/jev.v4.27066
25. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi:10.1126/science.aau6977
26. An Q, Ehlers K, Kogel K-H, van Bel AJE, Hückelhoven R. Multivesicular compartments proliferate in susceptible and resistant MLA12-barley leaves in response to infection by the biotrophic powdery mildew fungus. New Phytol. 2006;172(3):563–576. doi:10.1111/j.1469-8137.2006.01844.x
27. Cai Q, Qiao L, Wang M, et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2018;360(6393):1126–1129. doi:10.1126/science.aar4142
28. Agrawal GK, Jwa N-S, Lebrun M-H, Job D, Rakwal R. Plant secretome: unlocking secrets of the secreted proteins. Proteomics. 2010;10(4):799–827. doi:10.1002/pmic.200900514
29. Rutter BD, Innes RW. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 2017;173(1):728–741. doi:10.1104/pp.16.01253
30. Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;188:1–11. doi:10.1016/j.pharmthera.2018.02.013
31. An Q, van Bel AJ, Hückelhoven R. Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal Behav. 2007;2(1):4–7. doi:10.4161/psb.2.1.3596
32. Li X, Bao H, Wang Z, et al. Biogenesis and Function of Multivesicular Bodies in Plant Immunity. Front Plant Sci. 2018;9:979. doi:10.3389/fpls.2018.00979
33. Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464(7290):864–869. doi:10.1038/nature08849
34. Ou X, Wang H, Tie H, et al. Novel plant-derived exosome-like nanovesicles from Catharanthus roseus: preparation, characterization, and immunostimulatory effect via TNF-α/NF-κB/PU.1 axis. J Nanobiotechnology. 2023;21(1):160. doi:10.1186/s12951-023-01919-x
35. Zhang M, Viennois E, Prasad M, et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi:10.1016/j.biomaterials.2016.06.018
36. Zhuang X, Deng Z-B, Mu J, et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713. doi:10.3402/jev.v4.28713
37. Ju S, Mu J, Dokland T, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21(7):1345–1357. doi:10.1038/mt.2013.64
38. Berger E, Colosetti P, Jalabert A, et al. Use of Nanovesicles from Orange Juice to Reverse Diet-Induced Gut Modifications in Diet-Induced Obese Mice. Mol Ther Methods Clin Dev. 2020;18:880–892. doi:10.1016/j.omtm.2020.08.009
39. Teng Y, Ren Y, Sayed M, et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe. 2018;24(5):637–652.e8. doi:10.1016/j.chom.2018.10.001
40. Sundaram K, Miller DP, Kumar A, et al. Plant-Derived Exosomal Nanoparticles Inhibit Pathogenicity of Porphyromonas gingivalis. iScience. 2019;21:308–327. doi:10.1016/j.isci.2019.10.032
41. Sundaram K, Mu J, Kumar A, et al. Garlic exosome-like nanoparticles reverse high-fat diet induced obesity via the gut/brain axis. Theranostics. 2022;12(3):1220–1246. doi:10.7150/thno.65427
42. Cao M, Yan H, Han X, et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer. 2019;7(1):326. doi:10.1186/s40425-019-0817-4
43. Song H, Canup BSB, Ngo VL, Denning TL, Garg P, Laroui H. Internalization of Garlic-Derived Nanovesicles on Liver Cells is Triggered by Interaction With CD98. ACS Omega. 2020;5(36):23118–23128. doi:10.1021/acsomega.0c02893
44. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi:10.1016/s0092-8674(04)00045-5
45. Zhang L, Hou D, Chen X, et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2011;22(1):273–274. doi:10.1038/cr.2011.174
46. Xiao J, Feng S, Wang X, et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ. 2018;6:e5186. doi:10.7717/peerj.5186
47. Garcia-Oliveira P, Otero P, Pereira AG, et al. Status and Challenges of Plant-Anticancer Compounds in Cancer Treatment. Pharmaceuticals. 2021;14(2):157. doi:10.3390/ph14020157
48. Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661. doi:10.1021/acs.jnatprod.5b01055
49. Garg S, Kaul SC, Wadhwa R. Cucurbitacin B and cancer intervention: chemistry, biology and mechanisms (Review). Int J Oncol. 2018;52(1):19–37. doi:10.3892/ijo.2017.4203
50. Chen T, Ma B, Lu S, et al. Cucumber-Derived Nanovesicles Containing Cucurbitacin B for Non-Small Cell Lung Cancer Therapy. Int J Nanomed. 2022;17:3583–3599. doi:10.2147/ijn.s362244
51. Deng Z, Rong Y, Teng Y, et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol Ther. 2017;25(7):1641–1654. doi:10.1016/j.ymthe.2017.01.025
52. Vanduchova A, Anzenbacher P, Anzenbacherova E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J Med Food. 2019;22(2):121–126. doi:10.1089/jmf.2018.0024
53. Li Z, Wang H, Yin H, Bennett C, Zhang H-G, Guo P. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Sci Rep. 2018;8(1):14644. doi:10.1038/s41598-018-32953-7
54. Buchman JT, Hudson-Smith NV, Landy KM, Haynes CL. Understanding Nanoparticle Toxicity Mechanisms To Inform Redesign Strategies To Reduce Environmental Impact. Acc Chem Res. 2019;52(6):1632–1642. doi:10.1021/acs.accounts.9b00053
55. Liu J, Jiang F, Jiang Y, et al. Roles of Exosomes in Ocular Diseases. Int J Nanomed. 2020;15:10519–10538. doi:10.2147/ijn.s277190
56. Wang Q, Zhuang X, Mu J, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4:1867. doi:10.1038/ncomms2886
57. Lu M, Xing H, Yang Z, et al. Recent advances on extracellular vesicles in therapeutic delivery: challenges, solutions, and opportunities. Eur J Pharm Biopharm. 2017;119:381–395. doi:10.1016/j.ejpb.2017.07.010
58. Kamen DE, Crotts G, Narasimhan C, et al. An Intercompany Perspective on Compatibility and In-use Stability Studies to Enable Administration Of Biopharmaceutical Drug Products. J Pharm Sci. 2022;111(4):1092–1103. doi:10.1016/j.xphs.2021.09.043
59. Liu C, Yan X, Zhang Y, et al. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J Nanobiotechnology. 2022;20(1):206. doi:10.1186/s12951-022-01421-w
60. Chen Q, Li Q, Liang Y, et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm Sin B. 2022;12(2):907–923. doi:10.1016/j.apsb.2021.08.016
61. Hwang J-H, Park Y-S, Kim H-S, et al. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice. J Control Release. 2023;355:184–198. doi:10.1016/j.jconrel.2023.01.071
62. Chen X, Zhou Y, Yu J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol Pharm. 2019;16(6):2690–2699. doi:10.1021/acs.molpharmaceut.9b00246
63. Kim K, Park J, Sohn Y, et al. Stability of Plant Leaf-Derived Extracellular Vesicles According to Preservative and Storage Temperature. Pharmaceutics. 2022;14(2):457. doi:10.3390/pharmaceutics14020457
64. Han X, Wei Q, Lv Y, et al. Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol Ther. 2022;30(1):327–340. doi:10.1016/j.ymthe.2021.08.028
65. Zhang L, He F, Gao L, et al. Engineering Exosome-Like Nanovesicles Derived from Asparagus cochinchinensis Can Inhibit the Proliferation of Hepatocellular Carcinoma Cells with Better Safety Profile. Int J Nanomed. 2021;16:1575–1586. doi:10.2147/ijn.s293067
66. Yang M, Luo Q, Chen X, Chen F. Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J Nanobiotechnology. 2021;19(1):259. doi:10.1186/s12951-021-00995-1
67. Wang B, Guo X-J, Cai H, et al. Momordica charantia-derived extracellular vesicles-like nanovesicles inhibited glioma proliferation, migration, and invasion by regulating the PI3K/AKT signaling pathway. Journal of Functional Foods. 2022;90:104968. doi:10.1016/j.jff.2022.104968
68. Özkan İ, Koçak P, Yıldırım M, et al. Garlic (Allium sativum)-derived SEVs inhibit cancer cell proliferation and induce caspase mediated apoptosis. Sci Rep. 2021;11(1):14773. doi:10.1038/s41598-021-93876-4
69. Wongkaewkhiaw S, Wongrakpanich A, Krobthong S, Saengsawang W, Chairoungdua A, Boonmuen N. Induction of apoptosis in human colorectal cancer cells by nanovesicles from fingerroot (Boesenbergia rotunda (L.) Mansf.). PLoS One. 2022;17(4):e0266044. doi:10.1371/journal.pone.0266044
70. Kim K, Jung J-H, Yoo HJ, et al. Anti-Metastatic Effects of Plant Sap-Derived Extracellular Vesicles in a 3D Microfluidic Cancer Metastasis Model. J Funct Biomater. 2020;11(3):E49. doi:10.3390/jfb11030049
71. Potestà M, Roglia V, Fanelli M, et al. Effect of microvesicles from Moringa oleifera containing miRNA on proliferation and apoptosis in tumor cell lines. Cell Death Discov. 2020;6:43. doi:10.1038/s41420-020-0271-6
72. Mancuso C, Santangelo R. Panax ginseng and Panax quinquefolius: from pharmacology to toxicology. Food Chem Toxicol. 2017;107(Pt A):362–372. doi:10.1016/j.fct.2017.07.019
73. Kiefer D, Pantuso T. Panax ginseng. Am Fam Physician. 2003;68(8):1539–1542.
74. Chen D, Chen G, Sun Y, Zeng X, Ye H. Physiological genetics, chemical composition, health benefits and toxicology of tea (Camellia sinensis L.) flower: a review. Food Res Int. 2020;137:109584. doi:10.1016/j.foodres.2020.109584
75. Chen D, Ding Y, Chen G, Sun Y, Zeng X, Ye H. Components identification and nutritional value exploration of tea (Camellia sinensis L.) flower extract: evidence for functional food. Food Res Int. 2020;132:109100. doi:10.1016/j.foodres.2020.109100
76. Wang M, Wang S, Hu W, Wang Z, Yang B, Kuang H. Asparagus cochinchinensis: a review of its botany, traditional uses, phytochemistry, pharmacology, and applications. Front Pharmacol. 2022;13:1068858. doi:10.3389/fphar.2022.1068858
77. Dandawate PR, Subramaniam D, Padhye SB, Anant S. Bitter melon: a panacea for inflammation and cancer. Chin J Nat Med. 2016;14(2):81–100. doi:10.1016/s1875-5364(16)60002-x
78. Fang EF, Froetscher L, Scheibye-Knudsen M, Bohr VA, Wong JH, Ng TB. Emerging Antitumor Activities of the Bitter Melon (Momordica charantia). Curr Protein Pept Sci. 2019;20(3):296–301. doi:10.2174/1389203719666180622095800
79. Charu K, Yogita S, Sonali S. Neutraceutical potential of organosulfur compounds in fresh garlic and garlic preparations. Int J Pharm Biol Sci. 2014;5(1):112–126.
80. De Greef D, Barton EM, Sandberg EN, et al. Anticancer potential of garlic and its bioactive constituents: a systematic and comprehensive review. Semin Cancer Biol. 2021;73:219–264. doi:10.1016/j.semcancer.2020.11.020
81. Zhang Y, Liu X, Ruan J, Zhuang X, Zhang X, Li Z. Phytochemicals of garlic: promising candidates for cancer therapy. Biomed Pharmacother. 2020;123:109730. doi:10.1016/j.biopha.2019.109730
82. Eng-Chong T, Yean-Kee L, Chin-Fei C, et al. Boesenbergia rotunda: from Ethnomedicine to Drug Discovery. Evid Based Complement Alternat Med. 2012;2012:473637. doi:10.1155/2012/473637
83. Balakrishnan R, Cho D-Y, Su-Kim I, Choi D-K. Dendropanax Morbiferus and Other Species from the Genus Dendropanax: therapeutic Potential of Its Traditional Uses, Phytochemistry, and Pharmacology. Antioxidants. 2020;9(10):E962. doi:10.3390/antiox9100962
84. Kim K, Yoo HJ, Jung J-H, et al. Cytotoxic Effects of Plant Sap-Derived Extracellular Vesicles on Various Tumor Cell Types. J Funct Biomater. 2020;11(2):E22. doi:10.3390/jfb11020022
85. Pareek A, Pant M, Gupta MM, et al. Moringa oleifera: an Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects. Int J Mol Sci. 2023;24(3):2098. doi:10.3390/ijms24032098
86. clinicaltrials.gov. Available from: https://clinicaltrials.gov/.
87. Pinedo M, de la Canal L, de Marcos Lousa C. A call for Rigor and standardization in plant extracellular vesicle research. J Extracell Vesicles. 2021;10(6):e12048. doi:10.1002/jev2.12048
88. Linares R, Tan S, Gounou C, Arraud N, Brisson AR. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles. 2015;4:29509. doi:10.3402/jev.v4.29509
89. Rutter BD, Innes RW. Growing pains: addressing the pitfalls of plant extracellular vesicle research. New Phytol. 2020;228(5):1505–1510. doi:10.1111/nph.16725
90. Cho E-G, Choi S-Y, Kim H, et al. Panax ginseng-Derived Extracellular Vesicles Facilitate Anti-Senescence Effects in Human Skin Cells: an Eco-Friendly and Sustainable Way to Use Ginseng Substances. Cells. 2021;10(3):486. doi:10.3390/cells10030486
91. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi:10.1080/20013078.2018.1535750
92. Zhao R, Zhao T, He Z, Cai R, Pang W. Composition, isolation, identification and function of adipose tissue-derived exosomes. Adipocyte. 2021;10(1):587–604. doi:10.1080/21623945.2021.1983242
93. You JY, Kang SJ, Rhee WJ. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact Mater. 2021;6(12):4321–4332. doi:10.1016/j.bioactmat.2021.04.023
94. Xiao Q, Zhao W, Wu C, et al. Lemon-Derived Extracellular Vesicles Nanodrugs Enable to Efficiently Overcome Cancer Multidrug Resistance by Endocytosis-Triggered Energy Dissipation and Energy Production Reduction. Adv Sci. 2022;9(20):e2105274. doi:10.1002/advs.202105274
95. Niu W, Xiao Q, Wang X, et al. A Biomimetic Drug Delivery System by Integrating Grapefruit Extracellular Vesicles and Doxorubicin-Loaded Heparin-Based Nanoparticles for Glioma Therapy. Nano Lett. 2021;21(3):1484–1492. doi:10.1021/acs.nanolett.0c04753
96. Correction. Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites. Cancer Res. 2016;76(9):2845. doi:10.1158/0008-5472.can-16-0564
97. Teng Y, Mu J, Hu X, et al. Grapefruit-derived nanovectors deliver miR-18a for treatment of liver metastasis of colon cancer by induction of M1 macrophages. Oncotarget. 2016;7(18):25683–25697. doi:10.18632/oncotarget.8361
98. Wang Q, Zhuang X, Mu J, et al. Corrigendum: delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2016;7:11347. doi:10.1038/ncomms11347
99. Zhang M, Xiao B, Wang H, et al. Edible Ginger-derived Nano-lipids Loaded with Doxorubicin as a Novel Drug-delivery Approach for Colon Cancer Therapy. Mol Ther. 2016;24(10):1783–1796. doi:10.1038/mt.2016.159
100. Zhang M, Wang X, Han MK, Collins JF, Merlin D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine. 2017;12(16):1927–1943. doi:10.2217/nnm-2017-0196
101. Chen K, Yu B. Certain progress of clinical research on Chinese integrative medicine. Chinese Med J. 1999;112(10):934–937.
102. Chen K. The development of integrated traditional Chinese and Western medicine in China. Bulletin Chin Acad Sci. 1995;4:330–332.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.