Back to Journals » Drug Design, Development and Therapy » Volume 19
Homeopathy for Heteropathy: FSS and Its Components for the Treatment of Alzheimer’s Disease and Endometriosis
Authors Yu Y, Sun T, Zhang J , Wu S, Tong X, Zhao F, Fu X
Received 14 March 2025
Accepted for publication 14 May 2025
Published 10 June 2025 Volume 2025:19 Pages 5009—5032
DOI https://doi.org/10.2147/DDDT.S523737
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Tamer Ibrahim
Yang Yu,1 Tiantian Sun,2 Jingyu Zhang,2 Songquan Wu,1 Xin Tong,3 Fazheng Zhao,3 Xin Fu1
1School of Medicine, Lishui University, Lishui, 323000, Zhejiang Province, People’s Republic of China; 2School of Pharmacy, Heilongjiang University of Chinese Medicine, Harbin, 150040, Heilongjiang Province, People’s Republic of China; 3Zhejiang University of Traditional Chinese Medicine, Hangzhou, 310000, Zhejiang Province, People’s Republic of China
Correspondence: Xin Fu, Email [email protected]
Abstract: Foshou San (FSS), which is a traditional Chinese compound formula. So far, a variety of components have been isolated and identified from its complex composition. These findings endow it with diverse pharmacological activities. According to a number of studies, FSS has significant efficacy in treating cognitive impairment in Alzheimer’s disease (AD) and gynecological diseases like Endometriosis (EMs). Behind these curative effects, the specific chemical components of FSS play a crucial role. In this paper, the research progress of FSS in phytochemistry, pharmacology and pharmacokinetics are reviewed. Through comprehensive analysis of apoptosis regulation, oxidative stress and inflammation, ferroptosis, bile acid and intestinal flora, we further demonstrate the feasibility and potential of FSS in treating of AD and EMs. This not only reveals the potential mechanism of FSS, but also provides valuable experience and enlightenment for future research and application in related fields.
Keywords: Foshou San, component, alzheimer’s disease, endometriosis, homeopathy for heteropathy
Introduction
Foshou San (FSS), also called Guixiong Tang (GXT), is a classic prescription in the ancient medical work “Pu-ji-beng-shi-fang”, with the function of promoting blood circulation and removing blood stasis. It is composed of Angelica sinensis (AS) [Apiaceae; Angelica sinensis root] and Ligusticum chuanxiong (CX) [Apiaceae; Ligusticum chuanxiong rhizome] in a ratio of 2:1. AS is mainly planted in Gansu, China, with some also distributed in Yunnan, Sichuan, Shaanxi, and Hubei; CX is mainly planted in Sichuan, China, with some distribution in Yunnan, Guizhou, and Shaanxi. Their botanical characteristics are shown in Table 1. As a traditional Chinese compound formula, FSS is widely used in traditional medicine for gynecological and obstetric diseases such as fetal restlessness, postpartum lochia, abdominal pain, dysmenorrhea, as well as blood deficiency and blood stasis induced yellowing, dizziness, and body pain in regulating qi and blood. It has a long history of application and diverse practical experience.1 In recent years, its efficacy in treating Alzheimer’s disease (AD) has been discovered. AS is warm, sweet and pungent, nourishing blood and activating blood, regulating menstruation and relieving pain; CX is pungent and warm in nature, promoting blood circulation, removing blood stasis, promoting qi circulation and relieving pain, and is a qi medicine in blood. Both of them are commonly used drugs for enriching blood and promoting blood circulation in traditional Chinese medicine (TCM), which have the effects of promoting blood circulation, nourishing blood and promoting qi circulation. Therefore, taking both botanical drugs at the same time can enrich blood and dissipate blood stasis.
 |
Table 1 The Botanical Characteristics of Angelica Sinensis and Ligusticum Chuanxiong |
AD, a neurodegenerative disorder, can damage memory, cognition, and behavior, manifesting as dementia.2,3 According to TCM theory, it is caused by stagnation of liver qi and blood stasis. Endometriosis (EMs) refers to the disease caused by endometrial glands or stromal cells outside the inner wall of uterine cavity, which usually occurs in women of reproductive age.4 Hormone synthesis, immune inflammatory reaction, angiogenesis, invasion and metastasis, and apoptosis are all considered as important pathogenesis.5–7 According to the theory of TCM, “blood is unfavorable, water is produced”, which is believed to be caused by blood stasis blocking the circulation of qi and blood. Therefore, TCM believes that AD and EMs have common symptoms such as “blood stasis” and “qi stagnation”, which suggests that they can be treated with FSS. This reflects the multi-component, multi-target, and multi-therapeutic effects of TCM.
Emerging studies demonstrate that the combination of AS and CX in FSS exhibits significant synergistic effects in preventing and treating neurological disorders.8 Experimental evidence indicates that FSS decoction ameliorates neurobehavioral deficits, reduces cerebral edema severity, and decreases infarct volume in rat models of cerebral ischemia.9 Furthermore, FSS modulates the gut-liver-brain axis in APP/PS1 transgenic mice, rectifying intestinal dysbiosis while regulating alkaline phosphatase activity, lipopolysaccharide levels, and malondialdehyde concentrations, ultimately improving cognitive function in AD models.10 In addition, studies have found that FSS has the effect of promoting blood circulation and removing blood stasis,11–13 which can be used to treat AD and EMS. For example, verified through animal experiments and network pharmacology has14 found that a new Chinese medicine formula “improved FSS”, derived from FSS, can treat EMs by reducing angiogenesis, inhibiting invasion and metastasis, and regulating immunity.15,16 At present, our research on the treatment of diseases with TCM prescriptions is more in-depth. We found that Chinese medicine has the advantages of multi-target and multi-channel in treating diseases. After summarizing many literatures, we found that there are many common KEGG and GO enrichment pathways in the network pharmacological search and treatment of AD and EMs by FSS, such as cancer pathway, biological regulation, stimulation response cell projection pathway, and chromium binding pathway.17,18 In addition, a large number of clinical trials have shown that the main components of FSS, such as Ferulic acid (FA)19 and Ligustrazine20 have significant therapeutic effects on AD and EMs.21,22 FSS was originally developed for gynecological conditions, its recent experimental validation in AD therapeutics prompted our multidimensional analysis. We hope to use this to comprehensively elucidate the pharmacological mechanism of FSS and to establish a translational framework connecting traditional therapeutic uses with novel neurological applications. This dual perspective not only enhances our understanding of FSS’s polypharmacological effects but also provides methodological references for expanding TCM applications in modern medicine. About how FSS and its active components affect both AD and EMs, we drew Figure 1 to explain and summarize.
 |
Figure 1 How FSS and its active components affect both AD and EMs. |
Phytochemistry
Components of FSS
Due to the inherent complexity of TCM and its formulations, a multimodal analytical approach is required to comprehensive quality assessment. As an integrative identification technology, chromatographic fingerprinting has become pivotal in TCM quality control. This technique not only provides comprehensive chemical profiling but also enables quantitative analysis of characteristic components, thereby ensuring the authenticity, superior quality, and batch-to-batch consistency of crude drugs and their intermediate products. Additionally, research has found that compared to a single decoction of AS or CX, FSS does not produce any new components, but it helps to dissolve the active ingredients23 Therefore, we screened literature from the recent years on PubMed and CNKI using “Angelica sinensis (Danggui)”, “Ligusticum chuanxiong (Chuanxiong)”, “Foushou San”, and “component”, as the keywords. A total of 167 relevant components were collected, including Phthalides, Organic acids, Phenols (excluding phenolic acids), Nitrogen-containing components, etc. Then, we associated different types of components with AD and EMs to discover their roles in these diseases.
Phthalides
Phthalides, widely present in Apiaceae plants, are a class of organic components with special structures and properties. A total of 58 components were identified, including common FSS phthalide components such as N - butylphthalide, Ligustilide, and Senkyunolide. Research has found that phthalides have vasodilatory effects,24 which may be related to the blood activating and stasis removing effects of FSS. This suggests that phthalides are an important component of FSS in the treatment of AD. For example, Zhu et al found that Ligustilide can improve memory impairment in mice.25 N-butylphthalide can improve learning and memory in cognitively impaired rats, and can be used to treat cognitive dysfunction in neurodegenerative diseases, such as AD.26 Senkyunolide has the effects of improving inflammation, ischemic stroke, neuroprotection, and improving AD.27,28 The relevant components are listed in Table 2, and the corresponding structures are shown in Figure 2.
 |
Table 2 Phthalides in FSS |
 |
Figure 2 Continued. |
 |
Figure 2 Structure of Phthalide components. |
Organic Acids
Organic acids refer to certain organic components that are acidic, and the organic acids in FSS are mainly phenolic acids. It is known that the phenolic acids isolated from FSS include FA, Chlorogenic acid (CGA), Caffeic acid (CA) etc. They are believed to have analgesic, anti-inflammatory, antioxidant, and platelet aggregation inhibiting effects,32 among which FA has been proven to improve the symptoms of AD by preventing neurodegeneration in several brain regions, inhibiting the aggregation of Aβ oligomers, and exerting antioxidant, anti-inflammatory and anti-apoptotic effects.33 The relevant components are listed in Table 3, and the corresponding structures are shown in Figure 3.
 |
Table 3 Organic Acids in FSS |
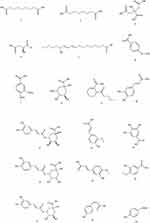 |
Figure 3 Continued. |
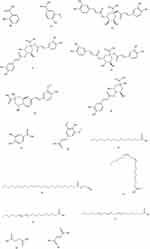 |
Figure 3 Structure of Organic Acids components. |
Phenols (Excluding Phenolic Acids)
Phenol components refer to substances with natural plant hormone activity, including phenolic aldehydes, phenolic esters, coumarins, flavonoids etc. Coumarin components are an important class of organic components. More than 10 kinds of coumarins, including Umbelliferone, Umbelliferone 6-carboxylic acid, Nodakeninand Nodakenetin, were isolated from AS, which have antihypertensive, neuroprotective, anti-amnesic, antioxidant, anti-diabetic and anti-AD effects.34 Flavonoids are a class of components that are widely found in plants and have a variety of biological activities, including antioxidant, anti-cancer, anti-inflammatory, hypolipidemic, cardiovascular, and cerebrovascular protection and other effects. The relevant components are listed in Table 4, and the corresponding structures are shown in Figure 4.
 |
Table 4 Phenols (Excluding Phenolic Acids) in FSS |
 |
Figure 4 Structure of Phenols (excluding phenolic acids) components. |
Nitrogen Components
This section includes Alkaloids, Amino acids, Amides etc. Alkaloids are a class of nitrogen-containing organic components primarily derived from plants, and Alkaloids have a cyclic structure with nitrogen atoms mostly embedded in the ring. They have significant physiological activity and are active components in Chinese botanical drugs. Alkaloids represented by Ligustrazine have significant effects in the treatment of AD and EMs.35,36 In addition, Amino acids and Amides are also believed to have a protective effect on blood vessels.25,26 The relevant components are listed in Table 5, and the corresponding structures are shown in Figure 5.
 |
Table 5 Nitrogen Components in FSS |
 |
Figure 5 Structure of Nitrogen components. |
Other Components
The components include Terpenes and their derivatives, Vitamins, Polysaccharides etc. Terpenes are volatile and aromatic oily components, and studies have found that terpenes can improve vascular function.29 Vitamins are a class of trace organic substances that humans and animals must obtain from food in order to maintain normal physiological functions, which play an important role in the process of human growth, metabolism and development, and can improve the body’s immunity. Recent studies have found that folic acid can alleviate symptoms of AD by reducing inflammation.37 It is worth noting that Polysaccharides, which are polymerized carbohydrates formed by the condensation and dehydration of multiple monosaccharide molecules, and their molecular structure is complex and large. Polysaccharides are widely distributed in nature, some of which constitute the components of animal and plant cell walls, such as cellulose; some are nutrients stored by animals and plants, such as glycogen and starch, and some have special biological activities. For example, Angelica sinensis polysaccharides (ASP) is considered to have unique effects in improving anemia, anti-inflammatory, antioxidant, immune regulation, and other aspects38 and Ligusticum chuanxiong polysaccharides have various biological activities such as promoting immunity.39 The relevant components are listed in Table 6, and the corresponding structures are shown in Figure 6.
 |
Table 6 Other Components in FSS |
 |
Figure 6 Structure of Other components.The structure of Ligusticum chuanxiong polysaccharides are unclear, so they are not listed. |
The Main Active Components and Chemical Structure of FSS
AS and CX, both belonging to the Apiaceae family, exhibit significant phytochemical similarities that underlie their frequent combination in TCM formulations. Literature analysis reveals that 16 common characteristic peaks in the FSS fingerprint predominantly originate from these two botanical drugs, corroborating their component consistency. Shared bioactive components include phenolic acids (CGA, CA, FA) and phthalide derivatives (Butylphthalide, Ligustilide, and Senkyunolide).41 Notably, CX possesses distinctive marker components such as Ligustrazine, which may contribute to its unique pharmacological properties. These phthalide derivatives and alkaloids demonstrate significant pharmacological activities in TCM preparations, playing crucial roles in ensuring therapeutic efficacy and medication safety. Particularly, the characteristic components Ligustrazine and Senkyunolide series are considered as key biomarkers underlying CX’s specific medicinal actions. The common components after FSS extraction are shown in Figure 7.
 |
Figure 7 Common components and chemical structures of FSS botanical drugs. |
Based on the above content, we believe that the most essential active ingredients are phthalides and organic acid compounds, as they are the most abundant and diverse in FSS. Therefore, we have included these two categories of compounds in Tables 2 and 3. Some of their components, such as CGA, CA, FA, and Butylphthalide, have been proven to play a key role in the treatment of AD and EMs. Additionally, emerging evidence highlights the indispensable role of polysaccharides in FSS-mediated therapeutic effects.
CGA regulates lysosomal function in SH-SY5Y cells derived from APP/PS1 AD model mice. This regulatory process inhibits the autophagy induced by Aβ25-35, thereby alleviating CA1 neuronal loss and cognitive deficits in vivo, providing a new idea for the treatment of AD.42 It is worth mentioning that CGA not only has a protective effect on the nervous system, but also has been found to have a targeted therapeutic effect on EMs. Through complex network analysis, scientists further confirmed the beneficial effect of CGA on EMs, opening up a new way for the treatment of this common gynecological disease.43 Similarly, CA can reduce cellular dysfunction, oxidative stress damage and transcriptional regulation in rat cortex, and has a significant therapeutic effect on AD.44 The mechanism of CA’s antioxidant activity may be related to the increase in the nuclear translocation of Nrf2 and the expression of the antioxidant enzymes glutathione peroxidase (GPx) and glutathione reductase (GR), which together reduce oxidative stress levels and protect the nervous system from damage.45 In addition, FA is believed to have anti AD effects through anti amyloidosis, anti-inflammatory, antioxidant, mitochondrial protection, and inhibition of cell apoptosis,46 and it can be used in combination with Ligustrazine and Fumarate to inhibit the invasion and metastasis of EMs through MMP/TIMP signaling pathway.47 Furthermore, Butylphthalide can regulate the level of inflammation in the body, and for patients with acute cerebral infarction, it can significantly improve neurological function, alleviate stroke symptoms, and improves prognosis in patients.48
Pharmacology
Regulation of Apoptosis
The normal human body maintains the stability of its internal environment by precisely regulating apoptosis. However, in neurodegenerative, endocrine, and metabolic diseases, improper activation of apoptosis can lead to cell loss and tissue damage,49 resulting in neuronal degeneration50 and endocrine imbalances. Fortunately, FSS and its active components have demonstrated significant therapeutic potential in modulating apoptosis. These effects are mediated through multiple mechanisms, including enhancing cellular antioxidant levels. For instance, FSS exerts neuroprotective effects by reducing reactive oxygen species (ROS), caspase-3, and Bax protein expression,51 thereby alleviating pathological damage in AD and EMs.
Among FSS components, ASP is particularly noteworthy. ASP alleviates AD and EMs pathology by regulating apoptosis through the Bcl-2, Caspase, and IAP protein families. It also exhibits diverse pharmacological effects, including anti-anemia, hepatoprotective, hypoglycemic, lipid-lowering, anti-inflammatory, anti-tumor, and antiviral properties. In AD models, ASP improves spatial learning and memory deficits by modulating apoptotic pathways, it suppresses pro-apoptotic Bax/Bad heterodimers, upregulates the anti-apoptotic Bcl-2/Bax ratio, and reduces caspase-3 and caspase-9 expression, thereby inhibiting mitochondrial apoptosis. Additionally, ASP enhances antioxidant activity, such as superoxide dismutase (SOD) and catalase (CAT) while lowering malondialdehyde (MDA) levels, further reducing neuronal apoptosis. Histological improvements in hippocampal CA1, CA3, and DG regions further support its neuroprotective role. Collectively, ASP inhibits Aβ 25–35 induced neuronal apoptosis in AD rats by downregulating caspase-3 and Bax, achieving therapeutic effects comparable to donepezil HCl.52
ASP also promotes hematopoiesis via the PI3K/Akt pathway, stimulating hematopoietic cell proliferation and differentiation.53 It enhances erythropoietin (EPO) production by stabilizing HIF-2α in the kidneys and liver, increasing serum EPO levels.54 Subsequent EPOR/JAK2/STAT5 and PI3K/Akt signaling upregulates Bcl-XL, Fam132b, and TFRC genes, while elevating the Bcl-2/Bax ratio in bone marrow-derived monocytes. This ratio correlates with anti-apoptotic capacity, enabling enhanced hematopoietic differentiation and mitigating EMs-associated bleeding complications.55
Notably, in addition to ASP, other active components in FSS also exhibited regulatory effects on apoptosis. For example, Ligustilide improves mitochondrial apoptosis in AD mouse model through PKA/AKAP1 signaling pathway56 and Butylphthalide inhibits neuronal apoptosis in mice by activating the Akt/mTOP and GDNF/GFRα1/Ret signaling pathway.57,58 Regarding EMs, CA, an important active ingredient in FSS, has been found to reduce ROS levels in ectopic endometrial cells and alleviate endometrial cell damage.59 In summary, FSS and its bioactive components regulate apoptosis through multiple pathways, offering novel therapeutic strategies for AD and EMs.
Improve Oxidative Stress and Inflammation
Oxidative stress is dominated by ROS and reactive nitrogen species (RNS), while inflammation is a defense system of the body against damaged tissues. Studies have shown that inflammation and oxidative stress can interact with each other and are considered important factors in disease development.60 Especially in AD and EMs, the occurrence of oxidative stress and inflammation plays a crucial role. In AD patients, the weaker the antioxidant system in the patient’s body, the more susceptible neurons are to damage.61 Studies have found that Dl-3-n-butylphthalamide can induce inflammation in APP/PS1 mice through the AGE-RAGE signaling pathway and Nrf2-TXNIP Trx axis.62,63 In recent years, some studies have shown that ASP can reduce free radical metabolism and inflammatory factor expression levels in AD patients. Choi M et al confirmed the ability of AS extract to alleviate cognitive deficits in mice injected with Aβ1-42 by increasing BDNF expression, ERK1/2 and CREB phosphorylation, inhibiting neuronal loss and neuroinflammatory responses, suggesting the therapeutic potential of AS in neurodegenerative diseases such as AD.64 FA, a natural antioxidant that can also improve cognitive impairment, is a recognized neuroprotective component for AD that reverses Aβ oligomer-induced morphological defects and reduces oxidation levels including protein oxidation, lipid peroxidation, and ROS assays.65 Vanillic acid, a FSS component, also has antioxidant and anti-inflammatory activities and can improve learning and memory disorders in AD rats,66 and Ligustrazine can inhibit the inflammatory response of human endometrial stromal cells through the STAT3/IGF2BP1/RELA axis, which is expected to be used in the treatment of EMs.35,67
EMs is an estrogen-dependent inflammatory disease in which oxidative stress, inflammation, and immunity play an important role in the development of EMs.68 In addition, TNF and IL-6 are inflammatory factors involved in the inflammatory response. High expression of TNF-α and IL-6 was detected in ectopic endometrium and ascites effusion.69 A variety of active components in FSS, such as CA and FA, have good anti-inflammatory effects.70,71 Z-ligustilide can attenuate lipopolysaccharide-induced inflammatory response by inhibiting the NF-κB pathway, inhibiting autophagy and accumulating DNA damage.72,73 The active component ASP in FSS can reduce LPS-induced inflammation and apoptosis by downregulating COX-1, downregulating cyclooxygenase, reducing the synthesis of PGs to inhibit leukocyte chemotaxis,74 reducing bradykinin production, inhibiting hyaluronidase, and achieving anti-inflammatory effects. Notably, Dai Y et al found that ovarian hyperoxidative stress in patients with EMs led to cumulus granulosa cell senescence.75 Therefore, modulating oxidative stress in the body is considered as a promising approach for the treatment of EMs.76,77 Navid Jamali’s experiment showed that CA can show antioxidant activity by increasing the nuclear translocation of Nrf2 and the expression of antioxidant enzymes GPx and GR, and achieve the therapeutic effect of oxidative stress on EMs cells by reducing oxidative stress.45
Emerging evidence clearly links oxidative stress to AD pathogenesis.78,79 In summary, FSS and its active components have demonstrated therapeutic potential for AD and EMs by inhibiting inflammatory factors and modulating oxidative stress responses.
Inhibit Ferroptosis
Iron overload in cells not only exacerbates the accumulation of toxic Aβ, leads to the formation of highly phosphorylated Tau protein, and directly induces oxidative damage in neurons,80 but also damages ovarian granulosa cells, oocytes, and embryos, leading to EMs-related infertility.81,82 Therefore, inhibition of ferroptosis can alleviate the symptoms of AD and EMs to some extent. Research has found that FSS can regulate ferroptosis to improve the symptoms of vascular dementia.83 In addition, it has been confirmed that AS and CX play important roles in regulating ferroptosis.84,85 For example, Ge et al found that CX can regulate ferroptosis through the JAK-STAT3 pathway.86 Furthermore, the active component of FSS has shown significant effects in regulating iron metabolism and inhibiting ferroptosis. The most noteworthy among them is ASP. ASP, as one of the main components of FSS, has shown significant therapeutic effects in regulating iron metabolism disorders, and studies have shown that it may be related to the regulation of hemagglutinin and EPO. Wang et al established a rat model of renal anemia and found that ASP stimulates endogenous EPO synthesis and activates the downstream EPOR pathway, which jointly promotes erythropoiesis and improves iron utilization.55 Inflammatory ferritin is an important cytokine that regulates iron balance in the body. It promotes iron release from cells into the extracellular space, thereby increasing iron ions in the plasma. At the same time, it decreases iron utilization by hematopoietic cells and decreases hematopoietic cell activity.87 Acidic ASP inhibits inflammatory ferroportin by blocking the IL-6/STAT3 and BMP/Smad pathways. In addition, it can increase the expression of ferroportin, mobilize iron in the liver and spleen, and increase serum iron levels.88 At the same time, too much hemagglutinin can lead to iron deposition in mononuclear macrophages, hindering the absorption of iron in the intestine. ASP has a strong inhibitory effect on the expression of hemagglutinin, and regulates iron homeostasis and increases pig iron supply. Similarly, Zhang et al and Liu et al further explored the molecular mechanism of ASP regulating ferritin and iron metabolism, and found that ASP could down-regulate the expression of ferritin by down-regulating C-EBP α, JAK/STAT, BMP/SMAD and ERK pathways, and by up-regulating the expression of transferrin, thereby promoting the transport of tissue iron to serum iron and further improving iron utilization.89,90 Unfortunately, there are few reports on FSS improving EMs symptoms by regulating ferroptosis and this gap in research represents a direction worthy of attention.
Regulates Intestinal Gut Microbiota and Bile Acid Metabolism
Bile acids, as products of cholesterol metabolism and clearance, are produced in the liver and further metabolized by intestinal bacteria. Imbalance of gut microbiota and abnormal bile acid metabolism have been confirmed to be one of the common causes of AD91,92 and EMs.93–95 This means that FSS can treat different diseases through improving gut microbiota imbalance and bile acid metabolism. In fact, numerous studies have confirmed that FSS can treat AD by improving gut microbiota and bile acid metabolism. For example, FSS can regulate the intestinal microbiota balance of APP/PS1 double transgenic AD mice, increase the number of lactobacilli in the intestine, reduce the abundance of Escherichia coli, and reduce the lipid peroxidation level in the liver, serum, brain and intestine of mice, which indicates that FSS can improve the cognitive function of mice by reducing lipid peroxidation levels and improving neuroinflammatory damage.96 In addition, animal experiments have shown that FA reduces non-alcoholic fatty liver disease (NAFLD) formation and lowers serum TC, TG, and LDL levels. It also alters gut microbiota composition particularly the Firmicutes/Bacteroidetes ratio—and decreases I3A production.97 These findings suggested that FA regulates bile acid metabolism via gut microbiota modulation, thereby alleviating AD and EMs symptoms.98 In summary, the improvement of gut microbiota imbalance and bile acid metabolism by FSS is a new direction for explaining the differential treatment of AD and EMs with FSS.
Pharmacokinetics
At present, there are relatively few reports on the pharmacokinetics of FSS components and flavorings in vivo, but research in this field is essential for a deeper understanding of the absorption, distribution, metabolism and excretion of FSS in living organisms. Pharmacokinetic studies can help us to predict the kinetic parameters of the main active components of drugs, such as bioavailability, apparent volume of distribution, half-life, clearance, etc., thus providing an important basis for drug screening and optimization.99 Previous studies have shown that the pharmacokinetic parameters of related components in plasma were determined by high-performance liquid chromatography (HPLC) by means of intragastric administration, such as intragastric administration of FSS decoction to rats and rabbits, and it was found that the concentration of active components such as FA and Ligustrazine in plasma was high, and the elimination half-life time was significantly shortened.100 Indicating that these components can be rapidly absorbed and exert their effects in the body. The pharmacokinetic study of Jiawei Foshou San in mice showed that the bioavailability of the formula in mice is generally high, and the absorption degree of each component in the pathological model of EMs is significantly better than that of normal rats, which provided strong evidence for the pharmacokinetic rationality of Jiawei FSS Capsule in the treatment of EMs.101
It is worth noting that Li et al discovered that AS extract, CX extract and AS-CX extract were administered to blood-deficient mice, and HPLC analysis showed that FA was well absorbed in blood-deficient rats and eliminated slowly. At the same time, CX can significantly prolong the distribution half-life of AS extract in blood-deficient rats, increase its partition volume and FA absorption, and prolong the half-life of FA in blood-deficient rats, which reveals the mutual promotion and auxiliary effect of AS and CX as a pair of botanical drugs.102 This suggests that FSS can exhibit stronger therapeutic effects compared to AS or CX alone. These findings provide strong evidence for the use of FSS in the treatment of AD and EMs.
Conclusion
This article reviews a large number of literature and summarizes the therapeutic potential of FSS and its bioactive components in the treatment of AD and EMs. The identification of multiple active plant-derived components in FSS formulations has demonstrated remarkable therapeutic efficacy against both AD and EMs, offering novel insights into FSS’s pharmacological mechanisms while exemplifying the TCM principle of treating diverse diseases through syndrome differentiation. The review further elucidates FSS’s therapeutic mechanisms through its modulation of critical biological processes including cellular apoptosis regulation, oxidative stress mitigation, anti-inflammatory actions, iron deposition inhibition, bile acid metabolism optimization, and gut microbiota modulation. These findings suggest FSS exerts its therapeutic effects via multi-target, multi-pathway interventions. Notably, the article highlights FSS’s potential influence on disease progression through gut microbiome regulation, proposing innovative perspectives for studying gut-brain-uterine axis disorders. As principal components of FSS, AS and CX demonstrate particular therapeutic significance. The abundant availability and wide distribution of these herbal constituents underscore TCM’s unique value in managing major diseases. Investigating the synergistic mechanisms of this classic botanical drug in treating AD and EMs not only enhances our understanding of TCM therapeutic principles, but also revitalizes the inheritance and development of traditional medicinal culture.
Although preliminary evidence supports FSS’s potential in AD and EMs management, several research gaps persist. Current literature shows limited investigation into FSS’s synergistic treatment of these comorbid conditions. Furthermore, the therapeutic contributions of numerous components of FSS is still uncharacterized, and requires systematic phytochemical analysis. Future research should prioritize well-designed pharmacological studies, comprehensive toxicological assessments, and randomized clinical trials to validate FSS’s medical applications. This provides a theoretical basis for clinical medication safety and the development of Chinese patent medicines.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81973746), the National Natural Science Foundation of China (Grant No. 82004404), the Science Foundation of Heilongjiang Province (Grant No. QC 2017114), Ph.D. Scientific Research Start-up Classic of Lishui University, (Grant NO. QD2421), Lishui Public Welfare Technology Application Research Plan Project (Grant No. 2024GYX37), National College Students Innovation and Entrepreneurship Training Program (Grant No. S202410352042).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Guo M, Liang L, Cao J, et al. Analysis of classic formula Foshousan based on ancient and modern literature. Chin J Exp Formul. 2024;2024:1–20; doi:10.13422/j.cnki.syfjx.20241215
2. Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179(2):312–339. doi:10.1016/j.cell.2019.09.001
3. Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol. 2021;17(3):157–172. doi:10.1038/s41582-020-00435-y
4. Li S, Wang L. Research progress of endometriosis. Chin Foreign Med Res. 2024;22(30):166–170.
5. Zulimirehmu A, Guo Y. Research progress of combination of traditional Chinese and Western medicine in the treatment of endometriosis of qi stagnation and blood stasis type. Chin Foreign Med Res. 2025;23(4):156–159.
6. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–275. doi:10.1038/nrendo.2013.255
7. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519. doi:10.1016/j.fertnstert.2012.06.029
8. Cao F, Xiang J, Tang D, et al. Anti-Parkinson mechanism of Foshou Powder based on factorial design. Chin Herbal Med. 2020;51(6):1559–1566.
9. Jia Z. Studies on Chemical Components Analyze of Foshousan Decoction and It’s Effects Against Cerebral Ischemia injure. Chengdu: Chengdu University of Traditional Chinese Medicine; 2014.
10. Lu J, Guo P, Liu X, et al. Herbal formula Foshou San attenuates Alzheimer’s disease related pathologies via the gut live train axis in APP/PS1 mouse model of Alzheimer’s disease. Evidence Based Comprehensive Alternative Med. 2019;20:1–14.
11. Wei H, Xiao Y, Tong Y, et al. Therapeutic effect of angelica and its compound formulas for hypertension and the complications: evidence mapping. Phytomedicine. 2019;59:152767. doi:10.1016/j.phymed.2018.11.027
12. Chen R, Wu P, Cai Z, et al. Puerariae Lobatae Radix with chuanxiong Rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain-gut barriers. J Nutr Biochem. 2019;65:101–114. doi:10.1016/j.jnutbio.2018.12.004
13. Lai X, Xiu C, Wang X, Fang J, Yang J, Lei Y. Efficacy of Renshen Sanqi Chuanxiong formula for preventing vascular aging. J Tradit Chin Med. 2019;39(6):780–793.
14. Chen Y, Wei J, Zhang Y, et al. Anti-endometriosis mechanism of Jiawei Foshou San based on network pharmacology. Front Pharmacol. 2018;9:811. doi:10.3389/fphar.2018.00811
15. Yang Y, Wang X, Chen G, Deng L, Xu X. Research of therapeutical effect and immunologic mechanism of Jiawei Foshou San on model rats of endometriosis. Zhongguo Zhong Yao Za Zhi. 2011;36(21):3001–3006.
16. Wang X, Yang Y, Chen G, Deng L, Xu X. Inhibitory effects of Jiawei Foshousan on angiogenesis of endometriosis in rats model and its mechanism. Chin Pharmacol Bull. 2011;27(3):350–354.
17. Zhao B, Wei J, Zhang C, Zhang Y, Xu X, Chen Y. Network Pharmacology-based Study on Mechanism of Foshou San in Endometriosis Treatment. New Trad Chin Med Clin Pharmacol. 2019;30(9):1047–1054.
18. Feng L, Guo P, Zhou L, Xie Q, Zhang Y, Guo X. An insight into the molecular mechanism of foshou powder against alzheimer’s disease based on systems pharmacology and experimental validation. New Trad Chin Med Clin Pharmacol. 2021;32(11):1622–1631.
19. Kudoh C, Hori T, Yasaki S, Ubagai R, Tabira T. Effects of ferulic acid and Angelica archangelica Extract (Feru-guard ®) on mild cognitive impairment: a multicenter, randomized, double-blind, placebo-controlled prospective trial. J Alzheimers Dis Rep. 2020;4(1):393–398. doi:10.3233/ADR-200211
20. Sun X, Ren L, Shi H. Influence of ligustrazine injection on chemokines, vegf and its receptors of patients with endometriosis. China Pharmaceut. 2017;26(20):51–53.
21. Zhuang L. Clinical Observation of Danggui Sini Tang in the Treatment of Endometriosis with Cold Coagulation and Stasis Type. Pract Gynecolog Endocrinol J. 2020;7(29):
22. Wang Q. Improving Effect of Ligustrazine Injection on Cognitive Function in Patients with Intracranial Aneurysm after Craniotomy. Explor Rat Drug Use China. 2019;16(1):69–71.
23. Jia Z, Chen M, Zhen D, Li P, Li H. Comparative Study on Effect Components in Single Decoction and Mixed Decoction of Foshousan. J Guangxi Univ Trad Chin Med. 2020;23(2):90–93.
24. Li W, Tang Y, Chen Y, Duan J. Advances in the chemical analysis and biological activities of chuanxiong. Molecules. 2012;17(9):10614–10651. doi:10.3390/molecules170910614
25. Zhu W, Zheng J, Cai W, et al. Ligustilide improves aging-induced memory deficit by regulating mitochondrial related inflammation in SAMP8 mice. Aging. 2020;12(6):5587. doi:10.18632/aging.102998
26. Tian A, Li W, Zai Q, Li H, Zhang RW. 3-N-Butyphthalide improves learning and memory in rats with vascular cognitive impairment by activating the SIRT1/BDNF pathway. Mol Med Rep. 2020;22(1):525–533. doi:10.3892/mmr.2020.11106
27. Hu P, Liu D, Zheng Q, Wu Q, Tang Y, Yang M. Elucidation of Transport Mechanism of Paeoniflorin and the Influence of Ligustilide, Senkyunolide I and Senkyunolide A on Paeoniflorin Transport through Mdck-Mdr1 Cells as Blood-Brain Barrier in Vitro Model. Molecules. 2016;21(3):300. doi:10.3390/molecules21030300
28. Zeng P, Su H, Ye C, et al. A tau pathogenesis-based network pharmacology approach for exploring the protections of Chuanxiong rhizoma in alzheimer’s disease. Front Pharmacol. 2022;13:877806. doi:10.3389/fphar.2022.877806
29. Tang F, Tan Y, Ao H, et al. Discovery of phthalides with vasodilating activity in stems and leaves of Ligusticum chuanxiong. Chin Herbal Med. 2020;51(5):1190–1195.
30. Chen Z, Dong Y, Hu K, et al. identification of chemical components of Angelicae Sinensis Radix-Chuanxiong Rhizoma herb pairs and absorbed components in serum of rats based on UPLC-Orbitrap-HRM. Drug Evaluation Research. 2024;47(4):776–791.
31. Jiao W, Wei J, Guo J, Yang Z, Yang X, Yang X. Identification of chemical components of Angelica sinensis using UPLC-Q-TOF/MS and its the effect and mechanism of activating blood circulation. Chinese Pharmacological Bulletin. 2025;41(1):147–156.
32. Zhang Q, Feng Y, Li C, et al. Rapid identification of phthalides and organic acids in Angelica sinensis based on UPLC-Q-TOF/MS. Chinese Pharmacy. 2022;33(5):579–585+591.
33. Di Giacomo S, Percaccio E, Gullì M, et al. Recent Advances in the Neuroprotective Properties of Ferulic Acid in Alzheimer’s Disease: a Narrative Review. Nutrients. 2022;14(18):3709. doi:10.3390/nu14183709
34. Ali MY, Seong SH, Jung HA, Choi JS. Angiotensin-I-Converting Enzyme Inhibitory Activity of Coumarins from Angelica decursiva. Molecules. 2019;24(21):3937. doi:10.3390/molecules24213937
35. Feng Y, Dong H, Zheng L. Ligustrazine inhibits inflammatory response of human endometrial stromal cells through the STAT3/IGF2BP1/RELA axis. Pharm Biol. 2023;61(1):666–673. doi:10.1080/13880209.2023.2195883
36. Zhang Q, Wang J, Zhu L, et al. Ligustrazine Attenuates Hyperhomocysteinemia-induced Alzheimer-like Pathologies in Rats. Curr Med Sci. 2021;41(3):548–554. doi:10.1007/s11596-021-2379-1
37. Chen H, Liu S, Ji L, et al. Folic Acid Supplementation Mitigates Alzheimer’s Disease by Reducing Inflammation: a Randomized Controlled Trial. Mediators Inflamm. 2016;2016:5912146. doi:10.1155/2016/5912146
38. Ren C, Luo Y, Li X, et al. Pharmacological action of Angelica sinensis polysaccharides: a review. Front Pharmacol. 2025;15:1510976. doi:10.3389/fphar.2024.1510976
39. Wang J, Wang L, Zhou H, et al. The isolation, structural features and biological activities of polysaccharide from Ligusticum chuanxiong: a review. Carbohydr Polym. 2022;285:118971. doi:10.1016/j.carbpol.2021.118971
40. Yang Y, Wang X, Liu F, Yang G, Li Q, Zhang L. Research Progress on Extraction, Purification, Structure and Activity of Angelica Polysaccharide. Chin Med J. 2024;26(10):989–1001.
41. Jia Z, Pei W, Wang X, Zhen H, Tang X. HPLC Fingerprint of Fushousan Decoction. Med Rev. 2013;32(11):1486–1490.
42. Gao L, Li X, Meng S, Ma T, Wan L, Xu S. Chlorogenic Acid Alleviates Aβ25-35-Induced Autophagy and Cognitive Impairment via the mTOR/TFEB Signaling Pathway. Drug Des Devel Ther. 2020;14:1705–1716. doi:10.2147/DDDT.S235969
43. Saltan G, Süntar I, Ozbilgin S, et al. Viburnum opulus L.: a remedy for the treatment of endometriosis demonstrated by rat model of surgically-induced endometriosis. J Ethnopharmacol. 2016;193:450–455. doi:10.1016/j.jep.2016.09.029
44. Colonnello A, Aguilera-Portillo G, Rubio-López LC, et al. Correction to: comparing the Neuroprotective Effects of Caffeic Acid in Rat Cortical Slices and Caenorhabditis elegans: involvement of Nrf2 and SKN-1 Signaling Pathways. Neurotox Res. 2020;37(3):779. doi:10.1007/s12640-019-00153-4
45. Jamali N, Mostafavi-Pour Z, Zal F, et al. Combination Effect of Caffeine and Caffeic Acid Treatment on the Oxidant Status of Ectopic Endometrial Cells Separated from Patients with Endometriosis. Iran J Med Sci. 2019;44(4):315–324. doi:10.30476/IJMS.2019.44970
46. Wang EJ, Wu MY, Lu JH. Ferulic Acid in Animal Models of Alzheimer’s Disease: a Systematic Review of Preclinical Studies. Cells. 2021;10(10):2653. doi:10.3390/cells10102653
47. Tan Y, Zhang C, Zhang Y, et al. Combination of ferulic acid, ligustrazine and tetrahydropalmatine inhibits invasion and metastasis through MMP/TIMP signaling in endometriosis. PeerJ. 2021;9:e11664. doi:10.7717/peerj.11664
48. Lv Y, Zhang Q, Rong L, Wei X, Liu H, Li Z. Butylphthalide soft capsules combined with modified tonic exercise therapy on neurological function and ability of daily living of patients with stroke hemiplegia. Am J Transl Res. 2021;13(12):13803–13810.
49. Vitale I, Pietrocola F, Guilbaud E, et al. Apoptotic cell death in disease-Current understanding of the NCCD 2023. Cell Death Differ. 2023;30(5):1097–1154. doi:10.1038/s41418-023-01153-w
50. Sharma VK, Singh TG, Singh S, Garg N, Dhiman S. Apoptotic Pathways and Alzheimer’s Disease: probing Therapeutic Potential. Neurochem Res. 2021;46(12):3103–3122. doi:10.1007/s11064-021-03418-7
51. Feng L, Guo X, Zhang Y, Chen Y. Mechanism of Foshousan for the amelioration of PC12 cells oxidative stress injury induced by sodium glutamate. Guangxi Medical Journal. 2023;45(12):1453–1457+1471.
52. Du Q, Zhu X, Si J. Angelica polysaccharide ameliorates memory impairment in Alzheimer’s disease rat through activating BDNF/TrkB/CREB pathway. Exp Biol Med. 2020;245(1):1–10. doi:10.1177/1535370219894558
53. Liu C, Li J, Meng F, et al. Polysaccharides from the root of Angelica sinensis promotes hematopoiesis and thrombopoiesis through the PI3K/AKT pathway. BMC Complement Altern Med. 2010;10:79. doi:10.1186/1472-6882-10-79
54. Jafari M, Ghadami E, Dadkhah T, Akhavan-Niaki H. PI3k/AKT signaling pathway: erythropoiesis and beyond. J Cell Physiol. 2019;234(3):2373–2385. doi:10.1002/jcp.27262
55. Wang K, Wu J, Xu J, et al. Correction of Anemia in Chronic Kidney Disease With Angelica sinensis Polysaccharide via Restoring EPO Production and Improving Iron Availability. Front Pharmacol. 2018;9:803. doi:10.3389/fphar.2018.00803
56. Zhang Q, Zhang X, Yang B, et al. Ligustilide-loaded liposome ameliorates mitochondrial impairments and improves cognitive function via the PKA/AKAP1 signaling pathway in a mouse model of Alzheimer’s disease. CNS Neurosci Ther. 2024;30(3):e14460. doi:10.1111/cns.14460
57. Li W, Wei D, Lin J, et al. Dl-3-n-Butylphthalide Reduces Cognitive Impairment Induced by Chronic Cerebral Hypoperfusion Through GDNF/GFRα1/Ret Signaling Preventing Hippocampal Neuron Apoptosis. Front Cell Neurosci. 2019;13:351. doi:10.3389/fncel.2019.00351
58. Xu J, Huai Y, Meng N, et al. L-3-n-Butylphthalide Activates Akt/mTOR Signaling, Inhibits Neuronal Apoptosis and Autophagy and Improves Cognitive Impairment in Mice with Repeated Cerebral Ischemia-Reperfusion Injury. Neurochem Res. 2017;42(10):2968–2981. doi:10.1007/s11064-017-2328-3
59. Jamali N, Mostafavi-Pour Z, Zal F, Kasraeian M, Poordast T, Nejabat N. Antioxidant ameliorative effect of caffeic acid on the ectopic endometrial cells separated from patients with endometriosis. Taiwan J Obstet Gynecol. 2021;60(2):216–220. doi:10.1016/j.tjog.2020.12.003
60. Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–1167. doi:10.1089/ars.2012.5149
61. Marttinen M, Takalo M, Natunen T, et al. Molecular Mechanisms of Synaptotoxicity and Neuroinflammation in Alzheimer’s Disease. Front Neurosci. 2018;12:963. doi:10.3389/fnins.2018.00963
62. Wang C, Xu Y, Wang X, Guo C, Wang T, Wang Z. Dl-3-n-Butylphthalide Inhibits NLRP3 Inflammasome and Mitigates Alzheimer’s-Like Pathology via Nrf2-TXNIP-TrX Axis. Antioxid Redox Signal. 2019;30(11):1411–1431. doi:10.1089/ars.2017.7440
63. Lu J, Zhang J, Wang X, et al. Dl-3-n-butylphthalide promotes microglial phagocytosis and inhibits microglial inflammation via regulating AGE-RAGE pathway in APP/PS1 mice. Brain Res Bull. 2024;212:110969. doi:10.1016/j.brainresbull.2024.110969
64. Choi M, Lee Y, Cho S. Angelica tenuissima Nakai Ameliorates Cognitive Impairment and Promotes Neurogenesis in Mouse Model of Alzheimer’s Disease. Chin J Integr Med. 2018;24(5):378–384. doi:10.1007/s11655-017-2812-2
65. Fu X, Wang Q, Wang Z, Kuang H, Jiang P. Danggui-Shaoyao-San: new Hope for Alzheimer’s Disease. Aging Dis. 2015;7(4):502–513. doi:10.14336/AD.2015.1220
66. Amin FU, Shah SA, Kim MO. Vanillic acid attenuates Aβ1-42-induced oxidative stress and cognitive impairment in mice. Sci Rep. 2017;7:40753. doi:10.1038/srep40753
67. Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515. doi:10.1155/2014/179515
68. Wang X, Ma Z, Song N. Inflammatory cytokines IL-6, IL-10, IL-13, TNF-α and peritoneal fluid flora were associated with infertility in patients with endometriosis. Eur Rev Med Pharmacol Sci. 2018;22(9):2513–2518. doi:10.26355/eurrev_201805_14899
69. Sittisart P, Chitsomboon B, Kaminski NE. Pseuderanthemum palatiferum leaf extract inhibits the proinflammatory cytokines, TNF-α and IL-6 expression in LPS-activated macrophages. Food Chem Toxicol. 2016;97:11–22. doi:10.1016/j.fct.2016.08.021
70. Choi KC, Son YO, Hwang JM, Kim BT, Chae M, Lee JC. Antioxidant, anti-inflammatory and anti-septic potential of phenolic acids and flavonoid fractions isolated from Lolium multiflorum. Pharm Biol. 2017;55(1):611–619. doi:10.1080/13880209.2016.1266673
71. Zhao L, Du J, Zhou H, Liu D, Gu M, Long F. Differences in Proinflammatory Property of Six Subtypes of Peroxiredoxins and Anti-Inflammatory Effect of Ligustilide in Macrophages. PLoS One. 2016;11(10):e0164586. doi:10.1371/journal.pone.0164586
72. Wang J, Du JR, Wang Y, Kuang X, Wang CY. Z-ligustilide attenuates lipopolysaccharide-induced proinflammatory response via inhibiting NF-kappaB pathway in primary rat microglia. Acta Pharmacol Sin. 2010;31(7):791–797. doi:10.1038/aps.2010.71
73. Qi H, Jiang Z, Wang C, et al. Sensitization of tamoxifen-resistant breast cancer cells by Z-ligustilide through inhibiting autophagy and accumulating DNA damages. Oncotarget. 2017;8(17):29300–29317. doi:10.18632/oncotarget.16832
74. Xie Y, Zhang H, Zhang Y, Wang C, Duan D, Wang Z. Chinese Angelica Polysaccharide (CAP) Alleviates LPS-Induced Inflammation and Apoptosis by Down-Regulating COX-1 in PC12 Cells. Cell Physiol Biochem. 2018;49(4):1380–1388. doi:10.1159/000493415
75. Dai Y, Lin X, Liu N, et al. Integrative analysis of transcriptomic and metabolomic profiles reveals abnormal phosphatidylinositol metabolism in follicles from endometriosis-associated infertility patients. J Pathol. 2023;260(3):248–260. doi:10.1002/path.6079
76. Harlev A, Gupta S, Agarwal A. Targeting oxidative stress to treat endometriosis. Expert Opin Ther Targets. 2015;19(11):1447–1464. doi:10.1517/14728222.2015.1077226
77. Clower L, Fleshman T, Geldenhuys WJ, Santanam N. Targeting Oxidative Stress Involved in Endometriosis and Its Pain. Biomolecules. 2022;12(8):1055. doi:10.3390/biom12081055
78. Spina E, Ferrari RR, Pellegrini E, et al. Mitochondrial Alterations, Oxidative Stress, and Therapeutic Implications in Alzheimer’s Disease: a Narrative Review. Cells. 2025;14(3):229. doi:10.3390/cells14030229
79. Bai R, Guo J, Ye XY, Xie Y, Xie T. Oxidative stress: the core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res Rev. 2022;77:101619. doi:10.1016/j.arr.2022.101619
80. Lei P, Ayton S, Bush AI. The essential elements of Alzheimer’s disease. J Biol Chem. 2021;296:100105. doi:10.1074/jbc.REV120.008207
81. Ng SW, Norwitz SG, Taylor HS, Norwitz ER. Endometriosis: the Role of Iron Overload and Ferroptosis. Reprod Sci. 2020;27(7):1383–1390. doi:10.1007/s43032-020-00164-z
82. Li Y, He Y, Cheng W, Zhou Z, Ni Z, Yu C. Double-edged roles of ferroptosis in endometriosis and endometriosis-related infertility. Cell Death Discov. 2023;9(1):306. doi:10.1038/s41420-023-01606-8
83. Wang J. Research on the Mechanism of Fo-Shou-San in Improving Vascular Dementia Against Ferroptosis. Guangzhou University of Traditional Chinese Medicine; 2023.
84. Gong G, Ganesan K, Liu Y, et al. Danggui Buxue Tang improves therapeutic efficacy of doxorubicin in triple negative breast cancer via ferroptosis. J Ethnopharmacol. 2024;323:117655. doi:10.1016/j.jep.2023.117655
85. Lou T, Wu H, Feng M, et al. Integration of metabolomics and transcriptomics reveals that Da Chuanxiong Formula improves vascular cognitive impairment via ACSL4/GPX4 mediated ferroptosis. J Ethnopharmacol. 2024;325:117868. doi:10.1016/j.jep.2024.117868
86. Ge Q, Wang Z, Yu J, et al. Chuanxiong Rhizoma regulates ferroptosis and the immune microenvironment in ischemic stroke through the JAK-STAT3 pathway. Sci Rep. 2024;14(1):31224. doi:10.1038/s41598-024-82486-5
87. Sangkhae V, Nemeth E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv Nutr. 2017;8(1):126–136. doi:10.3945/an.116.013961
88. Wang K, Wu J, Cheng F, Huang X, Zeng F, Zhang Y. Acidic Polysaccharide from Angelica sinensi Reverses Anemia of Chronic Disease Involving the Suppression of Inflammatory Hepcidin and NF-κB Activation. Oxid Med Cell Longev. 2017;2017:7601592. doi:10.1155/2017/7601592
89. Zhang Y, Cheng Y, Wang N, Zhang Q, Wang K. The action of JAK, SMAD and ERK signal pathways on hepcidin suppression by polysaccharides from Angelica sinensis in rats with iron deficiency anemia. Food Funct. 2014;5(7):1381–1388. doi:10.1039/c4fo00006d
90. Liu J, Zhang Y, You R, Zeng F, Guo D, Wang K. Polysaccharide isolated from Angelica sinensis inhibits hepcidin expression in rats with iron deficiency anemia. J Med Food. 2012;15(10):923–929. doi:10.1089/jmf.2012.2231
91. Angelucci F, Cechova K, Amlerova J, Hort J. Antibiotics, gut microbiota, and Alzheimer’s disease. J Neuroinflammation. 2019;16(1):108. doi:10.1186/s12974-019-1494-4
92. Chen C, Liao J, Xia Y, et al. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut. 2022;71(11):2233–2252. doi:10.1136/gutjnl-2021-326269
93. Xholli A, Cremonini F, Perugi I, Londero AP, Cagnacci A. Gut microbiota and endometriosis: exploring the relationship and therapeutic implications. Pharmaceuticals. 2023;16(12):1696. doi:10.3390/ph16121696
94. Colonetti T, Saggioratto MC, Grande AJ, et al. Gut and vaginal microbiota in the endometriosis: systematic review and meta-analysis. Biomed Res Int. 2023;2023:2675966. doi:10.1155/2023/2675966
95. Ata B, Yildiz S, Turkgeldi E, et al. The endobiota study: comparison of vaginal, cervical and gut microbiota between women with stage 3/4 endometriosis and healthy controls. Sci Rep. 2019;9(1):2204. doi:10.1038/s41598-019-39700-6
96. Lu J. Herbal Formula Fo Shou San Attenuates Alzheimer’s Disease-Related Pathologies via the Gut-Liver-Brain Axis in APP/PS1 Mouse Model of Alzheimer’s Disease. Guangzhou University of Traditional Chinese Medicine; 2019.
97. Ruan X, Ju C, Shen Y, et al. Suxiao Jiuxin pill promotes exosome secretion from mouse cardiac mesenchymal stem cells in vitro. Acta Pharmacol Sin. 2018;39(4):569–578. doi:10.1038/aps.2018.19
98. Ma Y, Chen K, Lv L, Wu S, Guo Z. Ferulic acid ameliorates nonalcoholic fatty liver disease and modulates the gut microbiota composition in high-fat diet fed ApoE-/- mice. Biomed Pharmacother. 2019;113:108753. doi:10.1016/j.biopha.2019.108753
99. Yan R, Chen Y, Yang Y. Pharmacokinetics of Chinese medicines: strategies and perspectives. Chin Med. 2018;13:24. doi:10.1186/s13020-018-0183-z
100. Liu X, Xue Y, Xie L, Ji H, Wang G. Pharmacokinetics of Ferric Acid in Rat after Oral Administration of Radix Angelica Sinensis, Rhizoma Chuanxiong and Their Compound Preparations. J China Pharmaceut Univ. 2003;2003:62–65.
101. Shang F. The Preparation and Pharmacokinetic Studies of the New Traditional Chinese Medicine Jiawei Foshou San Capsule. Southwest University; 2015.
102. Li W, Guo J, Tang Y, et al. Pharmacokinetic comparison of ferulic acid in normal and blood deficiency rats after oral administration of Angelica sinensis, Ligusticum chuanxiong and their combination. Int J Mol Sci. 2012;13(3):3583–3597. doi:10.3390/ijms13033583
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

