Back to Journals » International Journal of Nanomedicine » Volume 20
How Do Organelle-Targeting Nanotherapeutics Treat Inflammatory Diseases? A Comprehensive Review of the Literature
Authors Wang SH, Xu XL , Chen W
Received 24 January 2025
Accepted for publication 20 May 2025
Published 3 June 2025 Volume 2025:20 Pages 7133—7152
DOI https://doi.org/10.2147/IJN.S516260
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Farooq A. Shiekh
Si-Hui Wang,1,2 Xiao-Ling Xu,2 Wei Chen1
1Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, 200032, People’s Republic of China; 2Shulan International Medical College, Zhejiang Shuren University, Hangzhou, 310015, People’s Republic of China
Correspondence: Wei Chen, Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, 725 South WanPing Road, Shanghai, 200032, People’s Republic of China, Tel +86-021-64385700-3522, Email [email protected] Xiao-Ling Xu, Shulan International Medical College, Zhejiang Shuren University, 8 Shuren Street, Hangzhou, 310015, People’s Republic of China, Tel +86-571-88208435, Email [email protected]
Abstract: Inflammation is a protective response of the body, but when excessive or prolonged, it can contribute to disease progression and tissue damage. Identifying more effective and less toxic drugs for treating both acute and chronic inflammatory diseases is a major challenge. Organelle-targeting strategies, which deliver drugs directly to specific organelles, offer a promising solution by improving treatment efficiency and minimizing toxic effects on healthy cells. However, despite the potential of organelles as therapeutic targets, precise targeting remains challenging. This review systematically summarizes organelle-targeting nanodelivery strategies for major organelles—mitochondria, the endoplasmic reticulum, lysosomes, and the Golgi apparatus—and the research progress in evaluating the potential of these strategies for treating inflammation-related diseases. This study focuses on the applications of these strategies for the treatment of sepsis, inflammatory bowel disease, atherosclerosis, and osteoarthritis. Additionally, this review outlines future directions and key challenges in this field, aiming to provide a scientific reference for the application of organelle-targeting nanotherapeutics for the treatment of inflammatory diseases.
Keywords: nanomedicine, drug delivery system, organelle targeting, inflammatory diseases
Introduction
Inflammation is a complex biological process closely associated with almost all human diseases. As the initial response of the immune system to infection or tissue damage, inflammation eliminates pathogenic factors by enhancing tissue and organ homeostasis, maintaining structural and functional integrity, and promoting adaptation to disturbances.1,2 However, during the resolution of acute inflammation, the lack of negative regulators can lead to chronic inflammation, causing excessive tissue damage and sustained immune responses, such as those observed in common autoinflammatory or autoimmune diseases such as osteoarthritis (OA), atherosclerosis (AS), and inflammatory bowel disease (IBD). Although acute and chronic inflammation differ in their underlying mechanisms, both can result in significant tissue damage if not properly regulated, underscoring the importance of protecting tissues from inflammatory damage to improve clinical outcomes. For example, in patients with sepsis, systemic inflammation leading to multiorgan failure is the primary cause of death. In patients with severe sepsis, proinflammatory responses aimed at eliminating pathogens often result in tissue damage, whereas the anti-inflammatory response can increase susceptibility to secondary infections.3
As common inflammatory diseases, sepsis, IBD, AS, and OA differ in clinical presentation and pathological mechanisms but share similarities in inflammation and immune responses, which provides new ideas for research and treatment. Anti-inflammatory drugs, particularly glucocorticoids, are widely used to alleviate inflammatory symptoms by suppressing immune responses. However, as they lack direct antimicrobial activity, their use is often associated with an increased risk of infections due to immunosuppression. Additionally, poor drug accumulation at the target site and rapid clearance hinder conventional treatments. The heterogeneity of diseases may also contribute to uncertain therapeutic outcomes. To achieve more effective treatment and minimize off-target effects, new drug mechanisms and therapeutic strategies are increasingly shifting toward precision targeting.
For example, the recently proposed precision immunotherapy for sepsis is becoming a third major treatment pillar, alongside early appropriate antimicrobial treatment and organ function support.4 In addition, individualized antibiotic dosage adjustment has been shown to be crucial for improving treatment outcomes and reducing the risk of drug resistance in critically ill patients, reflecting the clinical value of precision medicine at the pharmacokinetic level.5 Similarly, new therapies for atherosclerotic vascular disease targeting low-density lipoprotein cholesterol, such as proprotein convertase subtilisin/kexin type 9 inhibitors combined with anti-inflammatory strategies, are gaining attention.6 For IBD, biological therapies such as anti-tumor necrosis factor, anti-interleukin-12/interleukin-23, and anti-alpha4beta7 integrin precisely modulate inflammatory pathways, minimizing systemic side effects and increasing treatment specificity.7 In OA, drug development focuses on targeting inflammatory pathways, cartilage metabolism, and bone remodeling, but while progress has been made with pain relievers such as nerve growth factor inhibitors, reversing structural changes remains challenging.8 Thus, developing safer and more effective therapies for acute and chronic inflammation continues to be a major challenge.
The progress of precision-targeted therapies has shifted medical treatments toward more personalized approaches. In recent years, organelle-targeting therapies with high sensitivity and precision have garnered increasing attention. Organelles are specialized structures in eukaryotic cells, and their dysfunction or disrupted interaction networks are closely linked to inflammation.9 Studying organelle functions and dynamics is crucial for understanding disease mechanisms and developing targeted therapies. While organelle-targeting strategies hold promise for reducing side effects and improving treatment efficiency, they remain challenging due to their lack of inherent drug specificity.
Nanotechnology breakthroughs provide innovative treatment and diagnostic opportunities for inflammatory diseases, especially in organelle-targeting therapies. Nanodelivery systems outperform traditional drugs by increasing bioavailability and efficacy and reducing side effects. In addition, by rationally designing nanomaterials, nanoparticles (NPs) can also be used to achieve efficient drug delivery and control drug release at specific sites. Currently, nanodelivery platforms can be broadly categorized into three types: organic carriers, inorganic carriers, and hybrid systems that integrate the advantages of both. Organic nanocarriers, such as liposomes, polymeric nanoparticles, dendrimers, and micelles, are typically composed of biocompatible and biodegradable materials. They offer excellent drug encapsulation efficiency, controllable release profiles, and flexible surface functionalization capabilities.10 Inorganic nanocarriers, including gold nanoparticles, mesoporous silica nanoparticles, and iron oxide nanoparticles, are valued for their superior physical and chemical stability, tunable size and architecture, and unique magnetic or optical properties, making them highly promising for therapeutic and diagnostic applications.11 Hybrid nanocarriers, which combine organic and inorganic components, integrate structural robustness with biological functionality, thereby overcoming the limitations of single-component systems in terms of delivery efficiency and biological adaptability. Together, these diverse nanoplatforms establish a critical foundation for achieving precision drug delivery at the organelle level.
Therefore, this review systematically summarizes recent advances in organelle-targeting nanodelivery strategies for key organelles, including mitochondria, the endoplasmic reticulum (ER), lysosomes, and the Golgi apparatus. We particularly focus on the applications of these strategies for the treatment of inflammatory diseases such as sepsis, IBD, AS, and OA. Compared with their well-established roles in oncology, organelle-specific nanodelivery systems remain relatively underexplored in the context of inflammation. To address this gap, we provide a targeted and comprehensive analysis of current strategies and therapeutic outcomes. In addition, we explore how emerging technologies such as precision medicine and artificial intelligence could be integrated to further optimize organelle-targeting therapies. This forward-looking perspective aims to guide future research and facilitate clinical translation in the treatment of inflammation-related diseases.
Current Advances in Targeting Vital Cell Organelles via Nanomedicine
Non-organelle-targeting drugs exhibit uneven intracellular distributions, which may reduce drug utilization efficiency while increasing toxicity and side effects to healthy cells. Furthermore, many diseases involve dysfunctions of specific organelles, making nontargeted drugs ineffective at precisely modulating these critical pathological pathways, thus limiting their therapeutic outcomes. These limitations highlight the importance and potential of organelle-targeting drugs. Researchers have developed various nanoscale delivery systems that target mitochondria, the endoplasmic reticulum, lysosomes, and the Golgi apparatus. These systems utilize ligand recognition, responsive materials, or specific physicochemical properties to achieve efficient and precise organelle targeting (Table 1). Here, we have outlined the key ligands that target mitochondria, the endoplasmic reticulum, lysosomes, and the Golgi apparatus (Figure 1).
 |
Table 1 Primary Targeting Strategies for Organelles |
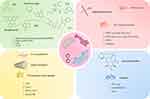 |
Figure 1 The background colors correspond to specific organelle-targeting strategies: green (mitochondria), red (endoplasmic reticulum), yellow (lysosome), and blue (Golgi apparatus). Adapted from Servier Medical Art (https://smart.servier.com/), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). |
Mitochondria-Targeting Nanostrategies
Mitochondria-targeting agents are a class of drugs or compounds that specifically target mitochondria and act on biological processes within mitochondria. Features such as large transmembrane potential and mitochondrial protein entry mechanisms are important for the design of mitochondria-targeting drugs. At present, the main ways to generate drug-targeting mitochondria are through lipophilic cation-mediated and mitochondrial protein input, as well as mitochondrial targeting by attaching nitro oxygen and cell-membrane-penetrating peptides to drugs.
Lipophilic Cations
Unlike other biofilms, mitochondria have a high degree of negative membrane potential due to the action of the electron transport chain. The high negative membrane potential and high lipid properties of mitochondria enable lipophilic cations to be targeted through the mitochondrial membrane and accumulate in the mitochondrial matrix. At present, the most studied delocalized lipophilic cations (DLCs) include triphenylphosphine (TPP), dequalinium (DQA), rhodamine, and anthocyanin dyes. As a representative DLC, TPP is widely used in the construction of mitochondria-targeting nanosystems. It consists of three phenyl groups attached to a phosphorus atom, forming a triphenylphosphine structure. This structure enhances the lipophilicity of TPP, facilitating membrane penetration and enabling it to target and accumulate in mitochondria through charge interactions.12 DQA can interfere with the mitochondrial membrane potential gradient, leading to increased mitochondrial permeability and the release of proapoptotic factors such as cytochrome c, resulting in selectivity for accumulation in cancer cell mitochondria.13 Other lipophilic cations, such as rhodamine (rhodamine 123) and anthocyanin derivatives (JC-1), are also used for mitochondrial colocalization.14,15 Additionally, mitochondria-targeting cations, such as pyridinium cations and F16 [(E)-4-(1H-Indol-3-ylvinyl)-N-methylpyridinium iodide], are commonly used in mitochondrial drug delivery systems.16,17
Mitochondria-Targeting Peptides
Peptide-based nanosystems are an emerging strategy for targeting mitochondria. These systems usually use specific peptide sequences or structural motifs to target mitochondria. Compared with DLC systems, peptide-based nanosystems have greater flexibility and designability, as peptide sequences can be tailored to specific needs. Mitochondria-penetrating peptides (MPPs), a subclass of cell-penetrating peptides (CPPs), can cross cell membranes and specifically deliver bioactive substances to mitochondria. The design of MPPs usually depends on the characteristics of the mitochondrial membrane, especially its negative potential.
A promising strategy to improve cellular entry and mitochondrial targeting involves combining CPPs with mitochondria-targeting sequences (MTSs). MTSs, typically found at the N-terminus of proteins, consist of 20–40 positively charged and hydrophobic amino acids, which are recognized by mitochondrial receptors for transport into the mitochondrial matrix.18 In the design of MPPs, the introduction of positively charged amino acids such as arginine and lysine can facilitate mitochondrial localization via electrostatic interactions.42 In addition to charge interactions, hydrophobic amino acids such as phenylalanine, tyrosine, and isoleucine aid in crossing cell membranes and enhancing interactions with mitochondrial membranes.43 Functional modification via charged natural amino acids and peptides is a promising approach for mitochondrial targeting.44
In addition to MPPs, Szeto-Schiller (SS) peptides, XJB peptides, and ATAP peptides are also used for the construction of mitochondria-targeting nanoplatforms. SS peptides, as mitochondria-targeting antioxidants, reduce reactive oxygen species (ROS) production and inhibit mitochondrial permeability transition. They can penetrate the plasma membrane and accumulate in the inner mitochondrial membrane (IMM), likely due to electrostatic interactions with IMM phospholipids and hydrophobic interactions with fatty acyl tails.45 Elamipretide (SS-31) is a promising mitochondria-targeting peptide that clears ROS and improves mitochondrial function, showing potential for treating mitochondrial dysfunction-related diseases.19 XJB-5-131 scavenges electrons to reduce ROS levels and prevent superoxide production.20 ATAP peptides induce apoptosis by targeting the mitochondrial membrane and bypassing Bcl-2 family protein-mediated protective mechanisms, making XJB peptides promising candidates for treating chemotherapy-resistant cancer.21
Endoplasmic Reticulum-Targeting Nanostrategies
ER dysfunction can cause the accumulation of misfolded proteins, leading to apoptosis. Therefore, targeted drug delivery to the ER is crucial for disease treatment. However, research on ER-targeting drug delivery is limited due to the few targetable characteristics of the ER and its complex 3D structure with varying thickness, making selective targeting difficult. Currently, strategies for targeting the ER focus mainly on ligand modifications, including ligand-modified carriers and small molecules or peptides with ER-targeting properties.
Small-Molecule Compounds
The direct targeting of the ER involves binding the subcellular targeting group to small-molecule drugs or reagents through chemical bonds. Sulfonamide ligands have been widely developed for the modification of small-molecule drugs and drug delivery carriers because of their low toxicity, high efficiency, and high selectivity. Currently, the widely used targeting ligands based on small molecules are mainly methanesulfonamide and its derivatives, including p-toluenesulfonamide and naphthylsulfonamide. Sulfonamide ligands can bind specifically and with high affinity to sulfonylurea receptors, which are potassium-selective ion channels highly expressed on the ER membrane.22 Naphthylsulfonamide and p-toluenesulfonamide share similar structural features, and both can target the ER by specifically binding to sulfonylurea receptors.23 In addition, the dansyl and tosyl groups in N-(2-aminoethyl)-5-(dimethylamino)naphthalene-1-sulfonamide can be used as typical sulfonyl ligands to improve the ER-targeting ability of drugs. Due to the millimolar concentration of calcium ions in the endoplasmic reticulum, which is much higher than that in the cytosol, anionic groups (such as carboxyl or phosphate groups) can serve as targeting moieties for modifying drugs or nanoparticles. These anionic groups achieve specific ER targeting by forming coordination bonds with calcium ions in the ER.24
Endoplasmic Reticulum-Targeting Peptides
Through extensive studies of protein transport, several signal peptides with ER specificity have been identified, typically located at the N-terminus of proteins. These peptides direct proteins to the ER by interacting with ER membrane receptors or chaperones. Common strategies for ER targeting involve inserting peptide sequences such as KDEL, KKXX, RARC, and Eriss. Among these, the KDEL sequence is the most widely used; it is recognized by KDEL receptors in the Golgi apparatus and directs proteins back to the ER via retrograde transport. For example, AuNP-KDEL nanostructures, formed by conjugating gold nanoparticles with KDEL peptides, can be rapidly internalized through clathrin-mediated pathways and directed to the ER via retrograde transport, thus avoiding lysosomal degradation.25 The KKXX signal peptide, typically located at the C-terminus of ER membrane proteins, functions similarly to the KDEL signal, targeting ER membrane proteins on transport vesicles to return to the ER.26 The short peptide RARC, which consists of arginine (R)-alanine (A)-arginine (R)-cysteine (C) and is rich in arginine, specifically interacts with ER proteins to facilitate ER targeting.27 The Eriss peptide, a 17-amino-acid sequence derived from the adenovirus E3-19K protein, also targets the ER.28 Pardaxin, a natural antimicrobial peptide, can localize to the ER, potentially due to its ability to form phosphatidylcholine vesicle pores, as phosphatidylcholine is the main phospholipid in the ER.29 In addition to targeting, pardaxin also induces ROS production and endoplasmic reticulum stress (ERS). Due to the high molecular weight of most ER-targeting peptides, nanocarriers or antibody-mediated strategies are often needed for effective ER localization.
Lysosome-Targeting Nanostrategies
Accurate lysosomal targeting in specialized cells may address autophagy dysregulation under pathological conditions. Most current studies focus on inducing mild lysosomal membrane permeabilization (LMP). Given that lysosomes play a key role in tumor invasion, metastasis, and resistance, LMP targeting has emerged as a promising therapeutic strategy for various cancers. Studies have shown that anionic NPs tend to attach to the surfaces of non-immune cells and are gradually internalized. In contrast, cationic NPs interact with cell membranes through electrostatic attraction, inducing membrane depolarization or the formation of hydrophilic pores, which leads to marked changes in membrane permeability.46 These distinct behaviors form the basis for designing mixed-charge NPs with enhanced cellular interactions. These [±] NPs selectively accumulate in tumor cells and compromise lysosomal membrane integrity over time, ultimately triggering lysosome-dependent apoptosis.30
pH-Responsive Fusion Peptides
Due to the significantly lower pH inside lysosomes than in the cytoplasm and other organelles, pH-responsive strategies have become a major research approach. Researchers have reported a variety of strategies for the precise delivery of nucleic acid drugs using the difference in pH between diseased and surrounding tissues, including the use of acid-labile chemical bonds, pH-sensitive sensory groups or pH-sensitive materials. However, developing nanoparticles that directly target lysosomes is challenging, as most nanoparticles undergo degradation via the endosomal/lysosomal pathway after internalization, losing their intended function; to prevent this, strategies to evade endosomal/lysosomal degradation have been developed. Lysosomal escape protein-based nanocarriers are special lysosome-targeting nanocarriers that help drugs escape lysosomal degradation, thereby improving bioavailability. For example, the influenza virus protein HA2, an amphipathic anionic peptide, undergoes a conformational change to a helical structure under low pH, promoting fusion with the endosomal membrane and releasing contents into the cytoplasm.31 Similarly, the developed synthetic peptide GALA has pH-sensitive and amphiphilic characteristics. Under neutral pH, GALA adopts a random coil conformation, but when the pH decreases to 5, GALA transitions into an amphipathic α-helix.32 In addition to the above fusion peptide, a new peptide has been proposed to fuse the CM 18 hybrid (KWKLFKKIGAVLKVLTTG, residues 1–7 of cecropin-A and residues 2–12 of melittin) with the arginine-rich motif of Tat 11. The results showed that the chimeric peptide effectively enhanced a variety of colocalized membrane-impermeable molecules.33 In addition, studies have shown that stearylated octaarginine (Stearyl-R8), a stearylated octaarginine peptide, acts as a potent cell-penetrating peptide with notable advantages in lysosomal targeting and escape, facilitating efficient gene delivery by promoting membrane fusion under acidic conditions and thereby enhancing transfection efficiency.34
Other Advances in Nanotherapeutics
Most DNA nanomaterials are naturally localized to lysosomes through caveolin endocytosis after being taken up by cells. This characteristic is the reason why DNA materials are often used for precise drug delivery targeted by lysosomes. Odom et al35 proposed a nanostructure system (HApt-AuNS) based on the combination of DNA aptamers and gold NPs, designed to target and sort human epidermal growth factor receptor 2 into lysosomes, where it is degraded by proteases under acidic conditions. Additionally, modifying the shape and surface properties of nanostructures can enhance their translocation across the lysosomal membrane. Given their exceptional ability to penetrate biological membranes and low toxicity, carbon nanotubes can be used to design coatings that increase nanodrug affinity for lysosomal lipid membranes, facilitating membrane permeabilization, pore formation, and subsequent release of nanoparticles into the cytoplasm.36
Golgi Apparatus-Targeting Nanostrategies
Golgi apparatus-targeting nanodrug delivery systems are still in the early stages of research and development. Currently, Golgi apparatus-targeting technology includes mainly the use of Golgi apparatus-specific fluorescent probes for diagnosis, and there are few reports on the application of Golgi apparatus-targeting technology for patient treatment.
Chondroitin Sulfate
Chondroitin sulfate (CS) is a typical sulfated glycosaminoglycan that specifically binds to CD44-overexpressing hepatic stellate cells.47 Due to its excellent biocompatibility, biodegradability, nonimmunogenicity, and low toxicity, CS has gained significant attention in drug delivery systems. When combined with nanoparticles, CS forms nanocarriers that show great potential for targeted drug delivery. Studies have shown that chondroitin sulfate nanomicelles exhibit high affinity and effectively target the Golgi apparatus in hepatic stellate cells. Thus, CS-based nanoparticles have been utilized in targeted therapies for chronic liver diseases and liver cancer.37,38
Golgi Apparatus-Targeting Peptides
Certain proteins or peptides contain specific signal sequences that guide them to the Golgi apparatus. Among them, the RS1-reg peptide follows the endocytic pathway, targeting vesicular transport to the trans-Golgi network without the need for endosomal escape, where it inhibits the activity of the glucose transporter SGLT1. Based on this discovery, Keller et al39 designed custom nanogels that specifically target RS1-reg to its action site within the trans-Golgi network. Under specific conditions, peptides can self-assemble into optimal sizes and shapes, facilitating the efficient transport of encapsulated drugs and enabling subcellular internalization and precise targeting. Li et al40 designed the convertible peptide C6RVRRF4KY, which self-assembles into nontoxic nanoparticles in aqueous medium and, upon targeting and cleavage by Flynn protease, transforms into left-handed helical fibers that mechanically disrupt the Golgi membrane in cancer cells. This nanomechanical disruption strategy not only demonstrates the advantage of subcellular organelle disruption in overcoming drug resistance in cancer therapy but also provides a novel therapeutic approach for combating multidrug-resistant bacteria and viruses. Furthermore, thiophosphate peptide pS1 designed by Tan undergoes enzymatic dephosphorylation to rapidly form self-assembled thiopeptides, which then interact with Golgi proteins to form disulfide bonds, enabling targeted delivery to the Golgi apparatus.41
Organelle-Targeting Nanodelivery Systems Improve Inflammatory Diseases
The study of organelle-targeting nanomedicine for treating inflammatory diseases not only facilitates the precise regulation of inflammatory responses but also provides new insights into the pathological mechanisms unresolved by traditional therapies. Inflammatory diseases can be broadly classified into systemic inflammation and localized chronic inflammation. Sepsis is a classic example of systemic inflammation, whereas IBD, AS, and OA represent localized chronic inflammation. These diseases encompass diverse inflammatory mechanisms, making them representative models for investigating organelle-targeting nanomedicine. In recent years, significant progress has been made in developing organelle-targeting nanotherapeutic strategies for these diseases. As shown in Table 2, organelle-targeting nanotherapeutics have demonstrated favorable efficacy in treating sepsis, IBD, AS, and OA. These studies not only clarify the mechanisms of nanomedicines in inflammation treatment but also provide valuable references and guidance for managing other inflammatory diseases.
 |
Table 2 Organelle Specific Drug Delivery for the Treatment of Inflammatory Diseases |
Sepsis
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection.67 Recent studies on the global burden of disease estimate that the incidence and mortality of sepsis are twice as high as previously thought.68 Current anti-inflammatory interventions are effective in treating sepsis; however, monotherapies have limited efficacy. Exploring multimodal therapies and achieving precise targeting, such as via the simultaneous inhibition of pathogen growth and suppression of the inflammatory response, may represent a viable strategy.
In sepsis, pathogens entering the bloodstream can induce immune disorders and activate macrophages to produce excessive ROS to eliminate invaders. However, the continued accumulation of ROS not only triggers transcriptional cascades but also leads to mitochondrial damage and the release of proinflammatory factors, thereby promoting uncontrolled inflammation. In recent years, mitochondria have received increasing attention as core organelles for the regulation of inflammation. Yu48 synthesized TPP-modified ceria NPs (Atv/PTP-TCeria NPs) to reduce ROS and improve sepsis-induced acute kidney injury. Similarly, another study based on mitochondria-targeting nanomaterials showed that TPP-AAV could inhibit the malonylation of VDAC2, thereby significantly alleviating sepsis-induced ferroptosis and myocardial dysfunction.49 These findings highlight the potential therapeutic significance of mitochondria-targeting nanomaterials in alleviating sepsis-related myocardial injury. Furthermore, as the first line of immune defense in the body, macrophages can differentiate into M1 (proinflammatory) and M2 (anti-inflammatory) macrophages. Overactivation of M1 macrophages leads to the progression of sepsis. Therefore, regulating the balance of macrophage polarization has become another important therapeutic target for inflammatory diseases. Studies have shown that decreased expression of the circRNA mSCAR (a mitochondrial circRNA that promotes M2 polarization by reducing mitochondria-derived ROS in the macrophages of mice with sepsis) is associated with excessive M1 polarization of macrophages. Li et al50 used TPP to deliver the TPP/circRNA complex in exosomes to mitochondria, regulating macrophage polarization to alleviate systemic inflammation and organ damage in sepsis.
The ER also plays an important role in sepsis-related inflammatory processes. Excessive production of ROS activates ERS, triggering cell apoptosis and the secretion of proinflammatory factors and thereby exacerbating immune dysregulation. Therefore, investigating macrophage ER-targeting strategies in response to ROS and pathogen infection may help mitigate ERS and immune dysregulation. Zhao et al51 developed a pathogen-responsive and macrophage ER-targeting nanoplatform consisting of mesoporous silica nanoparticles (MSNs) functionalized with ER-targeting peptides and a pathogen-responsive cap containing ROS-cleavable boronates and bovine serum albumin. This platform enables precise ER targeting and rapid antimicrobial peptide release in response to infection, offering a therapeutic approach for systemic infections.
Lysosomes are sites of bacterial degradation in monocytes/macrophages and hiding places for bacteria evading immune clearance. During sepsis, dysfunctional macrophages inhibit bacterial phagocytosis and lysosomal degradation, even facilitating bacterial immune evasion. Based on this, Zhao et al52 developed an NP system for monocyte/macrophage lysosomes (Alpha-MOF). Alpha-MOFs with the CD47 ligand on their surface target monocytes/macrophages. After internalization, they are located in the lysosome. The acidic environment dissolves the metal-organic framework, releasing zinc and calcium ions, which synergistically enhance bacterial phagocytosis and degradation. Furthermore, the latest studies have explored strategies for targeting lysosomes to combat intracellular small colony variants (SCVs) in severe infections such as peritonitis and sepsis. Xie53 constructed a nanogel system called NG@G1-mHAG2, encapsulating gentamicin and its nano-loaded form in a mannose-modified multilayer structure and destroying its membrane structure via the pH-triggered release of Ca²+ in lysosomes to achieve efficient lysosomal escape. This strategy not only reversed the antibiotic resistance of low-metabolism SCVs but also showed significant antibacterial and anti-inflammatory effects in a mouse model of peritonitis, which is expected to provide new targets for the treatment of sepsis.
Currently, some progress has been made in evaluating the use of inflammatory site-targeting and cell-targeting nanosystems for the treatment of sepsis, while relatively little research has been done on targeted therapies at the organelle level, an area that still needs to be further explored and developed to provide more precise and effective strategies for sepsis treatment.
Inflammatory Bowel Disease
IBD is a chronic, nonspecific, and recurrent inflammatory disorder. Common treatment strategies for IBD include anti-inflammatory drugs, immunosuppressants, and antibiotics, with fecal microbiota transplantation also being explored as a potential therapeutic option. Oral administration remains the preferred method for IBD treatment; however, accurately targeting the inflamed regions of the colon is challenging. In recent years, research into targeted nanomedicines for IBD treatment has made significant progress. As a novel drug delivery system, NPs leverage the permeability and retention properties of the inflamed intestine to deliver drugs precisely to a target site, thereby increasing drug bioavailability and reducing potential adverse effects.
In patients with IBD, impaired mitochondrial function leads to excessive ROS production, causing colonic mucosal damage and gut flora imbalance. Mitochondria-targeting antioxidants can scavenge ROS to protect mitochondria and maintain epithelial barrier function. However, the limited delivery specificity to the colon and the constraints of single-factor treatments restrict the efficacy of current therapeutics. Determining how to deliver drugs to the mitochondria of colon cells is crucial for optimizing treatment. Yuan et al54 developed an orally hierarchical delivery system (Res MT Lip@Gel) that regulates the intestinal microbiota and protects mitochondria to alleviate symptoms of IBD. The system uses mitochondria-targeting liposomes loaded with resveratrol and inulin gel. The liposomes are modified with the ALD5MTS sequence to target macrophages and epithelial cell mitochondria in the colon, scavenging ROS and promoting wound healing. Moreover, inulin regulates the gut microbiota, enhances probiotic growth, increases butyric acid production, and supports mitochondrial energy. This hierarchical targeted delivery system improves the interaction between host mitochondria and the gut microbiota, significantly alleviating IBD symptoms. Another study developed a dual-functional nanocarrier (AST@EGCG-Cys-TPP) with both mitochondria-targeting capability and glutathione responsiveness, enabling efficient delivery of the anti-inflammatory and antioxidant agents astaxanthin (AST) and epigallocatechin gallate (EGCG). This system precisely targets macrophage mitochondria, effectively alleviates oxidative stress, and reprograms the intestinal immune microenvironment.55 pH/ROS dual-responsive NPs also have potential for treating IBD. Chao et al69 developed a prodrug delivery system (CMCS-B-DMB) for the treatment of IBD, using demethyleneberberine conjugated with carboxymethyl chitosan through boric acid bonds as a pH/ROS dual-response trigger. This amphiphilic conjugate forms self-assembled nanomicelles in solution, exhibits strong anti-inflammatory effects in vivo, and is effective in treating IBD. Similarly, Zeeshan et al70 prepared and evaluated pH-sensitive nanoparticles loaded with glycyrrhizic acid (GA), which effectively delivered GA to inflamed colon tissue and improved mucosal inflammation over time. A nanocarrier with both pH-responsive release and precise mitochondrial targeting abilities not only protect the drug with gastric acid, preventing its degradation under strongly acidic conditions, and improves drug utilization but also greatly enhances the drug’s antioxidant and anti-inflammatory effects. Zhang et al56 designed a nanocarrier, cauliflower-like carrier (CC), composed of caseinate, chitosan-TPP, and sodium alginate. This nanocarrier improved the biocompatibility, stability, and targeted delivery of astaxanthin, effectively alleviating colitis symptoms in DSS-induced mouse models (). Similarly, in another study, hierarchical microspheres encapsulating astaxanthin nanoparticles modified with TPP+ also exhibited mitochondria-targeting capability, further suppressing macrophage-mediated inflammation and demonstrating potent therapeutic effects in IBD models.57
ERS is a key factor in IBD, but precise organelle targeting remains underexplored. Significant progress has been made in oral targeted therapies for inflammatory colon diseases, improving treatment efficacy and minimizing systemic toxicity by selectively targeting affected tissues. For example, Ruiping Zhang’s research team71 developed a biocompatible and IBD-targeting metabolic nanoregulator (TMNR). TMNR, consisting of melanin-gallium complexes encapsulated in thermosensitive, colitis-specific hydrogels, targets inflammatory areas, enhances retention, and provides a physical barrier. It also regulates cell and bacterial metabolism, scavenges ROS, and blocks inflammatory pathways, resulting in broad-spectrum antioxidant effects. Huang et al72 proposed the use of metal-free melanin nanozymes (MeNPs) as a promising treatment for IBD, offering excellent gastrointestinal stability and biocompatibility. MeNPs naturally target IBD lesions and alleviate key pathological features, including oxidative stress, ERS, apoptosis, inflammation, intestinal barrier damage, and microbiota imbalance. Min et al73 developed inflamed colon-targeting antioxidant nanotherapeutics (ICANs). ICANs consist of mesoporous silica NPs (MSNs) and ROS-scavenging cerium oxide NPs (CeNPs) coated with polyacrylic acid (PAA) for improved adhesion to inflamed colon tissue, providing targeted adhesion and ROS scavenging for the treatment of IBD.
Overall, few studies on organelle-targeting nanomedicines exist, as research has focused more on targeting inflammatory sites and cells. Existing studies have led to significant advancements in nanomedicine applications and lay the groundwork for future organelle-targeting research.
Atherosclerosis
AS is a chronic inflammatory disease affecting primarily elastic and muscular arteries. The formation of atherosclerotic plaques (cholesterol) can lead to vascular stenosis, and if the plaques become unstable, they may result in thrombosis, causing ischemic heart disease and stroke, making AS a leading cause of death and disability worldwide.74,75 Inflammation plays a central role throughout the development of AS. Evidence shows that proinflammatory cellular stress, including mitochondrial and ER stress, oxidative stress, inflammasome, autophagy, and heat shock proteins, induces atherosclerotic inflammation.76–81 Studies have confirmed that targeting inflammatory pathways can effectively treat AS and prevent its complications.82 Strategies such as inhibiting proinflammatory cytokines, blocking key inflammatory signaling pathways, and promoting inflammation resolution have been applied in anti-AS therapy. Additionally, NPs have been employed in AS treatment to promote cholesterol clearance, exert anti-inflammatory effects, mitigate oxidative stress, and inhibit aging.
Mitochondrial homeostasis is crucial in AS progression. Proteomic studies revealed a significant reduction in mitochondrial protein abundance during early AS.81 Mitochondria-targeting nanomedicines, created by conjugating the TPP-targeting moiety with anti-inflammatory and antioxidant agents, can concentrate in mitochondria, enhancing their function and reducing inflammation. Synthetic TPP derivatives, such as MitoQ and Mito-Esc, show promise in treating vascular injury, significantly reducing angiotensin II–induced plaque formation and delaying AS-related aging in long-term studies in mice.58,59 Liu et al60 developed nanoparticles by conjugating TPP+ with hydroxytyrosol, enabling hydroxytyrosol to cross biological membranes and accumulate in mitochondria, effectively protecting against hyperlipidemia-induced endothelial damage. Similarly, Banik et al61 designed mitochondria-targeting high-density lipoprotein (HDL)-mimicking polymer-lipid hybrid nanoparticles (T-HDL-NPs). These NPs consist of a hydrophobic core made of cholesteryl oleate and biodegradable polylactide-co-glycolic acid (PLGA) coated with a lipid layer containing stearyl-TPP for mitochondrial targeting. This design enhances extracellular and intracellular cholesterol transport, optimizes cholesterol binding and release, and has significant lipid-lowering and anti-inflammatory effects. In addition, a recent study developed an ROS-responsive simvastatin nanoprodrug (OPDH-SV) using a zwitterionic polymer.83 Although OPDH-SV does not carry a typical mitochondria-targeting moiety, the ROS-triggered release enables the drug to function effectively in oxidative environments; moreover, it achieves intercellular transport and mitochondrial targeting through a cellular “endocytosis-exocytosis” mechanism, thereby supporting mitochondrial homeostasis and demonstrating potent anti-inflammatory and lipid-lowering effects for the treatment of atherosclerosis. Notably, plaque regions are often characterized by a hypoxic microenvironment, which exacerbates mitochondrial dysfunction and triggers immune-inflammatory responses. A recently proposed nanotherapeutic strategy that remains effective under hypoxia offers new insights into organelle-targeting treatments for hypoxia-associated inflammatory diseases such as atherosclerosis.84 In addition to the conventional mitochondria-targeting moiety TPP, the mitochondria-targeting peptide SS-31 has also demonstrated promising therapeutic potential. Studies have shown that SS-31 effectively reduces atherosclerotic plaque size, suppresses oxidative stress, and significantly alleviates systemic inflammation, indicating its potential as a candidate drug for the prevention and treatment of atherosclerosis.62
Furthermore, emerging evidence highlights a causal link between lysosomal cholesterol accumulation and inflammation, indicating that a reduction in lysosomal cholesterol is a key target in the treatment of AS. During AS progression, monocytes are recruited beneath the vascular intima, where they differentiate into macrophages to clear residual lipids and cell debris. Lysosomal enzymes hydrolyze cholesterol esters into free cholesterol and fatty acids. However, excessive uptake of poorly hydrolyzable lipoproteins, such as oxidized low-density lipoprotein (ox-LDL), leads to lysosomal accumulation of unmetabolized cholesterol esters and free cholesterol; this impairs lysosomal and macrophage function, triggers the production of inflammatory mediators, exacerbates vascular inflammation, and accelerates AS progression. Since lysosomal function relies on an acidic environment, disruption of the H+ gradient compromises enzymatic activity. Restoring lysosomal acidity to enhance the degradation capacity of macrophages and mitigate AS represents a promising therapeutic approach. Zhang et al63 developed an acidic NP delivery system that targets macrophage lysosomes and maintains their acidic pH. When PLGA-based NPs are used as carriers, the system effectively restores macrophage autophagy-lysosomal degradation, enhances lysosomal function, and reduces apoptosis and inflammasome activation.
Long-term ERS is an important reason for the apoptosis of macrophages and possibly endothelial cells in advanced lesions.85 The additional ERS-mediated proinflammatory effects in these cells may also affect the formation of early AS.86 Unfortunately, due to the technical challenges of targeting organelles, research focused on ER-specific nanotherapeutics remains limited and has slow progress. Nevertheless, strategies aimed at alleviating ERS and other organelle dysfunctions through responsive nanosystems offer promising approaches for treating vascular injury in various cardiovascular diseases.87
Osteoarthritis
OA is the most common joint disease and is characterized by pain, inflammation, cartilage damage, and limited joint mobility, with its prevalence increasing with age.88 The mechanism of OA involves persistent excessive inflammation and irreversible destruction of cartilage, with mitochondria playing a key role in its pathogenesis. Mitochondrial dysfunction increases the production of ROS, resulting in mitochondrial DNA damage, impaired chondrocyte function, excessive apoptosis, reduced autophagy, and enhanced inflammation.89 As disease-modifying drugs for OA are limited, targeting oxidative stress pathways is a promising therapeutic strategy. Antioxidants such as vitamin C, vitamin E, glutathione, and plant polyphenols are emerging as potential treatments to reduce ROS and slow OA progression.90 Recent studies have shown that precious metals, metal oxides, carbides, and other nanoenzymes with the ability to scavenge ROS have great potential for the treatment of inflammatory diseases. Compared with natural enzymes, nanoenzymes have better resistance to enzymatic degradation and stability in vivo.91 Li’s team64 proposed a novel nanoenzyme, Mn3O4@PDA@Pd-SS31, which targets mitochondria, responds to near-infrared light, and scavenges mitochondrial ROS. This nanoenzyme restores mitochondrial function, reduces oxidative stress, inhibits inflammation, and promotes cartilage regeneration in OA (). Building on this, the team synthesized a new multifunctional Mn3O4/UIO-TPP nanoenzyme combining a Mn3O4 core and a TPP-targeting group to scavenge ROS and repair mitochondrial function. In cell and animal models, Mn3O4/UIO-TPP effectively reduced mitochondrial dysfunction, inflammation, and oxidative DNA damage, indicating its potential for OA treatment.65 Additionally, Huang et al92 developed a mitochondria-targeting rhein nanoprodrug (RPT NP) as a potential therapeutic option for OA. By covalently coupling rhein with TPP-PEG, the self-assembled NPs can increase the accumulation of rhein in mitochondria, boosting its antioxidant effects, inhibiting chondrocyte apoptosis, and slowing OA progression. Mitochondrial calcium overload is also an important driver of the inflammatory response and cellular dysfunction in osteoarthritis. Excessive calcium accumulation leads to loss of mitochondrial membrane potential, excessive production of ROS, and the development of energy metabolism disorders, thereby activating proinflammatory pathways and exacerbating joint tissue damage. A multifunctional mesoporous silica-based nanoparticle system (METP), modified with EGTA, TPP, and PEG, was shown to effectively target mitochondria and chelate excess calcium ions, thereby restoring mitochondrial calcium ion homeostasis.66 This approach reprogrammed the proinflammatory phenotype of macrophages, inhibited ROS production and mitochondrial metabolic shunts, and ultimately alleviated tissue inflammation associated with osteoarthritis, highlighting its therapeutic potential.
Advancements in nanotechnology and a better understanding of the OA microenvironment have led to the development of stimuli-responsive NP delivery systems targeting microenvironment signals in the joints of patients with OA. Specifically, pH-responsive NPs have been designed to exploit the weakly acidic environment of the joints of patients with OA. These systems remain stable under physiological conditions and release drugs explosively in acidic environments, significantly enhancing therapeutic effects. Zhang et al93 reported a pH-responsive MOF (MIL-101-NH2) for codelivering curcumin and small interfering RNA. Confocal laser scanning microscopy revealed that MIL-101-NH2@CCM-siCy5 could escape lysosomal capture and accumulate in the cytoplasm, with high concentrations of phosphate ions in the lysosome triggering the release of Fe³+, destabilizing the MOF structure and allowing interfering RNA to avoid enzyme degradation and escape. In summary, pH-responsive NP delivery systems offer a promising approach for improving drug delivery and therapeutic outcomes in osteoarthritis by exploiting the acidic environment of the joints of patients with OA.
Conclusion
Inflammatory responses are central pathological processes in various diseases, with organelles such as mitochondria, the ER, lysosomes, and the Golgi apparatus playing pivotal roles. Despite existing challenges, organelle-targeting nanomedicines have demonstrated significant potential in mitigating inflammation and elucidating its mechanisms. This review discusses the main nanocarriers and drugs targeting these four key organelles and summarizes the application of organelle-targeting nanotherapeutics for the treatment of sepsis, inflammatory bowel disease, atherosclerosis, and osteoarthritis, providing new perspectives and strategies for the treatment of inflammatory diseases.
As organelle dysfunction emerges as a key driver of inflammation, organelle-targeting nanomedicines offer significant potential for treating inflammation-related diseases through precision and versatility. The precise identification of inflammatory pathways at the subcellular level holds promise for revolutionizing diagnostics and informing new clinical guidelines. Additionally, organelle-targeting nanomedicines can increase therapeutic efficiency and specificity, advance precision medicine, and potentially reduce treatment costs.
While much attention has been given to mitochondria, the endoplasmic reticulum, lysosomes, and the Golgi apparatus in organelle-targeting strategies for treating inflammatory diseases, the importance of other organelles should not be overlooked.94 Notably, the nucleus also plays a pivotal role in regulating inflammation and cell fate. In recent years, nuclear-targeting nanodelivery systems have garnered increasing attention in cancer and gene therapy, with relevant reviews (eg, PMID: 38857762) summarizing peptide- and polymer-based nuclear localization strategies and their therapeutic potential. However, in inflammatory diseases such as sepsis, IBD, atherosclerosis, and osteoarthritis, nanodelivery research has focused primarily on organelles that are more directly involved in inflammatory regulation, including mitochondria, the endoplasmic reticulum, lysosomes, and the Golgi apparatus. Nuclear targeting remains underexplored in these contexts, possibly due to the lack of inflammation-specific nuclear markers, technical barriers to nuclear entry, and limited mechanistic understanding. Therefore, while this review emphasizes organelles with more established roles in inflammation, we also acknowledge the nucleus as a promising but underdeveloped target worthy of further investigation.
As the field continues to advance, it is important to recognize that organelle-targeting nanotherapeutics for inflammatory diseases, despite their potential, still face multiple technical and translational challenges. Here, we provide a brief perspective on the persistent challenges: (1) The dynamic and crowded intracellular environment poses significant challenges for organelle-specific drug delivery. While current research focuses on organelle-targeting nanomedicines, poor retention within organelles remains unresolved. Most nanomedicines enter cells via clathrin-mediated endocytosis but are often degraded in endosomes or lysosomes, highlighting the need to optimize endosomal escape mechanisms. The unique functions, membrane properties, and defenses of organelles such as mitochondria, the ER, and the Golgi apparatus require careful design consideration. Additionally, the development of stimuli-responsive nanodelivery systems to enhance targeting precision, regulate drug release, and minimize leakage during circulation remains a key research focus. (2) Current research on organelle targeting focuses largely on its effects in mitigating inflammation, with limited exploration of the underlying mechanisms, including drug-organelle interactions, uptake, and intracellular activity. Future studies should delve deeper into molecular regulatory mechanisms and identify new molecular targets to enable more precise and effective treatments for inflammatory diseases. (3) Timely suppression of excessive inflammation is crucial for health and survival. Inflammation resolution involves multiple mechanisms, including blocking proinflammatory pathways, producing anti-inflammatory mediators, and clearing or relocating inflammatory cells. Studies suggest that targeting a single proinflammatory mediator is often insufficient to halt or reverse inflammation progression. Given the coordinated interactions between organelles, developing combined organelle-targeting therapies represents a key direction in organelle-targeting nanomedicine research. Furthermore, intervention strategies integrating multiple cell types and multilevel targets are expected to achieve more comprehensive and precise inflammation regulation at the organelle level. Notably, enhancing the targeting specificity of nanodelivery systems toward particular cell types (eg, immune cells) is a critical prerequisite for efficient organelle delivery.95 Integrating cell-specific targeting with functional payload delivery further augments targeting precision and holds great promise for inflammatory modulation.96 (4) While some nanoparticles show promise in vitro and in animal studies, challenges remain for clinical application. Nanomaterials may accumulate in unintended tissues, posing risks of long-term toxicity or immunogenicity. Future research should focus on developing simpler, more efficient nanoparticles, prioritizing biodegradable and biocompatible materials (such as lipid- or polymer-based systems), and conducting comprehensive safety evaluations, including long-term in vivo studies, to meet the specificity and safety requirements for inflammation treatment.
Future Perspectives
Although this review focuses on inflammation, advancements in organelle-targeting strategies hold promise for developing more efficient and safer diagnostic and therapeutic approaches across various fields. Organelle-targeting nanomedicine may become a cornerstone of precision medicine, evolving from single-target therapies to system-level approaches such as multiorganelle delivery platforms to restore mitochondrial and lysosomal function or regulate ER stress and Golgi dysfunction. Combined with advanced diagnostic technologies, organelle-specific biomarkers, and imaging tools, this strategy could enable precise and rapid diagnosis, particularly in the early stages of diseases such as atherosclerosis. Additionally, organelle-targeting therapies are expected to evolve toward personalization, with nanoparticles tailored to patient profiles and cellular environments, driving the realization of individualized precision medicine. Furthermore, the integration of nanotechnology advancements with artificial intelligence has the potential to bridge the gap between laboratory research and clinical applications. Machine learning algorithms can be utilized to optimize nanoparticle design and drug delivery pathways, simulating how organelle-targeting therapies interact with cells to predict efficacy and side effects, thereby driving progress in nanomedicine.
In summary, organelle-targeting nanomedicine offers a novel approach for treating inflammatory diseases and has the potential to revolutionize diagnostics and therapies across various fields. While challenges remain in clinical translation, advancements in technology are expected to overcome these obstacles. In the future, organelle-targeting therapies will integrate deeply with precision medicine, enabling personalized nanoparticle design and combining diagnostic and therapeutic functions. This approach could transform the management of complex diseases, bridging the gap between exploratory research and large-scale clinical application.
Acknowledgments
This work was supported in part by the Construction of Traditional Chinese Medicine Inheritance and Innovation Development Demonstration Pilot Projects in Pudong New Area - High-Level Research-Oriented Traditional Chinese Medicine Hospital Construction Project (YC-2023-0901).
The organelle images used in the Graphical Abstract and Figure 1 were adapted from Servier Medical Art (https://smart.servier.com/), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).97
Disclosure
The authors declare no conflict of interest, financial or otherwise.
References
1. Medzhitov R. The spectrum of inflammatory responses. Science. 2021;374(6571):1070–1075. doi:10.1126/science.abi5200
2. Hazra N, Ray R, Banerjee A. Rapid targeting and imaging of mitochondria via carbon dots using an amino acid-based amphiphile as a carrier. Nanoscale. 2024;16(20):9827–9835. doi:10.1039/d4nr00665h
3. Angus DC, van der Poll T. Severe Sepsis and Septic Shock. N Engl J Med. 2013;369(9):840–851. doi:10.1056/NEJMra1208623
4. Giamarellos-Bourboulis EJ, Aschenbrenner AC, Bauer M, et al. The pathophysiology of sepsis and precision-medicine-based immunotherapy. Nat Immunol. 2024;25(1):19–28. doi:10.1038/s41590-023-01660-5
5. Shi A-X, Qu Q, Zhuang -H-H, et al. Individualized antibiotic dosage regimens for patients with augmented renal clearance: review. Front Pharmacol. 2023;14:1137975. doi:10.3389/fphar.2023.1137975
6. Momtazi-Borojeni AA, Sabouri-Rad S, Gotto AM, et al. PCSK9 and inflammation: a review of experimental and clinical evidence. Eur Heart J Cardiovasc Pharmacother. 2019;5(4):237–245. doi:10.1093/ehjcvp/pvz022
7. Hazel K, O’Connor A. Emerging treatments for inflammatory bowel disease. Ther Adv Chronic Dis. 2020;11:2040622319899297. doi:10.1177/2040622319899297
8. Smakaj A, Gasbarra E, Cardelli T, et al. Exploring Intra-Articular Administration of Monoclonal Antibodies as a Novel Approach to Osteoarthritis Treatment: a Systematic Review. Biomedicines. 2024;12(10):2217. doi:10.3390/biomedicines12102217
9. Kushwaha A, Kumar V, Agarwal V. Pseudomonas quinolone signal induces organelle stress and dysregulates inflammation in human macrophages. Biochim Biophys Acta Gen Subj. 2023;1867(2):130269. doi:10.1016/j.bbagen.2022.130269
10. Peng Y, Bariwal J, Kumar V, et al. Organic Nanocarriers for Delivery and Targeting of Therapeutic Agents for Cancer Treatment. Adv Ther. 2020;3(2):1900136. doi:10.1002/adtp.201900136
11. Xu Z, Chen X, Sun Z, et al. Recent progress on mitochondrial targeted cancer therapy based on inorganic nanomaterials. Mater Today Chem. 2019;12:240–260. doi:10.1016/J.MTCHEM.2019.02.004
12. Zielonka J, Joseph J, Sikora A, et al. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem Rev. 2017;117(15):10043–10120. doi:10.1021/acs.chemrev.7b00042
13. Pawar A, Korake S, Gajbhiye KR. Dequalinium-Derived Nanoconstructs: a Promising Vehicle for Mitochondrial Targeting. Curr Drug Deliv. 2021;18(8):1056–1063. doi:10.2174/1567201818999210120201252
14. Forster S, Thumser AE, Hood SR, et al. Characterization of rhodamine-123 as a tracer dye for use in in vitro drug transport assays. PLoS One. 2012;7(3):e33253. doi:10.1371/journal.pone.0033253
15. Sivandzade F, Bhalerao A, Cucullo L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Biol Protoc. 2019;9(1):e3128. doi:10.21769/BioProtoc.3128
16. Xie S, Cong Z, Wang W, et al. Mitochondria-targeting NIR AIEgens with cationic amphiphilic character for imaging and efficient photodynamic therapy. Chem Commun. 2023;59(18):2592–2595. doi:10.1039/d2cc06457j
17. Dubinin MV, Semenova AA, Nedopekina DA, et al. Effect of F16-Betulin Conjugate on Mitochondrial Membranes and Its Role in Cell Death Initiation. Membranes. 2021;11(5):352. doi:10.3390/membranes11050352
18. Avendaño-Monsalve MC, Mendoza-Martínez AE, Ponce-Rojas JC, et al. Positively charged amino acids at the N terminus of select mitochondrial proteins mediate early recognition by import proteins αβ’-NAC and Sam37. J Biol Chem. 2022;298(6):101984. doi:10.1016/j.jbc.2022.101984
19. Birk AV, Chao WM, Bracken C, et al. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol. 2014;171(8):2017–2028. doi:10.1111/bph.12468
20. Xun Z, Wipf P, McMurray CT. XJB-5-131 Is a Mild Uncoupler of Oxidative Phosphorylation. J Huntingtons Dis. 2022;11(2):141–151. doi:10.3233/jhd-220539
21. Ko JK, Choi KH, Peng J, et al. Amphipathic tail-anchoring peptide and Bcl-2 homology domain-3 (BH3) peptides from Bcl-2 family proteins induce apoptosis through different mechanisms. J Biol Chem. 2011;286(11):9038–9048. doi:10.1074/jbc.M110.198457
22. Choi JW, Choi SH, Hong ST, et al. Two-photon probes for the endoplasmic reticulum: its detection in a live tissue by two-photon microscopy. Chem Commun. 2020;56(25):3657–3660. doi:10.1039/d0cc00236d
23. Ghosh C, Nandi A, Basu S. Supramolecular self-assembly of triazine-based small molecules: targeting the endoplasmic reticulum in cancer cells. Nanoscale. 2019;11(7):3326–3335. doi:10.1039/c8nr08682f
24. Wan J, Sun L, Wu P, et al. Synthesis of indocyanine green functionalized comblike poly(aspartic acid) derivatives for enhanced cancer cell ablation by targeting the endoplasmic reticulum. Polym Chem. 2018;9(10):1206–1215. doi:10.1039/C7PY01994G
25. Wang G, Norton AS, Pokharel D, et al. KDEL peptide gold nanoconstructs: promising nanoplatforms for drug delivery. Nanomedicine. 2013;9(3):366–374. doi:10.1016/j.nano.2012.09.002
26. Stornaiuolo M, Lotti LV, Borgese N, et al. KDEL and KKXX retrieval signals appended to the same reporter protein determine different trafficking between endoplasmic reticulum, intermediate compartment, and Golgi complex. Mol Biol Cell. 2003;14(3):889–902. doi:10.1091/mbc.e02-08-0468
27. Liaci AM, Förster F. Take Me Home, Protein Roads: structural Insights into Signal Peptide Interactions during ER Translocation. Int J Mol Sci. 2021;22(21):11871. doi:10.3390/ijms222111871
28. Matsuo K, Yoshikawa T, Oda A, et al. Efficient generation of antigen-specific cellular immunity by vaccination with poly(gamma-glutamic acid) nanoparticles entrapping endoplasmic reticulum-targeted peptides. Biochem Biophys Res Commun. 2007;362(4):1069–1072. doi:10.1016/j.bbrc.2007.08.112
29. Huang TC, Lee JF, Chen JY. Pardaxin, an antimicrobial peptide, triggers caspase-dependent and ROS-mediated apoptosis in HT-1080 cells. Mar Drugs. 2011;9(10):1995–2009. doi:10.3390/md9101995
30. Borkowska M, Siek M, Kolygina DV, et al. Targeted crystallization of mixed-charge nanoparticles in lysosomes induces selective death of cancer cells. Nature Nanotechnol. 2020;15(4):331–341.
31. Lear JD, DeGrado WF. Membrane binding and conformational properties of peptides representing the NH2 terminus of influenza HA-2. J Biol Chem. 1987;262(14):6500–6505. doi:10.1016/S0021-9258(18)48270-1
32. Goormaghtigh E, De Meutter J, Szoka F, et al. Secondary structure and orientation of the amphipathic peptide GALA in lipid structures. An infrared-spectroscopic approach. Eur J Biochem. 1991;195(2):421–429. doi:10.1111/j.1432-1033.1991.tb15721.x
33. Salomone F, Cardarelli F, Di Luca M, et al. A novel chimeric cell-penetrating peptide with membrane-disruptive properties for efficient endosomal escape. J Control Release. 2012;163(3):293–303. doi:10.1016/j.jconrel.2012.09.019
34. El-Sayed A, Masuda T, Khalil I, et al. Enhanced gene expression by a novel stearylated INF7 peptide derivative through fusion independent endosomal escape. J Control Release. 2009;138(2):160–167. doi:10.1016/j.jconrel.2009.05.018
35. Lee H, Dam DH, Ha JW, et al. Enhanced Human Epidermal Growth Factor Receptor 2 Degradation in Breast Cancer Cells by Lysosome-Targeting Gold Nanoconstructs. ACS Nano. 2015;9(10):9859–9867. doi:10.1021/acsnano.5b05138
36. Iturrioz-Rodríguez N, González-Domínguez E, González-Lavado E, et al. A Biomimetic Escape Strategy for Cytoplasm Invasion by Synthetic Particles. Angew Chem Int Ed Engl. 2017;56(44):13736–13740. doi:10.1002/anie.201707769
37. Luo J, Zhang P, Zhao T, et al. Golgi apparatus-targeted chondroitin-modified nanomicelles suppress hepatic stellate cell activation for the management of liver fibrosis. ACS Nano. 2019;13(4):3910–3923.
38. Li H, Zhang P, Luo J, et al. Chondroitin Sulfate-Linked Prodrug Nanoparticles Target the Golgi Apparatus for Cancer Metastasis Treatment. ACS Nano. 2019;13(8):9386–9396. doi:10.1021/acsnano.9b04166
39. Keller T, Trinks N, Brand J, et al. Design of Nanohydrogels for Targeted Intracellular Drug Transport to the Trans-Golgi Network. Adv Healthc Mater. 2023;12(13):e2201794. doi:10.1002/adhm.202201794
40. Li RS, Liu J, Shi H, et al. Transformable Helical Self-Assembly for Cancerous Golgi Apparatus Disruption. Nano Lett. 2021;21(19):8455–8465. doi:10.1021/acs.nanolett.1c03112
41. Tan W, Zhang Q, Wang J, et al. Enzymatic Assemblies of Thiophosphopeptides Instantly Target Golgi Apparatus and Selectively Kill Cancer Cells*. Angew Chem Int Ed Engl. 2021;60(23):12796–12801. doi:10.1002/anie.202102601
42. Keskin A, Akdoğan E, Dunn CD. Evidence for Amino Acid Snorkeling from a High-Resolution, In Vivo Analysis of Fis1 Tail-Anchor Insertion at the Mitochondrial Outer Membrane. Genetics. 2017;205(2):691–705. doi:10.1534/genetics.116.196428
43. Fu YM, Zhang H, Ding M, et al. Selective amino acid restriction targets mitochondria to induce apoptosis of androgen-independent prostate cancer cells. J Cell Physiol. 2006;209(2):522–534. doi:10.1002/jcp.20766
44. Jeena MT, Palanikumar L, Go EM, et al. Mitochondria localization induced self-assembly of peptide amphiphiles for cellular dysfunction. Nat Commun. 2017;8(1):26. doi:10.1038/s41467-017-00047-z
45. Nguyen SV, Levintov L, Planalp RP, et al. Interactions and Transport of a Bioconjugated Peptide Targeting the Mitomembrane. Bioconjug Chem. 2024;35(3):371–380. doi:10.1021/acs.bioconjchem.3c00561
46. Biswas N, Bhattacharya R, Saha A, et al. Interplay of electrostatics and lipid packing determines the binding of charged polymer coated nanoparticles to model membranes. Phys Chem Chem Phys. 2015;17(37):24238–24247.
47. Mikami T, Kitagawa H. Chondroitin sulfate glycosaminoglycans function as extra/pericellular ligands for cell surface receptors. J Biochem. 2023;173(5):329–332. doi:10.1093/jb/mvac110
48. Yu H, Jin F, Liu D, et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics. 2020;10(5):2342–2357. doi:10.7150/thno.40395
49. She H, Tan L, Du Y, et al. VDAC2 malonylation participates in sepsis-induced myocardial dysfunction via mitochondrial-related ferroptosis. Int J Biol Sci. 2023;19(10):3143–3158. doi:10.7150/ijbs.84613
50. Fan L, Yao L, Li Z, et al. Exosome‐Based Mitochondrial Delivery of circRNA mSCAR Alleviates Sepsis by Orchestrating Macrophage Activation. Adv Sci. 2023;10(14):202205692. doi:10.1002/advs.202205692
51. Zhao Y, Liu S, Shi Z, et al. Pathogen infection-responsive nanoplatform targeting macrophage endoplasmic reticulum for treating life-threatening systemic infection. Nano Res. 2022;15(7):6243–6255. doi:10.1007/s12274-022-4211-z
52. Zhao Q, Gong ZJ, Wang JL, et al. A Zinc- and Calcium-Rich Lysosomal Nanoreactor Rescues Monocyte/Macrophage Dysfunction under Sepsis. Adv Sci. 2023;10(6):5097. doi:10.1002/advs.202205097
53. Xie S, Li Y, Cao W, et al. Dual-Responsive Nanogels with Cascaded Gentamicin Release and Lysosomal Escape to Combat Intracellular Small Colony Variants for Peritonitis and Sepsis Therapies. Adv Healthc Mater. 2024;13(14):e2303671. doi:10.1002/adhm.202303671
54. Xing L, Liu X, Wu L, et al. Orally hierarchical targeting delivery systems relieve colitis by protecting host mitochondria and modulating gut microbiota. Nano Today. 2024;55:102155. doi:10.1016/j.nantod.2024.102155
55. Zhang X, Su W, Chen Y, et al. Bi-functional astaxanthin macromolecular nanocarriers to alleviate dextran sodium sulfate-induced inflammatory bowel disease. Int J Biol Macromol. 2024;256(Pt 2):128494. doi:10.1016/j.ijbiomac.2023.128494
56. Zhang X, Zhao X, Tie S, et al. A smart cauliflower-like carrier for astaxanthin delivery to relieve colon inflammation. J Control Release. 2022;342:372–387. doi:10.1016/j.jconrel.2022.01.014
57. Xi PP, Wei XY, Xu YX, et al. Bioinspired micro-nano hierarchical delivery system for sequential targeted therapy of inflammatory bowel disease. Chem Eng J. 2024:498155215. doi:10.1016/j.cej.2024.155215.
58. Gioscia-Ryan RA, Battson ML, Cuevas LM, et al. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol. 2018;124(5):1194–1202. doi:10.1152/japplphysiol.00670.2017
59. Pulipaka S, Singuru G, Sahoo S, et al. Therapeutic efficacies of mitochondria-targeted esculetin and metformin in the improvement of age-associated atherosclerosis via regulating AMPK activation. GeroScience. 2023;46(2):2391–2408. doi:10.1007/s11357-023-01015-w
60. Liu X, Gao J, Yan Y, et al. Mitochondria-Targeted Triphenylphosphonium-Hydroxytyrosol Prevents Lipotoxicity-Induced Endothelial Injury by Enhancing Mitochondrial Function and Redox Balance via Promoting FoxO1 and Nrf2 Nuclear Translocation and Suppressing Inflammation via Inhibiting p38/NF-кB Pathway. Antioxidants. 2023;12(1):175. doi:10.3390/antiox12010175
61. Banik B, Wen R, Marrache S, et al. Core hydrophobicity tuning of a self-assembled particle results in efficient lipid reduction and favorable organ distribution. Nanoscale. 2017;10(1):366–377. doi:10.1039/c7nr06295h
62. Zhang M, Zhao H, Cai J, et al. Chronic administration of mitochondrion-targeted peptide SS-31 prevents atherosclerotic development in ApoE knockout mice fed Western diet. PLoS One. 2017;12(9):e0185688. doi:10.1371/journal.pone.0185688
63. Zhang X, Misra SK, Moitra P, et al. Use of acidic nanoparticles to rescue macrophage lysosomal dysfunction in atherosclerosis. Autophagy. 2023;19(3):886–903. doi:10.1080/15548627.2022.2108252
64. Li Y, Yang J, Chen X, et al. Mitochondrial-targeting and NIR-responsive Mn(3)O(4)@PDA@Pd-SS31 nanozymes reduce oxidative stress and reverse mitochondrial dysfunction to alleviate osteoarthritis. Biomaterials. 2024;305:122449. doi:10.1016/j.biomaterials.2023.122449
65. Zhang S, Cai J, Yao Y, et al. Mitochondrial-targeting Mn3O4/UIO-TPP nanozyme scavenge ROS to restore mitochondrial function for osteoarthritis therapy. Regen Biomater. 2023;10:rbad078. doi:10.1093/rb/rbad078
66. Lei X, Tan G, Wang Y, et al. Mitochondrial Calcium Nanoregulators Reverse the Macrophage Proinflammatory Phenotype Through Restoring Mitochondrial Calcium Homeostasis for the Treatment of Osteoarthritis. Int J Nanomed. 2023;18:1469–1489. doi:10.2147/ijn.S402170
67. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
68. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi:10.1016/S0140-6736(19)32989-7
69. Guo S, Shen C, Chen T, et al. A stimuli-responsive demethyleneberberine-conjugated carboxylmethyl chitosan prodrug for treatment of inflammatory bowel diseases. Mater Lett. 2024;357:135730. doi:10.1016/j.matlet.2023.135730
70. Zeeshan M, Ali H, Khan S, et al. Glycyrrhizic acid-loaded pH-sensitive poly-(lactic-co-glycolic acid) nanoparticles for the amelioration of inflammatory bowel disease. Nanomedicine. 2019;14(15):1945–1969. doi:10.2217/nnm-2018-0415
71. Xu T, Ning X, Wu J, et al. Metabolic Nanoregulator Remodels Gut Microenvironment for Treatment of Inflammatory Bowel Disease. ACS Nano. 2024;18(9):7123–7135. doi:10.1021/acsnano.3c11496
72. Huang Q, Yang Y, Zhu Y, et al. Oral Metal-Free Melanin Nanozymes for Natural and Durable Targeted Treatment of Inflammatory Bowel Disease (IBD). Small. 2023;19(19):e2207350. doi:10.1002/smll.202207350
73. Min DK, Kim YE, Kim MK, et al. Orally Administrated Inflamed Colon-Targeted Nanotherapeutics for Inflammatory Bowel Disease Treatment by Oxidative Stress Level Modulation in Colitis. ACS Nano. 2023;17(23):24404–24416. doi:10.1021/acsnano.3c11089
74. Gusev E, Sarapultsev A. Atherosclerosis and Inflammation: insights from the Theory of General Pathological Processes. Int J Mol Sci. 2023;24(9):7910. doi:10.3390/ijms24097910
75. Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J Am Coll Cardiol. 2020;76(25):2982–3021. doi:10.1016/j.jacc.2020.11.010
76. Sukhorukov VN, Khotina VA, Bagheri Ekta M, et al. Endoplasmic Reticulum Stress in Macrophages: the Vicious Circle of Lipid Accumulation and Pro-Inflammatory Response. Biomedicines. 2020;8(7):210. doi:10.3390/biomedicines8070210
77. Qu K, Yan F, Qin X, et al. Mitochondrial dysfunction in vascular endothelial cells and its role in atherosclerosis. Front Physiol. 2022;13:1084604. doi:10.3389/fphys.2022.1084604
78. Soehnlein O, Tall AR. AIMing 2 treat atherosclerosis. Nat Rev Cardiol. 2022;19(9):567–568. doi:10.1038/s41569-022-00755-0
79. Hua Y, Zhang J, Liu Q, et al. The Induction of Endothelial Autophagy and Its Role in the Development of Atherosclerosis. Front Cardiovasc Med. 2022;9:831847. doi:10.3389/fcvm.2022.831847
80. Poznyak AV, Orekhova VA, Sukhorukov VN, et al. Atheroprotective Aspects of Heat Shock Proteins. Int J Mol Sci. 2023;24(14):11750. doi:10.3390/ijms241411750
81. Herrington DM, Mao C, Parker SJ, et al. Proteomic Architecture of Human Coronary and Aortic Atherosclerosis. Circulation. 2018;137(25):2741–2756. doi:10.1161/CIRCULATIONAHA.118.034365
82. Kong P, Cui Z-Y, Huang X-F, et al. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduc Target Ther. 2022;7:131. doi:10.1038/s41392-022-00955-7
83. Yang H, Guo M, Guan Q, et al. ROS-responsive simvastatin nano-prodrug based on tertiary amine-oxide zwitterionic polymer for atherosclerotic therapy. J Nanobiotechnol. 2025;23(1):176. doi:10.1186/s12951-025-03232-1
84. Chen Y, Deng Y, Li Y, et al. Oxygen-Independent Radiodynamic Therapy: radiation-Boosted Chemodynamics for Reprogramming the Tumor Immune Environment and Enhancing Antitumor Immune Response. ACS Appl Mater Interfaces. 2024;16(17):21546–21556. doi:10.1021/acsami.4c00793
85. Huang A, Patel S, McAlpine C, Werstuck G. The Role of Endoplasmic Reticulum Stress-Glycogen Synthase Kinase-3 Signaling in Atherogenesis. Int J Mol Sci. 2018;19(6):1607. doi:10.3390/ijms19061607
86. McAlpine CS, Werstuck GH. The development and progression of atherosclerosis: evidence supporting a role for endoplasmic reticulum (ER) stress signaling. Cardiovasc Hematol Disord Drug Targets. 2013;13(2):158–164. doi:10.2174/1871529x11313020009
87. Li J, Zhang J, Yu P, et al. ROS-responsive & scavenging NO nanomedicine for vascular diseases treatment by inhibiting endoplasmic reticulum stress and improving NO bioavailability. Bioact Mater. 2024;37:239–252. doi:10.1016/j.bioactmat.2024.03.010
88. Mandl LA. Osteoarthritis year in review 2018: clinical. Osteoarthritis Cartilage. 2019;27(3):359–364. doi:10.1016/j.joca.2018.11.001
89. Blanco FJ, Rego-Pérez I. Mitochondria and mitophagy: biosensors for cartilage degradation and osteoarthritis. Osteoarthritis Cartilage. 2018;26(8):989–991. doi:10.1016/j.joca.2018.05.018
90. Mobasheri A, Biesalski HK, Shakibaei M, et al. Antioxidants and Osteoarthritis. In:Systems Biology of Free Radicals and Antioxidants (Berlin, Heidelberg: Springer Berlin Heidelberg). 2014:2997–3026. doi:10.1007/978-3-642-30018-9_130
91. Huang Y, Ren J, Qu X. Nanozymes: classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem Rev. 2019;119(6):4357–4412. doi:10.1021/acs.chemrev.8b00672
92. Huang H, Yang L, He H, et al. Construction of mitochondrial-targeting nano-prodrug for enhanced Rhein delivery and treatment for osteoarthritis in vitro. Int J Pharm. 2024;661:124397. doi:10.1016/j.ijpharm.2024.124397
93. Zhang ZJ, Hou YK, Chen MW, et al. A pH-responsive metal-organic framework for the co-delivery of HIF-2α siRNA and curcumin for enhanced therapy of osteoarthritis. J Nanobiotechnol. 2023;21(1):18. doi:10.1186/s12951-022-01758-2
94. Li Z, Fan J, Xiao Y, et al. Essential role of Dhx16-mediated ribosome assembly in maintenance of hematopoietic stem cells. Leukemia. 2024;38(12):2699–2708. doi:10.1038/s41375-024-02423-3
95. Yin D, Zhong Y, Ling S, et al. Dendritic-cell-targeting virus-like particles as potent mRNA vaccine carriers. Nat Biomed Eng. 2025;9(2):185–200. doi:10.1038/s41551-024-01208-4
96. Pei W, Zhang Y, Zhu X, et al. Multitargeted Immunomodulatory Therapy for Viral Myocarditis by Engineered Extracellular Vesicles. ACS Nano. 2024;18(4):2782–2799. doi:10.1021/acsnano.3c05847
97. Servier Medical Art. https://smart.servier.com/
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
How Nanotherapeutic Platforms Play a Key Role in Glioma? A Comprehensive Review of Literature

Yang Y, Cheng N, Luo Q, Shao N, Ma X, Chen J, Luo L, Xiao Z
International Journal of Nanomedicine 2023, 18:3663-3694
Published Date: 3 July 2023

