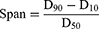Back to Journals » International Journal of Nanomedicine » Volume 19
Highly Drug-Loaded Nanoaggregate Microparticles for Pulmonary Delivery of Cyclosporin A
Authors Huang Y , Tang H, Meng X, Liu D, Liu Y, Chen B, Zou Z
Received 24 May 2024
Accepted for publication 8 July 2024
Published 24 July 2024 Volume 2024:19 Pages 7529—7546
DOI https://doi.org/10.2147/IJN.S470134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Yongpeng Huang, Hui Tang, Xiangyan Meng, Dongxin Liu, Yanli Liu, Bo Chen, Zhiyun Zou
State Key Laboratory of NBC Protection for Civilian, Beijing, People’s Republic of China
Correspondence: Bo Chen; Zhiyun Zou, Email [email protected]; [email protected]
Introduction: Nanoparticles have the advantages of improving the solubility of poorly water-soluble drugs, facilitating the drug across biological barriers, and reducing macrophage phagocytosis in pulmonary drug delivery. However, nanoparticles have a small aerodynamic particle size, which makes it difficult to achieve optimal deposition when delivered directly to the lungs. Therefore, delivering nanoparticles to the lungs effectively has become a popular research topic.
Methods: Nanoaggregate microparticles were used as a pulmonary drug delivery strategy for the improvement of the bioavailability of cyclosporine A (CsA). The nanoaggregate microparticles were prepared with polyvinyl pyrrolidone (PVP) as the excipient by combining the anti-solvent method and spray drying process. The physicochemical properties, aerodynamic properties, in vivo pharmacokinetics and inhalation toxicity of nanoaggregate microparticles were systematically evaluated.
Results: The optimal nanoparticles exhibited mainly spherical shapes with the particle size and zeta potential of 180.52 nm and − 19.8 mV. The nanoaggregate microparticles exhibited irregular shapes with the particle sizes of less than 1.6 μm and drug loading (DL) values higher than 70%. Formulation NM-2 as the optimal nanoaggregate microparticles was suitable for pulmonary drug delivery and probably deposited in the bronchiole and alveolar region, with FPF and MMAD values of 89.62% and 1.74 μm. In addition, inhaled NM-2 had Cmax and AUC0-∞ values approximately 1.7-fold and 1.8-fold higher than oral cyclosporine soft capsules (Neoral®). The inhalation toxicity study suggested that pulmonary delivery of NM-2 did not result in lung function damage, inflammatory responses, or tissue lesions.
Conclusion: The novel nanoaggregate microparticles for pulmonary drug delivery could effectively enhance the relative bioavailability of CsA and had great potential for clinical application.
Keywords: pulmonary drug delivery, dry powder inhalers, nanoaggregate microparticles, aerosol performance, pharmacodynamic study, inhalation toxicity
Graphical Abstract:

Introduction
In drug delivery system, nanoparticles have significant advantages over conventional microparticles and have attracted much attention. Firstly, nanoparticles can improve the solubility and dissolution of poorly water-soluble drugs. In the pharmaceutical industry, it is generally accepted that more than 40% of newly discovered drug candidates are poorly soluble in water. Recently, it has been reported that the new chemical entities have a percentage of up to 90%, and the compounds in development have a percentage of up to 75%.1,2 Nanoparticles can significantly improve the solubility and dissolution rate of drugs due to their larger specific surface area. Additionally, adjusting the physicochemical properties of the nanoparticles can regulate the pharmacokinetics of the drug.3 Secondly, nanoparticles can improve the ability of drug to penetrate mucus. Nanoparticles can help the drug cross biological barriers and deliver it directly to the target site. This increases the accumulation of drug in the lungs and allows it to be delivered to the site of the lesion, effectively reducing the non-specific toxicity.4 Finally, nanoparticle-based dry powder inhalers (DPIs) can significantly reduce phagocytosis by macrophages in pulmonary drug delivery. Particles with a geometric size of 1–2 µm are easily phagocytosed by macrophages,5 and nanoparticles decrease the likelihood of macrophage clearance.6 Additionally, nanoparticles can be deposited in the deep lung, resulting in better lung deposition rates and more precise treatment.
It should be noted that the direct use of drug nanoparticles for pulmonary drug delivery does not produce satisfactory results. To achieve effective particle deposition in the lungs, DPIs should have an aerodynamic diameter ranging from 1 to 5 µm.7 However, nanoparticles typically have small aerodynamic particle sizes, and most of the particles will be exhaled due to Brownian diffusion after inhalation.8 Therefore, effective delivery of nanoparticles to the lungs has been a popular research topic. Nanoaggregate microparticles are one of the most commonly used strategies, which are generally prepared by drying a suspension of primary nanoparticles dissolved with excipients using various drying methods.9 When nanoaggregate microparticles are deposited in the lungs, they will be redistributed into primary nanoparticles to produce therapeutic benefits. The production of nanoparticles involves primarily top-down and bottom-up methods. The top-down method involves reducing large drug particles into nanoparticles through mechanical stress.10 This method offers the advantages of simplicity and ease of large-scale preparation. However, it typically requires a significant amount of time to reduce particle size to less than 100 nm, and mechanical stress during preparation may inactivate peptide and protein drugs. The bottom-up method is a technique for recrystallizing and precipitating dissolved drugs by controlling the conditions. Its advantages include simple instrumentation, low energy consumption, and the ability to operate at room temperature. This method is especially suitable for drugs with poor thermal stability, and it is easier to obtain nanoparticles with a small and uniform particle size and distribution.11 Among the various bottom-up methods, the anti-solvent method is one of the simplest and most cost-effective techniques, which is particularly suitable for peptide and protein drugs with poor water solubility.12 Spray drying is a frequently used technique to prepare primary nanoparticles into nanoaggregate microparticles, which has the advantages of high preparation efficiency, continuous operation and controllable particle size. In addition, the morphology of nanoaggregate microparticles prepared by spray drying is mostly dimpled, wrinkled and hollow, which is conducive to reducing the aerodynamic particle size of DPIs and more appropriate for pulmonary drug delivery.13
During the preparation of nanoaggregate microparticles, mannitol and lactose are the most commonly used excipients, but they have their own shortcomings. Lactose is potentially capable of reacting with proteins in a Maillard reaction and is not appropriate for proteins.14 In addition, lactose is highly hygroscopic and may reduce the aerosol properties of DPIs. Mannitol is not reductive, but it can cause allergic reactions in some patients.15 Furthermore, mannitol is easily recrystallized, which may reduce the redispersing and aerosol properties of nanoaggregate microparticles.16 Using polymers as excipients has become a viable approach to solve these problems. Polyvinyl pyrrolidone (PVP) is a biodegradable and biocompatible polymer with the characteristics of non-toxic, an inert, pH stable and high temperature resistant, which plays an important role in a number of fields such as pharmaceutical preparations, biomedicine, cosmetics and food industry.17,18 As a non-ionic surfactant, PVP is soluble not only in water but also in organic solvents such as methanol and ethanol. It can be used to encapsulate both water-soluble and oil-soluble drugs and is widely used as an excipient in the preparation of oral solid forms, oral liquid forms, eye drops, injections, transdermal preparations, nasal sprays and so on.18
Cyclosporine A (CsA) is a commonly used immunosuppressant for organ transplantation.19 However, its poor water solubility results in low bioavailability when administered orally. As a result, large quantities of CsA must be used for an extended period of time after transplantation, which can lead to side effects including hepatotoxicity and nephrotoxicity.20,21 Thus, the design of novel dosage form of CsA with a low toxicity and high efficacy based on a new drug delivery system is of great value and application prospect. Currently, a large number of clinical trials have been conducted to evaluate the effect of nebulized inhalation of CsA solution in transplantation.22,23 Recently, DPIs have become a research hotspot in the development of new dosage form of CsA, and the reported technologies mainly include freeze-drying, spray drying, spray freeze-drying or a combination of multiple methods.24–28 The delivery strategies of these DPIs can be divided into traditional microparticles, while there are few reports on nanoaggregate microparticles to deliver CsA.
In this paper, we prepared nanoaggregate microparticles for pulmonary delivery of CsA by combining the anti-solvent method and spray drying process. We focused on the effects of the organic solvent type, the CsA concentration and the surfactant type upon the nanoparticle particle size and polydispersity index (PDI). We also systematically evaluated the physicochemical properties, aerodynamic properties, in vivo pharmacokinetics and inhalation toxicity of nanoaggregate microparticles.
Materials and Methods
Materials
Methanol and acetonitrile were supplied by Merck KGaA (Germany). Acetone was purchased from Beijing Chemical Industry Group Co., Ltd (China). Poloxamer 188, PEG 3000 and PEG 8000 were supplied by Shanghai Yuanye Bio-Technology Co., Ltd (China). Tween 20, sodium dodecyl sulfate (SDS) and PVP K30 were supplied by J&K Scientific Co., Ltd (China). Ethanol and hydroxypropyl methylcellulose (HPMC) were supplied by Shanghai Macklin Biochemical Co., Ltd (China). Polyvinyl alcohol (PVA) was purchased from Xiya Reagent (China). CsA was supplied by BioDuly (China). Cyclosporine D (CsD) and A549 cells were purchased from Meilunbio® (China). DMEM was purchased from Thermo Fisher Scientific Inc (USA).
Preparation of Nanoparticles
CsA nanoparticles were produced by means of the anti-solvent method. Briefly, CsA (5, 10, and 20 mg/mL) was dissolved in organic solvents (methanol, ethanol, acetonitrile and acetone) and passed through a filter (0.45 μm) to separate insoluble impurities. The resulting solution was added slowly to 100 mL of water with 0.1% surfactant at a speed of 1 mL/min under the condition of room temperature and 1000 r/min. CsA nanoparticles were produced by continuous stirring and sonication for 5 minutes after the dropwise addition was completed.
Preparation of Nanoaggregate Microparticles
CsA nanoaggregate microparticles were produced by using a spray drying process. Firstly, CsA nanoparticles underwent centrifugation at 14000 r/min for 30 minutes, and the obtained precipitate was washed three times with pure water to completely remove the surfactant. The precipitate was then re-dispersed by sonication with 100 mL of an aqueous solution of PVP (0.04, 0.08, and 0.12 mg/mL). Finally, the re-dispersed nanoparticles were dried with a B-290 spray dryer (BUCHI Ltd., Switzerland), resulting in the formation of CsA nanoaggregate microparticles. The closed-loop blowing mode was used to operate the spray dryer using a B-295 device (BUCHI Ltd., Switzerland). The dual-fluid 0.7 mm spray tip was utilized, and the entrance temperature was fixed at 170 °C. The injection rate was set to 20%, while the gas flow rate and aspirator rate were set to 414 L/h and 100%.
Particle Size and Distribution
The Zetasizer Nano ZS90 (Malvern Panalytical Ltd., UK) was used by the dynamic light scattering technique to analyze the zeta potential, PDI and particle size of CsA nanoparticles. The measurements were taken with the temperature of 25.0 ± 0.1 °C and intense light scattering angle of 90°.
The Mastersizer 3000 (Malvern Panalytical Ltd., UK) was applied by the laser diffraction analysis technique to analyze the particle size of CsA nanoaggregate microparticles. The measurements were taken with the aero dispersion units. D50 and Span were applied to define the particle size and distribution, respectively. The calculation of Span was performed according to Eq. (1).
Drug Loading (DL)
The HPLC method described in published literature was used to determine the DL of CsA in nanoaggregate microparticles.29 Briefly, nanoaggregate microparticles were dissolved in ethanol-water (50:50, v/v), and the resulting solution was injected into a 1260 Infinity II system (Agilent Technologies Inc., USA) to analyze the CsA content. The mobile phases and column were set to water-acetonitrile (40:60, v/v) and ZORBAX 300 SB C8 column (4.6 mm × 250 mm, 5.0 μm), respectively. The pump speed and column temperature were fixed at 1.0 mL/min and 60 °C, respectively. The injection volume and detection wavelength were set to 10 μL and 205 nm, respectively.
Scanning Electron Microscopy (SEM)
The surface structures of the particles were investigated using a SU8020 instrument (Hitachi Ltd., Japan). Before imaging, the particles were covered with a layer of gold by an MC 1000 ion sputtering system (Hitachi Ltd., Japan).
Powder X-Ray Diffraction (PXRD)
The crystalline structure of the particles was investigated by means of PXRD with the Cu-Kα radiation and D8 Advance diffractometer (Bruker, Germany). The tube current and tube voltage of 40 mA and 40 kV were used, respectively. At a scanning speed of 2°/min, the diffraction images were recorded in the region from 3 to 40° 2θ.
Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
The DSC curve of the particles was investigated by a Q 100 device (TA Instruments, USA). The DSC curve was recorded in the range of 40 °C to 250 °C with a temperature rise speed of 10 °C/min, and the nitrogen stream was set to 20 mL/min.
The TGA curve of the particles was investigated by a Q 500 device (TA Instruments, USA). The TGA curve was recorded in the range of 30 °C to 450 °C with a temperature rise speed of 10 °C/min, and the nitrogen stream was set to 60 mL/min.
In vitro Release Studies
Simulated lung fluid (SLF) was used to evaluate the in vitro release characteristics of the particles. Briefly, nanoaggregate microparticles (ca. 2 mg CsA) were carefully measured and transferred to 100 mL of SLF. The stirring speed and temperature of the SLF were controlled at 100 r/min and 37 °C, respectively, with an MR Hei-Tec device (Heidolph Instruments GmbH & Co., KG., Germany). The release of CsA from nanoaggregate microparticles was monitored at specific intervals using the HPLC method described above.
The SLF was developed in accordance with the published literature,30 which was made up of 0.01% dipalmitoylphosphatidylcholine, 0.19 g/L Glycine, 0.16 g/L Na3citrate·2H2O, 0.12 g/L NH4Cl, 0.15 g/L Na2HPO4, 0.18 g/L Na2SO4·10H2O, 0.10 g/L MgCl2, 2.70 g/L NaHCO3, 6.40 g/L NaCl and 0.26 g/L CaCl2·2H2O.
In vitro Aerosol Performance
A Next Generation Impactor (NGI, Copley Scientific Ltd., UK) was applied to investigate the simulated lung deposit of nanoaggregate microparticles at 90 L/min. All processes were performed in accordance with the Chapter <601> specification of United States Pharmacopoeia. The particles collected at each position of NGI were rinsed with ethanol-water (50:50, v/v), and CsA was detected using the HPLC method described above. The mass median aerodynamic diameter (MMAD) was employed to describe the aerodynamic diameter. The geometric standard deviation (GSD) was employed to characterize the aerodynamic diameter distribution. The fine particle fraction (FPF) was considered to be the fraction with the aerodynamic diameters less than 5 μm. MMAD, GSD and FPF were determined by means of the Inhalytix software.
Cell Viability Assays
In vitro cytotoxicity assay of nanoaggregate microparticles on A549 cells was investigated. A549 cells cultured in DMEM medium were seeded at 2000 colonies/well in a 96-well plate and grown at 37 °C for 24 h. The cells were exposed to nanoaggregate microparticles and then grown for 48 h, and the terminal concentration of CsA was as high as 100 μg/mL. Following this, cells were then grown for 1 h with 20 μL of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) applied to each well. The sample absorption was checked at 490 nm (630 nm as a reference standard) with a Multiskan GO device (Thermo Fisher Scientific Inc., USA), and the cytotoxicity on A549 cells was assessed according to Eq. (2).
Where, Asample refers to the absorbance of cells containing nanoaggregate microparticles, Acontrol refers to the absorbance of cells excluding nanoaggregate microparticles, and Ablank refers to the absorbance of original cells.
In vivo Pharmacodynamic Study
Pharmacodynamic Study of CsA in Rats
Eighteen male SD rats were separated at random into three groups: intravenous administration of cyclosporine soft capsules (Neoral®), oral administration of cyclosporine soft capsules, and inhalation administration of nanoaggregate microparticles. The doses of administration for all three groups were 0.4 mg CsA/kg in accordance with the published literature.29 The rats were anesthetized with isoflurane prior to inhalation administration of nanoaggregate microparticles, which were then delivered via the trachea into the lungs with the help of the particle delivery equipment (Huironghe Technology Co., Ltd., Beijing, China). At predetermined intervals, an aliquot (50 μL) of blood was obtained via intubation of the rat jugular vein for analysis using the HPLC-MS/MS method in accordance with the published literature.29
Prior to testing, blood samples were processed as follows: 50 μL of cyclosporine D (600 ng/mL, internal standard), 50 μL of ZnSO4 (0.5 mol/L) and 350 μL of methanol were transferred to the blood sample, and vortexed for 30s. The mixture was stored at –20 °C for 30 min and then centrifuged at 14000 r/min for 10 min. After filtration with a membrane (0.45 μm), the supernatant was infused into a 1290–6470 Triple Quad (Agilent Technologies Inc., USA) for analysis. The column and column temperature were set to ZORBAX 300 SB C8 column (2.1 mm × 100 mm, 1.8 μm) and 60 °C, respectively. The mobile phases were set to 0.1% formic acid containing 10 mmol/L ammonium formate (A) and acetonitrile (B). The pump speed and infused amount were fixed at 0.5 mL/min and 5 μL, respectively. The program for gradient elution was adjusted as shown below: 50%A (0–3 min); 50%A–30%A (3–5 min); 30%A–50%A (5–5.1 min); 50%A (5.1–7 min). The ESI+ mode and MRM program were applied. The precursor ion and product ion of internal standard were set to 609.5 and 156.2, respectively. The precursor ion and product ion of CsA were set to 602.5 and 156.2, respectively.
Residue Study of CsA in the Lungs of Rats
The residue of CsA in the lungs of rats after inhalation of nanoaggregate microparticles was further examined, and the method of inhalation administration and the doses of administration were consistent with those described above. At predetermined intervals, the residue of CsA in the alveolar macrophages was assessed by collecting bronchoalveolar lavage (BAL) fluid from the rats, and the residue of CsA in the lungs was assessed by dissecting and sampling the lung tissue.
The method used to collect the BAL samples was based on literature with minor changes.31 Briefly, 3 mL of PBS(pH = 7.4) was injected into the lungs and washed 3 times. The BAL cells were collected by centrifuging the BAL samples at 1700 r/min for 10 min, which were resuspended in 0.5 mL of saline and disrupted with the milling method. The suspension was diluted with 0.5 mL of methanol and then spun down at 14000 r/min for 10 min. Finally, the HPLC-MS/MS method described above was used to process and detect the supernatant.
The lungs were extracted with 8 mL of ethyl acetate after homogenization in 2 mL of saline. The mixture was centrifugated at 14000 r/min for 10 min to collect the organic phase. After three extractions, the organic phase was dried by a rotary evaporator, and 10 mL of acetonitrile was added to redissolve the residues. The solution was centrifugated at 14000 r/min for 5 min after storage at –20 °C for 30 min. Then, 50 μL of the supernatant was mixed with 50 μL of cyclosporine D (600 ng/mL) and 350 μL of acetonitrile. Finally, the above mentioned HPLC-MS/MS method was used to detect the sample.
Repeated Dose Inhalation Toxicity
Twelve male SD rats were separated at random into three groups: control group, low-dose group and high-dose group. The method of inhalation administration was consistent with that described above. The control group was treated with air. The doses of inhaled nanoaggregate microparticles for low-dose group and high-dose group were 0.4 and 0.8 mg CsA/kg, respectively. The rats were exposed to nanoaggregate microparticles through inhalation for 14 consecutive days and were administered once daily.
The animals were monitored daily for health and weight during the study. After the experiment, the Madlab-4C/501H instruments (Beijing Zhongshi Dichuang Technology Development Co., Ltd., China) was used to assess the pulmonary function of rats. Briefly, the fur of neck was plucked when 1% pentobarbital sodium was injected intraperitoneally to anesthetize the rat. Then, a 2 cm incision was cut from the neck to reveal the air tube. Finally, the FEV0.3/FVC value, which was defined as forced expiratory volume over 0.3 s divided by forced vital capacity, was measured by inserting the respiratory sensor into the air tube.
The BAL samples were obtained according to the method described above, which were used to assess cytokine levels with ELISA test. The rats were executed to collect specimens. Blood samples were obtained to assess the biochemical parameters. The tissues (kidney, lung, liver, pancreas and spleen) were obtained and weighed, which were placed in 10% formalin solutions and then embedded in paraffin for H&E staining to perform the histopathologic evaluation.
Statistical Analysis
All experimental data were shown as mean ± standard deviation (SD). Statistical significance was analyzed using t tests, and the value of P lower than 0.05 was deemed to be a statistically significant difference.
Results
Particle Size of Nanoparticles
Effect of Surfactant on Particle Size
The effect of surfactant on particle size and PDI of nanoparticles was investigated using ethanol as the solvent and the CsA concentration of 10 mg/mL. As shown in Figure 1A and B, the particle size of the nanoparticles prepared using the same surfactant basically tended to increase as the volume ratio of solvent to anti-solvent increased. Within the experimental range, the particle sizes were all less than 350 nm and the PDIs were all less than 0.3 when poloxamer 188 was used as the surfactant, indicating that the nanoparticles prepared with poloxamer 188 as the surfactant were more stable. Therefore, poloxamer 188 was used as the surfactant in subsequent studies.
 |
Figure 1 Influence of (A and B) surfactant, (C and D) organic solvent and (E and F) CsA concentration on particle size and PDI of nanoparticles (mean ± SD, n = 3). |
Effect of Organic Solvent on Particle Size
The effect of organic solvent, including methanol, ethanol, acetonitrile and acetone, on nanoparticle size and PDI was investigated using poloxamer 188 as the surfactant and the CsA concentration of 10 mg/mL. As shown in Figure 1C and D, the nanoparticles prepared with different solvents had significant differences in particle size and PDI. Under the same experimental conditions, the nanoparticles prepared using methanol as the solvent had the smallest particle size, with the values of less than 300 nm and PDI less than 0.3. These results suggested that CsA nanoparticles prepared with methanol as the solvent were superior.
Effect of CsA Concentration on Particle Size
The effect of CsA concentration on nanoparticle size and PDI was investigated using poloxamer 188 as the surfactant and methanol as the solvent. As shown in Figure 1E and F, under the same experimental conditions, the particle size of nanoparticles was smallest with the PDI less than 0.3 at a CsA concentration of 10 mg/mL. To achieve a high yield and particle size less than 200 nm, the CsA nanoparticles were finally prepared using methanol as the solvent, poloxamer 188 as the surfactant, CsA concentration of 10 mg/mL and solvent to anti-solvent volume ratio of 0.04. Moreover, the particle size was 180.52 ± 3.20 nm with PDI and zeta potential values of 0.08 ± 0.03 and −19.8 ± 0.4 mV, respectively.
Particle Size and DL of Nanoaggregate Microparticles
The nanoaggregate microparticles were prepared by using the spray drying method to dry the nanoparticles redispersed with PVP solution. The results of DL, particle size and distribution of nanoaggregate microparticles were shown in Table 1. The particle size and distribution of nanoaggregate microparticles exhibited an increasing tendency with the increase in PVP concentration. The particle sizes were all less than 1.6 µm and the Span values were all less than 2.3, indicating that nanoaggregate microparticles had the small geometric particle size and narrow distribution. Furthermore, nanoaggregate microparticles showed high DL values, none less than 70%. The DL values were all less than the theoretical values, which could be attributed to incomplete precipitation and recovery of nanoparticles during the treatment process.
 |
Table 1 Content, Particle Size and Distribution of Nanoaggregate Microparticles (Mean ± SD, n = 3) |
Appearance of Particles
Appearance of the nanoparticles and nanoaggregate microparticles was shown in Figure 2. The nanoparticles exhibited mainly spherical or nearly spherical shapes, and the individual particle sizes were basically consistent with the results of DLS detection. Completely different from nanoparticles in appearance and particle size, the nanoaggregate microparticles exhibited irregular shapes with wrinkled or concave surfaces and larger particle sizes, indicating that the nanoaggregate microparticles were successfully obtained. Additionally, such DPIs with these shapes could decrease particle interactions and enhance the flow and aerosol properties of the particles, which were beneficial for pulmonary drug delivery.32–34
PXRD
The PXRD curves of CsA, PVP and nanoaggregate microparticles were shown in Figure 3A. No typical crystalline peaks were found for CsA and PVP, suggesting that they were both in the amorphous state.35,36 Similar to CsA and PVP, the nanoaggregate microparticles (NM-1, NM-2, and NM-3) were all in the amorphous state, as evidenced by the absence of clearly visible crystalline peaks. The use of amorphous formulations was advantageous for the poorly water-soluble drugs because it could enhance their dissolving properties and pharmacological effect.37–39
TGA and DSC
The thermal stability and water content of nanoaggregate microparticles were evaluated by the TGA and DSC methods, and the results were shown in Figure 3B and C. The TGA curves of CsA, PVP and nanoaggregate microparticles did not exhibit any significant weight loss under 120 °C, suggesting the absence of significant water content. CsA and PVP started to show obvious weight loss at greater than 244 °C and 180 °C, respectively, indicating the beginning of their thermal decomposition. Nanoaggregate microparticles (NM-1, NM-2, and NM-3) exhibited multistage weight loss at temperatures above 200 °C as a result of the decomposition of PVP and drug, and the initial decomposition temperature decreased as the PVP content increased. The data suggested a minor reduction in the thermal stability of nanoaggregate microparticles in comparison to CsA.
The DSC curve of CsA displayed no strong endothermic peak, except for a weak endotherm at 132 °C, which represented its glass transition temperature.40 This suggested that CsA was in the amorphous state. The DSC curve of PVP displayed a broad and weak endothermic peak below 100 °C due to the loss of water. Additionally, a strong endothermic peak was observed at 224 °C caused by the decomposition of PVP, which was also confirmed by the TGA results. Interestingly, the DSC curves of nanoaggregate microparticles (NM-1, NM-2, and NM-3) all displayed no obvious endothermic peak compared to CsA and PVP over the experimental temperature range. The DSC results suggested that CsA, PVP and nanoaggregate microparticles were in the amorphous state, supporting the PXRD conclusions.
In vitro Release Studies
The dissolution property of the poorly water-soluble drug is a major consideration for the application in the clinic.41 As shown in Figure 3D, approximately 50% of CsA was rapidly released from nanoaggregate microparticles (NM-1, NM-2, and NM-3) within the first 30 min. The amount of dissolved CsA essentially reached equilibrium after 60 min, and no crystallization was observed within 180 min. The dissolution profiles of three nanoaggregate microparticles were similar, indicating that PVP did not significantly affect the dissolution rate of CsA. In addition, nanoaggregate microparticles improved the dissolution rate of drug compared to raw CsA. The results could be attributed to two factors. Firstly, PVP is commonly used as the excipient to enhance the dissolution properties of poorly water-soluble drugs, thereby increasing their bioavailability.36,42 Secondly, nanoaggregate microparticles had the smaller particle size, which is a commonly used strategy to enhance the dissolution properties of poorly water-soluble drugs.43,44
In vitro Aerosol Performance
The effective disposition of DPIs in the lungs is a critical factor in pulmonary drug delivery. Simulated drug lung deposition of nanoaggregate microparticles was assessed by NGI, and the results were presented in Figure 4 and Table 2. The throat and pre-separator of NGI mainly simulate the upper respiratory tract areas such as nose, pharynx and throat, while the impactor stages mainly simulate the lower respiratory tract areas such as bronchi, bronchioles and alveoli. The nanoaggregate microparticles in both throat and the pre-separator were found to have a deposition rate of below 5%, demonstrating that the nanoaggregate microparticles did not contain significantly larger particles and were easily able to be inhaled and transported to the trachea. In addition, the nanoaggregate microparticles were primarily precipitated in stage 3 to 5, predicting the small aerodynamic particle size and narrow distribution. As a crucial factor in evaluating the number of respirable particles of DPIs, the FPF values for nanoaggregate microparticles were calculated to be in the range of 82% to 90%. The MMAD values ranged from 1.7 to 2.1 μm, indicating that nanoaggregate microparticles were suitable for pulmonary drug delivery and ideal for deposit in the bronchiole and alveolar region.45–47 The GSD values were calculated to be less than 1.9, indicating that aerodynamic diameter distribution of nanoaggregate microparticles was narrow. Furthermore, the formulation of NM-2 exhibited the highest FPF value and the lowest MMAD and GSD values, making it the ideal DPIs for further research.
 |
Table 2 The Aerodynamic Results of Nanoaggregate Microparticles (Mean ± SD, n = 3) |
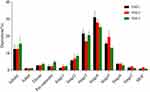 |
Figure 4 Simulated drug lung deposition of nanoaggregate microparticles (mean ± SD, n = 3). |
In vitro Cytotoxicity Assays
The cytotoxic effect was performed to evaluate the initial safety feature of nanoaggregate microparticles (NM-2) in human cells. The relationship between dose and cell viability of nanoaggregate microparticles on A549 cells was evaluated after 48 h of exposure. As shown in Figure 5, it was found that the nanoaggregate microparticles showed no in vitro cytotoxicity at a concentration of up to 100 µg/mL (equivalent to the level of CsA). Based on the lack of cytotoxicity, the nanoaggregate microparticles with PVP as the excipient were deemed to be safe for pulmonary drug delivery.
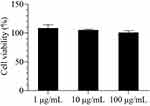 |
Figure 5 Cytotoxic effect of nanoaggregate microparticles on A549 cell line (mean ± SD, n = 3). |
In vivo Pharmacodynamic Study
The pharmacodynamic curves in rats were presented in Figure 6A, and pharmacodynamic parameters were presented in Table 3. The plasma level of CsA was significantly improved after inhaling nanoaggregate microparticles (NM-2) with the Cmax and AUC0-∞ values of 90.1 ng/mL and 956.4 ng·h/mL, respectively, which were about 1.7-fold and 1.8-fold increased over oral cyclosporine soft capsules. Inhaled nanoaggregate microparticles (NM-2) exhibited a slight increase and decrease in Tmax and T1/2 values compared to oral cyclosporine soft capsules, respectively, which were not very meaningful due to the minor change in values. In addition, the absolute bioavailability of inhaled nanoaggregate microparticles (NM-2) was 37.8%, which could be obtained by calculating its AUC0-∞ ratio to intravenous cyclosporine soft capsules. The pharmacodynamic results indicate that pulmonary delivery of nanoaggregate microparticles can effectively deliver CsA to the systemic circulation and achieve effective therapeutic concentration at a low systemic exposure dose compared to oral delivery, which has a good prospect for clinical application.
 |
Table 3 The Pharmacodynamic Parameters of CsA Formulations in Rats (Mean ± SD, n = 6) |
 |
Figure 6 (A) Pharmacodynamic curves of CsA in rats (mean ± SD, n = 6) and (B) residue of CsA in BAL cells and lungs of rats following inhaled nanoaggregate microparticles (mean ± SD, n = 4). |
In addition, the residue of CsA in BAL cells and lungs of rats following inhaled nanoaggregate microparticles (NM-2) was further investigated. As shown in Figure 6B, the amount of CsA in rat lungs could be rapidly decreased to a very low level when nanoaggregate microparticles were deposited into the moist lungs. This suggested that PVP as the excipient could be rapidly dissolved to release primary CsA nanoparticles, which were then rapidly introduced into the systemic circulation. Furthermore, the CsA level in BAL cells was extremely low, demonstrating that nanoaggregate microparticles could significantly escape phagocytosis by macrophages. It was related to the nature of nanoaggregate microparticles, which had the excellent MMAD value (1.7 μm) that allow them to be efficiently deposited into the deep lungs. Meanwhile, the released primary nanoparticles could significantly reduce macrophage phagocytosis, enabling efficient drug delivery.9,48
Inhalation Toxicity
PVP is considered to be a biocompatible polymer that is widely used in the pharmaceutical formulation and food industry, and the pharmacokinetics and toxicity of PVP has been extensively evaluated.49 However, it is worth noting that PVP has not been approved for use as an excipient for pulmonary drug delivery. On this basis, the repeated dose inhalation toxicity study for 14 days was conducted on nanoaggregate microparticles (NM-2) to examine the suitability for pulmonary drug delivery.
During the experiment, all the rats grew normally without any fatalities, and their body weight showed a uniform increase, indicating that inhaled nanoaggregate microparticles did not have any significant effect on the appetite or metabolism of rats (Figure 7A). The FEV0.3/FVC values of the rats in the experimental group were not significantly different from those of the control group, indicating that the deposition of nanoaggregate microparticles into the lungs did not have an impact on the respiratory system of rats during the experiment (Figure 7B). Compared with the control group, the weights of the five major tissues of rats in the experimental group remained within an acceptable range, indicating that nanoaggregate microparticles did not cause significant damage to the major tissues after entering the systemic circulation and being metabolized (Figure 7C). Moreover, H&E staining results of the major tissues exhibited no visible evidence of necrosis or lesions, indicating the good biocompatibility and safety of PVP as the excipient for pulmonary drug delivery (Figure 8). Biochemical parameters of rats were also assessed and most of them fluctuated within a small range compared to the control group, while a few parameters such as ALT, LDH and UA fluctuated within a larger range but still within acceptable limits (Table 4).
 |
Table 4 Evaluation of Biochemical Parameters in Rats (Mean ± SD, n = 4) |
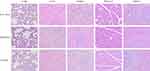 |
Figure 8 H&E staining of main tissues of rats to assess inhalation toxicity of nanoaggregate microparticles. |
Cytokines modulate the immune of host reactions to trauma, inflammation and infection, and several of them have a crucial function in the development of inflammation.50 IL-4 and IL-13 collaborate to promote the IgE generation from B cells, stimulate the development of eosinophilic inflammation, and enhance mucus hypersecretion, airway fibrosis, and re-molding.51 IL-5 enhances eosinophil differentiation from bone marrow and extends their survival in the airways, making it a key player in eosinophilic inflammation.52 TNF-α functionally induces a number of different inflammatory molecules and plays a crucial role in inflammatory responses and the development of certain autoimmune and inflammatory diseases.53 To further examine whether the inflammatory response occurred in the lungs after inhaled nanoaggregate microparticles, the cytokine levels of IL-4, IL-5, IL-13 and TNF-α were further assessed (Figure 7D–G). No significant differences were found in the levels of all cytokines between the low-dose and high-dose groups when compared to the control group, indicating that inhaled nanoaggregate microparticles did not cause a significant inflammatory response in the lungs, confirming the biocompatibility and safety of PVP as the excipient for pulmonary drug delivery. Similarly, Nair found no inflammatory response in rats exposed to the PVP solution at a concentration of 118 μg/mL for 8 h/day over a period of 30 days.54
In conclusion, PVP as the excipient was safe and biocompatible for pulmonary drug delivery with broad application prospects, and did not cause lung function damage, inflammatory responses, or tissue lesions.
Discussion
Pulmonary drug delivery has the characteristics of non-invasive administration, high blood drug concentration, rapid absorption rate, no first-pass effect and low enzyme activity, making it an effective drug delivery system for improving the bioavailability of CsA. In addition, pulmonary delivery of CsA can significantly reduce the systemic exposure dose, thereby reducing their toxic side effects.55 At present, the DPIs that have been reported for the delivery of CsA to the lungs are mainly microparticles. In comparison, nanoparticles are more promising DPIs, but their effective delivery to the lungs is a tricky problem because their aerodynamic diameter is small and easily exhaled. Therefore, nanoparticles should be transformed into micrometer-scale nanocomposite structures with aerodynamic diameters between 1 and 5 μm to optimize delivery efficiency.
In this systematic and broad investigation, novel nanoaggregate microparticles for pulmonary delivery of CsA were efficiently designed and successfully manufactured by combining the anti-solvent method and spray drying process. The anti-solvent method for the preparation of nanoparticles is not only suitable for organic small-molecule drugs but also for macromolecular drugs such as peptides and proteins. The optimal particle size was 180.52 nm when nanoparticles of CsA were prepared by the anti-solvent method. This was advantageous for pulmonary drug delivery as it was able to evade macrophage phagocytosis and pass directly from the alveoli into the systemic circulation.9,56 Spray drying is a common method for manufacturing DPIs because it can customize and optimize particle characteristics such as surface morphology and particle size.57,58 In this study, the nanoaggregate microparticles of CsA were further prepared by spray drying with PVP as the excipient. The SEM results further confirmed the successful preparation of nanoaggregate microparticles with wrinkled structures, as their geometric particle size was significantly larger than that of nanoparticles. In addition, the particles with this morphology were advantageous for pulmonary drug delivery due to their ability to improve the flowability of DPIs.
The PXRD and TGA results confirmed that the nanoaggregate microparticles had an amorphous structure and almost no water content. This type of DPIs had advantages for pulmonary drug delivery, as high moisture content could lead to particle aggregation and affect the aerosol properties of DPIs. It should be noted that particles with amorphous structure had high moisture absorption and poor stability, which might undergo crystalline transformation during storage.59 Therefore, future work should also consider long-term stability studies to evaluate their practical applicability in clinical settings.
The NGI results demonstrated the excellent aerosol properties of nanoaggregate microparticles, as reflected by significantly high FPF and MMAD values. In addition, the MMAD values were in the range of 1.7–2.1 µm for targeting the smaller airways. The in vivo pharmacokinetic results further confirmed that pulmonary delivery of nanoaggregate microparticles significantly improved the relative bioavailability of CsA. Moreover, the extremely low content of CsA in BAL cells demonstrated the significant escape of nanoaggregate microparticles from macrophage phagocytosis, which further reflected the advantages of nanoparticles in pulmonary drug delivery. The inhalation toxicity results have demonstrated that PVP has good safety and biocompatibility as an excipient for pulmonary drug delivery, which is also of great significance for the approval of new carrier excipient.
Conclusions
Nanoaggregate microparticles for pulmonary delivery of CsA were prepared by combining the anti-solvent method and spray drying process. The nanoaggregate microparticles exhibited high DL values, all above 70%. Formulation NM-2 as the optimal nanoaggregate microparticles was suitable for pulmonary drug delivery and probably deposited in the bronchiole and alveolar region, with FPF and MMAD values of 89.62% and 1.74 μm, respectively. In addition, the in vivo pharmacodynamic data suggested that pulmonary delivery of NM-2 in rats could effectively enhance the relative bioavailability of drug in comparison to oral delivery. The inhalation toxicity study suggested that pulmonary delivery of NM-2 did not result in lung function damage, inflammatory responses, or tissue lesions. In summary, the nanoaggregate microparticles for pulmonary drug delivery may be a feasible delivery strategy to enhance the bioavailability of CsA.
Ethics Approval and Consent to Participate
The Institutional Animal Care and Ethical Committee of State Key Laboratory of NBC Protection for Civilian gave approval to the animal testing (No. LAE-2023-02-001), and all experiments were done in compliance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Acknowledgments
The authors are grateful for State Key Laboratory of NBC Protection for Civilian.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5(5):442–453. doi:10.1016/j.apsb.2015.07.003
2. Rodriguez-Aller M, Guillarme D, Veuthey JL, Gurny R. Strategies for formulating and delivering poorly water-soluble drugs. J Drug Deliv Sci Tec. 2015;30:342–351. doi:10.1016/j.jddst.2015.05.009
3. Fattal E, Fay F. Nanomedicine-based delivery strategies for nucleic acid gene inhibitors in inflammatory diseases. Adv Drug Deliv Rev. 2021;175:113809. doi:10.1016/j.addr.2021.05.019
4. Jin Z, Gao Q, Wu K, Ouyang J, Guo W, Liang XJ. Harnessing inhaled nanoparticles to overcome the pulmonary barrier for respiratory disease therapy. Adv Drug Deliv Rev. 2023;202:115111. doi:10.1016/j.addr.2023.115111
5. Shetty N, Cipolla D, Park H, Zhou QT. Physical stability of dry powder inhaler formulations. Expert Opin Drug Deliv. 2020;17(1):77–96. doi:10.1080/17425247.2020.1702643
6. Gaikwad SS, Pathare SR, More MA, et al. Dry Powder Inhaler with the technical and practical obstacles, and forthcoming platform strategies. J Control Release. 2023;355:292–311. doi:10.1016/j.jconrel.2023.01.083
7. Guo Y, Bera H, Shi C, Zhang L, Cun D, Yang M. Pharmaceutical strategies to extend pulmonary exposure of inhaled medicines. Acta Pharm Sin B. 2021;11(8):2565–2584. doi:10.1016/j.apsb.2021.05.015
8. Ke WR, Chang RYK, Chan HK. Engineering the right formulation for enhanced drug delivery. Adv Drug Deliv Rev. 2022;191:114561. doi:10.1016/j.addr.2022.114561
9. Chan HW, Chow S, Zhang X, Zhao Y, Tong HHY, Chow SF. Inhalable nanoparticle-based dry powder formulations for respiratory diseases: challenges and strategies for translational research. AAPS Pharm Sci Tech. 2023;24(4):98. doi:10.1208/s12249-023-02559-y
10. Yue P, Zhou W, Huang G, et al. Nanocrystals based pulmonary inhalation delivery system: advance and challenge. Drug Deliv. 2022;29(1):637–651. doi:10.1080/10717544.2022.2039809
11. Zhang X, Xia Q, Gu N. Preparation of all-trans retinoic acid nanosuspensions using a modified precipitation method. Drug Dev Ind Pharm. 2006;32(7):857–863. doi:10.1080/03639040500534184
12. Sinha B, Müller RH, Möschwitzer JP. Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int J Pharm. 2013;453(1):126–141. doi:10.1016/j.ijpharm.2013.01.019
13. Cheow WS, Li S, Hadinoto K. Spray drying formulation of hollow spherical aggregates of silica nanoparticles by experimental design. Chem Eng Res Des. 2010;88(5):673–685. doi:10.1016/j.cherd.2009.11.012
14. Liang W, Pan HW, Vllasaliu D, Lam JKW. Pulmonary delivery of biological drugs. Pharmaceutics. 2020;12(11):1025. doi:10.3390/pharmaceutics12111025
15. Flume PA, Aitken ML, Bilton D, et al. Optimising inhaled mannitol for cystic fibrosis in an adult population. Breathe. 2015;11(1):39–48. doi:10.1183/20734735.021414
16. Lee YY, Wu JX, Yang M, Young PM, van den Berg F, Rantanen J. Particle size dependence of polymorphism in spray-dried mannitol. Eur J Pharm Sci. 2011;44(1–2):41–48. doi:10.1016/j.ejps.2011.06.002
17. Koczkur KM, Mourdikoudis S, Polavarapu L, Skrabalak SE. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015;44(41):17883–17905. doi:10.1039/C5DT02964C
18. Teodorescu M, Bercea M, Morariu S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: perspectives and challenges. Biotechnol Adv. 2019;37(1):109–131. doi:10.1016/j.biotechadv.2018.11.008
19. Zhu J, McKeon F. NF-AT activation requires suppression of Crm1-dependent export by calcineurin. Nature. 1999;398(6724):256–260. doi:10.1038/18473
20. Korolczuk A, Caban K, Amarowicz M, Czechowska G, Irla-Miduch J. Oxidative stress and liver morphology in experimental cyclosporine A-induced hepatotoxicity. Biomed Res Int. 2016;2016:5823271. doi:10.1155/2016/5823271
21. Wu Q, Wang X, Nepovimova E, Wang Y, Yang H, Kuca K. Mechanism of cyclosporine A nephrotoxicity: oxidative stress, autophagy, and signalings. Food Chem Toxicol. 2018;118:889–907. doi:10.1016/j.fct.2018.06.054
22. Iacono AT, Johnson BA, Grgurich WF, et al. A randomized trial of inhaled cyclosporine in lung-transplant recipients. N Engl J Med. 2006;354(2):141–150. doi:10.1056/NEJMoa043204
23. Behr J, Zimmermann G, Baumgartner R, et al. Lung deposition of a liposomal cyclosporine A inhalation solution in patients after lung transplantation. J Aerosol Med Pulm Drug Deliv. 2009;22(2):121–130. doi:10.1089/jamp.2008.0714
24. Matilainen L, Järvinen K, Toropainen T, et al. In vitro evaluation of the effect of cyclodextrin complexation on pulmonary deposition of a peptide, cyclosporin A. Int J Pharm. 2006;318(1–2):41–48. doi:10.1016/j.ijpharm.2006.03.009
25. Leung SS, Wong J, Guerra HV, Samnick K, Prud’homme RK, Chan HK. Porous mannitol carrier for pulmonary delivery of cyclosporine A nanoparticles. AAPS J. 2017;19(2):578–586. doi:10.1208/s12248-016-0039-3
26. Niwa T, Mizutani D, Danjo K. Spray freeze-dried porous microparticles of a poorly water-soluble drug for respiratory delivery. Chem Pharm Bull. 2012;60(7):870–876. doi:10.1248/cpb.c12-00208
27. Onoue S, Sato H, Ogawa K, et al. Inhalable dry-emulsion formulation of cyclosporine A with improved anti-inflammatory effects in experimental asthma/COPD-model rats. Eur J Pharm Biopharm. 2012;80(1):54–60. doi:10.1016/j.ejpb.2011.10.003
28. D’Angelo D, Quarta E, Glieca S, et al. An enhanced dissolving cyclosporin-A inhalable powder efficiently reduces SARS-CoV-2 infection in vitro. Pharmaceutics. 2023;15(3):1023. doi:10.3390/pharmaceutics15031023
29. Huang Y, Tang H, Liu D, et al. Cyclosporine A-loaded chitosan extra-fine particles for deep pulmonary drug delivery: in vitro and in vivo evaluation. J Control Release. 2023;362:243–256. doi:10.1016/j.jconrel.2023.08.050
30. Huang Y, Tang H, Meng X, et al. γ-Cyclodextrin metal-organic frameworks as the promising carrier for pulmonary delivery of cyclosporine A. Biomed Pharmacother. 2024;171:116174. doi:10.1016/j.biopha.2024.116174
31. Kim JK, Shin JH, Lee JS, et al. 28-Day inhalation toxicity of graphene nanoplatelets in Sprague-Dawley rats. Nanotoxicology. 2016;10(7):891–901. doi:10.3109/17435390.2015.1133865
32. Alhajj N, O’Reilly NJ, Cathcart H. Designing enhanced spray dried particles for inhalation: a review of the impact of excipients and processing parameters on particle properties. Powder Technol. 2021;384(1):313–331. doi:10.1016/j.powtec.2021.02.031
33. Chvatal A, Ambrus R, Party P, et al. Formulation and comparison of spray dried non-porous and large porous particles containing meloxicam for pulmonary drug delivery. Int J Pharm. 2019;559:68–75. doi:10.1016/j.ijpharm.2019.01.034
34. Party P, Bartos C, Farkas Á, Szabó-Révész P, Ambrus R. Formulation and in vitro and in silico characterization of “nano-in-micro” dry powder inhalers containing meloxicam. Pharmaceutics. 2021;13(2):211. doi:10.3390/pharmaceutics13020211
35. Suzuki H, Moritani T, Morinaga T, Seto Y, Sato H, Onoue S. Amorphous solid dispersion of cyclosporine A prepared with fine droplet drying process: physicochemical and pharmacokinetic characterization. Int J Pharm. 2017;519(1–2):213–219. doi:10.1016/j.ijpharm.2017.01.018
36. Zhang W, Zhang CN, He Y, et al. Factors affecting the dissolution of indomethacin solid dispersions. AAPS Pharm Sci Tech. 2017;18(8):3258–3273. doi:10.1208/s12249-017-0813-2
37. Chen L, Okuda T, Lu XY, Chan HK. Amorphous powders for inhalation drug delivery. Adv Drug Deliv Rev. 2016;100:102–115. doi:10.1016/j.addr.2016.01.002
38. Chen T, Zhuang B, Huang Y, et al. Inhaled curcumin mesoporous polydopamine nanoparticles against radiation pneumonitis. Acta Pharm Sin B. 2022;12(5):2522–2532. doi:10.1016/j.apsb.2021.10.027
39. Kim DH, Kim YW, Tin YY, et al. Recent technologies for amorphization of poorly water-soluble drugs. Pharmaceutics. 2021;13(8):1318. doi:10.3390/pharmaceutics13081318
40. Yang TT, Wen BF, Liu K, et al. Cyclosporine A/porous quaternized chitosan microspheres as a novel pulmonary drug delivery system. Artif Cells Nanomed Biotechnol. 2018;46(sup2):552–564. doi:10.1080/21691401.2018.1463231
41. Liu Y, Wu C, Hao Y, et al. Preparation of a novel starch-derived three-dimensional ordered macroporous carbon for improving the dissolution rate and oral bioavailability of water-insoluble drugs. J Pharm Biomed Anal. 2016;118:267–275. doi:10.1016/j.jpba.2015.11.003
42. Molaei MA, Osouli-Bostanabad K, Adibkia K, Shokri J, Asnaashari S, Javadzadeh Y. Enhancement of ketoconazole dissolution rate by the liquisolid technique. Acta Pharm. 2018;68(3):325–336. doi:10.2478/acph-2018-0025
43. Kocbek P, Baumgartner S, Kristl J. Preparation and evaluation of nanosuspensions for enhancing the dissolution of poorly soluble drugs. Int J Pharm. 2006;312(1–2):179–186. doi:10.1016/j.ijpharm.2006.01.008
44. Sampathi S, Prajapati S, Junnuthula V, Dyawanapelly S. Pharmacokinetics and anti-diabetic studies of gliclazide nanosuspension. Pharmaceutics. 2022;14(9):1947. doi:10.3390/pharmaceutics14091947
45. Jetzer MW, Morrical BD, Schneider M, Edge S, Imanidis G. Probing the particulate microstructure of the aerodynamic particle size distribution of dry powder inhaler combination products. Int J Pharm. 2018;538(1–2):30–39. doi:10.1016/j.ijpharm.2017.12.046
46. Scherließ R, Bock S, Bungert N, Neustock A, Valentin L. Particle engineering in dry powders for inhalation. Eur J Pharm Sci. 2022;172:106158. doi:10.1016/j.ejps.2022.106158
47. Wu X, Zhang W, Hayes DJ, Mansour HM. Physicochemical characterization and aerosol dispersion performance of organic solution advanced spray-dried cyclosporine A multifunctional particles for dry powder inhalation aerosol delivery. Int J Nanomed. 2013;8:1269–1283. doi:10.2147/IJN.S40904
48. Lazo REL, Mengarda M, Almeida SL, Caldonazo A, Espinoza JT, Murakami FS. Advanced formulations and nanotechnology-based approaches for pulmonary delivery of sildenafil: a scoping review. J Control Release. 2022;350:308–323. doi:10.1016/j.jconrel.2022.08.021
49. Kurakula M, GSNK R. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): as excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J Drug Deliv Sci Technol. 2020;60:102046. doi:10.1016/j.jddst.2020.102046
50. Barnes PJ. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2018;18(7):454–466. doi:10.1038/s41577-018-0006-6
51. Larose MC, Chakir J, Archambault AS, et al. Correlation between CCL26 production by human bronchial epithelial cells and airway eosinophils: involvement in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2015;136(4):904–913. doi:10.1016/j.jaci.2015.02.039
52. Drick N, Seeliger B, Welte T, Fuge J, Suhling H. Anti-IL-5 therapy in patients with severe eosinophilic asthma - clinical efficacy and possible criteria for treatment response. BMC Pulm Med. 2018;18(1):119. doi:10.1186/s12890-018-0689-2
53. Jang DI, Lee AH, Shin HY, et al. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci. 2021;22(5):2719. doi:10.3390/ijms22052719
54. Nair B. Final report on the safety assessment of polyvinylpyrrolidone (pvp). Int J Toxicol. 1998;17(4_suppl):95–130. doi:10.1177/109158189801700408
55. Sato H, Suzuki H, Yakushiji K, et al. Biopharmaceutical evaluation of novel cyclosporine A nano-matrix particles for inhalation. Pharm Res. 2016;33(9):2107–2116. doi:10.1007/s11095-016-1949-6
56. Wan KY, Weng J, Wong SN, Kwok PCL, Chow SF, Chow AHL. Converting nanosuspension into inhalable and redispersible nanoparticles by combined in-situ thermal gelation and spray drying. Eur J Pharm Biopharm. 2020;149:238–247. doi:10.1016/j.ejpb.2020.02.010
57. Zimmermann CM, Baldassi D, Chan K, et al. Spray drying siRNA-lipid nanoparticles for dry powder pulmonary delivery. J Control Release. 2022;351:137–150. doi:10.1016/j.jconrel.2022.09.021
58. Abu Elella MH, Al Khatib AO, Al-Obaidi H. Spray-dried nanolipid powders for pulmonary drug delivery: a comprehensive mini review. Pharmaceutics. 2024;16(5):680. doi:10.3390/pharmaceutics16050680
59. Qian S, Heng W, Wei Y, Zhang J, Gao Y. Coamorphous lurasidone hydrochloride–saccharin with charge-assisted hydrogen bonding interaction shows improved physical stability and enhanced dissolution with ph-independent solubility behavior. Cryst Growth Des. 2015;15(6):2920–2928. doi:10.1021/acs.cgd.5b00349
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.