Back to Journals » Journal of Pain Research » Volume 18
Hippocampal Functional Radiomic Features for Identification of the Cognitively Impaired Patients from Low-Back-Related Pain: A Prospective Machine Learning Study
Authors Yang Z , Liang X, Ji Y, Zeng W, Wang Y , Zhang Y, Zhou F
Received 30 June 2024
Accepted for publication 6 December 2024
Published 20 January 2025 Volume 2025:18 Pages 271—282
DOI https://doi.org/10.2147/JPR.S484680
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Michael A Ueberall
Ziwei Yang,1,2,* Xiao Liang,1,2,* Yuqi Ji,1,2 Wei Zeng,1,2 Yao Wang,1,2 Yong Zhang,3 Fuqing Zhou1,2
1Jiangxi Provincial Key Laboratory for Precision Pathology and Intelligent Diagnosis, Department of Radiology, the First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, 330006, People’s Republic of China; 2Neuroradiology Laboratory, Jiangxi Province Medical Imaging Research Institute, Nanchang, 330006, People’s Republic of China; 3Department of Pain Clinic, The First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi Province, 330006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yong Zhang, Department of Pain Clinic, The First Affiliated Hospital, Jiangxi Medical College, Nanchang University, 17 Yongwaizheng Street, Nanchang, Jiangxi, 330006, People’s Republic of China, Tel +86 791 8869 5036, Email [email protected] Fuqing Zhou, Jiangxi Provincial Key Laboratory for Precision Pathology and Intelligent Diagnosis, Department of Radiology, the First Affiliated Hospital, Jiangxi Medical College, Nanchang University, 17 Yongwaizheng Street, Nanchang, Jiangxi, 330006, People’s Republic of China, Tel +86 791 8869 5132, Email [email protected]
Purpose: To investigate whether functional radiomic features in bilateral hippocampi can identify the cognitively impaired patients from low-back-related leg pain (LBLP).
Patients and Methods: For this retrospective study, a total of 95 clinically definite LBLP patients (40 cognitively impaired patients and 45 cognitively preserved patients) were included, and all patients underwent functional MRI and clinical assessments. After calculating the amplitude of low-frequency fluctuations (ALFF), regional homogeneity (ReHo), voxel-mirrored homotopic connectivity (VMHC) and degree centrality (DC) imaging, the radiomic features (n = 819) of bilateral hippocampi were extracted from these images, respectively. After feature selection, machine learning models were trained. Finally, we further analyzed the relationship between the hippocampal functional radiomic features and clinical measures, to explore the clinical significance of these features.
Results: The combined radiomic features model logistic regression algorithm superior performance in distinguishing cognitively impaired patients from LBLP (AUC = 0.970, accuracy = 92.3%, sensitivity = 92.3%, specificity = 92.3%) compared to the other models. Additionally, radiomic wavelet features were correlated with Montreal Cognitive Assessment (MoCA) and Hamilton Anxiety Scale, present pain intensity scores in cognitively impaired LBLP patients (P < 0.05, with Bonferroni correction).
Conclusion: Hippocampal functional radiomic features are valuable for diagnosing cognitively impaired patients from LBLP.
Keywords: cognitive impairment, resting-state functional MRI, low-back-related leg pain, radiomic, logistic regression algorithm
Introduction
Low-back-related leg pain (LBLP) is one of the most common variations of low back pain. Individuals commonly experience pain that radiates to the leg, and it is believed to be associated with the severity of disease, increased disability, prolonged recovery, and higher health-related costs. Several studies have shown that chronic pain is associated with cognitive impairment.1,2 This decline in cognitive function has also been observed in patients with LBLP and has a negative impact on their quality of life and ability to maintain employment.3–5
Chronic pain patients with cognitive impairment may exhibit executive function deficits, particularly on working memory and emotional control subscales.6 A recent study has suggested that abnormalities of the mesolimbic dopamine system and the noradrenergic system in the locus coeruleus and microglial dysfunction in hippocampus play a crucial role in the cognitive impairment process.7,8 The Hippocampus is an important brain structure for cognitive decline. Studies of humans and animals both shown abnormalities in the hippocampus, including reduced volume, neurogenesis, and synaptic plasticity, reflecting certain functional abnormalities in cognitively impaired chronic pain patients.9,10 Recent resting-state functional magnetic resonance imaging (rs-fMRI) approaches have focused on the hippocampus, targeting altered intrinsic connectivity measures. Decreased hippocampal functional connectivity is related to anxiety, while increased connectivity involved in emotion regulation has been observed in chronic pain patients.11 The alterations of regional homogeneity (ReHo) in the hippocampus may be the result of chronic pain and then affect the cognitive function of patients.12 These findings indicate that hippocampus may play a specific role in the pathological process of the cognitively impaired LBLP (CI-LBLP).
Radiomics has traditionally been utilized as a method for morphological imaging analysis, which can use advanced imaging features to objectively and quantitatively describe phenotypic information for classification.13 Radiomic features include first-order features, textural features and wavelet features. Currently, studies have shown that there are significant abnormalities in hippocampal radiomic features, which served as a neuroimaging biomarker to distinguish between mild cognitive impairment (MCI) and Alzheimer’s disease (AD),14 as well as between cognitively impaired and cognitively preserved multiple sclerosis patients.15 In addition to the structural radiomic features of the hippocampus, the texture features of the hippocampus extracted from rs-fMRI have also been employed for the identification of cognitive impairment types.16 Moreover, previous study has found that the functional radiomic information contained in the hippocampus can better reflect the cognitive status of patients.16 It may assist in developing more appropriate and effective treatment plans for the cognitively impaired patients from those with LBLP in clinical settings, such as therapeutic exercise mentioned in certain studies.17
In this study, we hypothesize that hippocampal functional radiomic features would be disrupted and that these alterations might be employed in the classification of CI-LBLP patients from cognitively preserved LBLP (CP-LBLP) patients. In addition, correlation analysis between hippocampal functional radiomic features and clinical scores was performed in the patients to confirm that these radiomic features play an important role in the clinical symptoms of LBLP patients. This study aims to investigate the value of hippocampal functional radiomic features in distinguishing the cognitively impaired patients from those with LBLP and to provide valuable information for clinical practice.
Materials and Methods
Participants
This prospective recruitment study involved a total of 85 clinically diagnosed LBLP patients who were recruited from October 2021 to October 2023 at the First Affiliated Hospital of Jiangxi Medical College, Nanchang University. Inclusion criteria were as follow: (1) 20–65 years old; (2) radiological and clinical diagnosis, including reports of discogenic compression with at least 1 ruptured annulus fibrosus on lumbar computed tomography (CT) and/or magnetic resonance imaging (MRI); (3) visual analog scale (VAS) score ≥3; (4) getting ineffective prior conservative treatment, including medications without opioids and physical therapy (eg, non-steroidal anti-inflammatory drugs). Exclusion criteria were as follow: (1) any other non-discogenic causes of low-back-related leg pain (eg, spinal protrusion, lateral recess stenosis, spinal stenosis, piriformis syndrome, sciatica); (2) a history of major intracranial and spinal cord diseases or surgery and any contraindications; (3) image artifacts or incomplete clinical information. All participants were given written informed consent. A flowchart detailing exclusion by each criteria is presented in Figure 1. We used the G*Power tool to estimate the required sample size, and the number of patients included in both groups exceeded the estimated requirement. All subjects provided informed written consent for the procedures approved by the Medical Research Ethics Committee of the First Affiliated Hospital of Jiangxi Medical College, Nanchang University, in accordance with the Declaration of Helsinki, under the ethical number of (2022) CDYFYYLK(08–009).
 |
Figure 1 A flowchart detailing exclusion by each criteria. Abbreviation: MoCA, Montreal Cognitive Assessment. |
Clinical Assessment and Cognitive Groups
On the day of scanning, all participants underwent a series assessment, including (1) the present pain intensity (PPI) and visual analogue scale (VAS) used to evaluate both the intensity and quality of pain, see reference18,20,21 for their normal range and the significance of abnormal values; (2) the central sensitization inventory (CSI) used to help identify patients with central sensitivity syndrome; (3) the Montreal Cognitive Assessment (MoCA) (from 0 to 30) used to evaluate neuropsychological test for detecting cognitive decline or dementia; (4) the Hamilton Anxiety Rating Scale (HAMA) and Hamilton Depression Rating Scale (HAMD) used to evaluate anxiety symptoms and depressive symptoms, respectively; (5) the Brief Pain Inventory (BPI-17) questionnaire used to evaluate the severity and interference of pain with daily functioning.
After MoCA score adjusted for education, the total score of those with less than 12 years of education is added to one point.21 Patients with LBLP were categorized into cognitively impaired (CI, score less than 26) and cognitively preserved (CP, score of 26–30) subgroups.
MRI Data Acquisition
All MRI data were collected on a 3.0T MRI (Signa Pioneer, GE Healthcare, Milwaukee, CA, USA) with a 32-channel head/neck coil at our hospital. The whole-brain 3D T1-weighted acquisition parameters were as follows: 176 sagittal slices, repetition time (TR) = 8.0 ms, echo time (TE) = 3.0 ms, flip angle (FA) = 12°, slice thickness/gap = 1.0/0 mm, field of view (FOV) = 256 × 256 mm2, matrix = 256 × 256. Rs-fMRI was acquired using a gradient echo-planar imaging pulse sequence with the following parameters: 40 axial slices, TR = 2000 ms, TE = 25 ms, FA = 90°, slice thickness/gap = 3.5/0.7 mm, FOV = 190 × 190 mm2, matrix = 64 × 64, and 240 volumes. All subjects were asked to kept their eyes closed during scanning, avoid systematic thinking and refrain from falling asleep. Noise and head-motion were controlled by foam padding and earplugs. Additionally, the conventional T2-weighted and fluid attenuated inversion recovery (FLAIR) sequences are acquired to exclude subjects with anatomical brain abnormalities. Lumbar spine evaluation on CT/MRI was consistent with that previously reported.22
Data Preprocessing and rs-fMRI Derivatives/Connectivity Measures
All fMRI data were processed and analyzed using the toolbox for Data Processing & Analysis of Brain Imaging (DPABI v5.2; http://rfmri.org/dpabi) and Statistical Parametric Mapping (SPM12, http://www.fil.ion.ucl.ac.uk/spm). The main steps include the following: (1) Dicom to Nifti format, (2) delete initial 10 volumes (image) for data stabilization, (3) slice timing for signal corrected, (4) realignment for movements correction, in which head motion exceeded 3 mm translation or 3° rotation according to the criteria of Van Dijk et al,23 (5) co-registered 3D T1-weighted imaging to mean functional image, (6) segmented the transformed structural images into gray matter, white matter and cerebrospinal fluid with Dartel tool,24 (7) covariates such as linear drift, white matter signal, and cerebrospinal fluid were removed without global signal regression, (8) spatial normalization, resampling the image to 3 mm isotropic voxels and smoothing (FWMH kernel: 6 mm) except for ReHo and degree centrality calculation, (9) temporal filtering (0.01–0.1 hz) for further rs-fMRI-based indices calculations. Standardized fMRI data preprocessing steps are referenced.24 The amplitude of low frequency fluctuations (ALFF), regional homogeneity (ReHo), voxel-mirrored homotopic connectivity (VMHC) and degree centrality (DC) values were calculated based on previous studies using DPABI (detail information see Table S1).
Hippocampal Radiomic Feature Extraction and Selection
Python3.7.12 Pandas was used for data encapsulation, and then PyRadiomics was employed to extract the hippocampal radiomic features from amplitude of low-frequency fluctuations (ALFF), regional homogeneity (ReHo), voxel-mirrored homotopic connectivity (VMHC) and degree centrality (DC) images, as previously reported. The radiomic features encompassed three categories: (1) 18 first-order features to evaluate the signal level, (2) 73 textural features to describe the spatial distribution information, and (3) 728 wavelet features to combine the spatial and frequency characteristics (for detailed information, please refer to Table S2). In total, 819 hippocampal radiomic features were extracted from ReHo, ALFF, VMHC, and DC images, respectively.
To identify the optimal features, all features are first preprocessed by Z-score normalization prior to feature screening, and each dimension feature is linearly stretched between [0–1]. Subsequently, the spearman rank correlation test was employed to evaluate the linear correlation between individual features for redundancy elimination. Once two features have a stronger correlation, they will have a higher absolute value of the correlation coefficient. We selected one of the features for subsequent analysis when a Spearman correlation coefficient >0.9 between each feature. Finally, the least absolute shrinkage and selection operator (LASSO) regression was utilized for feature selection with non-zero coefficients serving as valuable predictors.
Hippocampal Functional Radiomic Model
Following the selection of hippocampal functional radiomic features, this study assessed the discriminative capability of various feature types in distinguishing between patients with CI-LBLP and CP-LBLP. Each radiomic feature of derivatives/connectivity measures captures different aspects of the information carried by rs-fMRI and may play different roles in the identification of CI-LBLP and CP-LBLP. Thus, a total of 15 combinations were created in this study, including (1) hippocampal radiomic features derived from single imaging metrics such as ReHo, ALFF, DC, and VMHC; (2) hippocampal radiomic features derived from two imaging metrics like ReHo + ALFF, ReHo + DC, ReHo + VMHC, ALFF + DC, ALFF + VMHC, and DC + VMHC; (3) hippocampal radiomic features derived from three imaging metrics including ReHo + ALFF + DC, ReHo + ALFF + VMHC, ReHo + DC + VMHC, and ALFF + DC + VMHC; and (4) hippocampal radiomic features derived from all four imaging metrics: ReHo + ALFF + DC + VMHC.
Two groups of LBLP patients were randomly divided into training set and test set at a ratio of 7:3. Then, machine learning classification models were developed using Python Scikit-learn (https://scikit-learn.org/stable/install.html) to differentiate patients between the CI and CP groups. The chosen features served as input vectors for support vector machine (SVM), logistic regression (LR), multilayer perceptron (MLP), NaiveBayes and k-nearest neighbors (KNN) to train the classification model. The diagnostic performance of the hippocampal functional radiomic models was evaluated by the area under the curve (AUC) of the receiver operating characteristic (ROC) curve, with quantitative indicators such as accuracy, sensitivity, and specificity. To estimate the clinical usefulness of the prediction models, decision curve analysis (DCA) was performed to assess the net benefit of the models in the test cohorts.
Statistical Analysis
IBM SPSS Statistics (Version 21.0, SPSS Inc., Chicago, Illinois, USA) was utilized for analyzing the clinical variables. Chi-square tests were used to compare categorical variables, while t-tests or the Mann–Whitney U-test were used for comparing quantitative variables to assess differences in clinical characteristics among patients (P < 0.05). To investigate the clinical correlations, a partial correlation analysis was conducted between the final selected hippocampal functional radiomic features and clinical information in both the CI group and CP group (P × n < 0.05, Bonferroni correction).
Results
Demographic and Clinical Data Analysis
Forty CI-LBLP patients and forty-five CP-LBLP patients were entered into the final analysis. The demographic and clinical information is shown in Table 1. In this study, the age of CI-LBLP patients was significantly older than that of CP-LBLP patients (P < 0.001), the proportion of female patients in the former group was higher (P = 0.021), and the interference of pain on daily activities was more obvious in CI-LBLP patients (P = 0.046).
 |
Table 1 Demographic and Clinical Characteristics of CI-LBLP and CP-LBLP Patients |
Hippocampal Functional Radiomic Features Selection and Model
(1) Hippocampal functional radiomic features selection
Following dimensionality reduction through Spearman rank correlation test and LASSO regression, a total of 31 hippocampal functional radiomic features were identified. These features consisted of 10 from DC, 5 from ALFF, 11 from ReHo, and 5 from VMHC (Figure 2 and Table S3).
(2) Hippocampal functional radiomic features models
Fifteen models were established based on the optimal features of the hippocampal radiomic features based on each machine learning algorithms. The combined radiomic features (DC + ReHo + VMHC) models based LR algorithm exhibited the highest accuracy (AUC = 0.970) in the validation set. Table 2 and Figure 3 show the ROC curves and the sensitivity, specificity, and accuracy of the training set and test set of hippocampal functional radiomic models developed by LR algorithms. The results of hippocampal functional radiomic models developed by other machine learning algorithms are shown in Table S4.
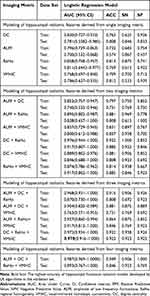 |
Table 2 The Classification Results of Hippocampal Functional radiomic Models Developed by LR Algorithms Between CI-LBLP and CP-LBLP Patients |
Correlation Analysis
In the CI-LBLP group, partial correlation analysis revealed found that a positive correlation between wavelet features (wavelet-HHH_ngtdm_Coarseness) from DC and MoCA score (r = −0.598, P = 0.001 × 8 < 0.05, with Bonferroni correction), as well as and a negative correlation bet ween wavelet features (wavelet-HLL_firstorder_TotalEnergy) from DC and HAMA score (r = −0.517, P = 0.001 × 8 < 0.05, with Bonferroni correction). Additionally, a negative correlation was observed between the wavelet features (wavelet-LLL_glszm_ZoneVariance) from ALFF and the PPI score (r = 0.568, P = 0.001 × 8 < 0.05, with Bonferroni correction) (Figure 4). For the CP-LBLP patients, the ReHo wavelet features (wavelet-HLL_firstorder_Skewness) show a positive correlation with HAMD score (r = 0.45, P = 0.002 × 8< 0.05, with Bonferroni correction), while ReHo wavelet features (wavelet-LHH_glcm_Idn) were negatively correlated with CSI score (r = −0.456, P = 0.002 × 8< 0.05, with Bonferroni correction) (Figure 4).
Discussion
In this study, we successfully developed a machine learning algorithm model based on multilevel functional radiomic features of the hippocampus. This model is capable of distinguishing cognitively impaired patients from those with LBLP. Furthermore, we found that the hippocampal functional radiomic features in CI-LBLP patients were significantly correlated with clinical pain intensity, cognitive status, and anxiety status scores.
Combined Modeling of Hippocampal Multilevel Functional Radiomic Features Rather Than Single Feature
In this study, 31 potential discriminative features were identified by using Spearman rank correlation test and LASSO regression. Except in the DC imaging has 2 “Gray Level Size Zone Matrix (glszm)” and 1 “Neighbouring Gray Tone Difference Matrix (ngtdm)” texture features, and the rest are “wavelet” features. In terms of distinguishing cognitively preserved patients from LBLP, the features that made the greatest contribution came from the wavelet features of DC imaging (HHL_glszm_SmallAreaEmphasis) and ReHo imaging (LHL_glcm_Correlation), respectively. Previous studies have confirmed that the wavelet transform features provided by PyRadiomics can help to improve the diagnostic efficiency of radiomic features.25,26 In this study, the optimization of valuable features may reveal the heterogeneity and spatial change rate in the strength of hippocampal functional connectivity strength, and help to understand the alteration of hippocampal function.
However, in this study, the AUC value of the machine learning algorithm model constructed by the radiomic features of a hippocampal single imaging metric (such as ReHo, etc.) was not high (AUC in the test set: 0.556–0.811), indicating that the altered higher order feature of hippocampal single function metric could not well distinguish cognitively impaired patients from those with LBLP. In previous studies including individuals with low back pain with radicular pain, these patients have demonstrated significant alterations in hippocampal volume,27 hippocampal structural covariance,28 and functional connectivity.29 It has been suggested that hippocampus can serve as a potential diagnostic biomarker for chronic back pain.30–32 However, its diagnostic efficiency is not high, which may be due to the limited information of included image or insufficient information mining, which inevitably ignores other potentially useful information.3,31,33
The combination of multilevel functional image features can improve classification performance, which seems to be a reasonable hypothesis in fMRI studies of chronic pain. Different types of features can provide complementary information to highlight the differences between the two groups of patients. Moreover, radiomic features further excavate its higher-order information, especially the wavelet features, which reflect the functional change patterns in different spaces. The combination of radiomic features from two functional images can significantly improve discrimination ability (AUC in the test set: 0.741–0.917). The improvement in diagnostic efficiency of machine learning models based on hippocampal radiomic features is mainly affected by the contribution degree from the features derived from DC and ReHo imaging. Combined with the radiomic feature of homotopy connectivity (VMHC), an exceptional discriminatory capacity can be attained, with an AUC of 0.970 and a sensitivity of 0.923. This thoroughly demonstrates that in the combination of DC and ReHo, VMHC radiomic features are more complementary than ALFF radiomic features.
Combined with the following findings: (1) neuroimaging studies have shown that there are varying degrees of morphological, (microscopic) structural, metabolic and functional changes in the hippocampus of patients with cognitive impairment;3 (2) recent studies using radiomics to evaluate hippocampal texture features are more effective than hippocampal volume measurement.26 We have reason to believe: based on the features of hippocampal functional radiomic modeling of machine learning algorithm, it can effectively distinguish cognitively impaired patients from those with LBLP.
Hippocampal Functional Radiomic Features and Clinical Relevance
According to the results of correlation analysis, DC imaging’s “wavelets” feature (wavelets-hhH_NGTDM_coarseness) has significant correlation with the MoCA scores of CI-LBLP patients. It represents the texture roughness of the rapid grayscale change between a DC’s pixel or voxel and its adjacent pixel or voxel. This suggests that the heterogeneity of degree centrality changes is closely associated with cognitive impairment in LBLP patients. In this study, it was also found that hippocampal functional radiomic features of patients in both groups were related to pain intensification and anxiety state, indicating that pain stimulation and pain-induced anxiety participated in the functional changes of the hippocampus.
Hippocampus is involved in pain processing, pain-related attention and anxiety, and stress response.34 Intrinsic connectivity between chronic pain and limbic systems, such as the hippocampus, has been proposed to explain the association between chronic pain and cognitive function.29 In the state of chronic pain, multiple factors such as nervous system sensitization, imbalance between ascending stimulation and descending inhibition, and dysregulation of regulatory circuits can aggravate the decline of cognitive functions related to the limbic system, such as executive function, language, memory and attention.3,29,31,35 Recent studies indicated tape containing magnetic particles (TCMP) and tape with magnetic particles (MPT) applied to the lumbar area have an immediate effect on the autonomic nervous system (ANS) and can reduce perceived chronic low back pain.25,35 The innovative application of this technique in comparative studies of CI-LBLP and CP-LBLP highlights the robust neurobiological foundation underlying the altered hippocampal functional radiomic. The differentiation between cognitively impaired patients and those with LBLP may provide valuable insights for clinical practice.
Limitations
There are several limitations to this study. First, this is a prospective recruitment study, the recruited LBLP patients come from the pain clinical department. When the patients first develop cognitive disorders, their family members are more willing to seek medical treatment, and with the prolongation of the disease course, they will gradually accept this reality or lose patience and reduce the behavior of seeking medical treatment, or transfer to the neurology department. In addition, age may be a risk factor for cognitive impairment. Second, as a pain study, the cognitive assessment was limited (only MoCA), and no specific scale assessment, such as memory scale, was performed. Further prospective studies with comprehensive clinical and specific cognitive information such as working memory to explore the clinical significance of these hippocampal imaging markers. Finally, as a preliminary, exploratory study, it was not verified by external data. The model requires careful application. This model based on the hippocampal function radiomic features still needs to be careful in clinical application.
Conclusion
In this study, based on the hippocampal functional radiomic features, an LR algorithm classifier was constructed, which can effectively distinguish cognitively impaired patients from those with LBLP, and is helpful to clinical practice and explain the pathologic mechanisms of patients.
Acknowledgments
To all of the participants in this study, we express our sincere thanks. This study was supported by the National Natural Science Foundation of China (82160331), Jiangxi Province Double Thousand Talent Plan (jxsq2023201039), and the Natural Science Foundation of Jiangxi Province (20202BABL206114). This project is implemented by the Jiangxi Clinical Research Center for Medical Imaging (20223BCG74001), and Jiangxi Province Key Laboratory for Precision Pathology and Intelligent Diagnosis (2024SSY06281). The funders had no role in the study design, data collection and analysis, the decision to publish or the preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Guo X, Hou C, Tang P, Li R. Chronic pain, analgesics, and cognitive status: a comprehensive Mendelian randomization study. Anesthesia Analg. 2023;137(4):896–905. doi:10.1213/ANE.0000000000006514
2. Zhang X, Gao R, Zhang C, et al. Evidence for cognitive decline in chronic pain: a systematic review and meta-analysis. Front Neurosci. 2021;15:737874. doi:10.3389/fnins.2021.737874
3. Pereira Nery ECH, Rocha NP, Cruz VT, Silva AG. Systematic review and meta-analysis on the association between chronic low back pain and cognitive function. Pain Pract. 2023;23(4):399–408. doi:10.1111/papr.13194
4. Apkarian AV, Sosa Y, Krauss BR, et al. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108(1–2):129–136. doi:10.1016/j.pain.2003.12.015
5. Luoto S, Taimela S, Hurri H, Alaranta H. Mechanisms explaining the association between low back trouble and deficits in information processing. A controlled study with follow-up. Spine. 1999;24(3):255–261. doi:10.1097/00007632-199902010-00011
6. Baker KS, Gibson S, Georgiou-Karistianis N, Roth RM, Giummarra MJ. Everyday executive functioning in chronic pain: specific deficits in working memory and emotion control, predicted by mood, medications, and pain interference. Clin J Pain. 2016;32(8):673–680. doi:10.1097/AJP.0000000000000313
7. Cao S, Fisher DW, Yu T, Dong H. The link between chronic pain and Alzheimer’s disease. J Neuroinflam. 2019;16(1):204. doi:10.1186/s12974-019-1608-z
8. Llorca-Torralba M, Borges G, Neto F, Mico JA, Berrocoso E. Noradrenergic Locus Coeruleus pathways in pain modulation. Neuroscience. 2016;338:93–113. doi:10.1016/j.neuroscience.2016.05.057
9. Neumann N, Domin M, Schmidt CO, Lotze M. Chronic pain is associated with less grey matter volume in the anterior cingulum, anterior and posterior insula and hippocampus across three different chronic pain conditions. Eur J Pain. 2023;27(10):1239–1248. doi:10.1002/ejp.2153
10. Vaculik MF, Noorani A, Hung -PS-P, Hodaie M. Selective hippocampal subfield volume reductions in classic trigeminal neuralgia. NeuroImage Clin. 2019;23:101911. doi:10.1016/j.nicl.2019.101911
11. Ruiz S, Buyukturkoglu K, Rana M, Birbaumer N, Sitaram R. Real-time fMRI brain computer interfaces: self-regulation of single brain regions to networks. Biol psychol. 2014;95:4–20. doi:10.1016/j.biopsycho.2013.04.010
12. Yu W, Wu X, Chen Y, et al. Pelvic pain alters functional connectivity between anterior cingulate cortex and hippocampus in both humans and a rat model. Front Systems Neurosci. 2021;15:642349. doi:10.3389/fnsys.2021.642349
13. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–577. doi:10.1148/radiol.2015151169
14. Yin TT, Cao MH, Yu JC, et al. T1-weighted imaging-based hippocampal radiomics in the diagnosis of alzheimer’s disease. Acad. Radiol. 2024;31:5183–5192. doi:10.1016/j.acra.2024.06.012
15. Meng M, Zhang CY, Li YM, et al. Independent and reproducible hippocampal radiomics biomarkers for multisite multiple sclerosis and neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2024;81:105146. doi:10.1016/j.msard.2023.105146
16. Wang L, Feng Q, Ge X, et al. Textural features reflecting local activity of the hippocampus improve the diagnosis of Alzheimer’s disease and amnestic mild cognitive impairment: a radiomics study based on functional magnetic resonance imaging. Front Neurosci. 2022;16:970245. doi:10.3389/fnins.2022.970245
17. Cuenca-Zaldivar JN, Fernández-Carnero J, Sánchez-Romero EA, et al. Effects of a therapeutic exercise protocol for patients with chronic non-specific back pain in primary health care: a single-group retrospective cohort study. J Clin Med. 2023;12(20):6478. doi:10.3390/jcm12206478
18. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill pain questionnaire (SF-MPQ-2). Pain. 2009;144(1):35–42. doi:10.1016/j.pain.2009.02.007
19. Kachooei AR, Ebrahimzadeh MH, Erfani-Sayyar R, Salehi M, Salimi E, Razi S. Short form-McGill pain questionnaire-2 (SF-MPQ-2): a cross-cultural adaptation and validation study of the Persian version in patients with knee osteoarthritis. Arch Bone Joint Surg. 2015;3(1):45–50.
20. Poquet N, Lin C. The brief pain inventory (BPI). J Physiother. 2016;62(1):52. doi:10.1016/j.jphys.2015.07.001
21. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
22. Wang Z, Wang Y, Ji Y, et al. Hypoconnectivity of the amygdala in patients with low-back-related leg pain linked to individual mechanical pain sensitivity: a resting-state functional MRI study. J Pain Res. 2023;16:3775–3784. doi:10.2147/JPR.S425874
23. Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59(1):431–438. doi:10.1016/j.neuroimage.2011.07.044
24. Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14(3):339–351. doi:10.1007/s12021-016-9299-4
25. Benítez-Martínez JC, García-Haba B, Fernández-Carnero S, et al. Effectiveness of transcutaneous neuromodulation on abductor muscles electrical activity in subjects with chronic low back pain: a randomized, controlled, crossover clinical trial. J Pain Res. 2023;16:2553–2566. doi:10.2147/JPR.S409028
26. Feng F, Wang P, Zhao K, et al. Radiomic features of hippocampal subregions in alzheimer’s disease and amnestic mild cognitive impairment. Front Aging Neurosci. 2018;10:290. doi:10.3389/fnagi.2018.00290
27. Ezzati A, Zammit AR, Lipton ML, Lipton RB. The relationship between hippocampal volume, chronic pain, and depressive symptoms in older adults. Psychiatry Res Neuroimaging. 2019;289:10–12. doi:10.1016/j.pscychresns.2019.05.003
28. Tsai CL, Chou KH, Lee PL, et al. Shared alterations in hippocampal structural covariance in subjective cognitive decline and migraine. Front Aging Neurosci. 2023;15:1191991. doi:10.3389/fnagi.2023.1191991
29. Mutso AA, Petre B, Huang L, et al. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J Neurophysiol. 2014;111(5):1065–1076. doi:10.1152/jn.00611.2013
30. Zhang X, Xu R, Ma H, Qian Y, Zhu J. Brain structural and functional damage network localization of suicide. Biol Psychiatry. 2024;95(12):1091–1099. doi:10.1016/j.biopsych.2024.01.003
31. Reckziegel D, Abdullah T, Wu B, et al. Hippocampus shape deformation: a potential diagnostic biomarker for chronic back pain in women. Pain. 2021;162(5):1457–1467. doi:10.1097/j.pain.0000000000002143
32. Mo F, Zhao H, Li Y, et al. Network localization of state and trait of auditory verbal hallucinations in schizophrenia. Schizophrenia Bull. 2024;2024:sbae020.
33. Cheng Y, Cai H, Liu S, et al. Brain network localization of gray matter atrophy and neurocognitive and social cognitive dysfunction in schizophrenia. Biol Psychiatry. 2024;97:148–156. doi:10.1016/j.biopsych.2024.07.021
34. Mokhtari T, Tu Y, Hu L. Involvement of the hippocampus in chronic pain and depression. Brain Sci Adv. 2019;5(4):288–298. doi:10.26599/BSA.2019.9050025
35. Sillevis R, Cuenca-Zaldívar JN, Fernández-Carnero S, García-Haba B, Sánchez Romero EA, Selva-Sarzo F. Neuromodulation of the autonomic nervous system in chronic low back pain: a randomized, controlled, crossover clinical trial. Biomedicines. 2023;11(6):1551. doi:10.3390/biomedicines11061551
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Hypoconnectivity of the Amygdala in Patients with Low-Back-Related Leg Pain Linked to Individual Mechanical Pain Sensitivity: A Resting-State Functional MRI Study
Wang Z, Wang Y, Ji Y, Yang Z, Pei Y, Dai J, Zhang Y, Zhou F
Journal of Pain Research 2023, 16:3775-3784
Published Date: 8 November 2023




