Back to Journals » International Journal of Nanomedicine » Volume 20
How Advanced are Self-Assembled Nanomaterials for Targeted Drug Delivery? A Comprehensive Review of the Literature
Authors Nsairat H , Lafi Z , Al-Najjar BO , Al-Samydai A, Saqallah FG, El-Tanani M , Oriquat GA, Sa’bi BM , Ibrahim AA, Dellinger AL, Alshaer W
Received 7 August 2024
Accepted for publication 22 January 2025
Published 19 February 2025 Volume 2025:20 Pages 2133—2161
DOI https://doi.org/10.2147/IJN.S490444
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Farooq A. Shiekh
Hamdi Nsairat,1 Zainab Lafi,1 Belal O Al-Najjar,1 Ali Al-Samydai,1 Fadi G Saqallah,2 Mohamed El-Tanani,1,3 Ghaleb Ali Oriquat,4 Bailasan Mohammad Sa’bi,4 Abed Alqader Ibrahim,5 Anthony Lee Dellinger,5 Walhan Alshaer6
1Pharmacological and Diagnostic Research Center, Faculty of Pharmacy, Al-Ahliyya Amman University, Amman, 19328, Jordan; 2Faculty of Pharmacy, Al-Zaytoonah University of Jordan, Amman, Jordan; 3College of Pharmacy, Ras Al Khaimah Medical and Health Sciences University, Ras Al Khaimah, United Arab Emirates; 4Pharmacological and Diagnostic Research Center, Faculty of Allied Medical Sciences, Al-Ahliyya Amman University, Amman, 19328, Jordan; 5Department of Nanoscience, Joint School of Nanoscience and Nanoengineering, University of North Carolina at Greensboro, Greensboro, NC, USA; 6Cell Therapy Center, The University of Jordan, Amman, 11942, Jordan
Correspondence: Anthony Lee Dellinger, Department of Nanoscience, Joint School of Nanoscience and Nanoengineering, University of North Carolina at Greensboro, P.O. Box 26170, 1400 Spring Garden Street, Greensboro, NC, 27402-6170, USA, Tel +1-336-334-5000, Email [email protected]
Abstract: The development of effective drug delivery systems is a key focus in pharmaceutical research, aiming to enhance therapeutic efficacy while minimizing adverse effects. Self-assembled nanostructures present a promising solution due to their tunable properties, biocompatibility, and ability to encapsulate and deliver therapeutic agents to specific targets. This review examines recent advancements in drug-based self-assembled nanostructures for targeted delivery applications, including drug-drug conjugates, polymeric-based architectures, biomolecules, peptides, DNA, squalene conjugates and amphiphilic drugs. Various strategies for fabricating these nanostructures are discussed, with an emphasis on the design principles and mechanisms underlying their self-assembly and potential for targeted drug delivery to specific tissues or cells. Furthermore, the integration of targeting ligands, stimuli-responsive moieties and imaging agents into these nanostructures is explored for enhanced therapeutic outcomes and real-time monitoring. Challenges such as stability, scalability and regulatory hurdles in translating these nanostructures from bench to bedside are also addressed. Drug-based self-assembled nanostructures represent a promising platform for developing next-generation targeted drug delivery systems with improved therapeutic efficacy and reduced side effects.
Keywords: self-assembly, targeted delivery, nanostructures, amphiphilic drugs, nanoconjugates
Introduction
Humans have utilized medicinal concoctions for thousands of years to improve the quality and longevity of life. In recent decades, the use of molecular therapeutic agents has increased significantly, especially in cancer diagnosis and treatment.1,2 However, with the increasing time and expense required for new drug development, interest has started to wane. The focus is increasingly shifting from the synthesis and discovery of new chemical entities to the development of innovative formulations and delivery systems for existing therapeutics, with the goal of enhancing clinical outcomes.3,4 In the context of cancer treatment, where numerous physiological, extracellular and intracellular barriers protect the body’s cells, drug delivery vehicles must be specifically engineered to circumvent the challenges. Nanostructured delivery systems have emerged as a promising strategy for improving drug bioavailability and precision targeting.5 This targeted drug delivery approach represents a significant advancement, enhancing therapeutic efficacy while minimizing systemic side effects.6,7
Among the many strategies in drug delivery, self-assembling supramolecular nanostructures have garnered considerable interest due to their tunable pharmacokinetic profiles and specificity in drug targeting.7 Drug-based self-assembled nanostructures represent a promising avenue for targeted delivery by utilizing the self-assembly phenomenon. Self-assembly refers to the spontaneous organization of molecules into well-defined structures.8 In these systems, therapeutic agents, such as drugs or bioactive molecules, autonomously organize into nanostructures or supramolecular assemblies stabilized through non-covalent interactions, including hydrogen bonding, hydrophobic interactions, or electrostatic forces. The primary goal is to develop nano-sized carriers or vehicles that enhance drug delivery efficiency, stability and targeting precision. By exploiting the intrinsic properties of the drugs themselves (wherein the drug serves as both the payload and the carrier), these systems eliminate the need for additional nondrug excipients, making them a compelling and efficient approach to advanced drug delivery.9–11
Drugs can be classified into synthetic compounds, steroids, peptides, sugars, nucleic acids and proteins based on the chemical structure of the active pharmaceutical ingredient (API).12 Numerous natural compounds and drugs exhibit the capability to self-assemble into nanostructures (Figure 1).12,13 For example, nucleic acids and peptides possess inherent structural properties and interactions that facilitate their self-assembly into nanostructures.14,15 This process is predominantly driven by noncovalent interactions, which enhance drug stability, enable controlled release and promote targeted delivery. Various types of drug self-assembly systems have been identified, including single-drug nano-assembled systems, drug-conjugate nano-assembled structures and multi-drug nano-assembled modules formed through the assembly of two or more drugs with additive conjugation.8
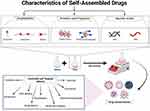 |
Figure 1 Schematic representation of self-assembled drug characteristics, formation and potential applications. |
Given that the drug-based self-assembled nanostructures do not comprise traditional nanomaterials, these systems have promising potential for targeted drug delivery without the side effects associated with carrier materials.14 This approach simplifies the production process by reducing the need for quality control and monitoring related to nanomaterial’s type, quantity and potential interactions with the API and the body.16,17
Drug delivery strategies focus on passive and active targeting, both relying on the enhanced permeability and retention (EPR) effect to accumulate drugs in cancerous tissues.18 Tumor mass pores can allow for drug nanocarriers to penetrate and new targeted systems that use ligands like polyunsaturated fatty acids, folic acid, hyaluronic acid or oligopeptides for tumor recognition have been leveraged previously. However, notable challenges have included limited success for small ligands, enzymatic degradation in circulation and unclear tumor-targeting mechanisms.19 Active targeting involves attaching ligands to nanocarriers or drugs to bind specific biomarkers on tumor cells, improving drug localization, effectiveness and reducing side effects and blood concentration variation.20
An important consideration in the design and application of nanostructures is the formation of the protein corona. This dynamic biomolecular layer adsorbs onto the surface of nanostructures upon their introduction to a biological environment. In doing so, this interaction can have a profound impact on the pharmacokinetics, biodistribution, cellular uptake and immunogenicity of the nanostructure. Accordingly, the formation of a protein corona can alter the targeting efficiency of nanostructures by masking ligands or modifying surface characteristics, which can impact their ability to bind specifically to target receptors. Moreover, this layer can result in off-target effects by directing nanostructures to unintended tissues or via triggering immune responses in the patient. Future work aimed at addressing these challenges will require novel design approaches, such as engineering stealth surfaces with polyethylene glycol (PEG) or zwitterionic coatings to minimize protein adsorption. Furthermore, functionalizing nanostructures with targeting ligands or “pre-coating” them with specific biomolecules may help optimize these biological interactions. As research in this space continues to advance, understanding the intricacies of the protein corona will be essential for enhancing stability, functionality and safety of these nanostructures.
This review highlights the potential of drug-based self-assembled nanostructures in delivering safe and effective targeted therapies. Detailed examples and references are provided to highlight the versatility of these systems, their broad range of applications and the promising future directions for their development.
What are Self-Assembled Nanomaterials?
Self-assembly is a naturally occurring process where individual components autonomously organize into well-defined structures.14 This process is primarily driven by non-covalent interactions such as hydrogen bonding, hydrophobic interactions, electrostatic forces and π–π stacking.16,17,21,22 These interactions, commonly observed in natural phenomena such as the formation of cell membrane phospholipids, have been effectively utilized by researchers in the design of advanced drug delivery systems.15 Many drugs, especially those with amphiphilic or polymeric properties, exhibit the ability to self-assemble in aqueous solutions.13 Amphiphilic drugs, including hormones, proteins and surfactants, can form diverse structures in solution, determined by their inherent amphiphilic characteristics.5 By harnessing these self-assembly principles, researchers can develop sophisticated and efficient drug delivery vehicles for advancing the field of targeted therapeutics.
Harnessing Nature: The Science Behind Self-Assembled Nanostructures
In biological systems, numerous natural molecules demonstrate the ability to spontaneously form self-assembled nanostructures.23 For example, the self-assembly of lipids into liposomes facilitates efficient drug encapsulation and targeted delivery.24 This phenomenon is also observed in various biological processes, such as lipid particles forming oil droplets in water, polypeptides assembling into functional hemoglobin, the complex structures of ribosomal RNA and proteins, viral capsid formation and the organization of lipid bilayers within cellular membranes.15,25,26
Natural materials such as collagen, cellulose, protamine and silk have the ability to self-assemble into highly ordered nanostructures, providing diverse functionalities.22 A prominent example of self-assembly in physical chemistry is the organization of amphiphilic molecules into micelles, rods or liposomes. In this process, the hydrophobic tails and hydrophilic heads of lipids naturally arrange to form bilayers.14 Micelles are particularly apt at encapsulating hydrophobic drugs, while liposomes are capable of encapsulating both hydrophobic and hydrophilic drugs.27 Specifically, the lipid bilayer of liposomes incorporates hydrophobic molecules within the membrane, while the aqueous core encapsulates hydrophilic molecules. This dual functionality makes liposomes a versatile and promising system for delivering a broad spectrum of therapeutic agents.
Micelles are sphere-shaped amphiphilic nanoparticles (NPs) characterized by a hydrophobic core and a hydrophilic shell. In aqueous solutions, blocks of copolymers can assemble into fiber-like micelles;28 however, at low concentrations, these particles remain dispersed. As the concentration increases, these particles organize into a structured arrangement, a behavior known as the critical micelle concentration (CMC).29 Understanding and determining the CMC of amphiphilic molecules is essential for the formation of stable and functional micelles.30
Distinct molecules, like DNA and RNA, can serve as integral components in molecular self-assembling systems. These nucleic acids inherently self-assemble into diverse structures and complex aggregates, driven by multivalent interactions.31 This process often initiates in a less organized state, such as a solution, random coil or disordered aggregate, and gradually progresses toward a highly structured final state, such as a crystal or a folded macromolecule. This transition is largely driven by energy minimization and culminates in the formation of well-ordered and stable structures.32
The natural phenomena of molecular self-assembly enable the development of diverse nanostructures for drug delivery systems, including polymeric micelles, liposomes, nanocapsules and peptide-based formations. These engineered nanostructures can undergo further modification through the conjugation of specific functional groups, which enhances their stability, solubility and targeting capabilities.14,24,25
Classification and Formation: From Molecules to Nanostructures
Nanostructures can be categorized based on their structural characteristics and the nature of their self-assembly processes, which may be dynamic or static and involve the self-assembly of atoms, molecules or colloids.33–36 The classifications further extend to drug-based nanostructures, encompassing lipid-based, polymer-based and peptide-based assemblies, as well as classifications by size, shape and surface properties.12 Understanding these categorizations is essential for optimizing the performance and functionality of nanostructures in targeted drug delivery applications.37
Nanoparticles can be assembled from a variety of materials, including metals, polymers, proteins and lipids.5,22 Typically classified within a size range from 1 to 100 nanometers, the small dimensions of NPs provide a substantial increase in surface area, enhancing their functionality as drug delivery platforms. Drug-based self-assembled nanostructures offer a promising strategy for achieving targeted delivery.8,9 Their diminutive size confers multiple advantages, including improved drug bioavailability, higher concentration, enhanced solubility and greater stability. Additionally, NP-based drug encapsulation can elevate therapeutic efficacy by enabling targeted delivery to specific cell types and tissues, thereby minimizing off-target toxicity.6,38
For materials or molecules to assemble into nanostructures, non-covalent driving forces and interactions play a pivotal role in establishing stable and functional delivery systems.21,22 These forces facilitate both intramolecular and intermolecular self-assembly under defined conditions, such as solvation in aqueous environments. The chemical structure of drug molecules directly effects the nature of these interactions, influencing how drug molecules engage with each other and the surrounding water molecules. As shown in Figure 2, key interactions include hydrophobic and electrostatic interactions, hydrogen bonding and π–π stacking of aromatic groups.39 Together, these forces collectively determine the stability and functionality of the resulting nanostructures, enabling precise control over their assembly and behavior within biological systems.
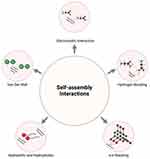 |
Figure 2 Schematic representation of the prevailing forces that drive self-assemble. |
Hydrophobic interactions are non-specific forces that are prevalent in biological systems. These interactions arise when molecules in aqueous environments rearrange themselves to bring hydrophobic regions into proximity, thereby minimizing contact with water and reducing the system’s overall free energy.40 Hydrophobic molecules naturally aggregate in aqueous solutions to achieve lower energy states.41 These interactions play a key role in the aggregation and self-assembly of NPs. In drug delivery, amphiphilic drugs or molecules that possess both hydrophobic and hydrophilic regions leverage these interactions to self-organize in aqueous environments, forming stable structures. This self-organization leads to the formation of micelles, liposomes and amphiphilic polymers, wherein the hydrophobic regions are sequestered within the core and shielded from the aqueous environment.8 This property represents an important determinant for the design of effective drug delivery systems.
Hydrophilic interactions are pivotal in stabilizing and functionalizing drug-based nanostructures. These interactions occur between the hydrophilic regions of drug molecules or their carriers, promoting the formation of a hydrated shell around the nanostructures, which is essential for their stability and solubility in aqueous environments.42 For example, in liposomes, the hydrophilic headgroups of lipid molecules orient themselves towards the aqueous surroundings, creating a stable aqueous core capable of encapsulating hydrophilic drugs. The presence of hydrophilic surfaces also helps maintain colloidal stability and prevents aggregation, which is often facilitated by the generation of a zeta potential around the nanostructure.43 A zeta potential exceeding ±30 mV is generally indicative of strong colloidal stability due to substantial electrostatic repulsions between particles. However, the threshold for stability depends on specific factors, including particle characteristics (eg, size, surface charge, and composition) and environmental conditions (eg, pH, ionic strength, and temperature). This electrostatic potential arises from charge distribution in the hydrophilic regions, creating a repulsive force that stabilizes the NPs in suspension. By harnessing hydrophilic interactions, researchers can design nanostructures that are more robust, stable, soluble and efficient, optimizing their use as delivery systems.
Electrostatic interactions between charged molecules represent another driving force that is crucial in the self-assembly process.44 These interactions occur when oppositely charged molecules attract each other, facilitating the formation of stable, self-assembled structures. For example, nanostructures can spontaneously organize through the electrostatic attraction between positively charged peptides or polymers and negatively charged nucleic acids.45 This fundamental principle is widely applied in the design of nanocarriers for drug delivery, where electrostatic interactions contribute to the stabilization and compaction of the therapeutic agents into functional delivery systems. These interactions play an important role in enhancing the structural integrity and overall performance of self-assembled nanostructures in biomedical applications.
Van der Waals forces are weak intermolecular forces that include both attractive and repulsive interactions between atoms, and molecules. Unlike covalent and ionic bonds, these forces are caused by the fluctuation in particle polarizations, which contribute to the stability of nanostructures.46 Despite their relatively weak nature, Van der Waals forces fulfill a critical function in the self-assembly of NPs and hydrophobic drugs with amphipathic nature.47 These forces facilitate the aggregation and stabilization of nanostructures, improving functionality and structural integrity.
Hydrogen bonding is a specific type of dipole–dipole interaction that occurs when a hydrogen atom is shared between electronegative atoms such as nitrogen, oxygen or fluorine. These bonds can form within a single molecule (intramolecular) or between different molecules (intermolecular), significantly influencing the physicochemical properties of polymeric compounds.48 For example, in the formation of double-stranded DNA, hydrogen bonds between adenine-thymine and guanine-cytosine base pairs ensure the stability and specificity of the helical structure. In proteins, hydrogen bonding is crucial for forming alpha-helices and beta-sheets, which are integral to their three-dimensional conformations. Hydrogen bonding is a key driving force for the self-assembly of various molecules, including polymers, peptides and nucleic acids.49,50 It stabilizes specific secondary structures by maintaining the spatial arrangement of these molecules, enabling the formation of functional and well-ordered assemblies.
Aromatic stacking, or π–π stacking, is a crucial interaction in the self-assembly of molecules containing aromatic rings. These interactions occur between the electron-rich π-systems of aromatic rings, leading to the formation of stable, stacked arrangements.51 Such stacking interactions are prevalent in molecules with multiple aromatic rings, such as DNA, where they contribute to the structural integrity of the double helix. Pi–pi (π–π) interactions are distinguished by their strong yet non-destructive nature, making them particularly effective in stabilizing self-assembled nanostructures. This stability is essential for enhancing the encapsulation efficiency and controlled release of therapeutic agents. For example, in nucleic acids and peptide-based conjugates, π–π stacking interactions between aromatic nitrogenous bases or aromatic amino acids serve a fundamental role that drives the formation, organization and structural integrity of self-assembled systems.52 These interactions are necessary for the design of drug delivery systems that rely on the precise and stable arrangement of molecules to function effectively within complex biological environments.
Certain polymers exhibit sensitivity to environmental changes, such as pH or temperature, which can trigger self-assemble processes. For example, pH-responsive polymers like poly(acrylic acid) (PAA) alter their conformation or charge state in response to pH fluctuations.53 This dynamic behavior allows these polymers to assemble or disassemble based on the prevailing pH conditions, making them versatile for tailored applications. Similarly, temperature-responsive polymers like poly(N-isopropylacrylamide) (PNIPAM) undergo a transition from a soluble to an insoluble state as temperature changes, initiating the process of self-assembly.54 These responsive properties enable precise control and modulation of polymer-based nanostructures, creating intelligent systems capable of specific responses under distinct environmental conditions. The adaptability of these responsive systems holds significant promise for various applications, particularly in drug delivery and the design of smart materials. By leveraging their environmental responsiveness, these polymers enable controlled drug release and improved targeting, laying the foundation for advancements in therapeutic and material science innovations.
Fabricating Nanostructures: Preparation Techniques for Precision Drug Delivery
Drugs can self-assemble through various mechanisms, requiring specific steps and conditions to ensure the successful formation of stable and functional nanostructures.55 Numerous methods, such as solvent evaporation, emulsification and thin-film hydration, can be used to prepare self-assembled nanostructures,56 thin-film hydration.57 Each technique offers control over critical parameters such as size, shape and drug loading capacity, which are critical for achieving targeted drug delivery.58,59 Thin-film hydration methods are suitable when the drug conjugate possesses either hydrophobic or amphipathic moieties, allowing for their dissolution in a suitable organic solvent.60 Next, the organic solvent is gently evaporated under reduced pressure to create a thin film layer. The obtained thin film is subsequently hydrated with an aqueous buffer solution to generate the nano-assembly. Reverse-phase evaporation methods are typically used as an alternative, forming a water-in-oil nano emulsion, whereas the injection methods involve dissolving the hydrophobic active agents with suitable solvent and then rapidly injected into an aqueous phase.61 The preparation process can generally be divided into four key steps:
(1) Selection of Materials: Identify appropriate drugs or materials capable of forming nanostructures, ensuring compatibility with self-assembly requirements and targeted applications.7
(2) Design and Formation: Develop the structural design and execute the formation of self-assembled particles under controlled conditions to achieve the desired nanostructures.
(3) Characterization and Optimization: Conduct physical and chemical characterization to assess the properties of the nanostructures and optimize their performance. Fundamental characterization techniques include dynamic light scattering (DLS), transmission electron microscopy (TEM), atomic force microscopy (AFM) and spectroscopy, which analyze particle size, morphology, stability, drug loading and release profiles.62 Surface modifications, such as covalent conjugation, electrostatic interactions or physical adsorption may be applied to improve targeting specificity, enhance stability or control drug release efficiency.63
(4) Biological Activity Assessment: Evaluate biological activity in both in vitro and in vivo systems to assess biocompatibility, efficiency, drug release, cellular uptake and targeting ability.64 In vitro studies may include release kinetics, cell viability assays and cellular uptake experiments. In vivo assessments focus on biodistribution, pharmacokinetics and therapeutic efficacy using relevant animal models systems to confirm the functional and therapeutic potential of the nanostructures.65,66
Engineering Effective Nanostructures: Morphology and Stability
Drugs (APIs) can be categorized based on their chemical structure into synthetic compounds, steroids, peptides, sugars, nucleic acids and proteins.12 Numerous natural compounds and drugs exhibit the capacity for self-assembly into nanostructures.13 For instance, nucleic acids and peptides inherently possess structural and interactive properties that facilitate their self-assembly into nanostructures.14,15 This process, predominantly driven by noncovalent interactions, contributes to enhanced drug stability, controlled release and targeted delivery. Drug self-assembled constructs can take diverse forms, including single drug nano-assembly modules, drug conjugate nano-assembled modules and multiple drug nano-assemblies formed by the assembly of two or more drugs with additive conjugation.8
The structural morphology and integrity of self-assembled drug nanostructures are critical determinants of their performance.62 Morphology pertains to the shape, size and arrangement of nanostructures, which may include spherical micelles, vesicles, fibers or hydrogels. Structural integrity, on the other hand, relates to the stability of these drug-based nanostructures and their capacity to preserve their configuration during storage and when interacting with biological environments.30
Structural morphology relates to the study of particle forms and shapes, which are influenced by material types and intermolecular forces. The shape and uniformity of particles serve a significant role in determining their performance and interactions with biological systems.67 Nanostructures can be engineered in various three-dimensional forms, including spheres, rods, fibers, vesicles and micelles.68 The selection of a specific shape is guided by the nature of the carrier, drug and targeting requirements. For example, spherical nanostructures are favored for their high drug loading capacity, controlled release and the potential for passive targeting through the EPR effect. Fibrous or nanotubular structures, in contrast, enhance cellular uptake and mimic the extracellular matrix, making them suitable for tissue engineering applications.69 Morphology can be modified through precise control of self-assembly conditions, including the selection of specific solvents, concentration, temperature and the inclusion of additives or surfactants. Particle morphology can be characterized using techniques such as electron microscopy, DLS, and small-angle X-ray scattering.70
Nanostructures must maintain their structural integrity and functionality without degradation or damage to ensure stability, efficient encapsulation and precise targeting capabilities of the formulated NPs.71 Common stability challenges during storage, such as aggregation, disintegration, phase separation and drug leakage, can compromise shelf life. Additionally, maintaining stability under varying physiological conditions, such as pH changes, enzymatic degradation and immune system opsonisation, is critical.72,73 To enhance the stability of nanostructures, it is important to optimize formulation parameters, incorporate additives and apply surface modifications. Various techniques can be used to evaluate the integrity of self-assembled nanostructures, such as fluorescence and infrared spectroscopy, and differential scanning calorimetry. Stability testing under relevant physiological conditions can be conducted through in vitro and in vivo release studies.16,72
Drug-Based Self-Assembly: A Novel Approach in Nanomedicine
Bioavailability is a major factor that influences the efficacy of anticancer therapies. Advanced drug delivery technologies, such as drug nanocarriers, have been developed to enhance the therapeutic potential of encapsulated chemotherapeutic agents by mitigating undesirable features and improving pharmacokinetics and tissue distribution.74–78 Despite their promise, many nano-therapies face significant hurdles in clinical translation, including low encapsulation efficiencies, compromised stability and the requirement for high volumes of non-drug excipients during NP production.79,80 Additionally, NPs are often sequestered by the reticuloendothelial system (RES), leading to off-target accumulation in organs such as liver, lung and brain. This sequestration can result in inflammation driven by NP-induced oxidative stress.81 Furthermore, the safety and toxicity profiles of some nanomaterials remain insufficiently characterized, posing challenges for regulatory approval and clinical application.82
These limitations are critical to achieving the appropriate therapeutic window.83,84 To overcome these constraints, a novel approach utilizing drug-based nanostructures has been developed. This approach leverages the ability of medications to self-assemble into supramolecular structures, forming stable nanocomposites. As described in Classification and Formation: From Molecules to Nanostructures, the primary driving mechanisms in self-assembly include noncovalent interactions (ie, van der Waals forces, hydrophobic effects, electrostatic interactions, hydrogen bonding, π–π stacking interactions, coordination bonding, and solvation and hydration forces).77 This strategy enables the development of self-delivering nanomedicines characterized by high and consistent drug content.85 The emergence of new nanocarriers that allow alternative dosing routes and reduced toxicity marks a significant progress in cancer treatment options.86 This approach confers novelty, especially when encapsulation enables high drug loading content, enhances anticancer activity and facilities targeted localization of drugs within cancer cells. Notably, this strategy offers the potential to mitigate drug resistance by engaging multiple pathways while reducing systemic toxicity compared to free drugs.
Amphiphilic Drugs: Dual-Function Molecules for Targeted Delivery
Amphiphilic drugs exhibit a dual nature, possessing both hydrophobic and hydrophilic characteristics and have an inherent capacity to self-organize into well-defined nanostructures.40,87 Early research in the 1950’s on drug self-aggregation demonstrated that penicillin and streptomycin salts could form colloidal micelles in aqueous solutions, as evidenced by surface tension measurements.88 Subsequent studies in 1971 utilizing 1H NMR confirmed that hydrophobic interactions drive this self-aggregation.89 This phenomenon was extended by Attwood and Argawal to include synthetic penicillins (ie, flucloxacillin and cloxacillin), which exhibited micellar properties in both water and isotonic saline.83 In drug delivery, the amphiphilic properties of these compounds dictate their potential for solubilization, bioavailability, integration into lipid membranes, transport characteristics and release kinetics from formulations.40 Moreover, amphiphilic drugs can be customized to interact with plasma proteins such as albumin or lipoprotein, enhancing their functionality and therapeutic potential.90,91
A limited number of studies have elucidated the capability of amphiphilic drugs to self-assemble into NP structures. Most recently, a study by Efthymiou et al (2021) demonstrated self-assembling properties of the hydrochloride salts of adiphenine, pavatrine and amitriptyline in aqueous solutions. Confirmed using small-angle X-ray scattering at concentrations above the CMC, these drugs formed micelles with an oblate spheroidal shape. While all three drugs exhibited a closed aggregation pattern, their amphiphilic nature resulted in pH sensitivity, leading to an increased micelle charge at higher drug concentrations.40 Despite offering several advantages over conventional methods, amphiphilic self-assembling drug delivery systems face competition from established technologies such as liposomes and polymeric NPs, each possessing distinct strengths and limitations. Recent advancements in computational tools and biomimetic approaches present promising opportunities to enhance the precision and efficiency of these systems, enabling more tailored design and optimized performance.
Barbosa et al (2008) explored the self-assembly behavior of two phenothiazine drugs, chlorpromazine (CPZ) and trifluoperazine (TFP), in aqueous solution using small-angle X-ray scattering (SAXS) and electron paramagnetic resonance (EPR). SAXS analysis demonstrated that CPZ molecules self-assembled into an orthorhombic cell basis configuration, forming nano-crystallites with aggregation numbers ranging from 60 to 80. Simulations of the EPR spectra using 5- and 16-doxyl stearic acids attached to aggregates provided insights into dynamic and magnetic characteristics.92 In related studies, edelfosine and fulvestrant were shown to self-assemble into nanostructures via the nanoprecipitation method, achieving high encapsulation efficiencies of 80% and 84%, respectively, for two hydrophobic agents. The resulting nanostructures had average sizes of 224.3 ± 1.8 nm and 247.3 ± 3.3, with zeta potentials of −17.3 ± 1.06 and −23.1 ± 3.51 mV. These nanostructures showed enhanced cellular uptake and penetration, with improved anticancer activity and the ability to induce apoptosis, particularly in estrogen receptor positive (ER+) breast cancer cell lines.93
Drug–Drug Conjugates
Self-assembling drug–drug conjugates represent an innovative approach in cancer therapy, aimed at enhancing drug delivery and therapeutic efficacy while minimizing adverse side effects.94 By applying the principles of nanotechnology and molecular design, researchers have developed systems that self-assemble into NPs, nanofibers, and other nanostructures capable of targeting specific tissues and releasing therapeutic agents in a controlled manner.95 These drug conjugates offer additional advantages beyond spontaneously forming NPs in aqueous environments, simplifying the preparation process, concentrating drugs within target tissues and potentially reducing systemic toxicity.96 Moreover, NPs derived from these conjugates can be engineered to enhance the penetration of small molecules through physiological barriers, thereby enhancing their pharmacological performance.
The concept of drug–drug conjugates is pivotal in contemporary pharmaceutical research, highlighting their ability to self-assemble through non-covalent interactions into NPs. Alternatively, self-assembly can be achieved by chemically modifying the drug into a prodrug, incorporating a non-toxic hydrophobic cleavable moiety. These prodrugs then assemble into nanoparticulates. This dual self-assembly mechanism highlights the versatility and potential of drug–drug conjugates in advancing nanomedicine.97 Building on these principles, Zhou et al developed a self-sufficient bi-prodrug nanomedicine technique to create a minimalist drug nanoplatform designed to enhance immunotherapeutic efficacy in chemotherapy. Gemcitabine (GEM) and 1-methyl-tryptophan (1MT), recognized for their bioactivity, were synthesized into a bi-prodrug molecule (GEM-1MT). These GEM-1MT bi-prodrug molecules demonstrated a unique ability to self-assemble into waste-free NPs for cancer treatment. This self-assembling capability significantly enhanced the overall therapeutic efficacy of combined chemo-immunotherapy. The bi-prodrug nanomedicine strategy introduces a novel approach for the deliberate design of straightforward drug nanoplatforms that improve the therapeutic outcomes of both immunotherapy and chemotherapy.
Numerous self-assembly inducers that can conjugate with drugs, such as hyperbranched poly(ether-ester), polyethylene glycol (PEG), hyaluronic acid, heparin and squalene, have been extensively reviewed previously by Fumagalli et al (2016).96 Expanding on these insights, Zhou et al (2023) investigated the self-assembly of bis(3-(pyridin-2-yl) phenyl) palladium(II) dimers for use in photo-dynamic therapy (PDT) as an anticancer treatment.98 The resulting self-assembled nanorods were evaluated against 3-dimensional A549 and A375 multicellular spheroidal models, yielding an EC50 value of 0.20 µM under irradiation. In vivo studies in mice with A375 tumors revealed high liver accumulation of the nanoconjugates, with lower levels in the heart, kidneys and lungs. This biodistribution pattern suggests prolonged bloodstream retention, leading to greater accumulation in tumor cells.98 Similarly, a platinum-containing prodrug was examined for its anticancer activity. This dimer was synthesized using cisplatin and a short peptide designed as a substrate for the phosphatase-catalyzed dephosphorylation. Upon dephosphorylation, the prodrug self-assembled into a nanotube hydrogel via π–π stacking and hydrogen bonding, forming structures with a diameter of 10 nm. This nanoconjugate effectively delayed cancer cell regrowth in 4T1 xenografted mice while significantly reducing liver and kidney accumulation and toxicity compared to free cisplatin. Furthermore, all treated mice maintained stable body weights throughout the treatment phase.99
Wang et al linked paclitaxel (PTX) dimers using a glutamic acid linker (Glu-PTX2), achieving a high PTX content of 88.9 wt.%. The Glu-PTX2 conjugates showed an ability to self-assemble into NPs (Glu-PTX2 NPs) in aqueous solution, significantly increasing their water solubility. These Glu-PTX2 NPs were internalized by cancer cells, where they exerted potent cytotoxicity. This innovative platform suggests that Glu-PTX2 NPs could serve as a promising alternative to free PTX with improved solubility and therapeutic efficacy.100
Continuing this trajectory, the second mitochondria-derived activator of caspases (SMAC), a pro-apoptotic protein, was conjugated with doxorubicin to create a self-assembling nanoconjugate that was capable of in vivo cleavage by cathepsin B, an enzyme highly expressed in cancer cells. These self-assembled nanoconjugates were stabilized through π–π stacking and hydrophobic intermolecular forces, resulting in the formation of spherical nanoparticulates with an average size of 221.8 nm. The SMAC-doxorubicin nanoconjugate demonstrated potential to address drug resistance in chemotherapy by delivering both therapeutic moieties simultaneously to their target sites in vitro. Furthermore, the nanoconjugate showed a 2.74-fold higher accumulation in Balb/c mice breast tumor within six hours of treatment.101
Doxorubicin–doxorubicin conjugates have been shown to self-assemble through π–π interactions. These di-doxorubicin conjugates were synthesized using either a disulfide linker102,103 or ester linkage.97 The resulting self-assembled nanoconjugates displayed an average size ranging from 75 to 180 nm with encapsulation efficiencies between 60% and 80%. Disulfide-linked conjugates showed higher cellular uptake, but lower cytotoxic activity compared to doxorubicin liposomes and free doxorubicin against MCF-7 cell lines. Notably, the disulfide-linked conjugates exhibited superior efficacy in female nu/nu mice xenografted with MCF-7 tumors, showing greater specificity for tumor cells over normal healthy cells.102,103 The release of doxorubicin from ester-linked nanoconjugate was found to be pH-dependent, with higher release rates in acidic microenvironments, thereby enhancing the targeting of cancer cells while sparing normal healthy cells. Further in vitro assessment using A549, HepG2, and MCF-7 cells revealed half-maximal inhibitory concentration (IC50) values of 7.69, 8.62, and 10.78 μg/mL, respectively.97 Recent advancements in the chemical modification of certain drugs, summarized in Table 1, highlight the potential for improved therapeutic performance through structural modifications.97–99,101–109
 |
Table 1 Overview of Drug–Drug Conjugates: Physical Characteristics, Applications and Key Findings |
The future of drug–drug conjugates represents a promising avenue in cancer treatment and holds great potential for improving therapeutic outcomes and overcoming drug resistance. However, to fully realize their clinical potential, it is imperative to overcome key challenges in formulation design, manufacturing and clinical translation. Progress in the field will depend on coordinated and collaborative efforts between researchers, clinicians and industry stakeholders to advance the technology and deliver its benefits to cancer patients.
Phytochemicals-Based Self-Assembly: Natural Solutions for Advanced Nanomedicine
In recent years, there has been a growing emphasis on investigating the active components present in traditional herbal medicine.110 Throughout history, compounds derived from plants, animals, fungi and microorganisms have served as pivotal elements in clinical drug discovery, particularly in the development of anticancer and anti-infective agents.111 The application of nanotechnology has emerged as a promising avenue for delivering natural phytochemicals and precisely regulating drug release within the body.112 Natural phytochemicals with self-assembly capabilities, such as flavonoids, terpenes,113 alkaloids114 and anthraquinones,13 have been identified as promising candidates for nanostructure development.115 These compounds exhibit an intrinsic ability to spontaneously organize monomers or multimers into well-defined nanostructures through noncovalent interactions in aqueous environments.116 This supramolecular noncovalent interaction, driven by the principle of minimum energy and the attractive forces between molecules, highlights the unique self-organizing behavior of these molecules.117 As a result, using natural phytochemicals as building blocks for the design of self-assembled functional nanostructures has emerged as a focal point in recent research.118 Among the diverse classes of phytochemicals (Figure 3), this section examines the distinctive types essential for self-assembly.
 |
Figure 3 2D chemical structure of different natural compounds examples with Self-Assembling Nanostructures properties (Prepared by ChemDraw®). |
Amongst this array of phytochemicals, steroids and terpenoids possess unique structural diversity and pharmacological significance. Steroids, which are characterized by a cyclopentane-poly(hydrophenanthrene) nucleus, possess a rigid hydrophobic backbone, flexible alkyl side chains, and multiple chiral centres. Terpenoids, the most abundant natural phytochemicals, utilize isoprene as a basic structural unit, with tetracyclic and pentacyclic triterpenes being the most common.119 Despite being homologous, these compounds exemplify how specific molecular architectures can influence self-assembly behavior and therapeutic potential.
Andrographolide, an active compound derived from Andrographis paniculata, is a terpenoid known for its notable anti-inflammatory and anticancer properties. Structurally, it is classified as a diterpene lactone, featuring a cis-1,3-diol configuration with hydroxyl groups at the 5-hydroxymethyl and 6-hydroxyl positions. Recent advancements in nanotechnology have explored its potential for drug delivery applications. For instance, Kim et al synthesized nanostructures by forming borate bonds between cis-1,3-diol of andrographolide and hydrophilic polymerized phenylboronic acid (pPBA), demonstrating pH-responsive controlled-release systems.120 Another approach utilizing glycyrrhizic acid as a building block to develop andrographolide-based nanostructures showed significant enhancements in both solubility and anticancer effects.121
Ginsenoside Rb1, a bioactive compound derived from Panax ginseng, has demonstrated the ability to self-assemble into stable NPs alongside anticancer drugs. These NPs feature hydrophilic head regions that facilitate aqueous connectivity while effectively isolating hydrophobic substances within the core. The stability and integrity of these nanostructures are further reinforced by π–π stacking interactions, which serve to create a robust system that does not induce toxicity or adverse effects, making ginsenoside Rb1-based green NPs a promising platform for the delivery of insoluble drugs.122
Steroids, which are abundant in biological systems, have been extensively explored for their potential in nanostructure self-assembly, particularly tetracyclic steroids characterized by their distinctive 6-6-6-5 ring structure. Sterols like ergosterol, known for their anticancer activity and self-assembly capability, have been utilized to create nanodrugs in combination with chlorin e6 (Ce6). These Ergo-Ce6 NPs exhibit remarkable phytotoxicity, increased blood circulation, excellent biocompatibility, prolonged tumor retention, biodegradability and low toxicity, making them a promising platform for targeted cancer therapies.123
Alkaloids are naturally occurring nitrogen-containing chemicals found in plants, fungi, and certain marine creatures. Alkaloids have a variety of chemical configurations and are frequently lipophilic, allowing them to interact efficiently with biological systems.124 Recently, alkaloids have garnered interest in drug delivery due to their propensity to conjugate with nanocarriers, which can result in stability, solubility and bioavailability enhancements.125 The ability of alkaloids to create self-assembled nanostructures allows for a more focused and controlled distribution that can lead to improved therapeutic efficacy with less adverse effects.126
Continuing this discussion of structurally diverse phytochemicals, alkaloids such as berberine exhibit remarkable self-assembly capabilities that contribute to their therapeutics. Berberine, a key antibacterial component of Coptis chinensis Franch, has gained attention for its ability to form nanostructures, driven by its polyaromatic ring structure and quaternary ammonium ions.127 Inspired by traditional Chinese medicine combinations, researchers successfully synthesized berberine-cinnamic acid NPs, which demonstrated superior inhibition of multidrug resistance compared to both berberine alone and control groups.13,128
Paclitaxel, a diterpene alkaloid derived from Taxus chinensis, is widely used in the treatment of breast and prostate cancers. However, its clinical application has been hindered by challenges such as water insolubility and multidrug resistance.129,130 To address these limitations, Cheng and Ji developed paclitaxel-sulfur-sulfur-Berberine (PTX-ss-BBR) NPs, which effectively accumulate in the mitochondrial and exhibit potent anticancer activity by inducing cell cycle arrest in the G2/M phase.131 Additionally, the combination of water-soluble vitamin E succinate with insoluble paclitaxel has been shown to self-assemble into paclitaxel-ss-VitE NPs through disulfide bonding. These NPs demonstrate significant pharmacological properties, including efficient hydrophobic drug loading and enhanced therapeutic efficacy.132 Another alkaloid, camptothecin, which is a quinoline alkaloid, has been shown to have potent antitumor activity, however has seen limited clinical utility due to challenges associated with poor solubility and stability. To overcome these obstacles, researchers have focused on developing effective nanodrug delivery systems. One such approach involves the self-assembly of hydroxycamptothecin and doxorubicin into hydroxycamptothecin-doxorubicin NPs. These NPs exhibit a morphological transition from nanorods to spherical particles over time, a process influenced by the molar ratio of doxorubicin to hydroxycamptothecin.133
Building on the self-assembly capabilities exhibited by alkaloids, flavonoids represent another class of natural compounds with significant pharmacological and structural potential. Known for their roles in managing cancer, neurodegenerative disorders and inflammatory diseases, flavonoids are increasingly recognized for their ability to self-assemble into functional nanostructures.134 This property enables them to serve as versatile building blocks in nanotechnology, further broadening the scope of natural phytochemicals in drug delivery and therapeutic applications.125 Baicalin and wogonoside, prominent bioactive flavones derived from Scutellaria baicalensis, exhibit self-assembly capabilities when combined with berberine, resulting in the formation of two distinct nanostructures.135 Li et al reported that baicalin-berberine NPs exhibited superior antibacterial activity compared to wogonoside-berberine nanofibers (NFs), a distinction attributed to differences in the spatial configuration and the underlying driving forces that influenced self-assembly mechanisms.136 While hydrophobic and electrostatic interactions predominantly drive the formation of baicalin-berberine NPs, the self-assembly of wogonoside-berberine NFs relies exclusively on potent hydrophobic forces. Notably, baicalin-berberine NPs initially form a one-dimensional unit, which subsequently evolves into a three-dimensional structure through interactions between hydrophilic glucuronic acid and the hydrophobic parent nucleus.136
Quinone compounds represent yet another diverse group of natural phytochemicals with self-assembling capabilities that have shown significant potential in nanomedicine. Quinones, encompassing benzoquinones, anthraquinones, phenanthrenequinones and naphthoquinones, are well recognized for their antibacterial and anticancer properties.137 Their propensity to form nanostructures stems from the presence of polyaromatic rings and hydrogen bonding interactions. Doxorubicin, a representative quinone compound with a hydrophilic amino sugar and a hydrophobic anthraquinone group, can form dimers or oligomers that act as building blocks for self-assembled nanodrugs.133 Anthraquinone, exemplified by rhein, exhibits self-assembly via hydrophilic, π–π-stacking, and hydrophobic interactions. In alkaline solutions, rhein molecules polymerize into dimers and aggregates, forming nanofibers through electrostatic repulsion.138 Furthermore, rhein can self-assemble with berberine into NPs through hydrogen bonds and π–π-stacking, creating a layered framework.13 Moreover, rhein hydrogels offer the advantage of molecular modification-free slow-release drug functions, introducing innovative concepts for nanomedicine design.137 Hypocrellin, a quinonoid derivative, demonstrates self-assembly with human serum albumin into NPs through hydrophobic interactions.139
Polysaccharides, the most abundant natural phytochemicals, exhibit antitumor, antioxidant and various therapeutic properties, making them promising candidates for nanostructure construction due to their biocompatibility, biodegradability and functional groups.140,141 While polysaccharides themselves do not inherently self-assemble, their multiple-hydroxyl groups facilitate hydrogen bonds, promoting orderly molecular arrangement. Researchers have enhanced the self-assembly potential of polysaccharides by modifying hydrophilic sugar groups with hydrophobic aromatic or alkyl chains, resulting in amphiphilic molecules that promote self-assembly in solutions.142,143 For instance, inulin, extracted from chicory root, forms spherical NPs and has shown potential in spinal cord injury treatment and dual cancer therapy when combined with curcumin.144–146 Similarly, pectin, another important polysaccharide, functions as a colon-specific drug-delivery material, effectively transporting drugs like dihydroartemisinin and hydroxycamptothecin to tumor sites.147
Beyond the commonly employed natural phytochemicals, unique molecular structures are gaining attention for their potential in nanostructure construction. Folic acid, composed of pterin, p-aminobenzoic acid and glutamic acid, exemplifies this trend with its self-assembly properties mediated through aromatic ring-driven stacking and nitrogen/oxygen atom interactions. Its hydrophilic groups, combined with its natural affinity for folate receptors on tumor cells, make folic acid an intriguing candidate for nanomedicine design.148 Leveraging these properties, researchers have developed folic acid-based NPs capable of delivering therapeutic agents such as doxorubicin with enhanced specificity. These NPs demonstrated increased endocytosis and reduced cytotoxicity toward normal cells.149,150 Similarly, ivy NPs (INPs), derived from Hedera helix adventitious roots, represent a new type of natural nanocarrier. Composed of arabinogalactan proteins, INPs combine water solubility, low viscosity and biocompatibility, making them highly compatible for drug delivery applications. INP-doxorubicin NPs, synthesized through electrostatic and hydrophobic interactions, have displayed potent therapeutic effects against cancer.151
Self-Assembled Peptide-Drug Conjugates: Multifunctional Nanostructures for Advanced Delivery
Self-assembling peptides, consisting of short amino acid sequences, possess the ability to spontaneously organize into complex and well-defined structures. These peptides have garnered significant interest in various fields, including medicine and drug delivery, due to their unique structural and functional properties.152 Through the precise design of self-assembling peptides, researchers can engineer nanostructures that serve as efficient drug delivery vehicles. These peptide-based nanostructures enhance drug solubility, improve stability and enable targeted delivery to specific cells or tissues. Moreover, by regulating the self-assembly process, it is possible to achieve controlled and sustained drug release, thereby improving therapeutic efficacy, and while minimizing potential negative side effects.49
Short peptides, naturally occurring molecules, play diverse roles in biological systems, functioning as hormones, pheromones, antibacterial and antifungal agents within innate immune systems, as well as in poisons and pesticides. Historically, the potential of peptides as scaffold hydrogel materials was never fully realized.153 However, a paradigm shift occurred in 1990 with the discovery of an ionic self-complementary peptide, identified as a repeating sequence within a yeast protein. This discovery highlighted the potential of self-assembling peptides to form structure materials. Today, peptides composed of the 20 natural amino acids are recognized for their intrinsic material properties.154 These simple and designer peptide-based scaffold hydrogels have since become commercially available, finding applications across a broad spectrum of fields.155
Peptides are now capable of being engineered to self-assemble into various nanostructures, including nanotubes, nano-fibers and nano-vesicles, customized to meet specific design and self-assembly conditions156 (Figure 4). Accordingly, the diverse capabilities of self-assembling peptides extend beyond scaffold hydrogels, offering immense potential in drug delivery applications. For example, the amphiphilic peptide-based biomaterial RADA16 demonstrates the adaptability of peptides in drug delivery. RAD16 is a peptide composed of 16 amino acids, featuring a repeating sequence of positively charged arginine (R), hydrophobic alanine (A) and negatively charged aspartic acid (D).157 In acidic aqueous solution, RADA16 spontaneously assembles into an extracellular matrix-like 3D structure within seconds upon interaction with physiological pH bodily fluids.158 Its high biocompatibility, low immunogenicity, benign breakdown products and ease of customization make RAD16 an excellent substrate for tissue engineering applications.159 RADA16 effectively functions as a delivery system for cells, therapeutic agents and bioactive factors. Its shear-thinning and thixotropic properties enable it to fill tissue gaps via injection without swelling. However, its lower mechanical strength and limited hydrophilicity pose notable drawbacks.159 To compensate for this constraint, researchers have modified RAD16 by incorporating several functional groups and polymers, significantly broadening its utility and advancing its application in the field of tissue engineering.157
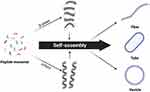 |
Figure 4 Peptides self-assembled into different nanostructures. Adapted from Fan T, Yu X, Shen B, Sun L. Peptide Self-Assembled Nanostructures for Drug Delivery Applications. J Nanomater. 2017;2017:1–16. Copyright © 2017 Taotao Fan et al. Creative Commons.94 |
Ren et al introduced an innovative injectable hydrogel strategy using an L-norvaline-based immunomodulating gelator designed to effectively inhibit the Arginase 1 (ARG1) pathway.160 The gelator, constructed as a diblock copolymer containing an L-norvaline-based polypeptide block, demonstrated the ability to form a thermally responsive injectable hydrogel through self-gelation within tumor microenvironments. This approach offers a promising platform for reversing ARG1-mediated immunosuppression, thereby amplifying the efficacy of immunotherapy.
The conjugation of anticancer drugs with small peptides offers various advantages, notably enhancing the aqueous solubility of the drugs and addressing a persistent challenge in drug delivery. This approach also enables improved biodistribution through targeted delivery and modified pharmacokinetics.160 Moreover, peptide-based drug conjugates can leverage the high affinity of peptides for specific receptors, significantly increasing the specificity and efficacy of drug delivery. The adaptable nature of peptide design, combined with the ability to incorporate peptide-based epitopes or functional other moieties, further allows for the development of drug nanostructures with enhance functionalities, such as targeting precision and stealth capabilities.161
Cheetham et al investigated the synthesis of self-assembling camptothecin amphiphiles by creating mono-, di-, and quad-camptothecin-butyl-disulfide-Tau peptide conjugates. They observed that the solubility of these conjugates decreased progressively from 2 to 1 to 1×10-4 M per liter as the number of camptothecin molecules increased, attributed to the rising molecular weight of the assembled conjugates. To address this challenge, further synthesis efforts focused on camptothecin-peptide conjugates (Sup35), wherein two hydrophobic amino acids were substituted with hydrophilic ones. This modification significantly improved the overall solubility of the di- and quad-camptothecin-peptide conjugates, resulting in the formation of nanotubes with average widths of 8.9 and 9.9 nm, respectively. Notably, the quad-camptothecin-Sup35 nanotubes exhibited an aqueous solubility exceeding 1 mol/L, resulting in a clear solution. This increased solubility allowed the nanotubes to self-assemble into longer nanofilaments, thereby facilitating targeted drug delivery. However, the incorporation of hydrophilic amino acids was found to produce nanotubes with increased average lengths of 1 µm, leading to increased viscosity of the solution with longer nanotubes.162 These characteristics call attention to the trade-offs associated with optimizing solubility and structural properties for efficient drug delivery.
In a recent study, researchers developed self-assembling doxorubicin NPs as a potential therapeutic strategy for breast cancer. The study synthesized an amphiphilic peptide dendrimer composed of a C18 hydrophobic alkyl chain and a hydrophilic poly-L-lysine peptide dendron. This amphiphilic dendrimer had a critical aggregation concentration (CAC) of 6.1 µM, reflecting its balanced hydrophobic and hydrophilic characteristics. Using a film-dispersion method, doxorubicin was encapsulated within the amphiphilic dendrimer, resulting in nanoassemblies with an average size of 73.0 nm. Drug release studies revealed that over 50% of the encapsulated doxorubicin was released at pH 7.4, while approximately 25% was released at pH 5.0 after 24 hours, highlighting the pH-responsive nature of the formulation. The efficacy of the self-assembled doxorubicin NPs was evaluated in vitro using MCF-7 and MCF-7R cell lines. The NPs demonstrated an IC50 of 4.4 µM in MCF-7 and 12.9 µM in MCF-7R, with the latter known for its resistance to doxorubicin. In comparison, free doxorubicin exhibited an IC50 of 6.6 µM in MCF-7 cells. Interestingly, in MCF-7R 3D-cultured tumor spheroids, the IC50 of the prepared self-assembling doxorubicin NPs was significantly higher at 89.1 µM, indicating reduced efficacy in the 3D tumor model compared to the 2D monolayer culture. However, the approach successfully overcame drug resistance in the MCF-7R cell line, likely due to enhanced cellular uptake of the nanoformulation.160 This study underscores the potential of self-assembling peptide dendrimers as a platform for overcoming multidrug resistance and improving therapeutic delivery of doxorubicin in breast cancer treatment.
Nucleic Acids as Self-Assembling Drug Delivery Systems
While self-assembly has been extensively investigated with materials like lipids, polymers and peptides, the use of nucleic acids (such as DNA or RNA) in self-assembling drug delivery systems represents an area of emerging biomedical research.163 Among these, DNA origami nanostructures have emerged as a particularly promising approach due to their simplicity, robustness and scalability. This methodology involves folding DNA into complex nanostructures using standard materials and protocols, enabling fast and efficient assembly. Remarkably, the technique can be performed with inexpensive and widely available equipment (eg, hot plates, water baths and laboratory burners), making it highly accessible for both existing and novel DNA origami designs.164
Transitioning into the use of nucleic acids as drug delivery systems, the design and engineering of nucleic acid nanostructures have emerged as an important innovation in the field of drug delivery. Since 1982, when Ned Seeman, the pioneer of DNA nanotechnology, proposed the potential of DNA to synthesize crystallized guest molecules as a structural framework, DNA has been recognized for more than its genetic role. It has also been identified as a versatile material for constructing nanoscale architectures.165,166 With its inherent complementary based on the Watson–Crick model and ease of fabrication, DNA serves as an ideal material for creating nanostructures with precise shapes and functions. By combining and self-assembling different DNA strands, predesigned configurations can be achieved, making DNA-based materials highly adaptable for a wide range of applications, including biosensing, drug delivery to targeted sites and advancements in nano- and microelectronics.167,168
Abbas et al developed a cisplatin-loaded deoxyribonucleic acid nano-thread (CPT-DNA-NT) with a diameter ranging from 50 to 150 nm and length of 300–600 nm. The DNA-NT was designed using a stiff-topology approach, employing a circular-scaffold to encapsulate CPT. The MTT assay revealed that the CPT-DNA-NT exhibited superior cytotoxicity against HeLa cells compared to free CPT, attributed to the depot-like release mechanisms of CPT following DNA-NT internalization. Additionally, the DNA-NT exhibited targeted cell internalization and controlled intracellular release of CPT, enhancing its therapeutic efficacy.167
Short interfering RNA (siRNA) and microRNA (miRNA) are nucleic acid molecules capable of regulating gene expression, offering therapeutic potential for a variety of diseases. Self-assembling nucleic acid carriers provide an innovative platform for the efficient delivery of siRNA and miRNA, allowing for the modulation of specific genes associated with specific pathological conditions.169,170 However, the delivery of miRNA presents significant challenges, as these molecules must traverse nuclease-rich blood compartments, where they are susceptible to rapid degradation. To address this, high-strength carriers are required to protect miRNA from enzymatic breakdown during blood circulation. While miRNA and siRNA have commendable potential as therapeutic agents, research on their co-delivery remains comparative, highlighting an area of opportunity for advancing nucleic acid-based therapies.171,172
Squalene Conjugates: A Versatile Platform for Drug Delivery
Squalene (SQ), a naturally occurring lipid recognized for its biocompatibility, plays a significant role in advancing medical treatments through its application in squalenoylation technology. This approach, characterized by the conjugation or binding of SQ to various nucleoside analogs shows efficaciousness against viruses and cancers through enhancing the pharmacological profile of the therapeutic compounds.173 This technique improves drug solubility and delivery, as well as having a meaningful impact toward the development of more effective treatments in virology and oncology.
Squalene, a polyunsaturated triterpene containing six isoprene units, serves as a biochemical precursor for cholesterol and other steroids. In drug delivery, SQ demonstrates its versatility as a foundational component in the synthesis of SQ-drug bioconjugates. These constructs exhibit remarkable self-assembly properties, forming NPs in aqueous environments without the need for additional carrier agents. This intrinsic capability highlights the significance of SQ-based systems in the development and evolution of targeted drug delivery platforms.96,174–177
Squalene is an abundant molecule in nature, particularly can be found in olives, shark liver oil, wheat germ and rice bran. Consequently, apart from being biosynthesized within cells, SQ is also ingested, serving as a fundamental component of the human diet that is predominantly synthesized in the liver and skin.175 The distribution of SQ throughout the bloodstream relies on very low-density lipoproteins (vLDLs) and low-density lipoproteins (LDLs), and its secretion by sebaceous glands underline the crucial role of SQ in both dietary intake and the regulation of internal biological processes.178,179
The enhanced solubility, stability and bioavailability of SQ have been therapeutically leveraged in formulations of drug conjugates or emulsions as a drug carrier.175 In drug delivery, self-assembly emerges as a key mechanism for creating nanostructures tailored for targeted therapeutic delivery. This process finds remarkable application in overcoming inherent limitations associated with certain potent therapeutic agents. For instance, gemcitabine, renowned for its efficacy against various tumor types,180 encounters challenges such as rapid metabolism post-administration, leading to a truncated biological half-life and necessitating higher doses for desired therapeutic outcomes.181 The SQ-conjugation techniques have emerged as a promising strategy to address such pharmacological constraints and challenges. For example, Squalenoyl-gemcitabine (SQgem) is a specific bioconjugate comprising SQ and gemcitabine, coupled at the amino group.182 This SQgem bioconjugate is distinguished by its notable ability to self-assemble into nanostructures within aqueous environments. X-ray diffraction results have shown the hexagonal molecular packing of SQgem, elucidating its structural intricacies resulting from the stacking of cylinders. These nanostructures, often exhibiting hexagonal or multifaceted shapes with internal reticular planes surrounded by an external shell, present an innovative precision medicine approach, facilitating targeted drug delivery.182
Compared to conventional gemcitabine formulations, in vitro studies of SQgem nano-assemblies have demonstrated markedly enhanced cytotoxic capabilities against various cancer cell lines, including KB-3 and MCF-7.174 SQgem nano-assemblies represent a potential delivery platform for reversing drug resistance within cancerous cells, a current challenge throughout oncology.183 Moreover, the integration of squalenoyl-polyethylene glycol into these nano-assemblies has been shown to amplify this resistance reversal effect, representing a significant stride toward improved cancer treatment strategies.184
Chemical synthesis and bioconjugation techniques can be considered as the primary approaches for conjugating SQ with drugs. In the chemical synthesis method, a chemical modification step applies on the SQ to allow the attachment to the drug molecules. For instance, SQ can be functionalized with reactive functional groups such as hydroxyl (OH) or carboxyl groups, which then can go through an esterification or amidation reaction to be coupled with a certain drug. Chemical synthesis offers precise control over the structure of the SQ-drug conjugate and allows for the incorporation of specific functionalities tailored to the desired application. For instance, squalenoyl-gemcitabine (SQ-Gem) was synthesized by covalently coupling gemcitabine with 1.1′,2-tris-nor-squalenic acid onto the amino group of the nucleoside heterocycle. This process involves the direct chemical modification of gemcitabine, a nucleoside analog, with a SQ-derivative, resulting in the formation of SQ-Gem.185
In a bioconjugation approach, SQ can be conjugated with a drug molecule containing an azide or alkyne functional group using click chemistry. An SQ-derivative functionalized with an alkyne group has been designed. This alkyne-functionalized SQ has then reacted with a drug molecule modified with an azide group using copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction. As an example, a diverse set of flexible linkers containing terminal azide groups was synthesized from SQ through a two-step process, resulting in a mixture of regiochemical and stereochemical isomers. These linkers were employed to tether either two high-affinity NDP-a-MSH ligands or two low-affinity MSH(4) ligands. Subsequently, the ligands underwent N-terminal acylation using N-hydroxysuccinimidoyl 5-hexynoate before being conjugated to the linkers through copper-catalyzed “click” 3+2 cyclization of the azide and alkyne functionalities. This demonstration highlights the utility of SQ in facilitating bioconjugation, particularly in linking ligands via click chemistry. Such an approach presents a versatile strategy for constructing multifunctional molecular architectures with potential implications in targeted drug delivery and therapeutic interventions.186
Recent advancements in the synthesis of SQ-drug conjugates have focused on enhancing efficiency, selectivity and scalability, while minimizing side reactions and toxic byproducts. New linker molecules have been designed that enable site-specific attachment of drugs to SQ, improving the homogeneity and stability of the conjugates. These linkers may incorporate cleavable bonds that facilitate drug release at the target site, enhancing therapeutic efficacy. In one study, a hemiaminal-based pH-sensitive linker was used to synthesize squalenoyl conjugates of sunitinib and semaxanib, two powerful antiangiogenic (pyrrolyl)methylidenyl-substituted oxindole multitarget tyrosine kinase inhibitors.179 The squalenoyl prodrugs bearing an acid-sensitive hemiaminal group were synthesized through direct alkylation of the NH group of the oxindole ring in each drug with 1,1,2-trisnorsqualenic acid chloromethyl ester. The prodrugs were prepared using a three-step sequence involving (i) N-alkylation with chloromethoxy-triisopropylsilane, (ii) desilylation, and acylation with trisnorsqualenic acid. According to their findings, these squalenoyl prodrugs could form nano-assemblies on their own in aqueous conditions without the aid of a surfactant. These squalenoyl sunitinib nano-assemblies were particularly cytotoxic to the human umbilical vein endothelial cell line (HUVEC), which is implicated in the development of tumor vessels, according to their in vitro results.187
The potential biomedical applications of SQ conjugates in the targeted delivery of diverse therapeutic agents encompass a wide array of treatments, including anti-cancer drugs, vaccines and nucleic acids. Recent studies have highlighted the effectiveness of SQ-based nanostructures, revealing promising results in both preclinical and clinical contexts. In targeted drug delivery, SQ conjugates have garnered significant attention for their ability to enhance the therapeutic efficacy of various agents, particularly in the treatment of cancer.175 By conjugating SQ with anti-cancer drugs, such as paclitaxel or doxorubicin, researchers have achieved improved drug solubility, stability and targeted delivery to tumor sites. For instance, Caron et al188 developed a series of novel lipid prodrugs of paclitaxel, aiming to address the limits caused by the systemic toxicity and limited water solubility of paclitaxel. These prodrugs consist of an SQ chain bound to the 2′-OH of paclitaxel through a 1.4-cis, cis-dienic linker, allowing them to self-assemble into NP systems while preserving efficient release of the free drug. The in vitro biological assessment of these squalenoyl–paclitaxel NPs showed notable cytotoxicity on several tumor cell lines, including A549 lung cell line, colon cell line HT-29, and KB 3.1 nasopharyngeal epidermoid cell line. In addition, the antitumor efficacy of the nano-assemblies constructed with the more active prodrugs was investigated on a human lung (A549) carcinoma xenograft model in mice. The prodrug bearing the cis,cis-deca-5,8-dienoyl linker showed comparable antitumor efficacy to the parent drug but much lower subacute toxicity, as observed in total body weight loss. These findings suggest that NPs incorporating squalenoyl paclitaxel prodrugs may be useful for replacing the toxic Cremophor EL and enhancing therapeutic outcomes in cancer treatment.188
SQ conjugates hold additional promise as carriers for nucleic acid-based therapeutics, including siRNA and messenger RNA (mRNA). By encapsulating nucleic acids within SQ-based NPs, these fragile molecules could be protected from degradation and facilitate their targeted delivery to specific cells or tissues. Recent advancements in the field of gene therapy have also witnessed the conjugation of the natural lipid SQ with siRNA, presenting a promising avenue for targeted gene silencing. For instance, in one study, SQ was conjugated with siRNA designed to target the junction oncogene RET/PTC1, commonly associated with papillary thyroid carcinoma (PTC).189 Through maleimide–sulfhydryl chemistry, the acyclic isoprenoid chain of SQ was covalently coupled with siRNA RET/PTC1 at the 3′-terminus of the sense strand. This conjugation resulted in the formation of amphiphilic molecules that self-organized into siRNA-SQ RET/PTC1 NPs in aqueous environments. Remarkably, these NPs exhibited stability in water and did not demonstrate any cytotoxicity in vitro.189
Of particular interest, in vivo studies utilizing mice xenografted with RET/PTC1 experimental models demonstrated that RET/PTC1-SQ NPs effectively inhibited tumor growth and suppressed both RET/PTC1 oncogene and oncoprotein expression following cumulative dose intravenous injections. This integration showcases how SQ conjugation with siRNA has been utilized to effectively deliver therapeutic payloads and achieve targeted gene silencing, exemplifying the potential applications of SQ-based nanostructures in gene therapy.189
Moreover, SQ conjugates have emerged as promising adjuvants for vaccine delivery, owing to their ability to enhance immune responses and improve antigen stability. By formulating vaccines with SQ-based adjuvants, such as SQ-in-water emulsions (eg, MF59), researchers have achieved enhanced immunogenicity and efficacy against infectious diseases, including influenza and COVID-19. Clinical studies have demonstrated the safety and efficacy of SQ-based adjuvants in boosting immune responses and improving vaccine efficacy, highlighting their potential for widespread use in vaccine development. The SQ-based oil-in-water emulsion vaccine adjuvant MF59 has been administered to more than 100 million people in over 30 countries according to Kim et al, whereby the results demonstrated efficacy in both seasonal and pandemic influenza vaccines. Their study demonstrated that immunization with MF59 or its mimetic AddaVax (AV) in combination with soluble antigen induces robust antigen-specific antibody and CD8 T cell responses in lymph nodes and non-lymphoid tissues. Surprisingly, antibody responses remain unaffected in RIPK3-kinase or Batf3 deficient mice, suggesting the involvement of RIPK3-independent pathways in antibody induction. These findings highlight the multifaceted mechanisms through which SQ emulsion-based vaccine adjuvants, such as MF59, elicit antigen-specific immune responses. By activating both RIPK3-dependent and -independent pathways, SQ-based adjuvants contribute to the enhancement of both cellular and humoral immune responses, highlighting their potential for improving vaccine efficacy and immunogenicity.190 Furthermore, a nanocomposites prodrug of squalenoyl-gemcitabine has been designed in combination with edelfosine. It was observed that these molecules spontaneously self-assembled as stable and monodisperse nanoassemblies with a size of 51 ± 1 nm in a surfactant/polymer free-aqueous suspension. This combination resulted in smaller particle size and a new supramolecular conformation, with higher stability and drug content, and an improved antitumor profile against patient-derived metastatic pediatric osteosarcoma cell line,84,191 as well as an optimized pharmacokinetic profile in mice.192
Emerging research of SQ conjugates will focus on developing novel conjugation strategies to expand the versatility and efficacy of SQ conjugates, optimizing nanostructure properties to enhance their biocompatibility, and targeting efficiency and exploring new therapeutic applications beyond traditional cancer therapy. These advancements hold promise for addressing unmet medical needs in various disease areas, including infectious diseases, autoimmune disorders, neurodegenerative diseases, and metabolic disorders (Figure 5). Moreover, advancements in SQ conjugate technology are expected to contribute significantly to personalized medicine by enabling precise control over drug targeting, release and therapeutic effect, ultimately leading to improved treatment outcomes and enhanced patient quality of life. On the other hand, the potential contamination of SQ in the anthrax vaccine (AVA) represents one topic of controversy, whereby SQ contamination in some vaccine lots have been implicated with various health problems. While studies from the FDA and Institute of Medicine (IOM) have not found a definitive link between SQ and the reported adverse effects, these concerns have raised alarms that have stalled greater utilization of SQ. Existing commercial therapeutic applications of SQ-based systems are limited to adjuvant functions, such as the FDA-approved seasonal influenza vaccine for ageing adults, Fluad® Quadrivalent, which contains an oil-in-water SQ emulsion. Another SQ containing adjuvant, AS03, used in vaccines, such as H5N1 influenza is not commercially available, but is part of the US Strategic National Stockpile and is only intended for use if public health officials deem administration necessary during a bird flu outbreak. Lastly, the complexity of production and requirements associated with consistent quality may represent a key hurdle in translating SQ-based systems into clinical applications.
Conclusion and Future Directions: The Scientific and Clinical Potential of Drug-Based Self-Assembled Nanostructures
In conclusion, drug-based self-assembled nanostructures hold immense promise as targeted drug delivery systems, offering a multifaceted approach to enhancing therapeutic efficacy and minimizing adverse effects. The inherent diversity of these self-assembled nanostructures allows for tailored design and delivery to specific targets. The integration of drug–drug conjugates, polymeric-based architectures, biomolecules, peptides, DNA, squalene conjugates and amphiphilic drugs further enriches the versatility of these systems to address complex biomedical challenges.
The incorporation of targeting ligands, stimuli-responsive moieties and imaging agents within these nanostructures opens new avenues for personalized medicine and real-time monitoring of therapeutic responses. Nonetheless, key challenges such as stability, scalability and regulatory compliance must be systematically addressed to enable the successful translation from bench to bedside.
Future research should prioritize refining fabrication techniques, improving biocompatibility and optimizing targeting strategies to achieve maximum therapeutic efficacy while minimizing off-target effects. Additionally, continued advancements in nanotechnology, materials science and bioinformatics will likely play critical roles in overcoming current limitations and driving progress in this evolving field.
As the field of drug-based self-assembled nanostructures continues to evolve, it is essential to identify and investigate emerging trends and future prospects that can drive innovation. These advancements encompass addressing current limitations as well as establishing new avenues for personalized medicine and targeted drug delivery. The integration of innovative technologies and interdisciplinary approaches in the presence of significant advancements in the field of nanoscience over the last two years, offers promising solutions to many longstanding challenges.
The protein corona, a layer of proteins that forms on nanostructures upon exposure to biological fluids, greatly influences nanostructure design by changing surface features, cell uptake and interactions with the immune system.193 These proteins may alter nanostructure size, charge and hydrophobicity, which can play a key function in how they are identified by and taken into cells. In some cases, the corona can promote or obstruct targeting, which can affect drug release and change the stability of the nanostructures. Moreover, the protein corona can trigger immune reactions, resulting in quicker clearance or inflammation, which can restrict therapeutic effectiveness.194 Greater understanding and better management of this biomolecular layer and its formation around nanostructures is an important area for future focus when developing efficient drug delivery. Nanostructures can reduce protein corona formation by first applying hydrophilic coatings, that give stealth characteristics to the nanostructure (ie, PEG). Pre-coating, fine-tuning the surface charge or size of the nanostructure represent alternative strategies with viability. Biocompatible materials and changes to the surface additionally lessen nonspecific protein attachment and detection by the immune system.195
Advancements in fabrication techniques represent a critical focus in this evolving field, with future research emphasizing the refinement of these methods. Emerging trends in 3D printing and microfluidics enable greater precision of the size, shape and surface characteristics of nanostructures, subsequently improving their consistency and functionality. While scalability concerns remain a challenge, the production of uniform nanostructures will benefit from advancements in manufacturing technologies. Continuous flow microfluidics, for instance, offer a pathway toward large-scale production of uniform nanostructures, meeting the quality and consistency demands essential for clinical translation and adoption. Equally important, addressing stability concerns, particularly degradation under physiological conditions, will require the use of more robust nanostructures, which can be achieved through advanced cross-linking strategies and encapsulation techniques.
Enhancing biodegradability and biocompatibility remains a priority in the development of drug-based nanostructures. Leveraging natural and bioinspired materials (ie, extracellular vesicles and biomimetic coatings) is expected to significantly improve the compatibility of these systems in biological environments. Moreover, the optimization of targeting strategies through the design of multifunctional ligands and advanced surface modification techniques offers a promising pathway to maximize therapeutic efficacy while minimizing off-target effects, further advancing the potential of nanostructures in precision medicine.
Commercial applications that incorporate stimuli-responsive elements, reacting to specific biological triggers (ie, pH, temperature, or enzymatic activity), have recently gained significant traction. In the field of self-assembled nanostructures, advances in responsive polymer design and nanotechnology will be crucial. These reactive systems will enhance the precision of drug delivery, creating a “smart delivery” approach that enables drug release exclusively at the target site, thereby minimizing systemic toxicity. Additionally, gastrointestinal tract targeted drug delivery systems with self-assembled nanostructures have gained attention for their ease of administration, offering significant quality of life improvements in treating various diseases. These systems enhance patient compliance and convenience, addressing a key challenge in modern medicine. By protecting sensitive therapeutics from acidic and enzymatic degradation of the gastrointestinal tract and stomach, these self-assembled systems offer an alternative delivery paradigm that utilizes easier and more accessible pathways. Similarly, addressing the challenges posed by the formation of a protein corona on NPs or potentially learning ways to leverage this biomolecular layer to enhance targeting precision will be essential in advancing drug-based self-assembled nanostructures.
The future of medicine will increasingly involve the integration of personalized approaches and the ability to amalgamate patient-specific data to design tailored nanostructures represents a significant advancement. Coupling these innovations with real-time monitoring capabilities through imaging agents and biosensors can provide continuous feedback on therapeutic responses, allowing for dynamic adjustments in treatment regimens. Furthermore, these approaches will play a crucial role in tracking patient compliance and providing new mechanisms to improve adherence to prescribed therapies.
Lastly, regulatory hurdles and safety concerns, such as immunogenicity and long-term biological effects, must be systematically addressed. Rigorous preclinical and clinical testing, along with the development of standardized evaluation protocols, are essential to ensure the safety and efficacy of these novel self-assembled nanostructure systems. Gaining regulatory approval can be challenging, especially if products with similar efficacy already exist for the same indication. Additionally, developing nanomedicines is fraught by a lack of standards and regulations in manufacturing practices, quality control, safety and efficacy evaluation. Currently, regulatory authorities such as the FDA in the US determine these geographic standards and provide guidance, although no specific regulatory standards governing their production or clinical translation have been devised. Therefore, the broader application of nanotechnology varies across regions and the use of a particular nanomedicine in one country could vary in others. As regulatory frameworks harmonize geographically and evolve, the pathway to market and clinical utility of nanomedicine will improve. Addressing these challenges and leveraging emerging trends will be essential in advancing the field of drug-based self-assembled nanostructures, ultimately leading to improved clinical treatment modalities and better patient outcomes.
Ultimately, drug-based self-assembled nanostructures represent an innovative platform in the evolution of targeted drug delivery systems, with the potential to revolutionize clinical treatment modalities and improve patient outcomes in diverse therapeutic areas. However, continued interdisciplinary collaboration and innovation will be essential in realizing the full clinical potential of these self-assembled nanostructures. The future potential of drug-based nanoconjugates requires additional considerations and addressing limitations related to stability concerns, biodistribution, immunogenicity, long-term implications and manufacturing complexity. Specifically, stability concerns (ie, degradation under certain conditions) could impact overall therapeutic efficacy and require further evaluation. Biodistribution parameters represent an important area of further investigation, to ensure targeted delivery with limited off-target effects. With continued interdisciplinary innovation and a focus on overcoming these limitations, the clinical translation of these nanostructures can herald a new era in precision medicine and personalized therapeutics.
Abbreviations
1MT, 1-methyl-tryptophan; API, Active pharmaceutical ingredient; ARG1, Arginase 1; Ce6, Chlorin e6; CMC, Critical micelle concentration; CPZ, Chlorpromazine; DLS, Dynamic light scattering; EPR, Enhanced permeability and retention; GEM, Gemcitabine; mV, Milli Volt; mRNA, messenger RNA; NPs, nanoparticles; PTX, Paclitaxel; pPBA, phenylboronic acid; PEG, Polyethylene glycol; RES, Reticuloendothelial system; siRNA, Small interfering RNA; SAXS, Small-angle X-ray scattering; SQ, Squalene; TEM, Transmission electron microscopy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Falzone L, Salomone S, Libra M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front Pharmacol. 2018;9:1300. doi:10.3389/fphar.2018.01300
2. Tulchinsky TH, Varavikova EA, Taylor R, Daniels J. A History of Public Health. The New Public Health. 2014;14:1–42. doi:10.1016/B978-0-12-415766-8.00001-X
3. Porter M, Lin RAN, Monroe M, Cui H. Self-Assembling Supramolecular Nanostructures for Drug Delivery. Soft Nanomater. 2018;19:1–25.
4. Liu Y, Wu Y, Luo Z, Li M. Designing supramolecular self-assembly nanomaterials as stimuli-responsive drug delivery platforms for cancer therapy. iScience. 2023;26(3):106279. doi:10.1016/j.isci.2023.106279
5. Harish V, Tewari D, Gaur M, et al. Review on Nanoparticles and Nanostructured Materials: bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials. 2022;12(3):457. doi:10.3390/nano12030457
6. Zhu Y, Liao L. Applications of nanoparticles for anticancer drug delivery: a review. J Nanosci Nanotechnol. 2015;15(7):4753–4773. doi:10.1166/jnn.2015.10298
7. Yadav S, Sharma AK, Kumar P. Nanoscale Self-Assembly for Therapeutic Delivery. Review. Front Bioeng Biiotechnol. 2020;8:127. doi:10.3389/fbioe.2020.00127
8. Messina PV, Besada-Porto JM, Ruso JM. Self-assembly drugs: from micelles to nanomedicine. Curr Top Med Chem. 2014;14(5):555–571. doi:10.2174/1568026614666140121112118
9. Müller RH, Gohla S, Keck CM. State of the art of nanocrystals–special features, production, nanotoxicology aspects and intracellular delivery. Eur J Pharm Biopharm. 2011;78(1):1–9. doi:10.1016/j.ejpb.2011.01.007
10. El-Masry TA, El-Nagar MMF, Oriquat GA, et al. Therapeutic efficiency of Tamoxifen/Orlistat nanocrystals against solid Ehrlich carcinoma via targeting TXNIP/HIF1-α/MMP-9/P27 and BAX/Bcl2/P53 signaling pathways. Biomed Pharmacother. 2024;180:117429. doi:10.1016/j.biopha.2024.117429
11. Alherz FA, El-Masry TA, Oriquat GA, et al. Hesperidin Nanoformulation: a Potential Strategy for Reducing Doxorubicin-Induced Renal Damage via the Sirt-1/HIF1-α/VEGF/NF-κB Signaling Cascade. Pharmaceuticals. 2024;17(9):1144. doi:10.3390/ph17091144
12. Mahoney A, Evans J. Comparing drug classification systems. AMIA Annu Symp Proc. 2008;1039.
13. Tian X, Wang P, Li T, et al. Self-assembled natural phytochemicals for synergistically antibacterial application from the enlightenment of traditional Chinese medicine combination. Acta Pharm Sin B. 2020;10(9):1784–1795. doi:10.1016/j.apsb.2019.12.014
14. Kashapov R, Gaynanova G, Gabdrakhmanov D, et al. Self-assembly of amphiphilic compounds as a versatile tool for construction of nanoscale drug carriers. Int J mol Sci. 2020;21(18):6961. doi:10.3390/ijms21186961
15. Xu X-Q, Xu X, Wang Y, Li Z-R, Han C. Self-assembled Natural Product-based Carrier-free Nanoplatforms for Efficient Bioactivity. J Explor Res in Pharmacol. 2022. doi:10.14218/jerp.2022.00050
16. Zhang A, Meng K, Liu Y, et al. Absorption, distribution, metabolism, and excretion of nanocarriers in vivo and their influences. Adv Colloid Interface Sci. 2020;284:102261. doi:10.1016/j.cis.2020.102261
17. Lombardi L, Falanga A, Del Genio V, Galdiero S. A New Hope: self-Assembling Peptides with Antimicrobial Activity. Pharmaceutics. 2019;11(4):166. doi:10.3390/pharmaceutics11040166
18. Nsairat H, Mahmoud IS, Odeh F, et al. Grafting of anti-nucleolin aptamer into preformed and remotely loaded liposomes through aptamer-cholesterol post-insertion. RSC Adv. 2020;10(59):36219–36229. doi:10.1039/D0RA07325C
19. Yan S, Na J, Liu X, Wu P. Different Targeting Ligands-Mediated Drug Delivery Systems for Tumor Therapy. Pharmaceutics. 2024;16(2):248. doi:10.3390/pharmaceutics16020248
20. Prajapati A, Rangra S, Patil R, et al. Receptor-Targeted Nanomedicine for Cancer Therapy. Receptors. 2024;3(3):323–361. doi:10.3390/receptors3030016
21. Shcherbina M, Chvalun S. Driving Forces of the Self-Assembly of Supramolecular Systems: partially Ordered Mesophases. Russ J Phys Chem A. 2018;92(6):1161–1170. doi:10.1134/S003602441806016X
22. He Y, Tang Y, Zhang Y, et al. Driving forces and molecular interactions in the self-assembly of block copolymers to form fiber-like micelles. Appl Phys Rev. 2022;9(2):1.
23. Mendes AC, Baran ET, Reis RL, Azevedo HS. Self‐assembly in nature: using the principles of nature to create complex nanobiomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(6):582–612. doi:10.1002/wnan.1238
24. Cadena-Nava RD, Comas-Garcia M, Garmann RF, Rao AL, Knobler CM, Gelbart WM. Self-assembly of viral capsid protein and RNA molecules of different sizes: requirement for a specific high protein/RNA mass ratio. J Virol. 2012;86(6):3318–3326. doi:10.1128/jvi.06566-11
25. Pokroy B, Epstein AK, Persson‐Gulda MC, Aizenberg J. Fabrication of bioinspired actuated nanostructures with arbitrary geometry and stiffness. Adv Mater. 2009;21(4):463–469. doi:10.1002/adma.200801432
26. Fernández-Rico C, Schreiber S, Oudich H, et al. Elastic microphase separation produces robust bicontinuous materials. Nature Mater. 2023;2023:1–7.
27. Pérez-Luna VH, González-Reynoso O. Encapsulation of Biological Agents in Hydrogels for Therapeutic Applications. Gels. 2018;4(3):61. doi:10.3390/gels4030061
28. Adamson AW, Gast AP. Physical Chemistry of Surfaces. Interscience publishers New York. 1967;Vol. 150.
29. Perumal S, Atchudan R, Lee W. A Review of Polymeric Micelles and Their Applications. Polymers. 2022;14(12):2510. doi:10.3390/polym14122510
30. Varma LT, Singh N, Gorain B, et al. Recent Advances in Self-Assembled Nanoparticles for Drug Delivery. Curr Drug Deliv. 2020;17(4):279–291. doi:10.2174/1567201817666200210122340
31. Wu J, Svensen N, Song W, et al. Self-Assembly of Intracellular Multivalent RNA Complexes Using Dimeric Corn and Beetroot Aptamers. J Am Chem Soc. 2022;144(12):5471–5477. doi:10.1021/jacs.1c13583
32. Cheng D, Jia F, Jiang Y-B, Conticello VP, Jiang T. Assembly of peptide nanostructures with controllable sizes. Nano Res. 2023;17(1):151–161. doi:10.1007/s12274-023-5970-x
33. Bergström LM. Thermodynamics of self-assembly. Appl Thermodynam Biological Mater Sci. 2011;11:289–314.
34. Weng J, Yang M, Wang W, Xu X, aZ T. Revealing Thermodynamics and Kinetics of Lipid Self-Assembly by Markov State Model Analysis. J Am Chem Soc. 2020;142(51):21344–21352. doi:10.1021/jacs.0c09343
35. Wang B, Zhang Y, Guo Z, Zhang L. Self-assembly of nanoparticles: static and dynamic. Mater Today. 2019;25:112–113. doi:10.1016/j.mattod.2019.04.001
36. Zhang J. Amphiphilic Membrane. In: Drioli E, Giorno L, editors. Encyclopedia of Membranes. Berlin Heidelberg: Springer; 2016:69–72.
37. Cheng X, Xie Q, Sun Y. Advances in nanomaterial-based targeted drug delivery systems. Front Bioeng Biotechnol. 2023;11:1177151. doi:10.3389/fbioe.2023.1177151
38. Shakiba A, Zenasni O, D. marquez M, Randall Lee T. Advanced drug delivery via self-assembled monolayer-coated nanoparticles. AIMS Bioengineering. 2017;4(2):275–299. doi:10.3934/bioeng.2017.2.275
39. Fu S, Li G, Zang W, Zhou X, Shi K, Zhai Y. Pure drug nano-assemblies: a facile carrier-free nanoplatform for efficient cancer therapy. Acta Pharm Sin B. 2022;12(1):92–106. doi:10.1016/j.apsb.2021.08.012
40. Efthymiou C, Bergström LM, Pedersen JN, Pedersen JS, Hansson P. Self-assembling properties of ionisable amphiphilic drugs in aqueous solution. J Colloid Interface Sci. 2021;600:701–710. doi:10.1016/j.jcis.2021.05.049
41. Fan Q, Ji Y, Wang J, et al. Self-assembly behaviours of peptide-drug conjugates: influence of multiple factors on aggregate morphology and potential self-assembly mechanism. R Soc Open Sci. 2018;5(4):172040. doi:10.1098/rsos.172040
42. Bellissent-Funel M-C. Hydrophilic–hydrophobic interplay: from model systems to living systems. CR Geosci. 2005;337(1):173–179. doi:10.1016/j.crte.2004.10.011
43. Lowry GV, Hill RJ, Harper S, et al. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ Sci Nano. 2016;3(5):953–965.
44. Al-Azzawi H, Alshaer W, Esawi E, et al. Multifunctional nanoparticles recruiting hyaluronic acid ligand and polyplexes containing low molecular weight protamine and ATP-Sensitive DNA motif for doxorubicin delivery. J Drug Delivery Sci Technol. 2022;69:103169. doi:10.1016/j.jddst.2022.103169
45. Mirtajaddini SA, Fathi Najafi M, Vaziri Yazdi SA, Kazemi Oskuee R. Preparation of Chitosan Nanoparticles as a Capable Carrier for Antigen Delivery and Antibody Production. Iran J Biotechnol. 2021;19(4):e2871. doi:10.30498/ijb.2021.247747.2871
46. Esquivel-Sirvent R. Finite-Size Effects of Casimir–van der Waals Forces in the Self-Assembly of Nanoparticles. Physics. 2023;5(1):322–330. doi:10.3390/physics5010024
47. Misra C, Paul RK, Thotakura N, Raza K. Chapter 8 - Biodegradable self-assembled nanocarriers as the drug delivery vehicles. In: Kesharwani P, Singh KK, editors. Nanoparticle Therapeutics. Academic Press; 2022:293–325.
48. Som A, Griffo A, Chakraborty I, et al. Strong and Elastic Membranes via Hydrogen Bonding Directed Self‐Assembly of Atomically Precise Nanoclusters. Small. 2022;18(34):2201707. doi:10.1002/smll.202201707
49. Habibi N, Kamaly N, Memic A, Shafiee H. Self-assembled peptide-based nanostructures: smart nanomaterials toward targeted drug delivery. Nano Today. 2016;11(1):41–60. doi:10.1016/j.nantod.2016.02.004
50. Wang N, Yu C, Xu T, et al. Self-assembly of DNA nanostructure containing cell-specific aptamer as a precise drug delivery system for cancer therapy in non-small cell lung cancer. J Nanobiotechnol. 2022;20(1):486. doi:10.1186/s12951-022-01701-5
51. Liang Y, Sun Y, Fu X, et al. The effect of π-conjugation on the self-assembly of micelles and controlled cargo release. Artif Cells Nanomed Biotechnol. 2020;48(1):525–532. doi:10.1080/21691401.2020.1725028
52. Profit AA, Felsen V, Chinwong J, Mojica ER, Desamero RZ. Evidence of π-stacking interactions in the self-assembly of hIAPP(22-29). Proteins. 2013;81(4):690–703. doi:10.1002/prot.24229
53. Roy SG, De P. pH responsive polymers with amino acids in the side chains and their potential applications. J Appl Polym Sci. 2014;131(20). doi:10.1002/app.41084
54. Shaibie NA, Ramli NA, Mohammad Faizal NDF, Srichana T, Mohd Amin MCI. Poly (N‐isopropylacrylamide)‐Based Polymers: recent Overview for the Development of Temperature‐Responsive Drug Delivery and Biomedical Applications. Macromol Chem Phys. 2023;224(20):2300157. doi:10.1002/macp.202300157
55. Kajbafvala A, Bahmanpour H, Maneshian MH, Li M. Self-Assembly Techniques for Nanofabrication. J Nanomater. 2013;2013(1):158517. doi:10.1155/2013/158517
56. Deshmukh R, Wagh P, Naik J. Solvent evaporation and spray drying technique for micro-and nanospheres/particles preparation: a review. Drying Technol. 2016;34(15):1758–1772. doi:10.1080/07373937.2016.1232271
57. Xiang B, Cao D-Y. Preparation of drug liposomes by thin-film hydration and homogenization. Liposome-Based Drug Delivery Systems. 2021;2021:25–35.
58. Kim S, Huang J, Lee Y, et al. Complexation and coacervation of like-charged polyelectrolytes inspired by mussels. Proc Natl Acad Sci. 2016;113(7):E847–E853.
59. Sing CE, Perry SL. Recent progress in the science of complex coacervation. Soft Matter. 2020;16(12):2885–2914. doi:10.1039/d0sm00001a
60. Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022;8(5):e09394. doi:10.1016/j.heliyon.2022.e09394
61. Sawant A, Kamath S, H G, Kulyadi G. Solid-in-Oil-in-Water Emulsion: an Innovative Paradigm to Improve Drug Stability and Biological Activity. AAPS Pharm Sci Tech. 2021;22(5). doi:10.1208/s12249-021-02074-y
62. Jo DH, Kim JH, Lee TG, Kim JH. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed Nanotechnol Biol Med. 2015;11(7):1603–1611. doi:10.1016/j.nano.2015.04.015
63. Kobayashi K, Wei J, Iida R, Ijiro K, Niikura K. Surface engineering of nanoparticles for therapeutic applications. Polym J. 2014;46(8):460–468. doi:10.1038/pj.2014.40
64. Pandey RP, Vidic J, Mukherjee R, Chang CM. Experimental Methods for the Biological Evaluation of Nanoparticle-Based Drug Delivery Risks. Pharmaceutics. 2023;15(2):612. doi:10.3390/pharmaceutics15020612
65. Chishti N, Jagwani S, Dhamecha D, Jalalpure S, Dehghan MH. Preparation, optimization, and in vivo evaluation of nanoparticle-based formulation for pulmonary delivery of anticancer drug. Medicina. 2019;55(6):294. doi:10.3390/medicina55060294
66. Bashir S, Hina M, Iqbal J, et al. Fundamental concepts of hydrogels: synthesis, properties, and their applications. Polymers. 2020;12(11):2702. doi:10.3390/polym12112702
67. Augenstreich J, Haanappel E, Ferré G, et al. The conical shape of DIM lipids promotes Mycobacterium tuberculosis infection of macrophages. Proc Natl Acad Sci U S A. 2019;116(51):25649. doi:10.1073/pnas.1910368116
68. Ridolfo R, Ede BC, Diamanti P, et al. Biodegradable, Drug-Loaded Nanovectors via Direct Hydration as a New Platform for Cancer Therapeutics. Small. 2018;14(32):1. doi:10.1002/smll.201703774
69. Wu J. The Enhanced Permeability and Retention (EPR) Effect: the Significance of the Concept and Methods to Enhance Its Application. J Personal Med. 2021;11(8):771. doi:10.3390/jpm11080771
70. Ridolfo R, Tavakoli S, Junnuthula V, Williams DS, Urtti A, van Hest JCM. Exploring the Impact of Morphology on the Properties of Biodegradable Nanoparticles and Their Diffusion in Complex Biological Medium. Biomacromolecules. 2021;22(1):126–133. doi:10.1021/acs.biomac.0c00726
71. Shariare MH, Khan MA, Al-Masum A, Khan JH, Uddin J, Kazi M. Development of Stable Liposomal Drug Delivery System of Thymoquinone and Its In Vitro Anticancer Studies Using Breast Cancer and Cervical Cancer Cell Lines. Molecules. 2022;27(19):6744. doi:10.3390/molecules27196744
72. Wang Y. Stability of Self-Assembling Drug Amphiphiles in Biological Environments. Johns Hopkins University; 2017.
73. Doole FT, Camp CP, Kim M. Tailoring the formation and stability of self-assembled structures from precisely engineered intrinsically disordered protein polymers: a comprehensive review. Giant. 2023;14:100158. doi:10.1016/j.giant.2023.100158
74. Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnol. 2007;2(12):751–760. doi:10.1038/nnano.2007.387
75. Hartshorn CM, Bradbury MS, Lanza GM, et al. Nanotechnology Strategies To Advance Outcomes in Clinical Cancer Care. ACS Nano. 2018;12(1):24–43. doi:10.1021/acsnano.7b05108
76. Odeh F, Nsairat H, Alshaer W, et al. Remote loading of curcumin-in-modified β-cyclodextrins into liposomes using a transmembrane pH gradient. RSC Adv. 2019;9(64):37148–37161. doi:10.1039/C9RA07560G
77. Nsairat H, Alshaer W, Odeh F, et al. Recent advances in using liposomes for delivery of nucleic acid-based therapeutics. OpenNano. 2023;11:100132. doi:10.1016/j.onano.2023.100132
78. Aljabali AAA, Obeid MA, Bakshi HA, et al. Synthesis, Characterization, and Assessment of Anti-Cancer Potential of ZnO Nanoparticles in an In Vitro Model of Breast Cancer. Molecules. 2022;27(6):1827. doi:10.3390/molecules27061827
79. Alshaer W, Nsairat H, Lafi Z, et al. Quality by Design Approach in Liposomal Formulations: robust Product Development. Molecules. 2023;28(1):10. doi:10.3390/molecules28010010
80. Khater D, Nsairat H, Odeh F, et al. Design, Preparation, and Characterization of Effective Dermal and Transdermal Lipid Nanoparticles: a Review. Cosmetics. 2021;8(2):39. doi:10.3390/cosmetics8020039
81. Bartucci R, Paramanandana A, Boersma YL, Olinga P, Salvati A. Comparative study of nanoparticle uptake and impact in murine lung, liver and kidney tissue slices. Nanotoxicology. 2020;14(6):847–865. doi:10.1080/17435390.2020.1771785
82. Dong C, Wu J, Chen Y, Nie J, Chen C. Activation of PI3K/AKT/mTOR Pathway Causes Drug Resistance in Breast Cancer. Front Pharmacol. 2021;12:628690. doi:10.3389/fphar.2021.628690
83. Lu Z-R, Qiao P. Drug Delivery in Cancer Therapy, Quo Vadis? Mol Pharmaceut. 2018;15(9):3603–3616. doi:10.1021/acs.molpharmaceut.8b00037
84. Rodríguez-Nogales C, Sebastián V, Irusta S, Desmaële D, Couvreur P, Blanco-Prieto MJ. A unique multidrug nanomedicine made of squalenoyl-gemcitabine and alkyl-lysophospholipid edelfosine. Eur J Pharm Biopharm. 2019;144:165–173. doi:10.1016/j.ejpb.2019.09.017
85. Ma W, Cheetham AG, Cui H. Building Nanostructures with Drugs. Nano Today. 2016;11(1):13–30. doi:10.1016/j.nantod.2015.11.003
86. Din FU, Aman W, Ullah I, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed. 2017;12:7291–7309. doi:10.2147/IJN.S146315
87. Xi J, Liu H. Recent Advances in the Design of Self‐Delivery Amphiphilic Drugs and Vaccines. Adv Ther. 2019;3(2):1900107. doi:10.1002/adtp.201900107
88. Hauser EA, Marlowe GJ. Colloidal phenomena of antibiotics. J Phys Colloid Chem. 1950;54(8):1077–1087. doi:10.1021/j150482a001
89. Thakkar AL, Wilham WL. Self-association of benzylpenicillin in aqueous solution: 1H nuclear magnetic resonance study. J Chem Soc D Chem Commun. 1971;7(7):320–322. doi:10.1039/c29710000320
90. Larsen MT, Kuhlmann M, Hvam ML, Howard KA. Albumin-based drug delivery: harnessing nature to cure disease. mol Cell Ther. 2016;4(1):3. doi:10.1186/s40591-016-0048-8
91. Sabnis N, Lacko AG. Drug delivery via lipoprotein-based carriers: answering the challenges in systemic therapeutics. Ther Deliv. 2012;3(5):599–608. doi:10.4155/tde.12.41
92. Barbosa LRS, Itri R, Caetano W, de Sousa Neto D, Tabak M. Self-Assembling of Phenothiazine Compounds Investigated by Small-Angle X-ray Scattering and Electron Paramagnetic Resonance Spectroscopy. J Phys Chem A. 2008;112(14):4261–4269. doi:10.1021/jp710332t
93. Nsairat H, Al-Sulaibi M, Alshaer W. PEGylated nanoassemblies composed of edelfosine and fulvestrant drugs: in vitro antiproliferative effect against breast cancer cells. J Drug Delivery Sci Technol. 2023;85:104612. doi:10.1016/j.jddst.2023.104612
94. Fan T, Yu X, Shen B, Sun L. Peptide Self-Assembled Nanostructures for Drug Delivery Applications. J Nanomater. 2017;2017:1–16. doi:10.1155/2017/4562474
95. Gupta N, Ansari A, Dhoke GV, et al. Computationally designed antibody-drug conjugates self-assembled via affinity ligands. Nat Biomed Eng. 2019;3(11):917–929. doi:10.1038/s41551-019-0470-8
96. Fumagalli G, Marucci C, Christodoulou MS, Stella B, Dosio F, Passarella D. Self-assembly drug conjugates for anticancer treatment. Drug Discov Today. 2016;21(8):1321–1329. doi:10.1016/j.drudis.2016.06.018
97. Li J, Li X, Liu P. Doxorubicin-doxorubicin conjugate prodrug as drug self-delivery system for intracellular pH-triggered slow release. Colloids Surf B. 2020;185:110608. doi:10.1016/j.colsurfb.2019.110608
98. Zhou XQ, Wang P, Ramu V, et al. In vivo metallophilic self-assembly of a light-activated anticancer drug. Nat Chem. 2023;15(7):980–987. doi:10.1038/s41557-023-01199-w
99. Liu H, Li Y, Lyu Z, et al. Enzyme-triggered supramolecular self-assembly of platinum prodrug with enhanced tumor-selective accumulation and reduced systemic toxicity. 10.1039/C4TB01563K. J Mater Chem B. 2014;2(47):8303–8309. doi:10.1039/C4TB01563K
100. Wang Z, Zhuang M, Sun T, Wang X, Xie Z. Self-assembly of glutamic acid linked paclitaxel dimers into nanoparticles for chemotherapy. Bioorg Med Chem Lett. 2017;27(11):2493–2496. doi:10.1016/j.bmcl.2017.03.101
101. Shim MK, Moon Y, Yang S, et al. Cancer-specific drug-drug nanoparticles of pro-apoptotic and cathepsin B-cleavable peptide-conjugated doxorubicin for drug-resistant cancer therapy. Biomaterials. 2020;261:120347. doi:10.1016/j.biomaterials.2020.120347
102. Wang Y, Wang X, Deng F, et al. The effect of linkers on the self-assembling and anti-tumor efficacy of disulfide-linked doxorubicin drug-drug conjugate nanoparticles. J Control Rel. 2018;279:136–146. doi:10.1016/j.jconrel.2018.04.019
103. Song Q, Wang X, Wang Y, et al. Reduction Responsive Self-Assembled Nanoparticles Based on Disulfide-Linked Drug-Drug Conjugate with High Drug Loading and Antitumor Efficacy. Mol Pharm. 2016;13(1):190–201. doi:10.1021/acs.molpharmaceut.5b00631
104. Fumagalli G, Stella B, Pastushenko I, et al. Heteronanoparticles by self-Assembly of Doxorubicin and Cyclopamine Conjugates. ACS Med Chem Lett. 2017;8(9):953–957. doi:10.1021/acsmedchemlett.7b00262
105. Pei Q, Hu X, Li Z, Xie Z, Jing X. Small molecular nanomedicines made from a camptothecin dimer containing a disulfide bond. 10.1039/C5RA18586F. RSC Adv. 2015;5(99):81499–81501. doi:10.1039/C5RA18586F
106. Ma W, Su H, Cheetham AG, et al. Synergistic antitumor activity of a self-assembling camptothecin and capecitabine hybrid prodrug for improved efficacy. J Control Release. 2017;263:102–111. doi:10.1016/j.jconrel.2017.01.015
107. Huang P, Wang D, Su Y, et al. Combination of small molecule prodrug and nanodrug delivery: amphiphilic drug-drug conjugate for cancer therapy. J Am Chem Soc. 2014;136(33):11748–11756. doi:10.1021/ja505212y
108. Fan M, Liang X, Li Z, Wang H, Yang D, Shi B. Chlorambucil gemcitabine conjugate nanomedicine for cancer therapy. Eur J Pharm Sci. 2015;79:20–26. doi:10.1016/j.ejps.2015.08.013
109. Duhem N, Danhier F, Pourcelle V, et al. Self-assembling doxorubicin-tocopherol succinate prodrug as a new drug delivery system: synthesis, characterization, and in vitro and in vivo anticancer activity. Bioconjug Chem. 2014;25(1):72–81. doi:10.1021/bc400326y
110. Ahmad Khan MS, Ahmad I. Chapter 1 - Herbal Medicine: current Trends and Future Prospects. In: Ahmad Khan MS, Ahmad I, Chattopadhyay D, editors. New Look to Phytomedicine. Academic Press; 2019:3–13.
111. Chopra B, Dhingra AK. Natural products: a lead for drug discovery and development. Phytother Res. 2021;35(9):4660–4702. doi:10.1002/ptr.7099
112. Salman Ul I, Ahmed BM, Mazhar U-I, Shehzad A, Lee SY. Switching from Conventional to Nano-natural Phytochemicals to Prevent and Treat Cancers: special Emphasis on Resveratrol. Curr Pharm Des. 2019;25(34):3620–3632. doi:10.2174/1381612825666191009161018
113. Bag BG, Majumdar R. Self-assembly of Renewable Nano-sized Triterpenoids. Chem Rec. 2017;17(9):841–873. doi:10.1002/tcr.201600123
114. Kellett K, Kantonen SA, Duggan BM, Gilson MK. Toward Expanded Diversity of Host–Guest Interactions via Synthesis and Characterization of Cyclodextrin Derivatives. J Solution Chem. 2018;47(10):1597–1608. doi:10.1007/s10953-018-0769-1
115. Wang Z, Chen J, Little N, Lu J. Self-assembling prodrug nanotherapeutics for synergistic tumor targeted drug delivery. Acta Biomater. 2020;111:20–28. doi:10.1016/j.actbio.2020.05.026
116. Whitesides GM, Grzybowski B. Self-Assembly at All Scales. Science. 2002;295(5564):2418–2421. doi:10.1126/science.1070821
117. Rybtchinski B. Adaptive Supramolecular Nanomaterials Based on Strong Noncovalent Interactions. ACS Nano. 2011;5(9):6791–6818. doi:10.1021/nn2025397
118. Cekan P, Hasegawa K, Pan Y, et al. RCC1-dependent activation of Ran accelerates cell cycle and DNA repair, inhibiting DNA damage-induced cell senescence. mol Biol Cell. 2016;27(8):1346–1357. doi:10.1091/mbc.E16-01-0025
119. Tholl D. Biosynthesis and Biological Functions of Terpenoids in Plants. In: Schrader J, Bohlmann J, editors. Biotechnology of Isoprenoids. Springer International Publishing; 2015:63–106.
120. Kim J, Lee J, Lee YM, Pramanick S, Im S, Kim WJ. Andrographolide-loaded polymerized phenylboronic acid nanoconstruct for stimuli-responsive chemotherapy. J Control Release. 2017;259:203–211. doi:10.1016/j.jconrel.2016.10.029
121. Li Y, Wang LF, Wang JL, Tu PF. Research on preparation process of andrographolide-glycyrrhizic acid polymeric micelles]. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi =. China J Chinese Materia Medica. 2018;43(1):79–85. doi:10.19540/j.cnki.cjcmm.20171107.001
122. Dai L, Liu K, Si C, et al. Ginsenoside nanoparticle: a new green drug delivery system. 10.1039/C5TB02305J. J Mater Chem B. 2016;4(3):529–538. doi:10.1039/C5TB02305J
123. Cheng J, Zhao H, Yao L, et al. Simple and Multifunctional Natural Self-Assembled Sterols with Anticancer Activity-Mediated Supramolecular Photosensitizers for Enhanced Antitumor Photodynamic Therapy. ACS Appl Mater Interfaces. 2019;11(33):29498–29511. doi:10.1021/acsami.9b07404
124. Andreani T, Cheng R, Elbadri K, et al. Natural compounds-based nanomedicines for cancer treatment: future directions and challenges. Drug Delivery Transl Res. 2024;14(10):2845–2916. doi:10.1007/s13346-024-01649-z
125. Lv Y, Li W, Liao W, et al. Nano-Drug Delivery Systems Based on Natural Products. Int j Nanomed. 2024;19:541–569. doi:10.2147/ijn.S443692
126. Naumenko E, Guryanov I, Gomzikova M. Drug Delivery Nano-Platforms for Advanced Cancer Therapy. Scientia Pharmaceutica. 2024;92(2):28. doi:10.3390/scipharm92020028
127. Li L, Chang L, Zhang X, et al. Berberine and its structural analogs have differing effects on functional profiles of individual gut microbiomes. Gut Microbes. 2020;11(5):1348–1361. doi:10.1080/19490976.2020.1755413
128. Huang X, Wang P, Li T, et al. Self-Assemblies Based on Traditional Medicine Berberine and Cinnamic Acid for Adhesion-Induced Inhibition Multidrug-Resistant Staphylococcus aureus. ACS Appl Mater Interfaces. 2020;12(1):227–237. doi:10.1021/acsami.9b17722
129. Enriquez-Navas PM, Kam Y, Das T, et al. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci Trans Med. 2016;8(327):327ra24–327ra24. doi:10.1126/scitranslmed.aad7842
130. Pienta KJ, Smith DC. Advances in Prostate Cancer Chemotherapy: a New Era Begins1. Ca a Cancer J Clinicians. 2005;55(5):300–318. doi:10.3322/canjclin.55.5.300
131. Cheng Y, Ji Y. Mitochondria-targeting nanomedicine self-assembled from GSH-responsive paclitaxel-ss-berberine conjugate for synergetic cancer treatment with enhanced cytotoxicity. J Control Release. 2020;318:38–49. doi:10.1016/j.jconrel.2019.12.011
132. Jiang M, Zhang R, Wang Y, et al. Reduction-sensitive Paclitaxel Prodrug Self-assembled Nanoparticles with Tetrandrine Effectively Promote Synergistic Therapy Against Drug-sensitive and Multidrug-resistant Breast Cancer. Mol Pharmaceut. 2017;14(11):3628–3635. doi:10.1021/acs.molpharmaceut.7b00381
133. Zhao Y, Chen F, Pan Y, et al. Nanodrug Formed by Coassembly of Dual Anticancer Drugs to Inhibit Cancer Cell Drug Resistance. ACS Appl Mater Interfaces. 2015;7(34):19295–19305. doi:10.1021/acsami.5b05347
134. Xu P, Marsafari M, Zha J, Koffas M. Microbial Coculture for Flavonoid Synthesis. Trends Biotechnol. 2020;38(7):686–688. doi:10.1016/j.tibtech.2020.01.008
135. Zhao Q, Zhang Y, Wang G, et al. A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellaria baicalensis. Sci Adv. 2016;2(4):e1501780. doi:10.1126/sciadv.1501780
136. Li T, Wang P, Guo W, et al. Natural Berberine-Based Chinese Herb Medicine Assembled Nanostructures with Modified Antibacterial Application. ACS Nano. 2019;13(6):6770–6781. doi:10.1021/acsnano.9b01346
137. Ying H-Z, Yu C-H, Chen H-K, et al. Quinonoids: therapeutic Potential for Lung Cancer Treatment. Biomed Res Int. 2020;2020(1):2460565. doi:10.1155/2020/2460565
138. Zheng J, Fan R, Wu H, et al. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nat Commun. 2019;10(1):1604. doi:10.1038/s41467-019-09601-3
139. Zhang C, Wu J, Liu W, Zheng X, Wang P. Natural-Origin Hypocrellin-HSA Assembly for Highly Efficient NIR Light-Responsive Phototheranostics against Hypoxic Tumors. ACS Appl Mater Interfaces. 2019;11(48):44989–44998. doi:10.1021/acsami.9b18345
140. Yang J, Han S, Zheng H, Dong H, Liu J. Preparation and application of micro/nanoparticles based on natural polysaccharides. Carbohydr Polym. 2015;123:53–66. doi:10.1016/j.carbpol.2015.01.029
141. Yu Y, Shen M, Song Q, Xie J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review. Carbohydr Polym. 2018;183:91–101. doi:10.1016/j.carbpol.2017.12.009
142. Xing R, Liu Y, Zou Q, Yan X. Self-assembled injectable biomolecular hydrogels towards phototherapy. Nanoscale. 2019;11(46):22182–22195. doi:10.1039/C9NR06266A
143. Allen C, Yu Y, Maysinger D, Eisenberg A. Polycaprolactone-b-poly(ethylene Oxide) Block Copolymer Micelles as a Novel Drug Delivery Vehicle for Neurotrophic Agents FK506 and L-685,818. Bioconjugate Chem. 1998;9(5):564–572. doi:10.1021/bc9702157
144. Zhang L, Li Y, Wang C, Li G, Zhao Y, Yang Y. Synthesis of methylprednisolone loaded ibuprofen modified inulin based nanoparticles and their application for drug delivery. Mater Sci Eng C. 2014;42:111–115. doi:10.1016/j.msec.2014.05.025
145. Fares MM, Salem Mt S. Dissolution enhancement of curcumin via curcumin–prebiotic inulin nanoparticles. Drug Dev Ind Pharm. 2015;41(11):1785–1792. doi:10.3109/03639045.2015.1004184
146. Jiménez-Sánchez M, Pérez-Morales R, Goycoolea FM, et al. Self-assembled high molecular weight inulin nanoparticles: enzymatic synthesis, physicochemical and biological properties. Carbohydr Polym. 2019;215:160–169. doi:10.1016/j.carbpol.2019.03.060
147. Liu Y, Zheng D, Ma Y, et al. Self-Assembled Nanoparticles Platform Based on Pectin-Dihydroartemisinin Conjugates for Codelivery of Anticancer Drugs. ACS Biomater Sci Eng. 2018;4(5):1641–1650. doi:10.1021/acsbiomaterials.7b00842
148. Gazzali AM, Lobry M, Colombeau L, et al. Stability of folic acid under several parameters. Eur J Pharm Sci. 2016;93:419–430. doi:10.1016/j.ejps.2016.08.045
149. Misra R, Mohanty S. Self-assembled liquid-crystalline folate nanoparticles for in vitro controlled release of doxorubicin. Biomed Pharmacother. 2015;69:326–336. doi:10.1016/j.biopha.2014.12.015
150. Wang D, Chen W, Li H, et al. Folate-receptor mediated pH/reduction-responsive biomimetic nanoparticles for dually activated multi-stage anticancer drug delivery. Int J Pharm. 2020;585:119456. doi:10.1016/j.ijpharm.2020.119456
151. Zhang M, Liu M, Prest H, Fischer S. Nanoparticles Secreted from Ivy Rootlets for Surface Climbing. Nano Lett. 2008;8(5):1277–1280. doi:10.1021/nl0725704
152. La Manna S, Di Natale C, Onesto V, Marasco D. Self-Assembling Peptides: from Design to Biomedical Applications. Int J mol Sci. 2021;22(23):12662. doi:10.3390/ijms222312662
153. Gelain F, Luo Z, Zhang S. Self-Assembling Peptide EAK16 and RADA16 Nanofiber Scaffold Hydrogel. Chem Rev. 2020;120(24):13434–13460. doi:10.1021/acs.chemrev.0c00690
154. Zhang S. Self-assembling peptides: from a discovery in a yeast protein to diverse uses and beyond. Protein Sci. 2020;29(11):2281–2303. doi:10.1002/pro.3951
155. Yang Z, Xu H, Zhao X. Designer Self-Assembling Peptide Hydrogels to Engineer 3D Cell Microenvironments for Cell Constructs Formation and Precise Oncology Remodeling in Ovarian Cancer. Adv Sci. 2020;7(9):1903718. doi:10.1002/advs.201903718
156. Li T, Lu X-M, Zhang M-R, Hu K, Li Z. Peptide-based nanomaterials: self-assembly, properties and applications. Bioact Mater. 2022;11:268–282. doi:10.1016/j.bioactmat.2021.09.029
157. Wang R, Wang Z, Guo Y, Li H, Chen Z. Design of a RADA16-based self-assembling peptide nanofiber scaffold for biomedical applications. Journal of Biomaterials Science, Polymer Edition. 2019;30(9):713–736. doi:10.1080/09205063.2019.1605868
158. Sankar S, O’Neill K, Bagot d’arc M, et al. Clinical Use of the Self-Assembling Peptide RADA16: a Review of Current and Future Trends in Biomedicine. Front Bioeng Biotechnol. 2021;9:679525. doi:10.3389/fbioe.2021.679525
159. Yao X, Hu Y, Lin M, et al. Self-assembling peptide RADA16: a promising scaffold for tissue engineering and regenerative medicine. Nanomedicine. 2023;18(19):1305–1326. doi:10.2217/nnm-2023-0161
160. Zhu D, Zhang H, Huang Y, et al. A Self-Assembling Amphiphilic Peptide Dendrimer-Based Drug Delivery System for Cancer Therapy. Pharmaceutics. 2021;13(7):1092. doi:10.3390/pharmaceutics13071092
161. Zhao C, Chen H, Wang F, Zhang X. Amphiphilic self-assembly peptides: rational strategies to design and delivery for drugs in biomedical applications. Colloids Surf B Biointerfaces. 2021;208:112040. doi:10.1016/j.colsurfb.2021.112040
162. Cheetham AG, Lin YA, Lin R, Cui H. Molecular design and synthesis of self-assembling camptothecin drug amphiphiles. Acta Pharmacol Sin. 2017;38(6):874–884. doi:10.1038/aps.2016.151
163. Kim J, Narayana A, Patel S, Sahay G. Advances in intracellular delivery through supramolecular self-assembly of oligonucleotides and peptides. Theranostics. 2019;9(11):3191–3212. doi:10.7150/thno.33921
164. Halley PD, Patton RA, Chowdhury A, Byrd JC, Castro CE. Low-cost, simple, and scalable self-assembly of DNA origami nanostructures. Nano Res. 2019;12(5):1207–1215. doi:10.1007/s12274-019-2384-x
165. Chen K, Zhang Y, Zhu L, et al. Insights into nucleic acid-based self-assembling nanocarriers for targeted drug delivery and controlled drug release. J Control Release. 2022;341:869–891. doi:10.1016/j.jconrel.2021.12.020
166. Xu F, Xia Q, Wang P. Rationally Designed DNA Nanostructures for Drug Delivery. Front Chem. 2020;8:751. doi:10.3389/fchem.2020.00751
167. Abbas M, Baig MMFA, Zhang Y, et al. RETRACTED: a DNA-based nanocarrier for efficient cancer therapy. J Pharm Anal. 2021;11(3):330–339. doi:10.1016/j.jpha.2020.03.005
168. Bathe M, Rothemund P. DNA Nanotechnology: a foundation for Programmable Nanoscale Materials. MRS Bulletin. 2017;42(12):882–888. doi:10.1557/mrs.2017.279
169. Lam JKW, Chow MYT, Zhang Y, Leung SWS. siRNA Versus miRNA as Therapeutics for Gene Silencing. mol Ther Nucleic Acids. 2015;4:e252. doi:10.1038/mtna.2015.23
170. Isenmann M, Stoddart MJ, Schmelzeisen R, Gross C, Della Bella E, Rothweiler RM. Basic Principles of RNA Interference: nucleic Acid Types and In Vitro Intracellular Delivery Methods. Micromachines. 2023;14(7):1321. doi:10.3390/mi14071321
171. Zare M, Pemmada R, Madhavan M, et al. Encapsulation of miRNA and siRNA into Nanomaterials for Cancer Therapeutics. Pharmaceutics. 2022;14(8):1620. doi:10.3390/pharmaceutics14081620
172. Dasgupta I, Chatterjee A. Recent Advances in miRNA Delivery Systems. Meth Protocols. 2021;4(1):10. doi:10.3390/mps4010010
173. Saha D, Testard F, Grillo I, et al. The role of solvent swelling in the self-assembly of squalene based nanomedicines. Soft Matter. 2015;11(21):4173–4179. doi:10.1039/C5SM00592B
174. Couvreur P, Stella B, Reddy LH, et al. Squalenoyl nanomedicines as potential therapeutics. Nano Lett. 2006;6(11):2544–2548. doi:10.1021/nl061942q
175. Reddy LH, Couvreur P. Squalene: a natural triterpene for use in disease management and therapy. Adv Drug Delivery Rev. 2009;61(15):1412–1426. doi:10.1016/j.addr.2009.09.005
176. Faul CF, Antonietti M. Ionic self‐assembly: facile synthesis of supramolecular materials. Adv Mater. 2003;15(9):673–683. doi:10.1002/adma.200300379
177. Desmaële D, Gref R, Couvreur P. Squalenoylation: a generic platform for nanoparticular drug delivery. J Control Release. 2012;161(2):609–618. doi:10.1016/j.jconrel.2011.07.038
178. Koivisto PV, Miettinen TA. Increased amounts of cholesterol precursors in lipoproteins after ileal exclusion. Lipids. 1988;23(10):993–996. doi:10.1007/BF02536349
179. Stewart M. Sebaceous Gland Lipids. 1992:100–105.
180. Hertel LW, Boder GB, Kroin JS, et al. Evaluation of the antitumor activity of gemcitabine (2′, 2′-difluoro-2′-deoxycytidine). Cancer Res. 1990;50(14):4417–4422.
181. Reid JM, Qu W, Safgren SL, et al. Phase I trial and pharmacokinetics of gemcitabine in children with advanced solid tumors. J Clin Oncol. 2004;22(12):2445–2451. doi:10.1200/JCO.2004.10.142
182. Reddy LH, Couvreur P. Novel approaches to deliver gemcitabine to cancers. Curr Pharm Des. 2008;14(11):1124–1137. doi:10.2174/138161208784246216
183. Reddy LH, Dubernet C, Mouelhi SL, Marque PE, Desmaele D, Couvreur P. A new nanomedicine of gemcitabine displays enhanced anticancer activity in sensitive and resistant leukemia types. J Control Release. 2007;124(1–2):20–27. doi:10.1016/j.jconrel.2007.08.018
184. Bekkara‐Aounallah F, Gref R, Othman M, et al. Novel PEGylated Nanoassemblies Made of Self‐Assembled Squalenoyl Nucleoside Analogues. Adv Funct Mater. 2008;18(22):3715–3725. doi:10.1002/adfm.200800705
185. Réjiba S, Reddy LH, Bigand C, Parmentier C, Couvreur P, Hajri A. Squalenoyl gemcitabine nanomedicine overcomes the low efficacy of gemcitabine therapy in pancreatic cancer. Nanomed Nanotechnol Biol Med. 2011;7(6):841–849. doi:10.1016/j.nano.2011.02.012
186. Jagadish B, Sankaranarayanan R, Xu L, et al. Squalene-derived flexible linkers for bioactive peptides. Bioorg Med Chem Lett. 2007;17(12):3310–3313. doi:10.1016/j.bmcl.2007.04.001
187. Buchy E, Valetti S, Mura S, et al. Synthesis and Cytotoxic Activity of Self‐Assembling Squalene Conjugates of 3‐[(Pyrrol‐2‐yl) methylidene]‐2, 3‐dihydro‐1H‐indol‐2‐one Anticancer Agents. Eur J Org Chem. 2015;2015(1):202–212. doi:10.1002/ejoc.201403088
188. Caron J, Maksimenko A, Wack S, et al. Improving the Antitumor Activity of Squalenoyl‐Paclitaxel Conjugate Nanoassemblies by Manipulating the Linker between Paclitaxel and Squalene. Adv Healthcare Mater. 2013;2(1):172–185. doi:10.1002/adhm.201200099
189. Raouane M, Desmaele D, Gilbert-Sirieix M, et al. Synthesis, characterization, and in vivo delivery of siRNA-squalene nanoparticles targeting fusion oncogene in papillary thyroid carcinoma. J Med Chem. 2011;54(12):4067–4076. doi:10.1021/jm2000272
190. Kim EH, Woodruff MC, Grigoryan L, et al. Squalene emulsion-based vaccine adjuvants stimulate CD8 T cell, but not antibody responses, through a RIPK3-dependent pathway. Elife. 2020;9:e52687. doi:10.7554/eLife.52687
191. Rodríguez-Nogales C, Moreno H, Zandueta C, et al. Combinatorial Nanomedicine Made of Squalenoyl-Gemcitabine and Edelfosine for the Treatment of Osteosarcoma. Cancers. 2020;12(7):1895. doi:10.3390/cancers12071895
192. Rodríguez-Nogales C, Mura S, Couvreur P, Blanco-Prieto MJ. Squalenoyl-gemcitabine/edelfosine nanoassemblies: anticancer activity in pediatric cancer cells and pharmacokinetic profile in mice. Int J Pharm. 2020;582:119345. doi:10.1016/j.ijpharm.2020.119345
193. Soliman MG, Martinez-Serra A, Antonello G, et al. Understanding the role of biomolecular coronas in human exposure to nanomaterials. Environ Sci Nano. 2024;11(11):4421–4448. doi:10.1039/D4EN00488D
194. Falahati M, Attar F, Sharifi M, et al. A health concern regarding the protein Corona, aggregation and disaggregation. Biochim Biophys Acta Gen Subj. 2019;1863(5):971–991. doi:10.1016/j.bbagen.2019.02.012
195. Chen D, Ganesh S, Wang W, Amiji M. Protein Corona-Enabled Systemic Delivery and Targeting of Nanoparticles. AAPS J. 2020;22(4):1. doi:10.1208/s12248-020-00464-x
196. Attwood D, Agarwal SP. Light scattering studies on micelle formation by some penicillins in aqueous solution. J Pharm Pharmacol. 1984;36(8):563–564. doi:10.1111/j.2042-7158.1984.tb04456.x
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.