Back to Journals » International Journal of Nanomedicine » Volume 20
How Advanced Is Nanomedicine for Atherosclerosis?
Authors Gu X, Du L, Lin R, Ding Z , Guo Z, Wei J, Li Y
Received 26 November 2024
Accepted for publication 27 February 2025
Published 17 March 2025 Volume 2025:20 Pages 3445—3470
DOI https://doi.org/10.2147/IJN.S508757
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Farooq A. Shiekh
Xiang Gu,1,* Lixin Du,1,* Ruifang Lin,2 Zehui Ding,2 Zhihua Guo,2 Jiaming Wei,2 Ya Li1
1School of Pharmacy, Hunan University of Chinese Medicine, Changsha, 410208, China; 2School of Chinese Medicine, Hunan University of Chinese Medicine, Changsha, 410208, China
*These authors contributed equally to this work
Correspondence: Jiaming Wei; Ya Li, Email [email protected]; [email protected]
Abstract: Advances in nanotechnology have opened new avenues for precision therapy, personalized medicine, and multifunctional theranostics in atherosclerosis (AS). This review provides a comprehensive overview of the role of nanoparticles (NPs) in precision medicine for AS, discussing their applications, challenges, and future prospects. The review first analyzes the current treatment landscape of AS and outlines potential biological targets for therapy. Various nanocarriers, including organic, inorganic, and hybrid systems, are evaluated for their therapeutic potential, with a focus on targeted drug delivery, anti-inflammatory therapy, vascular repair, plaque stabilization, and lipid clearance. Additionally, the review explores NP preparation methods, emphasizing strategies to enhance drug loading, stability, and controlled release. Finally, the translational challenges of NP-based therapies, including biocompatibility, large-scale production, regulatory hurdles, and clinical implementation, are critically analyzed. Future directions highlight the importance of interdisciplinary collaboration and technological innovation in advancing nanoparticle-based precision medicine for AS.
Keywords: nanoparticles, nanocarrier, nanomedicine, atherosclerosis, precision medicine
Introduction
Cardiovascular diseases (CVDs) are the leading cause of morbidity and mortality worldwide, claiming millions of lives annually and posing a severe threat to human health.1 Both the incidence and mortality of CVDs continue to rise.2 The pathogenesis of CVDs involves complex pathological cascades, including conditions such as Hypertension, Coronary artery disease, Myocardial infarction, and Heart failure.3,4 Among these, Atherosclerosis (AS) serves as the primary pathological basis and a pivotal factor in the intricate interplay of CVDs.5 AS is a systemic, multifactorial chronic disorder of large and medium arteries characterized by atheromatous plaque formation.6,7 In AS, low-density lipoprotein (LDL) accumulates beneath the vascular endothelium, undergoing oxidative modification to form oxidized LDL (ox-LDL). Ox-LDL stimulates endothelial cells to express adhesion molecules, attracting monocytes that migrate into the intima and differentiate into macrophages. These macrophages engulf ox-LDL, transforming into foam cells and causing intimal thickening. Endothelial dysfunction triggers inflammation, promoting smooth muscle cell proliferation and migration, leading to the formation of a fibrous cap. As the lesion progresses, plaques become prone to rupture, inducing platelet aggregation and thrombosis, further obstructing blood flow and driving AS development.8–11
In recent years, significant progress has been made in the treatment of AS, with pharmacological therapy remaining the primary approach.12 Current Western medicine treatments typically involve statins, antiplatelet agents, and antihypertensive drugs.13–15 Statins effectively lower LDL levels, slowing plaque formation and progression, while antiplatelet drugs like aspirin prevent thrombosis.16,17 Traditional Chinese Medicine (TCM) emphasizes principles like “activating blood circulation and resolving stasis” and “tonifying qi and unblocking collaterals”. Commonly used herbs, such as Salvia miltiorrhiza (Danshen) and Ligusticum chuanxiong (Chuanxiong), improve blood circulation, reduce inflammation, and have fewer side effects.18–20 Consequently, TCM is often employed as adjuvant therapy to slow disease progression and enhance overall patient health. Both Western medicine and TCM treatments are associated with adverse reactions, such as hepatotoxicity and nephrotoxicity, due to long-term medication use. Additionally, the bioavailability of active ingredients remains relatively low, and there are still limitations in slowing disease progression.21,22 Integrative therapy combining Western drugs and TCM offers new strategies for AS treatment by leveraging complementary effects. Nevertheless, challenges remain: Western drugs often lack specificity and pose risks of adverse effects with prolonged use, while the low bioavailability of active compounds in TCM restricts its effectiveness.23,24 Traditional delivery methods for both approaches fail to fully meet the demands of AS treatment, highlighting the urgent need for innovative drug delivery systems to enhance targeting, efficacy, and safety.
To address these challenges, nanomedicine has emerged as a promising solution. Nano drug delivery systems (NDDS), an application of nanotechnology in medicine, encapsulate drugs within nanocarriers to leverage their unique physicochemical properties. This approach enhances drug targeting, stability, and bioavailability, effectively overcoming the limitations of traditional delivery systems.25–27 Compared to conventional methods, NDDS offers multiple advantages. Nanocarriers can be engineered with precise size and surface modifications to achieve targeted delivery, allowing selective accumulation at lesion sites and significantly reducing systemic side effects.28 Among various nanotechnologies, nanoparticles (NPs) are the most extensively studied in the treatment and diagnosis of CVDs. NPs, composed of drugs and carriers, are small-sized particles known for their ease of synthesis, high tunability, and substantial drug-loading capacity. By optimizing particle size, surface modifications, and drug-loading strategies, NPs extend drug circulation time and enhance targeting efficiency to pathological sites.29,30 These properties position NPs as a potent tool to address the challenges in AS treatment.
The application of NPs in AS treatment holds significant promise, offering a potential pathway for efficient and low-toxicity therapeutic strategies, and is poised to become an innovative direction for future AS therapy. This review provides an overview of AS pathogenesis, discusses biological targets for AS treatment, summarizes the types of nanocarriers used for constructing NPs and their preparation methods, and systematically reviews recent advancements in the personalized treatment of AS using NPs. Finally, it highlights the challenges and obstacles in the clinical translation of NPs for AS and presents perspectives on future research in precision medicine.
Current Treatment for Atherosclerosis
AS is a chronic, progressive vascular disease characterized by lipid deposition, inflammatory responses, and fibrous tissue proliferation in the arterial walls, leading to the formation of atherosclerotic plaques that cause arterial narrowing and impaired blood flow.31 AS is a major contributor to cardiovascular diseases, including coronary artery disease, stroke, and peripheral artery disease.32 Effective therapeutic strategies can help slow or halt disease progression and reduce the risk of cardiovascular events. Currently, the treatment of AS involves a combination of lifestyle interventions, pharmacological therapies, and interventional procedures.33
Lifestyle interventions are crucial for early or mild AS patients. A low-saturated-fat and cholesterol diet helps slow AS progression, while regular aerobic exercise improves cardiovascular health, lowers blood pressure, enhances lipid metabolism, and increases High-Density Lipoprotein (HDL) levels.34 Pharmacological treatment plays a key role in controlling AS, with common medications including statins, ezetimibe, PCSK9 inhibitors, antiplatelet agents, and antihypertensive drugs.35 Statins, the first-line treatment, lower Low-Density Lipoprotein Cholesterol (LDL-C) by inhibiting HMG-CoA reductase and have anti-inflammatory effects that stabilize plaques and reduce cardiovascular events.36,37 Ezetimibe lowers blood lipids by inhibiting intestinal cholesterol absorption,38 and, when combined with statins, further reduces LDL-C.39 PCSK9 inhibitors enhance LDL-C clearance by increasing LDL receptor numbers.40 Antiplatelet drugs like aspirin and clopidogrel reduce thrombosis risk after plaque rupture.41 Antihypertensive medications, such as Angiotensin-Converting Enzyme (ACE) inhibitors, ARBs, β-blockers, and calcium channel blockers, effectively control blood pressure, easing cardiovascular stress and slowing AS progression.42,43 For patients with severe symptoms or inadequate pharmacological control, interventional treatments are effective, though they carry risks of infection, vessel damage, and restenosis, and cannot cure AS but only relieve symptoms.44
Key Drug Targets to Treat Atherosclerosis
Biological targets are biomolecules that interact with drugs to produce pharmacological effects. A deep understanding of AS pathogenesis is crucial for precisely identifying relevant biological targets, which is key to developing effective treatment strategies.45 AS involves multiple cellular responses and molecular signaling pathways. Therefore, targeting key pathological mechanisms such as inflammation, lipid metabolism, and endothelial dysfunction provides potential targets to inhibit plaque formation. Identifying these targets and developing targeted therapies may enable more precise interventions, advancing the personalized treatment of AS.
Inflammatory Cells
Due to the high abundance of macrophages in plaques and their ability to absorb drugs, macrophages are a common biological target.46 Macrophages engulf ox-LDL to form foam cells, releasing pro-inflammatory factors and matrix metalloproteinases (MMPs), which exacerbate local inflammation.47 The most common biological markers for macrophages are scavenger receptors, including type A and type B scavenger receptors,48 while apolipoprotein A1-derived peptides, which mimic LDL, are also common ligands for targeting macrophages.49 Therefore, inhibiting macrophage polarization, modulating immune responses, and targeting the inflammatory mediators they secrete can effectively suppress AS progression.
Endothelial Cells
Endothelial cells play a central role in vascular protection and pathological development. They secrete molecules such as NO, endothelin-1 (ET-1), Vascular Cell Adhesion Molecule-1 (VCAM-1), Intercellular Adhesion Molecule-1 (ICAM-1), and vascular endothelial growth factor (VEGF), which regulate vascular tone, permeability, and inflammation.50,51 In certain curved and branching areas of arterial vessels, the shear stress from blood flow can cause endothelial damage, leading to the formation of AS plaques. Stimulated endothelial cells in these regions express higher levels of the aforementioned molecules, promoting vasodilation to restore endothelial function.
Vascular Smooth Muscle Cells
Vascular smooth muscle cells (VSMCs) play a crucial role in AS, with their migration, proliferation, and secretion significantly influencing plaque formation and stability.52 VSMCs can migrate to the intima and proliferate extensively in response to various stimuli, secreting extracellular matrix to form the fibrous cap of the plaque, temporarily stabilizing it.53 However, under pathological conditions, VSMCs release pro-inflammatory and proliferative molecules, including platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), and MMPs. These molecules not only promote cell proliferation and migration but also weaken the stability of the fibrous cap by degrading the extracellular matrix.54
Non-Cellular Components
In AS lesions, non-cellular components primarily consist of the extracellular matrix (ECM) and lipid core, collectively determining plaque stability and progression. The ECM is a highly complex structure composed of collagen, elastin, hyaluronic acid, fibrinogen, and other macromolecules, with collagen being the most abundant non-cellular component. As a key structural element of the ECM, collagen regulates cellular responses and contributes to the strength and integrity of the fibrous cap.55 It provides essential mechanical support to blood vessels, maintaining their structural integrity and elasticity. Beyond its structural role, collagen interacts with integrins and other cell surface receptors, thereby modulating the behavior of vascular smooth muscle cells, macrophages, and endothelial cells.56,57 Strategies to mitigate collagen degradation or pharmacologically enhance its synthesis have shown potential in influencing AS progression. Elastin, predominantly found in the vascular wall and ECM, is essential for vascular elasticity and compliance.58 Hyaluronic acid (HA) degradation products act as inflammatory mediators, promoting monocyte infiltration, whereas fibrinogen, a key plasma coagulation protein, is converted into fibrin upon plaque rupture by thrombin, contributing to thrombus formation in conjunction with HA.59 The metabolic dysregulation of these non-cellular components directly impacts plaque progression and represents a critical therapeutic target for drug delivery in AS treatment.
How Precise Is Advancing Nanomedicine to Treat Atherosclerosis?
NPs have emerged as a research focus in AS treatment and diagnosis due to their unique biodistribution and targeting capabilities. Comprising drugs and carriers, NPs utilize diverse carrier types, including organic, inorganic, and hybrid nanomaterials. These carriers can be engineered for specific targeting and biocompatibility, enabling efficient penetration of AS lesions and demonstrating versatile advantages in AS applications.
Poly (Lactic-Co-Glycolic Acid) Nanoparticles
Poly (lactic-co-glycolic acid) (PLGA), a widely used biodegradable polymer, is renowned for its biocompatibility and controllable degradation rates, making it a common organic nanocarrier in NPs. It has been approved by the US Food and Drug Administration (FDA) for medical and pharmaceutical applications.60 In vivo, PLGA degrades into harmless small metabolites, such as lactic acid and CO₂, which are excreted via normal metabolic pathways, minimizing long-term toxicity risks.61,62 PLGA NPs exhibit strong drug encapsulation capabilities, effectively preserving drug activity. Their surface can be functionalized with targeting molecules (eg, antibodies, peptides) to enable site-specific drug delivery.63 Additionally, the physicochemical properties of PLGA NPs, such as size, shape, and surface charge, can be tailored to enhance adhesion and penetration into vascular walls or target cells. This is particularly beneficial for stabilizing AS plaques, reducing inflammation, and decreasing lipid deposition.64,65
Tang et al66 developed Vanillic acid-4’-hydroxy-3’-phospho-ketone (VHPK) peptide-modified PLGA nanoparticles encapsulating colchicine (VHPK-PLGA@COL) using a double emulsion method. Colchicine and PLGA were dissolved in chloroform as the organic phase, emulsified with an aqueous phase, followed by the addition of VHPK peptide for further sonication (schematic shown in Figure 1A). In vitro cytotoxicity assays showed cell viabilities of 95.0%, 78.1%, and 91.8% for the control, colchicine, and VHPK-PLGA@COL groups, respectively. Hemolysis tests further demonstrated that VHPK-PLGA@COL at various concentrations did not induce hemolysis (Figure 1B), indicating good biocompatibility and negligible cytotoxicity. To confirm the targeted ability of VHPK peptide-modified NPs, DiI-labeled nanoparticles were tested on Tumor Necrosis Factor Alpha (TNF-α)-activated HUVECs. VHPK-PLGA@COL exhibited significantly higher fluorescence signals and adhesion to activated HUVECs compared to unmodified PLGA@COL (Figure 1C). Additionally, upon intravenous injection into AS mouse models, ex vivo imaging showed lower VHPK-PLGA@COL distribution in major organs compared to PLGA@COL (Figure 1D) but stronger fluorescence signals in plaques (Figure 1E), highlighting its potential for targeted AS plaque delivery.
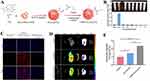 |
Figure 1 (A) Schematic illustration of VHPK-PLGA@COL preparation. (B) Results of hemolysis testing. (C) In vitro targeting results: stronger red fluorescence from DiI labeling indicates higher cellular affinity. (D) Fluorescence imaging of major organs ex vivo. (E) Fluorescence intensity of VHPK-PLGA@COL and PLGA@COL targeting AS plaques, *P<0.05, **P<0.01, ***P<0.001 compared with the Control. Adapted with permission from Tang J, Li T, Xiong X, et al. Colchicine delivered by a novel nanoparticle platform alleviates atherosclerosis by targeted inhibition of NF-κB/NLRP3 pathways in inflammatory endothelial cells. J Nanobiotechnology. 2023;21(1):460. https://creativecommons.org/licenses/by/4.0/.66 |
Building on the favorable effects of pioglitazone in AS treatment, Todaro et al67 developed PLGA NPs encapsulating pioglitazone (PGZ-NPs). They compared the effects of emulsion and nanoprecipitation methods on PGZ-NPs preparation and optimized the conditions for both. In the emulsion method, pH significantly influenced the particle size and zeta potential of the NPs, with the optimal pH identified as 6.2. For the nanoprecipitation method, the PLGA concentration critically impacted the encapsulation efficiency and drug-loading capacity, with the best results achieved at 10 mg/mL. Another study68 employed PLGA to co-encapsulate curcumin and piperine into dual-drug NPs to inhibit AS foam cell formation. Fourier Transform Infrared Spectroscopy (FTIR) analysis confirmed the presence of characteristic peaks from curcumin, piperine, and PLGA, indicating successful drug-polymer integration. The NPs exhibited a particle size of 181 ± 8.63 nm and a Polydispersity Index (PDI) of 0.21 ± 0.005, demonstrating good dispersity. In vitro and in vivo experiments showed that dual-drug NPs had superior efficacy compared to single-drug formulations, suggesting a synergistic enhancement of therapeutic effects under PLGA encapsulation.
Polyethylene Glycol Nanoparticles
Polyethylene Glycol (PEG), like PLGA, is a biocompatible polymer. Unlike PLGA, which degrades and is excreted from the body, PEG remains non-degradable and serves as a modifying molecule to extend the circulation time of drugs.69 The ethylene glycol groups in PEG form a hydration layer, enhancing its hydrophilicity and giving PEG-modified NPs an “invisibility” effect.70,71 D-PDMP, a sphingolipid biosynthesis inhibitor, holds promise for AS treatment but is rapidly cleared from the body, limiting its application. Researchers72 have encapsulated D-PDMP in PEG NPs to overcome this issue, significantly increasing its in vivo half-life from less than 1 h to 4 h and enhancing its therapeutic effects on AS and cardiac hypertrophy. In addition to AS treatment, PEG NPs are increasingly used for AS diagnosis. Lee et al73 synthesized a D-mannose-PEG nanoparticle (MAN-PEG-Ce6) for photodynamic diagnosis of AS. Results showed that MAN-PEG-Ce6 specifically internalized into macrophage-derived foam cells via receptor-mediated endocytosis. After laser irradiation, it significantly enhanced singlet oxygen production, showing great potential for AS imaging and diagnosis.
Due to the excellent hydrophilicity of PEG, a dual-purpose NP for both diagnosis and therapy has been developed. Lin et al74 synthesized pH-responsive ST/NCP-PEG NPs by polymerizing benzimidazole with PEG to encapsulate simvastatin. Dynamic light scattering (DLS) and transmission electron microscopy (TEM) showed that ST/NCP-PEG exhibited uniform size and spherical shape with an average diameter of 40 nm (Figure 2A). To elucidate its pH-responsive mechanism, FTIR analysis revealed that the stretching vibration of the imine bond at 1610 cm−1 significantly decreased when the pH dropped from 7.4 to 5.6, and a peak appeared at 1704 cm−1, indicating bond cleavage (Figure 2B). To verify the targeting effect of ST/NCP-PEG on AS plaques, after intravenous injection of Cy5-labeled ST/NCP-PEG, clear fluorescence signals were observed in the aortic regions isolated from ApoE−/− mice, indicating accumulation in the plaque area (Figure 2C and D). To assess its imaging performance, magnetic resonance imaging (MRI) was performed on 16-week-old ApoE−/− mice, showing a significant signal enhancement in the arterial vessel wall, indicating the presence of AS plaques (Figure 2E).
 |
Figure 2 (A) TEM images of ST/NCP-PEG. (B) FT-IR spectrum of ST/NCP-PEG at pH 5.6 and pH 7.4. (C) Ex vivo fluorescent imaging of the aortas. (D) Quantitative data analysis of Cy5-labeled fluorescent signals in the aorta, **P<0.01 compared with the control. (E) The MR images in ApoE−/− mice (the region of interest is outlined in the insert). Adapted with permission from Lin Y, Liu J, Chong SY, et al. Dual-function nanoscale coordination polymer nanoparticles for targeted diagnosis and therapeutic delivery in atherosclerosis. Small. 2024;20(47):e2401659. Copyright © 2024 The Authors. All rights reserved.74 |
Chitosan Nanoparticles
Chitosan, like PLGA and PEG, is a naturally derived polymer with excellent biocompatibility and biodegradability, offering unique applications in AS treatment.75,76 Its structure contains abundant amino groups that can acquire positive charges in different pH environments, enabling interactions with negatively charged drug molecules.77,78 Chitosan also exhibits adhesion and sustained release properties, facilitating drug loading and targeted delivery. It not only prolongs drug circulation time but also interacts directly with cell membranes, enhancing nanoparticle uptake in target tissues.79 Wang et al80 modified chitosan to prepare nanoparticles loaded with mangiferin, with FTIR and 1H-NMR analysis confirming successful encapsulation. Chitosan-mediated slow drug release followed the Baker-Lonsdale model. Additionally, Du et al81 developed HA-modified chitosan nanoparticles (HA@GRb1@CS NPs) to encapsulate ginsenoside Rb1, which exhibited sustained release in PBS at various pH values. Bioinformatics analysis suggested their potential therapeutic effects on cardiovascular diseases, particularly AS, by regulating targets like STAT3, JUN, and EGFR.
Statins are commonly used inhibitors in the treatment of AS, and nucleic acid drugs have also shown promising effects in AS clinical research. Jiang et al82 developed atorvastatin and chitosan NPs for encapsulating siRNA and 33pDNA, aiming for co-delivery of statins and nucleic acids. Atorvastatin NPs (GTANPs) were first prepared via an amide reaction, then siRNA and 33pDNA were assembled via electrostatic interactions (GTANPs/siRNA). In vitro release studies showed rapid initial release followed by slower, sustained release (Figure 3A), with no significant difference between the drug releases, indicating that the carrier’s encapsulation had negligible effect on drug release. For cellular uptake, GTANPs/siRNA was administered to L02 and RAW264.7 cells, and fluorescence analysis indicated effective internalization, with stronger fluorescence than other groups (Figure 3B and C), and reduced Baf60a expression in L02 cells (Figure 3D). Oil Red O staining of aortic plaques showed that all groups reduced plaque area, with the GTANPs/siRNA group showing only 7.2% plaque area, and also reducing Baf60a expression in the aorta (Figure 3E–G). Stability and degradation tests revealed minimal changes in particle size when pH was alternated between 7.4 and 2.0 (Figure 3H), indicating the nanoparticles’ stability and reduced degradation in the gastrointestinal tract.
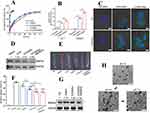 |
Figure 3 (A) In vitro release profiles of AVS in PBS. (B) Quantitative analysis of cellular uptake of TANPs/siRNA and GTANPs/siRNA by L02 and RAW264.7 cells, ***P < 0.001, vs the LPS treated RAW264.7 cells after treatment with saline. (C) Typical CLSM images of cellular uptake of GTANPs/siRNA by L02 and RAW264.7 cells. Scale bar represents 10 μm. (D) Western blotting and quantitative analysis of Baf60a protein levels in L02 cells. (E and F) Representative images and quantitative analysis of oil red O stained descending aorta, *P < 0.05, **P < 0.01, ##P < 0.01, and ###P < 0.001, vs mice in the saline group. (G) Western blotting and quantitative analysis of Baf60a protein levels in descending aorta. (H) TEM images of GTANPs/pDNA under various pH conditions. Scale bar represents 200 nm. Reprinted from Biomaterials, Jiang T, Xu L, Zhao M, et al. Dual targeted delivery of statins and nucleic acids by chitosan-based nanoparticles for enhanced antiatherosclerotic efficacy. Biomaterials. 280:121324.. Copyright © 2022, with permission from Elsevier.82 |
Zeolitic Imidazolate Frameworks Nanoparticles
In addition to natural polymers, metal-organic frameworks are increasingly used as carriers for NPs. Zeolitic Imidazolate Frameworks (ZIF-8), a metal-organic framework composed of zinc ions and 2-methylimidazole, features a highly porous and stable three-dimensional structure.83 This framework not only accommodates various drug molecules but also degrades rapidly in acidic environments, facilitating drug or active substance release, making it suitable for drug delivery in pathological microenvironments.84,85 ZIF-8’s pH-sensitive degradation allows rapid drug release in low pH environments, concentrating drug delivery at acidic lesions like AS plaques.86 Sheng et al87 developed ZIF-8 NPs loaded with losartan potassium (LP@ZIF-8) for anti-AS therapy. They found that LP@ZIF-8 activated autophagy to regulate lipid metabolism and effectively reduced Interleukin-6 (IL-6), Interleukin-1 Beta (IL-1β), and TNF-α levels. Another study88 loaded simvastatin into ZIF-8 and modified it with HA to create self-assembled NPs (SIM/ZIF-8@HA). TEM images showed that SIM/ZIF-8@HA exhibited narrow, uniform size with a diameter of approximately 200 nm (Figure 4A). ZIF-8 degraded under acidic conditions, enabling sustained drug release, especially after HA modification, which significantly enhanced the controlled release (Figure 4B). To assess immune evasion, 1.1’-Dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (DiD)-labeled SIM/ZIF-8@HA was incubated with macrophages, and fluorescence results indicated that macrophage phagocytosis was time-dependent, with HA modification promoting macrophage internalization of ZIF-8@HA via CD44-mediated endocytosis (Figure 4C and D). Hemolysis tests showed no hemolytic activity for SIM/ZIF-8@HA and SIM/ZIF-8, with hemolysis rates of 2.4% and 1.4%, respectively (Figure 4E and F). Oil Red O staining revealed that SIM/ZIF-8@HA more effectively inhibited AS lesions and reduced plaque lipid accumulation compared to SIM and SIM/ZIF-8 (Figure 4G).
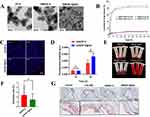 |
Figure 4 (A) TEM images of ZIF-8, SIM/ZIF-8, and SIM/ZIF-8@HA, scale bar = 200 nm. (B) In vitro drug release study of SIM from SIM/ZIF-8 and SIM/ZIF-8@HA under various pH conditions. (C) CLSM images of ZIF-8 and ZIF-8@HA internalized by RAW264.7 cells at 1 h or 3 h (scale bar = 40 µm). (D) Quantification of the cellular uptake of ZIF-8 and ZIF-8@HA in RAW264.7 cells at 1 h or 3 h, *p < 0.05, and ns, no significance. (E) Images and (F) statistics of the hemolysis test with SIM/ZIF-8 and SIM/ZIF-8@HA. (G) Aortic root cross-sections stained with ORO (scale bar = 500 and 100 μm, respectively). Used with permission of Biomaterials science, from Obaid EAMS, Wu S, Zhong Y, et al. pH-Responsive hyaluronic acid-enveloped ZIF-8 nanoparticles for anti-atherosclerosis therapy. Biomater Sci. 2022;10(17):4837–4847.; permission conveyed through Copyright Clearance Center, Inc.88 |
Liposome Nanoparticles
Liposomes are spherical vesicles formed by phospholipid bilayers, with a structure similar to cell membranes, capable of encapsulating both hydrophilic and lipophilic drugs.89,90 As one of the earliest studied and applied nanocarriers, liposomes offer excellent biocompatibility and low toxicity, making them safe for in vivo use. Their unique bilayer structure protects encapsulated drugs from degradation in the body and enhances targeting through surface modifications, thereby improving therapeutic precision.91,92 Additionally, liposomes exhibit good controlled drug release, prolonging circulation time and reducing side effects, particularly suitable for chronic diseases like AS.93 Dhanasekara et al94 synthesized liposome NPs using soybean lecithin, and in vitro uptake experiments showed high binding affinity with macrophage CD36 receptors, offering potential for AS molecular imaging. Shi et al95 designed Cholesteryl-9-carboxynonanoate-(125I‑iron oxide nanoparticle/Curcumin)-lipid-coated nanoparticles (MLNPs), demonstrating that MLNPs accurately accumulate in unstable plaques and efficiently deliver curcumin to macrophage inflammation sites. Photodynamic therapy has emerged as a new strategy for AS treatment, with researchers96 using the film dispersion method to design liposome NPs loaded with Ce6, then covalently crosslinking CD68 antibodies to the liposome surface. Flow cytometry results indicated that Ce6-loaded liposomes promoted intracellular uptake more effectively under laser irradiation. Furthermore, CD68-modified liposomes significantly enhanced cellular recognition and internalization. They also promoted foam cell autophagy by increasing LC3-I/LC3-II expression and decreasing p62 expression, while inhibiting migration of mouse aortic smooth muscle cells in vitro.
Albumin Nanoparticles
Albumin, a naturally occurring protein in the body, is non-immunogenic and has a long circulation time in the bloodstream, making it a promising drug carrier.97 Compared to liposomes, albumin carriers not only stabilize drug loading but also enable targeted delivery through receptor binding.98,99 Huang et al100 developed a dextran-bovine serum albumin conjugate (DB) and used it as an emulsifier to load Ce6, forming albumin NPs (UCNPs-Ce6@DB). Their results showed that UCNPs-Ce6@DB could recognize and bind to the class A scavenger receptors highly expressed on macrophages, promoting the uptake of foam cells derived from macrophages in AS and inhibiting the secretion of pro-inflammatory cytokines. Boada et al101 developed albumin NPs loaded with rapamycin, characterized by a particle size of 108 ± 2.3 nm and a zeta potential of −15.4 ± 14.4 mV. In vivo studies showed that treatment with these NPs reduced inflammation levels in mice and suppressed macrophage polarization.
Gold Nanoparticles
In addition to organic materials, inorganic carriers are commonly used in drug delivery due to their superior structural stability and functional versatility compared to organic carriers.102 Gold Nanoparticles (AuNPs) have become a prominent focus in AS research, particularly for imaging and diagnosis, due to their surface modifiability and photothermal properties.103,104 In 2016, Li et al105 developed a gold nanoparticle-based imaging probe, Tc-GNPs-Annexin V, for targeting apoptotic macrophages in AS. The probe, with a uniform size of 30.2 ± 2.9 nm, exhibited high labeling efficiency and good stability. Computed Tomography (CT) scans of AS gene knockout mice induced by a high-fat diet showed that the imaging probe accumulated more effectively in apoptotic macrophages with the addition of targeting molecules. Recently, AuNPs-PEI-siRNA was used for AS plaque targeting in imaging studies. Results showed that macrophages more readily interacted with AuNPs-PEI-siRNA, and tail vein injection into high-fat diet mice enhanced AS plaque labeling, with CD40 surface expression being a key targeting factor.106
Silver Nanoparticles
Similar to AuNPs, Silver Nanoparticles (AgNPs) also exhibit optical properties and surface modifiability, with additional anti-inflammatory and antimicrobial characteristics that provide unique advantages in AS treatment.107 As a metal material with enhanced photothermal conversion, AgNPs hold potential for macrophage-mediated hyperthermia treatment of arterial inflammation.108 However, AgNPs may also exert negative effects on AS treatment by negatively regulating inflammation and cholesterol uptake, both of which exacerbate AS.109 Therefore, careful control of their biotoxicity and biocompatibility is required. CpG, a toll-like receptor agonist, is used for AS therapy. Tang et al110 developed CpG-AgNPs by conjugating CpG to AgNPs, which reduced CpG degradation under the silver carrier, while leveraging the inherent antioxidant and anti-inflammatory properties of silver. This multifunctional nanomedicine can simultaneously promote macrophage phagocytosis and repolarization. In an AS mouse model, intravenous injection of CpG-AgNPs effectively targeted AS plaques, demonstrating therapeutic efficacy and excellent biocompatibility.
Ferric Ferrous Oxide Nanoparticles
Ferric Ferrous Oxide Nanoparticles (Fe₃O₄ NPs) possess superparamagnetism, allowing precise control under an external magnetic field. This property enables targeted drug delivery to diseased sites in the bloodstream,111,112 reducing side effects in healthy tissues serve both as a drug delivery carrier and an MRI contrast agent.113 Shen et al114 developnctional probto H₂O₂ by coupling Fe₃O₄ with citric acid and incorporating H₂O₂-responsive simvastatin-modified amphiphilic block copolymers and the targeting molecule ISO-1, forming the drug-loaded micelle PGMA-PEG-ISO-1-Sim@FC (PPIS@FC). Their study showed that PPIS@FC effectively releases drugs in the plaque’s unique microenvironment, with in vivo results confirming its targeting ability, MRI, and fluorescence imaging. Additionally, Huang et al115 developed Rap/Fe₃O₄@VHP-Lipo, a liposomal ncarrying rapamycin, modified with VCAM-1 on Fe₃O₄. In vitro studies showed superior biocompatibility and targeted collagen-binding characteristics, with MRI imaging performance. In vivo studies demonstrated its targeting ability in an AS mouse model, effectively treating lipid accumulation in plaques.
Prussian Blue Nanoparticles
Prussian Blue (PB), a prototype of mixed-valence transition metal hexacyanoferrates, is a deep blue pigment. Its structure consists of divalent and trivalent iron ions connected via cyanide groups, forming a uniform crystalline lattice. This unique lattice endows PB with exceptional properties, including photophysical, electrochemical, and electrochromic characteristics.116,117
To address the poor targeting of rosuvastatin, Liu et al118 developed PB-based NPs coated with macrophage membranes (MPR NPs). In vitro experiments demonstrated that MPR NPs effectively inhibited the TLR4/HIF-1α/NOD pathway, promoted cholesterol efflux, and reduced lipid deposition. In vivo studies in mice confirmed the therapeutic potential of MPR NPs for AS. In another study, researchers119 encapsulated colchicine within PB NPs, coated them with HA, and labeled them with Cy5.5, forming col@PBNP@HA. To evaluate its properties, a series of characterizations were conducted. TEM images revealed cubic structures with dispersed distribution, and compared to uncoated col@PBNP, col@PBNP@HA exhibited a gray membrane-like structure on the surface, confirming successful hyaluronic acid modification (Figure 5A). The particle size of col@PBNP@HA was measured as 166.1 ± 0.286 nm (Figure 5B). To assess ROS-scavenging activity, levels of inflammatory and oxidative stress markers were evaluated. Results showed col@PBNP@HA reduced malondialdehyde (MDA) levels (Figure 5C) and increased glutathione (GSH) levels (Figure 5D). Histological staining (HE, Oil Red O, and Masson) of aortic tissue sections was used to assess plaque regression (Figure 5E). HE staining revealed a significant reduction in plaque size, with col@PBNP@HA-treated plaques exhibiting the smallest necrotic areas. After col@PBNP@HA treatment, Masson staining indicated increased collagen content. Additionally, immunofluorescence was performed to examine P65 nuclear translocation. As shown in Figure 5F, P65 was primarily expressed in the cytoplasm of unstimulated cells, whereas LPS-induced inflammatory macrophages exhibited significant nuclear overexpression of P65, indicating its phosphorylation and translocation to the nucleus to initiate downstream inflammatory mediator transcription.
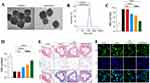 |
Figure 5 (A) Representative image obtained through TEM showcasing col@PBNP and col@PBNP@HA. (B) Hydrodynamic diameter distribution of col@PBNP and col@PBNP@HA. (C and D) Quantification of MDA and GSH in macrophages of different groups (**P < 0.01, ***P < 0.001, and ****P < 0.0001, compared with the LPS group. ####P < 0.0001, compared with the LPS + colchicine group). (E) Histopathological examination of the segments from aortic sinuses isolated from atherosclerotic mice models after various treatments (scale bar = 500 μm). (F) Representative images of Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) immunofluorescence staining following the incubation of inflammatory macrophages with colchicine or col@PBNP@HA (scale bar = 50 μm). Adapted with permission from Zhu Y, Fang Y, Wang Y, et al. Cluster of Differentiation-44-targeting prussian blue nanoparticles onloaded with colchicine for atherosclerotic plaque regression in a mice model. ACS Biomater Sci Eng. 2024;10(3):1530–1543. Copyright © 2024 American Chemical Society.119 |
Silica Nanoparticles
Mesoporous Silica (SiO2) NPs are characterized by their relatively small pore size, simple fabrication process, and high drug-loading capacity. Their surface, rich in silanol groups, is highly amenable to modification, offering excellent biocompatibility. These properties make SiO2 NPs a focus of interest in the development of novel nanomedicines.120–122 Pham et al123 designed CD9 antibody-modified SiO2 NPs, which demonstrated enhanced cellular uptake and reduced levels of Reactive Oxygen Species (ROS), TNF-α, and IL-6 in in vitro models of oxidized high-density lipoprotein-induced senescent foam macrophages and endothelial cells, thereby improving cellular viability. Similarly, Ma et al124 leveraged the properties of SiO2 to develop CD44 ligand-modified probes (HA-Gd2O₃@MSN) for the identification and diagnosis of AS. Cell Counting Kit-8 (CCK-8) assays, hemolysis tests, hematoxylin-eosin staining, and blood biochemical analyses confirmed the excellent biocompatibility of HA-Gd2O₃@MSN, while laser confocal microscopy, cellular MRI, flow cytometry, and immunohistochemistry demonstrated its strong targeting capability. Furthermore, Wang et al125 introduced a composite SiO2 NPs system (CMSN@SRT@Anti) for AS diagnosis and therapy. In vitro studies revealed significant uptake of CMSN@SRT@Anti by RAW264.7 macrophages and its ability to inhibit macrophage polarization. In vivo experiments showed that after 4 weeks of treatment, the serum total cholesterol levels, aortic plaque condition, and plaque area in mice were significantly improved, effectively alleviating AS symptoms.
Composite Hybrid Nanoparticles
Composite hybrid nanomaterials can consist of combinations of organic and inorganic materials or organic-organic materials. By integrating different materials into a single structure, these composites achieve enhanced stability, biocompatibility, and multifunctionality. This synergistic effect makes them particularly effective in precision drug delivery and targeted therapy.126 Groner et al developed PLGA and PEG-based NPs to improve the bioavailability of pioglitazone. The NPs had an approximate size of 85 nm, a PDI of 0.15, and an encapsulation efficiency of 55%, as determined by HPLC. Since pioglitazone targets PPAR-γ, an intracellular receptor, macrophage uptake experiments were conducted to assess the targeting affinity of the NPs. Results showed significant uptake by macrophages, which increased with the NPs concentration, reaching equilibrium at 150 μg/mL.127 Ye et al128 prepared an AS treatment and imaging probe (Fe-PFH-PLGA/CS-DS NPs) by loading dextran sulfate (DS) with PLGA and chitosan as carriers, and adding iron as a contrast agent. Using DiI labeling, they observed the targeting capability of the NPs in RAW264.7 cells. Fluorescence results showed strong adhesion of Fe-PFH-PLGA/CS-DS NPs to activated cells. CCK-8 assays indicated no significant effect on cell viability, suggesting that the carrier materials were safe and reliable.
In addition to combining various materials, extensive research has also focused on coating NPs with biomimetic membranes to enhance their targeting ability. The biomembrane coating acts like a “biomimetic suit”, improving the biocompatibility and circulation time of drugs in vivo, thereby enabling multifunctional therapeutic effects.129 Li et al130 synthesized a novel NPs (PM/Fe₃O₄@PLGA) by first preparing PLGA polymers loaded with Fe₃O₄ (Fe₃O₄@PLGA) via emulsion solvent evaporation, followed by coating with platelet membranes (PM) extracted using a low-osmolarity centrifugation method, as shown in Figure 6A. DLS results revealed that after coating with PM, the NPs’ diameter increased from 244.13 nm to 280.33 nm, corresponding to the bilayer membrane thickness, and the zeta potential shifted from −17.2 mV to −27.3 mV (Figure 6B and C). TEM confirmed the “core-shell” structure of PM/Fe₃O₄@PLGA (Figure 6D). After labeling PM/Fe₃O₄@PLGA and Fe₃O₄@PLGA with DiI (1,1’-Dioctadecyl-3,3,3’,3’-Tetramethylindocarbocyanine Perchlorate) and incubating with cells for 4 h, the PM/Fe₃O₄@PLGA group showed strong red fluorescence signals on the cell surface, while the non-targeted Fe₃O₄@PLGA group only showed green fluorescence (Figure 6E). To investigate the ability of PM/Fe₃O₄@PLGA to detect AS plaques, they established an AS mouse model for MRI, as shown in Figure 6F. MRI of 25 mice revealed an area under the curve (AUC) of 0.954 ± 0.026. Receiver operating characteristic (ROC) analysis indicated an optimal cutoff of 8493.5, with sensitivity and specificity both at 88%, indicating that this nanoprobe exhibits excellent diagnostic capability in distinguishing atherosclerotic plaques. (Figure 6G).
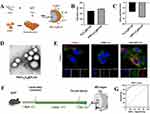 |
Figure 6 (A) Schematic illustration of the preparation of PM/Fe3O4@PLGA. (B) Particle size of Fe3O4@PLGA and PM/Fe3O4@PLGA measured by DLS. (C) Zeta potential distribution of Fe3O4@PLGA and PM/Fe3O4@PLGA. (D) TEM images of Fe3O4@PLGA and PM/Fe3O4@PLGA. (Scale bar = 100 nm). (E) Cell uptake of DiI NPs and PM/DiI NPs after incubation with foam cells for 4 h. (scale bar = 10 μm). (F). Experiment setup in MRI of the vulnerable atherosclerotic plaque in vivo. (G) ROC curve showing the performance of different plaques (stable plaque, venerable plaque) in terms of true positive rate and false positive rate. Adapted with permission from Li Y, Wang Y, Xia Z, et al. Noninvasive platelet membrane-coated Fe3O4 nanoparticles identify vulnerable atherosclerotic plaques. Smart Med. 2024;3(2):e20240006. © 2024 The Authors. Smart Medicine published by Wiley-VCH GmbH on behalf of Wenzhou Institute, University of Chinese Academy of Sciences.130 |
Preparation Methods of Nanoparticles
The preparation method of NPs is a key factor determining their structure and function. Different preparation techniques directly influence the particle size, morphology, dispersibility, drug-loading efficiency, and stability of NPs, which in turn significantly impact their therapeutic effectiveness in AS treatment.131 Additionally, precise optimization of these preparation processes allows for fine-tuned control over NPs, enhancing their targeted delivery efficiency and reducing drug toxicity to normal tissues. Choosing an appropriate preparation method is not only fundamental for achieving the desired structure and function of NPs but also crucial for advancing precision treatment of AS. Currently, major NPs preparation methods include emulsion, ultrasonic dispersion, ion gelation, nanoprecipitation, and self-assembly. The characteristics of each method are summarized and discussed below (Table 1).
 |
Table 1 Preparation Methods and Characteristics of NPs Commonly Used for AS Treatment |
Emulsion
Emulsion methods involve dispersing hydrophobic drugs or materials in the aqueous phase to form nanoemulsions, which are then solidified into NPs by solvent evaporation or coagulation.136 Emulsions can be classified as oil-in-water (O/W) or water-in-oil (W/O), suitable for drugs and carriers with different polarities. Variants of emulsion methods include the emulsion-evaporation method and double emulsion method. The emulsion-evaporation method is used for hydrophobic drug nanocarriers, where organic solvent evaporation solidifies the emulsion into particles. The double emulsion method, used for encapsulating water-soluble drugs, forms a water-in-oil-in-water (W/O/W) structure to improve encapsulation efficiency.137 Wu et al138 used the double emulsion method to prepare PLGA NPs for targeted delivery of chemokine CCR2 to macrophages in AS lesions, achieving a drug encapsulation rate of 79.37%. Manesh et al139 employed the emulsion-evaporation method to prepare imatinib-loaded NPs, with FTIR analysis confirming drug-carrier conjugation and in vitro release studies showing sustained release of imatinib.
Ultrasonic Dispersion
Ultrasonic dispersion140 utilizes high-frequency ultrasonic vibrations to break down large particles into nanoscale ones. The cavitation effect generated by ultrasound in a liquid medium forms numerous tiny gas bubbles, which rapidly collapse under pressure changes, releasing significant energy to disperse aggregated particles into smaller NPs. This method is often combined with emulsification, and by controlling the ultrasonic frequency and duration, the size of NPs can be effectively regulated. Apolipoprotein A1 is a recognized biomarker for AS and other cardiovascular diseases, making its detection critical for AS diagnosis. Sun et al141 used ultrasonic dispersion to synthesize a nanocomposite material, Ce-MOF@AuNPs, which exhibited high selectivity, good stability, and reproducibility, making it suitable for AS diagnosis and detection.
Ion Gelation
Ion gelation is a method for forming NPs through ionic crosslinking, commonly used to prepare polysaccharide or protein-based NPs with good biocompatibility, particularly those using chitosan as a carrier.142 In this process, the charged polymer solution rapidly crosslinks upon contact with the corresponding crosslinking agent through electrostatic interactions, forming stable nanogels.143 This method can encapsulate various active molecules, including water-soluble drugs and proteins. Nguyen et al144 used chitosan as a carrier for miRNA delivery via ion gelation, optimizing the process to obtain NPs of 150–200 nm. Their study showed that miRNA could be effectively delivered to macrophages via NPs, where it regulated cholesterol efflux to alleviate AS lesions.
Nanoprecipitation
Nanoprecipitation is a method that forms NPs by inducing solute precipitation through the mixing of a solvent and nonsolvent. Typically, hydrophobic drugs and polymers are dissolved in an organic solvent and then slowly added to an aqueous or other nonsolvent phase. This sudden decrease in solubility causes the drug and polymer to deposit on the droplet surface, ultimately forming stable NPs. One study145 used this method to develop an environmentally friendly magnetic mesoporous NP, coating Fe₃O₄ with ZIF to enhance its surface area and chemical stability, with potential applications in AS treatment.
Self-Assembly
Self-assembly is a widely used preparation method in recent years. It relies on non-covalent interactions, such as electrostatic forces, hydrophobic interactions, and hydrogen bonds, to spontaneously arrange molecules into nanostructures. This process typically occurs under mild conditions, where amphiphilic molecules with hydrophobic or hydrophilic groups aggregate in aqueous or organic phases to form stable, multifunctional NPs.146,147 Wu et al148 mixed tannic acid with polyoxylamine in equal proportions and added the mixture to deionized water within a dialysis bag, where NPs spontaneously formed under stirring. In vitro studies showed that tannic acid NPs inhibited LPS-induced macrophage inflammation by scavenging ROS, downregulating pro-inflammatory cytokines, and modulating macrophage polarization. Zhao et al149 also exploited the self-assembly properties of simvastatin to develop a fibrin-targeted delivery system for co-delivering simvastatin and ticagrelor. Both in vitro and in vivo experiments demonstrated that the nanoparticle drug delivery system exhibited good stability, reduced off-target drug leakage, and showed excellent anti-inflammatory and antioxidant effects for treating AS.
Applications of Nanoparticles in Atherosclerosis Treatment
Targeted Drug Delivery
The core advantage of NPs in drug delivery lies in their targeting ability. On one hand, formulating drugs with carriers into NPs can reduce drug clearance in the body, prolonging their therapeutic duration.150 On the other hand, surface modification with targeting molecules, such as antibodies, ligands, or small molecules, enables specific delivery to AS lesion sites.151 This targeted approach reduces drug toxicity to normal tissues, enhances therapeutic efficacy, and minimizes systemic side effects associated with conventional drug treatments. AS lesions are often deep and difficult to access, but targeted delivery via NPs not only increases drug concentration at the affected sites but also improves drug stability in the body.
Bruton’s tyrosine kinase (BTK) has emerged as a novel therapeutic target for AS. Wang et al152 introduced a poly(dopamine)-based nanoparticle drug delivery system to deliver the BTK inhibitor Ibrutinib to AS plaques with active targeting properties. This system utilizes the pH sensitivity of poly(dopamine) for controlled drug release, while inhibiting NF-κB pathway activation in B cells and NOD-like Receptor Pyrin Domain Containing 3 (NLRP3) inflammasome activation in macrophages within plaques. Li et al153 designed a biomimetic NP system (MM/MTXNPs) based on hyaluronic acid and macrophage membranes to deliver methotrexate (MTX), as shown in Figure 7A. TEM imaging revealed that NPs without macrophage membrane (MTXNPs) were spherical and uniform, whereas MM/MTXNPs exhibited a clear “core-shell” structure (Figure 7B), with the core being MTXNPs and the shell being the macrophage membrane. SDS-PAGE showed no protein bands for MTXNPs, but MM/MTXNPs displayed protein bands similar to total macrophage proteins and macrophage vesicles, confirming successful membrane coating (Figure 7C). After co-incubating DiD-labeled MM/MTXNPs and MTXNPs with macrophages for 4 h, a reduction in red fluorescence accumulation was observed in MM/DiDNPs-treated cells compared to MTXNPs, indicating that macrophage membranes can help NPs evade macrophage phagocytosis (Figure 7D). Cytotoxicity assays showed a gradual decrease in cell viability as MTX concentration increased (Figure 7E), with cell viability remaining above 80% even at 30 μM MTX, and MM/MTXNPs exhibiting higher viability than MTXNPs. Hemolysis tests revealed significant hemolysis in the positive control, while MTXNPs, MM/MTXNPs, and negative controls showed no hemolysis, with RBCs deposited at the tube bottom (Figure 7F). To evaluate in vivo circulation, fluorescence intensity was measured in blood samples from mice injected with MM/MTXNPs and MTXNPs, showing significantly higher and sustained fluorescence from MM/MTXNPs for up to 48 h (Figure 7G). Oil Red O staining of aortic tissues post-treatment showed that MM/MTXNPs were the most effective, with reduced lipid deposition and enhanced drug targeting due to the macrophage membrane coating (Figure 7H).
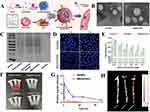 |
Figure 7 (A) Schematic of MM/MTXNPs fabrication. (B) TEM images of MTXNPs and MM/MTXNPs (the scale bar is 100nm). (C) SDS-PAGE images of membrane proteins of total macrophage proteins, macrophage vesicles, MM/MTXNPs and MTXNPs. (D) Laser confocal images of uptake of DiDNPs and MM/DiDNPs by macrophages (DAPI-stained nuclei in blue, fluorescently labeled nanoparticles in red, the scale bar is 20 μm). (E) Cell viability of macrophages after treatment with MTX, MTXNPs and MM/MTXNPs. (F) Pictures of hemolysis tests including the positive control, negative control, MTXNPs and MM/MTXNPs. (G) Fluorescence intensity of MTXNPs and MM/MTXNPs circulating in mice at different times. (H) Vascular fluorescence images of MTXNPs and MM/MTXNPs in mice after 24 h of circulation in vivo. Adapted with permission from Fontana F, Molinaro G, Moroni S, et al. Biomimetic platelet-cloaked nanoparticles for the delivery of anti-inflammatory curcumin in the treatment of atherosclerosis. Adv Healthc Mater. 2024;13(15):e2302074.154 |
Anti-Inflammatory Therapy
Inflammation plays a crucial role in the pathogenesis of AS, as persistent inflammation not only promotes plaque formation and progression but also increases the risk of plaque rupture and thrombosis. Therefore, targeting inflammation is a key direction in AS precision medicine. The controlled release property of NPs allows anti-inflammatory drugs to be continuously released at the lesion site, maintaining therapeutic concentrations and enhancing treatment efficacy. Curcumin, an effective anti-inflammatory drug for AS, was formulated into NPs with a platelet membrane coating by Fontana et al.154 The study demonstrated that the NPs exhibited cell compatibility with endothelial cells, smooth muscle cells, and immune cells without inducing immune activation. Real-time fluorescence qPCR and ELISA analysis of endothelial cell-related factors confirmed the anti-inflammatory effects, showing reduced expression of NF-κB, Transforming Growth Factor Beta 1 (TGF-β1), IL-6, and IL-1β in inflammatory cells. In another study,155 red blood cell membrane-coated probucol was used to create biomimetic NPs (RP-PU). In vitro experiments showed good sustained-release properties and excellent biocompatibility. RP-PU reduced collagen fibers in aortic root sections and inhibited foam cell formation, decreasing the expression of ICAM-1 and MCP-1, thus delaying lesion development.
Vascular Repair and Regeneration
The goal of vascular repair and regeneration is to restore endothelial integrity, promote neovascularization, and repair damaged vessel walls, thereby halting or reversing the pathological progression of AS.156 Due to their targeting, efficient delivery, and multifunctionality, NPs show great potential in vascular repair and regeneration. Endothelial progenitor cells (EPCs) are associated with the prognosis of CVDs and play a critical role in the development of AS. Wei et al157 explored the potential of superparamagnetic iron oxide NPs (USPION)-labeled EPCs for non-invasive monitoring and evaluated the therapeutic outcomes in an AS rabbit model. The study found that labeled EPCs formed capillary-like structures, expressing high levels of CD31, CD34, and VEGFR2. Histopathological analysis revealed USPION-stained EPCs in the vascular lesions of the rabbit model one day post-transplantation.
Stabilizing Plaques and Clearing Lipids
The core pathology of AS is the formation and instability of arterial plaques, with plaque rupture often leading to acute cardiovascular events. Therefore, stabilizing plaques and clearing lipids have become important therapeutic targets for AS. NPs can efficiently deliver drugs to plaque sites, reducing the expression of inflammatory cytokines and inflammation cell infiltration, thereby improving plaque stability.158 Additionally, surface-modified NPs can specifically target and clear excess lipids in the vasculature. These NPs can also enhance macrophage lipid clearance, promoting the transformation of foam cells into anti-inflammatory and lipid-excreting phenotypes, accelerating lipid metabolism within plaques.159
Src homology 2 domain-containing phosphatase-1 inhibitor (SHP-1i) is a small molecule inhibitor of the CD47 downstream pathway that mediates macrophage phagocytosis, enhancing the clearance of apoptotic cells and reducing cholesterol accumulation. Sha et al160 designed macrophage membrane-coated SHP-1i liposomal biomimetic NPs (MM@Lips-SHP1i) for lipid clearance in AS. Macrophage membranes were extracted using a low-osmolarity lysis method, while SHP-1i liposomes were prepared using the film dispersion method, followed by mechanical co-extrusion to form MM@Lips-SHP1i. MM@Lips-SHP1i had a size of 199.1 nm and a zeta potential of −11.18 mV. UV-visible spectroscopy confirmed characteristic absorption peaks at 230 nm, 320 nm, and 490 nm for SHP1i, and MM@Lips-SHP1i retained these peaks, indicating successful encapsulation of SHP1i (Figure 8A–C). The nanoparticles showed good stability after 7 d in PBS (Figure 8D). Studies revealed that MM@Lips-SHP1i could mitigate the effects of LPS on macrophages, suppressing inflammation and significantly reducing TNF-α, IL-6, and IFN-γ levels (Figure 8E–G). DCFH-DA fluorescence probes showed that MM@Lips-SHP1i had the lowest green fluorescence, indicating the most effective ROS inhibition (Figure 8H–I). Additionally, MM@Lips-SHP1i effectively inhibited ox-LDL-induced macrophage apoptosis, reducing late and early apoptotic cells to 5.36% and 9.94%, respectively (Figure 8J).
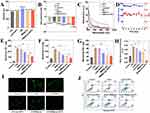 |
Figure 8 (A) Hydrodynamic diameters of Lips, Lips-SHP1i, MM, MM@Lips and MM@Lips-SHP1i. (B) Zeta potential of SHP1i, Lips, Lips-SHP1i, MM, MM@Lips and MM@Lips-SHP1i. (C) UV–vis absorption spectra of SHP1i, Lips, Lips-SHP1i, MM, MM@Lips and MM@Lips-SHP1i. (D) The size stability of MM@Lips-SHP1i NPs over a span of 7 days in PBS. (E–G) The levels of pro-inflammatory cytokines, including TNF-α, IL-6 and IFN-γ in RAW264.7 supernatant after different treatments, *P<0.05, **P<0.01, ***P<0.001 compared with the Control. (H) Analysis for mean DCFH-DA fluorescence intensity. (I) Fluorescence images observed the effect of different nanoparticles on ROS generation in RAW264.7 cells. The cells were stained with DCFH-DA fluorescent probe and photographed under the fluorescence microscope. (J) Flow cytometric analysis of cell apoptosis after different treatments determined by the Annexin V-FITC/PI assay. Adapted with permission from Sha X, Dai Y, Chong L, et al. Pro-efferocytic macrophage membrane biomimetic nanoparticles for the synergistic treatment of atherosclerosis via competition effect. J Nanobiotechnology. 2022;20(1):506. https://creativecommons.org/licenses/by/4.0/.160 |
Gene Therapy
Gene therapy, as a strategy targeting the root causes of diseases, can effectively influence the pathogenesis of AS by modifying or regulating gene expression. NPs provide a protective barrier for gene drugs, preventing degradation in the bloodstream while enabling efficient accumulation at plaque sites through the modification of specific targeting ligands, such as antibodies or peptides targeting endothelial cells or macrophages.161 Additionally, the multifunctional properties of NPs allow for the combination of gene therapy with other treatment modalities to achieve synergistic effects. Distasio et al162 designed and developed branched poly(β-amino ester) NPs containing plasmid DNA encoding Interleukin-10 (IL-10), which were modified with a specific targeting peptide, VHPK. Their study showed that IL-10-loaded NPs specifically targeted inflamed plaques, promoting endothelial cell gene transcription and reducing inflammation levels. Bai et al163 prepared NPs containing microRNA-146a oligonucleotides, and their research confirmed that these microRNA NPs could regulate the NF-κB pathway, targeting A-type scavenger receptors on plaque macrophages and endothelial cells, thereby effectively treating AS.
Multifunctional Integrated Diagnosis and Therapy
The limitations of single therapeutic strategies in addressing the complex pathological mechanisms of AS are increasingly apparent. Multifunctional integrated NPs can combine diagnostic and therapeutic functions by integrating drug delivery, targeted modification, and imaging, effectively addressing the complex pathology of AS for personalized diagnosis and treatment. Zhang et al164 developed a pH-responsive NP coupled with profilin-1 antibody (PFN1) using the specificity of superparamagnetic iron oxide. Their study showed that these NPs readily bind to vascular smooth muscle cells and accumulate in plaques to exert anti-AS effects. Ma et al165 prepared NPs modified with hyaluronic acid to encapsulate rosuvastatin for AS mouse studies, demonstrating that NPs administration led to plaque regression, achieving simultaneous therapy and imaging.
The multifunctional integration of NPs is reflected not only in the combination of diagnosis and therapy but also in the integration of multiple therapeutic functions during the treatment process. Luo et al166 proposed the construction of polydopamine NPs encapsulating NLRP3 inhibitor (CY-09) and conjugated with anti-CD47 antibodies—DNPC-αCD47 NPs—to achieve synergistic treatment both intracellularly and extracellularly, thereby restoring macrophage function. Briefly, DNPC-αCD47 NPs were prepared by stepwise stirring, as shown in Figure 9A, with an average particle size of 320 nm and a zeta potential of −35 mV (Figure 9B and C). HPLC analysis of CY-09 release in vitro revealed a release rate of 60% within 12 h in PBS (Figure 9D). To investigate the cellular uptake and endocytic pathway of DNPC-αCD47 NPs, Ce6 was encapsulated in the NPs and detected by flow cytometry. The results showed that the fluorescence intensity increased with incubation time and DNPC-αCD47 NPs concentration, indicating that DNPC-αCD47 NPs were rapidly and easily absorbed by macrophages (Figure 9E and F). Co-localization analysis using confocal microscopy after co-incubation with lysosome probes revealed no significant co-localization, suggesting that DNPC-αCD47 NPs can escape from the lysosome into the cytoplasm (Figure 9G). In vitro immunofluorescence and WB results showed a reduction in NLRP3 expression, with the DNPC-αCD47 NPs group exhibiting stronger inhibition (Figure 9H and I). In vivo experiments demonstrated that all drug treatments reduced AS plaque to varying degrees, with Oil Red O staining showing a significant reduction in necrotic areas of aortic plaques in the DNPC-αCD47 NPs group (Figure 9J).
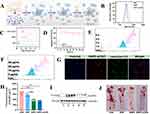 |
Figure 9 (A) Schematic illustration of the preparation of DNP, DNPC and DNPC-αCD47 NPs. (B and C) Size and zeta potentials of DNPC-αCD47 NPs. (D) CY-09 release curve of DNPC, DNPC-αCD47 and free CY-09. (E–F) Fluorescence intensity of macrophages after incubation with DNPC-αCD47 at different times or different concentrations. (G) Co-localization of DNPC-αCD47 and lysotracker (Scale bar: 10 μm). (H) Semi-quantitative analysis of expression area of NLRP3, ***P<0.001 compared with the Control. (I) Expression of NLRP3 protein by Western blot (Lane1: Blank, Lane2: LPS + ATP, Lane3: LPS +ATP + DNP, Lane4: LPS + ATP + DNPC, Lane5: LPS + ATP + DNPC-αCD47 NPs). (J) Representative images of oil red O stained descending aorta. Adapted with permission from Luo Q, Dai L, Li J, et al. Intracellular and extracellular synergistic therapy for restoring macrophage functions via anti-CD47 antibody-conjugated bifunctional nanoparticles in atherosclerosis. Bioact Mater. 2024;34:326–337. https://creativecommons.org/licenses/by/4.0/.166 |
The Future Prospective
Despite significant advances in the application of NPs in precision medicine for AS, clinical translation remains challenging. A critical concern is the long-term biocompatibility and safety of NPs, which require further in-depth evaluation.167 Most studies are still limited to small animal models, and the successful translation of these findings into clinical practice remains a key hurdle.168 Biocompatibility, essential for minimizing immune responses and toxicity, is a major determinant of NPs safety. Insufficient biocompatibility may trigger allergic reactions, tissue damage, or chronic inflammation. Additionally, the biodegradability of NPs is crucial; ideally, they should undergo enzymatic or metabolic degradation post-drug delivery to prevent prolonged accumulation and potential toxicity. However, different NP types—metallic, polymeric, and lipid-based—exhibit varying degradation rates, metabolic pathways, and biocompatibility profiles. Thus, systematic assessment of NP degradation products is essential to ensure long-term safety in clinical applications.
The fabrication of NPs requires further optimization to enhance drug loading capacity, stability, and controlled release, thereby improving clinical applicability. As the manufacturing process directly influences drug encapsulation, in vivo stability, and therapeutic efficacy, future research should focus on developing more efficient and precise fabrication strategies. Current techniques face challenges such as limited drug loading, uneven distribution, and suboptimal release kinetics, hindering clinical translation. Additionally, drug–carrier compatibility issues may further compromise encapsulation efficiency and therapeutic performance. Stability remains a critical concern, as NPs are susceptible to aggregation or degradation under physiological conditions, including variations in solvent composition, temperature, and pH, particularly during circulation. To address this, advanced surface modification techniques, such as hydrophilic polymer coatings, biomimetic membranes, and stimuli-responsive layers, should be explored to enhance in vivo stability and biocompatibility. Controlled drug release remains another key area for optimization, as many existing NPs exhibit either premature or delayed release, limiting therapeutic precision. Emerging strategies, including stimuli-responsive NPs triggered by pH, temperature, enzymes, or magnetic fields, as well as hybrid carrier systems, offer promising solutions for on-demand drug delivery.169 However, further refinement is needed to ensure precise drug release tailored to specific pathological conditions. Future advancements in NPs fabrication should prioritize a balance between drug loading efficiency, stability, and controlled release. Multidisciplinary collaboration integrating nanotechnology, materials science, and pharmaceutics will be essential to overcoming current limitations, ultimately advancing the clinical application of NPs in precision medicine and personalized therapy.
The challenges faced by nanocarriers are not only technological bottlenecks but also provide new perspectives and opportunities for researchers. The safety concerns drive scientists to explore innovative biomaterials and intelligent applications. In the future, the development of biomimetic materials and smart nanocarriers will be key breakthroughs, such as stimuli-responsive nanocarriers and targeted delivery systems, which can be customized based on the pathological features of different diseases to meet personalized treatment needs. For example, disease-specific microenvironment-responsive nanoparticles, designed for conditions like low pH or peroxidase activity, can enhance drug targeting and enable precise release, minimizing damage to healthy tissues.170,171 This technological advancement not only offers new insights for atherosclerosis treatment but also provides important implications for precision treatment of other diseases, promoting the integration of precision and personalized medicine. Multifunctional nanoplatforms are a promising research direction,172 capable of integrating disease diagnosis, treatment, and monitoring, ensuring precise drug release during imaging and diagnosis, and evaluating therapeutic effects in real-time. Through this integration, nanoplatforms can play a role in early disease detection and dynamically regulate drug release during treatment, ensuring more personalized and efficient therapies.173 The continuous evolution of this technology will pave new paths for precision medicine and elevate personalized treatment to a higher level.
NPs have opened broad prospects for precision medicine in AS treatment. With the continuous advancement of nanotechnology, NPs not only enable efficient targeted drug delivery but also integrate imaging and real-time monitoring, providing more precise solutions for personalized treatment. By improving drug bioavailability, stability, and controlled release, NPs can maximize therapeutic effects while minimizing impact on healthy tissues, thus reducing side effects. In the future, with the optimization of nanofabrication technologies and the innovation of multifunctional NPs platforms, NPs will become more refined and intelligent in AS treatment. Through biomimetic modifications, adaptive delivery systems, and intelligent-responsive carriers, NPs are expected to be personalized according to patients’ pathological characteristics, achieving precise and effective treatment strategies. Moreover, interdisciplinary integration and technological innovation will further propel the use of NPs in early diagnosis, targeted therapy, and efficacy monitoring of AS, making them key tools in precision medicine. In summary, the widespread application of NPs is driving a deep shift from traditional AS treatments to precision medicine, offering safer and more effective solutions to patients and improving quality of life. As research progresses, nanotechnology is expected to become a breakthrough in AS treatment, advancing cardiovascular disease therapy into a new era of precision and personalization.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This article was supported by the National Natural Science Foundation of China (82174343), Key Discipline Project on Chinese Pharmacology of Hunan University of Chinese Medicine (202302), Postgraduate Scientific Research Innovation Project of Hunan Province (CX20230830), Hunan University of Chinese Medicine 2023 Provincial Undergraduate Innovation and Entrepreneurship Training Program Project (S202310541090), Hunan University of Chinese Medicine 2023 Undergraduate Research Innovation Project (2023BKS098), Scientific Research Topics of Hunan Provincial Health and Wellness Commission (D202303017861) and Hunan Province Key Research Project in Traditional Chinese Medicine (A2023017).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Soppert J, Lehrke M, Marx N, et al. Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv Drug Deliv Rev. 2020;159:4–33. doi:10.1016/j.addr.2020.07.019
2. Fischer MA, Vondriska TM. Clinical epigenomics for cardiovascular disease: diagnostics and therapies. J mol Cell Cardiol. 2021;154:97–105. doi:10.1016/j.yjmcc.2021.01.011
3. Heidary Moghaddam R, Samimi Z, Moradi SZ, et al. Naringenin and naringin in cardiovascular disease prevention: a preclinical review. Eur J Pharmacol. 2020;887:173535. doi:10.1016/j.ejphar.2020.173535
4. Di Fusco SA, Aquilani S, Spinelli A, et al. The polypill strategy in cardiovascular disease prevention: it’s time for its implementation. Prog Cardiovasc Dis. 2023;79:37–40. doi:10.1016/j.pcad.2023.03.003
5. Yanai H, Adachi H, Hakoshima M, et al. Metabolic-dysfunction-associated steatotic liver disease-its pathophysiology, association with atherosclerosis and cardiovascular disease, and treatments. Int J mol Sci. 2023;24(20):15473. doi:10.3390/ijms242015473
6. Tucker B, Goonetilleke N, Patel S, et al. Colchicine in atherosclerotic cardiovascular disease. Heart. 2024;110(9):618–625. doi:10.1136/heartjnl-2023-323177
7. Jebari-Benslaiman S, Larrea-Sebal A, Benito-Vicente A, et al. Cardiovascular disease, atherosclerosis and familial hypercholesterolemia: from molecular mechanisms causing pathogenicity to new therapeutic approaches. Int J mol Sci. 2023;24(8):7659. doi:10.3390/ijms24087659
8. Zheng WC, Chan W, Dart A, et al. Novel therapeutic targets and emerging treatments for atherosclerotic cardiovascular disease. Eur Heart J Cardiovasc Pharmacother. 2024;10(1):53–67. doi:10.1093/ehjcvp/pvad074
9. Vasan RS, Pan S, Larson MG, et al. Arteriosclerosis, atherosclerosis, and cardiovascular health: joint relations to the incidence of cardiovascular disease. Hypertension. 2021;78(5):1232–1240. doi:10.1161/HYPERTENSIONAHA.121.18075
10. Gnanenthiran SR, Agarwal A, Patel A. Frontiers of cardiovascular polypills: from atherosclerosis and beyond. Trends Cardiovasc Med. 2023;33(3):182–189. doi:10.1016/j.tcm.2021.12.013
11. Abramson BL, Grégoire J. The challenges of contemporary atherosclerotic cardiovascular disease management. Can J Cardiol. 2024;40(8S):S1–S3. doi:10.1016/j.cjca.2024.03.019
12. Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci. 2018;132(12):1243–1252. doi:10.1042/CS20180306
13. Poznyak AV, Bharadwaj D, Prasad G, et al. Renin-angiotensin system in pathogenesis of atherosclerosis and treatment of CVD. Int J mol Sci. 2021;22(13):6702. doi:10.3390/ijms22136702
14. Xu S, Ilyas I, Little PJ, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. 2021;73(3):924–967. doi:10.1124/pharmrev.120.000096
15. Zarzycka B, Nicolaes GA, Lutgens E. Targeting the adaptive immune system: new strategies in the treatment of atherosclerosis. Expert Rev Clin Pharmacol. 2015;8(3):297–313. doi:10.1586/17512433.2015.1025052
16. Gawaz M, Geisler T, Borst O. Current concepts and novel targets for antiplatelet therapy. Nat Rev Cardiol. 2023;20(9):583–599. doi:10.1038/s41569-023-00854-6
17. Lordan R, Tsoupras A, Zabetakis I. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: potential role of antiplatelet agents. Blood Rev. 2021;45:100694. doi:10.1016/j.blre.2020.100694
18. Zhi W, Liu Y, Wang X, et al. Recent advances of traditional Chinese medicine for the prevention and treatment of atherosclerosis. J Ethnopharmacol. 2023;301:115749. doi:10.1016/j.jep.2022.115749
19. Ma X, Zhang L, Gao F, et al. Salvia miltiorrhiza and Tanshinone IIA reduce endothelial inflammation and atherosclerotic plaque formation through inhibiting COX-2. Biomed Pharmacother. 2023;167:115501. doi:10.1016/j.biopha.2023.115501
20. Wang J, Zhang Y, Feng X, et al. Tanshinone IIA alleviates atherosclerosis in LDLR mice by regulating efferocytosis of macrophages. Front Pharmacol. 2023;14:1233709. doi:10.3389/fphar.2023.1233709
21. Zhang Y, Li D, Jia Z, et al. Zhizi-Chuanxiong herb pair alleviates atherosclerosis progression in ApoE-/- mice by promoting the methylation of FGFR3 to inhibit MAPK/ERK-mediated apoptosis. J Ethnopharmacol. 2024;319(Pt 1):117188. doi:10.1016/j.jep.2023.117188
22. Wang C, Niimi M, Watanabe T, et al. Treatment of atherosclerosis by traditional Chinese medicine: questions and quandaries. Atherosclerosis. 2018;277:136–144. doi:10.1016/j.atherosclerosis.2018.08.039
23. Liu H, Zhu L, Chen L, et al. Therapeutic potential of traditional Chinese medicine in atherosclerosis: a review. Phytother Res. 2022;36(11):4080–4100. doi:10.1002/ptr.7590
24. Jing J, Zhu C, Gong R, et al. Research progress on the active ingredients of traditional Chinese medicine in the intervention of atherosclerosis: a promising natural immunotherapeutic adjuvant. Biomed Pharmacother. 2023;159:114201. doi:10.1016/j.biopha.2022.114201
25. Li B, Shao H, Gao L, et al. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: a review. Drug Deliv. 2022;29(1):2130–2161. doi:10.1080/10717544.2022.2094498
26. Rai S, Singh N, Bhattacharya S. Concepts on smart nano-based drug delivery system. Recent Pat Nanotechnol. 2022;16(1):67–89. doi:10.2174/1872210515666210120113738
27. Huang L, Huang XH, Yang X, et al. Novel nano-drug delivery system for natural products and their application. Pharmacol Res. 2024;201:107100. doi:10.1016/j.phrs.2024.107100
28. Lv W, Liu Y, Li S, et al. Advances of nano drug delivery system for the theranostics of ischemic stroke. J Nanobiotechnology. 2022;20(1):248. doi:10.1186/s12951-022-01450-5
29. Najahi-Missaoui W, Arnold RD, Cummings BS. Safe nanoparticles: are we there yet? Int J mol Sci. 2020;22(1):385. doi:10.3390/ijms22010385
30. Cho H, Huh KM, Cho HJ, et al. Beyond nanoparticle-based oral drug delivery: transporter-mediated absorption and disease targeting. Biomater Sci. 2024;12(12):3045–3067. doi:10.1039/D4BM00313F
31. Gong Z, Yang H, Gao L, et al. Mechanisms of wogonoside in the treatment of atherosclerosis based on network pharmacology, molecular docking, and experimental validation. BMC Complement Med Ther. 2025;25(1):28. doi:10.1186/s12906-025-04760-x
32. Cheng G, Liu J, Zhang H, et al. Sortilin as a culprit in the atherosclerosis plaque progression: evidence from clinical and experimental studies. Curr Mol Med.
33. Zhu L, Liu Z, Liu J, et al. NCOA4 linked to endothelial cell ferritinophagy and ferroptosis:a key regulator aggravate aortic endothelial inflammation and atherosclerosis. Redox Biol. 2025;79:103465. doi:10.1016/j.redox.2024.103465
34. Men J, Li H, Cui C, et al. Fecal bacteria transplantation replicates aerobic exercise to reshape the gut microbiota in mice to inhibit high-fat diet-induced atherosclerosis. PLoS One. 2025;20(2):e0314698. doi:10.1371/journal.pone.0314698
35. Mey L, Bonaterra GA, Hoffmann J, et al. PAC1 agonist maxadilan reduces atherosclerotic lesions in hypercholesterolemic ApoE-deficient mice. Int J mol Sci. 2024;25(24):13245. doi:10.3390/ijms252413245
36. Wang Y, Ohishi H, Wu R, et al. Prophylactic and therapeutic effects of EsV3 on atherosclerotic lesions in ApoE-/- mice. BMC Cardiovasc Disord. 2025;25(1):54. doi:10.1186/s12872-025-04497-y
37. Tan H, Liu L, Qi Y, et al. Atorvastatin attenuates endothelial cell injury in atherosclerosis through inhibiting ACSL4-mediated ferroptosis. Cardiovasc Ther. 2024;2024:5522013. doi:10.1155/2024/5522013
38. Tang F, Tian LH, Zhu XH, et al. METTL3 -mediated m6A modification enhances lncRNA H19 stability to promote endothelial cell inflammation and pyroptosis to aggravate atherosclerosis. FASEB J. 2024;38(20):e70090. doi:10.1096/fj.202401337RR
39. Ali AH, Younis N, Abdallah R, et al. Lipid-lowering therapies for atherosclerosis: statins, fibrates, ezetimibe and PCSK9 monoclonal antibodies. Curr Med Chem. 2021;28(36):7427–7445. doi:10.2174/0929867328666210222092628
40. Abduljabbar MH. PCSK9 inhibitors: focus on evolocumab and its impact on atherosclerosis progression. Pharmaceuticals. 2024;17(12):1581. doi:10.3390/ph17121581
41. Donadini MP, Calcaterra F, Romualdi E, et al. The link between venous and arterial thrombosis: is there a role for endothelial dysfunction? Cells. 2025;14(2):144. doi:10.3390/cells14020144
42. Strohm L, Ubbens H, Mihalikova D, et al. CD40-TRAF6 inhibition suppresses cardiovascular inflammation, oxidative stress and functional complications in a mouse model of arterial hypertension. Redox Biol.
43. Zhou QY, Pan JQ, Liu W, et al. Angiotensin II: a novel biomarker in vascular diseases. Clin Chim Acta. 2025;568:120154. doi:10.1016/j.cca.2025.120154
44. Urbanowicz TK, Gąsecka A, Olasińska-Wiśniewska A, et al. Plasma leukocyte-derived extracellular vesicles are related to target vessel revascularization in off-pump coronary artery bypass grafting. Postepy Kardiol Interwencyjnej. 2024;20(4):433–442. doi:10.5114/aic.2024.145576
45. Man AWC, Li H, Xia N. Circadian rhythm: potential therapeutic target for atherosclerosis and thrombosis. Int J mol Sci. 2021;22(2):676. doi:10.3390/ijms22020676
46. Wang C, Li Z, Liu Y, et al. Exosomes in atherosclerosis: performers, bystanders, biomarkers, and therapeutic targets. Theranostics. 2021;11(8):3996–4010. doi:10.7150/thno.56035
47. Wilson HM. The intracellular signaling pathways governing macrophage activation and function in human atherosclerosis. Biochem Soc Trans. 2022;50(6):1673–1682. doi:10.1042/BST20220441
48. Li Y, Zhou M, Li H, et al. Macrophage P2Y6 receptor deletion attenuates atherosclerosis by limiting foam cell formation through phospholipase Cβ/store-operated calcium entry/calreticulin/scavenger receptor A pathways. Eur Heart J. 2024;45(4):268–283. doi:10.1093/eurheartj/ehad796
49. Duan M, Chen H, Yin L, et al. Mitochondrial apolipoprotein A-I binding protein alleviates atherosclerosis by regulating mitophagy and macrophage polarization. Cell Commun Signal. 2022;20(1):60. doi:10.1186/s12964-022-00858-8
50. Yang J, Xu H, Chen K, et al. Platelets-derived miR-200a-3p modulate the expression of ET-1 and VEGFA in endothelial cells by targeting MAPK14. Front Physiol. 2022;13:893102. doi:10.3389/fphys.2022.893102
51. Shafi O. Switching of vascular cells towards atherogenesis, and other factors contributing to atherosclerosis: a systematic review. Thromb J. 2020;18:28. doi:10.1186/s12959-020-00240-z
52. Chen R, McVey DG, Shen D, et al. Phenotypic switching of vascular smooth muscle cells in atherosclerosis. J Am Heart Assoc. 2023;12(20):e031121. doi:10.1161/JAHA.123.031121
53. Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118(4):692–702. doi:10.1161/CIRCRESAHA.115.306361
54. Zhang T, Wu S, Xu R, et al. Musashi-2 binds with Fbxo6 to induce Rnaset2 ubiquitination and chemokine signaling pathway during vascular smooth muscle cell phenotypic switch in atherosclerosis. Cell Signal. 2023;111:110869. doi:10.1016/j.cellsig.2023.110869
55. San Antonio JD, Jacenko O, Fertala A, et al. Collagen structure-function mapping informs applications for regenerative medicine. Bioengineering. 2020;8(1):3. doi:10.3390/bioengineering8010003
56. Osidak MS, Osidak EO, Akhmanova MA, et al. Fibrillar, fibril-associated and basement membrane collagens of the arterial wall: architecture, elasticity and remodeling under stress. Curr Pharm Des. 2015;21(9):1124–1133. doi:10.2174/1381612820666141013122906
57. Smith KA, Lin AH, Stevens AH, et al. Collagen molecular damage is a hallmark of early atherosclerosis development. J Cardiovasc Transl Res. 2023;16(2):463–472. doi:10.1007/s12265-022-10316-y
58. Di Nubila A, Dilella G, Simone R, et al. Vascular extracellular matrix in atherosclerosis. Int J mol Sci. 2024;25(22):12017. doi:10.3390/ijms252212017
59. Konieczyńska M, Natorska J, Undas A. Thrombosis and aging: fibrin clot properties and oxidative stress. Antioxid Redox Signal. 2024;41(4–6):233–254. doi:10.1089/ars.2023.0365
60. Wang Y, Qin B, Xia G, et al. FDA’s poly (Lactic-Co-Glycolic Acid) research program and regulatory outcomes. AAPS J. 2021;23(4):92. doi:10.1208/s12248-021-00611-y
61. Rocha CV, Gonçalves V, da Silva MC, et al. PLGA-based composites for various biomedical applications. Int J mol Sci. 2022;23(4):2034. doi:10.3390/ijms23042034
62. Chereddy KK, Payen VL, Préat V. From a classic drug carrier to a novel therapeutic activity contributor. J Control Release. 2018;289:10–13. doi:10.1016/j.jconrel.2018.09.017
63. El-Hammadi MM, Arias JL. Recent advances in the surface functionalization of PLGA-based nanomedicines. Nanomaterials. 2022;12(3):354. doi:10.3390/nano12030354
64. Dou Y, Chen Y, Zhang X, et al. Non-proinflammatory and responsive nanoplatforms for targeted treatment of atherosclerosis. Biomaterials. 2017;143:93–108. doi:10.1016/j.biomaterials.2017.07.035
65. Jan N, Bostanudin MF, Moutraji SA, et al. Unleashing the biomimetic targeting potential of platelet-derived nanocarriers on atherosclerosis. Colloids Surf B Biointerfaces. 2024;240:113979. doi:10.1016/j.colsurfb.2024.113979
66. Tang J, Li T, Xiong X, et al. Colchicine delivered by a novel nanoparticle platform alleviates atherosclerosis by targeted inhibition of NF-κB/NLRP3 pathways in inflammatory endothelial cells. J Nanobiotechnology. 2023;21(1):460. doi:10.1186/s12951-023-02228-z
67. Todaro B, Moscardini A, Luin S. Pioglitazone-Loaded PLGA nanoparticles: towards the most reliable synthesis method. Int J mol Sci. 2022;23(5):2522. doi:10.3390/ijms23052522
68. Pillai SC, Borah A, MNT L, et al. Co-delivery of curcumin and bioperine via PLGA nanoparticles to prevent atherosclerotic foam cell formation. Pharmaceutics. 2021;13(9):1420. doi:10.3390/pharmaceutics13091420
69. D’souza AA, Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv. 2016;13(9):1257–1275. doi:10.1080/17425247.2016.1182485
70. Tenchov R, Sasso JM, Zhou QA. PEGylated lipid nanoparticle formulations: immunological safety and efficiency perspective. Bioconjug Chem. 2023;34(6):941–960. doi:10.1021/acs.bioconjchem.3c00174
71. Guo C, Yuan H, Wang Y, et al. The interplay between PEGylated nanoparticles and blood immune system. Adv Drug Deliv Rev. 2023;200:115044. doi:10.1016/j.addr.2023.115044
72. Mishra S, Bedja D, Amuzie C, et al. Improved intervention of atherosclerosis and cardiac hypertrophy through biodegradable polymer-encapsulated delivery of glycosphingolipid inhibitor. Biomaterials. 2015;64:125–135. doi:10.1016/j.biomaterials.2015.06.001
73. Lee SY, Kim JH, Song JW, et al. Macrophage-mannose-receptor-targeted photoactivatable agent for in vivo imaging and treatment of atherosclerosis. Int J Pharm. 2024;654:123951. doi:10.1016/j.ijpharm.2024.123951
74. Lin Y, Liu J, Chong SY, et al. Dual-function nanoscale coordination polymer nanoparticles for targeted diagnosis and therapeutic delivery in atherosclerosis. Small. 2024;20(47):e2401659. doi:10.1002/smll.202401659
75. Yu Y, Luo T, Liu S, et al. Chitosan oligosaccharides attenuate atherosclerosis and decrease non-HDL in ApoE-/- Mice. J Atheroscler Thromb. 2015;22(9):926–941. doi:10.5551/jat.22939
76. Sriamornsak P, Dass CR. Chitosan nanoparticles in atherosclerosis-development to preclinical testing. Pharmaceutics. 2022;14(5):935. doi:10.3390/pharmaceutics14050935
77. Hooshyar MR, Raygan S, Mehdinavaz Aghdam R. Investigating layer-by-layer chitosan-dextran sulfate-coated mesoporous silica as a pH-sensitive drug delivery system. J Mater Sci Mater Med. 2024;35(1):29. doi:10.1007/s10856-024-06797-9
78. Dash R, Yadav M, Biswal J, et al. Modeling of chitosan modified PLGA atorvastatin-curcumin conjugate (AT-CU) nanoparticles, overcoming the barriers associated with PLGA: an approach for better management of atherosclerosis. Int J Pharm. 2023;640:123009. doi:10.1016/j.ijpharm.2023.123009
79. Hirpara MR, Manikkath J, Sivakumar K, et al. Long circulating PEGylated-chitosan nanoparticles of rosuvastatin calcium: development and in vitro and in vivo evaluations. Int J Biol Macromol. 2018;107(Pt B):2190–2200. doi:10.1016/j.ijbiomac.2017.10.086
80. Wang Y, Karmakar T, Ghosh N, et al. Targeting mangiferin loaded N-succinyl chitosan-alginate grafted nanoparticles against atherosclerosis - A case study against diabetes mediated hyperlipidemia in rat. Food Chem. 2022;370:131376. doi:10.1016/j.foodchem.2021.131376
81. Du L, Xiao Y, Wei Q, et al. Preparation, evaluation, and bioinformatics study of hyaluronic acid-modified ginsenoside rb1 self-assembled nanoparticles for treating cardiovascular diseases. Molecules. 2024;29(18):4425. doi:10.3390/molecules29184425
82. Jiang T, Xu L, Zhao M, et al. Dual targeted delivery of statins and nucleic acids by chitosan-based nanoparticles for enhanced antiatherosclerotic efficacy. Biomaterials. 2022;280:121324. doi:10.1016/j.biomaterials.2021.121324
83. Abdelhamid HN. Zeolitic imidazolate frameworks (ZIF-8) for biomedical applications: a review. Curr Med Chem. 2021;28(34):7023–7075. doi:10.2174/0929867328666210608143703
84. Wang Q, Sun Y, Li S, et al. Synthesis and modification of ZIF-8 and its application in drug delivery and tumor therapy. RSC Adv. 2020;10(62):37600–37620. doi:10.1039/D0RA07950B
85. Ge Y, Wang K, Liu J, et al. A ZIF-8-based multifunctional intelligent drug release system for chronic osteomyelitis. Colloids Surf B Biointerfaces. 2022;212:112354. doi:10.1016/j.colsurfb.2022.112354
86. Wang Y, Zeng M, Fan T, et al. Biomimetic ZIF-8 nanoparticles: a novel approach for biomimetic drug delivery systems. Int J Nanomed. 2024;19:5523–5544. doi:10.2147/IJN.S462480
87. Sheng J, Zu Z, Zhang Y, et al. Targeted therapy of atherosclerosis by zeolitic imidazolate framework-8 nanoparticles loaded with losartan potassium via simultaneous lipid-scavenging and anti-inflammation. J Mater Chem B. 2022;10(31):5925–5937. doi:10.1039/D2TB00686C
88. Obaid EAMS, Wu S, Zhong Y, et al. pH-Responsive hyaluronic acid-enveloped ZIF-8 nanoparticles for anti-atherosclerosis therapy. Biomater Sci. 2022;10(17):4837–4847. doi:10.1039/D2BM00603K
89. Almeida B, Nag OK, Rogers KE, et al. Recent progress in bioconjugation strategies for liposome-mediated drug delivery. Molecules. 2020;25(23):5672. doi:10.3390/molecules25235672
90. Guimarães D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571. doi:10.1016/j.ijpharm.2021.120571
91. Lee J, De La Torre AL, Rawlinson FL, et al. Characterization of stealth liposome-based nanoparticles Encapsulating the ACAT1/SOAT1 Inhibitor F26: efficacy and toxicity studies in vitro and in wild-type mice. Int J mol Sci. 2024;25(17):9151. doi:10.3390/ijms25179151
92. Adiguzel S, Karamese M, Kugu S, et al. Doxorubicin-loaded liposome-like particles embedded in chitosan/hyaluronic acid-based hydrogels as a controlled drug release model for local treatment of glioblastoma. Int J Biol Macromol. 2024;278(Pt 4):135054. doi:10.1016/j.ijbiomac.2024.135054
93. Aizik G, Grad E, Golomb G. Monocyte-mediated drug delivery systems for the treatment of cardiovascular diseases. Drug Deliv Transl Res. 2018;8(4):868–882. doi:10.1007/s13346-017-0431-2
94. Dhanasekara CS, Zhang J, Nie S, et al. Nanoparticles target intimal macrophages in atherosclerotic lesions. Nanomedicine. 2021;32:102346. doi:10.1016/j.nano.2020.102346
95. Shi Z, Huang J, Chen C, et al. Lipid nanoparticles encapsulating curcumin for imaging and stabilization of vulnerable atherosclerotic plaques via phagocytic “eat-me. Signals J Control Release. 2024;373:265–276. doi:10.1016/j.jconrel.2024.07.027
96. Zou L, Zhang Y, Cheraga N, et al. Chlorin e6 (Ce6)-loaded plaque-specific liposome with enhanced photodynamic therapy effect for atherosclerosis treatment. Talanta. 2023;265:124772. doi:10.1016/j.talanta.2023.124772
97. Lei C, Liu XR, Chen QB, et al. Hyaluronic acid and albumin based nanoparticles for drug delivery. J Control Release. 2021;331:416–433. doi:10.1016/j.jconrel.2021.01.033
98. Wu B, Wang J, Chen Y, et al. Inflammation-targeted drug delivery strategies via albumin-based systems. ACS Biomater Sci Eng. 2024;10(2):743–761. doi:10.1021/acsbiomaterials.3c01744
99. Qu N, Song K, Ji Y, et al. Albumin nanoparticle-based drug delivery systems. Int J Nanomed. 2024;19:6945–6980. doi:10.2147/IJN.S467876
100. Huang J, Xu S, Liu L, et al. Targeted treatment of atherosclerosis with protein-polysaccharide nanoemulsion co-loaded with photosensitiser and upconversion nanoparticles. J Drug Target. 2023;31(10):1111–1127. doi:10.1080/1061186X.2023.2284093
101. Boada C, Zinger A, Tsao C, et al. Rapamycin-loaded biomimetic nanoparticles reverse vascular inflammation. Circ Res. 2020;126(1):25–37. doi:10.1161/CIRCRESAHA.119.315185
102. Jiao M, Zhang P, Meng J, et al. Recent advancements in biocompatible inorganic nanoparticles towards biomedical applications. Biomater Sci. 2018;6(4):726–745. doi:10.1039/C7BM01020F
103. Pattnaik S, Thalluri C, Swain K. Rise of gold nanoparticles as carriers of therapeutic agents. Acta Chim Slov. 2023;70(4):467–478. doi:10.17344/acsi.2023.8216
104. Ali SH, Ali H, Aziz MA. Computational identification of PDL1 inhibitors and their cytotoxic effects with silver and gold nanoparticles. Sci Rep. 2024;14(1):26610. doi:10.1038/s41598-024-77868-8
105. Li X, Wang C, Tan H, et al. Gold nanoparticles-based SPECT/CT imaging probe targeting for vulnerable atherosclerosis plaques. Biomaterials. 2016;108:71–80. doi:10.1016/j.biomaterials.2016.08.048
106. Huang C, Huang W, Meng Y, et al. T1-weighted MRI of targeting atherosclerotic plaque based on CD40 expression on engulfed USPIO’s cell surface. Biomed Mater. 2024;19(2):10.1088/1748–605X/ad1df6.
107. Peng X, Liu J, Ming C, et al. AgFeS2 nanoparticles as a novel photothermal platform for effective artery stenosis therapy. Nanoscale. 2020;12(20):11288–11296. doi:10.1039/D0NR01587C
108. Peng X, Liu J, Li B, et al. Janus Ag/Ag2S beads as efficient photothermal agents for the eradication of inflammation and artery stenosis. Nanoscale. 2019;11(42):20324–20332. doi:10.1039/C9NR04804A
109. Younis NK, Ghoubaira JA, Bassil EP, et al. Metal-based nanoparticles: promising tools for the management of cardiovascular diseases. Nanomedicine. 2021;36:102433. doi:10.1016/j.nano.2021.102433
110. Tang C, Wang H, Guo L, et al. CpG-Conjugated silver nanoparticles as a multifunctional nanomedicine to promote macrophage efferocytosis and repolarization for atherosclerosis therapy. ACS Appl Mater Interfaces. doi:10.1021/acsami.3c11227
111. Segers FME, Ruder AV, Westra MM, et al. Magnetic resonance imaging contrast-enhancement with superparamagnetic iron oxide nanoparticles amplifies macrophage foam cell apoptosis in human and murine atherosclerosis. Cardiovasc Res. 2023;118(17):3346–3359. doi:10.1093/cvr/cvac032
112. Qu G, Wu Q, Zhao B, et al. The promotion effect of novel magnetic nanoparticles on atherosclerotic plaque vulnerability in apolipoprotein E-/- mice. Toxicology. 2019;419:24–31. doi:10.1016/j.tox.2019.03.002
113. Huang X, Lin C, Luo C, et al. Fe3O4@M nanoparticles for MRI-targeted detection in the early lesions of atherosclerosis. Nanomedicine. 2021;33:102348. doi:10.1016/j.nano.2020.102348
114. Shen M, Jiang H, Li S, et al. Dual-modality probe nanodrug delivery systems with ROS-sensitivity for atherosclerosis diagnosis and therapy. J Mater Chem B. 2024;12(5):1344–1354. doi:10.1039/D3TB00407D
115. Huang C, Huang W, Zhang L, et al. Targeting peptide, fluorescent reagent modified magnetic liposomes coated with rapamycin target early atherosclerotic plaque and therapy. Pharmaceutics. 2022;14(5):1083. doi:10.3390/pharmaceutics14051083
116. Wang Y, Liang Z, Liang Z, et al. Advancements of Prussian blue-based nanoplatforms in biomedical fields: progress and perspectives. J Control Release. 2022;351:752–778. doi:10.1016/j.jconrel.2022.10.007
117. Zhou H, You P, Liu H, et al. Artemisinin and Procyanidins loaded multifunctional nanocomplexes alleviate atherosclerosis via simultaneously modulating lipid influx and cholesterol efflux. J Control Release. 2022;341:828–843. doi:10.1016/j.jconrel.2021.12.021
118. Liu D, Yang A, Li Y, et al. Targeted delivery of rosuvastatin enhances treatment of hyperhomocysteinemia-induced atherosclerosis using macrophage membrane-coated nanoparticles. J Pharm Anal. 2024;14(9):100937. doi:10.1016/j.jpha.2024.01.005
119. Zhu Y, Fang Y, Wang Y, et al. Cluster of Differentiation-44-targeting prussian blue nanoparticles onloaded with colchicine for atherosclerotic plaque regression in a mice model. ACS Biomater Sci Eng. 2024;10(3):1530–1543. doi:10.1021/acsbiomaterials.3c01518
120. Huang Y, Li P, Zhao R, et al. Silica nanoparticles: biomedical applications and toxicity. Biomed Pharmacother. 2022;151:113053. doi:10.1016/j.biopha.2022.113053
121. Xu B, Li S, Shi R, et al. Multifunctional mesoporous silica nanoparticles for biomedical applications. Signal Transduct Target Ther. 2023;8(1):435. doi:10.1038/s41392-023-01654-7
122. Kudaibergen D, Park HS, Park J, et al. Silica-based advanced nanoparticles for treating ischemic disease. Tissue Eng Regen Med. 2023;20(2):177–198. doi:10.1007/s13770-022-00510-z
123. Pham LM, Kim EC, Ou W, et al. Targeting and clearance of senescent foamy macrophages and senescent endothelial cells by antibody-functionalized mesoporous silica nanoparticles for alleviating aorta atherosclerosis. Biomaterials. 2021;269:120677. doi:10.1016/j.biomaterials.2021.120677
124. Ma R, Hao L, Cheng J, et al. Hyaluronic acid-modified mesoporous silica nanoprobes for target identification of atherosclerosis. Biochem Biophys Res Commun. 2024;702:149627. doi:10.1016/j.bbrc.2024.149627
125. Wang Q, Wang Y, Liu S, et al. Theranostic nanoplatform to target macrophages enables the inhibition of atherosclerosis progression and fluorescence imaging of plaque in ApoE(-/-) mice. J Nanobiotechnology. 2021;19(1):222. doi:10.1186/s12951-021-00962-w
126. Chen Y, Chen Z, Duan J, et al. H2O2-responsive VEGF/NGF gene co-delivery nano-system achieves stable vascularization in ischemic hindlimbs. J Nanobiotechnology. 2022;20(1):145. doi:10.1186/s12951-022-01328-6
127. Groner J, Tognazzi M, Walter M, et al. Encapsulation of pioglitazone into polymer-nanoparticles for potential treatment of atherosclerotic diseases. ACS Appl Bio Mater. 2023;6(6):2111–2121. doi:10.1021/acsabm.2c01001
128. Ye M, Zhou J, Zhong Y, et al. SR-A-Targeted phase-transition nanoparticles for the detection and treatment of atherosclerotic vulnerable plaques. ACS Appl Mater Interfaces. 2019;11(10):9702–9715. doi:10.1021/acsami.8b18190
129. Gu Y, Du L, Wu Y, et al. Biomembrane-modified biomimetic nanodrug delivery systems: frontier platforms for cardiovascular disease treatment. Biomolecules. 2024;14(8):960. doi:10.3390/biom14080960
130. Li Y, Wang Y, Xia Z, et al. Noninvasive platelet membrane-coated Fe3O4 nanoparticles identify vulnerable atherosclerotic plaques. Smart Med. 2024;3(2):e20240006. doi:10.1002/SMMD.20240006
131. Duong VA, Nguyen TT, Maeng HJ. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules. 2020;25(20):4781. doi:10.3390/molecules25204781
132. Jin M, Chen X, Zheng L, et al. Astaxanthin-loaded polylactic acid-glycolic acid nanoparticles alleviates atherosclerosis by suppressing macrophage ferroptosis via the NRF2/SLC7A11/GPX4 pathway. Arch Biochem Biophys.
133. Deng Y, Liu L, Li Y, et al. pH-sensitive nano-drug delivery systems dual-target endothelial cells and macrophages for enhanced treatment of atherosclerosis. Drug Deliv Transl Res.
134. Wei M, Jiang Q, Bian S, et al. Dual-mode-driven nanomotors targeting inflammatory macrophages for the MRI and synergistic treatment of atherosclerosis. J Nanobiotechnology. 2025;23(1):54. doi:10.1186/s12951-025-03136-0
135. Chyu KY, Zhao X, Zhou J, et al. Immunization using ApoB-100 peptide-linked nanoparticles reduces atherosclerosis. JCI Insight. 2022;7(11):e149741. doi:10.1172/jci.insight.149741
136. Kouhjani M, Jaafari MR, Kamali H, et al. Microfluidic-assisted preparation of PLGA nanoparticles loaded with insulin: a comparison with double emulsion solvent evaporation method. J Biomater Sci Polym Ed. 2024;35(3):306–329. doi:10.1080/09205063.2023.2287247
137. de Oliveira BM, Teodoro JBM, Ambrósio JBM, et al. Zinc pthalocyanine loaded poly (lactic acid) nanoparticles by double emulsion methodology for photodynamic therapy against 9 L/LacZ gliosarcoma cells. J Biomater Sci Polym Ed. 2022;33(1):93–109. doi:10.1080/09205063.2021.1980359
138. Wu Z, Chen C, Luo J, et al. EGFP-EGF1-conjugated poly (lactic-co-glycolic acid) nanoparticles as a carrier for the delivery of CCR2- shRNA to atherosclerotic macrophage in vitro. Sci Rep. 2020;10(1):19636. doi:10.1038/s41598-020-76416-4
139. Esfandyari-Manesh M, Abdi M, Talasaz AH, et al. S2P peptide-conjugated PLGA-Maleimide-PEG nanoparticles containing Imatinib for targeting drug delivery to atherosclerotic plaques. Daru. 2020;28(1):131–138. doi:10.1007/s40199-019-00324-w
140. Zhou J, Niu C, Huang B, et al. Platelet membrane biomimetic nanoparticles combined with UTMD to improve the stability of atherosclerotic plaques. Front Chem. 2022;10:868063. doi:10.3389/fchem.2022.868063
141. Sun B, Li G, Wu Y, et al. Ce-MOF@Au-Based Electrochemical Immunosensor for Apolipoprotein A1 Detection Using Nanobody Technology. ACS Appl Mater Interfaces. 2024;16(43):58405–58416. doi:10.1021/acsami.4c14027
142. Chen L, Wang C, Wu Y. Cholesterol (Blood lipid) lowering potential of rosuvastatin chitosan nanoparticles for atherosclerosis: preclinical study in rabbit model. Acta Biochim Pol. 2020;67(4):495–499. doi:10.18388/abp.2020_5186
143. Xiao J, Li N, Xiao S, et al. Comparison of selenium nanoparticles and sodium selenite on the alleviation of early atherosclerosis by inhibiting endothelial dysfunction and inflammation in apolipoprotein E-Deficient mice. Int J mol Sci. 2021;22(21):11612. doi:10.3390/ijms222111612
144. Nguyen MA, Wyatt H, Susser L, et al. Delivery of MicroRNAs by chitosan nanoparticles to functionally alter macrophage cholesterol efflux in vitro and in vivo. ACS Nano. 2019;13(6):6491–6505. doi:10.1021/acsnano.8b09679
145. Asadzadeh F, Poursattar Marjani A. Revolutionizing acridine synthesis: novel core-shell magnetic nanoparticles and Co-Zn zeolitic imidazolate framework with 1-aza-18-crown-6-ether-Ni catalysts. Sci Rep. 2024;14(1):25739. doi:10.1038/s41598-024-75591-y
146. Liu Y, Yuan H, Liu Y, et al. Multifunctional nanoparticle-VEGF modification for tissue-engineered vascular graft to promote sustained anti-thrombosis and rapid endothelialization. Front Bioeng Biotechnol. 2023;11:1109058. doi:10.3389/fbioe.2023.1109058
147. Pan H, Palekar RU, Hou KK, et al. Anti-JNK2 peptide-siRNA nanostructures improve plaque endothelium and reduce thrombotic risk in atherosclerotic mice. Int J Nanomed. 2018;13:5187–5205. doi:10.2147/IJN.S168556
148. Wu H, Sheng J, Wang Z, et al. Tannic acid-poloxamer self-assembled nanoparticles for advanced atherosclerosis therapy by regulation of macrophage polarization. J Mater Chem B. 2024;12(19):4708–4716. doi:10.1039/D3TB01157G
149. Zhao R, Ning X, Wang M, et al. A ROS-responsive simvastatin nano-prodrug and its fibronectin-targeted co-delivery system for atherosclerosis treatment. ACS Appl Mater Interfaces. 2022;14(22):25080–25092. doi:10.1021/acsami.2c02354
150. Zong Q, He C, Long B, et al. Targeted delivery of nanoparticles to blood vessels for the treatment of atherosclerosis. Biomedicines. 2024;12(7):1504. doi:10.3390/biomedicines12071504
151. Pang AS, Dinesh T, Pang NY, et al. Nanoparticles as drug delivery systems for the targeted treatment of atherosclerosis. Molecules. 2024;29(12):2873. doi:10.3390/molecules29122873
152. Wang H, Zhao R, Peng L, et al. A dual-function CD47-targeting nano-drug delivery system used to regulate immune and anti-inflammatory activities in the treatment of atherosclerosis. Adv Healthc Mater. 2024;13(22):e2400752. doi:10.1002/adhm.202400752
153. Li B, He M, Xu Z, et al. Biomimetic ROS-responsive hyaluronic acid nanoparticles loaded with methotrexate for targeted anti-atherosclerosis. Regen Biomater. 2024;11:rbae102. doi:10.1093/rb/rbae102
154. Fontana F, Molinaro G, Moroni S, et al. Biomimetic platelet-cloaked nanoparticles for the delivery of anti-inflammatory curcumin in the treatment of atherosclerosis. Adv Healthc Mater. 2024;13(15):e2302074. doi:10.1002/adhm.202302074
155. Liang X, Li H, Zhang A, et al. Red blood cell biomimetic nanoparticle with anti-inflammatory, anti-oxidative and hypolipidemia effect ameliorated atherosclerosis therapy. Nanomedicine. 2022;41:102519. doi:10.1016/j.nano.2022.102519
156. Ma Y, Song D, Yuan J, et al. Alisol A inhibits and stabilizes atherosclerotic plaques by protecting vascular endothelial cells. Front Pharmacol. 2024;15:1493948. doi:10.3389/fphar.2024.1493948
157. Wei H, Tan T, Cheng L, et al. MRI tracing of ultrasmall superparamagnetic iron oxide nanoparticle‑labeled endothelial progenitor cells for repairing atherosclerotic vessels in rabbits. Mol Med Rep. 2020;22(4):3327–3337. doi:10.3892/mmr.2020.11431
158. Fang F, Ni Y, Yu H, et al. Inflammatory endothelium-targeted and cathepsin responsive nanoparticles are effective against atherosclerosis. Theranostics. 2022;12(9):4200–4220. doi:10.7150/thno.70896
159. Patel Y, Manturthi S, Tiwari S, et al. Development of pro-resolving and pro-efferocytic nanoparticles for atherosclerosis therapy. ACS Pharmacol Transl Sci. 2024;7(10):3086–3095. doi:10.1021/acsptsci.4c00292
160. Sha X, Dai Y, Chong L, et al. Pro-efferocytic macrophage membrane biomimetic nanoparticles for the synergistic treatment of atherosclerosis via competition effect. J Nanobiotechnology. 2022;20(1):506. doi:10.1186/s12951-022-01720-2
161. Zakirov FH, Zhang D, Grechko AV, et al. Lipid-based gene delivery to macrophage mitochondria for atherosclerosis therapy. Pharmacol Res Perspect. 2020;8(2):e00584. doi:10.1002/prp2.584
162. Distasio N, Dierick F, Ebrahimian T, et al. Design and development of branched poly(ß-aminoester) nanoparticles for Interleukin-10 gene delivery in a mouse model of atherosclerosis. Acta Biomater. 2022;143:356–371. doi:10.1016/j.actbio.2022.02.043
163. Bai Q, Xiao Y, Hong H, et al. Scavenger receptor-targeted plaque delivery of microRNA-coated nanoparticles for alleviating atherosclerosis. Proc Natl Acad Sci U S A. 2022;119(39):e2201443119. doi:10.1073/pnas.2201443119
164. Zhang S, Xu W, Gao P, et al. Construction of dual nanomedicines for the imaging and alleviation of atherosclerosis. Artif Cells Nanomed Biotechnol. 2020;48(1):169–179. doi:10.1080/21691401.2019.1699823
165. Ma Q, Wu S, Yang L, et al. Hyaluronic acid-guided cerasome nano-agents for simultaneous imaging and treatment of advanced atherosclerosis. Adv Sci. 2023;10(5):e2202416. doi:10.1002/advs.202202416
166. Luo Q, Dai L, Li J, et al. Intracellular and extracellular synergistic therapy for restoring macrophage functions via anti-CD47 antibody-conjugated bifunctional nanoparticles in atherosclerosis. Bioact Mater. 2024;34:326–337. doi:10.1016/j.bioactmat.2023.12.024
167. Ji W, Zhang Y, Deng Y, et al. Nature-inspired nanocarriers for improving drug therapy of atherosclerosis. Regen Biomater. 2023;10:rbad069. doi:10.1093/rb/rbad069
168. Lin L, Chen L, Yan J, et al. Advances of nanoparticle-mediated diagnostic and theranostic strategies for atherosclerosis. Front Bioeng Biotechnol. 2023;11:1268428. doi:10.3389/fbioe.2023.1268428
169. Wang A, Yue K, Zhong W, et al. Targeted delivery of rapamycin and inhibition of platelet adhesion with multifunctional peptide nanoparticles for atherosclerosis treatment. J Control Release.
170. Ni H, Zhou H, Liang X, et al. Reactive oxygen species-responsive nanoparticle delivery of small interfering ribonucleic acid targeting olfactory receptor 2 for atherosclerosis Theranostics. ACS Nano. 2024;18(34):23599–23614. doi:10.1021/acsnano.4c07988
171. He Z, Chen Q, Duan X, et al. Reactive oxygen species-responsive nano-platform with dual-targeting and fluorescent lipid-specific imaging capabilities for the management of atherosclerotic plaques. Acta Biomater. 2024;181:375–390. doi:10.1016/j.actbio.2024.05.011
172. Nankivell V, Vidanapathirana AK, Hoogendoorn A, et al. Targeting macrophages with multifunctional nanoparticles to detect and prevent atherosclerotic cardiovascular disease. Cardiovasc Res. 2024;120(8):819–838. doi:10.1093/cvr/cvae099
173. Tu Y, Ma X, Chen H, et al. Molecular imaging of matrix metalloproteinase-2 in atherosclerosis using a smart multifunctional PET/MRI nanoparticle. Int J Nanomed. 2022;17:6773–6789. doi:10.2147/IJN.S385679
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

