Back to Journals » Journal of Experimental Pharmacology » Volume 16
Histopathological Evaluation of Wound Healing and Anti-Inflammatory Effects of Granola Potato Peel Ethanol Extract in Rat Oral Mucosa
Authors Santoso AW , Amalia E , Sari KI, Takarini V, Sufiawati I
Received 17 July 2024
Accepted for publication 9 October 2024
Published 24 October 2024 Volume 2024:16 Pages 377—395
DOI https://doi.org/10.2147/JEP.S487373
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Abdelwahab Omri
Astrid Widhowaty Santoso,1,* Eri Amalia,2,* Kartika Indah Sari,3 Veni Takarini,4 Irna Sufiawati5,*
1Oral Medicine Residency Program, Faculty of Dentistry, Universitas Padjadjaran, Bandung, Indonesia; 2Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, Indonesia; 3Department of Oral Biology, Faculty of Dentistry, Universitas Padjadjaran, Bandung, Indonesia; 4Department of Dental Material Science and Technology, Faculty of Dentistry, Universitas Padjadjaran, Bandung, Indonesia; 5Department of Oral Medicine, Faculty of Dentistry, Universitas Padjadjaran, Bandung, Indonesia
*These authors contributed equally to this work
Correspondence: Irna Sufiawati, Department of Oral Medicine, Faculty of Dentistry, Universitas Padjadjaran, Jalan Sekeloa No. 1, Bandung, West Java, 40132, Indonesia, Tel +62-22-2504985, Email [email protected]
Introduction: Oral mucosal wounds present significant clinical challenges due to their susceptibility to infection, inflammation, and delayed healing. The limitation of standard anti-inflammatory drugs (both steroidal and non-steroidal) highlights the urgent need for plant-derived alternative therapies. Granola potato (Solanum tuberosum L.) from Pangalengan, West Java, Indonesia, has shown promise due to its bioactive compounds. However, its potential for wound healing and anti-inflammatory effects, specifically for oral mucosal wounds, remains largely unexplored.
Purpose: To evaluate the wound healing and anti-inflammatory activity of Granola potato peel ethanol extract (GPPEE) on the oral mucosa of Wistar rats based on histopathological analysis.
Materials and Methods: Forty-eight Wistar rats were wounded on the palatal mucosa using a 4 mm punch biopsy and subsequently divided into four groups: placebo gel, 0.1% triamcinolone acetonide ointment (TCA), 4% GPPEE gel, and 6% GPPEE gel. The rats were euthanized on days 0, 1, 3, 7, and 14. Histopathological parameters assessed included fibroblast proliferation, collagen deposition, angiogenesis, and the presence of inflammatory cells.
Results: Phytochemical screening revealed the presence of phenolic compounds, flavonoids, tannins, and alkaloids in the Granola potato peel ethanol extract (GPPEE). Significant differences in the number of inflammatory cells were observed on days 1, 3, 7, and 14 (p< 0.05), with the groups treated with 4% and 6% GPPEE gel initially exhibiting pro-inflammatory effects on day 3, followed by significant anti-inflammatory effects on days 7 and 14. The 6% GPPEE gel treatment demonstrated a notable increase in fibroblasts on days 1, 7, and 14 (p< 0.05), as well as collagen deposition on days 7 and 14 (p< 0.05). However, no significant difference was observed in angiogenesis (p> 0.05).
Conclusion: The application of 4% and 6% GPPEE gel demonstrated superior wound healing efficacy compared to 0.1% TCA and exhibited comparable anti-inflammatory activity to 0.1% TCA.
Keywords: anti-inflammatory, granola, oral ulcer, potato peel extract, Solanum tuberosum, wound healing
Introduction
Wound healing is a meticulously regulated process to restore tissue integrity following an injury. Regardless of the tissue type affected, this process is divided into four overlapping phases: hemostasis, inflammation, proliferation, and remodeling. Each phase involves specific cellular and molecular mechanisms, including various signaling pathways.1 The oral cavity environment is highly dynamic, rendering some treatments less effective for managing oral mucosal wounds.2
Oral ulceration is the most prevalent form of oral mucosal wound lesions, frequently leading patients to seek care from a dentist or oral medicine specialist. Oral ulceration may present as a single lesion or in clusters. It can occur at various sites within the oral cavity, including the buccal mucosa, labial mucosa, tongue, gingiva, and palate.3 Several causes of oral ulceration exist, including inflammatory conditions (eg, traumatic ulcers, aphthous stomatitis), viral infections (eg, herpes simplex virus, herpes zoster), bacterial infections (eg, syphilis, tuberculosis), autoimmune diseases (eg, pemphigus vulgaris, mucous membrane pemphigoid, oral lichen planus), and malignancies.4 Oral traumatic ulcers are among the most common lesions, with a prevalence of approximately 24% in the population, followed by recurrent aphthous stomatitis (RAS), which has a prevalence of 20%.5
The treatment of oral ulceration depends on the severity of the lesion, however, in most cases, the primary goal is to reduce pain and the duration of the ulcer by decreasing the local immune response and preventing secondary infections. Topical corticosteroids, topical anesthetics, and analgesics are recommended treatments, but long-term use of these medications may lead to drug resistance, an imbalance in oral flora, and secondary fungal infections.2 Therefore, due to the growing incidence of adverse effects associated with synthetic drugs, there is increasing interest in developing plant-based or herbal medicines as promising alternatives.6
Potatoes (Solanum tuberosum L.) rank as the fourth most significant agricultural product globally, with an annual production of approximately 388 million tons, half of which is consumed fresh. According to the Food and Agriculture Organization (FAO), potato production exceeded 300 million tons in 2016.7 The substantial consumption of processed potato products, such as fries, potato chips, and frozen potatoes, generates large quantities of potato peel waste annually.8 Traditionally, this waste is used for low-value applications like animal feed, fertilizer, or biogas production, leading to the underutilization of the nutritious components in potato peels. These peels are rich in antioxidants, antibacterial agents, apoptotic inducers, possess chemopreventive and anti-inflammatory properties.7
Potato peels are a rich source of phytochemicals with biological activity, including phenolic acids and flavonoids.9,10 According to Albishi et al, phenolic compounds are more abundant in potato peels compared to potato flesh.11 The anti-inflammatory properties of flavonoids are attributed to their role as antioxidants, which neutralize free radicals that mediate the inflammatory process.12 Phenolic compounds also exhibit potential antimicrobial, antioxidant, and regenerative properties, making them promising bioactive compounds for application in wound dressings.13
Our previous study investigated the efficacy of Granola potato peel ethanol extract gel (GPPEE) at concentrations of 2%, 4%, and 6% for promoting wound contraction in the gingival mucosa of Wistar rats. The results showed that the 6% GPPEE gel produced the most significant wound contraction, as measured macroscopically by wound length on the 3rd, 7th, and 14th days.14 However, our previous study only observed wound healing at the macroscopic level without accompanying microscopic observations such as fibroblast count, inflammatory count, and angiogenesis. Therefore, our recent study was conducted to evaluate the wound healing and anti-inflammatory properties of Granola potato peel ethanol extract gel at the microscopic level. To the best of the authors’ knowledge, this is the first study to analyze the wound healing and anti-inflammatory activity of ethanol extract from the peel of the Granola variety of potato, sourced from Pangalengan, West Java, through histopathological examination. This study aims to evaluate the wound healing and anti-inflammatory properties of ethanol extract gel derived from Granola potato peel by assessing epithelialization, angiogenesis, and inflammation in Wistar rat models.
Materials and Methods
Ethical Approval
This study received approval from the Health Research Ethics Committee (HREC) of Universitas Padjadjaran, with the ethical clearance number 1236/UN.KEP/EC/2023. The guideline followed by the welfare of the laboratory animal was The Eighth Edition of the Guide for the Care and Use of Laboratory Animals (NRC 2011).
Plant Material
Granola variety potatoes were obtained from Pangalengan, Bandung, West Java, Indonesia, during the dry season. The samples used in this study were potato peels (Figure 1). The identification of the potato plants was conducted at the Generasi Biologi Indonesia Laboratory (Certificate No: 08.159/Genbinesia/I/2023).
Preparation of Extract
The potato peels were dried in an oven at 40°C for 24 hours. The dried peels were then ground to an approximate size of 4 mm. A total of 300 grams of the ground potato peels were subjected to extraction using the maceration method with 96% ethanol as the solvent. The sample-to-solvent ratio was maintained at 1:10. The maceration process was carried out for 72 hours, with the solvent refreshed every 24 hours. The extract was then concentrated using a rotary evaporator at 75 rpm and 45°C. Subsequently, the concentrated extract was further thickened using a water bath at 60°C.
Preliminary Phytochemical Analysis
Phytochemical screening of the GPPEE was conducted to identify the major classes of compounds present in the extract using standard protocols. The extract underwent qualitative analysis, with visual observations of color changes and precipitate formation following the addition of specific chemical reagents. This process was used to detect the presence of alkaloids, phenolics, flavonoids, saponins, tannins, steroids, and triterpenoids. The tube test method was employed for these analyses.
Phenolic Test
A total of 0.2 grams of the sample was extracted with 5 mL of methanol, then filtered using filter paper and transferred to another tube. Subsequently, 2–3 drops of 5% FeCl₃ were added. A positive result for phenolic compounds was indicated by a color change to dark blue or dark green.
Flavonoid Test
Concentrated HCl and Mg reagent: A total of 0.2 grams of the sample was extracted with 5 mL of methanol, then filtered using filter paper and transferred to another tube. Subsequently, 1–2 drops of concentrated hydrochloric acid (HCl) were added, and the solution was shaken and mixed with a small amount of Mg powder. The mixture was shaken again. A positive result for flavonoids was indicated by a color change to orange and the formation of abundant foam.
2N H2SO4 reagent: A total of 0.2 grams of the sample was extracted with 5 mL of methanol, then filtered using filter paper and transferred to another tube. Subsequently, 2–3 drops of 2N sulfuric acid (H2SO₄) were added. A positive result for flavonoids was indicated by a color change to yellow, red, or brown.
10% NaOH reagent: A total of 0.2 grams of the sample was extracted with 5 mL of methanol, then filtered using filter paper and transferred to another tube. Subsequently, 2–3 drops of 10% sodium hydroxide (NaOH) were added. A positive result for flavonoids was indicated by a color change to yellow, red, green, or brown.
Saponin Test
A total of 0.2 grams of the sample was weighed and dissolved in 5 mL of distilled water. The test tube was then sealed with plastic wrap and shaken vigorously. A positive result for saponins was indicated by the formation of abundant foam.
Tanin Test
A total of 0.2 grams of the sample was extracted with 5 mL of methanol, then filtered using filter paper and transferred to another tube. Subsequently, 2–3 drops of 1% FeCl₃ were added. A positive result for tannins was indicated by a color change to dark blue or dark green.
Triterpenoid and Steroid Tests
A total of 0.2 grams of the sample was weighed and dissolved in 5 mL of ammoniacal chloroform, then filtered using filter paper. The filtrate was placed on a spot plate and allowed to dry. Subsequently, one drop of concentrated sulfuric acid (H2SO₄) and one drop of anhydrous acetic acid were added. A positive result for triterpenoids was indicated by a color change to brown or red, while a positive result for steroids was indicated by a color change to green, purple, or blue.
Alkaloid Test
A total of 0.2 grams of the sample was weighed and dissolved in 5 mL of ammoniacal chloroform, then filtered using filter paper. The solution was transferred to another test tube and fractionated using 2N sulfuric acid (H2SO₄). The sulfuric acid fraction was transferred to another test tube, and 2–3 drops of Dragendorff’s reagent were added. A positive result for alkaloids was indicated by the formation of a red precipitate.
Cytotoxicity Assay
The cytotoxicity assay was conducted using the CV-1 cell line (ATCC CCL-70) derived from monkey kidney fibroblasts. CV-1 cells were cultured in liquid Roswell Park Memorial Institute Medium (RPMI) supplemented with 10% Fetal Bovine Serum (FBS, Gibco 10270–106) and antibiotics at a concentration of 50 μL/50 mL (Sigma Aldrich P4333). The culture was maintained at 37°C in a humidified atmosphere with 5% CO2.
The CV-1 cell plates were treated with GPPEE at concentrations of 1000 μg/mL, 500 μg/mL, 250 μg/mL, 125 μg/mL, 62.5 μg/mL, 31.25 μg/mL, 15.63 μg/mL, and 7.81 μg/mL. Cell viability was assessed using the Presto Blue Cell Viability Reagent (Thermofisher A13262), and incubated for 1–2 hours. Absorbance was measured to determine the reduction of resazurin to resorufin. The absorbance was read at a wavelength of 570 nm using a Multimode Reader. The cell viability percentage was calculated using the formula: Viability % = (Absorbance of treated group / Absorbance of the control group) x 100%.
Wound Healing Study
Animals
Male albino Wistar rats (Rattus norvegicus), aged 6–8 weeks and weighing between 150–250 grams, were used in this study. The rats were maintained under optimal conditions with controlled humidity and a 12-hour light/dark cycle. Prior to treatment, the rats were acclimatized for 7 days. During acclimatization, the rats were fed standardized pellets containing low fiber (5%), protein (20%), and fat (5–10%), and provided with water ad libitum. The cages and water dispensers were cleaned regularly.
Preparation of Wound Healing Ointment
The gel was prepared by dispersing Carbomer 940 into distilled water, followed by homogenization. Methylparaben and propylparaben were then added to the mixture and stirred until homogeneous. Subsequently, the Granola potato peel ethanol extract was dissolved in glycerin and combined with the pre-prepared gel base. The mixture was then homogenized again until a uniform gel formulation was achieved.14,15 The gel concentration used in this study was determined based on previous research literature, with modifications made to align with the objectives of this study.16–18 Gel formulations are shown in Table 1.
 |
Table 1 GPPEE Gel Formulation |
Animal Groups
Based on Federer’s sample size calculation formula,14,19 a total of 48 rats were used in this study. The rats were randomly divided into four test groups (n = 12 per group) as follows:
Group 1 (G1): Wounded and treated with placebo gel (negative control)
Group 2 (G2): Wounded and treated with 0.1% triamcinolone acetonide ointment (positive control)
Group 3 (G3): Wounded and treated with 4% Granola potato peel ethanol extract gel
Group 4 (G4): Wounded and treated with 6% Granola potato peel ethanol extract gel
Excision Wound Model
A total of 48 rats were anesthetized intraperitoneally using ketamine (45 mg/kg) and xylazine (0.35 mg/kg) before the induction of oral ulceration. Antisepsis of the rat palatal mucosa was performed using a 10 mm round cotton swab moistened with 0.2% chlorhexidine gluconate mouthwash solution. Oral ulcers were induced in the median palatal region using a single-use punch biopsy (Mentok Co., Ltd., India) with a diameter of 4 mm and an excision depth of approximately 2 mm. The mucoperiosteal specimens were dissected using sharp instruments.20,21 After the punch biopsy procedure, the ulcer induction area was cleaned using 0.9% NaCl solution administered with a syringe fitted with a blunt end. The area was then dried using sterile gauze while gently applying pressure to control bleeding. Once the bleeding ceased, each group was treated with the respective topical agent according to their assigned treatment group.22 Without touching the wound, 1 mL of the test topical agent was directly applied to the wound using a syringe with a blunt cannula until the wound was completely covered.20 Induction of the palatal mucosal wound is shown in Figure 2.
Hematoxylin-Eosin (HE) Staining and Scoring
Gingival tissue samples were collected on days 0, 1, 3, 7, and 14. The histological preparation of the rat oral mucosa was performed using the paraffin method and Hematoxylin-Eosin (HE) staining. The first step involved decalcification by immersing the specimens in 14% EDTA solution, followed by fixation, dehydration, and clearing processes. Subsequently, the tissues were embedded in paraffin and sectioned. The tissue sections were stained with Hematoxylin-Eosin (HE). The number of inflammatory cells, fibroblasts, and new blood vessels was manually counted using a binocular microscope. Complete re-epithelialization is characterized by a continuous, newly formed epithelial layer that fully covers the wound surface. Clinically, it is indicated by minimal or no defects on the tissue surface.23–25
Masson’s Trichrome Staining for Collagen
Masson’s Trichrome staining was performed on 5 µm thick tissue sections following standard procedures to determine collagen synthesis in each group. The tissue sections with wounds were stained with Weigert’s hematoxylin for approximately 10 minutes and rinsed under running tap water for 5 minutes, followed by a wash with distilled water. Subsequently, the sections were stained with Biebrich scarlet for 5 minutes and rinsed with distilled water.
The slides were then immersed in freshly prepared phosphotungstic/phosphomolybdic acid for 10 minutes. Following this, the slides were transferred directly to aniline blue for 5 minutes and rinsed with distilled water. The slides were then dipped in 1% acetic acid for 1 minute and rinsed with distilled water. Finally, the sections were dehydrated in absolute ethanol, cleared with xylene, and mounted with DPX. In the stained sections, nuclei appeared black, cytoplasm, muscle, and erythrocytes were red, and collagen was stained blue.
Statistical Analysis
Data analysis was performed with a confidence level of 95% (α = 0.05). For normally distributed data, parametric statistical analysis was conducted using One-Way ANOVA to assess variance. If the data were not normally distributed, the Kruskal–Wallis test was used for variance analysis. Post hoc analysis was performed using Bonferroni correction or t-tests as appropriate.
Results
Phytochemical Screening Results
Qualitative phytochemical screening of the GPPEE indicated strong positive results for phenolic compounds and alkaloids, moderate positive results for flavonoids (using 10% NaOH reagent), and weak positive results for tannins and triterpenoids (Table 2).
 |
Table 2 Phytochemical Screening Results of GPPEE |
Cytotoxicity Assay Results
The antiproliferative test results indicated that the IC5○ value of the Granola potato peel ethanol extract was 235.80 μg/mL (with cisplatin used as a reference at an IC5○ concentration of 44 μM). According to Figure 3, the percentage of cell viability remained above 70% even after the administration of 100 mL of GPPEE at 125 μg/mL for 24 hours. However, with increasing concentrations of the GPPEE, cell viability began to decrease at 250 μg/mL, dropping to only 6.05% at a concentration of 1000 μg/mL.
 |
Figure 3 Curve of GPPEE cytotoxicity test on CV-1 cells. |
According to Figure 4, the microscopic observations of CV-1 cells after 24 hours of incubation in different conditions were analyzed. These conditions included the control (media only), 2% DMSO media, cisplatin, and various concentrations of GPPEE (7.81 μg/mL, 15.63 μg/mL, 31.25 μg/mL, 62.50 μg/mL, 125 μg/mL, 250 μg/mL, 500 μg/mL, and 1000 μg/mL). The cells were observed under a microscope at 400x magnification. Viable cells were identified as cylindrical or elongated and adhered to the substrate, while non-viable cells appeared round and large.
 |
Figure 4 Documentation of CV-1 cell morphology following treatment with GPPEE. |
Wound Healing Evaluation
Based on the clinical examination of the wound (Figure 5), gradual healing was observed in all treatment groups. Macroscopically, the wound area was covered by fibrin tissue on days 1 and 3, and the wound edges appeared irregular and began to migrate towards the center of the wound. The fibrin covering the center of the wound began to diminish from day 7 to day 14. By day 14, the wound defect had fully closed, although slight redness was still visible in the placebo gel group.
 |
Figure 5 Clinical observations of palatal ulcer induction in Wistar rats were conducted on days 0, 1, 3, 7, and 14. |
Histopathological analysis (4x magnification) showed that the ulcer wound appearance on day 0 was similar across all treatment groups (Figure 6), with a defect and loss of epithelium. On day 1, there was a thickening of the tissue in the wound area and the beginning of inflammatory cell infiltration. By day 3, granulation tissue with denser inflammatory cells and migrating edge epithelium towards the center was observed in the groups treated with 0.1% TCA (G2) and 6% GPPEE gel (G4). On day 7, gradual epithelial proliferation to close the wound was noted, starting from the wound margins in all groups, with the most significant results in the 0.1% TCA (G2) and 6% GPPEE gel (G4) groups. By day 14, complete epithelialization was observed in all groups, but the 0.1% triamcinolone acetonide (G2) and 6% GPPEE gel (G4) groups showed lower inflammatory cell infiltration compared to the other groups.
 |
Figure 6 Histological images of wound healing using Hematoxylin-Eosin (HE) staining at 4x magnification. Abbreviations: F, fibroblasts; IC, inflammatory cells; BV, blood vessels. |
Effect of GPPEE Gel on the Number of Inflammatory Cells
Based on the research findings, both the 4% and 6% concentrations of the GPPEE gel exhibited pro-inflammatory effects on days 1 and 3 of observation, similar to the group treated with 0.1% TCA (G2). The 6% GPPEE gel (G4) demonstrated the highest pro-inflammatory activity during the initial period (days 1 and 3), followed by a marked anti-inflammatory effect, as evidenced by a significant reduction in the number of inflammatory cells on days 7 and 14, compared to the 0.1% TCA group (G2). In contrast, the 4% GPPEE gel (G3) continued to exhibit pro-inflammatory effects on day 7, which then diminished by day 14. Results are shown in Table 3 and Figure 7.
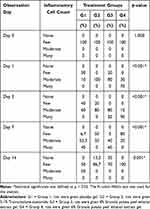 |
Table 3 Differences in the Number of Inflammatory Cells Between Treatment Groups on Days 0, 1, 3, 7, and 14 |
 |
Figure 7 Histological images of wound healing using Hematoxylin-Eosin (HE) staining at 400x magnification. Abbreviations: F, fibroblasts; IC, inflammatory cells; BV, blood vessels. |
Effect of GPPEE Gel on the Number of Fibroblast
On day 0 of observation, significant differences were found between G3 and G1, G3 and G2, and G4 and G3, indicating that G3 had a higher number of fibroblasts compared to G1, G2, and G4. On day 1, significant differences were observed between G4 and G2, as well as between G3 and G2, with G4 (6% GPPEE gel) showing a lower number of fibroblasts compared to G2 (0.1% TCA). On day 3, there were no significant differences between the groups. On day 7, significant differences were noted between G3 and G2, G4 and G2, and G3 and G1, suggesting that G3 and G4 continued significantly stimulate on fibroblast proliferation compared to G2 and G1. On day 14, significant differences were observed between G2 and G1, as well as between G3 and G2. These results indicate that G2 showed a significant increase in the number of fibroblasts compared to G1, and G3 showed a higher number of fibroblasts compared to G2. Results are detailed in Table 4 and Figure 7.
 |
Table 4 Differences in the Number of Fibroblast Between Treatment Groups on Days 0, 1, 3, 7, and 14 |
Effect of GPPEE Gel on Angiogenesis
Based on the observations, all groups showed an increase in the number of new blood vessels from day 1 to day 14. The groups treated with 4% and 6% GPPEE gel exhibited a temporary decrease in the number of blood vessels on day 3, followed by an increase on days 7 and 14. However, this increase did not show a statistically significant difference compared to the other treatment groups. Results are shown in Table 5 and Figure 7.
 |
Table 5 Differences in New Blood Vessel Formation (Angiogenesis) Between Treatment Groups on Observation Days 0, 1, 3, 7, and 14 |
Effect of GPPEE Gel on the Number of Collagen
Based on the observations, the percentage of collagen on days 0, 1, and 3 showed an increase in each group, although there was no statistically significant difference between the groups. On day 7, the groups treated with GPPEE gel at both 4% and 6% concentrations showed a significant increase in collagen percentage compared to the placebo gel and 0.1% TCA groups. However, on day 14, only the 6% Granola potato peel extract gel group showed a significant increase in collagen compared to the other treatment groups. Results are shown in Table 6 and Figure 8.
 |
Table 6 Differences in Collagen Percentage Between Treatment Groups on Observation Days 0, 1, 3, 7, and 14 |
 |
Figure 8 Histological images of wound healing using Masson’s Trichrome (MT) staining to observe collagen content. Yellow arrows indicate collagen stained in blue. (Magnification 100x). |
Discussion
The potatoes used in this study were sourced from potato farms in Pangalengan, Bandung Regency, West Java, and cultivated at the same location to ensure consistent plant quality. The part used in this research was the potato peel. Phytochemical screening of the ethanol extract of Granola potato peel revealed the presence of phenolic compounds, flavonoids, alkaloids, terpenoids, and tannins. This finding is consistent with previous studies that reported higher levels of phenolic compounds and flavonoids in potato peels compared to potato flesh.7,9–11,26,27 According to Akyol et al, potatoes are an excellent source of phenolic compounds, with a higher total phenol content than other fruits and vegetables such as carrots, onions, and tomatoes. The phenolic compounds found in potatoes include phenolic acids and flavonoids, such as flavonols, flavanols, and anthocyanins. The chemical compounds in potato peels exhibit significant anti-inflammatory properties, which are the second most prominent trait after their antioxidant capabilities.9
Wound healing begins with the hemostasis and inflammatory phases. Hemostasis in the wound area typically occurs within a few minutes to several hours after injury.28 The inflammatory response is a natural and essential process that is highly coordinated and necessary for healing. This phase facilitates healing at the wound base by removing necrotic tissue, debris, and bacterial contaminants, while also recruiting and activating fibroblasts.29 Alongside the initial hemostasis phase, the wound undergoes inflammatory infiltration immediately in response to chemokines in the injured area. The inflammatory response peaks between 24 and 48 hours post-injury and can last up to one week.30 In our study, inflammatory cells began to appear in small numbers in the wound area across all groups on day 0 of observation. The number of inflammatory cells subsequently increased on days 1 and 3 of observation. The groups treated with 4% and 6% GPPEE, as well as the positive control group (0.1% TCA), exhibited a higher number of inflammatory cells compared to the negative control group (placebo gel). This suggests that the active compounds in GPPEE may influence the inflammatory process. The faster increase in inflammatory cells compared to the negative control group indicates that GPPEE may accelerate the inflammatory phase.
Flavonoids in potato peels act as anti-inflammatory agents. The anti-inflammatory mechanism of flavonoids involves the inhibition of cyclooxygenase and lipoxygenase pathways, as well as the suppression of histamine release and leukocyte accumulation, which are crucial in combating inflammation and allergic symptoms. Additionally, flavonoids inhibit the secretion of arachidonic acid and lysosomal enzymes.31 Garcia-Lafuente et al found that most flavonoids are potent inhibitors of arachidonic acid, phospholipase A2, cyclooxygenase, and nitric oxide synthase (NOS). This inhibition reduces the production of prostaglandins, leukotrienes, and nitric oxide (NO), which are key inflammatory mediators.32
Flavonoids decrease the release of arachidonic acid metabolites and chemokines, thereby reducing leukocyte infiltration and edema. Additionally, Ferrali et al reported that flavonoids chelate iron and inhibit complement system activation, thus reducing inflammation. Flavonoids also chelate transition metals, thereby decreasing the formation of reactive oxygen species (ROS).31 Furthermore, a study by Li et al found that flavonoids can reduce the expression of pro-inflammatory cytokines such as IL-6, IL-8, TNF-α, IL-1, and monocyte chemoattractant protein-1 (MCP-1) in RAW macrophages, peripheral blood mononuclear cells, and Jurkat T cells.31
The flavonoid content in potato peel extract is associated with anti-inflammatory, antibacterial, and antioxidant activities. As anti-inflammatory agents, flavonoids can shorten the inflammation stage, allowing for a quicker transition to the proliferation and remodeling stages, thus accelerating the wound healing process. The antibacterial activity of flavonoids also supports wound healing. As antibacterial agents, flavonoids can form complexes with bacterial cell membrane proteins. Flavonoids act as antioxidants by binding to free radicals, thereby preventing oxidative stress.33 Free radicals are reactive molecules with antibacterial properties in the form of reactive oxygen species (ROS). However, excessive ROS can cause damage to the wound area, slowing the healing process.34 Therefore, the antioxidant activity of flavonoids in plant extracts can help prevent oxidative stress.34,35 Flavonoids can donate electrons to ROS, preventing ROS from taking electrons from tissue-forming molecules such as DNA, proteins, and lipids.33
From days 5 to 7, fibroblasts begin to form new collagen and glycosaminoglycans. In addition, re-epithelialization occurs as cells migrate from the periphery of the wound and adjacent edges. Initially, only a thin layer of epithelial cells forms, but over time, a thicker and more durable layer bridges the wound. Furthermore, neovascularization takes place through angiogenesis, which is the formation of new blood vessels from existing ones, and vasculogenesis, the formation of new blood vessels from endothelial progenitor cells (EPC).36 Fibroblast proliferation contributes to the occurrence of angiogenesis.37 Vascular restructuring in wounds commences immediately following injury, with heightened activity during the proliferative phase. This process supplies the oxygen and nutrients essential for cell migration, proliferation, and the synthesis of extracellular matrix components. Secreted mediators, including VEGF and angiopoietins, stimulate endothelial cell proliferation and contribute to the restructuring of the vascular system at the wound site.38 Once collagen fibers are established on the fibrin framework, the wound enters the maturation phase. Wound contraction begins, facilitated by the ongoing deposition of fibroblasts and myofibroblasts.36
On day 7, the number of inflammatory cells in the GPPEE gel and 0.1% TCA treatment groups was lower compared to the negative control group (placebo gel). Meanwhile, the number of fibroblasts and the amount of collagen in the 4% and 6% GPPEE gel treatment groups were higher than in both the negative control (placebo gel) and 0.1% TCA groups. This suggests that the 4% and 6% GPPEE gels may have a stimulatory effect on fibroblasts and collagen, which are critical components of the wound healing process. Although the number of new blood vessels increased across all groups, no significant difference was observed.
From days 7 to 21, the maturation or remodeling phase begins. Excess collagen is degraded, and wound contraction reaches its peak around the third week. Wound contraction is significantly greater in secondary healing compared to primary healing. The maximal tensile strength of the incision is achieved approximately 11 to 14 weeks after injury.36 By day 14 of observation, the number of fibroblasts in each group began to decrease; however, the amount of collagen in the 4% and 6% GPPEE gel groups continued to increase compared to the negative control group (placebo gel) and the positive control group (0.1% TCA). This suggests that the Granola potato peel extract contains compounds that may enhance collagen production in the tissue. The number of inflammatory cells in the positive control group (0.1% TCA) and the 4% and 6% GPPEE gel groups was minimal or absent, while the negative control group (placebo gel) still exhibited mild to moderate levels of inflammatory cells. This indicates that both concentrations of GPPEE gel can shorten the inflammatory phase, stimulate the proliferation phase, and potentially prevent the development of chronic inflammation.
The proliferation and migration of fibroblasts to the wound area play a crucial role during the re-epithelialization process to restore tissue integrity. The proliferation phase also includes angiogenesis in newly formed cells and tissues, the formation of granulation tissue, and the deposition of collagen produced by fibroblasts.39 The high antioxidant activity in potato peels can be attributed to the presence of vitamin C, flavonoids, polyphenols, and anthocyanins.40 Vitamin C is an antioxidant that promotes collagen production, playing a vital role in skin health and facilitating wound healing by stimulating fibroblast formation. Increased fibroblast activity can accelerate the wound healing process.41
According to research conducted by Rosas-Cruz et al on the wound healing activity of potato ointment, the phytoconstituents in potatoes (both in the peel and flesh) exhibit antioxidant, anti-inflammatory, and antimicrobial properties. These phytoconstituents are responsible for the enhanced wound closure observed up to day 7 of treatment. Groups treated with potato ointment showed accelerated wound closure compared to the negative and positive control groups (Neomycin, Polymyxin B, and Bacitracin ointment). Histological analysis of rat skin in the study revealed that the groups treated with potato ointment demonstrated progressive and significant wound healing, characterized by the presence of fibroblasts and the absence of crusts or eschar on days 7 observation. This activity was more pronounced in the specimens from the group treated with 2% potato ointment, which showed a clear increase in fibroblast content horizontally arranged, indicating advanced reparative processes. In addition, no fibroblasts were present in the crusts or eschar, further highlighting the effectiveness of the treatment.18 These findings are consistent with our results on the 7th day, where significant wound healing was observed using GPPEE gel concentrations. However, in our study, we used different concentrations, including 4% and 6% of GPPEE gel. The 6% GPPEE gel demonstrated the most effective wound healing, as indicated by the higher number of fibroblasts. Also, increased collagen deposition, new blood vessel formation, and reduced inflammatory cells support our findings regarding the wound healing process on days 1, 3, 7, and 14. To date, the research literature on the anti-inflammatory and wound-healing activities of potato peel extract, specifically with indicators such as fibroblast activity, collagen deposition, new blood vessel formation, and inflammatory cell reduction, remains limited.
Phenolic compounds, especially flavonoids, have garnered significant interest as wound care agents due to their anti-inflammatory, angiogenic, re-epithelialization, and antioxidant properties.42,43 According to Ibrahim et al and Shedoeva et al, flavonoid compounds stimulate collagen synthesis in fibroblast cell cultures on the skin.44,45 In a study by Lodhi et al, which observed increased collagen cross-linking in wounds by determining hydroxyproline in an in vivo diabetic rat model after topical application of Martynia annua and Tephrosia purpurea extracts containing luteolin, histopathological analysis on day 18 post-treatment confirmed that flavonoids can stimulate collagen synthesis and participate in cross-link formation as collagen matures. This was indicated by the proliferation of angioblasts and fibroblasts with the infiltration of a large number of lymphocytes, macrophages, and few neutrophils. Additionally, dense fibrous tissue and blood capillaries were found, with capillary arrangements parallel to the fibrous tissue.46
Based on a study by Balderas-Cordero et al on the wound healing potential of propolis on the skin tissue of Wistar rats, it was concluded that the presence of flavonoid compounds can accelerate the transition from type III to type I collagen. This results in a better fiber arrangement in the tissue, re-epithelialization of the wound area, tensile strength, and mechanical stability of the newly formed tissue, as well as an increase in the number of myofibroblasts on day 14. The transition from type III to type I collagen was observed using Herovici staining.47 Furthermore, according to Almeida et al, flavonoid compounds can inhibit MMP (an enzyme that breaks down collagen), thereby increasing the rate and amount of collagen needed for the formation of the new wound matrix and accelerating the wound healing process.48
The presence of glycoalkaloid compounds in Granola potato peels is closely associated with cell death. Several studies have shown that glycoalkaloids in the Solanaceae family exhibit cytotoxic effects on healthy and cancerous cells.49–52 Solanine and chaconine are the two primary glycoalkaloids found in potatoes. These compounds exhibit fungicidal, antibacterial, and anti-inflammatory activities, primarily by inhibiting the NF-κB signaling pathway.49,53 A cytotoxicity test of Granola potato peel ethanol extract (GPPEE) on CV-1 cells (ATCC CCL-70) was conducted, using the inhibitory concentration of 50% (IC50) as the parameter. IC50 refers to the concentration required to inhibit 50% of cell proliferation. The number of viable fibroblasts in the sample groups reflects the level of toxicity. An increase in the percentage of living fibroblasts indicates higher cell viability and fibroblast proliferation.54,55 The IC50 value of GPPEE was 235.80 μg/mL, compared to the IC50 value of cisplatin, which was 44 μM (13.2 μg/mL). This suggests that GPPEE was non-toxic and may possess proliferative ability.
Conclusion
Granola potato peel ethanol extract gel at concentrations of 4% and 6% shows superior wound healing activity compared to 0.1% triamcinolone acetonide, a commonly used anti-inflammatory agent. This is evidenced by increased fibroblast proliferation (cells important for wound healing), collagen deposition (a protein that helps rebuild tissue), and blood vessel formation, particularly from day 7 onward. Additionally, the Granola potato peel ethanol extract gel at 4% and 6% concentrations exhibit anti-inflammatory effects comparable to 0.1% triamcinolone acetonide. More studies are needed to explore the underlying mechanisms and potential biomarkers (a biological molecule) associated with the wound healing and anti-inflammatory effects of the GPPEE gel. Investigating these aspects could provide deeper insights into its therapeutic potential and optimize its application in medical practice.
Acknowledgments
The authors extend their gratitude to the Central Laboratory staff at Universitas Padjadjaran, the Animal Laboratory staff at the Faculty of Medicine Universitas Jenderal Achmad Yani, and the botanist, Heri Santoso, S.Si., from Generasi Biologi Indonesia for their invaluable technical support. This research was funded by the Academic Leadership Grant from Universitas Padjadjaran (Grant No. 1549/UN6.3.1/PT.00/2023).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Waasdorp M, Krom BP, Bikker FJ, van Zuijlen PPM, Niessen FB, Gibbs S. The bigger picture: why oral mucosa heals better than skin. Biomolecules. 2021;11(8):1–22. doi:10.3390/biom11081165
2. Arundina I, Diyatri I, Surboyo MDC, Monica E, Afanda NM. Growth factor stimulation for the healing of traumatic ulcers with liquid rice hull smoke. J Taibah Univ Med Sci. 2021;16(3):431–439. doi:10.1016/j.jtumed.2021.01.003
3. Minhas S, Sajjad A, Kashif M, Taj F, Waddani Al H, Khurshid Z. Oral ulcers presentation in systemic diseases: an update. Open Access Maced J Med Sci. 2019;7(19):3341–3347. doi:10.3889/oamjms.2019.689
4. Yogarajah S, Setterfield J. Mouth ulcers and diseases of the oral cavity. Medicine. 2021;49(7):407–413. doi:10.1016/j.mpmed.2021.04.003
5. Légeret C, Furlano R. Oral ulcers in children- a clinical narrative overview. Ital J Pediatr. 2021;47(1):144. doi:10.1186/s13052-021-01097-2
6. Coondoo A, Phiske M, Verma S, Lahiri K. Side-effects of topical steroids: a long overdue revisit. Indian Dermatol Online J. 2014;5(4):416–425. doi:10.4103/2229-5178.142483
7. Javed A, Ahmad A, Tahir A, Shabbir U, Nouman M, Hameed A. Potato peel waste—its nutraceutical, industrial and biotechnological applications. AIMS Agric Food. 2019;4(3):807–823. doi:10.3934/agrfood.2019.3.807
8. Pacifico D, Lanzanova C, Pagnotta E, et al. Sustainable use of bioactive compounds from Solanum tuberosum and Brassicaceae wastes and by-products for crop protection—a review. Molecules. 2021;26(8):2174. doi:10.3390/molecules26082174
9. Sampaio SL, Petropoulos SA, Dias MI, et al. Phenolic composition and cell-based biological activities of ten coloured potato peels (Solanum tuberosum L.). Food Chem. 2021;363:130360. doi:10.1016/j.foodchem.2021.130360
10. Silva-Beltrán NP, Chaidez-Quiroz C, López-Cuevas O, et al. Phenolic compounds of potato peel extracts: their antioxidant activity and protection against human enteric viruses. J Microbiol Biotechnol. 2017;27(2):234–241. doi:10.4014/jmb.1606.06007
11. Albishi T, John JA, Al-Khalifa AS, Shahidi F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J Funct Foods. 2013;5(2):590–600. doi:10.1016/j.jff.2012.11.019
12. Wahyudi IA, Ramadhan FR, Wijaya RIK, Ardhani R, Utami TW. Analgesic, anti-inflammatory and anti-biofilm-forming activity of potato (Solanum tuberosum L.) Peel Extract. Indones. J. Cancer Chemoprevent. 2020;11(1):30–35. doi:10.14499/indonesianjcanchemoprev11iss1pp30-35
13. Ghuman S, Ncube B, Finnie JF, et al. Antioxidant, anti-inflammatory and wound healing properties of medicinal plant extracts used to treat wounds and dermatological disorders. South Afr J Bot. 2019;126:232–240. doi:10.3390/biom11081165
14. Tiarasanti F, Sufiawati I, Amalia E, Sari KI, Zubaedah C, Takarini V. The effects of potato (Solanum tuberosum L. vs. Granola; Solanaceae) peel extract gel on gingival wound healing in Wistar rats. J Exp Pharmacol. 2024;16:25–35. doi:10.2147/JEP.S443355
15. Farid N, Kalsum U, Yustisi J, Wahyuli R. Formulasi Sediaan Gel Basis HPMC Ekstrak Etanol Daun Jarak Cina (Jatropha multifida) Sebagai Penembuhan Luka Sayat pada Tikus Putih (Rattus norvegicus). Sasambo Journal of Pharmacy. 2020;1(2):57–62. doi:10.29303/sjp.v1i2.25
16. Bhinge SD, Bhutkar MA, Randive DS, et al. Formulation development and evaluation of antimicrobial polyherbal gel. Ann Pharm Françaises. 2017;75(5):349–358. doi:10.1016/j.pharma.2017.04.006
17. Harahap NI, Nainggolan M, Harahap U. Formulation and evaluation of herbal antibacterial gel containing ethanolic extract of mikania micrantha kunth leaves. Asian J Pharm Clin Res. 2018;11(3):429–431. doi:10.22159/ajpcr.2018.v11i3.22211
18. Rosas-Cruz GP, Silva-Correa CR, Calderón-Peña AA, et al. Wound healing activity of an ointment from Solanum tuberosum L. “Tumbay Yellow Potato” on Mus musculus Balb/c. Pharmacogn J. 2020;12(6):1268–1275. doi:10.5530/PJ.2020.12.175
19. Federer WT. Principles of statistical design with special reference to experiment and treatment design. Iowa State Univ. 1984;1983-07:77–104.
20. Hammad HM, Hammad MM, Abdelhadi IN, Khalifeh MS. Effects of topically applied agents on intra-oral wound healing in a rat model: a clinical and histomorphometric study. Int J Dent Hyg. 2011;9(1):9–16. doi:10.1111/j.1601-5037.2009.00410.x
21. Amaliya A, Muhaimina RK, Susanto A, Sutjiatmo AB. Histological assessment of palatal donor site wound healing after application of moringa oleifera Lamarck leaf extract in rats. Eur J Dent. 2019;13(2):248–254. doi:10.1055/s-0039-1695065
22. Qi W, Dong N, Wu L, et al. Promoting oral mucosal wound healing using a DCS-RuB2A2 hydrogel based on a photoreactive antibacterial and sustained release of BMSCs. Bioact Mater. 2022;23:53–68. doi:10.1016/j.bioactmat.2022.10.027
23. Van De Vyver M, Boodhoo K, Frazier T, et al. Histology scoring system for murine cutaneous wounds. Stem Cells Dev. 2021;30(23):1141–1152. doi:10.1089/scd.2021.0124
24. Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014;3(7):445–464. doi:10.1089/wound.2013.0473
25. Gupta A, Kumar P. Assessment of the histological state of the healing wound. Plast Aesthetic Res. 2015;2(5):239–242. doi:10.4103/2347-9264.158862
26. Mishra T, Raigond P, Thakur N, Dutt S, Singh B. recent updates on healthy phytoconstituents in potato: a nutritional depository. Potato Res. 2020;63(3):323–343. doi:10.1007/s11540-019-09442-z
27. Akyol H, Riciputi Y, Capanoglu E, Caboni MF, Verardo V. Phenolic compounds in the potato and its byproducts: an overview. Int J Mol Sci. 2016;17(6):835. doi:10.3390/ijms17060835
28. Ellis S, Lin EJ, Tartar D. Immunology of wound healing. Curr Dermatol Rep. 2018;7(4):350–358. doi:10.1007/s13671-018-0234-9
29. Shukla SK, Sharma AK, Gupta V, Yashavarddhan MH. Pharmacological control of inflammation in wound healing. J Tissue Viability. 2019;28(4):218–222. doi:10.1016/j.jtv.2019.09.002
30. Toma AI, Fuller JM, Willett NJ, Goudy SL. Oral wound healing models and emerging regenerative therapies. Transl Res. 2021;236(404):17–34. doi:10.1016/j.trsl.2021.06.003
31. Al-Khayri JM, Sahana GR, Nagella P, Joseph BV, Alessa FM, Al-Mssallem MQ. Flavonoids as potential anti-inflammatory molecules: a review. Molecules. 2022;27(9):2901. doi:10.3390/molecules27092901
32. García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res off J Eur Histamine Res Soc. 2009;58(9):537–552. doi:10.1007/s00011-009-0037-3
33. Budiawan A, Purwanto A, Puradewa L, Cahyani ED, Purwaningsih CE. Wound healing activity and flavonoid contents of purslane (Portulaca grandiflora) of various varieties. RSC Adv. 2023;13(15):9871–9877. doi:10.1039/d3ra00868a
34. Priyadarshi A, Keshri GK, Gupta A. Hippophae rhamnoides L. leaf extract diminishes oxidative stress, inflammation and ameliorates bioenergetic activation in full-thickness burn wound healing. Phytomedicine Plus. 2022;2(3):100292. doi:10.1016/j.phyplu.2022.100292
35. Asante-Kwatia E, Adjei S, Jibira Y, et al. Amphimas pterocarpoides harms.: an Evaluation of flavonoid and phenolic contents, wound healing, anthelmintic and antioxidant activities of the leaves and stem bark. Heliyon. 2021;7(11):e08261. doi:10.1016/j.heliyon.2021.e08261
36. Bowden LG, Byrne HM, Maini PK, Moulton DE. A morphoelastic model for dermal wound closure. Biomech Model Mechanobiol. 2016;15(3):663–681. doi:10.1007/s10237-015-0716-7
37. Cialdai F, Risaliti C, Monici M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front Bioeng Biotechnol. 2022;10:958381. doi:10.3389/fbioe.2022.958381
38. Gushiken LFS, Beserra FP, Bastos JK, Jackson CJ, Pellizzon CH. Cutaneous wound healing: an update from physiopathology to current therapies. Life. 2021;11(7):665. doi:10.3390/life11070665
39. Muniandy K, Gothai S, Tan WS, et al. In vitro wound healing potential of stem extract of Alternanthera sessilis. Evid Based Complement Alternat Med. 2018;2018:3142073. doi:10.1155/2018/3142073
40. Jimenez-Champi D, Romero-Orejon FL, Moran-Reyes A, Muñoz AM, Ramos-Escudero F. Bioactive compounds in potato peels, extraction methods, and their applications in the food industry: a review. CyTA - J Food. 2023;21(1):418–432. doi:10.1080/19476337.2023.2213746
41. Hidayat W, Sufiawati I, Satari MH, Lesmana R, Ichwan S. Pharmacological activity of chemical compounds of potato peel waste (Solanum tuberosum L.) in vitro: a scoping review. J Exp Pharmacol. 2024;16:61–69. doi:10.2147/JEP.S435734
42. Carvalho MTB, Araújo-Filho HG, Barreto AS, Quintans-Júnior LJ, Quintans JSS, Barreto RSS. Wound healing properties of flavonoids: a systematic review highlighting the mechanisms of action. Phytomedicine. 2021;90:153636. doi:10.1016/j.phymed.2021.153636
43. Zulkefli N, Che Zahari CNM, Sayuti NH, et al. Flavonoids as potential wound-healing molecules: emphasis on pathways perspective. Int J Mol Sci. 2023;24(5):4607. doi:10.3390/ijms24054607
44. Ibrahim’Izzah N, Wong SK, Mohamed IN, et al. Wound healing properties of selected natural products. Int J Environ Res Public Health. 2018;15(11):2360. doi:10.3390/ijerph15112360
45. Shedoeva A, Leavesley D, Upton Z, Fan C. Wound Healing and the Use of Medicinal Plants. Evid Based Complement Alternat Med. 2019;2019:2684108. doi:10.1155/2019/2684108
46. Lodhi S, Singhai AK. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua Linn. on streptozotocin induced diabetic rats. Asian Pac J Trop Med. 2013;6(4):253–259. doi:10.1016/S1995-7645(13)60053-X
47. Balderas-Cordero D, Canales-Alvarez O, Sánchez-Sánchez R, Cabrera-Wrooman A, Canales-Martinez MM, Rodriguez-Monroy MA. Anti-inflammatory and histological analysis of skin wound healing through topical application of Mexican propolis. Int J Mol Sci. 2023;24(14):11831. doi:10.3390/ijms241411831
48. de Almeida EB, Cordeiro Cardoso J, Karla de Lima A, et al. The incorporation of Brazilian propolis into collagen-based dressing films improves dermal burn healing. J Ethnopharmacol. 2013;147(2):419–425. doi:10.1016/j.jep.2013.03.031
49. Winkiel MJ, Chowański S, Słocińska M. Anticancer activity of glycoalkaloids from Solanum plants: a review. Front Pharmacol. 2022;13:979451. doi:10.3389/fphar.2022.979451
50. da Silva DC, Andrade PB, Valentão P, Pereira DM. Neurotoxicity of the steroidal alkaloids tomatine and tomatidine is RIP1 kinase- and caspase-independent and involves the eIF2α branch of the endoplasmic reticulum. J Steroid Biochem Mol Biol. 2017;171:178–186. doi:10.1016/j.jsbmb.2017.03.009
51. Fujimaki J, Sayama N, Shiotani S, et al. The steroidal alkaloid tomatidine and tomatidine-rich tomato leaf extract suppress the human gastric cancer-derived 85As2 cells in vitro and in vivo via modulation of interferon-stimulated genes. Nutrients. 2022;14(5):1023. doi:10.3390/nu14051023
52. Yu T, Wu Q, You X, et al. Tomatidine Alleviates Osteoporosis by Downregulation of p53. Med Sci Monit Int Med J Exp Clin Res. 2020;26:e923996. doi:10.12659/MSM.923996
53. Shin J-S, Lee K-G, Lee -H-H, et al. α-solanine isolated from Solanum tuberosum L. cv Jayoung Abrogates LPS-induced inflammatory responses Via NF-κB Inactivation in RAW 264.7 macrophages and endotoxin-induced shock model in mice. J Cell Biochem. 2016;117(10):2327–2339. doi:10.1002/jcb.25530
54. Nevozhay D. Cheburator software for automatically calculating drug inhibitory concentrations from in vitro screening assays. PLoS One. 2014;9(9):e106186. doi:10.1371/journal.pone.0106186
55. Nirwana I, Munadziroh E, Yogiartono RM, Thiyagu C, Ying CS, Dinaryanti A. Cytotoxicity and proliferation evaluation on fibroblast after combining calcium hydroxide and ellagic acid. J Adv Pharm Technol Res. 2021;12(1):27–31. doi:10.4103/japtr.JAPTR_154_20
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



