Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 18
Histopathological Subtypes and Clinical Presentation of Seborrheic Keratosis: A 15-Year Retrospective Analysis of 1,169 Cases in Hainan, China
Authors Luo W, Liang Y, Yang X, Wu W , Lu J
Received 13 January 2025
Accepted for publication 18 March 2025
Published 24 March 2025 Volume 2025:18 Pages 721—727
DOI https://doi.org/10.2147/CCID.S517318
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Wen Luo,1,2,* Yujia Liang,1,* Xianxu Yang,1 Weiwei Wu,1 Jiejie Lu1
1Department of Dermatology, Affiliated Dermatology Hospital of Hainan Medical University, The Fifth People’s Hospital of Hainan Province, Haikou, Hainan, People’s Republic of China; 2Hainan Medical University, Haikou, Hainan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weiwei Wu, Department of Dermatology, the Fifth People’s Hospital of Hainan Province, 8, Road of LongHua, LongHua District, Haikou, Hainan, 570125, People’s Republic of China, Email [email protected] Jiejie Lu, Department of Dermatology, the Fifth People’s Hospital of Hainan Province, 8, Road of LongHua, LongHua District, Haikou, Hainan, 570125, People’s Republic of China, Email [email protected]
Background: Seborrheic keratosis (SK), the most common benign epithelial tumor, frequently presents with cosmetic concerns despite its benign nature. The clinicopathological features of SK may demonstrate notable geographical variability. Diagnosis remains challenging due to varied clinical presentations.
Objective: This study aimed to investigate the clinicopathological characteristics of SK through a comprehensive analysis of a large patient cohort.
Methods: We conducted a retrospective analysis of clinical and pathological data from 1,169 patients diagnosed with SK between 1 January 2009 and 31 December 2023. Histopathological subtypes were categorized, and a descriptive analysis of demographic and clinical characteristics was performed.
Results: A total of 1,169 SK specimens were identified. The most frequent histopathological subtype was the acanthotic type (79.86%, 960 cases), followed by melanoacanthoma (7.98%, 92 cases), adenoid (5.32%, 64 cases), hyperkeratotic (4.90%, 59 cases), irritated (1.74%, 21 cases), and clonal (0.004%, 6 cases). The male-to-female ratio was 1.3:1, with a higher prevalence in men. SK most commonly affected the head, face, and neck, which accounted for 54.58% of cases (656/1,169), followed by the trunk (28.87%, 347 cases) and extremities (11.48%, 138 cases). The perineal area accounted for 4.48% (54 cases), while mucosal and plantar lesions were rare. The acanthotic subtype predominated across all anatomical sites, including mucosal and plantar regions. The age group most frequently diagnosed with SK was 40– 59 years, with the acanthotic, melanoacanthoma, and adenoid types being particularly prevalent in this cohort. The clinical-pathological diagnostic concordance rate was 55.69%, indicating a relatively high rate of diagnostic discrepancy based on visual examination alone.
Conclusion: Our study confirms the acanthotic subtype as the most common histological type of SK, predominantly observed in patients aged 40– 59 years. The head, face, and neck emerged as the most frequently involved sites. Ultraviolet radiation may play a significant role in SK pathogenesis.
Keywords: seborrheic keratosis, retrospective study, Hainan, China
Introduction
Seborrheic keratosis (SK) is a benign, age-related cutaneous tumor that typically presents as round or oval papules and plaques of varying sizes. In the United States, it is estimated that approximately 83.8 million people are affected by SK.1 A multicenter German study suggested that SK ranks as the third most prevalent dermatological condition among individuals over 65 years of age.2 Furthermore, an epidemiological survey conducted in a community in Shanghai, China, revealed a 100% prevalence of SK in individuals aged over 60 years.3
SK is predominantly diagnosed clinically. Histopathological examination to serve as the gold standard for diagnosing SK. Non-invasive dermatological imaging modalities-such as dermoscopy and reflectance confocal microscopy (RCM)—have demonstrated the potential to enhance diagnostic accuracy and are increasingly adopted in clinical practice. The histological classification of SK encompasses the following subtypes: acanthotic, melanoacanthoma, hyperkeratotic, adenoid, irritated, and clonal type. The histological diversity of seborrheic keratosis subtypes may contribute to elevated clinical misdiagnosis rates. Histopathological re-evaluation of clinically diagnosed SK cases in Guizhou, China revealed 21.9% misidentification, with 26.1% of these misdiagnosed cases representing cutaneous malignancies.4 The clonal type of SK dermoscopically mimics melanoma and basal cell carcinoma (BCC) by the presence of globule-like structures,5 highlighting SK’s capacity to clinically simulate malignant neoplasms.
Epidemiological studies have demonstrated a significant association between UV exposure and SK incidence, with higher UV indices correlating with greater SK prevalence.6–8 Hainan Province, the southernmost region of China, experiences elevated solar radiation and high ultraviolet (UV) radiation levels, which are thought to contribute to a higher incidence of SK. However, large-scale epidemiological data on SK in high UV radiation regions such as Hainan remain sparse. To address this gap, this retrospective study analyzed clinical parameters (including age of onset, age at diagnosis, and lesion distribution) and histopathological subtypes of SK cases diagnosed at the Department of Dermatology, Fifth People’s Hospital of Hainan Province, aiming to identify distinct clinical manifestations associated with specific histological type. The findings provide insights into clinicopathological correlations in SK, particularly in high-UV-exposure regions, and enhance diagnostic accuracy for dermatologists and pathologists.
Materials and Methods
A retrospective review was conducted using the dedicated database of pathological biopsies from the Fifth People’s Hospital of Hainan Province, covering the period from 1 January 2009 to 31 December 2023. Patients diagnosed with SK based on histopathological examination were included in the study. Clinical and pathological data, including gender, age at diagnosis, age of onset, lesion distribution, and histopathological subtype, were collected and reviewed. Cases with incomplete or uncertain clinical and pathological data were excluded from the analysis.
To ensure the accuracy of histopathological diagnoses, two experienced dermatopathologists independently reviewed the pathological slides. In instances discrepancies arose between the two pathologists, a third expert was consulted to reach a consensus, and the final diagnosis was determined through discussion.
The study protocol was approved by the Ethics Committee of the Fifth People’s Hospital of Hainan Province, and the research was conducted in accordance with the ethical standards outlined in the Declaration of Helsinki.
Statistical Analysis
A descriptive analysis was performed on patients clinically diagnosed with SK, encompassing demographic variables (sex, age at diagnosis), lesion distribution patterns, and clinicopathological characteristics. Normally distributed variables (eg age at diagnosis) were expressed as mean ± standard deviation (SD) with 95% confidence intervals [CI]. Group comparisons were analyzed using Student’s t-test, with Welch’s t-test applied when heterogeneity of variance was confirmed by Levene’s test (α=0.05). All statistical analyses were conducted using SPSS 27.0 (IBM Corp., Armonk, NY) with two-tailed significance set at P<0.05.
Results
A total of 1,430 cases diagnosed as seborrheic keratosis (SK) through pathological biopsy were initially identified between 1 January 2009 and 31 December 2023. Following a review of the clinical data, 261 cases were excluded due to incomplete information or recurrence of previously diagnosed lesions. Consequently, 1,169 cases were included in the final study cohort (Figure 1). Over a 15-year period, this center collected a total of 21,810 skin biopsy cases, of which 1,430 were diagnosed as SK (6.55%). The incidence of SK has demonstrated a sustained upward trajectory (Figure 2), attributable to regional population aging and enhanced public awareness of dermatological conditions.
 |
Figure 1 Strobe flow diagram. |
 |
Figure 2 Incidence and Gender Differences of Seborrheic Keratosis. |
The predominant pathological subtype identified was the acanthotic type, accounting for 960 cases (79.86%), followed by melanoacanthoma (92 cases, 7.98%), adenoid type (64 cases, 5.32%), hyperkeratotic type (59 cases, 4.90%), irritated type (21 cases, 1.74%), and clonal type (6 cases, 0.004%).
This study analyzed a cohort of 1,169 patients diagnosed with SK, encompassing a total of 1,202 pathology reports, which included cases with lesions at multiple sites. In terms of lesion distribution, head, face, and neck were the most frequently affected regions, with 656 cases (54.58%). The trunk followed as the second most prevalent site, with 347 cases (28.87%), while the extremities accounted for 138 cases (11.48%), and the perianal region comprised 54 cases (4.48%). Among these common sites, the acanthotic type of SK was overwhelmingly predominant, with 956 cases (79.53%). Four cases of SK were identified on mucosal surfaces, including 3 cases of the acanthotic type and 1 case of melanoacanthoma. Additionally, 3 cases were observed on the soles of the feet: one of the acanthotic type, 1 of melanoacanthoma, and 1 of the hyperkeratotic type (Table 1).
 |
Table 1 Pathological Types and Locations of Seborrheic Keratosis |
The age at diagnosis ranged from 20 to 94 years, with no cases diagnosed in individuals under 20 years of age. The most common age group for SK was 40–59 years, which accounted for 556 cases (52.11%). Histopathological subtypes appear to demonstrate significant differences in age distribution at time of diagnosis. Melanoacanthoma, acanthotic, and adenoid types were most prevalent in the 40–59 age group. In contrast, the hyperkeratotic and irritated types were more common in the 60–79 age group, comprising 39.29% and 45.00% of cases, respectively. For the clonal subtype, 50.00% of patients were in the 20–39 age group (Table 2). The clonal subtype was associated with a comparatively earlier mean age of onset (44.00 ± 9.14 years; mean ± SD) among individuals diagnosed with SK. (Table 3)
 |
Table 2 Age Distribution of Seborrheic Keratosis Diagnosis |
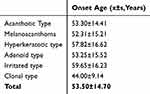 |
Table 3 Onset Age of Seborrheic Keratosis by Pathological Type |
Among the SK patients, 828 were male (57.90%) and 602 were female (42.10%), with a male-to-female ratio of 1.37:1. In the gender distribution of SK patients, males were the predominant.
Among the 1,169 patients diagnosed with SK, 647 were clinically diagnosed, with 522 patients being misdiagnosed. The clinical-pathological concordance rate for SK was 55.69%. SK is frequently misdiagnosed as naevus pigmentosa (284 cases, 24.2%), skin malignancies (120 cases, 10.2%, including melanoma, basal cell carcinoma, squamous cell carcinoma, etc)., and warts (63 cases, 5.3%, including common warts, condyloma acuminatum, and flat warts). Among the pathological subtypes, the misdiagnosis rates were particularly high for melanoacanthoma and clonal type, reaching 50% and 49.45%, respectively. (Table 4)
 |
Table 4 Missed Diagnosis Rates for Different Pathological Types of Seborrheic Keratosis |
Discussion
This study presents a comprehensive analysis of clinical and pathological data on SK collected over a 15-year period. The pathological classification of SK varies significantly across studies and geographical regions.9,10 In accordance with Andrews’ Diseases of the Skin: Clinical Dermatology, we categorized SK into six histopathological subtypes: acanthotic, hyperkeratotic, adenoid, clonal, irritated, and melanoacanthoma.11 Consistent with prior research, the acanthotic type was the most prevalent, accounting for 960 cases (80%), thereby supporting the widespread recognition of its predominance in SK.
Although SK can occur in any area covered by hair, it is rare on the palms, soles, or mucosal surfaces.9 In our study, we identified seven cases in atypical locations: three cases of palmoplantar SK and four cases involving mucosal surfaces (one in the perianal region, two on the lips, and one on the penile region). This anatomical distribution aligns with previous case reports documenting SK development on mucosal surfaces.12–14
The role of human papillomavirus (HPV) in the pathogenesis of SK continues to be debated. A study examining genital and extragenital SK using HPV-PCR found that 28 of 40 genital SK samples (70%) were HPV-positive, suggesting a potential pathogenic link between HPV and genital SK.15 In our study, one patient with SK located on the penile region exhibited vacuolated cells in the upper spinous layer (koilocytic phenomenon) on pathological examination, indicative of HPV infection. Subsequent HPV-PCR analysis confirmed the presence of HPV6 DNA in the affected tissue, further supporting the association between HPV infection and SK in this case.16
Current evidence suggests UV radiation exposure constitutes a risk factor for both SK and cutaneous malignancies.17 SK typically occurs on areas such as head, neck, chest, and back,8 which are frequently exposed to sunlight. Our findings align with this distribution pattern, with SK lesions on the head, face, and neck comprising 54.58% of cases. This supports UV radiation as a significant etiological factor in SK pathogenesis, which corroborates similar observations reported in studies from Australia and South Korea.7,8 In Australia’s high-UV environment, SK demonstrates male predominance (male-to-female ratio 1.3:1) with lesions predominantly located in sun-exposed head and neck regions.18 These epidemiological patterns align with our cohort’s observations.
Our study revealed a year-by-year increase in the incidence of SK, which could suggest a correlation with regional population aging and enhanced public awareness of dermatological conditions. Existing clinical perspectives posit that SK exhibits age-dependent patterns, with incidence rates demonstrating a progressive elevation in tandem with advancing age. Empirical data from Hainan Province indicate a accelerated growth rate in the elderly population relative to the working-age cohort, substantiating the region’s transition into a demographic aging society. This demographic aging pattern may constitute one etiological contributor to the observed secular trend of escalating SK incidence.19
In our investigation, the majority of SK diagnoses clustered within the 40–59 age cohort. This epidemiological divergence from conventional dermatological trends may be partially attributable to age-stratified health literacy. Middle-aged cohorts (40–59 age) exhibit elevated socioeconomic parameters, superior educational attainment, and more stringent clinical expectations regarding dermatological diagnostic precision and therapeutic intervention efficacy compared to geriatric populations.
Our findings further underscore a significant clinical challenge: the clinical-pathological concordance rate for SK diagnosis was 55.69%, indicating a high rate of diagnostic discrepancy in clinical practice. This diagnostic dilemma persists due to frequent misinterpretations of SK presentations as cutaneous malignancies, melanocytic nevi, or verrucous lesions by clinicians. Additionally, the appearance of multiple SK lesions over a short period—referred to as the Leser-Trélat sign(LTS).20 LTS predominantly affects the elderly population, with a median age of 61 years and no significant gender predilection. Approximately half of cases are associated with adenocarcinomas, followed by lymphoproliferative disorders (20%), and less commonly carcinomas of the breast, lung, bladder, renal, prostate, ovarian, or nasopharyngeal origin.21 Therefore, the appearance of LTS necessitates comprehensive oncological evaluation, including abdominal CT scans and tumor marker profiling to rule out underlying malignancies.
Our findings demonstrate that the head, face, and neck represent the most frequently involved anatomical regions for SK. Biopsies in these aesthetically sensitive areas may result in scarring, which can profoundly impact patient quality of life. Therefore, non-invasive techniques are critical in dermatological practice. Dermoscopy and confocal microscopy serve as valuable diagnostic tools, enhancing diagnostic accuracy and reducing diagnostic uncertainty.22,23 Recent advancements in artificial intelligence (AI) technology have enabled researchers to develop AI-driven diagnostic algorithms for differentiating actinic keratosis (AK) from SK. These systems serve as a supplementary tool for dermatologists in clinical practice, potentially enhancing diagnostic precision and workflow efficiency, thereby optimizing clinical workflows.24
In summary, This study systematically delineates the clinical manifestations, histopathological subtype distribution patterns, and epidemiological profile of SK. It establishes the necessity for early diagnostic protocols and provides critical epidemiological benchmarks for developing region-specific prevention and management strategies in high UV-exposure areas of China.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of the Fifth People’s Hospital of Hainan Province. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from the patients prior to study commencement.
Acknowledgments
The authors express their gratitude to the personnel of the Department of Pathology.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Construction Project of Hainan Province Clinical Medical Center.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55(3):490–500. doi:10.1016/j.jaad.2006.05.048
2. Hahnel E, Blume-Peytavi U, Trojahn C, et al. Prevalence and associated factors of skin diseases in aged nursing home residents: a multicentre prevalence study. BMJ Open. 2017;7(9):e018283. doi:10.1136/bmjopen-2017-018283
3. Huang J, Zhang L, Shi L, et al. An epidemiological study on skin tumors of the elderly in a community in Shanghai, China. Sci Rep. 2023;13(1):4441. doi:10.1038/s41598-023-29012-1
4. Zhang J, Wang Y, Zhang W, et al. Clinical misdiagnosis of cutaneous malignant tumors as melanocytic nevi or seborrheic keratosis: a retrospective analysis of a Chinese population. Clin Cosmet Invest Dermatol. 2024;17:465–476. doi:10.2147/CCID.S451288
5. Minagawa A. Dermoscopy-pathology relationship in seborrheic keratosis. J Dermatol. 2017;44(5):518–524. doi:10.1111/1346-8138.13657
6. Jackson JM, Alexis A, Berman B, et al. Current understanding of seborrheic keratosis: prevalence, etiology, clinical presentation, diagnosis, and management. J Drugs Dermatol. 2015;14(10):1119–1125.
7. Yeatman JM, Kilkenny M, Marks R. The prevalence of seborrheic keratoses in an Australian population: does exposure to sunlight play a part in their frequency? Br J Dermatol. 1997;137(3):411–414.
8. Kwon OS, Hwang EJ, Bae JH, et al. Seborrheic keratosis in the Korean males: causative role of sunlight. Photodermatol Photoimmunol Photomed. 2003;19(2):73–80. doi:10.1034/j.1600-0781.2003.00025.x
9. Barthelmann S, Flaig M, Berenice ML. Seborrheic keratosis. J Dtsch Dermatol Ges. 2023;21(2):265–277.
10. Kim BR, Chae JB, Choi CW, et al. Quantitative comparison of the histological subtypes of seborrheic keratosis using computer-aided image analysis. J Cutan Pathol. 2017;44(10):903–905. doi:10.1111/cup.13004
11. Odom RB, James WD, Berger TG. Andrews’ Diseases of the Skin: Clinical Dermatology.
12. Gulias-Cañizo R, Aranda-Rábago J, Rodríguez-Reyes AA. Seborrheic keratosis of the conjunctiva: a case report. Arch Soc Esp Oftalmol. 2006;81(4):217–219. doi:10.4321/s0365-66912006000400008
13. Kim JH, Bae HW, Lee KK, et al. Seborrheic keratosis of the conjunctiva: a case report. Korean J Ophthalmol. 2009;23(4):306–308. doi:10.3341/kjo.2009.23.4.306
14. Charles NC, Belinsky I. Pigmented inflamed seborrheic keratosis of the bulbar conjunctiva. Ophthalmic Plast Reconstr Surg. 2023;39(3):e89–e91. doi:10.1097/IOP.0000000000002346
15. Tardío JC, Bancalari E, Moreno A, et al. Genital seborrheic keratoses are human papillomavirus-related lesions. A linear array genotyping test study. APMIS. 2012;120(6):477–483. doi:10.1111/j.1600-0463.2011.02853.x
16. Sun LB, Lu JJ, Wu WW. Human papillomavirus 6 (HPV6) DNA positive in penile seborrheic keratosis: a case report. Chin J Dermatol Venereol. 2024;38(5):558–561.
17. Gaffney DC, Muir JB, De’Ambrosis B. Malignant change in seborrhoeic keratoses in a region with high solar ultraviolet levels. Australas J Dermatol. 2014;55(2):142–144. doi:10.1111/ajd.12035
18. Lim C. Seborrhoeic keratoses with associated lesions: a retrospective analysis of 85 lesions. Australas J Dermatol. 2006;47(2):109–113. doi:10.1111/j.1440-0960.2006.00258.x
19. Xie JJ, Xie YY, Wang DH. Health service demands of middle-aged and elderly populations in Hainan Province under demographic aging. Chin J Gerontol. 2018;38(18):4546–4550.
20. Kirchberger MC. Gastrointestinal: eruptive seborrheic keratoses: sign of Leser-Trélat in gastric adenocarcinoma. J Gastroenterol Hepatol. 2019;34(12):2058. doi:10.1111/jgh.14727
21. Silva JA, Mesquita KC, Igreja AC, et al. Paraneoplastic cutaneous manifestations: concepts and updates. An Bras Dermatol. 2013;88(1):9–22. doi:10.1590/s0365-05962013000100001
22. Sahin MT, Oztürkcan S, Ermertcan AT, et al. A comparison of dermoscopic features among lentigo senilis/initial seborrheic keratosis, seborrheic keratosis, lentigo maligna and lentigo maligna melanoma on the face. J Dermatol. 2004;31(11):884–889. doi:10.1111/j.1346-8138.2004.tb00621.x
23. Ahlgrimm-Siess V, Cao T, Oliviero M, et al. Seborrheic keratosis: reflectance confocal microscopy features and correlation with dermoscopy. J Am Acad Dermatol. 2013;69(1):120–126. doi:10.1016/j.jaad.2012.12.969
24. Reddy S, Giri D, Patel R. Artificial intelligence-based distinction of actinic keratosis and seborrheic keratosis. Cureus. 2024;16(4):e58692. doi:10.7759/cureus.58692
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Management of SARS-CoV-2 Omicron Variant Community Screenings in Shanghai, China: A Cross-Sectional Study
Chen K, Xu B, Tang Y, Cao J, Wang R, Tian Y, Gao C, Chu M
Risk Management and Healthcare Policy 2023, 16:111-120
Published Date: 2 February 2023

